Abstract
Due to its abundant source, good biocompatibility, low price and mild crosslinking process, alginate is an ideal selection for tissue engineering applications. In this work, alginate vascular conduits were fabricated through a coaxial extrusion-based system. However, due to the inherent weak mechanical properties of alginate, the vascular conduits are not capable of biomimicking natural vascular system. In this paper, multiwall carbon nanotubes (MWCNT) were used to reinforce vascular conduits. Mechanical, dehydration, swelling and degradation tests were performed to understand influences of MWCNT reinforcement. The unique mechanical properties together with perfusion and diffusional capability are two important factors to mimic the nature. Thus, perfusion experiments were also conducted to explore the MWCNT reinforcement effect. In addition, cell viability and tissue histology were conducted to evaluate the biological performance of conduits both in short and long term for MWCNT reinforcement.
Keywords: Artificial Vascular Constructs, Carbon Nanotubes, Biofabrication, Tissue Engineering
1. Introduction
Vascular tissue fabrication is an important research focus in tissue engineering. Researchers aim to biomimic the natural vascular system, which is considered to provide oxygen and nutrients to cells in engineered tissues as well as takes away the waste. Due to the lack of an efficient vascular system, biofabrication of functional thick tissues remains a challenge in tissue engineering. Researchers have proved that inclusion of an artificial vascular network resulted in great improvement in cell viability and provided enough oxygen and nutrients for surrounding cells [1-3].
In the past decade, several methodologies have been proposed for the fabrication of small-diameter vascular substitutes [4-7], including decellularized tissues [8-11], cell sheets [12], biodegradable synthetic polymer-based constructs [13, 14] and natural biomaterial-based blood vessel grafts [15-17]. Decellularized tissues have several advantages, including being entirely composed of natural extracellular matrix (ECM) and having biocompatibility and appealing mechanical properties [18]. However, significant shrinkage was observed during the decellularization process [19]. Cell sheet approach provides the best burst pressure result so far [20], yet the cell sheet approach has limitations in fabrication of small diameter and long length vascular substitutes [12]. Major problem with synthetic polymer-based approach is due to the lack of specific reactive groups within the surface chemistry where difficulties are shown in cell attachment and signaling [21]. Besides, the byproducts of synthetic polymer are usually toxic or acidic during the biodegradation process, which further devastates the cell culture environment [22]. Natural biomaterials with great biocompatibility and degradability provide an ideal substrate for cell attachment and proliferation. However, as an inherent weakness, the mechanical properties of natural biomaterials are limited. Several researchers contributed to the development of fabrication of natural material-based, small-diameter vascular substitutes [15, 16, 23, 24]. None of them was capable to fabricate vasculature conduits with smooth and well defined walls, and in any desired length with controllable dimensions.
To address the inherent weak mechanical properties of natural polymers, in this work, carbon nanotubes were used and reinforced into artificial vascular conduits. Previous studies have shown that carbon nanotube reinforcement was capable of increasing the mechanical strength of materials significantly [25, 26]. Several experiments have been conducted to apply carbon nanotube in tissue engineering. Results showed that carbon nanotube was a suitable substrate for cells to grow on [27-29]. Mazzatenta et al. [30] demonstrated that carbon nanotubes are capable of promoting the tissue-specific development of seeded cells. Verdejo et al. [31] showed that carbon nanotubes have a positive influence on the increase of osteoblastic cell differentiation and proliferation.
In this paper, multiwall carbon nanotubes (MWCNTs) were used to reinforce alginate vascular conduits. Alginate vascular conduits used in this work were fabricated using an extrusion based deposition system. Compared to other methods available for vascular conduit fabrication, this method has a small fabrication time span, the capability to fabricate vascular conduits in any length, and acceptable biocompatibility [32-34]. Mechanical tests were carried out on vascular conduits with and without MWCNT reinforcement. To evaluate their tensile strength, burst pressure of vascular conduits was studied. Also, experiments were performed to evaluate the influence of MWCNT reinforcement on the dehydration, swelling and degradation properties of vascular conduits. Perfusion experiments were carried out to explore the effect of MWCNT reinforcement since perfusion and permeability capability is another important property of the natural vascular system in addition to its unique mechanical property. To test the biological performance of the conduits, cell viability and tissue histology experiments were also performed.
2. Materials and Methods
2.1. Materials
Sodium alginate (purchased from Sigma Aldrich, United Kingdom) and calcium chloride (purchased from Sigma Aldrich, Japan) were used to form the hydrogel. 4% (w/v) sodium alginate solution was prepared in deionized water and placed in a shaker for 10 hours at 120 rpm. Similarly, 4% (w/v) calcium chloride solution was prepared using deionized water. Pristine MWCNTs were purchased from Nano Labs Inc., USA. For preparation of the CNT solution, a functionalization method and an acid-washing treatment with 70% nitric acid (HNO3) were used to oxidize the pristine CNTs. CNTs (100 mg) were dispersed by sonication in 250 ml for 1 hour. The mixture of MWCNT-HNO3 was refluxed at 140° C for 1.5 hours while stirring. Then, the mixture was cooled down to room temperature. To evaluate the reinforcement effect, 0.5% and 1% (w/v) MWCNT solutions were dissolved in 4% (w/v) alginate solutions.
2.2. Fabrication
A new extrusion-based system (see Figure 1A) was used in this paper to fabricate vascular conduits. The vascular conduit fabrication system consists of a co-axial nozzle unit (see Figure 1B), a pressure regulator (EFD® Nordson, East Providence, RI, USA) and a syringe pump (New Era Pump System Inc., Farmingdale, NY, USA). In this paper, vascular conduits reinforced with 0.5% and 1% MWCNT were fabricated and used. 4% (w/v) alginate vascular conduits were used as the control group in all experiments since 4% alginate had good mechanical properties as well as acceptable biocompatibility [32, 35]. All fabrication parameters other than MWCNT concentration were fixed during fabrication. The alginate dispensing pressure and calcium chloride dispensing rates were set at 17 kPa and 16 ml/min, respectively.
Figure 1.
