Abstract
GTP cyclohydrolase (GCH1) is rate limiting for tetrahydrobiopterin (BH4) synthesis, where BH4 is a cofactor for nitric oxide (NO) synthases and aromatic hydroxylases. GCH1 polymorphisms are implicated in the pathophysiology of pain, but have not been investigated in African populations. We examined GCH1 and pain in sickle cell anemia where GCH1 rs8007267 was a risk factor for pain crises in discovery (n = 228; odds ratio [OR] 2.26; P = 0.009) and replication (n = 513; OR 2.23; P = 0.004) cohorts. In vitro, cells from sickle cell anemia subjects homozygous for the risk allele produced higher BH4. In vivo physiological studies of traits likely to be modulated by GCH1 showed rs8007267 is associated with altered endothelial dependent blood flow in females with SCA (8.42% of variation; P = 0.002). The GCH1 pain association is attributable to an African haplotype with where its sickle cell anemia pain association is limited to females (OR 2.69; 95% CI 1.21–5.94; P = 0.01) and has the opposite directional association described in Europeans independent of global admixture. The presence of a GCH1 haplotype with high BH4 in populations of African ancestry could explain the association of rs8007267 with sickle cell anemia pain crises. The vascular effects of GCH1 and BH4 may also have broader implications for cardiovascular disease in populations of African ancestry.
Introduction
Sickle cell disease (SCD) is a Mendelian disorder where the clinical hallmark is spontaneous acute painful episodes, presumably precipitated by occlusion of post-capillary venules by erythrocytes deformed by polymerized intracellular sickle hemoglobin (HbS). Pain is believed to develop in response to tissue hypoxia, reperfusion injury and inflammation. Prior to sickle cell specific therapies, 39% of patients had <1 severe painful episodes annually, while another 5% had three or more episodes per year that represented a third of all events requiring physician treatment [1]. Severe pain is the reason for 90% of SCD hospitalizations, and hospitalizations for pain are a marker of severity and a mortality risk [1–3].
There is considerable variability in SCD painful episodes, including a heritable subphenotype with more frequent episodes [4,5]. Few reasons for this variability have been identified, however erythrocytic factors inhibiting HbS polymerization like fetal hemoglobin (HbF) concentration and concurrent α-thalassemia carrier status are indirectly are associated with pain frequency [1,6,7]. In addition, non-erythrocytic factors, like adhesive proteins, including adhesion molecule expression on endothelial cells, leukocytes or red cells are also determinants of vasoocclusion in mice [8]. Moreover, genetic loci directly associated with SCD acute painful episodes have not been identified.
Painful stimuli can produce both acute and chronic responses in the nervous system [9]. A subset of individuals develops persistent pain hypersensitivity for unknown reasons, although both environmental and genetic determinants are thought to contribute. Different strategies including rodent, human twin, genome wide screens, and studies of candidate genes have shown that pain hypersensitivity is heritable with contributions by individual genes [10–13]. In particular, a genome-wide search for pain modulating loci using expression microarrays in dorsal nerve root ganglion cells from neuropathic and inflammatory pain models identified an association with tetrahydrobiopterin (BH4) and the GCH1 gene encoding the enzyme GTP cyclohydrolase (GTPCH) [13]. GTPCH is the rate-limiting enzyme for the synthesis of BH4, a critical cofactor for coupling nitric oxide synthases (NOS) and aromatic hydroxylases that synthesize tyrosine, dopamine and serotonin [14]. Modulation of BH4 contributes to functional pain responses where inhibition of GTPCH ameliorates neuropathic pain and intrathecal BH4 produces hyperalgesia [13]. Furthermore, a GCH1 haplotype present in 15% of those of European ancestry has been associated with a pain protective effect in experimental and some clinical pain phenotypes, but not others [13,15–19]. This reduced expression haplotype also modulates nitric oxide (NO), vascular responses and autonomic function [13,20,21]. Studies in populations of African ancestry have been limited [17,21].
To study the relationship between variation in GCH1, BH4 production and pain in African Americans, we examined patients with homozygous SCD or sickle cell anemia (SCA). Alleles previously associated with protection from pain sensitivity were examined. Genetic and biochemical studies were used to further refine the basis for these observations. Finally, forearm plethysmography, an in vivo physiologic functional test, was used to explore specific associations with vascular function that could be modulated by GCH1.
Methods
Study populations
Three hundred eighty subjects 18 years of age and older with SCD were recruited to the IRB approved Bethesda Sickle Cell Cohort Study at NIH (Clinical-Trials.gov Identifier: NCT00011648). Subjects provided informed consent allowing (1) clinical evaluation and (2) analysis of genomic DNA. Cases were defined by ≥1 visits to an emergency department (or other acute care facility) or hospitalization for the treatment of acute sickle cell pain during the 12 months prior to evaluation as a patient reported outcome. Controls were those with no such painful events. This classification was chosen because these criteria have previously been used to demonstrate the association between frequent painful events and mortality and as an endpoint in two SCA placebo controlled, efficacy trials [3,22,23]. Two hundred eighty one subjects with SCA were included in the case control discovery cohort [24]. The replication study used SCA subjects from the cooperative study of sickle cell disease (CSSCD; ClinicalTrials.gov Identifier: NCT00005277). Severe pain crisis in the CSSCD was defined by any visit to a physician for treatment of sickle cell related pain lasting more than 2 hr [1].
Discovery cohort exploratory vascular endophenotypes
Forearm blood flow studies using venous occlusion strain gauge plethysmography in 21 SCA subjects before and after intra-arterial infusions of acetycholine (Ach), sodium nitroprusside, and NG-monomethyl-l-arginine (L-NMMA, an NO synthase inhibitor) have been described (Clinicaltrials.gov Identifier: NCT00009581) [25]. Normalized data from this study was combined with normalized data from a second study of 20 nonoverlapping SCA subjects (Clinicaltrials.gov Identifier: NCT00072826).
