Abstract
Matrix metalloproteinases (MMPs) are proteolytic enzymes belonging to the family of zinc-dependent endopeptidases that are capable of degrading almost all the proteinaceous components of the extracellular matrix (ECM). It is known that MMPs play a role in a number of renal diseases, such as, various forms of glomerulonephritis and tubular diseases, including some of the inherited kidney diseases. In this regard, ECM accumulation is considered to be a hallmark morphologic finding of diabetic nephropathy, which not only is related to the excessive synthesis of matrix proteins, but also to their decreased degradation by the MMPs. In recent years, increasing evidence suggest that there is a good correlation between the activity or expression of MMPs and progression of renal disease in patients with diabetic nephropathy in humans and in various experimental animal models. In such a diabetic milieu, the expression of MMPs is modulated by high glucose, advanced glycation end products (AGEs), TGF-β, reactive oxygen species (ROS), transcription factors and some of the microRNAs. In this review, we focused on the structure and functions of MMPs, and their role in the pathogenesis of diabetic nephropathy.
Keywords: Diabetic nephropathy, extracellular matrix, metalloproteinases, TGF-β
INTRODUCTION
Diabetic nephropathy (DN) is one of the severe forms of microangiopathies in patients with diabetes mellitus, and this reno-vascular complication is the most prevalent cause of end-stage renal failure (ESRD) in the Western world. Only 20% of the diabetic patients with ESRD have a survival rate up to five years [1, 2]. A better understanding of the pathogenesis of DN is needed to facilitate the development of more effective and early therapeutic strategies to prevent ominous pathologic changes in kidney, which if not dealt with at an early stage would have an irreversible outcome. In addition, there are several other risk factors which further contribute to the progression of DN with worsening outcome, and they include glomerular hypertension, hyperglycemia, proteinuria, hyperlipidemia and heredity [3–8]. Although, abundant research has been carried out over a period of three decades to delineate the pathogenesis of DN a well-defined underlying unifying mechanism has yet to be emerged.
Morphological changes in the kidney seen in patients with type 1 and 2 diabetes are quite similar and they are confined to all the three compartments of the kidney, i.e., glomerulus, tubulo-interstitium and intrarenal vasculature [9–11]. The classical changes are seen in the glomerular compartment, and they include glomerular hypertrophy, thickening of glomerular basement membrane (GBM) and mesangial expansion with the formation of Kimmelstiel-Wilson nodules [12–14]. The changes usually accompanied with glomerular lesions include tubulo-interstitium undergoing fibrosis while there is also arteriolar thickening and hyalinization in the vascular compartment of the kidney. Basically, there is an imbalance of extracellular matrix (ECM) synthesis and degradation, which most likely is responsible for accumulation for excessive matrices in various compartments of the kidney [13, 15]. Thus, the genesis of Kimmelstiel-Wilson lesion, a diagnostic feature of DN, is not only due to the excessive synthesis of ECM glycoproteins but also to a decreased degradation by matrix metallo-proteinases (MMP) [16]. In this regard, numerous studies have been carried out since the discovery of MMPs to address their intra-renal dysregulation, i.e., perturbation in their expression and activity, in the progression of DN. In this article, we review the structure and function of MMPs and their relevance in the pathogenesis of DN, while also focusing on the role of their interactive partners, i.e., Tissue Inhibitor of Metallo-Proteinases (TIMPs) in the progression of DN.
MATRIX METALLOPROTEINASES
Structure, Classification and Functions
In general, Matrix Metallo-proteinases (MMPs) include a Zn++ ion binding site that is essential for their zinc-dependent proteolytic activities. These proteinases constitute a much larger superfamily of zinc peptidases having topological similarities among its members or groups and they are globally known as “metzincins” [17]. Within the superfamily, there is a category of MMPs with more than 28 members and 6 subgroups. Overall their domain structure is comprised of a propeptide, Zn++ binding catalytic domain, four hemopexin-like domains, transmembrane domain and a cytoplasmic domain. Tridimensional structural analyses of the MMPs indicate that topologically they share the catalytic domain consisting of an orderly sequential arrangement of twisted five-stranded β-sheets and three α-helices. Among these include certain metzincins, such as, MMP-8, adamlysin II, astacin and bacterial alkaline protease [18]. Another characteristic of the metzincins include a highly conserved motif containing three histidines (HEXXHXXGXXH) that bind zinc at the catalytic site and a methionine turn following the active catalytic site [10]. This catalytic site motif has essential histidine (H), glutamic acid (E) and glycine (G) residues, while X is a variable amino acid residue [19, 20]. Based on this amino acid sequence of the catalytic motifs, the family of metzincins has been further extended to include certain other proteases, such as, astacins, reprolysins (ADAMs) and serralysins, besides the MMPs, thus making the classification and functionalities of metzincins highly complex and at times confusing [21–23]. Certainly, under the broad category of metzincins, the MMPs, a multigene family with shared domain structure, are believed to play an essential role in the breakdown of extracellular matrix (ECM) in order to maintain a proper balance in its synthesis and degradation [24]. This process of turnover is essential for morphogenesis, embryonic development, reproduction, remodeling of tissues under normal and pathologic states, the prototype of latter being neoplastic and inflammatory diseases [24, 25]. As indicated above that for the large part MMPs share a core structure made up of a prodomain, a catalytic domain, a hinge region and a hemopexin-like domain (Hpx); however, a few variations do exist. For instance, MMP2 (gelatinases A) and MMP9 (gelatinases B) have three repeats of a fibronectin type II-like motif in the metalloproteinase domain [26–28]. MMP7, MMP26 and MMP23 lack the linker peptide and the Hpx domain [29]. MMP-23 has a distinct cysteine-rich domain and an IL-1 receptor-like domain following the metalloproteinase domain instead of the Hpx domain [30]. MT4-MMP (membrane-Type 4 matrix metalloproteinases) is endowed with unique hydrophobicity properties at its C-terminus that are akin to some of the GPIanchored proteins, such as human uPAR, NCAM120 and Thy-1 [31].
On the basis of substrate specificity and sequence homology, MMPs have been subclassified into six groups (see Table 1): Collagenases (MMP1, MMP8, MMP13 and MMP18) [32–35], Gelatinases (MMP2 and MMP9) [36, 37], Stromelysins (MMP3, MMP10 and MMP11) [38–40], Matrilysins (MMP7 and MMP26) [39, 41, 42], Membrane-type matrix metalloproteinases (MT-MMP14, -MMP15, -MMP16, -MMP17, -MMP24 and –MMP25) [43–49] and other MMPs (MMP12, MMP19, MMP20, MMP21, MMP23, MMP27 and MMP28) [25, 50–58]. Except for the MT-MMPs most of the MMPs are secreted out and have extracellular distribution; however, recent evidence suggest that MMP1, MMP2 and MMP11 also have intracellular expression where they may interact with cytosolic proteins to modulate various biological processes [59–61]. Whether cellular or extracellular or transmembrane localization it is now well accepted that MMPs regulate a variety of physiological and pathological processes, including embryonic development, tissue homeostasis, inflammatory, vascular and autoimmune disorders, carcinogenesis and fibrogenesis in various organ systems [26, 62–85].
Table 1.
The Matrix Metalloproteinase (MMP) and Substrate As Well As Action
| Group | MMPs | Enzyme | Substrate(s) | Activated by | Action(s) Pro-fibrotic Anti- fibrotic |
|---|---|---|---|---|---|
| Collagenases | MMP1 | Collagenase-1 | Collagen (I–III, VII, X); entactin, IL-1β; IGFBP-2,3; perlectan; pro-IL-1β; aggrecan; versican; perlecan; serpins | MMP-3,10; PDGF; plasmin; hyperglycemia; inflammatory cytokines; kallikrein; chymase |
Yes [158] |
| MMP8 | Collagenase-2 | Collagen (I–III, VII, X); aggrecan; fibronectin; pro-TNF-α; IGF-BP, | hyperglycemia; MMP-3,10; plasmin | Yes [79] | |
| MMP13 | Collagenase-3 | Collagens (I, II, III); gelatin; aggrecan; tenascin | MMP-2,3,10,14,15; plasmin, TGF-β | Yes [74] Yes [85] |
|
| MMP18 (Xenopus) | Collagenase-4 | not determined | not determined | ||
| Gelatinase | MMP2 | Gelatinase A | Collagen type (I, II, III, IV, V, VII, X, XI, XIV); gelatin; aggrecan; laminin; fibronectin; elastin; MMP-9; MMP-13 | hyperglycemia; MMP-1,7,13,14,15,16,24,25; NF-ΚB; plasmin; TGF-β tryptase; CTGF thrombin | Yes [224] Yes [77] |
| MMP9 | Gelatinase B | Collagen (IV, V, VII, XI, XIV); gelatin; fibronectin; elastin; pro-IL-8; pro-TNF-α |
hyperglycemia; MMP-2,3,13; TGF-β; TNF-α; VEGF; AP-1; NF-ΚB; plasmin | Yes [75] Yes [77] |
|
| Stromelysins | MMP3 | Stromelysins-1 | Collagens (III, IV, V, IX, X), proteoglycans, fibronectin, elastin | plasmin; kallikrein; chymase; tryptase | Yes [78] |
| MMP10 | Stromelysins-2 | Collagens (III, IV, V); fibronectin, proteoglycan; aggrecan, gelatin | Elastase; hyperglycemia; cathepsin; plasmin; | Yes [133] | |
| MMP11 | Stromelysins-3 | Collagen IV; fibronectin; proteoglycans; laminin; IGFBP-1 | Furin | Not determined | |
| Matrilysins | MMP7 | Matrilysins-1 | Collagens (IV, X); fibronectin; E-cadherin; entactin | hyperglycemia; Wnt/β catenin MMP-3; plasmin | Yes [80] Yes [81] |
| MMP26 | Matrilysins-2 | Collagen IV; fibronectin; fibrinogen; gelatin | Pro-MMP9 | Not determined | |
| Membrane-type | MMP14 | MT1-MMP | Collagen (I, II, III), casein; laminin; fibronectin; nidogen; aggreca; tenascin; perlecan | TGF-β; hyperglycemia; Plasmin; furin; E-cadherin | Yes [62] Yes [77] |
| MMP15 | MT2-MMP | MMP2; fibronectin, laminin; tissue transglutaminase | not determined | Not determined | |
| MMP16 | MT3-MMP | Collagen III; fibronectin; MMP2; tissue transglutaminase | furin | Not determined | |
| MMP17 | MT4-MMP | Gelatin; fibronectin; proMMP2; fibrillin | not determined | Not determined | |
| MMP24 | MT5-MMP | Collagen I; fibronectin; gelatin | hyperglycemia; | Not determined | |
| MMP25 | MT6-MMP | Collagen IV; gelatin; fibrin; laminin; proMMP9 | not determined | Not determined | |
| Other MMPs | MMP12 | Macrophase metalloelastase | Collagen IV; elastin; gelatin; plasminogen; fibronectin | Plasmin | Yes [82] |
| MMP19 | Aggrecan; fibronectin; gelatin; nidogen; cartilage oligomeric matrix protein | Trypsin | Yes [83] | ||
| MMP20 | Enamelysin | Aggrecan; amelogenin; cartilage oligomeric matrix protein | not determined | Not determined | |
| MMP21 | Gelatin; α-1-antitrypsin | not determined | Not determined | ||
| MMP23 | not determined | not determined | |||
| MMP27 | not determined | not determined | |||
| MMP28 | Epilysin | Neural cell adhesion molecule (NCAM); casein | not determined | Yes [84] |
Regulation of MMPs
Under physiological conditions, in order for MMPs to exert their full effect following transcriptional induction and translation and biochemical activation, they need to be expressed in a coordinated manner with a highly restricted spatiotemporal distribution, suggesting they do not target their cellular or extracellular substrates indiscriminately, although they can act synergistically to degrade a broad spectrum of ECM glycoproteins. Besides, cell and time specific transcriptional, translation and post-translation modulation of MMPs followed by secretion at specific target sites their enzymatic activities are also regulated by some of the known endogenous metalloproteinase inhibitors known as TIMPs while they may be other additional MMP inhibitors that are yet to be discovered. Normally, the activities of most of the matrixins are negligible or they have very low expression in healthy intact tissues, and this general biologic characteristic may be related to the sharing of common cis-elements in the promoters regions of MMPs, meaning thereby that their expression is tightly controlled at the transcriptional level depending upon the biologic state of a given cell type. Furthermore the literature information indicate that a multitude of signaling intermediates are involved in the activation of transcription factors, such as, NF-ΚB, Ap-1, AP-2, MAPK, STAT, SMAD and PEA3 in order for MMPs to exert their effects [29, 63, 86, 87]. In this regard, Wnt/β-catenin signaling controlling MMP7 and MMP9 expression has been described in rodent animal models, and this regulatory mechanism among many others may be relevant to the pathobiology of kidney injury [88, 89]. Interestingly, among humans there are also genetic variations which influence the level of MMP gene expression that ultimately may dictate the progression of a given disease process. In fact, the relationship between some of the diseases and single nucleotide polymorphisms (SNPs) of MMPs’ promoters has been described. For instance, recently it has been shown that rs3025058 at position-1612 in MMP3 and rs2276109 at position −82 in MMP12 are associated with an increased susceptibility to cardiovascular disease [90]. In addition, Kure et al. demonstrated that the variants at the MMP3/MMP12 locus are associated with a reduced risk of diabetic nephropathy in patients with type 1 diabetes [91].
Although MMPs expression seems to be heavily modulated at the transcriptional level, a series of post-translation regulation mechanisms have also been identified in recent years. Miriam et al. reported that there are transcripts that harbor specific sequences in their 5′- or 3′-untranslated regions (UTRs) of MMPs to which UTR-binding proteins can associate to regulate their corresponding mRNAs levels [92]. For instance, AU-rich elements present in 3’-UTR of MMP1 and MMP9 heavily determine their mRNA stability and ultimately the biological effects [93, 94]. Rydziel et al. reported that cortisol modulates mRNA stability via AU-rich sequences of 3’-UTR region in MMP13 in osteoblasts [95].
An additional gene regulation of MMPs’ expression and subsequent activation of various signaling pathways and targeting other genes and organ system by microRNAs (miRNAs) has recently attracted the attention of many investigators. Zhihong Yang et al. found that the miR-127 inhibits the expression of MMP13 protein both by repressing the 3'-UTR activity and inhibiting MMP13/TGF-β signaling in human hepatocellular carcinoma [96]. Similarly, miR-133a was found to down-regulate MMP14 protein expression with decreased frequency of lung cancer metastasis. Likewise, Lin SY et al. [97] demonstrated that over-expression of miR-92a could repress the expression of RECK, a MMP inhibitor, by post-transcriptional regulation in H1299 cells.
