Supplemental digital content is available in the text.
Abstract
Background
We conducted a randomized and unblinded 2×2 sequential-factorial trial, composed of an induction arm (part 1) comparing single-dose (SD) versus divided-dose rabbit antithymocyte globulin (rATG), and a maintenance arm (part 2) comparing tacrolimus minimization versus withdrawal. We report the long-term safety and efficacy of SD-rATG induction in the context of early steroid withdrawal and tacrolimus minimization or withdrawal.
Methods
Patients (n=180) received 6 mg/kg rATG, SD or four alternate-day doses (1.5 mg/kg/dose), with early steroid withdrawal and tacrolimus or sirolimus maintenance. After 6 months targeted maintenance levels were tacrolimus, 2 to 4 ng/mL and sirolimus, 4 to 6 ng/mL or, if calcineurin inhibitor–withdrawn, sirolimus 8 to 12 ng/mL with mycophenolate mofetil 2 g two times per day. Primary endpoints were renal function (abbreviated modification of diet in renal disease) and chronic graft histopathology (Banff). Secondary endpoints included patient survival, graft survival, biopsy-proven rejection, and infectious or noninfectious complications.
Results
Follow-up averaged longer than 4 years. Tacrolimus or sirolimus and mycophenolate mofetil exposure was identical between groups. The SD-rATG associated with improved renal function (2-36 months; P<0.001) in deceased donor recipients. The SD-rATG associated with quicker lymphocyte, CD4 T cell, and CD4-CD8 recovery and fewer infections. Cox multivariate hazard modeling showed divided-dose–rATG (P=0.019), deceased donor (P=0.003), serious infection (P=0.0.018), and lower lymphocyte count (P=0.001) associated with increased mortality. Patients with all four covariates showed a 27-fold increased likelihood of death (P=0.00002). Chronic graft histopathology, rejection rates, and death-censored graft survival were not significantly different between groups.
Conclusion
The SD-rATG induction improves the 3-year renal function in recipients of deceased donor kidneys. This benefit, along with possibly improved patient survival and fewer infections suggest that how rATG is administered may impact its efficacy and safety.
Modern immunosuppressants reduce rejection with minimally improved graft survival.1 Reduced calcineurin inhibitor (CNI) exposure associates with improved early renal graft function, but after prolonged CNI exposure, even complete withdrawal may not improve renal function because of irreversible renal injury,2 and diminished CNI use may risk late or subclinical rejection, antibody-mediated injury,3-8 or graft loss.9-12 In addition, steroid-mediated metabolic derangements contribute to morbidity among patients with functioning grafts. In a large meta-analysis, cardiovascular risk-factor reduction was associated with steroid withdrawal, with a small increased risk of acute rejection that did not impact patient and graft survival.13-15
Both CNI and steroid minimization or avoidance may be facilitated by maintenance immunosuppression that includes a mammalian target of rapamycin inhibitor (mTORi; e.g., sirolimus, everolimus).16 However, these drugs present their own clinical challenges.17-20 Although early replacement of CNI with mTORi may improve renal function, patients with glomerular filtration rate (GFR) less than 40 mL per min respond with proteinuria and deteriorating function.21 However, mTORi have advantages as well (e.g., antineoplastic properties), and the potent mTORi-CNI synergy can reduce the risk of rejection with minimized CNI and steroid exposure.16, 22, 23 Adverse mTORi wound healing effects can be minimized by delayed introduction, an approach that minimizes sirolimus dose-dependent side effects (e.g., mouth ulcers, hyperlipidemia, proteinuria).18
Since 1999, our goal has been to prevent kidney rejection while avoiding immunosuppressant side effects through early steroid withdrawal (ESW) and reduced CNI and mTORi maintenance. Our long-term blood-level targets were: tacrolimus, 6-8 ng/mL and sirolimus, 8-12 ng/mL. Reducing the risk of early acute rejection with this ESW protocol depended on profound lymphocyte depletion with rabbit antithymocyte globulin (rATG), four 1.5 mg/kg doses on alternate days.24 Although patient and graft survivals were not different, renal function did not improve compared to 8 to 12 ng/mL of tacrolimus and 1 g two times per day of mycophenolate mofetil (MMF), with or without prednisone.14,16,25-27 We speculated that further CNI reduction, or even withdrawal, was needed to achieve renal function improvement.
The rATG is usually administered as a series of small doses spaced at 1-day or 2-day intervals. However, more intensive administration (fewer, larger doses) may confer more comprehensive lymphocyte depletion, both peripherally and in secondary lymphoid structures.28 Improved early renal function with deceased donor kidneys was reported when rATG administration was initiated before reperfusion,29 and a nonrandomized experience with single-dose (SD) rATG induction seemed to enable CNI minimization and even complete withdrawal.30-33 Impressed by its possible benefits, we designed a randomized, nonblinded trial of SD rATG induction.
The 2×2 factorial trial design we used, although uncommon in transplantation studies, has been recommended as resolving multiple hypotheses with a minimum investment of patients, time, and effort.34,35 This design allowed efficient detection of the independent effects of SD versus divided-dose (DD) rATG induction and CNI minimization versus withdrawal. The primary endpoints of the trial were renal function and protocol biopsy histopathology, with secondary endpoints that included patient, graft, and rejection-free survival, and infectious and noninfectious complications (Fig. 1).
FIGURE 1.
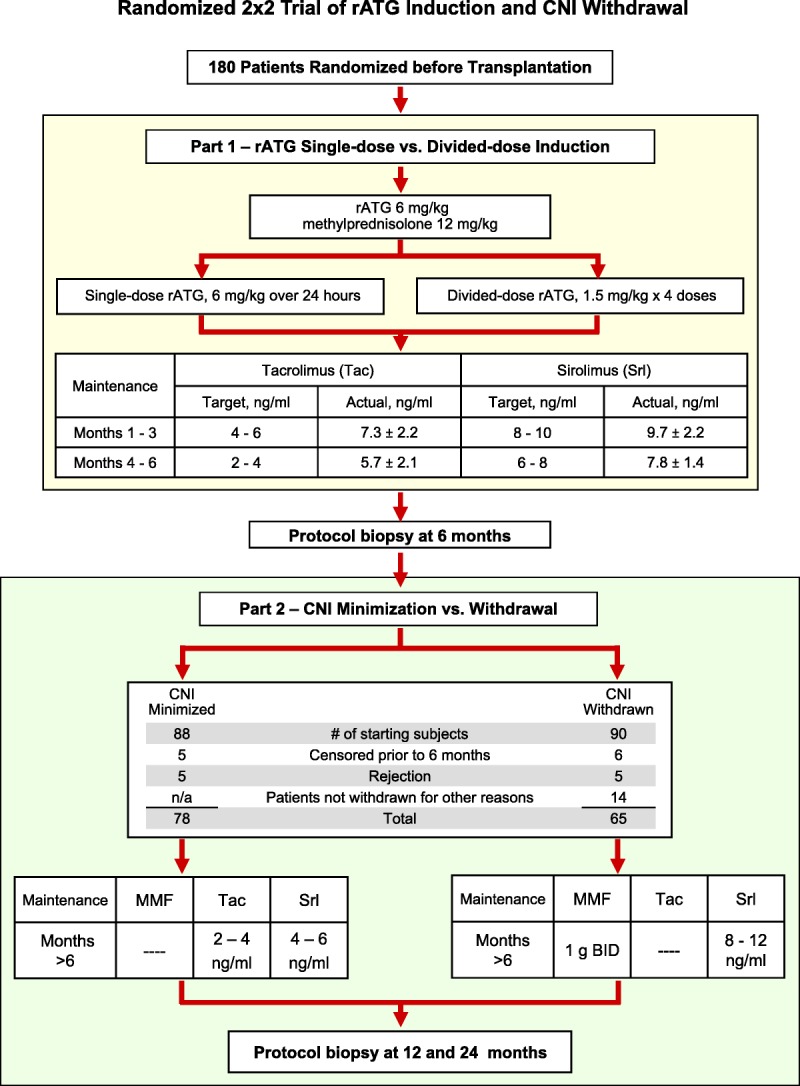
2×2 factorial trial of rATG induction dosing and CNI withdrawal. Between April 20, 2004, and April 14, 2009, at the University of Nebraska Medical Center, 180 recipients of renal transplants were enrolled in a single center, prospective, randomized, unblinded 2×2 factorial trial of single-dose versus divided-dose rATG induction (6 mg/kg over 24 hr vs. 1.5 mg/kg × 4 alternate-day doses) followed after 6 months by CNI minimization or CNI withdrawal and replacement with MMF (IRB # 286–03; ClinicalTrials.gov #NCT00556933). Two patients not meeting enrollment criteria were consented and randomized in error, but were identified before transplantation and removed from participation in the trial. Sirolimus introduction was delayed in favor of MMF use until weeks 3 to 6 in patients with ATN-DGF or who were at high risk for wound complications (e.g., truncal obesity). We determined that 160 patients would provide 80% power with a two-sided 0.05 α level to detect a 10% difference in calculated GFR during the first year.34 (Enrollment was raised to 180 during the trial to guarantee sufficient patients to undergo eventual CNI withdrawal.) Randomization included stratification by race (white/Asian vs. non-white/Asian), donor type (living vs. deceased), and whether listed for eventual pancreas transplantation. Randomized assignments for both part 1 and part 2 treatment were contained in sequentially numbered, sealed envelopes opened after obtaining consent for trial participation to maintain allocation concealment. All analyses are intent-to-treat. MMF, mycophenolate mofetil; GFR, glomerular filtration rate; ATN, acute tubular necrosis; DGF, delayed graft function; rATG, rabbit antithymocyte globulin; CNI, calcineurin inhibitor.
