Abstract
BACKGROUND AND PURPOSE
H2O2 is widely understood to regulate intracellular signalling. In airway epithelia, H2O2 stimulates anion secretion primarily by activating an autocrine PGE2 signalling pathway via EP4 and EP1 receptors to initiate cytic fibrosis transmembrane regulator (CFTR)-mediated Cl− secretion. This study investigated signalling downstream of the receptors activated by H2O2.
EXPERIMENTAL APPROACH
Anion secretion by differentiated bronchial epithelial cells was measured in Ussing chambers during stimulation with H2O2, an EP4 receptor agonist or β2-adrenoceptor agonist in the presence and absence of inhibitors of ACs and downstream effectors. Intracellular calcium ([Ca2+]I) changes were followed by microscopy using fura–2-loaded cells and PKA activation followed by FRET microscopy.
KEY RESULTS
Transmembrane adenylyl cyclase (tmAC) and soluble AC (sAC) were both necessary for H2O2 and EP4 receptor-mediated CFTR activation in bronchial epithelia. H2O2 and EP4 receptor agonist stimulated tmAC to increase exchange protein activated by cAMP (Epac) activity that drives PLC activation to raise [Ca2+]i via Ca2+ store release (and not entry). Increased [Ca2+]i led to sAC activation and further increases in CFTR activity. Stimulation of sAC did not depend on changes in [HCO3−]. Ca2+-activated apical KCa1.1 channels and cAMP-activated basolateral KV7.1 channels contributed to H2O2-stimulated anion currents. A similar Epac-mediated pathway was seen following β2-adrenoceptor or forskolin stimulation.
CONCLUSIONS AND IMPLICATIONS
H2O2 initiated a complex signalling cascade that used direct stimulation of tmACs by Gαs followed by Epac-mediated Ca2+ crosstalk to activate sAC. The Epac-mediated Ca2+ signal constituted a positive feedback loop that amplified CFTR anion secretion following stimulation of tmAC by a variety of stimuli.
Tables of Links
| TARGETS | |
|---|---|
| GPCRsa | Transportersd |
| β2-adrenoceptor | Ca2+-ATPases |
| EP1 receptor | Enzymese |
| EP4 receptor | Adenylyl cyclases |
| Ligand-gated ion channelsb | COX |
| IP3 receptor | Epac |
| Ryanodine receptor | PKA |
| Ion channelsc | PLCε |
| CFTR | |
| KCa1.1 | |
| KV7.1 |
| LIGANDS | |
|---|---|
| 2-APB | Clotrimazole |
| 8-pCPT-2′-O-Me cAMP | Forskolin |
| Albuterol | H2O2 |
| Amiloride | IP3 |
| ATP | Paxilline |
| cAMP | PGE2 |
| Clofilium |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,b,c,d,eAlexander et al., 2013a,b,c,d,e).
Introduction
Ion secretion and absorption by bronchial epithelia are essential elements of the mucociliary clearance mechanism, providing the osmotic conditions needed for appropriate water movement across the mucosa that results in ample luminal fluid for ciliary beating and mucus hydration (Boucher, 2007). Control of ion fluxes at the bronchial mucosal surface is complex and multifaceted. H2O2 is known to either directly or indirectly regulate several important components at the apical side of bronchial epithelial cells including a cAMP-regulated epithelial cell membrane Cl− channel [cytic fibrosis transmembrane regulator (CFTR)] (Cowley and Linsdell, 2002b), and Na+ and K+ channels (Liu et al., 2009; 2010; Ma, 2011; Downs et al., 2013). In airway epithelia, H2O2 stimulates anion secretion primarily by activating an autocrine PGE2 signalling pathway via EP4 and EP1 receptors, leading to CFTR-mediated Cl− secretion (Conner et al., 2010; 2013; Jones et al., 2012). Pharmacological analysis of H2O2 stimulation shows that the majority of the anion secretion response occurs through CFTR and that EP4 receptor signalling is the predominant stimulator as the majority of the H2O2-induced response was blocked by EP4 receptor antagonist (Conner et al., 2013). Both EP1 and EP4 receptors are GPCRs, with EP4 believed to act primarily through Gαs and EP1 to act primarily through Gq. As CFTR anion secretion is regulated by PKA phosphorylation, it is expected that H2O2 stimulation of CFTR will mostly occur through Gαs activation of transmembrane AC (tmAC), but that Gq-mediated increased intracellular [Ca2+] ([Ca2+]I) will also activate, although to a lesser extent, the Ca2+ activated tmACs and soluble AC (sAC) (Litvin et al., 2003).
A complex set of regulatory signals control CFTR gating. PKA phosphorylation is a key element, although other kinases and phosphatases (e.g. Chappe et al., 2003; Billet et al., 2013) are essential. CFTR is regulated by interaction with a large number of other proteins found in complexes containing CFTR (e.g. Kunzelmann and Mehta, 2013). Regulation of CFTR by extracellular signals is further adjusted via compartmentalization of cAMP signalling by actin cytoskeleton (Fanelli et al., 2008) and through co-localization with A-kinase anchor proteins and associated PDE activity (e.g. Sun et al., 2000).
The studies of H2O2 and GPCR-mediated activation of CFTR, described here, show that H2O2-mediated signalling to alter anion secretion occurs through both tmAC and sAC activities. In addition, the data show that cAMP not only drives PKA activity directly, but also leads to increased [Ca2+]i via exchange protein activated by cAMP (Epac) that, in turn, provides an amplified cAMP response through further increases in Ca2+-stimulated ACs, including sAC.
Methods
Solutions
For intact airway epithelial cells, the apical and basolateral bath solution (Krebs–Henseleit, KH) consisted of: 118 mM NaCl, 25 mM NaHCO3, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM NaH2PO4⋅H2O, 1.2 mM CaCl2⋅2 H2O, 5.5 mM glucose, pH 7.35 when gassed with 95% O2–5% CO2. For permeabilization of the basolateral membrane, the apical solution consisted of: 145 mM NaGluconate, 3.3 mM NaH2PO4, 0.8 mM Na2HPO4, 1.2 mM MgCl2⋅6 H2O, 4 mM CaCl2, 10 mM glucose and 10 mM HEPES, pH 7.35. The corresponding basolateral solution consisted of: 145 mM NaCl, 3.3 mM NaH2PO4, 0.8 mM Na2HPO4, 1.2 mM MgCl2⋅6 H2O, 1.2 mM CaCl2, 10 mM mannitol and 10 mM HEPES, pH 7.35 (Cowley and Linsdell, 2002b). Bicarbonate-free buffer consisted of: 133 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM NaH2PO4⋅H2O, 1.2 mM CaCl2⋅2 H2O, 5.5 mM glucose and 10 mM HEPES, pH 7.35.
Cell culture and lentivirus infection
All experiments used fully differentiated normal human bronchial epithelial (NHBE) cells in air–liquid interface (ALI) culture. Human lungs, not to be used for transplant, were obtained from the Life Alliance Organ Recovery Association following appropriate consent as determined by the Institution Review Board and in accordance with the Declaration of Helsinki. Cells were isolated from human lungs, cultured and differentiated at an ALI as described previously (Nlend et al., 2002; Fulcher et al., 2005). All experiments were performed using date- and lung-matched cultures. Cells were judged to be differentiated by the presence of beating cilia and mucus secretion (14–28 days at the ALI). For expression of shRNA, undifferentiated cells were infected with pLKO.1-based third-generation lentiviruses encoding shRNA for sAC (SHC002, non-targeted; TRCN0000078370, exon 2; TRCN0000078369, exon 15; Sigma-Aldrich) and selected with 1 μg·mL−1 puromycin until uninfected control cultures were dead and then puromycin removed. Cultures were then redifferentiated at the ALI before analysis.
Ussing chambers
Differentiated NHBE ALI cultures on Snapwells (Corning, Inc., Corning, NY, USA) were rinsed and mounted in KH buffer in EasyMount Ussing chambers (Physiologic Instruments, San Diego, CA, USA) at 37°C. The KH buffer was maintained at pH 7.35 when gassed with 95% O2/5% CO2. All experiments were performed in the presence of 10 μM amiloride in the apical chamber to block effects of the sodium channel. To monitor short circuit current (ISC), the transepithelial membrane potential was clamped at 0 mV with a six-channel voltage clamp (model VCC MC2, Physiologic Instruments) using Ag/AgCl electrodes in agar bridges. Signals were digitized and recorded with DAQplot software (VVI Software, College Station, PA, USA) via a LabJack A/D converter (LabJack Corp., Lakewood, CO, USA). The input resistance of each filter was measured by application of 1 mV bipolar pulses of 2 s duration. For permeabilization experiments, the appropriate solutions were bubbled with air and the cells were incubated with basolateral 100 μM nystatin before exposure to H2O2. Inhibitors were added 20–50 min before stimulation with H2O2 or agonists. For BAPTA experiments, 10 μM BAPTA-AM was added to mucosal and serosal compartments in KH buffer for 1 h before stimulation.
