Abstract
Among vertebrates, startle responses are a ubiquitous method for alerting, and avoiding or escaping from alarming or dangerous stimuli. In zebrafish larvae, fast escape behavior is easily evoked through either acoustic or tactile stimuli. For example, a light touch to the head will excite trigeminal neurons that in turn excite a large reticulospinal neuron in the hindbrain called the Mauthner cell (M-cell). The M-cell action potential then travels down the contralateral trunk of the larva exciting motoneurons, which subsequently excite the entire axial musculature, producing a large amplitude body bend away from the source of the stimulus. This body conformation is known as the “C-bend” due to the shape of the larva during the behavior. As a result of the semi-synchronized activation of the M-cell, the population of motor neurons, and the axial trunk muscles, a large field potential is generated and can be recorded from free-swimming or fixed-position larvae.
Undergraduate laboratories that record field potentials during escape responses in larval zebrafish are relatively simple to setup and allow students to observe and study the escape reflex circuit. Furthermore, by testing hypotheses, analyzing data and writing journal-style laboratory reports, students have multiple opportunities to learn about many neuroscience topics including vertebrate reflexes; sensory transduction; synaptic-, neuro-, and muscle-physiology; the M-cell mediated escape response; and the zebrafish as a model organism. Here, we detail the equipment, software, and recording setup necessary to observe field potentials in an undergraduate teaching lab. Additionally, we discuss potential advanced laboratory exercises and pedagogical outcomes. Finally, we note possible low-cost alternatives for recording field potentials.
Keywords: zebrafish, Mauthner cells, field potentials, electromyography (EMG), electrophysiology, neurophysiology, escape responses, motor behavior
The startle response of larval zebrafish provides an excellent opportunity for an undergraduate neuroscience laboratory. Shared with all vertebrates in one form or another, the startle response, also called the escape response, is one of the zebrafish’s most readily demonstrated behaviors. When confronted with an abrupt or noxious stimulus, a fish will rapidly bend its body away from the stimulus and quickly swim several body lengths from the source (Eaton et al., 1977a). Zebrafish larvae develop this coordinated escape behavior around 2 days post fertilization (dpf) and continue to display the behavior throughout development and adulthood (Eaton et al., 1977b).
The startle response is characterized by an initial high-amplitude body bend called the “C-bend” or “C-start,” named after the larva’s body shape during the movement (Figure 1; see Olson et al., 2010). This fast onset behavior is primarily initiated by activation of one of a pair of large reticulospinal neurons located in the hindbrain known as the Mauthner cells or M-cells (Zottoli, 1977; Liu and Fetcho, 1999). Following activation of sensory receptors, excitatory neurotransmission onto the M-cell leads to propagation of an action potential down its long axon, which in turn excites primary motor neurons in the contralateral trunk of the fish (Figure 2; see Eaton et al., 2001). Motor neuron excitation and action potential firing then leads to acetylcholine neurotransmitter release onto axial trunk muscles, whose collective excitation and contraction produces the stereotyped C-bend. The synchronized and coordinated muscle contraction together with excitation of the M-cell and motoneurons, generates a large extracellular “field” potential that can be recorded from zebrafish larvae (Eaton and Farley, 1975; Prugh et al., 1982; Issa et al., 2011).
Figure 1.

The escape response of a 5 day post fertilization zebrafish larva captured with a high-speed camera. In the first frame on the left, the zebrafish is startled and an escape response is initiated. In the second frame the C-bend can be seen. The next two frames show the larva swimming away from the source of the stimulus. The upper right corner of each frame indicates the time from stimulus onset.
Figure 2.
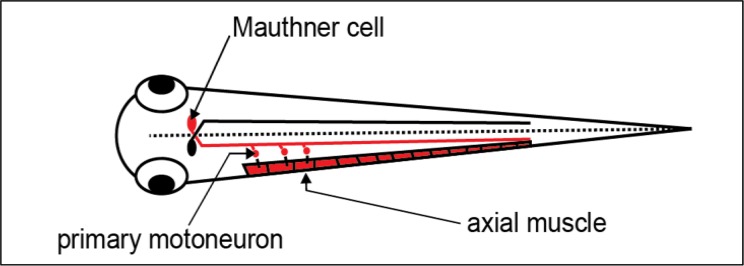
The Mauthner cell synapses on primary motoneurons. This diagram shows a dorsal view of the zebrafish larva and the M-cell’s soma and long axon. For clarity, connections between the Mauthner cell and primary motor neurons are only shown on one side of the larval. The Mauthner cells receive extensive sensory input, and their axons cross the midline (dashed line) to synapse on contralateral primary motoneurons down the length of the trunk. For clarity, cell bodies of primary motor neurons are only shown for three muscle units. Action potentials from the M-cell excite the motoneurons, whose action potentials in turn release acetylcholine, exciting the axial trunk muscles. The coordinated muscle activation results in the C-bend behavioral response that occurs at the beginning of the fast escape response. And the concerted excitation of the M-cell, motor neurons, and skeletal muscles together contribute to the field potential measured in this article.