We previously reported the 6-month (part 1) interim results of this trial, showing the early tolerability of SD rATG induction and its association with superior (first week only) renal function among deceased donor recipients. However, this improvement did not sustain significance throughout the remainder of the initial 6-month analysis. Given that the average follow-up was less than 2 years, this initial report could not address the longer-term safety and efficacy of SD induction. In this article, we report the long-term results of part 1 of the trial, with increased enrollment and average follow-up over 4 years, enabling a robust assessment of longer-term safety and efficacy of induction with SD rATG. Part 2 of this 2×2 factorial trial, the long-term results of complete CNI withdrawal versus minimization, will be reported in a separate article.
RESULTS
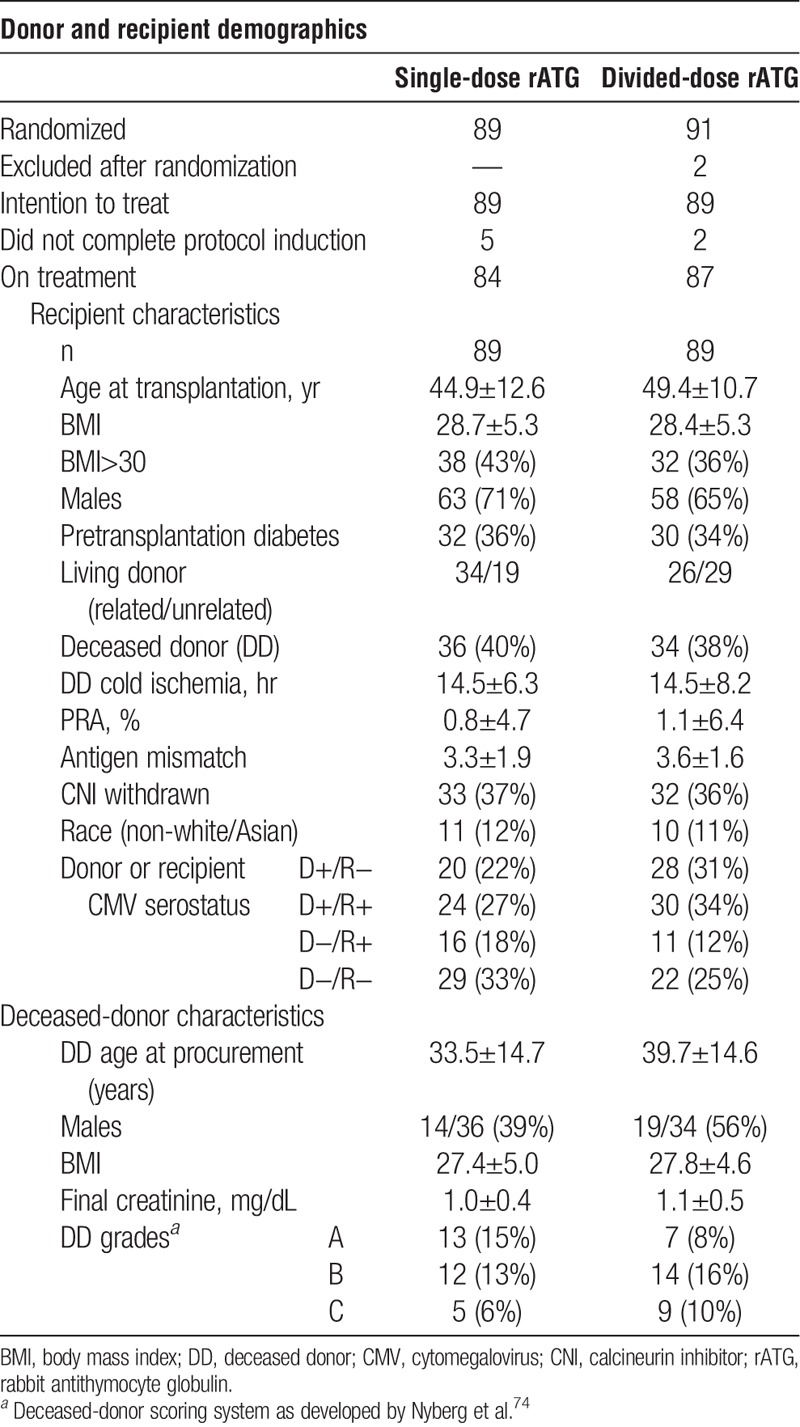
Patient Enrollment and Recipient and Donor Characteristics
Follow-up after transplantation averaged 51.8±15.1 months, with no patient followed less than 2 years. Patients excluded are presented in Table S1 (SDC, http://links.lww.com/TP/B12). Four previously transplanted patients were enrolled and distributed equally between the study arms. Patients were censored for reasons that included withdrawal of consent, scheduled pancreas transplantation, transfer of care, or death (Table 1 and Table S1, SDC, http://links.lww.com/TP/B12). Causes of patient renal failure are detailed in Table S2 (SDC, http://links.lww.com/TP/B12).
TABLE 1.
Complications and infections after rATG induction
With the multiplicity of possible demographic comparisons, there was a 64% chance that at least one of the differences between the SD and DD groups would be significant when in fact no difference actually exists. To maintain the family-wise collective type-1 error rate over all 20 demographic comparisons at 0.05, a Bonferroni critical value adjustment was calculated for the individual tests, none of which were significant. Despite the lack of significant demographic differences between the trial arms, factors potentially impacting study outcomes have been included as covariates in all of our numerical modeling (e.g., patient age, pretransplant diabetes, hypertension).
Analysis for Interaction Between rATG Induction and CNI Withdrawal
Validation of the 2×2 trial design included numerical modeling of primary and secondary endpoints and possible covariates, which showed that CNI withdrawal status did not interact significantly with rATG induction regimen, justifying independent analysis of the two treatments.35
rATG and Steroid Exposure and Acute Tubular Necrosis-Delayed Graft Function
The total rATG administered was similar between groups: induction (SD, 5.8±0.9 mg/kg vs. DD, 5.9±0.6 mg/kg; P=0.72) to treat acute tubular necrosis (ATN), delayed graft function (DGF), or poor early renal function (SD, 4.1±1.4 mg/kg vs. DD, 2.8±1.8 mg/kg; P=0.15) or cumulative dose (SD, 6.2±1.5 mg/kg vs. DD, 6.1±1.2 mg/kg; P=0.85). Total steroid exposure was SD, 12.2±2.9 mg/kg and DD, 12.1±2.4 mg/kg (P=0.84).
All patients receiving additional rATG and steroid to treat ATN-DGF (n=12) or poor early renal function (n=5) received deceased donor kidneys (SD vs. DD, 9 vs. 8; P=0.55).
Maintenance Immunosuppression, Therapeutic Drug Monitoring, and Antiviral Prophylaxis
Because of obesity or ATN-DGF, MMF (instead of sirolimus) was in use at day 14 in 39 (44%) SD and 44 (49%) DD patients (P=0.55), with 90% of all patients being initiated on sirolimus by week 6. Only 5% of the patients proved unable to tolerate or afford sirolimus in the long term. Overall exposure to MMF was not statistically different between groups (P=0.99).
Three of the 12 patients with ATN-DGF did not receive sirolimus; sirolimus was initiated in the remaining nine at 5 to 306 days (82±106). One sirolimus-free patient died on day 39; the remaining 11 all achieved a serum creatinine less than 3.0 mg/dL after 8 to 46 days (23.1±12.0).
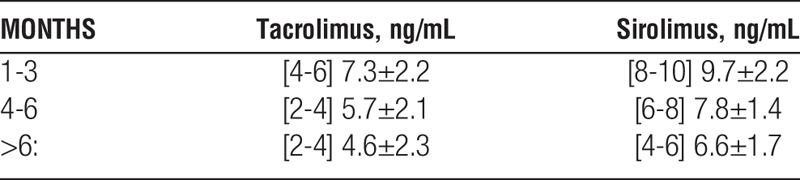
Mean immunosuppressant trough levels of both tacrolimus and sirolimus declined over time in accordance with the study design; however, levels tended to be at or just above the upper limit of the [target]:
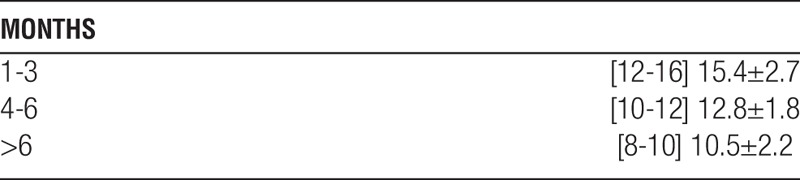
The combined tacrolimus+sirolimus levels (ng/mL) were:
Actual sirolimus level among patients withdrawn from CNI: [8-12] 10.4±1.4, with an average MMF dose of 1.9±0.3 g per day. An equal number of patients underwent CNI withdrawal in each induction group (SD vs. DD, 32/89 vs. 33/89; P=1.00).
Mean immunosuppressant trough levels and antiviral prophylaxis exposure were not statistically different between the induction groups at any time.
Renal Function
In contrast to our interim report,36 we now show superior renal function among all patients and deceased donor recipients for 3 years after transplantation. We designed the trial to take full advantage of repeated serum creatinines collected throughout the follow-up (General Linear Model of repeated measures), but we can show superior renal function among SD patients months 36 to 38 (all patients, P=0.04; deceased donor recipients, P=0.06), but not living-donor recipients (P=0.35).
