Abstract
Objective
Though there is growing evidence of brain abnormalities among individuals with Conduct Disorder (CD), the structural neuroimaging literature is mixed and frequently aggregates cortical volume rather than differentiating cortical thickness from surface area. The current study assesses CD-related differences in cortical thickness, surface area, and gyrification as well as volume differences in subcortical structures critical to neurodevelopmental models of CD (amygdala; striatum) in a carefully characterized sample. We also examined whether group structural differences were related to severity of callous-unemotional (CU) traits in the CD sample.
Method
Participants were 49 community adolescents (aged 10-18 years); 22 with CD and 27 healthy comparison youth. Structural MRI was collected and the FreeSurfer image analysis suite was used to provide measures of cortical thickness, surface area and local gyrification as well as subcortical (amygdala and striatum) volumes.
Results
Youths with CD showed reduced cortical thickness in superior temporal cortex. There were also indications of reduced gyrification in ventromedial frontal cortex particularly for youth with CD without comorbid Attention Deficit Hyperactivity Disorder. There were no group differences in cortical surface area. However, the youth with CD also showed reduced amygdala and striatum (putamen and pallidum) volumes. Right temporal cortical thickness was significantly inversely related to severity of CU traits.
Conclusions
Youths with CD show reduced cortical thickness within superior temporal regions, some indication of reduced gyrification within ventromedial frontal cortex and reduced amygdala and striatum (putamen and pallidum) volumes. These results are discussed with reference to neurobiological models of CD.
Keywords: conduct disorder, antisocial, amygdala, striatum, cortical thickness
Introduction
Conduct Disorder (CD) is characterized by increased aggression and antisocial behavior.(1) Moreover, prognosis is poor; many adolescents with CD are at increased risk for later mental health and adjustment problems.(2,3) Neurodevelopmental theories have attributed the emergence of CD and other antisocial disorders to dysfunction in a number of cortical and subcortical regions.(4,5) Three notably interconnected, core areas considered particularly important are the amygdala, striatum and ventromedial frontal cortex(4) though regions such as superior temporal cortex and anterior and posterior cingulate cortex may also be implicated(5). In essence, the suggestion is that amygdala dysfunction leads to impaired stimulus-reinforcement learning, including reinforcement learning from social reinforcers such as sad, fearful and happy expressions(4). Relatedly, the striatal dysfunction disrupts prediction error signaling (the individual is less sensitive to the divergence between expected reinforcement and that received)(6). Prediction error signaling is a critical trigger for reinforcement-based learning(7). Ventromedial frontal cortex represents the expected value of objects and actions(8). Dysfunction in this region means that the individual is less able to choose actions that will optimize reward/minimize punishment. In short, the dysfunction seen in CD leads to an individual who socializes poorly (due to impaired stimulus-reinforcement learning and reduced responsiveness to distress cues) and is impaired in learning and representing the expected value of objects and actions. Reduced sensitivity to the distress of others leads to an increased risk for goal-directed aggression and reduced guilt/empathy and the decision making deficits both contribute to the selection of non-optimal antisocial behaviors and, as a consequence of frustration, increase the risk for reactive aggression(4).
Functional implications have centered on the amygdala9-11 (particularly in the contex of diminished responsivenss to the distress of others12), the caudate and ventromedial frontal cortex (in the context of decision making paradigms6,13-16). With respect to structural MRI, several studies have reported reduced amygdala volume in this population(17-20) though two did not(21, 22). Moreover, cortical volume(17,19,23) and thickness(24) are more generally reduced within temporal cortex in youth with CD. There have also been reports of insula abnormalities–either when indexed by volume,(18-20) cortical thickness(25) or folding(24)—though this is not always seen.(21,22) Ventromedial prefrontal cortex reductions have also been reported—again either when indexed by volume,(17) cortical thickness(25) or folding(24)—though again this is not always seen(18-20,22)—one study reported increases in volume in this region.(21) Reduced caudate volume has been reported twice(19,20) but the absence of replications may only reflect a lack of investigations targeting this area.
Inconsistencies in structural imaging findings may reflect the methodology used. With two very recent exceptions,(24,25) all published structural brain imaging studies of youth with CD have focused exclusively on indexing cortical gray matter volume.(17-22) This is problematic as cortical gray matter volume is a function of both cortical thickness and cortical surface area and these indices are increasingly viewed as separable endophenotypes for understanding the relationship of genetic influences on brain structure and function.(26-28) Because volumetric techniques may obscure the degree to which each factor contributes to cortical gray matter volume differences,(26) focusing on more specific measures of brain structure abnormality might help clarify previously discrepant findings in CD.
Moreover, there has been considerable heterogeneity in the cases investigated; ranging from patients with CD assessed by clinicians(19,24) to cases of CD as identified by computerized self-report(25) to individuals with extensive drug use.(22) In addition, groups have also not always been carefully matched for variables that are associated with individual differences in brain structure (e.g., IQ24). Finally, prior studies have not focused on youth with CD with callous-unemotional (CU) traits. Youth with CD are not considered to be a homogeneous group.(29,30) Indeed, the advisory committee on attention-deficit hyperactivity disorder (ADHD) and Disruptive Behavior Disorders for the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) has proposed modifications of the CD diagnosis to include a CU specifier. As such, increased work in these areas to provide clarity is critical.