Microscopy
To assess changes in [Ca2+]i, differentiated NHBE ALI cultures were loaded with fura-2 AM (10 μM) in Dulbecco's PBS containing 1% glucose and 10% FBS for 2 h at room temperature. Cultures were washed and mounted in a perfusion chamber in KH buffer at room temperature and ratiometric images captured and quantified as described previously (Lieb et al., 2002).
To assess changes in PKA activation, NHBE cells were transduced with lentiviruses expressing PKA subunit FRET sensors (Zaccolo and Pozzan, 2008) as described previously (Schmid et al., 2006). Undifferentiated cultures were transduced and then redifferentiated at the ALI before experiments as fully differentiated cultures were resistant to viral transduction. Fully differentiated cultures were mounted in a perfusion chamber at room temperature and FRET signals (cyan fluorescent protein/yellow fluorescent protein ratios) were acquired and quantified using MetaFluor software as described previously (Schmid et al., 2010).
Data analysis
Changes in ISC were normalized to lung- and date-matched control cultures that were assayed simultaneously with experimental cultures and expressed as fraction of control ΔISC. This normalization controlled for variations in the magnitude of responses among lung donors. Replicate cultures from each lung donor and then all donors were averaged to give mean values for the fraction of control ΔISC. Mean values were compared by one-way anova and if significant differences were obtained, by the Tukey's Kramer honestly significant difference test. EC50 and IC50 values were calculated by nonlinear regression fit of the log of agonist concentrations versus normalized ΔISC responses.
Reagents
All reagents were from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise noted. DMEM, Ham's nutrient F-12 and HBSS were purchased from Gibco, Life Technologies (Grand Island, NY, USA). Clotrimazole, CFTRinh-172, forskolin, KH7, SQ22536, MDL-12,330A (Enzo Life Sciences, Farmingdale, NY, USA), BAPTA-AM, fura-2 AM (Invitrogen, Carlsbad, CA, USA), U-73343, gallein, 3-[5-(tert.-butyl)isoxazol-3-yl]-2-[2-(3-chlorophenyl)hydrazono]-3-oxopropanenitrile (ESI-09) and 8-pCPT-2′-O-methyl- adenosine 3′,5′-cyclic monphosphate-acetoxy methyl ester (8-pCPT-2′-O-Me-cAMP) were dissolved in DMSO. Both ESI-09 and 8-pCPT-2′-O-Me-cAMP were from Biolog, (Bremen, Germany). Amiloride, H89, clofilium, 4,4′-dinitrostilbene-2,2′-disulphonic acid and BaCl2 were dissolved in distilled H2O. Cay10598 (Cayman Chemicals, Ann Arbor, MI, USA) and U-73122 were dissolved in ethanol. Paxilline and 2-aminoethyl diphenyl borinate (2-APB) were dissolved in methanol. Nystatin was dissolved in distilled H2O (or bath solution) and sonicated before use.
Results
Role of tmACs and sAC in H2O2-mediated CFTR activation
H2O2-mediated anion secretion by bronchial epithelial cells is known to occur through stimulation of both EP4 and EP1 PG receptors (Joy and Cowley, 2008; Jones et al., 2012; Conner et al., 2013). The magnitude and duration of the H2O2 response depends on the concentration, with higher H2O2 leading to transient changes and lower H2O2 leading to sustained increases in ISC (Conner et al., 2013), and both sustained and transient changes occurring through activation of COX and stimulation of EP1 and EP4 receptors to increase CFTR activity (Joy and Cowley, 2008; Jones et al., 2012; Conner et al., 2013). Using receptor antagonists, EP4 stimulation was shown to contribute the larger proportion of CFTR activation (Conner et al., 2013). To examine the EP4 receptor signalling mechanisms that lead to H2O2-mediated CFTR activation, MDL-12,330A and KH7, inhibitors of tmAC and sAC, respectively (Hess et al., 2005; Bitterman et al., 2013), were used to block H2O2-stimulated CFTR currents (in the presence of amiloride to eliminate Na+ channel contributions) in fully differentiated NHBE cells mounted in Ussing chambers (Figure 1). MDL-12,330A inhibited H2O2-mediated stimulation of CFTR currents with an IC50 of 6.2 μM that was nearly identical to its inhibition of forskolin stimulation (IC50 = 7.3 μM) suggesting that inhibition of H2O2 stimulation occurred by blocking tmACs (Figure 1A). However, KH7 also inhibited 1 mM H2O2 stimulation of anion secretion with an IC50 = 2 μM, similar to the published values for sAC (Hess et al., 2005; Bitterman et al., 2013). Similar results were obtained using a lower concentration of H2O2 (Supporting Information Fig. S1). KH7 did inhibit forskolin stimulation of CFTR currents only at higher concentrations and did not block all activity (IC50 = 26 μM; Figure 1B). To rule out the possibility that MDL-12,330A also inhibits sAC with an IC50 equivalent to its action against tmAC and that sAC is responsible for all activation of CFTR via H2O2, SQ 22536 was used and showed that the H2O2 response was also sensitive to this tmAC inhibitor (data not shown). These data suggest that inhibition of tmACs and sAC were involved in the H2O2 response and that inhibition of either could block the majority of the response.
Figure 1.
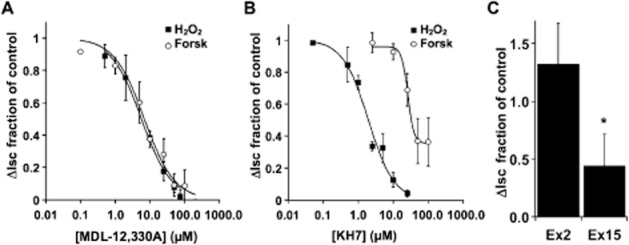
H2O2 stimulates both tmAC and sAC. Fully differentiated NHBE cells in ALI culture were mounted in Ussing chambers and stimulated with either H2O2 (1 mM) or forskolin (10 μM) in the presence of various concentrations of the tmAC inhibitor MDL-12,330A (A, n = 3–6 lung donors at each concentration) or various concentrations of the sAC inhibitor KH7 (B, 3–6 lung donors at each concentration). (C) NHBE cells were infected with shRNA expressing lentiviruses targeted to either exon 2 or exon 15 of sAC or with non-targeted lentiviruses. After differentiation, cultures were mounted in Ussing chambers and stimulated with H2O2 (1 mM). Compared with non-target controls and exon 2-targeted cultures, the response of exon 15 targeted cultures was significantly reduced (n = 5 cultures from two lung donors, *P < 0.05).
To confirm a role for sAC, undifferentiated NHBE cells were infected with lentivirus encoding sAC-specific shRNAs, directed to either exon 2 or exon 15, or non-targeted shRNA. Following redifferentiation, the CFTR response to H2O2 of NHBE cultures infected with shRNA targeted to sAC exon 15 was reduced when compared with control cultures infected with non-targeted shRNA virus. Exon 2 sAC shRNA was not different from the control (Figure 1C; P < 0.05, n = 5 cultures from each of two lung donors). sAC mRNA undergoes a variety of alternative splices (Chen et al., 2014) and is a rare message in bronchial epithelia that renders assessment of both mRNA and protein reductions after knock-down impossible. It is not clear which alternatively spliced form of sAC plays a role in the response, and the lack of an effect by the exon 2-targeted shRNA may simply be due to the efficacy of this shRNA sequence. However, the clear reduction of the physiological response to H2O2 following infection with shRNA to sAC strongly supports the pharmacological data mentioned earlier that indicated sAC plays an essential role in CFTR stimulation in addition to tmAC.