Many undergraduate neuroscience laboratories rely on dissected preparations using the cockroach, earthworm, crayfish, fruit fly or other organism to teach electrophysiology and neurophysiology (Olivo, 2003; Krans et al., 2005; Johnson et al., 2007; Ramos et al., 2007; Kladt et al., 2010; Vilinsky and Johnson, 2012; Dagda et al., 2013). Here, we describe a method to record field potentials from intact zebrafish during startle responses using both free-swimming or fixed-position larvae, providing students an opportunity to follow a physical stimulus through the nervous system to a behavioral outcome.
MATERIALS AND METHODS
Animal welfare
Since the experiments described here are performed on a vertebrate organism, instructors are encouraged to obtain any necessary approval from their Institutional Animal Care and Use Committee (IACUC). All the animal protocols described here were approved by the IACUC at Amherst College under assurance number 3925-1 with the Office of Laboratory Animal Welfare.
Zebrafish husbandry
Adult zebrafish (Danio rerio) are small, simple to care for, and most importantly, they are easy to breed and produce a large number of fertilized embryos (often >100) with each mating. Due to the popularity of the zebrafish as a model organism, many colleges and universities already have zebrafish facilities, which can provide larvae for weekly experiments throughout a semester. For institutions without access to a zebrafish facility, zebrafish can be found at most local pet stores (Westerfield, 2000). Caring for zebrafish is significantly easier than most vertebrate animals and there are a wide variety of resources for information on zebrafish husbandry. We recommend the Zebrafish Book and the ZFIN Protocol Wiki (see Table 1 and Westerfield, 2000).
Table 1:
Zebrafish husbandry and supplies.
| http://zfin.org/zf_info/zfbook/zfbk.html (husbandry guidelines) |
| https://wiki.zfin.org/display/prot/ZFIN+Protocol+Wiki |
| https://www.aaalac.org/accreditation/refresources/zebrafishreference.pdf |
| Adult male and female zebrafish for breeding (4 to 6 pairs) |
| Aquarium or fish facility |
| Mating chambers (for male-female pairs of zebrafish) http://aquatichabitats.com/consumables/breeding-and-nursery/info/breeding-tank-sets/ |
| Tea strainer (for collecting fertilized eggs) http://adaptivesciencetools.com/le3indiststs.html |
| Petri dishes: large (100×15 mm) and small (35 or 60 mm) |
| Plastic transfer pipettes (8 mL) |
| Incubator kept at 28.5 °C for raising embryos |
| Embryo medium (for rinsing and raising zebrafish embryos) http://zfin.org/zf_info/zfbook/chapt10.html-wptohtml16 |
| Zebrafish larvae between 2 and 7 dpf (for experiments) |
Zebrafish mating and embryo collection
Zebrafish mating is coordinated with the daily light-dark cycle, (generally 14 hours light and 10 hours dark), and mating will typically occur in the morning when lights are turned on. To breed zebrafish, place a male and female adult in a mating chamber in the afternoon. Note that to determine the sex of the adults, there is an excellent guide on the Zebrafish in the Classroom website (Liang et al., 2011). In order to obtain enough embryos for these experiments, it is a good idea to set up at least three to four male-female pairs of zebrafish. While one mating will generally produce enough eggs for an entire class, multiple pairings ensure you will get at least one pair of zebrafish to successfully mate.
Fertilized embryos are collected from the breeding tank after removing the mating pair. If not using a purpose built mating chamber, clean marbles can be placed at the bottom of a fish tank prior to mating to allow the fertilized embryos to fall into the cracks, protecting them from being consumed by the adults. Embryos are collected by pouring the liquid contents of the mating chamber through a tea strainer. Next, embryo media is used to rinse the embryos from the tea strainer into a Petri dish. Fertilized embryos should be kept at ∼28.5 °C, where temperatures above this point cause embryos to develop faster, while temperatures below this point cause them to develop more slowly. We note that temperatures outside of the 25–33°C range will cause embryos to develop abnormally or die (Kimmel et al., 1995).
The rapid development and the transparency of larval zebrafish are great assets for neuroscience teaching laboratories. Within a few days after fertilization, zebrafish larvae already display sensory responses and robust swimming and startle behaviors (Saint-Amant and Drapeau, 1998; Brustein et al., 2003). They also have a relatively simple nervous system that is well characterized and homologous to other higher vertebrates. Another ideal aspect of working with larvae is that they do not need to be fed prior to 8 dpf as they subsist on the contents of their yolk sac. To minimize nitrogenous waste and growth of harmful organisms, Petri dishes should have the embryo medium replaced daily and should be cleaned of unfertilized embryos and hatched chorions that will accumulate between the second and third days after fertilization. Starting at 2 dpf, field potentials can be recorded from zebrafish larvae (Prugh et al., 1982; Issa et al., 2011).
