Abstract
Background
Mucins are implicated in survival in various cancers, but there have been no report addressed on survival in appendiceal carcinoma, an uncommon disease with different clinical and pathological features from those of other colon cancers. We aimed to investigate the clinical implications of expression of mucins in appendiceal carcinoma.
Methods
Expression profiles of MUC1, MUC2, MUC3, MUC4, MUC5AC, MUC6, MUC16 and MUC17 in cancer tissue were examined by immunohistochemistry in 108 cases of surgically resected appendiceal carcinoma.
Results
The following relationships of mucins with clinicopathologic factors were identified: MUC1 with positive lymphatic invasion (p = 0.036); MUC2 with histological type (mucinous carcinoma, p<0.001), superficial invasion depth (p = 0.007), negative venous invasion (p = 0.003), and curative resection (p = 0.019); MUC3 with non-curative resection (p = 0.017); MUC5AC with histological type (mucinous carcinoma, p = 0.002), negative lymphatic invasion (p = 0.021), and negative venous invasion (p = 0.022); and MUC16 with positive lymph node metastasis (p = 0.035), positive venous invasion (p<0.05), and non-curative resection (p = 0.035). A poor prognosis was related to positive lymph node metastasis (p = 0.04), positive lymphatic invasion (p = 0.02), positive venous invasion (p<0.001), non-curative resection (p<0.001), and positive expression of MUC3 (p = 0.004). In multivariate analysis, positive venous invasion (HR: 6.93, 95% CI: 1.93–24.96, p = 0.003), non-curative resection (HR: 10.19, 95% CI: 3.05–34.07, p<0.001) and positive MUC3 expression (HR: 3.37, 95% CI: 1.13–10.03, p = 0.03) were identified as significant independent prognostic factors in patients with appendiceal carcinoma.
Conclusions
Expression of MUC3 in appendiceal carcinoma is an independent factor for poor prognosis and a useful predictor of outcome in patients with appendiceal carcinoma after surgery.
Introduction
Appendiceal cancer is rare in the United States, with an age-adjusted incidence of 0.12 cases per 1,000,000 people per year [1], and a rate among intestinal cancers of 0.7%, compared to 1.5% for small bowel carcinoma and 97.8% for colon carcinoma in the Surveillance, Epidemiology and End Results (SEER) registry [2]. A similar rarity of appendiceal carcinoma is also found in Japan, with incidences of 0.2% in the Japanese Society for Cancer of the Colon and Rectum Registry and 0.08% in the Japanese Autopsy Annual Database of Colorectal Cancer [3]. The disease differs from cancers at other sites in the colon, with clinical presentation of acute abdominal symptoms suggestive of appendicitis [4], [5] and peritoneal mucinous carcinomatosis. The 5-year survival rate for appendiceal carcinoma after surgery is 46–64% [5]–[7]. Curative surgical resection is required for improving survival, and the pathological characteristics of the tumor affect prognosis. Among histological types, patients with non-mucinous carcinoma have poorer survival than those with mucinous carcinoma [5], [8], and those with signet ring cell carcinoma also have poor survival [1]. Cases with a high histological grade have poorer survival than low grade cases [6], [7]. Thus, prognostic factors in appendiceal carcinoma have included curative resection [6], primary tumor status [6], histological type [1], [5], [6], [8], and histological grade [6], [7], [9].
Mucins are high molecular weight glycoproteins having core protein backbones by O-glycosidic linkages with oligosaccharides [10]. Eighteen core proteins for human mucins (MUC1-MUC8, MUC12, MUC13, MUC15-17, MUC19-21) have been identified. The first cloned, MUC1, has been reported to be one of the most important human tumor antigens, namely, the second ranking next to WT1 [11]. Yonezawa et al. showed that MUC1 and/or MUC4 expression is related to a poorer prognosis for various human cancers, whereas MUC2 expression is related to a better prognosis [10], [12]. Aberrant expression of MUC3, MUC4, MUC5AC and MUC6 is found in pancreatic intraepithelial neoplasia [13], [14], and MUC16 and MUC17 are expressed in pancreatobiliary and small intestinal cancers [15]–[17] and have high prognostic value [15], [17]–[19]. Mucin expression also occurs in appendiceal carcinoma [20]–[26], however, there is no study for the relationship between mucin expression and survival of over 100 surgically-treated patients with appendiceal carcinoma.
The aim of this study was to investigate whether expression of mucins (MUC1, MUC2, MUC3, MUC4, MUC5AC, MUC6, MUC16 and MUC17) has prognostic significance in patients with appendiceal carcinoma using surgical specimens collected from multiple centers.
Materials and Methods
Patients and Tissue Specimens
Between 1991 and 2013, 108 resected specimens of appendiceal carcinoma were collected from 23 hospitals in Japan: Toyota Kosei Hospital, Kagoshima Medical Association Hospital, Imakiire General Hospital, Chutoen General Medical Center, Japanese Red Cross Nagoya Daini Hospital, Toyohashi Municipal Hospital, Handa City Hospital, Meijo Hospital, Anjo Kosei Hospital, Japanese Red Cross Nagoya Daiichi Hospital, Sakashita Hospital, Shizuoka Saiseikai General Hospital, Tokai Hospital, Kiryu Kosei General Hospital, Kamiiida Daiichi General Hospital, Yamashita Hospital, Tsushima City Hospital, Minami Seikyo Hospital, Toyota Memorial Hospital, Tosei General Hospital, Nagoya Tokushukai General Hospital, Saiseikai Matsusaka General Hospital, and Mie Prefectural General Medical Center.
This study was conducted in accordance with the guiding principles of the Declaration of Helsinki. Informed, written consent was obtained from 10 patients, and was approved by the Ethics Committees of Kagoshima-shi Medical Association Hospital (KMAH 2011-02-02), Japanese Red Cross Nagoya Daini Hospital (IRB20140128-7), Toyota Memorial Hospital (1211-4), and Saiseikai Matsusaka General Hospital (52-2013). For the other patients without informed consent, the Institutional Review Board of Toyota Kosei Hospital (22-ST04), the Ethics Committees of Imakiire General Hospital (119-2013), Toyohashi Municipal Hospital (43-2011), Japanese Red Cross Nagoya Daiichi Hospital (26-2013), Sakashita Hospital (1-2013), Shizuoka Saiseikai General Hospital (25-3-02), Tsushima City Hospital (2013-06), Toyota Memorial Hospital (1211-4), Tosei General Hospital (420-2013), Chutoen General Medical Center, Handa City Hospital, Kiryu Kosei General Hospital, Kamiiida Daiichi General Hospital, and Yamashita Hospital, and the hospital directors of Meijo Hospital, Anjo Kosei Hospital, Tokai Hospital, Minami Seikyo Hospital, Nagoya Tokushukai General Hospital, and Mie Prefectural General Medical Center (no specified number in these eleven hospitals) waived the need for written informed consent from the participants, and gave us their approval for use of the resected specimens, under the strict condition of privacy protection of the personal information of the patients.
