Abstract
Vascularization of thick engineered tissue and organ constructs like the heart, liver, pancreas or kidney remains a major challenge in tissue engineering. Vascularization is needed to supply oxygen and nutrients and remove waste in living tissues and organs through a network that should possess high perfusion ability and significant mechanical strength and elasticity. In this paper, we introduce a fabrication process to print vascular conduits directly, where conduits were reinforced with carbon-nanotubes (CNTs) to enhance their mechanical properties and bioprintability. In vitro evaluation of printed conduits encapsulated in human coronary artery smooth muscle cells (HCASMCs) was performed to characterize the effects of CNT reinforcement on the mechanical, perfusion and biological performance of the conduits. Perfusion and permeability, cell viability, extracellular matrix formation and tissue histology were assessed and discussed, and it was concluded that CNT-reinforced vascular conduits provided a foundation for mechanically appealing constructs where CNTs could be replaced with natural protein nanofibers for further integration of these conduits in large-scale tissue fabrication.
1. Introduction
Artificial blood vessels save the lives of many patients, especially in bypass applications such as shunts for dialysis or treatment of blood vessel failure; they also can be used as supplement vessels for the fabrication of engineered thick tissues [1]. Based on the application of artificial vascular tissues, several features are required, including perfusability, mechanical elasticity and strength for pulsatile stress and suture retention, diffusion properties, and the capability to transport nutrients, oxygen, and waste [2–3]. In order to develop vascular constructs biomimetically, one should thus consider incorporating these requirements during the fabrication process by choosing proper biomaterials and fabrication methods [2, 4].
Hydrogels are among the most commonly employed matrices in tissue engineering because they are biocompatible, able to facilitate nutrient/oxygen transport, and are highly hydrated three-dimensional (3D) networks that structurally resemble the extracellular matrix (ECM) [2, 4–5]. A suitable hydrogel for fabricating vascular conduits needs to satisfy several design parameters to promote the formation of functional new tissues—for instance, physical parameters, biological properties, and biocompatibility [2, 5–6]. Natural polymers, such as collagen, alginate, and chitosan, have better biocompatibility than synthetic polymer gels. However, they have limitations in mechanical properties (physical parameters), which are very important design criteria in tissue engineering [6]. Therefore, modifying their properties by means of adding other polymers or fibers can be helpful. One of the most common natural hydrogels is alginate, which is appropriate for fabricating tissue construct because it is a natural polymer, is abundant in nature, is highly biocompatible, and has low toxicity and macromolecular properties similar to those of natural ECM [7]. It has a crosslinking-based gelation property that enables printing through a coaxial nozzle unit. When biomaterial solution and crosslinker solution are fed simultaneously, the gelation of alginate vascular conduits occurs immediately, providing tubular conduits. The process of coaxial printing allows printing vascular conduits in any desired shape in 3D [8]. In this work, the goal is to modify alginate properties by adding carbon nanotubes to prepare composite solutions for fabricating vascular conduits. The composite solution consists of a biocompatible polymer as a matrix and is reinforced with fibers.
Determining the orientation, de-aggregation, and dispersion of reinforcement by fibers can increase the strength and resistance to deformation of the composite vasculature [3, 6–7]. By homogenously dispersing reinforced fibers, the interfacial interaction with the matrix can increase, resulting in the enhancement of the mechanical properties of the composite [3, 7–9]. One approach to improving the mechanical properties of alginate is the integration of carbon nanotubes (CNTs), which are among the strongest materials known and possess a simple structure [10–12]. In particular, single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWNTs) have unique mechanical strength and stiffness, a high aspect ratio, are lightweight, and are flexible and low-density [10]. Therefore, CNTs have outstanding potential as reinforcements in composite materials. Several researchers used CNTs in their studies to enhance mechanical properties and evaluated cell viability and proliferation in tissue scaffolds. Yildirim et al. investigated whether SWCNT-incorporated scaffolds had better cell attachment and proliferation, thus having more viable cells after seven days in comparison with non-reinforced scaffolds [13]. Tissue scaffolds were fabricated by means of a freeform fabrication technique through layer-by-layer deposition of material. The mechanical testing showed that the composite reinforced with 1% SWCNT had higher tensile strength. Mattson et al. successfully used CNT as suitable substrates for nerve cell growth [14]. In that study, the flat substrate of MWCNTs was coated with a 4-hydroxynonenal bioactive molecule to create multiple neuritis. Then neurons were grown on that substrate and generated a number of branches. However, neurons that were grown on the substrates without CNTs could generate only a few branches since they did not resemble the cellular surfaces and ECM in the body. Hu et al. used the same method as Mattson et al. did [14] to incorporate CNTs as a substructure in order to culture the hippocampal neuronal growth. The coated surface with MWCNTs was able to control the neuritis outgrowth, generate longer neuritis length, and control the branching pattern [15]. In addition, the study by Lobo et al. showed that it was possible to achieve higher cell viability and cell adhesion on MWCNT films [16].