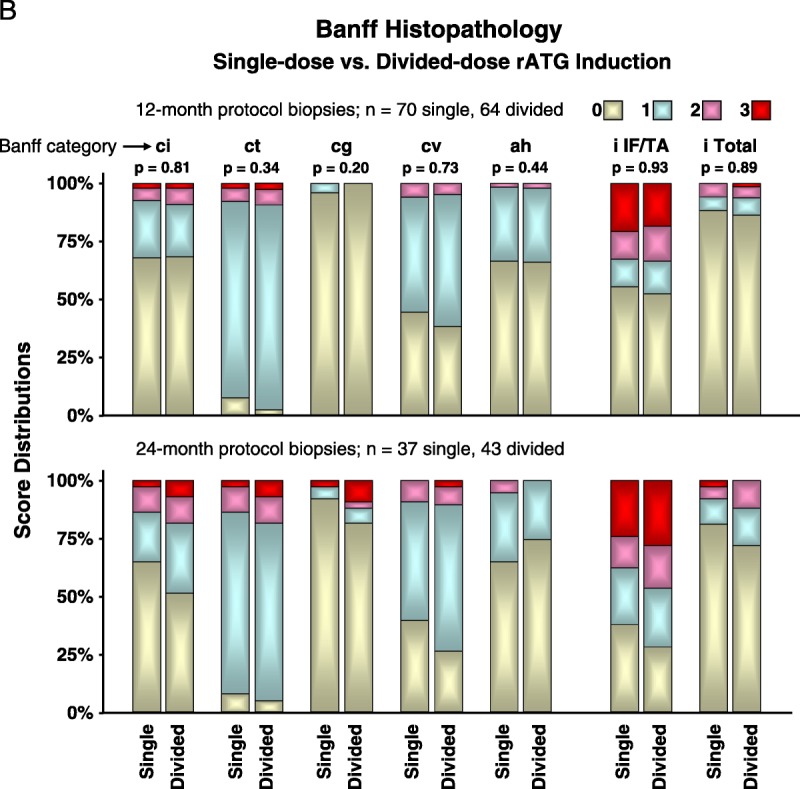
Histopathology in 12-Month and 24-Month Protocol Biopsies
At 12 months, rATG induction protocol had no independent effect on the five Banff categories of chronic renal injury (singly or together). There also was no difference in Banff scores for inflammation in areas of interstitial fibrosis or tubular atrophy or inflammation throughout the biopsy. Protocol biopsies collected at 24 months are presented only graphically (Fig. 2B).
FIGURE 2.
(continued)
Patient Survival, Graft Survival, and Rejection
There were significantly fewer deaths among kidney recipients who received SD rATG induction; causes of patient death are presented in Table S3 (SDC, http://links.lww.com/TP/B12). No statistically significant differences were present between induction groups in death-censored or rejection-free graft survival (see Fig. 3A, Banff acute scores; Table S3, SDC, http://links.lww.com/TP/B12).
FIGURE 3.
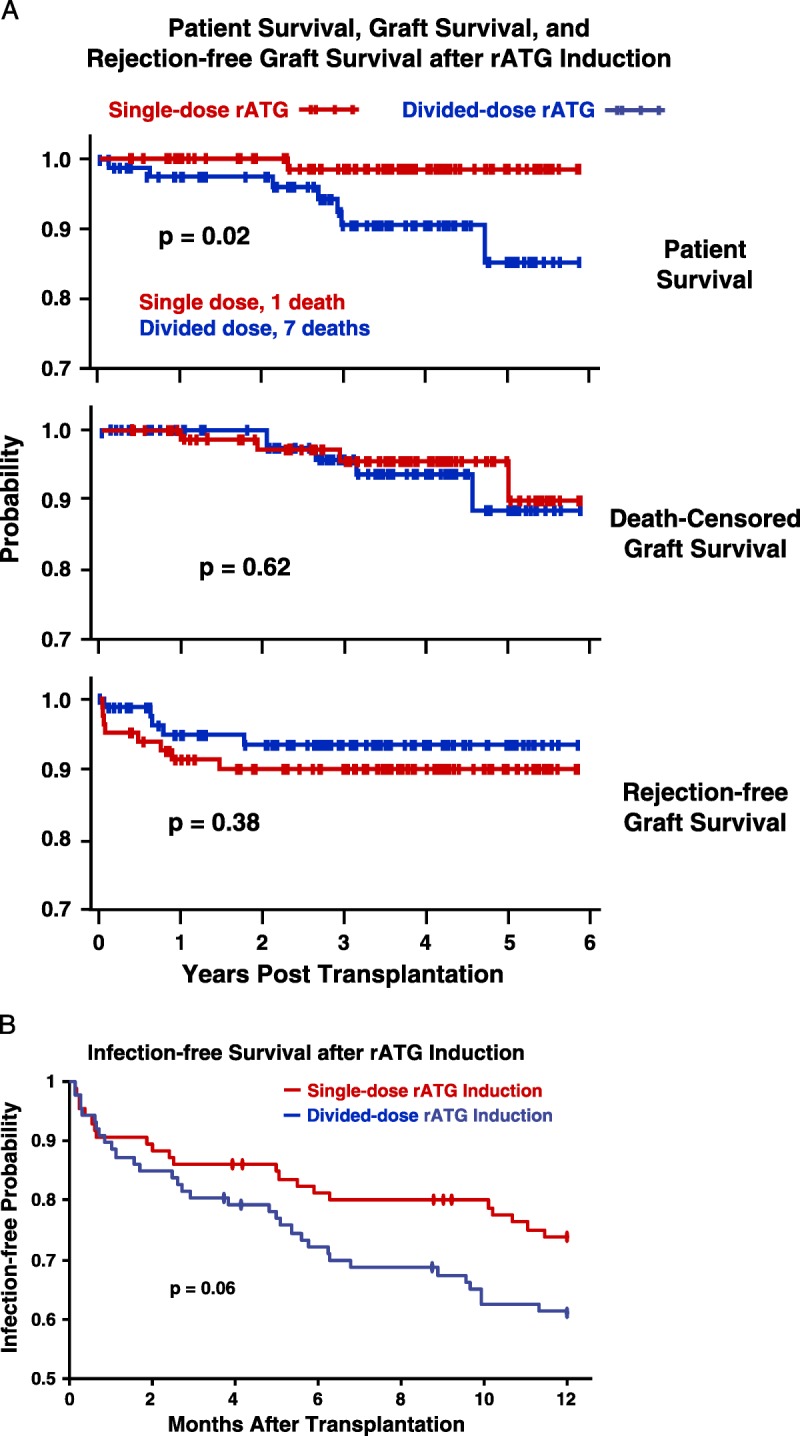
A, Rates of patient survival, graft survival, and rejection were compared with Kaplan-Meier analyses and log-rank tests. Patient deaths occurred at an average of 2.3±1.4 years after transplantation, of causes that included myocardial infarction, cancer, sepsis, drug overdose, and pulmonary embolus (Table S2, SDC, http://links.lww.com/TP/B12). Rejection was confirmed by ultrasound-guided biopsies (biopsy-proven acute rejection) graded according to Banff 1997 or 2005 criteria.73. There were eight acute cellular rejection episodes in the single-dose group; five Banff grade IA, three grade IB. There were five cellular rejections in the divided-dose group; 1 grade IA, 3 grade IB, 1 grade IIA. B, There were eight episodes of suspicious or borderline rejection in each group not included. There were three instances of DSA+ AMR observed among our study patients, all in the divided-dose rATGgroup, at 9 days, 9months, and 14 months after transplantation. Only one of these grafts has been lost, at 2 years in the patient who experienced AMR at 14 months. The treatment of acute cellular rejection was guided by specific Banff classification (Table S4, SDC, http://links.lww.com/TP/B12). (B) Kaplan-Meier estimates of likelihood of infection after rATG induction and renal transplantation. The first of any infection after transplantation was scored, including pneumonia, abscess, UTI, bacteremia, “other” bacterial, BK (viruria or disease),CMV, Epstein-Barr virus, post-transplantation lymphoproliferative disorder, “other” viral, and fungal. C, In both rATG induction groups, lymphocyte counts immediately declined steeply after rATG infusion, but recovered significantly more rapidly in the single-dose group. D and E, T-cell subset data were obtained from only the first 80 patients because of the cost. Although CD8 numbers recovered rapidly and equally in both groups, CD4 counts and the CD4-to-CD8 ratio recovered significantly faster in the single-dose group. DSA+, donor-specific antibody positive; AMR, antibody-mediated rejection; rATG, rabbit antithymocyte globulin; CMV, cytomegalovirus; UTI, urinary tract infection.
