Oomycete effectors hijack host catalases to regulate plant programmed cell death and H2O2 homeostasis.
Abstract
Plant pathogenic oomycetes, such as Phytophthora sojae, secrete an arsenal of host cytoplasmic effectors to promote infection. We have shown previously that P. sojae PsCRN63 (for crinkling- and necrosis-inducing proteins) induces programmed cell death (PCD) while PsCRN115 blocks PCD in planta; however, they are jointly required for full pathogenesis. Here, we find that PsCRN63 alone or PsCRN63 and PsCRN115 together might suppress the immune responses of Nicotiana benthamiana and demonstrate that these two cytoplasmic effectors interact with catalases from N. benthamiana and soybean (Glycine max). Transient expression of PsCRN63 increases hydrogen peroxide (H2O2) accumulation, whereas PsCRN115 suppresses this process. Transient overexpression of NbCAT1 (for N. benthamiana CATALASE1) or GmCAT1 specifically alleviates PsCRN63-induced PCD. Suppression of the PsCRN63-induced PCD by PsCRN115 is compromised when catalases are silenced in N. benthamiana. Interestingly, the NbCAT1 is recruited into the plant nucleus in the presence of PsCRN63 or PsCRN115; NbCAT1 and GmCAT1 are destabilized when PsCRN63 is coexpressed, and PsCRN115 inhibits the processes. Thus, PsCRN63/115 manipulates plant PCD through interfering with catalases and perturbing H2O2 homeostasis. Furthermore, silencing of catalase genes enhances susceptibility to Phytophthora capsici, indicating that catalases are essential for plant resistance. Taken together, we suggest that P. sojae secretes these two effectors to regulate plant PCD and H2O2 homeostasis through direct interaction with catalases and, therefore, overcome host immune responses.
To infect host plants, phytopathogens secrete a battery of effectors to interfere with the plant immune system, resulting in effector-triggered susceptibility (Hann et al., 2010; Dou and Zhou, 2012; Rafiqi et al., 2012). A growing number of effectors that contribute to pathogen virulence have been reported. For example, to suppress host immune responses, bacterial pathogens deliver effector proteins into the host cytoplasm through a specialized type III secretion apparatus (Hann et al., 2010). Filamentous fungi and oomycetes secrete effectors, which are either translocated into the host cytoplasm or act in the plant apoplast to interfere with a wide range of host physiological processes (Kamoun, 2006; Dou and Zhou, 2012; Rafiqi et al., 2012). The effectors secreted by these distinct pathogen groups can target diverse host immune signaling pathways, such as plant programmed cell death (PCD; Dou and Zhou, 2012).
Phytopathogenic oomycetes cause some of the most destructive plant diseases globally. For example, Phytophthora sojae causes soybean (Glycine max) root and stem rot, leading to substantial annual yield losses (Tyler, 2007). Many Phytophthora spp. pathogens are hemibiotrophs that mostly initiate the infection cycle as biotrophs but switch to a necrotrophic lifestyle at later stages. However, little is known about the molecular mechanisms that promote each phase, nor those that regulate the transition between the two stages. Recent advances in effector biology revealed that this process is likely determined by the sequential delivery of functionally distinct effectors into host plant cells (Koeck et al., 2011). For example, to establish a biotrophic interaction with the host, Phytophthora infestans produces SUPPRESSOR OF NECROSIS1 at the early infection stages and suppresses PCD triggered by other elicitors, and it later switches to a necrotrophic lifestyle (Kelley et al., 2010). P. sojae delivers effectors with contrasting biological activities to sequentially promote infection (Wang et al., 2011). Oomycete pathogens can secrete hundreds of cytoplasmic effectors, including RxLRs (for Arg, any amino acid, Leu, and Arg) and CRNs (for crinkling- and necrosis-inducing proteins). However, it is difficult to predict their functions due to a lack of sequence similarity to known enzymes or proteins. Two notable exceptions are the P. sojae avirulence protein 3b (AVR3b), which contains a domain with similarity to Nudix hydrolases (Dong et al., 2011), and P. infestans CRN8, which exhibits kinase activity (van Damme et al., 2012).
The identification of host targets remains an essential approach to elucidate effector functions. The functional mechanisms of several P. infestans effectors have been discovered using this strategy. PiAVR3a manipulates plant immunity by interacting with and stabilizing the host E3 ligase CMPG1 (for a U-box E3 ligase with a conserved Cys/Met/Pro/Gly amino acid motif in its sequence; Bos et al., 2010). PiAVRblb1 may target the plant lectin receptor kinase LecRK-I.9 to interfere with the cell wall and plasma membrane continuum and promote infection (Bouwmeester et al., 2011). PiAVRblb2 prevents the secretion of the plant immune protease C14 by interacting with it at the haustorial interface (Bozkurt et al., 2011). A P. infestans RxLR effector, PITG_03192, interacts with NAC (an acronym for NAM [No Apical Meristem], ATAF1-2, and CUC2 [Cup-Shaped Cotyledon]) transcriptional factors in Nicotiana benthamiana and prevents their relocalization from the endoplasmic reticulum to the nucleus (McLellan et al., 2013). The avirulence effector PiAVR2 associates with plant phosphatase BSU-LIKE PROTEIN1, and the complex can be recognized by the resistance protein R2, resulting in effector-triggered immunity (Saunders et al., 2012). However, the targets of the P. sojae effectors and oomycete CRN effectors have not been reported.
CRNs, a major class of oomycete cytoplasmic effectors, were originally identified following an in planta functional expression screen for candidate secreted proteins of P. infestans (Torto et al., 2003). Transient expression of several CRNs causes PCD in plants (Schornack et al., 2010; Rajput et al., 2014). Analogous to RxLR effectors, the N-terminal region of CRN proteins contains a conserved FLAK motif, which is known to be required for CRN effector translocation into the host plant cells (Schornack et al., 2010). Similar to RxLR effectors, the C termini of CRNs are diverse and required for virulence (Haas et al., 2009). The majority of CRN effectors are thought to cause PCD in plants; however, recent studies demonstrated that few CRNs induce PCD in plants and that the majority of CRNs suppress PCD induced by distinct elicitors (Shen et al., 2013; Stam et al., 2013). PsCRN63 and PsCRN115 were identified in P. sojae and found to be essential for the full virulence of the pathogen. Although sharing 95.7% identity at the amino acid level, they possess contrasting biological activities on N. benthamiana and soybean. Specifically, PsCRN63 induces PCD while PsCRN115 blocks it (Liu et al., 2011). The underlying mechanisms, however, remain unknown.
Hydrogen peroxide (H2O2), a reactive oxygen species (ROS), is a regulator of many physiological processes, including disease resistance (Petrov and Van Breusegem, 2012). The biological function of H2O2 is dependent on its concentration. Generally speaking, a low level of H2O2 serves as a signaling molecule, whereas high levels provoke cell death (Gough and Cotter, 2011). Inhibition of host H2O2-mediated resistance by pathogen effectors may be an effective strategy to suppress plant immunity (Hemetsberger et al., 2012). Catalases present in the majority of living organisms convert H2O2 into water and oxygen. In Arabidopsis (Arabidopsis thaliana), the CATALASE gene family includes three members (CAT1, CAT2, and CAT3). CAT2 and CAT3 are localized to the peroxisome and function as major H2O2 scavengers that contribute to ROS homeostasis (Du et al., 2008). LESION SIMULATING DISEASE1 (LSD1) interacts physically and genetically with catalases to regulate PCD in Arabidopsis (Li et al., 2013). Thus, catalases may play an important role in plant H2O2 homeostasis, PCD, and resistance.
Here, we report that both PsCRN63 and PsCRN115 of P. sojae interacted directly with catalases and relocated these enzymes from the peroxisomes to the nucleus in N. benthamiana. PsCRN63 promotes plant PCD, which may result from its ability to down-regulate catalase stability and up-regulate H2O2 levels. PsCRN115, however, counteracts the functions of PsCRN63. Our results suggest that PsCRN63 and PsCRN115 interact in opposing manners to regulate H2O2 homeostasis and PCD.
RESULTS
Expression of PsCRN63 or PsCRN63/115 Promotes the in Planta Growth of Phytophthora capsici
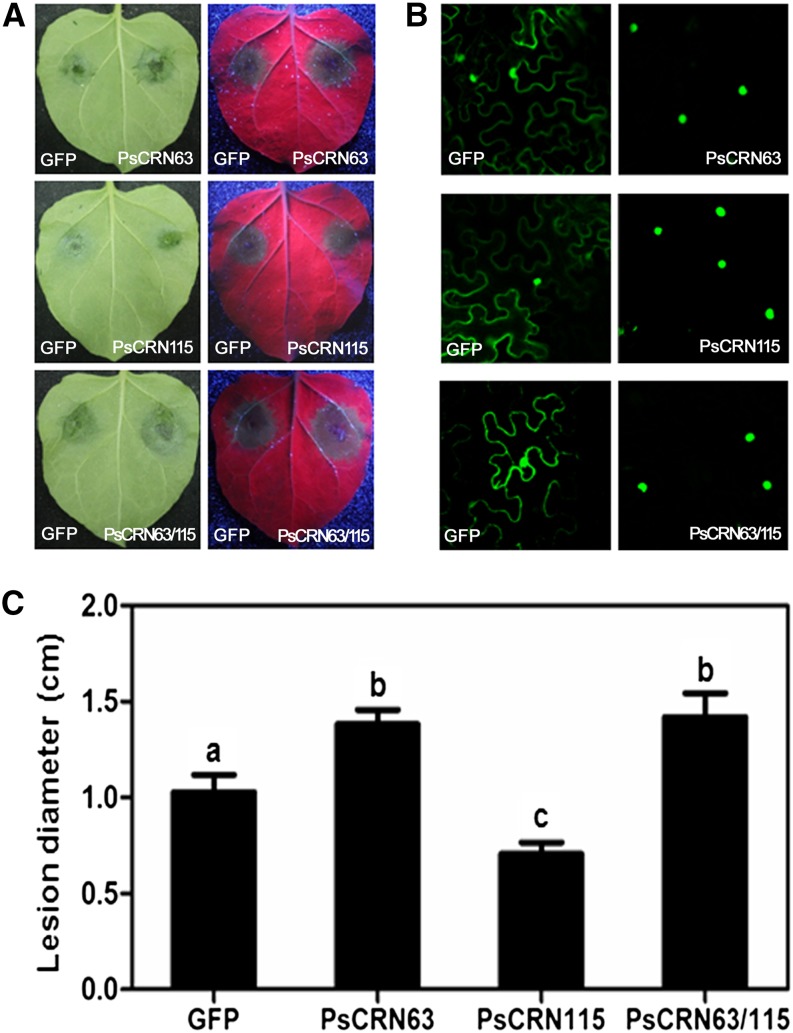
Silencing of PsCRN63/115 jointly reduced P. sojae virulence (Liu et al., 2011). To further determine the role of PsCRN63 and PsCRN115 during pathogen infection, we used an in planta transient assay. In this assay, pBinGFP2 empty (GFP alone) was expressed in one-half of an N. benthamiana leaf via agroinfiltration, and PsCRN63 and PsCRN115 were expressed as GFP fusions separately or coexpressed in the other half of the leaf. Leaves were inoculated with P. capsici zoospores 12 h after infiltration. The average lesion diameters were recorded at 24 h after inoculation (Fig. 1). All of the inoculated leaves showed typical water-soaked lesions (Fig. 1A), suggesting that PsCRN63 and PsCRN115 could not impair P. capsici penetration. However, the results clearly showed that, compared with the GFP expression control, the expression of PsCRN63 in N. benthamiana cells significantly enhanced P. capsici colonization. In contrast, the expression of PsCRN115 promoted resistance to the Phytophthora spp. pathogen. Interestingly, coexpression of both CRN effectors enhanced the susceptibility of N. benthamiana to P. capsici infection (Fig. 1). To confirm the expression of the effector genes, the GFP fluorescence of the fusion proteins was observed using a confocal laser scanning microscope, and nuclear localization of fluorescent signals showed that GFP-effector fusion proteins were expressed (Fig. 1B). These results suggest that PsCRN63 alone or together with PsCRN115 can act within plant cells to promote effector-triggered susceptibility.
