Abstract
Tumor ablation is a minimally invasive technique that is commonly used in the treatment of tumors of the liver, kidney, bone, and lung. During tumor ablation, thermal energy is used to heat or cool tissue to cytotoxic levels (less than −40°C or more than 60°C). An additional technique is being developed that targets the permeability of the cell membrane and is ostensibly nonthermal. Within the classification of tumor ablation, there are several modalities used worldwide: radiofrequency, microwave, laser, high-intensity focused ultrasound, cryoablation, and irreversible electroporation. Each technique, although similar in purpose, has specific and optimal indications. This review serves to discuss general principles and technique, reviews each modality, and discusses modality selection.
Keywords: Microwave, radiofrequency, cryoablation, laser, HIFU, IRE
Introduction
Tumor ablation is a minimally invasive technique that is commonly used in the treatment of tumors of the liver, kidney, bone, and lung. It is an important option for people who have failed chemotherapy or radiotherapy or are not surgical candidates. Ablation is also being considered a potential first-line treatment in many patients with small hepatocellular carcinomas or benign tumors in the liver.
Most ablation systems comprise a generator and a needlelike device that delivers the energy directly to the targeted tissue to cause acute cellular necrosis. Radio-frequency (RF), microwave (MW), laser, and high-intensity focused ultrasound (HIFU) systems apply energy to heat the tissue to at least 60 C for maximum efficacy.1 Cryoablation systems cool the tissue to less than −40°C to cause tissue necrosis. Irreversible electroporation (IRE) is an ostensibly nonthermal technique. Targeted tissues can be accessed percutaneously, laparoscopically, through a celiotomy incision, or endoscopically. A notable exception is HIFU, which can be completed with a specialized ultrasound probe extracorporeally. Thus, tumor ablation is largely a minimally invasive technique but can also be useful as an adjuvant to surgery.
Each technique, although similar in purpose, has specific and optimal indications. This review serves to discuss general ablation principles, commonly encountered ablation modalities, tissue-ablation interactions, modality selection, and general ablation techniques.
General Ablation Principles
As previously stated, thermal ablation is completed by heating or cooling the targeted tissue to cytotoxic levels. Generally, cytotoxic temperature less than −40°C or more than 60°C cause complete necrosis in most tissues, although temperature sensitivity can vary based on cell type.1 Tumor cells are generally more sensitive to heating than normal cells owing to variations in sensitivity to tissue hypoxia and pH.1–3 Temperatures slightly more than −40°C or less than 60°C can also cause tissue destruction, but longer treatment times are required.
Hyperthermic ablation aims to cause acute coagulative necrosis within the targeted tissue. At temperatures up to 41°C, blood vessels dilate, blood flow increases, and the heat shock response is initiated.1 The heat shock response is a process of rapid gene expression that aims to combat the thermally induced damage. This includes the production of heat shock proteins, which may confer increased thermal resistance in tissues that survive initial thermal damage.4 From 42°C–46°C, irreversible damage occurs, and after 10 minutes, significant necrosis occurs. From 46°C–52°C, the time to cell death decreases owing to a combination of microvascular thrombosis, ischemia, and hypoxia. At high enough temperatures (>60°C), proteins denature and the plasma membrane melts so that cell death is nearly instantaneous.
Hypothermic ablations destroy cells with temperatures less than −40°C through ice crystal formation and osmotic shock. As tissue is cooled, cellular metabolism breaks down. As the temperature further decreases, ice begins to form outside of the cell leading to a hyperosmotic extracellular space, which causes outflow of intracellular fluid and cell dehydration. Upon thawing, there is a reversal of the osmotic gradient, which causes influx of extracellular fluid into the cell leading to cell swelling and membrane rupture.5 If there is rapid cooling, ice crystals can form within the cell and, owing to negative thermal expansion, expand the cell, causing irreversible cell membrane damage. During cryoablation, the cells closest to the cryoprobe experience rapid cooling and intracellular ice crystal formation, whereas the more peripheral cells cool slower and are susceptible to cell death from osmotic shock, as previously described.
IRE is an ostensibly nonthermal technique. Its main mechanism of action uses a strong electrical current to form permanent nanopores within the cell membrane. These small nanopores then induce cell apoptosis, programmed cell death.6 Short pulses of electric current are administered to the target tissue at brief intervals, which has been reported to eliminate tissue heating and any thermally induced effects.6,7
Ablation Modalities
RF
RF ablations create a simple electrical circuit through the body, using an oscillating electrical current to produce resistive heating within the tissues surrounding an interstitial electrode. Because tissues are poor conductors of electricity, current flowing through tissues leads to ionic agitation and production of frictional heat. Therefore, the areas closest to the electrode experience the highest current and thus, a greater rise in temperature. Tissues farther away from the electrode are heated primarily by thermal conduction.8 The circuit is completed by a dispersive electrode typically placed on the skin of the patient in a monopolar system or by a second interstitial electrode in the case of bipolar RF ablation.
Most currently available RF systems operate with monopolar electrodes. Monopolar electrodes come in 3 general varieties: straight needlelike, multitined, or multitined expandable. Expandable multitined and non-deployable multitined electrodes increase the electrode-tissue contact surface area and disperse the current over a greater volume, which can also increase the ablation zone size. Bipolar systems employ 2 interstitial electrodes between which current oscillates. The 2 electrodes are either within the same applicator or on separate applicators. Current flow is restricted between the 2 electrodes which limits cooling from heat sinks and increases heating in tissues between the electrodes.
Although RF ablation has been shown to be clinically effective against small tumors (<2 cm), it is hampered by difficulties in heating physics.9,10 As tissues become dehydrated and charred, or water vapor is generated near 100°C, the electrical impedance of the tissue increases rapidly, effectively limiting the flow of electrical current. That is, RF ablation tends to be a self-limiting process. Cooling the electrode with circulating water can decrease temperatures at the electrode-tissue interface, reducing the amount of charring and improving current flow over time.11
In addition, the RF generator output is generally controlled to counteract charring. Impedance-controlled systems either initialize power to relatively low level (20–50 W) and increase until a maximum impedance is achieved or initialize the power to a high level and suspend power output temporarily when the impedance rapidly increases. This power pulsing algorithm allows the tissue to cool and rehydrate as needed, which decreases the average impedance of the tissue and allows greater energy deposition. Power pulsing can also be sequentially performed with multiple electrodes to create multiple independent ablations or one large ablation, increasing procedural efficiency.12,13 Temperature-controlled systems aim to achieve a preset target temperature at the tip(s) of the electrode(s). Power is gradually increased until the target temperature is obtained. Power is then modulated to maintain the target temperature for the duration the ablation, typically 10–45 minutes.