Primary appendiceal carcinomas that were clinically and pathologically diagnosed by surgeons and pathologists were included in the study. Possible cecum cancers with invasion of the appendix, metastatic cancer to the appendix, or carcinoid of the appendix were excluded. Samples were collected from 55 males and 53 females with a mean age of 65 years (range 23–95). The surgical procedures are shown as Table 1. Mucinous peritonitis was found in laparotomy in 14 cases. Of the 108 patients, 34 died, and the causes of death were the primary disease in 30, another disease in 3, and an unknown cause in 1. All specimens were fixed in formalin, embedded in paraffin and cut into 4-μm -thick sections for immunohistochemistry (IHC), in addition to hematoxylin and eosin (HE) staining.
Table 1. Surgical Procedure.
| Procedure | No.patients |
| Primary resection only | 88 |
| Type of colectomy | |
| Appendectomy | 20 |
| Resection of the cecum | 3 |
| Ileocecal resection | 56 |
| Right colectomy | 3 |
| Right hemicolectomy | 6 |
| Combined resection | |
| Rectosigmoid colon | 1 |
| Uterus and adnexa | 1 |
| Liver | 1 |
| Elective resectiona | 20 |
| Type of colectomy | |
| Ileocecal resection | 15 |
| Right colectomy | 1 |
| Right hemicolectomy | 3 |
| Mucinous tumor resection | 1 |
| Combined resection | |
| Retroperitoneum, uterus, right adnexa and rectum | 1 |
| Lymph node dissection | |
| Performed | 81 |
| Not performed | 27 |
| Curability | |
| Curative resection | 64 |
| Non-curative resection | 41 |
| Unknown | 3 |
Elective resection after pathological diagnosis of appendiceal carcinoma using the resected specimen at the first surgery.
Immunohistochemistry
MUC1 was detected by a monoclonal antibody (MAb) DF3 (mouse IgG, Toray-Fuji Bionics, Tokyo, Japan), MUC2 by MAb Ccp58 (Novocastra Reagents, Leica Biosystems, Newcastle Upon Tyne, UK), MUC3 by MAb mMUC3-1 (generated by K. Rousseau and D. M. Swallow), MUC4 by MAb 8G7 (generated by S. K. Batra), MUC5AC by MAb CLH2 (Novocastra), MUC6 by MAb CLH5 (Novocastra), MUC16 by MAb OC125 (Acris Antibodies GmbH, Herford, Germany), and MUC17 by a polyclonal anti-human MUC17 (rabbit IgG, generated by S. K. Batra).
IHC was performed using the immunoperoxidase method. Antigen retrieval was performed using CC1 antigen retrieval buffer (pH8.5, EDTA, 100°C, 30 min, Ventana Medical Systems, Tucson, AZ, USA). Sections were incubated with a primary antibody (DF3 diluted 1∶50, 37°C, 32 min; Ccp58 diluted 1∶200, 37°C, 24 min; 8G7 diluted 1∶3000, 37 °C, 32 min; CLH2 diluted 1∶100, 37°C, 24 min; CLH5 diluted 1∶100, 37°C, 24 min; OC125 diluted 1: 100, 37°C, 24 min; anti-human MUC17 diluted 1: 100) in phosphate-buffered saline (PBS) pH 7.4 with 1% bovine serum albumin, and stained on a Benchmark XT automated slide stainer using a diaminobenzidine detection kit (ultraView DAB, Ventana Medical Systems). For MUC3 staining, sections were treated at 100°C for 10 min in 0.01 M citrate buffer at pH 6.0, and then reduced with 0.01 M dithiothreitol in 0.1 M Tris/HCl buffer (pH 8.0) for 30 min at room temperature and alkylated with 0.025 M iodoacetamide in 0.1 M Tris/HCl buffer (pH 8.0) for 30 min [13], [17], [27]. They were incubated with mMUC3-1 at 4°C for 16 h and stained by avidin-biotin complex method. Reaction products were not present when hybridoma culture medium, normal mouse serum, normal rabbit serum, or PBS was used instead of primary antibodies.
Evaluation of Staining
The results were evaluated based on the percentage of positively stained carcinoma cells. Staining of the following components was evaluated: membrane and cytoplasm for MUC1 and MUC16; supranuclear area for MUC2; membrane for MUC3, cytoplasm for MUC4, MUC5AC and MUC6; and supranuclear area, cytoplasm and membrane for MUC17. Carcinoma cells are considered to be stained positively when at least one of the components was positive. A tumor was considered positive if more than 5% of carcinoma cells were stained, based on our previous use of 5% as the cutoff for mucin expression [17], [28]–[33].
Statistical Analysis
Associations between mucin expression profiles and clinicopathological factors were examined by chi-square test. Postoperative survival was calculated using the Kaplan-Meier method. Differences in survival curves were compared by log-rank test. A Cox proportional hazard analysis was used to estimate hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) in multivariate analysis. P<0.05 was considered significant.
