The terminal step in cocaine biosynthesis is catalyzed by an acyltransferase that utilizes benzoyl-CoA and methylecgonine as substrates and is localized to the spongy mesophyll.
Abstract
The esterification of methylecgonine (2-carbomethoxy-3β-tropine) with benzoic acid is the final step in the biosynthetic pathway leading to the production of cocaine in Erythoxylum coca. Here we report the identification of a member of the BAHD family of plant acyltransferases as cocaine synthase. The enzyme is capable of producing both cocaine and cinnamoylcocaine via the activated benzoyl- or cinnamoyl-Coenzyme A thioesters, respectively. Cocaine synthase activity is highest in young developing leaves, especially in the palisade parenchyma and spongy mesophyll. These data correlate well with the tissue distribution pattern of cocaine as visualized with antibodies. Matrix-assisted laser-desorption ionization mass spectral imaging revealed that cocaine and cinnamoylcocaine are differently distributed on the upper versus lower leaf surfaces. Our findings provide further evidence that tropane alkaloid biosynthesis in the Erythroxylaceae occurs in the above-ground portions of the plant in contrast with the Solanaceae, in which tropane alkaloid biosynthesis occurs in the roots.
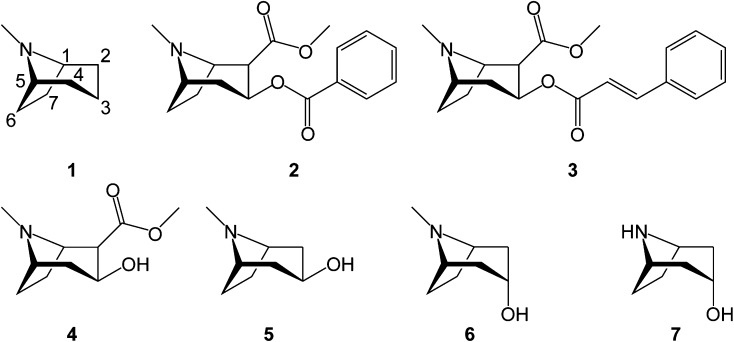
One of the most widely known plant alkaloids is cocaine, the benzoate ester of 2-carbomethoxy-3β-tropine (methylecgonine). Cocaine belongs to the tropane class of alkaloids defined by a common chemical substructure, the azabicyclo[3.2.1]octane skeleton (Fig. 1). Esterifications and hydroxylations of the tropane skeleton are common in nature, and more than 200 tropane alkaloids (TAs) with drastically different pharmacological activities are known to exist (Pollini et al., 2006). The physiological effects of these compounds have been ascribed to various features of the tropane skeleton and its substituents. The methylated nitrogen atom mimics that of acetylcholine and thereby leads to inhibition of muscarinic acetylcholine receptors (Schmeller et al., 1995). Binding to dopamine receptors is mediated by the stereochemistry of substituents at the C-2 and C-3 positions (Carroll et al., 1992a; Kelkar et al., 1994), with the strongest affinity being found for compounds containing an aromatic ring connected directly or indirectly to the 3β position (Carroll et al., 1992b). This in turn explains why cocaine exhibits both anesthetic and euphorigenic properties. The main source of cocaine is the South American Erythroxylum coca, a shrub or small tree cultivated for religious and medicinal purposes for more than 8,000 years (Dillehay et al., 2010). The result of long-term cultivation and selection for increasing alkaloid content has given rise to several cultivars containing up to 1% dry weight of cocaine in the leaves (Plowman and Rivier, 1983).
Figure 1.
Structures of selected TAs: numbered tropane nucleus (1), cocaine (2), cinnamoylcocaine (3), methylecgonine (4), pseudotropine (5), tropine (6), and nortropine (7).
Many years of in vivo biosynthetic studies of cocaine have led to a proposed pathway (Supplemental Fig. S1) beginning first with Orn or Arg, which produces the polyamine N-methylputrescine (Humphrey and O’Hagan, 2001). After oxidation of N-methylputrescine to 4-methylaminobutanal, which undergoes spontaneous cyclization to an N-methyl-Δ1-pyrrolinium cation, the equivalent of two acetyl units are added. Some debate remains regarding whether these carbons are supplied via acetate, acetoacetate, or malonate (Leete et al., 1991; Robins et al., 1997). The oxobutanoic acid intermediate formed by this condensation then cyclizes to form a tropane intermediate called methylecgonone (Jirschitzka et al., 2013). In the penultimate biosynthetic step to cocaine, methylecgonone is reduced to form methylecgonine. This reaction is catalyzed by the enzyme methylecgonone reductase (Jirschitzka et al., 2012).
The last step in cocaine biosynthesis is the esterification of methylecgonine with a benzoyl moiety hypothesized to utilize benzoyl-CoA as the activated acyl donor (Leete et al., 1988). This moiety was found to be derived from cinnamic acid, but it was not determined whether it arises via benzoyl-CoA or benzaldehyde (Leete et al., 1988; Bjorklund and Leete, 1992). Enzyme activities responsible for the acetylation of other TAs were purified, but no structural genes were isolated (Robins et al., 1991; Rabot et al., 1995). In plants, acylation reactions of secondary metabolites are performed by several acyltransferase families, namely the tyramine N-hydroxycinnamoyltransferase/serotonin N-hydroxycinnamoyltransferase, BAHD, and serine carboxypeptidase-like acyltransferases (Kang et al., 2006; Mugford and Osbourn, 2010). However, of these three groups, only the BAHD acyltransferases are known to utilize activated acyl-CoA thioesters (D’Auria, 2006). In this study, we report the identification and characterization of the enzymes responsible for the last biosynthetic step in the formation of cocaine in E. coca. These convert methylecgonine, a molecule with little physiological activity, into the pharmacologically active cocaine (Williams et al., 1977).
RESULTS
Formation of Cocaine and Cinnamoylcocaine in Aerial Tissues Utilizes CoA Esters
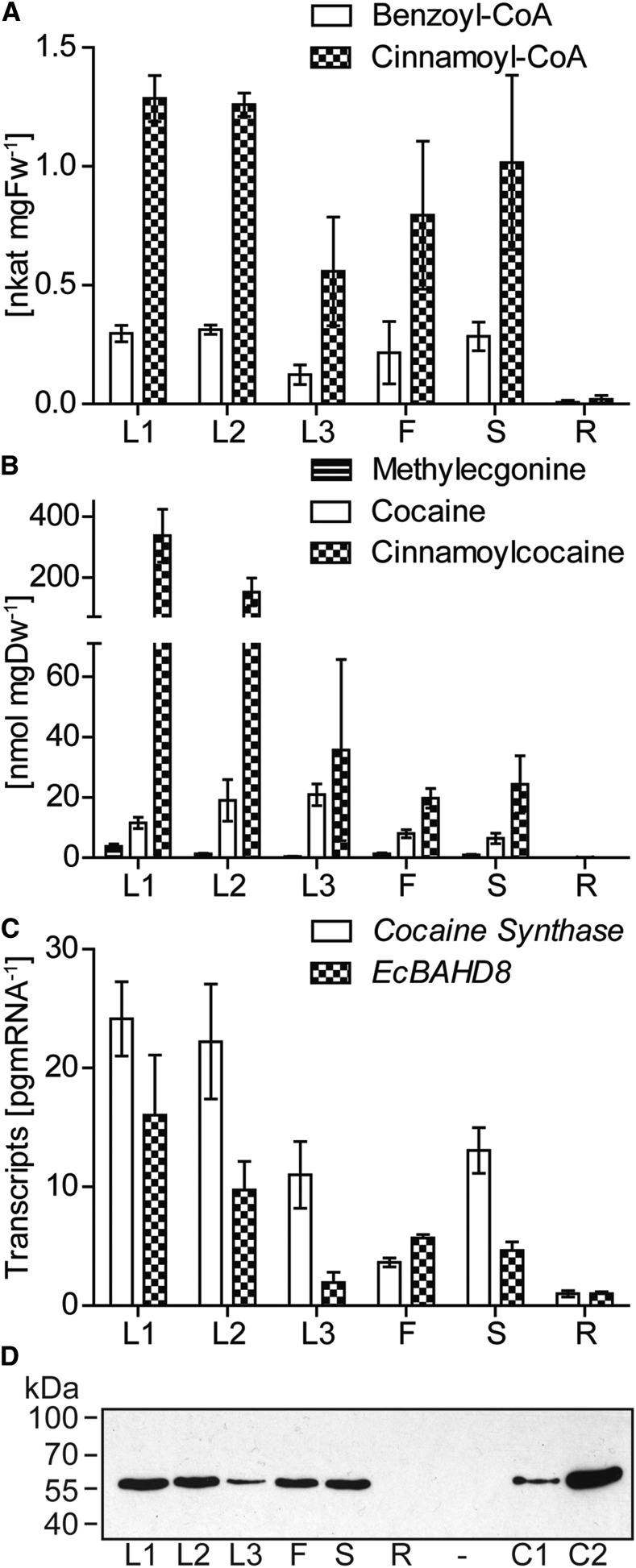
To investigate the nature of the reaction forming cocaine or its cinnamoyl analog abundant in young leaves, enzyme assays were performed on crude plant E. coca extracts using methylecgonine and either cinnamoyl-CoA or benzoyl-CoA as cosubstrates. Ester-forming activities were found in all tissue extracts with the exception of the roots (Fig. 2A). The highest activities were present in the early stages of leaf development (leaf stage 1 [L1], rolled young expanding leaves; and leaf stage 2 [L2], unrolled, expanding leaves) with 313 ± 20 pkat mg−1 fresh weight for benzoyl-CoA and 1285 ± 97 pkat mg−1 fresh weight for cinnamoyl-CoA as substrate. Activities in stem, flower, and leaf stage 3 (L3; mature, nonexpanding leaves) extracts were reduced by comparison. The levels of TAs in leaves correlate well with enzyme activities (Fig. 2B) with cocaine, cinnamoylcocaine, and methylecgonine highest in the early leaf stages (L1 and L2) followed by L3 stem and flower. None of these three metabolites were detected in the roots.
Figure 2.
Cocaine synthase enzyme activity, protein, and gene transcript levels compared with the amounts of reaction substrates and products in different E. coca organs and developmental stages. A, Cocaine- and cinnamoylcocaine-forming enzyme activity in desalted E. coca protein preparations. Enzymatic assays were performed using methylecgonine with either benzoyl-CoA or cinnamoyl-CoA as cosubstrates. Values displayed are means ± sd of three technical replicates from each of three biological replicates. B, Quantification of methylecgonine, cocaine, and cinnamoylcocaine. Values displayed are means ± sd of at least three biological replicates. C, Absolute quantification of RNA transcripts of cocaine synthase (EcBAHD7) and EcBAHD8 in E. coca. Values displayed are means ± sd of three technical replicates from each of three biological replicates. D, Levels of cocaine synthase protein determined by immunoblotting. Samples consisting of 15 μg of protein extracted from each organ as well as 75 ng (C1) and 150 ng (C2) recombinant Strep-tagged cocaine synthase (50.7 kD) were run on SDS-PAGE and gels were blotted onto filters. The filters were first probed with anticocaine synthase antibodies, followed by incubation with secondary antibodies conjugated to HRP. Polyclonal antibodies recognize cocaine synthase as well as EcBAHD8 (Supplemental Fig. S2B). Bands were visualized by chemiluminescence. L1 are young expanding leaves, still rolled after emerging from bud; L2 are larger expanding leaves, unrolled; and L3 are mature, nonexpanding leaves. DW, Dry weight, F, flower; FW, fresh weight; R, root; S, stem.