The primary purpose of this study was to identify cortical thickness, surface area, and/or local gyrification differences in a well-characterized sample of youth with CD. Given the previous cortical thickness(24,25) and gyrification(24) studies, we predicted that youth with CD would show reduced cortical thickness and gyrification within ventromedial frontal cortex, insula and/or superior temporal and parietal cortices. In addition, we were interested in determining whether there might be subcortical volume differences particularly within structures that are thought, when dysfunctional, to influence the development of CD; i.e., amygdala and striatum.(4, 5) Indeed, considerable fMRI data has shown dysfunction in both regions in CD.(6,9,10,13-16,31) We thus predicted that youth with CD would show reduced amygdala and striatum volumes relative to comparison youth. Our final prediction concerned CU traits. CU traits have been shown to modulate BOLD responses within the amygdala to social cues.(9,10) Therefore, we predicted that group differences in cortical thickness and subcortical volumes would be particularly marked in those youth with elevated CU traits.
Method
Participants
Forty-nine youths participated: 22 youths with CD and 27 healthy comparison youths (Table 1). Youths were recruited from the community through newspaper ads, fliers, and referrals from area mental health practitioners. Statements of informed assent and consent were obtained from participating children and parents. This study was approved by the NIMH IRB.
Table 1.
Demographic characteristics
| Whole Sample Mean (SD) |
IQ-Matched Sample Mean (SD) |
|||
|---|---|---|---|---|
| CD (n=22) | TD (n=27) | CD (n=16) | TD (n=16) | |
| Age | 15.04 (1.91) | 14.85 (2.19) | 14.89 (2.27) | 14.96 (2.01) |
| Full-Scale IQa | 94.05 (10.68) |
110.96 (12.39) |
99.13 (7.05) | 103.56 (9.99) |
| Sex Ratio (M:F) | 16:6 | 17:10 | 11:5 | 10:6 |
| Race (White:Non- White)b |
4:18 | 11:15 | 4:12 | 2:13 |
| ICU traitsc | 38.59 (10.10) |
14.85 (8.20) | 40.86 (11.35) |
17.88 (8.66) |
| APSDc | 28.29 (5.56) | 5.60 (3.45) | 28.40 (6.21) | 6.13 (3.79) |
| Handedness (R:L) | 22:5 | 18:4 | 12:4 | 14:2 |
| ADHD Co- morbidity (no ADHD:ADHD) |
10:12 | 27:0 | 8:8 | 16:0 |
| Psychotropic medication usage at time of scan (no meds:meds) |
13:9 | 27:0 | 11:5 | 16:0 |
Note: ADHD = attention-deficit/hyperactivity disorder.
In the whole sample only, IQ was significantly higher in the typically developing (TD) group than in the conduct disorder (CD) group.
In the TD groups, information on race could not be obtained from one participant.
In both the whole and the IQ-matched samples, Inventory of Callous-Unemotional (ICU) and Antisocial Process Screening Device (APSD) scores were significantly higher in the CD group than in the TD group.
All youths and parents completed Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS32); assessments conducted by a doctoral-level clinician as part of a comprehensive psychiatric and psychological assessment. The K-SADS has demonstrated good validity and inter-rater reliability (kappa >0.75 for all diagnoses32). The K-SADS assesses for substance abuse and substance dependence but, due to exclusion criteria, no children in either group met criteria for these diagnoses. IQ was assessed with the Wechsler Abbreviated Scale of Intelligence (two-subtest form). Exclusion criteria were autism spectrum disorders, Tourette’s syndrome, lifetime history of psychosis, depression, bipolar disorder, generalized, social or separation anxiety disorder, PTSD, neurologic disorder, history of head trauma, history of substance abuse, and IQ<70. In addition, parents completed the Antisocial Process Screening Device, APSD,(33) a measure of psychopathic traits (Table 1). Youths meeting K-SADS criteria for Conduct Disorder were included in the CD group, while comparison subjects did not meet criteria for any K-SADS diagnosis. 82% (18/22) of the CD patients had engaged in illegal/violent behavior and 64% (14/22) of the sample had legal involvement (arrests/charges).
The groups did not differ significantly in terms of age, sex ratio, handedness, or racial composition (ps>.05; Table 1). However, the healthy comparison group had a significantly higher IQ [t(48)=5.05, p<.001]. Because of this, two analyses were conducted. The first examined the whole sample. The second focused on IQ matched groups of youth with CD and typically developing youth (N=16 in each group).
Study Measures
Inventory of Callous-Unemotional Traits (ICU34). The ICU is a 24-item parent-report scale designed to assess callous and unemotional traits in youth. The ICU was derived from the callous-unemotional (CU) scale of the widely used APSD.(33) The construct validity of the ICU has been supported in community and juvenile justice samples.(35,36)
MRI parameters
Participants were scanned using a 1.5-T GE Signa scanner. A high-resolution anatomical scan (3-dimensional spoiled gradient recalled acquisition in a steady state; repetition time=9 milliseconds; echo time=2.872 milliseconds; 24cm field of view; 20° flip angle; 124 axial slices; thickness, 1.5 mm; 256×256 matrix) was obtained covering the whole brain.
