Abstract
Direct visualization shows enhanced embolism of xylem samples when they are collected under tension.
Embolism resistance is a critically important trait for evaluating the ability of plants to survive and recover from drought periods and predicting future drought-induced forest decline (Choat et al., 2012). However, recent publications have provided evidence that some measurement techniques used to evaluate the hydraulic function and vulnerability to cavitation of plant organs may be prone to artifacts (Sperry et al., 2012; Cochard et al., 2013; Torres-Ruiz et al., 2014; Trifilò et al., 2014). The discovery of these artifacts has raised questions regarding the reliability of some previously published plant hydraulics data, in particular data relating to the refilling of embolized xylem conduits while the xylem is under tension. In this context, Wheeler et al. (2013) reported that sampling plant organs by cutting while the xylem is under tension can induce artificial increases in the degree of embolism at the moment of sample excision, even when cuts are made under water. The methodology applied by Wheeler et al. (2013), however, did not allow the visualization of embolized or functional vessels, and native embolism levels could not be determined in intact plants before any cutting was done.
Whereas Scoffoni and Sack (2014) showed that the artifact described by Wheeler et al. (2013) has no impact on leaf xylem hydraulic conductance, there is some uncertainty about its importance in stems or shoots (Trifilò et al., 2014; Venturas et al., 2014). The results of Wheeler et al. (2013) indicate that more embolism could be induced by cutting samples that are under midrange xylem tension (e.g. at midday or under conditions of water stress). Potential overestimation of embolism due to changes in the xylem tension during the day has important implications for our understanding of plant water relations, since they could erroneously suggest that daily patterns of embolism formation and repair are routine in many woody plant species. Debate continues regarding the implications of a cutting artifact for the existence of a mechanism that allows plants to repair embolism while the xylem is under tension, so-called novel refilling (Salleo et al., 1996; Cochard and Delzon, 2013; Sperry, 2013; Delzon and Cochard, 2014). To avoid the excision artifact, Wheeler et al. (2013) recommended the relaxation of the xylem tension prior to excision by rehydrating plant tissue for anywhere between 2 min and 2 h. However, recent results from Trifilò et al. (2014) indicated that the rehydration procedures used by Wheeler et al. (2013) for relaxing the samples might favor xylem refilling and embolism repair (rehydration artifact), suggesting that the artifact resides in the relaxing procedure rather than in the cutting procedure. In light of these data, the assessment of the artifact described by Wheeler et al. (2013) using noninvasive techniques on intact plants, such as direct observation using x-ray microtomography (micro-CT; McElrone et al., 2013; Cochard et al., 2014) or magnetic resonance imaging (Choat et al., 2010; Zwieniecki et al., 2013), is useful to visually assess changes in embolism after cutting stems.
DISENTANGLING CURRENT CONTROVERSIES BY DIRECT VISUALIZATION USING MICRO-CT
Micro-CT is a noninvasive technique that allows researchers to directly visualize xylem conduits at high resolution and determine the degree of embolism and embolism spreading patterns (Brodersen et al., 2013). By using this technique, Cochard et al. (2014) recently found a lower amount of embolism in droughted Laurus nobilis stems than was reported previously for this species using indirect techniques (Cochard, 2002; Salleo et al., 2006).
We used two different Synchrotron-based micro-CT facilities (the Swiss Light Source [SLS] in Villigen, Switzerland, and the Advanced Light Source [ALS] in Berkeley, CA) to test the excision artifact described by Wheeler et al. (2013). Specifically, we examined the impact of cutting xylem under tension and under water in three woody and one herbaceous species: Acer campestre, Eucalyptus pauciflora, Juglans microcarpa, and Helianthus annuus. By direct visualizations, we tested whether (1) the degree of embolism of the samples varied before and after cutting them (underwater) for all four species and (2) how much xylem tension affected the degree of embolism observed in E. pauciflora, A. campestre, and J. microcarpa after cutting under water. All experiments were carried out on intact plants except for A. campestre, in which cut branches were used. Intact seedlings were scanned at approximately 10 cm above the base of the stem. For A. campestre branches, the scans before cuttings were done at a minimum distance corresponding to three internodes from the cut end (enough to avoid any open vessels at the basal end). All postcut scans were done immediately after cutting under water at the same location as the first scan. All scans were completed in approximately 5 min using the continuous tomography setting at 17 and 20 keV at the ALS and SLS facilities, respectively. The degree of embolism was calculated by comparing the amount of xylem area embolized before and after cutting with the total xylem area. Cutting under water was done a few centimeters away from the scanned area.