Setting up the recording chamber
A free-swimming larva is placed within a drop of distilled water at the center of a Sylgard-lined dish with two embedded stainless-steel insect pins (see Figure 3B). A small amount of modeling clay can help hold the dish in place beneath a dissection microscope. In addition to recording field potentials from free-swimming larvae, potentials can be measured from larvae that have their head and upper torso mounted in ∼2% low melting point agarose. The agarose should be dissolved in distilled water by heating it in pulses with a conventional microwave. Once in solution, the agarose should be placed in a 40°C water bath or heat block to keep from gelling. Larvae should never be exposed to temperatures above 40°C, so it is important to let the temperature of the agarose solution reach equilibrium with the water bath or heat block. Using a transfer pipette, larvae are moved into the agarose solution, and then a drop of the agarose solution containing the larva is placed on a clean slide or Petri dish. As the solution solidifies, the fish is manipulated dorsal-side up using fine-tipped forceps. Once the agarose has set, trim the agarose away from the trunk using a razor blade and dissection microscope. The mounted larva should then be placed in the Sylgard-lined recording chamber and covered in distilled water. Tactile stimuli are then delivered to the head of the larva using a non-conductive glass or plastic probe.
Figure 3.
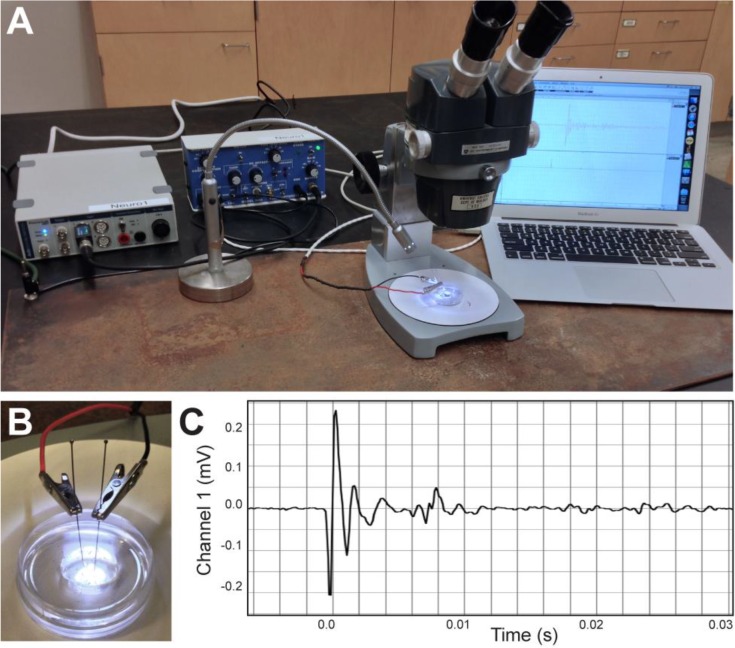
Recoding setup and representative field potential trace. (A) Field potential recording setup: DAQ device, differential amplifier, laptop computer, LED light, steel plate and microscope. (B) A Sylgard-lined dish with embedded stainless-steel insect pins and a drop of distilled water. (C) A single representative trace of a field potential from a 5 dpf zebrafish larva in response to a touch stimulus. The waveform of the field potential results from the coordinated neural and muscle activity that generates the C-bend at the onset of the startle response. The subsequent smaller changes in amplitude likely represent smaller, uncoordinated muscle activity while the larva swims away from the stimulus.
Connecting the recording equipment
An excellent way for students to become familiar with the equipment and learn the basics of electrophysiology is to have them connect their own recording stations (see Table 2 for list of equipment). Before students begin, instructors can deliver a brief tutorial on the acquisition hardware and software, including a review of concepts such as field potentials, differential amplifiers, signal gain, filtering, and sampling rates.
Table 2.
Recording equipment and software.
| LabChart software (AD Instruments) |
| PowerLab 26T DAQ device (AD Instruments) |
| Model 3000 AC/DC differential amplifier (A-M Systems) |
| DB15 cable (A-M Systems) with stainless steel alligator clips |
| Boekel Tackiwax (VWR) or Sylgard 184 (Dow Corning) |
| Low melting point agarose (Sigma Aldrich, A9414) |
| Stainless steel insect pins (size 000; 0.25 mm diameter) |
| Fiber optic light source and dissection microscope |
We would like to note that teaching labs already set up to record from earthworms, crayfish, and other small critters most likely have all the electrophysiology equipment necessary for these recordings. We have listed our equipment in the interest of providing a specific example; however, this is only one of many possible recording setups.
Students begin by connecting the differential amplifier and data acquisition (DAQ) device to a grounded power strip. Next, they connect the analog output of the differential amplifier to one of the analog inputs on the DAQ using a BNC cable and attach the recording input cable to the input connection of the differential amplifier. For our setup, we soldered stainless-steel alligator clips to the ends of a DB15 input cable, which then clip to the stainless-steel insect pins embedded in the recording dish (Figure 3B).
Students then select the amplifier settings needed to isolate field potentials generated by the zebrafish larva during escape responses. The amplifier should be set to differential mode, the high pass filter to 300-Hz, and the low pass filter at 3-kHz. If the amplifier has a dedicated notch filter, this can be used to help eliminate 60-Hz electrical noise. The amplifier’s gain should be set to maximize the amplitude of the field potential (e.g., 5,000X or 10,000X).