Since the discovery of MMPs, the investigators over the last 2–3 decades have been exploring to develop inhibitors that could directly neutralize the activities of MMPs at the intra- or extra-cellular target sites, although several endogenous inhibitors of MMP, known as TIMPs, that are capable of modulating cellular homeostasis have been identified [98, 99]. The TIMPs family includes at least four 20 – 29 kDa secreted proteins (TIMP1 - 4) that exhibit specificities for different MMPs [26, 29]. Allison et al. found that TIMP1 mRNA expression is quite low in mouse kidney, while TIMP2 and TIMP3 mRNA are expressed at high levels, while TIMP4 is undetectable [100]. Interestingly, almost all the known MMPs can be inhibited by TIMP1, 2 and 3 [101]. However, recent studies show that although TIMP1 is a poor inhibitor of MT1-MMP, MMP19 and ADAM17, it can be transformed to an active inhibitor of ADAM17 when substitution of threonine is made at residue 98 with leucine (T98L) [99, 102]. Additionally, TIMP4 is involved in suppressing the activity of MMP1, MMP2, MMP3, MMP7 and MMP9 [103]. Furthermore, besides having inhibitory effect on MMPs, the TIMPs are endowed with other biological activities, including cell growth, migration, invasion, antiangiogenesis, anti- and pro-apoptosis and synaptic plasticity [104–109]. TIMPs as a major MMPs inhibitor was considered increasing ECM accumulation and renal fibrosis as it can reduce the degradation of matrix proteins [110–112]. However, Allison A et al. found that the degree of renal interstitial fibrosis did not diminish in TIMP1 deficiency mice [100]. Furthermore, TIMP3 prevented glomerular and tubulointerstitial from age-depended renal injury [113]. Recent study has also shown that aggravated renal damage in response to lack of TIMP3 but not TIMP2 in UUO model, which accompanyed by the increase expression of collagen type I/III and activites of capase-3 [114]. Microarray analysis suggested that significant difference gene expression in TIMP3−/− mice compared to TIMP2−/− mice after UUO. Moreover, TIMP3 is a pivotal negative modulator of renal TNFα which induced renal fibrosis and injury in UUO [113]. Conceivably, several other proteins also have inhibitory activity targeting the MMPs, such as, RECK, TFPI-2, PCPE and α2-Macroglobulin, however, still much more research is needed to characterize their biological properties and functions [115].
MMPs AND DIABETIC NEPHROPATHY
The spatial-temporal localization and delineation of functions of MMPs in the kidney is quite complex, and that to some extent may be related to a given MMP involved in a particular disease process and animal specie. Certainly, the balance of ECM synthesis and degradation/turnover is one of the most significant processes for maintaining the glomerular structural and functional integrity [116, 117]. The MMPs may affect ECM turnover via several different mechanisms. Abnormal MMP expression or activity will directly render into an altered ECM turnover, and such an abnormal matrix is likely to influence heavily the behavior of glomerular cells [118]. Besides the ECM degradation or turnover, MMP2 can modulate the biology of the glomerular mesangial cells upon exogenous reconstitution of active MMP2 to antisense MMP RNA-induced quiescent cells, and in doing so they rapidly reverted to a pro-inflammatory phenotype [119]. MMPs also can activate or release some of the growth factors, such as insulin like growth factors, tumor necrosis factor (TNF-α) and heparin-binding-epidermal growth factor, and such a modulation is associated with profound pathologic changes that adversely affect cellular homeostasis in various diseases [120–123]. During the past few years, extensive investigatory efforts have delineated the role of MMPs in a variety of kidney diseases, including acute kidney injury, diabetic nephropathy, tubulo-interstitial fibrosis and various forms of glomerulonephritis [26]. The MMPs have long been identified as critical mediators of ECM degradation and turnover, but increasing evidence support that they in conjunction with TIMPs play an important role in the progression of diabetic nephropathy.
The Expression of MMPs in Patients with Diabetic Nephropathy
Traditional thinking is that hyperglycemia down-regulates the expression and activity of MMPs in patients with diabetic nephropathy (DN), as a result there is a decreased ECM degradation and accumulation in the matrices leading to mesangial expansion with the evolution of Kimmelstiel-Wilson lesions. It is well established that high glucose ambience adversely affects ECM degradation or turnover and enzymatic activities of MMPs and TIMPs in patients with DN. Surprisingly, circulating TIMP1, TIMP2 and MMP2 have also been found to be decreased in patients with DN [124]. However, there are conflicting reports suggesting that MMP2, MMP9, MMP7, MMP8, MMP14 as well as TIMP1 are increased in the serum or urine from diabetic patients [125–128]. Similarly, there are variations in the renal tissue expression of MMP2 in the biopsy specimen of patients with DN. Sekiuchi et al. reported that MMP2 is weakly expressed in the glomerular capillary loops and Bowman's capsules along with TIMP2 in the mesangial area, suggesting that MMP-2 most likely is involved in the turnover of glomerular basement membrane [129]. Del Prete et al. demonstrated a dramatic decrease in MMP2 gene expression in glomeruli of patients with type 2 diabetes [130]. However, another study showed that MMP2 protein and related enzyme activity were up-regulated in kidneys of patients with diabetes, as assessed by Western blot analysis and ELISA methods [126]. Similarly, MMP7 mRNA expression was reduced in renal tissues from patients with DN compared with control [131], while by immuno- histochemical staining methods they were found to be up-regulated [89]. In addition, these unique changes in the MMP7 expression in renal tubular epithelia may be in some ways related to Wnt/β-catenin signaling in the kidney [89]. Furthermore, by in situ hybridization, a significantly down-regulated expression of MMP3 and TIMP1 mRNA in intrinsic glomerular cells and tubular epithelia was observed in patients with DN. Tubular expression was found to correlate with the extent of interstitial injury, while it was inversely proportional to the glomerular mesangial expansion [132]. MT5-MMP expression was detected only in the kidney by Western blot analyses and it was noted to be significantly increased in patients with DN [126]. Romanic et al. reported that MT5-MMP was highly expressed in epithelia of proximal and distal convoluted tubules in diabetic patients. Furthermore, the expression of MT5-MMP was associated with tubular atrophy, which incidentally worsens the progression of DN [126]. In another study MMP10 levels were reported to be high in diabetic patients and positively correlated with the degree of damage related to nephropathy in diabetic patients [133]. Taken together, MMPs and TIMPs expression in DN patients is somewhat variable, and their role in kidney pathology seems to be still very much confounding. But one can safely assume that the imbalance in MMP-TIMP expression or activation could lead to abnormal ECM deposition which decidedly remains as one of the hallmark of DN.
Albuminuria or microalbuminuria as a clinical biomarker of DN is matter that has been a critical issue for several years [134, 135]. Numerous studies demonstrated a strong correlation between the MMPs expression and the degree of albuminuria or the severity of clinical symptoms. It has also been reported that urinary TIMP1 levels remarkably increase in diabetic patients, and they are associated with the severity of diffuse glomerulosclerosis and urinary N-acetyl-β-glucoaminidase (NAG) excretion [136]. Another study demonstrated that circulating MMP7 levels were up-regulated in the micro- as well as macro-albuminuric groups and had negative correlation with glomerular filtration rate in patients with diabetes [137]. Interestingly, Sekiuchi et al. reported that MMP9 expression was concentrated in the stalk mesangial of the glomerulus in patients with DN, as delineated by the immuno-histochemical studies, suggesting that MMP9 may play a critical role in the turnover of the mesangial matrix in DN [129]. In another recent study, Szu-Yuan Li et al. observed that MMP-9 was dramatically increased in the glomeruli of diabetic mice, and MMP-9 deficiency attenuates diabetic nephropathy by modulation of podocyte functions and dedifferentiation. Moreover, they found, in diabetic patients, the upregulation of urinary MMP-9 concentrations occurred earlier than the onset of microalbuminuria. Collectively, these results suggest MMP-9 play a role in the development of diabetic nephropathy [138]. In addition, the presence of MMP9 in urine of DN patients was detected where they were seen as high molecular weight complexes that were significantly higher than healthy adults [125]. Moreover, a positive correlation has been well documented between the urinary excretion of MMP9 and the magnitude of albuminuria and renal lesions in patients with type 2 diabetes mellitus [139, 140]. Ebihara et al. [139] discovered that the plasma MMP9 levels were elevated before the onset of albuminuria, and they were dramatically decreased following treatment with angiotensin-converting enzyme inhibitor in patients with type 2 diabetes patients with high risk for development of diabetic nephropathy. In addition, a recent study has shown that elevated MMP9 levels in T2DM patients had strongest association with age, BMI, hyperglycaemia, blood pressure, HbA1c and progression of diabetes [127]. Furthermore, plasma concentration of MMP9 in diabetic patients has been significantly correlated with the aberrant shedding of podocytes in the urine, which is reduced by treating with Trandolapril [141]. These findings are in accord with other studies [125, 139, 140"], and the literature data suggest that urinary MMP9 may be a useful marker for diagnosing the degree of renal damage in early phases of DN. On the other hand, Toni et al. demonstrated that serum MMP-9 concentration correlates with the incidence of DN [141], and they hypothesized that the patients with A21 allele of the MMP9 gene may be protective with respect to the occurrence and progression of DN [142]. Importantly, findings of another recent study suggested that MMPs could serve as a potential target for the therapeutic intervention in DN. Aggarwal et al. demonstrated that compared with angiotensin-converting enzyme inhibitors (ACEIs) and or angiotensin receptor blockers (ARBs), doxycycline (an inhibitor of MMP) can effectively reduce proteinuria in adult patients with DN, but unfortunately it is effective only for a short duration, i.e., less than 12 weeks [143].
The Expression of MMPs in Animal Model of Diabetes
Rodent studies have provided further insights into the potential contribution of MMPs and TIMPs in animal models of diabetic nephropathy (DN). Interestingly, an altered expression and enzyme activity of MMPs and TIMPs have been reported in DN models. But the quandary is that both up- and down-regulation has been described for MMP-2 expression or enzyme activity in renal tissues in rodent models of diabetes [111, 144, 145"]. Fornoni et al. investigated the MMP2 mRNA expression and activity in mesangial cells derived from kidneys of ROP mouse (reduced nephron number) that rapidly develop glomerulosclerosis and from parental B6 mouse strain that are resistant to develop glomerulosclerosis [144]. Exposure of cells to high glucose (HG) ambience led to a comparable increase in the MMP2 mRNA expression and activity in both the ROP and B6 mice mesangial cells, but this altered expression was reversible when glucose concentration was reduced from 25 mmol/L to 6 mmol/L in B6 mice cells only. These intriguing observations suggested that there is a potential role of MMP2 in the progression of DN in the model of sclerosing phenotype ROP mice under HG ambience [144]. Similarly, renal MMP2 expression and activity have been observed to be increased mainly after 16 weeks STZ induced mice when the sclerosing process is overtly seen. In addition, transgenic studies by Takamiya et al. indicate that MMP2-KO mice have relatively high serum levels of BUN and Creatinine and increased urinary excretion of albumin and NAG compared to their wild-type littermates [146]. Their studies also highlighted that changes in MMP-2 in diabetic mice may be time dependent, and the increased renal expression and activity of MMP2 may be a protective compensatory mechanism during the early phases of DN, while it may be operationally ineffective in later sclerosing phases. MMP9 is another protease which has been investigated with respect to its role in the DN with mixed variable results and contradictions. It is of interest to note that non-specific pharmacological inhibition of MMP9 with doxycycline were found to be associated with amelioration of renal injury [143]. In addition, it is also reported that Simvastatin protects kidney damage of diabetic rat through the suppression of MMP9 expression [147]. Along these lines, the expression of MMP9 mRNA and protein were found to be up-regulated compared with the control group in 16 weeks of Kkay mice with glomerular sclerosing lesions [148]. Similarly, Szu li et al. reported reduced glomerular hyperfiltration and glomerular basement membrane thickening in diabetic MMP9−/− mice than diabetic WT mice [138]. In vitro, overexpression of MMP9 upregulated the expression of mesenchymal markers protein and induced podocyte dedifferentiation and increased the ECM synthesis [138]. Conflicting results were reported in another study with 24 weeks of STZ rat where increased collagens IV in the glomerulus and tubulo-interstitium was noted to be associated with decreased matrix degradation activity and reduced MMP9 mRNA expression in the kidney. Furthermore, the changes in MMPs and type IV collagen accumulation were attenuated by ACE inhibitor, Perindopril [149]. Taken together, these observations suggest that the MMPs expression level were inconformity at different phases of DN. It was reported that MMP-9 directly processed TGF-β into an active ligand [150] and it also regulated the release of growth factors like VEGF [151]. Furthermore, MMP9 may act on pro-inflammatory pathway through induction of TNF-α [152, 153] and IL-1β [72, 154] in an early phase of diabetic rat or mouse. These data indicates that expression of MMP9 was increased in early phase of DN, which directly promoted the degradation of basal membrane protein of kidney and stimulated secretion of activate growth factors as well, culminating in renal lesion. However, in later phase of DN, the expression of MMP9 was negatively regulated by feedback mechanism leading to decreased degradation of ECM and renal tissue fibrosis under DN condition eventually. On the other hand, Most likely, in DN it is the high glucose ambience or hyperglycemic environment that down-regulate MMP3, MMP7, MMP14 and TIMP3 in various diabetic animal models [131, 155–157]. The direct relevance of MMPs in DN is provided by the studies showing suppression of excessive type 1 collagen accumulation and renal fibrosis following MMP1 plasmid DNA gene delivery via microspheres implanted in the DN mouse model [158]. Along these lines conceivably gene disruption of another protease, i.e., MMP-10, may prevent renal damage in diabetic mice since reduced expansion of mesangial matrix and interstitial macrophage accumulation were observed in MMP10−/− mice [133]. Taken all together the above observations, it seems that the altered expression and dysfunction of MMPs and TIMPs may be related to the development of DN, however, as to how the differential expression of various MMPs and TIMPs influence the renal disease progression in diabetic patients remains somewhat enigmatic. High glucose, AGEs, ROS, hypoxia, TGF-β and miRNA et al. probably be involved in regulating the expression and activity of MMPs. We would depict them one by one in the following text.
High Glucose and MMPs
A large body of data focusing on the role of MMPs in the DN is available in the literature, however, the specific mechanism(s) in regard to their modulation are not clear rather they are somewhat complex. There are several mechanisms by which dysregulation in renal MMPs and TIMPs expression or activity in kidney could contribute to the development of progressive DN. As previously mentioned, MMPs expression is tightly regulated by various mechanisms that include transcription and post-transcription. Evidence indicates that HG may regulate MMP gene expression via various transcription factors, such as, AP-1 and NF-κB [159, 160] or depending upon the tissue levels of growth factor cytokines, such as, TGF-β and CTGF [161, 162]. Since AP-1 is made up of Fos and Jun families of transcription factors its role may be dependent upon the dimeric composition that most likely would influence the downstream signaling pathways [163]. There are two AP-1 sites in the promoters of MMP1 and MMP3 and the consensus AP-1 sequences at the second site is variable, which may explain why the differential effects by these MMPs following the induction by HG are observed [164]. Differential regulation was also observed following HG induced increased levels of PDGF which exclusively and negatively regulate the MMP3 expression while sparing the MMP1 [164]. Similarly, although MMPs expression is increased in human mesangial cells under prolonged HG ambience associated high MMP2 activity while that of MMP9 decrease under such an environment. These opposite catalytic effects are interesting and may be related to the compositional differences in the promoter sequences of these two gelatinases [165]. In addition, MMP2 does not have a TGF-β inhibitor element (TIE) and AP-1 sites, whereas they are included in the MMP9 promoter [166]. In this regard, Alison K et al. study suggested that the increased MMP9 mRNA level induced by hyperglycemia might mediated by AP-1 and NF-κB transcription factors [164]. Recently, Bai Y et al. investigated the ERK1/2 MAPK signaling pathway and its role in MMPs expression in murine podocytes exposed to HG ambience [167]. HG-induced increase in MMP9 activity in earlier phases of culture required activation of ERK1/2 MAPK signaling and that is associated with downstream decreased collagen IV collagen expression, suggesting blocking of the ERK pathway is critical in the modulation of MMP9 expression and thereby the ECM turnover.
