Abstract
BACKGROUND AND PURPOSE
Parkinson's disease (PD) is usually diagnosed clinically from classical motor symptoms, while definitive diagnosis is made postmortem, based on the presence of Lewy bodies and nigral neuron cell loss. α-Synuclein (ASYN), the main protein component of Lewy bodies, clearly plays a role in the neurodegeneration that characterizes PD. Additionally, mutation in the SNCA gene or copy number variations are associated with some forms of familial PD. Here, the objective of the study was to evaluate whether olesoxime, a promising neuroprotective drug can prevent ASYN-mediated neurotoxicity.
EXPERIMENTAL APPROACH
We used here a novel, mechanistically approachable and attractive cellular model based on the inducible overexpression of human wild-type ASYN in neuronally differentiated human neuroblastoma (SHSY-5Y) cells. This model demonstrates gradual cellular degeneration, coinciding temporally with the appearance of soluble and membrane-bound ASYN oligomers and cell death combining both apoptotic and non-apoptotic pathways.
KEY RESULTS
Olesoxime fully protected differentiated SHSY-5Y cells from cell death, neurite retraction and cytoplasmic shrinkage induced by moderate ASYN overexpression. This protection was associated with a reduction in cytochrome c release from mitochondria and caspase-9 activation suggesting that olesoxime prevented ASYN toxicity by preserving mitochondrial integrity and function. In addition, olesoxime displayed neurotrophic effects on neuronally differentiated SHSY-5Y cells, independent of ASYN expression, by promoting their differentiation.
CONCLUSIONS AND IMPLICATIONS
Because ASYN is a common underlying factor in many cases of PD, olesoxime could be a promising therapy to slow neurodegeneration in PD.
Tables of Links
| TARGETS | |
|---|---|
| Caspase 3 | Caspase-9 |
| Caspase 7 |
| LIGANDS |
|---|
| Doxycycline |
| RA, all-trans retinoic acid (tretinoin) |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guideto PHARMACOLOGY 2013/14 (Alexander et al., 2013).
Introduction
Parkinson's disease (PD) is an age-related neurodegenerative disease with unknown aetiology. PD is usually diagnosed based on classical motor symptoms resulting from the death of dopaminergic neurons in the substantia nigra although other parts of the nervous system are also affected (Davie, 2008). PD is definitively diagnosed on the basis of postmortem brain histopathology showing within the substantia nigra the presence of Lewy bodies, whose main protein component is α-synuclein (ASYN). Either mutations in the SNCA gene (Polymeropoulos et al., 1997; Kruger et al., 1998; Kasten and Klein, 2013; Singleton et al., 2013) or copy number variations (Singleton et al., 2003) are associated with some forms of familial PD. Additionally, polymorphisms in and around the SNCA gene are associated with a higher risk for sporadic PD (Maraganore et al., 2006; Pankratz et al., 2009). Taken together, ASYN clearly plays a role in PD pathogenesis.
The molecular determinants underlying ASYN secretion and aggregation, intracellular toxicity and transmission of pathology are still unclear. ASYN oligomers may cause toxicity through formation of pore-like channels (Volles et al., 2001) or by other mechanisms such as impairment of proteasome-mediated protein degradation (Chen et al., 2005; Sharma et al., 2006) or disruption of endoplasmic reticulum/Golgi trafficking resulting in endoplasmic reticulum stress (Smith et al., 2005; Cooper et al., 2006). Furthermore, in vitro and in vivo studies of the effects of overexpression of either ‘normal’ or familial mutant forms of ASYN have reported mitochondrial abnormalities (Hsu et al., 2000; Martin et al., 2006; Devi et al., 2008) and increased oxidative stress (Hsu et al., 2000; Kumar et al., 2005).
A novel cellular model using a human neuroblastoma cell line, SHSY-5Y, (Vekrellis et al., 2009) appears to be an attractive system for studying the pathogenic effects of ASYN overexpression, intracellular accumulation or secretion. This model, based on the inducible overexpression of wild-type, human ASYN in neuronally differentiated human cells, demonstrates gradual cellular degeneration, coinciding temporally with the appearance of soluble and membrane-bound ASYN oligomers. Interestingly, the death pathway activated in the presence of high levels of ASYN combined both the intrinsic apoptotic machinery engaging the mitochondria and non-apoptotic features (Vekrellis et al., 2009). Assuming this model system recapitulates relevant events underlying PD pathophysiology, it offers a valuable tool to identify potential therapeutic targets and screen small molecules for their ability to prevent ASYN neurotoxicity.
Olesoxime (cholest-4-en-3-one, oxime; TRO19622) is a low MW compound that rescues motor neurons from neurotrophic factor deprivation or Fas-induced cell death (Bordet et al., 2007; Sunyach et al., 2012; Yang et al., 2013). This drug also inhibits neuronal cell death induced by the topoisomerase-I inhibitor camptothecin by preventing mitochondrial permeabilization and release of pro-apoptotic factors (Gouarne et al., 2013). Unlike brain-derived neurotrophic factor (BDNF), which also rescues neurons from both trophic factor deprivation and camptothecin intoxication, olesoxime does not activate prosurvival kinases nor does it simply inhibit apoptosis, but also exerts potent neurotrophic effects such as promoting neurite outgrowth in multiple preclinical neurodegeneration models (Bordet et al., 2007; 2008; Xiao et al., 2009; Rovini et al., 2010; Sunyach et al., 2012) and actively promotes oligodendrocyte maturation and myelination (Magalon et al., 2012). Here, the objective of the study was to evaluate whether olesoxime could prevent neuronal cell death induced by ASYN accumulation in neuronally differentiated SHSY-5Y cells.
Methods
Cell culture
The generation of stable Tet-Off SHSY-5Y human neuroblastoma cell lines conditionally expressing ASYN or β-galactosidase (β-gal) was previously described (Vekrellis et al., 2009). Cells were cultured in RPMI 1640 medium (Invitrogen, Grand Island, NY, USA) supplemented with 10% FBS (Thermo Scientific, Waltham, MA, USA), penicillin (100 U·mL−1), streptomycin (100 μg·mL−1), 1 mM GlutaMAX™ (Gibco, Grand Island, NY, USA) and maintained in 250 μg·mL−1 G418 and 50 μg·mL−1 Hygromycin B. Stock cultures were always kept in the presence of 0.5 μg·mL−1 doxycycline to switch off ASYN or β-gal expression. Cultures were maintained at 37°C in a humidified atmosphere with 5% CO2.