In this paper, MWCNT-reinforced alginate vascular conduits were printed using a coaxial bioprinting process. Mechanical properties were evaluated and compared with vascular conduits made of plain alginate with different concentrations of alginate and dosages of MWCNTs. In addition, the elongation and diffusion rate of the vascular conduit were investigated. Furthermore, cell viability tests and tissue histology studies were conducted to evaluate effect of MWCNTs on cell viability, matrix deposition and the tissue generation process.
2. Materials and Methods
2.1 Materials
Sodium alginate (purchased from Sigma Aldrich, United Kingdom) and calcium chloride (CaCl2) (purchased from Sigma Aldrich, Japan) were used to fabricate vascular conduits. Solutions of 3–4% (w/v) sodium alginate were dissolved in deionized water and placed in a shaker for 10 hours at 120 rpm. Similarly, 4% (w/v) calcium chloride solutions were prepared using deionized water [8]. These concentrations were preferred due to the manufacturability of functional conduits based on our earlier experiments.
Pristine MWCNTs were purchased from Nano Labs Inc, USA. For preparation of the CNT solution, a functionalization method and an acid-washing treatment of nitric acid 70% (HNO3) were used to oxidize the pristine CNTs [17]. Carbon nanotubes (100 mg) were dispersed by sonication in 250 ml for 1 hour. Then, the mixture of MWCNT-HNO3 was refluxed at 140° C for 1.5 hours while stirring. Next, the mixture was cooled down to room temperature. The concentration of the suspension was 70% (w/v).
For the first four composite samples, 1% (w/v) and 0.5% (w/v) MWCNT were dissolved in alginate solutions with 3% and 4% concentrations, and each sample was placed on a stirrer for one day until the CNTs were dissolved homogenously. Two samples of alginate only with concentrations of 3% and 4% (w/v) were also produced.
2.2 Cell Preparation
Human coronary artery smooth muscle cells (HCASMCs) (Life Technologies, MA, USA) were used in this study to test cell viability when encapsulated in carbon-nanotube-reinforced vascular conduits. The HCASMCs were cultured at 37° C in 5% CO2 in smooth muscle cell growth media (Life Technologies, MA, USA) supplemented with smooth muscle cell growth supplement (Life Technologies, MA, USA), 100 μg/μl penicillin, 100 μg/ml streptomycin, and 2.5 μg/μl Fungizone. The culture media was changed every other day. Cells were harvested until we achieved a sufficient amount for bioprinting. After harvesting, cells were centrifuged down, re-suspended in a 4% sodium alginate and 1% MWCNT solution, and gently mixed by a vortex mixer to get uniform distribution. The cell-seeding density used in this study was 10×106 cells/ml.
2.3 Fabrication of Carbon Nanotube-reinforced Vascular Conduits
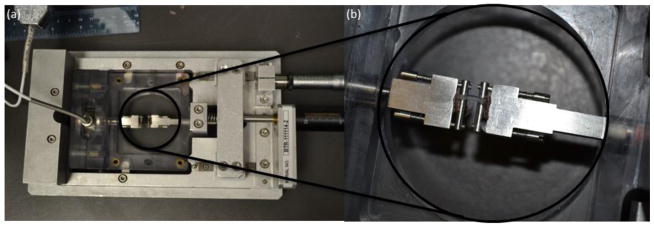
The experimental setup (see figs. 1a and 1b) consisted of a single-arm robotic printer (EFD Nordson, East Providence, RI) with a motion unit, a coaxial nozzle (see figs. 1a and 1c), a syringe pump (New Era Pump System Inc., Farmingdale, RI, and a pressure regulator (EFD Nordson, East Providence, RI) for biomaterial and CaCl2 solution as the cross-linker. The coaxial nozzle was mounted on the bioprinter unit.
Fig. 1.
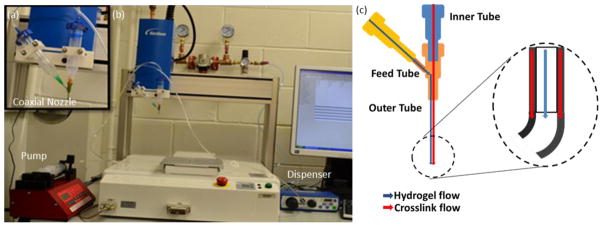
The experimental setup for fabricating vascular conduits: (a) the coaxial nozzle system, (b) the bioprinter platform and (c) cross-sectional view of the coaxial nozzle assembly.