H2O2 stimulates sAC through increased [Ca2+]i
sAC activity is stimulated by both Ca2+ and HCO3− (Litvin et al., 2003). H2O2 leads to increases in [Ca2+]i in a number of cell types (e.g. Hayashi et al., 1989; Rice et al., 1992; Meyer et al., 1996; Nakazaki et al., 2000). To assess whether H2O2 treatment of NHBE cells raised [Ca2+]i, fluorescence changes in fura–2-loaded cells were followed by microscopy and showed a transient increase in [Ca2+]i (Figure 2A). As sAC is stimulated by increases in [Ca2+]i, NHBE cultures were loaded with BAPTA-AM before being placed into Ussing chambers and treated with H2O2. BAPTA loading significantly reduced H2O2-mediated CFTR activation (Figure 2B and C), supporting the idea that increased [Ca2+]i was the signal for sAC activation. The PLC inhibitor U-73122, but not the mostly inactive analogue U-73343 (e.g. Horowitz et al., 2005), blocked H2O2-mediated CFTR activity increases at the expected concentration (Figure 2D and E). The IP3 receptor antagonist 2-APB also blocked H2O2 stimulation (Figure 2F) suggesting that IP3-mediated Ca2+ release was an important element in sAC activation following H2O2. Interestingly, neither nominal outside Ca2+ nor exchange of Ca2+ for Sr2+ in external buffers reduced the H2O2-mediated CFTR current, suggesting that Ca2+ entry did not play a significant role in increasing [Ca2+]i (Figure 2F).
Figure 2.
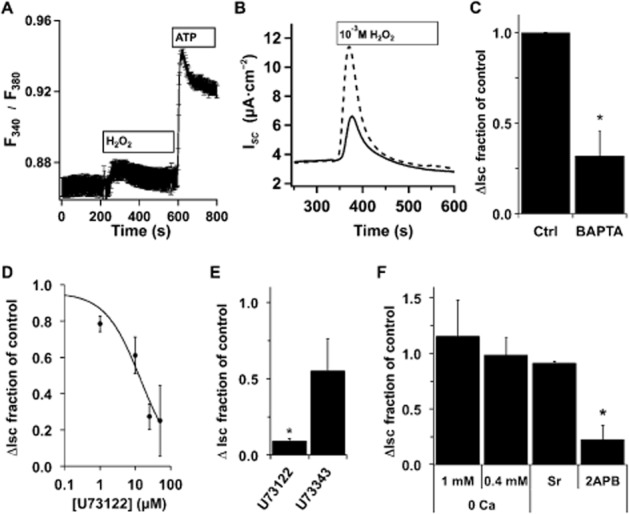
H2O2-mediated [Ca2+]i increases stimulate anion secretion. (A) Fully differentiated NHBE cells in ALI culture were loaded with fura-2AM and mounted in a perfusion chamber on a Nikon E600fn microscope (Nikon Inc., Melville, NY, USA). H2O2 (400 μM) led to an increase in [Ca2+]i. Subsequent perfusion with ATP (10 μM) led to the expected robust response (ratio plotted is the mean of 16 cells from 1 donor ± SEM, representative of experiments with three individual lungs). For comparison of ATP-stimulated ISC changes, see Supporting Information Fig. S3. (B) Representative ISC traces of fully differentiated NHBE cells in ALI culture loaded with 25 μM BAPTA-AM (solid trace) before they were mounted in Ussing chambers and stimulated with H2O2 (1 mM) compared with control (unloaded cells; dashed trace). (C) BAPTA-loaded cultures had a reduced anion secretion response to 1 mM H2O2 (mean ± SEM, n = 6 lung donors, two to three cultures per donor, *P < 0.05). (D) NHBE ALI cultures in Ussing chambers were pretreated with different concentrations of the PLC inhibitor U73122 and then stimulated with H2O2 (1 mM) in the presence of inhibitor. U73122 led to a concentration-dependent decrease in anion secretion with an apparent IC50 = 10 μM (n = 3–4 lung donors at each concentration). (E) Comparison of U73122 (25 μM) with the less active isomer U73343 (25 μM) showed specificity. (F) NHBE ALI cultures were mounted in Ussing chambers and stimulated with H2O2 (1 or 0.4 mM) in the presence or absence of extracellular Ca2+, in the presence of Sr2+ instead of Ca2+ or in the presence of 2-APB (200 μM). Neither removal of Ca2+ nor substitution of Ca2+ with Sr2+ significantly reduced anion secretion (n = 3 lung donors), while addition of the IP3 receptor antagonist 2-APB significantly reduced anion secretion (n = 5 lung donors, *P < 0.05).
CO2 and HCO3− also stimulate sAC activity (Litvin et al., 2003). To evaluate their role in H2O2-mediated sAC activation, H2O2 stimulation of CFTR activity in Ussing chambers was measured in HCO3− free buffer in the presence and absence of acetazolamide (1 mM) to inhibit carbonic anhydrase. Neither HCO3−-free buffers (Figure 3) nor carbonic anhydrase inhibition (data not shown) reduced the amplitude of H2O2-stimulated CFTR ISC, although the time to return to baseline was extended compared with HCO3−-containing buffers. However, KH7 similarly reduced anion currents in these conditions compared with controls (Figure 3B). Thus, sAC stimulation after H2O2 treatment appeared to depend only on changes in [Ca2+]i and not CO2/HCO3−.
Figure 3.
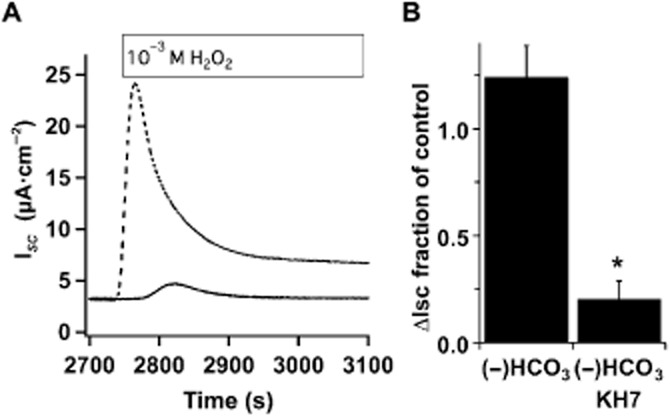
H2O2 stimulation of sAC is not HCO3− dependent. (A) Representative ISC traces of fully differentiated NHBE cells in an ALI culture mounted in an Ussing chamber containing KH without HCO3−, buffered with 10 mM HEPES pH 7.4 and stimulated with 1 mM H2O2 in the absence (dashed trace) or presence of KH7 (solid trace, 25 μM). (B) Removal of HCO3− did not reduce anion secretion in response to H2O2 (n = 5 lungs), and the H2O2 response was sensitive to KH7 in the absence of bicarbonate (n = 3 lungs, *P < 0.05).
Mechanism of H2O2-stimulated increases in [Ca2+]I
The majority of the H2O2 stimulation of anion currents appears to be due to signalling through EP4 receptors (Conner et al., 2013) that acts primarily through Gαs to stimulate tmAC, but not sAC (Braun et al., 1977; Buck et al., 1999). Although the EP1 receptor also contributes to H2O2 activation of CFTR via Gq stimulation of PLC, it appears to be responsible for only a small fraction of the H2O2 response (Conner et al., 2013). Thus, it was initially surprising that KH7 inhibition of sAC blocked such a large portion of the H2O2-mediated stimulation of anion secretion, as sAC is insensitive to G-protein stimulation (Buck et al., 1999). Several possible mechanisms were examined that might explain the apparent H2O2-mediated EP4 receptor signalling through increased [Ca2+]i and sAC.
PGs have been shown to signal through receptor coupling to Gβγ to regulate Ca2+ channels. Gallein (200 μM), an inhibitor of Gβγ activity, was added before H2O2 stimulation and measurement of anion secretion showed no effect with short or long pre-incubation (1–18 h; data not shown). To rule out direct H2O2 action on sAC or on IP3 receptors (Zheng and Shen, 2005), cultures were treated with an EP4 receptor agonist (Cay10598, 50 nM) instead of H2O2. The EP4 receptor stimulates anion secretion primarily through CFTR (Figure 4A). KH7 or MDL-12,330A (both at 25 μM) blocked nearly 75% of the EP4 receptor-mediated response (Figure 4B and C). The concentration-dependence of KH7 inhibition confirmed its specificity in EP4 agonist-stimulated cells (IC50 = 8 μM, Figure 4D). Thus, direct H2O2 stimulation of downstream effectors was not responsible for KH7 sensitivity.
Figure 4.