Genotyping and sequencing
Genotyping in the discovery cohort used a combination of Taqman genotyping assays (Applied Biosystems) and automated DNA sequencing [13]. CSSCD genotyping was performed with the Illumina Human610-Quad SNP array with 592,652 autosomal SNPs [26]. Two common SNPs, rs8007267 and rs2878172, genotyped in the NIH discovery cohort were available in the replication study. Illumina assays were performed according to the manufacturer’s protocol with genotypes determined with BeadStudio Software.
Whole blood assays for biopterins
SCA subjects homozygous for rs8007267 had blood drawn for whole blood forskolin assays as described previously [13]. Blood was treated with 10 µM forskolin and incubated for 24 hr at 37°C in six-well cell culture plates (Falcon). After incubation, blood was centrifuged at 3,000 rpm for 10 min. Plasma was removed and stored at −80°C. BH4 and total biopterin levels were determined individually from each plasma sample by high-performance liquid chromatography followed by serial electrochemical and fluorescent detection, as described elsewhere [20].
Population admixture
Population admixture in the CSSCD was assessed with genotype data for 411 SNP ancestry informative markers (AIMs) defined among SCA cases and controls for whom genome-wide data was available. AIMs were selected from a larger set (n = 2,251) identified by calculating δ and FST values between YRI and CEU (Hap-Map population from Utah with European ancestry) define populations for the entire HapMap Phase 1–3 data release [27].
Statistical analysis
Statistical analysis was performed using Instat (Graph Pad Software, San Diego, CA), Prism (Graph Pad Software), Phase 2.0 (http://stephenslab.uchicago.edu/software.html), Microsoft Excel (Microsoft Corporation, Redmond, WA), Haploview 3.32 (wwwbroad.mit.edu/mpg/haploview), SVS (Golden Helix, Bozeman, MT) and Eigensoft 4.2 [28]. Continuous data were normalized to Z-scores as described in the NIST/SEMATECH e-Handbook of Statistical Methods (http://www.itl.nist.gov/div898/handbook/). Comparison of categorical data was implemented by SVS with output measures including crude odds ratios (ORs) and 95% confidence intervals (95% CIs). P values are presented as crude values without statistical correction and FDR adjusted values. Categorical comparisons were made by Chi-square analysis and continuous data comparisons were made using paired t tests, Alternate Welch’s t tests, Mann–Whitney tests or two-way ANOVA (plethysmography) where appropriate.
Results
Pain crises requiring hospital based treatment as a phenotype
Severe vasoocclusion was defined by patient reported hospital based treatment among 228 adults with SCA from a discovery cohort (81.1% with available data from a total of 281 subjects) with a mean age of 34.0 years (standard deviation 11.5). One hundred fifty five experienced 1 or more severe acute painful episodes during the year prior to evaluation, where associations were observed between painful events and hemoglobin, LDH, and ferritin (Table I). Subsequent analyses used a conservative categorical classification into a pain crisis case group (≥1 severe acute painful events) and a low pain crisis control group (0 severe vasoocclusive events) [2].
TABLE I.
Clinical Associations With Severe Pain Events in the Discovery Cohort
| Characteristic | Severe pain cases; n = 155 | No severe pain controls; n = 73 | P value |
|---|---|---|---|
| ER/hospitalizations (SD) | 6.3 (10.9) | 0.0 (0.0) | – |
| Age, yrs (SD) | 32.4 (10.0) | 34.9 (13.6) | 0.42 |
| Male, n (%) | 78 (50.3%) | 31 (42.5%) | 0.76 |
| Hydroxyurea treatment, n (%) | 69 (44.5%) | 24 (32.9%) | 0.09 |
| HbF, % (SD) | 9.0 (6.5) | 7.2 (5.1) | 0.10 |
| Ferritin, mcg L−1 (SD) | 1145.0 (1540.4) | 828.4 (1440.2) | 0.03 |
| LDH, IU L−1 (SD) | 362.3 (151.6) | 420.6 (149.0) | 0.0008 |
| WBC 109 L−1 (SD) | 10.9 (2.8) | 10.6 (2.8) | 0.87 |
| Hemoglobin, g dL−1 (SD) | 9.3 (1.5) | 8.3 (1.6) | <0.0001 |
| MDRD GFR, mL/min./1.73m2 | 160.6 (72.4) | 156.8 (81.6) | 0.78 |
| α-thalassemia, n (%) | 39 (32.8%) | 14 (31.8%) | 0.71 |
GCH1 and vasoocclusive pain crises in sickle cell anemia
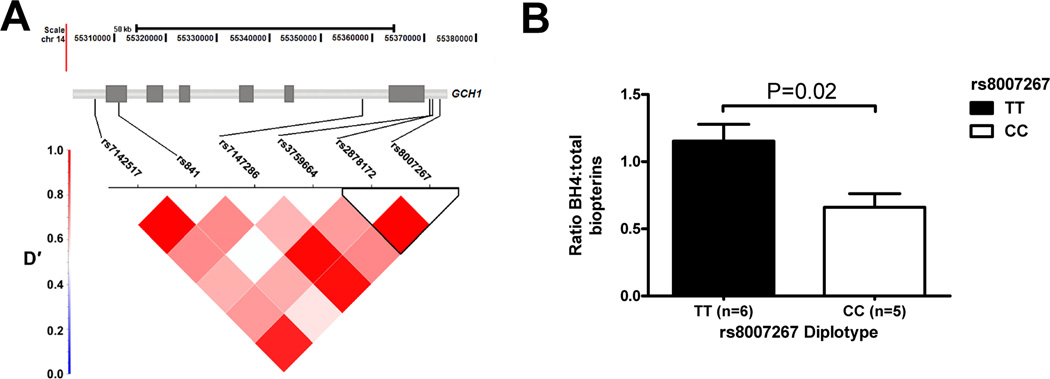
Three out of six GCH1 SNPs were associated with the pain crisis group using a dominant genetic model comparing cases to controls (Table II). All markers were in Hardy–Weinberg equilibrium (Supporting Information Table SI). Two SNPs (rs8007267 and rs7147286), located in the upstream promoter and the first intron (Fig. 1A), had statistically significant association with the case group after correction for false discovery (Table II). These SNPs are in strong linkage disequilibrium (LD; Fig. 1A), indicating that these associations could be attributable to a haplotype. A six marker haplotype was associated with severe painful events (CCACGT haplotype frequency cases 0.252 versus controls 0.162; OR 1.74, 95% CI 1.04–2.92; uncorrected P = 0.03). Unlike previous reports in a European population, the rs8007267 T allele was more prevalent in the SCA population overall and among those in the pain group [13,18,19]. We replicated this association in adults with SCA from the CSSCD using two markers present on an Illumina SNP array, rs8007267 and rs2878172, which were genotyped in the discovery cohort. The rs8007267 major allele again was associated with severe painful episodes and an OR of 2.23 (Table II; 95% CI 1.29–3.84; unadjusted P = 0.0039). Multiple logistic regression with backward elimination in the discovery cohort demonstrated rs8007267, hemoglobin and ferritin were the only variables from Table I independently associated with the painful episode case group (Table III).