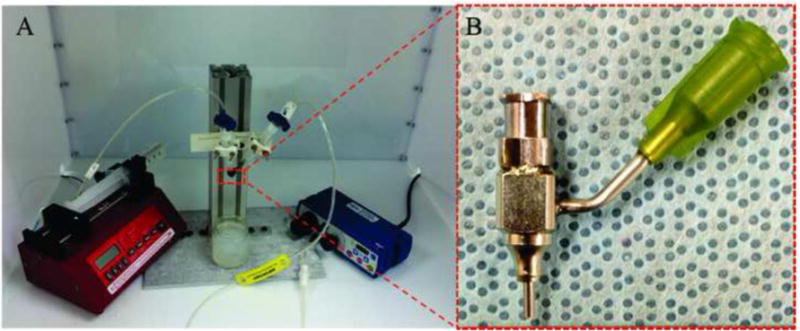
Experimental setup: (A) extrusion-based system, and, (B) coaxial nozzle unit.
2.3. Dehydration, Swelling and Degradation Tests
Upon fabrication, vascular conduits were soaked in 4% (w/v) calcium chloride for 30 min to ensure full crosslinking. The vascular conduits were dehydrated at room temperature for 4 days. The dehydrated vascular conduits were then soaked in phosphate buffered saline (PBS) for swelling and degradation. The shrinkage rate by weight (SRW), diameter shrinkage rate (DSR), volume shrinkage rate (VSR) and swelling ratio (SR) were calculated using the following equations:
| (1) |
| (2) |
| (3) |
| (4) |
Where Wo is the original sample weight after fabrication, Wi is the instant sample weight at the measurement moments, and Wd is the dehydrated sample weight. Do and Dd are the diameter of original sample after fabrication and the dehydrated sample, respectively.
2.4. Mechanical Testing
Upon fabrication, vascular conduits were soaked in the calcium chloride solution overnight. Soaking the samples in the calcium chloride solution minimized the effect of residence time on the samples. A Biotense perfusion bioreactor (ADMET, Inc. Norwood, MA) with a 2 N load cell was used to evaluate the tensile test. Each sample was a maximum of 30 mm long and was mounted on the rectangular mini sandpaper in order to prevent slippage during the test. By applying mechanical load, samples were ruptured in the middle or near the edge. Displacement and load information data were recorded by means of a data acquisition system (MTestQuattro System, ADMET, Inc. Norwood, MA).
The burst pressure (BP) was calculated from mechanical testing results by rearranging the Laplace law for a pressurized thin-walled hollow cylinder [36]:
| (5) |
Where BP is the estimated burst pressure (mmHg); T represents the wall thickness (μm) of vascular conduits; and LD represents the lumen diameter (μm).
2.5. Perfusion and Permeability Study
To test the media perfusion and permeability capabilities of vascular conduits, a customized system was developed (see Figure 2A). The media perfusion system consists of three parts: a cell media reservoir, a pump (Cole-Parmer, IL, USA) and a perfusion chamber with a clear cover to prevent evaporation. Cell media was perfused from the media reservoir, through the pump and the vascular conduit, and pumped back to the media reservoir. Needles inserted into the fabricated vascular conduits were selected depending on the lumen diameter of vascular conduits. Surgery clips were used to fix ends to prevent leakage (see Figure 2B).
Figure 2.
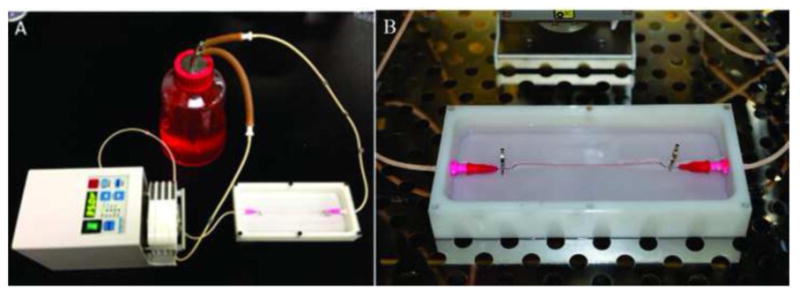
Experimental setup for perfusion test: (A) perfusion system consists of three parts, a cell media reservoir, a peristaltic pump and a perfusion chamber, and (B) a conduit under perfusion.
2.6. Cell Preparation
Human umbilical vein smooth muscle cells (HUVSMCs) (Life Technologies, MA, USA) were used in this study to test cell viability when encapsulated in carbon-nanotube-reinforced vascular conduits as well as control group. Cells were cultured at 37° C in 5% CO2 in smooth muscle cell growth media (Life Technologies, MA, USA) supplemented with smooth muscle cell growth supplement (Life Technologies, MA, USA), 100 μg/μl penicillin, 100 μg/ml streptomycin and 2.5 μg/μl Fungizone. Culture media was replenished every other day. Cells were harvested upon 70-80% confluent, and were centrifuged down and re-suspended in 4% (w/v) sodium alginate solution containing 1% MWCNTs. The cellular solution was gently mixed with a vortex mixer until uniformity was reached. The density of cells used in this study was 10×106 cells/ml. Cellular sodium alginate solution was loaded into the extrusion unit along with the crosslinker solution. When two solutions were extruded through the coaxial nozzle unit, sodium alginate through the sheath section and the crosslinker through the core section, crosslinking started forming vascular conduits.
2.7. Cell Viability Test
To check the biocompatibility of MWCNT-reinforced vascular conduits, cell viability analysis was performed to compare cell survival rate between control group and MWCNT-reinforced ones by LIVE/DEAD staining, and semi-quantification of relative live cell ratio. In general, vascular conduits were stained with calcium acetoxymethylester (calcein AM) and ethidiumhomodimer-2 (Invitrogen), at a concentration of 1.0 mM each. Calcein AM is metabolized in living cells to form a bright green fluorescent product that accumulates in the cytosol. Ethidiumhomodimer is a red fluorophore that stains the DNA of nonviable cells but cannot penetrate living cells with intact plasma membranes. After a 30-minute incubation, the staining medium was aspirated, and new medium was added to wash off any residual stains on the vascular conduit surface before fluorescent illumination. Vascular conduits were then imaged under a Leica fluorescent microscope (North Central Instrument, Germany). ImageJ (National Institutes of Health, Bethesda, MD) was used for automated counting of red and green-stained cells in each image, and percentages of viable cells were calculated as described in previous study [37].