ASYN-induced neurotoxicity assay and treatments
ASYN or β-gal-expressing SHSY-5Y cells were seeded into 96-well plates at low or high density (3000 or 30 000 cells·cm−2) in the presence or absence of doxycycline (Sigma Aldrich, Seelze, Germany). Twenty-four hours after seeding, neuronal differentiation was induced by addition of 10 μM all-trans retinoic acid (RA; Sigma-Aldrich). At the same time cells were treated with test compounds in 0.5% final DMSO (control cultures received DMSO only). For all experiments, the entire medium was changed every 2 days and all drugs were replaced at the same concentration.
Assessment of survival
The survival of differentiated ASYN and β-gal cells, with or without doxycycline, was assessed at various time points after RA addition. Surviving cells were labelled with 2 μg·mL−1 calcein-AM (Invitrogen) for 30 min at 37°C. Subsequently, fluorescence images of each well of the 96-well plates were acquired using a Plate Runner HD® (Trophos, Marseille, France) and analysed using Tina® software as previously described (Gouarne et al., 2013). When plated at ‘low density’, individual cells in each well were counted and survival was analysed. When plated at ‘high density’, the global fluorescence of surviving cells in each well was measured. Cell survival was also evaluated by counting the number of intact nuclei in a hemocytometer after cells were lysed in a detergent-containing solution (Rukenstein et al., 1991; Farinelli et al., 1998).
Neurite length and cell body area measurement
Calcein fluorescence images obtained from low-density cultures were processed to obtain additional cell morphology parameters. Quantification of neurite length and cell body area in large surviving ASYN cells was done using MetaMorph® Neurite Outgrowth Application Module (Molecular Devices, Sunnyvale, CA, USA) from exported 16-bit TIFF files acquired using the Plate Runner HD as previously described (Bordet et al., 2007) or using images acquired with a fluorescence microscope. Parameters for defining cell body area and neurites were optimized according to the software instructions and verified by comparing the images generated by MetaMorph with the original fluorescence images.
Caspase assay
Caspase activation was assessed on differentiated ASYN cells seeded at a density of 30 000 cells·cm−2 (high density model) in 96-well plates using Caspase-Glo® proluminescent substrates of caspases (Promega, Madison, WI, USA). Caspase-9 and caspase-3/7 activation was measured according to manufacturer's instructions.
Western immunoblotting
Mitochondrial fractions and total protein extracts were prepared from ASYN cells seeded at a density of 30 000 cells·cm−2 (high-density model) after 6 days of differentiation, with or without doxycycline. For mitochondrial fractions, cells were lysed in cold mitochondria buffer [210 mM mannitol, 70 mM sucrose, 1 mM EDTA, 10 mM HEPES, pH 7.5, 0.04% digitonin, 1 mM DTT and complete protease inhibitor cocktail (Roche, Mannheim, Germany) and centrifuged 5 min at 520× g at 4°C. Supernatant was collected and then centrifuged at 9600× g at 4°C for 30 min. The mitochondria-enriched pellet was then resuspended in mitochondria buffer. For total protein extracts, cells were lysed in CelLytic™ mammalian lysis buffer (Sigma-Aldrich). Total protein content was determined using the Micro quick start™ Bradford kit (Biorad, Hercules, CA, USA) and a fixed amount in micrograms was loaded and separated on precast NuPAGE® 4–12% bis-tris SDS-PAGE (Invitrogen), and transferred by electrophoresis onto nitrocellulose membrane (Pierce, Rockford, IL, USA). Membranes were blocked for 1 h in 10 mM Tris (pH 7.4), 150 mM NaCl and 0.2% Tween 20 with 5% (w/v) dry skim milk powder and then incubated overnight with primary antibodies of interest at 4°C. After washing, membranes were incubated for 1 h with appropriate HRP-conjugated secondary antibodies (Pierce) and then developed by an enhanced chemiluminescence system according to the manufacturer's instructions (SuperSignal® West Dura Chemiluminescent Substrate, Pierce). Autoradiography signals were quantified using ImageJ software.
Primary antibodies and dilutions used were: monoclonal mouse anti-ASYN (1:10 000; BD bioscience, San Jose, CA, USA 610787), monoclonal mouse anti-α-tubulin (1:1000; Sigma-Aldrich T9026), monoclonal mouse anti-cytochrome c (1:1000; BD Pharmingen, Franklin Lakes, NJ, USA 556433), polyclonal rabbit anti-TOM20 (1:20 000; Santa Cruz, CA, USA sc11415), polyclonal rabbit anti-beta tubulin III (Tuj1) (1:25 000; Sigma-Aldrich T2200). Secondary antibodies used were HRP-conjugated goat anti-mouse IgG (1:50 000; Pierce 31430) and HRP-conjugated goat anti-rabbit IgG (1:50 000; Pierce 31460).
Data analysis
Data are expressed as means ± SEM from at least three independent experiments. Comparisons between two groups were performed using an unpaired Student's t-test. Comparisons among several groups were conducted using one-way anova. A two-way anova was performed to analyse differences between treatments and experimental groups. A P value of less than 0.05 was considered statistically significant.
Materials
Olesoxime was synthesized by Synkem (Chenôve, France). Drugs were dissolved in DMSO to prepare 10 mM stock solutions. DMSO was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Results
Moderate ASYN overexpression induces cell mortality in differentiated SHSY-5Y cells
We first characterized the effect of ASYN overexpression on survival in both low- and high-density differentiated SHSY-5Y cell cultures. Indeed, two different cell-density models were necessary for the different methods used to assess and dissect the potential therapeutic benefit of olesoxime. For morphology studies, it was necessary to use sparse cell cultures (3000 cells·cm−2, low-density model) in order to observe each cell and identify their neurites while for other assays, such as Western blot, a large quantity of cell protein was needed, which was not possible using sparse cell cultures. For these studies, we used high-density cultures (30 000 cells·cm−2) in order to collect sufficient protein. In the two models, differentiation was induced by addition of 10 μM RA the day after seeding (day 0). In the absence of doxycycline, cell death became evident after 4 to 5 days of differentiation, depending on density, and gradually increased with time. By 5 days after the addition of RA, ASYN overexpression (doxycycline withdrawal) significantly reduced cell numbers counted in low-density cultures (143 ± 14 vs. 267 ± 24 calcein-positive cells, in the absence or in the presence of doxycycline, respectively, P < 0.0001). By 6 days of differentiation, we estimated that 50% of cells had died in high-density cultures while very few cells were still detectable in low-density cultures (Figure 1A and B). Representative images of calcein-positive neurons in low-density cultures after 5 days of differentiation in the presence or in absence of doxycycline show the effects of ASYN overexpression on cell morphology as a consequence of ASYN toxicity (Figure 1C). These effects were quantified by measuring cell body area and neurite length. Four cell categories were defined depending on cell body area in terms of pixel area (a pixel measures 10 × 10 μm): 5–10 pixels; 10–15 pixels; 15–20 pixels and >20 pixels. Importantly, there was a reduction preferentially of the largest differentiated cells (cell body area over 20 pixels) as a consequence of ASYN overexpression (Figure 1E), a result confirming that ASYN affects only differentiated SHSY-5Y cell survival as previously described by Vekrellis et al (2009). Cell body area and neurite length were measured in surviving cells having cell body area over 20 pixels. Interestingly, this revealed neurite retraction and cell body shrinkage concomitant with cell death that was significant after 4 days of differentiation and increased with time. By day 5, average cell body area was 33.7 ± 0.7 versus 30.0 ± 0.5 pixels (P < 0.0001) and neurite length was 3.4 ± 0.2 versus 2.6 ± 0.1 pixels (P < 0.0001) in the presence or absence of doxycycline respectively (Figure 1D and F).