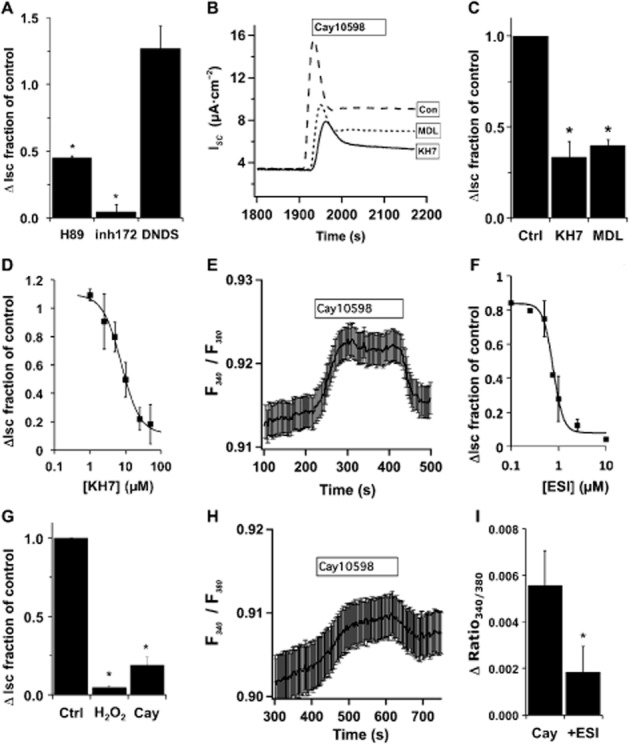
EP4 stimulation of CFTR activates sAC through Epac and increased [Ca2+]i. (A) NHBE ALI cultures in Ussing chambers were stimulated with Cay10598 (50 nM) in the presence or absence of H89 (10 μM), CFTRinh172 (10 μM) or 4,4′-dinitro-stilbene-2,2′-disulphonic acid (DNDS; 100 μM) (n = 4 lung donors for each inhibitor, *P < 0.05). Both the kinase inhibitor H89 and the CFTR inhibitor blocked EP4 receptor-mediated anion secretion, while DNDS has no effect, consistent with CFTR activation. (B) ISC traces of NHBE ALI cultures mounted in Ussing chambers and stimulated with Cay10598 (50 nM) in the presence or absence of KH7 (25 μM) or MDL12,330A (25 μM). (C) Anion secretion was significantly reduced by each inhibitor (n = 5 lung donors, *P < 0.05). (D) NHBE ALI cultures were stimulated with Cay10598 in the presence of different concentrations of KH7 (n = 3–6 lung donors at each concentration, apparent IC50 = 8 μM). (E) NHBE ALI cultures were loaded with fura2-AM, mounted in a perfusion chamber and imaged by epifluorescence microscopy. Addition of Cay10598 (100 nM) to the perfusate increased [Ca2+]i that returned towards baseline after removal of the agonist (representative trace is the mean fura-2 ratio recorded from regions of interest in 16 cells from a single lung donor ± SEM, and is representative of experiments with three individual donors). (F) The Epac inhibitor ESI-09 blocked H2O2-induced changes in ISC with an apparent IC50 = 0.8 μM (n = 1–5 lung donors at each concentration), and ESI-09 (10 μM) attenuated Cay10598-induced anion secretion (G; n = 4 lung donors, *P < 0.05 compared with control no ESI-09). (H) Pretreatment with ESI-09 (10 μM) also attenuated changes in Cay10598-induced fura-2 fluorescence (representative trace of the mean fura-2 ratio recorded from regions of interest in 19 cells from a single donor ± SEM, and is representative of experiments with three individual donors). (I) Cay10598-induced fura-2 fluorescence changes were significantly altered by ESI-09 (10 μM; n = 4 lung donors, P < 0.05).
To demonstrate that the EP4 receptor signalled to increase [Ca2+]i, NHBE cultures were loaded with fura-2 and stimulated with Cay10598. An increase in the 340 nm/380 nm fluorescence ratio confirmed that EP4 receptor activation led to an increase in [Ca2+]i (Figure 4E). In agreement, U-73122 also blocked Cay10598 responses (IC50 = 8 μM, data not shown). Based on the data presented earlier, a large fraction of H2O2-mediated EP4 signalling most likely results from increased [Ca2+]i that activates sAC despite EP4 receptors coupling to Gαs and not Gq. cAMP can stimulate PLC through exchange protein directly activated by cAMP (Epac) (Schmidt et al., 2001) and Epac1 is expressed in fully differentiated NHBE cells (Supporting Information Fig. S2). ESI-09, a specific Epac inhibitor (Almahariq et al., 2013), blocked H2O2 with the expected IC50 (0.8 μM, Figure 4F) and also blocked EP4 receptor stimulated anion secretion (10 μM) (Figure 4G), suggesting that increased [Ca2+]i could be due to activation of PLC (shown earlier) by Epac. Pre-incubation and inclusion of ESI-09 during perfusion of fura–2-loaded cells attenuated Cay10598-mediated increases in [Ca2+]i (Figure 4H and I). Thus, the data support the idea that EP4 receptor stimulation of tmAC not only produces cAMP to stimulate CFTR anion secretion directly by PKA, but also amplifies the response by activation of Epac to increase [Ca2+]i and activate sAC to further increase cAMP production. Interestingly, ESI-09 blocked a large portion of H2O2-stimulated anion secretion suggesting that a significant portion of PKA-stimulated CFTR activity was a result of Ca2+-promoted cAMP production by sACs and tmACs.
It was possible that other Gαs-coupled receptor cAMP signals might also activate Epac and lead to Ca2+-mediated sAC stimulation and similar amplification. In fact, albuterol (10 μM) stimulation of β2-adrenoceptors to activate CFTR in NHBE cultures was partially sensitive to KH7 treatment (Figure 5A). Figure 1B shows that the KH7 partially inhibited forskolin-stimulated CFTR activity at a higher dose despite being ineffective against tmAC (Bitterman et al., 2013). Given the intricacies of CFTR activity regulation by association with complexes containing regulatory components and with tmAC (Namkung et al., 2010), and based on the clear specificity of KH7 for sAC over tmAC (Bitterman et al., 2013), it was possible that the partial KH7 inhibition of forskolin responses reflected activation of sAC via Epac-mediated [Ca2+]i transients. To assess whether forskolin-stimulated cAMP increases were due to Epac stimulation, NHBE cultures were transduced with PKA-GFP fusion proteins as FRET sensors to report PKA activation (Zaccolo and Pozzan, 2008). Increased PKA activation in response to forskolin was partially inhibited by pre-incubation with ESI-09 consistent with the hypothesis that a portion of the forskolin-induced CFTR response was Epac mediated (Figure 5B and C). Thus, the data support a role for sAC in activation of CFTR currents independent of bicarbonate changes (Wang et al., 2005) and during stimulation by GPCRs rather than only contributing to basal CFTR activity (Sun and Bonanno, 2002).
Figure 5.
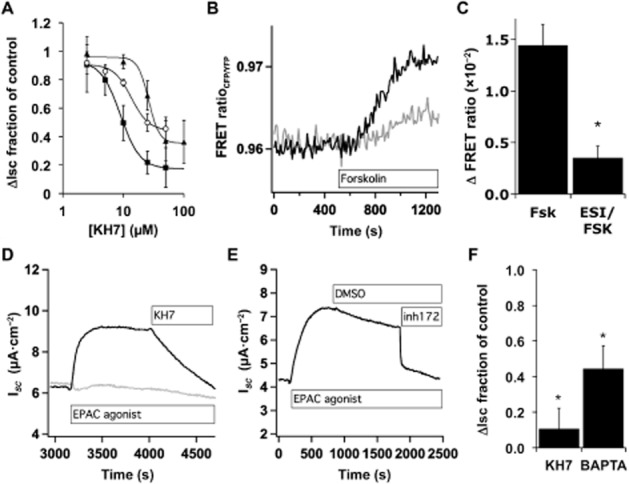
Epac inhibition partially blocks forskolin stimulation of CFTR. (A) NHBE ALI cultures in Ussing chambers were stimulated with Cay10598 (100 nM, squares), albuterol (10 μM, circles) or forskolin (10 μM, triangles) in the presence of KH7. Forskolin trace is from Figure 1A. Values plotted are the mean ± SEM, n = 3–6 lung donors at each point. (B) NHBE ALI cultures that were transduced with lentiviruses encoding fluorescent PKA subunits, mounted in a perfusion chamber in the presence or absence of ESI-09 (10 μM) and imaged by epifluorescence microscopy during forskolin (10 μM) stimulation. Shown are example traces from a single control cell (black trace) and a single-cell pretreated with ESI-09 (grey trace). (C) Maximum forskolin-induced changes in the FRET ratio are shown with and without ESI-09 (mean ± SEM, n = 17, eight to nine cells from each of two donors). (D, E) NHBE ALI cultures in Ussing chambers were stimulated with 8-pCPT-2′-O-Me-cAMP (20 μM) and the increased ISC was blocked by addition of KH7 (25 μM, D), by BAPTA loading (D, grey trace) and by CFTRinh172 (5 μM, E). (F) NHBE ALI cultures in Ussing chambers stimulated with 8-pCPT-2′-O-Me-cAMP and treated with KH7 or were stimulated with 8-pCPT-2′-O-Me-cAMP with or without loading with BAPTA-AM (n = 3 lung donors).