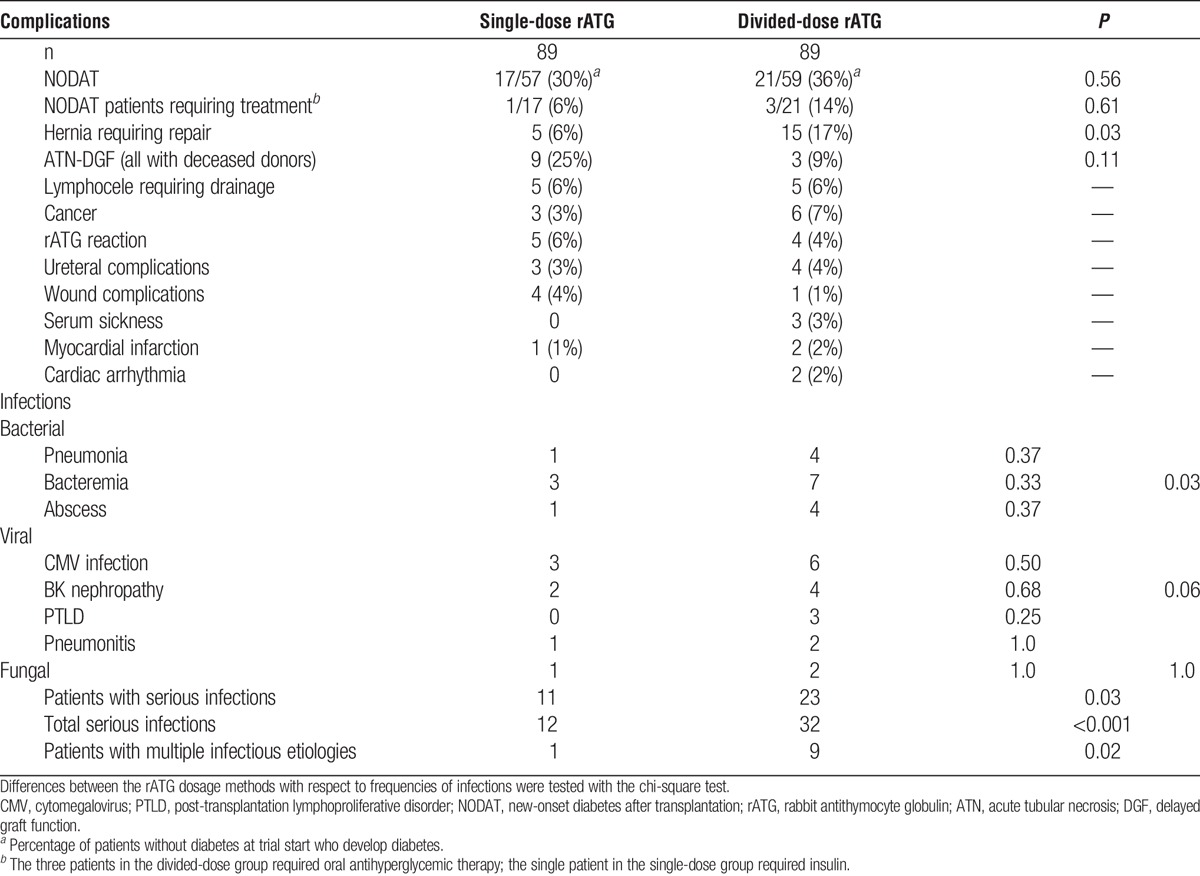
Noninfectious Complications
Composited rATG infusion complication rates were not statistically different between induction groups. Hernias requiring repair were more frequent among DD patients. The frequency of hernia risk factors (e.g., age, body mass index, diabetes, sirolimus exposure) did not explain this difference. Interestingly, more patients with polycystic kidney disease were randomized to the DD group (P=0.012), but patients who developed hernias showed an equal incidence of polycystic kidney disease within each induction group (P=0.61) (Table 2).
TABLE 2.
Complications after rATG induction
Infectious Complications
Tables 2 and 3 summarize the most serious infections or infection sequelae (e.g., post-transplantation lymphoproliferative disorder [PTLD]). Thirty-four patients demonstrated serious infectious outcomes among 178 patients (19%), 73% (P<0.001) of these were DD patients. Patients with abscesses or pneumonia are not included among those listed with bacteremia to avoid duplication of events; those with bacteremia had associated cardiopulmonary signs indicative of sepsis, typically from line sepsis or urosepsis.
TABLE 3.
Organisms and sources of bacterial and fungal infectious complications
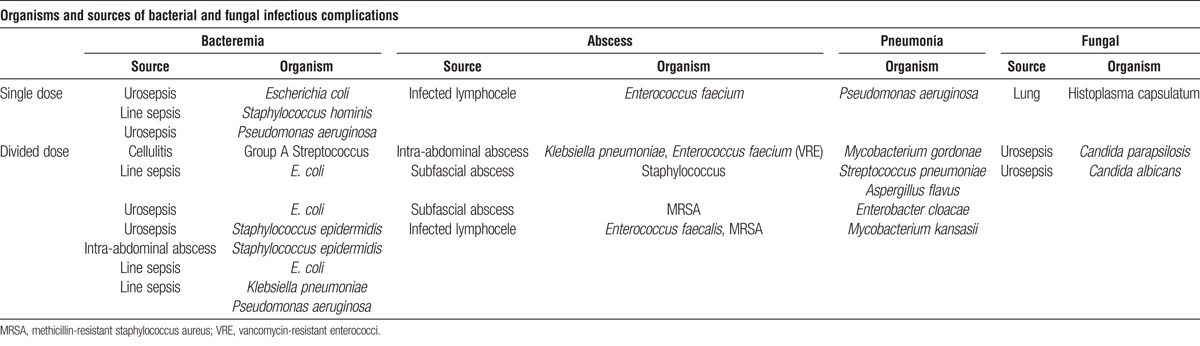
Donor-recipient cytomegalovirus (CMV) serostatus profiles were not statistically different between induction groups (P=0.28) (Table 1). Cytomegalovirus infection or disease occurred exclusively in D+ and R− patients (9 and 48; 19%). Only one patient (DD) showed tissue-invasive CMV disease (enteritis/colitis); the remaining eight demonstrated CMV syndrome. Cases of PTLD, included here as sequelae of Epstein-Barr virus infection, were all monoclonal B-cell lymphomas.
Frequencies of multiple infections (two or more infectious origins, e.g., bacterial, viral, fungal) were lower among SD patients (1 vs. 9; P=0.02). Time-to-first infection was significantly earlier after DD induction. There were more infections-per-patient among those in either group treated with additional rATG and steroid (n=21) for ATN-DGF or rejection; 1.1±0.31 versus 0.57±0.08 (P=0.03). Multiple infections (both life-threatening and others) were also more common after additional rATG; 38% vs. 13% (P<0.01). After receiving additional rATG, DD patients developed more infections than those receiving the single dose (1.6±0.49 vs. 0.5±0.27; P=0.06).
Immunological Impact of rATG Induction Regimen
After induction, the rate of total lymphocyte recovery was significantly faster in SD patients. The projected return to preinduction values for patients receiving the single dose was 6 years, and double dose, 14 years. This difference was primarily because of the more rapid recovery of the CD4 (but not CD8) subset in patients receiving the single dose (Fig. 3C-E).
FIGURE 3.
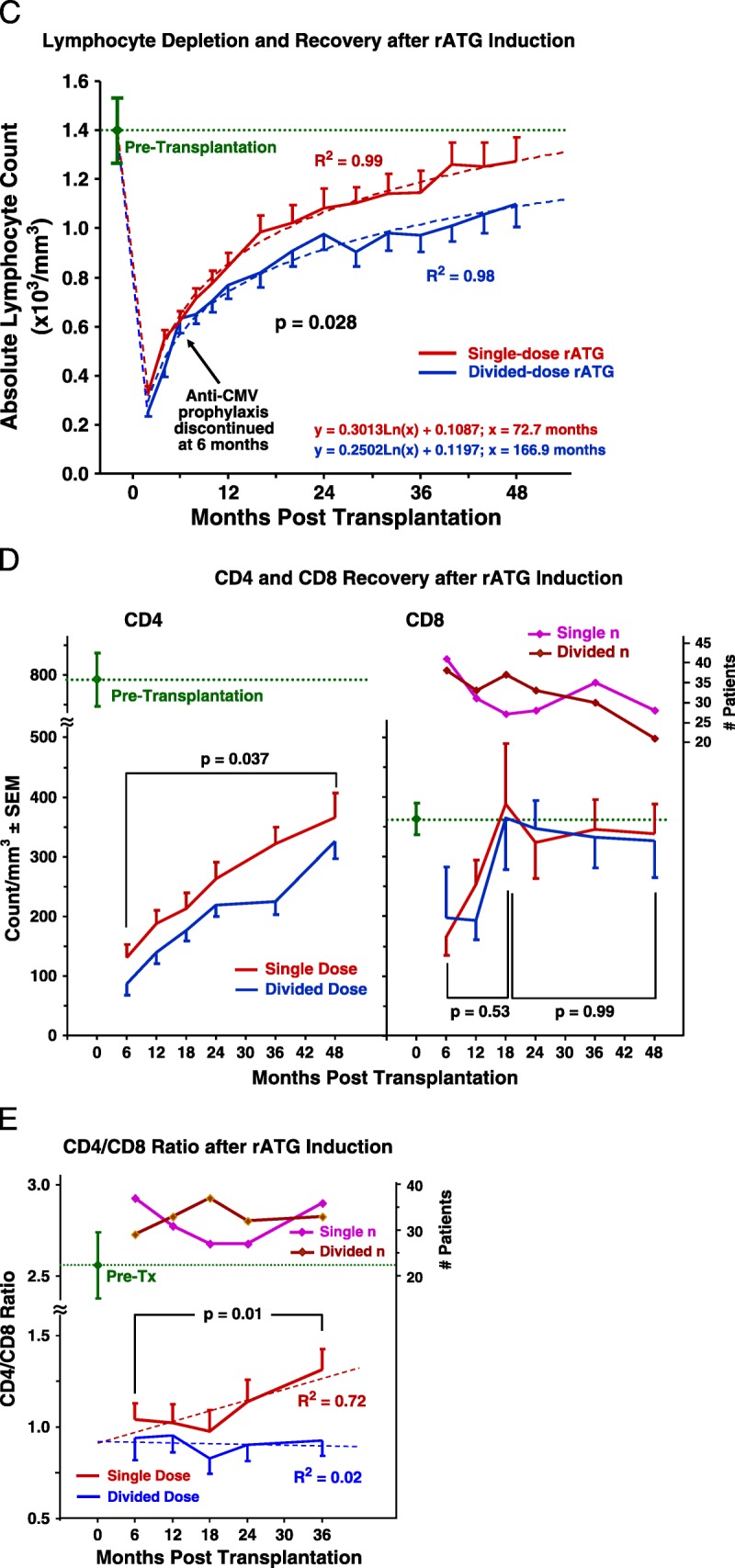
(continued)
Multivariate Analysis of Infectious Complications and Mortality
In a multivariate regression model of infectious complications, SD rATG induction (P=0.046) and higher 12-month absolute lymphocyte counts (P=0.033) associated with significantly fewer occurrences of severe viral infection (CMV infection or disease, BK nephropathy, and PTLD). In this model, additional associates of severe viral infection were increased age (P=0.042), pretransplant diabetes (P=0.015), and additional rATG (P=0.047). The risk of multiple infections decreased in association with rising absolute CD4 counts (P=0.06) and CD4-CD8 (P=0.05).