Figure 1.
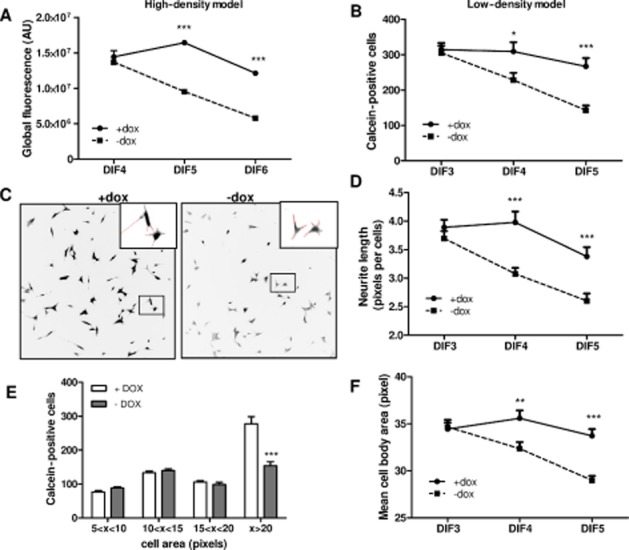
Moderate wild-type ASYN overexpression is toxic in neuronally differentiated SHSY-5Y cells. ASYN cells were seeded in 96-well plates at 30 000 cells·cm−2 (high density or ∼10 000 cells per well) (A) or 3000 cells·cm−2 (low density or ∼1000 cells·per well) (B–F) in the presence or absence of doxycycline (dox). Differentiation was induced by addition of 10 μM RA the day after seeding. Surviving neurons were labelled with calcein and global fluorescence was quantified (A) or neurons were individually counted (B) with Trophos' Plate Runner HD. (C) Representative inverse black and white images of surviving neurons labelled with calcein were acquired with a fluorescence microscope (10× objective). Neurites defined by MetaMorph are depicted in red in the insets. (E) Cell distribution according to cell body area after 5 days differentiation (DIF 5). In surviving large neurons, quantification of neurite length per cell (D) and mean cell body area (F) was measured using MetaMorph software. Mean ± SEM (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 compared with DMSO-dox.
Olesoxime prevents ASYN induced toxicity of differentiated ASYN cells
Treatment of ASYN-expressing cells with olesoxime during the differentiation process reversed ASYN-induced toxicity in a dose-dependent manner in both high- and low-density cultures (Figure 2A and B). Interestingly, we observed that when differentiated SHSY-5Y cells were cultured in the presence of doxycycline, that is in absence of ASYN induction or toxicity, olesoxime dose-dependently increased the number of large calcein-positive cells (Figure 2B, open bars). We also evaluated the effects of olesoxime on the three other cell populations: 5–10 pixels, 10–15 pixels, 15–20 pixels (Supporting Information Fig. S1). The results showed that in the presence or absence of ASYN induction, olesoxime decreased the number of 5–10 and 10–15 pixels cells (two-way anova, ASYN effect P < 0.0001) while there was little or no effect on the number of 15–20 pixels cells. In the large cell population (>20 pixels) both the overall cell body area and the total neurite length per cell were significantly increased by olesoxime treatment in a similar dose-dependent manner, effects that were more pronounced do the effects on survival in ASYN-expressing cells (filled bars in Figure 2C and D) although, olesoxime also increased neurite length and cell body area in differentiated SHSY-5Y cells even in the absence of ASYN overexpression (open bars in Figure 2C and D).
Figure 2.
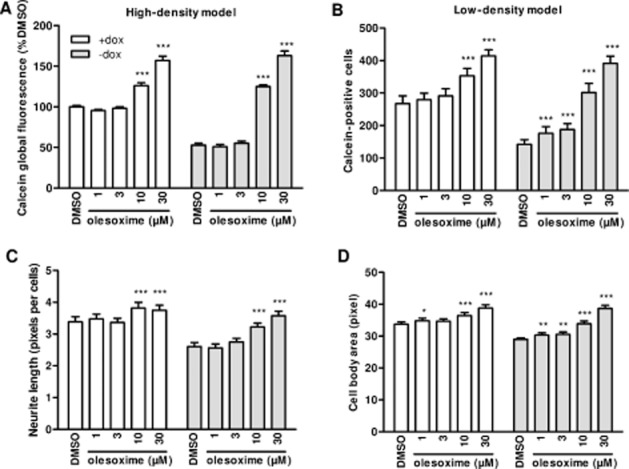
Olesoxime protects differentiated SHSY-5Y cells from ASYN-induced toxicity. ASYN cells seeded in 96-well plates at 30 000 cells·cm−2 (high density) (A) or 3000 cells·cm−2 (low density) (B–D), with or without doxycycline (dox), were differentiated with 10 μM RA starting 24 h after seeding. Olesoxime or DMSO treatment started at the same time. Surviving neurons were detected by calcein labelling and global fluorescence was quantified after 6 days of differentiation (DIF6) (A), or large cells (above 20 pixels) were individually counted after 5 days of differentiation (DIF5) (B) with Trophos' Plate Runner HD. In surviving large neurons, quantification of neurite length per cell (C) and mean cell body area (D) was measured using MetaMorph. Mean ± SEM (n = 3) *P < 0.05, **P < 0.01, ***P < 0.001 compared with respective DMSO groups (±dox).