Figure 1.
Expression of PsCRN63 or PsCRN63/PsCRN115 in planta enhanced the susceptibility of N. benthamiana to P. capsici. A, Leaf phenotypes upon P. capsici infection. A. tumefaciens was used to transiently express the indicated CRN effectors (PsCRN63 or PsCRN115 alone or coexpressing both effectors) as GFP fusions on one-half of an N. benthamiana leaf and free GFP on the other. Leaves were infected with P. capsici zoospores 12 h after infiltration. Typical photographs were taken 24 h after inoculation under normal light (left) and UV light (right). B, Nuclear localization of GFP florescence signals in the infiltrated leaves. The infiltrated leaves were detached at 24 h after infiltration, and GFP fluorescence was detected using a confocal laser scanning microscope. C, Average lesion diameters of N. benthamiana leaves expressing CRN effectors or GFP. Error bars represent se calculated from at least 11 independent biological replicates. The letters indicate significant differences (P < 0.01) determined by a nonparametric Kruskal-Wallis test.
PsCRN63/115 Associates with CATs from N. benthamiana and Soybean
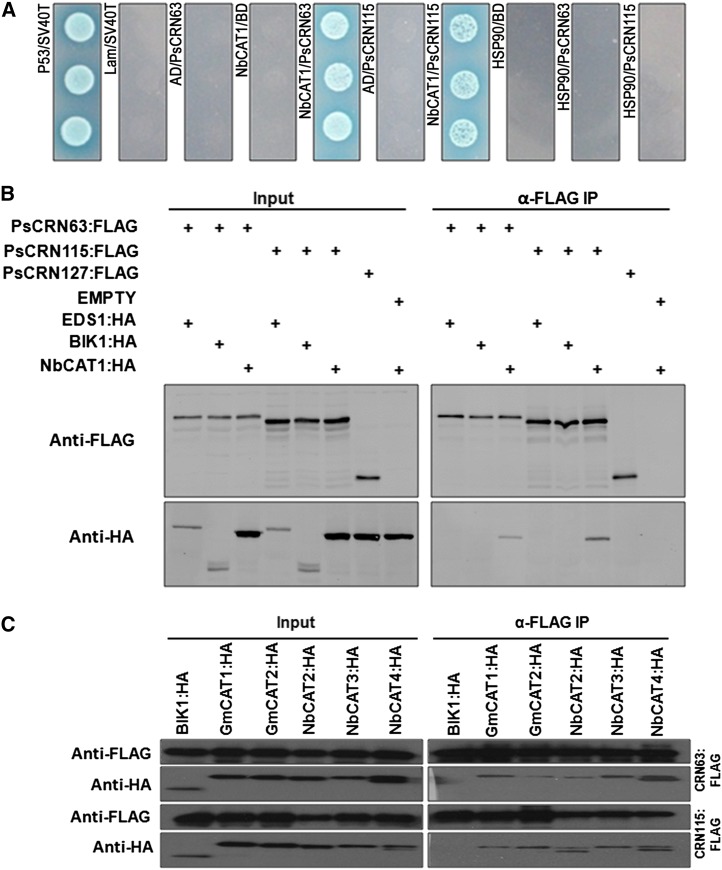
To investigate the molecular mechanisms underlying the regulation of plant PCD and resistance responses by PsCRN63 and PsCRN115, we identified potential protein targets in N. benthamiana using in planta coimmunoprecipitation (co-IP) followed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis (Supplemental Table S1). We identified two proteins, a homolog of HSP90 (NbHSP90) and a catalase (NbCAT1), that may associate with PsCRN63/115 because they were not found in the GFP control (Supplemental Table S1). We then examined the interaction between these two candidates and the CRN effectors using a yeast (Saccharomyces cerevisiae) two-hybrid assay. Figure 2A shows that NbCAT1, but not HSP90, interacted with both PsCRN63 and PsCRN115. Furthermore, co-IP was conducted to confirm the interaction between NbCAT1 and these two CRN effectors in Arabidopsis protoplasts. The result showed that both PsCRN63 and PsCRN115 were associated with NbCAT1 (Fig. 2B). In contrast, PsCRN127, a randomly selected CRN effector from P. sojae, did not coprecipitate with NbCAT1. PsCRN63 or PsCRN115 did not coprecipitate with two other Arabidopsis proteins, BOTRYTIS-INDUCED KINASE1 (BIK1) and ENHANCED DISEASE SUSCEPTIBILITY1 (EDS1), indicating that interactions between PsCRN63/115 and NbCAT1 were specific.
Figure 2.
PsCRN63 and PsCRN115 interact with plant catalases. A, Interaction between PsCRN63 and PsCRN115 (lacking the predicated signal peptides) with NbCAT1 in yeast two-hybrid assays. Yeast transformants expressing the indicated genes were plated on synthetic dextrose/-Trp-Leu-His-adenine medium containing 5-bromo-4-chloro-3-indolyl-α-d-galactopyranoside. B, co-IP of PsCRN63 or PsCRN115 with NbCAT1. PsCRN63:FLAG or PsCRN115:FLAG was transiently coexpressed with NbCAT1 in Arabidopsis protoplasts. The co-IP experiment was performed using anti-HA antibodies, and the isolated protein was analyzed by immunoblotting using anti-FLAG antibodies to detect PsCRN63 or PsCRN115 and anti-HA antibodies to detect NbCAT1. The P. sojae effector CRN127 and Arabidopsis BIK1 and EDS1 were used as negative controls. C, co-IP of PsCRN63 or PsCRN115 with other catalase homologs encoded by N. benthamiana and soybean. PsCRN63:FLAG or PsCRN115:FLAG was transiently coexpressed with the indicated catalases in Arabidopsis protoplasts. The co-IP experiment was performed using anti-HA antibodies, and the isolated protein was analyzed by immunoblotting using anti-FLAG antibodies to detect PsCRN63 or PsCRN115 and anti-HA antibodies to detect catalases. Arabidopsis BIK1:HA was used as a negative control.
The search for genomic sequences homologous to NbCAT1 (NbS00013764g0007.1) revealed seven putative CATs in N. benthamiana genome databases and four in soybean genome databases. Protein sequence alignment showed that catalases are conserved among N. benthamiana and soybean (Supplemental Fig. S1). We cloned three other CATs (NbS00003826g0005.1, NbCAT2; NbS00015038g0018.1, NbCAT3; and NbS00006116g0019.1, NbCAT4) from N. benthamiana and two (Glyma14g39810.1, GmCAT1; and Glyma04g01920.1, GmCAT2) from soybean and then examined the interactions between the CRN effectors and these catalases using co-IP. The results showed that both CRN effectors interacted with all of the tested proteins, including four from N. benthamiana and two from soybean (Fig. 2C). Our results suggested that PsCRN63/115 interacted with plant catalases.