MW
MW ablation uses dielectric hysteresis to produce heat in tissue. When electromagnetic energy (MW range: 300 MHz–300 GHz) is applied to a tissue, polar molecules such as water continuously attempt to align with the oscillating electromagnetic field. The inability of such molecules to keep up with the rapidly oscillating field leads to energy absorption and tissue heating. Thus, tissues that have high water content (eg, liver and kidney) are heated most readily during MW ablation. Unlike RF ablation, MW energy is not an electrical current but rather a propagating electromagnetic field. This makes MW ablation useful in tissues with poor electrical conductance, such bone, lung, and ablated tissue. Additionally, because MW fields can overlap in tissue, multiple applicators can be used simultaneously to create larger ablations (Fig. 1).
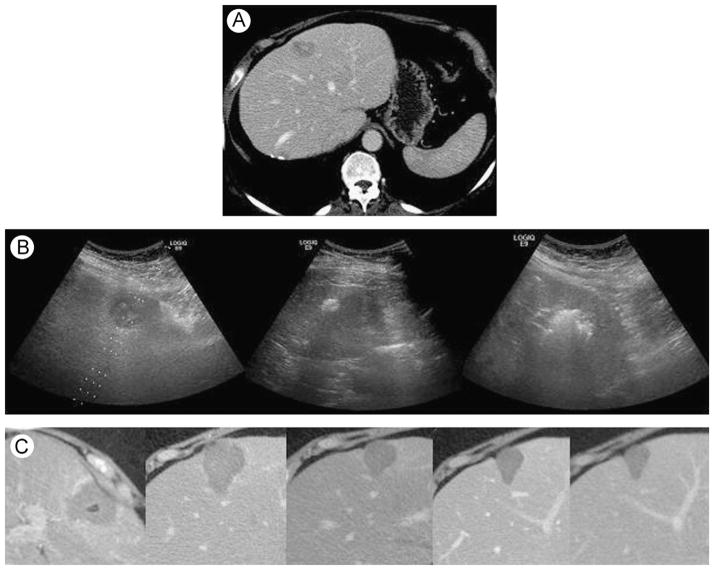
Figure 1.
Microwave ablation for metastatic disease. Two gas-cooled microwave antennas were used. (A) Preablation CT. (B) Ultrasound images during procedure: before, early, and final. (C) Postablation CT images: day 0, 1 month, 6 months, and 12 months. CT, computed tomography.
Most ablation antennas use a straight needlelike design and operate at a frequency of either 915 MHz or 2.45 GHz. Although some studies have concluded that one frequency is preferable to another, some of those studies have been flawed in design and data analysis; specifically, some conclusions are based on unequal power deposition into the tissue. A survey of the literature and more controlled studies reveal that both 915 MHz and 2.45 GHz systems are capable of creating large, clinically useful ablation zones, but that not all systems are equal.14–21 In addition, there is also evidence that tissue contraction may cause underestimation of the extent of tissue damage, especially with MW systems.22
As with other thermal ablation techniques, MW heating is susceptible to heat sink from adjacent vessels. However, because MW energy heats tissues so effectively, it is not as susceptible as other thermal techniques. Especially for new users, the increased power offered by MW energy should be used with a measure of caution, as unintended consequences such as skin burn have been reported.23 Thus, the antenna shaft must be cooled to prevent tissue damage from the skin entry site to the target ablation zone. Two shaft-cooling options are available on today’s MW devices: (1) water cooling, as also seen with some RF devices and (2) gas cooling with CO2.24 MW ablations should also be monitored diligently as they progress to avoid ablating adjacent structures, as the ablation zone can grow quickly.
A growing body of literature describing the efficacy and safety of MW ablation in ex vivo and in vivo studies, with an increasing number of clinical studies, has developed.25–28 However, long-term data are still lacking, especially with the newer higher power systems. As with any new technique or device, the lack of experience may negatively affect initially the short-term results until the operator becomes proficient with the new device. Study design, power-delivery settings, experimental model, and data analysis techniques should also be interpreted carefully when evaluating a MW ablation system for clinical use.
Laser
Interstitial laser ablation is an additional hyperthermic ablation technique. The devices and systems used for laser tumor ablation are similar to those used for other clinical laser treatments. Light generated by neodymium:yttrium aluminum garnet lasers (wavelength of 1064 nm) are applied to the target tissue using a fiberoptic applicator. External cooling may be used if the applied power is sufficient to cause applicator heating. Applicators with a scattering or diffusing tip are often used to increase the volume of ablation. The light energy is then scattered and absorbed by the tissue and converted into heat.29,30 As the scattering transmission of light energy decreases in charred or desiccated tissue, laser ablation is hampered by a similar self-limiting nature like RF ablation. Therefore, most systems employ arrays of applicators to increase the ablation zone size at the expense of increased invasiveness, greater procedural complexity, and higher monetary cost.31,32 One advantage to using laser fiber applicators is that most are magnetic resonance imaging compatible, allowing for preprocedural planning and intraprocedural treatment monitoring using a variety of temperature-sensitive techniques.29,30,33,34 Such techniques are not widely available and do increase procedural cost, however.