AGEs and MMPs
Advanced glycation or glycol-oxidation end products, known as AGEs, are aberrantly synthesized molecules that can be one of the major factors influencing the progression of DN. Hyperglycemia leads to the generation of AGEs which is basically as a result of condensation of sugar and free amino group with the formation of a labile Schiff base which undergoes further complex intra-molecular modification to generate complex toxic AGEs. Such derivatization can occur between sugar and lipids as well, and their formation can be initiated in both intra- and extracellular compartments [168–170]. Several forms of AGEs derivatives have been described in renal injury related to diabetes, and morphologic changes that are often associated with it include glomerular and tubular basement membrane thickening, capillary aneurysmal dilatation, mesangial expansion with formation of Kimmelstiel-Wilson nodules and arteriolar thickening and hyalinosis [171–173]. Biologically active AGEs can be highly reactive in the intra- and extra-celluar compartments and their downstream injurious effects include increased oxidative stress, alterations in the molecular conformations of the molecules and aberrant expression of cytokines and growth factors [171, 174, 175]. As a result the AGE formation can alter the functionalities of several important extracellular matrix molecules, such as, type I collagen, type III collagen, type IV collagen and fibronectin and laminin [176–179]. Furthermore, AGEs can modulate the intracellular signaling pathways and gene and protein expression by interacting with their receptors, i.e., RAGE [180]. In this regard, a significant reduction in the gene and protein expression of MMP7 was observed in patients with DN and STZ-animal model system and in mesangial cells in vitro subjected to HG ambience, and these effects were blocked by neutralizing TGF-β antibodies and Aminoguanidine, an inhibitor AGEs formation [131]. Interestingly, AGEs can modulate the expression of certain growth factors belonging to CNN family. Broadly speaking the latter include six members: CYR61 (Cysteine-Rich61, CYR61/CCN1), CTGF (Connective Tissue Growth Factor, CTGF/CCN2), NOV (Nephroblastoma Over-expressed, NOV/CCN3), WNT1-Inducible Signalling Pathway proteins 1, 2 and 3 (WISP1, -2 and -3 or CCN4, -5 and -6) [181]. They have been shown to regulate various biological processes, such as, cellular activity, fibrosis, inflammation and angiogenesis. Hughes et al. reported that AGEs increase the CCNs mRNA and protein levels in retina of rats with STZ induced rat and that were reduced following the treatment with Aminoguanidine [181]. Recently, Wang X et al. described that CCN-2 mRNA and protein expression were increased by glycated ECM, which in turn can up-regulate the expression of fibronectin and TIMP-1 in human renal mesangial cells, and this circuitous cycle may play a role in evolution of phenotypic changes seen in the glomerulus in DN [182]. Additionally, the AGEs via AGE:RAGE interaction can activate PKC, MAPK and NF-κB, which can in turn modulate the expression of TGF-β and subsequently of MMPs. Such ligand: receptor interactions can also generate reactive oxygen species (ROS), which then can regulate the MMPs’ expression via modulation of various transcription factors [16]. Interestingly, Mclennan SV et al. demonstrated that glycation of ECM certainly increases the gene expression of MMP-2, but decreases TGF-β mRNA and activity in human fetal mesangial cells, suggesting that glycation of matrix affects MMP2 is independent of TGF-β initiated signaling pathway [183].
ROS, Hypoxia and MMPs
The excessive generation of ROS and the development of oxidative stress (OS) are regarded as the key events influencing the pathogenesis of DN [184, 185]. The ROS are known to modulate various biological processes, such as, cell proliferation and cell adhesion, as well as certain pathobiologic processes, such as, atherosclerosis and immunologically-mediated inflammatory disorders [186–188]. The activation of AGEs and ROS are intricately interwoven and thus may amplify the signaling cellular events in a hyperglycemic environment. It is known that over-production of ROS by HG in renal cells though AGE:RAGE interaction or glucose auto-oxidation or metabolism modulate the activation of PKC, NF-κB and AP-1, as well as increase the TGF-β activity, thereby promoting a further increase in the expression of ECM proteins [189]. Moreover, this increase in the activity of these signaling molecules and expression of cytokines and ECM can be effectively reduced with the treatment by antioxidants [189–192]. Uemura S et al. suggested that MMPs activity is tightly correlated with the cellular redox balance. They indicated that the expression of MMP9 in the vasculature is redox-sensitive since it can be reduced by various antioxidants, such as, N-acetyl-L-cysteine (NAC) [193]. On one hand, excessive generation of ROS could increase the expression of some of the key transcription factors, such as, AP-1 or NF-κB, that are important for the gene activation of MMPs in a vast number of disease processes [194], while on the other hand, oxidant stress also may regulate MMPs by direct oxidation of crucial cysteine residues within the DNA-binding domain [194, 195]. Masash et al. demonstrated that MT1-MMP plays a vital role in NADPH oxidase-dependent signaling pathways that are initiated following AGE:RAGE interaction [196]. They demonstrated that the gene disruption of MT1-MMP remarkably blocks the NADPH oxidase activity and ROS generation in smooth muscle cells [196]. Furthermore, mitochondrial-derived ROS may also regulate MMP-9 activation or expression via activation of ERK-1/2 signal cascade in vascular endothelial cells in hyper- homocysteinemic (HHCY) state [197]. Although HHCY state induces MMP-9 in myocytes it negatively regulates their contractile functions, apparently related to the perturbation in the mitochondrial homeostasis [198]. In line with this contention, other MMPs, i.e., MMP2, has been shown to negatively regulate mitochnodrial functions in states of oxidant stress [199]. Similarly, gene disruption of MMP-9 in mice led to the amelioration of mitochondrial dysfunctions and development of retinopathy in diabetic milieu [200]. Like the evidence that increased MMP-9 activity in vitro in bovine carotid artery endothelial cells (BAECs) induced by HG can be inhibited by antioxidants there is also evidence that insulin infusion in vivo has an inhibitory effect on the increased expression of MMP9 in patients with hyperglycemia, which in part may be related to the insulin capacity to exert antioxidant and anti-inflammatory effects besides lowering the blood sugar levels [201].
In recent years the notion that in DN increases oxidative stress is commonly associated with hypoxia has attracted the attention of many investigators [16, 202]. The hypoxic injury seems to be an overwhelming feature of several other renal diseases beside DN [203–206]. Kidneys from diabetic animals have increased renal oxygen consumption [207]. Conceivably, it is related to the increased metabolic rate and oxygen demand in various target tissues which will inevitably lead to kidney tissue hypoxia and potentially contribute to the progression of diabetic nephropathy [208]. The tubule-interstitial hypoxia is a most significant early event in diabetes [206]. Recent studies have delineated that the tubule-interstitial damage, including tubular cells apoptosis in patients with DN, is related to hypoxia-induced injury or at least it would certainly contribute to the onset of the development of DN [16, 206, 209]. There are literature reports which suggest that hypoxic stimuli boost the ECM synthesis and deceleration of its turnover with up-regulation of α1 chain of type 1 collagen as well as that of the tissue inhibitor of TIMP-1, and down-regulation of collagenase in human renal cortical fibroblasts when subjected to relative anoxic ambience [210]. Furthermore, they found that the increased expression of TIMP1 induced by hypoxia was associated with activation of transcription factor known as hypoxia-inducible factor 1(HIF-1)[210]]. Moreover, HIF-1-mediated hypoxic injury in tubular epithelial cells involves CTGF since the promoter of this growth factor includes hypoxia response elements [211]. Hypoxia also decreases the expression of MMP-9 and MMP-2 activity in human cortical fibroblasts under normal as well as HG ambience [212]. However, the cells derived from 2/3 of patients only had increased MMP9 and MMP2 expression under HG ambience, suggesting that effects of hypoxia and HG may be independent from each other with respect to MMP activities, and hypoxia may be a better predictable parameter with respect to kidney injury [212].
TGF-β and MMPs
Numerous studies have demonstrated a vital role of TGF-β in aberrant accumulation of ECM in glomerular and tubulo-interstitial compartments in DN [116, 213, 214]. The overexpression of TGF-β mRNA and protein in diabetic kidney in both experimental models and human has been observed [215]. By in situ hybridization and immunehistochemistry, besides TGF-β, an increased mRNA and protein expression of type IV collagen in both glomeruli and tubulo-interstitium of diabetic rats has been seen [216]. Furthermore, expression of specific matrix proteins induced by TGF-β was seen to be increased in glomeruli of diabetic rat as well of patients with DN [215]. TGF-β initiates its fibrogenic effects by stimulating matrix synthesis of various integral ECM proteins, such as, fibronectin, collagens and proteoglycans [217, 218]. At the same time there is inhibition of matrix degradation by dampening the synthesis of proteases and boosting the levels of TIMPs [219] while modulating the cellular matrix receptor (integrin) expression to strengthen cell-matrix connections [220].
Experimental data support that HG regulates MMP and TIMP expression, possibly through TGF-b axis [221]. Singh R et al. found a decrease of MMP2 activity that was conceivably regulated by TGF-β1 in rat mesangial cells under HG ambience [161]. They also reported that TIMP2 and TGF-β1 levels were increased following HG stimulation, while secreted MMP2 protein capable of degrading type IV collagen was decreased, and this effect was blocked partially by neutralizing anti-TGF-β1 antibody. Baricos et al. found that these effects were conceivably due to the blocking of the conversion of latent MMP-2 to active form in human mesangial by cells TGF-β1 [222]. In addition, studies by McLennan et al. suggested MMP2 and TIMP1 gene expression were increased, while MMP-9 mRNA was decreased in diabetic rat kidney and these events are associated with altered transcriptional activities of TGF-β [149]. Furthermore, TGF-β1 stimulates the synthesis of MMP14 and MMP2, which is required for epithelial-mesenchymal transformation (EMT) in the rat remnant kidney model of progressive renal fibrosis [223]. Cheng and Lovett et al. also found that active MMP2 could induce EMT alone in vitro and amplify the transformation course by proteolytic generation of TGF-β1 thought paracrine way [223]. In addition, transgenic mice highly expressing active MMP2 in renal proximal tubular epithelial cells exhibited glomerulosclerosis, extensive mononuclear cell infiltration, tubular interstitial fibrosis and developing renal fuction dysregulation compared with control [224]. This proteolytic gelatinase cleaves type IV collagen, a major component of basement membranes and maintenance the epithelial phenotype of proximal tubular eptihelial cells [225], leading to TBM destruction which is significant to trigger EMT. Nuclear run-off analyses suggest that the TGF-β regulates MMP2 expression at both transcriptional and post transcriptional levels by early increase in MMP2 transcription while at the same time increasing the half-life of the MMP2 mRNA in human fibroblasts [226] or through increased stability of the MMP2 proenzyme [227]. In addition, TGF-β has also been found to increase MMP-9 mRNA stability [227]. On the other hand CM et al. reported that the TGF-β decreased the synthesis of collagenase in fetal rat calvarial bone cells and human fibroblasts, whereas the TIMP mRNA levels were found to be increased [228]. Similarly, TGF-β1 also was found to suppress the transcriptional activity of stromelysin [229, 230]. Moreover, TGF-β inhibits ECM related gene expression by associating Fos- containing protein complex with the TGF-β1 inhibitory elements (TIE) localized in the promoter regions of stromelysin [231]. This is in accord with other studies which suggest that TGF-β could affect several MMPs that contain AP-1 sites in their promoter regions that are intricately involved in the regulation of MMPs expression [163].
TGF-β also reduces MMP-7 gene and protein expression and also the activity in mesangial cells cultured under high glucose ambience as indicated above, and the effect is abolished with TGF-β neutralizing antibody in STZ-induced diabetic rats in vivo as well [131]. A recent in vitro study reported that TGF-β induced Wnt1 expression, activated β-catenin and up-regulated Wnt target genes, such as, MMP7 and MMP9 [88]. In addition, TGF-β and Smad4 signaling pathway down-regulated renal ECM degradation in DN in rats with STZ induced diabetes, and this was accompanied by diminished MMP3 mRNA expression but increased both TIMP-1 and collagen III mRNA [155]. Another intriguing observation reported by MClennan SV and Martell SK indicated that glycation of matrix perturbs the balance between MMP2 and its inhibitors and may not be related to TGF-β activity in the accumulation of ECM at the target sites [183]. Overall, it seems that the effect of TGF-β on the MMPs’ and TIMPs’ expression is differential depending upon their regulation during different pathobiological processes.
MiRNA and MMPs
MiRNAs are a family of a small non-protein-encoding RNAs which control gene expression by inhibiting target mRNAs [232]. Several miRNAs, such as, miR-216A, miR-217, miR-192, miR-377, miR-21 and miR-29c are believed to play a significant role in the progression of DN [233]. Almost all of these miRNA are increased by HG or TGF-β in mouse or human mesangial cells [234–238]. MiRNA has also been found to take part in some of the biological processes, such as, ECM accumulation, resulting in aberrant tissue phenotype. Recently, Alvarez et al. demonstrated that an increase in miR-1207-5p, under hyperglycemia conditions, contributes to ECM accumulation in the kidney [233]. Under in vitro conditions, expression of miRNA1207-5p is markedly up-regulated in kidney cells with HG, while TGF-β stimulation enhanced the protein levels of major ECM glycoproteins [233]. Recently, Wang et al. elucidated that hyperglycemia-induced over-expression of miR-21 conceivably perturbs the balance between MMP and TIMP activities in DN [239]. Interestingly, in their studies they noted that the expression of miR-21 was significantly increased in KK-ay mice compared with control mice, which positively correlated with the expression of collagen IV, TIMP1 and FN; however, it negatively correlated with MMP9 protein levels. Furthermore, the antagmir-21 up-regulated the MMP9 protein levels, which suggests that miR-21 directly influence MMP9 expression. There is evidence that hyperglycemia induces an increase of TGF-β/Smad 2, which could promote miR-21 overexpression [240, 241]. Likewise, TIMP3 expression is also regulated by numerous of miRNAs including miR-21, miR-181b, miR-182, miR-216 and miR-221/2 [242]. Among various miRNAs, the miR-217 has been shown to modulate TIMP3 expression indirectly via SirT1 small interference RNA inhibition [242]. In addition, miR-21 was found to be increased both in human kidney biopsies from diabetic patients and in mesangial cells under HG conditions. Interestingly, miR-21 levels increase and TIMP3 level decrease were detected almost at the same time point under HG ambience [242]. Taken together, these data indicate that miRNA can directly or indirectly regulate MMP:TIMP balance, the perturbations in which would likely contribute to the aberrant ECM synthesis and renal fibrosis in DN.