Obtaining a recording
Once the electrophysiology hardware is connected, students should connect the DAQ device to the computer (e.g., via USB cable), power on the equipment, and start the data acquisition software. The software settings should be set similarly to those used for extracellular recordings from earthworms and cockroaches. Setting the acquisition software to record continuously can function as a digital oscilloscope, but it can also make it challenging to find and review evoked field potentials. Instead, setting the software to work via “triggering” will allow data to be collected once the signal amplitude goes above a set threshold value. The field potential should reach this value at its onset, though it may take some trial and error to determine the best threshold value to use. By using triggering, data can be collected for a discrete time window before and after the field potential, which allows for easy comparison across multiple recordings.
Trouble-shooting
Students may encounter trouble with their recordings, and this is an excellent opportunity for them to use a multimeter (set to the “continuity” setting) to trouble-shoot their preparation. In particular, students can evaluate the grounding and test the electrical connections of their setup. To minimize electrical noise, the recordings can be performed on a steel plate that is placed underneath the recording setup and that grounds the amplifier, DAQ device, microscope, and other equipment (see Figure 3A). Use of a Faraday cage will also greatly reduce 60-Hz noise caused by poorly grounded nearby electrical equipment and fluorescent lights. Using laptop computers on battery power can also help minimize noise, especially when recording setups do not have Faraday cages (see George, 2006). While electrical connections may pose a problem, one common issue seen with this preparation is not rinsing the larva in distilled water prior to placing it in distilled water in the recording dish. The high resistance of distilled water is essential to recording robust field potentials (Issa et al., 2011).
RESULTS
Recording activity from free-swimming zebrafish
Multiple sensory systems can excite an escape response in zebrafish larvae including auditory and tactile stimuli. Perhaps the easiest method to elicit an escape response is to use a glass probe to gently touch the head of the larval zebrafish. A light touch to the head of a zebrafish activates trigeminal neurons, while a touch to the tail will activate touch receptors known as Rohon-Beard neurons, both of which synapse on the M-cell and evoke startle responses (Kimmel et al., 1990). To examine this response in a free-swimming larva, we rinsed a larva in distilled water and placed it into a drop of distilled water that surrounded the two stainless-steel insect pins in our recording dish (see Figure 3B). We then evoked an escape response with a light touch (Figure 3C) using a glass probe fashioned by heating the tip of a Pasteur pipette with a Bunsen burner until the end melted closed. Alternatively, any probe made of nonconductive material, like the tip of a plastic pipette will work.
Field recordings of escape responses from larval zebrafish are subject to the position of the larva relative to the recording electrodes. The polarity of the potential is dependent on the location of the larva in relation to the recording electrodes and will sometimes be reversed. The amplitude of the field potential is also dependent on the distance of the larva from the electrodes. Students can be encouraged to develop hypotheses about the effect that the position of the recording electrodes will have on measured field potentials (such as size, shape, or polarity). Next, we present a student-designed experiment to examine this variability.
Testing the effect of larval position and distance from electrodes on the field potential
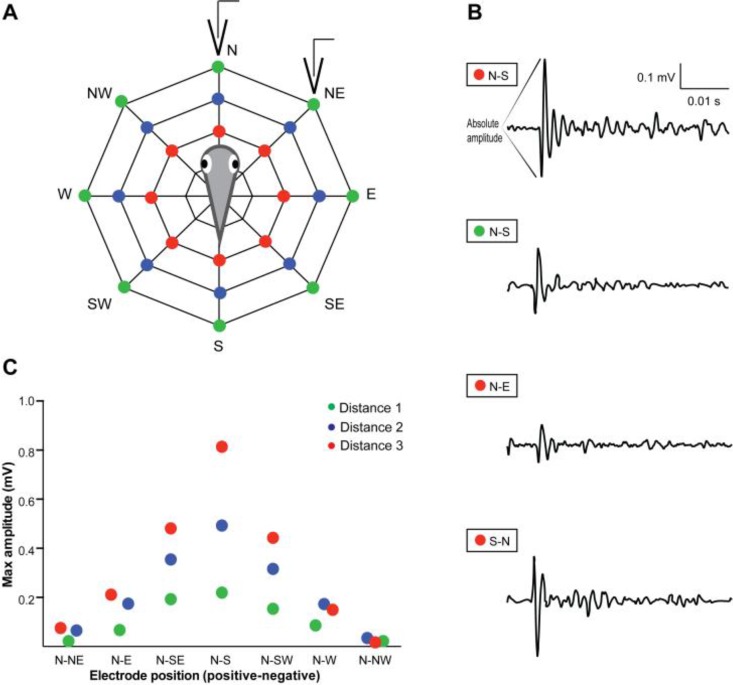
In order to track the position of the electrodes relative to the larva, we drew concentric octagons on a piece of paper that was pasted to the bottom of the recording dish. The lines were visible through the transparent dish and the intersections served as reference points for placing the stainless steel pins and clipping the recording electrodes (see Figure 4A).
Figure 4.