Results
MUC expression in carcinomas
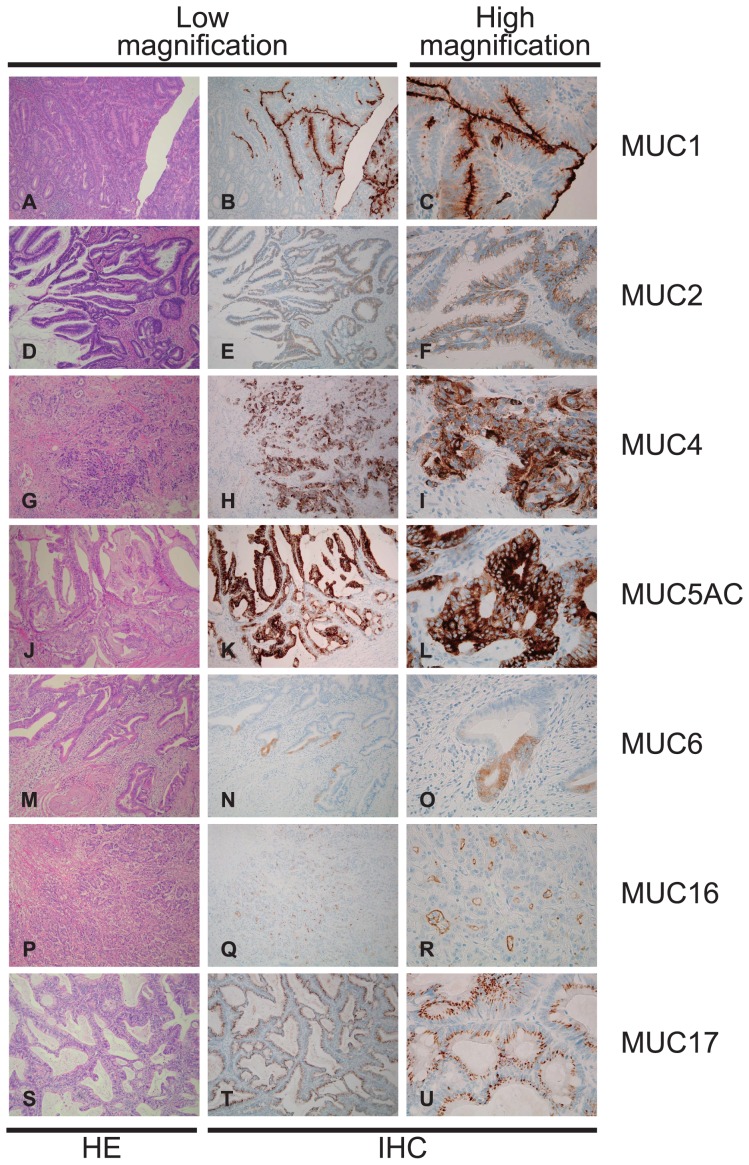
In the 108 cases, the positive expression rates (more than 5% of carcinoma cells stained) of each mucin antigen were MUC1, 47.2% (51/108); MUC2, 71.3% (77/108); MUC3, 18.5% (20/108); MUC4, 93.5% (101/108); MUC5AC, 50.0% (54/108); MUC6, 4.6% (5/108) MUC16, 16.7% (18/108) and MUC17, 86.1% (93/108). Representative mucin expression patterns in cancer tissues are shown in Fig. 1 (MUC3) and Fig. 2 (MUC1, MUC2, MUC4, MUC5AC, MUC6, MUC16 and MUC17). In appendiceal carcinoma cells, MUC3 showed membrane expression in the cell apexes (Fig. 1A–D); MUC1 showed membrane expression (Fig. 2A–C); MUC2 showed supranuclear expression (Fig. 2D–F); MUC4 (Fig. 2G–I), MUC5AC (Fig. 2J–L), and MUC6 (Fig. 2M–O) showed cytoplasmic expression; MUC16 showed membrane expression (Fig. 2P–R), and MUC17 showed supranuclear expression (Fig. 2S–U).
Figure 1. Histological features of appendiceal carcinoma.
(A, C) Hematoxylin and eosin stain. (B, D) Immunohistochemistry. MUC3 showed membrane expression in the cell apexes in appendiceal carcinoma.
Figure 2. In appendiceal carcinoma cells (A, D, G, J, M, P and S), MUC1 showed membrane expression (B and C); MUC2 showed supranuclear expression (E and F); MUC4 (H and I), MUC5AC (K and L) and MUC6 (N and O) showed cytoplasmic expression; MUC16 showed membrane expression (Q and R); and MUC17 (T and U) showed supranuclear expression. HE, hematoxylin and eosin stain; IHC, immunohistochemical stain.
Relationship of MUC Expression in Cancer Cells with Clinicopathological Features
Relationships between mucin expression and clinicopathological features are summarized in Table 2. MUC1 expression was related to lymphatic invasion (higher in positive lymphatic invasion, p = 0.036); MUC2 expression was related to histological type (higher for mucinous carcinoma, p<0.001), invasion depth (higher in the superficial area than the musclaris propria, p = 0.007), venous invasion (higher for negative venous invasion, p = 0.003), and curability (higher in curative resection, p = 0.019); MUC3 expression was related to curability (higher in non-curative resection, p = 0.017); MUC5AC expression was related to histological type (higher in mucinous carcinoma, p = 0.002), lymphatic invasion (higher for negative lymphatic invasion, p = 0.021), and venous invasion (higher in negative venous invasion, p = 0.022); and MUC16 expression was related to lymph node metastasis (higher in positive lymph node metastasis, p = 0.035), venous invasion (higher for positive venous invasion, p<0.05), and curability (higher in non-curative resection, p = 0.035).
Table 2. Summary of the Data on the Expression of MUC1, MUC2, MUC3, MUC4, MUC5AC, MUC6, MUC16 and MUC17 in Clinicopathological Features of Appendiceal Carcinoma (n = 108).
| MUC1 | MUC2 | MUC3 | MUC4 | ||||||||||
| Category | No. patients (%) | Negative | Positive | P Value | Negative | Positive | P Value | Negative | Positive | P Value | Negative | Positive | P Value |
| Age (yrs) | 0.483 | 0.132 | 0.517 | 0.41 | |||||||||
| ≥65 | 61 (56.5) | 34 (55.7) | 27 (44.3) | 14 (23) | 47 (77) | 51 (83.6) | 10 (16.4) | 5 (8.2) | 56 (91.8) | ||||
| <65 | 47 (43.5) | 23 (48.9) | 24 (51.1) | 17 (36.2) | 30 (63.8) | 37 (78.7) | 10 (21.3) | 2 (4.3) | 45 (95.7) | ||||
| Gender | 0.126 | 0.928 | 0.686 | 0.659 | |||||||||
| Men | 55 (50.9) | 33 (60) | 22 (40) | 16 (29.1) | 39 (70.9) | 44 (80) | 11 (20) | 3 (5.5) | 52 (94.5) | ||||
| Women | 53 (49.1) | 24 (45.3) | 29 (54.7) | 15 (28.3) | 38 (71.7) | 44 (83) | 9 (17) | 4 (7.5) | 49 (92.5) | ||||
| Histological typea | 0.597 | <0.001 | 0.052 | 0.111 | |||||||||
| pap, well, mod | 68 (63) | 34 (50) | 34 (50) | 17 (25) | 51 (75) | 54 (79.4) | 14 (20.6) | 7 (10.3) | 61 (89.7) | ||||
| por, sig | 19 (17.6) | 12 (63.2) | 7 (36.8) | 14 (73.7) | 5 (26.3) | 19 (100) | 0 (0) | 0 (0) | 19 (100) | ||||
| muc | 21 (19.4) | 11 (52.4) | 10 (47.6) | 0 (0) | 21 (100) | 15 (71.4) | 6 (28.6) | 0 (0) | 21 (100) | ||||
| Tumor depthb | 0.305 | 0.007 | 0.103 | 0.123 | |||||||||
| m, sm, mp | 26 (24.1) | 16 (61.5) | 10 (38.5) | 2 (7.7) | 24 (92.3) | 24 (92.3) | 2 (7.7) | 0 (0) | 26 (100) | ||||
| ss, se, si | 82 (75.9) | 41 (50) | 41 (50) | 29 (35.4) | 53 (64.6) | 64 (78) | 18 (22) | 7 (8.5) | 75 (91.5) | ||||
| Lymph node metastasisc | 0.304 | 0.056 | 0.756 | 0.946 | |||||||||
| Negative | 55 (67.9) | 30 (54.5) | 25 (45.5) | 12 (21.8) | 43 (78.2) | 45 (81.8) | 10 (18.2) | 4 (7.3) | 51 (92.7) | ||||
