Abstract
MicroRNAs (miRNAs) are a class of small, conserved, tissue-specific regulatory non-coding RNAs that modulate a variety of biological processes and play a fundamental role in pathogenesis of major human diseases, including nonalcoholic fatty liver disease (NAFLD). However, the association between inter-individual differences in susceptibility to NAFLD and altered miRNA expression is largely unknown. In view of this, the goals of the present study were (i) to determine whether or not individual differences in the extent of NAFLD-induced liver injury are associated with altered miRNA expression, and (ii) assess if circulating blood miRNAs may be used as potential biomarkers for the noninvasive evaluation of the severity of NAFLD. A panel of seven genetically diverse strains of inbred male mice (A/J, C57BL/6J, C3H/HeJ, 129S/SvImJ, CAST/EiJ, PWK/PhJ, and WSB/EiJ) were fed a choline- and folate-deficient (CFD) diet for 12 weeks. This diet induced liver injury in all mouse strains; however, the extent of NAFLD-associated pathomorphological changes in the livers was strain-specific, with A/J, C57BL/6J, and C3H/HeJ mice being the least sensitive and WSB/EiJ mice being the most sensitive. The morphological changes in the livers were accompanied by differences in the levels of hepatic and plasma miRNAs. The levels of circulating miR-34a, miR-122, miR-181a, miR-192, and miR-200b miRNAs were significantly correlated with a severity of NAFLD-specific liver pathomorphological features, with the strongest correlation occurring with miR-34a. These observations suggest that the plasma levels of miRNAs may be used as biomarkers for noninvasive monitoring the extent of NAFLD-associated liver injury and susceptibility to NAFLD.
Keywords: Inter-individual differences, microRNAs, mouse, nonalcoholic fatty liver disease
INTRODUCTION
The incidence of nonalcoholic fatty liver disease (NAFLD) is increasing dramatically in the United States and developed countries (Tiniakos et al., 2010; Bellentani et al., 2010). It is estimated that 25%–30% of adults in the United States have NAFLD (Pascale et al., 2010; Day, 2011) and that NAFLD accounts for 39% of newly diagnosed cases of chronic liver disease (Pascale et al., 2010). NAFLD is composed of several related liver disorders ranging from uncomplicated steatosis to non-alcoholic steatohepatitis (NASH). More importantly, NAFLD is considered to be a major risk factor for the development of other chronic liver diseases, such as fibrosis, cirrhosis, and hepatocellular carcinoma (Siegel and Zhu, 2009; Starley et al., 2010; Welzel et al., 2011). The underlying molecular mechanisms involved in the etiology and pathogenesis of NAFLD are only partially understood and pharmacotherapy options are limited (Cohen et al., 2011). Supportive treatment strategies for NAFLD are focused on managing the metabolic syndrome and chronic inflammation in the liver; thus, early detection of the disease is critical for improved prognosis and prevention of more serious liver illnesses. A recent report by Paie et al. in a small cohort of patients with NAFLD demonstrated that isolated steatosis does progress to NASH accompanied by a worsening of the metabolic syndrome (Pais et al., 2011), and suggested the crucial need for continued hepatic monitoring in patients diagnosed with uncomplicated NAFLD. Unfortunately, at the present time, a liver biopsy is the only method for the diagnosis and assessment of disease progression (Sanyal et al., 2011).
Even though the incidence of NAFLD in the general population is appreciable, it is estimated that only 10% of those diagnosed will progress to liver fibrosis or cirrhosis (Siegel and Zhu, 2009). In addition, the incidence of NAFLD varies greatly among racial groups (Browning et al., 2004) and NAFLD is a highly heritable trait (Schwimmer et al., 2011). A recent genome-wide association study (GWAS) of the subjects with histopathologicaly-confirmed NAFLD identified several single nucleotide polymorphisms as potential genetic modifiers of the NAFLD activity score, fibrosis, inflammation, and serum alanine aminotransferase (ALT) levels (Chalasani et al., 2010). Because the pathogenesis of NAFLD is complex and involves dysregulation of several interdependent physiological processes, including lipid metabolism, insulin resistance, immune response, inflamation, oxidative stress, and apoptosis (Marra et al., 2008; Larter et al., 2010), additional studies in human populations or animal models of the human population (Rusyn et al., 2010) are needed to elucidate the genetic determinants of NAFLD.
Investigating the molecular basis of how genetic factors influence the susceptibility to NAFLD in humans is frequently impractical and always very complex, whereas using relevant animal models may substantially overcome many limitations of human-only studies. Several studies have demonstrated an inter-strain variability in the susceptibility of mice to NAFLD, NASH, and hepatocarcinogenesis induced by either high-fat diet, or methionine- and choline-deficient diet (Yamazaki et al., 2008; Hill-Baskin et al., 2009). Each of these dietary models has some advantages and limitations. For instance, feeding high-energy diets compromises the metabolic status and induces obesity but does not uniformly cause liver injury (Maher, 2011). In contrast, feeding either a methionine- and choline-deficient or choline-deficient diet to rats or mice causes liver injury similar to NAFLD but does not attain the compromised metabolic status observed in patients.
Previous reports have convincingly demonstrated that dysregulation of miRNA expression is an important early event in the pathogenesis of NAFLD in humans (Cheung et al., 2008) and mice (Wang et al., 2009a). However, the association between inter-individual differences in susceptibility to NAFLD and altered miRNA expression is largely unknown.
To explore further the potential mechanisms of inter-individual differences in susceptibility and severity of NAFLD-related liver injury, we fed a panel of seven genetically diverse inbred mouse strains that are parental lines in the Collaborative Cross (Aylor et al., 2011) the choline- and folate-deficient (CFD) diet that consistently induces fat-related liver injury resembling pathomorphological features of human NAFLD (Maher, 2011). We determined the inter-strain variability in severity of NAFLD induced by the CFD diet and its association with aberrations in miRNA expression. We also assessed whether or not circulating blood miRNAs may be used as potential biomarkers for noninvasive evaluation of liver injury in NAFLD. Because miRNA expression in mouse liver varies little among inbred strains (Gatti et al., 2011) the establishment of circulatory miRNA biomarkers as noninvasive diagnostics of the severity of NAFLD may fill a critical gap in clinical practice.