The field potential amplitude is dependent on electrode location relative to the larva. (A) Diagram of the experimental setup where electrodes were placed at three distances from a fixed-position larva. The red, blue and green dots represent increasing distances from the zebrafish, respectively. For each distance, the positive electrode was fixed at the N position, while the negative electrode was placed at different angles around the larva. The electrodes represented on the diagram correspond to the “N-NE” position at distance “1”. (B) Sample traces of field potentials obtained at electrode positions N-S, distance 3; N-S, distance 1; N-E, distance 3; S-N, distance 3 (from top to bottom, respectively). Scale for all traces indicated at top right corner. Note that the electrodes were reversed for recording the bottom potential and this resulted in the flipped polarity of the field potential. (C) Plot of the absolute amplitude of the initial large-amplitude, biphasic portion of each field potential at a given recording distance and electrode position.
We then fixed a 5 dpf larva in 2% low-melt agarose at the center of a recording dish along its rostro-caudal axis and collected a series of field recordings by varying the electrode location. For example, the alligator clip for the positive electrode was connected to a pin located at the N position, at distance 1, and the negative electrode was then clipped to the other available positions along distance 1. Here we chose to use one easily quantifiable feature of the field potential, the absolute size of the initial large-amplitude biphasic waveform. We measured this as the voltage difference between the initial highest and lowest peaks (see Figure 3C and Figure 4B for example). Our results for a series of pin locations at three different distances and at eight different angles were then plotted for comparison (Figure 4C). We waited approximately one minute in between consecutive trials to avoid habituation of the escape reflex—another student experiment could examine this. Our results indicate that the absolute amplitude of the field potential is greatest when the electrodes are closest to the larva and positioned along its rostro-caudal axis, and the amplitude varies linearly with distance and angle away from this optimum location. Furthermore, we found that the polarity of the response is reversed when the negative electrode is closer to the anterior end of the larva and the positive electrode is toward to the posterior end (see Figure 4C, bottom trace). Students can be encouraged to think about what this means in terms of the biological signal being generated by the animal.
DISCUSSION
Recording field potentials from the electrophysiological signals generated by the larval zebrafish during an escape response is an excellent tool for use in neuroscience teaching laboratories. Many advanced electrophysiology labs rely on more expensive equipment, complex dissections, and often prove to be difficult for students to obtain successful recordings. Importantly, the techniques described here should provide all students with their own data to analyze. Furthermore, several excellent pedagogical goals can be accomplished through this laboratory, including the formulation of a testable hypothesis, the collection, analysis, and representation of quantifiable data, and perhaps the subsequent creation of a journal-style laboratory report discussed below.
Potential experiments and classroom exercises
The escape response and field recordings of the underlying electrophysiological signals can be used for hypothesis-based, student-driven experiments. Providing students with an in-depth lecture at the onset of the laboratory can position students to think critically about many aspects of the laboratory. Here we provide several different avenues for potential experiments and laboratory exercises.
Given the importance of the startle response for survival from potential threats, the M-cell receives synaptic input from multiple senses, including auditory, lateral line, visual, and somatosensory systems (Zottoli, 1977; Zottoli et al., 1995; Korn and Faber, 2005; Kohashi and Oda, 2008; Mu et al., 2012; Stewart et al., 2013). For instance, the M-cells receive direct synaptic input from the afferent neurons of the ear (Kimmel et al., 1981). Thus, a brief tone or vibration (via a light tap to the recording dish) can also be used evoke an escape response (Nakayama and Oda, 2004; Kohashi et al., 2012). This well-studied auditory startle response is also modulated by the lateral line, an additional sensory system in fish and amphibians that utilizes hair cells to detect low frequency water motion. The lateral line is composed of collections of hair cells located at regular intervals along the length of the body and around the head, and its function is vital for the timing of M-cell activity (Mirjany et al., 2011; Mirjany and Faber, 2011; Stewart et al., 2013). Additionally, the visual system has been shown to modulate the activity of M-cells. Visual stimuli when paired with auditory stimuli increase the probability of an M-cell response (Mu et al., 2012). Altogether, students have a variety of sensory systems to examine with respect to the startle response, though these types of experiments may require multi-week projects.
Importantly, zebrafish larvae are amenable to many genetic manipulations, including mRNA knock-down using morpholinos (http://www.gene-tools.com), forward and reverse mutagenesis screens, enhancer trap screens (Kawakami et al., 2010), and GAL4/UAS transgenic tools (Halpern et al., 2008). There is also a repository (available at http://zebrafish.org/zirc/home/guide.php) of an expanding number of mutant and transgenic fish lines available to the community (Bradford et al., 2011). Furthermore, the power of optogenetics and optophysiology are being increasingly harnessed using larval zebrafish (Del Bene and Wyart, 2012; Akerboom et al., 2013; Portugues et al., 2013). For example, our laboratory recently expressed the light-activated ion channel, Channelrhodopsin-2 (ChR2) in zebrafish hair cells of the ear and lateral line. These transgenic fish display an escape response when ChR2 is activated by ∼470-nm light (Monesson-Olson et al., 2014). The experiment in the Results section was performed using this line and a 10-ms pulse of ∼470 nm light via an inexpensive blue LED (for LED design see Pulver et al., 2011). Adding optogenetics to an undergraduate laboratory is a powerful tool (also see Pulver et al., 2011) and provides an excellent opportunity to teach about this rapidly developing field of neuroscience (for review see Smedemark-Margulies and Trapani, 2013).