| Positive | 26 (32.1) | 11 (42.3) | 15 (57.7) | 11 (42.3) | 15 (57.7) | 22 (84.6) | 4 (15.4) | 2 (7.7) | 24 (92.3) | ||||
| Lymphatic invasion | 0.036 | 0.083 | 0.854 | 0.202 | |||||||||
| Negative | 56 (51.9) | 35 (62.5) | 21 (37.5) | 12 (21.4) | 44 (78.6) | 46 (82.1) | 10 (17.9) | 2 (3.6) | 54 (96.4) | ||||
| Positive | 52 (48.1) | 22 (42.3) | 30 (57.7) | 19 (36.5) | 33 (63.5) | 42 (80.8) | 10 (19.2) | 5 (9.6) | 47 (90.4) | ||||
| Venous invasion | 0.153 | 0.003 | 0.256 | 0.114 | |||||||||
| Negative | 75 (69.4) | 43 (57.3) | 32 (42.7) | 15 (20) | 60 (80) | 59 (78.7) | 16 (21.3) | 3 (4) | 72 (96) | ||||
| Positive | 33 (30.6) | 14 (42.4) | 19 (57.6) | 16 (48.5) | 17 (51.5) | 29 (87.9) | 4 (12.1) | 4 (12.1) | 29 (87.9) | ||||
| Curabilityd | 0.164 | 0.019 | 0.017 | 0.831 | |||||||||
| Curative resection | 64 (61) | 37 (57.8) | 27 (42.2) | 13 (20.3) | 51 (79.7) | 57 (89.1) | 7 (10.9) | 4 (6.2) | 60 (93.8) | ||||
| Non-curative resection | 41 (39) | 18 (43.9) | 23 (56.1) | 17 (41.5) | 24 (58.5) | 29 (70.7) | 12 (29.3) | 3 (7.3) | 38 (92.7) | ||||
pap, papillary adenocarcinoma; well, well differentiated adenocarcinoma; mod, moderately differentiated adenocarcinoma; por, poorly differentiated adenocarcinoma; sig, signet-ring cell carcinoma; muc, mucinous carcinoma.
m, mucosa; sm, submucosa; mp, muscularis propria; ss, subserosa, se, serosa; si, invasion to other organ.
27 cases without lymph node dissection were excluded.
3 cases with unknown details regarding curative or non-curative resection were excluded.
Relationship of Clinicopathological Factors and Mucin Expression with Survival
The 5-year overall survival rate and median survival period were 62.4% and 2.1 years, respectively. Log-rank tests showed that positive lymph node metastasis (p = 0.04), positive lymphatic invasion (p = 0.02), positive venous invasion (p<0.001), and non-curative resection (p<0.001) were significantly related to a worse prognosis (Table 3). Positive expression of MUC3 (p = 0.004) was also significantly related to a worse prognosis (Table 3, Fig. 3), but survival was not correlated with expression of MUC1, MUC2, MUC4, MUC5AC, MUC6, MUC16 and MUC17.
Table 3. Survival in Patients with Appendiceal Carcinoma by the Log-Rank Test (n = 108).
| No.patients | 5-year survival rate | P Value | |
| Category | (%) | (%) | |
| Age (yrs) | 0.054 | ||
| <65 | 47 (43.5) | 72.4 | |
| ≥65 | 61 (56.5) | 53.6 | |
| Gender | 0.296 | ||
| Men | 55 (50.9) | 57.4 | |
| Women | 53 (49.1) | 65.9 | |
| Histological typea | |||
| pap, well, mod | 68 (63) | 67.7 | 0.226 |
| por, sig | 19 (17.6) | 48.8 | |
| muc | 21 (19.4) | 60.3 | |
| Tumor depthb | 0.066 | ||
| m, sm, mp | 26 (24.1) | 84 | |
| ss, se, si | 82 (75.9) | 57.6 | |
| Lymph node metastasisc | 0.04 | ||
| Negative | 55 (67.9) | 76.1 | |
| Positive | 26 (32.1) | 47.7 | |
| Lymphatic invasion | 0.02 | ||
| Negative | 56 (51.9) | 77.6 | |
| Positive | 52 (48.1) | 47 | |
| Venous invasion | <0.001 | ||
| Negative | 75 (69.4) | 73.7 | |
| Positive | 33 (30.6) | 35.4 | |
| Curabilityd | <0.001 | ||
| Curative resection | 64 (61) | 83 | |
| Non-curative resection | 41 (39) | 28.5 | |
| MUC1 | 0.626 | ||
| Negative | 57 (52.8) | 63.1 | |
| Positive | 51 (47.2) | 62 | |
| MUC2 | 0.072 | ||
| Negative | 31 (28.7) | 51.3 | |
| Positive | 77 (71.3) | 66.5 | |
| MUC3 | 0.004 | ||
| Negative | 88 (81.5) | 69.1 | |
| Positive | 20 (18.5) | 38.8 | |
| MUC4 | 0.467 | ||
| Negative | 7 (6.5) | 47.6 | |
| Positive | 101 (93.5) | 63.4 | |
| MUC5AC | 0.433 | ||
| Negative | 54 (50) | 59.1 | |
| Positive | 54 (50) | 66.1 | |
| MUC6 | 0.698 | ||
| Negative | 103 (95.4) | 61.6 | |
| Positive | 5 (4.6) | 75 | |
| MUC16 | 0.061 | ||
| Negative | 90 (83.3) | 65.4 | |
| Positive | 18 (16.7) | 48.1 | |
| MUC17 | 0.5 | ||
| Negative | 15 (13.9) | 67.5 | |
| Positive | 93 (86.1) | 61.9 |
pap, papillary adenocarcinoma; well, well differentiated adenocarcinoma; mod, moderately differentiated adenocarcinoma; por, poorly differentiated adenocarcinoma; sig, signet-ring cell carcinoma; muc, mucinous carcinoma.
m, mucosa; sm, submucosa; mp, muscularis propria; ss, subserosa, se, serosa; si, invasion to other organ.
27 cases without lymph node dissection were excluded.
3 cases with unknown details regarding curative or non-curative resection were excluded.
Figure 3. Correlation between mucin expression and the cumulative survival rate.