The coaxial nozzle had three sections: a feed tube, an outer tube, and an inner tube (see fig. 1c). The composite solution was fed from the feed tube, and the inner tube was used to feed the crosslinker solution. The composite solution flowed into the space between the inner and outer tubes (see fig. 1c). A dispenser was connected to the syringe barrel, which contained the composite solution, and the syringe pump was connected to the barrel with the calcium chloride solution (see fig. 1d). The sodium alginate was dispensed from the sheath section of the nozzle, while the crosslink solution was dispensed from the core section by compressed air. When two solutions contacted, the vascular conduits were formed and printed. The coaxial assembly used in this study had a 25 gauge (260 μm inner diameter (I.D.), 510 μm outer diameter (O.D.)) inner needle and a 14 gauge (1600 μm I.D., 2110 μm O.D.) outer needle. The dispensing rate for the CaCl2 solution was 16 ml/min and 12 ml/min for 4% and 3% plain alginate, respectively. The dispensing pressure for composite solution was 20.7 kPa and 13.8 kPa for 4% and 3% alginate, respectively.
2.4 Mechanical Testing
All printed vascular conduits were soaked in a calcium chloride solution for 24 hours in order to minimize the effect of residence time in the CaCl2 solution. Three different random segments for each sample were fabricated to evaluate mechanical characterization using a Biotense Perfusion Bioreactor (ADMET, Inc. Norwood, MA) (see fig. 2).
Fig. 2.
Biotense Perfusion Bioreactor: (a) tensile test system, (b) sample grips
The mechanical testing unit consisted of a linear actuator, sample grips, a bioreactor frame, and a 250 g load cell. The load cell and closed-loop servo-controlled actuator can measure a maximum tensile load of 2 N and provide a stroke of 25 mm, respectively. The samples were mounted in the grips between pieces of sandpaper (to minimize slip), leaving a sample gauge length of 6–8 mm for mechanical loading. The vascular conduits were loaded to failure at a rate of 10 mm/min. Samples that failed at the grips were discarded from analysis. Load-displacement data was recorded at 1 Hz through a data acquisition system (MTestQuattro System, ADMET, Inc., Norwood, MA).
2.5 Perfusion Test
The main function of vascular conduits is to provide nutrients and oxygen to surrounding tissues as well as take away the waste. In order to develop vascular conduits biomimetically, it is essential to evaluate the permeability capability of the engineered vascular conduits. Thus, a perfusion system, which consisted of a media reservoir, a peristaltic pump (ISMATEC, IDEX Corporation, Glattbrugg Switzerland), and a perfusion chamber (with a cover to prevent evaporation) (see fig. 3a), was developed. A peristaltic pump was selected to provide pulsatile media flow, and cell media was perfused from the media reservoir, through the pump and the vascular conduit, and pumped back to the media reservoir. Gauge 25 (0.25 mm I.D., 0.52 mm O.D.) needles were inserted into the fabricated vascular conduits. Surgery clips were used to fix the vascular conduits during perfusion without leakage. Several combinations of fabrication parameters (i.e., composite dispensing pressure and CaCI2 dispensing rate) were tested to obtain the ideal core diameter to obtain a best match for a gauge 25 needle. The criteria for the fabrication parameter selection was that the lumen diameter of the dispensed vascular conduit should be exactly same as the size of a gauge 25 needle’s outer diameter, such that the needle could be inserted into the vascular conduit tightly and no leakage should be allowed at the connection of the conduit and the needle. The original length of the perfusable vascular conduits was fixed at 8 cm. Perfusion experiments were conducted with a 20 ml/min perfusion flow rate for 1 hour. Elongation and diffusion rate measurements were conducted immediately after 1 hour of perfusion. The diffusion rate and elongation were measured for 4% alginate vascular conduits, 4% alginate-0.5% MWCNT-reinforced vascular conduits, and 4% alginate-1% MWCNT vascular conduits.
Fig. 3.
