Abstract
BACKGROUND AND PURPOSE
AMG 139 is a human anti-IL-23 antibody currently in a phase II trial for treating Crohn's disease. To support its clinical development in humans, in vitro assays and in vivo studies were conducted in cynomolgus monkeys to determine the pharmacology, preclinical characteristics and safety of this monoclonal antibody.
EXPERIMENTAL APPROACH
The in vitro pharmacology, pharmacokinetics (PK), pharmacodynamics and toxicology of AMG 139, after single or weekly i.v. or s.c. administration for up to 26 weeks, were evaluated in cynomolgus monkeys.
KEY RESULTS
AMG 139 bound with high affinity to both human and cynomolgus monkey IL-23 and specifically neutralized the biological activity of IL-23 without binding or blocking IL-12. After a single dose, linear PK with s.c. bioavailability of 81% and mean half-life of 8.4–13 days were observed. After weekly s.c. dosing for 3 or 6 months, AMG 139 exposure increased approximately dose-proportionally from 30 to 300 mg·kg−1 and mean accumulation between the first and last dose ranged from 2- to 3.5-fold. Peripheral blood immunophenotyping, T-cell-dependent antigen responses and bone formation markers were not different between AMG 139 and vehicle treatment. No adverse clinical signs, effects on body weight, vital signs, ophthalmic parameters, clinical pathology, ECG, organ weights or histopathology were observed in the monkeys with the highest dose of AMG 139 tested (300 mg·kg−1 s.c. or i.v.).
CONCLUSIONS AND IMPLICATIONS
The in vitro pharmacology, PK, immunogenicity and safety characteristics of AMG 139 in cynomolgus monkeys support its continued clinical development for the treatment of various inflammatory diseases.
Tables of Links
| TARGETS |
|---|
| Other protein targetsa |
| TNF-α |
| Catalytic receptorsb |
| IL-12 receptor |
| IL-23 receptor |
| LIGANDS | |
|---|---|
| CD40 ligand | IL-6 |
| GM-CSF | IL-12 |
| IFN-γ | IL-18 |
| IL-2 | IL-23 |
| IL-4 | Ustekinumab |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,bAlexander et al., 2013a,b).
Introduction
IL-23 is a member of the IL-12 family of heterodimeric cytokines and is composed of the IL-23 specific p19 subunit and the common subunit p40, which it shares with IL-12 (Oppmann et al., 2000; Hunter, 2005; Kastelein et al., 2007). Similarly, the heterodimeric cell surface transmembrane IL-12 and IL-23 receptor complexes share a common subunit, IL-12 receptor β1 subunit, that associates with IL-12 receptor β2 subunit or the IL-23 receptor for IL-12 and IL-23 signalling, respectively, which allows for control of distinct biological pathways.
IL-12 and IL-23 are produced by antigen-presenting cells such as dendritic cells and macrophages in response to inflammation or infection. While IL-12 acts on naïve T-cells to induce their differentiation into T-helper 1 cells which produce IFN-γ and other pro-inflammatory cytokines, IL-23 acts on immune cells, including T-helper 17, γδ T-cells, natural killer (NK) cells, dendritic cells, macrophages and innate lymphoid cells to induce production of cytokines such as IL-17, IL-6, TNF-α and GM-CSF (Langrish et al., 2005; Bettelli et al., 2007; Kastelein et al., 2007; Buonocore et al., 2010; Geremia et al., 2011).
Increased expression of IL-23 is found in the target tissue of inflammatory/autoimmune diseases including Crohn's disease, ulcerative colitis, psoriasis, rheumatoid arthritis and multiple sclerosis (Lee et al., 2004; Schmidt et al., 2005; Vaknin-Dembinsky et al., 2006; Li et al., 2007; Guo et al., 2013). Furthermore, genome-wide association studies and targeted single nucleotide polymorphism analyses have revealed an association between specific polymorphisms in genes encoding the unique IL-23 receptor subunit (IL-23 receptor) and IL-12/23 p40 subunit (IL-12B) and susceptibility to Crohn's disease, ulcerative colitis, psoriasis and psoriatic and rheumatoid arthritis (Duerr et al., 2006; Cargill et al., 2007; Ellinghaus et al., 2012; Song et al., 2012; Zhu et al., 2012).
The involvement of IL-23 in inflammatory/autoimmune disease is strongly supported by animal models demonstrating that IL-23-deficient mice (IL-23p19−/−) are resistant to experimentally induced autoimmune encephalitis and collagen-induced arthritis and that IL-23 neutralizing antibodies are efficacious in a number of inflammatory bowel disease and skin inflammation models (Cua et al., 2003; Murphy et al., 2003; van der Fits et al., 2009; Blumberg et al., 2010; Cox et al., 2012). Clinical trials with ustekinumab and briakinumab, which target the common p40 subunit shared by IL-12 and IL-23, in psoriasis and Crohn's disease, and tildrakizumab and guselkumab, which target the p19 subunit of IL-23, in psoriasis highlight the potential of IL-23 signalling blockade in human disease (Mannon et al., 2004; Kimball et al., 2012; Langley et al., 2012; Sandborn et al., 2012; Traczewski and Rudnicka, 2012; Reichert, 2013).
Studies using IL-12/23p40 and IL-12p35 null mice suggest a dominant role for IL-12 in host defence against intracellular pathogens and in tumour immune surveillance (Fieschi and Casanova, 2003; Bowman et al., 2006; Langowski et al., 2006; Meeran et al., 2006). Consistent with the recognized role of IL-12 in cancer suppression (Meeran et al., 2006; Teng et al., 2012; Yuzhalin and Kutikhin, 2012), emerging data from clinical trials with briakinumab and ustekinumab suggest a possible association between dual inhibition of IL-12 and IL-23 and the development of certain malignancies (Reich et al., 2011; Gordon et al., 2012). While a meta-analysis of four phase II/III randomized ustekinumab trials with up to 4 years of follow-up did not demonstrate an increased risk of malignancies compared with the general or psoriasis populations (Reich et al., 2012), there have been post-marketing reports of rapid appearance of cutaneous squamous cell carcinomas in patients with pre-existing risk factors receiving ustekinumab (Food and Drug Administration, 2014). In contrast to IL-12 inhibition, IL-23 inhibition is associated with a reduced tumour incidence and faster elimination of injected tumour cells (Langowski et al., 2006; Teng et al., 2010).
In addition, safety concerns have been raised for ustekinumab and/or briakinumab regarding the incidence of serious infections and major adverse cardiovascular events, although this is still controversially discussed (Ryan et al., 2011; Langley et al., 2012; Tzellos et al., 2012; 2013; Papp et al., 2013a).
Based on the mechanism of action, experimental data and clinical experience with the IL-12/23p40 antagonists, an IL-23-specific antagonist may provide similar or greater efficacy than the anti-IL-12/23p40 antibodies without the (potential) risks associated with blocking IL-12. Therefore, AMG 139, a human IgG2 monoclonal antibody, was developed to specifically bind to IL-23p19 and block binding of IL-23 to the IL-23 receptor. Currently, this antibody is in or has recently completed clinical trials in patients with Crohn's disease and moderate to severe psoriasis (http://Clinicaltrials.gov NCT01258205 and NCT01094093 respectively).
In this manuscript, we describe the in vitro pharmacology as well as the pharmacokinetic (PK) and safety results from four cynomolgus monkey studies. The data obtained demonstrate that the in vitro pharmacology, PK and safety profile of AMG 139 in cynomolgus monkeys support its clinical development.
Methods
Test article
AMG 139 is a human monoclonal IgG2 antibody. It was manufactured at Amgen Inc. (Thousand Oaks, CA, USA) by expression in a CHO cell line in accordance with Good Manufacturing Practices. For studies in cynomolgus monkeys, the test sample was supplied at a nominal concentration of 70 mg·mL−1 with pharmaceutically acceptable excipients, pH 5.2 and stored at −60 to −80°C. The vehicle control sample was formulated with the same excipients and packaged and stored like the active test sample.
Material
Recombinant human and cynomolgus monkey IL-23 and GM-CSF were generated by the Protein Sciences Department (Amgen Inc., Seattle, WA, USA). Native human IL-23 (huIL-23) was generated by culturing human monocytes in the presence of GM-CSF and IL-4 for 7 days to generate monocyte-derived dendritic cells (MoDCs). MoDCs were then stimulated with CD40 ligand for 48 h, which induced IL-23 but not IFN-γ production. Similarly, native murine IL-23 was generated by culturing murine bone marrow-derived dendritic cells as described for native huIL-23.