MATERIALS AND METHODS
Animals and experimental design
Male A/J, C57BL/6J, C3H/HeJ, 129S1/SvImJ, CAST/EiJ, PWK/PhJ, and WSB/EiJ mice (6 weeks of age) were obtained from the Jackson Laboratory (Bar Harbor, ME). These strains were selected because they provide an excellent representation of the broad genetic diversity and their genomes have been fully sequenced (Yang et al., 2011). The mice were housed in sterilized cages in a temperature-controlled room (24°C) with a 12 h light/dark cycle, and given ad libitum access to purified water and NIH-31 pelleted diet. At 8 weeks of age, mice from each strain were allocated randomly into control and experimental groups. Mice in the experimental groups were maintained on the CFD diet, a diet lacking choline and folic acid (Diet #519541, choline and folate deficient, iron supplemented, and L-amino acid defined diet; Dyets, Inc., Bethlehem, PA) for 12 weeks. Mice in the control groups received the same diet supplemented with 0.4% methionine, 0.3% choline bitartrate, and 2 mg/kg folic acid. Diets were stored at 4°C before use and given ad libitum with replacement twice a week. Body weights of the mice were recorded weekly. Five experimental and five control mice from each strain were euthanized by exsanguination following deep isoflurane anesthesia 12 weeks after diet initiation. Blood was collected into BD vacutainer EDTA containing blood collection tubes (BD Biosciences, Franklin Lakes, NJ) by direct puncture of the heart. Plasma was isolated by centrifugation at 3,000 rpm for 10 minutes at 4°C according to the manufacturer’s instructions and stored at −80°C. The livers were excised and a slice of the median lobe was fixed in neutral buffered formalin for 48 h for histopathological examination. The remaining liver was snap-frozen immediately in liquid nitrogen and stored at −80°C for subsequent analyses. All experimental procedures were reviewed and approved by the National Center for Toxicological Research Animal Care and Use Committee.
Biochemical analyses
Serum triglyceride, ALT, aspartate aminotransferase (AST), lactate dehydrogenase (LDH), total cholesterol, and glucose levels were measured using an ACE Alera® Clinical Chemistry System (Alfa Wassermann Inc., West Caldwell, NJ) according to the manufacturer’s protocol.
Tissue processing, histological analysis and criteria for pathology assessment
After 48 h, a slice of the median lobe of the liver that was fixed in 10% neutral buffered formalin was trimmed, processed, and embedded in infiltrating media (Surgipath Formula R®, Leica Biosystems, Richmond, IL), sectioned at approximately 5 microns, mounted on a glass slide, and stained with hematoxylin and eosin. The liver sections were examined histopathologically for NAFLD-specific lesions, including cytoplasmic alteration, steatosis, hepatocellular degeneration, inflammation, hepatocellular karyocytomegaly, and oval cell proliferation, and graded using a severity score system for each of the morphological parameters as follows: grade 0, absent; grade 1, minimal; grade 2, mild; grade 3, moderate; and grade 4, severe changes. Total liver pathology scores were calculated as the mean severity for all of the NAFLD-specific lesions detected in the livers of the mouse strains.
RNA extraction and miRNA expression analysis by quantitative reverse transcription real-time PCR (qRT-PCR)
Total RNA was extracted from liver tissue using miRNAeasy Mini kits (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Total RNA (100 ng) was used for qRT-PCRs of miR-122, miR-192, miR-34a, miR-200b, miR-221, and miR-181a using TaqMan miRNA assays (Applied Biosystems, Foster City, CA), according to the manufacturer’s instructions. SnoRNA202 was used as an endogenous control. The relative amount of each miRNA was measured using the 2−ΔΔCt method (Schmittgen and Livak, 2008). All qRT-PCR reactions were conducted in triplicate and repeated twice.
Total plasma RNA, including miRNA, was isolated using QIAzol reagent (Qiagen) according to the manufacturer’s instructions with minor modifications. In brief, 700 μl of QIAzol reagent containing 1.2 μg of carrier MS2 RNA (Roche Diagnostics Corporation, Indianapolis, IN) was added to 100 μl of each plasma sample. The sample was mixed in a tube, spiked with 4 μl of 0.5 μM cel-miR-54 miRNA (Qiagen), and 140 μl of chloroform was added. After mixing vigorously for 15 s, the sample was then centrifuged at 12,000 g for 15 min. The upper aqueous phase was carefully transferred to a new collection tube, and precipitated with 2 volumes of isopropanol, centrifuged at 18,000 g for 15 min, and washed with 75 % ethanol. After air drying, the RNA was dissolved in 30 μl RNase-free water. The quality and quantity of the RNA was evaluated using a NanoDrop 200c spectrophotometer (Thermo Fisher Scientific, Waltham, MA). The efficiency of small RNA isolation was monitored by determining the amount of spiked miRNA recovered by using TaqMan miRNA assays (Applied Biosystems). Total RNA (1.5 μl per reaction) was used for qRT-PCRs of miR-122, miR-34a, miR-200b, miR-192, miR-221, and miR-181a using TaqMan miRNA assays (Applied Biosystems), according to the manufacturer’s instructions. The relative amount of each miRNA was measured using the 2−ΔΔCt method and normalized to mmu-miR-16, a ubiquitous non-liver-specific miRNA.
Statistical analyses
Results are presented as mean ± S.D. Clinical chemistry values, body weights, and miRNA levels were analyzed by two-way analysis of variance (ANOVA), with pair-wise comparisons being made by the Student-Newman-Keuls method. When necessary, the data were natural log transformed before conducting the analyses to maintain a more equal variance or normal data distribution. Histopathology scores were evaluated by one-way ANOVA, using strain as the fixed factor. Pearson product-moment correlation coefficients were used to determine the relationship between miRNA levels and histopathology scores. P-values <0.05 were considered significant.
RESULTS
Inter-strain differences in liver pathology elicited by a choline- and folate-deficient diet
Over the course of this 12 week study, all mice fed the CFD diet gained weight, with the 129S1/SvImJ mice gaining significantly more than the methyl-sufficient control mice, and the WSB/EiJ mice gaining significantly less than the methyl-sufficient control mice (Table 1). These results are in contrast to some of the previous reports that found a substantial loss of the body weight, often between 20–40%, as a result of feeding mice various formulations of “lipogenic methyl-deficient diets”, e.g., methionine- and choline-deficient, or choline-deficient diets (Larter and Yeh, 2008; Ariz et al., 2010; Hebbard and George, 2011). This is considered as one of the major disadvantages of the mouse model of NAFLD induced by these diets (Larter and Yeh, 2008; Ariz et al., 2010; Hebbard and George, 2011). A significant increase, 1.5–3-fold, in relative liver weight was observed in six of the seven strains fed the CFD diet (Table 1).
Table 1.