TABLE II.
GCH1 Marker Associations With Severe Pain Crises in Sickle Cell Anemia
| NIH discovery cohort (155 cases vs. 73 controls) | CSSCD replication (313 cases vs. 200 controls) | ||||
|---|---|---|---|---|---|
| Marker | Major allele (+strand) |
Dominant model OR (95% CI) | P | Dominant model OR (95% CI) | P |
| rs8007267 | T | 1.98 (1.11–3.53) | 0.02a | 2.23 (1.29–3.84) | 0.004a |
| rs2878172 | G | 2.33 (1.23–4.41) | 0.01b | 0.82 (0.57–1.19) | 0.30 |
| rs3759664 | C | 0.84 (0.47–1.49) | 0.56 | – | – |
| rs7147286 | A | 2.13 (1.21–3.78) | 0.009a | – | – |
| rs841 | C | 1.31 (0.74–2.31) | 0.38 | – | – |
| rs7142517 | C | 1.13 (0.45–2.79) | 0.82 | – | – |
False discovery P value≤ 0.05.
False discovery P value≤ 0.10.
Figure 1.
GCH1 haplotype defined by SNP rs8007267 and in vitro BH4 production. (A) Location of 6 variants typed in the discovery cohort and pattern of linkage disequlibrium (LD, measured as D′) across the GCH1 locus. Rs8007267 and rs2878172 form a haplotype block in strong LD with rs7147286. (B) Ratio of plasma BH4 to total biopterin from whole blood treated with 10 µM forskolin for 24 hr in sickle cell anemia subjects.
TABLE III.
Characteristics Associated With Severe Pain Crises by Logistic Regression in the Discovery Cohort
| Independent variablea | Beta | Standard error | Odds ratiob | P value |
|---|---|---|---|---|
| Hemoglobin | 0.7110 | 0.1747 | 2.04 | 7.35 × 10−5 |
| Ferritin | 0.4138 | 0.1597 | 1.51 | 0.008 |
| rs8007267 | 0.8144 | 0.3198 | 2.26 | 0.009 |
Covariates from the discovery cohort were rank order normalized and converted to z scores prior to analysis. Age, sex, hydroxyurea treatment, percent fetal hemoglobin, and LDH were removed for nonsignificance during stepwise backward elimination.
Odds ratio is given for each standard deviation change in the covariate except for rs8007267 where a dominant genetic model was used.
In vitro BH4 production associated with GCH1 marker rs8007267
To determine the functional relevance of this GCH1 association, we measured pterins in supernatants from forskolin stimulation assays performed on whole blood from SCA patients, where rs8007267 risk factor (T allele) homozygotes had a significantly higher ratio of BH4 to total biopterin (P = 0.02; Fig. 1B).
Sex specificity of the vasoocclusive painful episode association
Sex is a known determinant for SCD pain crisis susceptibility and also pain sensitivity unrelated to SCD [1,29]. We observed significant unadjusted associations with rs8007267, rs2878172, and rs7147286 when the analysis was limited to females (Supporting Information Table SII). Comparisons were not significant in males. No association was detectable between vasoocclusive painful events and sex or as a sex–genotype interaction by regression analysis (data not shown). Analysis restricted to 77 female pain cases and 42 female controls in the Discovery cohort again showed association of the CCACGT haplotype with the severe painful event group (Table III; OR 2.69; 95% CI 1.21–5.94; unadjusted P = 0.01), while a significant association was not observed in males (data not shown).