2.8. Tissue Histology
In addition to short term effect of cell viability of MWCNTs reinforcement, vascular conduits were cultured for a prolonged time period, followed by immunohistochemistry examination. After 6 weeks of in vitro culture in smooth muscle cell differentiation media, frozen vascular conduits were fixed and sectioned at 5 μm for histological examination for markers specific to smooth muscle cells. Verhoeff–VanGiesen was used to visualize collagen deposition. Collagen stains with a red color. All the procedures was carried out per the manufacturers' instructions. Sample slides were examined under an Olympus BX-61 Brightfield- Fluorescent microscope (Olympus America, Melville, NY, USA) at different magnifications.
2.9. Statistical Analysis
In fabrication experiments, more than 50 pieces of data were obtained by measuring different vascular conduits and different sections of vascular conduits under a digital microscope (Motic®, BA310, Motic Incorporation Inc., Canada). The data shown in Figure 3 were an average of all 50 pieces of data. The statistical analysis was carried out using Minitab 16. The statistical difference analysis among groups was conducted by a two-tailed student's t-test. Groups with a significance level of p<0.05 were considered as significant. In dehydration, swelling, degradation, tensile testing and cell viability experiments, the sample number n=3.
Figure 3.
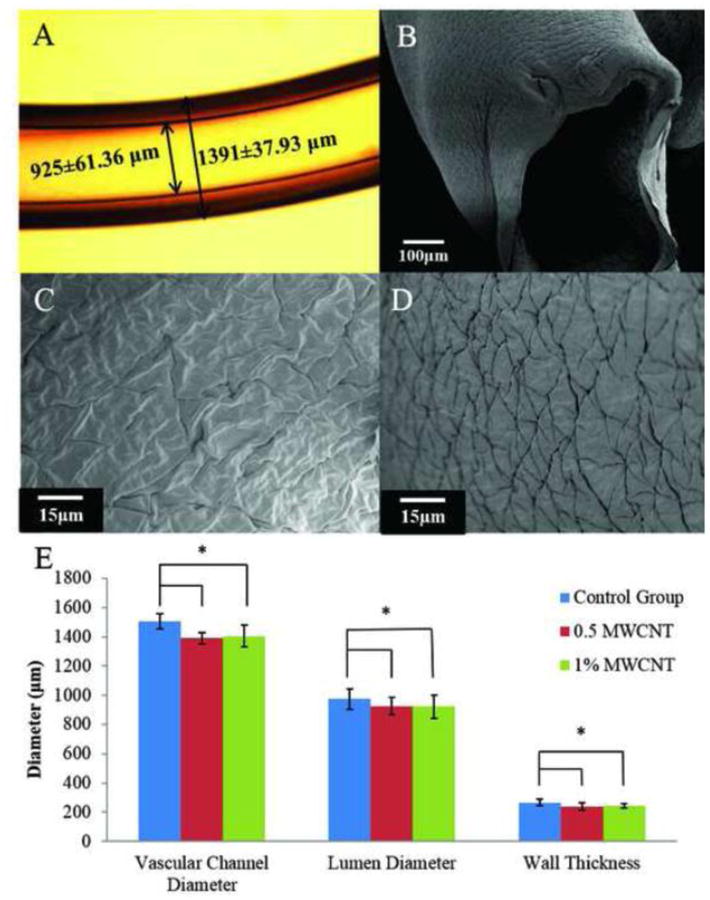
Fabrication of vascular conduits: (A) vascular conduit made of 4% (w/v) alginate with 1% WMCNT reinforcement, (B) dehydrated vascular conduit, (C) SEM image of dehydrated vascular conduit inner wall surface, (D) SEM image of dehydrated vascular conduit outer wall surface, and (E) dimensional characterization of vascular conduits.
3. Results and Discussion
3.1. Fabrication
A microscopic image of a vascular conduit is shown in Figure 3A. Uniform, smooth and well defined walls were observed. Figure 3B shows a scanning electron microscopy (SEM) image of a dehydrated vascular conduit. After dehydration, vascular conduits still maintained the lumen shape. Figure 3C-D show the inner wall surface and outer wall surface of the dehydrated vascular conduits. The results show that there were significant statistical differencesinvascularconduitdimensionsbetweentheMWCNTreinforcementgroupsandthecontrolgroupusing the same fabrication parameters (see Figure 3E). Conduits with MWCNT reinforcement tended to have smaller vascular diameter, lumen diameter and wall thickness. However, between the 0.5% (w/v) MWCNT reinforcement group and the 1% (w/v) MWCNT reinforcement group, there were no significant differences in vascular conduit diameter, lumen diameter and wall thickness.
3.2. Dehydration, Swelling and Degradation Test
Experiments were conducted for dimensional characterization of dehydrated samples (see Figure 4A). Only vascular conduit diameters were measured on the dehydrated conduits because, after dehydration, vascular conduit wall thickness and lumen section were not visible under the microscopy. No statistically significant difference was observed between groups. Figure 4B shows the vascular conduit shrinkage rate by weight. There was a clear difference between the reinforcement groups and the control group, whereas the shrinkage rate of 1% and 0.5% (w/v) MWCNT reinforcement groups were close to each other (61.48% and 62.30%, respectively). Shrinkage rate by diameter only represents the shrinkage in uniaxial direction. The ionically cross-linked alginate is a homogeneous structure, and thus the shrinkage process should possess isotropic homogeneity, which means shrinkage rates along all axes are same.
Figure 4.
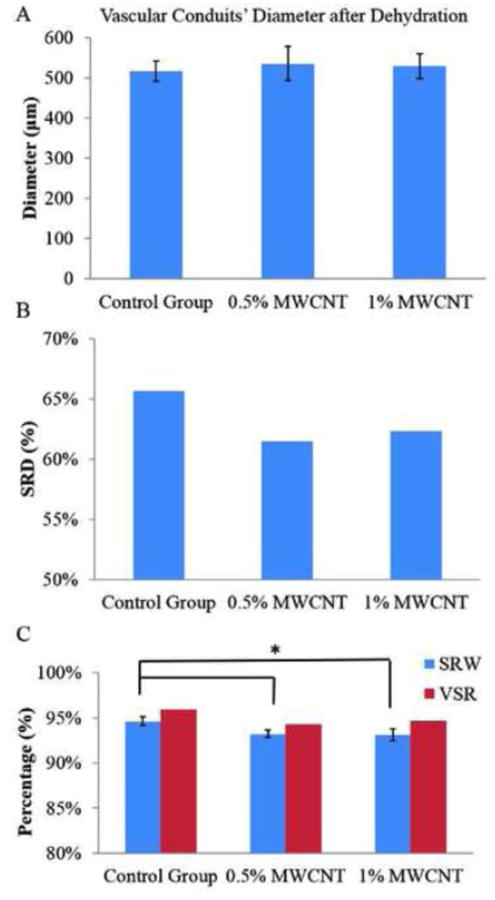
Influence of MWCNT reinforcement on vascular conduit dehydration process:(A)vascular conduit diameter after dehydration, (B) SRD, and (C) SRW and VSR.