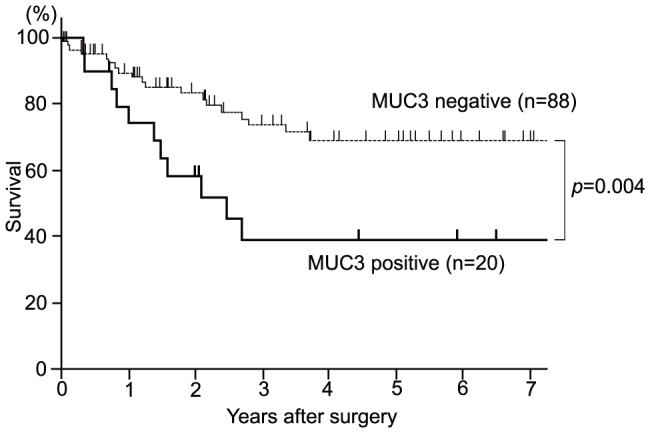
In the study of the correlation between mucin expression and the cumulative survival rate in patients with appendiceal carcinoma using the Kaplan-Meier method, the survival rate of patients with a positive expression of MUC3 were poorer than those of patients with negative expression of MUC3 (p = 0.004).
Multivariate Analysis of Prognostic Factors
The above results identified lymph node metastasis, lymphatic invasion, venous invasion, curative resection and MUC3 expression as candidates for prognostic factors. In multivariate analysis using a Cox proportional hazard model, positive venous invasion (HR: 6.93, 95% CI: 1.93–24.96, p = 0.003), non-curative resection (HR: 10.19, 95% CI: 3.05–34.07, p<0.001), and positive MUC3 expression (HR: 3.37, 95% CI: 1.13–10.03, p = 0.03) were identified as significant independent prognostic factors in patients with appendiceal carcinoma (Table 4).
Table 4. Multivariate Analysis of Prognostic Factors.
| Category | Hazard Ratio | 95% Confidence Interval | P Value |
| Lymph node metastasis | 0.511 | ||
| Negative | 1 | ||
| Positive | 1.41 | 0.51–3.91 | |
| Lymphatic invasion | 0.488 | ||
| Negative | 1 | ||
| Positive | 1.67 | 0.39–7.11 | |
| Venous invasion | 0.003 | ||
| Negative | 1 | ||
| Positive | 6.93 | 1.93–24.96 | |
| Curability | <0.001 | ||
| Curative resection | 1 | ||
| Non-curative resection | 10.19 | 3.05–34.07 | |
| MUC3 | 0.03 | ||
| Negative | 1 | ||
| Positive | 3.37 | 1.13–10.03 |
Discussion
In this study, the rates of positive expression were MUC1, 47.2%; MUC2, 71.3%; MUC3, 18.5%; MUC4, 93.5%; MUC5AC, 50.0%; MUC6, 4.6%; and MUC16, 16.7% in 108 cases of appendiceal carcinoma. In colorectal carcinoma, these rates are MUC1, 24–32% [10], [34]; MUC2, 38% [10]; MUC3, 74% [34]; MUC4, 94% [35]; MUC5AC, 34–50% [36], [37]; MUC6, 39% [37]; and MUC16, 64% [18]. The MUC17 expression rate in colon cancer is unknown, but is lower than that in normal epithelium [38]. In appendiceal carcinoma, MUC3, MUC6 and MUC16 had lower expression, MUC2 expression was markedly higher, and MUC1 expression was higher than the respective rates in colorectal carcinoma. These differences indicate the distinct characteristics of appendiceal carcinoma compared to other colorectal cancers. We also previously examined mucin expression in small intestinal carcinoma, and found positive expression rates of MUC1, 51.7%; MUC2, 26.7%; MUC3, 55.0%; MUC4, 51.7%; MUC5AC, 33.3%; MUC6, 10.0%; and MUC16, 8.3% (MUC17 expression was not examined) [17]. Appendiceal carcinoma was MUC1-positive in about half of the cases, similarly to small intestinal carcinoma, but other mucin profiles were different. Thus, with regard to mucin expression, appendiceal carcinoma may have a different carcinogenesis mechanism compared with other colorectal or small intestinal carcinomas.
Expression of MUC3 has been examined in malignancies of the pancreas, periampullary site, bile duct, kidney, salivary gland, lung, and breast, with examining tumor progression and prognosis [13], [39]–[44]. Duncan et al. [34] showed that MUC3 did not affect on survival in colorectal cancer. However, in appendiceal carcinoma, we firstly indicated that MUC3 had impact on survival.
MUC3 maps to a mucin cluster on chromosome 7q22 and is a membrane-bound mucin with tandem repeats of 17 amino acids (HSTPSFTS- SITTTETTS) [10]. IHC of MUC3 (mMUC3-1) in formalin-fixed paraffin-embedded specimens has been developed as a specific method for epitope retrieval [13], [17], [27]. We found that clear linear staining of the surface of villi in the normal mucosa of the small intestine is a good positive control for MUC3 staining [17], [27]. Other studies have used different antibodies, including 1143/B7 [34], [39], [44], [45] and M3P [40], and some have evaluated both membranous and cytoplasmic expression [34], [44], [45]. Using the 1143/B7 antibody, Aloysius et al [39] showed that MUC3 membranous expression is an independent prognostic factor in periampullary cancer. The use of different antibodies and evaluation of different expression patterns might give different results for MUC3, and the association of tumor behavior with results from each MUC3 antibody will be an interesting area for future study.
MUC3 is associated with a poor prognosis in appendiceal carcinoma, but the molecular mechanism of MUC3 in carcinogenesis is uncertain. Epigenetically, expression of MUC3A is contributed by promoter hypomethylation [46]. Cysteine-rich domains of MUC3 promote cell migration and inhibit apoptosis [47], and the MUC3 C-terminal domain undergoes autoproteolysis at its SEA module, which maintains its availability for potentiation of signaling modulated by HER/ErbB2 phosphorylation to promote migration and invasion [48]. Enhanced MUC3 expression by a tetrameric branched peptide with a conserved TFLK motif inhibits bacteria adherence [49], and expression of MUC3 is altered in inflammatory bowel disease and correlated with disease activity and the extent of inflammation [50]. Thus, MUC3 has several potential roles in malignant and inflammatory cells and these effects might be implicated in the poor prognosis of MUC3-positive patients with appendiceal carcinoma.
Expression of MUC1, MUC2, MUC4, MUC5AC, MUC6, MUC16 and MUC17 was not related to survival in appendiceal carcinoma. MUC1 expression is related to a poor prognosis of various human neoplasms and plays an important role in tumor invasion and metastasis [10], [12], but in our cases MUC1 expression was only related to positive lymphatic invasion. Mucinous carcinoma has high MUC2 expression compared to other adenocarcinomas in the pancreas, bile duct, ovary, breast [10], [12] and colorectum [51]. MUC2 expression is also related to a better prognosis of neoplasms in the stomach, pancreas and bile duct [10], [12]. The role of MUC2 in mucinous carcinoma suggests that production of this type of mucin may act as a barrier to cancerous extension, resulting in the indolent nature of many tumors [10]. In the current study, MUC2 expression was associated with mucinous carcinoma, consistent with a previous report [51], and with superficial invasion depth, negative venous invasion, and curative resection, but not with a better prognosis in appendiceal carcinoma.