Using pharmacology, students can compare responses in the presence of drugs that modulate many aspects of the reflex. Paralytics and other ion channel blockers can be used to generate dose-response curves and inhibition assays (see for example the effects of alpha-bungarotoxin, Issa et al., 2011). Another example could have students block the glycine receptor, resulting in paralysis during these developmental stages (Hirata et al., 2005). Furthermore, McKeown et al. describe a set of teaching labs investigating glycine neurotransmitter function in zebrafish that could be complemented by field recordings of the escape response (2009). Importantly, treating whole larvae with drugs and chemicals is straightforward as most agents can be added directly to the surrounding medium (often with 0.1 % DMSO to aid in carrying the drug across the skin).
The startle response can also be compared across different stimulus modalities, including using an electric field pulse to excite the M-cell directly (see Methods section in Tabor et al., 2014), as direct M-cell activation can evoke the C-bend behavior (Zottoli, 1977; Eaton et al., 1981). We note that fixed larvae are best for quantitative comparisons of the field potential. Novel stimuli can also be assayed for their ability to trigger startle responses. For example, application of noxious odorants or pulsing cold or hot liquids onto the larva can be used to examine whether there are olfactory or temperature-sensitive inputs onto the M-cell. Within these potential experiments, students can also examine the relationship between stimulus strength and duration on the threshold and magnitude of the escape response.
Pedagogical outcomes
Through initial lectures at the start of the lab, potentially coinciding with syllabus lectures, students are able to learn about and observe a vertebrate reflex behavior that involves multiple sensory systems, neural circuitry, synaptic physiology, muscle physiology, and the electrophysiological methods used by researchers to study these processes. Indeed, many of the papers cited here provide useful assignments both for background reading of techniques and for forming the basis of student-developed hypotheses. In its simplest form, an introductory laboratory will expose students to the equipment and software necessary for later, more in depth experiments. This introduction will also give students an appreciation for the power of the zebrafish model organism, which is presently used in many basic research laboratories around the world.
For a journal-style lab report, students provide an Introduction section that introduces the experiment and justifies their hypotheses, write a concise Materials and Methods section (in the passive voice), generate a Results section that includes well-designed Figures and proper Figure Legends, and finally, they craft a Discussion section with its requisite components. Students are also reminded about plagiarism and instructed on how to include proper citations and a References section. Feedback from students that have created this form of lab report have remarked both on how well they have learned the subject matter and how much their writing was improved—which typically results from a required first draft of the report, and which could further be improved through peer to peer editing and reviewing. Many students also note that they gained an appreciation for the published research articles that they read in their various courses. Finally, the lab report allows for assessment of student learning goals that may have been set at the onset of the laboratory.
Low-cost equipment and software options
We note that similar field potentials could be recorded from other aquatic vertebrates such as medaka, tadpoles, or goldfish (see for example Featherstone et al., 1991). Also, we have found that field potentials can be observed using a SpikerBox (a small, inexpensive amplifier from Backyard Brains https://backyardbrains.com/; Marzullo and Gage, 2012). Using a SpikerBox together with their free acquisition application called Spike Recorder (https://backyardbrains.com/products/spikerecorder), students are able to record and analyze field potentials similar to those described here. These open-source tools provide an excellent low-cost setup for laboratories with a limited budget. Open-source options also highlight the potential for student-driven Makerspace projects (http://makerspace.com/) and could include using open-source programming (http://code.org/) together with Arduino (http://www.arduino.cc/) or Raspberry Pi (http://www.raspberrypi.org/) resources for creating their own acquisition hardware.
Acknowledgments
This work was supported in part by a Faculty Research Award from the H. Axel Schupf ’57 Fund for Intellectual Life to JGT and by The Kresge Foundation.