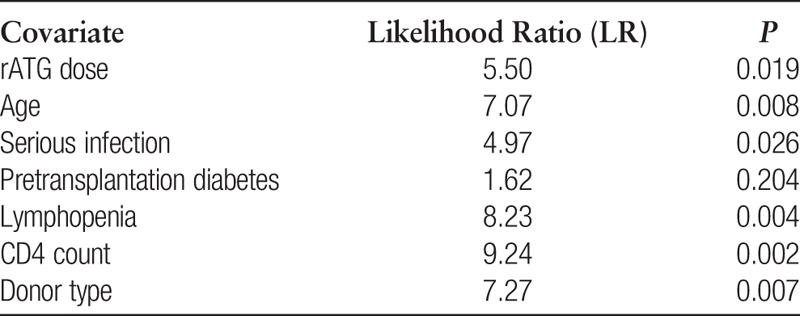
Univariate Cox hazard analyses of patient mortality resulted in the following:
The six significant covariates were combined into Cox multivariate hazard models and sequentially analyzed for their incremental contributions to LR statistics (the estimation algorithm for hypertension or cardiovascular disease failed because of excessive prevalence). The final optimized model comprised four covariates; DD rATG (P=0.019), deceased donor (P=0.003), serious infection (P=0.0.018), and lower lymphocyte count (P=0.001), all significantly associated with more hazard of mortality. Patients with all four conditions showed a 27-fold increased likelihood of death (P=0.00002).
DISCUSSION
The earliest description of depleting lymphocytes in vivo with antilymphocyte serum was by Metchnikoff in 1899, and its impact on skin homograft survival in rats was reported by Woodruff in 1963.37,38 In 1966, Monaco reported that horse antidog serum reduced allograft rejection in transplanted dogs.39 Najarian and Starzl effectively treated kidney rejection in humans with horse antihuman globulin.40,41 Kaden (1992-1998) reported less ATN-DGF after induction using rATG-Fresenius.42 The efficacy of induction with a single 9 mg/kg bolus of rATG-Fresenius versus two 20-mg doses of basiliximab was supported by Kyllonen in work reported in 2007, and SD rATG induction before weaning recipients to CNI monotherapy was reported by Starzl and Shapiro.31,33,43 In 2009, Kaden summarized a large retrospective experience of SD rATG-Fresenius compared against an unmatched historical control group and concluded that the DD showed worse patient survival and graft function.30
No previous randomized trial has compared SD versus DD rATG induction as we have, and despite its limitations (single-center, open-label, primarily low immunologic risk patients), our previous reports indicated superior early graft function, overall equivalent early safety,36 reduced early hyperglycemia and hypomagnesemia, and delayed development of NODAT.44 The long-term data now seem to show that SD rATG associates with superior patient survival, improved early (all donor types), and long-term deceased-donor graft function, fewer infections, and fewer multiple infections. Our trial was in retrospect not adequately powered to detect a difference in histopathology between induction groups, due both to a slower rate of chronic injury development than that reported previously and patient unwillingness to undergo protocol biopsy at 24 months.45,46
We selected the 2×2 trial design to increase statistical power by avoiding a four-group analysis. Because the trial involves an initial treatment (rATG induction) followed by a second treatment (CNI withdrawal after six months), it is proper to analyze each treatment separately if there is no significant confounding interaction between treatments and patients receiving each treatment are distributed equally between groups, as is true here.34,35
The unexpected mortality difference between rATG induction groups was not because of the differences in demographics or exposure to total rATG, steroid, maintenance agents (tacrolimus, sirolimus, MMF), or antiviral prophylaxis (valganciclovir). The possibility that alternate-day administration of steroids (vs. over 24 hr) might account for the increased incidence of serious infections, multiple infections, and increased infections and mortality observed in the divided dose group is extremely unlikely in that the lymphocyte, CD4, and CD4-CD8 recovery rates that probably cause those outcomes are acutely and chronically influenced by rATG but not steroid administration. In our interim report, we did not observe any statistically significant differences in early complication rates between induction groups.36
The increased mortality among DD patients is consistent with that group’s increased infections, multiple infections, and more severe and prolonged CD4 T-cell depletion. The risk of multiple infections associated with the severity of CD4 T-cell lymphopenia, and in a multivariate regression model of infectious complications, SD rATG induction associated with higher 12-month absolute lymphocyte counts and significantly fewer occurrences of severe viral infection. An association has been shown between increased infections, mortality, morbidity, and the extent and duration of CD4 depletion after rATG induction.47 Of the seven deaths among our DD rATG patients, most were from sepsis (43%) or cancer (29%), an outcome consistent with the observations of Ducloux et al.,47 “….prolonged polyclonal anti-thymocyte globulin–induced CD4 T-cell lymphopenia is an independent risk factor for death (P=0.001)”. In renal transplant patients, fewer CD4-to-CD8 ratio because of any origin increased the risk of life-threatening infections.48
Four patients in the divided dose group showed cardiac complications of a significant arrhythmia or myocardial infarction as compared to one patient in the SD group. Interestingly, lymphopenia, especially CD4 lymphopenia, also has been associated with accelerated progression of coronary atherosclerosis and cardiac-related mortality after renal transplantation.49-51
Alemtuzumab induction also has a profound and prolonged impact on lymphocyte populations. A review of more than 7,000 deceased donor renal transplants in the SRTR database indicated increased graft loss and patient death with alemtuzumab versus rATG induction.52 However, in a prospective, randomized trial comparing alemtuzumab and rATG induction, no such difference was observed.53 This difference may reflect the administration of two doses of alemtuzumab to most of the patients in the SRTR data set and the current practice of SD alemtuzumab induction in the patients in the prospective, randomized trial.
The total exposure to rATG in this study was in keeping with that used at many transplant programs.25-27 An equal number of patients in each of our induction groups received additional rATG (ATN-DGF, poor early function, rejection), yet still there were more serious infections in the DD rATG group, suggesting greater immune suppression.
How could administering rATG as a single dose produce the benefit to function we are reporting here? Given the polyclonal nature of rATG, this effect may not be directly related to T-cell depletion.54,55 For example, investigators have identified rATG antibodies with affinities to endovascular adhesion molecules necessary to leukocyte homing and trafficking, suggesting that rATG may be able to reduce ischemia-reperfusion injury.7,56-61 A recent report by Urbanova et al.62 showed that 3 months after induction, thymoglobulin rATG, in contrast to rATG-Fresenius, decreased the expression of genes involved in the nuclear factor-kappa B (NF-κB) pathway (TLR4, MYD88, and CD209), along with CD80 and CTLA4 (costimulation), NLRP1 (apoptosis), CCR10 (chemoattraction), and CLEC4C (dendritic cell function). In an experimental model of kidney reperfusion injury, NF-κB upregulation correlated with the severity of injury, and inhibitors and antagonists of NF-κB had a beneficial effect.63,64 These findings suggest that rATG may have an impact on early renal allograft inflammation, and that this impact may persist.
We have also found that up to 10% of rATG antibodies recognize and inactivate heparanase, and another 5% recognize and inactivate interleukin (IL)-2.44 Heparanase in the kidney, released with reperfusion, degrades basement membrane heparan sulfate, allowing edema and cell infiltration. Extensive loss of heparan sulfate chains occurs within minutes of reperfusing rodent autocardiac and xenocardiac grafts.65,66 Because hypoxia upregulates heparanase expression,67 ischemic organs may be “primed” to release heparanase. In addition to degrading the endothelial cell glycocalyx, heparanase releases heparan sulfate-bound IL-2 found in tissues and lining blood vessels.68,69 Local increases in IL-2 induce lymphocyte proliferation, neutrophil activation, and endothelial cell permeability.70,71
The idea that the benefit of rATG induction in reducing inflammation or reperfusion injury might be dose-related is supported by an observation reported in our initial article that the amount of rATG administered before kidney reperfusion associated with improved renal function (P=0.025) (but not less ATN-DGF) during the first 5 days posttransplantation. (SD group vs. DD group, 0.61±0.26 vs. 0.16±0.06 mg/kg; P<0.0001).36 Kyllonen et al.31 reported a significant reduction in ATN-DGF rates when the full rATG induction dose (9 mg/kg, rATG-Fresenius) was administered before reperfusion.
We hypothesize that SD rATG induction results in a higher concentration of rATG at reperfusion, and more extensively decreases NF-κB and blocks adhesion molecules, heparanase, and IL-2 that are undoubtedly expressed and released to a greater extent in deceased donor kidneys. This hypothesis is consistent with the greater benefit to renal function of SD rATG in deceased versus living-donor kidneys and is undergoing direct evaluation in our ongoing STAT multicenter clinical trial (STAT trial; Single-dose vs. Traditional Administration Thymoglobulin) (Trials.gov #NCT00906204).