To confirm a role for Epac in increasing [Ca2+]i and stimulating sAC and CFTR, NHBE cultures were stimulated with the Epac agonist 8-pCPT-2′-O-Me-cAMP (20 μM) and then treated with either KH7 (Figure 5D and 5F) or CFTRinh172 (Figure 5E). Both blocked the Epac agonist-induced ISC. BAPTA-AM loading of cells prior to stimulation reduced the 8-pCPT-2′-O-Me-cAMP-induced ISC (Figure 5D and F). Thus, Epac1 activation appears to mimic, in part, the stimulation of ISC seen following H2O2 treatment or stimulation of Gαs-coupled receptors.
Role of potassium channels in H2O2-stimulated anion secretion
Apical, PKA-stimulated CFTR Cl− secretion from bronchial epithelia is known to rely on both apical and basolateral K+ channel activity that provides a driving force for Cl− secretion (e.g. Mall et al., 2000; Cowley and Linsdell, 2002a,b,; Manzanares et al., 2011), and both increased [Ca2+]i and cAMP/PKA are key in regulating these channels. Previously, others have shown that sAC stimulates basolateral K+ secretion via Kv7.1 from colon epithelia following epinephrine stimulation of the β2-adrenergic receptor that also works via Gαs, but through an unknown mechanism (Halm et al., 2010). Inclusion of the K+ channel inhibitors Ba2+ (5 mM, nonspecific) and clofilium (100 μM, Kv7.1 containing complexes), but not clotrimazole (30 μM, KCa channels) in the serosal compartment of NHBE ALI cultures abrogated the H2O2-stimulated ISC in Ussing chambers (Figure 6A and B) confirming the published data of others (e.g. Cowley and Linsdell, 2002a). However, none of the compounds added to the apical compartment had an effect on H2O2-stimulated currents (data not shown). Thus, as previously reported, a cAMP-activated basolateral K+ channel (response was clofilium sensitive, Cowley and Linsdell, 2002a,b), but not a Ca2+-activated K+ channel (response was clotrimazole insensitive) was needed for apical Cl− secretion. However, cAMP from sAC activity was not acting to stimulate basolateral K+ channel activity following H2O2 treatment, as H2O2 stimulation of basolaterally permeabilized cells was still sensitive to KH7 (Figure 6C). Interestingly, basolateral permeabilization eliminated the MDL-12,330A sensitivity of H2O2-stimulated CFTR activity suggesting that H2O2-stimulated tmAC activity might predominantly stimulate basolateral K+ channels (Figure 6C).
Figure 6.
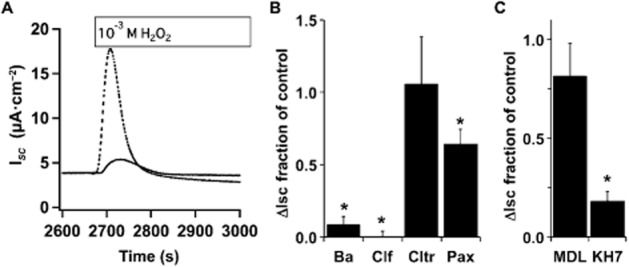
H2O2-mediated stimulation of CFTR via sAC occurs in basolaterally permeabilized cells. (A) Representative ISC traces of NHBE cells in ALI culture mounted in Ussing chambers and stimulated with H2O2 (1 mM) with (solid trace) or without Ba2+ (dashed trace, 5 mM). (B) NHBE ALI cultures mounted in Ussing chambers and stimulated with H2O2 in the presence or absence of basolateral Ba2+ (5 mM), clofilium (100 μM), clotrimazole (30 μM) or apical paxilline (4 μM). Compared with controls basolateral Ba2+ and clofilium and apical paxilline blocked H2O2 responses while clotrimazole had no effect (n = 5–8 lung donors, *P < 0.05). (C) NHBE cells in ALI culture were mounted in buffer containing 100 μM nystatin to permeabilize the basolateral membrane and with or without MDL-12,330A (75 μM) or KH7 (20 μM). After stabilization of baseline ISC, H2O2 was added to the apical compartment. KH7 inhibited H2O2 stimulation while MDL-12,330A inhibition was reduced by basolateral membrane permeabilization (n = 3 lung donors, *P < 0.05 compared with controls).
As increased [Ca2+]i was seen after H2O2 treatment and as the KCa1.1 channel was recently shown to play a role in NHBE apical ion secretion (Manzanares et al., 2011), the effect of a specific KCa1.1 channel blocker, paxilline, was tested [the KCa1.1 channel is insensitive to Ba2+ (Miller et al., 1987; Neyton and Miller, 1988)]. Paxilline (4 μM) blocked about 30% of the H2O2-stimulated ISC when included in the mucosal compartment (Figure 6B) suggesting that the KCa1.1 channel was activated and played a role in H2O2-stimulated apical Cl− secretion. As expected from previous reports, paxilline only inhibited a fraction of the H2O2-stimulated current. These data suggest that in bronchial epithelia sAC does not play a role in basolateral K+ secretion as shown for intestinal epithelia, and that apical KCa1.1 channels may be stimulated by increased [Ca2+]i during H2O2 stimulation.
Discussion and conclusions
H2O2 stimulates bronchial epithelial cells to increase CFTR anion secretion and presumably increase available fluid on the mucosal surface. H2O2 is produced by Duox1 and Duox2 in NHBE cells and the H2O2 concentrations in the range used in these experiments are possibly encountered during inflammation and/or with impaired H2O2 consumption (El-Chemaly et al., 2003; Conner et al., 2013). H2O2 is produced as a substrate for lactoperoxidase (Conner et al., 2002; Geiszt et al., 2003) and also serves to influence signalling in cells (Strengert et al., 2014). During periods of high H2O2 production by Duox (Harper et al., 2005; Gattas et al., 2009) or loss of lactoperoxidase activity (e.g. following cigarette smoke exposure Reznick et al., 2003), increased H2O2 may therefore stimulate anion secretion as a compensatory mechanism to maintain adequate fluid levels at the airway surface.
H2O2 stimulation operates primarily through an autocrine pathway that activates EP4 receptors and to a lesser extent through activation of EP1 receptors (Conner et al., 2013). The present study demonstrates that both tmAC and sAC are activated by H2O2 through these G-protein coupled PG receptors and that the EP4 receptor, which primarily signals through Gαs, also activates sAC through Epac-stimulated Ca2+ crosstalk which serves to amplify cAMP signalling in a positive feedback loop to further increase CFTR anion secretion (Figure 7). The studies also suggest that, in bronchial epithelial cells, stimulation of β2-adrenoceptor results in tmAC-catalysed cAMP increases to activate Epac and increase [Ca2+]i to stimulate sAC and thus extends the previously reported effects of Epac after β2-adrenoceptor stimulation (Schmidt et al., 2001). Thus, this cAMP amplification may be a more general mechanism to indirectly couple tmAC and sAC activities in bronchial epithelial cells and amplify GPCR cAMP signals. Calcium dependence of the amplification loop provides a pathway for termination of the signal via re-equilibration of [Ca2+]i.
Figure 7.