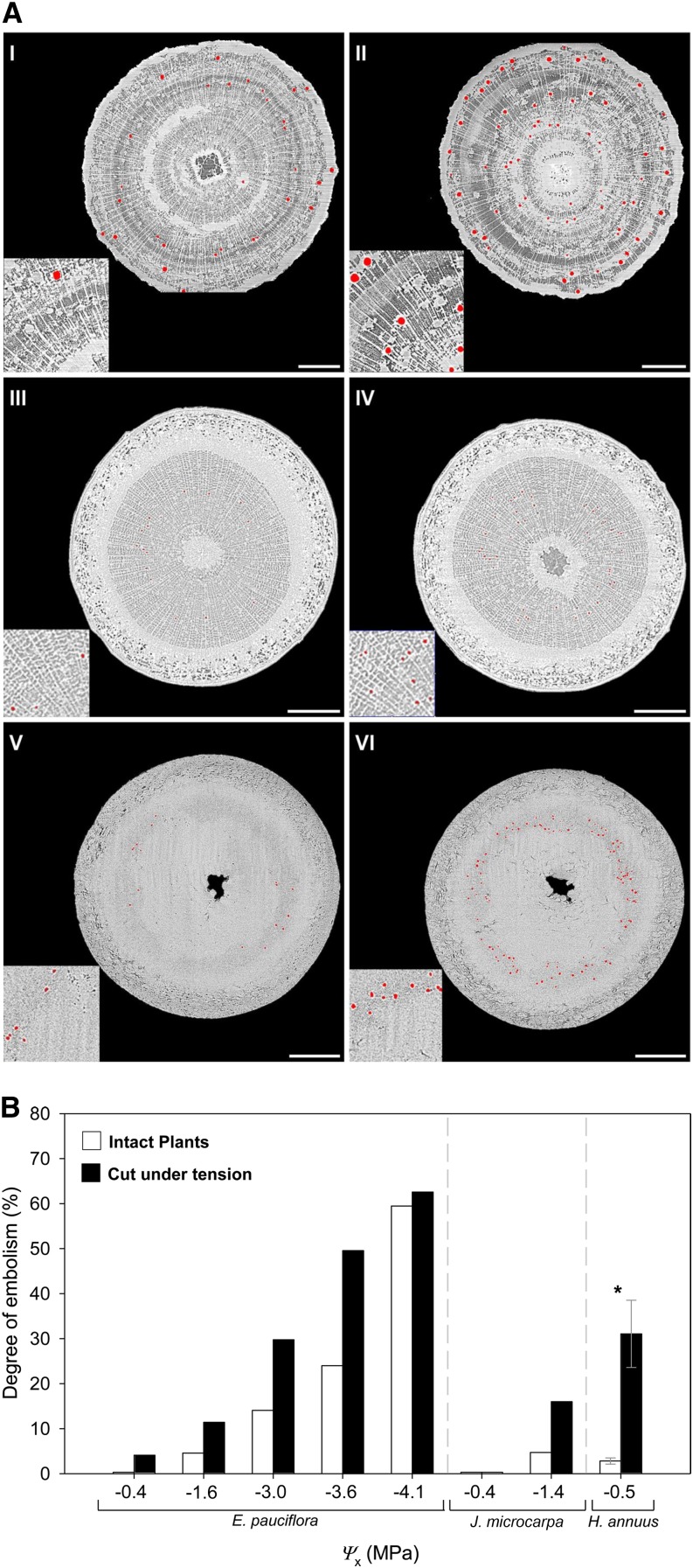
For all the species studied, images showed an increase in the degree of embolism after cutting while the xylem was under tension, whether the measurements were done on intact plants (Fig. 1A) or cut branches (A. campestre). For A. campestre, the degree of artificially increased embolism was 3.8% and 26.3% at a xylem tension (i.e. xylem water potential [Ψx]) of −3 and −3.5 MPa, respectively. This is consistent with the excision artifact suggested by Wheeler et al. (2013). The percentages of embolism introduced by cutting are lower than those reported by Wheeler et al. (2013), who quantified the degree of embolism in longer xylem segments using a hydraulic method. The effect of vessel size was not taken into account in our percentage embolism estimates so, if the widest and longest vessels were the ones to embolize first, this would result in a higher loss of conductance (Cochard et al., 2013). Although these increases were relatively low, they clearly support the suggestions that the standard practice of cutting branches underwater to preserve the endogenous state of embolism can provoke the formation of bubbles into the vascular system, providing implications for accurate measurements of embolism. Although more access to micro-CT facilities is required for confirmation, the variability in our results suggests that the intensity of the cutting artifact is not constant within or between species even at similar xylem tensions, probably being affected by other factors (e.g. vessel diameter or amount of air in the pith). In E. pauciflora, J. microcarpa, and H. annuus, the possibility of working with intact seedlings allowed us to measure the occurrence of this excision artifact (i.e. increases in the degree of embolism compared with the intact stem) not only in woody species but also in herbaceous plants (Fig. 1). In addition, results from E. pauciflora and J. microcarpa showed how this artifact is influenced by the xylem tension: none or negligible increases were observed at low xylem tensions (i.e. Ψx of approximately −0.4 MPa) and when the degree of native embolism was relatively high (Fig. 1B). The embolism induced by cutting, therefore, was a function of the species, the xylem tension, and the degree of native embolism at the moment of the sample excision.
Figure 1.
A, Transverse sections based on consecutive micro-CT scans for E. pauciflora (I and II), J. microcarpa (III and IV), and H. annuus (V and VI) at Ψx of −3.6, −1.4, and −0.4 MPa, respectively. Images show the amount of cavitated or air-filled conduits before (intact seedling; left) and after (right) cutting the sample under water without releasing the xylem tension. Both images were scanned at the same height to allow before and after comparison. Cavitated or air-filled vessels are observed as red. Insets show the same magnified areas (2×) of each section. Bars = 500 