The FreeSurfer image analysis suite was used to generate a cortical surface model providing measures of cortical thickness, surface area(37,38) and local gyrification(39) at each vertex. Initial steps in this surface-based, multi-step and semi-automated morphometric pipeline included visual inspection of data for motion artifacts, transformation to Talairach space, intensity normalization for correction of magnetic field inhomogeneities and removal of non-brain tissues (e.g. skull stripping). Cortical thickness representations were constructed using these procedures based on the entire 3D magnetic resonance volume. Cortical thickness was quantified at each surface location or vertex as the distance (in mm) from the gray/white boundary to the pial surface.(38) Spatial intensity gradients across tissue classes were used to create maps that are capable of detecting submillimeter differences between groups because they are not restricted to the voxel resolution of the original data. The resulting surface models generated were reviewed for accuracy and manually edited in all cases due to over-inclusion of white matter around the optic nerve. This method of cortical thickness measurement has been validated against post-mortem brains and histological analysis,(40) hand tracings,(41,42) and other automated methods of quantification(43) and has shown good reliability across sites and platforms(44) (for detailed description of surface area calculation, see45). In short, surface area is quantified by assigning an area to each vertex equal to the average of its surrounding triangles. When the vertex areas are summed over all vertices, the total is equal to the sum of the areas of the triangles. Finally, tessellated hull surface and pial surface contours derived from FreeSurfer provide vertex-based surfaces for calculating local gyrification. Using each vertex as the center point of a spherical 25mm region of interest, the ratio of the convoluted pial surface to the closely fitting hull perimeter provides a fine-grain local gyrification metric.(39) Vertex-level cortical thickness, surface area, and gyrification values were obtained and mapped onto a normalized cortical surface. These cortical maps were smoothed with a 15mm (for cortical thickness and surface area) or 5mm (for gyrification) full width at half maximum kernel.
Lateralized subcortical volumes (of the caudate, putamen, pallidum, and amygdala) were also calculated using FreeSurfer’s automatic segmentation pipeline.(46) The pipeline performed a skull strip on each subject’s structural scan. The remaining voxels were assigned one of 37 neuroanatomical labels derived from probabilistic information based on a labeled training set. The technique used a registration method that accurately accounted for a wide variety of anatomical variability and has demonstrated accuracy rates comparable to manual labeling(46) and exhibits good reliability.(47)
Hippocampal-amygdala boundaries created by the FreeSurfer segmentation pipeline were each manually inspected in order to be conservative.(48) Corrections were made in tkmedit (a program within the FreeSurfer suite) when the boundaries of the segmentation did not align with the amygdala borders defined by previous work.(49,50) Only 2 scans in the CD group and 5 scans in the control group required manual edits. The rater was not blind to group assignment; however, the pattern of results reported below remained the same if unedited amygdala volumes were utilized instead. The hippocampal-amygdala border was first viewed in the sagittal plane and then in the axial plane to verify that the pipeline correctly labeled the voxels on either side of the hippocampal-amygdala transition area. Incorrectly labeled voxels were relabeled to avoid overestimation of the amygdala volume by accidental inclusion of any of the hippocampal voxels. The anterior amygdaloid area was viewed in the axial plane and manual edits were performed if the amygdala volume was overestimated.
Statistical analysis
Group differences in cortical thickness, surface area, and gyrification were examined on the surface maps vertex by vertex using a least squares general linear model (with and without the inclusion of intracranial volume, age, and IQ as covariates). To control for multiple comparisons, cluster correction was completed using Monte Carlo simulation with 10,000 iterations (vertex-wise threshold of p<.05). For more details, see: http://ftp.nmr.mgh.harvard.edu/fswiki/FsTutorial/QdecGroupAnalysis. Subcortical volumes were compared between groups using one-way analysis of covariance with intracranial volume (on which groups did not differ; p>.5) as a covariate and subsequently including age and IQ as additional covariates. Given significant levels of co-morbidity with ADHD, we re-ran the analyses and compared the typically developing group with both the youth with CD and co-morbid ADHD (N=12) and youth without co-morbid ADHD (N=10) separately. Our final analysis examined the relationship between cortical thickness/subcortical volumes in the regions showing CD-typically developing group differences and severity of CU traits.
Results
Initially, the potential influence of sex differences was tested; however, no significant interactions with sex were found; hence, the main results below include comparisons of groups composed of both males and females. Furthermore, re-running the analyses including males only (given the small number of females) provides the same pattern of results as reported below.
Cortical Thickness, Surface Area, and Gyrification
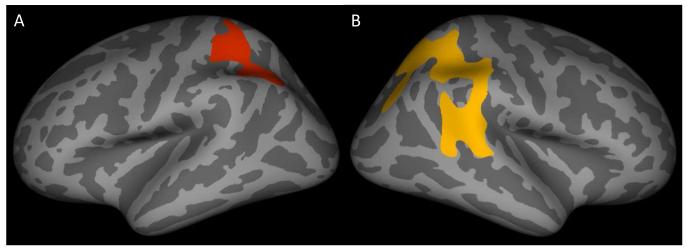
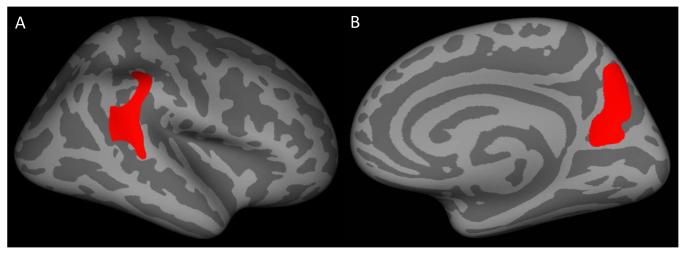
Decreased cortical thickness was found in two large clusters of posterior cortices, which were located in left superior/inferior parietal cortex and right superior temporal/parietal cortex (Table S1, available online; Figures 1A/1B; cluster corrected p<.05). Similarly, thinner cortex was also found within the IQ-matched samples of youth with CD as compared to typically developing children. As in the larger group analysis, right superior temporal cortex was a site of thinner cortex, but differing from the earlier analyses was the finding of thinner right precuneus extending into posterior cingulate cortex (Table S2, available online; Figures 2A/2B). No significant differences were detected in regions outside of these areas, and no brain regions were significantly thicker in the youth with CD as compared with the typically developing group. Diminished cortical thickness in youth with CD was not the result of tissue compartment (i.e., grey matter to white matter) substitution, as white matter volumes were not different between the groups.
