Abstract
Importance
Proteus syndrome is an extremely rare disorder of mosaic postnatal overgrowth affecting multiple tissues including bone, soft tissue and skin. It typically presents in early childhood with asymmetric and progressive skeletal overgrowth that leads to severe distortion of the skeleton and disability. The genetic basis has recently been identified as a somatic activating mutation in the AKT1 gene, which encodes an enzyme mediating cell proliferation and apoptosis.
Observations
We present a 33-year-old man that developed plantar cerebriform collagenomas on the soles of both feet and varicose veins in early childhood, in the absence of any skeletal or other connective tissue abnormality. Although the patient did not meet the diagnostic criteria for Proteus syndrome, he was found to have the c.49G>A, p.Glu17Lys AKT1 mutation in lesional skin but not in his blood.
Conclusions and Relevance
To our knowledge, this is the mildest molecularly confirmed patient with Proteus syndrome, occurring in the absence of the characteristic skeletal overgrowth. These findings extend the spectrum of Proteus syndrome pathology and suggest that somatic mutations late in development and restricted in distribution cause subtle clinical presentations that do not meet the published clinical criteria.
Proteus syndrome is a rare overgrowth disorder that is caused by somatic mosaicism in the AKT1 gene.1 It is characterised by disproportionate and progressive overgrowth affecting multiple tissues including bone, soft tissue and skin. The 4 common skin lesions seen in this condition include epidermal nevi, vascular malformations, lipomas and the characteristic plantar cerebriform collagenoma (also known as a cerebriform connective tissue nevus). Affected individuals typically have striking asymmetrical skeletal enlargement that results in significant disfigurement and loss of function. Due to the progressive nature and variability of the disorder, making a clinical diagnosis of Proteus syndrome can be difficult. We present a patient with a very mild form of Proteus syndrome whose manifestations do not meet the published clinical criteria, which raises important questions about diagnosing mosaic disorders.
REPORT OF A CASE
We report on a 33-year-old man who presented to us with cerebriform tumors over the plantar surfaces of both feet (Figure 1A). The lesions were first noticed around the age of 4-years and grew progressively during childhood and adolescence. Overgrowth under his toes led to problems with walking and he had debulking surgery at the age of 16-years. During adulthood there has been no further enlargement and he does not suffer any functional impairment. He also has severe varicose veins in his legs, which similarly developed in early childhood. Venous duplex ultrasonography at the age of 16-years showed severely dilated varicose veins as a result of valvular reflux at the saphenofemoral junction and he underwent stripping of the long saphenous veins. There is no family history of plantar cerebriform collagenomas or varicose veins occurring in childhood. Examination showed asymmetrical, soft, cerebriform plaques with prominent gyriform-like sulci over the plantar surfaces of both feet (Figure 1B). There were prominent varicose veins in both legs. He had no clinical evidence of skeletal abnormalities, dysregulation of adipose tissue, or of any other notable skin lesion.
Figure 1.