IL-2, IL-4, IL-12, IL-18 (human and/or murine), and the IFN-γ (human, mouse and primate) and mouse IL-17 elisa kits were purchased from R&D systems (Minneapolis, MN, USA). Cell culture media and supplements were from Invitrogen (Carlsbad, CA, USA). The luciferase assay system was from Promega (Madison, WI, USA). Fcγ fragment-specific Cy5-conjugated goat anti-human IgG was purchased from Jackson ImmunoResearch (West Grove, PA, USA).
In vitro pharmacology studies
Kinetic exclusion assay (KinExA)
To assess the binding interaction of AMG 139 with human and cynomolgus monkey IL-23, the equilibrium KD was determined using the KinExA method as described previously (Rathanaswami et al., 2008). Briefly, 50 pM AMG 139 was incubated with IL-23 (concentrations ranging from 0.39 to 800 pM) and allowed to equilibrate for 72 h at room temperature. The pre-incubated mixtures were passed over human or cynomolgus monkey IL-23-coated beads mounted in the flow cell of a KinExA machine (Sapidyne Instruments Inc., Boise, ID, USA) to allow capturing of unbound AMG 139 to the solid support. To determine the association rate (Ka), 200 pM AMG 139 was mixed with 300 pM human or cynomolgus monkey IL-23 and the unbound AMG 139 concentrations were measured at fixed time intervals (46.5 min) over 13.3 h using human or cynomolgus monkey IL-23-coated beads, respectively, as described earlier. For both KD and Ka, bead-bound antibodies were detected using Fcγ fragment-specific Cy5-conjugated goat anti-human IgG. The fluorescence signals were measured and the KinExA software was used for curve fitting to obtain KD and Ka values and their 95% confidence intervals (CIs).
COS reporter assay
To determine the neutralizing capacity and species specificity of AMG 139 for IL-23, COS cells stably transfected with huIL-12 receptor β1 subunit and huIL-23 receptor (COS-pool 12) were transiently transfected with a STAT1/4-luciferase reporter construct using FUGENE 6 from Roche (Indianapolis, IN, USA) according to the manufacturer's instructions. After 18 h of transfection, cells were detached using Versene from Invitrogen and seeded in 96-well plates at a density of ∼10 000 cells per well. AMG 139 with serial diluted concentrations was pre-incubated with human native and recombinant, cynomolgus monkey or rat IL-23 at room temperature for 20 min, added to the COS pool-12 cells, at a final concentration of 2 ng·mL−1 for human native and recombinant and cynomolgus monkey IL-23 (the predetermined EC90) or 100 ng·mL−1 rat IL-23 (the predetermined EC65) and 0.02 pM – 84 nM for AMG 139. Then after being incubated at 37°C for 5 h, the culture medium was removed and cells were lysed with Glo Lysis buffer (100 μL per well) at room temperature for 5 min. Fifty microlitres of the lysate from each well were transferred to an assay plate and 50 μL Bright-Glo luciferase substrate were added into each well and luminescence was measured for 5 s on a Microlumat Plus LB luminometer (Berthold, Bad Wildbad, Germany). Medium alone served as a negative control.
NK cell assay
To assess the potency of AMG 139 at inhibiting IL-23-induced bioactivity, IL-2-activated NK cells were stimulated with IL-23 (10 ng·mL−1 recombinant human or cynomolgus monkey, or 20 ng·mL− native human; the predetermined EC50 to EC90) and IL-18 (20 ng·mL−1) in the presence of serially diluted AMG 139. NK cells were isolated from human peripheral blood of healthy donors by negative selection (Miltenyi Biotech, Auburn, CA, USA) and cultured for 7 days in RPMI 1640 and 10% FBS supplemented with 10 ng·mL−1 IL-2. After 7 days, cells were centrifuged and resuspended in medium at a concentration of 1 × 106 cells·mL−1. IL-23 and IL-18 were incubated with AMG 139 for 30 min at room temperature, before the addition of 1 × 105 NK cells per well for final concentrations of 10 ng·mL−1 IL-23, 20 ng·mL−1 IL-18 and 0 to 300 ng·mL−1 AMG 139. After 24 h, IFN-γ in the supernatant was measured using an elisa according to the manufacturer's protocol.
Whole blood assay
To assess AMG 139-mediated inhibition of IL-23-induced activity in whole blood, blood samples from healthy human volunteers and cynomolgus monkeys were collected using Refludan (Bayer, Pittsburgh, PA, USA) as an anticoagulant. The assay was performed as described for the NK cell assay with pre-incubation of recombinant human IL-23 (1 ng·mL−1; the predetermined EC90 value), IL-18 (20 ng·mL−1) and IL-2 (5 ng·mL−1) with 0 to 300 ng·mL−1 AMG 139. The final blood was 60% in volume. For the cynomolgus monkey whole blood assay, recombinant human IL-23 was substituted by recombinant cynomolgus monkey IL-23 (1 ng·mL−1). Human and cynomolgus monkey IFN-γ was measured in the supernatant by elisas according to the manufacturer's protocol.
In vivo studies
Animals
Studies in naïve male and female cynomolgus monkeys (Macaca fascicularis; 3 to 8.8 years old; 2.6 to 9.1 kg for male and 2.1 to 5.4 kg for female) were conducted at facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (Frederick, MD, USA) based on regulations outlined in the United States Department of Agriculture Animal Welfare Act (Title 9, Code of Federal Regulations Parts 1, 2 and 3) (Animal and Plant Health Inspection Service USA, 2005), the conditions specified in the Guide for the Care and Use of Laboratory Animals (National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals et al., 2011) and in compliance with the Testing Facilities' Animal Welfare Assurance (A4112-01) on file at the National Institute of Health. The protocols were reviewed and approved by the Testing Facilities' Institutional Animal Care and Use Committee. The multiple dose studies were conducted under current Good Laboratory Practice (GLP) standards (FDA, 1979; MHLW, 1997; OECD, 1997). A total of 130 monkeys were used in this studies. Animals were housed in stainless steel cages in a controlled environment (18–29°C; 30–70% relative humidity) and offered Certified Primate Diet #2055C (Harlan Laboratories, Indianapolis, IN, USA) daily and tap water ad libitum. As part of the Testing Facilities' environmental enrichment programme, small bits of primate treats and cage-enrichment devices were supplied. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Cynomolgus monkeys were randomly assigned to treatment groups for the single-dose pharmacokinetics and the three GLP toxicology studies. Blood samples were collected as outlined in Table 1.
Table 1.
AMG 139 treatments and procedures for studies in cynomolgus monkeys
| Study | n | Groups | Treatment | Sample collection for PK |
|---|---|---|---|---|
| Single dose | 6 males | 2 animals per group | 3 mg·kg−1 i.v. 3 mg·kg−1 s.c. 8.63 mg·kg−1 s.c. Single dose | Samples were collected pre-dose and at 1, 4, 8, 24, 72, 96, 120, 168, 240, 336, 504, 840 and 1176 h post-dose; an additional sample at 0.25 h was collected for the i.v. group |
| 14-day GLP | 16 males 16 females | 3–5 animals per group per sex | Vehicle control s.c. 30 mg·kg−1 s.c. 100 mg·kg−1 s.c. 300 mg·kg−1 s.c. QW for 2 weeks | Blood samples were collected pre-dose and at 4, 24, 48, 72 and 168 h post-dose on study days 1 and 8 of the dosing phase; the pre-dose sample collected prior to the second dose on day 8 is equivalent to 168 h post-dose for day 1. Blood samples from recovery monkeys (two monkeys from vehicle control and 300 mg·kg−1 group) were collected on study days 28, 56, 84 and 98 days |
| 3-month GLP | 26 males 26 females | 4–6 animals per group per sex | Vehicle control s.c. and i.v. 30 mg·kg−1 s.c. 100 mg·kg−1 s.c. 300 mg·kg−1 s.c. 300 mg·kg−1 i.v. QW for 13 weeks | Serum samples were collected pre-dose and 0.5, 4, 24, 48, 72 and 168 h post-dose on study days 1 and 92. In addition, samples were collected from animals pre-dose on study days 15, 29 and 57. Blood samples from recovery monkeys (two monkeys per sex from vehicle control and the 300 mg·kg−1 dose groups) were collected on study days 126, 154, 182, 210, 238, 266, 295, 322, 350, 378, 406, 434 and 462. |
| 6-month GLP | 20 males 20 females | 4–6 animals per group per sex | Vehicle control s.c. 30 mg·kg−1 s.c. 100 mg·kg−1 s.c. 300 mg·kg−1 s.c. QW for 26 weeks | Serum samples were collected pre-dose and at approximately 0.5, 4, 24, 48, 72 and 168 h post-dose on study days 1 and 176. In addition, blood samples were collected pre-dose and at 48 h post-dose on study days 29, 57, 92 and 134. Blood samples from recovery animals (2/sex/group) were collected on study day 211. |
PK, pharmacokinetics; QW, once-weekly.