Figure 1.
Inflated surface maps (dark gray=sulci; light gray=gyri) of the surviving clusters (p<.05) showing thinner cortex in the whole sample of youths with conduct disorder as compared to typically developing controls: (A) left superior/inferior parietal cortex and (B) right superior temporal/parietal cortex. Note: See Table S1, available online, for information on the surviving clusters.
Figure 2.
Inflated surface maps (dark gray=sulci; light gray=gyri) of the surviving clusters (p<.05) showing thinner cortex in the IQ-matched sample of youths with conduct disorder as compared to typically developing controls: (A) right superior temporal cortex and (B) right precuneus. Note: See Table S2, available online, for information on the surviving clusters.
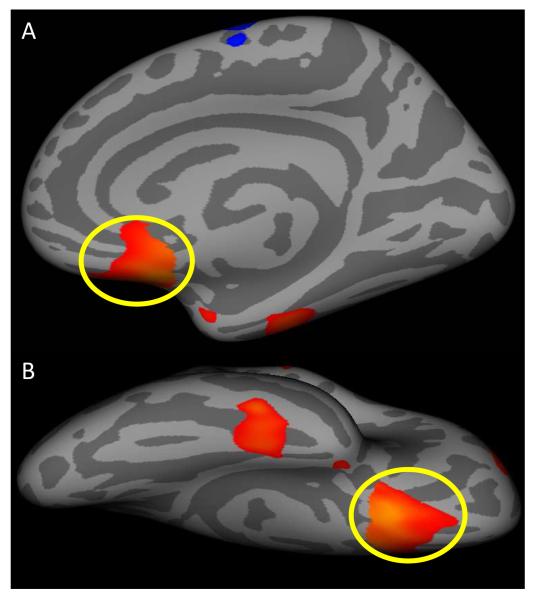
There were no group differences with respect to surface area. However, there were weak indications of group differences in gyrification within right ventromedial frontal cortex, a region of a priori predicted group differences (Figure 3). It is important to note though that this reduction in gyrification in ventromedial frontal cortex did not survive cluster correction. Moreover, the group difference was muted to trend levels when comparing the IQ-matched groups (F=3.28, p=.08).
Figure 3.
(A) Medial and (B) ventral views displayed on inflated surface maps (dark gray=sulci; light gray=gyri) of the uncorrected (p<.05) right ventromedial prefrontal region (circled in yellow) showing decreased gyrification in the whole sample of youths with conduct disorder as compared to typically developing controls.
Including intracranial volume, age, and IQ as covariates resulted in a highly similar pattern of findings to those reported above for cortical thickness, surface area, and gyrification.
Subcortical volumes
When examining group differences for subcortical volumes using the whole sample after covarying intracranial volume, we found that the youth with CD showed reduced amygdala (LH and RH: Fs>7.15, ps<.01), putamen (LH and RH: Fs>11.73, ps<.001), pallidum (RH: F=4.50, p<.05) and caudate volumes (LH: F=4.75, p<.05). Importantly, most of these regions remained significantly smaller in the CD group following the introduction of age and IQ as additional covariates: amygdala (RH: F=6.11, p<.05), putamen (LH and RH: Fs>7.71, ps<.01), pallidum (RH: F=5.28, p<.05). Moreover, when examining the groups matched for IQ after covarying intracranial volume, we again found that the youth with CD showed reduced amygdala (LH: F=5.38, p<.05; RH: F=7.73, p<.01), putamen (LH and RH: Fs>11.76, ps<.01) and pallidum (LH and RH: Fs>4.91, ps<.05) volumes.
Co-morbidity with ADHD
Both the youth with CD and co-morbid ADHD and youth without co-morbid ADHD showed significantly thinner cortex (p<.05) than the typically developing children in all of the extracted clusters derived from CD-typically developing group differences (both matched and unmatched analyses). Notably, when compared to one another, the youth with CD and co-morbid ADHD and those with CD without co-morbid ADHD showed no group differences in cortical thickness for any of these regions.
The presence of co-morbid ADHD did affect the gyrification finding. Youth with CD without comorbid ADHD showed significantly reduced gyrification in the right ventromedial cortex relative to the typically developing youth (F=14.60, p=.001) and the CD group with co-morbid ADHD (F=6.15, p=.02). Gyrification in the typically developing youth and the youth with CD with comorbid ADHD did not significantly differ (F=1.37, p=.25).
Similar to earlier reported subcortical results, we found that the youth with CD without co-morbid ADHD (N=10) showed reduced amygdala (LH and RH: Fs>5.47, ps<.05), putamen (LH and RH: Fs>8.11, ps<.01), and caudate volumes (LH: F=5.73, p<.05) compared to the typically developing controls (N=27). However, relative to typically developing controls, the youth with CD and co-morbid ADHD (N=12) exhibited significant subcortical volumetric reductions limited to the putamen (LH and RH: Fs>5.00, ps<.05). Finally, as with cortical thickness above, subcortical volumes for the youth with CD and co-morbid ADHD did not differ significantly from those in the CD group without co-morbid ADHD.