Cocaine Synthase Is a BAHD Acyltransferase
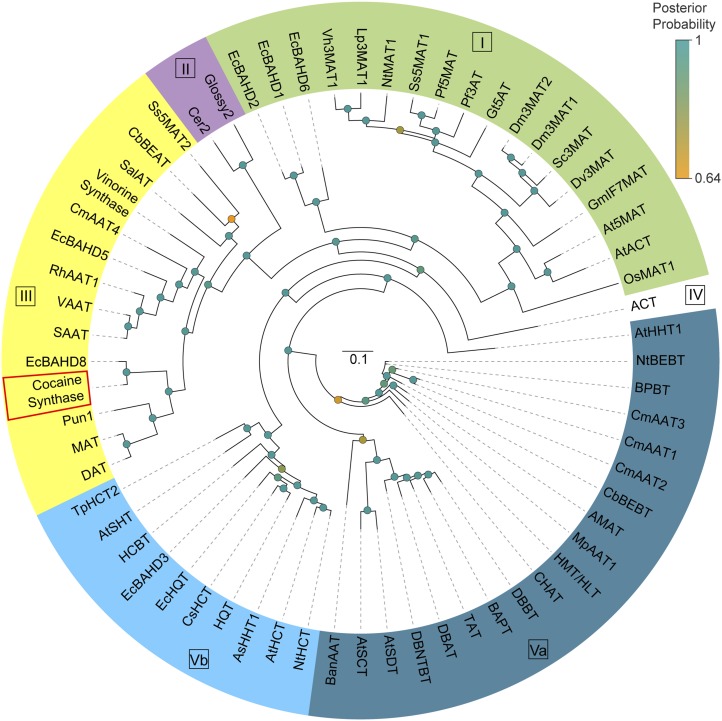
The involvement of acyl-CoA thioesters in cocaine and cinnamoylcocaine formation pointed to the likelihood of catalysis by a BAHD acyltransferase. In previous work, six BAHD acyltransferases were reported from an E. coca λZAPII complementary DNA (cDNA) library (Torre et al., 2013). The screening of a transcriptome database made from E. coca early leaf tissues (L1 and L2) yielded two more BAHD sequences designated EcBAHD7 and EcBAHD8. All eight EcBAHDs were heterologously expressed in Escherichia coli and the resulting recombinant proteins were purified using nickel-chelate chromatography. Verification of protein expression for all eight EcBAHDs was achieved using immunoblot analysis with anti-His antibodies (Supplemental Fig. S2A). The recombinant purified proteins were then tested in enzyme assays using the substrates methylecgonine and benzoyl-CoA. Of the eight individual proteins tested, only EcBAHD7 and EcBAHD8 exhibited ester-forming activity. A sequence alignment of EcBAHD7 and BAHD8 reveals that both enzymes share 77.3% identity at the amino acid level. Both EcBAHD7 and EcBAHD8 contain recognizable BAHD motifs including the DFGWG motif found near the C terminus as well as the HxxxD motif, which is critical for catalytic function (D’Auria, 2006). Phylogenetic analysis revealed that EcBAHD7 and EcBAHD8 belong to clade III of the BAHD superfamily (Fig. 3). Within this clade, EcBAHD7 and EcBAHD8 cluster with three other BAHDs involved in alkaloid biosynthesis. Two of these enzymes, deacetylvindoline-4-O-acetyltransferase and minovincinine-19-O-acetyltransferase from Madagascar periwinkle (Catharanthus roseus), are involved in the formation of vindoline and minovincine, respectively, both monoterpenoid indole alkaloids, whereas Pungency1 was shown via gene knockout experiments to be involved in capsaicin formation in pepper (Capsicum annuum; St-Pierre et al., 1998; Laflamme et al., 2001; Stewart et al., 2005).
Figure 3.
A circular Bayesian phylogram of BAHD acyltransferases involved in plant secondary metabolism. Selected BAHD acyltransferases were aligned using the CLUSTAL X program with standard settings for protein alignment. A phylogram was generated using the MRBAYES program and visualized using FigTree software. The different clades of BAHD acyltransferases are depicted according to D’Auria (2006). The posterior probabilities appear as the shaded circles shown at each node. Please refer to Supplemental Table S1 for an explanation of the abbreviated names. Accession numbers are as follows: cocaine synthase (accession no. KC140149) and EcBAHD8 (accession no. KC140150). Bar = 0.1 expected amino acid substitutions per site.
Enzyme Kinetics and Immunoprecipitation Reveal BAHD7 as Cocaine Synthase
Heterologous expression of EcBAHD7 and EcBAHD8 for biochemical characterization was carried out in Saccharomyces cerevisiae because overall enzyme activities were higher than in E. coli. The native EcBAHD7 protein with the addition of StrepTagII (10 amino acids added) was analyzed via gel sizing chromatography and yielded a single peak corresponding to a size of 43.9 kD. These data suggest that EcBAHD7, like other characterized acyltransferases, is monomeric (D’Auria, 2006). The pH optimum of the heterologously expressed EcBAHD7 protein was determined to be 9.4 when catalyzing the esterification of methylecgonine with benzoyl-CoA as the acyl donor. The enzyme activities were reduced to 39% and 44% of maximum activity at a pH of 9.1 or 10.4, respectively. Similar pH optima were also obtained for the heterologously expressed EcBAHD8.
The EcBAHD7 Km values for methylecgonine and benzoyl-CoA were 369 ± 26 µm and 93 ± 7 µm, respectively (Table I), whereas the reaction with methylecgonine and cinnamoyl-CoA gave a lower Km value for methylecgonine (62 ± 11 µm) but a similar value for the CoA ester (103 ± 8 µm for cinnamoyl-CoA). For EcBAHD8, Km values for methylecgonine and cinnamoyl-CoA were similar to those EcBAHD7, whereas those for methylecgonine and benzoyl-CoA were divergent. Furthermore, substrate inhibition was observed for the CoA thioesters in EcBAHD8 kinetic assays with Ki values of 5 ± 1 mm and 14 ± 3 mm for benzoyl-CoA and cinnamoyl-CoA, respectively. Comparison of catalytic efficiencies (kcat/Km) revealed that EcBAHD7 is over 1,000-fold more efficient than EcBAHD8 when using methylecgonine and benzoyl-CoA as substrates. The difference increases to 5,000-fold when comparing the catalytic efficiencies of these two enzymes using methylecgonine and cinnamoyl-CoA as substrates. Because EcBAHD7 is far more efficient at catalyzing the production of cocaine or cinnamoylcocaine compared with the activity of EcBAHD8, we designated EcBAHD7 as the true cocaine synthase in E. coca.
Table I. Kinetic parameters of EcCS and another E. coca BAHD acyltransferase, EcBAHD8.
Values preceding the ± sign represent sds from two technical replicates. N/A, Not available.
| Substrate | Apparent Km | Apparent kcat | kcat/Km | Apparent Ki |
|---|---|---|---|---|
| µm | s−1 | M−1 s−1 | mm | |
| EcCS | ||||
| Methylecgonine | 370 ± 26 | 9.7 ± 0.24 | 26,000 | N/A |
| Benzoyl-CoA | 93 ± 6.9 | 7.6 ± 0.17 | 82,000 | N/A |
| Methylecgonine | 62 ± 11 | 19 ± 1 | 306,000 | N/A |
| Cinnamoyl-CoA | 103 ± 8 | 46 ± 0.02 | 450,000 | N/A |
| EcBAHD8 | ||||
| Methylecgonine | 840 ± 64 | 0.02 ± 0.005 | 24 | N/A |
| Benzoyl-CoA | 6.9 ± 0.81 | 0.02 ± 0.004 | 2,900 | 5.5 ± 0.69 |
| Methylecgonine | 57 ± 6 | 0.003 ± 1.0 E-04 | 53 | N/A |
| Cinnamoyl-CoA | 93 ± 7 | 0.005 ± 1.1 E-04 | 54 | 14 ± 3 |
When cocaine synthase was tested with various alcohol substrates, ester formation was achieved only when using methylecgonine or pseudotropine (no C-2 carbomethoxy function) and not with tropine (3α-OH) or nortropine (3α-OH, no N-methyl group) as substrates. The specific activity of the reaction of methylecgonine and benzoyl-CoA was 4.03 pkat mg−1. Changing the CoA thioester to cinnamoyl-CoA, hexanoyl-CoA, or p-coumaroyl-CoA resulted in reductions in enzyme activities of 73%, 81%, and 99%, respectively. The relative activity of cocaine synthase with benzoyl-CoA and pseudotropine was 80% that of cocaine synthase with methylecgonine and benzoyl-CoA. The product formed from this reaction, tropacocaine, was estimated using cocaine as a standard reference.
Polyclonal antibodies were produced against the heterologously expressed cocaine synthase protein and used to investigate its localization (see below) as well as to confirm its identity as the cocaine-forming activity in planta by immunoprecipitation. Cocaine synthase activity in E. coca L2 protein extracts measured at 3.41 pkat mg−1 was subsequently reduced to 1.23 pkat mg−1, whereas preimmune serum did not reduce enzyme activity at all. Immunoprecipitated proteins were then separated on a protein gel and subsequent protein sequencing analysis identified the cocaine synthase protein within the sample, but not in the precipitate formed by the preimmune serum. EcBAHD8 was not detected in any of these samples.
Cocaine Synthase Transcript and Protein Levels Are Highest in Young Leaves
Both cocaine synthase and EcBAHD8 gene expression were higher in the early leaf stages (L1 and L2) than in mature leaves, stems, and flowers (Fig. 2C). They were almost completely absent in roots. Cocaine synthase transcript levels were generally at least 2-fold greater than those of EcBAHD8. The pattern of cocaine synthase protein levels in different organs, as evaluated by immunoblot analysis, generally followed the transcript pattern, with highest amounts in youngest leaves and no protein detected in roots (Fig. 2D).