Shanmugam et al. [35] found that MUC4 expression (≥ 75%) is a poor prognostic factor in colorectal cancer. However, high MUC4 expression (≥ 75%) in appendiceal carcinoma was not significantly related to survival (data not shown). A cut-off value of more than 5% for MUC4 expression detected with antibody 8G7 is significantly related to survival in many tumors [30]–[32], [52]. Kocer et al. [36] found that MUC5AC expression is associated with a better prognosis in colorectal carcinoma, and we also found that MUC5AC expression was related to favorable clinicopathological factors such as negative lymphatic invasion and negative venous invasion. MUC6 expression is a useful marker of pancreatobiliary neoplasms [53]–[55], but has no relationship with clinicopathological factors or survival. MUC16 expression is a poor prognostic factor in cholangiocarcinoma and small intestinal cancer [15], [17], and was related to positive lymph node metastasis, positive venous invasion and non-curative resection in appendiceal carcinoma in the current study.
MUC17 expression is related to tumor progression in pancreatic cancer [19], but was not related to clinicopathological factors or survival in appendiceal carcinoma. MUC17 and MUC3 are similarly expressed on the apical surface of intestinal epithelia, are both present in glycocalyx, and are both located on chromosome 7q22 [56], [57]. MUC17 and MUC3A both have promoter methylation sites, but those are different (−179 to +52 in MUC17 and −345 to −75 in MUC3A) [58]. Regarding histone modification, histone H3-K9 is more highly acetylated in MUC17-positive cells, whereas H3-K9 does not play a critical role in MUC3A regulation [58]. In the molecular structures, MUC17 and MUC3 both have an N-terminal large mucin domain, a SEA domain, a transmembrane domain, a cytoplasmic tail, and PDZ-binding motifs [59], [60], but their molecular function is different. In enterocytes, in response to carbachol, MUC17 is relocated from the apical membrane to an intracellular vesicular pool distinct from classical endosomes; this behavior is specific for MUC17, and does not occur for MUC3 [60], [61]. The current study showed a different IHC staining pattern, with MUC17 in the supranuclear area and MUC3 in the membrane, and different clinical significance. The differences in biological behavior between MUC17 and MUC3 may be due to differences in promoters and regulators, or in the structure and domains. Further studies are needed to determine the differences in the roles of these mucins in carcinogenesis.
We emphasize that this study is base on a large collection (n = 108) of a very rare appendiceal carcinoma. Furthermore, we prove that MUC3 only affected the survival, while other mucins with prognostic potentials in many malignancies have little importance. These new data would have a significant clinical impact. The patients with positive MUC3 expression of appendiceal carcinoma, should be followed-up carefully after surgery.
In conclusion, we found that expression of MUC3 in appendiceal carcinoma is an independent poor prognostic factor. MUC3 is a useful predictor of outcome in patients after surgery, and the key mucin for tumor progression in this rare tumor.
Acknowledgments
The authors thank Dr. Dallas M. Swallow and Dr. Suzanne Crawley (Galton Laboratory, University College London, London, UK) for providing anti-MUC3 antibody and for valuable discussions. We also thank Mr. Y. Atsuchi, Ms. C. Baba, Mr. S. Matuo, Ms. Y. Nishimura and Ms. S. Yoshimura for their technical assistance, and Ms. Y. Tokura for her assistance with ethics in the institutional review board.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This study was supported in part by Princess Takamatsu Cancer Research Fund (11-24319) to S. Yonezawa; by JSPS KAKENHI Grants-in-Aid for Scientific Research (B) 26290048 to S. Yonezawa and Scientific Research (C) 24590447 to M. Higashi; by the Kodama Memorial Foundation, Japan to S. Yokoyama; and by USPHS grant CA163120 from the National Institutes of Health to S. K. Batra. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. McCusker ME, Cote TR, Clegg LX, Sobin LH (2002) Primary malignant neoplasms of the appendix: a population-based study from the surveillance, epidemiology and end-results program, 1973–1998. Cancer 94:3307–3312. [DOI] [PubMed] [Google Scholar]
- 2. Gustafsson BI, Siddique L, Chan A, Dong M, Drozdov I, et al. (2008) Uncommon cancers of the small intestine, appendix and colon: an analysis of SEER 1973–2004, and current diagnosis and therapy. International journal of oncology 33:1121–1131. [PubMed] [Google Scholar]
- 3. Ozawa H (2012) Statistics of appendiceal malignant tumors: data from the JSCCR Registry and the Japan Autopsy Annual Database. Daichougan Frontier (in Japanese) 5:150–153. [Google Scholar]
- 4. Benedix F, Reimer A, Gastinger I, Mroczkowski P, Lippert H, et al. (2010) Primary appendiceal carcinoma-epidemiology, surgery and survival: results of a German multi-center study. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology 36:763–771. [DOI] [PubMed] [Google Scholar]
- 5. Nitecki SS, Wolff BG, Schlinkert R, Sarr MG (1994) The natural history of surgically treated primary adenocarcinoma of the appendix. Annals of surgery 219:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ito H, Osteen RT, Bleday R, Zinner MJ, Ashley SW, et al. (2004) Appendiceal adenocarcinoma: long-term outcomes after surgical therapy. Diseases of the colon and rectum 47:474–480. [DOI] [PubMed] [Google Scholar]
- 7. Ko YH, Park SH, Jung CK, Won HS, Hong SH, et al. (2010) Clinical characteristics and prognostic factors for primary appendiceal carcinoma. Asia-Pacific journal of clinical oncology 6:19–27. [DOI] [PubMed] [Google Scholar]
- 8. Kabbani W, Houlihan PS, Luthra R, Hamilton SR, Rashid A (2002) Mucinous and nonmucinous appendiceal adenocarcinomas: different clinicopathological features but similar genetic alterations. Mod Pathol 15:599–605. [DOI] [PubMed] [Google Scholar]
- 9. Overman MJ, Fournier K, Hu CY, Eng C, Taggart M, et al. (2013) Improving the AJCC/TNM staging for adenocarcinomas of the appendix: the prognostic impact of histological grade. Annals of surgery 257:1072–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yonezawa S, Higashi M, Yamada N, Yokoyama S, Kitamoto S, et al. (2011) Mucins in human neoplasms: clinical pathology, gene expression and diagnostic application. Pathol Int 61:697–716. [DOI] [PubMed] [Google Scholar]