Even if there is no mechanistic rationale for an effect of olesoxime to delay or reduce the expression of ASYN in this Tet-Off cell model, we wanted to confirm that there was no effect on the level of ASYN expression at a time when cell death is well underway, 6 days after doxycycline removal. Western blotting of cell lysates obtained from cells cultured at high density in 6-well plates showed that in the presence of doxycycline, ASYN levels were very low, but detectable. Inducing ASYN expression by removing doxycycline resulted in similarly elevated ASYN protein levels in the presence or absence of olesoxime (Figure 3). Therefore, the protective effect conferred by olesoxime could not be attributed to a reduction in total ASYN protein levels. Indeed, using another neurotoxicity assay described with this model (Vekrellis et al., 2009), olesoxime was found to rescue SHSY-5Y neuronal cells from ASYN-induced cell death when added 3–5 days following RA-mediated differentiation in SHSY-5Y cells already expressing ASYN (RA differentiation started 6 days after doxycycline removal; see Supporting Information Fig. S2).
Figure 3.
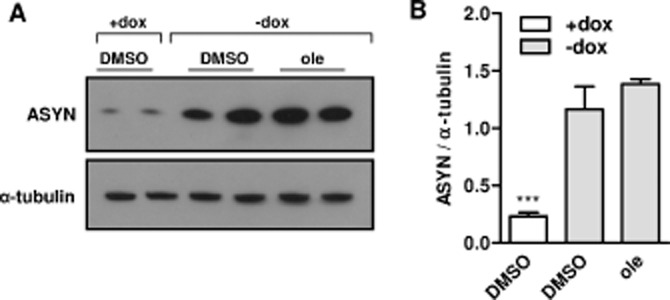
Olesoxime does not modify ASYN expression in ASYN cells. ASYN cells seeded in 6-well plates at 30 000 cells·cm−2 (high density) with or without doxycycline (dox) were differentiated with 10 μM RA and treated with olesoxime (ole; 30 μM). After 6 days of differentiation and treatment, cells were lysed and total proteins were separated by SDS-PAGE and blotted onto nitrocellulose membranes. Blots were incubated with antibodies against ASYN and α-tubulin proteins. (A) A representative experiment is shown. (B) Intensity of signals was quantified using ImageJ software. Mean ± SEM (n = 4). ***P < 0.001 compared with DMSO -dox, one-way anova followed by Bonferroni post test.
Olesoxime has trophic or protective effects on differentiated SHSY-5Y cells
To further explore the effect of olesoxime on differentiated SHSY-5Y cells in the absence of ASYN toxicity, we treated differentiated SHSY-5Y cells conditionally expressing β-gal with olesoxime. Representative images of calcein-positive neurons observed by fluorescence microscopy after 5 days of differentiation are presented in Figure 4A. We observed that olesoxime-treated cells had a different morphology, compared with DMSO-treated cells. This was reflected in the cell distribution as a function of cell body area, which was increased in the presence of olesoxime. Indeed, we measured a dose-dependent increase in the number of large cells (>20 pixels) and a concomitant dose-dependent decrease in the number of smaller cells (<15 pixels) (Figure 3B), although the total number of cells was not increased by olesoxime treatment (data not shown). Interestingly, these effects were associated with a positive effect of olesoxime on neurite length (Figure 4C). Additionally, olesoxime accelerated expression of a neuronal differentiation marker, class III β-tubulin (Tuj1 monoclonal antibody). This effect is reflected by the higher level of Tuj1 expression in olesoxime compared with DMSO-treated cells by the first day of differentiation induced by RA and the absence of olesoxime effect on Tuj1 expression when cells were fully differentiated by day 3 (Figure 4D).
Figure 4.
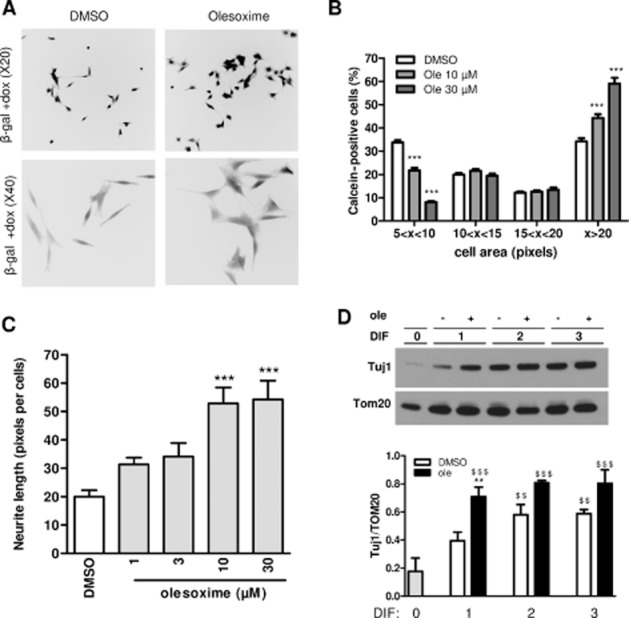
Olesoxime accelerates β-gal SHSY-5Y differentiation and has a neurotrophic effect. β-gal-expressing SHSY-5Y cells seeded in 96-well plates at 3000 cells·cm−2 (low density) (A–C), or 30 000 cells·cm−2 (high density) (D) in the presence of doxycycline (dox), were differentiated with 10 μM RA 24 h after seeding and treated with olesoxime (ole) or DMSO at the same time. (A) Representative reverse black and white images of calcein-labelled neurons treated with DMSO or olesoxime (30 μM) were acquired using a fluorescence microscope (20× or 40× objective in the upper or lower figures respectively). (B) Calcein-labelled neurons in low-density cultures were individually counted with Trophos' Plate Runner HD after 5 days differentiation and cell distribution was classified according to cell body area. One representative experiment is shown. ***P < 0.001 compared with DMSO. (C) Neurite length per cell in olesoxime-treated cultures relative to DMSO controls was determined using images acquired using a fluorescence microscope (20x objective) and MetaMorph software. One representative experiment is shown. ***P < 0.001 compared with DMSO. (D) Expression of β-tubulin III using Tuj1 monoclonal antibody in differentiated SHSY-5Y cells treated with DMSO or olesoxime (30 μM) at different time points was monitored by immunoblotting. A representative experiment is shown and Tuj1-labelled protein levels were quantified using ImageJ and expressed relative to TOM20 protein levels. Mean ± SEM (n = 4). $$P < 0.01 $$$P < 0.001 compared with DIF0, **P < 0.05 compared with DMSO.