PsCRN63 and PsCRN115 Interact with Catalase in the Plant Nucleus
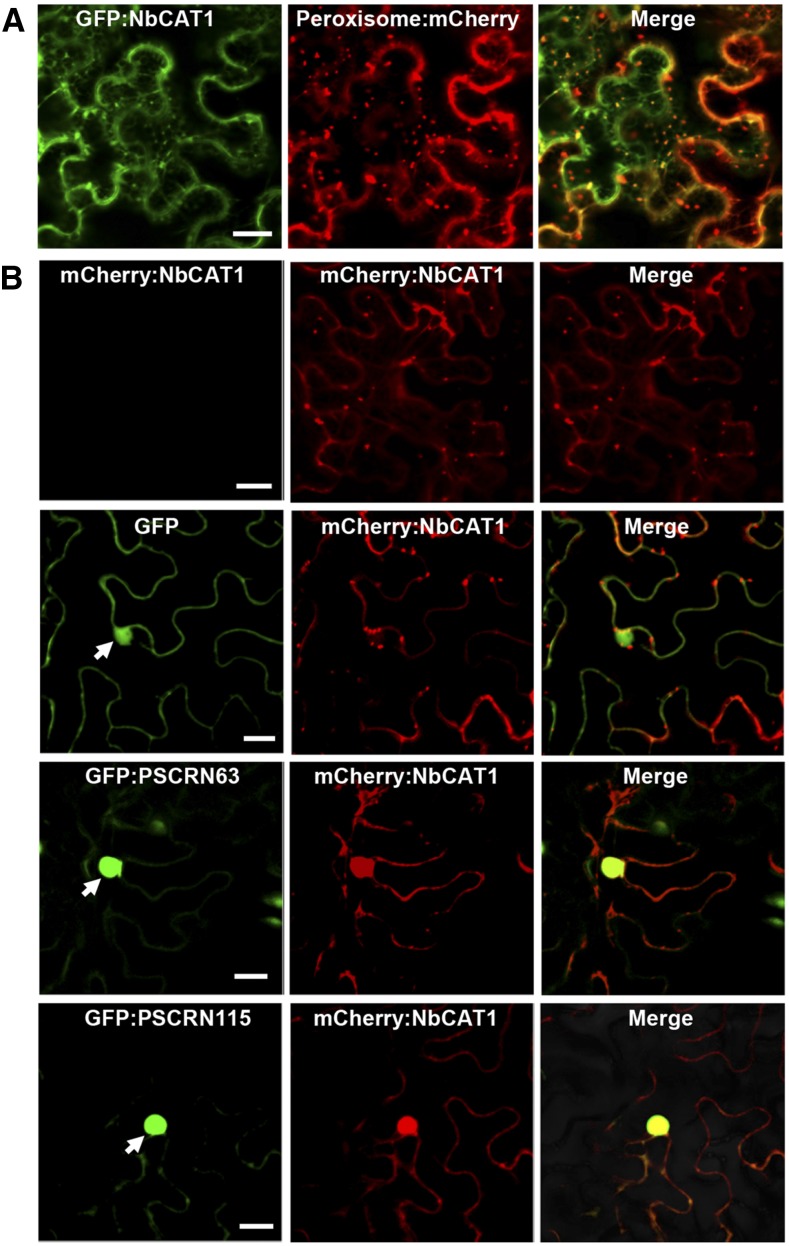
PsCRN63 and PsCRN115 are localized to the plant cell nucleus (Liu et al., 2011). However, catalases are usually enriched in the cytoplasm and peroxisomes (Mhamdi et al., 2010). We found that N. benthamiana NbCAT1 colocalized with a peroxisome marker protein (Nelson et al., 2007; Fig. 3A), confirming the peroxisome localization of NbCAT1. To determine the subcellular site(s) where the two CRN effectors interact with a plant catalase, we transiently coexpressed GFP:CRN and mCherry:NbCAT1 fusions via agroinfiltration in N. benthamiana leaves. Strong mCherry fluorescent signals were detected in the nucleus, overlapping with the GFP:CRN signals (Fig. 3B), indicating that NbCAT1 was relocated to the nucleus by PsCRN63 or PsCRN115. Transient expression of GFP:PsCRN63 fusion protein induced cell death in N. benthamiana leaves, and the cell death could be suppressed by GFP:PsCRN115, indicating that the functionality of the GFP-tagged effectors was not affected (Supplemental Fig. S2). These results suggested that, although both PsCRN63 and PsCRN115 can relocate NbCAT1 from the peroxisomes to the nucleus, the two effectors play different roles in plant cell death. These roles were explored further, as described below.
Figure 3.
PsCRN63 and PsCRN115 recruit catalases into the nucleus. A, Subcellular localization of NbCAT1 in N. benthamiana. Agrobacterial cells containing GFP:NbCAT1 and the peroxisome marker vector PX-RK were coinfiltrated into N. benthamiana leaves, and photographs were taken at 2 d after infiltration. Bar = 50 μm. B, Subcellular localization of NbCAT1. Agrobacteria harboring mCherry:NbCAT1 were infiltrated alone or coinfiltrated with agrobacterial cells harboring GFP, GFP:PsCRN63, or GFP:PsCRN115 into N. benthamiana leaves. Photographs were taken at 2 d after infiltration. Bars = 50 μm.
PsCRN63 Promotes H2O2 Accumulation in N. benthamiana and PsCRN115 Inhibits This Activity
Since H2O2 is a critical trigger of plant cell death (Petrov and Van Breusegem, 2012), we tested whether the transient expression of PsCRN63 and/or PsCRN115 affects H2O2 accumulation in N. benthamiana leaves. As shown in Figure 4, H2O2 accumulation was significantly up-regulated in leaves expressing PsCRN63 as compared with its level in leaves expressing GFP; this up-regulation was not observed in PsCRN115-expressing leaves. Thus, PsCRN63, but not PsCRN115, promoted H2O2 accumulation in N. benthamiana. Coexpression of PsCRN63 and PsCRN115 did not alter H2O2 accumulation levels as compared with the GFP expression controls (Fig. 4B), suggesting that PsCRN115 inhibited the capacity of PsCRN63 to promote H2O2 accumulation. Since PsCRN63 and PsCRN115 have contrasting roles in manipulating PCD (Liu et al., 2011; Supplemental Fig. S2), we suggest that PsCRN63 induces plant PCD by promoting H2O2 accumulation, whereas PsCRN115 suppresses the process by inhibiting PsCRN63-induced H2O2 accumulation.
Figure 4.
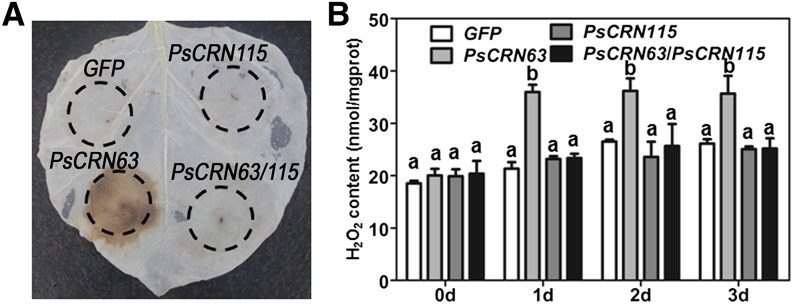
PsCRN63 promotes H2O2 accumulation, whereas PsCRN115 suppresses it, in N. benthamiana. A, H2O2 production in N. benthamiana leaves. DAB staining was performed at 2 d after infiltration with agrobacterial cells containing the constructs GFP, PsCRN63, PsCRN115, and PsCRN63/PsCRN115. B, H2O2 contents in N. benthamiana leaves expressing the constructs in A using the H2O2 detection kit. N. benthamiana leaves were collected at the indicated time points. Error bars represent se from at least five biological replicates. For each time point, the same letter indicates no significant difference between values and different letters indicate significant differences between values (P < 0.01, nonparametric Kruskal-Wallis test).
PsCRN63-Induced PCD Is Alleviated by Transient Expression of CAT Genes
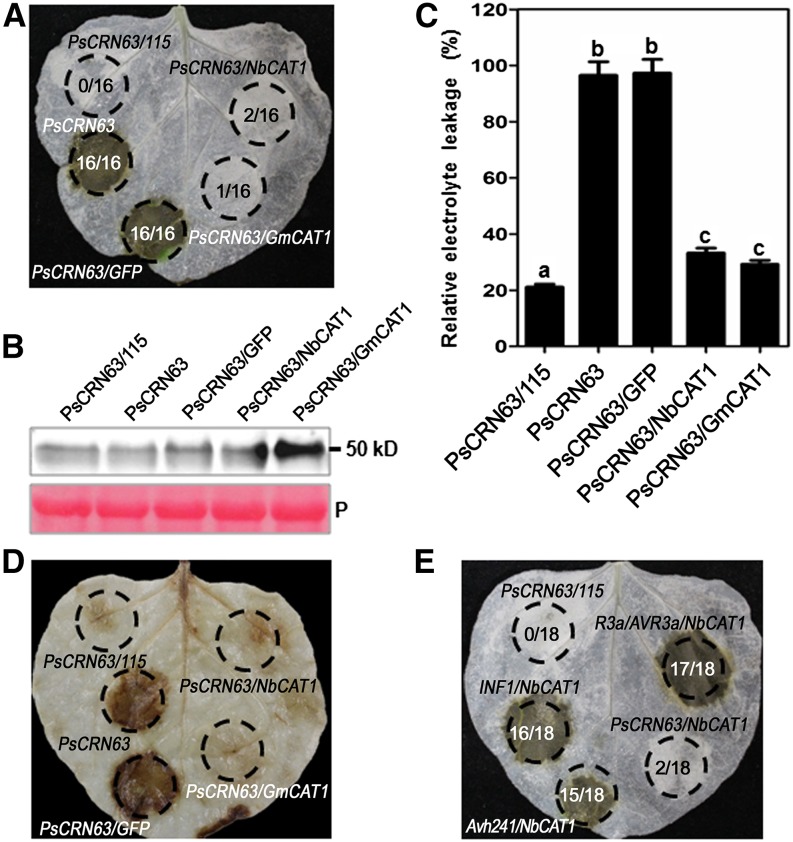
Since catalases are responsible for the degradation of H2O2 (Du et al., 2008), we postulated that overexpression of catalases should alleviate the PsCRN63-triggered high accumulation of H2O2 and PCD. To test this postulation, we infiltrated N. benthamiana leaves with PsCRN63, PsCRN63/PsCRN115, PsCRN63/GFP, PsCRN63/NbCAT1, and PsCRN63/GmCAT1 (Fig. 5A). PsCRN63 was expressed successfully in all cases (Fig. 5B). PsCRN63-triggered PCD could be alleviated by transient coexpression of catalases from both N. benthamiana and soybean as well as by coexpression of PsCRN115 (Fig. 5A). Consistent with these results, coexpression of the plant catalases (NbCAT1 and GmCAT1) or PsCRN115 with PsCRN63 decreased electrolyte leakage as compared with the coexpression of PsCRN63 and PsCRN63/GFP (Fig. 5C). Collectively, these data showed that the PCD-inducing activity of PsCRN63 was inhibited by coexpression of its interacting protein catalases as well as of PsCRN115.
Figure 5.
Transient expression of the NbCAT1 and GmCAT1 genes alleviates PsCRN63-triggered plant PCD. A, Suppression of PsCRN63-induced PCD by NbCAT1 and GmCAT1. GFP was used as a negative control and PsCRN115 as a positive control. Leaves of N. benthamiana were infiltrated with A. tumefaciens carrying the corresponding constructs. The photographs was taken at 4 d after infiltration. The numbers in each circled area represent summary data showing the number of infiltrated area(s) exhibiting cell death over the total number of leaf areas infiltrated with a particular construct or combination of constructs. B, The presence of the PsCRN63 protein was confirmed by immunoblotting using anti-HA antibodies. P, Ponceau S staining of the membrane to show equal loading. C, Quantification of PCD by measurement of electrolyte leakage. N. benthamiana leaves were agroinfiltrated with the corresponding constructs. Electrolyte leakage from the infiltrated leaf discs was measured as a percentage of leakage from boiled discs at 4 d after infiltration. Error bars represent se calculated from five biological replicates. The same letter indicates no significant difference between values, and different letters indicate significant differences between values (P < 0.01, nonparametric Kruskal-Wallis test). D, H2O2 production in N. benthamiana leaves as determined by DAB staining. N. benthamiana leaves were infiltrated with the corresponding constructs, and the measurement was performed at 2 d after infiltration. E, Specific suppression of PsCRN63 triggered by transient expression of NbCAT1. P. infestans INF1, P. sojae Avh241, and R3a/AVR3a were used in this assay to test the specificity of PCD suppression. Photographs in D and E were taken at 4 d after infiltration. The numbers in each circled area represent summary data showing the number of infiltrated area(s) exhibiting cell death over the total number of leaf areas infiltrated with a particular construct or combination of constructs.