REFERENCES
- Akerboom J, et al. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front Mol Neurosci. 2013;6:2. doi: 10.3389/fnmol.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford Y, et al. ZFIN: enhancements and updates to the Zebrafish Model Organism Database. Nucleic Acids Res. 2011;39:D822–D829. doi: 10.1093/nar/gkq1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustein E, Saint-Amant L, Buss RR, Chong M, McDearmid JR, Drapeau P. Steps during the development of the zebrafish locomotor network. J Physiol-Paris. 2003;97:77–86. doi: 10.1016/j.jphysparis.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dagda RK, Thalhauser RM, Dagda R, Marzullo TC, Gage GJ. Using crickets to introduce neurophysiology to early undergraduate students. J Undergrad Neurosci Educ. 2013;12:A66–A74. [PMC free article] [PubMed] [Google Scholar]
- Del Bene F, Wyart C. Optogenetics: a new enlightenment age for zebrafish neurobiology. Dev Neurobiol. 2012;72:404–414. doi: 10.1002/dneu.20914. [DOI] [PubMed] [Google Scholar]
- Eaton RC, Bombardieri RA, Meyer DL. The Mauthner-initiated startle response in teleost fish. J Exp Biol. 1977a;66:65–81. doi: 10.1242/jeb.66.1.65. [DOI] [PubMed] [Google Scholar]
- Eaton RC, Farley RD. Mauthner neuron field potential in newly hatched larvae of the zebra fish. J Neurophysiol. 1975;38:502–512. doi: 10.1152/jn.1975.38.3.502. [DOI] [PubMed] [Google Scholar]
- Eaton RC, Farley RD, Kimmel CB, Schabtach E. Functional development in the Mauthner cell system of embryos and larvae of the zebra fish. J Neurobiol. 1977b;8:151–172. doi: 10.1002/neu.480080207. [DOI] [PubMed] [Google Scholar]
- Eaton RC, Lavender WA, Wieland CM. Identification of Mauthner-initiated response patterns in goldfish: evidence from simultaneous cinematography and electrophysiology. J Comp Physiol. 1981;144:521–531. [Google Scholar]
- Eaton RC, Lee RK, Foreman MB. The Mauthner cell and other identified neurons of the brainstem escape network of fish. Prog Neurobiol. 2001;63:467–485. doi: 10.1016/s0301-0082(00)00047-2. [DOI] [PubMed] [Google Scholar]
- Featherstone D, Drewes CD, Coats JR. Short communication: noninvasive detection of electrical events during the startle response in larval medaka. J Exp Biol. 1991;158:583–589. [Google Scholar]
- George S. Data acquisition and display for electrophysiology: PC oscilloscopes. J Undergrad Neurosci Educ. 2006;5:R11–R14. [PMC free article] [PubMed] [Google Scholar]
- Halpern ME, Rhee J, Goll MG, Akitake CM, Parsons M, Leach SD. Gal4/UAS transgenic tools and their application to zebrafish. Zebrafish. 2008;5:97–110. doi: 10.1089/zeb.2008.0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Saint-Amant L, Downes GB, Cui WW, Zhou W, Granato M, Kuwada JY. Zebrafish bandoneon mutants display behavioral defects due to a mutation in the glycine receptor beta-subunit. Proc Natl Acad Sci U S A. 2005;102:8345–8350. doi: 10.1073/pnas.0500862102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa FA, O’Brien G, Kettunen P, Sagasti A, Glanzman DL, Papazian DM. Neural circuit activity in freely behaving zebrafish (Danio rerio) J Exp Biol. 2011;214:1028–1038. doi: 10.1242/jeb.048876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BR, Hauptman SA, Bonow RH. Construction of a simple suction electrode for extracellular recording and stimulation. J Undergrad Neurosci Educ. 2007;6:A21–A26. [PMC free article] [PubMed] [Google Scholar]
- Kawakami K, Abe G, Asada T, Asakawa K, Fukuda R, Ito A, Lal P, Mouri N, Muto A, Suster ML, Takakubo H, Urasaki A, Wada H, Yoshida M. zTrap: zebrafish gene trap and enhancer trap database. BMC Dev Biol. 2010;10:105. doi: 10.1186/1471-213X-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Hatta K, Metcalfe WK. Early axonal contacts during development of an identified dendrite in the brain of the zebrafish. Neuron. 1990;4:535–545. doi: 10.1016/0896-6273(90)90111-r. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Sessions SK, Kimmel RJ. Morphogenesis and synaptogenesis of the zebrafish Mauthner neuron. J Comp Neurol. 1981;198:101–120. doi: 10.1002/cne.901980110. [DOI] [PubMed] [Google Scholar]
- Kladt N, Hanslik U, Heinzel HG. Teaching basic neurophysiology using intact earthworms. Neurosci Educ. 2010;9:A20–A35. [PMC free article] [PubMed] [Google Scholar]
- Kohashi T, Nakata N, Oda Y. Effective sensory modality activating an escape triggering neuron switches during early development in zebrafish. J Neurosci. 2012;32:5810–5820. doi: 10.1523/JNEUROSCI.6169-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohashi T, Oda Y. Initiation of Mauthner- or non-Mauthner-mediated fast escape evoked by different modes of sensory input. J Neurosci. 2008;28:10641–10653. doi: 10.1523/JNEUROSCI.1435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn H, Faber DS. The Mauthner cell half a century later: a neurobiological model for decision-making? Neuron. 2005;47:13–28. doi: 10.1016/j.neuron.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Krans JL, Rivlin PK, Hoy RR. Demonstrating the temperature sensitivity of synaptic transmission in a Drosophila mutant. J Undergrad Neurosci Educ. 2005;4:A27–A33. [PMC free article] [PubMed] [Google Scholar]
- Liang JO, et al. Zebrafish in the classroom. 2011. Direct Website Submission ( http://www.zfic.org).