Callous-Unemotional Traits and Structural Differences
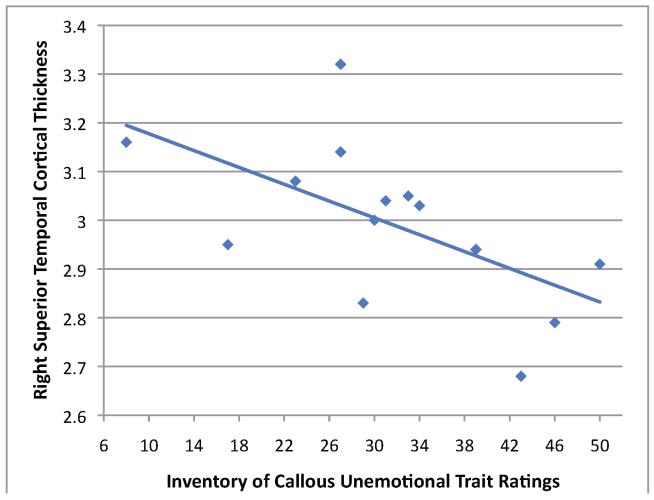
There was a significant inverse relationship between level of CU traits in the IQ-matched group of youth with CD and mean cortical thickness of the region of superior temporal cortex depicted in Figure 2A (r=-.60, p=.02; Figure 4). However, no other significant relationships were found.
Figure 4.
Within the IQ-matched group of youth with conduct disorder, scatterplot of the correlation between mean cortical thickness in the right superior temporal cortex cluster surviving cluster correction (as depicted in Figure 2A) and parent-rated callous-unemotional traits (R2=.36).
Discussion
The current study examined cortical thickness, surface area, local gyrification, and volumes of several critical subcortical structures in a sample of youth with CD. This study found reduced cortical thickness within extensive regions of infero-parietal cortex (extending into temporal-parietal junction, superior temporal cortex and precuneus/posterior cingulate cortex in the right hemisphere), in the youth with CD relative to the typically developing group, after applying a cluster correction. These remained present (albeit concentrating in superior temporal cortex and precuneus extending into posterior cingulate cortex) in smaller, carefully matched groups of CD and typically developing youth. While surface area was comparable between groups, we found indications of reduced gyrification within right ventromedial cortex among the youth with CD. Finally, the youth with CD showed smaller striatal (putamen) and amygdala volumes than the typically developing youth.
The cortical thickness data showed notable similarity with those of Hyatt et al.(22) In this previous study, patients with CD also showed thinner cortex in an extensive region of inferoparietal cortex. However, this previous study did not match groups for IQ. In the current study, when the groups were matched for IQ, the group differences in parietal cortex were mostly lost. In contrast, those for superior temporal cortex were not. Interestingly, the region of superior temporal cortex showing cortical thinning here in the youth with CD is proximal to a region displaying thinner cortex in a sample of adults with psychopathy.(51) Indeed, it was cortical thickness within this region that correlated with callous-unemotional traits, the core emotional component of psychopathy, in the current study.
Despite the robustness of these findings, their interpretation remains unclear. Both of the major neurobiological positions on CD/psychopathy have considered superior temporal cortex to be potentially dysfunctional.(5,52) However, neither has suggested a computational account of what functional processes might be disrupted by any putative dysfunction. They could reflect a developmental consequence of the functional deficits in the amygdala, a region where dysfunction is consistently implicated in psychopathy;(52) i.e., there might be no specific temporal cortical dysfunction but rather simply morphological impact. Alternatively, the results may relate to the well documented impairment in stimulus-reinforcement learning;(52) i.e., the capacity to learn some stimuli are associated with positive outcomes and others with negative outcomes. Stimulus-reinforcement learning involves the integrated functioning of the amygdala and temporal cortex–as such the function/structure of both of these highly interconnected regions may be inevitably compromised in individuals with psychopathy. Finally, it is also possible that there may be specific computational processes, as yet unidentified, that are reliant on temporal cortex and which are compromised in individuals with this disorder. With respect to this last point, it should be noted that patients with autism also show thinner cortex within these temporal regions.(28) Autism is another disorder of social cognition albeit one marked by notably different computational impairments than psychopathy; e.g., Theory of Mind (the capacity to include the mental states of others; i.e., their beliefs, intentions and knowledge in one’s own internal representation of their identity) is impaired in autism but intact in psychopathy.(52) It is possible that there is/are currently unidentified social cognitive function(s), reliant on superior temporal cortex, which are dysfunctional in both disorders.
Amygdala dysfunction is considered central to understanding the basis of the deficits underlying CD.(4) In particular, it is argued that amygdala dysfunction compromises the individual’s ability to learn the reinforcement value (including social reinforcement value determined by the facial responses of others) of stimuli and actions.(4) Supporting the suggestion of amygdala dysfunction in this population, the youth with CD in the current sample showed reduced amygdala volumes relative to the typically developing youth. This is consistent with several functional imaging studies where youth with CD have shown reduced amygdala responses to the fear and sadness of others(9-12) and during moral judgment.(31) It is also in line with several previous structural imaging studies investigating CD.(17-20) Two previous structural imaging studies did not report reduced amygdala volumes in CD.(21,22) However, one involved subclinical cases(21) while the second involved a population with extensive recreational drug experience.(22) Given that amygdala volumes did not correlate with CU trait ratings in the CD group, it may be that amygdala volumetric reductions are associated with CD more broadly, though limited statistical power might also be the culprit. Future work with a larger sample size and including a CD group without elevated CU trait ratings would assist in clarifying this point.
There has been a growing recent interest in striatal abnormalities in youth with CD as well as in adults with psychopathy.(6,13,14) Thus, three studies reported atypical responses to reinforcement in the caudate of youth with CD consistent with a fundamental dysfunction in prediction error signaling(6,13,14) More recently, three structural imaging studies have reported striatal abnormalities in youth with CD(19,20) and adults with psychopathy.(53) The current study, like the previous studies of youth with CD,(19,20) revealed reduced striatal volumes (particularly within the putamen in the current study). However, the study with adults with psychopathic tendencies found increased caudate volumes.(53) Whether this reflects developmental differences or a chance result is currently unclear.