Rs8007267 and in vivo plethsmography: Endothelial function
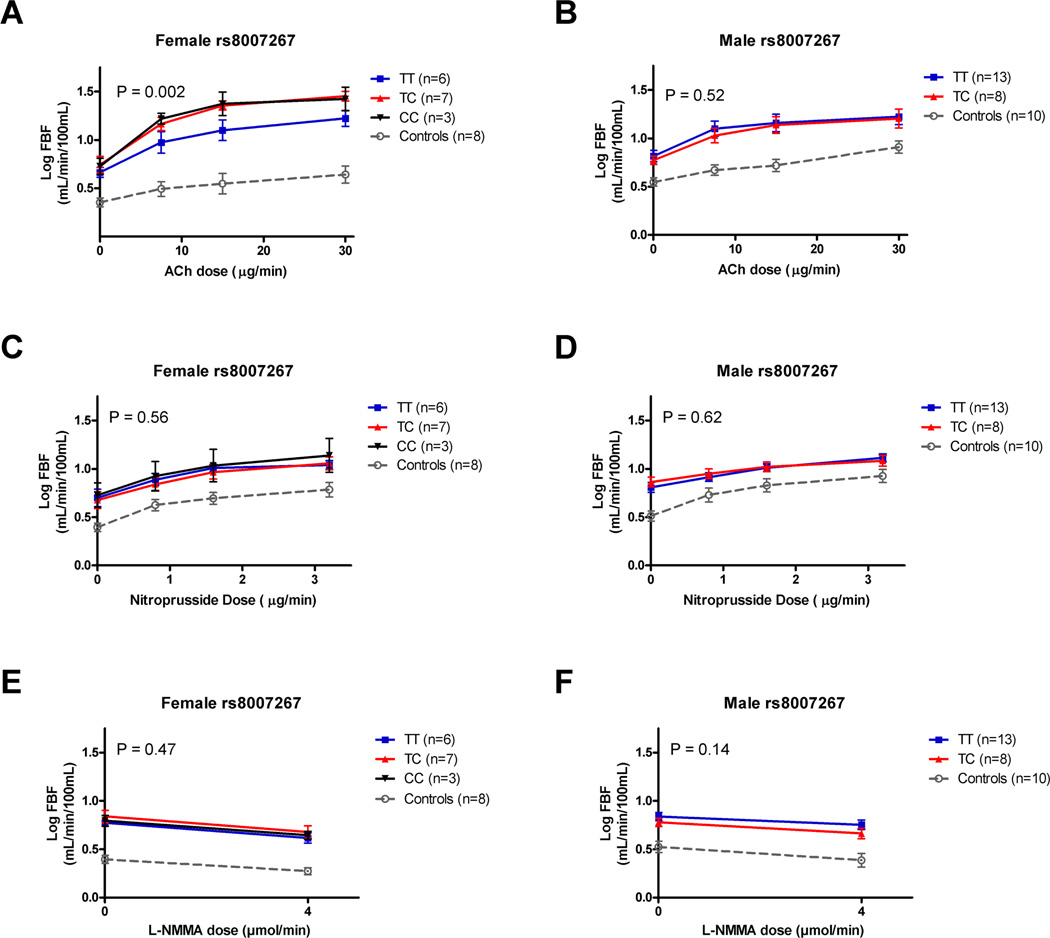
In vitro data indicated rs8007267 was associated with differences in forskolin induced BH4 production, presumably due to differences in GCH1 enzymatic activity (Fig. 1B). In addition, informatic analysis of DNA sequence adjacent to rs8007267 shows phylogenetic conservation within a known Encode project DNase I hypersensitivity site (Supporting Information Fig. S1). Thus, this SNP was further examined for associations with in vivo vascular phenotypes in an exploratory analysis as has been performed in Europeans with coronary artery disease [20]. We examined rs8007267 diploid genotypes among 37 subjects who had undergone forearm blood flow measurements before and after intra-arterial infusions of Ach, nitroprusside, and L-NMMA. Vasodilatory response to Ach infusion is a key indicator of endothelial function. Rs8007267 accounted for 2.15% of the variation in Ach response (data not shown; two way ANOVA, P = 0.08). The rs8007267 effect size was considerably larger in females (8.42% of variation; P = 0.002; Fig. 2A), while there was no association in males (P = 0.52; Fig. 2B). Rs8007267 was not associated endothelium independent vasomotor dilation in response to the NO donor nitroprusside in all 37 subjects (two way ANOVA, P = 0.94; data not shown). This SNP accounted for 1.4% of the variation in nitroprusside dilator response in females (two way ANOVA P = 0.56, Fig. 2C) and 0.21% of the response variation in males (two way ANOVA P = 0.62; Fig. 2D). These results suggest the rs8007267 risk allele is specifically associated with endothelial function. Rs8007267 did not influence basal NO production (response to L-NMMA; P = 0.47), and this was not influenced by sex (Fig. 2E,F). However, L-NMMA did significantly reduce blood flow for both females (25.5% of variation attributed to L-NMMA dose; P = 0.004) and males (9.3% of variation; P = 0.05) consistent with previous observations that males with SCA have diminished NO responsiveness [25].
Figure 2.
Exploratory analysis of GCH1 sex specific in vivo plethysmography responses to Ach (acetylcholine), nitroprusside and L-NMMA in sickle cell anemia. (A) Forearm flow responses after intra-arterial infusion of Ach in females with sickle cell anemia by rs8007267 diploid genotype. (B) Ach blood flow responses in males by rs8007267 diploid genotype. (C–D) Forearm flow responses to infusion of nitroprusside in females and males, respectively. (E–F) Forearm flow responses in females and males to L-NMMA were not associated with rs8007267 genotypes.