Swelling and degradation tests were conducted to explore the influence of carbon nanotube reinforcement on vasculature conduits. Figure 5A shows the swelling ratio curve for the control group and the 1% MWCNT reinforcement group. The control group has a slightly higher swelling ratio than 1% MWCNT reinforcement group, but the difference was not statistically significant due to the relatively low MWCNT concentration. The maximum swelling ratio for the control group and the 1% MWCNT reinforcement group were 521±30.17% and 477±44.67%, respectively (see Figure 5B). No statistical difference was observed between groups. However, the difference between the calculated Wmax/Wo for the control group and the 1% (w/v) MWCNT reinforcement group was obvious. The Wmax/Wo ratio represents the conduit's liquid reabsorption capacity. The Wmax/Wo ratio for control group and 1% (w/v) MWCNT reinforcement group were 30.17% and 44.67%, respectively. Thus, during the swelling process, the dehydrated conduits in control group and 1% MWCNT reinforcement group reabsorbed PBS up to 30.17% and 44.67% of conduits' original weight.
Figure 5.
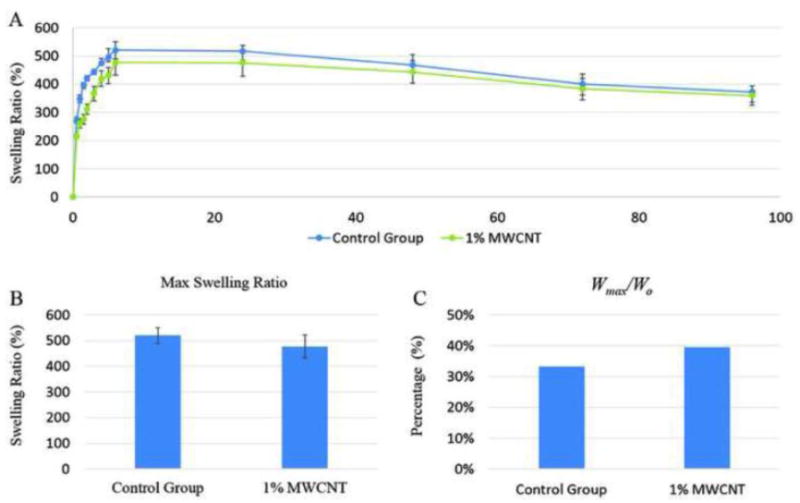
Influence of MWCNT reinforcement on vascular conduit swelling process: (A) swelling ratio curve, (B) maximum swelling ratio and (C) vascular conduit liquid reabsorption capacity.
3.3. Mechanical Testing
Mechanical tensile testing results are presented in Figure 6. The tensile strengths for the control group, the 0.5% MWCNT reinforcement group and the 1% MWCNT reinforcement group were 382±19kPa, 420±22kPa and 422±23kPa, respectively. Figure 6A indicates that different MWCNT concentrations could change vascular conduits' mechanical properties. These differences in the tensile strength could be determined by the distribution, dispersion and alignment of the MWCNT in alginate, which could affect the physical and mechanical properties and the orientation of nanomaterial along the vascular conduits. However, there was no statistically significant difference between the tensile strengths of these two groups.
Figure 6.
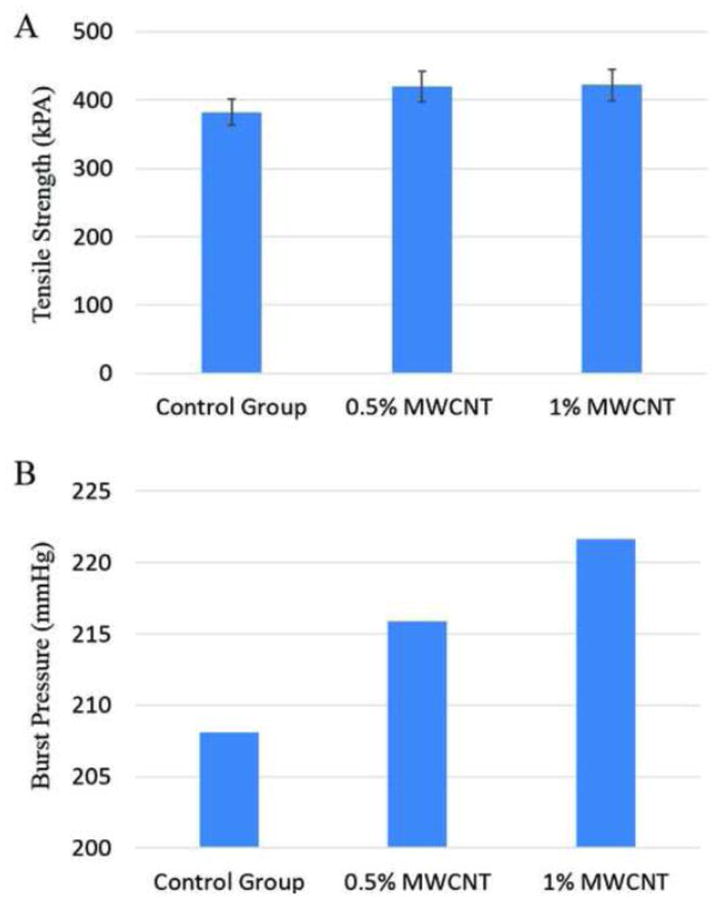
Mechanical Tests: (A) tensile strength, and (B) estimated burst pressure.
Based on Equation 5, estimated burst pressure values are plotted in Figure 6B. The estimated burst pressure for the control group, 0.5% (w/v) MWCNT reinforced group and 1% MWCNT reinforced group were 208.14 mmHg, 215.89 mmHg and 221.65 mmHg, respectively. The 0.5% and 1% (w/v) MWCNT reinforcement increased burst pressure by 3.7% and 6.5%, respectively.