Measurement of serum AMG 139 concentrations
Serum samples were analysed for AMG 139 using a validated assay employing the electrochemiluminescent technology from MSD® (Meso Scale Discovery, Rockville, MD, USA). Briefly, murine anti-AMG 139, 1F2 Mab antibody (Amgen Inc., CA), was passively adsorbed to Multi-Array® 96-well high-bind microplates (MSD). After removing excess antibody, wells were blocked with StartingBlock™ T20 buffer (Thermo Scientific, Rockford, IL, USA). Standards, quality controls and dilutional quality controls, if applicable, were performed by spiking AMG 139 into 100% cynomolgus monkey serum, samples and blanks were loaded into wells after the pretreatment with StartingBlock T20 buffer. After a wash step, a murine anti-AMG 139, 1A4.1 Mab ruthenium-conjugated detection antibody (Amgen Inc., CA), was added to the wells. After a final wash step, a quarter of the diluted tripropylamine read buffer (4X MSD Read Buffer T, MSD) was added to the wells before taking a reading on the Sector Imager 6000 (MSD). The ruthenium label emitted light at 620 nm, when electrically stimulated by electrodes located in the bottom of the microplate and co-reacted with the tripropylamine buffer to enhance the electrochemiluminescent signals. The lower limit of quantification was 25 ng·mL−1 and the nominal dynamic assay range was 25 to 16 000 ng·mL−1.
Immunogenicity assay
Serum samples were assessed for anti-AMG 139 antibodies using a validated immunoassay (MSD; electrochemiluminescence platform). The lower limit of reliable detection (LLRD) was 40 ng·mL−1 and tolerated up to 100 μg·mL−1 of AMG 139 at the LLRD. Samples that tested positive for binding antibodies were further assessed for anti-AMG 139 neutralizing antibodies using a cell-based competitive reporter gene bioassay (similar to the COS reporter assay described earlier). The assay sensitivity of neat-pooled naïve cynomolgus monkey serum was 5 μg·mL−1 AMG 139, but the presence of AMG 139 in concentrations greater than 330 ng·mL−1 may compromise assay performance.
Safety evaluations and T-cell-dependent antibody responses assay
General observations of animals were performed twice daily. In addition, cage side observations were conducted 2 to 3 h post-dose to assess acute toxicity. Other observations included daily assessment of food consumption, weekly assessment of body weight, ophthalmic examinations, vital sign measurements, ECGs and clinical and anatomic pathology evaluations.
To assess potential drug effects on immune function, the T-cell-dependent antigen response was evaluated. Briefly, animals received a dose of Keyhole limpet hemocyanin (KLH; 1 mg) on study days 30 and 60 in the 3-month GLP study. Blood was collected before the first KLH immunization on study day 30 and on study days 37, 40 and 45 before the second KLH dose on study day 60 and on study days 67, 70, 75, 81 and 88. Samples were analysed for anti-KLH-IgM (up to study day 45, data on file at Amgen Inc., CA) and anti-KLH-IgG (all samples) using a validated elisa.
PK data analyses
Non-compartmental analyses were performed using WinNonlin® (Enterprise version 5.1.1, 2006, Pharsight®, a Certara™ Company, Sunnyvale, CA, USA). The following PK parameters after single or multiple i.v. or s.c. administrations were determined: observed maximum concentration in serum (Cmax), area under the serum AMG 139 concentration-time curve within the dosing interval (AUCτ for multiple dosing) or extrapolated to infinity (AUC∞ for single dosing), clearance (CL for i.v. dosing; CL/F for s.c. dosing). The rate constant for the terminal log-linear phase of the concentration-time curve (λz) was estimated using linear regression. The terminal phase elimination half-life (t1/2,z) was calculated as ln2/λz. For the single-dose study, the volume of distribution at steady state (Vss) was calculated as Vss = CL × MRT with MRT being the mean residence time calculated as MRT = AUMC∞/AUC∞, where AUMC∞ is the area under the first-moment-time curve extrapolated to infinity. Descriptive statistics [n, mean (median for tmax) and SD (range for tmax)] were calculated for each cohort or dose level.
Results
In vitro pharmacology
Binding affinity of AMG 139 to human and cynomolgus monkey IL-23
AMG 139 bound to human and cynomolgus monkey IL-23 with high affinity; KD values were 0.138 and 6.0 pM respectively. The association rates of AMG 139 with human and cynomolgus monkey IL-23 were 7.27 × 105 M−1·s−1 (95% CI 6.12 ×105–8.55 ×105 M−1·s−1) and 5.34 × 105 M−1·s−1 (95% CI 4.35 × 105–6.46 × 105 M−1·s−1) (data on file at Amgen Inc., CA).
Blocking activity and specificity of AMG 139 for IL-23
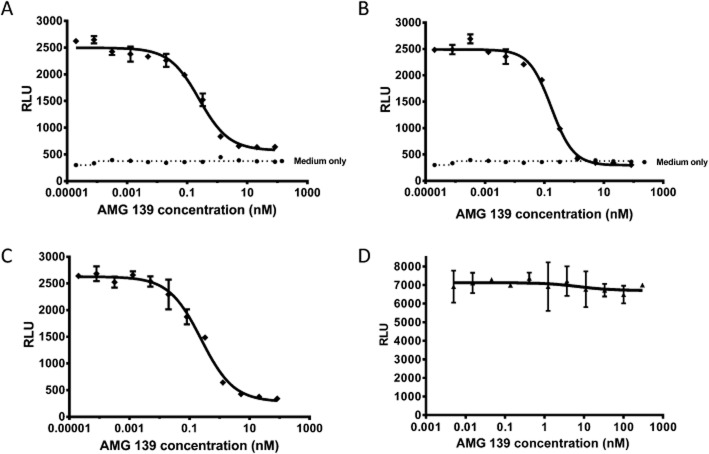
AMG 139 potently and completely inhibited recombinant human and cynomolgus monkey IL-23 but not rat IL-23 or mouse IL-23 (data on file at Amgen Inc., CA) in COS cells stably transfected with the IL-23 receptor and IL-12 receptor β1 subunit and transiently transfected with a STAT-luciferase reporter (Figure 1), demonstrating strong cross-reactivity between human and cynomolgus monkey but not rat or mouse IL-23. Native human ligand was produced from MoDCs following stimulation with CD40 ligand for 48 h. The supernatant was then added to transfected COS cells in the presence or absence of AMG 139. In screening for antibodies to IL-23, some antibodies bound and potently inhibited recombinant IL-23 but not native IL-23. Therefore, demonstrating comparable inhibition of native ligand was a crucial step in identifying the antibodies most likely to inhibit IL-23 in vivo. Comparable inhibition for AMG 139 was observed with human native and human recombinant IL-23 with IC50 values of 284 ± 48 and 188 ± 25 pM (mean ± SD, n = 3 independent experiments) respectively. For cynomolgus monkey IL-23, IC50 values were 250 ± 6 pM (mean ± SD, n = 3 independent experiments).
Figure 1.
AMG 139 inhibits IL-23 activity in a COS cell reporter gene assay. COS-pool 12 cells (expressing huIL-12 receptors β1 subunit and huIL-23 receptors) were transiently transfected with a STAT1/4-luciferase reporter for 48 h. At the day of the experiment, COS-pool 12 cells were incubated with native human IL-23 (A), recombinant human IL-23 (B), recombinant cynomolgus monkey IL-23 (C) or recombinant rat IL-23 (D) in the presence of various concentrations of AMG 139. AMG 139 was pre-incubated with IL-23 for 20 min prior to addition to the cells. After 5 h, relative light units (RLU) were measured as an indicator of the STAT1/4-luciferase reporter using the Bright-Glo luciferase as described in the Methods section. Data are representative of n = 3 (A–C) or n = 2 (D) independent experiments. Values are expressed as mean ± SEM for duplicated measures.