Inter-strain differences in pathology endpoints in mice fed the choline- and folate-deficient diet.
| Strain | Group | Body weight (g) | Change of body weight (g) | Relative liver weight (%) | ALT (U/L) | AST (U/L) | LDH (U/L) | Triglycerides (mg/dl) | Cholesterol (mg/dl) | Glucose (mg/dl) |
|---|---|---|---|---|---|---|---|---|---|---|
| A/J | Control | 25.4±1.51 | 6.62±1.26 | 4.16±0.25 | 162.8±85.0 | 203.2±16.9 | 1180±280 | 175.2±11.7 | 94.0±4.5 | 214.4±17.2 |
| MCFD diet | 23.2±2.41 | 5.02±1.02 | 4.76±0.45 | 118.4±7.7 | 204.8±32.3 | 1287±231 | 94.0±7.1* | 116.4±15.7 | 194.8±16.5 | |
| C57BL/6J | Control | 30.8±1.22 | 11.88±1.02 | 4.17±0.22 | 49.6±8.3 | 135.6±26.6 | 1148±475 | 188.0±38.8 | 102.4±21.5 | 203.6±39.2 |
| MCFD diet | 30.7±2.98 | 11.90±3.40 | 6.39±0.95* | 194.8±50.6* | 209.2±36.1† | 1430±293 | 110.8±13.8† | 114.8±14.1 | 274.4±31.3 | |
| C3H/HeJ | Control | 32.4±1.80 | 11.50±1.92 | 4.19±0.14 | 57.6±13.0 | 129.6±21.6 | 1149±438 | 166.4±23.0 | 153.2±4.8 | 197.6±9.8 |
| MCFD diet | 34.8±4.33 | 13.40±3.40 | 7.44±0.47* | 266.4±54.6* | 259.2±54.5* | 1472±336 | 126.0±10.7* | 149.6±8.9 | 202.0±23.5 | |
| 129S1/SvImJ | Control | 27.2±2.22 | 7.88±1.26 | 3.42±0.19 | 191.2±92.4 | 368.0±99.1 | 1480±235 | 171.2±18.6 | 138.4±7.6 | 248.0±12.7 |
| MCFD diet | 27.7±1.30 | 10.14±1.21* | 7.60±2.05* | 336.4±68.6 | 455.6±50.4 | 2415±631 | 101.6±19.9* | 122.6±11.6 | 219.0±19.4 | |
| CAST/EiJ | Control | 16.9±0.29 | 4.46±0.51 | 4.16±0.44 | 184.8±66.9 | 255.8±65.4 | 1227±198 | 233.2±22.5 | 83.6±5.9 | 278.8±25.9 |
| MCFD diet | 17.2±0.23 | 4.75±0.68 | 7.92±0.24* | 375.5±60.1* | 374.5±60.7† | 2316±574* | 104.5±11.8* | 57.0±24.3 | 329.0±36.5† | |
| PWK/PhJ | Control | 18.6±1.27 | 2.86±1.49 | 4.75±0.83 | 89.6±28.2 | 197.2±62.4 | 1066±199 | 162.4±12.1 | 84.4±5.4 | 199.6±22.7 |
| MCFD diet | 20.1±1.18 | 1.96±0.73 | 13.2±0.72* | 760.8±74.4* | 576.0±66.0* | 2529±313* | 106.0±8.4* | 33.4±8.4* | 170.0±4.5 | |
| WSB/EiJ | Control | 21.1±0.80 | 6.84±0.51 | 4.26±0.05 | 103.6±32.1 | 329.2±73.5 | 1184±343 | 108.4±5.5 | 114.4±3.0 | 195.6±15.5 |
| MCFD diet | 19.3±0.85 | 4.54±0.57† | 7.60±0.34* | 745.2±42.2* | 617.6±71.0* | 3090±301* | 113.2±11.7 | 30.0±3.0* | 153.6±6.4* |
Significantly different from age-matched control mice (p-value ≤ 0.01);
Significantly different from age-matched control mice (p-value ≤ 0.05).
Serum triglycerides, cholesterol, and glucose levels were affected by the methyl donor-deficient diet in a strain-dependent manner (Table 1). A decrease in triglycerides was significant in five of the seven strains tested and a greater than 50% reduction was observed in CAST/EiJ mice. The magnitude of change in serum cholesterol and glucose levels was less pronounced. Only two strains, PWK/PhJ, and WSB/EiJ, exhibited a greater than 50% significant reduction in cholesterol and a significant decrease in serum glucose concentrations was found in WSB/EiJ mice only.
Feeding the CFD diet resulted in a significant increase in the activity of serum enzyme markers of liver injury, ALT and AST, in C57BL/6J, C3H/HeJ, CAST/EiJ, PWK/PhJ, and WSB/EiJ strains (Table 1), which was significantly correlated with the degree of liver injury (Supplementary Figure 1). Serum activity of LDH was increased significantly in CAST/EiJ, PWK/PhJ, and WSB/EiJ strains by 1.9, 2.4, and 2.6-fold, respectively.
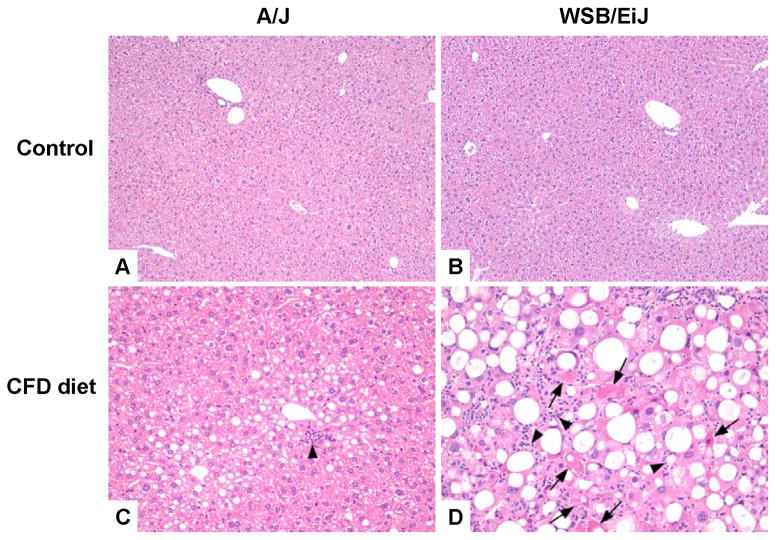
Histopathological evaluation of liver injury revealed the presence of NAFLD-associated pathomorphological features, including cytoplasmic alteration, steatosis, necrosis, inflammation, oval cell hyperplasia, and karyomegaly in all mice fed the CFD diet (Table 2). Interestingly, the magnitude of the liver injury varied greatly among strains with the extent of total liver injury increasing in the following order according to the total liver pathology scores: A/J ≈ C57BL/6J ≈ C3H/HeJ < 129S1/SvImJ ≈ CAST/EiJ < PWK/PhJ < WSB/EiJ. Lipid accumulation in hepatocytes was characterized by microvesicular, macrovesicular, and most commonly mixed (microvesicular and macrovesicular) patterns of fat deposition. Hepatocytes with microvesicular fat accumulation appeared foamy, with the cytoplasm partially or completely filled with numerous small lipid vacuoles, which did not displace the nucleus to the periphery. This pattern of steatosis was most pronounced in A/J mice (Figure 1C). Macrovesicular fatty change was morphologically characterized by hepatocytes mostly containing a moderately large to large and well-defined single rounded vacuole within each cell. The nucleus and cytoplasm were displaced to the periphery in such cells. This pattern was commonly observed in strains exhibiting the greatest degree of liver injury, such as WSB/EiJ mice (Figure 1D).