Olesoxime reduces ASYN-induced cytochrome c release and caspase activation in differentiated SHSY-5Y cells
Similar to the time course of cell death (Figure 1A), and as previously reported (Vekrellis et al., 2009), we observed a progressive increase in caspase-9 activation (Figure 6A) following RA differentiation in ASYN cells. Based on this time course, we measured the effect of olesoxime on cytochrome c release and caspase activation after 6 days of differentiation, the same time point of survival measurement (Figure 2A). As previously reported (Vekrellis et al., 2009), ASYN overexpression decreased cytochrome c abundance in mitochondria-enriched fractions from differentiated ASYN cells and this was dose-dependently restored to control levels by olesoxime treatment (Figure 5A and B), suggesting that olesoxime treatment prevented mitochondrial membrane permeabilization and pro-apoptotic factor release. By contrast, olesoxime had no significant effect on TOM20 expression (Figure 5D), which allowed us to use this mitochondrial outer membrane protein to correct eventual sample discrepancies or differences in mitochondrial isolation efficiency between experimental conditions. Olesoxime treatment dose-dependently reduced caspase-9 activation over the same concentration range shown to prevent cell death (Figure 6B). Interestingly, at the highest concentration tested, 30 μM, olesoxime also reduced basal caspase-9 and caspase-3/7 activity present even in the absence of ASYN overexpression (cells maintained in the presence of doxycycline) (open bars, Figure 6B and C).
Figure 6.
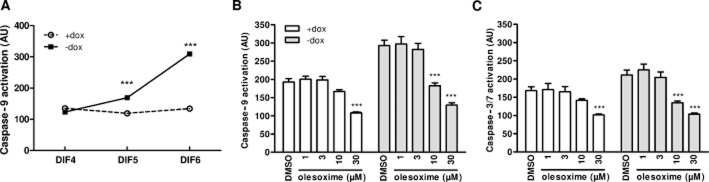
Olesoxime prevents ASYN-induced caspase-9 activation. ASYN cells seeded in 96-well plates at 30 000 cells·cm−2 (high density) with or without doxycycline (dox) were differentiated with 10 μM RA 24 h after seeding and treated at the same time with DMSO or increasing concentrations of olesoxime. (A) Caspase-9 activity was assayed between 4 days of differentiation (DIF4) and DIF6. (B,C) Caspase-9 and caspase-3/7 activity was assessed after DIF6. (B) Olesoxime treatment dose-dependently decreased caspase-9 activation because of ASYN expression (−dox) but also decreased basal caspase-9 activity (+dox). (C) ASYN expression did not significantly increase caspase-3/7 activation (DMSO ± dox); however, olesoxime treatment decreased basal caspase-3/7 activity in the presence or absence of ASYN expression (±dox). Mean ± SEM (n = 3). ***P < 0.001 compared with respective DMSO groups (±dox).
Figure 5.
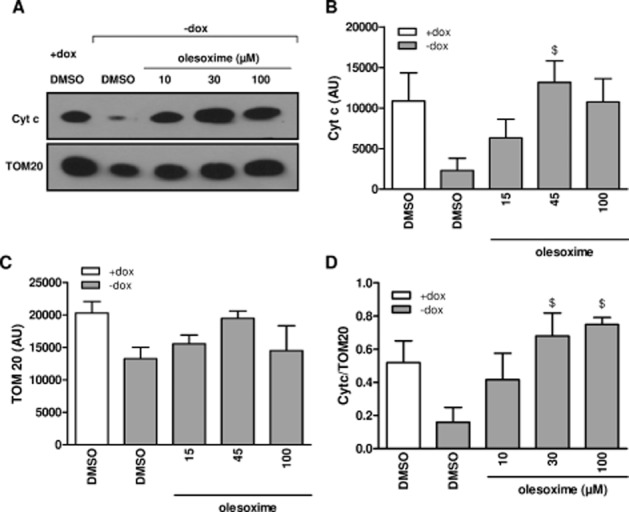
Olesoxime prevents ASYN-induced cytochrome c (Cyt c) release. ASYN cells seeded in 6-well plates at 30 000 cells·cm−2 (high density) with or without doxycycline (dox) were differentiated with 10 μM RA 24 h after seeding and treated with olesoxime or DMSO. Enriched mitochondrial fractions were prepared after 6 days of differentiation to assess Cyt c protein and TOM20 levels using Western blotting. (A) Representative Western blot experiment is shown. (B) Cyt c protein levels and (C) TOM20 protein levels were quantified using ImageJ and (D) Cyt c expressed relative to TOM20 protein levels. Mean ± SEM (n = 3). $P < 0.05 compared with DMSO-dox condition.
Discussion
In this study, we used a human neuronal model exhibiting pathogenic effects of ASYN overexpression (Vekrellis et al., 2009) to assess the potential therapeutic benefit of olesoxime. Olesoxime was originally identified by its ability to rescue trophic-factor deprived rat primary motor neurons (Bordet et al., 2007), a protection confirmed in a similar human-induced pluripotent stem cell model of motor neuron disease (Yang et al., 2013). Recently, we reported that olesoxime also protects primary cortical neurons from camptothecin (topoisomerase I inhibitor) intoxication as efficiently as BDNF. Unlike BDNF, olesoxime does not activate prosurvival kinases such as Akt or ERK; however, both BDNF and olesoxime preserve mitochondrial membrane integrity and prevent pro-apoptotic factor release, which allowed olesoxime to preserve mitochondrial function delaying camptothecin-induced mitochondrial respiratory failure (Gouarne et al., 2013).
The cell death that occurs specifically in neuronally differentiated ASYN cells proceeds by a non-canonical cell death pathway combining both apoptotic and non-apoptotic features: activation of the apoptotic pathway engaging mitochondria with mitochondrial membrane potential reduction, cytochrome c release and apoptosome assembly with activation of caspase-9. However, apoptosis was arrested at a point downstream of mitochondrial participation and caspase-3 activation and cell death was not accompanied by DNA laddering or apoptosis-inducing factor translocation to the nucleus (Vekrellis et al., 2009).
Here, we show that olesoxime completely and dose-dependently protected human neuronal cells from ASYN toxicity. The prosurvival effects in this model were associated with a decrease in mitochondrial cytochrome c release and inhibition of caspase-9 activation. We have previously shown that olesoxime treatment inhibits caspase activation without inhibiting caspase activity (Gouarne et al., 2013). From this, we conclude that olesoxime prevents ASYN toxicity by preserving mitochondrial integrity and function, as observed in the camptothecin-induced neurodegeneration model. Olesoxime is a cholesterol-like compound which concentrates in mitochondria possibly by binding to the mitochondrial cholesterol transporter, the translocator protein as well as the voltage-dependent anion channel (VDAC) (Bordet et al., 2010). We hypothesize that the mitochondrial integrity is preserved by olesoxime-mediated effects on protein–protein interactions located at the outer mitochondrial membrane (e.g. VDAC interaction with hexokinases, Bax and/or other Bcl-2 family proteins or the adenine nucleotide carrier in the inner mitochondrial membrane protein), either directly or indirectly, through the modulation of the mitochondrial membrane fluidity (Eckmann et al., 2014).