While we did see group differences in the structural integrity of ventromedial prefrontal cortex in this study, they were not robust. Thus, while youth with CD showed reduced gyrification within this region, the finding did not survive cluster correction. Interestingly, the presence/absence of comorbid ADHD had an impact on the findings. It was only youth with CD without comorbid ADHD who showed reduced ventromedial frontal cortex gyrification relative to healthy youth. Moreover, this reduction in the youth with CD without comorbid ADHD was also seen relative to the youth with CD with comorbid ADHD. Structural neuroimaging work on patients with ADHD has implicated a developmental delay in frontal, parietal and temporal cortical development.(54-56) However, there has been relatively little work implicating ventromedial prefrontal cortex. As such these ventromedial prefrontal cortex results, especially considering the inconsistency in findings in previous structural imaging work in CD,(17-22,24,25) should be treated with some caution.
We did not see group differences in cortical thickness for the insula. However, it should be noted that the previous literature is somewhat mixed. Reduced insula volume has been reported in three studies(18-20) but was not seen in a further two studies.(21,22) Similarly, reduced insular cortical thickness was seen in CD in one study(25) but not another.(24) Moreover, it should be noted the results of fMRI studies of insula responsiveness in CD have been mixed. There are indications of reduced insula responsiveness to expected value information in the context of decision making paradigms(6,16,57) even if the insula response to punishment information may be intact.(14) There have also been indications of reduced insula responses to emotional expressions(11) though this is often not seen.(9,10,12) In short, there are reasons to believe that some (though not all) functions of the insula are disrupted in CD and it is possible that in some (though not all) patients these impairments also manifest structurally.
Several caveats should be considered with respect to the current data. First, we did not include an ADHD comparison group. This was because previous work had indicated that youths with ADHD do not present with the pathophysiology found in youth with CD.(10,14,15) However, mitigating this limitation, it is important to note that our subsequent group analysis excluding youth with CD and comorbid ADHD revealed extremely similar results for the cortical thickness, gyrification, and subcortical volumetric analyses. Second, the patient and control groups significantly differed in terms of their IQs. Mitigating this limitation, the results of the initial analyses across metrics survive post-hoc IQ covariation, and subsequent analyses with an IQ matched sample again identified highly similar regions showing significant group differences. Although sex differences in neuroanatomy during childhood and adolescence are well-established(58) and brain structural abnormalities have been demonstrated in females with CD(59), the present study included too few females to adequately address the question of sex differences in brain structure among youth with CD. To this end, similar anatomical work with larger samples of youth with CD is needed, given that additional statistical power may allow detection of subtler abnormalities and possible sex differences.
In summary, youth with CD showed reduced cortical thickness within superior temporal cortex, some indication of reduced gyrification within ventromedial frontal cortex and reduced amygdala and striatum (putamen and pallidum) volumes. These results are consistent with models stressing that dysfunction of the amygdala in response to distress cues and the ventromedial prefrontal cortex and striatum in reinforcement related processing during decision making contribute to the development of CD.(4) Moreover, they stress the importance of determining what the (or whether there are) functional implications of the reduced cortical thickness within superior temporal cortex are.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program at NIMH, NIH under grant number 1-ZIA-MH002860-08. Ethics approval for this study was granted by the NIH Combined Neuroscience Institutional Review Board under protocol number 05-M-0105.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental material cited in this article is available online.
Disclosure: Drs. Wallace, White, Sinclair, Hwang, Martin, and Blair, and Ms. Robustelli report no biomedical financial interests or potential conflicts of interest.
References
- 1.Frick PJ, Stickle TR, Dandreaux DM, Farell JM, Kimonis ER. Callous-unemotional traits in predicting the severity and stability of conduct problems and delinquency. J Abnorm Child Psychol. 2005;33:471–87. doi: 10.1007/s10648-005-5728-9. [DOI] [PubMed] [Google Scholar]
- 2.Fergusson DM, Boden JM, Horwood LJ. Classification of behavior disorders in adolescence: Scaling methods, predictive validity, and gender differences. J Abnorm Psychol. 2010;119:699–712. doi: 10.1037/a0018610. [DOI] [PubMed] [Google Scholar]
- 3.Robins LN. Deviant Children Grow Up. Williams and Wilkins; Baltimore: 1966. [Google Scholar]
- 4.Blair RJR. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends Cogn Sci. 2007;11(9):387–92. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Anderson NE, Kiehl KA. The psychopath magnetized: insights from brain imaging. Trends Cogn Sci. 2012;16(1):52–60. doi: 10.1016/j.tics.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White SF, Pope K, Sinclair S, Fowler KA, Brislin SJ, Williams WC, et al. Disrupted expected value and prediction error signaling in youths with disruptive behavior disorders during a passive avoidance task. Am J Psychiatry. 2013;170(3):315–23. doi: 10.1176/appi.ajp.2012.12060840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rescorla Wagner. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Black A, Prokasy W, editors. Classical Conditioning II: Current Research and Theory. II. Appleton Crofts; New York: 1972. pp. 64–99. [Google Scholar]