In summary, the results from Part 1 of our 2×2 factorial trial suggest that rATG dosing strategy impacts both the safety and efficacy of lytic induction in renal transplantation. Both strategies of rATG induction at the least allow minimization of CNI and mTORi to the levels achieved in the trial.
Our data support the concept that how rATG is administered has an effect on its complication profile. However, an ironclad cause-and-effect relationship cannot be established because neither patient survival nor infection rates were used to power this trial. Our observations warrant definitive assessment in a larger, appropriately powered multicenter trial.
MATERIALS AND METHODS
Study Patients and Endpoints
Adult primary and selected previous renal transplant recipients (nonimmunologic causes of graft loss) over age 18 years were eligible for study participation. Patients excluded were: older than 65 years, panel-reactive antibodies greater than 75%, human leukocyte antigen-identical, or required steroids. Expanded-criteria donors and donation after cardiac death donors were excluded from the trial.
Primary endpoints were renal function by calculated glomerular filtration rate (abbreviated modification of diet in renal disease)72 and acute and chronic renal histopathology (based on Banff ’05 criteria). Secondary endpoints included patient survival, graft survival, biopsy-proven rejection, and infectious and noninfectious complications.
Power Analysis, Statistics, and Patient Randomization
The power analysis is described in Figure 1. Individual figures and tables include details of statistical analyses. PASS software (NCSS LLC, Kaysville, UT), SAS software (SAS Institute, Cary, NC), and SPSS software (IBM Corporation, Armonk, NY).
Assessment of Graft Function and Renal Histopathology
Details are presented in the respective legends (Fig. 2A and B).
FIGURE 2.
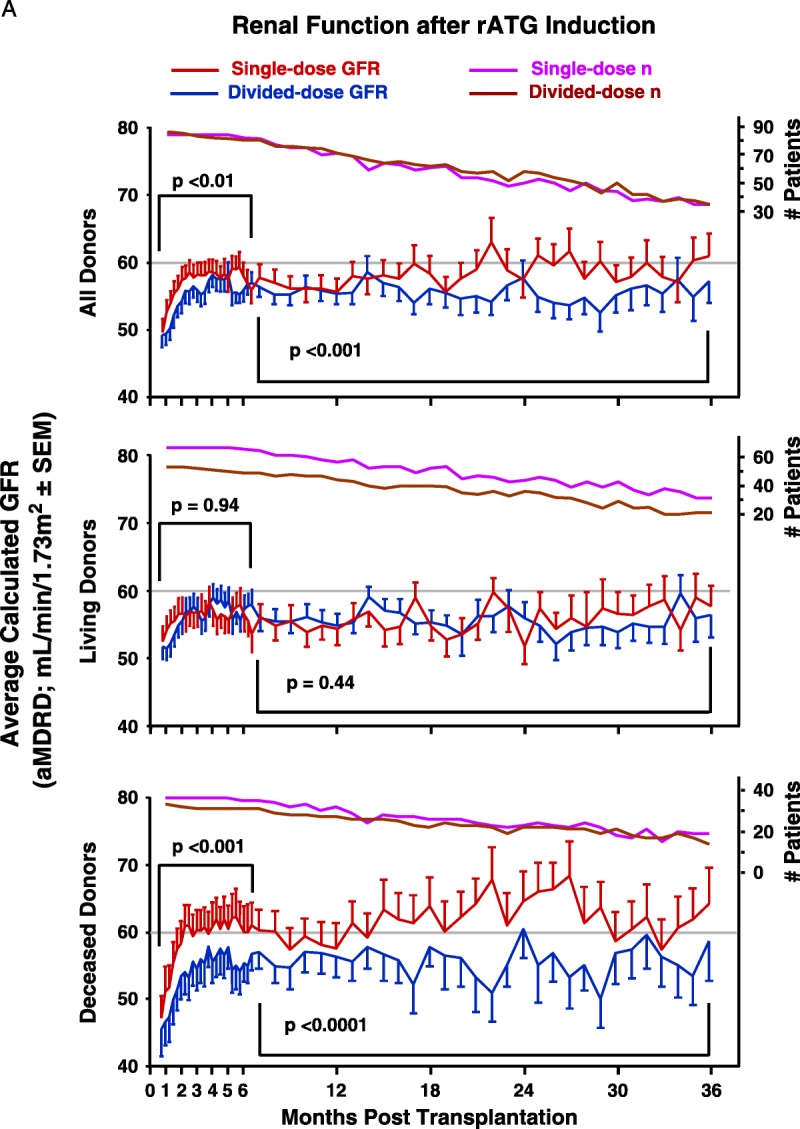
A, Graft function was assessed by using the aMDRD equation to estimate GFR,57,72 using all blood draws that provided serum creatinine measurements. Greater blood draw frequency during the first 6 months allowed weekly averages for each group to be compared, followed by monthly averages after 6 months. The GFR between treatment groups was compared and analyzed using a general linear model for repeated measures with maximum likelihood estimation, an approach sensitive to differences between small groups despite large intrapatient variance over time. Ordinary least-squares regression was not used because in the context of repeated measures, it can fail to recognize significant effects in the model because of faulty estimation of the covariance structure of the data.75 Although more patients in the single-dose group developed ATN-DGF, this trend did not reach statistical significance (P=0.11). This likely reflects our failure early in the trial to administer sufficient steroids to single-dose recipients; total steroid exposure, after our initial 20 patients, was increased from 6 mg/kg to 12 mg/kg. B, Frequencies for each induction group’s individual Banff categories were compared by Kruskal-Wallis rank testing. At 12 months, among single-dose recipients, there were five instances of suspicious or borderline rejection and nine among single-dose recipients. Among 24-month protocol biopsies, there were five instances of suspicious or borderline rejection in single-dose recipients and eight in the divided-dose group. There were no observations of recurrent disease among the protocol biopsies. aMDRD, abbreviated modification of diet in renal disease; GFR, glomerular filtration rate; ATN, acute tubular necrosis; DGF, delayed graft function.
rATG and Steroids; Induction and ATN-DGF
All recipients received 6 mg/kg intravenous rATG (Genzyme Corporation, Cambridge, MA) beginning at transplantation, a single infusion over 24 hr or four 1.5 mg/kg infusions on alternate days (Fig. 1).36 The total steroid administered was 12 mg/kg, in 3 mg/kg doses with DD rATG, and 3 mg/kg Q6 over 24 hr with SD rATG.
Except to treat rejection, steroids were administered only in association with rATG. In cases of ATN-DGF (dialysis within seven days of transplantation), up to six additional 1 mg/kg doses of rATG (with 1 mg/kg methylprednisolone) were given. To prevent excessive exposure to rATG and steroids, we capped exposure at 100 kg.
Maintenance Immune Suppression
Discretionary clinical judgment is required to achieve the best sequence and rate of introduction of maintenance agents to suit each patient’s circumstances. Initiating tacrolimus and sirolimus depends partly on early renal graft function. In patients with ATN-DGF, monotherapy with MMF (500 mg orally, two times per day) is initiated early (POD 1-3) and is replaced with CNI and sirolimus when graft function improves (sCr<3.0). Similarly, in obese patients (BMI>32 or truncal obesity), MMF is used until sirolimus can safely be initiated (3-6 weeks). Target blood levels were:
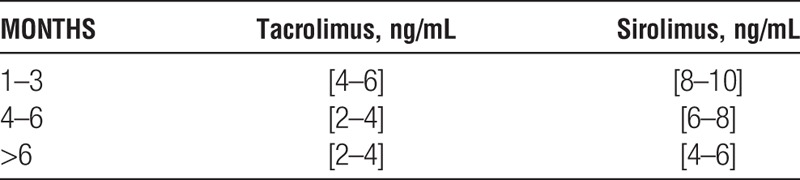
Combined tacrolimus+sirolimus target levels [ng/mL] were:
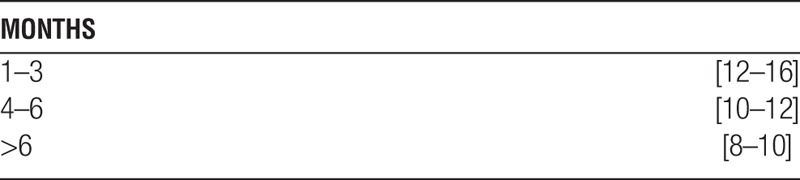
Patients who underwent CNI withdrawal after six months received MMF (1 g two times per day) with a sirolimus target level of 8 to 12 ng/mL.
Although never formally validated, adding blood levels of the two maintenance agents was performed to avoid both agents being at the low end of their targeted range, thereby preventing the combined exposure from being inadequate in the context of ESW, a practice used by others.27
Viral, Fungal, and Pneumocystis Prophylaxis
All recipients received oral valganciclovir and clotrimazole for 3 months after transplantation; CMV− recipients with a CMV+ donor received valganciclovir 3 months longer. Patients treated with rATG for rejection received a repeated course of the antiviral prophylaxis used at induction.
Pneumocystis prophylaxis was with trimethoprim-sulfamethoxazole or, if allergic to sulfa, diamino-diphenyl sulfone (Dapsone) or aerosolized pentamidine diisethionate for 3 months after transplantation.