Table 2.
Summary of the type and extent of hepatic lesions in mice fed the choline- and folate-deficient diet for 12 weeks.
| Strain | Group | Lesion | |||||
|---|---|---|---|---|---|---|---|
| Karyomegaly | Steatosis | Necrosis, hepatocyte | Inflammation | Oval cell hyperplasia | Cytoplasmic alteration | ||
| A/J | Control | 0/5 | 0/5 | 0/5 | 1/5 (0.2 ± 0.5) | 0/5 | 5/5 (1.6 ± 0.6) |
| MCFD diet | 0/5 | 5/5 (1.4 ± 0.6) | 0/5 | 5/5 (1.0 ± 0.0) | 1/5 (0.2 ± 0.5) | 4/5 (1.2 ± 0.8) | |
| C57BL/6J | Control | 0/5 | 0/5 | 1/5 (0.2 ± 0.5) | 1/5 (0.2 ± 0.5) | 0/5 | 2/5 (0.8 ± 1.1) |
| MCFD diet | 0/5 | 5/5 (1.8 ± 0.5) | 0/5 | 5/5 (1.4 ± 0.6) | 3/5 (0.6 ± 0.6) | 3/5 (0.6 ± 0.6) | |
| C3H/HeJ | Control | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| MCFD diet | 0/5 | 5/5 (2.6 ± 0.6) | 0/5 | 5/5 (1.0 ± 0.0) | 0/5 | 0/5 | |
| 129S1/SvImJ | Control | 0/5 | 0/5 | 0/5 | 1/5 (0.2 ± 0.5) | 0/5 | 5/5 (1.8 ± 0.5) |
| MCFD diet | 0/5 | 5/5 (3.8 ± 0.5) | 0/5 | 5/5 (1.4 ± 0.6) | 1/5 (0.2 ± 0.5) | 0/5 | |
| CAST/EiJ | Control | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 2/5 (0.8 ± 1.1) |
| MCFD diet | 4/4 (1.0 ± 0.0) | 4/4 (3.5 ± 0.6) | 0/4 | 3/4 (0.8 ± 0.5) | 1/4 (0.3 ± 0.5) | 3/4 (1.0 ± 0.8) | |
| PWK/PhJ | Control | 0/5 | 0/5 | 0/5 | 1/5 (0.2 ± 0.5) | 0/5 | 1/5 (0.2 ± 0.5) |
| MCFD diet | 5/5 (1.4 ± 0.6) | 5/5 (3.8 ± 0.5) | 5/5 (1.0 ± 0.0) | 5/5 (2.0 ± 0.0) | 5/5 (1.2 ± 0.5) | 5/5 (4.0 ± 0.0) | |
| WSB/EiJ | Control | 0/5 | 0/5 | 0/5 | 1/5 (0.2 ± 0.5) | 0/5 | 4/5 (1.0 ± 0.7) |
| MCFD diet | 5/5 (2.0 ± 0.0) | 5/5 (4.0 ± 0.0) | 5/5 (3.8 ± 0.5) | 5/5 (3.8 ± 0.5) | 5/5 (3.6 ± 0.6) | 5/5 (2.2 ± 0.5) | |
Lesion prevalence.
(Mean severity).
Figure 1. Representative hematoxylin and eosin staining of liver tissues from control A/J and WSB/EiJ mice (A,B) mice fed the choline- and folate-deficient diet (C,D).
(A) Representative liver section from control A/J mice. (B) Representative liver section from control WSB/EiJ mice. (C) Representative liver section from A/J mice fed the CFD diet for 12 weeks. Minimal steatosis and inflammation (arrows) in the livers. (D) Representative liver section from WSB/EiJ mice fed the CFD for 12 weeks. Severe steatosis, inflammation (arrowheads) and necrosis (arrows) of hepatocytes in the livers.
Inflammatory foci (Figures 1C and 1D, marked by arrowheads) were composed of a mixed population of polymorphonuclear and mononuclear cells, mainly neutrophils, macrophages, and lymphocytes. Single-cell necrosis was one of the most common histopathological observations in both of the PWK/PhJ and WSB/EiJ mouse strains, but was most severe in the WSB/EiJ mice. Karyomegaly, characterized as 2–4 times larger than typical hepatocyte nuclei, was found in CFD diet-fed mice from PWK/PhJ and WSB/EiJ strains (Figure 1; compare larger sized nuclei in Figures 1C and 1D). Hyperplasia of oval cells, stem cells of bile ductular epithelium that appeared as single or double rows of oval- or round-shaped cells organized in linear arrays in liver sinusoids, was also observed in choline- and folate- deficient PWK/PhJ and WSB/EiJ mice.