- 8.O’Doherty JP. Contributions of the ventromedial prefrontal cortex to goal-directed action selection. Ann N Y Acad Sci. 2011;1239:118–29. doi: 10.1111/j.1749-6632.2011.06290.x. [DOI] [PubMed] [Google Scholar]
- 9.White SF, Marsh AA, Fowler KA, Schechter JC, Adalio C, Pope K, et al. Reduced amygdala response in youths with disruptive behavior disorders and psychopathic traits: decreased emotional response versus increased top-down attention to nonemotional features. Am J Psychiatry. 2012;169(7):750–8. doi: 10.1176/appi.ajp.2012.11081270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marsh AA, Finger EC, Mitchell DG, Reid ME, Sims C, Kosson DS, et al. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatry. 2008;165(6):712–20. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- 11.Passamonti L, Fairchild G, Goodyer IM, Hurford G, Hagan CC, Rowe JB, et al. Neural abnormalities in early-onset and adolescence-onset conduct disorder. Arch Gen Psychiatry. 2010;67(7):729–38. doi: 10.1001/archgenpsychiatry.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones AP, Laurens KR, Herba CM, Barker GJ, Viding E. Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. Am J Psychiatry. 2009;166:95–102. doi: 10.1176/appi.ajp.2008.07071050. [DOI] [PubMed] [Google Scholar]
- 13.Finger EC, Marsh AA, Blair KS, Reid ME, Sims C, Ng P, et al. Disrupted reinforcement signaling in the orbitofrontal cortex and caudate in youths with conduct disorder or oppositional defiant disorder and a high level of psychopathic traits. Am J Psychiatry. 2011;168(2):152–62. doi: 10.1176/appi.ajp.2010.10010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finger EC, Marsh AA, Mitchell DG, Reid ME, Sims C, Budhani S, et al. Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Arch Gen Psychiatry. 2008;65(5):586–94. doi: 10.1001/archpsyc.65.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubia K, Smith AB, Halari R, Matsukura F, Mohammad M, Taylor E, et al. Disorder-specific dissociation of orbitofrontal dysfunction in boys with pure conduct disorder during reward and ventrolateral prefrontal dysfunction in boys with pure ADHD during sustained attention. Am J Psychiatry. 2009;166(1):83–94. doi: 10.1176/appi.ajp.2008.08020212. [DOI] [PubMed] [Google Scholar]
- 16.Crowley TJ, Dalwani MS, Mikulich-Gilbertson SK, Du YP, Lejuez CW, Raymond KM, et al. Risky decisions and their consequences: Neural processing by boys with antisocial substance disorder. PLoS ONE. 2010;5(9):e12835. doi: 10.1371/journal.pone.0012835. doi: 10.1371/journal.pone.0012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huebner B, Vloet TD, Marx I, Konrad K, Fink GR, Herpertz SC, et al. Morphometric brain abnormalities in boys with Conduct Disorder. J Am Acad Child Adolesc Psychiatry. 2008;47:540–7. doi: 10.1097/CHI.0b013e3181676545. [DOI] [PubMed] [Google Scholar]
- 18.Sterzer P, Stadler C, Poustka F, Kleinschmidt A. A structural neural deficit in adolescents with conduct disorder and its association with lack of empathy. Neuroimage. 2007;37(1):335–42. doi: 10.1016/j.neuroimage.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 19.Fairchild G, Passamonti L, Hurford G, Hagan CC, von dem Hagen EA, van Goozen SH, et al. Brain structure abnormalities in early-onset and adolescent-onset conduct disorder. Am J Psychiatry. 2011;168(6):624–33. doi: 10.1176/appi.ajp.2010.10081184. [DOI] [PubMed] [Google Scholar]
- 20.Fairchild G, Hagan CC, Walsh ND, Passamonti L, Calder AJ, Goodyer IM. Brain structure abnormalities in adolescent girls with conduct disorder. J Child Psychol Psychiatry. 2013;54(1):86–95. doi: 10.1111/j.1469-7610.2012.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Brito SA, Mechelli A, Wilke M, Laurens KR, Jones AP, Barker GJ, et al. Size matters: Increased grey matter in boys with conduct problems and callous-unemotional traits. Brain. 2009;132:843–52. doi: 10.1093/brain/awp011. [DOI] [PubMed] [Google Scholar]
- 22.Dalwani M, Sakai JT, Mikulich-Gilbertson SK, Tanabe J, Raymond K, McWilliams SK, et al. Reduced cortical gray matter volume in male adolescents with substance and conduct problems. Drug Alcohol Depend. 2011;118(2-3):295–305. doi: 10.1016/j.drugalcdep.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krusei MJP, Casanova MF, Mannheim G, Johnson-Bilder A. Reduced temporal lobe volume in early onset conduct disorder. Psychiatry Res: Neuroimaging. 2004;132:1–11. doi: 10.1016/j.pscychresns.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Hyatt CJ, Haney-Caron E, Stevens MC. Cortical thickness and folding deficits in conduct-disordered adolescents. Biol Psychiatry. 2012;72(3):207–14. doi: 10.1016/j.biopsych.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fahim C, He Y, Yoon U, Chen J, Evans A, Perusse D. Neuroanatomy of childhood disruptive behavior disorders. Aggress Behav. 2011;37(4):326–37. doi: 10.1002/ab.20396. [DOI] [PubMed] [Google Scholar]