To test further whether overexpressed catalases suppress PsCRN63-induced PCD by preventing H2O2 accumulation, we assayed H2O2 levels in these infiltrated leaves using 3,3′-diaminobenzidine (DAB) staining. Very weak staining was observed in leaf areas infiltrated with PsCRN63/NbCAT1 and PsCRN63/GmCAT1 compared with those infiltrated with PsCRN63 and PsCRN63/GFP (PsCRN63/PsCRN115 was used as the positive control; Fig. 5D). These results indicated that overexpression of plant catalases can alleviate PsCRN63-triggered PCD by suppressing H2O2 levels.
To explore whether catalases also suppress PCD triggered by other inducers, we used elicitin infestin1 (INF1) from P. infestans (Kamoun et al., 1998), avirulence homolog241 (Avh241) from P. sojae (Yu et al., 2012), R/AVR pair potato (Solanum tuberosum) R3a (Huang et al., 2005), and P. infestans AVR3a (Armstrong et al., 2005). Interestingly, catalases from N. benthamiana and soybean did not inhibit PCD triggered by these inducers (Fig. 5E), indicating that the suppression of PsCRN63-induced PCD by catalases is specific. We also noticed that PsCRN115 did not suppress PCD triggered by these inducers (Liu et al., 2011), indicating that the catalases and PsCRN115 are specifically involved in regulating the PCD-triggering activity of PsCRN63.
Catalases Are Required for the PCD-Suppressing Activity of PsCRN115
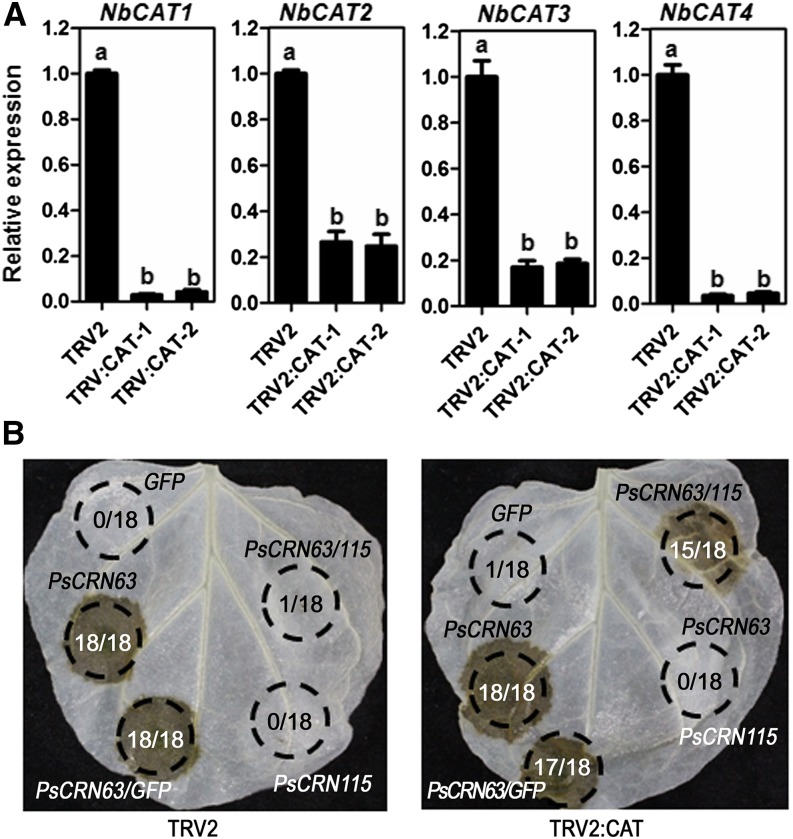
To further test the specific interactions between catalases, PsCRN115, and PsCRN63 in regulating PCD, we silenced the CAT genes in N. benthamiana using virus-induced gene silencing (VIGS). Quantitative reverse transcription (RT)-PCR results showed that four selected CAT members were simultaneously down-regulated in the silenced lines compared with the control lines (Fig. 6A). The down-regulated expression of catalases did not negatively impact the PCD-triggering function of PsCRN63 (Fig. 6B). This is consistent with the above results showing that an elevated expression of catalases suppressed the PCD-triggering function of PsCRN63. However, the role of PsCRN115 inhibiting PsCRN63-triggered PCD was blocked in the catalase-silenced lines (Fig. 6B), indicating that this unique role of PsCRN115 was dependent on plant catalases. In contrast, transient expression of GFP did not trigger PCD at 4 d after infiltration (Fig. 6B).
Figure 6.
The suppression of PsCRN63-triggered PCD of PsCRN115 is dependent on plant catalases. A, Relative CAT gene expression levels in VIGS and control N. benthamiana determined by real-time PCR. TRV2:CAT-1 and TRV2:CAT-2 represent two independent VIGS plants, and TRV2 represents the empty vector control plants. NbEF1α and NbACTIN genes were used as reference genes. The same letter indicates no significant difference between values, and different letters indicate significant differences between values (P < 0.01, nonparametric Kruskal-Wallis test). B, Loss of PsCRN115 suppression of PsCRN63-triggered PCD in CAT-silenced N. benthamiana. Agrobacterial cells carrying the PsCRN115 construct were infiltrated into CAT-silenced N. benthamiana leaves 12 h prior to infiltration with agrobacterial cells harboring the PsCRN63 construct. Photographs were taken at 4 d after infiltration. GFP was used as a negative control. The numbers in each circled area represent summary data showing the number of infiltrated area(s) exhibiting cell death over the total number of leaf areas infiltrated with a particular construct or combination of constructs.
PsCRN63 Affects the Stability of Catalase Proteins
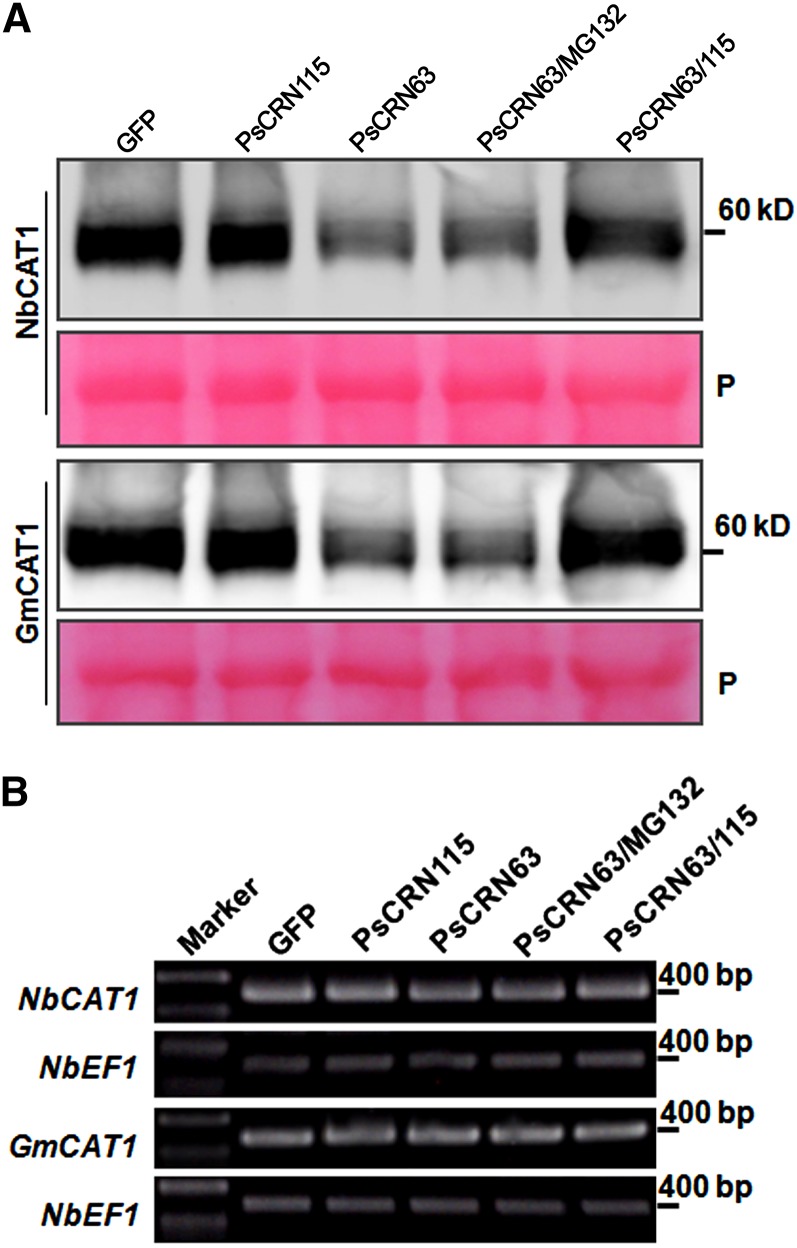
The above results showing that PsCRN63-induced PCD was alleviated by overexpression of catalases and that PsCRN63 still induced PCD in catalase-silenced plants raised the possibility that one mechanism of PsCRN63 induction of PCD is decreased levels of catalases in the presence of PsCRN63. Furthermore, PsCRN115 may counteract this effect by maintaining or elevating the catalase levels. To test these possibilities, we investigated whether these CRN effectors affected catalase stability. We coinfiltrated the Agrobacterium tumefaciens strain carrying the GFP:CRN construct with that containing the CAT:HA (for hemagglutinin) construct into N. benthamiana leaves and extracted total proteins from the treated leaves 2 d after infiltration. Western-blot analysis revealed that the protein level of NbCAT1:HA or GmCAT1:HA was significantly lower when PsCRN63 was coexpressed than in the control in which GFP was coexpressed (Fig. 7A). When catalase was coexpressed with PsCRN63 and PsCRN115, the protein stability in infiltrated leaves was recovered compared with those expressing only PsCRN63 (Fig. 7A), suggesting that PsCRN115 protects catalases from destabilization by PsCRN63. To test whether the degradation of catalases by PsCRN63 was dependent on the 26S proteasome, we analyzed their stability in the presence of MG132, which is an inhibitor of the 26S proteasome. The result showed that the degradation of catalases was not alleviated by treatment with MG132 compared with that treated with dimethyl sulfoxide (Fig. 7A). Thus, destabilization of catalases by PsCRN63 is likely independent of the 26S proteasome pathway.