HIFU
HIFU uses ultrasound waves to heat quickly tissue to cytotoxic levels. HIFU is like conventional diagnostic ultrasound, only with increased intensity (720 mW/cm2 compared to 100–10,000 W/cm2).35 This high-intensity energy is focused in the region of interest leading to acoustic wave absorption causing rapid ablative heating of the target tissue. Although the mechanism of cell damage in HIFU ablation is primarily thermal, HIFU can also induce mechanical effects in the tissue. High-intensity acoustic pulses can either lead to the formation of cavitations or induce the cavitations to expand and contract. Cavitations that grow rapidly and then collapse release shock waves into the surrounding tissues causing mechanical cell injury.1,36
Four types of HIFU devices have been developed: extracorporeal, transrectal, interstitial, and percutaneous.37,38 Extracorporeal devices are used primarily for benign and more superficial tumors not encumbered by bone or air in the ultrasound window such as uterine fibroids.39,40 Transrectal devices have been evaluated for prostate cancer treatment, whereas interstitial devices are used for biliary and esophageal tumors.35 Percutaneous devices can be used for deeper lesions, although these devices remain in early development stages and are not widely available for clinical use. HIFU can also be used for targeted drug or gene therapy using DNA or drug-containing microbubbles.41
Of all the ablation modalities considered here, HIFU is the only noninvasive option. The cytotoxic heating and mechanical effects on the targeted tissues can be performed through intact skin or mucosa. This makes HIFU a very attractive option for many cancers. However, non-invasive treatment with HIFU does have several limitations. The lesions treated most effectively by HIFU are mostly superficial owing to limitations in ultrasound penetrance through many tissues. Additionally, the high-intensity ultrasound waves are subject to scatter and reflection, which can lead to injury of tissues adjacent to the targeted area, such as skin burns, damage to peripheral nerves, or bowel injury.41,42 HIFU is also limited in areas that are subject to respiration motion, owing to lack of precision, or have overlying bone, because of sonic shadowing.41 Coagulation, desiccation, and vapor formation are also detrimental to ultrasound energy propagation, so most HIFU treatments need careful planning to ensure tumor coverage.43
Cryoablation
Cryoablation destroys tumors by cooling them to cytotoxic temperatures. Most devices today use the Joule-Thomson effect—a change in temperature in response to a change in gas pressure—to create rapid cooling inside the cryoprobe. At the most distal end of the cryoprobe, there is a small chamber into which gas passes from a narrower pathway proximally. This expansion of the gas at the distal tip of the probe can lead to temperatures as low as −140°C when using argon.44 Cryoablation is commonly used in the treatment of renal cancers and metastatic osseous lesions.
One of the primary benefits of cryoablation is the high visibility of the ice ball on ultrasound, computed tomography, and magnetic resonance imaging (Fig. 2). This allows precise monitoring of treatment progress, and potentially improved precision in tissues near sensitive or critical structures. However, the lethal isotherm actually lies inside of the visualized ice ball. In a recent study by Georgiades et al, the lethal isotherm was placed at 1.15 ± 0.51 mm inside of the margin of visualized ice ball in swine renal tissue, but this may be dependent on tissue type and blood flow conditions.45,46 Healing after cryoablation also appears to be faster and more complete than after hyperthermic ablation. However, cryoablation has been associated with potentially severe systemic reactions (cryoshock), relatively small ablations compared with more contemporary technologies, and potentially greater risk for bleeding complications due to the lack of coagulation during the cryoablation procedure.47–49 For these reasons, cryoablation has found limited utility in treating hepatocellular carcinomas owing to common comorbidities such as cirrhosis, poor liver function, and clotting disorders. Cryoablation is more commonly used in the treatment of renal masses, metastatic tumors in the liver, and bone lesions and is of increasing interest for lung and breast tumors as well.
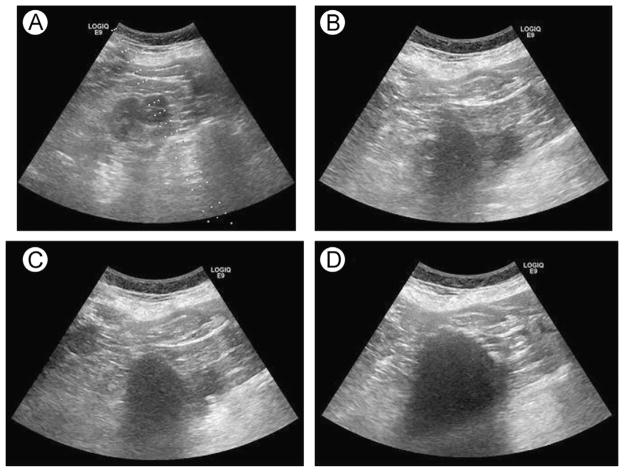
Figure 2.
Ultrasound images from a cryoablation of a renal cell carcinoma. (A) Preablation, (B) early ablation, (C) midablation, and (D) final ablation.
IRE
IRE is an ostensibly nonthermal ablation modality.50 IRE uses pulses of electrical current (up to 3 kV/cm) transmitted through straight needlelike electrodes that last several microseconds to milliseconds to cause irreversible damage to the cell membrane and initiation of apoptosis. As IRE treatments consist of pulses that last several microseconds to milliseconds, extreme precision must be used, as adjustments during treatments are impossible. The ablation zones created by IRE have sharp well-defined ablative margins between nonablated tissue and ablated tissue allowing precise control over the ablation zone.6,51,52
Unlike thermal ablation techniques, IRE is not susceptible to heat sink from nearby vessels; the ablation zone can be precisely controlled and the treatment time is short, several minutes or less. Additionally, the ablation zone resolves relatively rapidly as there is little scarring and damage to the underlying tissue scaffolding, such as the adjacent vessels and bile ducts.6,52 However, current IRE devices require the use of multiple applicators with precise parallel alignment to create moderate ablation sizes (2 applicators to create a 2.5-cm ablation zone).53 The increased number of applicators and precise positioning requirement negatively affects the cost, complexity, and invasiveness of IRE procedures compared with other ablation procedures. Intraprocedural imaging for IRE ablations is also quite different from that of traditional thermal ablation with a delay of up to minutes before changes correlating with the ablation zone are visible by ultrasound.54 IRE also can require general anesthesia with paralytics, as the electrical currents can cause alterations in ion transport, which can induce cardiac arrhythmias and provoke muscle contraction.55,56 Initial studies have shown moderate efficacy of IRE in preclinical and small scale, well-controlled clinical trials. However, confirmatory long-term, large sample clinical studies are still needed to confirm the safety and effectiveness of IRE.54,57–59
Tissue-Ablation Interactions
Fundamental tissue properties such as electrical conductivity, thermal conductivity, dielectric permittivity, and heat capacity as well as blood perfusion rate have substantial effect on the growth of ablation zones. Electrical conductivity is an important factor in the success of RF ablation and IRE. RF ablation relies on the flow of current through tissue to cause adequate heating to cause cell death. IRE relies on similar changes in electric charge around the cell to induce cell death. The production and flow of electrical current is therefore most affected by tissue hydration status and ion content. Thus, tissues with high water and ion contents (eg, liver) would more effectively transmit current, whereas those with lower water and ion contents (eg, lung and fat) would have higher electrical impedance. Additionally, as the ablation progresses, the tissue can become dehydrated and charred, which can increase tissue impedance to electrical current flow. MW ablation is not similarly limited by tissue conductance, as the propagation of MW energy is not dependent on electrical current. Rather, MW propagation is described by the complex permittivity of the tissue.60 Laser light penetration and ultrasound wave propagation is also limited by tissue desiccation, charring, and carbonization.29,30,61 HIFU can also be affected by suboptimal ultrasound wave transmission though low-density media such as fat and gas.