- 26.Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19(11):2728–35. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, et al. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53(3):1135–46. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallace GL, Dankner N, Kenworthy L, Giedd JN, Martin A. Age-related temporal and parietal cortical thinning in autism spectrum disorders. Brain. 2010;133:3745–54. doi: 10.1093/brain/awq279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pardini DA, Frick PJ, Moffitt TE. Building an evidence base for DSM-5 conceptualizations of oppositional defiant disorder and conduct disorder: introduction to the special section. J Abnorm Psychol. 2010;119(4):683–8. doi: 10.1037/a0021441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blair RJR. Neuro-cognitive models of aggression, the Antisocial Personality Disorders and Psychopathy. J Neurol Neurosurg Psychiatry. 2001;71:727–31. doi: 10.1136/jnnp.71.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marsh AA, Finger EC, Fowler KA, Jurkowitz IT, Schechter JC, Yu HH, et al. Reduced amygdala-orbitofrontal connectivity during moral judgments in youths with disruptive behavior disorders and psychopathic traits. Psychiatry Res. 2011;194(3):279–86. doi: 10.1016/j.pscychresns.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADSPL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 33.Frick PJ, Hare RD. The antisocial process screening device. Multi-Health Systems; Toronto: 2001. [Google Scholar]
- 34.Frick PJ. Unpublished rating scale. University of New Orleans; 2004. Inventory of callous–unemotional traits. [Google Scholar]
- 35.Essau CA, Sasagawa S, Frick PJ. Callous-unemotional traits in a community sample of adolescents. Assessment. 2006;13:459–69. doi: 10.1177/1073191106287354. [DOI] [PubMed] [Google Scholar]
- 36.Kimonis ER, Frick PJ, Skeem JL, Marsee MA, Cruise K, Munoz LC. Assessing callous– unemotional traits in adolescent offenders: validation of the Inventory of Callous–Unemotional Traits. Int J Law Psychiatry. 2008;31:241–52. doi: 10.1016/j.ijlp.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 38.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97(20):11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schaer M, Cuadra MB, Tamarit L, Lazeyras F, Eliez S, Thiran JP. A surface-based approach to quantify local cortical gyrification. IEEE Trans Med Imaging. 2008;27(2):161–70. doi: 10.1109/TMI.2007.903576. [DOI] [PubMed] [Google Scholar]
- 40.Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, et al. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58(5):695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- 41.Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60(9):878–88. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- 42.Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14(7):721–30. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- 43.Clarkson MJ, Cardoso MJ, Ridgway GR, Modat M, Leung KK, Rohrer JD, et al. A comparison of voxel and surface based cortical thickness estimation methods. Neuroimage. 2011;57(3):856–65. doi: 10.1016/j.neuroimage.2011.05.053. [DOI] [PubMed] [Google Scholar]
- 44.Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32(1):180–94. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 45.Winkler AM, Sabuncu MR, Yeo BT, Fischl B, Greve DN, Kochunov P, et al. Measuring and comparing brain cortical surface area and other areal quantities. Neuroimage. 2012;61(4):1428–43. doi: 10.1016/j.neuroimage.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 47.Jovicich J, Czanner S, Han X, Salat D, van der Kouwe A, Quinn B, et al. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage. 2009;46:177–92. doi: 10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dewey J, Hana G, Russell T, Price J, McCaffrey D, Harezlak J, et al. Reliability and validity of MRI-based automated volumetry software relative to auto-assisted manual measurement of subcortical structures in HIV-infected patients from a multisite study. Neuroimage. 2010;51(4):1334–44. doi: 10.1016/j.neuroimage.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Convit A, McHugh P, Wolf OT, de Leon MJ, Bobinski M, De Santi S, et al. MRI volume of the amygdala: a reliable method allowing separation from the hippocampal formation. Psychiatry Res. 1999;90(2):113–23. doi: 10.1016/s0925-4927(99)00007-4. [DOI] [PubMed] [Google Scholar]
- 50.Doty TJ, Payne ME, Steffens DC, Beyer JL, Krishnan KR, LaBar KS. Age-dependent reduction of amygdala volume in bipolar disorder. Psychiatry Res. 2008;163(1):84–94. doi: 10.1016/j.pscychresns.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ly M, Motzkin JC, Philippi CL, Kirk GR, Newman JP, Kiehl KA, et al. Cortical thinning in psychopathy. Am J Psychiatry. 2012;169(7):743–9. doi: 10.1176/appi.ajp.2012.11111627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blair RJR. Fine cuts of empathy and the amygdala: dissociable deficits in psychopathy and autism. Quarterly Journal of Experimental Psychology. 2008;61(1):157–70. doi: 10.1080/17470210701508855. [DOI] [PubMed] [Google Scholar]
- 53.Glenn AL, Raine A, Yaralian PS, Yang Y. Increased volume of the striatum in psychopathic individuals. Biol Psychiatry. 2010;67(1):52–8. doi: 10.1016/j.biopsych.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shaw P, Malek M, Watson B, Sharp W, Evans A, Greenstein D. Development of cortical surface area and gyrification in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;72(3):191–7. doi: 10.1016/j.biopsych.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci USA. 2007;104(49):19649–54. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Almeida Montes LG, Prado Alcantara H, Martinez Garcia RB, De La Torre LB, Avila Acosta D, Duarte MG. Brain cortical thickness in ADHD: age, sex, and clinical correlations. J Atten Disord. 2013;17(8):641–54. doi: 10.1177/1087054711434351. [DOI] [PubMed] [Google Scholar]
- 57.White SF, Brislin SJ, Meffert H, Sinclair S, Blair RJ. Callous-unemotional traits modulate the neural response associated with punishing another individual during social exchange: a preliminary investigation. J Pers Disord. 2013;27(1):99–112. doi: 10.1521/pedi.2013.27.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36(4):1065–73. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fairchild G, Hagan CC, Walsh ND, Passamonti L, Calder AJ, Goodyer IM. Brain structure abnormalities in adolescent girls with conduct disorder. J Child Psychol Psychiatry. 2013;54(1):86–95. doi: 10.1111/j.1469-7610.2012.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.