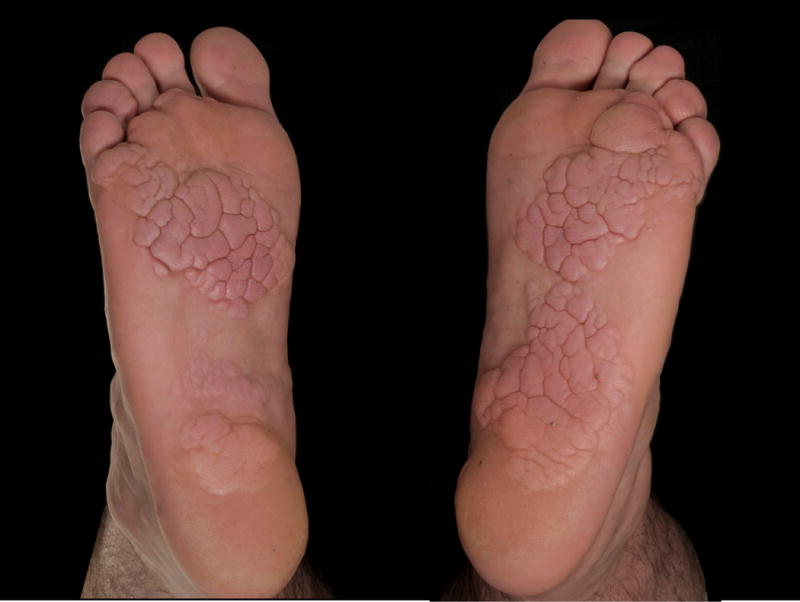
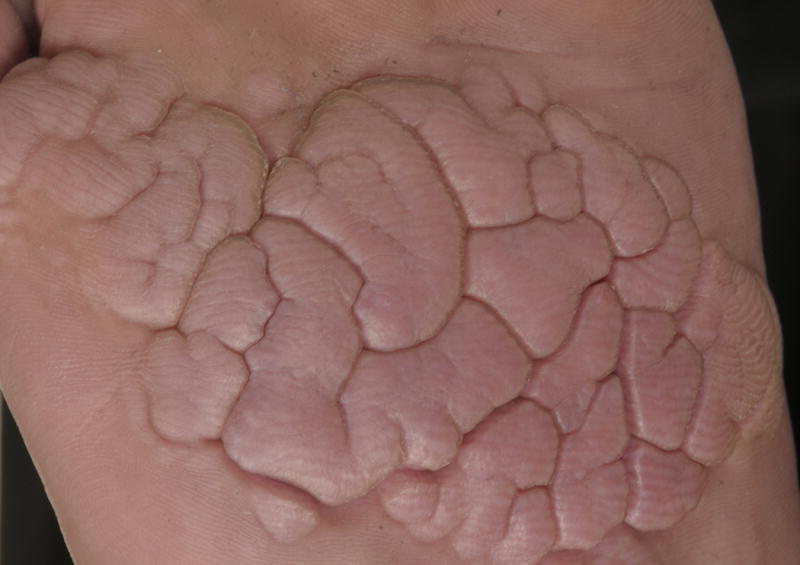
Plantar cerebriform tumors.
A, Bilateral plantar involvement.
B, Prominent gyriform-like sulci.
A skin biopsy taken from the left sole showed a markedly thickened dermis, consisting of thick collagen fibres arranged in a haphazard orientation (Figure 2A). An Elastic van-Gieson (EVG) stain demonstrated a reduction in elastic fibres in the reticular dermis, with fragmented elastic fibres seen on high power. These histological findings are consistent with a collagenoma. In addition, large calibre, irregular thick-walled vessels with valves were seen at the interface of the deep dermis and subcutaneous tissue. Their appearances, together with their staining pattern on EVG (Figure 2B) and lack of immunostaining with D2-40, suggest they represent veins. To further investigate his varicose veins he underwent a magnetic resonance venogram, which showed marked dilatation of the superficial and deep perforator veins in the legs and feet consistent with deep venous malformations. Radiographs of the limbs and feet showed no evidence of skeletal abnormalities.
Figure 2.