Figure 7.
PsCRN63 destabilizes catalase in N. benthamiana. A, Analysis of the protein stability of NbCAT1 and GmCAT1 using western blotting. Total protein was extracted from each sample collected and immunoblotted using anti-HA antibodies to detect NbCAT1 or GmCAT1. Agrobacterial cells carrying the NbCAT1:HA or GmCAT1:HA construct were coinfiltrated into N. benthamiana leaves with cells carrying GFP, PsCRN63, or PsCRN115 (without the secretory signal peptide). In both experiments, leaves were treated with MG132 (100 μm; as indicated) or dimethyl sulfoxide (other lanes) 1 d after infiltration with agrobacterial cells. The infiltrated leaves were harvested 1 d after treatment with MG132 or dimethyl sulfoxide. P, Ponceau S staining of the membrane to show equal loading. B, Transcriptional levels of each gene determined by RT-PCR. Total RNA was isolated from infiltrated N. benthamiana leaves. The expression levels of NbCAT1 or GmCAT1 in infiltrated leaves were detected by RT-PCR. N. benthamiana NbEF1α was used as a reference gene.
To exclude the possibility that the destabilization of catalase by PsCRN63 was a general consequence of PCD, we stained the total proteins used in the western-blot experiment. Clear and sharp protein bands were observed for all samples tested, indicating that the degradation of catalases was not due to quality problem of the total proteins (Fig. 7A). To test whether the difference in the catalase protein contents in the presence and absence of PsCRN63 was caused by differential transcriptional expression of CAT genes, we analyzed the NbCAT1 and GmCAT1 mRNA abundance using RT-PCR and found no significant difference in the transcription levels of these genes among samples (Fig. 7B).
Taken together, these data suggest that PsCRN63 may induce PCD by reducing the stability of catalases and that PsCRN115 may suppress PsCRN63-triggered PCD by suppressing its catalase-destabilizing activity. How can this model explain the observation that PsCRN63 could still induce PCD in the catalase-silenced plants? It is possible that catalase accumulation needs to reach a threshold low level to allow high enough H2O2 accumulation to trigger PCD and that PsCRN63 acted to reduce catalase in the silenced plants to reach this threshold low level. Another possibility is that PsCRN63 interacts with a yet-to-be identified host factor, in addition to reducing the catalase level (and elevating H2O2), to trigger PCD. Whether relocating the catalase to the nucleus to interact with this putative factor remains an outstanding question.
Plant Catalases Contribute to Resistance against Phytophthora spp. Pathogens
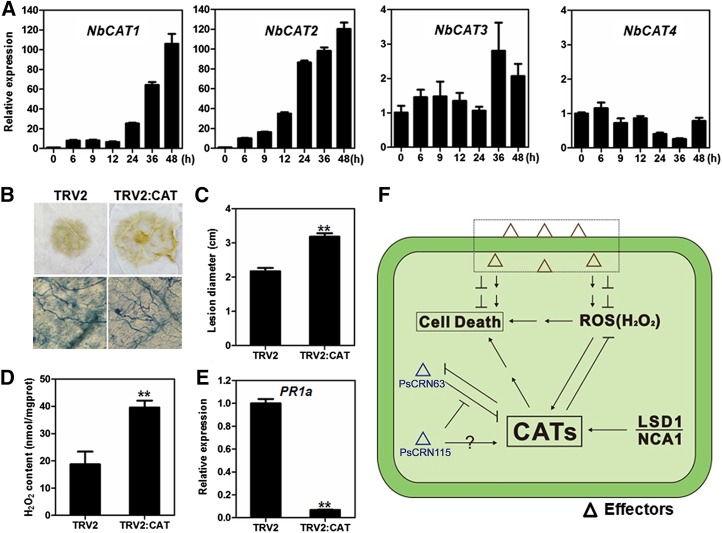
To determine whether CAT genes are responsive to Phytophthora spp. infection, we used real-time PCR to detect CAT gene expression in N. benthamiana leaves challenged with P. capsici zoospores. The expression levels of NbCAT1 and NbCAT2 were significantly up-regulated in response to P. capsici inoculation, indicating that the catalases may be involved in plant resistance to Phytophthora spp. pathogens (Fig. 8A). However, the expression of NbCAT3 and NbCAT4 was not induced during Phytophthora spp. infection (Fig. 8A).
Figure 8.
Silencing of CAT genes in N. benthamiana reduces resistance to P. capsici. A, Relative expression of CAT genes in N. benthamiana at 0, 6, 9, 12, 24, 36, and 48 h after inoculation with P. capsici. Relative expression levels were standardized to that of the NbEF1α and NbACTIN reference genes. B, Comparison of P. capsici lesions on N. benthamiana leaves expressing the TRV2:CAT or TRV2 construct. The top row shows decolorized infected leaves at 24 h after inoculation. The bottom row shows Trypan Blue staining of lesions, revealing a higher hyphal density in the CAT-silenced N. benthamiana. C, Average lesion diameters of N. benthamiana leaves expressing the TRV2:CAT or TRV2 construct. Error bars represent se calculated from 12 biological replicates (**P < 0.01, Student’s t test). D, H2O2 production in CAT-silenced and control N. benthamiana. Error bars represent se calculated from four biological replicates (**P < 0.01, Student’s t test). E, Relative expression levels of the PR1a gene in CAT-silenced N. benthamiana and control lines. Error bars represent se calculated from three biological replicates (**P < 0.01, Student’s t test). F, Model for the manipulation of plant PCD by PsCRN63 and PsCRN115 through interactions with plant CATs. Pathogens produce effectors to induce or suppress PCD and ROS in plant cells. P. sojae secretes PsCRN63 to interact with plant catalases, which elevates H2O2 levels in plant cells and causes PCD. At the same time, P. sojae produces relatively lower amounts of PsCRN115 to suppress the PsCRN63-induced PCD by recovering the catalase activity. To overcome this manipulation by the pathogen, plants up-regulate the expression of catalases, which in turn suppresses the PsCRN63 activity and leads to resistance against the oomycete pathogen P. sojae.
To examine the role of catalases in resistance to Phytophthora spp. pathogens, we silenced four CAT genes simultaneously in N. benthamiana using the VIGS approach (Fig. 5A). The inoculation assay results showed that the catalase-silenced plants were more susceptible to P. capsici than the TRV2 control lines (Fig. 8, B and C). To investigate the effect of silencing on plant H2O2 accumulation, we measured the H2O2 content in the catalase-silenced lines; as expected, the H2O2 level was higher than in control plants (Fig. 8D). Expression of the PATHOGENESIS-RELATED 1a (PR1a) gene, a marker for salicylic acid-mediated plant defense, was significantly down-regulated in the catalase-silenced plants compared with the control lines (Fig. 8E). Therefore, we showed that catalases positively regulate plant resistance to Phytophthora spp. pathogens.
DISCUSSION AND CONCLUSION
PCD plays a central role in plant innate immune systems. Recognition of the pathogen by plant nucleotide-binding oligomerization domain-like receptors often leads to the hypersensitive response (a form of PCD). The hypersensitive response may prevent pathogen growth and/or generate long-range signals for systemic acquired resistance, although disease resistance and the hypersensitive response can be uncoupled in some cases (Coll et al., 2011). Phytophthora spp. pathogens likely evade host PCD during the biotrophic phase but promote it during the necrotrophic phase. A wide range of secreted proteins from Phytophthora spp. pathogens have been identified as modulators of host PCD (Kamoun, 2006). However, the mechanisms by which host PCD is manipulated by these proteins remain unknown. We previously demonstrated that PsCRN63 and PsCRN115, although sharing high sequence similarity, have contrasting roles in manipulating plant PCD. Expression of PsCRN63 and PsCRN115 is jointly required for full virulence (Liu et al., 2011). Here, we show that expression of PsCRN63 or PsCRN63/115 together promotes the in planta growth of P. capsici. PsCRN63 and PsCRN115 both interact with N. benthamiana and soybean catalases, the essential enzymes for the plant antioxidative system. The expression of NbCAT1 or GmCAT1 specifically blocks PsCRN63-induced PCD in N. benthamiana, and PsCRN63-induced PCD is associated with ROS accumulation. Silencing of NbCAT genes in N. benthamiana compromises the suppression of PsCRN63-induced PCD by PsCRN115. NbCAT1 and NbCAT2 genes are highly expressed during N. benthamiana-P. capsici interactions, and silencing of NbCAT genes accelerates pathogen infection in N. benthamiana. These results indicate that PsCRN63/PsCRN115 may regulate plant PCD and immune responses by interacting with plant catalases.
Plant catalase, a type of peroxisomal enzyme decomposing H2O2, plays essential roles in maintaining H2O2 homeostasis and regulating PCD in plant cells (Mhamdi et al., 2010; Fig. 8F). It was reported that the Arabidopsis cat2 mutant exhibits increasingly intense DAB staining after infiltration with avirulent bacteria (Simon et al., 2010) and shows spreading necrotic lesions in a daylength-related manner (Chaouch et al., 2010). Little genetic evidence shows that CATs are involved in disease resistance. We observed that CAT-deficient N. benthamiana leaves, although accumulating higher levels of H2O2, were more susceptible to P. capsici. We inferred that CATs could be essential components for disease resistance by maintaining proper H2O2 levels in N. benthamiana.