Interestingly, we also observed that olesoxime accelerates RA-induced neuronal differentiation, indicated by earlier expression of neuron-specific class III β-tubulin and increased neurite length and cell body area at 5 days of differentiation, in the presence of the compound. We also observed that olesoxime prevented cell death initiated by RA differentiation in SHSY-5Y cells already expressing ASYN (Supporting Information Fig. S2) even when added 3 or 5 days after RA addition. This result suggests that trophic activity is not essential for the protective effect of olesoxime in SHSY-5Y cells confronted with ASYN toxicity. Moreover, this result is consistent with the neuroprotective activity already observed with olesoxime in differentiated primary neuronal cells cultured in the presence of neurotrophic factors such as primary rat motor neurons intoxicated by FAS ligand (Sunyach et al., 2012) or primary cortical neurons intoxicated by camptothecin (Gouarne et al., 2013). Additionally, olesoxime also prevented basal cell death in neuronal SHSY-5Y cells. We think this effect results from both the trophic activity of olesoxime and a protection against leaky expression of ASYN that occurs even when doxycycline is present in the culture medium (about twofold higher basal ASYN expression was detected in ASYN SHSY-5Y cells in the presence of doxycycline compared with β-gal SHSY-5Y cells) (Vekrellis et al., 2009). We can observe that in the absence of ASYN overexpression (+doxycycline condition), the number of small cells is decreased in the presence of olesoxime and the number of large cells was increased (Supporting Information Fig. S1). We assumed that the large cells are differentiated cells and as expected, large neuronal cells (>20 pixels) are predominantly affected by ASYN expression, whereas small cells (<20 pixels) were not affected by ASYN toxicity (Vekrellis et al., 2009). In small cells, we assume there are two cell type populations: (i) differentiated cells which are undergoing cell death and are therefore shrinking; and (ii) cells that are not fully differentiated and are therefore less sensitive to ASYN toxicity. Interestingly, we have previously characterized the effects of olesoxime on differentiated and non-differentiated SK-N-SH cells, the parent of the SHSY-5Y cell line (Rovini et al., 2010). Olesoxime had no effect on the cytotoxicity of microtubule-targeting chemotherapeutic agents in proliferative SK-N-SH cells but prevented neurotoxicity induced by these agents in differentiated neuronal cells.
Mitochondrial preservation may not completely explain all the benefits of olesoxime observed in this model where both apoptotic and non-apoptotic pathways are implicated. Other compounds with protective effects in this model include two AMP-activated protein kinase activators (metformin and 5-aminoimidazole-4-carboxamide riboside), the antioxidant ascorbic acid, the tyrosine hydroxylase inhibitor α-methyltyrosine and the caspase inhibitor zVAD-fmk, but their effects were only moderate (Vekrellis et al., 2009; Dulovic et al., 2013). Only the sugar scyllo-inositol, described to neutralize the inhibitory effect of Aβ oligomers on hippocampal long-term potentiation and to improve memory deficits in mice (McLaurin et al., 2000; Townsend et al., 2006), displayed a complete protective effect in this model. Some or all of these compounds may reduce oxidative stress either directly or indirectly. Olesoxime also reduces oxidative stress and mitochondria-generated reactive oxygen species (C. Gouarné, unpubl. data), which may contribute to its neuroprotective effects against ASYN intoxication. In addition, it should be noted that olesoxime protects against exogenously activated cell death pathways, such as Fas ligand-induced motor neuron cell death (Sunyach et al., 2012). This pathway was recently implicated in an ASYN oligodendrocyte toxicity model (Kragh et al., 2013), suggesting further potential therapeutic applications where olesoxime's neuroprotection may be beneficial.
In conclusion, the results presented here show that olesoxime provides significant prosurvival benefits in a human neuronal model of ASYN-mediated toxicity. Because ASYN is a common underlying factor in many cases of PD and related synucleinopathies, olesoxime could be a promising therapy to slow neurodegeneration in such diseases. The compound, which is formulated for once-a-day oral administration, has already been studied and found to be safe and well tolerated in healthy volunteers and patients for up to 2 years in a number of clinical studies (Lenglet et al., 2014). Moreover, olesoxime has recently been shown to prevent the loss of neuromuscular function for 2 years in a clinical trial in non-ambulatory spinal muscular atrophy patients (Dessaud et al., 2014; Trophos, 2014) and thus is a clinically validated neuroprotective compound. This clinical proof of concept reinforces the interest to explore olesoxime as a drug candidate in other neurodegenerative diseases where neurotrophins are considered as a therapeutic solution such as PD. Initiation of clinical trials could begin if further evidence for efficacy against ASYN-mediated neurotoxicity emerges from animal models and as soon as reliable endpoints for neuroprotection in PD patients are available.
Acknowledgments
This work was supported by the European Union under the 7th Framework Program for RTD – Project MEFOPA – Grant Agreement HEALTH-2009-241791.
Glossary
- ASYN
α-synuclein
- β-gal
β -galactosidase
- BDNF
brain-derived neurotrophic factor
- dox
doxycycline
- PD
Parkinson's disease
- RA
retinoic acid
- VDAC
voltage-dependent anion channel
Author contributions
C. G. designed and performed the experiments, analysed the data and wrote the manuscript. J. T. performed the experiments and analysed the data. M. G. P. performed the experiments and analysed data. V. D. performed the experiments and analysed the data. M. S. performed the experiments and analysed the data. G. T. performed the experiments and analysed the data. M. X. designed and performed the experiments, provided the materials, analysed the data and wrote the manuscript. L. S. provided the materials, designed the experiments, analysed the data and wrote the manuscript. T. B. designed the experiments, analysed the data and wrote the manuscript. R. M. P. designed the experiments, analysed the data and wrote the manuscript.