- 11. Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, et al. (2009) The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res 15:5323–5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yonezawa S, Goto M, Yamada N, Higashi M, Nomoto M (2008) Expression profiles of MUC1, MUC2, and MUC4 mucins in human neoplasms and their relationship with biological behavior. Proteomics 8:3329–3341. [DOI] [PubMed] [Google Scholar]
- 13. Park HU, Kim JW, Kim GE, Bae HI, Crawley SC, et al. (2003) Aberrant expression of MUC3 and MUC4 membrane-associated mucins and sialyl Le(x) antigen in pancreatic intraepithelial neoplasia. Pancreas 26:e48–54. [DOI] [PubMed] [Google Scholar]
- 14. Kim GE, Bae HI, Park HU, Kuan SF, Crawley SC, et al. (2002) Aberrant expression of MUC5AC and MUC6 gastric mucins and sialyl Tn antigen in intraepithelial neoplasms of the pancreas. Gastroenterology 123:1052–1060. [DOI] [PubMed] [Google Scholar]
- 15. Higashi M, Yamada N, Yokoyama S, Kitamoto S, Tabata K, et al. (2012) Pathobiological implications of MUC16/CA125 expression in intrahepatic cholangiocarcinoma-mass forming type. Pathobiology 79:101–106. [DOI] [PubMed] [Google Scholar]
- 16. Kitamoto S, Yokoyama S, Higashi M, Yamada N, Matsubara S, et al. (2012) Expression of MUC17 is regulated by HIF1alpha-mediated hypoxic responses and requires a methylation-free hypoxia responsible element in pancreatic cancer. PLoS One 7:e44108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shibahara H, Higashi M, Koriyama C, Yokoyama S, Kitazono I, et al. (2014) Pathobiological implications of mucin (MUC) expression in the outcome of small bowel cancer. PLoS One 9:e86111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Streppel MM, Vincent A, Mukherjee R, Campbell NR, Chen SH, et al. (2012) Mucin 16 (cancer antigen 125) expression in human tissues and cell lines and correlation with clinical outcome in adenocarcinomas of the pancreas, esophagus, stomach, and colon. Human pathology 43:1755–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hirono S, Yamaue H, Hoshikawa Y, Ina S, Tani M, et al. (2010) Molecular markers associated with lymph node metastasis in pancreatic ductal adenocarcinoma by genome-wide expression profiling. Cancer science 101:259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O'Connell JT, Hacker CM, Barsky SH (2002) MUC2 is a molecular marker for pseudomyxoma peritonei. Mod Pathol 15:958–972. [DOI] [PubMed] [Google Scholar]
- 21. Yajima N, Wada R, Yamagishi S, Mizukami H, Itabashi C, et al. (2005) Immunohistochemical expressions of cytokeratins, mucin core proteins, p53, and neuroendocrine cell markers in epithelial neoplasm of appendix. Human pathology 36:1217–1225. [DOI] [PubMed] [Google Scholar]
- 22. Mall AS, Chirwa N, Govender D, Lotz Z, Tyler M, et al. (2007) MUC2, MUC5AC and MUC5B in the mucus of a patient with pseudomyxoma peritonei: biochemical and immunohistochemical study. Pathol Int 57:537–547. [DOI] [PubMed] [Google Scholar]
- 23. Yoon SO, Kim BH, Lee HS, Kang GH, Kim WH, et al. (2009) Differential protein immunoexpression profiles in appendiceal mucinous neoplasms: a special reference to classification and predictive factors. Mod Pathol 22:1102–1112. [DOI] [PubMed] [Google Scholar]
- 24. Suzuki J, Kazama S, Kitayama J, Uozaki H, Miyata T, et al. (2009) Signet ring cell carcinoma of the appendix manifesting as colonic obstruction and ovarian tumors: report of a case. Surgery today 39:235–240. [DOI] [PubMed] [Google Scholar]
- 25. Chu PG, Chung L, Weiss LM, Lau SK (2011) Determining the site of origin of mucinous adenocarcinoma: an immunohistochemical study of 175 cases. Am J Surg Pathol 35:1830–1836. [DOI] [PubMed] [Google Scholar]
- 26. Chang MS, Byeon SJ, Yoon SO, Kim BH, Lee HS, et al. (2012) Leptin, MUC2 and mTOR in appendiceal mucinous neoplasms. Pathobiology 79:45–53. [DOI] [PubMed] [Google Scholar]
- 27. Higashi M, Goto M, Saitou M, Shimizu T, Rousseau K, et al. (2010) Immunohistochemical study of mucin expression in periampullary adenomyoma. J Hepatobiliary Pancreat Sci 17:275–283. [DOI] [PubMed] [Google Scholar]
- 28. Higashi M, Yonezawa S, Ho JJ, Tanaka S, Irimura T, et al. (1999) Expression of MUC1 and MUC2 mucin antigens in intrahepatic bile duct tumors: its relationship with a new morphological classification of cholangiocarcinoma. Hepatology 30:1347–1355. [DOI] [PubMed] [Google Scholar]
- 29. Tamada S, Goto M, Nomoto M, Nagata K, Shimizu T, et al. (2002) Expression of MUC1 and MUC2 mucins in extrahepatic bile duct carcinomas: its relationship with tumor progression and prognosis. Pathol Int 52:713–723. [DOI] [PubMed] [Google Scholar]
- 30. Shibahara H, Tamada S, Higashi M, Goto M, Batra SK, et al. (2004) MUC4 is a novel prognostic factor of intrahepatic cholangiocarcinoma-mass forming type. Hepatology 39:220–229. [DOI] [PubMed] [Google Scholar]
- 31. Saitou M, Goto M, Horinouchi M, Tamada S, Nagata K, et al. (2005) MUC4 expression is a novel prognostic factor in patients with invasive ductal carcinoma of the pancreas. J Clin Pathol 58:845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tamada S, Shibahara H, Higashi M, Goto M, Batra SK, et al. (2006) MUC4 is a novel prognostic factor of extrahepatic bile duct carcinoma. Clin Cancer Res 12:4257–4264. [DOI] [PubMed] [Google Scholar]
- 33.Hamada T, Nomura M, Kamikawa Y, Yamada N, Batra SK, et al. (2012) DF3 epitope expression on MUC1 mucin is associated with tumor aggressiveness, subsequent lymph node metastasis, and poor prognosis in patients with oral squamous cell carcinoma. Cancer. [DOI] [PubMed]
- 34. Duncan TJ, Watson NF, Al-Attar AH, Scholefield JH, Durrant LG (2007) The role of MUC1 and MUC3 in the biology and prognosis of colorectal cancer. World journal of surgical oncology 5:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shanmugam C, Jhala NC, Katkoori VR, Wan W, Meleth S, et al. (2010) Prognostic value of mucin 4 expression in colorectal adenocarcinomas. Cancer 116:3577–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kocer B, Soran A, Erdogan S, Karabeyoglu M, Yildirim O, et al. (2002) Expression of MUC5AC in colorectal carcinoma and relationship with prognosis. Pathol Int 52:470–477. [DOI] [PubMed] [Google Scholar]