CONCLUSIONS AND PERSPECTIVE
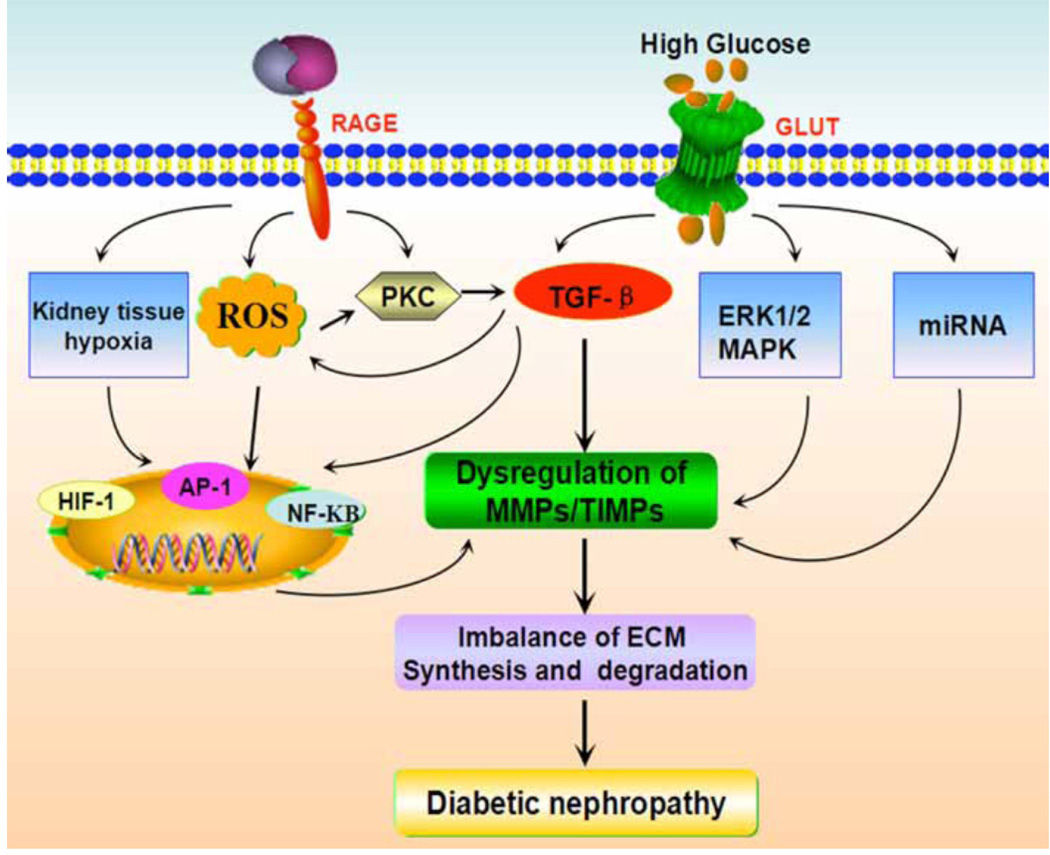
DN reflects a pathological hallmark of overproduction and expansion ECM in the kidney [117]. Therefore, the MMP family which can influence the balance between synthesis and degradation of ECM proteins may play a significant role in the progression of DN. The MMP superfamily includes of various proteases, including collagenases, gelatinases, stromelysins, matrilysins and membrane type- collagenases [25]. The research efforts are ongoing in discovering a detailed understanding of regulation of the MMPs/TIMP in hyperglycemic milieu. Experimental and clinical studies revealed that expression of MMPs/TIMPs in vitro or in vivo is somewhat discordant and inconsistent; importantly, the regulatory mechanism(s) that how hyperglycemia modulates MMPs and TIMPs have not yet been completely understood. These contradictions may be explained by the difference in samples obtained, cell types and the effect of varying therapies given to humans for the treatment of diabetes, as well as the duration of DN that impact MMPs expression [243]. Certainly, the influence of hyperglycemia on MMP and TIMP expression effectively reinforce the degradation arm of MMP/TIMP system that ultimately lead to increase ECM accumulation and pathological disorders, resulting in reno-vascular complications of diabetes [243]. As shown in (Fig. 1), under diabetic condition, HG and AGE induce hypoxia, cellular ROS level, activation of PKC and TGF-β signaling pathway, leading to dysregulation of MMT/TIMPs expression via various transcription factor(s). In addition, HG can also regulate MMT/TIMPs expression through ERK1/2 MAPK and miRNA, which results in imbalance of ECM synthesis and degradation, leading to the progression of DN. However, as discussed in this review, the role of MMPs in DN is intriguingly complex and the mechanisms are still elusive. Continued research may be required to fully understand the underlying genetic and molecular regulation of MMPs in DN, and undoubtedly it will help us further expound the pathogenesis of DN.
Fig. (1). Regulatory mechanism involving MMPs in the diabetic nephropathy.
Schematic drawing depicting the conceivable regulatory mechanism(s) in MMPs/TIMPs regulation by high glucose and advanced glycation end products (AGEs). Hyperglycemia and AGEs may induce ROS and TGF-β as well kidney tissue hypoxia. The ROS, hypoxia and high glucose lead to the activation of NF-κB, AP-1 and HIF-1 and thereby transcription of some of the MMPs. Inside the cells, the AGEs and ROS can activate PKC, which in turn can modulate the expression of TGF-β. TGF-β stimulates synthesis of matrix glycoproteins and modulation of MMPs/TIMPS via transcriptional mechanisms. High glucose also can active ERK1/2MAPK signaling and miRNAs, leading to alter MMPs expression. In addition, the mechanical stretch, besides dysregulation of MMPs and TIMPs, lead to increased extracellular matrix synthesis and its accumulation and decreased degradation, the hallmark features of diabetic nephropathy.
Abbreviations: MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinase; ECM, extracellular matrix; AP-1, activator protein 1; NF-κB, nuclear factor kappa B; ROS, reactive oxygen species; TGF-β, transforming growth factor β; ERK1/2, extracellular-signal-regulated kinases1/2; MAPKs, mitogen-activated protein kinases.
Consensus of recent studies suggest that MMPs may serve as sensitive and clinically useful biomarkers for predicting pathological changes seen in DN. In future, MMP9 may become a new biomarker of renal injury like Neutrophil gelatinase- associated lipocalin (NGAL). A research study with Type 1 diabetic patients has indicated that the changes in urine NGAL and MMP9 are concomitant, and both of them were positively correlated with albuminuria [244]. Urine MMP2 concentrations of type 1 diabetic subjects were correlated with several clinic parameters which predict increased risk for DN (renal hyperfiltration, microalbuminuria, duration of disease, and higher hemoglobin A1c levels) [245]. Furthermore, another study suggested the use of MMP-inhibitors as preventive therapeutic agents for patients with Alport syndrome before the onset of kidney disease could be advantageous, but if delayed from this early time frame the inhibition may result in detrimental progression of kidney injury [246]. If similar mechanisms are operative in the development of DN, then very early intervention with anti-MMP treatment in DN patients before the onset of microalbuminuria could prevent or retard the progression of DN [243]. Certainly, further studies are needed to define the redundancies and specificities of MMP regulators along with robust research efforts to fully comprehend the mechanism(s) involved in MMPs’ regulation under hyperglycemia ambience in concert with a major effort to search for the drugs that could ultimately lead to potentially effective, and efficient therapeutic measures.
ACKNOWLEDGEMENTS
This work was supported by grants from the Creative Research Group Fund of the National Foundation Committee of Natural Sciences of China (81270812, and 81370832), the Doctoral Fund of Ministry of Education of China (20110162110012), the Furong Scholars Fund from Hunan Province Education Department, Program for Changjiang Scholars and Innovative Research Team in University (IRT1195), and a grant from the National Institutes of Health (DK-60635).
DISCLOSURES
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Rychlik I, Miltenberger-Miltenyi G, Ritz E. The drama of the continuous increase in end-stage renal failure in patients with type II diabetes mellitus. Nephrol. Dial. Transplant. 1998;13(Suppl 8):6–10. doi: 10.1093/ndt/13.suppl_8.6. [DOI] [PubMed] [Google Scholar]
- 2.Marcelli D, Spotti D, Conte F, Limido A, Lonati F, Malberti F, Locatelli F. Prognosis of diabetic patients on dialysis: analysis of Lombardy Registry data. Nephrol. Dial. Transplant. 1995;10(10):1895–1900. [PubMed] [Google Scholar]
- 3.Bruno G, Merletti F, Biggeri A, Bargero G, Ferrero S, Pagano G, Cavallo Perin P, Casale Monferrato S. Progression to overt nephropathy in type 2 diabetes: the Casale Monferrato Study. Diabetes Care. 2003;26(7):2150–2155. doi: 10.2337/diacare.26.7.2150. [DOI] [PubMed] [Google Scholar]
- 4.Boright AP, Paterson AD, Mirea L, Bull SB, Mowjoodi A, Scherer SW, Zinman B, Group DER. Genetic variation at the ACE gene is associated with persistent microalbuminuria and severe nephropathy in type 1 diabetes: the DCCT/EDIC Genetics Study. Diabetes. 2005;54(4):1238–1244. doi: 10.2337/diabetes.54.4.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fagot-Campagna A, Nelson RG, Knowler WC, Pettitt DJ, Robbins DC, Go O, Welty TK, Lee ET, Howard BV. Plasma lipoproteins and the incidence of abnormal excretion of albumin in diabetic American Indians: the Strong Heart Study. Diabetologia. 1998;41(9):1002–1009. doi: 10.1007/s001250051023. [DOI] [PubMed] [Google Scholar]
- 6.Hostetter TH. Hyperfiltration and glomerulosclerosis. Semin. Nephrol. 2003;23(2):194–199. doi: 10.1053/anep.2003.50017. [DOI] [PubMed] [Google Scholar]
- 7.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N. Engl. J. Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 8.Robert Turne. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 9.Dalla Vestra M, Saller A, Bortoloso E, Mauer M, Fioretto P. Structural involvement in type 1 and type 2 diabetic nephropathy. Diabetes Metab. 2000;26(Suppl 4):8–14. [PubMed] [Google Scholar]
- 10.Osterby R. Glomerular structural changes in type 1 (insulin-dependent) diabetes mellitus: causes, consequences, and prevention. Diabetologia. 1992;35(9):803–812. doi: 10.1007/BF00399925. [DOI] [PubMed] [Google Scholar]
- 11.Osterby R, Gall MA, Schmitz A, Nielsen FS, Nyberg G, Parving HH. Glomerular structure and function in proteinuric type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36(10):1064–1070. doi: 10.1007/BF02374500. [DOI] [PubMed] [Google Scholar]
- 12.Mogensen CE, Christensen CK, Vittinghus E. The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes. 1983;32(Suppl 2):64–78. doi: 10.2337/diab.32.2.s64. [DOI] [PubMed] [Google Scholar]
- 13.Tervaert TW, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, Ferrario F, Fogo AB, Haas M, de Heer E, Joh K, Noel LH, Radhakrishnan J, Seshan SV, Bajema IM, Bruijn JA. Pathologic classification of diabetic nephropathy. J. Am. Soc. Nephrol. 2010;21(4):556–563. doi: 10.1681/ASN.2010010010. [DOI] [PubMed] [Google Scholar]
- 14.Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC. Structural-functional relationships in diabetic nephropathy. J. Clin. Invest. 1984;74(4):1143–1155. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolset SO, Reinholt FP, Jenssen T. Diabetic nephropathy and extracellular matrix. J. Histochem. Cytochem. 2012;60(12):976–986. doi: 10.1369/0022155412465073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanwar YS, Sun L, Xie P, Liu FY, Chen S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu. Rev. Pathol. 2011;6:395–423. doi: 10.1146/annurev.pathol.4.110807.092150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bode W, Gomis-Ruth FX, Stockler W. Astacins, serralysins, snake venom and matrix metalloproteinases exhibit identical zinc-binding environments (HEXXHXXGXXH and Met-turn) and topologies and should be grouped into a common family, the 'metzincins'. FEBS Lett. 1993;331(1–2):134–140. doi: 10.1016/0014-5793(93)80312-i. [DOI] [PubMed] [Google Scholar]
- 18.Stocker W, Grams F, Baumann U, Reinemer P, Gomis-Ruth FX, McKay DB, Bode W. The metzincins--topological and sequential relations between the astacins, adamalysins, serralysins, and matrixins (collagenases) define a superfamily of zinc-peptidases. Protein Sci. 1995;4(5):823–840. doi: 10.1002/pro.5560040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stocker W, Ng M, Auld DS. Fluorescent oligopeptide substrates for kinetic characterization of the specificity of Astacus protease. Biochemistry. 1990;29(45):10418–10425. doi: 10.1021/bi00497a018. [DOI] [PubMed] [Google Scholar]
- 20.Murphy GJ, Murphy G, Reynolds JJ. The origin of matrix metalloproteinases and their familial relationships. FEBS Lett. 1991;289(1):4–7. doi: 10.1016/0014-5793(91)80895-a. [DOI] [PubMed] [Google Scholar]
- 21.Bode W, Gomis-Ruth FX, Huber R, Zwilling R, Stocker W. Structure of astacin and implications for activation of astacins and zinc-ligation of collagenases. Nature. 1992;358(6382):164–167. doi: 10.1038/358164a0. [DOI] [PubMed] [Google Scholar]
- 22.Jiang W, Bond JS. Families of metalloendopeptidases and their relationships. FEBS Lett. 1992;312(2–3):110–114. doi: 10.1016/0014-5793(92)80916-5. [DOI] [PubMed] [Google Scholar]
- 23.Stocker W, Gomis-Ruth FX, Bode W, Zwilling R. Implications of the three-dimensional structure of astacin for the structure and function of the astacin family of zinc-endopeptidases. Eur. J. Biochem. 1993;214(1):215–231. doi: 10.1111/j.1432-1033.1993.tb17915.x. [DOI] [PubMed] [Google Scholar]
- 24.Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids. 2011;41(2):271–290. doi: 10.1007/s00726-010-0689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catania JM, Chen G, Parrish AR. Role of matrix metalloproteinases in renal pathophysiologies. Am. J. Physiol. Renal Physiol. 2007;292(3):F905–F911. doi: 10.1152/ajprenal.00421.2006. [DOI] [PubMed] [Google Scholar]
- 26.Tan RJ, Liu Y. Matrix metalloproteinases in kidney homeostasis and diseases. Am. J. Physiol. Renal Physiol. 2012;302(11):F1351–F1361. doi: 10.1152/ajprenal.00037.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy G, Nguyen Q, Cockett MI, Atkinson SJ, Allan JA, Knight CG, Willenbrock F, Docherty AJ. Assessment of the role of the fibronectin-like domain of gelatinase A by analysis of a deletion mutant. J. Biol. Chem. 1994;269(9):6632–6636. [PubMed] [Google Scholar]
- 28.Shipley JM, Doyle GA, Fliszar CJ, Ye QZ, Johnson LL, Shapiro SD, Welgus HG, Senior RM. The structural basis for the elastolytic activity of the 92-kDa and 72-kDa gelatinases. Role of the fibronectin type II-like repeats. J. Biol. Chem. 1996;271(8):4335–4341. doi: 10.1074/jbc.271.8.4335. [DOI] [PubMed] [Google Scholar]
- 29.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park HI, Ni J, Gerkema FE, Liu D, Belozerov VE, Sang QX. Identification and characterization of human endometase (Matrix metalloproteinase-26) from endometrial tumor. J. Biol. Chem. 2000;275(27):20540–20544. doi: 10.1074/jbc.M002349200. [DOI] [PubMed] [Google Scholar]
- 31.Itoh Y, Kajita M, Kinoh H, Mori H, Okada A, Seiki M. Membrane type 4 matrix metalloproteinase (MT4-MMP, MMP-17) is a glycosylphosphatidylinositol-anchored proteinase. J. Biol. Chem. 1999;274(48):34260–34266. doi: 10.1074/jbc.274.48.34260. [DOI] [PubMed] [Google Scholar]
- 32.McCawley LJ, Matrisian LM. Matrix metalloproteinases: they're not just for matrix anymore! Curr. Opin. Cell Biol. 2001;13(5):534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- 33.Gross J. How tadpoles lose their tails: path to discovery of the first matrix metalloproteinase. Matrix Biol. 2004;23(1):3–13. doi: 10.1016/j.matbio.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Van Lint P, Libert C. Matrix metalloproteinase-8: cleavage can be decisive. Cytokine Growth Factor Rev. 2006;17(4):217–223. doi: 10.1016/j.cytogfr.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Leeman MF, Curran S, Murray GI. The structure, regulation, and function of human matrix metalloproteinase-13. Crit. Rev. Biochem. Mol. Biol. 2002;37(3):149–166. doi: 10.1080/10409230290771483. [DOI] [PubMed] [Google Scholar]