Blocking activity of AMG 139 in IL-23-induced IFN-γ production in human NK cells
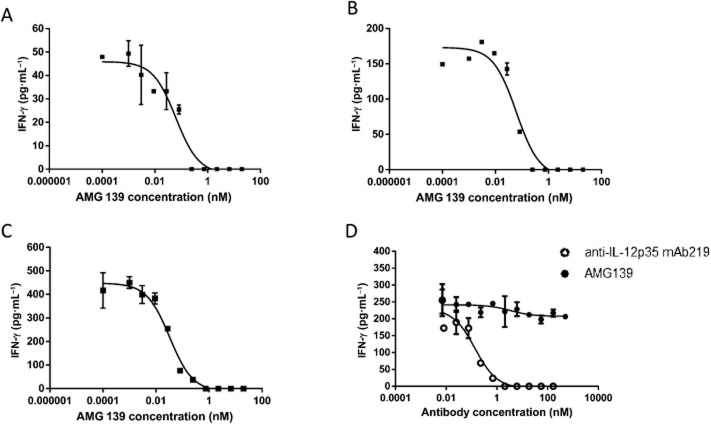
IL-23 in combination with IL-18 induces IFN-γ production in primary human NK cells. AMG 139 potently inhibited this induction by human native and recombinant IL-23 and recombinant cynomolgus monkey IL-23 with IC50 values of 238 ± 248, 93 ± 64 and 71 ± 66 pM (mean ± SD, n = 3–6 donors) respectively. In addition, IFN-γ production by NK cells can also be induced by IL-12; AMG 139 did not inhibit this activity even at a concentration of 0.5 μM. These data demonstrate that AMG 139 is specific for IL-23 and does not inhibit IL-12 signalling. Conversely, the positive control IL-12p35 antibody, mAB219, potently inhibited IL-12-mediated IFN-γ production (Figure 2).
Figure 2.
AMG 139 inhibits IL-23 activity in NK cells. Human NK cells were incubated with native human IL-23 (A), recombinant human IL-23 (B), recombinant cynomolgus monkey IL-23 (C) or recombinant human IL-12 (D) in the presence of various concentrations of AMG 139 (A–D) or the positive control IL-12p35 antibody mAb219 (D). AMG 139 or mAb219 was incubated with IL-23 or 0.3 ng·mL−1 IL-12, for 30 min prior to addition to the cells. After 24 h in culture, the supernatant was collected and IFN-γ was measured via elisa. Results are representative of at least three experiments, except (A) which is n = 2. Data are presented as mean ± SD.
Blocking activity of AMG 139 on IL-23-induced IFN-γ production in human and cynomolgus monkey whole blood
IL-23 will also induce IFN-γ production in human and cynomolgus monkey whole blood. AMG 139 inhibited IL-23-induced IFN-γ production with IC50 values of 28 ± 20 and 125 ± 256 pM (mean ± SD, n = 8–9 donors) for recombinant human and cynomolgus monkey IL-23 in human whole blood, respectively, demonstrating that the presence of serum does not affect the ability of AMG 139 to bind its target and block the signalling pathway. AMG 139 also potently inhibited recombinant cynomolgus IL-23-induced IFN-γ production in cynomolgus monkey whole blood with an IC50 value of 91 ± 95 pM (mean ± SD, n = 4 donors) (Figure 3).
Figure 3.
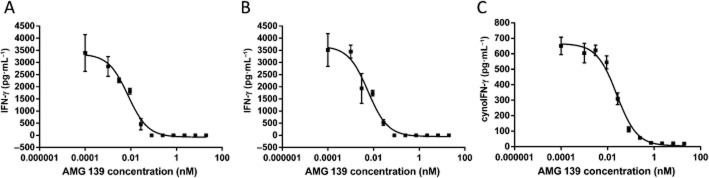
AMG 139 inhibits IL-23 activity in human (A and B) and cynomolgus monkey (C) whole blood. Whole blood was incubated with recombinant human IL-23 (A) or recombinant cynomolgus monkey IL-23 (B and C) in the presence of various concentrations of AMG 139; AMG 139 was pre-incubated with IL-23 for 30 min prior to addition to the cells. After 24 h in culture, the supernatant was collected and IFN-γ was measured via elisa. Results are representative of experiments conducted in blood from four to eight donors; results are represented as mean ± SD.
Cynomolgus monkey studies
PK and toxicokinetics
In a single-dose study in cynomolgus monkeys, serum AMG 139 concentrations declined in a biphasic manner after administration of 3 mg·kg−1 i.v. After s.c. administration, AMG 139 was rapidly absorbed with a median tmax of 3.5–4 days. The mean bioavailability after 3 mg·kg−1 s.c. was approximately 81%. AMG 139 exhibited approximately dose-proportional increases in exposure (Cmax and AUC∞) across the investigated dose range of 3 to 8.63 mg·kg−1. The mean terminal half-life ranged from 8.4 to 13 days (Figure 4A, Table 2). In one animal, after the 3 mg·kg−1 s.c. dose, a rapid decline in serum AMG 139 concentrations was observed >504 h post-dose. This increased elimination rate might be due to the formation of anti-drug antibodies (ADAs); however, ADA measurements were not performed in this single-dose study.
Figure 4.
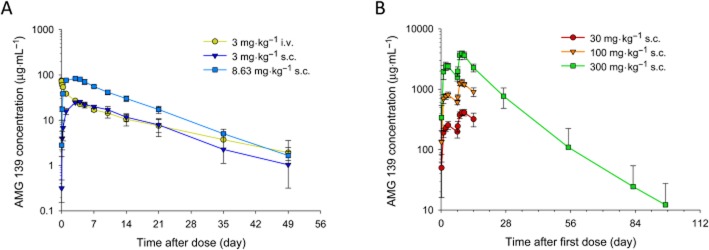
Mean (±SD) serum concentration-time profiles of AMG 139. Cynomolgus monkeys were administered a single i.v. or s.c. (A) or two-dose s.c. (B) of AMG 139.
Table 2.