3.4. Perfusion and Permeability Study
Results after one hour of media perfusion show that all vascular conduits elongated from 8 cm to 8.4±0.05 cm, which was caused by the weight of the conduits. The weight of the perfused media as well as the weight of the conduits themselves stretched the conduits toward the bottom of the perfusion chamber. It was observed that elongation started as media perfusion started and terminated in five minutes. Elongation terminated as long as the vascular conduit reached the bottom of the perfusion chamber. Diffusion rates are shown in Figure 7. The diffusion rates for the control group, 0.5% MWCNT reinforcement group and 1% MWCNT reinforcement group were 7.2±0.48 μl/min, 8.4±0.68 μl/min and 7±0.71 μl/min, respectively. No statistical difference was observed among those three groups, which might be due to the negligible effect of MWCNT reinforcement on the alginate polymer structure. Firstly, the concentrations of MWCNT used in alginate reinforcement are relatively low, which were only 0.5% and 1%. According to the manufacturer, the diameter of carbon MWCNT is around 30 nm, whereas the alginate pore size is in the rage of 30-450 nm [38]. Compared with alginate pores, the size of MWCNT is small and thus barely had an effect on permeability.
Figure 7.
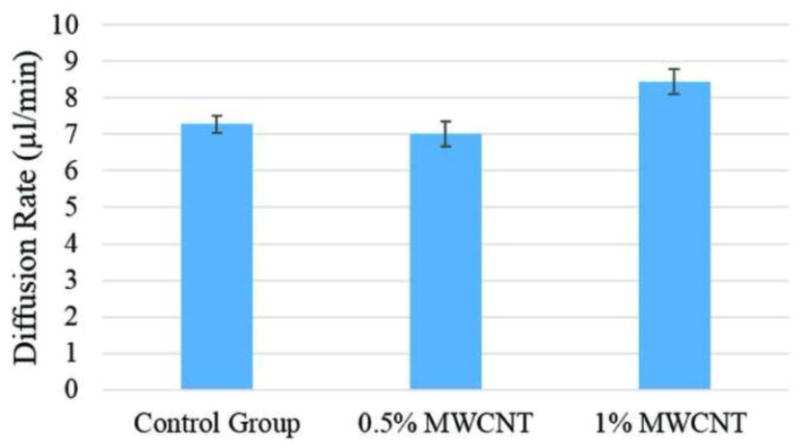
Perfusion capability of vascular conduits.
3.5. Cell Viability
No significant difference was observed between the control group and the MWCNT reinforcement group immediately after printing as well as 3 days post in vitro culture. As shown in Figure 8A, on day 0, immediately after fabrication, cell viability decreased in both groups compared to viability of control group, which was 75 ± 4% and 72 ± 1.6% in plain alginate and MWCNT-reinforced group, respectively. Nevertheless, no significant difference was observed in the cell viability of these two groups. Right after bioprinting, MWCNT-reinforced conduits had 53.5 ± 1.3% cell viability, which was similar to that of the control group with viability of 54 ± 1.4%. On day three, cell viability of both groups significantly increased compared to the viability on day 0, where MWCNT-reinforced group reached 71 ± 1.7%, and plain alginate group reached 69 ± 1.2%.
Figure 8.
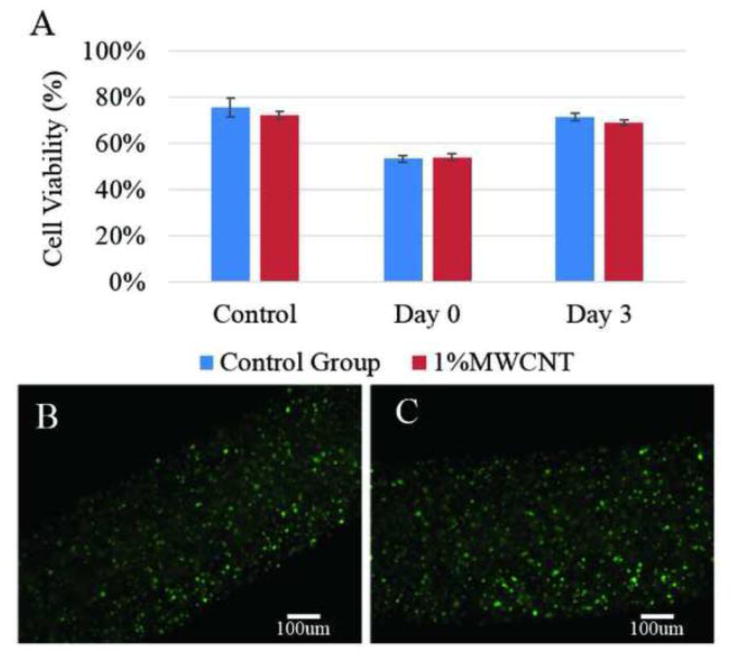
Cell viability study: (A) cell viability over time, (B) fluorescence microscopy image of a three days cultured plain alginate conduit, and (C) fluorescence microscopy image of a three days cultured 1% (w/v) MWCNT reinforced vascular conduit.
3.6. Tissue Histology
Immunohistochemistry study was taken to evaluate long term biocompatibility of MWCNT reinforcement by checking cell morphology and tissue specific extra cellular matrix formation. Evident differences were observed between MWCNT-reinforced conduits and control group. Damaged cells with broken nucleus presented in MWCNT group (Figure 9A) throughout the histology section, however majority of cells shown normal encapsulated cell morphology with rounded nucleus and good integrity in control group (Figure 9B). In addition, in control group, cells were able to migrate towards the peripheral side of the channel wall, and deposit extra cellular matrix, forming a lining of cell-matrix sheet around the channel (Figure 9D). However, no visible matrix formation was observed in MWCNT-reinforced conduits (Figure 9C).
Figure 9.