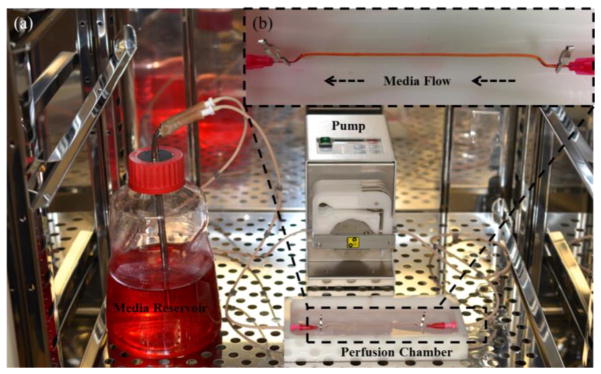
(a) Perfusion system, (b) with media flow through the vascular conduit
2.6 Cell Viability
Immediately after printing, samples were washed with HBSS supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 2.5 μg/ml fungizone for sterilization before incubation. After washing, vascular conduits were cultured at 37° C in 5% CO2. Cell viability assays were performed immediately after printing, as well as after three days in vitro culture to evaluate cell survival. Plain vascular conduits with alginate served as a control group. Vascular conduits 5 cm in length were printed for each sample. For cell viability analysis, samples underwent fluorescent microscopic examination. Vascular conduits were stained with calcium acetoxymethylester (calcein AM) and ethidiumhomodimer-2 (Invitrogen) at a concentration of 1.0 mM each. Calcein AM stains live cells for green, while ethidiumhomodiumer-2 stains dead cells red. After a 30-minute incubation period, vascular conduits were imaged under a Leica fluorescent microscope (Leica Microsystems Inc., Buffalo Grove, IL, USA). Images were collected from three different locations randomly chosen from each sample. ImageJ (National Institutes of Health, Bethesda, MD, USA) was used for automated counting of red- and green-stained HCASMCs in each image, and percentages of viable cells were calculated. Cellular droplets prior to printing were used for initial control for all groups.
2.7 Tissue Histology
Histochemistry was applied to sections of vasculature. After 6 weeks of in vitro culturing in smooth muscle cell differentiation media, frozen vascular conduits were fixed and sectioned at 5 μm for histological examination for markers specific to smooth muscle cells. Verhoeff–Van Giesen method was used to visualize elastic fiber formation and collagen deposition. Elastic fibers stain a blue black to black color, while collagen stains a red color. Samples were examined under an Olympus BX-61 Brightfield-Fluorescent microscope (Olympus America, Melville, NY, USA) at different magnifications.
2.8 Statistical Analysis
The statistical significance of experimental data for the mechanical and perfusion tests was determined by one-way analysis of variance (ANOVA) with a significance level of p<0.05 in Minitab 16. The paired-wise test was combined with the Tukey post-hoc test at a significance level of p<0.05 and used for the cell viability study in the Statistical Package for the Social Sciences (SPSS). Three samples were used for each experimental group (n=3).
3. Results
3.1 Fabrication
In this study, vascular conduits were fabricated using MWCNT-reinforced alginate and plain alginate (see fig. 4a). During the fabrication, the alginate dispensing pressure was set at 17.2 kPa, and the calcium chloride dispensing rate was set at 16 ml/min. The printed vascular structures were obtained in the form of conduits with well-defined tubular wall and lumen (see fig. 4b). The average inner and outer diameters of the fabricated vascular conduits were 461±68 μm and 930±43 μm, respectively. Vascular conduits were fabricated at more than a meter long with successful perfusion capability, demonstrating their functionality. Conduits could be manufactured at any desired length and pattern through printing without producing any blockage or rupture that resulted in a leak or burst. Successful perfusion through a meter-long vascular conduit was achieved as shown in Fig. 4c. In addition, they were highly permeable, which enabled diffusion of media in a radial direction similar to natural blood vessels. Moreover, they could be printed layer by layer, and a 3D printed structure with eight layers or more was achieved (data not shown). A vascular conduit with controllable dimensions in the micro- and sub-millimeter scales can be printed by altering the process parameters. For example, fig. 4b shows the wall and lumen under light microscopy. The wall dimensions could be controlled by controlling the process parameters, instantaneously resulting in varying diameters across the length.
Fig. 4.
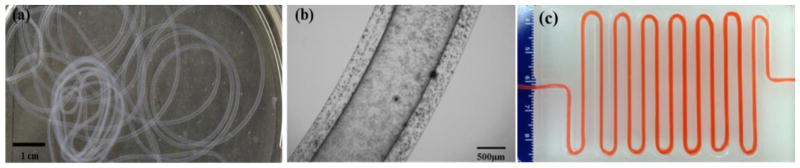
Sample vascular conduits: (a) printed vascular conduits, (b) a zoomed image under light microscopy showing the wall and lumen, and (c) cell media successfully perfused through a meter-long printed conduit.