Non-compartmental pharmacokinetic parameters for AMG 139
| Single dose | n = 2 per group | 3 mg·kg−1 i.v. | 3 mg·kg−1 s.c. | 8.63 mg·kg−1 s.c. | |
|---|---|---|---|---|---|
| C0/Cmax (μg·mL−1) | 75.1 | 25.5 | 83.5 | ||
| tmax (day) | 0.0104 | 4.0 | 3.5 | ||
| AUC∞ (μg·day·mL−1) | 517 | 435 | 1160 | ||
| CL (mL·day−1·kg−1)a | 6.12 | 7.63 | 7.63 | ||
| V (mL·kg−1)b | 97.5 | 84.4 | 93.0 | ||
| t1/2 (day) | 13.5 | 8.65 | 8.41 |
| 14-day GLP | n = 6–10 per group | 30 mg·kg−1 s.c. | 100 mg·kg−1 s.c. | 300 mg·kg−1 s.c. | |
|---|---|---|---|---|---|
| tmax (day) | 3.0 (2.0–3.0) | 3.0 (1.0–3.0) | 2.0 (2.0–3.0) | ||
| Day 1 | Cmax (μg·mL−1) | 255 (37.8) | 813 (89.5) | 2 480 (323) | |
| AUCτ (μg·day·mL−1) | 1 450 (208) | 4 700 (486) | 13 400 (1880) | ||
| Day 8 | tmax (day) | 2.5 (2.0–3.0) | 2.0 (2.0–3.0) | 2.0 (1.0–2.0) | |
| Cmax (μg·mL−1) | 417 (46.5) | 1 330 (135) | 3 940 (407) | ||
| AUCτ (μg·day·mL−1) | 2 550 (397) | 7 630 (698) | 21 800 (2730) | ||
| AR | 1.76 (0.173) | 1.63 (0.141) | 1.64 (0.131) |
| 3-month GLP | n = 7–12 per group | 30 mg·kg−1 s.c. | 100 mg·kg−1 s.c. | 300 mg·kg−1 s.c. | 300 mg·kg−1 i.v. |
|---|---|---|---|---|---|
| Day 1 | tmax (day) | 3.0 (3.0–3.0) | 3.0 (2.0–3.0) | 3.0 (1.0–3.0) | 0.02 (0.02–0.167) |
| Day 92 | C0/Cmax (μg·mL−1) | 293 (26.5) | 1 060 (136) | 3 230 (334) | 9 370 (970) |
| AUCτ (μg·day·mL−1) | 1 710 (139) | 6 120 (822) | 17 600 (2060) | 28 000 (3130) | |
| tmax (day) | 1.5 (0.02–3.0) | 1.0 (1.0–3.0) | 1.0 (1.0–3.0) | 0.167 (0.02–0.167) | |
| C0/Cmax (μg·mL−1) | 929 (283) | 3 250 (652) | 7 500 (987) | 14 800 (1300) | |
| AUCτ (μg·day·mL−1) | 5 430 (1510) | 18 400 (3520) | 41 500 (6220) | 53 500 (8210) | |
| AR | 3.10 (0.831) | 3.01 (0.491) | 2.37 (0.393) | 1.91 (0.250) |
| 6-month GLP | n = 8–12 per group | 30 mg·kg−1 s.c. | 100 mg·kg−1 s.c. | 300 mg·kg−1 s.c. | |
|---|---|---|---|---|---|
| Day 1 | tmax (day) | 3.0 (2.0–7.0) | 3.0 (2.0–3.0) | 2.0 (2.0–3.0) | |
| Cmax (μg·mL−1) | 285 (61.3) | 993 (99.5) | 2 910 (363) | ||
| AUCτ (μg·day·mL−1) | 1 670 (342) | 5 760 (748) | 16 000 (1310) | ||
| Day 176 | tmax (day) | 2.0 (0.167–3.0) | 1.5 (0.167–3.0) | 2.0 (1.0–3.0) | |
| Cmax (μg·mL−1) | 1 010 (370) | 2 090 (507) | 5 900 (1030) | ||
| AUCτ (μg·day·mL−1) | 5 980 (2350) | 11 900 (2710) | 32 100 (5600) | ||
| AR | 3.52 (1.12) | 2.07 (0.390) | 2.03 (0.410) |
Results are presented as means with SD in parenthseses.
CL is reported for i.v. dosing, CL/F for s.c. dosing.
Vss is reported for i.v. dosing, Vz/F for s.c. dosing.
AR, accumulation ratio for AUC; AUCτ, area under the concentration-time curve from time zero to the last observed concentration; C0, extrapolated concentration at time zero for i.v. dosing; Cmax, maximum observed concentration for s.c. dosing; tmax, time to Cmax, expressed as median (minimum–maximum).
Multiple dose toxicokinetics were assessed in 2-week, 3-month and 6-month GLP toxicology studies in cynomolgus monkeys. In the 2-week study, AMG 139 was administered once weekly for 2 weeks followed by a 3-month treatment-free phase. The exposure to AMG 139, as assessed by Cmax and AUCτ, increased approximately dose-proportionally for doses between 30 and 300 mg·kg−1. The median tmax ranged from 2.0 to 3.0 days (Figure 4B, Table 2). In the 3-month study, AMG 139 was administered to cynomolgus monkeys once weekly for 14 weeks followed by a 12-month treatment-free phase. On study days 1 and 92, the exposure to AMG 139 increased dose-proportionally across the investigated dose range. The mean accumulation ratio (based on AUC) after 14 weekly doses, ranged from 2.37 to 3.10 for s.c. and 1.91 for i.v. administration (Figure 5A, Table 2). The estimated bioavailability after 300 mg·kg−1 s.c. administration was 63% on study day 1 and 78% on study day 92. In the 6-month study, AMG 139 was administered once weekly via s.c. injection for 6 months followed by an 11-week treatment-free phase. Exposure increased approximately dose-proportionally over the 30 to 300 mg·kg−1 dose range. The mean accumulation ratios ranged from 2.0 to 3.5-fold after 6 months of weekly AMG 139 injections (Figure 5B, Table 2). For all studies, no or only minor, non-statistically significant gender differences were observed for the AMG 139 exposure parameters Cmax and AUC.
Figure 5.
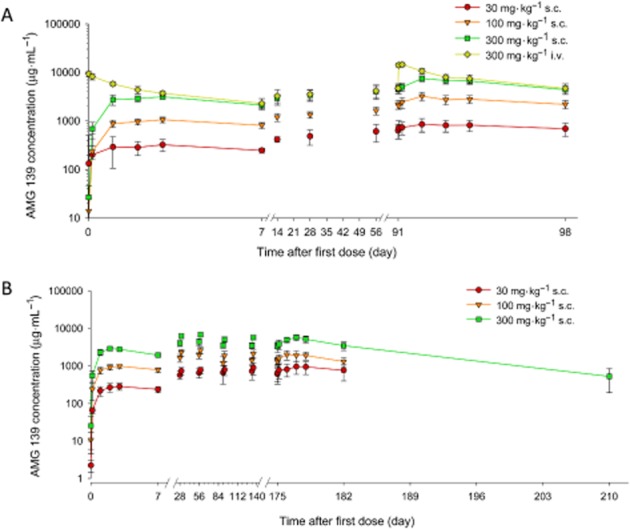
Mean (±SD) serum concentration-time profiles in cynomolgus monkeys after multiple i.v. or s.c. doses of AMG 139. Cynomolgus monkeys received 13 i.v. or s.c. (A) or 26 s.c. doses of AMG 139 (B) or vehicle every week.
Immunogenicity
In the 2-week study, all animals tested negative for ADAs during the dosing phase. During the post-treatment phase, two of four animals in the 300 mg·kg−1 s.c. dose group tested positive for anti-AMG 139 neutralizing antibodies (on day 82 and 96 post-first dose), which appeared to have decreased the exposure to AMG 139. Anti-AMG 139 antibodies during the treatment period were detected in four of 28 animals (two of eight, one of eight, one of 12 and zero of 12 in the 30, 100 and 300 mg·kg−1 s.c. and the 300 mg·kg−1 i.v. dose groups, respectively) and in four of 28 animals (one of eight, two of eight and one of 12 in the 30, 100 and 300 mg·kg−1 s.c. dose group, respectively) in the 3- and 6-month studies respectively. In the latter study, one of four monkeys (300 mg·kg−1 s.c. dose group) developed anti-AMG 139 binding antibodies in the treatment-free phase. No neutralizing antibodies were detected in either of these latter studies and the anti-AMG 139 binding antibodies did not appear to decrease the exposure of AMG 139 in these animals. It should be noted that high circulating levels of the drug could have prevented the detection of anti-AMG 139 antibodies, as the assays testing for binding and neutralizing antibodies were only accurate for up to 100 μg·mL−1 and 330 ng·mL−1 AMG 139 respectively.
Safety evaluation
In all studies, AMG 139 was well-tolerated; no AMG 139-related clinical signs or changes in food consumption, body weight, physical parameters, ECGs, ophthalmic tests, serum chemistry, haematology, peripheral blood phenotyping, bone formation markers or coagulation parameters were observed after up to and including 300 mg·kg−1 AMG 139, administered s.c. or i.v. AMG 139-related microscopic changes were limited to a minor increase in intensity of cellular infiltrates at the s.c. injection site relative to controls. These findings were reversed in the 2-week study. Based on these observations, the no-observed-adverse-effect level of AMG 139 in cynomolgus monkeys was determined to be 300 mg·kg−1 s.c. or i.v., the highest dose tested. In addition, no changes in the T-cell-dependent antigen response assay against KLH compared with the placebo control were observed (Figure 6).
Figure 6.