Conflict of interest
All authors except for L. S. and M. X. are/were employees of Trophos, which discovered and is developing olesoxime. T. B. and R. M. P. are named as inventors on patents covering olesoxime and its use.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
http://dx.doi.org/10.1111/bph.12939
Figure S1 Olesoxime reduced the number of small cells (<15 pixels) in presence or absence of ASYN. ASYN cells seeded in 96-well plates at 3000 cells·cm−2 (low density) with or without doxycycline (dox),were differentiated with 10 μM RA starting 24 h after seeding. Olesoxime or DMSO treatment started at the same time. Surviving neurons were detected by calcein labelling after 5 days of differentiation (DIF 5) and (A) cells with 5–10 pixels were individually counted. (B) Cells with 10–15 pixels were individually counted, (C) cells with 15–20 pixels were individually counted, (D) cells with >20 pixels were individually counted. Mean ± SEM (n = 3) *P < 0.05, **P < 0.01, ***P < 0.001 compared with respective DMSO groups (±dox).
Figure S2 Olesoxime protects neuronally differentiated cells against ASYN-induced toxicity in a time- and dose-dependent manner. ASYN cells cultured for 4 days in the presence or absence of doxycycline (dox) were seeded into 24-well plates at 18 000 cells·cm−2 and maintained in the presence or absence of dox. Differentiation was initiated with 10 μM RA 24 h after seeding. Olesoxime or DMSO treatment started at day 3 (A) or day 5 (B) following RA addition and the culture medium was replenished every 48 h. Seven days after the start of neuronal differentiation, survival was assessed by lysing cells remaining in each well and counting the number of intact nuclei using a hemocytometer as previously described (Vekrellis et al., 2009). The number of intact nuclei counted is expressed as the mean ± SEM (n = 3). *P < 0.05, **P < 0.01, olesoxime treatment compared with respective DMSO groups (±dox); one-way anova followed by Bonferroni post test.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordet T, Buisson B, Michaud M, Drouot C, Galéa P, Delaage P, et al. Identification and characterization of cholest-4-en-3-one, oxime (TRO19622), a novel drug candidate for amyotrophic lateral sclerosis. J Pharmacol Exp Ther. 2007;322:709–720. doi: 10.1124/jpet.107.123000. [DOI] [PubMed] [Google Scholar]
- Bordet T, Buisson B, Michaud M, Abitbol JL, Marchand F, Grist J, et al. Specific antinociceptive activity of cholest-4-en-3-one, oxime (TRO19622) in experimental models of painful diabetic and chemotherapy-induced neuropathy. J Pharmacol Exp Ther. 2008;326:623–632. doi: 10.1124/jpet.108.139410. [DOI] [PubMed] [Google Scholar]
- Bordet T, Berna P, Abitbol JL, Pruss RM. Olesoxime (TRO19622): a novel mitochondrial-targeted neuroprotective compound. Pharmaceuticals. 2010;3:345–368. doi: 10.3390/ph3020345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Thorpe J, Keller JN. Alpha-synuclein alters proteasome function, protein synthesis, and stationary phase viability. J Biol Chem. 2005;280:30009–30017. doi: 10.1074/jbc.M501308200. [DOI] [PubMed] [Google Scholar]
- Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, et al. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie CA. A review of Parkinson's disease. Br Med Bull. 2008;86:109–127. doi: 10.1093/bmb/ldn013. [DOI] [PubMed] [Google Scholar]
- Dessaud E, André C, Scherrer B, Berna P, Pruss R, Cuvier V, et al. AAN annual meeting abstracts 2014, emerging science abstracts: a phase II study to assess safety and efficacy of olesoxime (TRO19622) in 3–25 year old spinal muscular atrophy (SMA) patients. Neurology. 2014;83:34–40. [Google Scholar]
- Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem. 2008;283:9089–9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulovic M, Jovanovic M, Xilouri M, Stefanis L, Harhaji-Trajkovic L, Kravic-Stevovic T, et al. The protective role of AMP-activated protein kinase in alpha-synuclein neurotoxicity in vitro. Neurobiol Dis. 2013;63C:1–11. doi: 10.1016/j.nbd.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Eckmann J, Clemens LE, Eckert SH, Hagl S, Yu-Taeger L, Bordet T, et al. Mitochondrial membrane fluidity is consistently increased in different models of Huntington disease: restorative effects of olesoxime. Mol Neurobiol. 2014;50:107–118. doi: 10.1007/s12035-014-8663-3. [DOI] [PubMed] [Google Scholar]
- Farinelli SE, Greene LA, Friedman WJ. Neuroprotective actions of dipyridamole on cultured CNS neurons. J Neurosci. 1998;18:5112–5123. doi: 10.1523/JNEUROSCI.18-14-05112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouarne C, Giraudon-Paoli M, Seimandi M, Biscarrat C, Tardif G, Pruss RM, et al. Olesoxime protects embryonic cortical neurons from camptothecin intoxication by a mechanism distinct from BDNF. Br J Pharmacol. 2013;168:1975–1988. doi: 10.1111/bph.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu LJ, Sagara Y, Arroyo A, Rockenstein E, Sisk A, Mallory M, et al. alpha-synuclein promotes mitochondrial deficit and oxidative stress. Am J Pathol. 2000;157:401–410. doi: 10.1016/s0002-9440(10)64553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten M, Klein C. The many faces of alpha-synuclein mutations. Mov Disord. 2013;28:697–701. doi: 10.1002/mds.25499. [DOI] [PubMed] [Google Scholar]
- Kragh CL, Fillon G, Gysbers A, Hansen HD, Neumann M, Richter-Landsberg C, et al. FAS-dependent cell death in alpha-synuclein transgenic oligodendrocyte models of multiple system atrophy. PLoS ONE. 2013;8:e55243. doi: 10.1371/journal.pone.0055243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Kumar B, Nahreini P, Hanson AJ, Andreatta C, Prasad JE, Prasad KN. Selenomethionine prevents degeneration induced by overexpression of wild-type human alpha-synuclein during differentiation of neuroblastoma cells. J Am Coll Nutr. 2005;24:516–523. doi: 10.1080/07315724.2005.10719498. [DOI] [PubMed] [Google Scholar]
- Lenglet T, Lacomblez L, Abitbol JL, Ludolph A, Mora JS, Robberecht W, et al. A phase II-III trial of olesoxime in subjects with amyotrophic lateral sclerosis. Eur J Neurol. 2014;21:529–536. doi: 10.1111/ene.12344. [DOI] [PubMed] [Google Scholar]
- Magalon K, Zimmer C, Cayre M, Khaldi J, Bourbon C, Robles I, et al. Olesoxime accelerates myelination and promotes repair in models of demyelination. Ann Neurol. 2012;71:213–226. doi: 10.1002/ana.22593. [DOI] [PubMed] [Google Scholar]