Population admixture and HapMap haplotype analysis
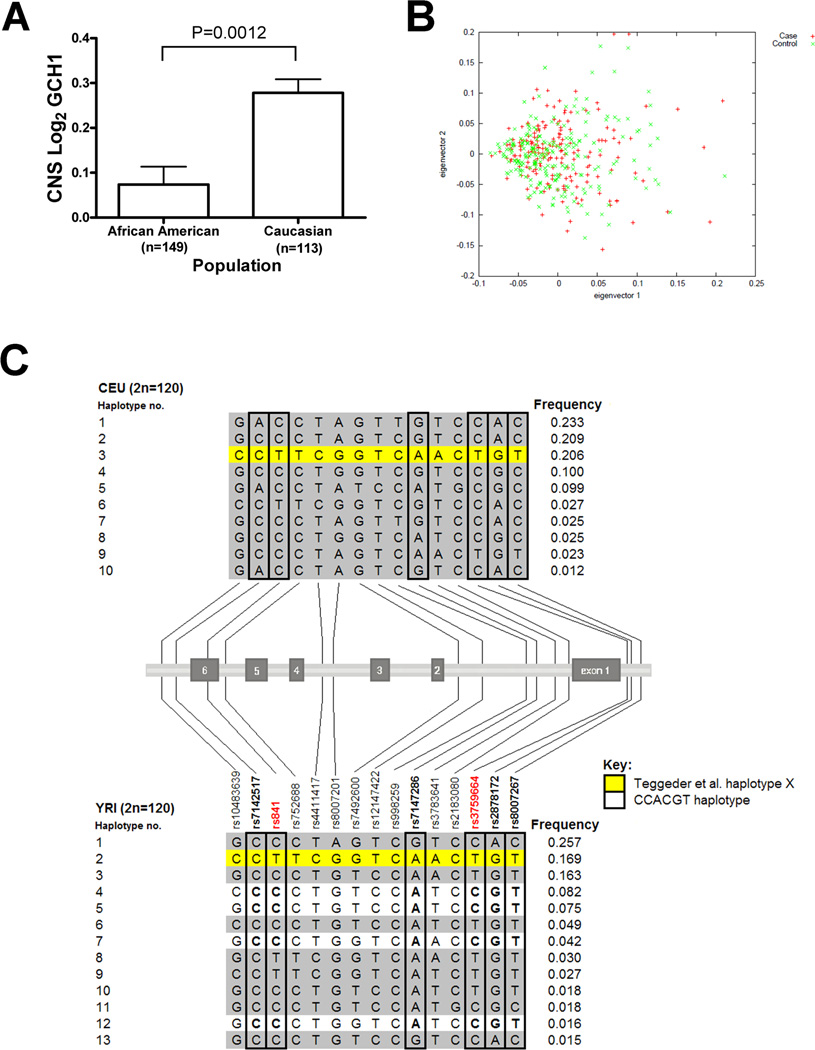
Results thus far suggested this rs8007267 allele was a marker for increased BH4 production and susceptibility to pain in SCA, in contrast to prior studies in Caucasians where the same allele was a marker for the opposite effects, specifically, reduced pain and lower BH4 [13]. We hypothesized that the GCH1 association in SCA might be attributed to population specific differences in gene expression, and therefore we compared GCH1 expression in postmortem brains between Caucasians and African Americans [30], where there was a threefold expression difference (P = 0.0012; Fig. 3A). To determine if this difference might be due to population admixture, we calculated median δ (0.151) and FST (0.115) values for 97 markers from Hap-Map CEU and YRI subjects across 96,304 base pairs of the GCH1 locus. Comparison to 67 common markers across the same base pair interval for BCL11A (median δ = 0.112 and FST = 0.037), a known SCA HbF modulator [7], demonstrated evidence of significantly more admixture in GCH1 than BCL11A (δ pairwise comparison P = 0.01 and FST pairwise comparison P < 0.0001, Supporting Information Figs. S2 and S3, respectively). BCL11A SNPs associated with HbF had minimal differences between populations (rs11886868 FST = 0.012; rs4671393 FST = 0.058; rs7557939 FST = 0.001). As African American populations have a variable degree of admixture, we reanalyzed the GCH1 association in the CSSCD by comparing eigenvectors from genome-wide AIMs (n = 411) among pain cases and controls (Fig. 3B). There were no significant differences between cases and controls (X2 = 1.105, P = 0.58), suggesting that the association was not confounded by undetected population admixture.
Figure 3.
Population effects on GCH1 expression, admixture and haplotype distribution. (A) A threefold difference in basal GCH1 expression is observed in post-mortem brains from African Americans and Caucasians (P = 0.0012) from a previously described dataset [30]. (B) Comparison of eigenvectors generated from 411 genome wide AIMs among cases and controls in the CSSCD replication cohort. There was no significant difference in global admixture between cases and controls (X2 = 1.11, P = 0.58). (C) GCH1 haplotypes in the CEU and YRI HapMap populations using 15 markers that overlap with those from the current association study (n = 6) and Teggeder et al. (n = 13) [13]. The pain protective haplotype X is present in both populations, while P haplotypes are only detected in the YRI. One or more of the P haplotypes are associated with susceptibility to pain crises in females with SCA.
GCH1 haplotypes in the CEU and YRI HapMap populations were determined for 15 markers that overlap with those from Teggeder et al. (n = 13) and this study (n = 6) (Fig. 3C) [13]. The previously described pain protective haplotype X [13,20] frequency was 20.6% (±0.4%) in the CEU, and it was also common in Yorubans (frequency 16.9% ± 1.1%). The GCH1 pain risk CCACGT haplotype defined by this study was present on four different YRI 15 marker “P” haplotypes with a cumulative haplotype frequency of 21.8% (Fig. 3C). P haplotypes were distinguished from haplotype X by the rs841 C allele, and P haplotypes were not detected in the CEU population.
Discussion
Diminished pain perception is associated with a loss of function GCH1 haplotype and reduced BH4 production in Europeans [13,14,18,19], although these observations have not been replicated in all pain phenotypes or in populations of African ancestry [17,31,32]. GCH1 alleles previously associated with protection from pain [13,19] are correlated with more frequent acute painful episodes in SCA. Biochemical study of BH4 production in vitro suggested this is associated with a genetic marker that defines a previously unidentified haplotype prevalent in populations of African ancestry. Exploratory in vivo physiologic studies further demonstrated associations with endothelial dependent vascular responses in females. These data highlight novel relationships among disease, sex, and population specific effects of GCH1 on pain and vascular function, and further support a role for GCH1 in pain modulation and BH4 dependent nociception [14,33]. GCH1 is also the first gene in SCA that does not directly modulate hematologic parameters and is directly associated with painful episodes. Taken together, we propose sickle vasoocclusion is influenced not only by erythrocytic factors like HbF and intracellular interactions, but also genetic variability in nonerythrocytic genes and possibly vascular reactivity.
We utilized hospital-based treatment for sickle cell painful episodes as a phenotype, although this definition does not completely represent the daily burden of acute or chronic pain in SCA [34]. This definition has been a useful indicator of overall disease morbidity and mortality and has served as a reasonable endpoint for clinical trials and genetic association studies [1–3,22,23,34]. This phenotype also appears to represent a heritable trait (pain crisis rate correlation coefficient 0.683) comparable to HbF (correlation coefficient 0.579) based upon correlation among sibling pairs from the CSSCD [5]. Pain crises as a patient reported outcome in this cohort correlate with hemoglobin and mortality as observed in the CSSCD [1–3,7] and the proportion with acute episodes requiring hospitalization is comparable to other reports [1,35]. Furthermore, GCH1 replicates in an independent cohort with documented, robust pain crisis data [1].