- 36.Sopata I, Dancewicz AM. Presence of a gelatin-specific proteinase and its latent form in human leucocytes. Biochim. Biophys. Acta. 1974;370(2):510–523. doi: 10.1016/0005-2744(74)90112-0. [DOI] [PubMed] [Google Scholar]
- 37.Templeton NS, Stetler-Stevenson WG. Identification of a basal promoter for the human Mr 72,000 type IV collagenase gene and enhanced expression in a highly metastatic cell line. Cancer Res. 1991;51(22):6190–6193. [PubMed] [Google Scholar]
- 38.Chin JR, Murphy G, Werb Z. Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase. Biosynthesis, isolation, characterization, and substrates. J. Biol. Chem. 1985;260(22):12367–12376. [PubMed] [Google Scholar]
- 39.Muller D, Quantin B, Gesnel MC, Millon-Collard R, Abecassis J, Breathnach R. The collagenase gene family in humans consists of at least four members. Biochem. J. 1988;253(1):187–192. doi: 10.1042/bj2530187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Basset P, Bellocq JP, Wolf C, Stoll I, Hutin P, Limacher JM, Podhajcer OL, Chenard MP, Rio MC, Chambon P. A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature. 1990;348(6303):699–704. doi: 10.1038/348699a0. [DOI] [PubMed] [Google Scholar]
- 41.Woessner JF, Jr, Taplin CJ. Purification and properties of a small latent matrix metalloproteinase of the rat uterus. J. Biol. Chem. 1988;263(32):16918–16925. [PubMed] [Google Scholar]
- 42.de Coignac AB, Elson G, Delneste Y, Magistrelli G, Jeannin P, Aubry JP, Berthier O, Schmitt D, Bonnefoy JY, Gauchat JF. Cloning of MMP-26. A novel matrilysin-like proteinase. Eur. J. Biochem. 2000;267(11):3323–3329. doi: 10.1046/j.1432-1327.2000.01363.x. [DOI] [PubMed] [Google Scholar]
- 43.d'Ortho MP, Will H, Atkinson S, Butler G, Messent A, Gavrilovic J, Smith B, Timpl R, Zardi L, Murphy G. Membrane-type matrix metalloproteinases 1 and 2 exhibit broad-spectrum proteolytic capacities comparable to many matrix metalloproteinases. Eur. J. Biochem. 1997;250(3):751–757. doi: 10.1111/j.1432-1033.1997.00751.x. [DOI] [PubMed] [Google Scholar]
- 44.Barbolina MV, Stack MS. Membrane type 1-matrix metalloproteinase: substrate diversity in pericellular proteolysis. Semin. Cell Dev. Biol. 2008;19(1):24–33. doi: 10.1016/j.semcdb.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takino T, Sato H, Shinagawa A, Seiki M. Identification of the second membrane-type matrix metalloproteinase (MT-MMP-2) gene from a human placenta cDNA library. MT-MMPs form a unique membrane-type subclass in the MMP family. J. Biol. Chem. 1995;270(39):23013–23020. doi: 10.1074/jbc.270.39.23013. [DOI] [PubMed] [Google Scholar]
- 46.Matsumoto S, Katoh M, Saito S, Watanabe T, Masuho Y. Identification of soluble type of membrane-type matrix metalloproteinase-3 formed by alternatively spliced mRNA. Biochim. Biophys. Acta. 1997;1354(2):159–170. doi: 10.1016/s0167-4781(97)00120-6. [DOI] [PubMed] [Google Scholar]
- 47.Llano E, Pendas AM, Freije JP, Nakano A, Knauper V, Murphy G, Lopez-Otin C. Identification and characterization of human MT5-MMP, a new membrane-bound activator of progelatinase a overexpressed in brain tumors. Cancer Res. 1999;59(11):2570–2576. [PubMed] [Google Scholar]
- 48.Pei D. Leukolysin/MMP25/MT6-MMP: a novel matrix metalloproteinase specifically expressed in the leukocyte lineage. Cell Res. 1999;9(4):291–303. doi: 10.1038/sj.cr.7290028. [DOI] [PubMed] [Google Scholar]
- 49.Pendas AM, Knauper V, Puente XS, Llano E, Mattei MG, Apte S, Murphy G, Lopez-Otin C. Identification and characterization of a novel human matrix metalloproteinase with unique structural characteristics, chromosomal location, and tissue distribution. J. Biol. Chem. 1997;272(7):4281–4286. doi: 10.1074/jbc.272.7.4281. [DOI] [PubMed] [Google Scholar]
- 50.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ. Res. 2003;92(8):827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 51.Kadoglou NP, Daskalopoulou SS, Perrea D, Liapis CD. Matrix metalloproteinases and diabetic vascular complications. Angiology. 2005;56(2):173–189. doi: 10.1177/000331970505600208. [DOI] [PubMed] [Google Scholar]
- 52.Hernandez-Perez M, Mahalingam M. Matrix metalloproteinases in health and disease: insights from dermatopathology. Am. J. Dermatopathol. 2012;34(6):565–579. doi: 10.1097/DAD.0b013e31821e8744. [DOI] [PubMed] [Google Scholar]
- 53.Kerkela E, Saarialho-Kere U. Matrix metalloproteinases in tumor progression: focus on basal and squamous cell skin cancer. Exp. Dermatol. 2003;12(2):109–125. doi: 10.1034/j.1600-0625.2003.120201.x. [DOI] [PubMed] [Google Scholar]
- 54.Banda MJ, Werb Z. Mouse macrophage elastase. Purification and characterization as a metalloproteinase. Biochem. J. 1981;193(2):589–605. doi: 10.1042/bj1930589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shapiro SD, Griffin GL, Gilbert DJ, Jenkins NA, Copeland NG, Welgus HG, Senior RM, Ley TJ. Molecular cloning, chromosomal localization, and bacterial expression of a murine macrophage metalloelastase. J. Biol. Chem. 1992;267(7):4664–4671. [PubMed] [Google Scholar]
- 56.Impola U, Toriseva M, Suomela S, Jeskanen L, Hieta N, Jahkola T, Grenman R, Kahari VM, Saarialho-Kere U. Matrix metalloproteinase-19 is expressed by proliferating epithelium but disappears with neoplastic dedifferentiation. Int. J. Cancer. 2003;103(6):709–716. doi: 10.1002/ijc.10902. [DOI] [PubMed] [Google Scholar]
- 57.Boyd S, Virolainen S, Parssinen J, Skoog T, van Hogerlinden M, Latonen L, Kyllonen L, Toftgard R, Saarialho-Kere U. MMP-10 (Stromelysin-2) and MMP-21 in human and murine squamous cell cancer. Exp. Dermatol. 2009;18(12):1044–1052. doi: 10.1111/j.1600-0625.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- 58.Saarialho-Kere U, Kerkela E, Jahkola T, Suomela S, Keski-Oja J, Lohi J. Epilysin (MMP-28) expression is associated with cell proliferation during epithelial repair. J. Invest. Dermatol. 2002;119(1):14–21. doi: 10.1046/j.1523-1747.2002.01790.x. [DOI] [PubMed] [Google Scholar]
- 59.Limb GA, Matter K, Murphy G, Cambrey AD, Bishop PN, Morris GE, Khaw PT. Matrix metalloproteinase-1 associates with intracellular organelles and confers resistance to lamin A/C degradation during apoptosis. Am. J. Pathol. 2005;166(5):1555–1563. doi: 10.1016/S0002-9440(10)62371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kwan JA, Schulze CJ, Wang W, Leon H, Sariahmetoglu M, Sung M, Sawicka J, Sims DE, Sawicki G, Schulz R. Matrix metalloproteinase-2 (MMP-2) is present in the nucleus of cardiac myocytes and is capable of cleaving poly (ADP-ribose) polymerase (PARP) in vitro. FASEB J. 2004;18(6):690–692. doi: 10.1096/fj.02-1202fje. [DOI] [PubMed] [Google Scholar]
- 61.Luo D, Mari B, Stoll I, Anglard P. Alternative splicing and promoter usage generates an intracellular stromelysin 3 isoform directly translated as an active matrix metalloproteinase. J. Biol. Chem. 2002;277(28):25527–25536. doi: 10.1074/jbc.M202494200. [DOI] [PubMed] [Google Scholar]
- 62.Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. The FEBS journal. 2011;278(1):16–27. doi: 10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]
- 63.Vincenti MP, Brinckerhoff CE. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002;4(3):157–164. doi: 10.1186/ar401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Airola K, Karonen T, Vaalamo M, Lehti K, Lohi J, Kariniemi AL, Keski-Oja J, Saarialho-Kere UK. Expression of collagenases-1 and-3 and their inhibitors TIMP-1 and-3 correlates with the level of invasion in malignant melanomas. Br. J. Cancer. 1999;80(5–6):733–743. doi: 10.1038/sj.bjc.6690417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, Arthur MJ. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J. Clin. Invest. 1998;102(3):538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gutierrez-Fernandez A, Fueyo A, Folgueras AR, Garabaya C, Pennington CJ, Pilgrim S, Edwards DR, Holliday DL, Jones JL, Span PN, Sweep FC, Puente XS, Lopez-Otin C. Matrix metalloproteinase-8 functions as a metastasis suppressor through modulation of tumor cell adhesion and invasion. Cancer Res. 2008;68(8):2755–2763. doi: 10.1158/0008-5472.CAN-07-5154. [DOI] [PubMed] [Google Scholar]
- 67.Balbin M, Fueyo A, Knauper V, Pendas AM, Lopez JM, Jimenez MG, Murphy G, Lopez-Otin C. Collagenase 2 (MMP-8) expression in murine tissue-remodeling processes. Analysis of its potential role in postpartum involution of the uterus. J. Biol. Chem. 1998;273(37):23959–23968. doi: 10.1074/jbc.273.37.23959. [DOI] [PubMed] [Google Scholar]
- 68.Kudo Y, Iizuka S, Yoshida M, Tsunematsu T, Kondo T, Subarnbhesaj A, Deraz EM, Siriwardena SB, Tahara H, Ishimaru N, Ogawa I, Takata T. Matrix metalloproteinase-13 (MMP-13) directly and indirectly promotes tumor angiogenesis. J. Biol. Chem. 2012;287(46):38716–38728. doi: 10.1074/jbc.M112.373159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front. Biosci. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 70.Nguyen M, Arkell J, Jackson CJ. Human endothelial gelatinases and angiogenesis. Int. J. Biochem. Cell Biol. 2001;33(10):960–970. doi: 10.1016/s1357-2725(01)00007-3. [DOI] [PubMed] [Google Scholar]
- 71.Nagase H. Cell surface activation of progelatinase A (proMMP-2) and cell migration. Cell Res. 1998;8(3):179–186. doi: 10.1038/cr.1998.18. [DOI] [PubMed] [Google Scholar]
- 72.Schonbeck U, Mach F, Libby P. Generation of biologically active IL-1 beta by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1 beta processing. J. Immunol. 1998;161(7):3340–3346. [PubMed] [Google Scholar]
- 73.Illman SA, Lehti K, Keski-Oja J, Lohi J. Epilysin (MMP-28) induces TGF-beta mediated epithelial to mesenchymal transition in lung carcinoma cells. J. Cell Sci. 2006;119(Pt 18):3856–3865. doi: 10.1242/jcs.03157. [DOI] [PubMed] [Google Scholar]
- 74.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer. 2002;2(3):161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 75.Wang X, Zhou Y, Tan R, Xiong M, He W, Fang L, Wen P, Jiang L, Yang J. Mice lacking the matrix metalloproteinase-9 gene reduce renal interstitial fibrosis in obstructive nephropathy. Am. J. Physiol. Renal Physiol. 2010;299(5):F973–F982. doi: 10.1152/ajprenal.00216.2010. [DOI] [PubMed] [Google Scholar]
- 76.Cornish TC, Bagnasco SM, Macgregor AM, Lu J, Selvin E, Halushka MK. Glomerular protein levels of matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 are lower in diabetic subjects. J. Histochem. Cytochem. 2009;57(11):995–1001. doi: 10.1369/jhc.2009.954107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Uchio-Yamada K, Manabe N, Goto Y, Anann S, Yamamoto Y, Takano K, Ogura A, Matsuda J. Decreased expression of matrix metalloproteinases and tissue inhibitors of metalloproteinase in the kidneys of hereditary nephrotic (ICGN) mice. J. Vet. Med. Sci. 2005;67(1):35–41. doi: 10.1292/jvms.67.35. [DOI] [PubMed] [Google Scholar]
- 78.Yamashita CM, Dolgonos L, Zemans RL, Young SK, Robertson J, Briones N, Suzuki T, Campbell MN, Gauldie J, Radisky DC, Riches DW, Yu G, Kaminski N, McCulloch CA, Downey GP. Matrix metalloproteinase 3 is a mediator of pulmonary fibrosis. Am. J. Pathol. 2011;179(4):1733–1745. doi: 10.1016/j.ajpath.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Craig VJ, Quintero PA, Fyfe SE, Patel AS, Knolle MD, Kobzik L, Owen CA. Profibrotic activities for matrix metalloproteinase-8 during bleomycin-mediated lung injury. J. Immunol. 2013;190(8):4283–4296. doi: 10.4049/jimmunol.1201043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moinzadeh P, Krieg T, Hellmich M, Brinckmann J, Neumann E, Muller-Ladner U, Kreuter A, Dumitrescu D, Rosenkranz S, Hunzelmann N. Elevated MMP-7 levels in patients with systemic sclerosis: correlation with pulmonary involvement. Exp. Dermatol. 2011;20(9):770–773. doi: 10.1111/j.1600-0625.2011.01321.x. [DOI] [PubMed] [Google Scholar]
- 81.Manicone AM, Huizar I, McGuire JK. Matrilysin (Matrix Metalloproteinase-7) regulates anti-inflammatory and antifibrotic pulmonary dendritic cells that express CD103 (alpha(E)beta(7)-integrin) Am. J. Pathol. 2009;175(6):2319–2331. doi: 10.2353/ajpath.2009.090101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chan MF, Li J, Bertrand A, Casbon AJ, Lin JH, Maltseva I, Werb Z. Protective effects of matrix metalloproteinase-12 following corneal injury. J. Cell Sci. 2013;126(Pt 17):3948–3960. doi: 10.1242/jcs.128033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu G, Kovkarova-Naumovski E, Jara P, Parwani A, Kass D, Ruiz V, Lopez-Otin C, Rosas IO, Gibson KF, Cabrera S, Ramirez R, Yousem SA, Richards TJ, Chensny LJ, Selman M, Kaminski N, Pardo A. Matrix metalloproteinase-19 is a key regulator of lung fibrosis in mice and humans. Am. J. Respir. Crit. Care Med. 2012;186(8):752–762. doi: 10.1164/rccm.201202-0302OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gharib SA, Johnston LK, Huizar I, Birkland TP, Hanson J, Wang Y, Parks WC, Manicone AM. MMP28 promotes macrophage polarization toward M2 cells and augments pulmonary fibrosis. J. Leukoc. Biol. 2014;95(1):9–18. doi: 10.1189/jlb.1112587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nkyimbeng T, Ruppert C, Shiomi T, Dahal B, Lang G, Seeger W, Okada Y, D'Armiento J, Gunther A. Pivotal role of matrix metalloproteinase 13 in extracellular matrix turnover in idiopathic pulmonary fibrosis. PLoS One. 2013;8(9):e73279. doi: 10.1371/journal.pone.0073279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pendas AM, Balbin M, Llano E, Jimenez MG, Lopez-Otin C. Structural analysis and promoter characterization of the human collagenase-3 gene (MMP13) Genomics. 1997;40(2):222–233. doi: 10.1006/geno.1996.4554. [DOI] [PubMed] [Google Scholar]
- 87.Westermarck J, Kahari VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13(8):781–792. [PubMed] [Google Scholar]