- 37. Walsh MD, Clendenning M, Williamson E, Pearson SA, Walters RJ, et al. (2013) Expression of MUC2, MUC5AC, MUC5B, and MUC6 mucins in colorectal cancers and their association with the CpG island methylator phenotype. Mod Pathol 26:1642–1656. [DOI] [PubMed] [Google Scholar]
- 38. Senapati S, Ho SB, Sharma P, Das S, Chakraborty S, et al. (2010) Expression of intestinal MUC17 membrane-bound mucin in inflammatory and neoplastic diseases of the colon. J Clin Pathol 63:702–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Aloysius MM, Zaitoun AM, Awad S, Ilyas M, Rowlands BJ, et al. (2010) Mucins and CD56 as markers of tumour invasion and prognosis in periampullary cancer. Br J Surg 97:1269–1278. [DOI] [PubMed] [Google Scholar]
- 40. Mall AS, Tyler MG, Ho SB, Krige JE, Kahn D, et al. (2010) The expression of MUC mucin in cholangiocarcinoma. Pathology, research and practice 206:805–809. [DOI] [PubMed] [Google Scholar]
- 41. Leroy X, Gouyer V, Ballereau C, Zerimech F, Huet G, et al. (2003) Quantitative RT-PCR assay for MUC3 and VEGF mRNA in renal clear cell carcinoma: relationship with nuclear grade and prognosis. Urology 62:771–775. [DOI] [PubMed] [Google Scholar]
- 42. Lee JH, Lee JH, Kim A, Kim I, Chae YS (2005) Unique expression of MUC3, MUC5AC and cytokeratins in salivary gland carcinomas. Pathol Int 55:386–390. [DOI] [PubMed] [Google Scholar]
- 43. Nguyen PL, Niehans GA, Cherwitz DL, Kim YS, Ho SB (1996) Membrane-bound (MUC1) and secretory (MUC2, MUC3, and MUC4) mucin gene expression in human lung cancer. Tumour Biol 17:176–192. [DOI] [PubMed] [Google Scholar]
- 44. Rakha EA, Boyce RW, Abd El-Rehim D, Kurien T, Green AR, et al. (2005) Expression of mucins (MUC1, MUC2, MUC3, MUC4, MUC5AC and MUC6) and their prognostic significance in human breast cancer. Mod Pathol 18:1295–1304. [DOI] [PubMed] [Google Scholar]
- 45. Furuya C, Kawano H, Yamanouchi T, Oga A, Ueda J, et al. (2012) Combined evaluation of CK5/6, ER, p63, and MUC3 for distinguishing breast intraductal papilloma from ductal carcinoma in situ. Pathol Int 62:381–390. [DOI] [PubMed] [Google Scholar]
- 46. Kitamoto S, Yamada N, Yokoyama S, Houjou I, Higashi M, et al. (2010) Promoter hypomethylation contributes to the expression of MUC3A in cancer cells. Biochemical and biophysical research communications 397:333–339. [DOI] [PubMed] [Google Scholar]
- 47. Ho SB, Dvorak LA, Moor RE, Jacobson AC, Frey MR, et al. (2006) Cysteine-rich domains of muc3 intestinal mucin promote cell migration, inhibit apoptosis, and accelerate wound healing. Gastroenterology 131:1501–1517. [DOI] [PubMed] [Google Scholar]
- 48. Peng Z, He Y, Yang Y, Zhu R, Bai J, et al. (2010) Autoproteolysis of the SEA module of rMuc3 C-terminal domain modulates its functional composition. Archives of biochemistry and biophysics 503:238–247. [DOI] [PubMed] [Google Scholar]
- 49. Pan Q, Tian Y, Li X, Ye J, Liu Y, et al. (2013) Enhanced membrane-tethered mucin 3 (MUC3) expression by a tetrameric branched peptide with a conserved TFLK motif inhibits bacteria adherence. J Biol Chem 288:5407–5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dorofeyev AE, Vasilenko IV, Rassokhina OA, Kondratiuk RB (2013) Mucosal barrier in ulcerative colitis and Crohn's disease. Gastroenterology research and practice 2013:431231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li L, Huang PL, Yu XJ, Bu XD (2012) Clinicopathological Significance of Mucin 2 Immuno-histochemical Expression in Colorectal Cancer: A Meta-Analysis. Chinese journal of cancer research = Chung-kuo yen cheng yen chiu 24:190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hamada T, Wakamatsu T, Miyahara M, Nagata S, Nomura M, et al. (2012) MUC4: a novel prognostic factor of oral squamous cell carcinoma. Int J Cancer 130:1768–1776. [DOI] [PubMed] [Google Scholar]
- 53. Shibahara H, Tamada S, Goto M, Oda K, Nagino M, et al. (2004) Pathologic features of mucin-producing bile duct tumors: two histopathologic categories as counterparts of pancreatic intraductal papillary-mucinous neoplasms. Am J Surg Pathol 28:327–338. [DOI] [PubMed] [Google Scholar]
- 54. Goto M, Shibahara H, Tamada S, Hamada T, Oda K, et al. (2005) Aberrant expression of pyloric gland-type mucin in mucin-producing bile duct carcinomas: a clear difference between the core peptide and the carbohydrate moiety. Pathol Int 55:464–470. [DOI] [PubMed] [Google Scholar]
- 55. Basturk O, Khayyata S, Klimstra DS, Hruban RH, Zamboni G, et al. (2010) Preferential expression of MUC6 in oncocytic and pancreatobiliary types of intraductal papillary neoplasms highlights a pyloropancreatic pathway, distinct from the intestinal pathway, in pancreatic carcinogenesis. Am J Surg Pathol 34:364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kim YS, Ho SB (2010) Intestinal goblet cells and mucins in health and disease: recent insights and progress. Current gastroenterology reports 12:319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ho SB, Luu Y, Shekels LL, Batra SK, Kandarian B, et al. (2010) Activity of recombinant cysteine-rich domain proteins derived from the membrane-bound MUC17/Muc3 family mucins. Biochimica et biophysica acta 1800:629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yamada N, Kitamoto S, Yokoyama S, Hamada T, Goto M, et al. (2011) Epigenetic regulation of mucin genes in human cancers. Clinical epigenetics 2:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pelaseyed T, Hansson GC (2011) CFTR anion channel modulates expression of human transmembrane mucin MUC3 through the PDZ protein GOPC. Journal of cell science 124:3074–3083. [DOI] [PubMed] [Google Scholar]
- 60. Pelaseyed T, Bergstrom JH, Gustafsson JK, Ermund A, Birchenough GM, et al. (2014) The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunological reviews 260:8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pelaseyed T, Gustafsson JK, Gustafsson IJ, Ermund A, Hansson GC (2013) Carbachol-induced MUC17 endocytosis is concomitant with NHE3 internalization and CFTR membrane recruitment in enterocytes. American journal of physiology Cell physiology 305:C457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.