Effect of a choline- and folate-deficient diet on hepatic microRNA expression profile
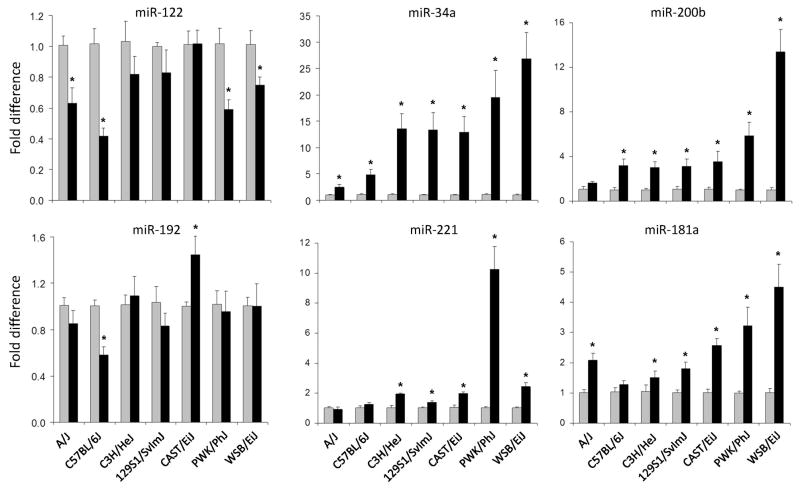
Dysregulation of hepatic expression of miR-34a, miR-122, miR-181a, miR-192, miR-200b, and miR-221 was previously reported to be a common feature in human (Cheung et al., 2008) and mouse NAFLD (Wang et al., 2009a; Pogribny et al., 2010). Because an inter-strain difference in liver injury phenotype elicited by the CFD diet was observed in this study, we investigated whether or not hepatic expression of these miRNAs may reflect variability in the degree of NAFLD or may be associated with strain-specific susceptibility to liver injury. The expression of miR-122 was down-regulated by 25 to 60% in the livers of A/J, C57BL/6J, PWK/PhJ, and WSB/EiJ mice fed the CFD diet (Figure 2). No change was observed in C3H/HeJ, 129S1/SvlmJ, or CAST/EiJ mice. MiR-192 was down-regulated by 50% only in C57BL/6J mice and slightly up-regulated in CAST/EiJ mice.
Figure 2. Expression changes of miR-122, miR-34a, miR-200b, miR-192, miR-221, and miR-181a miRNAs in the livers of control mice and mice fed the choline- and folate-deficient diet.
The miRNA expression data are presented as fold change of each miRNA normalized to that of snoRNA202 in the livers of mice fed the CFD diet compared to control mice (n = 5, mean ± SED). Gray bars – control groups; black bars – CFD diet groups.
* - Significantly different from age-matched control mice.
The expression of miR-34a, miR-200b, and miR-181a was significantly increased in CFD diet-fed mice of a majority of strains in a strain-dependent manner, with the maximum response occurring in WSB/EiJ mice (Figure 2). Up-regulation of hepatic expression of miR-221 was most pronounced (>10-fold) in PWK/PhJ mice, with a smaller, but significant, increases being observed in C3H/HeJ, 129S1/SvlmJ, CAST/EiJ, and WSB/EiJ mice. In contrast, feeding the CFD diet did not affect the expression of a ubiquitous non-liver-specific miRNA let-7c in all but WSB/EiJ mice (Supplementary Figure 2).
Effect of a choline- and folate-deficient diet on plasma microRNA expression profile
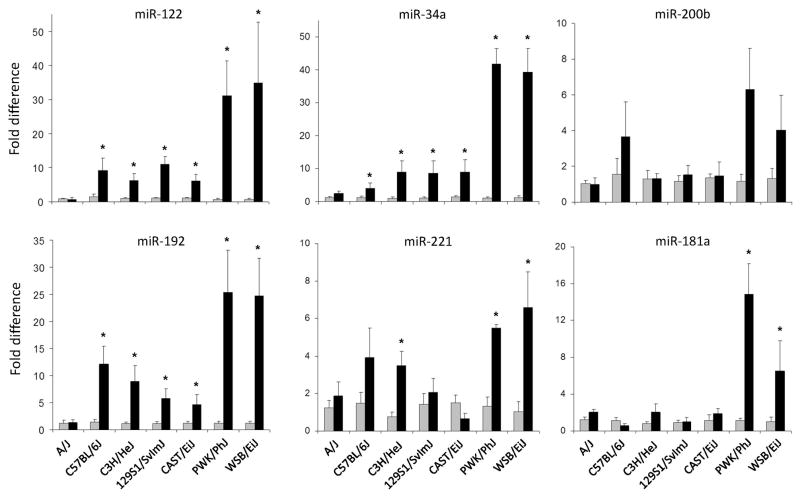
Plasma levels of miRNAs have been shown to be correlated with the degree of liver injury in response to drug treatment (Wang et al., 2009b; Larerza et al., 2009). We evaluated the differences in patterns of miRNA changes in plasma of mice fed control or CFD diet. Figure 3 demonstrates that there were strain-specific differences in the levels of miRNAs in the mice fed the CFD diet. Notably, in A/J mice, one of the strains with the lowest extent of methyl donor-deficient diet-induced liver injury, none of the miRNAs was increased by feeding the CFD diet. In contrast, in plasma of PWK/PhJ and WSB/EiJ mice, strains that exhibited the greatest degree of liver injury, each of miRNAs, with the exception of miR-200b was significantly increased by choline and folate deficiency.
Figure 3. Levels of miR-122, miR-34a, miR-200b, miR-192, miR-221, and miR-181a miRNAs in plasma of control mice and mice fed the choline- and folate-deficient diet.
The miRNA expression data are presented as fold change of each miRNA normalized to that of normalized to mmu-miR-16, a ubiquitous non-liver-specific miRNA in plasma of mice fed the CFD diet compared to control mice. (n = 5, mean ± SED). Gray bars – control groups; black bars – CFD diet groups.
* - Significantly different from age-matched control mice.
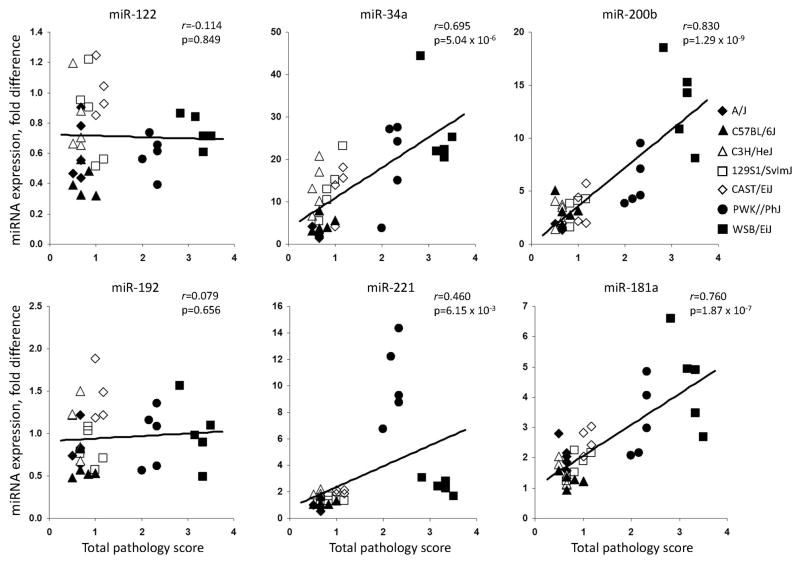
Because strain-dependent effects of treatment on miRNA levels in liver and plasma were observed, we tested whether the inter-strain difference in the degree of liver injury correlated with miRNA expression. Figure 4 shows that the induction of miR-34a, miR-181a, miR-200b and miR-221 in the livers is significantly correlated with the histopathology score in CFD diet-fed mice, with the strongest correlations being observed with miR-181a and miR-200b. Interestingly, expression of miR-122 and miR-192 in the livers, two of the most abundant hepatic miRNAs (Gatti et al., 2011), did not correlate with the extent of liver injury.