- 88.Wang D, Dai C, Li Y, Liu Y. Canonical Wnt/beta-catenin signaling mediates transforming growth factor-beta1-driven podocyte injury and proteinuria. Kidney Int. 2011;80(11):1159–1169. doi: 10.1038/ki.2011.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.He W, Tan RJ, Li Y, Wang D, Nie J, Hou FF, Liu Y. Matrix metalloproteinase-7 as a surrogate marker predicts renal Wnt/beta-catenin activity in CKD. J. Am. Soc. Nephrol. 2012;23(2):294–304. doi: 10.1681/ASN.2011050490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ye S. Influence of matrix metalloproteinase genotype on cardiovascular disease susceptibility and outcome. Cardiovasc. Res. 2006;69(3):636–645. doi: 10.1016/j.cardiores.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 91.Kure M, Pezzolesi MG, Poznik GD, Katavetin P, Skupien J, Dunn JS, Mychaleckyj JC, Warram JH, Krolewski AS. Genetic variation in the matrix metalloproteinase genes and diabetic nephropathy in type 1 diabetes. Mol. Genet. Metab. 2011;103(1):60–65. doi: 10.1016/j.ymgme.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fanjul-Fernandez M, Folgueras AR, Cabrera S, Lopez-Otin C. Matrix metalloproteinases: evolution, gene regulation and functional analysis in mouse models. Biochim. Biophys. Acta. 2010;1803(1):3–19. doi: 10.1016/j.bbamcr.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 93.Akool el S, Kleinert H, Hamada FM, Abdelwahab MH, Forstermann U, Pfeilschifter J, Eberhardt W. Nitric oxide increases the decay of matrix metalloproteinase 9 mRNA by inhibiting the expression of mRNA-stabilizing factor HuR. Mol. Cell. Biol. 2003;23(14):4901–4916. doi: 10.1128/MCB.23.14.4901-4916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vincenti MP. The matrix metalloproteinase (MMP) and tissue inhibitor of metalloproteinase (TIMP) genes. Transcriptional and posttranscriptional regulation, signal transduction and cell-type-specific expression. Methods Mol. Biol. 2001;151:121–148. doi: 10.1385/1-59259-046-2:121. [DOI] [PubMed] [Google Scholar]
- 95.Rydziel S, Delany AM, Canalis E. AU-rich elements in the collagenase 3 mRNA mediate stabilization of the transcript by cortisol in osteoblasts. J. Biol. Chem. 2004;279(7):5397–5404. doi: 10.1074/jbc.M311984200. [DOI] [PubMed] [Google Scholar]
- 96.Yang Z, Zhang Y, Wang L. A feedback inhibition between miRNA-127 and TGFbeta/c-Jun cascade in HCC cell migration via MMP13. PLoS One. 2013;8(6):e65256. doi: 10.1371/journal.pone.0065256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lin HY, Chiang CH, Hung WC. STAT3 upregulates miR-92a to inhibit RECK expression and to promote invasiveness of lung cancer cells. Br. J. Cancer. 2013;109(3):731–738. doi: 10.1038/bjc.2013.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): Positive and negative regulators in tumor cell adhesion. Semin. Cancer Biol. 2010;20(3):161–168. doi: 10.1016/j.semcancer.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006;69(3):562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 100.Eddy AA, Kim H, Lopez-Guisa J, Oda T, Soloway PD. Interstitial fibrosis in mice with overload proteinuria: deficiency of TIMP-1 is not protective. Kidney Int. 2000;58(2):618–628. doi: 10.1046/j.1523-1755.2000.00208.x. [DOI] [PubMed] [Google Scholar]
- 101.Thomas GT, Lewis MP, Speight PM. Matrix metalloproteinases and oral cancer. Oral Oncol. 1999;35(3):227–233. doi: 10.1016/s1368-8375(99)00004-4. [DOI] [PubMed] [Google Scholar]
- 102.Lee MH, Rapti M, Knauper V, Murphy G. Threonine 98, the pivotal residue of tissue inhibitor of metalloproteinases (TIMP)-1 in metalloproteinase recognition. J. Biol. Chem. 2004;279(17):17562–17569. doi: 10.1074/jbc.M312589200. [DOI] [PubMed] [Google Scholar]
- 103.Skiles JW, Gonnella NC, Jeng AY. The design, structure, and therapeutic application of matrix metalloproteinase inhibitors. Curr. Med. Chem. 2001;8(4):425–474. doi: 10.2174/0929867013373417. [DOI] [PubMed] [Google Scholar]
- 104.Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim. Biophys. Acta. 2010;1803(1):55–71. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bond M, Murphy G, Bennett MR, Amour A, Knauper V, Newby AC, Baker AH. Localization of the death domain of tissue inhibitor of metalloproteinase-3 to the N terminus. Metalloproteinase inhibition is associated with proapoptotic activity. J. Biol. Chem. 2000;275(52):41358–41363. doi: 10.1074/jbc.M007929200. [DOI] [PubMed] [Google Scholar]
- 106.Hoegy SE, Oh HR, Corcoran ML, Stetler-Stevenson WG. Tissue inhibitor of metalloproteinases-2 (TIMP-2) suppresses TKR-growth factor signaling independent of metalloproteinase inhibition. J. Biol. Chem. 2001;276(5):3203–3214. doi: 10.1074/jbc.M008157200. [DOI] [PubMed] [Google Scholar]
- 107.Khokha R, Waterhouse P, Yagel S, Lala PK, Overall CM, Norton G, Denhardt DT. Antisense RNA-induced reduction in murine TIMP levels confers oncogenicity on Swiss 3T3 cells. Science. 1989;243(4893):947–950. doi: 10.1126/science.2465572. [DOI] [PubMed] [Google Scholar]
- 108.Alexander CM, Werb Z. Targeted disruption of the tissue inhibitor of metalloproteinases gene increases the invasive behavior of primitive mesenchymal cells derived from embryonic stem cells in vitro. J. Cell Biol. 1992;118(3):727–739. doi: 10.1083/jcb.118.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Martin DC, Fowlkes JL, Babic B, Khokha R. Insulin-like growth factor II signaling in neoplastic proliferation is blocked by transgenic expression of the metalloproteinase inhibitor TIMP-1. J. Cell Biol. 1999;146(4):881–892. doi: 10.1083/jcb.146.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tang WW, Feng L, Xia Y, Wilson CB. Extracellular matrix accumulation in immune-mediated tubulointerstitial injury. Kidney Int. 1994;45(4):1077–1084. doi: 10.1038/ki.1994.144. [DOI] [PubMed] [Google Scholar]
- 111.Wu K, Setty S, Mauer SM, Killen P, Nagase H, Michael AF, Tsilibary EC. Altered kidney matrix gene expression in early stages of experimental diabetes. Acta Anat. (Basel) 1997;158(3):155–165. doi: 10.1159/000147926. [DOI] [PubMed] [Google Scholar]
- 112.Cruzado JM, Lloberas N, Torras J, Riera M, Fillat C, Herrero-Fresneda I, Aran JM, Alperovich G, Vidal A, Grinyo JM. Regression of advanced diabetic nephropathy by hepatocyte growth factor gene therapy in rats. Diabetes. 2004;53(4):1119–1127. doi: 10.2337/diabetes.53.4.1119. [DOI] [PubMed] [Google Scholar]
- 113.Kassiri Z, Oudit GY, Kandalam V, Awad A, Wang X, Ziou X, Maeda N, Herzenberg AM, Scholey JW. Loss of TIMP3 enhances interstitial nephritis and fibrosis. J. Am. Soc. Nephrol. 2009;20(6):1223–1235. doi: 10.1681/ASN.2008050492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang Z, Famulski K, Lee J, Das SK, Wang X, Halloran P, Oudit GY, Kassiri Z. TIMP2 and TIMP3 have divergent roles in early renal tubulointerstitial injury. Kidney Int. 2014;85(1):82–93. doi: 10.1038/ki.2013.225. [DOI] [PubMed] [Google Scholar]
- 115.Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J. Cell Sci. 2002;115(Pt 19):3719–3727. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- 116.Bruijn JA, Roos A, de Geus B, de Heer E. Transforming growth factor-beta and the glomerular extracellular matrix in renal pathology. J. Lab. Clin. Med. 1994;123(1):34–47. [PubMed] [Google Scholar]
- 117.Mason RM, Wahab NA. Extracellular matrix metabolism in diabetic nephropathy. J. Am. Soc. Nephrol. 2003;14(5):1358–1373. doi: 10.1097/01.asn.0000065640.77499.d7. [DOI] [PubMed] [Google Scholar]
- 118.Marti HP. The role of matrix metalloproteinases in the activation of mesangial cells. Transpl. Immunol. 2002;9(2–4):97–100. doi: 10.1016/s0966-3274(02)00006-0. [DOI] [PubMed] [Google Scholar]
- 119.Turck J, Pollock AS, Lee LK, Marti HP, Lovett DH. Matrix metalloproteinase 2 (gelatinase A) regulates glomerular mesangial cell proliferation and differentiation. J. Biol. Chem. 1996;271(25):15074–15083. doi: 10.1074/jbc.271.25.15074. [DOI] [PubMed] [Google Scholar]
- 120.Fowlkes JL, Thrailkill KM, Serra DM, Suzuki K, Nagase H. Matrix metalloproteinases as insulin-like growth factor binding protein-degrading proteinases. Prog. Growth Factor Res. 1995;6(2–4):255–263. doi: 10.1016/0955-2235(95)00017-8. [DOI] [PubMed] [Google Scholar]
- 121.Ii M, Yamamoto H, Adachi Y, Maruyama Y, Shinomura Y. Role of matrix metalloproteinase-7 (matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Exp. Biol. Med. (Maywood) 2006;231(1):20–27. doi: 10.1177/153537020623100103. [DOI] [PubMed] [Google Scholar]
- 122.Fowlkes JL, Serra DM, Nagase H, Thrailkill KM. MMPs are IGFBP-degrading proteinases: implications for cell proliferation and tissue growth. Ann. N. Y. Acad. Sci. 1999;878:696–699. doi: 10.1111/j.1749-6632.1999.tb07765.x. [DOI] [PubMed] [Google Scholar]
- 123.Zhuang S, Kinsey GR, Rasbach K, Schnellmann RG. Heparin-binding epidermal growth factor and Src family kinases in proliferation of renal epithelial cells. Am. J. Physiol. Renal Physiol. 2008;294(3):F459–F468. doi: 10.1152/ajprenal.00473.2007. [DOI] [PubMed] [Google Scholar]
- 124.Rysz J, Banach M, Stolarek RA, Pasnik J, Cialkowska-Rysz A, Koktysz R, Piechota M, Baj Z. Serum matrix metalloproteinases MMP-2 and MMP-9 and metalloproteinase tissue inhibitors TIMP-1 and TIMP-2 in diabetic nephropathy. Journal of nephrology. 2007;20(4):444–452. [PubMed] [Google Scholar]
- 125.Lauhio A, Sorsa T, Srinivas R, Stenman M, Tervahartiala T, Stenman UH, Gronhagen-Riska C, Honkanen E. Urinary matrix metalloproteinase -8, -9, -14 and their regulators (TRY-1, TRY-2, TATI) in patients with diabetic nephropathy. Ann. Med. 2008;40(4):312–320. doi: 10.1080/07853890801923746. [DOI] [PubMed] [Google Scholar]
- 126.Romanic AM, Burns-Kurtis CL, Ao Z, Arleth AJ, Ohlstein EH. Upregulated expression of human membrane type-5 matrix metalloproteinase in kidneys from diabetic patients. Am. J. Physiol. Renal Physiol. 2001;281(2):F309–F317. doi: 10.1152/ajprenal.2001.281.2.F309. [DOI] [PubMed] [Google Scholar]
- 127.van der Zijl NJ, Hanemaaijer R, Tushuizen ME, Schindhelm RK, Boerop J, Rustemeijer C, Bilo HJ, Verheijen JH, Diamant M. Urinary matrix metalloproteinase-8 and -9 activities in type 2 diabetic subjects: A marker of incipient diabetic nephropathy? Clin. Biochem. 2010;43(7–8):635–639. doi: 10.1016/j.clinbiochem.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 128.Gharagozlian S, Svennevig K, Bangstad HJ, Winberg JO, Kolset SO. Matrix metalloproteinases in subjects with type 1 diabetes. BMC Clin. Pathol. 2009;9:7. doi: 10.1186/1472-6890-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sekiuchi M, Kudo A, Nakabayashi K, Kanai-Azuma M, Akimoto Y, Kawakami H, Yamada A. Expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of matrix metalloproteinases 2 and 1 in the glomeruli of human glomerular diseases: the results of studies using immunofluorescence, in situ hybridization, and immunoelectron microscopy. Clin. Exp. Nephrol. 2012;16(6):863–874. doi: 10.1007/s10157-012-0633-3. [DOI] [PubMed] [Google Scholar]
- 130.Del Prete D, Anglani F, Forino M, Ceol M, Fioretto P, Nosadini R, Baggio B, Gambaro G. Down-regulation of glomerular matrix metalloproteinase-2 gene in human NIDDM. Diabetologia. 1997;40(12):1449–1454. doi: 10.1007/s001250050848. [DOI] [PubMed] [Google Scholar]
- 131.McLennan SV, Kelly DJ, Schache M, Waltham M, Dy V, Langham RG, Yue DK, Gilbert RE. Advanced glycation end products decrease mesangial cell MMP-7: a role in matrix accumulation in diabetic nephropathy? Kidney Int. 2007;72(4):481–488. doi: 10.1038/sj.ki.5002357. [DOI] [PubMed] [Google Scholar]
- 132.Suzuki D, Miyazaki M, Jinde K, Koji T, Yagame M, Endoh M, Nomoto Y, Sakai H. In situ hybridization studies of matrix metalloproteinase-3, tissue inhibitor of metalloproteinase-1 and type IV collagen in diabetic nephropathy. Kidney Int. 1997;52(1):111–119. doi: 10.1038/ki.1997.310. [DOI] [PubMed] [Google Scholar]
- 133.Toni M, Hermida J, Goni MJ, Fernandez P, Parks WC, Toledo E, Montes R, Diez N. Matrix metalloproteinase-10 plays an active role in microvascular complications in type 1 diabetic patients. Diabetologia. 2013;56(12):2743–2752. doi: 10.1007/s00125-013-3052-4. [DOI] [PubMed] [Google Scholar]
- 134.Jindal A, Garcia-Touza M, Jindal N, Whaley-Connell A, Sowers JR. Diabetic kidney disease and the cardiorenal syndrome: old disease, new perspectives. Endocrinol. Metab. Clin. North Am. 2013;42(4):789–808. doi: 10.1016/j.ecl.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fineberg D, Jandeleit-Dahm KA, Cooper ME. Diabetic nephropathy: diagnosis and treatment. Nat. Rev. Endocrinol. 2013;9(12):713–723. doi: 10.1038/nrendo.2013.184. [DOI] [PubMed] [Google Scholar]
- 136.Kanauchi M, Nishioka H, Nakashima Y, Hashimoto T, Dohi K. Role of tissue inhibitors of metalloproteinase in diabetic nephropathy. Nihon Jinzo Gakkai shi. 1996;38(3):124–128. [PubMed] [Google Scholar]
- 137.Ban CR, Twigg SM, Franjic B, Brooks BA, Celermajer D, Yue DK, McLennan SV. Serum MMP-7 is increased in diabetic renal disease and diabetic diastolic dysfunction. Diabetes Res. Clin. Pract. 2010;87(3):335–341. doi: 10.1016/j.diabres.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 138.Li SY, Huang PH, Yang AH, Tarng DC, Yang WC, Lin CC, Chen JW, Schmid-Schonbein G, Lin SJ. Matrix metalloproteinase-9 deficiency attenuates diabetic nephropathy by modulation of podocyte functions and dedifferentiation. Kidney Int. 2014 doi: 10.1038/ki.2014.67. [DOI] [PubMed] [Google Scholar]
- 139.Ebihara I, Nakamura T, Shimada N, Koide H. Increased plasma metalloproteinase-9 concentrations precede development of microalbuminuria in non-insulin-dependent diabetes mellitus. Am. J. Kidney Dis. 1998;32(4):544–550. doi: 10.1016/s0272-6386(98)70015-0. [DOI] [PubMed] [Google Scholar]