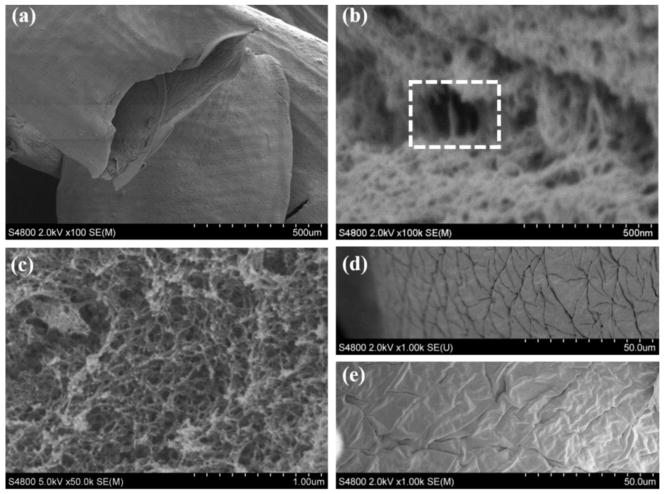
Scanning electron microscope (SEM) figures of vascular conduits with 1% MWCNT and plain alginate are shown in fig. 5. Figure 5a shows the lumen of vascular conduits, where an acceptable cylindricity was obtained, demonstrating the structural integrity of the conduits. Figure 5b shows the structure of a MWCNT-reinforced composite vascular conduit at the fracture, where fibrous MWCNTs are highlighted within the dashed rectangle. A similar structure did not appear in a 4% plain alginate conduit, as presented in fig. 5c. In both cases, sponge-like shapes were obtained at the fracture. The inner and outer walls of the vascular conduit are illustrated in figs. 5d and 5e, respectively. Deformation was observed to some extend during the dehydration process.
Fig. 5.
SEM images of vascular conduits: (a) structural integrity showing tubular shape, (b) 4% alginate reinforced with 1% MWCNTs with highlighted fibrous MWCNT at the fracture site of vascular conduits (see the dashed rectangle), (c) 4% plane alginate vasculature at the fracture site without appearance of fibrous shape in the spongy architecture, (d) inner wall of vascular conduit reinforced with 1% MWCNT, and (e) outer wall of vascular conduit reinforced with 1% MWCNT.
3.2 Mechanical Characterization of Vascular Conduits
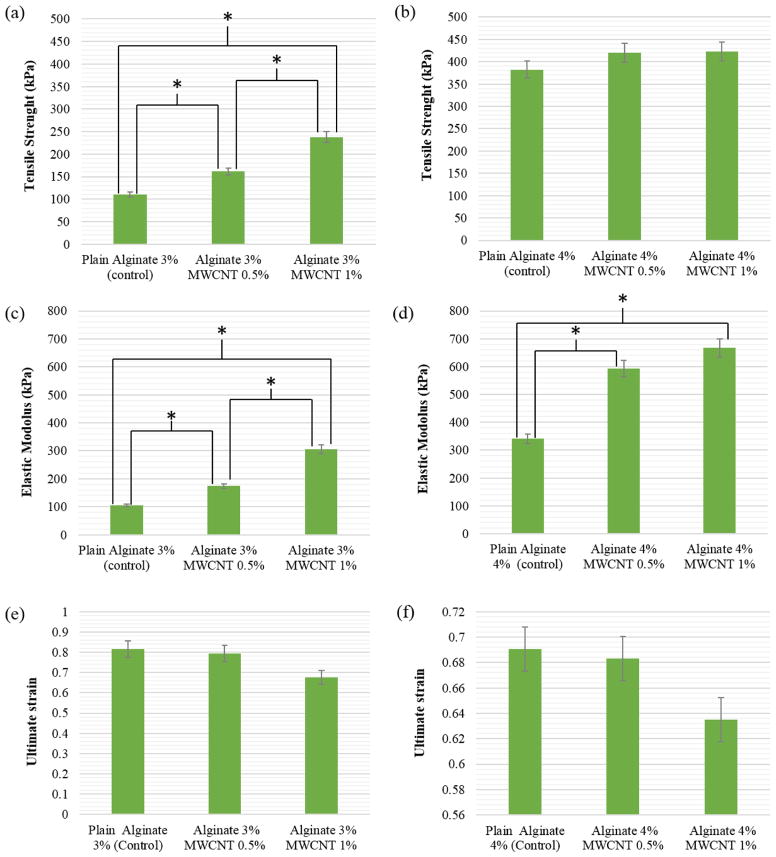
The results of the tensile stress test showing tensile strength, elastic modulus, and ultimate strain of vascular conduits for all types of samples are presented in fig. 6. From the plots, one can compare the mechanical properties of samples with and without MWCNT and different concentrations of biomaterial solution. Figure 6a shows that the tensile strength increased significantly with each addition of MWCNT from 110±5.8 kPa in 3% alginate only gels to 161±24 kPa in gels with 0.5% MWCNT and 238±14 kPa in gels with 1% MWCNT. The tensile strength of 4% alginate also increased with the inclusion of MWCNT from 382±19 kPa in alginate alone gels to 420±22 kPa in gels 0.5% MWCNT to 422±22 kPa in gels with 1% MWCNT (see fig. 6b). As presented in fig. 6c, elastic modulus for 3% alginate gels also increased significantly with the addition of MWCNT from 105±7.5 kPa to 174±13 kPa and 305± 17.5 kPa, for alginate alone, alginate with 0.5% MWCNT, and alginate with 1.0% MWCNT, respectively. Using the same reinforcement percentages, elastic modulus also increased from 341±23 kPa to 593±28 kPa and 667±35 kPa for 4% alginate (fig. 6d). For groups in Figs. 6c and 6d, there was a significant difference in the elastic modulus between plain alginate and the reinforced ones; however, no significant difference was observed in the elastic modulus between the reinforced ones with different MWCNT concentrations for 4% alginate.