Tissue heating or cooling is affected by adjacent vasculature, as large blood vessels dissipate thermal energy. Thus, thermal ablation size and cytotoxic effectiveness decreases with the proximity and the size of adjacent vessels. Increased local recurrence rates of tumors adjacent to large vessels (>3 mm) demonstrate the significant effect of thermal energy sinks.62 MW ablations seem to be less susceptible to the effects of heat sink. Bhardwaj et al evaluated MW, RF, and cryoablations histologically and found no perivascular hepatocyte survival with MW ablation but did find perivascular hepatocyte survival within the ablated volume for cryoablation and conspicuous perivascular hepatocyte survival within the RF ablations.63 Yu et al examined MW ablations and found that the thermal injury from the ablation extended to the vessel wall for all the ablations, but they did see distortion of the perivascular margin in approximately one-third of the ablations. The extent of the heat sink effect significantly correlated with the size of the vessel.64 Multiple studies have also examined the effects of modulating hepatic perfusion and have found that the ablation size increases with decreased blood flow.8,65,66 Developing methods to either decrease blood flow or increase heating efficacy may be vital to optimizing perivascular ablation lethality.
Ablations within the lung are susceptible to unique energy-tissue interactions. Like other tissues, lung ablations are affected by heat sinks from the surrounding pulmonary vasculature. Additionally, air flow due to respiration provides a secondary heat sink. Aerated lung tissue can also act as an insulator, limiting the conductance of thermal and electric energy, and cause incomplete treatment of the tumor if not offset by procedural techniques such as increasing ablation time or power or using multiple applicators.27,67,68 MW ablation, which does not rely on electrical current conductance through tissue, has produced ablations 25% larger in mean diameter compared with RF in the lung.27
Ablation Modality Selection
Choosing the most appropriate modality is vital to the success of any ablation. The type of tissue to be ablated and the size of the lesion are 2 important factors in this decision. Generally, RF ablation is appropriate for the treatment of lesions of the liver and kidney that are less than 2 cm in diameter. Treatment efficacy has been shown to decrease for larger tumors in multiple series.10,69–72 MW ablation may be applicable to a broader spectrum of tissues, including lung, liver, kidney, and bone. Furthermore, newer generation MW systems may be more effective for larger tumors, but longer-term clinical data are needed to evaluate the role of tumor size on MW ablation efficacy. Cryoablation has historically been contraindicated for use against primary tumors of the liver, especially in patients with severe cirrhosis, but it has been used successfully against smaller (<2 cm) lesions of the lung, liver, breast, prostate, and bone.49,73,74
IRE may theoretically be an interesting option for perivascular tumors owing to its nonthermal technique.7 The noninvasive, high-precision nature of HIFU is attractive in stationary or superficial regions, such as the prostate or uterus, but so far has had limited applicability in other organs. Finally, although the clinical data supporting RF, MW, laser, and cryoablation are relatively robust for many indications, further clinical data are needed to support the use of IRE and HIFU in human subjects.
Ablation Procedure Techniques
In additional to appropriate modality choice, certain techniques can be used to optimize ablation results. The first is the use of multiple applicators to increase the ablation size.32,46,75,76 The simultaneous use of multiple applicators has been shown to result in thermal synergy between the applicators, as the cumulative effect of the overlapping ablations zones leads to increased temperatures.12 Additionally, tissue perfusion–mediated effects are decreased as the adjacent tissues coagulate and become ischemic.12,77,78 Using multiple applicators can also increase the ease of applicator placement, as the placement of subsequent applicators can be adjusted based on the placement of the prior applicators to ensure that the targeted tissue is encompassed within the ablation zone (Fig. 3).
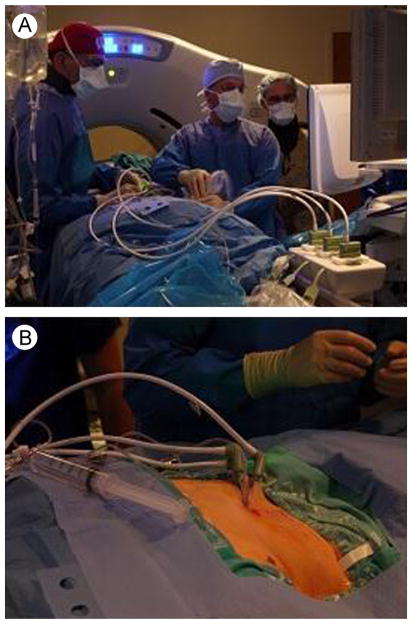
Figure 3.
Images from within the ablation suite. (A) Operator working together with ultrasound technologist. (B) Insertion of 3 gas-cooled microwave antennas. Hydrodissection was performed before ablation. (Color version of figure is available online.)