Figure 4. Correlation plots of total liver pathology scores and induction of miRNAs in the livers of mice fed the choline- and folate-deficient diet.
Total liver pathology scores represent the mean severity for all of the lesions detected in the liver of mouse. Each symbol represents individual animal in the CFD diet group in respected mouse strain.
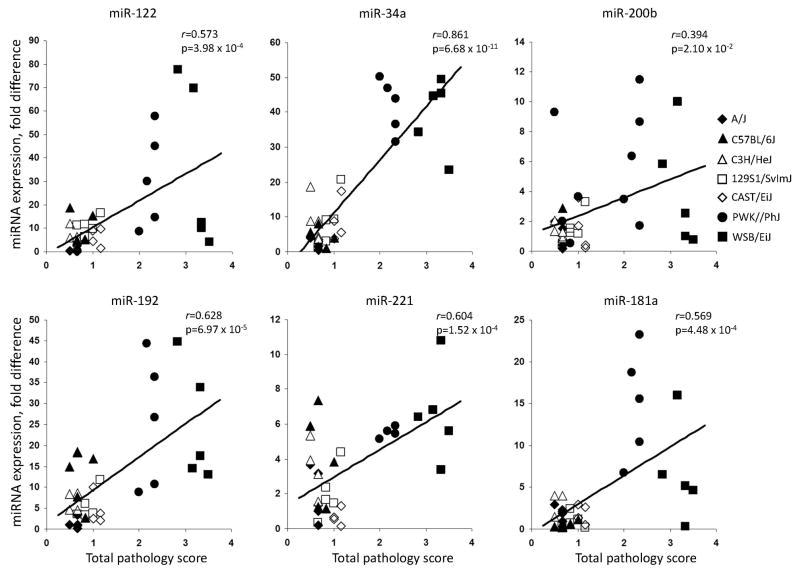
Figure 5 shows that plasma levels of each of the miRNAs were significantly correlated with the degree of liver injury induced by the CFD diet, with strongest correlation occurring with miR-34a, whereas level of circulating miRNA let-7c did not differ (Supplementary Figure 2).
Figure 5. Correlation plots of total liver pathology scores and induction of miRNA levels in plasma of mice fed the choline- and folate-deficient diet.
Total liver pathology scores represent the mean severity for all of the lesions detected in the liver of mouse. Each symbol represents individual animal in the CFD diet group in respected mouse strain.
DISCUSSION
The results of the present study demonstrate that feeding a multistrain panel of inbred mice the CFD diet for 12 weeks resulted in strain-specific changes in biochemical indicators of liver injury in plasma (Table 1) and accumulation of microscopically observed lesions in the livers similar to human NAFLD (Table 2, Figure 1). The previous comprehensive studies have established that dietary-based models of NAFLD induced by methionine- and choline-deficient or choline-deficient diets are the best models to study mechanisms of liver injury in NAFLD, despite the fact that some of the features do not resemble pathogenesis of NAFLD in humans (Larter and Yeh, 2008; Ariz et al., 2010; Hebbard and George, 2011; Maher, 2011). One of the major disadvantage of the mouse NAFLD model induced by methionine- and choline-deficient or choline-deficient diets is associated with significant loss of body weight (Larter and Yeh, 2008; Ariz et al., 2010; Hebbard and George, 2011). The results of the present study showing that feeding the methionine-containing CFD diet induced liver injury without the loss of body weight, overcome this shortcoming of the commonly used methionine- and choline-deficient or choline-deficient diets.
The magnitude of biochemical indicators of liver injury in plasma and the extent of histomorphological changes in the livers ranged, with the order being: A/J ≈ C57BL/6J ≈ C3H/HeJ < 129S1/SvImJ ≈ CAST/EiJ < PWK/PhJ < WSB/EiJ. More importantly, the pathomorphological alterations in the livers were accompanied by different expression patterns of hepatic miR-122, miR-181a, miR-192, miR-34a, miR-200b, and miR-221.
miRNA-122 is the most abundant and highly liver-specific miRNA, accounting for 70% of all miRNAs in the adult liver (Girard et al., 2008; Lewis and Jopling, 2010). The expression of hepatic miR-122 has been reported to decrease significantly in individuals with viral- and alcohol-induced liver injuries and NASH (Cheung et al., 2008; Morita et al., 2011) and to be inversely correlated with the severity of liver histopathology. Similar down-regulation of miR-122 has been reported in experimental animals with NAFLD (Wang et al., 2008b; Pogribny et al., 2010). However, the results of present study demonstrate clearly the existence of inter-strain variability in expression of hepatic miR-122 in response to feeding the CFD diet. Specifically, down-regulation of miR-122 was found only in the livers of A/J, C57BL/6J, PWK/PhJ, and WSB/EiJ mice only. More importantly, while changes in miR-122 expression in the livers may be reflective of liver injury, the down-regulation of this miRNA was not associated with severity of liver injury. This was evidenced by the fact that changes in hepatic miR-122 expression did not correlate with the magnitude of pathomorphological changes in the livers of choline- and folate-deficient mice (Figure 4). Likewise, the expression of another highly abundant and liver-specific miRNAs, miR-192, was not associated with the severity of liver damage induced by a CFD diet. In contrast, expression of miR-34a, miR-200b, miR-221 and miR-181a miRNAs increased across strains, with the greatest magnitude being found in sensitive PWK/PhJ, and WSB/EiJ strains. More importantly, up-regulation of miR-34a, miR-181a, miR-200b, and miR-221 in the livers of CFD diet-fed mice strongly correlated with a severity of NAFLD-related pathomorphological changes in the livers.
The most notable finding in this study is that plasma levels of each of the miRNAs were significantly correlated with the severity of liver injury induced by the CFD diet, with strongest correlation occurring with miR-34a.