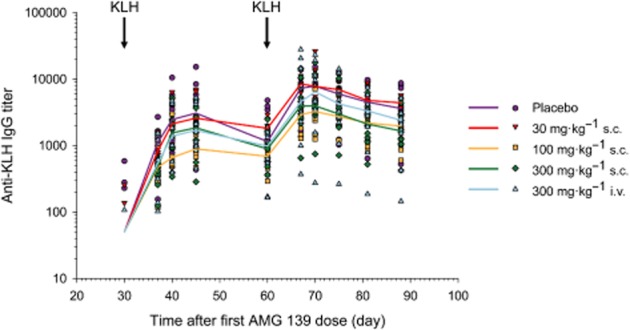
Kinetics of the anti-KLH IgG response in monkeys following immunization with KLH. Male and female cynomolgus monkeys were given a dose of KLH at 1 mg per animal on day 30 and 60. Serum anti-KLH IgG titres were measured by elisa. Symbols represent individual results. Lines represent the group median. Responses in the AMG 139-treated animals (combined sexes) were comparable with the responses of the controls after both the primary and secondary immunization with KLH when animals with pre-existing anti-KLH titres were excluded. Median values below the assay cut-off point were set to one-half of the assay cut-point.
Discussion and conclusions
AMG 139, a human monoclonal IgG2 antibody that targets IL-23, has been developed as a therapeutic agent for treatment of autoimmune/inflammatory diseases. AMG 139 is currently in a phase II clinical trial in patients with Crohn's disease and recently completed a phase I clinical trial in patients with psoriasis. As part of the preclinical development of AMG 139, in vitro assays and in vivo studies were conducted in cynomolgus monkeys to determine the pharmacology, PK and safety of this human monoclonal antibody.
In vitro experiments demonstrated that AMG 139 binds human IL-23 and cynomolgus monkey IL-23 with high potencies; affinities (dissociation constant) of 0.138 and 6 pM, respectively, were estimated. Furthermore, AMG 139 inhibited human (recombinant and native) and recombinant cynomolgus monkey IL-23 with similar IC50 values in a COS cell-based luciferase reporter assay and suppressed IL-23-induced IFN-γ production in primary human NK cells and in human and cynomolgus monkey whole blood. The comparable results between whole blood cell assays and the other cellular assays demonstrate that the presence of serum does not affect AMG 139's ability to bind its target and inhibit signalling. Most importantly, the similar inhibitory potencies for human and cynomolgus monkey IL-23 suggest that AMG 139 has comparable in vitro pharmacodynamic properties in both species, which supports the use of cynomolgus monkeys as a relevant toxicology model for this antibody.
Our data further demonstrated that AMG 139 is specific for IL-23 and does not interact with IL-12 signalling. This is especially important because of the predominant role of IL-12 in defence against intracellular pathogens and tumour immune surveillance (Fieschi and Casanova, 2003; Bowman et al., 2006; Langowski et al., 2006; Meeran et al., 2006; Schulz et al., 2008) and the controversially debated safety concerns (increased risk for malignancies and major adverse cardiovascular events) raised for the dual IL-12/23 inhibitors briakinumab and ustekinumab in recent clinical trials (Lee et al., 1999; Reddy et al., 2010; Gordon et al., 2012; Lin et al., 2012; Tzellos et al., 2012; Papp et al., 2013b). Targeting IL-23 alone may offer a better benefit : risk profile compared with dual inhibition of IL-12 and IL-23.
The PK of AMG 139 in cynomolgus monkeys were characterized by a biphasic decline in serum concentration after administration of a single i.v. dose and rapid absorption after s.c. administrations. The observed clearance of 6.1 to 7.6 mL·kg−1·day−1 is in the range of what has been previously reported for monoclonal antibodies in cynomolgus monkeys (Hotzel et al., 2012). With 84 to 98 mL·kg−1, the volume of distribution was slightly higher than the reported plasma volume of 45 mL·kg−1 in cynomolgus monkeys suggesting that this antibody also distributes to extracellular fluid (Davies and Morris, 1993).
The PK of monoclonal antibodies often appear non-linear because of binding to the specific target; at high antibody concentrations, this target binding will be saturated and linear elimination predominates (Dong et al., 2011). In contrast to many other monoclonal antibodies, no non-linear kinetics as a result of target-mediated disposition were observed for AMG 139, which is not surprising as the levels of IL-23 are expected to be low in cynomolgus monkeys and healthy human subjects (Nogueira et al., 2010; Gagliardi et al., 2011). Further research is needed to determine whether target-mediated disposition can affect AMG 139's PK under pathophysiological conditions with increased concentrations of the target IL-23p19 in plasma and/or tissue. Insights into the effect of disease on target-mediated disposition has been addressed in a single ascending dose in healthy volunteers and patients with psoriasis (http://Clinicaltrials.gov NCT01094093) and a multiple ascending dose study in healthy volunteers and subjects with Crohn's disease (http://Clinicaltrials.gov NCT01258205, ongoing).
Of note, the AMG 139 exposure parameters Cmax and AUC were lower after 6-month dosing than after 3-month dosing. Although the exact mechanism is not clear, this discrepancy might be due to high between-study and/or between-animal variability often observed for monoclonal antibodies (Dirks and Meibohm, 2010) or other non-observed or non-observable covariates (e.g. changes in target expression or physiological changes). In addition, the longer duration of weekly AMG 139 treatment in the 6-month study may have resulted in a higher rate of immunogenic response and thus lower AMG 139 exposure at the last week of the dosing phase compared with the 3-month study. Although a similar rate of immunogenicity was detected in both studies, high levels of circulating AMG 139 might have interfered with the immunogenicity assay leading to under-detection of the anti-AMG 139 antibodies and hence an inability to more definitively assess its potential effect on the AMG 139 exposure. Across all the multiple dose studies, 10 out of 90 actively treated monkeys (11.1%) developed anti-AMG 139 antibodies. Immunogenicity in monkeys was anticipated because AMG 139 is a human antibody and would be expected to be immunogenic in non-human species. Interestingly, except for two animals in the 2-week toxicology study, no neutralizing anti-AMG 139 antibodies were detected and no effect on AMG 139 exposure was observed. The incidence of anti-AMG 139 antibodies appeared to decrease with increasing dose of AMG 139. Such an inverse relationship between dose and the incidence of immunogenicity has previously been observed for other antibody therapies (e.g. for AMG 181 or infliximab) (Wagner et al., 2003; Pan et al., 2013a).
The multiple dose toxicology studies in cynomolgus monkeys indicate that once-weekly i.v. or s.c. administration for up to 6 months was well tolerated at doses up to 300 mg·kg−1; only injection site reactions were observed, which is a common side effect after s.c. administration of biological agents.
The data obtained in the present study were used to inform further clinical development of AMG 139. Based on the observed linear elimination and dose-proportionality of this antibody in cynomolgus monkeys and the exposure parameters (AUC and Cmax) at the no-observed-adverse-effect level of 300 mg·kg−1 s.c. (the highest dose tested), monkey PK parameters were allometrically scaled to predict human exposure and safety margins (Dong et al., 2011). Based on these calculations, doses up to 700 mg i.v. are predicted to be safe in humans. Indeed, preliminary analysis of the first-in-human study in healthy volunteers indicates that AMG 139 is well- tolerated and that the observed exposure parameters were well predicted using the allometric scaling approach (within twofold).
Due to the low expression levels of the target (IL-23) in the central circulation, an effect of AMG 139 on the biomarkers evaluated in healthy cynomolgus monkeys, the only non-human species to which AMG 139 cross-reacts, could not be demonstrated. However, proof-of-concept clinical studies in patients with psoriasis and Crohn's disease are currently being performed (http://clinicaltrial.gov NCT01094093 and NCT01258205); results from these studies will be reported separately.
To infer efficacious concentration for clinical trials in psoriasis patients, the in vitro IC50 values were used: AMG 139 concentrations higher than the in vitro IC90 values (between approximately 0.038 and 0.38 μg·mL−1) were targeted. The preliminary results indicated that after a single dose of 21 mg in patients with moderate to severe psoriasis, the AMG 139 concentrations were sustained above 0.1 μg·mL−1 for approximately 16 weeks and this was associated with a reduction in psoriasis area severity index score and an improvement in psoriasis disease markers within the 16-week post-dosing period [(Pan et al., 2013b) and data on file at Amgen Inc., CA]. Detailed results from the first-in-human study of AMG 139 will be reported separately.
Conclusions
Given the role of IL-23 in the pathogenesis of autoimmune/inflammatory diseases, our pharmacology, PK and safety data suggest that AMG 139-mediated IL-23 blockade is a promising therapeutic approach and support further clinical development.