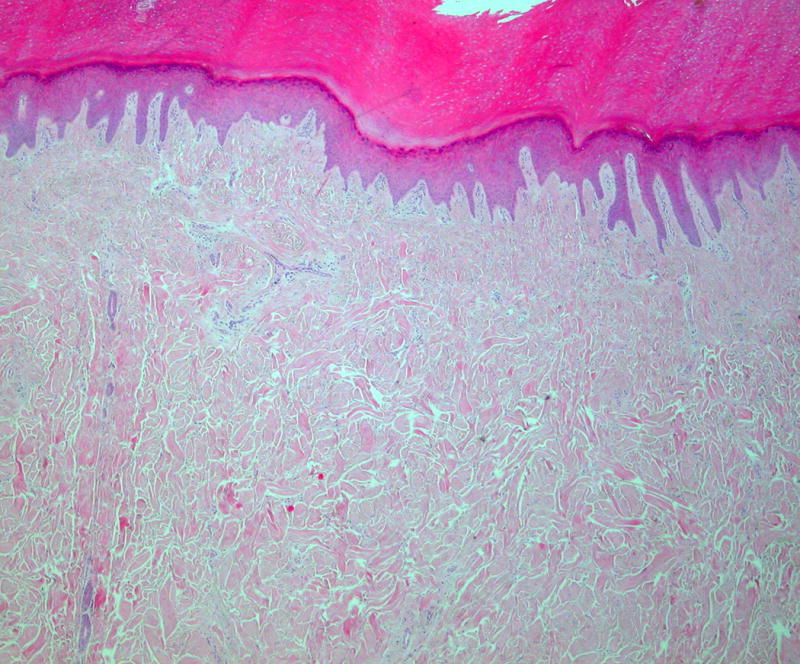
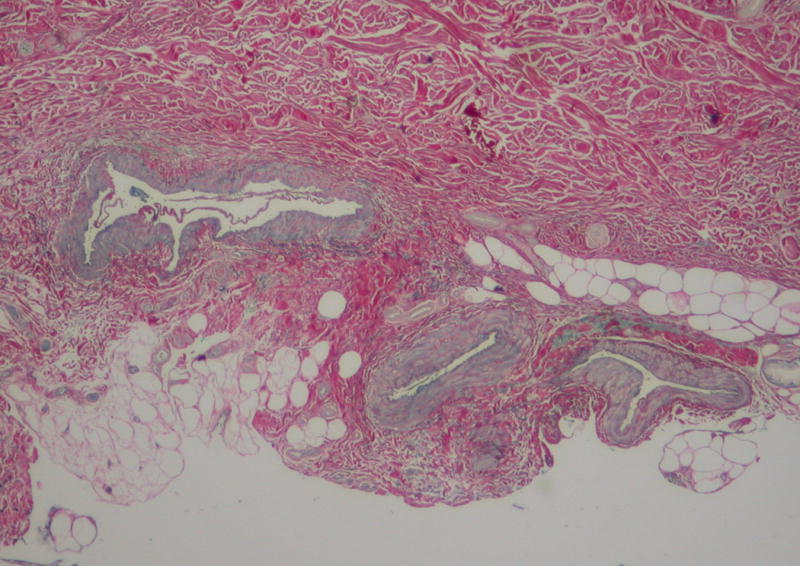
Histopathological findings of skin biopsy.
A, Markedly thickened dermis consisting of thick collagen fibres arranged in a haphazard orientation (hematoxylin and eosin stain).
B, Large calibre, irregular thick-walled veins at the interface of the deep dermis and subcutaneous tissue (EVG stain).
To assess for the AKT1 c.49G>A mutation in this patient, DNA was isolated from a collagenoma skin biopsy and from peripheral blood, and the mutation level was assessed using a custom-designed, quantitative restriction enzyme assay as described previously.1 The mutation was found at a level of 3% in the collagenoma DNA sample, but was not detected in the peripheral blood sample, consistent with somatic mosaicism.
COMMENT
Proteus syndrome is a rare, sporadic disorder (incidence of <1 case per 1 million population) characterised by patchy or segmental overgrowth of diverse tissues of all germ layers, most commonly affecting the skeleton, skin, adipose, and central nervous systems. The condition is named after the mythical Greek sea-god who was able to assume many forms. Somatic mosaicism was hypothesised as the cause due to the sporadic occurrence, mosaic distribution of lesions and variable extent of involvement.2 Joseph Merrick, a man who lived in the late 19th century and known as the Elephant man, is thought to have suffered with the Proteus syndrome.