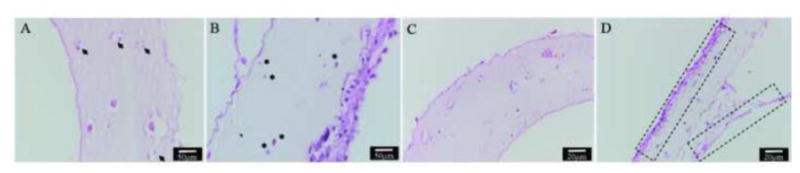
Immunohistochemistry: (A) damaged cells with disintegrated nucleus presented in the longitudinal section of MWCNT-reinforced conduits (shown by arrow head); (B) most of the cells in control group were undamaged showing regular morphology (shown by asterisks); (C) no visible matrix deposition was present in MWCNT-reinforced conduits; (D) substantial amount of extra cellular matrix was deposited along the peripheral part of the wall of the control group (shown by dashed boxes).
4. Discussion
The proposed coaxial extrusion-based system enabled fabrication of vascular conduits, which can be used as supplement vasculature for scale-up tissue fabrication. In general, the proposed fabrication setup produces vascular conduits in any length with controllable dimensions from 500 μm to 2 mm in diameter. Conduit and lumen diameter could be easily controlled by controlling process parameters and the coaxial nozzle configurations. The unique mechanical property is an important factor to mimic the natural vascular constructs. However due to inherent weak mechanical properties of alginate, vascular conduits were not mechanically capable of mimicking the natural vascular constructs. Thus, carbon nanotube reinforcement was used in this work to improve the mechanical properties of alginate conduits. Multiwall carbon nanotube reinforcement improved the tensile strength and burst pressure by 10.5% and 6.5%, respectively. The improvement in tensile strength demonstrated that the stress was transferred between the alginate matrix and MWCNTs reinforced in the vascular conduit's composites. In addition, the external stress was effectively transferred from the alginate matrix to the reinforced MWCNTs through the better bonded interfaces in alginate-reinforced MWCNT composites. The mechanical improvement of MWCNT reinforcement was not as remarkable as expected due to the relatively low concentration of MWCNT in 4% alginate along with difficulties to control MWCNT alignment in conduits. Increasing MWCNT concentration increased the mechanical properties further and this would be more significant if lower alginate concentration was used; however, increasing MWCNT concentration decreases extrusion ability of the precursor solution resulting in nozzle clogging. In addition, increasing MWCNT concentration can be more detrimental in long-term performance of cells in culture as discussed later in details. The distribution, dispersion and alignment of the MWCNT in the composite could improve the physical and mechanical properties of conduits as well. Compared with natural vascular, where burst pressure is around 3561 mmHg [39], further improvement will be necessary for transplantation purposes; however, the proposed reinforced vascular conduits would be sufficient to provide enough oxygenation for in vitro scale-up tissue and organ model fabrication.
The effect of carbon nanotube reinforcement on conduits' fabrication, dehydration, swelling, degradation and perfusion properties were also explored in this paper. Results showed that MWCNT reinforcement have small effect on conduits' swelling ratio, degradation rate and perfusion capability, which was due to relatively low concentration of MWCNTs in the conduits. Whereas MWCNT reinforcement significantly influenced the dimensions of fabricated conduits. Conduits with reinforcement tended to have smaller vascular diameter, lumen diameter and wall thickness, which was due to the inclusion of MWCNTs that changed the alginate solution viscosity significantly. With MWCNT reinforcement, the alginate solution viscosity was increased, and thus under the same pressure, the solution was ejected with a smaller dispensing rate. Also, MWCNT reinforcement had a significant effect on the vascular conduits' shrinkage rate by weight and liquid absorption capacity. The Wmax/Wo ratio for control group and 1% (w/v) MWCNT reinforcement group, were 30.17% and 44.67%, respectively. The fact that the value of Wmax/Wo for the control group was smaller than that of the 1% (w/v) MWCNT reinforcement group does not indicate that reinforcement group had a higher liquid absorption capacity. Multiwall carbon nanotubes are not hydrophilic, which means they do not absorb liquid during the swelling process. The liquid reabsorption capacity of 4% alginate was around 33%. If one assumes that Wo of conduits is a gram and the weight of MWCNT in conduits is b gram, for control group, the maximum swelling weight is 0.33a, and the value of Wmax/Wo is 33%. However, for the 1% (w/v) MWCNT reinforcement group, the value of Wois 0.33(a-b)+b, and the calculated Wmax/Wo is 0.33+0.67b/a.
In terms of biocompatibility, there was no significant difference for cell viability in the short term between plain and MWCNT-reinforced vascular conduits. Although decreased viability was observed immediately after fabrication, which might be largely due to the shear stress-induced damage caused during extrusion process, as elaborated in our previous study [32], cells were able to recover and grow with increased viability not long after fabrication in both groups. This may indicate that MWCNT reinforcement would not cause impaired cell function at least for short term. Nevertheless, evident differences were observed after over 6 week in vitro culture between MWCNT-reinforcement and plain conduits, with impaired cell growth, migration, and extra cellular matrix synthesis for MWCNT reinforcement. This could be explained by the toxicity effect of MWCNTs in the long run that restricted cells to perform their biological functions properly. Thus, very limited ECM formation was observed in the reinforced group in long term in vitro culture. In contrast, smooth muscle cells in the control group produced larger amount of ECM, particularly along the periphery of the conduits in addition to some matrix formation inside the lumen wall. We can speculate that encapsulated smooth muscle cells migrated toward the media due to growth factors. It is also possible that the altered hydrophobic, swelling and permeability properties due to MWCNT reinforcement, although not statistically significant, could cause profound difference in cellular response, as other studies have shown that matrix rigidity would direct differential cell growth and migration pattern for various cell types [40,41] as well as matrix hydrophobicity[42]. In general, encapsulated cells in alginate network were confined and could not migrate and proliferate throughout alginate network easily resulting in limited matrix formation. For future studies, more biologically active materials, like collagen and fibrin can be generated into nano-scale fibers by different means such as electro-spinning, and be integrated into vascular conduits fabrication for enhancing mechanical properties, while maintaining, or even improving their biocompatibility.