- 140.Tashiro K, Koyanagi I, Ohara I, Ito T, Saitoh A, Horikoshi S, Tomino Y. Levels of urinary matrix metalloproteinase-9 (MMP-9) and renal injuries in patients with type 2 diabetic nephropathy. J. Clin. Lab. Anal. 2004;18(3):206–210. doi: 10.1002/jcla.20024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nakamura T, Ushiyama C, Suzuki S, Hara M, Shimada N, Ebihara I, Koide H. Urinary excretion of podocytes in patients with diabetic nephropathy. Nephrol. Dial. Transplant. 2000;15(9):1379–1383. doi: 10.1093/ndt/15.9.1379. [DOI] [PubMed] [Google Scholar]
- 142.Maeda S, Haneda M, Guo B, Koya D, Hayashi K, Sugimoto T, Isshiki K, Yasuda H, Kashiwagi A, Kikkawa R. Dinucleotide repeat polymorphism of matrix metalloproteinase-9 gene is associated with diabetic nephropathy. Kidney Int. 2001;60(4):1428–1434. doi: 10.1046/j.1523-1755.2001.00945.x. [DOI] [PubMed] [Google Scholar]
- 143.Aggarwal HK, Jain D, Talapatra P, Yadav RK, Gupta T, Kathuria KL. Evaluation of role of doxycycline (a matrix metalloproteinase inhibitor) on renal functions in patients of diabetic nephropathy. Ren. Fail. 2010;32(8):941–946. doi: 10.3109/0886022X.2010.502606. [DOI] [PubMed] [Google Scholar]
- 144.Fornoni A, Striker LJ, Zheng F, Striker GE. Reversibility of glucose-induced changes in mesangial cell extracellular matrix depends on the genetic background. Diabetes. 2002;51(2):499–505. doi: 10.2337/diabetes.51.2.499. [DOI] [PubMed] [Google Scholar]
- 145.Zhang SX, Wang JJ, Lu K, Mott R, Longeras R, Ma JX. Therapeutic potential of angiostatin in diabetic nephropathy. J. Am. Soc. Nephrol. 2006;17(2):475–486. doi: 10.1681/ASN.2005020217. [DOI] [PubMed] [Google Scholar]
- 146.Takamiya Y, Fukami K, Yamagishi S, Kaida Y, Nakayama Y, Obara N, Iwatani R, Ando R, Koike K, Matsui T, Nishino Y, Ueda S, Cooper ME, Okuda S. Experimental diabetic nephropathy is accelerated in matrix metalloproteinase-2 knockout mice. Nephrol. Dial. Transplant. 2013;28(1):55–62. doi: 10.1093/ndt/gfs387. [DOI] [PubMed] [Google Scholar]
- 147.Yao XM, Ye SD, Zai Z, Chen Y, Li XC, Yang GW, Wang YX, Chen K. Simvastatin protects diabetic rats against kidney injury through the suppression of renal matrix metalloproteinase-9 expression. J. Endocrinol. Invest. 2010;33(5):292–296. doi: 10.1007/BF03346588. [DOI] [PubMed] [Google Scholar]
- 148.Qing-Hua G, Ju-Ming L, Chang-Yu P, Zhao-Hui L, Xiao-Man Z, Yi-Ming M. The kidney expression of matrix metalloproteinase-9 in the diabetic nephropathy of Kkay mice. J. Diabetes Complications. 2008;22(6):408–412. doi: 10.1016/j.jdiacomp.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 149.McLennan SV, Kelly DJ, Cox AJ, Cao Z, Lyons JG, Yue DK, Gilbert RE. Decreased matrix degradation in diabetic nephropathy: effects of ACE inhibition on the expression and activities of matrix metalloproteinases. Diabetologia. 2002;45(2):268–275. doi: 10.1007/s00125-001-0730-4. [DOI] [PubMed] [Google Scholar]
- 150.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14(2):163–176. [PMC free article] [PubMed] [Google Scholar]
- 151.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell Biol. 2000;2(10):737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chan PS, Kanwar M, Kowluru RA. Resistance of retinal inflammatory mediators to suppress after reinstitution of good glycemic control: novel mechanism for metabolic memory. J. Diabetes Complications. 2010;24(1):55–63. doi: 10.1016/j.jdiacomp.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kiaei M, Kipiani K, Calingasan NY, Wille E, Chen J, Heissig B, Rafii S, Lorenzl S, Beal MF. Matrix metalloproteinase-9 regulates TNF-alpha and FasL expression in neuronal, glial cells and its absence extends life in a transgenic mouse model of amyotrophic lateral sclerosis. Exp. Neurol. 2007;205(1):74–81. doi: 10.1016/j.expneurol.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 154.Kowluru RA, Odenbach S. Role of interleukin-1beta in the pathogenesis of diabetic retinopathy. Br. J. Ophthalmol. 2004;88(10):1343–1347. doi: 10.1136/bjo.2003.038133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yang Q, Xie RJ, Yang T, Fang L, Han B, Zhang GZ, Cheng ML. Transforming growth factor-beta1 and Smad4 signaling pathway down-regulates renal extracellular matrix degradation in diabetic rats. Chin. Med. Sci. J. 2007;22(4):243–249. [PubMed] [Google Scholar]
- 156.Boucher E, Mayer G, Londono I, Bendayan M. Expression and localization of MT1-MMP and furin in the glomerular wall of short- and long-term diabetic rats. Kidney Int. 2006;69(9):1570–1577. doi: 10.1038/sj.ki.5000316. [DOI] [PubMed] [Google Scholar]
- 157.Fiorentino L, Cavalera M, Menini S, Marchetti V, Mavilio M, Fabrizi M, Conserva F, Casagrande V, Menghini R, Pontrelli P, Arisi I, D'Onofrio M, Lauro D, Khokha R, Accili D, Pugliese G, Gesualdo L, Lauro R, Federici M. Loss of TIMP3 underlies diabetic nephropathy via FoxO1/STAT1 interplay. EMBO Mol. Med. 2013;5(3):441–455. doi: 10.1002/emmm.201201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Aoyama T, Yamamoto S, Kanematsu A, Ogawa O, Tabata Y. Local delivery of matrix metalloproteinase gene prevents the onset of renal sclerosis in streptozotocin-induced diabetic mice. Tissue Eng. 2003;9(6):1289–1299. doi: 10.1089/10763270360728206. [DOI] [PubMed] [Google Scholar]
- 159.Hsieh HL, Chi PL, Lin CC, Yang CC, Yang CM. Up-regulation of ROS-Dependent Matrix Metalloproteinase-9 from High-Glucose-Challenged Astrocytes Contributes to the Neuronal Apoptosis. Mol. Neurobiol. 2014 doi: 10.1007/s12035-013-8628-y. [DOI] [PubMed] [Google Scholar]
- 160.Lee SJ, Bae SS, Kim KH, Lee WS, Rhim BY, Hong KW, Kim CD. High glucose enhances MMP-2 production in adventitial fibroblasts via Akt1-dependent NF-kappaB pathway. FEBS Lett. 2007;581(22):4189–4194. doi: 10.1016/j.febslet.2007.07.058. [DOI] [PubMed] [Google Scholar]
- 161.Singh R, Song RH, Alavi N, Pegoraro AA, Singh AK, Leehey DJ. High glucose decreases matrix metalloproteinase-2 activity in rat mesangial cells via transforming growth factor-beta1. Exp. Nephrol. 2001;9(4):249–257. doi: 10.1159/000052619. [DOI] [PubMed] [Google Scholar]
- 162.Huang CN, Chan KC, Lin WT, Su SL, Wang CJ, Peng CH. Hibiscus sabdariffa inhibits vascular smooth muscle cell proliferation and migration induced by high glucose--a mechanism involves connective tissue growth factor signals. J. Agric. Food Chem. 2009;57(8):3073–3079. doi: 10.1021/jf803911n. [DOI] [PubMed] [Google Scholar]
- 163.Kaminska B, Pyrzynska B, Ciechomska I, Wisniewska M. Modulation of the composition of AP-1 complex and its impact on transcriptional activity. Acta Neurobiol. Exp. (Wars.) 2000;60(3):395–402. doi: 10.55782/ane-2000-1358. [DOI] [PubMed] [Google Scholar]
- 164.Death AK, Fisher EJ, McGrath KC, Yue DK. High glucose alters matrix metalloproteinase expression in two key vascular cells: potential impact on atherosclerosis in diabetes. Atherosclerosis. 2003;168(2):263–269. doi: 10.1016/s0021-9150(03)00140-0. [DOI] [PubMed] [Google Scholar]
- 165.Abdel Wahab N, Mason RM. Modulation of neutral protease expression in human mesangial cells by hyperglycaemic culture. Biochem. J. 1996;320(Pt 3):777–783. doi: 10.1042/bj3200777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gaire M, Magbanua Z, McDonnell S, McNeil L, Lovett DH, Matrisian LM. Structure and expression of the human gene for the matrix metalloproteinase matrilysin. J. Biol. Chem. 1994;269(3):2032–2040. [PubMed] [Google Scholar]
- 167.Bai Y, Wang L, Li Y, Liu S, Li J, Wang H, Huang H. High ambient glucose levels modulates the production of MMP-9 and alpha5(IV) collagen by cultured podocytes. Cell. Physiol. Biochem. 2006;17(1–2):57–68. doi: 10.1159/000091464. [DOI] [PubMed] [Google Scholar]
- 168.Busch M, Franke S, Ruster C, Wolf G. Advanced glycation end-products and the kidney. Eur. J. Clin. Invest. 2010;40(8):742–755. doi: 10.1111/j.1365-2362.2010.02317.x. [DOI] [PubMed] [Google Scholar]
- 169.Schrijvers BF, De Vriese AS, Flyvbjerg A. From hyperglycemia to diabetic kidney disease: the role of metabolic, hemodynamic, intracellular factors and growth factors/cytokines. Endocr. Rev. 2004;25(6):971–1010. doi: 10.1210/er.2003-0018. [DOI] [PubMed] [Google Scholar]
- 170.Degenhardt TP, Thorpe SR, Baynes JW. Chemical modification of proteins by methylglyoxal. Cell. Mol. Biol. (Noisyle-grand) 1998;44(7):1139–1145. [PubMed] [Google Scholar]
- 171.Wendt T, Tanji N, Guo J, Hudson BI, Bierhaus A, Ramasamy R, Arnold B, Nawroth PP, Yan SF, D'Agati V, Schmidt AM. Glucose, glycation, and RAGE: implications for amplification of cellular dysfunction in diabetic nephropathy. J. Am. Soc. Nephrol. 2003;14(5):1383–1395. doi: 10.1097/01.asn.0000065100.17349.ca. [DOI] [PubMed] [Google Scholar]
- 172.Huang Y, Lin S, Zhou J. Gene expression of receptor for advanced glycosylation end products and its modulation by aminoguanidine in diabetic kidney tissue. Chin. Med. J. (Engl.) 1998;111(8):698–704. [PubMed] [Google Scholar]
- 173.Goh SY, Cooper ME. Clinical review: The role of advanced glycation end products in progression and complications of diabetes. J. Clin. Endocrinol. Metab. 2008;93(4):1143–1152. doi: 10.1210/jc.2007-1817. [DOI] [PubMed] [Google Scholar]
- 174.Williams ME. New therapies for advanced glycation end product nephrotoxicity: current challenges. Am. J. Kidney Dis. 2003;41(3) Suppl 1:S42–S47. doi: 10.1053/ajkd.2003.50083. [DOI] [PubMed] [Google Scholar]
- 175.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 176.Tanaka S, Avigad G, Brodsky B, Eikenberry EF. Glycation induces expansion of the molecular packing of collagen. J. Mol. Biol. 1988;203(2):495–505. doi: 10.1016/0022-2836(88)90015-0. [DOI] [PubMed] [Google Scholar]
- 177.Charonis AS, Reger LA, Dege JE, Kouzi-Koliakos K, Furcht LT, Wohlhueter RM, Tsilibary EC. Laminin alterations after in vitro nonenzymatic glycosylation. Diabetes. 1990;39(7):807–814. doi: 10.2337/diab.39.7.807. [DOI] [PubMed] [Google Scholar]
- 178.Forbes JM, Cooper ME, Oldfield MD, Thomas MC. Role of advanced glycation end products in diabetic nephropathy. J. Am. Soc. Nephrol. 2003;14(8) Suppl 3:S254–S258. doi: 10.1097/01.asn.0000077413.41276.17. [DOI] [PubMed] [Google Scholar]
- 179.Throckmorton DC, Brogden AP, Min B, Rasmussen H, Kashgarian M. PDGF and TGF-beta mediate collagen production by mesangial cells exposed to advanced glycosylation end products. Kidney Int. 1995;48(1):111–117. doi: 10.1038/ki.1995.274. [DOI] [PubMed] [Google Scholar]
- 180.Oldfield MD, Bach LA, Forbes JM, Nikolic-Paterson D, McRobert A, Thallas V, Atkins RC, Osicka T, Jerums G, Cooper ME. Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE) J. Clin. Invest. 2001;108(12):1853–1863. doi: 10.1172/JCI11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hughes JM, Kuiper EJ, Klaassen I, Canning P, Stitt AW, Van Bezu J, Schalkwijk CG, Van Noorden CJ, Schlingemann RO. Advanced glycation end products cause increased CCN family and extracellular matrix gene expression in the diabetic rodent retina. Diabetologia. 2007;50(5):1089–1098. doi: 10.1007/s00125-007-0621-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang X, McLennan SV, Twigg SM. CCN-2 is up-regulated by and mediates effects of matrix bound advanced glycated end-products in human renal mesangial cells. J. Cell Commun. Signal. 2011;5(3):193–200. doi: 10.1007/s12079-011-0137-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.McLennan SV, Martell SK, Yue DK. Effects of mesangium glycation on matrix metalloproteinase activities: possible role in diabetic nephropathy. Diabetes. 2002;51(8):2612–2618. doi: 10.2337/diabetes.51.8.2612. [DOI] [PubMed] [Google Scholar]
- 184.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404(6779):787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 185.Ceriello A, Morocutti A, Mercuri F, Quagliaro L, Moro M, Damante G, Viberti GC. Defective intracellular antioxidant enzyme production in type 1 diabetic patients with nephropathy. Diabetes. 2000;49(12):2170–2177. doi: 10.2337/diabetes.49.12.2170. [DOI] [PubMed] [Google Scholar]
- 186.Werner E. GTPases and reactive oxygen species: switches for killing and signaling. J. Cell Sci. 2004;117(Pt 2):143–153. doi: 10.1242/jcs.00937. [DOI] [PubMed] [Google Scholar]
- 187.Djordjevic VB. Free radicals in cell biology. Int. Rev. Cytol. 2004;237:57–89. doi: 10.1016/S0074-7696(04)37002-6. [DOI] [PubMed] [Google Scholar]
- 188.Topham MK, Carveth HJ, McIntyre TM, Prescott SM, Zimmerman GA. Human endothelial cells regulate polymorphonuclear leukocyte degranulation. FASEB J. 1998;12(9):733–746. doi: 10.1096/fasebj.12.9.733. [DOI] [PubMed] [Google Scholar]
- 189.Ha H, Lee HB. Reactive oxygen species as glucose signaling molecules in mesangial cells cultured under high glucose. Kidney Int. Suppl. 2000;77:S19–S25. doi: 10.1046/j.1523-1755.2000.07704.x. [DOI] [PubMed] [Google Scholar]
- 190.Catherwood MA, Powell LA, Anderson P, McMaster D, Sharpe PC, Trimble ER. Glucose-induced oxidative stress in mesangial cells. Kidney Int. 2002;61(2):599–608. doi: 10.1046/j.1523-1755.2002.00168.x. [DOI] [PubMed] [Google Scholar]
- 191.Du X, Stocklauser-Farber K, Rosen P. Generation of reactive oxygen intermediates, activation of NF-kappaB, and induction of apoptosis in human endothelial cells by glucose: role of nitric oxide synthase? Free Radic. Biol. Med. 1999;27(7–8):752–763. doi: 10.1016/s0891-5849(99)00079-9. [DOI] [PubMed] [Google Scholar]
- 192.Manabe E, Handa O, Naito Y, Mizushima K, Akagiri S, Adachi S, Takagi T, Kokura S, Maoka T, Yoshikawa T. Astaxanthin protects mesangial cells from hyperglycemia-induced oxidative signaling. J. Cell. Biochem. 2008;103(6):1925–1937. doi: 10.1002/jcb.21583. [DOI] [PubMed] [Google Scholar]