Acknowledgments
This work and the studies presented herein were financially funded by Amgen Inc., Thousand Oaks, CA, USA. The authors would like to thank Amgen Therapeutic Discovery, Inflammation Research, Medical Sciences, Pharmacokinetics and Drug Metabolism (PKDM), and Comparative Biology and Safety Sciences (CBSS) colleagues and Amgen contractors for their kind assistance in the antibody generation, production and purification, the conduct of the studies, as well as data management and processing. Financial support for this study and publication was provided by Amgen Inc. AMG 139 is being co-developed by MedImmune, LLC (a wholly owned subsidiary of AstraZeneca plc.) and Amgen Inc., and may be referred to as AMG 139 or MEDI2070.
Glossary
- ADA
anti-drug antibodies
- CI
confidence interval
- Cmax
maximum observed concentration
- GLP
good laboratory practice
- Ka
association constant
- KLH
Keyhole limpet hemocyanin
- moDCs
monocyte-derived dendritic cells
- NK
natural killer cell
- PK
pharmacokinetic
Author contributions
W. J. P., M. J. H., J. P. G., T. J. G., K. J. N., M. W. T., W. H. T., J. E. T. and W. A. R. participated in the research design. Y. S., Y. Z., K. J. N., A. N. U-R., S. P. P., C. D. K., A. C., L. S., S. A. B., M. W. T. and J. E. T. conducted the experiments. A. C., T. J. G., J. C. O. and J. E. T. contributed new reagents or analytic tools. K. K., W.J. P., M. J. H., J. P. G., A. C., W. A. R., S. A. B., Y. S. and Y. Z. performed the data analysis. K. K., W. J. P., J. M. G., W. H. T. and J. E. T. wrote or contributed to the writing of the manuscript.
Conflict of interest
This work and the studies presented herein were financially funded by Amgen Inc., Thousand Oaks, CA, USA. The authors are current or former employees or contractors and shareholders of Amgen Inc. AMG 139 is being co-developed by MedImmune, LLC (a wholly owned subsidiary of AstraZeneca plc.) and Amgen Inc., and may be referred to as AMG 139 or MEDI2070.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, McGrath JC, et al. The Concise Guide to PHARMACOLOGY 2013/14: Overview. Br J Pharmacol. 2013a;170:1449–1458. [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Catalytic receptors. Br J Pharmacol. 2013b;170:1676–1705. doi: 10.1111/bph.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Animal and Plant Health Inspection Service USA . In: Animal Welfare Act and Animal Welfare Regulations. Agriculture USDA, editor. Washington, DC: US Department of Agriculture; 2005. [Google Scholar]
- Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007;19:652–657. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg H, Dinh H, Dean C, Jr, Trueblood ES, Bailey K, Shows D, et al. IL-1RL2 and its ligands contribute to the cytokine network in psoriasis. J Immunol. 2010;185:4354–4362. doi: 10.4049/jimmunol.1000313. [DOI] [PubMed] [Google Scholar]
- Bowman EP, Chackerian AA, Cua DJ. Rationale and safety of anti-interleukin-23 and anti-interleukin-17A therapy. Curr Opin Infect Dis. 2006;19:245–252. doi: 10.1097/01.qco.0000224818.42729.67. [DOI] [PubMed] [Google Scholar]
- Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargill M, Schrodi SJ, Chang M, Garcia VE, Brandon R, Callis KP, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet. 2007;80:273–290. doi: 10.1086/511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JH, Kljavin NM, Ota N, Leonard J, Roose-Girma M, Diehl L, et al. Opposing consequences of IL-23 signaling mediated by innate and adaptive cells in chemically induced colitis in mice. Mucosal Immunol. 2012;5:99–109. doi: 10.1038/mi.2011.54. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10:1093–1095. doi: 10.1023/a:1018943613122. [DOI] [PubMed] [Google Scholar]
- Dirks NL, Meibohm B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:633–659. doi: 10.2165/11535960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Dong JQ, Salinger DH, Endres CJ, Gibbs JP, Hsu CP, Stouch BJ, et al. Quantitative prediction of human pharmacokinetics for monoclonal antibodies: retrospective analysis of monkey as a single species for first-in-human prediction. Clin Pharmacokinet. 2011;50:131–142. doi: 10.2165/11537430-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinghaus D, Ellinghaus E, Nair RP, Stuart PE, Esko T, Metspalu A, et al. Combined analysis of genome-wide association studies for Crohn disease and psoriasis identifies seven shared susceptibility loci. Am J Hum Genet. 2012;90:636–647. doi: 10.1016/j.ajhg.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA. 1979. The nonclinical laboratory studies good laboratory practice regulations united states health and human services food and drug administration.
- Fieschi C, Casanova JL. The role of interleukin-12 in human infectious diseases: only a faint signature. Eur J Immunol. 2003;33:1461–1464. doi: 10.1002/eji.200324038. [DOI] [PubMed] [Google Scholar]
- van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182:5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration. 2014. Ustekinumab Label. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/125261s114lbl.pdf (accessed 1/6/2014)
- Gagliardi MC, Starnino S, Teloni R, Mariotti S, Dal Conte I, Di Carlo A, et al. Circulating levels of interleukin-17A and interleukin-23 are increased in patients with gonococcal infection. FEMS Immunol Med Microbiol. 2011;61:129–132. doi: 10.1111/j.1574-695X.2010.00759.x. [DOI] [PubMed] [Google Scholar]
- Geremia A, Arancibia-Cárcamo CV, Fleming MP, Rust N, Singh B, Mortensen NJ, et al. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med. 2011;208:1127–1133. doi: 10.1084/jem.20101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon KB, Langley RG, Gottlieb AB, Papp KA, Krueger GG, Strober BE, et al. A phase III, randomized, controlled trial of the fully human IL-12/23 mAb briakinumab in moderate-to-severe psoriasis. J Invest Dermatol. 2012;132:304–314. doi: 10.1038/jid.2011.304. [DOI] [PubMed] [Google Scholar]
- Guo YY, Wang NZ, Zhao S, Hou LX, Xu YB, Zhang N. Increased interleukin-23 is associated with increased disease activity in patients with rheumatoid arthritis. Chin Med J. 2013;126:850–854. [PubMed] [Google Scholar]
- Hotzel I, Theil F-P, Bernstein LJ, Prabhu S, Deng R, Quintana L, et al. A strategy for risk mitigation of antibodies with fast clearance. MAbs. 2012;4:753–760. doi: 10.4161/mabs.22189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5:521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG NC3Rs Reporting Guidelines Working Group. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball AB, Papp KA, Wasfi Y, Chan D, Bissonnette R, Sofen H, et al. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2012;27:1535–1545. doi: 10.1111/jdv.12046. [DOI] [PubMed] [Google Scholar]
- Langley RG, Papp K, Gottlieb AB, Krueger GG, Gordon KB, Williams D, et al. Safety results from a pooled analysis of randomized, controlled phase II and III clinical trials and interim data from an open-label extension trial of the interleukin-12/23 monoclonal antibody, briakinumab, in moderate to severe psoriasis. J Eur Acad Dermatol Venereol. 2012;27:1252–1261. doi: 10.1111/j.1468-3083.2012.04705.x. [DOI] [PubMed] [Google Scholar]
- Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Trepicchio WL, Oestreicher JL, Pittman D, Wang F, Chamian F, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125–130. doi: 10.1084/jem.20030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TS, Yen HC, Pan CC, Chau LY. The role of interleukin 12 in the development of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19:734–742. doi: 10.1161/01.atv.19.3.734. [DOI] [PubMed] [Google Scholar]
- Li Y, Chu N, Hu A, Gran B, Rostami A, Zhang GX. Increased IL-23p19 expression in multiple sclerosis lesions and its induction in microglia. Brain. 2007;130(Pt 2):490–501. doi: 10.1093/brain/awl273. [DOI] [PubMed] [Google Scholar]
- Lin Y, Huang Y, Lu Z, Luo C, Shi Y, Zeng Q, et al. Decreased plasma IL-35 levels are related to the left ventricular ejection fraction in coronary artery diseases. PLoS ONE. 2012;7:e52490. doi: 10.1371/journal.pone.0052490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannon PJ, Fuss IJ, Mayer L, Elson CO, Sandborn WJ, Present D, et al. Anti-interleukin-12 antibody for active Crohn's disease. N Engl J Med. 2004;351:2069–2079. doi: 10.1056/NEJMoa033402. [DOI] [PubMed] [Google Scholar]
- Meeran SM, Mantena SK, Meleth S, Elmets CA, Katiyar SK. Interleukin-12-deficient mice are at greater risk of UV radiation-induced skin tumors and malignant transformation of papillomas to carcinomas. Mol Cancer Ther. 2006;5:825–832. doi: 10.1158/1535-7163.MCT-06-0003. [DOI] [PubMed] [Google Scholar]
- MHLW. 1997. The Japanese good laboratory practice standards for safety studies on drugs pharmaceutical affairs bureau the ministry of health labour and welfare.
- Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research (U.S.), National Academies Press (U.S.) Guide for the Care and Use of Laboratory Animals. 8th edn. Washington, DC: National Academies Press; 2011. [Google Scholar]
- Nogueira E, Hamour S, Sawant D, Henderson S, Mansfield N, Chavele KM, et al. Serum IL-17 and IL-23 levels and autoantigen-specific Th17 cells are elevated in patients with ANCA-associated vasculitis. Nephrol Dial Transplant. 2010;25:2209–2217. doi: 10.1093/ndt/gfp783. [DOI] [PubMed] [Google Scholar]
- OECD. 1997. Principles on good laboratory practice: the organisation for economic cooperation and development.
- Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- Pan WJ, Hsu H, Rees WA, Lear SP, Lee F, Foltz IN, et al. Pharmacology of AMG 181, a human anti-alpha4 beta7 antibody that specifically alters trafficking of gut-homing T cells. Br J Pharmacol. 2013a;169:51–68. doi: 10.1111/bph.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WJ, Rees WA, Towne JE, Gibbs JP, Colbert A, Goletz TJ, et al. Clinical pharmacology, safety, and effects of anti-IL-23 antibody AMG 139. 2013b. UEG Week. Available at: https://www.ueg.eu/education/document-detail/?name=clinical_pharmacology_safety_and_effects_of_anti-il-23_antibody_amg_139&file=103131 (accessed 15/6/2014)
- Papp KA, Griffiths CE, Gordon K, Lebwohl M, Szapary PO, Wasfi Y, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013a;168:844–854. doi: 10.1111/bjd.12214. [DOI] [PubMed] [Google Scholar]
- Papp KA, Sundaram M, Bao Y, Williams DA, Gu Y, Signorovitch JE, et al. Effects of briakinumab treatment for moderate to severe psoriasis on health-related quality of life and work productivity and activity impairment: results from a randomized phase III study. J Eur Acad Dermatol Venereol. 2013b;28:790–798. doi: 10.1111/jdv.12177. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathanaswami P, Babcook J, Gallo M. High-affinity binding measurements of antibodies to cell-surface-expressed antigens. Anal Biochem. 2008;373:52–60. doi: 10.1016/j.ab.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Reddy M, Torres G, McCormick T, Marano C, Cooper K, Yeilding N, et al. Positive treatment effects of ustekinumab in psoriasis: analysis of lesional and systemic parameters. J Dermatol. 2010;37:413–425. doi: 10.1111/j.1346-8138.2010.00802.x. [DOI] [PubMed] [Google Scholar]
- Reich K, Langley RG, Papp KA, Ortonne JP, Unnebrink K, Kaul M, et al. A 52-week trial comparing briakinumab with methotrexate in patients with psoriasis. N Engl J Med. 2011;365:1586–1596. doi: 10.1056/NEJMoa1010858. [DOI] [PubMed] [Google Scholar]
- Reich K, Papp KA, Griffiths CE, Szapary PO, Yeilding N, Wasfi Y, et al. An update on the long-term safety experience of ustekinumab: results from the psoriasis clinical development program with up to four years of follow-up. J Drugs Dermatol. 2012;11:300–312. [PubMed] [Google Scholar]
- Reichert JM. Antibodies to watch in 2013: mid-year update. mAbs. 2013;5:513–517. doi: 10.4161/mabs.24990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C, Leonardi CL, Krueger JG, Kimball AB, Strober BE, Gordon KB, et al. Association between biologic therapies for chronic plaque psoriasis and cardiovascular events: a meta-analysis of randomized controlled trials. JAMA. 2011;306:864–871. doi: 10.1001/jama.2011.1211. [DOI] [PubMed] [Google Scholar]
- Sandborn WJ, Gasink C, Gao LL, Blank MA, Johanns J, Guzzo C, et al. Ustekinumab induction and maintenance therapy in refractory Crohn's disease. N Engl J Med. 2012;367:1519–1528. doi: 10.1056/NEJMoa1203572. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Giese T, Ludwig B, Mueller-Molaian I, Marth T, Zeuzem S, et al. Expression of interleukin-12-related cytokine transcripts in inflammatory bowel disease: elevated interleukin-23p19 and interleukin-27p28 in Crohn's disease but not in ulcerative colitis. Inflamm Bowel Dis. 2005;11:16–23. doi: 10.1097/00054725-200501000-00003. [DOI] [PubMed] [Google Scholar]
- Schulz SM, Köhler G, Schutze N, Knauer J, Straubinger RK, Chackerian AA, et al. Protective immunity to systemic infection with attenuated Salmonella enterica serovar enteritidis in the absence of IL-12 is associated with IL-23-dependent IL-22, but not IL-17. J Immunol. 2008;181:7891–7901. doi: 10.4049/jimmunol.181.11.7891. [DOI] [PubMed] [Google Scholar]
- Song GG, Bae SC, Choi SJ, Ji JD, Lee YH. Associations between interleukin-23 receptor polymorphisms and susceptibility to rheumatoid arthritis: a meta-analysis. Mol Biol Rep. 2012;39:10655–10663. doi: 10.1007/s11033-012-1955-7. [DOI] [PubMed] [Google Scholar]
- Teng MW, Andrews DM, McLaughlin N, von Scheidt B, Ngiow SF, Möller A, et al. IL-23 suppresses innate immune response independently of IL-17A during carcinogenesis and metastasis. Proc Natl Acad Sci U S A. 2010;107:8328–8333. doi: 10.1073/pnas.1003251107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng MW, Vesely MD, Duret H, McLaughlin N, Towne JE, Schreiber RD, et al. Opposing roles for IL-23 and IL-12 in maintaining occult cancer in an equilibrium state. Cancer Res. 2012;72:3987–3996. doi: 10.1158/0008-5472.CAN-12-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traczewski P, Rudnicka L. Briakinumab for the treatment of plaque psoriasis. Biodrugs. 2012;26:9–20. doi: 10.2165/11595940-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Tzellos T, Kyrgidis A, Trigoni A, Zouboulis CC. Association of ustekinumab and briakinumab with major adverse cardiovascular events: an appraisal of meta-analyses and industry sponsored pooled analyses to date. Dermatoendocrinol. 2012;4:320–323. doi: 10.4161/derm.23100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzellos T, Kyrgidis A, Zouboulis CC. Re-evaluation of the risk for major adverse cardiovascular events in patients treated with anti-IL-12/23 biological agents for chronic plaque psoriasis: a meta-analysis of randomized controlled trials. J Eur Acad Dermatol Venereol. 2013;27:622–627. doi: 10.1111/j.1468-3083.2012.04500.x. [DOI] [PubMed] [Google Scholar]
- Vaknin-Dembinsky A, Balashov K, Weiner HL. IL-23 is increased in dendritic cells in multiple sclerosis and down-regulation of IL-23 by antisense oligos increases dendritic cell IL-10 production. J Immunol. 2006;176:7768–7774. doi: 10.4049/jimmunol.176.12.7768. [DOI] [PubMed] [Google Scholar]
- Wagner CL, Schantz A, Barnathan E, Olson A, Mascelli MA, Ford J, et al. Consequences of immunogenicity to the therapeutic monoclonal antibodies ReoPro and Remicade. Dev Biol (Basel) 2003;112:37–53. [PubMed] [Google Scholar]
- Yuzhalin AE, Kutikhin AG. Interleukin-12: clinical usage and molecular markers of cancer susceptibility. Growth Factors. 2012;30:176–191. doi: 10.3109/08977194.2012.678843. [DOI] [PubMed] [Google Scholar]
- Zhu KJ, Zhu CY, Shi G, Fan YM. Association of IL23R polymorphisms with psoriasis and psoriatic arthritis: a meta-analysis. Inflamm Res. 2012;61:1149–1154. doi: 10.1007/s00011-012-0509-8. [DOI] [PubMed] [Google Scholar]