It has been suggested that plasma miRNAs may be potential biomarkers to diagnose and monitor diseases (Cortez and Calin, 2009), including several types of liver pathologies, ranging from drug-induced liver injury to chronic viral hepatitis and NAFLD (Wang et al., 2008b; Laterza et al., 2009; Bihrer et al., 2011; Cermelli et al., 2011). Specifically, the increased levels of plasma miR-122 alone or the combination of miR-122 and miR-192 have been used to detect liver injury induced by traditional liver toxicants, including trichlorobromomethane, carbon tetrachloride, and acetaminophen in rats and mice (Wang et al., 2008b; Laterza et al., 2009). Importantly, several recent clinical studies have demonstrated that the levels of circulating miR-122, miR-192, and miR-34a are significantly higher in patients with acetoaminophen-induced acute liver injury, NAFLD, and chronic hepatitis C infection suggesting that plasma miRNAs may be useful biomarkers to monitor ongoing damage of hepatocytes and the magnitude of necroinflammation (Cermelii et al., 2011; Starkey Lewis et al., 2011). The results of the present study showing a high and significant correlation between the levels of miR-122, miR-181a, miR-192, miR-34a, miR-200b, and miR-221 in plasma and a severity of NAFLD-specific pathomorphological changes in the livers of mice fed the CFD diet reinforce this suggestion. More importantly, the lack of changes in the plasma level of ubiquitous non-liver-specific miRNA let-7c indicates that these circulating miRNAs may be potential liver-specific biomarkers for noninvasive evaluation of liver injury in NAFLD. In addition, a strong correspondence between correlation of the levels on miRNAs and the activity of ALT and AST in plasma with the extent of liver injury suggests that miRNAs may serve as independent robust biomarkers of liver injury (Figure 5 and Supplementary Figure 1).
In conclusion, the results of our study demonstrate that development of NAFLD in mice induced by a choline- and folate-deficient diet is associated with altered expression of hepatic miRNAs and their subsequent elevation in plasma. More importantly, the severity of NAFLD is strongly associated with levels of circulating miR-122, miR-181a, miR-192, miR-34a, miR-200b, and miR-221 that mirror the magnitude of NAFLD-associated liver injury. These results suggest that circulating miRNAs may be applied in human population-based studies as sensitive genetic background-independent indicators for noninvasive monitoring the development and extent of NAFLD-associated liver injury. Additionally, they can be used as potential noninvasive indicators of susceptibility to NAFLD liver injury.
Supplementary Material
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- LDH
lactate dehydrogenase
- miRNAs
microRNAs
- NAFLD
nonalcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- qRT-PCR
quantitative reverse transcription real-time PCR
Footnotes
Conflict of Interest: The authors declare that there are no conflicts of interest
The views expressed in this manuscript do not necessarily represent those of the U.S. Food and Drug Administration.
References
- Ariz U, Mato JM, Lu SC, Martines-Chantar ML. Nonalcoholic steatohepatitis, animal models, and biomarkers: what is new? Methods Mol Biol. 2010;593:109–136. doi: 10.1007/978-1-60327-194-3_6. [DOI] [PubMed] [Google Scholar]
- Aylor DL, Valdar W, Foulds-Mathes W, Buus RJ, Verdugo RA, Baric RS, Ferris MT, Frelinger JA, Heise M, Frieman MB, Gralinski LE, Bell TA, Didion JD, Hua K, Nehrenberg DL, Powell CL, Steigerwalt J, Xie Y, Kelada SNP, Collins FC, Yang IV, Schwartz DA, Branstetter LA, Chesler EJ, Miller DR, Spence J, Liu EY, McMillan L, Sarkar A, Wang J, Wang W, Zhang Q, Broman KW, Korstanje R, Durrant C, Mott R, Iraqi FA, Pomp D, Threadgill D, Pardo-Manuel de Villena F, Churchill GA. Genetic analysis of complex traits in the emerging collaborative cross. Genome Res. 2011;21:1213–1222. doi: 10.1101/gr.111310.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellentani S, Scaqlioni F, Marino M, Begogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28:155–161. doi: 10.1159/000282080. [DOI] [PubMed] [Google Scholar]
- Bihrer V, Friedrich-Rust M, Kronenberger B, Forestier N, Haupenthal J, Shi Y, Peveling-Oberhag J, Radeke HH, Sarrazin C, Herrmann E, Zeuzem S, Waidmann O, Piiper A. Serum miR-122 as biomarker of necroinflammation in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 2011;106:1663–1669. doi: 10.1038/ajg.2011.161. [DOI] [PubMed] [Google Scholar]
- Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- Cermelli S, Ruggieri A, Marrero JA, Ioannou GN, Beretta L. Circulating microRNAs in patients with chronic hepatitis C and non-alcoholic fatty liver disease. PLoS One. 2011;6:e23937. doi: 10.1371/journal.pone.0023937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalasani N, Guo X, Loomba R, Goodarzi MO, Haritunians T, Kwon S, Cui J, Taylor KD, Wilson L, Cummings OW, Chen YD, Rotter JI Nonalcoholic Steatohepatitis Clinical Research Network. Genome-wide association study identifies variants associated with histologic features of nonalcoholic fatty liver disease. Gastroenterology. 2010;139:1567–1576. doi: 10.1053/j.gastro.2010.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung O, Puri P, Eicken C, Contos MJ, Mirshahi F, Maher JW, Kellum JM, Min H, Luketic VA, Sanyal AJ. Nonalcoholic steatohepatitis is associated with altered hepatic MicroRNA expression. Hepatology. 2008;48:1810–1820. doi: 10.1002/hep.22569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2001;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez MA, Calin GA. MicroRNA identification in plasma and serum: a new tool to diagnose and monitor diseases. Expert Opin Biol Ther. 2009;9:703–711. doi: 10.1517/14712590902932889. [DOI] [PubMed] [Google Scholar]
- Day CP. Non-alcoholic fatty liver disease: a massive problem. Clin Med. 2011;11:176–178. doi: 10.7861/clinmedicine.11-2-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti DM, Lu L, Williams RW, Sun W, Wright FA, Threadgill DW, Rusyn MicroRNA expression in the livers of inbred mice. Mutat Res. 2011;714:126–133. doi: 10.1016/j.mrfmmm.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard M, Jacquemin E, Munnich A, Lyonnet S, Henrion-Caude A. miR-122, a paradigm for the role of microRNAs in the liver. J Hepatol. 2008;48:648–656. doi: 10.1016/j.jhep.2008.01.019. [DOI] [PubMed] [Google Scholar]