- Maraganore DM, de Andrade M, Elbaz A, Farrer MJ, Ioannidis JP, Kruger R, et al. Collaborative analysis of alpha-synuclein gene promoter variability and Parkinson disease. JAMA. 2006;296:661–670. doi: 10.1001/jama.296.6.661. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Pan Y, Price AC, Sterling W, Copeland NG, Jenkins NA, et al. Parkinson's disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J Neurosci. 2006;26:41–50. doi: 10.1523/JNEUROSCI.4308-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin J, Golomb R, Jurewicz A, Antel JP, Fraser PE. Inositol stereoisomers stabilize an oligomeric aggregate of Alzheimer amyloid beta peptide and inhibit a beta-induced toxicity. J Biol Chem. 2000;275:18495–18502. doi: 10.1074/jbc.M906994199. [DOI] [PubMed] [Google Scholar]
- Pankratz N, Wilk JB, Latourelle JC, DeStefano AL, Halter C, Pugh EW, et al. Genomewide association study for susceptibility genes contributing to familial Parkinson disease. Hum Genet. 2009;124:593–605. doi: 10.1007/s00439-008-0582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl. Acids Res. 2014;42(Database Issue):D1098–1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Rovini A, Carre M, Bordet T, Pruss RM, Braguer D. Olesoxime prevents microtubule-targeting drug neurotoxicity: selective preservation of EB comets in differentiated neuronal cells. Biochem Pharmacol. 2010;80:884–894. doi: 10.1016/j.bcp.2010.04.018. [DOI] [PubMed] [Google Scholar]
- Rukenstein A, Rydel RE, Greene LA. Multiple agents rescue PC12 cells from serum-free cell death by translation- and transcription-independent mechanisms. J Neurosci. 1991;11:2552–2563. doi: 10.1523/JNEUROSCI.11-08-02552.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Brandis KA, Herrera SK, Johnson BE, Vaidya T, Shrestha R, et al. alpha-Synuclein budding yeast model: toxicity enhanced by impaired proteasome and oxidative stress. J Mol Neurosci. 2006;28:161–178. doi: 10.1385/JMN:28:2:161. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, et al. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer MJ, Bonifati V. The genetics of Parkinson's disease: progress and therapeutic implications. Mov Disord. 2013;28:14–23. doi: 10.1002/mds.25249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WW, Jiang H, Pei Z, Tanaka Y, Morita H, Sawa A, et al. Endoplasmic reticulum stress and mitochondrial cell death pathways mediate A53T mutant alpha-synuclein-induced toxicity. Hum Mol Genet. 2005;14:3801–3811. doi: 10.1093/hmg/ddi396. [DOI] [PubMed] [Google Scholar]
- Sunyach C, Michaud M, Arnoux T, Bernard-Marissal N, Aebischer J, Latyszenok V, et al. Olesoxime delays muscle denervation, astrogliosis, microglial activation and motoneuron death in an ALS mouse model. Neuropharmacology. 2012;62:2346–2352. doi: 10.1016/j.neuropharm.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Townsend M, Shankar GM, Mehta T, Walsh DM, Selkoe DJ. Effects of secreted oligomers of amyloid beta-protein on hippocampal synaptic plasticity: a potent role for trimers. J Physiol. 2006;572(Pt 2):477–492. doi: 10.1113/jphysiol.2005.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trophos AT. 2014. Trophos will present results of pivotal phase II/III study of olesoxime in spinal muscular atrophy patients at the American Academy of Neurology (AAN). Press release.
- Vekrellis K, Xilouri M, Emmanouilidou E, Stefanis L. Inducible over-expression of wild type alpha-synuclein in human neuronal cells leads to caspase-dependent non-apoptotic death. J Neurochem. 2009;109:1348–1362. doi: 10.1111/j.1471-4159.2009.06054.x. [DOI] [PubMed] [Google Scholar]
- Volles MJ, Lee SJ, Rochet JC, Shtilerman MD, Ding TT, Kessler JC, et al. Vesicle permeabilization by protofibrillar alpha-synuclein: implications for the pathogenesis and treatment of Parkinson's disease. Biochemistry. 2001;40:7812–7819. doi: 10.1021/bi0102398. [DOI] [PubMed] [Google Scholar]
- Xiao WH, Zheng FY, Bennett GJ, Bordet T, Pruss RM. Olesoxime (cholest-4-en-3-one, oxime): analgesic and neuroprotective effects in a rat model of painful peripheral neuropathy produced by the chemotherapeutic agent, paclitaxel. Pain. 2009;147:202–209. doi: 10.1016/j.pain.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YM, Gupta SK, Kim KJ, Powers BE, Cerqueira A, Wainger BJ, et al. A small molecule screen in stem-cell-derived motor neurons identifies a kinase inhibitor as a candidate therapeutic for ALS. Cell Stem Cell. 2013;12:713–726. doi: 10.1016/j.stem.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Olesoxime reduced the number of small cells (<15 pixels) in presence or absence of ASYN. ASYN cells seeded in 96-well plates at 3000 cells·cm−2 (low density) with or without doxycycline (dox),were differentiated with 10 μM RA starting 24 h after seeding. Olesoxime or DMSO treatment started at the same time. Surviving neurons were detected by calcein labelling after 5 days of differentiation (DIF 5) and (A) cells with 5–10 pixels were individually counted. (B) Cells with 10–15 pixels were individually counted, (C) cells with 15–20 pixels were individually counted, (D) cells with >20 pixels were individually counted. Mean ± SEM (n = 3) *P < 0.05, **P < 0.01, ***P < 0.001 compared with respective DMSO groups (±dox).
Figure S2 Olesoxime protects neuronally differentiated cells against ASYN-induced toxicity in a time- and dose-dependent manner. ASYN cells cultured for 4 days in the presence or absence of doxycycline (dox) were seeded into 24-well plates at 18 000 cells·cm−2 and maintained in the presence or absence of dox. Differentiation was initiated with 10 μM RA 24 h after seeding. Olesoxime or DMSO treatment started at day 3 (A) or day 5 (B) following RA addition and the culture medium was replenished every 48 h. Seven days after the start of neuronal differentiation, survival was assessed by lysing cells remaining in each well and counting the number of intact nuclei using a hemocytometer as previously described (Vekrellis et al., 2009). The number of intact nuclei counted is expressed as the mean ± SEM (n = 3). *P < 0.05, **P < 0.01, olesoxime treatment compared with respective DMSO groups (±dox); one-way anova followed by Bonferroni post test.