Cocaine Synthase and Its Product Cocaine Are Concentrated in the Palisade Parenchyma
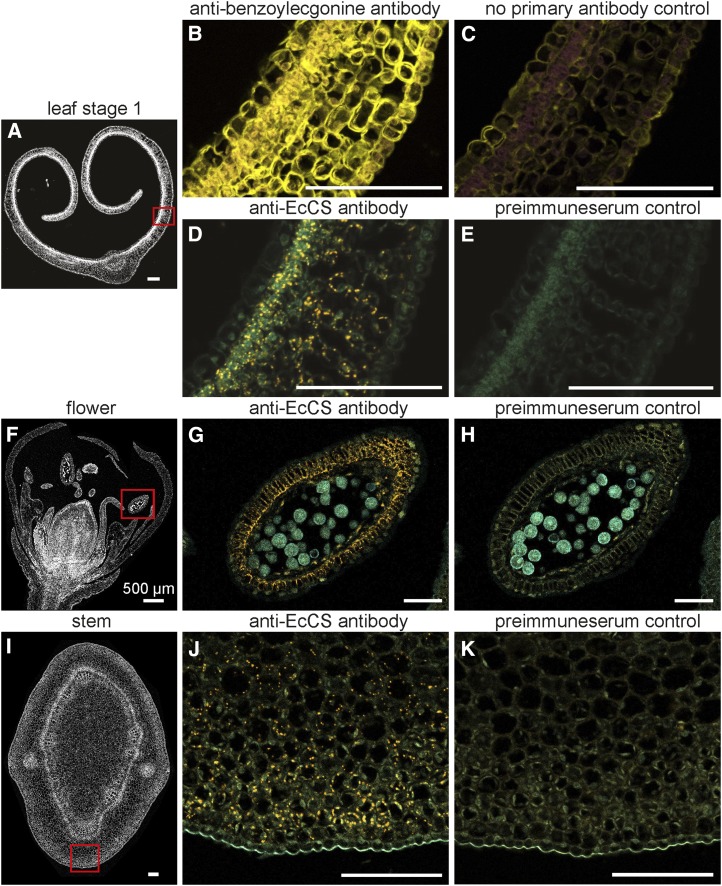
Based on the immunoblot results, leaves, flowers, and stems were used for tissue-level immunolocalization experiments. In addition, anticocaine antibodies were used to localize cocaine in the same tissues (Fig. 4). Antibodies were visualized by fluorescence methods (secondary antibody conjugated to horseradish peroxidase [HRP] assayed with fluorescent substrate) and all panels are overlays of fluorophore and autofluorescence channels. Preimmune serum was employed as a negative control and the fluorescence signal obtained from these samples was found to be unspecific. Cocaine was localized throughout the leaf with highest levels in the palisade layer, lower levels in the spongy mesophyll, and lowest levels in the upper and lower epidermis. A similar pattern was observed for the cocaine synthase protein with strongest signals in the palisade and a diffuse distribution in the spongy mesophyll. In the flower, cocaine synthase accumulated in the tapetum cells of the anther, and in the green stems accumulation was present in the ground tissue.
Figure 4.
Immunolocalization of cocaine and cocaine synthase. Fluorescence micrographs of immunolabeled cross sections of different E. coca organs. A, Overview of L1 cross section with the region of interest marked by a red rectangle. B and C, L1 cross section immunolabeled with antibenzoylecgonine antibodies and no primary antibody, respectively. Fluorescence signal of secondary antibody shown in yellow. Background autofluorescence shown in purple. D and E, L1 cross section immunolabeled with polyclonal anticocaine synthase antibodies and preimmune serum, respectively. Fluorescence signal of secondary antibody shown in orange. Background autofluorescence shown in cyan. F, Overview of flower cross section with the region of interest marked by a red rectangle. G and H, Flower cross section immunolabeled with polyclonal anticocaine synthase antibodies and preimmune serum, respectively. Fluorescence signal of secondary antibody shown in orange. Background autofluorescence shown in cyan. I, Overview of stem cross section with the region of interest marked by a red rectangle. J and K, Stem cross section immunolabeled with polyclonal anticocaine synthase antibodies and preimmune serum, respectively. Fluorescence signal of secondary antibody shown in orange. Background autofluorescence shown in cyan. Single sections were probed with primary antibody (anticocaine, anticocaine synthase, or preimmune serum) and secondary antibody (anti-rabbit conjugated to HRP) and subsequently assayed with fluorescent tyramide substrate. Excitation of fluorophore for cocaine imaging was at 543 nm and detection using a 585- to 615-nm band-pass filter. Plant autofluorescence was excited at 488 nm and detected using a 505-nm low-pass filter. For imaging of cocaine synthase excitation of fluorophore was at 561 nm and detection using 585- to 614-nm channels. Plant autofluorescence was excited at 488 nm and detected using 495- to 534-nm channels. All pictures are overlays of fluorophore and autofluorescence channels. Bars = 100 µm (unless otherwise indicated).
Cocaine and Cinnamoylcocaine Are Differentially Distributed within the Leaf
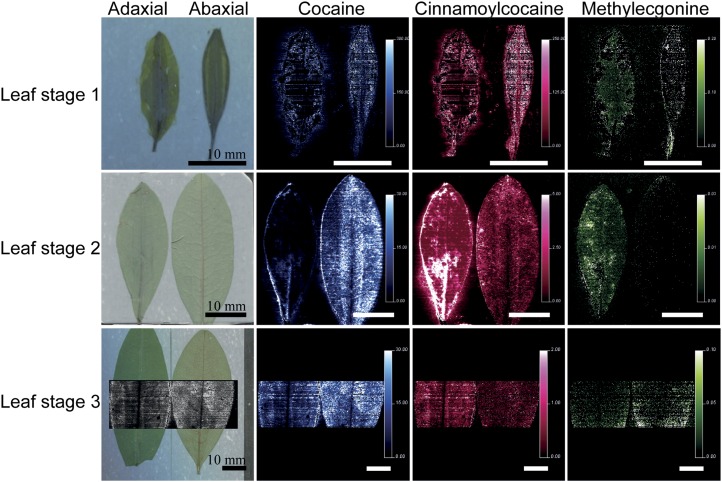
To determine the patterns of accumulation for methylecgonine in E. coca leaves, cinnamoylcocaine, and cocaine, matrix-assisted laser-desorption ionization (MALDI) imaging techniques were employed. The three compounds were detected by selected reaction monitoring (SRM) acquisition mode and their identities were further confirmed by enhanced product ion tandem mass spectrometry (MS/MS) experiments. Cocaine gave the highest signal of all three TAs, followed by cinnamoylcocaine and methylecgonine (Fig. 5; Supplemental Fig. S3). In leaf stages L2 and L3, there was a difference in intensity between the adaxial and abaxial surfaces, with the cocaine signal intensity highest on the abaxial surface and the cinnamoylcocaine signal higher on the adaxial surface. The methylecgonine signal intensity was overall very low, but was higher in the adaxial leaf surface of L2 and the abaxial leaf surface of L3 compared with the opposite leaf surface. An equal signal intensity of all three metabolites can be observed in adaxial and abaxial leaf surfaces of L1. When signal intensity was high, an uneven distribution of compounds could be observed within the leaf, with highest amounts being associated with the peripheral and central veins.
Figure 5.
MALDI MS imaging of E. coca leaves of different developmental stages for TAs. Optical images (left) and MALDI MS imaging of E. coca leaves of different developmental stages for TAs. MALDI-SRM/MS images are based on traces of spectra in the SRM mode specific for each TA. A black and white image based on the SRM trace for cocaine is overlaid on the optical image for L3, which corresponds to the area analyzed by MALDI for this sample and highlights details that can be expected with high spatial resolution MS imaging of intact leaves. Trace for cocaine is shown in blue at mass-to-charge ratio (m/z) 304 > m/z 182, cinnamoylcocaine trace is shown in pink at m/z 330 > m/z 182, and methylecgonine trace is shown in green at m/z 200 > m/z 182 in leaf stages L1 to L3. The images are displayed after normalization with the SRM trace of cocaine-d3 applied as a standard in solution with the matrix. The vertical color scale represents the respective SRM signal intensity ratio of the analyte over the reference compound cocaine-d3: White pixels give the highest signal, whereas dark pixels give the lowest signal. Horizontal bars represent a 10-mm scale for each image. MS image resolution is pixels of 50 × 50 µm.
DISCUSSION
TAs are commonly modified via esterification of the hydroxyl function at the C-3 position of the tropane ring. This feature is found in TAs of species from all of the four major plant lineages known to produce these compounds (Jirschitzka et al., 2013). The esterification of TAs in E. coca was first suggested to proceed via CoA-activated thioesters (Leete et al., 1988), and two CoA-dependent acyltransferases involved in TA modification were purified from Datura stramonium, a member of the Solanaceae. However, the sequence of the proteins and their corresponding genes were not reported (Robins et al., 1991). Here we established that the cocaine synthase reaction in E. coca uses benzoyl-CoA and methylecgonine as substrates. Given the CoA-dependent nature of the enzyme reaction as well as the properties reported for the tigloyl-CoA:pseudotropine acyltransferase from D. stramonium, we hypothesized that cocaine synthase is most likely a member of the BAHD acyltransferase superfamily (Rabot et al., 1995).
BAHD acyltransferases are well known for participating in the modification of secondary metabolites producing both esters and amides (D’Auria, 2006). A total of eight BAHD-like acyltransferases were subsequently identified in our databases, and two of these exhibited cocaine synthase activity. One of the two (EcBAHD7) was shown to be the native cocaine synthase in E. coca based on its greater activity with benzoyl-CoA and methylecgonine and the results of immunoprecipitation followed by protein sequencing. Another E. coca BAHD acyltransferase was recently reported to make 4-coumaroylquinate, a compound that is likely to be involved in the storage of cocaine and cinnamoylcocaine in planta (Torre et al., 2013). The activities of the remaining five BAHD-like enzymes remain to be determined. It is expected that the E. coca genome should contain more members of the BAHD acyltransferase family since Arabidopsis (Arabidopsis thaliana) and Populus trichocarpa contain approximately 60 and 150 unique members, respectively (D’Auria, 2006; Tuominen et al., 2011).
Although cocaine synthase and EcBAHD8 share over 77% sequence identity at the amino acid level, cocaine synthase has a much higher efficiency for the production of both cocaine and cinnamoylcocaine (1,000-fold and 5,000-fold differences, respectively). The two enzymes share similar Michaelis-Menten constants for methylecgonine and either benzoyl- or cinnamoyl-CoA, and the large catalytic efficiency differences are attributable to the very low turnover numbers of EcBAHD8. The two purified TA acyltransferases from D. stramonium show similar simple Michaelis-Menten kinetics for their respective substrates. Substrate inhibition kinetics was observed for EcBAHD8 with benzoyl- or cinnamoyl-CoA. However the high Ki values of 5 mm and 14 mm, respectively, are considerably higher than the natural concentration of CoA thioesters reported in plants (Perera et al., 2009; Qualley et al., 2012).
The apparent kcat values determined for methylecgonine and cinnamoyl-CoA for cocaine synthase differ by a factor of 2 (Table I). It is not unexpected to find differences in the apparent Vmax of a dual substrate enzyme when performing the type of discontinuous assays reported here (Segel, 1993). In addition, the biochemical characterization of many other BAHD family members also reported a difference in the Vmax or kcat of their respective dual substrates. For example, a 2-fold difference in the Vmax of the enzyme deacetylvindoline-4-O-acetyltransferase for its substrates acetyl-CoA and deacetylvindoline was observed (Laflamme et al., 2001). The enzyme anthraniloyl-CoA:methanol acyltransferase had a 6-fold difference in kcat for the dual substrates anthraniloyl-CoA and methanol. In the cases in which the apparent kcat values of BAHD enzymes for their dual substrates are different from one another, the higher value is always ascribed to the CoA substrate. The reported crystal structure for vinorine synthase, a BAHD member involved in indole alkaloid biosynthesis, suggested that binding order of substrates is independent (Ma et al., 2005). Although a second structural study on a BAHD anthocyanin malonyltransferase also determined that binding order is likely independent because of separate binding sites for the two substrates, a slight conformational change was observed in the native enzyme Dm3MaT3, suggesting that an induced fit model for catalysis is possible (Unno et al., 2007). The production of cinnamoylcocaine by cocaine synthase may also involve an induced fit mechanism in which the binding of cinnamoyl-CoA facilitates a conformational change in the enzyme. This can only be confirmed by performing detailed structural studies.