CATs have opposite roles in manipulating plant PCD (Fig. 8F). On the one hand, CATs prevent PCD by detoxifying H2O2, which is supported by a large body of evidence (Chaouch et al., 2010; Mhamdi et al., 2010). LSD1, a negative regulator of PCD, interacts with CATs to regulate light-dependent runaway PCD and hypersensitive-type PCD (Li et al., 2013). On the other hand, CATs may promote autophagy-dependent PCD in Arabidopsis (Juul et al., 2010; Hackenberg et al., 2013). Plant catalases enter into an active state by one molecule of H2O2. Arabidopsis cat2 mutants compromise hydroxyurea (an antineoplastic drug)-induced PCD, in which hydroxyurea interacts directly with catalase and inhibits its H2O2 decomposition activities (Juul et al., 2010). This may be because catalase activation by H2O2 generates a signal to activate autophagy-dependent PCD. Arabidopsis no catalase activity1 (nca1) mutants exhibit extremely low catalase activities, and both nca1 and cat2 mutants attenuate avirulence protein Rpm1-triggerred PCD, suggesting that CAT acts as a molecular link between ROS and the promotion of autophagy-dependent PCD (Hackenberg et al., 2013). Thus, plant CATs may act as fine-tuning regulators of H2O2 homeostasis and H2O2-mediated PCD. In this regard, we speculate that P. sojae produces these two cytoplasmic effectors to perturb H2O2 homeostasis and H2O2-mediated PCD by interacting with the CATs (Fig. 8F). Consistently, silencing PsCRN63/115 in P. sojae leads to a stronger PCD in host cells at the early infection stage than the wild-type control (Liu et al., 2011).
Interestingly, it has also been shown that the cucumber mosaic virus (CMV) 2b protein interferes directly with plant catalase activity to induce PCD, which appears to promote CMV infection (Inaba et al., 2011). Similar to the 2b protein, we show that PsCRN63 induces PCD by promoting H2O2 accumulation. However, the PsCRN63-CAT interaction may decrease the stability of the catalase proteins, which was not observed for the 2b-CAT interaction, suggesting that P. sojae PsCRN63 interference with catalases differs from that of the CMV 2b protein. The regulation of plant PCD by modulating H2O2 homeostasis has been adopted by other plant pathogens (Hemetsberger et al., 2012). That case offers an example of convergent evolution, in which these evolutionarily distant organisms have evolved the ability to interfere with the same targets.
The interaction between PsCRN63/115 and plant catalases appears to favor the pathogen. These two effectors together are required for the full pathogenesis of P. sojae by suppressing host defense responses, and these two highly similar proteins exhibit contrasting roles in the manipulation of host PCD (Liu et al., 2011). P. sojae is a hemibiotroph and typically switches from biotrophic to necrotrophic lifestyle at 16 to 24 h following the invasion of host tissues (Tyler, 2007). PsCRN63 is highly expressed in each of the tested stages and induced during the late infection stages, whereas PsCRN115 is weakly expressed and slightly down-regulated at all infection stages (Liu et al., 2011), indicating that PsCRN63 may play a dominant role during the interactions. PsCRN63 reduces the stability of catalase proteins. Consequently, the PsCRN63-CAT interaction partially compromises H2O2 scavenging by catalases, which enhances H2O2 accumulation and triggers PCD (Fig. 8F). Considering its expression patterns during infection, we suggest that PsCRN63-induced PCD by association with CATs may contribute to a hemibiotrophic lifestyle. By contrast, PsCRN115 also exhibits the ability to suppress PCD induced by P. sojae necrosis-inducing protein (Liu et al., 2011), which transcribes during the transition from biotrophy to necrotrophy and is highly expressed during late stages of a compatible interaction (Qutob et al., 2006). Silencing of CAT genes in N. benthamiana compromises the PCD-suppressing activity of PsCRN115, suggesting that catalases are required for the biological function of PsCRN115 (Fig. 8F). A possible explanation is that PsCRN115 suppresses PsCRN63-induced PCD by protecting plant catalases through competitive binding of catalases.
Overexpression of catalase alleviates PsCRN63-induced PCD, indicating that plants may evade the effects imposed by PsCRN63 through increasing expression of CAT genes (Fig. 8F). However, it does not affect PCD triggered by other inducers, such as Avh241, INF1, and R3a/AVR3a, indicating that catalases specifically suppress PsCRN63-triggered PCD. An explanation is that PsCRN63-induced PCD is dependent on the H2O2 signaling pathway, while the other inducers are independent of this pathway. Furthermore, silencing catalases in N. benthamiana leads to increased disease lesions when challenged with P. capsici, reinforcing the importance of catalases in plant immunity and of manipulating catalases in Phytophthora spp. pathogenesis.
In conclusion, PsCRN63 and PsCRN115, although with high sequence similarity, play opposite roles in regulating plant cell death. Both effector proteins interact directly with plant catalases, which remove H2O2, suggesting that PsCRN63 and PsCRN115 modulate host cell death by perturbing H2O2 homeostasis. Indeed, PsCRN63 may alter the subcellular localization of catalases and reduce the stability of catalase proteins, therefore enhancing H2O2 accumulation in plant cells. PsCRN115 suppresses these processes, although it also recruits catalases in the plant nucleus. Furthermore, silencing catalases in N. benthamiana led to increased disease lesions when challenged with P. capsici, reinforcing the importance of manipulating catalases in Phytophthora spp. pathogenesis. Although it cannot be completely excluded that PsCRN63 and PsCRN115 may have other targets that are also involved in plant PCD, our results provide evidence that Phytophthora spp. pathogens may produce two CRN effectors to interfere directly with plant ROS homeostasis to manipulate PCD by hijacking plant catalases.
MATERIALS AND METHODS
Transient Expression
Agrobacterium tumefaciens strain GV3101 harboring different constructs was grown in liquid Luria-Bertani medium at 28°C in a shaking incubator at 200 rpm. After 24 h, bacterial cells were collected by centrifugation at 3,500g for 5 min, washed with 10 mm MgCl2 three times, and resuspended in infiltration medium (10 mm MgCl2, 10 mm MES, pH 5.6, and 150 μm acetosyringone) to an optical density at 600 nm of 0.4 prior to infiltration into the entire leaf or leaf sections. In experiments where different coinoculation ratios were tested, the total amount of each construct combination was an optical density at 600 nm of 0.4.
Phytophthora capsici Culture Conditions and Inoculation Procedures
The P. capsici isolates used in the study were routinely cultured at 25°C in the dark on 10% (v/v) V8 juice agar plates. Zoospores were obtained by incubating mycelial plugs in 10% (v/v) V8 broth at 25°C for 2 d and then washed three times with sterilized water. After 12 h, numerous sporangia developed. To release zoospores, the cultures were treated in cold sterilized water for 0.5 h followed by incubation at 25°C for 1 h (Zhang et al., 2012). Infection assays were performed using droplet inoculations of zoospore solutions of the P. capsici isolate (10 μL of a 50,000 zoospores mL−1 solution) on detached Nicotiana benthamiana leaves. To compare CAT-silenced N. benthamiana and the empty vector control, detached leaves at 10 d after infiltration were challenged with P. capsici zoospores. To evaluate the virulence function of PsCRN63 or PsCRN115, N. benthamiana leaves were agroinfiltrated with the binary vector pBinGFP2 (expressing free GFP) on one-half of the leaf and the recombinant pBinGFP2 vector containing CRN effector was agroinfiltrated on the other half of the same leaf. The infiltrated leaves were inoculated with P. capsici zoospores 12 h after infiltration. The lesion size was measured 24 h after inoculation. The assay was repeated at least three times.
co-IP Experiments and LC-MS/MS Analysis
For co-IP experiments, total protein was extracted from N. benthamiana leaves 2 to 5 d after infiltration of the PVX:PsCRN63:HA or PVX:PsCRN115:HA construct and subjected to immunoprecipitation using the HA Tag IP/Co-IP Kit (Pierce), as per the manufacturer’s protocol.
The preparation of peptides for LC-MS/MS was performed as follows. Briefly, each protein sample was cleaned and digested in gel using sequencing-grade modified trypsin. The resulting peptide mixtures were analyzed by an LC-MS/MS system, in which HPLC using a 75-µm i.d. reverse-phase C18 column was coupled with an ion-trap mass spectrometer. The mass spectrometric data acquired were used to search a nonredundant protein database. The output from the database search was validated manually before reporting.
Arabidopsis Protoplast Isolation, Transfection, and co-IP
The Columbia ecotype of Arabidopsis (Arabidopsis thaliana) was used for protoplast isolation. The plants were grown for 4 to 5 weeks in a 10-h-light/14-h-dark photoperiod at 22°C. Leaves (approximately 0.5 g) from plants were collected and cut into 0.5- to 1-mm strips with a fresh razor blade. Leaf sections were transferred to a petri dish containing 10 mL of enzyme solution (1% [w/v] cellulose R10, 0.2% [w/v] macerozyme R10, 0.4 m mannitol, 20 mm KCl, 10 mm CaCl2, and 0.1% [w/v] bovine serum albumin) and gently shaken (40 rpm on a platform shaker) for 3 h. The protoplasts were filtered (200-μm nylon mesh) and pelleted at 100g for 3 min. The protoplasts were then washed twice with W5 solution (154 mm NaCl, 125 mm CaCl2, 5 mm KCl, and 2 mm MES, pH 5.7), repelleted and resuspended gently in W5 solution, and incubated on ice for 30 min. The protoplasts were collected and resuspended in MMg solution (0.4 m mannitol, 15 mm MgCl2, and 4 mm MES, pH 5.7) at 3 × 105 protoplasts mL−1 for experiments requiring transfected genes. Protoplasts were transfected by a polyethylene glycol method. Briefly, 6 × 104 protoplasts in 0.2 mL of MMg solution were mixed at room temperature with 10 μg of supercoiled plasmid. An equal volume of polyethylene glycol 4000 solution (40% [w/v] polyethylene glycol 4000, 0.2 m mannitol, and 0.1 m CaCl2) was added, and the mixture was incubated at room temperature for 7 min. After incubation, 1 mL of W5 solution was added slowly and pelleted by centrifugation at 100g for 3 min. The protoplasts were washed twice with W5 solution and incubated for 16 h under weak light. For the co-IP assay, the total proteins were extracted from Arabidopsis protoplasts expressing the indicated genes in extraction buffer (50 mm HEPES-KOH, pH 7.5, 150 mm KCl, 1 mm EDTA, 0.5% [v/v] Triton X-100, 1 mm dithiothreitol, and 1× protease inhibitor cocktail). After centrifugation at 13,000 rpm for 15 min, anti-FLAG antibody-couple beads (Sigma-Aldrich) were subsequently added and incubated for 4 h at 4°C. After washing the beads six times with extraction buffer, immunoprecipitates were analyzed by western-blot analysis.