μm. B, Degree of embolism in E. pauciflora, J. microcarpa, and H. annuus at different Ψx in intact plants (white columns) and after cutting them under tension and under water (black columns). Due to limited access to Synchrotron micro-CT facilities, only one replication per Ψx was carried out except for H. annuus (n = 3). Columns indicate averaged values, error bars represent se, and the asterisk indicates a significant difference (P < 0.05).
IMPLICATIONS FOR PLANT PHYSIOLOGY AND ECOLOGY
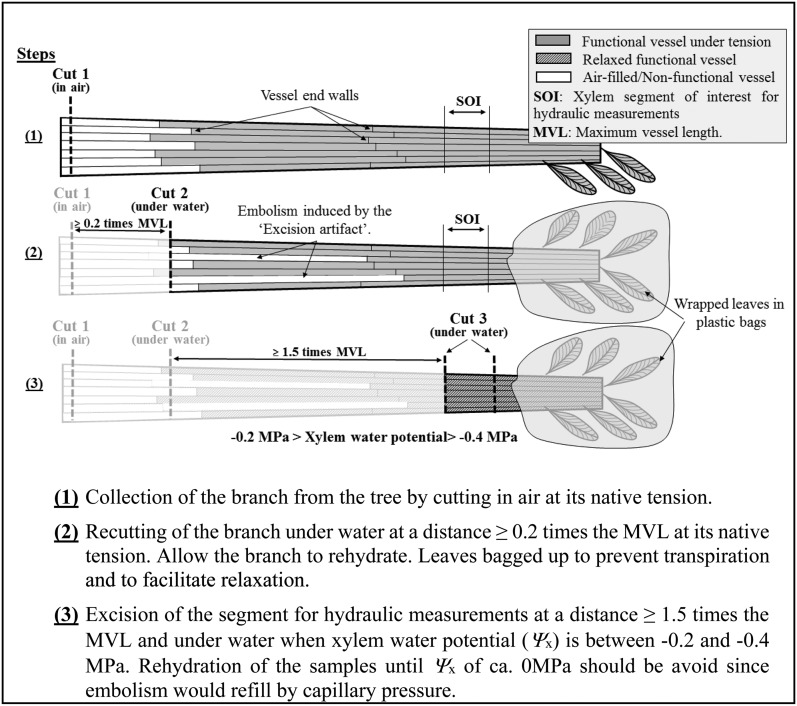
Our results confirm that the excision artifact could have a significant impact on estimates of embolism at the stem level. This indicates that we must carefully reassess the accuracy of some previous results, especially those obtained from samples collected under tension (e.g. at midday, from dehydrating branches, and from water-stressed plants) and from long-vesseled species in which vessels could be open in the measurement segment. Artifactual increases in the degree of embolism can be misleading for two reasons: (1) they may suggest an erroneously high vulnerability to cavitation for some species; and (2) the variation of the xylem tension throughout the day would induce artifactual increases in the degree of embolism that could be interpreted as the occurrence of diurnal cycles of embolism formation and repair. Therefore, protocols of invasive techniques should be improved and validated by direct visualization for accurate evaluation of the hydraulic function or vulnerability curves. To minimize the excision artifact, the following protocol to collect and prepare samples should be followed (Fig. 2): if branches are initially harvested from a tree by cutting in air (cut 1), a second cut (cut 2) should be done under water at a minimum distance of 0.2 times the maximum vessel length (MVL; Ewers and Fisher, 1989) of the species to hydraulically reconnect 50% of the vessels and then release the tension of the xylem by immersing the cut end in water and allowing the branch to rehydrate. Once the Ψx is between −0.2 and −0.4 MPa, the xylem segment of interest for hydraulic measurements should be excised under water (cut 3) at a minimum distance of 1.5 times the MVL from the initial cut to remove any artificial embolism generated during cut 1 and cut 2. Alternatively, one can determine the hydraulically weighted vessel length instead of the MVL (Cochard et al., 1994). After cut 3, the measurement on the segment of interest should be carried out immediately to avoid any possible effect of capillary pressure that will eventually passively refill embolized vessels.
Figure 2.
Experimental protocol to avoid the excision artifact suggested by Wheeler et al. (2013) at the time of collecting xylem segments for hydraulic measurements.
Our results highlight the importance of both validating xylem embolism data and invasive hydraulic techniques by direct observation in intact plants for an improved understanding of water transport physiology in plants.