CMV and BK Viral Surveillance
In CMV− recipients transplanted with kidneys from CMV+ donors, blood viral DNA load was checked at 2 weeks, and then monthly for 6 months after valganciclovir was discontinued. Patients with significant leukopenia (white blood cells<2,000) were routinely screened for CMV viremia or infection. All patients underwent urine screening for BK virus at months 1, 3, 6, 9, and 12, and then annually for 5 years. If urine screening showed more than 9 million viral DNA copies, blood was screened also. Patients with renal dysfunction and BK viremia underwent renal biopsy; asymptomatic patients with BK viremia more than 2,000 plasma BK DNA copies per milliliter also were biopsied. This threshold for significant BK viremia is higher than that now used by the authors.
Diagnosis and Treatment of Rejection
Rejection was confirmed by ultrasound-guided biopsies (biopsy-proven acute rejection) graded according to Banff 1997 or 2005 criteria.73 The treatment of rejection was guided by specific Banff classification (Table S4, SDC, http://links.lww.com/TP/B12).
Assessment of Complications and Infections
Specific criteria defined each complication (Table S5, SDC, http://links.lww.com/TP/B12). Complications were analyzed both individually and as sets occurring after rATG induction, early (rATG reaction, cardiac dysrhythmia, myocardial infarction, and serum sickness) or late (wound complications, lymphocele drainage, and hernia repair). Transplant infectious disease specialists (A.K. and D.F.) assessed infections using standard criteria.
Supplementary Material
Footnotes
This work was supported by the Ann Goldstein-Cheryl Cooper New Frontiers in Transplant Medicine Fund, a Research Support Fund grant from the Nebraska Medical Center and the University of Nebraska Medical Center, and a research grant fromGenzyme Corporation, which partially funded clinical research nurse time.
The authors declare no conflicts of interest.
The trial reported in this article is registered at ClinicalTrials.gov, #NCT00556933.
R.B.S. designed and supervised the trial. K.W.F. read the study protocol biopsies. C.D.M. participated in the medical management of study patients and data review. J.T.L. participated in medical management of study patients and data review. A.C.K. reviewed and analyzed infectious complications of study patients. D.F.F. reviewed infectious complications of study patients. J.P.S. performed the statistical analyses. T.H.R. participated in chart review, data collection, article preparation, and editing. K.J.N. participated in study database management. J.Y.S. participated in chart review, data collection, and article preparation. A.M.K. maintained IRB documents. T.M. participated in patient management and article review. L.E.W. participated in data and article review, patient management, and heparan sulfate studies.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1. Kasiske BL, Gaston RS, Gourishankar S, et al. Long-term deterioration of kidney allograft function. Am J Transplant 2005; 5: 1405. [DOI] [PubMed] [Google Scholar]
- 2. Flechner SM, Kobashigawa J, Klintmalm G. Calcineurin inhibitor-sparing regimens in solid organ transplantation: focus on improving renal function and nephrotoxicity. Clin Transplant 2008; 22: 1. [DOI] [PubMed] [Google Scholar]
- 3. Gaston RS, Cecka JM, Kasiske BL, et al. Evidence for antibody-mediated injury as a major determinant of late kidney allograft failure. Transplantation 2010; 90: 68. [DOI] [PubMed] [Google Scholar]
- 4. Haririan A, Kiangkitiwan B, Kukuruga D, et al. The impact of c4d pattern and donor-specific antibody on graft survival in recipients requiring indication renal allograft biopsy. Am J Transplant 2009; 9: 2758. [DOI] [PubMed] [Google Scholar]
- 5. Hidalgo LG, Campbell PM, Sis B, et al. De novo donor-specific antibody at the time of kidney transplant biopsy associates with microvascular pathology and late graft failure. Am J Transplant 2009; 9: 2532. [DOI] [PubMed] [Google Scholar]
- 6. Kedainis RL, Koch MJ, Brennan DC, et al. Focal C4d+ in renal allografts is associated with the presence of donor-specific antibodies and decreased allograft survival. Am J Transplant 2009; 9: 812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Einecke G, Sis B, Reeve J, et al. Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant 2009; 9: 2520. [DOI] [PubMed] [Google Scholar]
- 8. Mao Q, Terasaki PI, Cai J, et al. Analysis of HLA class I specific antibodies in patients with failed allografts. Transplantation 2007; 83: 54. [DOI] [PubMed] [Google Scholar]
- 9. Mulay AV, Cockfield S, Stryker R, et al. Conversion from calcineurin inhibitors to sirolimus for chronic renal allograft dysfunction: a systematic review of the evidence. Transplantation 2006; 82: 1153. [DOI] [PubMed] [Google Scholar]
- 10. Ekberg H, Bernasconi C, Tedesco-Silva H, et al. Calcineurin inhibitor minimization in the Symphony study: observational results 3 years after transplantation. Am J Transplant 2009; 9: 1876. [DOI] [PubMed] [Google Scholar]
- 11. Troppmann C, Gruessner RW, Matas AJ, et al. Results with renal transplants performed after previous solitary pancreas transplants. Transplant Proc 1994; 26: 448. [PubMed] [Google Scholar]
- 12. Mannon RB, Matas AJ, Grande J, et al. Inflammation in areas of tubular atrophy in kidney allograft biopsies: a potent predictor of allograft failure. Am J Transplant 2010; 10: 2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matas AJ. Minimization of steroids in kidney transplantation. Transpl Int 2009; 22: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Woodle ES, First MR, Pirsch J, et al. A prospective, randomized, double-blind, placebo-controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann Surg 2008; 248: 564. [DOI] [PubMed] [Google Scholar]
- 15. Knight SR, Morris PJ. Steroid avoidance or withdrawal after renal transplantation increases the risk of acute rejection but decreases cardiovascular risk. A meta-analysis. Transplantation 2010; 89: 1. [DOI] [PubMed] [Google Scholar]
- 16. Matas AJ, Granger D, Kaufman DB, et al. Steroid minimization for sirolimus-treated renal transplant recipients. Clin Transplant 2011; 25: 457. [DOI] [PubMed] [Google Scholar]
- 17. Srivastava A, Muruganandham K, Vinodh PB, et al. Post-renal transplant surgical complications with newer immunosuppressive drugs: mycophenolate mofetil vs. m-TOR inhibitors. Int Urol Nephrol 2010; 42: 279. [DOI] [PubMed] [Google Scholar]
- 18. Miles CD, Skorupa JY, Sandoz JP, et al. Albuminuria after renal transplantation: maintenance with sirolimus/low-dose tacrolimus vs. mycophenolate mofetil/high-dose tacrolimus. Clin Transplant 2011; 25: 898. [DOI] [PubMed] [Google Scholar]
- 19. Pengel LH, Liu LQ, Morris PJ. Do wound complications or lymphoceles occur more often in solid organ transplant recipients on mTOR inhibitors? A systematic review of randomized controlled trials. Transpl Int 2011; 24: 1216. [DOI] [PubMed] [Google Scholar]
- 20. Nashan B, Citterio F. Wound healing complications and the use of mammalian target of rapamycin inhibitors in kidney transplantation: a critical review of the literature. Transplantation 2012; 94: 547. [DOI] [PubMed] [Google Scholar]
- 21. Lebranchu Y, Thierry A, Toupance O, et al. Efficacy on renal function of early conversion from cyclosporine to sirolimus 3 months after renal transplantation: concept study. Am J Transplant 2009; 9: 1115. [DOI] [PubMed] [Google Scholar]
- 22. Salgo R, Gossmann J, Schofer H, et al. Switch to a sirolimus-based immunosuppression in long-term renal transplant recipients: reduced rate of (pre-)malignancies and nonmelanoma skin cancer in a prospective, randomized, assessor-blinded, controlled clinical trial. Am J Transplant 2010; 10: 1385. [DOI] [PubMed] [Google Scholar]
- 23. Bunnapradist S, Vincenti F. Transplantation: to convert or not to convert: lessons from the CONVERT trial. Nat Rev Nephrol 2009; 5: 371. [DOI] [PubMed] [Google Scholar]
- 24. Brennan DC, Daller JA, Lake KD, et al. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med 2006; 355: 1967. [DOI] [PubMed] [Google Scholar]
- 25. Matas AJ, Kandaswamy R, Humar A, et al. Long-term immunosuppression, without maintenance prednisone, after kidney transplantation. Ann Surg 2004; 240: 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kandaswamy R, Melancon JK, Dunn T, et al. A prospective randomized trial of steroid-free maintenance regimens in kidney transplant recipients—an interim analysis. Am J Transplant 2005; 5: 1529. [DOI] [PubMed] [Google Scholar]
- 27. Suszynski TM, Gillingham KJ, Rizzari MD, et al. Prospective randomized trial of maintenance immunosuppression with rapid discontinuation of prednisone in adult kidney transplantation. Am J Transplant 2013; 13: 961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Preville X, Flacher M, LeMauff B, et al. Mechanisms involved in antithymocyte globulin immunosuppressive activity in a nonhuman primate model. Transplantation 2001; 71: 460. [DOI] [PubMed] [Google Scholar]