Yeast Two-Hybrid Assay
The yeast (Saccharomyces cerevisiae) two-hybrid assay was performed according to the Matchmaker Two-Hybrid System 3 protocol (Clontech). PsCRN63 and PsCRN115 lacking the signal peptides were amplified with the primer pairs CRN63F_EcoRI/CRN63R_BamHI and CRN115F_EcoRI/CRN63R_BamHI, respectively (Supplemental Table S2). The PCR products were inserted into pGBKT7 as the bait vectors. NbCAT1 was amplified using the primer pair NbC1-Y-F/NbC1-Y-R (Supplemental Table S2) and inserted into pGADT7 as the prey vector. The resulting pGADT7-NbCAT1 and each bait vector were cotransformed into the yeast strain AH109. Transformants that appeared on synthetic dextrose/-Trp-Leu medium were isolated and assayed for growth on synthetic dextrose/-Trp-Leu-His-adenine plates supplemented with 5-bromo-4-chloro-3-indolyl-α-d-galactopyranoside.
Sequence Analysis
The homologous catalases in N. benthamiana and soybean (Glycine max) were identified by BLAST using the N. benthamiana genome database (http://solgenomics.net/organism/Nicotiana_benthamiana/genome) or the soybean genome database (http://www.plantgdb.org/GmGDB/cgi-bin/blastGDB.pl). Sequence alignment was performed using MUSCLE software (Edgar, 2004).
Confocal Laser Scanning Microscopy
N. benthamiana leaf tissue was mounted in water under a coverslip at 48 h after infiltration with A. tumefaciens carrying the corresponding constructs. Images were captured using the Zeiss LSM 710 confocal laser scanning microscope. The GFP constructs were excited at 488 nm, and the mCherry constructs were excited at 561 nm. Images were processed using the Zeiss LSM 710 confocal laser scanning microscope and Adobe Photoshop software packages.
DAB Staining and H2O2 Measurement
H2O2 was detected in situ using DAB staining (Thordal-Christensen et al., 1997) with some modifications. N. benthamiana leaves were infiltrated with the recombinant A. tumefaciens suspension. Detached leaf samples were collected 3 d after infiltration with 1 mg mL−1 DAB solution. The sampled leaves were placed in a plastic box within a constant-temperature incubator for 4 h; they were then fixed in 95% (v/v) ethanol and photographed.
Quantitative measurement of H2O2 production was performed using the H2O2 detection kit (Jiancheng Bioengineering Institute). The principle of the kit is to generate a stable chelating material through the interaction between H2O2 and molybdic acid. The optical density of this material was measured at 405 nm. The infiltrated N. benthamiana leaves were harvested, and H2O2 accumulation was assayed following the manufacturer’s instructions.
Protein Extraction and Western Blotting
Proteins were transiently expressed by A. tumefaciens in N. benthamiana leaves and harvested 2 d after infiltration. Immunoblot analyses were performed on protein extracts prepared by grinding 400 mg of leaf tissues in 1 mL of extraction buffer (50 mm HEPES-KOH, pH 7.5, 150 mm KCl, 1 mm EDTA, 0.5% [v/v] Triton X-100, 1 mm dithiothreitol, and 1× protease inhibitor cocktail). Suspensions were mixed and centrifuged at 12,000g for 15 min at 4°C. Protein concentration was measured using the Bradford assay. Proteins from the sample lysates were fractionated using SDS-PAGE. After electrophoresis, proteins were transferred from the gel to an Immobilon PSQ polyvinylidene difluoride membrane. The membrane was washed in PBST (phosphate-buffered saline with 0.1% [v/v] Tween 20) for 2 min, and the membrane was then blocked in PBSTM (phosphate-buffered saline with 0.1% [v/v] Tween 20 and 5% [w/v] nonfat dry milk) for 1 h. Mouse anti-HA monoclonal antibodies (Sigma-Aldrich) were added to the PBSTM at a ratio of 1:5,000 and incubated at room temperature for 90 min, followed by three washes (5 min each) with PBST. The membrane was then incubated with goat anti-mouse IRDye 800CW (Odyssey [no. 926-32210]; Li-Cor) at a ratio of 1: 8,000 in PBSTM at room temperature for 30 min with shaking. The membrane was washed three times (5 min each) with PBST and then visualized using Odyssey with excitation at 700 and 800 nm.
Measurement of Ion Leakage
Ion leakage is an indicator of PCD. In this study, PCD was assayed based on measuring ion leakage from leaf discs. For each measurement, five leaf discs (9 mm in diameter) were floated on 5 mL of distilled water for 3 h at room temperature. The conductivity of the bathing solution was then measured using a conductivity meter (Con 700; Consort) to obtain value A. The leaf discs were then transferred to the bathing solution and boiled in sealed tubes for 25 min, after which the conductivity was measured again to obtain value B. For each measurement, ion leakage was expressed as a percentage: (value A/value B) × 100.
VIGS of Catalases in N. benthamiana
We used the TRV-based VIGS system, which uses bipartite sense RNA1 and RNA2 viruses (Liu et al., 2002), to silence CAT genes in N. benthamiana. The NbCAT1 fragment was amplified by PCR and then cloned into pTRV2. Primer pairs NbCF_SmaI and NbCR_XbaI (Supplemental Table S2) were used to amplify NbCAT1. The construct was sequenced to confirm cloning accuracy. Wild-type N. benthamiana plants were grown in a growth chamber under a 16-h-light/8-h-dark photoperiod at 23°C. At the three- to four-leaf stage, the N. benthamiana plants were selected for agroinfiltration. Prior to agroinfiltration, A. tumefaciens GV3101 cells carrying pTRV1 and pTRV2 constructs were collected and resuspended in infiltration medium (10 mm MgCl2, 10 mm MES, pH 5.6, and 150 μm acetosyringone) and mixed in a 1:1 ratio. Plants were kept in high humidity for 24 h and then grown for approximately 2 weeks after infiltration with TRV. The fully expanded six to eight leaves of the silenced plants were then used for real-time PCR and inoculation assays. VIGS of the N. benthamiana phytoene desaturase was used as an indicator of effective silencing (Liu et al., 2002). The empty TRV2 vector was used as the negative control.
RNA Extraction and Transcript Level Analysis
Total RNA was isolated using Trizol reagent (Invitrogen) from N. benthamiana leaves. Total RNA was treated with DNase I (TaKaRa) before being used for complementary DNA (cDNA) synthesis. To generate first-strand cDNA, 1 μg of total RNA was reverse transcribed in a 20-μL volume using the PrimeScript RT reagent kit according to the manufacturer’s instructions (TaKaRa).
For gene transcript level analysis, SYBR Green quantitative RT-PCR assays were conducted. Primer pairs (Supplemental Table S2) were designed for each of the selected genes. The N. benthamiana elongation factor 1α (NbEF1α) and NbACTIN genes were used as internal controls. Quantitative RT-PCR was performed in a 20-μL reaction including 20 ng of cDNA, 0.2 μm gene-specific primer, 0.4 μL of ROX Reference Dye, 10 μL of SYBR Premix ExTaq (TaKaRa), and 6.8 μL of deionized water. Reactions were performed on an ABI PRISM 7500 Fast Real-Time PCR System (Applied Biosystems) under the following conditions: 95°C for 30 s, then 40 cycles at 95°C for 5 s and 60°C for 34 s, followed by 95°C for 15 s and 60°C for 1 min, and then 95°C for 15 s to obtain melt curves. The relative transcript abundance of each gene was determined using the delta-delta Ct method employing the formula: relative expression = 2−[ΔCt test-ΔCt calibrator] where Ct refers to the threshold cycle, test indicates the treated sample, and calibrator indicates the control sample. Gene expression was normalized against the geometric average of two housekeeping genes (NbEF1α and NbACTIN; Vandesompele et al., 2002).
Trypan Blue Staining
For the detection of cell death, Trypan Blue staining was performed. A Trypan Blue stock solution was prepared by mixing 10 g of phenol, 10 mL of glycerol, 10 mL of lactic acid, 10 mL of distilled water, and 0.02 g of Trypan Blue. N. benthamiana leaves soaking in Trypan Blue solution were boiled in a water bath for 2 min and incubated for 1 d. After destaining in saturated chloral hydrate solution (2.5 g of chloral hydrate per 1 mL of water) for 2 d, samples were equilibrated with 70% (v/v) glycerol for photography.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Protein sequence alignment of the catalases from N. benthamiana and G. max.
Supplemental Figure S2. Cell-death manipulation assay of CRN:GFP fusion proteins.
Supplemental Table S1. Plant proteins that may associate with PsCRN63 or PsCRN115 after co-IP as identified by mass spectrometry.
Supplemental Table S2. Oligonucleotides used for PCR and plasmid construction.
Supplementary Material
Acknowledgments
We thank Biao Ding (Ohio State University) for editing the article, and Brett Tyler (Oregon State University) and Xiaofeng Cui (Shanghai Institutes for Biological Sciences) for constructive suggestions.
Glossary
- PCD
programmed cell death
- H2O2
hydrogen peroxide
- ROS
reactive oxygen species
- co-IP
coimmunoprecipitation
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- VIGS
virus-induced gene silencing
- RT
reverse transcription
- DAB
3,3′-diaminobenzidine
- cDNA
complementary DNA
Footnotes
This work was supported by the National Natural Science Foundation of China (grant nos. 31371894 and 31301613) and the Natural Science Foundation of Jiangsu Province (grant no. BK2012027).