Fig. 6.
Comparison of (a) tensile strength of 3% alginate with 0.5% and 1% MWCNT-reinforced 3% alginate, (b) tensile strength of 4% plain alginate with 0.5% and 1% MWCNT-reinforced 4% alginate, (c) elastic modulus of 3% plain alginate with 0.5% and 1% MWCNT-reinforced 3% alginate, (d) elastic modulus of 4% plain alginate with 0.5% and 1% MWCNT-reinforced 4% alginate, (e) ultimate strain of 3% plain alginate with 0.5% and 1% MWCNT-reinforced 3% alginate, (f) ultimate strain of 4% plain alginate with 0.5% and 1% MWCNT-reinforced 4% (* represents statistically significant difference p<0.05).
In contrast to tensile strength and elastic modulus, ultimate strain decreased (though not significantly) for both 3% and 4% alginate gels as MWCNT concentration was increased (see fig. 6e, 6f). In 3% alginate gels, ultimate strain decreased from 0.82 ± 0.18 to 0.79 ± 0.03 and 0.68 ± 0.18 for gel alone, gel with 0.5% MWCNT and gel with 1% MWCNT, respectively. In 4% alginate gels, ultimate strain decreased from 0.69 ± 0.09 to 0.68 ± 0.13 and 0.64 ± 0.22 for gel alone, gel with 0.5% MWCNT and gel with 1% MWCNT, respectively.
Samples with higher concentration of alginate and MWCNT had higher tensile strength and elastic modulus and lower ultimate strain compared to samples with lower concentrations of these components. Differences in these mechanical properties can be explained in part by the properties of the alginate itself. The higher the concentration and viscosity of the alginate solution, the stronger are the intermolecular interactions between polymer chains and the more entanglements form [18]. Therefore, one would expect that the 4% alginate gel’s increased tensile strength and elastic modulus and decreased ultimate strain can be attributed to greater cohesion between the polymer chains due to more physical entanglements. These could be due to distribution, dispersion and alignment of the MWCNT in alginate, which could affect the physical and mechanical properties and the orientation of nanomaterial along the vascular conduits. The addition of reinforcing MWCNT also affected the mechanical properties, although much more so in 3% alginate gels than in 4% alginate gels. The distribution and dispersion of the MWCNT in alginate appears to affect the microstructure of the gel, which in turn can control the physical properties of the vascular conduits. The incorporation of MWCNT in a lower alginate solution likely improved the mechanical properties of the conduits more substantially than in the higher concentration alginate solutions due to the presence of fewer polymer chain entanglements. If the ratio of MWCNT to alginate concentration had been maintained for both 3% and 4% alginate gels it is possible that the inclusion of MWCNT would have produced a stronger affect in the 4% gels.
3.3 Perfusion Study
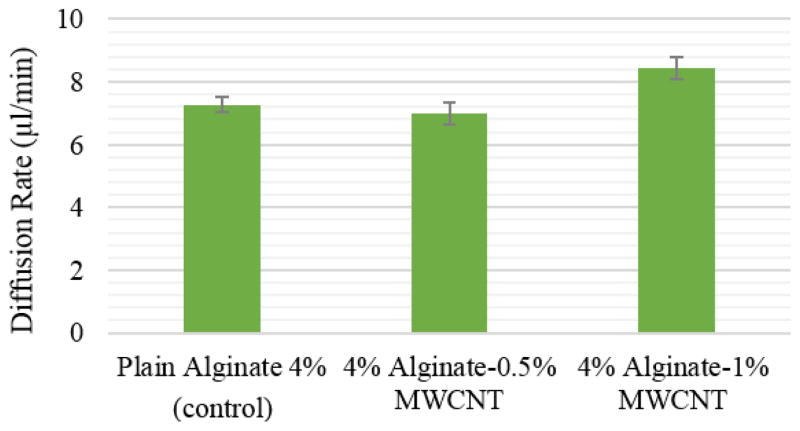
The results of the perfusion study show that all vascular conduits elongated from 8 cm to 8.4±0.05 cm after one hour of media perfusion. This was primarily caused by the weight of vascular conduits. Due to gravity, the weight of the perfused media as well as the weight of the vascular conduit itself stretched the conduit toward the bottom of the perfusion chamber. Elongation terminated as long as the vascular conduit reached the bottom of perfusion chamber. The diffusion rates for three hours of perfusion are shown in fig. 7. The diffusion rate for 4% plain alginate, 4% plain alginate-0.5% MWCNT, and 4% alginate-1% MWCNT were 7.2±0.48 μl/min, 8.4±0.68 μl/min, and 7±0.71 μl/min, respectively. Although the 4% alginate to 1% MWCNT group had the highest diffusion rate, no statistical difference was observed among those three groups, which might be due to the negligible effect of MWCNT reinforcement on the permeability of the alginate polymer structure. In other words, the pore size of alginate polymer, which is approximately 30–450 nm for 4% [20], is much greater than the diameter of MWCNT (approximately 30 nm, according to the manufacturer’s specifications), and hence the diffusion of media was not affected by the reinforcement of MWCNTs at low concentration. However, one can speculate that the diffusion rate could decrease if the reinforcement concentration increased significantly.