- 29. Goggins WC, Pascual MA, Powelson JA, et al. A prospective, randomized, clinical trial of intraoperative versus postoperative Thymoglobulin in adult cadaveric renal transplant recipients. Transplantation 2003; 76: 798. [DOI] [PubMed] [Google Scholar]
- 30. Kaden J, May G, Volp A, et al. Improved long-term survival after intra-operative single high-dose ATG-Fresenius induction in renal transplantation: a single centre experience. Ann Transplant 2009; 14: 7. [PubMed] [Google Scholar]
- 31. Kyllonen LE, Eklund BH, Pesonen EJ, et al. Single bolus antithymocyte globulin versus basiliximab induction in kidney transplantation with cyclosporine triple immunosuppression: efficacy and safety. Transplantation 2007; 84: 75. [DOI] [PubMed] [Google Scholar]
- 32. Shapiro R, Basu A, Tan H, et al. Kidney transplantation under minimal immunosuppression after pretransplant lymphoid depletion with Thymoglobulin or Campath. J Am Coll Surg 2005; 200: 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Starzl TE, Murase N, Abu-Elmagd K, et al. Tolerogenic immunosuppression for organ transplantation. Lancet 2003; 361: 1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gore SM. Graft survival after renal transplantation: agenda for analysis. Kidney Int 1983; 24: 516. [DOI] [PubMed] [Google Scholar]
- 35. Winer BJ, Brown DR, Michels KM. Statistical principles in experimental design. 3rd ed New York: McGraw-Hill; 1991: xiii. [Google Scholar]
- 36. Stevens RB, Mercer DF, Grant WJ, et al. Randomized trial of single-dose versus divided-dose rabbit anti-thymocyte globulin induction in renal transplantation: an interim report. Transplantation 2008; 85: 1391. [DOI] [PubMed] [Google Scholar]
- 37. Metchnikoff E. Recherches sur l’influence de l’organisme sur les toxins: toxine tetanique et leukocytes. Ann Inst Pasteur 1898; 12: 263. [Google Scholar]
- 38. Woodruff M, Forman B. Effect of antilymphocytic serum on suspensions of lymphocytes in vitro. Nature 1951; 168: 35. [DOI] [PubMed] [Google Scholar]
- 39. Monaco AP, Abbott WM, Othersen HB, et al. Antiserum to lymphocytes: prolonged survival of canine renal allografts. Science 1966; 153: 1264. [DOI] [PubMed] [Google Scholar]
- 40. Najarian JS, Simmons RL, Gewurz H, et al. Anti-serum to cultured human lymphoblasts: preparation, purification and immunosuppressive properties in man. Ann Surg 1969; 170: 617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Starzl TE, Marchioro TL, Porter KA, et al. The use of heterologous antilymphoid agents in canine renal and liver homotransplantation and in human renal homotransplantation. Surg Gynecol Obstet 1967; 124: 301. [PMC free article] [PubMed] [Google Scholar]
- 42. Kaden J, Strobelt V, May G. Short and long-term results after pretransplant high-dose single ATG-fresenius bolus in cadaveric kidney transplantation. Transplant Proc 1998; 30: 4011. [DOI] [PubMed] [Google Scholar]
- 43. Shapiro R, Basu A, Tan H, et al. Kidney transplantation under minimal immunosuppression after pretransplant lymphoid depletion with Thymoglobulin or Campath. J Am Coll Surg 2005; 200: 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stevens RB, Lane JT, Boerner BP, et al. Single-dose rATG induction at renal transplantation: superior renal function and glucoregulation with less hypomagnesemia. Clin Transplant 2012; 26: 123. [DOI] [PubMed] [Google Scholar]
- 45. Nankivell BJ, Borrows RJ, Fung CL, et al. The natural history of chronic allograft nephropathy. N Engl J Med 2003; 349: 2326. [DOI] [PubMed] [Google Scholar]
- 46. Nankivell BJ, Borrows RJ, Fung CL, et al. Calcineurin inhibitor nephrotoxicity: longitudinal assessment by protocol histology. Transplantation 2004; 78: 557. [DOI] [PubMed] [Google Scholar]
- 47. Ducloux D, Courivaud C, Bamoulid J, et al. Prolonged CD4 T cell lymphopenia increases morbidity and mortality after renal transplantation. J Am Soc Nephrol 2010; 21: 868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schooley RT, Hirsch MS, Colvin RB, et al. Association of herpesvirus infections with T-lymphocyte-subset alterations, glomerulopathy, and opportunistic infections after renal transplantation. N Engl J Med 1983; 308: 307. [DOI] [PubMed] [Google Scholar]
- 49. Ducloux D, Challier B, Saas P, et al. CD4 cell lymphopenia and atherosclerosis in renal transplant recipients. J Am Soc Nephrol 2003; 14: 767. [DOI] [PubMed] [Google Scholar]
- 50. Tse K, Tse H, Sidney J, et al. T cells in atherosclerosis. Int Immunol 2013; 25: 615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Aukrust P, Yndestad A, Sandberg WJ, et al. T cells in coronary artery disease: different effects of different T-cell subsets. J Am Coll Cardiol 2007; 50: 1459. [DOI] [PubMed] [Google Scholar]
- 52. Sureshkumar KK, Thai NL, Hussain SM, et al. Influence of induction modality on the outcome of deceased donor kidney transplant recipients discharged on steroid-free maintenance immunosuppression. Transplantation 2012; 93: 799. [DOI] [PubMed] [Google Scholar]
- 53. Hanaway MJ, Woodle ES, Mulgaonkar S, et al. Alemtuzumab induction in renal transplantation. N Engl J Med 2011; 364: 1909. [DOI] [PubMed] [Google Scholar]
- 54. LaCorcia G, Swistak M, Lawendowski C, et al. Polyclonal rabbit antithymocyte globulin exhibits consistent immunosuppressive capabilities beyond cell depletion. Transplantation 2009; 87: 966. [DOI] [PubMed] [Google Scholar]
- 55. Mohty M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia 2007; 21: 1387. [DOI] [PubMed] [Google Scholar]
- 56. Mehrabi A, Mood Zh A, Sadeghi M, et al. Thymoglobulin and ischemia reperfusion injury in kidney and liver transplantation. Nephrol Dial Transplant 2007; 22: viii54. [DOI] [PubMed] [Google Scholar]
- 57. Bunnag S, Einecke G, Reeve J, et al. Molecular correlates of renal function in kidney transplant biopsies. J Am Soc Nephrol 2009; 20: 1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mueller TF, Reeve J, Jhangri GS, et al. The transcriptome of the implant biopsy identifies donor kidneys at increased risk of delayed graft function. Am J Transplant 2008; 8: 78. [DOI] [PubMed] [Google Scholar]
- 59. Mueller TF, Einecke G, Reeve J, et al. Microarray analysis of rejection in human kidney transplants using pathogenesis-based transcript sets. Am J Transplant 2007; 7: 2712. [DOI] [PubMed] [Google Scholar]
- 60. Gaber AO, Monaco AP, Russell JA, et al. Rabbit antithymocyte globulin (thymoglobulin): 25 years and new frontiers in solid organ transplantation and haematology. Drugs 2010; 70: 691. [DOI] [PubMed] [Google Scholar]
- 61. Mueller TF. Mechanisms of action of thymoglobulin. Transplantation 2007; 84: S5. [Google Scholar]
- 62. Urbanova M, Brabcova I, Girmanova E, et al. Differential regulation of the nuclear factor-kappaB pathway by rabbit antithymocyte globulins in kidney transplantation. Transplantation 2012; 93: 589. [DOI] [PubMed] [Google Scholar]
- 63. Sanz AB, Sanchez-Nino MD, Ramos AM, et al. NF-kappaB in renal inflammation. J Am Soc Nephrol 2010; 21: 1254. [DOI] [PubMed] [Google Scholar]
- 64. Rangan GK, Wang Y, Tay YC, et al. Inhibition of nuclear factor-kappaB activation reduces cortical tubulointerstitial injury in proteinuric rats. Kidney Int 1999; 56: 118. [DOI] [PubMed] [Google Scholar]
- 65. Stevens RB, Platt JL. The pathogenesis of hyperacute xenograft rejection. Am J Kidney Dis 1992; 20: 414. [DOI] [PubMed] [Google Scholar]
- 66. Stevens RB, Wang YL, Kaji H, et al. Administration of nonanticoagulant heparin inhibits the loss of glycosaminoglycans from xenogeneic cardiac grafts and prolongs graft survival. Transplant Proc 1993; 25 (1 Pt 1): 382. [PubMed] [Google Scholar]
- 67. Navarro FP, Fares RP, Sanchez PE, et al. Brain heparanase expression is up-regulated during postnatal development and hypoxia-induced neovascularization in adult rats. J Neurochem 2008; 105: 34. [DOI] [PubMed] [Google Scholar]
- 68. Chappell D, Jacob M, Rehm M, et al. Heparinase selectively sheds heparan sulphate from the endothelial glycocalyx. Biol Chem 2008; 389: 79. [DOI] [PubMed] [Google Scholar]
- 69. Miller JD, Clabaugh SE, Smith DR, et al. Interleukin-2 is present in human blood vessels and released in biologically active form by heparanase. Immunol Cell Biol 2012; 90: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Downie GH, Ryan US, Hayes BA, et al. Interleukin-2 directly increases albumin permeability of bovine and human vascular endothelium in vitro. Am J Respir Cell Mol Biol 1992; 7: 58. [DOI] [PubMed] [Google Scholar]
- 71. Li J, Gyorffy S, Lee S, et al. Effect of recombinant human interleukin 2 on neutrophil adherence to endothelial cells in vitro. Inflammation 1996; 20: 361. [DOI] [PubMed] [Google Scholar]
- 72. Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461. [DOI] [PubMed] [Google Scholar]
- 73. Solez K, Colvin RB, Racusen LC, et al. Banff ’05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’). Am J Transplant 2007; 7: 518. [DOI] [PubMed] [Google Scholar]
- 74. Nyberg SL, Matas AJ, Kremers WK, et al. Improved scoring system to assess adult donors for cadaver renal transplantation. Am J Transplant 2003; 3: 715. [DOI] [PubMed] [Google Scholar]
- 75. Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. Springer Verlag; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