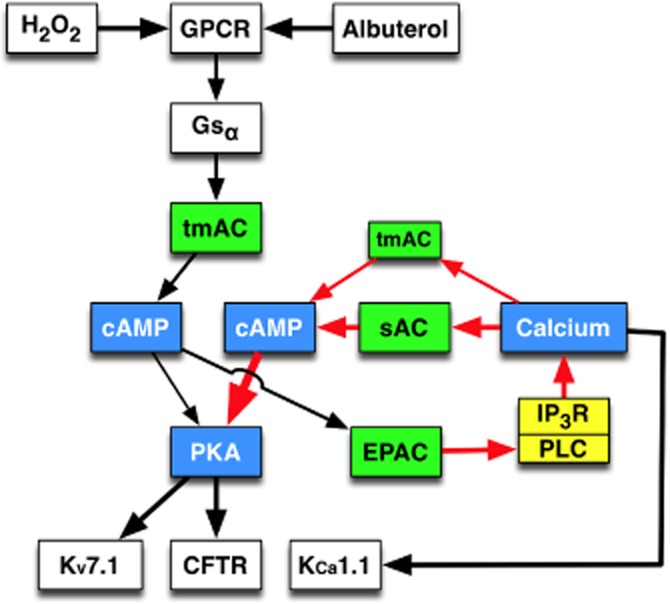
Proposed model for H2O2 stimulation of CFTR. In differentiated NHBE cells, H2O2 activates the GPCRs EP1 and EP4. EP4, that appears responsible for the majority of the H2O2 response (Conner et al., 2013), and albuterol both activate Gαs to stimulate tmAC. Increased cAMP activates PKA to stimulate CFTR-mediated Cl− secretion and basolateral Kv7.1 K+ secretion. Also, cAMP activation of Epac initiates a Ca2+ signal presumably through PLC stimulation. This Epac-dependent Ca2+ signal stimulates both tmAC and sAC to amplify the cAMP/PKA pathway.
The role of sAC was confirmed by both KH7 sensitivity and by shRNA-mediated reduction in CFTR activity. Several shRNA sequences were examined and one targeted to exon 15 significantly reduced the H2O2 response. sAC is alternatively spliced in airway epithelia (Chen et al., 2014), but the exact forms playing a role here are not known. However, the lack of effect using exon 2-targeted sequence may reflect an ineffective shRNA sequence rather than presence or absence of this exon in transcripts. Despite these data supporting a sAC role, the CFTR activity was not sensitive to removal of HCO3− in the presence or absence of carbonic anhydrase inhibitor suggesting that sAC regulation by HCO3− is not fully understood.
The initial observations that inhibitors of both tmAC and sAC inhibited H2O2-mediated anion secretion were expected as H2O2 was shown previously to stimulate both EP1 and EP4 receptors (Jones et al., 2012; Conner et al., 2013) that act through Ca2+ and cAMP respectively. On the other hand, it was surprising that both inhibitors were able to block nearly all H2O2 stimulation and that both inhibitors substantially blocked EP4 receptor stimulation that is believed to work through Gαs activation of tmAC. The observations that an Epac inhibitor was able to nearly abolish the H2O2 response and to block the EP4 receptor-meidated response were equally unexpected as CFTR is activated by PKA phosphorylation. The model suggested in Figure 7 can account for the dual sAC and tmAC dependence of the CFTR response as the initial cAMP generated by Gαs stimulation would be necessary to initiate the activation of sAC that contributes a large fraction of cAMP for PKA phosphorylation of CFTR. Consistent with this idea, inhibition of either PLC or the IP3 receptor to interrupt the Ca2+ signal blocked nearly all of the EP4 receptor-mediated and H2O2 responses. PLC is a multifunctional family of lipases and PLCε is known to be stimulated downstream of Epac (Schmidt et al., 2001) consistent with the data presented here. Together, these data suggested that an amplification mechanism was at work that included Epac activity and PLC-driven increases in [Ca2+]i.
It is known that Gαs-coupled receptors can increase intracellular Ca2+ through a variety of mechanisms in addition to Epac. For example, PG signalling has been shown to be coupled through Gβγ (Speirs et al., 2010), but Gβγ did not appear to mediate H2O2 stimulation as it was unaffected by gallein. Stimulation of the EP4 receptor was previously shown to activate Epac in rat neointimal formation (Yokoyama et al., 2008), and an Epac inhibitor supported a role for Epac in increasing [Ca2+]i that results in stimulation of both sAC and tmAC and increased activation of CFTR. Recently, the action of the Epac inhibitor ESI-09 was suggested to be due to a non-specific thermal instability (Rehmann, 2013). The IC50 for ESI-09 effects on H2O2 signalling was 0.8 μM well below the ESI-09 concentration (50–100 μM) demonstrated to have induced thermal instability.
Epinephrine stimulation of β-adrenergic receptor was previously shown to activate a sAC-dependent K+, but not Cl−, secretion pathway in colon epithelium (Halm et al., 2010). This sAC-dependent pathway affected basolateral K+ channels; however, basolaterally permeabilized bronchial epithelial cells retained KH7 sensitivity of H2O2 stimulation suggesting that basolateral KV7.1 found in bronchial epithelia was not the sAC-sensitive component of H2O2-stimulated anion currents. Conversely, H2O2-stimulated CFTR currents lost sensitivity to tmAC inhibitor after basolateral permeabilization suggesting that tmAC was perhaps responsible for regulating basolateral K+ secretion after H2O2 stimulation.
Clearly, regulation of [Ca2+]i is complex. For example, in addition to Epac regulation of [Ca2+]i, H2O2 might also alter either plasma membrane or ER Ca2+ ATPases or the ryanodine receptor (RyR) that is expressed in airway epithelia. It is possible that H2O2 stimulates anion secretion through other pathways in addition to Ca2+ activation of sAC. H2O2 is known to inhibit phosphotyrosine phosphatases and to potentiate tyrosine kinase activities that are known to stimulate CFTR (Billet and Hanrahan, 2013; Billet et al., 2013), and H2O2 can also stimulate KCa1.1 channels (Liu et al., 2009) to increase the driving force for Cl− exit. In addition, others have speculated that H2O2 directly activates sAC in sperm during fertilization (Rivlin et al., 2004). Thus, although the data presented here support the idea that H2O2 stimulates sAC through EP4 receptor activation and increased [Ca2+]i, we cannot rule out that H2O2 is additionally stimulating anion secretion through other pathways.
Acknowledgments
This work was supported by National Institutes of Health grants HL066125 to G. E. C., HL-60644 and HL-89399 to M. S., and by FAMRI grants CIA 123060 to G. E. C. and CIA 103027 to M. S. The authors thank Nathalie Baumlin and Monica Valencia Gattas for excellent technical assistance and Drs Irene Litosch and Gregory Holt for very helpful discussions.
Glossary
- AC
adenylyl cyclase
- 2-APB
2-aminoethyl diphenyl borinate
- 8-pCPT-2′-O-Me-cAMP
8-pCPT-2′-O-methyl- adenosine 3′,5′-cyclic monphosphate-acetoxy methyl ester
- ALI
air–liquid interface
- ESI-09
3-[5-(tert.-butyl)isoxazol-3-yl]-2-[2-(3-chlorophenyl)hydrazono]-3-oxopropanenitrile
- ISC
short circuit current
- KH
Krebs–Henselait buffer
- NHBE
normal human bronchial epithelia
Author contributions
P. I. collected the research data, M. S. and G. E. C. were responsible for the research design, and all the authors contributed to the writing of the paper.
Conflict of interest
None.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
http://dx.doi.org/10.1111/bph.12934
Figure S1 Fully differentiated NHBE cells in ALI culture were mounted in Ussing chambers and stimulated with 400 μM H2O2 (example trace in panel a). Panel b, H2O2 (400 μM) stimulation of anion currents after preincubation and in the presence of various concentrations of the the sAC inhibitor KH7 showed an apparent IC50 = 1.4 μM (n = 1–3 lung donors at each concentration).
Figure S2 Total RNA was extracted from fully differentiated NHBE cells in ALI culture derived from two lung donors (E.Z.N.A Omega Bio-Tek, Norcross, GA, USA), reverse transcribed (iScript cDNA synthesis kit; Bio-Rad, Hercules, CA, USA) and assayed for presence of Epac1 (TaqMan assay Hs00183449_m1), MUC5AC (TaqMan assay Hs01365601_m1) and GAPDH as a reference mRNA (TaqMan assay 4352934E). The data demonstrate expression of Epac1 at a level slightly less than the differentiation product Muc5AC.
Figure S3 Fully differentiated NHBE cells in ALI culture were mounted in Ussing chambers and stimulated with 10 μM ATP. The trace shows an initial abrupt ISC peak thought to be primarily Ca2+ activated Cl− current and a later, more gradual response believed to be a sum of CFTR and Ca2+ activated Cl− currents (e.g. Namkung et al., 2010).