Fig. 7.
Effect of MWCNT concentration on diffusion rate of vascular conduits.
3.4 Cell Viability
As demonstrated in fig. 8a, the printing process caused some cell damage; quantitative red-fluorescent-labeled dead cells were observed from the fluorescence image along the conduit channel wall. Compared with the initial control, cell viability was decreased from 75.6±0.04% to 53.3±0.01% for plain alginate vascular conduits, and from 72.2±0.02% to 53.9±0.01% for MWCNT-reinforced upon printing (see fig. 8a). Nevertheless, cells were able to recover from damage during in vitro culture after the printing process, as cell viability in day 3 reached 71.3±0.02% and 68.8±0.01% for plain alginate and MWCNT-reinforced vascular conduits, respectively. These percentages were close to the initial control group. This demonstrated that cells were not completely dead (see fig. 8b); instead they might be damaged to some extent and able to recover and grow in culture.
Fig. 8.
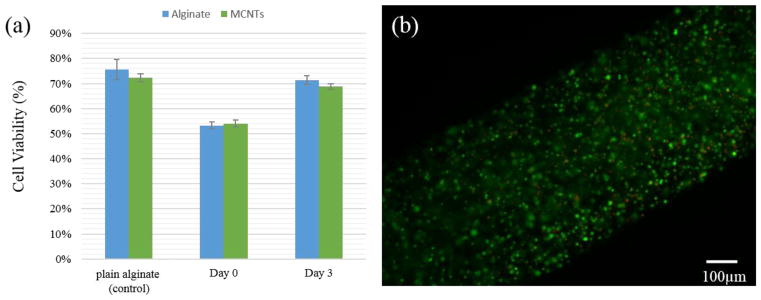
(a) Cell viability over time in plain alginate vascular conduits and MWCNT-reinforced ones, (b) fluorescent microscopic image from three-day cultured MWCNT-reinforced vascular conduit showed most of the cells are viable (green), and a minimal amount of dead cells (red) were also observed.
No significant difference was observed between the control group and the MWCNT-reinforced ones. Thus, the low concentration of MWCNT reinforcement in this research did not affect the viability of HCASMC in short-term in vitro culture; however, biodegradable nanofibers are preferred for long-term culture or in vivo studies while the living environment cannot absorb CNTs by natural means.
3.5 Tissue Histology
The tissue histology study showed different characteristics between CNT-free (positive control group) and CNT-reinforced conduits. In CNT-reinforced conduits, damaged cells within the conduit wall can be easily identified by broken cell nuclei, as indicated by the red arrowheads in figs. 9a and 9b. In CNT-free conduits, the majority of the cells were intact, with rounded nuclei uniformly distributed within the conduit wall (see fig. 9c, blue arrowheads). Some live cells were still observed, as shown in fig. 9b. In addition, a number of cells migrated to the outermost section of the conduit wall and proliferated, forming a well-aligned cellular layer with substantial ECM formation, as shown in the dashed box in fig. 9c. In CNT-reinforced conduits, almost no ECM formation was observed, and elastin and collagen were stained negative. On the other hand, plain conduits stained positive for collagen as shown in fig. 9c and stained positive for elastin in some other histological sections (data not shown here).
Fig. 9.
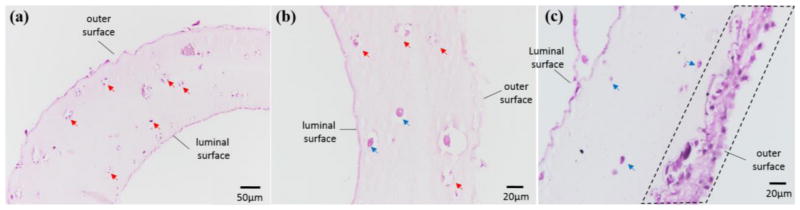
CNT-reinforced conduits: (a) transverse section showing damaged cells (shown with red arrows) within conduit wall, (b) radial longitudinal section showing damaged cells (shown with red arrows) within conduit wall, (c) positive control group without CNT reinforcement, where cells were healthy (shown with blue arrows) and produced substantial matrix in the outer section of the wall in 6 weeks (highlighted in dashes).