Acknowledgments
We thank Dr. Sara Irvine, Dr. Kevin Mader, Dr. Alberto Astolfo, and Gordan Mikuljan (SLS) and Drs. Dula Parkinson and Alastair MacDowell (ALS), whose outstanding efforts have made the experiments possible. We also thank Jochen Schenk (California State University, Fullerton) for assistance with the research at the SLS and for comments on an earlier draft of the article, and Italo Cuneo, Ashley Eustis, and Thorsten Knipfer for assistance with research at the ALS.
Glossary
- micro-CT
x-ray microtomography
- SLS
Swiss Light Source
- ALS
Advanced Light Source
- Ψx
xylem water potential
- MVL
maximum vessel length
Footnotes
This work was supported by the Alexander von Humboldt Foundation (fellowship to B.C.), the PitBulles project (French National Research Agency grant no. 2010 Blanc 171001 to H.C. and E.B.), the China Scholarship Council (to S.L.), and the German Research Foundation (to S.J. and H.M.).
References
- Brodersen CR, McElrone AJ, Choat B, Lee EF, Shackel KA, Matthews MA (2013) In vivo visualizations of drought-induced embolism spread in Vitis vinifera. Plant Physiol 161: 1820–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choat B, Drayton WM, Brodersen C, Matthews MA, Shackel KA, Wada H, McElrone AJ (2010) Measurement of vulnerability to water stress-induced cavitation in grapevine: a comparison of four techniques applied to a long-vesseled species. Plant Cell Environ 33: 1502–1512 [DOI] [PubMed] [Google Scholar]
- Choat B, Jansen S, Brodribb TJ, Cochard H, Delzon S, Bhaskar R, Bucci SJ, Feild TS, Gleason SM, Hacke UG, et al. (2012) Global convergence in the vulnerability of forests to drought. Nature 491: 752–755 [DOI] [PubMed] [Google Scholar]
- Cochard H. (2002) A technique for measuring xylem hydraulic conductance under high negative pressures. Plant Cell Environ 25: 815–819 [Google Scholar]
- Cochard H, Badel E, Herbette S, Delzon S, Choat B, Jansen S (2013) Methods for measuring plant vulnerability to cavitation: a critical review. J Exp Bot 64: 4779–4791 [DOI] [PubMed] [Google Scholar]
- Cochard H, Delzon S (2013) Hydraulic failure and repair are not routine in trees. Ann Sci 70: 659–661 [Google Scholar]
- Cochard H, Delzon S, Badel E (July 18, 2014) X-ray microtomography (micro-CT): a reference technology for high-resolution quantification of xylem embolism in trees. Plant Cell Environ http://dx.doi.org/10.1111/pce.12391 [DOI] [PubMed] [Google Scholar]
- Cochard H, Ewers FW, Tyree MT (1994) Water relations of a tropical vine-like bamboo (Rhipidocladum racemiflorum): root pressures, vulnerability to cavitation and seasonal changes in embolism. J Exp Bot 45: 1085–1089 [Google Scholar]
- Delzon S, Cochard H (2014) Recent advances in tree hydraulics highlight the ecological significance of the hydraulic safety margin. New Phytol 203: 355–358 [DOI] [PubMed] [Google Scholar]