Sex differences in pain sensitivity are documented in both the general population and SCA [1,11,29], however, the GCH1 association in females was not evident by interaction test. On the other hand, the exploratory association with vascular phenotypes in females is biologically plausible, as sex differences in endothelial dependent vasodilation are observed in SCA [25] and normal subjects [36]. Moreover, while the GCH1 pain association does not show a significant interaction with sex, the vascular function phenotype shows sex-specific association, and future SCA pain studies will need to include sufficient numbers of both male and female subjects.
Population admixture potentially confounds studies of genetic modifiers, although few candidate gene studies have considered this important variable [37]. GCH1 has expression and allele frequency differences between populations (Fig. 3A,C), although our genetic and biochemical data suggest a robust association for GCH1. Rs8007267 T alleles mark common haplotypes in Africans that are associated with higher GCH1 expression and pain. Fine mapping and resequencing will help to determine if the relative proportions of different haplotypes are consistent with our HapMap analysis and the extent to which local admixture is present across GCH1.
Physiologic effects of GCH1 and BH4 on non-NO vasodilators in vascular homeostasis and vasoocclusion in SCA are poorly understood. Murine studies suggest NO bioavailability indirectly regulates other endothelial vasodilators and produces paradoxical vascular constriction due an altered ratio of NO to non-NO vasodilators [38,39]. Vascular responses are also attributed to non-NO vasodilators in SCA forearm flow studies [25,40] and Ach induced vasodilation in human skin [41]. SCA blood flow studies show impaired NO responses in men, while women have higher NO bioavailability and exaggerated Ach induced vasorelaxation [25,42]. This is not observed in normal volunteers, suggesting responses are heightened in SCD. Furthermore, associations are not observed for L-NMMA responses which reflect basal NO production (Fig. 2E,F). In Europeans, the rs8007267 GCH1 haplotype X demonstrates correlation between low BH4 and diminished Ach response [20]. In Africans, the same allele is associated with higher BH4 (Fig. 1B) and paradoxically, the least vessel dilation in women with sickle cell (Fig. 2A). NO generation producing vasodilation is expected with high BH4 based upon studies in Europeans [20], but high NO also reduces production of non-NO vasodilators (e.g., prostaglandins) in normal states [43] and in SCA [25,38–40]. Pain and vascular phenotypes in women with SCA may be due to intact vessel responses to NO donors or increased generation of reactive oxygen and nitrogen species [44]. We speculate that NO is required for modulation of BH4 dependent vascular tone, although GCH1 and NO may do this indirectly through other endothelial vasodilators or NO metabolites [33,41,43]. The net effect of the African GCH1 pain haplotype in SCA is paradoxical vasoconstriction, similar to that observed in mice [38,39] and humans with SCA (Fig. 2A). We hypothesize that GCH1 variants associated with diminished vessel diameter or flow velocity in SCA could also promote erythrocyte adhesion and vasoocclusion as another variable in the model of vasoocclusion [8,45]. Moreover, additional work is necessary to define the interaction between BH4 and vascular responses during vasoocclusion. Additional study is also needed to determine if GCH1 variants also have effects in common vascular diseases among populations of African ancestry.
Moreover, pilot studies originally suggested that inhaled NO decreased pain crisis duration, but these observations were not confirmed in a controlled clinical trial [46]. Sildenafil amplifies NO signaling through guanylate cyclase by inhibition of phosphodiesterase 5. In a multicenter trial of sickle cell cardiopulmonary disease defined by echocardiography, sildenafil treatment was associated with more frequent hospitalizations for painful episodes than placebo treated patients [47]. This result, combined with animal data and results from our study suggest NO could enhance pain signaling [33,43]. The GCH1 pain haplotype may link vessel tone, vaso-occlusive pain and the BH4/NO pathway mechanistically in females with SCA. Alternatively, BH4 is also a cofactor for catecholamine and serotonin synthesis where either of these pathways could also underlie the associations with pain and vascular response. Indeed, epinephrine has been shown to promote cell adhesion and vaso-occlusion in murine models [48]. Overall, this association, if validated, might aid in the identification of targets for personalized therapies to modulate sickle cell acute painful events.
Supplementary Material
TABLE IV.
GCH1 Haplotype Pain Crisis Association Restricted to Females with Sickle Cell Anemia
| Haplotypea | Overall frequency | Case frequency | Control frequency | OR (95% CI) | P value |
|---|---|---|---|---|---|
| CCACGT | 0.257 | 0.270 | 0.128 | 2.69 (1.21–5.94) | 0.01 |
| CCATGT | 0.193 | 0.186 | 0.156 | 1.27 (0.59–2.73) | 0.54 |
| CTATGT | 0.162 | 0.143 | 0.188 | 0.73 (0.34–1.54) | 0.41 |
| CCGCAC | 0.149 | 0.136 | 0.158 | 0.86 (0.39–1.88) | 0.70 |
Analysis restricted to 119 females including 77 cases and 42 controls from the discovery cohort.
Acknowledgments
The authors thank the study participants and the nursing staff from the NIH Clinical Center. This work is in memory of Dr. Mitchell B. Max (1949–2008).
Contract grant sponsor: Intramural Research Programs of NHLBI; Contract grant numbers: 1 ZIA HL006012; 1 ZIA HL006160.
Contract grant sponsor: National Heart, Lung and Blood Institute (NHLBI); Contract grant number: HL R01 87681, HL 068970, T32 HL007501..
Footnotes
Additional Supporting Information may be found in the online version of this article.
Conflict of interest: Nothing to report.