The plantar cerebriform collagenoma is a connective tissue nevus associated with a proliferation of collagen in the dermis that leads to a distinctive cerebriform appearance. It is the most characteristic skin finding in Proteus syndrome, although it is not seen in all patients. The typical site of involvement is the plantar aspect of the feet, but it can also develop over the palms and more rarely over the trunk, arms and face.3 In contrast to epidermal nevi and vascular malformations, which are usually seen in the first month of life, the plantar cerebriform collagenoma usually develops during the first 2 years of life.4 It is an extremely rare cutaneous finding and due to its specificity for Proteus syndrome, it is the only category A sign (cerebriform connective tissue nevus) in the diagnostic criteria.5 Some authors have considered the plantar cerebriform collagenoma to be pathognomonic for Proteus syndrome. However, there are a few reports of isolated plantar cerebriform collagenoma occurring in the absence of any other feature of Proteus syndrome, with all but one case displaying small unilateral plaques or nodules.6–13 Most of these patients did not have the typical cerebriform appearance seen in Proteus syndrome.8–13 Of note, the only patient with bilateral plantar involvement6 was later found to have Proteus syndrome.5 All of these reports preceded the identification of the genetic basis of Proteus syndrome, which was found to be a somatic activating mutation in the oncogene AKT1.1 This gene is involved in the PI3K-AKT-mTOR signalling pathway and encodes an enzyme mediating cell proliferation and apoptosis. Mutations in several genes involved in the PI3K-AKT-mTOR pathway that lead to upregulation are now known to be responsible for a number of hamartoma or overgrowth syndromes (Figure 3). In Proteus syndrome the constitutive activation of the AKT1 protein is thought to underlie the overgrowth and tumour susceptibility seen in affected individuals.1 To date, all patients with a clinical diagnosis of Proteus syndrome have the same c.49G>A, p.Glu17Lys AKT1 mutation.
Figure 3.
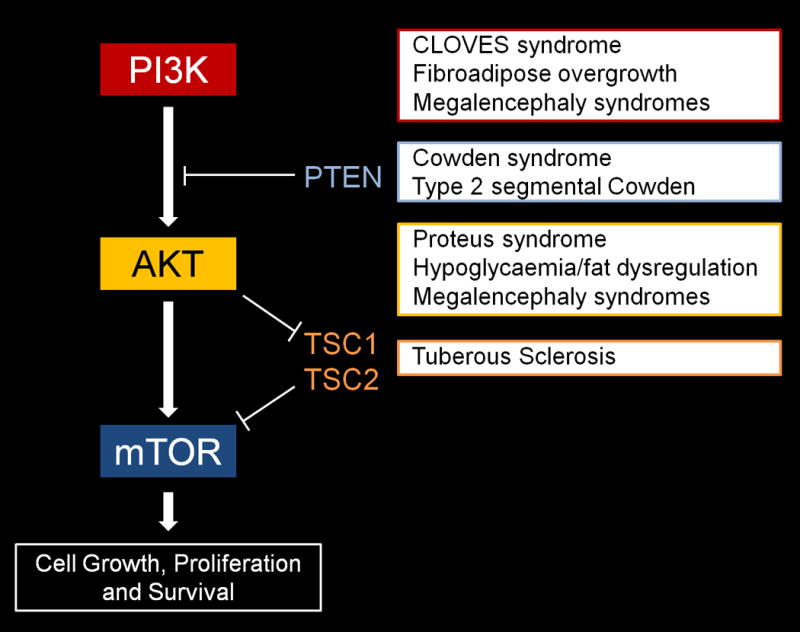
PI3K-AKT-mTOR signalling pathway and associated hamartoma/overgrowth syndromes (PI3K subsumes PIK3CA and PIK3R2, AKT subsumes AKT1-3).
Prior to the discovery of the molecular basis of Proteus syndrome, it was necessary to use only clinical features to diagnose the disorder. This led to a significant degree of confusion and misdiagnosis, which was addressed by formal diagnostic criteria which were updated in 2006 (Table 1).14 The present patient does not strictly meet the current diagnostic criteria, as he only meets 2 of the 3 general criteria, which are mandatory, with features that are mosaic and sporadic in nature, but not progressive. Of the specific criteria, his plantar cerebriform collagenoma is a criterion from category A, whilst his varicose veins, which are the result of deep venous malformations, constitute a single criterion from category C. Therefore, he meets the specific criteria but not the mandatory general criteria for a clinical diagnosis. Despite this, the unusual clinical features and development in early childhood raised our suspicion that this could be a forme fruste of Proteus syndrome. This was confirmed by the finding of the c.49G>A, p.Glu17Lys AKT1 mutation in lesional skin but not in his blood. The limited involvement in this patient suggests the somatic mutation may have occurred later in fetal development (compared to more typically affected patients) as an early postzygotic mutation would give rise to more extensive abnormal cell lineages.
Table 1.