- Ewers FW, Fisher JB (1989) Techniques for measuring vessel lengths and diameters in stems of woody plants. Am J Bot 76: 645–656 [Google Scholar]
- McElrone AJ, Choat B, Parkinson D, MacDowell A, Brodersen CR (2013) Utilization of high resolution computed tomography to visualize the three dimensional structure and function of plant vasculature. J Vis Exp 74: e50162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salleo S, Lo Gullo MA, De Paoli D, Zippo M (1996) Xylem recovery from cavitation induced embolism in young plants of Laurus nobilis: a possible mechanism. New Phytol 132: 47–56 [DOI] [PubMed] [Google Scholar]
- Salleo S, Trifilò P, Lo Gullo MA (2006) Phloem as a possible major determinant of rapid cavitation reversal in stems of Laurus nobilis (laurel). Funct Plant Biol 33: 1063–1074 [DOI] [PubMed] [Google Scholar]
- Scoffoni C, Sack L (September 26, 2014) Are leaves ‘freewheelin’? Testing for a Wheeler-type effect in leaf xylem hydraulic decline. Plant Cell Environ http://dx.doi.org/10.1111/pce.12413 [DOI] [PubMed] [Google Scholar]
- Sperry J. (2013) Cutting-edge research or cutting-edge artefact? An overdue control experiment complicates the xylem refilling story. Plant Cell Environ 36: 1916–1918 [DOI] [PubMed] [Google Scholar]
- Sperry JS, Christman MA, Torres-Ruiz JM, Taneda H, Smith DD (2012) Vulnerability curves by centrifugation: is there an open vessel artefact, and are ‘r’ shaped curves necessarily invalid? Plant Cell Environ 35: 601–610 [DOI] [PubMed] [Google Scholar]
- Torres-Ruiz JM, Cochard H, Mayr S, Beikircher B, Diaz-Espejo A, Rodriguez- Dominguez CM, Badel E, Fernández JE (2014) Vulnerability to cavitation in Olea europaea current-year shoots: further evidence of an open-vessel artifact associated with centrifuge and air-injection techniques. Physiol Plant 152: 465–474 [DOI] [PubMed] [Google Scholar]
- Trifilò P, Raimondo F, Lo Gullo MA, Barbera PM, Salleo S, Nardini A (2014) Relax and refill: xylem rehydration prior to hydraulic measurements favours embolism repair in stems and generates artificially low PLC values. Plant Cell Environ 37: 2491–2499 [DOI] [PubMed] [Google Scholar]
- Venturas MD, Mackinnon ED, Jacobsen AL, Pratt RB (November 11, 2014) Excising stem samples under water at native tension does not induce xylem cavitation. Plant Cell Environ http://dx.doi.org/10.1111/pce.12461 [DOI] [PubMed] [Google Scholar]
- Wheeler JK, Huggett BA, Tofte AN, Rockwell FE, Holbrook NM (2013) Cutting xylem under tension or supersaturated with gas can generate PLC and the appearance of rapid recovery from embolism. Plant Cell Environ 36: 1938–1949 [DOI] [PubMed] [Google Scholar]
- Zwieniecki MA, Melcher PJ, Ahrens ET (2013) Analysis of spatial and temporal dynamics of xylem refilling in Acer rubrum L. using magnetic resonance imaging. Front Plant Sci 4: 265. [DOI] [PMC free article] [PubMed] [Google Scholar]