Group authorship information: Conceived of the study: IB and JGT. Recruited subjects: DA, GJK, and JGT. Statistical analysis: ZW, MD, JM, VN, and JGT. CSSCD genotyping and analysis: VN, JM, SWH, and MHS. Lab studies: IB, VY, DSD, LD, LF, KD, DA. Biopterin assays: CC. Supervision of lab studies: IB, KC, MHS, DG, and JGT. Wrote the paper: IB, VY, DD and JGT. All authors revised the paper for content.
References
- 1.Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. Rates and risk factors. N Engl J Med. 1991;325:11–16. doi: 10.1056/NEJM199107043250103. [DOI] [PubMed] [Google Scholar]
- 2.Darbari DS, Hildesheim M, Wang Z, et al. Severe painful vaso-occlusive crises and mortality in a contemporary adult sickle cell anemia cohort study. PLOS One. 2013;8:e79923. doi: 10.1371/journal.pone.0079923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 4.Alexander N, Higgs D, Dover G, Serjeant GR. Are there clinical phenotypes of homozygous sickle cell disease? Br J Haematol. 2004;126:606–611. doi: 10.1111/j.1365-2141.2004.05025.x. [DOI] [PubMed] [Google Scholar]
- 5.Lettre G. The search for genetic modifiers of disease severity in the beta-hemoglobinopathies. Cold Spring Harb Perspect Med. 2012;2:a015032. doi: 10.1101/cshperspect.a015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higgs DR, Aldridge BE, Lamb J, et al. The interaction of alpha-thalassemia and homozygous sickle-cell disease. N Engl J Med. 1982;306:1441–1446. doi: 10.1056/NEJM198206173062402. [DOI] [PubMed] [Google Scholar]
- 7.Lettre G, Sankaran VG, Bezerra MA, et al. DNA polymorphisms at the BCL11A, HBS1L-MYB, and beta-globin loci associate with fetal hemoglobin levels and pain crises in sickle cell disease. Proc Natl Acad Sci USA. 2008;105:11869–11874. doi: 10.1073/pnas.0804799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaul DK, Fabry ME. In vivo studies of sickle red blood cells. Microcirculation. 2004;11:153–165. [PubMed] [Google Scholar]
- 9.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lariviere WR, Wilson SG, Laughlin TM, et al. Heritability of nociception. III. Genetic relationships among commonly used assays of nociception and hypersensitivity. Pain. 2002;97:75–86. doi: 10.1016/s0304-3959(01)00492-4. [DOI] [PubMed] [Google Scholar]
- 11.Mogil JS, Sorge RE, LaCroix-Fralish ML, et al. Pain sensitivity and vasopressin analgesia are mediated by a gene-sex-environment interaction. Nat Neurosci. 2011;14:1569–1573. doi: 10.1038/nn.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neely GG, Hess A, Costigan M, et al. A genome-wide Drosophila screen for heat nociception identifies alpha2delta3 as an evolutionarily conserved pain gene. Cell. 2010;143:628–638. doi: 10.1016/j.cell.2010.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tegeder I, Costigan M, Griffin RS, et al. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med. 2006;12:1269–1277. doi: 10.1038/nm1490. [DOI] [PubMed] [Google Scholar]
- 14.Latremoliere A, Costigan M. GCH1, BH4 and pain. Curr Pharm Biotechnol. 2011;12:1728–1741. doi: 10.2174/138920111798357393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell CM, Edwards RR, Carmona C, et al. Polymorphisms in the GTP cyclohydrolase gene (GCH1) are associated with ratings of capsaicin pain. Pain. 2009;141:114–118. doi: 10.1016/j.pain.2008.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dabo F, Gronbladh A, Nyberg F, et al. Different SNP combinations in the GCH1 gene and use of labor analgesia. Mol Pain. 2010;6:41. doi: 10.1186/1744-8069-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim H, Dionne RA. Lack of influence of GTP cyclohydrolase gene (GCH1) variations on pain sensitivity in humans. Mol Pain. 2007;3:6. doi: 10.1186/1744-8069-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lotsch J, Klepstad P, Doehring A, Dale O. A GTP cyclohydrolase 1 genetic variant delays cancer pain. Pain. 2010;148:103–106. doi: 10.1016/j.pain.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 19.Tegeder I, Adolph J, Schmidt H, et al. Reduced hyperalgesia in homozygous carriers of a GTP cyclohydrolase 1 haplotype. Eur J Pain. 2008;12:1069–1077. doi: 10.1016/j.ejpain.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Antoniades C, Shirodaria C, Van Assche T, et al. GCH1 haplotype determines vascular and plasma biopterin availability in coronary artery disease effects on vascular superoxide production and endothelial function. J Am Coll Cardiol. 2008;52:158–165. doi: 10.1016/j.jacc.2007.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Rao F, Zhang K, et al. Discovery of common human genetic variants of GTP cyclohydrolase 1 (GCH1) governing nitric oxide, autonomic activity, and cardiovascular risk. J Clin Invest. 2007;117:2658–2671. doi: 10.1172/JCI31093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ataga KI, Reid M, Ballas SK, et al. Improvements in haemolysis and indicators of erythrocyte survival do not correlate with acute vasoocclusive crises in patients with sickle cell disease: A phase III randomized, placebo-controlled, double-blind study of the Gardos channel blocker senicapoc (ICA-17043) Br J Haematol. 2011;153:92–104. doi: 10.1111/j.1365-2141.2010.08520.x. [DOI] [PubMed] [Google Scholar]
- 23.Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the multicenter study of hydroxyurea in sickle cell anemia. N Engl J Med. 1995;332:1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 24.Taylor JG, Ackah D, Cobb C, et al. Mutations and polymorphisms in hemoglobin genes and the risk of pulmonary hypertension and death in sickle cell disease. Am J Hematol. 2008;83:6–14. doi: 10.1002/ajh.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gladwin MT, Schechter AN, Ognibene FP, et al. Divergent nitric oxide bioavailability in men and women with sickle cell disease. Circulation. 2003;107:271–278. doi: 10.1161/01.cir.0000044943.12533.a8. [DOI] [PubMed] [Google Scholar]