- 193.Uemura S, Matsushita H, Li W, Glassford AJ, Asagami T, Lee KH, Harrison DG, Tsao PS. Diabetes mellitus enhances vascular matrix metalloproteinase activity: role of oxidative stress. Circ. Res. 2001;88(12):1291–1298. doi: 10.1161/hh1201.092042. [DOI] [PubMed] [Google Scholar]
- 194.Nelson KK, Melendez JA. Mitochondrial redox control of matrix metalloproteinases. Free Radic. Biol. Med. 2004;37(6):768–784. doi: 10.1016/j.freeradbiomed.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 195.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 196.Kamioka M, Ishibashi T, Ohkawara H, Nagai R, Sugimoto K, Uekita H, Matsui T, Yamagishi S, Ando K, Sakamoto T, Sakamoto N, Takuwa Y, Wada I, Shiomi M, Maruyama Y, Takeishi Y. Involvement of membrane type 1-matrix metalloproteinase (MT1-MMP) in RAGE activation signaling pathways. J. Cell. Physiol. 2011;226(6):1554–1563. doi: 10.1002/jcp.22492. [DOI] [PubMed] [Google Scholar]
- 197.Moshal KS, Singh M, Sen U, Rosenberger DS, Henderson B, Tyagi N, Zhang H, Tyagi SC. Homocysteine-mediated activation and mitochondrial translocation of calpain regulates MMP-9 in MVEC. Am. J. Physiol. Heart Circ. Physiol. 2006;291(6):H2825–H2835. doi: 10.1152/ajpheart.00377.2006. [DOI] [PubMed] [Google Scholar]
- 198.Moshal KS, Tipparaju SM, Vacek TP, Kumar M, Singh M, Frank IE, Patibandla PK, Tyagi N, Rai J, Metreveli N, Rodriguez WE, Tseng MT, Tyagi SC. Mitochondrial matrix metalloproteinase activation decreases myocyte contractility in hyperhomocysteinemia. Am. J. Physiol. Heart Circ. Physiol. 2008;295(2):H890–H897. doi: 10.1152/ajpheart.00099.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhou HZ, Ma X, Gray MO, Zhu BQ, Nguyen AP, Baker AJ, Simonis U, Cecchini G, Lovett DH, Karliner JS. Transgenic MMP-2 expression induces latent cardiac mitochondrial dysfunction. Biochem. Biophys. Res. Commun. 2007;358(1):189–195. doi: 10.1016/j.bbrc.2007.04.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kowluru RA, Mohammad G, dos Santos JM, Zhong Q. Abrogation of MMP-9 gene protects against the development of retinopathy in diabetic mice by preventing mitochondrial damage. Diabetes. 2011;60(11):3023–3033. doi: 10.2337/db11-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Garg R, Chaudhuri A, Munschauer F, Dandona P. Hyperglycemia, insulin, and acute ischemic stroke: a mechanistic justification for a trial of insulin infusion therapy. Stroke. 2006;37(1):267–273. doi: 10.1161/01.STR.0000195175.29487.30. [DOI] [PubMed] [Google Scholar]
- 202.Fine LG, Orphanides C, Norman JT. Progressive renal disease: the chronic hypoxia hypothesis. Kidney Int. Suppl. 1998;65:S74–S78. [PubMed] [Google Scholar]
- 203.Mimura I, Nangaku M. The suffocating kidney: tubulointerstitial hypoxia in end-stage renal disease. Nature reviews. Nephrology. 2010;6(11):667–678. doi: 10.1038/nrneph.2010.124. [DOI] [PubMed] [Google Scholar]
- 204.Nangaku M. Hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. Nephron. Experimental nephrology. 2004;98(1):e8–e12. doi: 10.1159/000079927. [DOI] [PubMed] [Google Scholar]
- 205.Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J. Am. Soc. Nephrol. 2006;17(1):17–25. doi: 10.1681/ASN.2005070757. [DOI] [PubMed] [Google Scholar]
- 206.Singh DK, Winocour P, Farrington K. Mechanisms of disease: the hypoxic tubular hypothesis of diabetic nephropathy. Nat. Clin. Pract. Nephrol. 2008;4(4):216–226. doi: 10.1038/ncpneph0757. [DOI] [PubMed] [Google Scholar]
- 207.Palm F, Cederberg J, Hansell P, Liss P, Carlsson PO. Reactive oxygen species cause diabetes-induced decrease in renal oxygen tension. Diabetologia. 2003;46(8):1153–1160. doi: 10.1007/s00125-003-1155-z. [DOI] [PubMed] [Google Scholar]
- 208.Hansell P, Welch WJ, Blantz RC, Palm F. Determinants of kidney oxygen consumption and their relationship to tissue oxygen tension in diabetes and hypertension. Clin. Exp. Pharmacol. Physiol. 2013;40(2):123–137. doi: 10.1111/1440-1681.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kumar D, Robertson S, Burns KD. Evidence of apoptosis in human diabetic kidney. Mol. Cell. Biochem. 2004;259(1–2):67–70. doi: 10.1023/b:mcbi.0000021346.03260.7e. [DOI] [PubMed] [Google Scholar]
- 210.Norman JT, Clark IM, Garcia PL. Hypoxia promotes fibrogenesis in human renal fibroblasts. Kidney Int. 2000;58(6):2351–2366. doi: 10.1046/j.1523-1755.2000.00419.x. [DOI] [PubMed] [Google Scholar]
- 211.Higgins DF, Biju MP, Akai Y, Wutz A, Johnson RS, Haase VH. Hypoxic induction of Ctgf is directly mediated by Hif-1. Am. J. Physiol. Renal Physiol. 2004;287(6):F1223–F1232. doi: 10.1152/ajprenal.00245.2004. [DOI] [PubMed] [Google Scholar]
- 212.Qi W, Poronnik P, Young B, Jackson CJ, Field MJ, Pollock CA. Human cortical fibroblast responses to high glucose and hypoxia. Nephron. Physiology. 2004;96(4):p121–p129. doi: 10.1159/000077383. [DOI] [PubMed] [Google Scholar]
- 213.Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, Chugh S, Danesh FR. Diabetic nephropathy: mechanisms of renal disease progression. Exp. Biol. Med. (Maywood) 2008;233(1):4–11. doi: 10.3181/0705-MR-134. [DOI] [PubMed] [Google Scholar]
- 214.Ziyadeh FN. Mediators of diabetic renal disease: the case for tgf-Beta as the major mediator. J. Am. Soc. Nephrol. 2004;15(Suppl 1):S55–S57. doi: 10.1097/01.asn.0000093460.24823.5b. [DOI] [PubMed] [Google Scholar]
- 215.Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc. Natl. Acad. Sci. U.S.A. 1993;90(5):1814–1818. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Park IS, Kiyomoto H, Abboud SL, Abboud HE. Expression of transforming growth factor-beta and type IV collagen in early streptozotocin-induced diabetes. Diabetes. 1997;46(3):473–480. doi: 10.2337/diab.46.3.473. [DOI] [PubMed] [Google Scholar]
- 217.Ignotz RA, Massague J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J. Biol. Chem. 1986;261(9):4337–4345. [PubMed] [Google Scholar]
- 218.Bassols A, Massague J. Transforming growth factor beta regulates the expression and structure of extracellular matrix chondroitin/dermatan sulfate proteoglycans. J. Biol. Chem. 1988;263(6):3039–3045. [PubMed] [Google Scholar]
- 219.Edwards DR, Murphy G, Reynolds JJ, Whitham SE, Docherty AJ, Angel P, Heath JK. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J. 1987;6(7):1899–1904. doi: 10.1002/j.1460-2075.1987.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Border WA, Noble NA. Evidence that TGF-beta should be a therapeutic target in diabetic nephropathy. Kidney Int. 1998;54(4):1390–1391. doi: 10.1046/j.1523-1755.1998.00127.x. [DOI] [PubMed] [Google Scholar]
- 221.McLennan SV, Fisher E, Martell SY, Death AK, Williams PF, Lyons JG, Yue DK. Effects of glucose on matrix metalloproteinase and plasmin activities in mesangial cells: possible role in diabetic nephropathy. Kidney Int. Suppl. 2000;77:S81–S87. doi: 10.1046/j.1523-1755.2000.07713.x. [DOI] [PubMed] [Google Scholar]
- 222.Baricos WH, Cortez SL, Deboisblanc M, Xin S. Transforming growth factor-beta is a potent inhibitor of extracellular matrix degradation by cultured human mesangial cells. J. Am. Soc. Nephrol. 1999;10(4):790–795. doi: 10.1681/ASN.V104790. [DOI] [PubMed] [Google Scholar]
- 223.Cheng S, Lovett DH. Gelatinase A (MMP-2) is necessary and sufficient for renal tubular cell epithelial-mesenchymal transformation. Am. J. Pathol. 2003;162(6):1937–1949. doi: 10.1016/S0002-9440(10)64327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Cheng S, Pollock AS, Mahimkar R, Olson JL, Lovett DH. Matrix metalloproteinase 2 and basement membrane integrity: a unifying mechanism for progressive renal injury. FASEB J. 2006;20(11):1898–1900. doi: 10.1096/fj.06-5898fje. [DOI] [PubMed] [Google Scholar]
- 225.Zeisberg M, Bonner G, Maeshima Y, Colorado P, Muller GA, Strutz F, Kalluri R. Renal fibrosis: collagen composition and assembly regulates epithelial-mesenchymal transdifferentiation. Am. J. Pathol. 2001;159(4):1313–1321. doi: 10.1016/S0002-9440(10)62518-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Overall CM, Wrana JL, Sodek J. Transcriptional and post-transcriptional regulation of 72-kDa gelatinase/type IV collagenase by transforming growth factor-beta 1 in human fibroblasts. Comparisons with collagenase and tissue inhibitor of matrix metalloproteinase gene expression. J. Biol. Chem. 1991;266(21):14064–14071. [PubMed] [Google Scholar]
- 227.Sehgal I, Thompson TC. Novel regulation of type IV collagenase (matrix metalloproteinase-9 and -2) activities by transforming growth factor-beta1 in human prostate cancer cell lines. Mol. Biol. Cell. 1999;10(2):407–416. doi: 10.1091/mbc.10.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Overall CM, Wrana JL, Sodek J. Transforming growth factor-beta regulation of collagenase, 72 kDa-progelatinase, TIMP and PAI-1 expression in rat bone cell populations and human fibroblasts. Connect. Tissue Res. 1989;20(1–4):289–294. doi: 10.3109/03008208909023899. [DOI] [PubMed] [Google Scholar]
- 229.Matrisian LM, Leroy P, Ruhlmann C, Gesnel MC, Breathnach R. Isolation of the oncogene and epidermal growth factor-induced transin gene: complex control in rat fibroblasts. Mol. Cell. Biol. 1986;6(5):1679–1686. doi: 10.1128/mcb.6.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kerr LD, Olashaw NE, Matrisian LM. Transforming growth factor beta 1 and cAMP inhibit transcription of epidermal growth factor- and oncogene-induced transin RNA. J. Biol. Chem. 1988;263(32):16999–17005. [PubMed] [Google Scholar]
- 231.Kerr LD, Miller DB, Matrisian LM. TGF-beta 1 inhibition of transin/stromelysin gene expression is mediated through a Fos binding sequence. Cell. 1990;61(2):267–278. doi: 10.1016/0092-8674(90)90807-q. [DOI] [PubMed] [Google Scholar]
- 232.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nature reviews. Genetics. 2004;5(7):522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 233.Alvarez ML, Khosroheidari M, Eddy E, Kiefer J. Role of MicroRNA 1207-5P and Its Host Gene, the Long Non-Coding RNA Pvt1, as Mediators of Extracellular Matrix Accumulation in the Kidney: Implications for Diabetic Nephropathy. PLoS One. 2013;8(10):e77468. doi: 10.1371/journal.pone.0077468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Long J, Wang Y, Wang W, Chang BH, Danesh FR. MicroRNA-29c is a signature microRNA under high glucose conditions that targets Sprouty homolog 1, and its in vivo knockdown prevents progression of diabetic nephropathy. J. Biol. Chem. 2011;286(13):11837–11848. doi: 10.1074/jbc.M110.194969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kato M, Arce L, Wang M, Putta S, Lanting L, Natarajan R. A microRNA circuit mediates transforming growth factor-beta1 autoregulation in renal glomerular mesangial cells. Kidney Int. 2011;80(4):358–368. doi: 10.1038/ki.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Long J, Wang Y, Wang W, Chang BH, Danesh FR. Identification of microRNA-93 as a novel regulator of vascular endothelial growth factor in hyperglycemic conditions. J. Biol. Chem. 2010;285(30):23457–23465. doi: 10.1074/jbc.M110.136168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wang Q, Wang Y, Minto AW, Wang J, Shi Q, Li X, Quigg RJ. MicroRNA-377 is up-regulated and can lead to increased fibronectin production in diabetic nephropathy. FASEB J. 2008;22(12):4126–4135. doi: 10.1096/fj.08-112326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kato M, Wang L, Putta S, Wang M, Yuan H, Sun G, Lanting L, Todorov I, Rossi JJ, Natarajan R. Post-transcriptional up-regulation of Tsc-22 by Ybx1, a target of miR-216a, mediates TGF-{beta}-induced collagen expression in kidney cells. J. Biol. Chem. 2010;285(44):34004–34015. doi: 10.1074/jbc.M110.165027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wang J, Gao Y, Ma M, Li M, Zou D, Yang J, Zhu Z, Zhao X. Effect of miR-21 on Renal Fibrosis by Regulating MMP-9 and TIMP1 in kk-ay Diabetic Nephropathy Mice. Cell Biochem. Biophys. 2013;67(2):537–546. doi: 10.1007/s12013-013-9539-2. [DOI] [PubMed] [Google Scholar]
- 240.Lo CS, Chen ZH, Hsieh TJ, Shin SJ. Atrial natriuretic peptide attenuates high glucose-activated transforming growth factor-beta, Smad and collagen synthesis in renal proximal tubular cells. J. Cell. Biochem. 2008;103(6):1999–2009. doi: 10.1002/jcb.21590. [DOI] [PubMed] [Google Scholar]
- 241.Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454(7200):56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Fiorentino L, Cavalera M, Mavilio M, Conserva F, Menghini R, Gesualdo L, Federici M. Regulation of TIMP3 in diabetic nephropathy: a role for microRNAs. Acta Diabetol. 2013;50(6):965–969. doi: 10.1007/s00592-013-0492-8. [DOI] [PubMed] [Google Scholar]
- 243.Thrailkill KM, Clay Bunn R, Fowlkes JL. Matrix metalloproteinases: their potential role in the pathogenesis of diabetic nephropathy. Endocrine. 2009;35(1):1–10. doi: 10.1007/s12020-008-9114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Thrailkill KM, Moreau CS, Cockrell GE, Jo CH, Bunn RC, Morales-Pozzo AE, Lumpkin CK, Fowlkes JL. Disease and gender-specific dysregulation of NGAL and MMP-9 in type 1 diabetes mellitus. Endocrine. 2010;37(2):336–343. doi: 10.1007/s12020-010-9308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Thrailkill KM, Bunn RC, Moreau CS, Cockrell GE, Simpson PM, Coleman HN, Frindik JP, Kemp SF, Fowlkes JL. Matrix metalloproteinase-2 dysregulation in type 1 diabetes. Diabetes Care. 2007;30(9):2321–2326. doi: 10.2337/dc07-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zeisberg M, Khurana M, Rao VH, Cosgrove D, Rougier JP, Werner MC, Shield CF, 3rd, Werb Z, Kalluri R. Stage-specific action of matrix metalloproteinases influences progressive hereditary kidney disease. PLoS Med. 2006;3(4):e100. doi: 10.1371/journal.pmed.0030100. [DOI] [PMC free article] [PubMed] [Google Scholar]