- Liu KS, Fetcho JR. Laser ablations reveal functional relationships of segmental hindbrain neurons in zebrafish. Neuron. 1999;23:325–335. doi: 10.1016/s0896-6273(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Marzullo TC, Gage GJ. The SpikerBox: a low cost, open-source bioamplifier for increasing public participation in neuroscience inquiry. PLoS ONE. 2012;7:e30837. doi: 10.1371/journal.pone.0030837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirjany M, Faber DS. Characteristics of the anterior lateral line nerve input to the Mauthner cell. J Exp Biol. 2011;214:3368–3377. doi: 10.1242/jeb.056226. [DOI] [PubMed] [Google Scholar]
- Mirjany M, Preuss T, Faber DS. Role of the lateral line mechanosensory system in directionality of goldfish auditory evoked escape response. J Exp Biol. 2011;214:3358–3367. doi: 10.1242/jeb.052894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown KA, Downes GB, Hutson LD. Modular laboratory exercises to analyze the development of zebrafish motor behavior. Zebrafish. 2009;6:179–185. doi: 10.1089/zeb.2008.0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monesson-Olson BD, Browning-Kamins J, Aziz-Bose R, Kreines F, Trapani JG. Optical stimulation of zebrafish hair cells expressing channelrhodopsin-2. PLoS One. 2014;9:e96641. doi: 10.1371/journal.pone.0096641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y, Li X, Zhang B, Du J. Visual input modulates audiomotor function via hypothalamic dopaminergic neurons through a cooperative mechanism. Neuron. 2012;75:688–699. doi: 10.1016/j.neuron.2012.05.035. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Oda Y. Common sensory inputs and differential excitability of segmentally homologous reticulospinal neurons in the hindbrain. J Neurosci. 2004;24:3199–3209. doi: 10.1523/JNEUROSCI.4419-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivo RF. An online lab manual for neurophysiology. J Undergrad Neurosci Educ. 2003;2:A16–A22. [PMC free article] [PubMed] [Google Scholar]
- Olson BD, Sgourdou P, Downes GB. Analysis of a zebrafish behavioral mutant reveals a dominant mutation in atp2a1/SERCA1. Genesis. 2010;48:354–361. doi: 10.1002/dvg.20631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugues R, Severi KE, Wyart C, Ahrens MB. Optogenetics in a transparent animal: circuit function in the larval zebrafish. Curr Opin Neurobiol. 2013;23:119–126. doi: 10.1016/j.conb.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Prugh JI, Kimmel CB, Metcalfe WK. Noninvasive recording of the Mauthner neurone action potential in larval zebrafish. J Exp Biol. 1982;101:83–92. doi: 10.1242/jeb.101.1.83. [DOI] [PubMed] [Google Scholar]
- Pulver SR, Hornstein NJ, Land BL, Johnson BR. Optogenetics in the teaching laboratory: using channelrhodopsin-2 to study the neural basis of behavior and synaptic physiology in Drosophila. Adv Physiol Educ. 2011;35:82–91. doi: 10.1152/advan.00125.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos RL, Moiseff A, Brumberg JC. Utility and versatility of extracellular recordings from the cockroach for neurophysiological instruction and demonstration. J Undergrad Neurosci Educ. 2007;5:A28–A34. [PMC free article] [PubMed] [Google Scholar]
- Saint-Amant L, Drapeau P. Time course of the development of motor behaviors in the zebrafish embryo. J Neurobiol. 1998;37:622–632. doi: 10.1002/(sici)1097-4695(199812)37:4<622::aid-neu10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Smedemark-Margulies N, Trapani JG. Tools, methods, and applications for optophysiology in neuroscience. Front Mol Neurosci. 2013;6:18. doi: 10.3389/fnmol.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart WJ, Cardenas GS, McHenry MJ. Zebrafish larvae evade predators by sensing water flow. J Exp Biol. 2013;216:388–398. doi: 10.1242/jeb.072751. [DOI] [PubMed] [Google Scholar]
- Tabor KM, Bergeron SA, Horstick EJ, Jordan DC, Aho V, Porkka-Heiskanen T, Haspel G, Burgess HA. Direct activation of the Mauthner cell by electric field pulses drives ultra-rapid escape responses. J Neurophysiol. 2014;112:834–844. doi: 10.1152/jn.00228.2014. Available at: http://jn.physiology.org/content/early/2014/05/16/jn.00228.2014 [Accessed July 1, 2014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilinsky I, Johnson KG. Electroretinograms in Drosophila: a robust and genetically accessible electrophysiological system for the undergraduate laboratory. J Undergrad Neurosci Educ. 2012;11:A149–A157. [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book A guide for the laboratory use of zebrafish (Danio rerio) 4th ed. Eugene, OR: University of Oregon Press; 2000. [Google Scholar]
- Zottoli SJ. Correlation of the startle reflex and Mauthner cell auditory responses in unrestrained goldfish. J Exp Biol. 1977;66:243–254. doi: 10.1242/jeb.66.1.243. [DOI] [PubMed] [Google Scholar]
- Zottoli SJ, Bentley AP, Prendergast BJ, Rieff HI. Comparative studies on the Mauthner cell of teleost fish in relation to sensory input. Brain Behav Evol. 1995;46:151–164. doi: 10.1159/000113268. [DOI] [PubMed] [Google Scholar]