5. Conclusion
In this paper, we performed a thorough investigation on the influence of MWCNT reinforcement on in-vitro performance of vascular conduits. The results show that MWCNT reinforcement had a significant effect on the dimensions of fabricated vascular conduits, vascular conduits' shrinkage rate by weight and liquid absorption capacity. Multi-walled carbon nanotube reinforcement did not affect swelling ratio, degradation rate and perfusion capability significantly. Although MWCNT reinforcement increased the tensile strength and estimated burst pressure for vascular conduits, further improvement is needed to reach the mechanical properties of natural blood vessels. Long-term culturing with expected elastin and collagen deposition would enhance the mechanical properties. In terms of biocompatibility, MWCNT reinforcement did not affect cell viability in the short term compared with plain alginate conduits, but it impeded cell survival as well as motility and ability to synthesis extracellular matrix in the long term. For future work, we will fabricate collagen and elastin nanofibers using electrospinning process and reinforce them replacing MWCNT used in this paper. This will possibly improve both mechanical and biological performances of the vascular conduits.
Highlights.
Multiwall carbon nanotubes (MWCNTs) were reinforced in vascular conduits.
Improved mechanical properties were obtained with MWCNT reinforcement.
Dehydration, swelling, degradation and perfusion tests were performed.
Cell viability was not affected in short term when MWCNTs were reinforced.
Tissue histology showed less matrix deposition in MWCNT-reinforced conduits.
Acknowledgments
This research has been supported by the National Institutes of Health (NIH) and the Institute for Clinical and Translational Science (ICTS) grant number ULIRR024979. The authors would like to thank Prof. Edward Sander (Biomedical Engineering Department, University of Iowa), and his student Aribet M. De Jesus for helping with the tensile stress test. The authors also thank Prof. David Cwiertny (Civil and Environmental Engineering, University of Iowa) for providing MWCNT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ling Y, Rubin J, Deng Y, Huang C, Demirci U, Karp J JM, et al. A cell-laden microfluidic hydrogel. Lab on a Chip. 2007;7:756–62. doi: 10.1039/b615486g. [DOI] [PubMed] [Google Scholar]
- 2.Lee W, Lee V, Polio S, Keegan P, Lee JH, Fischer K, et al. On-demand three-dimensional free form fabrication of multi-layered hydrogel scaffold with fluidic channels. Biotechnology and Bioengineering. 2010;105:1178–86. doi: 10.1002/bit.22613. [DOI] [PubMed] [Google Scholar]
- 3.Choi NW, Cabodi M, Held B, Gleghorn JP, Bonassar LJ, Stroock AD. Microfluidic scaffolds for tissue engineering. Nat Mater. 2007;6:908–15. doi: 10.1038/nmat2022. [DOI] [PubMed] [Google Scholar]
- 4.Peck M, Gebhart D, Dusserre N, McAllister TN, L'Heureux N. The evolution of vascular tissue engineering and current state of the art. Cells Tissues Organs. 2012;195:144–58. doi: 10.1159/000331406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Derham C, Yow H, Ingram J, Fisher J, Ingham E, Korrosis SA, et al. Tissue Engineering Small-Diameter Vascular Grafts: Preparation of a Biocompatible Porcine Ureteric Scaffol. Tissue Engineering. 2008;14:1871–82. doi: 10.1089/ten.tea.2007.0103. [DOI] [PubMed] [Google Scholar]
- 6.Hoerstrup SP, Zünd G, Sodian R, Schnell AM, Grünenfelder J, Turina MI. Tissue engineering of small caliber vascular grafts. European Journal Cardio-Thoracic Surgery. 2001;20:164–9. doi: 10.1016/s1010-7940(01)00706-0. [DOI] [PubMed] [Google Scholar]
- 7.Naito Y, Shinoka T, Duncan D, Hibino N, Solomon D, Cleary M, et al. Vascular tissue engineering: Towards the next generation vascular grafts. Advanced Drug Delivery Reviews. 2011;63:312–23. doi: 10.1016/j.addr.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Gui L, Muto A, Chan SA, Breuer CK, Niklason LE. Development of decellularized human umbilical arteries as small-diameter vascular grafts. Tissue Engineering. 2009;15:2665–76. doi: 10.1089/ten.tea.2008.0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahl SLM, Koh J, Prabhakar V, Niklason LE. Decellularized Native and Engineered Arterial Scaffolds for Transplantation. Cell Transplantation. 2003;12:659–66. doi: 10.3727/000000003108747136. [DOI] [PubMed] [Google Scholar]
- 10.Sheridan WS, Duffy GP, Murphy BP. Mechanical characterization of a customized decellularized scaffold for vascular tissue engineering. Journal of the Mechanical Behavior of Biomedical Materials. 2012;8:58–70. doi: 10.1016/j.jmbbm.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt CE, Baier JM. A cellular vascular tissues: natural biomaterials for tissue repair and tissue engineering. Biomaterials. 2000;21:2215–31. doi: 10.1016/s0142-9612(00)00148-4. [DOI] [PubMed] [Google Scholar]
- 12.Isenberg BC, Backman DE, Kinahan ME, Jesudason R, Suki B, Stone PJ, et al. Micropatterned cell sheets with defined cell and extracellular matrix orientation exhibit anisotropic mechanical properties. Journal of Biomechanics. 2012;45:756–61. doi: 10.1016/j.jbiomech.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Caves JM, Kumar VA, Martinez AM, Kim J, Ripberger CM, Haller CA, et al. The use of microfiber composites of elastin-like protein matrix reinforced with synthetic collagen in the design of vascular grafts. Biomaterials. 2010;31:7175–82. doi: 10.1016/j.biomaterials.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe M, Shin'oka T, Tohyama S S, Hibino N, Konuma T, Matsumura G, et al. Tissue-engineered vascular autograft: inferior vena cava replacement in a dog model. Tissue Engineering. 2001;7:429–39. doi: 10.1089/10763270152436481. [DOI] [PubMed] [Google Scholar]
- 15.Norotte C, Marga FS, Niklason LE, Forgacs G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials. 2009;30:5910–7. doi: 10.1016/j.biomaterials.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozawa F, Ino K, Takahashi Y, Shiku H, Matsue T. Electrodeposition of alginate gels for construction of vascular-like structures. Journal of Bioscience and Bioengineering. 2013;115:459–61. doi: 10.1016/j.jbiosc.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Stegemann JP, Nerem RM. Phenotype Modulation in Vascular Tissue Engineering Using Biochemical and Mechanical Stimulation. Annals of Biomedical Engineering. 2003;31:391–402. doi: 10.1114/1.1558031. [DOI] [PubMed] [Google Scholar]