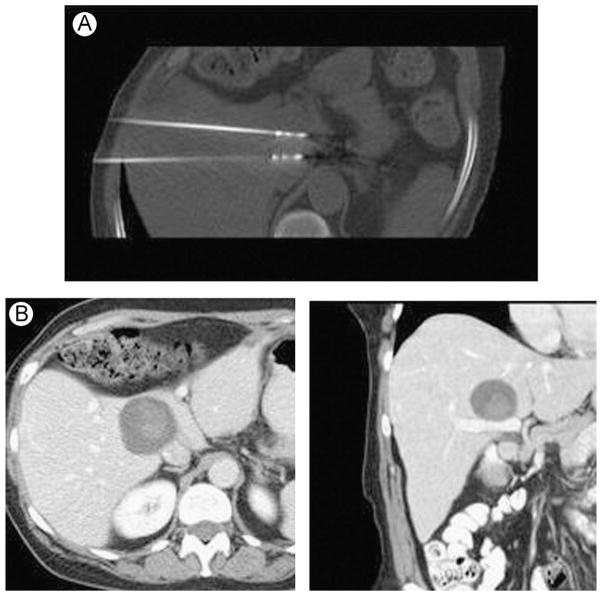
If multiple applicators are to be used, the spacing between those applicators can be optimized to avoid clefts within the ablation zone and to obtain the maximal ablation size. In general, spacing between applicators should not exceed 2 cm to avoid incomplete ablation of the intervening tissue. The geometry of the tumor to be treated is also an important factor to take into account during the preablation planning. The size and shape of the tumor would dictate the number of probes and the orientation of the probes needed to achieve an appropriate ablative margin (0.5–1.0 cm).79–81 Ablation zone patterns can vary in size and shape (from oblong to spherical) depending on the device, applicator, and tissue interactions, so a solid understanding of each device is necessary to optimize each treatment (Fig. 4). Specifically, RF ablation electrodes have historically been placed less than 2.0 cm apart when in a triangular array, and MW antennas have been placed up to 1.5 cm apart in a linear array and 2.0 cm in a triangular array.75,82 Perhaps counterintuitively, placing MW antennas in a linear array can still produce spherical ablations zones owing to the combination of electromagnetic and thermal effects (Fig. 5). Cryoprobes should be placed less than 2.0 cm apart as distances of 2.0 cm allow for nonlethal cooling to occur at the center of the ablations.46,83 There is mounting evidence that smaller interantenna spacing may increase ablation zone confluence without substantially affecting overall ablation zone size.84
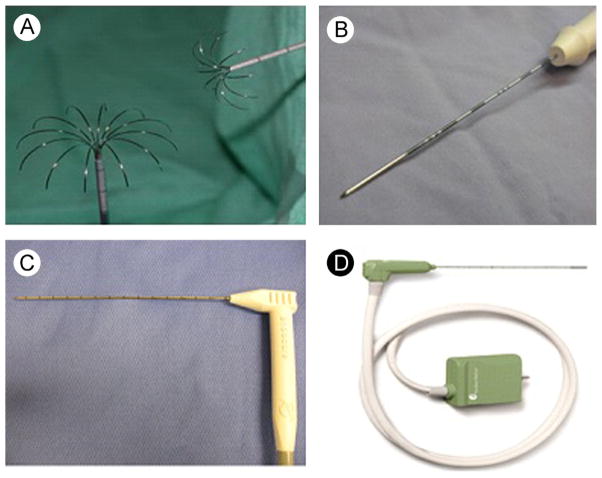
Figure 4.
Thermal ablation applicators. (A) Deployable RF electrode, (B) single water-cooled RF electrode, (C) cryoprobe, and (D) gas-cooled microwave antenna. (Color version of figure is available online.)
Figure 5.
Microwave ablation with 2 antennas in a linear array. (A) Intraprocedural CT image; (B) CT images from 6-month follow-up. CT, computed tomography.
Conclusion
Tumor ablation is an important technique in the treatment of a variety of tumors. Its minimally invasive nature and technical success has increased its clinical relevance and has made it an accepted oncologic treatment modality. In general, we recommend that both a hypothermic and hyperthermic device be available to have widespread treatment abilities. A user’s choice in system would be determined by the most common clinical indications that need to be treated. If only small liver tumors are to be treated, RF or laser would likely be sufficient. MW systems can adequately treat tumors that RF currently covers plus tumors in tissue of high impedance (lung and bone), high perfusion (kidney), or larger tumors. IRE offers a nonthermal alternative, whereas HIFU presents a noninvasive ablation option, but with limited clinical data to support their use to date. For nearly every modality, some degree of additional study is needed to verify treatment efficacy and provide appropriate treatment guidelines for human subjects.
References
- 1.Nikfarjam M, Muralidharan V, Christophi C. Mechanisms of focal heat destruction of liver tumors. J Surg Res. 2005:208–223. doi: 10.1016/j.jss.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Vanagas T, Gulbinas A, Pundzius J, et al. Radiofrequency ablation of liver tumors (I): Biological background. Medicina (Kaunas) 2010:13–17. [PubMed] [Google Scholar]
- 3.Overgaard J. Influence of extracellular pH on the viability and morphology of tumor cells exposed to hyperthermia. J Natl Cancer Inst. 1976;56:1243–1250. doi: 10.1093/jnci/56.6.1243. [DOI] [PubMed] [Google Scholar]
- 4.Richter K, Haslbeck M, Buchner J. The heat shock response: Life on the verge of death. Mol Cell. 2010:253–266. doi: 10.1016/j.molcel.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998:171–186. doi: 10.1006/cryo.1998.2115. [DOI] [PubMed] [Google Scholar]
- 6.Lee EW, Thai S, Kee ST. Irreversible electroporation: A novel image-guided cancer therapy. Gut Liver. 2010;4(suppl 1):S99–S104. doi: 10.5009/gnl.2010.4.S1.S99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davalos RV, Mir IL, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng. 2005;33:223–231. doi: 10.1007/s10439-005-8981-8. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed M, Brace CL, Lee FT, Jr, et al. Principles of and advances in percutaneous ablation. Radiology. 2011;2011:351–369. doi: 10.1148/radiol.10081634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82–89. doi: 10.1002/hep.21933. [DOI] [PubMed] [Google Scholar]
- 10.Gervais DA, McGovern FJ, Arellano RS, et al. Radiofrequency ablation of renal cell carcinoma: Part 1, Indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. Am J Roentgenol. 2005;185:64–71. doi: 10.2214/ajr.185.1.01850064. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg SN, Gazelle GS, Solbiati L, et al. Radiofrequency tissue ablation: Increased lesion diameter with a perfusion electrode. Acad Radiol. 1996;3:636–644. doi: 10.1016/s1076-6332(96)80188-7. [DOI] [PubMed] [Google Scholar]
- 12.Brace CL, Sampson LA, Hinshaw JL, et al. Radiofrequency ablation: Simultaneous application of multiple electrodes via switching creates larger, more confluent ablations than sequential application in a large animal model. J Vasc Interv Radiol. 2009;20:118–124. doi: 10.1016/j.jvir.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JM, Han JK, Kim HC, et al. Multiple-electrode radiofrequency ablation of in vivo porcine liver: Comparative studies of consecutive monopolar, switching monopolar versus multipolar modes. Invest Radiol. 2007;42:676–683. doi: 10.1097/RLI.0b013e3180661aad. [DOI] [PubMed] [Google Scholar]