Compared with nearly all other characterized BAHD enzymes whose pH optima range from 5.5 to 7.5, the pH optima for the acylation of TAs range from 9 to 9.5 (Rabot et al., 1995; Boswell et al., 1999). At pH values above 8, the nitrogen present in the tropane ring is uncharged, which may be important for substrate binding and the acid base-catalyzed reaction mechanism of enzymes of this family. The alcohol cosubstrates of most other BAHD enzymes lack any group with potential charge at physiological pH values, and thus may function optimally at lower pH values.
The ability of cocaine synthase to accept other CoA thioesters, albeit at very low rates, may explain the trace amounts of other acylated methylecgonine derivatives found in E. coca such as hexanoylecgonine methyl ester (Casale and Moore, 1996a, 1996b). However, cocaine synthase is very specific for the alcohol cosubstrate catalyzing the esterification of only 3β-hydroxyl substrates. This fits well with the properties of the previous enzyme in the E. coca TA pathway, methylecgonone reductase, which only produces the 3β-tropane alcohol methylecgonine and not the corresponding 3α-compound (Jirschitzka et al., 2012). Specificity for the alcohol containing substrate was also observed for the other TA acyltransferases previously characterized (Boswell et al., 1999).
The distribution of alkaloids within plants is dependent upon key factors such as availability of substrate for biosynthesis, localization of biosynthetic genes, enzyme expression, and transport. The interaction of these factors may lead to a complex distribution pattern. For example, for TAs produced in the Solanaceae, the core biosynthetic pathway is in the roots, but these metabolites largely accumulate in the above-ground organs (Ziegler and Facchini, 2008). The benzylisoquinoline alkaloids in opium poppy (Papaver somniferum) are biosynthesized in sieve elements of phloem throughout the plant, whereas accumulation is mainly in specialized laticifers (Samanani et al., 2006). In E. coca, on the other hand, the accumulation and biosynthesis of TAs appear to occur within the same tissues. In previous work, cocaine was found to be synthesized in shoots, not roots (Docimo et al., 2012), and methylecgonone reductase, the penultimate enzyme in cocaine biosynthesis, was found to be localized to the palisade parenchyma and spongy mesophyll of the leaves (Jirschitzka et al., 2012). In this study, we demonstrated that cocaine synthase, like methylecgonone reductase, is localized to the parenchyma and spongy mesophyll of the leaf, whereas the storage of cocaine and related metabolites occurs in the same tissue. Curiously, cocaine synthase can also be found in the anther tapetum like the enzyme 6β-hydroxylase, which is involved in TA biosynthesis in Atropa belladonna (Suzuki et al., 1999). The function of TAs is in the anther tapetum is not clear, but this tissue is also known to accumulate chalcones, flavonols, and anthocyanins, which are involved in pollen pigmentation (Beerhues et al., 1993). The hydroxycinnamoyl-CoA thioesters required for flavonoid biosynthesis could also serve as substrates for cocaine synthase. Cocaine has been considered to act as a defense against insect herbivores (Blum et al., 1981; Nathanson et al., 1993), so its accumulation in leaves is easy to understand; however, its presence in pollen is enigmatic.
Cocaine synthase is responsible for the production of both cocaine and cinnamoylcocaine in E. coca, producing mostly cinnamoylcocaine in young leaves and increasing amounts of cocaine as leaves mature. This is most likely attributable to the different kinetic parameters of the two substrates (cinnamoyl-CoA being favored over benzoyl-CoA) and changes in the availability of cinnamoyl-CoA and benzoyl-CoA during leaf development. Young coca leaves are high in flavonoids whose biosynthesis requires large pools of hydroxycinnamoyl-CoA thioesters (Johnson et al., 1998, 2002). As the leaf develops, the need for cinnamoyl-CoA declines and more of this thioester can be converted into benzoyl-CoA (Klempien et al., 2012).
MALDI mass spectrometry (MS) imaging has been used to map metabolites within plant tissues (Burrell et al., 2007; Kaspar et al., 2011; Lee et al., 2012) as well as to monitor cocaine and its metabolites in single intact hair samples from humans for toxicological screening (Porta et al., 2011). The resolution of MS can distinguish among individual TAs based on their Mr, an improvement over the less specific recognition abilities of antibodies for these metabolites. MS imaging was employed to determine the general pattern of TA distribution in E. coca because it requires minimal sample preparation and does not alter the integrity of large samples, such as whole leaves. In mature leaf stages, we demonstrated that cocaine was preferentially distributed on the abaxial (bottom) surface, whereas cinnamoylcocaine was preferentially distributed on the adaxial (top) surface. To our knowledge, no other MS imaging study of plant leaf tissues has reported such differences between the adaxial and abaxial leaf surfaces (Mullen et al., 2005; Shroff et al., 2008; Ibáñez et al., 2010; Vrkoslav et al., 2010). Although differences between the surfaces could arise from ionization suppression as a result of varying thickness of the waxy layer or other components, such matrix effects should be the same for cocaine and cinnamoylcocaine. Thus, the distinct distributions of these two alkaloids may be a real phenomenon. The larger amount of cinnamoylcocaine on the adaxial surface may reflect the increased supply of cinnamoyl-CoA there as a result of increased formation of UV-absorbing flavonoids compared with the abaxial surface. The preferential distribution of TAs in veins is in accord with a study of TAs in D. stramonium (Solanaceae) leaves using indirect desorption electrospray ionization (ESI) imaging MS (Thunig et al., 2011).
In summary, we have determined that cocaine and other TAs in E. coca are formed by the acylation of the 3β-hydroxyl function of methylecgonine catalyzed by cocaine synthase, a member of the BAHD acyltransferase family. To our knowledge, this is the first report showing that a BAHD member is involved in TA production in plants, but we predict that the dominant esters of TAs found in other plant families such as Proteaceae, Brassicaceae, Rhizophoraceae, Convolvulaceae, and Solanaceae will be also formed by the action of a BAHD acyltransferase. It is still unclear whether TA formation was found in common ancestors of these groups or has arisen independently in the evolution of each lineage. Isolation of these acyltransferases may help determine whether this enzyme family has been recruited more than once to TA biosynthesis. The independent evolutionary origin of TAs in different lineages is supported by our results confirming that TA biosynthesis in E. coca occurs in the above-ground tissues, in stark contrast with the root location of this pathway in members of the Solanaceae.
MATERIALS AND METHODS
Chemicals and Plant Material
Methylecgonine (2-carbomethoxy-3β-tropine) was purchased from LGC Standards. Cocaine, cocaine-d3, cinnamoylcocaine, and methylecgonine for MALDI MS imaging were provided by Lipomed. All other chemicals were purchased from Sigma-Aldrich, Carl Roth, or Merck and had the highest available quality. Water was supplied by a Milli-Q Synthesis System (Millipore). Seeds of Erythroxylum coca were obtained from the botanical garden in Bonn, Germany. The seeds were removed from the surrounding pulp and germinated in Perlite. The plants were grown at 22°C under a 12-h-light/12-h-dark photoperiod, with humidity of 65% and 70%, respectively, and were fertilized once a week with Ferty 3 (15-10-15) and Wuxal Top N (Planta). A voucher specimen was deposited at the Herbarium Haussknecht (JE) at Friedrich Schiller University in Jena, Germany. Leaves for MALDI MS imaging experiments were collected from E. coca at the botanical garden in Zurich, Switzerland.
Cloning, Heterologous Expression, and Purification of Cocaine Synthase and EcBAHD8
Previously, six BAHD acyltransferases were identified from an E. coca young leaf λZAPII cDNA library (Torre et al., 2013). Using these six sequences, a BLAST search was performed on an in-house 454 cDNA sequencing database of E. coca young leaf tissue yielding two additional sequences: BAHD7 (designated EcCS) and BAHD8, respectively. The open reading frames of EcCS (GenBank accession no. KC140149) and EcBAHD8 (GenBank accession no. KC140150) were amplified from E. coca L2 cDNA using primer pairs EcCS_EC_Fwd/EcCS_EC_Rev and EcBAHD8_EC_Fwd/EcBAHD8_EC_Rev (Supplemental Table S2), respectively, and were gateway cloned into the Escherichia coli expression vector pH9GW as previously described (Jirschitzka et al., 2012). The expression vectors were introduced into E. coli BL21(DE3; Invitrogen) and the bacterial culture was grown in Luria-Bertani medium supplemented with 50 μg mL−1 of kanamycin at 37°C with shaking at 220 rpm until an OD600 of 0.4 to 0.5 was reached. Protein expression was induced by addition of 1 mm isopropyl β-d-1-thiogalactopyranoside with further cultivation at 18°C for 24 h. The cells were resuspended in 50 mm Bis-Tris buffer, pH 8, supplemented with 10% (v/v) glycerol and 5 mm dithiothreitol and disrupted by sonication. The lysate was centrifuged at 15,000g at 4°C for 15 min and the soluble fractions were tested for enzymatic activity. The assay buffer consisted of 50 mm Bis-Tris propane buffer, pH 8.0, supplemented with 10% (v/v) glycerol, 5 mm Tris(2-carboxyethyl)phosphine hydrochloride (TCEP), 1 mm methylecgonine, 1 mm benzoyl-CoA, and 10 μL of protein extract in a total volume of 100 μL. The assay was stopped after 2 h with 10 μL of 1 n HCl.
The assays were analyzed by liquid chromatography-ion trap MS on an 1100 series HPLC device (Agilent Technologies) coupled to an Esquire 6000 ESI-Ion Trap mass spectrometer (Bruker Daltonics) operated in positive ionization mode in the m/z range from 50 to 500 as follows: Skimmer voltage, 42.4 V; capillary exit voltage, 110 V; capillary voltage, −4,000 V; nebulizer pressure, 35 pounds per square inch (psi); drying gas, 9 L min−1; and gas temperature, 330°C. Elution was accomplished using a Nucleodur Sphinx RP column (25 cm × 4.6 mm, 5 µm; Macherey-Nagel) with a gradient of 0.2% (v/v) formic acid (solvent A) and methanol (solvent B) at a flow rate of 1 mL min−1 at 25°C as follows: 45% to 65% B (5 min), 65% to 100% B (0.1 min), 100% B (1.9 min), 100% to 45% B (0.1 min), and 45% B (3.9 min). Flow coming from the column was diverted in a 4:1 ratio before reaching the ESI unit. The molecular masses of cocaine (m/z 304) and cinnamoylcocaine (m/z 330) were monitored, and the area under the product peak was used for activity comparison.