- 26.Solovieff N, Milton JN, Hartley SW, et al. Fetal hemoglobin in sickle cell anemia: Genome-wide association studies suggest a regulatory region in the 5’ olfactory receptor gene cluster. Blood. 2010;115:1815–1822. doi: 10.1182/blood-2009-08-239517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenberg NA, Li LM, Ward R, Pritchard JK. Informativeness of genetic markers for inference of ancestry. Am J Hum Genet. 2003;73:1402–1422. doi: 10.1086/380416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patterson N, Price AL, Reich D. Population structure and eigenanalysis. PLoS Genet. 2006;2:e190. doi: 10.1371/journal.pgen.0020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gureje O, Von Korff M, Simon GE, Gater R. Persistent pain and well-being: A World Health Organization study in primary care. JAMA. 1998;280:147–151. doi: 10.1001/jama.280.2.147. [DOI] [PubMed] [Google Scholar]
- 30.Colantuoni C, Lipska BK, Ye T, et al. Temporal dynamics and genetic control of transcription in the human prefrontal cortex. Nature. 2011;478:519–523. doi: 10.1038/nature10524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holliday KL, Nicholl BI, Macfarlane GJ, et al. Do genetic predictors of pain sensitivity associate with persistent widespread pain? Mol Pain. 2009;5:56. doi: 10.1186/1744-8069-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lazarev M, Lamb J, Barmada MM, et al. Does the pain-protective GTP cyclohydrolase haplotype significantly alter the pattern or severity of pain in humans with chronic pancreatitis? Mol Pain. 2008;4:58. doi: 10.1186/1744-8069-4-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miclescu A, Gordh T. Nitric oxide and pain: “Something old, something new”. Acta Anaesthesiol Scand. 2009;53:1107–1120. doi: 10.1111/j.1399-6576.2009.02054.x. [DOI] [PubMed] [Google Scholar]
- 34.Smith WR, Penberthy LT, Bovbjerg VE, et al. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008;148:94–101. doi: 10.7326/0003-4819-148-2-200801150-00004. [DOI] [PubMed] [Google Scholar]
- 35.Brousseau DC, Owens PL, Mosso AL, et al. Acute care utilization and rehospitalizations for sickle cell disease. JAMA. 2010;303:1288–1294. doi: 10.1001/jama.2010.378. [DOI] [PubMed] [Google Scholar]
- 36.Forte P, Kneale BJ, Milne E, et al. Evidence for a difference in nitric oxide biosynthesis between healthy women and men. Hypertension. 1998;32:730–734. doi: 10.1161/01.hyp.32.4.730. [DOI] [PubMed] [Google Scholar]
- 37.da Silva MC, Zuccherato LW, Lucena FC, et al. Extensive admixture in Brazilian sickle cell patients: Implications for the mapping of genetic modifiers. Blood. 2011;118:4493–4495. doi: 10.1182/blood-2011-06-361915. author reply 4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaul DK, Liu XD, Chang HY, et al. Effect of fetal hemoglobin on microvascular regulation in sickle transgenic-knockout mice. J Clin Invest. 2004;114:1136–1145. doi: 10.1172/JCI21633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaul DK, Zhang X, Dasgupta T, Fabry ME. Arginine therapy of transgenic-knockout sickle mice improves microvascular function by reducing non-nitric oxide vasodilators, hemolysis, and oxidative stress. Am J Physiol Heart Circ Physiol. 2008;295:H39–H47. doi: 10.1152/ajpheart.00162.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nath KA, Katusic ZS, Gladwin MT. The perfusion paradox and vascular instability in sickle cell disease. Microcirculation. 2004;11:179–193. doi: 10.1080/10739680490278592. [DOI] [PubMed] [Google Scholar]
- 41.Kellogg DL, Jr, Zhao JL, Coey U, Green JV. Acetylcholine-induced vasodilation is mediated by nitric oxide and prostaglandins in human skin. J Appl Physiol. 2005;98:629–632. doi: 10.1152/japplphysiol.00728.2004. [DOI] [PubMed] [Google Scholar]
- 42.Belhassen L, Pelle G, Sediame S, et al. Endothelial dysfunction in patients with sickle cell disease is related to selective impairment of shear stress-mediated vasodilation. Blood. 2001;97:1584–1589. doi: 10.1182/blood.v97.6.1584. [DOI] [PubMed] [Google Scholar]
- 43.Guhring H, Gorig M, Ates M, et al. Suppressed injury-induced rise in spinal prostaglandin E2 production and reduced early thermal hyperalgesia in iNOS-deficient mice. J Neurosci. 2000;20:6714–6720. doi: 10.1523/JNEUROSCI.20-17-06714.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muscoli C, Cuzzocrea S, Ndengele MM, et al. Therapeutic manipulation of peroxynitrite attenuates the development of opiate-induced antinociceptive tolerance in mice. J Clin Investig. 2007;117:3530–3539. doi: 10.1172/JCI32420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaul DK, Fabry ME, Nagel RL. Microvascular sites and characteristics of sickle cell adhesion to vascular endothelium in shear flow conditions: Pathophysiological implications. Proc Natl Acad Sci USA. 1989;86:3356–3360. doi: 10.1073/pnas.86.9.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gladwin MT, Kato GJ, Weiner D, et al. Nitric oxide for inhalation in the acute treatment of sickle cell pain crisis: A randomized controlled trial. JAMA. 2011;305:893–902. doi: 10.1001/jama.2011.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Machado RF, Barst RJ, Yovetich NA, et al. Hospitalization for pain in patients with sickle cell disease treated with sildenafil for elevated TRV and low exercise capacity. Blood. 2011;118:855–864. doi: 10.1182/blood-2010-09-306167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zennadi R, Moeller BJ, Whalen EJ, et al. Epinephrine-induced activation of LW-mediated sickle cell adhesion and vaso-occlusion in vivo. Blood. 2007;110:2708–2717. doi: 10.1182/blood-2006-11-056101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.