- 14.Lubner MG, Brace CL, Hinshaw JL, et al. Microwave tumor ablation: Mechanism of action, clinical results, and devices. J Vasc Interv Radiol. 2010;21(suppl 8):S192–S203. doi: 10.1016/j.jvir.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu NC, Lu DS, Raman SS, et al. Hepatocellular carcinoma: Microwave ablation with multiple straight and loop antenna clusters—Pilot comparison with pathologic findings. Radiology. 2006;239:269–275. doi: 10.1148/radiol.2383041592. [DOI] [PubMed] [Google Scholar]
- 16.Chiang J, Wang P, Brace CL. Computational modelling of microwave tumour ablations. Int J Hyperthermia. 2013;29:308–317. doi: 10.3109/02656736.2013.799295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strickland AD, Clegg PJ, Cronin NJ, et al. Experimental study of large-volume microwave ablation in the liver. Br J Surg. 2002;89:1003–1007. doi: 10.1046/j.1365-2168.2002.02155.x. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Cheng Z, Dong L, et al. Comparison of temperature curve and ablation zone between 915- and 2450-MHz cooled-shaft microwave antenna: Results in ex vivo porcine livers. Eur J Radiol. 2012;81:553–557. doi: 10.1016/j.ejrad.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Hines-Peralta AU, Pirani N, Clegg P, et al. Microwave ablation: Results with a 2.45-GHz applicator in ex vivo bovine and in vivo porcine liver. Radiology. 2006;239:94–102. doi: 10.1148/radiol.2383050262. [DOI] [PubMed] [Google Scholar]
- 20.Brace CL, Laeseke PF, Sampson LA, et al. Microwave ablation with a single small-gauge triaxial antenna: In vivo porcine liver model. Radiology. 2007;242:435–440. doi: 10.1148/radiol.2422051411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castle SM, Salas N, Leveillee RJ. Initial experience using microwave ablation therapy for renal tumor treatment: 18-month follow-up. Urology. 2011;77:792–797. doi: 10.1016/j.urology.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 22.Brace CL, Diaz TA, Hinshaw JL, et al. Tissue contraction caused by radiofrequency and microwave ablation: A laboratory study in liver and lung. J Vasc Interv Radiol. 2010:1280–1286. doi: 10.1016/j.jvir.2010.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolf FJ, Grand DJ, Machan JT, et al. Microwave Ablation of lung malignancies: Effectiveness, CT findings, and safety in 50 patients. Radiology. 2008;247:871–879. doi: 10.1148/radiol.2473070996. [DOI] [PubMed] [Google Scholar]
- 24.Knavel EM, Hinshaw JL, Lubner MG, et al. High-powered gas-cooled microwave ablation: Shaft cooling creates an effective stick function without altering the ablation zone. Am J Roentgenol. 2012;198:W260–W265. doi: 10.2214/AJR.11.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brace CL. Microwave ablation technology: What every user should know. Curr Probl Diagn Radiol. 2009;38:61–67. doi: 10.1067/j.cpradiol.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu J, Liang P, Yu XL, et al. US-guided percutaneous microwave ablation of renal cell carcinoma: Intermediate-term results. Radiology. 2012;263:900–908. doi: 10.1148/radiol.12111209. [DOI] [PubMed] [Google Scholar]
- 27.Durick NA, Laeseke PF, Broderick LS, et al. Microwave ablation with triaxial antennas tuned for lung: Results in an in vivo porcine model. Radiology. 2008;247:80–87. doi: 10.1148/radiol.2471062123. [DOI] [PubMed] [Google Scholar]
- 28.Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: What are the differences? Curr Probl Diagn Radiol. 2009;38:135–143. doi: 10.1067/j.cpradiol.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gough-Palmer AL, Gedroyc WM. Laser ablation of hepatocellular carcinoma—A review. World J Gastroenterol. 2008;14:7170–7174. doi: 10.3748/wjg.14.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pacella CM, Francica G, Di Costanzo GG. Laser ablation for small hepatocellular carcinoma. Radiol Res Pract. 2011;2011:595627. doi: 10.1155/2011/595627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veenendaal LM, de Jager A, Stapper G, et al. Multiple fiber laser-induced thermotherapy for ablation of large intrahepatic tumors. Photomed Laser Surg. 2006;24:3–9. doi: 10.1089/pho.2006.24.3. [DOI] [PubMed] [Google Scholar]
- 32.Steger AC, Lees WR, Shorvon P, et al. Multiple-fibre low-power interstitial laser hyperthermia: Studies in the normal liver. Br J Surg. 1992;79:139–145. doi: 10.1002/bjs.1800790215. [DOI] [PubMed] [Google Scholar]
- 33.Kickhefel A, Rosenberg C, Weiss CR, et al. Clinical evaluation of MR temperature monitoring of laser-induced thermotherapy in human liver using the proton-resonance-frequency method and predictive models of cell death. J Magn Reson Imaging. 2011;33:704–712. doi: 10.1002/jmri.22499. [DOI] [PubMed] [Google Scholar]
- 34.Stollberger R, Ascher PW, Huber D, et al. Temperature monitoring of interstitial thermal tissue coagulation using MR phase images. J Magn Reson Imaging. 1998;8:188–196. doi: 10.1002/jmri.1880080132. [DOI] [PubMed] [Google Scholar]
- 35.Zhou YF. High intensity focused ultrasound in clinical tumor ablation. World J Clin Oncol. 2011;2:8–27. doi: 10.5306/wjco.v2.i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tezel A, Mitragotri S. Interactions of inertial cavitation bubbles with stratum corneum lipid bilayers during low-frequency sonophoresis. Biophys J. 2003;85:3502–3512. doi: 10.1016/S0006-3495(03)74770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deardorff DL, Diederich CJ. Axial control of thermal coagulation using a multi-element interstitial ultrasound applicator with internal cooling. IEEE Trans Ultrason Ferroelectr Freq Control. 2000;47:170–178. doi: 10.1109/58.818759. [DOI] [PubMed] [Google Scholar]
- 38.Kinsey AM, Tyreus PD, Rieke V, et al. Interstitial ultrasound applicators with dynamic angular control for thermal ablation of tumors under MR-guidance. Conf Proc IEEE Eng Med Biol Soc. 2004;4:2496–2499. doi: 10.1109/IEMBS.2004.1403719. [DOI] [PubMed] [Google Scholar]