For obtaining sufficient protein for biochemical characterization, the open reading frame of EcCS was synthesized as a codon-optimized version designated EcCSopt by GenScript and supplied in pUC57 vector (GenScript; Supplemental Fig. S4). EcCSopt was amplified from the pUC57 vector using the primer pair EcCSopt_SC_Fwd/EcCSopt_SC_Rev (Supplemental Table S2) and was gateway cloned into the Saccharomyces cerevisieae expression vector pYes-NStrep-GW (EcBAHD8 was also gateway cloned into pYes-NStrep-GW, using the pDONR207 clone from the initial gateway cloning into pH9GW). The vector pYes-NStrep-GW is a modified pYes-DEST52 (Invitrogen) S. cerevisieae expression vector that was built in house, to facilitate the production of recombinant proteins carrying an N-terminal StrepTagII and a thrombin cleavage site. Furthermore, EcCSopt and EcBAHD8 were amplified using the primer pairs EcCSopt_SC_Fwd/EcCSopt_SC_C-Strep_Rev and EcBAHD8_SC_Fwd/EcBAHD8_SC_C-Strep_Rev (Supplemental Table S2) and were gateway cloned into the S. cerevisieae expression vector pYes-DEST52 to facilitate the production of recombinant proteins carrying a C-terminal StrepTagII. The expression constructs were introduced into S. cerevisieae Inv-Sc1 (Invitrogen) and the protein was expressed as described in the manufacturer’s manual. For protein purification, the cells were resuspended in 100 mm Tris-HCl, pH 8, supplemented with 150 mm NaCl, 1 mm EDTA, 5 mm TCEP, and 1 mm phenylmethylsulfonyl fluoride (PMSF), and lysed using a pressure cell homogenizer (Stansted Fluid Power). The lysate was centrifuged at 15,000g at 4°C for 15 min and the soluble protein was purified using FPLC. After filtration of the sample using a 0.45-μm pore size filter (Millipore), the filtrate was directly loaded onto a StrepTrap HP column (GE Healthcare) using an FPLC machine (ÄKTApurifier; GE Healthcare). Recombinant protein was eluted using 100 mm Tris-HCl, pH 8, supplemented with 150 mm NaCl, 1 mm EDTA, 5 mm TCEP, 1 mm PMSF, and 2.5 mm desthiobiotin. Glycerol was added to a final concentration of 10% (v/v), and the mix was aliquoted and stored at −20°C and used directly for enzyme assays. The purity of recombinant protein was evaluated by SDS-PAGE gel electrophoresis followed by colloidal Coomassie staining. Protein concentration was measured using the Bradford protein assay (Bio-Rad) according to the manufacturer’s manual. Equivalent amounts of N-terminal and C-terminal StrepTagII-tagged recombinant protein were tested for enzyme activity, but no significant difference was observed. N-terminal StrepTagII-tagged protein expressed in S. cerevisieae was used for enzyme kinetics.
Extraction of Protein from E. coca Tissues
Fresh E. coca tissues were harvested and ground using a mortar and pestle precooled with liquid nitrogen. The plant powder was mixed in a 1:5 ratio with 100 mm Tris-HCl, pH 8, supplemented with 10% (v/v) glycerol, 2% (w/v) polyvinylpolypyrrolidone, 50 mm Na2S2O5, 5 mm dithiothreitol, and 1 mm PMSF. The resulting emulsion was mixed and incubated for 10 min on ice followed by centrifugation at 16,000g at 4°C for 10 min. The supernatant is referred to as the plant extract, and was used directly for immunoblotting analysis. For plant activity assays, the plant extracts were desalted into 100 mm Tris-HCl, pH 8, supplemented with 10% (v/v) glycerol and 5 mm TCEP using Ilustra Nap-5 columns (GE Healthcare) according to the manufacturer’s instructions. Protein concentration was measured using the Bradford protein assay (Bio-Rad) according to the manufacturer’s manual.
Synthesis of Cinnamoyl-CoA
The synthesis of the activated cinnamic acid was performed as described (Pabsch et al., 1991) with some modifications. Fifty millimoles of cinnamic acid was added to a solution of 120 mmol of carbonyldiimidazole in tetrahydrofuran. The reaction mixture was stirred for 5 h at reflux. After cooling to room temperature, any solids were filtered off. The solvent was removed by a rotary evaporator. The pale-yellow solid was washed with 10 mL of water and dried in vacuo. Then, 0.16 mmol of activated cinnamic acid was dissolved in 700 µL of 50 mm NaHCO3 and added slowly to a solution of 0.13 mmol of CoA in 500 µL of 50 mm NaHCO3 at 3°C. Acetone was added until the precipitate dissolved completely. The mixture was stirred for 24 h at 3°C. Afterward, the acetone was removed with N2. The CoA ester was purified as previously described (Torre et al., 2013) and fractions were analyzed by UV HPLC. The absorption at 260 nm was used for quantification with CoA tritium salt for calibration. The fraction with absorption at 260 nm (6.3 min) was further analyzed by liquid chromatography-ion trap MS on an 1100 series HPLC device (Agilent Technologies) coupled to an Esquire 6000 ESI-Ion Trap mass spectrometer (Bruker Daltonics) operated in negative ionization mode in the m/z range from 100 to 1,000 as follows: skimmer voltage, 42.4 V; capillary exit voltage, 123.7 V; capillary voltage, −4,500 V; nebulizer pressure, 35 psi; drying gas, 10 L min−1; and gas temperature, 330°C. Elution was accomplished using a Nucleodur Sphinx RP column (25 cm × 4.6 mm, 5 µm; Macherey-Nagel) with a gradient of 20 mm (v/v) ammonium acetate (solvent A) and acetonitrile (solvent B) at a flow rate of 1 mL min−1 at 25°C as follows: 15% to 29% B (7 min), 29% to 90% B (1 min), 90% B (2 min), 90% to 15% B (0.1 min), and 15% B (4.9 min). Flow coming from the column was diverted in a 4:1 ratio before reaching the ESI unit.
Measurement of Methylecgonine, Cinnamoylcocaine, and Cocaine in Plant Tissue
E. coca leaves of all three leaf stages (L1, L2, and L3), flower, stem, and roots were ground to a fine powder in liquid N2. TAs were extracted 1:10 (w/v) using 0.1% (v/v) formic acid and 30% (v/v) methanol at room temperature for 15 min. Samples were centrifuged at 4,000g for 15 min. The supernatant was filtered using 0.45-µm syringe filters and adjusted to pH 8 with saturated sodium carbonate solution followed by re-extraction with 3× chloroform. Phase separation was achieved using Chromabond PTS columns (Macherey-Nagel). The organic phase was vacuum dried and solved in water for analysis. Measurements were taken for six replicates of each sample. Analysis was done as described in the section on enzyme assays for kinetic analysis of enzymes and plant activity determination using authentic standards of methylecgonine, cocaine, and cinnamoylcocaine.
EcCS and EcBAHD8 Localization in E. coca Organs
Protein from different plant organs was extracted as described above. Equal amounts (15 μg) of protein were immunoblotted as described previously with the following exceptions (Jirschitzka et al., 2012). After blocking, the membranes were incubated with 1:1,000 anti-EcCS and 1:5,000 anti-rabbit HRP-conjugated antibodies (Sigma-Aldrich) in blocking solution.
For immunohistochemistry, selected fresh tissues were harvested and fixed for 4 h at room temperature in 50% (v/v) ethanol, 5% (v/v) acetic acid, and 3.7% (v/v) formaldehyde using vacuum infiltration. Samples were rinsed three times for 10 min in phosphate-buffered saline (PBS; 2.7 mm KCl, 137 mm NaCl, 1.8 mm KH2PO4, and 10 mm Na2HPO4, adjusted to pH 7.4 with 1 n KOH). Samples were dehydrated in an ascending series of ethanol solutions (10% [v/v], 30% [v/v], 50% [v/v], 70% [v/v], and 90% [v/v]) each time for 1 h. Samples were left in 96% (v/v) ethanol overnight, followed by an ascending series in Roti-Histol (Carl Roth), 25% (v/v), 50% (v/v), 75% (v/v), and 100% for 1 h each. The following steps were performed at 60°C. Roti-Histol (Carl Roth) was exchanged with liquid Paraplast X-Tra (Carl Roth) over a course of 4 d by replacing one-half of the solution with liquid Paraplast X-Tra every 12 h. Remaining Roti-Histol was allowed to evaporate in an open container at 60°C for 5 h. Single plant organs were embedded in aluminum molds and left to settle at room temperature for 30 min, before being transferred to 4°C for storage. Samples were cut on a rotary microtome to obtain 10-μm slices that were adhered to poly(Lys)-coated slides (Thermo Scientific) at 42°C overnight. To prepare for antibody treatment, slides were incubated in Roticlear (Carl Roth) and rehydrated by a descending ethanol series (2× 100%, 95% [v/v], 70% [v/v], 50% [v/v], 30% [v/v], 15% [v/v], and 2× water) for 2 min at each concentration. Endogenous peroxidases were blocked with 3% (v/v) hydrogen peroxide in PBS for 30 min. Slides were washed twice with PBS and once with phosphate-buffered saline supplemented with 0.1% (v/v) Tween 20 (PBT) for 5 min each, and blocked overnight with 1% (w/v) bovine serum albumin (BSA) in PBT at 4°C.
For localization of EcCS, slides were incubated with 1:100 anti-EcCS antibody in 1% (w/v) BSA in PBT overnight at 4°C in a humid chamber. For localization of cocaine, slides were incubated with 1:100 sheep antibenzoylecgonine antibodies (made to the demethylated metabolite of cocaine [certificate of analysis available in the Supplemental Data]; RayBiotech) in 1% (w/v) BSA in PBT overnight at 4°C in a humid chamber. Specificity of the antibodies was determined by the manufacturer using a fluorescent polarization immunoassay according to the protocol established for similar antibodies (Colbert et al., 1986). After washing slides with PBT, incubation with 1:100 anti-rabbit HRP-conjugated antibody (Sigma-Aldrich) or 1:100 anti-sheep HRP-conjugated antibody (RayBiotech) in 1% (w/v) BSA in PBT for 1 h was performed for localization of EcCS or cocaine, respectively. For fluorescent staining, Tyramide Signal Amplification (TSA) Kit no. 24 (Invitrogen) was used according to the manufacturer’s manual, with a developing time of 30 min. For cocaine localization, slides were imaged using a Zeiss LSM510 confocal microscope and a ×20 objective lens (Plan-Apochromat 20×/0.8 M27; Carl Zeiss). TSA fluorescence was excited at 543 nm and detected using a 585- to 615-nm band-pass filter. Plant autofluorescence was excited at 488 nm and detected using a 505-nm low-pass filter. For EcCS localization, slides were imaged using a Zeiss LSM710 confocal microscope and a ×20 objective lens (EC Plan-Neofluar ×20/0.5 M27; Carl Zeiss). TSA fluorescence was excited at 561 nm and detected using 585- to 614-nm λ channels. Plant autofluorescence was excited at 488 nm and detected using 495- to 534-nm λ channels. Overview pictures were obtained by taking tile pictures of whole tissue sections. TSA fluorescence and plant autofluorescence were imaged simultaneously, overlaid using ImageJ (open source; National Institutes of Health), and transferred to Illustrator CS6 (Adobe Systems) without further manipulation.
EcCS Antibody Production
Purified recombinant EcCS protein carrying an N-terminal StrepTagII, produced in S. cerevisieae as described above, was used to produce polyclonal antibodies in rabbits. Antibodies were affinity purified using epoxy-activated Sepharose conjugated to the same recombinant protein that was used for immunization (Davids Biotechnologie). Specificity of the purified antibodies to EcCS compared with other E. coca BAHD proteins was assessed by immunoblotting of recombinant proteins expressed in E. coli using the pH9GW vector as described above (Supplemental Fig. S2B).