- Hebbard L, George J. Animal models of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2011;8:35–44. doi: 10.1038/nrgastro.2010.191. [DOI] [PubMed] [Google Scholar]
- Hill-Baskin AE, Markiewski MM, Buchner DA, Shao H, DeSantis D, Hsiao G, Subramaniam S, Berger NA, Croniger C, Lambris JD, Nadeau JH. Diet-induced hepatocellular carcinoma in genetically predisposed mice. Hum Mol Genet. 2010;18:2975–2988. doi: 10.1093/hmg/ddp236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larter CZ, Yeh MM. Animal models of NASH: getting both pathology and metabolic context right. J Gastroenterol Hepatol. 2008;23:1635–1648. doi: 10.1111/j.1440-1746.2008.05543.x. [DOI] [PubMed] [Google Scholar]
- Larter CZ, Chitturi S, Heydet D, Farrel GC. A fresh look at NASH pathogenesis. Part 1: the metabolic movers. J Gastroenterol Hepatol. 2010;25:672–690. doi: 10.1111/j.1440-1746.2010.06253.x. [DOI] [PubMed] [Google Scholar]
- Laterza OF, Lim L, Garrett-Engele PW, Vlasakova K, Muniappa N, Tanaka WK, Johnson JM, Sina JF, Fare TL, Sistare FD, Glaab WE. Plasma microRNAs as sensitive and specific biomarkers of tissue injury. Clin Chem. 2009;55:1977–1983. doi: 10.1373/clinchem.2009.131797. [DOI] [PubMed] [Google Scholar]
- Lewis AP, Jopling CL. Regulation and biological function of the liver-specific miR-122. Biochem Soc Trans. 2010;38:1553–1557. doi: 10.1042/BST0381553. [DOI] [PubMed] [Google Scholar]
- Marra F, Gastaldelli A, Svegliati Baroni G, Tell G, Tiribelli C. Molecular basis and mechanisms of progression of non-alcoholic steatohepatitis. Trends Mol Med. 2008;14:72–81. doi: 10.1016/j.molmed.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Maher JJ. New insights from rodent models of fatty liver disease. Antioxid Redox Signal. 2011;15:535–550. doi: 10.1089/ars.2010.3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Taketomi A, Shirabe K, Umeda K, Kayashima H, Ninomiya M, Uchiyama H, Soejima Y, Maehara Y. Clinical significance and potential of hepatic microRNA-122 expression in hepatitis C. Liver Int. 2011;31:474–484. doi: 10.1111/j.1478-3231.2010.02433.x. [DOI] [PubMed] [Google Scholar]
- Pais R, Pascale A, Fedchuck L, Charlotte F, Poynard T, Ratziu V. Progression from isolated steatosis to steatohepatitis and fibrosis in nonalcoholic fatty liver disease. Clin Res Hepatol Gastroenterol. 2011;35:23–28. doi: 10.1016/j.gcb.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Pascale A, Pais RA, Ratziu V. An overview of nonalcoholic steatohepatitis: past, present and future directions. J Gastrointestin Liver Dis. 2010;19:415–423. [PubMed] [Google Scholar]
- Pogribny IP, Tryndyak VP, Bagnyukova TV, Melnyk S, Montgomery B, Ross SA, Latendresse JR, Rusyn I, Beland FA. Hepatic epigenetic phenotype predetermines individual susceptibility to hepatic steatosis in mice fed a lipogenic methyl-deficient diet. J Hepatol. 2009;51:176–186. doi: 10.1016/j.jhep.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogribny IP, Starlard-Davenport A, Tryndyak VP, Han T, Ross SA, Rusyn I, Beland FA. Difference in expression of hepatic microRNAs miR-29c, miR-34a, miR-155, and miR-200b is associated with strain-specific susceptibility to dietary nonalcoholic steatohepatitis in mice. Lab Invest. 2010;90:1437–1446. doi: 10.1038/labinvest.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusyn I, Gatti DM, Wiltshire T, Kleeberger SR, Threadgill DW. Toxicogenetics: population-based testing of drug and chemical safety in mouse models. Pharmacogenomics. 2010;11:1127–1136. doi: 10.2217/pgs.10.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, Ratziu V, McCullough A. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54:344–353. doi: 10.1002/hep.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwimmer JB, Celedon MA, Lavine JE, Salem R, Campbell N, Schork NJ, Shiehmorteza M, Yokoo T, Chavez A, Middleton MS, Sirlin CB. Heritability of nonalcoholic fatty liver disease. Gastroenterology. 2011;136:1585–1592. doi: 10.1053/j.gastro.2009.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Siegel AB, Zhu AX. Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link. Cancer. 2009;115:5651–5661. doi: 10.1002/cncr.24687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey Lewis PJ, Dear J, Platt V, Simpson KJ, Craig DG, Antoine DJ, French NS, Dhaun N, Webb DJ, Costello EM, Neoptolemos JP, Moggs J, Goldring CE, Park BK. Circulating microRNAs as potential markers of human drug-induced liver injury. Hepatology. 2011;54:1767–1776. doi: 10.1002/hep.24538. [DOI] [PubMed] [Google Scholar]
- Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol. 2010;5:145–171. doi: 10.1146/annurev-pathol-121808-102132. [DOI] [PubMed] [Google Scholar]
- Wang B, Majumder S, Nuovo G, Kutay H, Volinia S, Patel T, Schmittgen TD, Croce C, Ghoshal K, Jacob ST. Role of microRNA-155 at early stages of hepatocarcinogenesis induced by choline-deficient and amino acid-defined diet in C57BL/6 mice. Hepatology. 2009;50:1152–1161. doi: 10.1002/hep.23100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Zhang S, Marzolf B, Troisch P, Brightman A, Hu Z, Hood LE, Galas DJ. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc Natl Acad Sci USA. 2009;106:4402–4407. doi: 10.1073/pnas.0813371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, McGlynn KA. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463–471. doi: 10.1002/hep.24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y, Kakizaki S, Takizawa D, Ichikawa T, Sato K, Takagi H, Mori M. Interstrain differences in susceptibility to non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2008;23:276–282. doi: 10.1111/j.1440-1746.2007.05150.x. [DOI] [PubMed] [Google Scholar]
- Yang H, Wang JR, Didion JP, Buus RJ, Bell TA, Welsh CE, Bonhomme F, Yu AH, Nachman MW, Pialek J, Tucker P, Boursot P. Subspecific origin and haplotype diversity in the laboratory mouse. Nat Genet. 2011;43:648–655. doi: 10.1038/ng.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.