The diagnostic criteria for Proteus syndrome
| General Criteria |
|
| Specific Criteria |
Category A
|
Category B
|
Category C
|
| A diagnosis of Proteus syndrome requires all 3 general criteria plus: 1 criterion from category A, or 2 criteria from category B, or 3 criteria from category C (Adapted from Biesecker, 2006)14 |
The results of our analysis suggests the diagnostic criteria are limited in their ability to account for patients with mild manifestations, as progressive changes (the third general criterion) are more applicable to the skeletal system. There is evidence that although the plantar cerebriform collagenoma grows progressively during childhood, it remains stable in adulthood as seen in the patient reported here.15 More generally, the clinical manifestations of patients with mosaic disorders is intrinsically limited by the initial mutational event – if that event occurs early in development and affects many tissues, the manifestations will be widespread and recognizable.16 If that event occurs late in development, the manifestations will be more anatomically limited and mild in degree. In fact, it is theoretically possible that a patient could harbour only a few cells or even a single cell with the AKT1 c.49G>A p.Glu17Lys mutation, which may have little or no clinical consequences. This then begs the question of what the lower boundary of a clinical diagnosis of Proteus syndrome should be. While it is tempting to fall back entirely on the molecular findings, it would make little sense to diagnose a patient with a small, isolated cerebriform collagenoma and no other manifestations as Proteus syndrome. This same mutation is known to occur as a part of the genomic mutational landscape of a number of cancers including breast, colorectal and ovarian.17 Patients with these tumors would clearly not be termed as having Proteus syndrome. This issue has also arisen with other genes and phenotypes in dermatology. An example of this that was recently reviewed16 is the mosaic mutation of the Gly12 residue of HRAS, which has been found in the mosaic state in benign keratinocytic epidermal nevi and bladder cancer, and in constitutional or germline state in the congenital malformation disorder Costello syndrome. Again, it would be highly problematic to suggest that patients with only the skin lesion or bladder cancer have Costello syndrome. Therefore, it is clear that our preconceptions about clinical and molecular diagnosis are not readily transferrable from germline to mosaic disorders, and novel approaches may be necessary. In the case of Proteus syndrome and other phenotypes with both germline and mosaic mutations, such efforts to reclassify phenotypic and molecular diagnosis will await more data on the range of these manifestations.
Acknowledgments
The authors are indebted to Hadley Bloomhardt for performing the molecular assays.
Funding/Support: This report was supported in part by the National Institutes of Health
| Funding/sponsor was involved? | |
| Design and conduct of the study | No |
| Collection, management, analysis and interpretation of data | No |
| Preparation, review, or approval of the manuscript | Yes (all NIH manuscript are submitted for approval, which is routinely granted) |
| Decision to submit the manuscript for publication | No |
Financial disclosure: Drs Lindhurst and Biesecker receive royalties from Genentech Corp. for development of Proteus patient cell lines.
Footnotes
Author contributions: Drs Wee and Biesecker had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Acquisition of data: Drs Wee, Lindhurst and Biesecker. Drafting of the manuscript: Drs Wee and Biesecker. Critical revision of the manuscript for important intellectual content: Drs Biesecker, Chong, Mortimer and Holden. Obtained funding: Drs Lindhurst and Biesecker are funded by the Intramural Research Program of the National Human Genome Research Institute. Administrative, technical, or material support: Drs Lindhurst and Chong. Study supervision: Drs Holden, Biesecker and Mortimer. Conflict of Interest: Drs Lindhurst and Biesecker receive royalties from Genentech Corp. for development of Proteus patient cell lines.
- Employment
- Consultancies:
- Honoraria: Dr. Biesecker receives honoraria from Wiley-Blackwell for editorial duties, which has no relationship to this manuscript.