Immunoprecipitation
Protein from L2 leaves of E. coca was extracted as described above. Protein was desalted into immunoprecipitation buffer (50 mm Bis-Tris, pH 8, supplemented with 10% [v/v] glycerol, 1 mm TCEP, 100 mm NaCl, 1 mm PMSF, and 0.5% [v/v] Nonidet P-40). Protein concentration was measured as described earlier. One hundred microliters of Protein A-coupled agarose beads (GE Healthcare) was prepared as described in the manual. Five-hundred nanograms of protein was adjusted to 700 μL with immunoprecipitation buffer. Five micrograms of anti-EcCS antibody and 20 μL of agarose bead slurry were added to the protein sample and incubated at 4°C for 1 h. The immunoprecipitate, represented by the agarose beads, was separated from the supernatant by centrifugation at 12,000g for 20 s at 4°C. The supernatant was kept as the immunoprecipitation supernatant sample. The immunoprecipitate was washed three times with 1 mL of immunoprecipitation buffer, and the wash solutions were discarded. Enzyme assays on the immunoprecipitate and supernatant were analyzed on an HPLC 1200 series device (Agilent) coupled to an API 3200 tandem mass spectrometer (Applied Biosystems) equipped with a turbospray ion source, using a ZORBAX RRHT Eclipse XDB-C18 column (5 cm × 4.6 mm × 1.8 μm; Agilent). Separation was achieved in 7 min at 20°C and a flow rate of 800 μL min−1, using 0.05% (v/v) formic acid (A) and acetonitrile (B) as the mobile phase as follows: 90% A (0.5 min), 90% to 30% A (3.5 min), 30% to 0% A (0.1 min), 0% A (0.7 min), 0% to 90% A (0.1 min), and 90% A (2.1 min). The spectrometer operated in positive ionization mode as follows: injection volume, 5 μL; curtain gas, 30 psi; turbo heater temperature, 700°C; nebulizing gas, 60 psi; heating gas, 70 psi; collision gas, 6 psi; and ion spray, 5,000 eV. Analytes were monitored by multiple reaction monitoring (MRM) as follows: cocaine m/z 304.3 → 182.3 (collision energy [CE], 26 V and declustering potential [DP], 45 V). Quantification was based on a standard curve of authentic cocaine. Both Q1 and Q3 quadrupoles were maintained at unit resolution.
Cocaine Synthase Substrate Specificity
Enzyme assays were performed as described in the section on enzyme assays for kinetic analysis of enzymes and plant activity determination with the exception that various CoA esters were used for the esterification reaction with methylecgonine. In addition to benzoyl and cinnamoyl-CoA, acetyl, acetoacyl, coumaroyl, hexanoyl, and malonyl-CoA were tested. Because of the lack of standards for the corresponding esters, the product ion was predicted and the peak area of the product peaks in MS analysis was compared with the peak area of cocaine, assuming that ionization of the molecules as well as the response of the detector are similar for the ester products. Quantification was based on an authentic standard curve for cocaine. Atropine served as the internal standard and enzyme assays (100 µL) were diluted with 900 µL of methanol, spiked with internal standard before injection.
Analysis was carried out on an HPLC 1200 series device (Agilent) coupled to an API 3200 tandem mass spectrometer (Applied Biosystems) equipped with a turbospray ion source, using a ZORBAX RRHT Eclipse XDB-C18 column (5 cm × 4.6 mm × 1.8 μm; Agilent). Separation was achieved in 7 min, at 20°C and a flow rate of 800 μL min−1, using 0.05% (v/v) formic acid (A) and acetonitrile (B) as the mobile phase as follows: 90% A (0.5 min), 90% to 30% A (3.5 min), 30% to 0% A (0.1 min), 0% A (0.7 min), 0% to 90% A (0.1 min), and 90% A (2.1 min). The spectrometer operated in positive ionization mode as follows: injection volume, 5 μL; curtain gas, 30 psi; turbo heater temperature, 700°C; nebulizing gas, 60 psi; heating gas, 70 psi; collision gas, 6 psi; and ion spray, 5,000 eV. Analytes were monitored by MRM as follows: cocaine m/z 304.3 → 182.3 (CE, 26 V and DP, 45 V), cinnamoylcocaine m/z 330.3 → 182.3 (CE, 26 V and DP, 45 V), methylecgonine m/z 200.2 → 182.3 (CE, 23 V and DP, 31 V), acetyl product m/z 242.3 → 182.3 (CE, 26 V and DP, 45 V), acetoacyl product m/z 284.3 → 182.3 (CE, 26 V and DP, 45 V), coumaroyl product m/z 346.3 → 182.3 (CE, 26 V and DP, 45 V), hexanoyl product m/z 298.3 → 182.3 (CE, 26 V and DP, 45 V), malonyl product m/z 286.3 → 182.3 (CE, 26 V and DP, 45 V), and atropine m/z 290.1 → 124.1 (CE, 31 V and DP, 51 V). Quantification was based on a standard curve of authentic cocaine and cinnamoylcocaine, taking the signal from the internal standard into account. Both Q1 and Q3 quadrupoles were maintained at unit resolution.
Size-Exclusion Chromatography
The protein size of EcCS was determined using an ÄKTApurifier (GE Healthcare) equipped with a HiLoad 16/60 Superdex 200 prep-grade column (GE Healthcare). Recombinant protein was expressed in S. cerevisieae and purified as described earlier. The running buffer consisted of 50 mm Bis-Tris, pH 8.0, supplemented with 150 mm NaCl. The column was calibrated using the Gel Filtration LMW Calibration Kit (GE Healthcare) as described in the manufacturer’s instructions. Samples were loaded at a flow rate of 0.5 mL min−1. Protein was eluted at a flow rate of 1 mL min−1 for 1.5 column volumes while collecting fractions of 6 mL.
Synthesis of [13C7]Cocaine
[U-13C]benzoyl-CoA was prepared as described (Beuerle and Pichersky, 2002) with the exception that benzoic acid was isotopically labeled. [13C7]benzoic acid was obtained from Isotec. An enzymatic assay (200 µL) containing 50 mm potassium phosphate buffer, pH 8, supplemented with 1 mm TCEP, 1 mg mL−1 BSA, 0.1 mm [13C7]benzoyl-CoA, 0.5 mm methylecgonine, and 8 µg of purified EcCS was left to react overnight at room temperature. Another 8 µg of purified EcCS was added and left to react at room temperature for 12 h. The solution was basified using 20 µL of 1 n NaOH and extracted three times with 500 µL of chloroform. The chloroform phase was dried down under nitrogen flow and resuspended in 200 µL of 10% (v/v) ethanol supplemented with 0.1% (v/v) formic acid. To determine the concentration for use as internal standard, dilutions of the product were analyzed on an API 3200 tandem mass spectrometer (Applied Biosystems) as described below.
Enzyme Assays for Kinetic Analysis of Enzymes and Plant Activity Determination
Protein concentration and incubation parameters were chosen so that the reaction velocity was linear with respect to enzyme concentration and incubation time for all enzyme assays. Standard assays contained 50 mm Gly-NaOH, pH 9.4, 1 mg mL−1 BSA, 1 mm TCEP, 1 mm methylecgonine, 1 mm benzoyl- or cinnamoyl-CoA, and the enzyme preparation. All reactions were carried out at 20°C in a Primus 96 Plus (MWG Biotech) PCR cycler. For determination of the pH optimum, 50 mm Gly-NaOH buffer covering the range from pH 8.6 to 10.4 was used. Enzyme and substrate concentrations were varied during the characterization process. For kinetic assays, one substrate was held constant at saturating conditions while the cosubstrate concentration was varied. Reactions (50 μL) were stopped after 10 min by adding 5 μL of 1 n HCl, and 5 µL of 2.5 µm [13C7]cocaine was added as an internal standard. One hundred microliters of chloroform was added to the assays and shaken in a paint shaker for 3 min to remove the protein from the assay. The aqueous phase was transferred into fresh HPLC vials and subjected to liquid chromatography-MS/MS analysis.
Analysis was carried out on an HPLC 1200 series device (Agilent) coupled to an API 3200 tandem mass spectrometer (Applied Biosystems) equipped with a turbospray ion source, using a ZORBAX RRHT Eclipse XDB-C18 column (5 cm × 4.6 mm × 1.8 μm; Agilent). Separation was achieved in 7 min, at 20°C and a flow rate of 800 μL min−1, using 0.05% (v/v) formic acid (A) and acetonitrile (B) as the mobile phase as follows: 90% A (0.5 min), 90% to 30% A (3.5 min), 30% to 0% A (0.1 min), 0% A (0.7 min), 0% to 90% A (0.1 min), and 90% A (2.1 min). The spectrometer was operated in positive ionization mode as follows: injection volume, 5 μL; curtain gas, 30 psi; turbo heater temperature, 700°C; nebulizing gas, 60 psi; heating gas, 70 psi; collision gas, 6 psi; and ion spray, 5,000 eV. Analytes were monitored by MRM as follows: cocaine m/z 304.3 → 182.3 (CE, 26 V and DP, 45 V), cinnamoylcocaine m/z 330.3 → 182.3 (CE, 26 V and DP, 45 V), methylecgonine m/z 200.2 → 182.3 (CE, 23 V and DP, 31 V), and 13C7-cocaine m/z 311.3 → 182.3 (CE, 26 V and DP, 45 V). Quantification was based on a standard curve of authentic cocaine and cinnamoylcocaine, taking the signal from the internal standard into account. Both Q1 and Q3 quadrupoles were maintained at unit resolution. For kinetic analysis of EcBAHD8 with cinnamoyl-CoA and methylecgonine, the analysis was carried out as described above except for the following changes. An API5000 tandem mass spectrometer (Applied Biosystems) was used and all chromatographic and mass spectrometer parameters were the same, except for the DP settings, which were as follows: cocaine, 75 V; cinnamoylcocaine, 75 V; methylecgonine, 61 V; and 13C7-cocaine, 75 V. Analyst 1.5 software (Applied Biosystems) was used for data acquisition and processing. Calculations and fitting of kinetic curves was performed using GraphPad Prism 5 (GraphPad Software).
Quantitative Real-Time PCR Analysis
For relative quantification, experiments were performed as described (Docimo et al., 2012). Primer pairs targeting the EcCS and EcBAHD8 transcripts EcCS_qPCR_Fwd/EcCS_qPCR_Rev and EcBAHD8_qPCR_Fwd/EcBAHD8_qPCR_Rev (Supplemental Table S2) were designed. Standard curve analysis showed a PCR efficiency of 95.5% and 88.3% and R2 values of 0.9983 and 0.9992 for EcCS and EcBAHD8 primer pairs, respectively. Expression of the genes was normalized to gene 6409 and gene 10131 (Docimo et al., 2012) expression using qBase (version 1.3.5; Hellemans et al., 2007).
For absolute quantification experiments, pDONR207 plasmids harboring EcCS or EcBAHD8 obtained during the cloning procedure were quantified using NanoDrop 2000c (NanoDrop Technologies). Standard curves of plasmids ranging from 10 to 109 plasmids per assay were run in parallel to quantitative PCR assays as described (Docimo et al., 2012). Standard curve analysis showed a PCR efficiency of 78.8% and 85.8% and R2 values of 1.000 and 0.998 for EcCS and EcBAHD8 primer pairs, respectively. The data were normalized to the input amount of the total RNA in the original cDNA synthesis reaction.