References
- Armstrong MR, Whisson SC, Pritchard L, Bos JI, Venter E, Avrova AO, Rehmany AP, Böhme U, Brooks K, Cherevach I, et al. (2005) An ancestral oomycete locus contains late blight avirulence gene Avr3a, encoding a protein that is recognized in the host cytoplasm. Proc Natl Acad Sci USA 102: 7766–7771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos JIB, Armstrong MR, Gilroy EM, Boevink PC, Hein I, Taylor RM, Zhendong T, Engelhardt S, Vetukuri RR, Harrower B, et al. (2010) Phytophthora infestans effector AVR3a is essential for virulence and manipulates plant immunity by stabilizing host E3 ligase CMPG1. Proc Natl Acad Sci USA 107: 9909–9914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester K, de Sain M, Weide R, Gouget A, Klamer S, Canut H, Govers F (2011) The lectin receptor kinase LecRK-I.9 is a novel Phytophthora resistance component and a potential host target for a RXLR effector. PLoS Pathog 7: e1001327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozkurt TO, Schornack S, Win J, Shindo T, Ilyas M, Oliva R, Cano LM, Jones AME, Huitema E, van der Hoorn RAL, et al. (2011) Phytophthora infestans effector AVRblb2 prevents secretion of a plant immune protease at the haustorial interface. Proc Natl Acad Sci USA 108: 20832–20837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouch S, Queval G, Vanderauwera S, Mhamdi A, Vandorpe M, Langlois-Meurinne M, Van Breusegem F, Saindrenan P, Noctor G (2010) Peroxisomal hydrogen peroxide is coupled to biotic defense responses by ISOCHORISMATE SYNTHASE1 in a daylength-related manner. Plant Physiol 153: 1692–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll NS, Epple P, Dangl JL (2011) Programmed cell death in the plant immune system. Cell Death Differ 18: 1247–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S, Yin W, Kong G, Yang X, Qutob D, Chen Q, Kale SD, Sui Y, Zhang Z, Dou D, et al. (2011) Phytophthora sojae avirulence effector Avr3b is a secreted NADH and ADP-ribose pyrophosphorylase that modulates plant immunity. PLoS Pathog 7: e1002353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou D, Zhou JM (2012) Phytopathogen effectors subverting host immunity: different foes, similar battleground. Cell Host Microbe 12: 484–495 [DOI] [PubMed] [Google Scholar]
- Du YY, Wang PC, Chen J, Song CP (2008) Comprehensive functional analysis of the catalase gene family in Arabidopsis thaliana. J Integr Plant Biol 50: 1318–1326 [DOI] [PubMed] [Google Scholar]
- Edgar RC. (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32: 1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough DR, Cotter TG (2011) Hydrogen peroxide: a Jekyll and Hyde signalling molecule. Cell Death Dis 2: e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BJ, Kamoun S, Zody MC, Jiang RHY, Handsaker RE, Cano LM, Grabherr M, Kodira CD, Raffaele S, Torto-Alalibo T, et al. (2009) Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans. Nature 461: 393–398 [DOI] [PubMed] [Google Scholar]
- Hackenberg T, Juul T, Auzina A, Gwizdz S, Malolepszy A, Van Der Kelen K, Dam S, Bressendorff S, Lorentzen A, Roepstorff P, et al. (2013) Catalase and NO CATALASE ACTIVITY1 promote autophagy-dependent cell death in Arabidopsis. Plant Cell 25: 4616–4626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann DR, Gimenez-Ibanez S, Rathjen JP (2010) Bacterial virulence effectors and their activities. Curr Opin Plant Biol 13: 388–393 [DOI] [PubMed] [Google Scholar]
- Hemetsberger C, Herrberger C, Zechmann B, Hillmer M, Doehlemann G (2012) The Ustilago maydis effector Pep1 suppresses plant immunity by inhibition of host peroxidase activity. PLoS Pathog 8: e1002684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, van der Vossen EA, Kuang H, Vleeshouwers VG, Zhang N, Borm TJ, van Eck HJ, Baker B, Jacobsen E, Visser RG (2005) Comparative genomics enabled the isolation of the R3a late blight resistance gene in potato. Plant J 42: 251–261 [DOI] [PubMed] [Google Scholar]
- Inaba J, Kim BM, Shimura H, Masuta C (2011) Virus-induced necrosis is a consequence of direct protein-protein interaction between a viral RNA-silencing suppressor and a host catalase. Plant Physiol 156: 2026–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juul T, Malolepszy A, Dybkaer K, Kidmose R, Rasmussen JT, Andersen GR, Johnsen HE, Jørgensen JE, Andersen SU (2010) The in vivo toxicity of hydroxyurea depends on its direct target catalase. J Biol Chem 285: 21411–21415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoun S. (2006) A catalogue of the effector secretome of plant pathogenic oomycetes. Annu Rev Phytopathol 44: 41–60 [DOI] [PubMed] [Google Scholar]
- Kamoun S, van West P, Vleeshouwers VG, de Groot KE, Govers F (1998) Resistance of Nicotiana benthamiana to Phytophthora infestans is mediated by the recognition of the elicitor protein INF1. Plant Cell 10: 1413–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley BS, Lee SJ, Damasceno CMB, Chakravarthy S, Kim BD, Martin GB, Rose JKC (2010) A secreted effector protein (SNE1) from Phytophthora infestans is a broadly acting suppressor of programmed cell death. Plant J 62: 357–366 [DOI] [PubMed] [Google Scholar]
- Koeck M, Hardham AR, Dodds PN (2011) The role of effectors of biotrophic and hemibiotrophic fungi in infection. Cell Microbiol 13: 1849–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chen L, Mu J, Zuo J (2013) LESION SIMULATING DISEASE1 interacts with catalases to regulate hypersensitive cell death in Arabidopsis. Plant Physiol 163: 1059–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Ye W, Ru Y, Yang X, Gu B, Tao K, Lu S, Dong S, Zheng X, Shan W, et al. (2011) Two host cytoplasmic effectors are required for pathogenesis of Phytophthora sojae by suppression of host defenses. Plant Physiol 155: 490–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Marathe R, Dinesh-Kumar SP (2002) Tobacco Rar1, EDS1 and NPR1/NIM1 like genes are required for N-mediated resistance to tobacco mosaic virus. Plant J 30: 415–429 [DOI] [PubMed] [Google Scholar]
- McLellan H, Boevink PC, Armstrong MR, Pritchard L, Gomez S, Morales J, Whisson SC, Beynon JL, Birch PRJ (2013) An RxLR effector from Phytophthora infestans prevents re-localisation of two plant NAC transcription factors from the endoplasmic reticulum to the nucleus. PLoS Pathog 9: e1003670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhamdi A, Queval G, Chaouch S, Vanderauwera S, Van Breusegem F, Noctor G (2010) Catalase function in plants: a focus on Arabidopsis mutants as stress-mimic models. J Exp Bot 61: 4197–4220 [DOI] [PubMed] [Google Scholar]
- Nelson BK, Cai X, Nebenführ A (2007) A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J 51: 1126–1136 [DOI] [PubMed] [Google Scholar]
- Petrov VD, Van Breusegem F (2012) Hydrogen peroxide: a central hub for information flow in plant cells. AoB Plants 2012: pls014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qutob D, Kemmerling B, Brunner F, Küfner I, Engelhardt S, Gust AA, Luberacki B, Seitz HU, Stahl D, Rauhut T, et al. (2006) Phytotoxicity and innate immune responses induced by Nep1-like proteins. Plant Cell 18: 3721–3744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiqi M, Ellis JG, Ludowici VA, Hardham AR, Dodds PN (2012) Challenges and progress towards understanding the role of effectors in plant-fungal interactions. Curr Opin Plant Biol 15: 477–482 [DOI] [PubMed] [Google Scholar]
- Rajput NA, Zhang M, Ru Y, Liu T, Xu J, Liu L, Mafurah JJ, Dou D (2014) Phytophthora sojae effector PsCRN70 suppresses plant defenses in Nicotiana benthamiana. PLoS ONE 9: e98114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders DGO, Breen S, Win J, Schornack S, Hein I, Bozkurt TO, Champouret N, Vleeshouwers VGAA, Birch PRJ, Gilroy EM, et al. (2012) Host protein BSL1 associates with Phytophthora infestans RXLR effector AVR2 and the Solanum demissum immune receptor R2 to mediate disease resistance. Plant Cell 24: 3420–3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schornack S, van Damme M, Bozkurt TO, Cano LM, Smoker M, Thines M, Gaulin E, Kamoun S, Huitema E (2010) Ancient class of translocated oomycete effectors targets the host nucleus. Proc Natl Acad Sci USA 107: 17421–17426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D, Liu T, Ye W, Liu L, Liu P, Wu Y, Wang Y, Dou D (2013) Gene duplication and fragment recombination drive functional diversification of a superfamily of cytoplasmic effectors in Phytophthora sojae. PLoS ONE 8: e70036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C, Langlois-Meurinne M, Bellvert F, Garmier M, Didierlaurent L, Massoud K, Chaouch S, Marie A, Bodo B, Kauffmann S, et al. (2010) The differential spatial distribution of secondary metabolites in Arabidopsis leaves reacting hypersensitively to Pseudomonas syringae pv. tomato is dependent on the oxidative burst. J Exp Bot 61: 3355–3370 [DOI] [PubMed] [Google Scholar]
- Stam R, Jupe J, Howden AJM, Morris JA, Boevink PC, Hedley PE, Huitema E (2013) Identification and characterisation CRN effectors in Phytophthora capsici shows modularity and functional diversity. PLoS ONE 8: e59517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang ZG, Wei YD, Collinge DB (1997) Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11: 1187–1194 [Google Scholar]
- Torto TA, Li S, Styer A, Huitema E, Testa A, Gow NAR, van West P, Kamoun S (2003) EST mining and functional expression assays identify extracellular effector proteins from the plant pathogen Phytophthora. Genome Res 13: 1675–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler BM. (2007) Phytophthora sojae: root rot pathogen of soybean and model oomycete. Mol Plant Pathol 8: 1–8 [DOI] [PubMed] [Google Scholar]
- van Damme M, Bozkurt TO, Cakir C, Schornack S, Sklenar J, Jones AM, Kamoun S (2012) The Irish potato famine pathogen Phytophthora infestans translocates the CRN8 kinase into host plant cells. PLoS Pathog 8: e1002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: RESEARCH0034.1–RESEARCH0034.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Han C, Ferreira AO, Yu X, Ye W, Tripathy S, Kale SD, Gu B, Sheng Y, Sui Y, et al. (2011) Transcriptional programming and functional interactions within the Phytophthora sojae RXLR effector repertoire. Plant Cell 23: 2064–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Tang J, Wang Q, Ye W, Tao K, Duan S, Lu C, Yang X, Dong S, Zheng X, et al. (2012) The RxLR effector Avh241 from Phytophthora sojae requires plasma membrane localization to induce plant cell death. New Phytol 196: 247–260 [DOI] [PubMed] [Google Scholar]
- Zhang M, Meng Y, Wang Q, Liu D, Quan J, Hardham AR, Shan W (2012) PnPMA1, an atypical plasma membrane H+-ATPase, is required for zoospore development in Phytophthora parasitica. Fungal Biol 116: 1013–1023 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.