- 39.Ren X-L, Zhou X-D, Yan R-L, et al. Sonographically guided extracorporeal ablation of uterine fibroids with high-intensity focused ultrasound: Midterm results. J Ultrasound Med. 2009;28:100–103. doi: 10.7863/jum.2009.28.1.100. [DOI] [PubMed] [Google Scholar]
- 40.Taran FA, Tempany CM, Regan L, et al. Magnetic resonance-guided focused ultrasound (MRgFUS) compared with abdominal hysterectomy for treatment of uterine leiomyomas. Ultrasound Obstet Gynecol. 2009;34:572–578. doi: 10.1002/uog.7435. [DOI] [PubMed] [Google Scholar]
- 41.Kim YS, Rhim H, Choi MJ, et al. High-intensity focused ultrasound therapy: An overview for radiologists. Korean J Radiol. 2008;9:291–302. doi: 10.3348/kjr.2008.9.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li JJ, Xu GL, Gu MF, et al. Complications of high intensity focused ultrasound in patients with recurrent and metastatic abdominal tumors. World J Gastroenterol. 2007;13:2747–2751. doi: 10.3748/wjg.v13.i19.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roberts WW, Hall TL, Ives K, et al. Pulsed cavitational ultrasound: A noninvasive technology for controlled tissue ablation (histotripsy) in the rabbit kidney. J Urol. 2006;175:734–738. doi: 10.1016/S0022-5347(05)00141-2. [DOI] [PubMed] [Google Scholar]
- 44.Kim C, O’Rourke AP, Mahvi DM, et al. Finite-element analysis of ex vivo and in vivo hepatic cryoablation. IEEE Trans Biomed Eng. 2007;54:1177–1185. doi: 10.1109/TBME.2006.889775. [DOI] [PubMed] [Google Scholar]
- 45.Georgiades C, Rodriguez R, Azene E, et al. Determination of the nonlethal margin inside the visible “ice-ball” during percutaneous cryoablation of renal tissue. Cardiovasc Intervent Radiol. 2013;36:783–790. doi: 10.1007/s00270-012-0470-5. [DOI] [PubMed] [Google Scholar]
- 46.Littrup PJ, Jallad B, Vorugu V, et al. Lethal isotherms of cryoablation in a phantom study: Effects of heat load, probe size, and number. J Vasc Interv Radiol. 2009;20:1343–1351. doi: 10.1016/j.jvir.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee FT, Jr, Mahvi DM, Chosy SG, et al. Hepatic cryosurgery with intraoperative US guidance. Radiology. 1997;202:624–632. doi: 10.1148/radiology.202.3.9051005. [DOI] [PubMed] [Google Scholar]
- 48.Yang Y, Wang C, Lu Y, et al. Outcomes of ultrasound-guided percutaneous argon-helium cryoablation of hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2012;19:674–684. doi: 10.1007/s00534-011-0490-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seifert JK, Morris DL. World survey on the complications of hepatic and prostate cryotherapy. World J Surg. 1999;23:109–113. doi: 10.1007/pl00013173. discussion 13–4. [DOI] [PubMed] [Google Scholar]
- 50.Faroja M, Ahmed M, Appelbaum L, et al. Irreversible electroporation ablation: Is all the damage nonthermal? Radiology. 2013;266:462–470. doi: 10.1148/radiol.12120609. [DOI] [PubMed] [Google Scholar]
- 51.Lee EW, Chen C, Prieto VE, et al. Advanced hepatic ablation technique for creating complete cell death: Irreversible electroporation. Radiology. 2010;255:426–433. doi: 10.1148/radiol.10090337. [DOI] [PubMed] [Google Scholar]
- 52.Lee EW, Loh CT, Kee ST. Imaging guided percutaneous irreversible electroporation: Ultrasound and immunohistological correlation. Technol Cancer Res Treat. 2007;6:287–294. doi: 10.1177/153303460700600404. [DOI] [PubMed] [Google Scholar]
- 53.Adeyanju OO, Al-Angari HM, Sahakian AV. The optimization of needle electrode number and placement for irreversible electroporation of hepatocellular carcinoma. Radiol Oncol. 2012;46:126–135. doi: 10.2478/v10019-012-0026-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmidt CR, Shires P, Mootoo M. Real-time ultrasound imaging of irreversible electroporation in a porcine liver model adequately characterizes the zone of cellular necrosis. Int Hepato Pancreat Biliary Assoc. 2012;14:98–102. doi: 10.1111/j.1477-2574.2011.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deodhar A, Dickfeld T, Single GW, et al. Irreversible electroporation near the heart: Ventricular arrhythmias can be prevented with ECG synchronization. Am J Roentgenol. 2011:W330–W335. doi: 10.2214/AJR.10.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomson KR, Cheung W, Ellis SJ, et al. Investigation of the safety of irreversible electroporation in humans. J Vasc Interv Radiol. 2011:611–621. doi: 10.1016/j.jvir.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 57.Martin RC, 2nd, McFarland K, Ellis S, et al. Irreversible electroporation therapy in the management of locally advanced pancreatic adenocarcinoma. J Am Coll Surg. 2012:361–369. doi: 10.1016/j.jamcollsurg.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 58.Olweny EO, Kapur P, Tan YK, et al. Irreversible electroporation: Evaluation of nonthermal and thermal ablative capabilities in the porcine kidney. Urology. 2013;81:679–684. doi: 10.1016/j.urology.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 59.Narayanan G, Hosein PJ, Arora G, et al. Percutaneous irreversible electroporation for downstaging and control of unresectable pancreatic adenocarcinoma. J Vasc Interv Radiol. 2012;23:1613–1621. doi: 10.1016/j.jvir.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 60.Wang P, Brace CL. Tissue dielectric measurement using an interstitial dipole antenna. IEEE Trans Biomed Eng. 2012;59:115–121. doi: 10.1109/TBME.2011.2167622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Skinner MG, Iizuka MN, Kolios MC, et al. A theoretical comparison of energy sources—microwave, ultrasound and laser—for interstitial thermal therapy. Phys Med Biol. 1998;43:3535–3547. doi: 10.1088/0031-9155/43/12/011. [DOI] [PubMed] [Google Scholar]