Phylogenetic Analysis
Analysis of protein sequences (Supplemental Table S1 includes the accession numbers) was performed as previously described (Torre et al., 2013). The following enzymes and their corresponding GenBank accession numbers were added to the analysis: EcCS (cocaine synthase; accession no. KC140149) and EcBAHD8 (accession no. KC140150).
MALDI Imaging
Standard and matrix solutions (α-cyano-4-hydroxycinnamic acid [CHCA]) were dissolved at a concentration of 10 mg mL−1 in MeOH:H2O:HCOOH (75:25:0.1 [v/v/v]). Deuterated cocaine (cocaine-d3) was added to the matrix solution (final concentration of 200 ng mL−1) and sprayed simultaneously with the matrix. Matrix solutions were kept in amber glass bottles to prevent their degradation by UV light and stored at 4°C between uses.
E. coca leaf samples were collected at three different stages of maturity: L1, L2, and L3. After being cut at the base of the stem, samples were then placed between two sheets of aluminum foil (to maintain their flat shape), and submerged immediately in liquid nitrogen for flash freezing. In addition, a set of L2 and L3 leaves was dipped in chloroform for 5 s prior to flash freezing to remove the waxy cuticle. The adaxial surface of coca leaves has a thick waxy cuticle containing hentriacontane as one of the major lipid components (Leete et al., 1988). Because wax components in the cuticle of Arabidopsis (Arabidopsis thaliana) are known to decrease the abundance of ions corresponding to flavonoids present within the leaf (Cha et al., 2008), cuticle was removed from some leaves. The chloroform treatment of L2 and L3 leaves did not change the abaxial and adaxial distribution pattern of the TAs (Supplemental Fig. S5). Frozen samples were then stored at −80°C prior to the analysis.
After drying in a desiccator under vacuum for 20 min at room temperature, intact leaf samples were mounted onto the stainless steel MALDI plate (OPTI TOF 384-well insert [123 × 81 mm] or OPTI TOF 192 well insert [44 × 44 mm]; AB SCIEX) using a double-sided adhesive tape (Plasto). Two intact leaves from the same maturity stage were placed on the MALDI plate in order to analyze both sides of the leaves in the same run. Approximately 12 mL of CHCA solution was manually sprayed using a 0.18-mm Custom Micron CM-C Plus airbrush (ANEST IWATA) held at a distance of 20 cm from the plate. N2 was used as the nebulizer gas (purity > 99.995%; Messer) and operated at a constant pressure of 0.5 bars. For the semiautomated spraying, the airbrush was fixed on a motorized XY two-axis stage (Zaber Technologies) to control the sprayer motion and speed. The sprayer was placed at a distance of 25 cm from the target plate and moved linearly at 15 mm s−1. Thirty layers of CHCA matrix solution containing the internal standard (solution of 200 ng mL−1) were applied.
Acquisitions were performed on a triple quadrupole linear ion trap mass spectrometer (AB SCIEX) equipped with a MALDI source and a frequency-tripled Nd:YAG laser 355 nm (elliptical beam shape of 100 × 200 µm). MS and MS/MS images were acquired in positive ionization mode. General operating conditions were as follows: data acquisition mode, line scan in rastering mode; repetition rate laser, 1,000 Hz; laser energy, 60 µJ; MALDI source and q0 region pressures were of 1 torr and 8 millitorrs, respectively; vacuum gauge in q2, 2.4 × 10−5 torr (nitrogen); DP, 70 V; and entrance potential, 10 V. Quadrupole resolution was set to unit for Q1 and Q3.
SRM data were acquired by simultaneously monitoring the following transitions: m/z 304 > m/z 182 for cocaine (CE, 35 eV), m/z 307 > m/z 185 for cocaine-d3 (CE, 35 eV), m/z 330 > m/z 182 for cinnamoylcocaine (CE, 40 eV), and m/z 200 > m/z 182 for methylecgonine (CE, 40 eV). The dwell time was set to 500 ms (TST = 2.020 s) and the distance between two line scans was 1 mm (rastering speed of 0.5 mm s−1), resulting in MALDI-SRM/MS-based images with pixels of 1 × 1 mm for the four compounds. To generate MALDI-SRM/MS images at higher resolution (i.e. pixels of 50 × 50 µm2), the plate speed was kept constant at 0.5 mm s−1, the dwell time was decreased to 20 ms, and the distance between two line scans was set to 50 µm.
MS/MS spectra in the enhanced product ion scan mode were acquired at a plate speed of 1 mm s−1 and the distance between two line scans was of 2 mm (pixels of 2 × 2 mm2). The conditions for each compounds were as follows: (1) for cocaine: precursor ion, m/z 304; CE, 35 eV; scan range, m/z 80 to 310; and TST, 1.991 s; (2) for cinnamoylcocaine: precursor ion, m/z 330; CE, 35 eV; scan range, m/z 80 to 340; and TST, 2.051 s; and (3) for methylecgonine: precursor ion, m/z 200; CE, 45 eV; scan range, m/z 80 to 210; and TST, 1.991 s. Reference spectra were acquired from a MALDI spot where 1 µL of a standard solution with the three analytes at a concentration of 100 ng mL−1 mixed 1:1 (v/v) with CHCA (10 mg mL−1) was spotted onto the target plate. Spectra for blank samples and for structural confirmation at the different maturation stages were generated by summing six vertical adjacent pixels over selected regions.
The MALDI source and its laser were controlled using a custom M3Q Server software based on a LabView platform (AB SCIEX). Analyst 1.5 software (AB SCIEX) was used for mass spectrometer control and for data collection. PeakView software (version 1.0.0.3; AB SCIEX) was used for raw data processing. A dedicated script was provided by Eva Duchoslav (AB SCIEX) to convert raw MS data files into an .img file that is compatible with the TissueView software (version 1.0; AB SCIEX) for MS and MS/MS image generation and processing.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers EcCS (KC140149) and EcBAHD8 (KC140150).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Cocaine biosynthesis pathway.
Supplemental Figure S2. Expression and antibody verification.
Supplemental Figure S3. MALDI MS imaging of E. coca leaves of different developmental stages for tropane alkaloids.
Supplemental Figure S4. Alignment of CS and CSopt nucleotide sequence.
Supplemental Figure S5. MALDI MS imaging of chloroform-treated E. coca leaves of different developmental stages for tropane alkaloids.
Supplemental Table S1. Details of the abbreviated BAHD acyltransferases used for Figure 3.
Supplemental Table S2. Primer sequences used for cocaine synthase and EcBAHD8.
Supplementary Material
Acknowledgments
We thank Drs. Heiko Vogel and Ewald Grosse-Wilde for assistance with assembling the 454 databases, Drs. Natalie Wielsch and Aleš Svatoš for assistance with protein sequencing, Jana Pastuscheck and Ina Haupt for technical assistance, and Elke Goschala, Andreas Weber, and the gardening staff of the Max Planck Institute for Chemical Ecology for assistance in growing the plants.
Glossary
- TA
tropane alkaloid
- cDNA
complementary DNA
- HRP
horseradish peroxidase
- MALDI
matrix-assisted laser-desorption ionization
- SRM
selected reaction monitoring
- MS/MS
tandem mass spectrometry
- MS
mass spectrometry
- ESI
electrospray ionization
- TCEP
Tris(2-carboxyethyl)phosphine hydrochloride
- psi
pounds per square inch
- PMSF
phenylmethylsulfonyl fluoride
- PBS
phosphate-buffered saline
- PBT
phosphate-buffered saline supplemented with 0.1% (v/v) Tween 20
- BSA
bovine serum albumin
- MRM
multiple reaction monitoring
- CE
collision energy
- DP
declustering potential
- CHCA
α-cyano-4-hydroxycinnamic acid
Footnotes
Articles can be viewed without a subscription.