- 18.Courtman DM, Pereira CA, Kashef V, McComb D, Lee JM, Wilson GJ. Development of a pericardial acellular matrix biomaterial: biochemical and mechanical effects of cell extraction. Journal of Biomedical Materials Research. 1994;28:655–66. doi: 10.1002/jbm.820280602. [DOI] [PubMed] [Google Scholar]
- 19.Sung HW, Hsu CS, Chen HC, Hsu HL, Chang Y, Lu JH, et al. Fixation of various porcine arteries with an epoxy compound. Artificial Organs. 1997;21:50–8. doi: 10.1111/j.1525-1594.1997.tb00699.x. [DOI] [PubMed] [Google Scholar]
- 20.L'Heureux N, Pâquet S, Labbé R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. The FASEB Journal. 1998;12:47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 21.Dee KC, Pulelo DA, Bizios R. An introduction to tissue biomaterial interactions. Hoboken, NJ: Wiley-Liss, Publications; 2002. [Google Scholar]
- 22.Hollinger JO. An Introduction a to Biomaterial. second. CRC Press, Taylor & Francis Group; 2011. [Google Scholar]
- 23.Weinberg CB, Bell E. A blood vessel model constructed form collagen and cultured vascular cells. Science. 1986;231:397–400. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- 24.Xu C, Chai W, Huang Y, Markwald RR. Scaffold-free inkjet printing of three-dimensional zigzag cellular tubes. Biotechnology and Bioengineering. 2012;109 doi: 10.1002/bit.24591. [DOI] [PubMed] [Google Scholar]
- 25.Bu H, Kjøniksen AL, Knudsen KD, NystrÖm B. Rheological and structural properties of aqueous alginate during gelation via the Ugi multicomponent condensation reaction. Biomacromolecules. 2004;5:1470–9. doi: 10.1021/bm049947+. [DOI] [PubMed] [Google Scholar]
- 26.Coleman JN, Khan U, Blau WJ, Gun'ko TK. Small but strong: a review of the mechanical properties of carbon nanotube–polymer composites. Carbon. 2006;44:1624–52. [Google Scholar]
- 27.Mattson MP, Haddon RC, Rao AM. Molecular functionalization of carbon nanotubes and use as substrates for neuronal growth. Journal of Molecular Neuroscience. 2000;14:175–82. doi: 10.1385/JMN:14:3:175. [DOI] [PubMed] [Google Scholar]
- 28.Hu H, Ni Y, Montana V, Haddon RC, Parpura V. Chemically functionalized carbon nanotubes as substrates for neuronal growth. Nano letters. 2004;4:507–11. doi: 10.1021/nl035193d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lobo AO, Antunes EF, Machado AHA, Pacheco-Soares C, Trava-Airoldi VJ, Corat EJ. Cell viability and adhesion on as grown multi-wall carbon nanotube films. Materials Science and Engineering: C. 2008;28:264–9. [Google Scholar]
- 30.Mazzatenta A, Giugliano M, Campidelli S. Interfacing neurons with carbon nanotubes: electrical signal transfer and synaptic stimulation in cultured brain circuits. Journal of Biomedical Materials Research Part A. 2009;88:65–73. doi: 10.1523/JNEUROSCI.1051-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verdejo R, Jell G, Safinia L, Bismarck A, Stevens MM, Shaffer MS. Reactive polyurethane carbon nanotube foams and their interactions with osteoblasts. Journal of Biomedical Materials Research Part A. 2009;88:4818–27. doi: 10.1002/jbm.a.31698. [DOI] [PubMed] [Google Scholar]
- 32.Yu Y, Zhang Y, Ozbolat IT. Characterization of Cell Viability and Functionality in Bioprintable Cellular Vessel-like Microfluidic Channels. Journal of Biomechanical Engineering. 2013;135:901011–9. doi: 10.1115/1.4024575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Yu Y, Ozbolat IT. Direct Bioprinting of Vessel-like Tubular Microfluidic Channels. Journal of Nanotechnology in Engineering and Medicine. 2013;4:020902. [Google Scholar]
- 34.Zhang Y, Yu Y, Chen H, Ozbolat IT. Characterization of printable cellular micro-fluidic channels for tissue engineering. Biofabrication. 2013;5:025004. doi: 10.1088/1758-5082/5/2/025004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cuadros TR, Skurtys O, Aguilera JM. Mechanical properties of calcium alginate fibers produced with a microfluidic device. Carbohydrate Polymers. 2012;89:1198–206. doi: 10.1016/j.carbpol.2012.03.094. [DOI] [PubMed] [Google Scholar]
- 36.Gauvin R, Guillemette M, Galbraith T, Bourget JM, Larouche D, Marcoux H, et al. Mechanical Properties of Tissue-Engineered Vascular Constructs Produced Using Arterial or Venous Cells. Tissue Engineering Part A. 2010;17:2049–59. doi: 10.1089/ten.TEA.2010.0613. [DOI] [PubMed] [Google Scholar]
- 37.Chan AW, Neufeld RJ. Modeling the controllable pH-responsive swelling and pore size of networked alginate based biomaterials. Biomaterials. 2009;30:6119–29. doi: 10.1016/j.biomaterials.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 38.Chan AW, Neufeld RJ. Modeling the controllable pH-responsive swelling and pore size of networked alginate based biomaterials. Biomaterials. 2009;30:6119–29. doi: 10.1016/j.biomaterials.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 39.Konig G, McAllister TN, Dusserre N, Garrido SA, Iyican C, Marini A, et al. Mechanical properties of completely autologous human tissue engineered blood vessels compared to human saphenous vein and mammary artery. Biomaterials. 2009;30:1542–50. doi: 10.1016/j.biomaterials.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peyton SR, Putnam AJ. Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. Journal of Cellular Physiology. 2005;204:198–209. doi: 10.1002/jcp.20274. [DOI] [PubMed] [Google Scholar]
- 41.Ulrich TA, de Juan Pardo EM, Kumar S. The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Research. 2009;69:4167–74. doi: 10.1158/0008-5472.CAN-08-4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ayala R, Zhang C, Yang D, Hwang Y, Aung A, Shroff SS, et al. Engineering the cell–material interface for controlling stem cell adhesion, migration, and differentiation. Biomaterials. 2011;32:3700–11. doi: 10.1016/j.biomaterials.2011.02.004. [DOI] [PubMed] [Google Scholar]