- Speakers bureau
- Stock ownership or options
- Expert testimony
- Grants
- Patents filed, received, pending, or in preparation
- Royalties: As above for Lindhurst and Biesecker
- Donation of medical equipment
References
- 1.Lindhurst MJ, Sapp JC, Teer JK, et al. A mosaic activating mutation in AKT1 associated with the Proteus syndrome. N Engl J Med. 2011;365(7):611–9. doi: 10.1056/NEJMoa1104017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Happle R. Lethal genes surviving by mosaicism: a possible explanation for sporadic birth defects involving the skin. J Am Acad Dermatol. 1987;16(4):899–906. doi: 10.1016/s0190-9622(87)80249-9. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen D, Turner JT, Olsen C, Biesecker LG, Darling TN. Cutaneous manifestations of proteus syndrome: correlations with general clinical severity. Arch Dermatol. 2004;140(8):947–53. doi: 10.1001/archderm.140.8.947. [DOI] [PubMed] [Google Scholar]
- 4.Twede JV, Turner JT, Biesecker LG, Darling TN. Evolution of skin lesions in Proteus syndrome. J Am Acad Dermatol. 2005;52(5):834–8. doi: 10.1016/j.jaad.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 5.Biesecker LG, Happle R, Mulliken JB, et al. Proteus syndrome: diagnostic criteria, differential diagnosis, and patient evaluation. Am J Med Genet. 1999;84(5):389–95. doi: 10.1002/(sici)1096-8628(19990611)84:5<389::aid-ajmg1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 6.Uitto J, Bauer EA, Santa Cruz DJ, Holtmann B, Eisen AZ. Decreased collagenase production by regional fibroblasts cultured from the skin of a patient with connective tissue nevi of the collagen type. J Invest Dermatol. 1982;78(2):136–40. doi: 10.1111/1523-1747.ep12506265. [DOI] [PubMed] [Google Scholar]
- 7.Botella-Estrada R, Alegre V, Sanmartin O, Ros C, Aliaga A. Isolated plantar cerebriform collagenoma. Arch Dermatol. 1991;127(10):1589–90. [PubMed] [Google Scholar]
- 8.Martinez W, Arnal F, Capdevila A, Almagro M. Isolated plantar cerebriform collagenoma. Pediatr Dermatol. 1994;11(1):84–5. doi: 10.1111/j.1525-1470.1994.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 9.Jones MS, Helm KF. A solitary warty plaque. Isolated cerebriform collagenoma Arch Dermatol. 1997;133(7):909–10. 912–3. doi: 10.1001/archderm.1997.03890430131020. [DOI] [PubMed] [Google Scholar]
- 10.Choi JC, Lee MW, Chang SE, et al. Isolated plantar collagenoma. Br J Dermatol. 2002;146(1):164–5. doi: 10.1046/j.1365-2133.2002.4513_2.x. [DOI] [PubMed] [Google Scholar]
- 11.Nico MM, Valente NY, Machado KA. Isolated plantar collagenoma. Acta Derm Venereol. 2003;83(2):144. doi: 10.1080/00015550310007571. [DOI] [PubMed] [Google Scholar]
- 12.Nelson AA, Ruben BS. Isolated plantar collagenoma not associated with Proteus syndrome. J Am Acad Dermatol. 2008;58(3):497–9. doi: 10.1016/j.jaad.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Khanna D, Goel K, Khurana N. Isolated plantar cerebriform collagenoma. Indian J Dermatol Venereol Leprol. 2012;78(5):666. doi: 10.4103/0378-6323.100562. [DOI] [PubMed] [Google Scholar]
- 14.Biesecker L. The challenges of Proteus syndrome: diagnosis and management. Eur J Hum Genet. 2006;14(11):1151–7. doi: 10.1038/sj.ejhg.5201638. [DOI] [PubMed] [Google Scholar]
- 15.Beachkofsky TM, Sapp JC, Biesecker LG, Darling TN. Progressive overgrowth of the cerebriform connective tissue nevus in patients with Proteus syndrome. J Am Acad Dermatol. 2010;63(5):799–804. doi: 10.1016/j.jaad.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biesecker LG, Spinner NB. A genomic view of mosaicism and human disease. Nat Rev Genet. 2013;14(5):307–20. doi: 10.1038/nrg3424. [DOI] [PubMed] [Google Scholar]
- 17.Carpten JD, Faber AL, Horn C, et al. A transforming mutation in the pleckstrin homology domain of the AKT1 in cancer. Nature. 2007;448(7152):439–44. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]