References
- Beerhues L, Rittscher M, Schopker H, Schwerdtfeger C, Wiermann R (1993) The significance of the anther tapetum in the biochemistry of pollen pigmentation – an overview. Plant Syst Evol 7: 117–125 [Google Scholar]
- Beuerle T, Pichersky E (2002) Enzymatic synthesis and purification of aromatic coenzyme A esters. Anal Biochem 302: 305–312 [DOI] [PubMed] [Google Scholar]
- Bjorklund JA, Leete E (1992) Biosynthesis of the benzoyl moiety of cocaine from cinnamic acid via (R)-(+)-3-hydroxy-3-phenylpropanoic acid. Phytochemistry 31: 3883–3887 [Google Scholar]
- Blum MS, Rivier L, Plowman T (1981) Fate of cocaine in the Lymantriid Eloria noyesi, a predator of Erythroxylum coca. Phytochemistry 20: 2499–2500 [Google Scholar]
- Boswell HD, Dräger B, McLauchlan WR, Portsteffen A, Robins DJ, Robins RJ, Walton NJ (1999) Specificities of the enzymes of N-alkyltropane biosynthesis in Brugmansia and Datura. Phytochemistry 52: 871–878 [DOI] [PubMed] [Google Scholar]
- Burrell M, Earnshaw C, Clench M (2007) Imaging matrix assisted laser desorption ionization mass spectrometry: a technique to map plant metabolites within tissues at high spatial resolution. J Exp Bot 58: 757–763 [DOI] [PubMed] [Google Scholar]
- Carroll FI, Gao Y, Abraham P, Lewin AH, Lew R, Patel A, Boja JW, Kuhar MJ (1992a) Probes for the cocaine receptor. Potentially irreversible ligands for the dopamine transporter. J Med Chem 35: 1813–1817 [DOI] [PubMed] [Google Scholar]
- Carroll FI, Lewin AH, Boja JW, Kuhar MJ (1992b) Cocaine receptor: biochemical characterization and structure-activity relationships of cocaine analogues at the dopamine transporter. J Med Chem 35: 969–981 [DOI] [PubMed] [Google Scholar]
- Casale JF, Moore JM (1996a) Lesser alkaloids of cocaine-bearing plants II. 3-Oxo-substituted tropane esters: detection and mass spectral characterization of minor alkaloids found in South American Erythroxylum coca var. coca. J Chromatogr A 749: 173–180 [Google Scholar]
- Casale JF, Moore JM (1996b) Lesser alkaloids of cocaine-bearing plants III. 2-Carbomethoxy-3-oxo substituted tropane esters: detection and gas chromatographic-mass spectrometric characterization of new minor alkaloids found in South American Erythroxylum coca var. coca. J Chromatogr A 756: 185–192 [Google Scholar]
- Cha S, Zhang H, Ilarslan HI, Wurtele ES, Brachova L, Nikolau BJ, Yeung ES (2008) Direct profiling and imaging of plant metabolites in intact tissues by using colloidal graphite-assisted laser desorption ionization mass spectrometry. Plant J 55: 348–360 [DOI] [PubMed] [Google Scholar]
- Colbert DL, Smith DS, Landon J, Sidki AM (1986) Single-reagent polarisation fluoroimmunoassay for the cocaine metabolite, benzoylecgonine, in urine. Ann Clin Biochem 23: 37–41 [DOI] [PubMed] [Google Scholar]
- D’Auria JC. (2006) Acyltransferases in plants: a good time to be BAHD. Curr Opin Plant Biol 9: 331–340 [DOI] [PubMed] [Google Scholar]
- Dillehay TD, Rossen J, Ugent D, Karathanasis A, Vásquez V, Netherly PJ (2010) Early Holocene coca chewing in northern Peru. Antiquity 84: 939–953 [Google Scholar]
- Docimo T, Reichelt M, Schneider B, Kai M, Kunert G, Gershenzon J, D’Auria JC (2012) The first step in the biosynthesis of cocaine in Erythroxylum coca: the characterization of arginine and ornithine decarboxylases. Plant Mol Biol 78: 599–615 [DOI] [PubMed] [Google Scholar]
- Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J (2007) qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 8: R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey AJ, O’Hagan D (2001) Tropane alkaloid biosynthesis. A century old problem unresolved. Nat Prod Rep 18: 494–502 [DOI] [PubMed] [Google Scholar]
- Ibáñez AJ, Scharte J, Bones P, Pirkl A, Meldau S, Baldwin IT, Hillenkamp F, Weis E, Dreisewerd K (2010) Rapid metabolic profiling of Nicotiana tabacum defence responses against Phytophthora nicotianae using direct infrared laser desorption ionization mass spectrometry and principal component analysis. Plant Methods 6: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirschitzka J, Dolke F, D'Auria JC (2013) Increasing the pace of new discoveries in tropane alkaloid biosynthesis. InGiglioli-Guivarc'h N, ed, New Light on Alkaloid Biosynthesis and Future Prospects, Vol 68 Elsevier, London, pp 39–72 [Google Scholar]
- Jirschitzka J, Schmidt GW, Reichelt M, Schneider B, Gershenzon J, D’Auria JC (2012) Plant tropane alkaloid biosynthesis evolved independently in the Solanaceae and Erythroxylaceae. Proc Natl Acad Sci USA 109: 10304–10309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EL, Schmidt WF, Cooper D (2002) Flavonoids as chemotaxonomic markers for cultivated Amazonian coca. Plant Physiol Biochem 40: 89–95 [Google Scholar]
- Johnson EL, Schmidt WF, Norman HA (1998) Flavonoids as markers for Erythroxylum taxa: E. coca var. ipadu and E. novogranatense var. truxillense. Biochem Syst Ecol 26: 743–759 [Google Scholar]
- Kang S, Kang K, Chung GC, Choi D, Ishihara A, Lee DS, Back K (2006) Functional analysis of the amine substrate specificity domain of pepper tyramine and serotonin N-hydroxycinnamoyltransferases. Plant Physiol 140: 704–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar S, Peukert M, Svatos A, Matros A, Mock H-P (2011) MALDI-imaging mass spectrometry - An emerging technique in plant biology. Proteomics 11: 1840–1850 [DOI] [PubMed] [Google Scholar]
- Kelkar SV, Izenwasser S, Katz JL, Klein CL, Zhu N, Trudell ML (1994) Synthesis, cocaine receptor affinity, and dopamine uptake inhibition of several new 2 beta-substituted 3 beta-phenyltropanes. J Med Chem 37: 3875–3877 [DOI] [PubMed] [Google Scholar]
- Klempien A, Kaminaga Y, Qualley A, Nagegowda DA, Widhalm JR, Orlova I, Shasany AK, Taguchi G, Kish CM, Cooper BR, et al. (2012) Contribution of CoA ligases to benzenoid biosynthesis in petunia flowers. Plant Cell 24: 2015–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme P, St-Pierre B, De Luca V (2001) Molecular and biochemical analysis of a Madagascar periwinkle root-specific minovincinine-19-hydroxy-O-acetyltransferase. Plant Physiol 125: 189–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Perdian DC, Song Z, Yeung ES, Nikolau BJ (2012) Use of mass spectrometry for imaging metabolites in plants. Plant J 70: 81–95 [DOI] [PubMed] [Google Scholar]
- Leete E, Bjorklund JA, Couladis MM, Kim SH (1991) Late intermediates in the biosynthesis of cocaine: 4-(1-methyl-2-pyrrolidinyl)-3-oxobutanoate and methyl ecgonine. J Am Chem Soc 113: 9286–9292 [Google Scholar]
- Leete E, Bjorklund JA, Sung HK (1988) The biosynthesis of the benzoyl moiety of cocaine. Phytochemistry 27: 2553–2556 [Google Scholar]
- Ma X, Koepke J, Panjikar S, Fritzsch G, Stöckigt J (2005) Crystal structure of vinorine synthase, the first representative of the BAHD superfamily. J Biol Chem 280: 13576–13583 [DOI] [PubMed] [Google Scholar]
- Mugford ST, Osbourn A (2010) Evolution of serine carboxypeptidase-like acyltransferases in the monocots. Plant Signal Behav 5: 193–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen AK, Clench MR, Crosland S, Sharples KR (2005) Determination of agrochemical compounds in soya plants by imaging matrix-assisted laser desorption/ionisation mass spectrometry. Rapid Commun Mass Spectrom 19: 2507–2516 [DOI] [PubMed] [Google Scholar]
- Nathanson JA, Hunnicutt EJ, Kantham L, Scavone C (1993) Cocaine as a naturally occurring insecticide. Proc Natl Acad Sci USA 90: 9645–9648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabsch K, Rao NN, Wandrey C, Petersen M, Alfermann AW (1991) Chemo-enzymatic synthesis of rosmarinic acid. Recl Trav Chim Pays-Bas 110: 199–205 [Google Scholar]
- Perera MADN, Choi S-Y, Wurtele ES, Nikolau BJ (2009) Quantitative analysis of short-chain acyl-coenzymeAs in plant tissues by LC-MS-MS electrospray ionization method. J Chromatogr B Analyt Technol Biomed Life Sci 877: 482–488 [DOI] [PubMed] [Google Scholar]
- Plowman T, Rivier L (1983) Cocaine and cinnamoylcocaine content of Erythroxylum species. Ann Bot 51: 641–659 [Google Scholar]
- Pollini GP, Benetti S, De Risi C, Zanirato V (2006) Synthetic approaches to enantiomerically pure 8-azabicyclo[3.2.1]octane derivatives. Chem Rev 106: 2434–2454 [DOI] [PubMed] [Google Scholar]
- Porta T, Grivet C, Kraemer T, Varesio E, Hopfgartner G (2011) Single hair cocaine consumption monitoring by mass spectrometric imaging. Anal Chem 83: 4266–4272 [DOI] [PubMed] [Google Scholar]
- Qualley AV, Cooper BR, Dudareva N (2012) Profiling hydroxycinnamoyl-coenzyme A thioesters: unlocking the back door of phenylpropanoid metabolism. Anal Biochem 420: 182–184 [DOI] [PubMed] [Google Scholar]
- Rabot S, Peerless ACJ, Robins RJ (1995) Tigloyl-CoA:pseudotropine acyl transferase—an enzyme of tropane alkaloid biosynthesis. Phytochemistry 39: 315–322 [Google Scholar]
- Robins RJ, Abraham TW, Parr AJ, Eagles J, Walton NJ (1997) The biosynthesis of tropane alkaloids in Datura stramonium: the identity of the intermediates between N-methylpyrrolinium salt and tropinone. J Am Chem Soc 119: 10929–10934 [Google Scholar]
- Robins RJ, Bachmann P, Robinson T, Rhodes MJC, Yamada Y (1991) The formation of 3 alpha- and 3 beta-acetoxytropanes by Datura stramonium transformed root cultures involves two acetyl-CoA-dependent acyltransferases. FEBS Lett 292: 293–297 [DOI] [PubMed] [Google Scholar]
- Samanani N, Alcantara J, Bourgault R, Zulak KG, Facchini PJ (2006) The role of phloem sieve elements and laticifers in the biosynthesis and accumulation of alkaloids in opium poppy. Plant J 47: 547–563 [DOI] [PubMed] [Google Scholar]
- Schmeller T, Sporer F, Sauerwein M, Wink M (1995) Binding of tropane alkaloids to nicotinic and muscarinic acetylcholine receptors. Pharmazie 50: 493–495 [PubMed] [Google Scholar]
- Segel IH. (1993) Rapid equilibrium bireactant and terreactant systems. InEnzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems. John Wiley, New York, pp 273–345 [Google Scholar]
- Shroff R, Vergara F, Muck A, Svatoš A, Gershenzon J (2008) Nonuniform distribution of glucosinolates in Arabidopsis thaliana leaves has important consequences for plant defense. Proc Natl Acad Sci USA 105: 6196–6201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre B, Laflamme P, Alarco AM, De Luca V (1998) The terminal O-acetyltransferase involved in vindoline biosynthesis defines a new class of proteins responsible for coenzyme A-dependent acyl transfer. Plant J 14: 703–713 [DOI] [PubMed] [Google Scholar]
- Stewart C Jr, Kang BC, Liu K, Mazourek M, Moore SL, Yoo EY, Kim BD, Paran I, Jahn MM (2005) The Pun1 gene for pungency in pepper encodes a putative acyltransferase. Plant J 42: 675–688 [DOI] [PubMed] [Google Scholar]
- Suzuki K, Yun DJ, Chen XY, Yamada Y, Hashimoto T (1999) An Atropa belladonna hyoscyamine 6beta-hydroxylase gene is differentially expressed in the root pericycle and anthers. Plant Mol Biol 40: 141–152 [DOI] [PubMed] [Google Scholar]
- Thunig J, Hansen SH, Janfelt C (2011) Analysis of secondary plant metabolites by indirect desorption electrospray ionization imaging mass spectrometry. Anal Chem 83: 3256–3259 [DOI] [PubMed] [Google Scholar]
- Torre JC, Schmidt GW, Paetz C, Reichelt M, Schneider B, Gershenzon J, D’Auria JC (2013) The biosynthesis of hydroxycinnamoyl quinate esters and their role in the storage of cocaine in Erythroxylum coca. Phytochemistry 91: 177–186 [DOI] [PubMed] [Google Scholar]
- Tuominen LK, Johnson VE, Tsai C-J (2011) Differential phylogenetic expansions in BAHD acyltransferases across five angiosperm taxa and evidence of divergent expression among Populus paralogues. BMC Genomics 12: 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unno H, Ichimaida F, Suzuki H, Takahashi S, Tanaka Y, Saito A, Nishino T, Kusunoki M, Nakayama T (2007) Structural and mutational studies of anthocyanin malonyltransferases establish the features of BAHD enzyme catalysis. J Biol Chem 282: 15812–15822 [DOI] [PubMed] [Google Scholar]
- Vrkoslav V, Muck A, Cvacka J, Svatos A (2010) MALDI imaging of neutral cuticular lipids in insects and plants. J Am Soc Mass Spectrom 21: 220–231 [DOI] [PubMed] [Google Scholar]
- Williams N, Clouet DH, Missa AL, Mule S (1977) Cocaine and metabolites: relationship between pharmacological activity and inhibitory action on dopamine uptake into striatal synaptosomes. Prog Neuropsychopharmacol 1: 265–269 [Google Scholar]
- Ziegler J, Facchini PJ (2008) Alkaloid biosynthesis: metabolism and trafficking. Annu Rev Plant Biol 59: 735–769 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.