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ligand-gated ion channels. Br J Pharmacol. 2013b;170:1582–1606. doi: 10.1111/bph.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ion channels. Br J Pharmacol. 2013c;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Transporters. Br J Pharmacol. 2013d;170:1706–1796. doi: 10.1111/bph.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013e;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almahariq M, Tsalkova T, Mei FC, Chen H, Zhou J, Sastry SK, et al. A novel EPAC-specific inhibitor suppresses pancreatic cancer cell migration and invasion. Mol Pharmacol. 2013;83:122–128. doi: 10.1124/mol.112.080689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billet A, Hanrahan JW. The secret life of CFTR as a calcium-activated chloride channel. J Physiol. 2013;591(Pt 21):5273–5278. doi: 10.1113/jphysiol.2013.261909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billet A, Luo Y, Balghi H, Hanrahan JW. Role of tyrosine phosphorylation in the muscarinic activation of the cystic fibrosis transmembrane conductance regulator (CFTR) J Biol Chem. 2013;288:21815–21823. doi: 10.1074/jbc.M113.479360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman JL, Ramos-Espiritu L, Diaz A, Levin LR, Buck J. Pharmacological distinction between soluble and transmembrane adenylyl cyclases. J Pharmacol Exp Ther. 2013;347:589–598. doi: 10.1124/jpet.113.208496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher RC. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu Rev Med. 2007;58:157–170. doi: 10.1146/annurev.med.58.071905.105316. [DOI] [PubMed] [Google Scholar]
- Braun T, Frank H, Dods R, Sepsenwol S. Mn2+-sensitive, soluble adenylate cyclase in rat testis. Differentiation from other testicular nucleotide cyclases. Biochim Biophys Acta. 1977;481:227–235. doi: 10.1016/0005-2744(77)90155-3. [DOI] [PubMed] [Google Scholar]
- Buck J, Sinclair ML, Schapal L, Cann MJ, Levin LR. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc Natl Acad Sci U S A. 1999;96:79–84. doi: 10.1073/pnas.96.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappe V, Hinkson DA, Zhu T, Chang XB, Riordan JR, Hanrahan JW. Phosphorylation of protein kinase C sites in NBD1 and the R domain control CFTR channel activation by PKA. J Physiol. 2003;548(Pt 1):39–52. doi: 10.1113/jphysiol.2002.035790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Baumlin N, Buck J, Levin LR, Fregien N, Salathe M. A soluble adenylyl cyclase form targets to axonemes and rescues beat regulation in sAC knockout mice. Am J Respir Cell Mol Biol. 2014 doi: 10.1165/rcmb.2013-0542OC. doi: 10.1165/rcmb.2013-0542OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner GE, Salathe M, Forteza R. Lactoperoxidase and hydrogen peroxide metabolism in the airway. Am J Respir Crit Care Med. 2002;166(12 Pt 2):S57–S61. doi: 10.1164/rccm.2206018. [DOI] [PubMed] [Google Scholar]
- Conner GE, Ivonnet P, Salathe M. Hydrogen peroxide activates prostanoid receptors to increase anion secretion by human bronchial epithelial cells. Am J Respir Crit Care Med. 2010;181:A4227. [Google Scholar]
- Conner GE, Ivonnet P, Gelin M, Whitney P, Salathe M. H2O2 stimulates cystic fibrosis transmembrane conductance regulator through an autocrine prostaglandin pathway, using multidrug-resistant protein-4. Am J Respir Cell Mol Biol. 2013;49:672–679. doi: 10.1165/rcmb.2013-0156OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley EA, Linsdell P. Characterization of basolateral K+ channels underlying anion secretion in the human airway cell line Calu-3. J Physiol. 2002a;538:747–757. doi: 10.1113/jphysiol.2001.013300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley EA, Linsdell P. Oxidant stress stimulates anion secretion from the human airway epithelial cell line Calu-3: implications for cystic fibrosis lung disease. J Physiol. 2002b;543:201–209. doi: 10.1113/jphysiol.2002.022400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs CA, Kumar A, Kreiner LH, Johnson NM, Helms MN. H2O2 regulates lung epithelial sodium channel (ENaC) via ubiquitin-like protein Nedd8. J Biol Chem. 2013;288:8136–8145. doi: 10.1074/jbc.M112.389536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Chemaly S, Salathe M, Baier S, Conner GE, Forteza R. Hydrogen peroxide-scavenging properties of normal human airway secretions. Am J Respir Crit Care Med. 2003;167:425–430. doi: 10.1164/rccm.200206-531OC. [DOI] [PubMed] [Google Scholar]
- Fanelli T, Cardone RA, Favia M, Guerra L, Zaccolo M, Monterisi S, et al. Beta-oestradiol rescues DeltaF508CFTR functional expression in human cystic fibrosis airway CFBE41o- cells through the up-regulation of NHERF1. Biol Cell. 2008;100:399–412. doi: 10.1042/BC20070095. [DOI] [PubMed] [Google Scholar]
- Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods Mol Med. 2005;107:183–206. doi: 10.1385/1-59259-861-7:183. [DOI] [PubMed] [Google Scholar]
- Gattas MV, Forteza R, Fragoso MA, Fregien N, Salas P, Salathe M, et al. Oxidative epithelial host defense is regulated by infectious and inflammatory stimuli. Free Radic Biol Med. 2009;47:1450–1458. doi: 10.1016/j.freeradbiomed.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiszt M, Witta J, Baffi J, Lekstrom K, Leto TL. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 2003;17:1502–1504. doi: 10.1096/fj.02-1104fje. [DOI] [PubMed] [Google Scholar]
- Halm ST, Zhang J, Halm DR. beta-Adrenergic activation of electrogenic K+ and Cl− secretion in guinea pig distal colonic epithelium proceeds via separate cAMP signaling pathways. Am J Physiol Gastrointest Liver Physiol. 2010;299:G81–G95. doi: 10.1152/ajpgi.00035.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper RW, Xu C, Eiserich JP, Chen Y, Kao CY, Thai P, et al. Differential regulation of dual NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2 cytokines in respiratory tract epithelium. FEBS Lett. 2005;579:4911–4917. doi: 10.1016/j.febslet.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Miyata H, Watanabe H, Kobayashi A, Yamazaki N. Effects of hydrogen peroxide on action potentials and intracellular Ca2+ concentration of guinea pig heart. Cardiovasc Res. 1989;23:767–773. doi: 10.1093/cvr/23.9.767. [DOI] [PubMed] [Google Scholar]
- Hess KC, Jones BH, Marquez B, Chen Y, Ord TS, Kamenetsky M, et al. The ‘soluble’ adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev Cell. 2005;9:249–259. doi: 10.1016/j.devcel.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz LF, Hirdes W, Suh BC, Hilgemann DW, Mackie K, Hille B. Phospholipase C in living cells: activation, inhibition, Ca2+ requirement, and regulation of M current. J Gen Physiol. 2005;126:243–262. doi: 10.1085/jgp.200509309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CL, Li T, Cowley EA. The prostaglandin E(2) type 4 receptor participates in the response to acute oxidant stress in airway epithelial cells. J Pharmacol Exp Ther. 2012;341:552–563. doi: 10.1124/jpet.111.187138. [DOI] [PubMed] [Google Scholar]
- Joy AP, Cowley EA. 8-iso-PGE2 stimulates anion efflux from airway epithelial cells via the EP4 prostanoid receptor. Am J Respir Cell Mol Biol. 2008;38:143–152. doi: 10.1165/rcmb.2006-0295OC. [DOI] [PubMed] [Google Scholar]
- Kunzelmann K, Mehta A. CFTR: a hub for kinases and crosstalk of cAMP and Ca2+ FEBS J. 2013;280:4417–4429. doi: 10.1111/febs.12457. [DOI] [PubMed] [Google Scholar]
- Lieb T, Frei CW, Frohock JI, Bookman RJ, Salathe M. Prolonged increase in ciliary beat frequency after short-term purinergic stimulation in human airway epithelial cells. J Physiol. 2002;538(Pt 2):633–646. doi: 10.1113/jphysiol.2001.013222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvin TN, Kamenetsky M, Zarifyan A, Buck J, Levin LR. Kinetic properties of ‘soluble’ adenylyl cyclase. Synergism between calcium and bicarbonate. J Biol Chem. 2003;278:15922–15926. doi: 10.1074/jbc.M212475200. [DOI] [PubMed] [Google Scholar]