- 62.Lu DS, Raman SS, Limanond P, et al. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol. 2003;14:1267–1274. doi: 10.1097/01.rvi.0000092666.72261.6b. [DOI] [PubMed] [Google Scholar]
- 63.Bhardwaj N, Strickland AD, Ahmad F, et al. A comparative histological evaluation of the ablations produced by microwave, cryotherapy and radiofrequency in the liver. Pathology. 2009;41:168–172. doi: 10.1080/00313020802579292. [DOI] [PubMed] [Google Scholar]
- 64.Yu NC, Raman SS, Kim YJ, et al. Microwave liver ablation: Influence of hepatic vein size on heat-sink effect in a porcine model. J Vasc Interv Radiol United States. 2008;19:1087–1092. doi: 10.1016/j.jvir.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 65.Goldberg SN, Hahn PF, Tanabe KK, et al. Percutaneous radio-frequency tissue ablation: Does perfusion-mediated tissue cooling limit coagulation necrosis? J Vasc Interv Radiol. 1998;9:101–111. doi: 10.1016/s1051-0443(98)70491-9. [DOI] [PubMed] [Google Scholar]
- 66.Aschoff AJ, Merkle EM, Wong V, et al. How does alteration of hepatic blood flow affect liver perfusion and radiofrequency-induced thermal lesion size in rabbit liver? J Magn Reson Imaging. 2001;13:57–63. doi: 10.1002/1522-2586(200101)13:1<57::aid-jmri1009>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 67.Morrison PR, vanSonnenberg E, Shankar S, et al. Radiofrequency ablation of thoracic lesions: Part 1, experiments in the normal porcine thorax. Am J Roentgenol. 2005;184:375–380. doi: 10.2214/ajr.184.2.01840375. [DOI] [PubMed] [Google Scholar]
- 68.Steinke K, Glenn D, King J, et al. Percutaneous pulmonary radio-frequency ablation: Difficulty achieving complete ablations in big lung lesions. Br J Radiol. 2003;76:742–745. doi: 10.1259/bjr/35823935. [DOI] [PubMed] [Google Scholar]
- 69.Van Tilborg AA, Meijerink MR, Sietses C, et al. Long-term results of radiofrequency ablation for unresectable colorectal liver metastases: A potentially curative intervention. Br J Radiol. 2011;84:556–565. doi: 10.1259/bjr/78268814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kuvshinoff BW, Ota DM. Radiofrequency ablation of liver tumors: Influence of technique and tumor size. Surgery. 2002;132:605–611. doi: 10.1067/msy.2002.127545. discussion 11–2. [DOI] [PubMed] [Google Scholar]
- 71.Gervais DA, Arellano RS, McGovern FJ, et al. Radiofrequency ablation of renal cell carcinoma: Part 2, Lessons learned with ablation of 100 tumors. Am J Roentgenol. 2005;185:72–80. doi: 10.2214/ajr.185.1.01850072. [DOI] [PubMed] [Google Scholar]
- 72.Best SL, Park SK, Yaacoub RF, et al. Long-term outcomes of renal tumor radio frequency ablation stratified by tumor diameter: Size matters. J Urol. 2012;187:1183–1189. doi: 10.1016/j.juro.2011.11.096. [DOI] [PubMed] [Google Scholar]
- 73.Seifert JK, France MP, Zhao J, et al. Large volume hepatic freezing: Association with significant release of the cytokines interleukin-6 and tumor necrosis factor a in a rat model. World J Surg. 2002;26:1333–1341. doi: 10.1007/s00268-002-6139-5. [DOI] [PubMed] [Google Scholar]
- 74.Brace C. Thermal tumor ablation in clinical use. IEEE Pulse. 2011;2:28–38. doi: 10.1109/MPUL.2011.942603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Laeseke PF, Lee FT, Jr, van der Weide DW, et al. Multiple-antenna microwave ablation: Spatially distributing power improves thermal profiles and reduces invasiveness. J Interv Oncol. 2009;2:65–72. [PMC free article] [PubMed] [Google Scholar]
- 76.Lee J, Lee JM, Yoon JH, et al. Percutaneous radiofrequency ablation with multiple electrodes for medium-sized hepatocellular carcinomas. Korean J Radiol. 2012;13:34–43. doi: 10.3348/kjr.2012.13.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laeseke PF, Sampson LA, Haemmerich D, et al. Multiple-electrode radiofrequency ablation creates confluent areas of necrosis: In vivo porcine liver results. Radiology. 2006;2006:116–124. doi: 10.1148/radiol.2411051271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brace CL, Laeseke PF, Sampson LA, et al. Microwave ablation with multiple simultaneously powered small-gauge triaxial antennas: Results from an in vivo swine liver model. Radiology. 2007;244:151–156. doi: 10.1148/radiol.2441052054. [DOI] [PubMed] [Google Scholar]
- 79.Kim YS, Lee WJ, Rhim H, et al. The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (>2 and <5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. Am J Roentgenol. 2010;195:758–765. doi: 10.2214/AJR.09.2954. [DOI] [PubMed] [Google Scholar]
- 80.Shimada K, Sakamoto Y, Esaki M, et al. Role of the width of the surgical margin in a hepatectomy for small hepatocellular carcinomas eligible for percutaneous local ablative therapy. Am J Surg. 2008;195:775–781. doi: 10.1016/j.amjsurg.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 81.Nakazawa T, Kokubu S, Shibuya A, et al. Radiofrequency ablation of hepatocellular carcinoma: Correlation between local tumor progression after ablation and ablative margin. Am J Roentgenol. 2007;188:480–488. doi: 10.2214/AJR.05.2079. [DOI] [PubMed] [Google Scholar]
- 82.Laeseke PF, Sampson LA, Haemmerich D, et al. Multiple-electrode radiofrequency ablation creates confluent areas of necrosis: In vivo porcine liver results. Radiology. 2006;241:116–124. doi: 10.1148/radiol.2411051271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Permpongkosol S, Nicol TL, Khurana H, et al. Thermal maps around two adjacent cryoprobes creating overlapping ablations in porcine liver, lung, and kidney. J Vasc Interv Radiol. 2007;18:283–287. doi: 10.1016/j.jvir.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 84.Laeseke PF, Sampson LA, Frey TM, et al. Multiple-electrode radio-frequency ablation: Comparison with a conventional cluster electrode in an in vivo porcine kidney model. J Vasc Interv Radiol. 2007;18:1005–1010. doi: 10.1016/j.jvir.2007.05.010. [DOI] [PubMed] [Google Scholar]