- Liu B, Gan L, Sun X, Zhu Y, Tong Z, Xu H, et al. Enhancement of BK(Ca) channel activity induced by hydrogen peroxide: involvement of lipid phosphatase activity of PTEN. Biochim Biophys Acta. 2009;1788:2174–2182. doi: 10.1016/j.bbamem.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Liu B, Sun X, Zhu Y, Gan L, Xu H, Yang X. Biphasic effects of H(2)O(2) on BK(Ca) channels. Free Radic Res. 2010;44:1004–1012. doi: 10.3109/10715762.2010.495126. [DOI] [PubMed] [Google Scholar]
- Ma HP. Hydrogen peroxide stimulates the epithelial sodium channel through a phosphatidylinositide 3-kinase-dependent pathway. J Biol Chem. 2011;286:32444–32453. doi: 10.1074/jbc.M111.254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mall M, Wissner A, Schreiber R, Kuehr J, Seydewitz HH, Brandis M, et al. Role of K(V)LQT1 in cyclic adenosine monophosphate-mediated Cl(−) secretion in human airway epithelia. Am J Respir Cell Mol Biol. 2000;23:283–289. doi: 10.1165/ajrcmb.23.3.4060. [DOI] [PubMed] [Google Scholar]
- Manzanares D, Gonzalez C, Ivonnet P, Chen RS, Valencia-Gattas M, Conner GE, et al. Functional apical large conductance, Ca2+-activated, and voltage-dependent K+ channels are required for maintenance of airway surface liquid volume. J Biol Chem. 2011;286:19830–19839. doi: 10.1074/jbc.M110.185074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TN, Gloy J, Hug MJ, Greger R, Schollmeyer P, Pavenstadt H. Hydrogen peroxide increases the intracellular calcium activity in rat mesangial cells in primary culture. Kidney Int. 1996;49:388–395. doi: 10.1038/ki.1996.57. [DOI] [PubMed] [Google Scholar]
- Miller C, Latorre R, Reisin I. Coupling of voltage-dependent gating and Ba++ block in the high-conductance, Ca++-activated K+ channel. J Gen Physiol. 1987;90:427–449. doi: 10.1085/jgp.90.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazaki M, Kakei M, Yaekura K, Koriyama N, Morimitsu S, Ichinari K, et al. Diverse effects of hydrogen peroxide on cytosolic Ca2+ homeostasis in rat pancreatic beta-cells. Cell Struct Funct. 2000;25:187–193. doi: 10.1247/csf.25.187. [DOI] [PubMed] [Google Scholar]
- Namkung W, Finkbeiner WE, Verkman AS. CFTR-adenylyl cyclase I association responsible for UTP activation of CFTR in well-differentiated primary human bronchial cell cultures. Mol Biol Cell. 2010;21:2639–2648. doi: 10.1091/mbc.E09-12-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J, Miller C. Discrete Ba2+ block as a probe of ion occupancy and pore structure in the high-conductance Ca2+-activated K+ channel. J Gen Physiol. 1988;92:569–586. doi: 10.1085/jgp.92.5.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nlend MC, Bookman RJ, Conner GE, Salathe M. Regulator of G-protein signaling protein 2 modulates purinergic calcium and ciliary beat frequency responses in airway epithelia. Am J Respir Cell Mol Biol. 2002;27:436–445. doi: 10.1165/rcmb.2002-0012OC. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl. Acids Res. 2014;42:D1098–1106. doi: 10.1093/nar/gkt1143. (Database Issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehmann H. Epac-inhibitors: facts and artefacts. Sci Rep. 2013;3:3032–3037. doi: 10.1038/srep03032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick AZ, Klein I, Eiserich JP, Cross CE, Nagler RM. Inhibition of oral peroxidase activity by cigarette smoke: in vivo and in vitro studies. Free Radic Biol Med. 2003;34:377–384. doi: 10.1016/s0891-5849(02)01297-2. [DOI] [PubMed] [Google Scholar]
- Rice KL, Duane PG, Archer SL, Gilboe DP, Niewoehner DE. H2O2 injury causes Ca(2+)-dependent and -independent hydrolysis of phosphatidylcholine in alveolar epithelial cells. Am J Physiol. 1992;263(4 Pt 1):L430–L438. doi: 10.1152/ajplung.1992.263.4.L430. [DOI] [PubMed] [Google Scholar]
- Rivlin J, Mendel J, Rubinstein S, Etkovitz N, Breitbart H. Role of hydrogen peroxide in sperm capacitation and acrosome reaction. Biol Reprod. 2004;70:518–522. doi: 10.1095/biolreprod.103.020487. [DOI] [PubMed] [Google Scholar]
- Schmid A, Bai G, Schmid N, Zaccolo M, Ostrowski LE, Conner GE, et al. Real-time analysis of cAMP-mediated regulation of ciliary motility in single primary human airway epithelial cells. J Cell Sci. 2006;119(Pt 20):4176–4186. doi: 10.1242/jcs.03181. [DOI] [PubMed] [Google Scholar]
- Schmid A, Sutto Z, Schmid N, Novak L, Ivonnet P, Horvath G, et al. Decreased soluble adenylyl cyclase activity in cystic fibrosis is related to defective apical bicarbonate exchange and affects ciliary beat frequency regulation. J Biol Chem. 2010;285:29998–30007. doi: 10.1074/jbc.M110.113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Evellin S, Weernink PA, von Dorp F, Rehmann H, Lomasney JW, et al. A new phospholipase-C-calcium signalling pathway mediated by cyclic AMP and a Rap GTPase. Nat Cell Biol. 2001;3:1020–1024. doi: 10.1038/ncb1101-1020. [DOI] [PubMed] [Google Scholar]
- Speirs CK, Jernigan KK, Kim SH, Cha YI, Lin F, Sepich DS, et al. Prostaglandin Gbetagamma signaling stimulates gastrulation movements by limiting cell adhesion through Snai1a stabilization. Development. 2010;137:1327–1337. doi: 10.1242/dev.045971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strengert M, Jennings R, Davanture S, Hayes P, Gabriel G, Knaus UG. Mucosal reactive oxygen species are required for antiviral response: role of Duox in influenza a virus infection. Antioxid Redox Signal. 2014;20:2695–2709. doi: 10.1089/ars.2013.5353. [DOI] [PubMed] [Google Scholar]
- Sun F, Hug MJ, Bradbury NA, Frizzell RA. Protein kinase A associates with cystic fibrosis transmembrane conductance regulator via an interaction with ezrin. J Biol Chem. 2000;275:14360–14366. doi: 10.1074/jbc.275.19.14360. [DOI] [PubMed] [Google Scholar]
- Sun XC, Bonanno JA. Expression, localization, and functional evaluation of CFTR in bovine corneal endothelial cells. Am J Physiol Cell Physiol. 2002;282:C673–C683. doi: 10.1152/ajpcell.00384.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lam CS, Wu F, Wang W, Duan Y, Huang P. Regulation of CFTR channels by HCO(3) – sensitive soluble adenylyl cyclase in human airway epithelial cells. Am J Physiol Cell Physiol. 2005;289:C1145–C1151. doi: 10.1152/ajpcell.00627.2004. [DOI] [PubMed] [Google Scholar]
- Yokoyama U, Minamisawa S, Quan H, Akaike T, Suzuki S, Jin M, et al. Prostaglandin E2-activated Epac promotes neointimal formation of the rat ductus arteriosus by a process distinct from that of cAMP-dependent protein kinase A. J Biol Chem. 2008;283:28702–28709. doi: 10.1074/jbc.M804223200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 2002;295:1711–1715. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Shen X. H2O2 directly activates inositol 1,4,5-trisphosphate receptors in endothelial cells. Redox Rep. 2005;10:29–36. doi: 10.1179/135100005X21660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Fully differentiated NHBE cells in ALI culture were mounted in Ussing chambers and stimulated with 400 μM H2O2 (example trace in panel a). Panel b, H2O2 (400 μM) stimulation of anion currents after preincubation and in the presence of various concentrations of the the sAC inhibitor KH7 showed an apparent IC50 = 1.4 μM (n = 1–3 lung donors at each concentration).
Figure S2 Total RNA was extracted from fully differentiated NHBE cells in ALI culture derived from two lung donors (E.Z.N.A Omega Bio-Tek, Norcross, GA, USA), reverse transcribed (iScript cDNA synthesis kit; Bio-Rad, Hercules, CA, USA) and assayed for presence of Epac1 (TaqMan assay Hs00183449_m1), MUC5AC (TaqMan assay Hs01365601_m1) and GAPDH as a reference mRNA (TaqMan assay 4352934E). The data demonstrate expression of Epac1 at a level slightly less than the differentiation product Muc5AC.
Figure S3 Fully differentiated NHBE cells in ALI culture were mounted in Ussing chambers and stimulated with 10 μM ATP. The trace shows an initial abrupt ISC peak thought to be primarily Ca2+ activated Cl− current and a later, more gradual response believed to be a sum of CFTR and Ca2+ activated Cl− currents (e.g. Namkung et al., 2010).