4. Discussions
Our experiments with printing CNT-reinforced vascular conduits were successfully demonstrated using sodium alginate because of its appealing gelation properties, which were highly suitable for the coaxial printing process. Using printing, complex shapes in 3D can be fabricated through layer-by-layer stacking of vascular conduits. In general, vascular conduits with an external diameter ranging from 500 μm to 2 mm and a lumen diameter ranging from 150 μm to 1 mm can be printed using the demonstrated system. One of the major advantages of the introduced bioprinting process is that it enables direct printing of the vascular conduits without the need for any temporary support material that is to be removed thereafter, such as thermo-sensitive hydrogels, i.e., agarose and collagen.
In order to fabricate functional vascular conduits, one should ensure that the mechanical, structural, biological and perfusion capabilities of vascular conduits are acceptable. In our experiments, we reached an 8.2±0.3 μL/min diffusion rate within three hours of perfusion, which enabled high viability of encapsulated cells due to the super-diffusive properties of alginate. As alginate concentration increases, mesh network size diminishes, and a compact structure forms, resulting in slower diffusion of the media [19]. In the meantime, mechanical and structural characteristics of the vascular conduits decrease as the material concentration increases. In general, viability and cell migration capability decrease as the concentration of alginate increases. Thus, there is a tradeoff between functionality and the cell viability and biological performance of the conduits. Our initial trials with low concentrations of alginate, such as 2.5%, showed that the bioprinting system did not produce structurally well-defined, mechanically acceptable perfusable conduits. Most of the time, the vascular conduits collapsed when low concentration ranges were used. Therefore, nano-fiber reinforcement (such as natural polymers and proteins) might be ideal for enhancing the mechanical, structural and perfusion characteristics of vascular conduits with biologically reasonable properties.
Carbon nanotube reinforcement increased the ultimate tensile stress by 11% and the modulus of elasticity by 94%. It decreased the ultimate strain by 18%. We achieved 70% cell viability three days post-printing that increased thereafter in short-term culture. In general, cells proliferate and reach over 90% viability in a week [16]. Particularly, the reinforcement enhanced the mechanical properties of low-concentration alginate. The improvement of ultimate tensile stress and elastic modulus demonstrated that the stress was transferred between the hydrogel matrix and the carbon nanotubes reinforced in the vascular conduit’s composites. In addition, the external stress was effectively transferred from the alginate matrix to the reinforced MWCNTs through the better bonded interfaces in alginate-reinforced MWCNT composites [22–23]. The interfacial adhesion between CNTs and the matrix is one of the crucial factors affecting the mechanical properties of the composites. In fact, MWCNTs have a greater affinity to the polymer matrix, resulting in a significant improvement in mechanical properties [22–23].
As demonstrated in the histology images, cells within CNT-reinforced conduits were damaged and underwent cell death. They were not able to repopulate or to produce ECM in the long term. Nevertheless, in plain vascular conduits (the positive control group), cells were largely intact and were able to migrate and proliferate towards the outer section of the channel wall. We can speculate that it was probably guided by the media gradient during culture, since the outer section interacted with cell culture media extensively, especially smooth muscle cell growth factors. Also, repopulated cells were able to produce intercellular ECM and form a sheet covering the outer section of the channel wall. Some matrix formation was observed in the inner surface of the wall as a lining, and that was separated from the wall during the cutting process of the histology study. However, matrix formation in the inner surface could be improved by keeping the pulsatile media flow through the lumen. In this way, HCASMCs could align their proliferation with respect to the pulsatile flow as in the natural blood vessels. Although a cell viability test showed acceptable cell viability in CNT-reinforced conduits in short-term culture, only a limited number of cells were able to survive and carry out their functions properly in long-term culture.
Despite their benefit in improving the mechanical properties of conduits, CNTs have side effects such as toxicity in long-term culture or in vivo testing [21]. Thus, in order to exploit the mechanical benefits of printed fiber composites, other non-toxic materials should be investigated. Biodegradable materials, such as polylactide, polyglycolide, and its derivatives, can be added to alginate in the form of nanofibers to increase the mechanical strength of conduits while also increasing biocompatibility for cell growth and function [24]. Alternatively, short extruded collagen fibers that exist on the mm-micron scale could be added for a similar effect [25]. Collagen type-1 and elastin are the essential components of natural blood vessels that give ultimate mechanical properties (strength and elasticity). Matrix deposition including these proteins was observed in the positive control group, and reinforcing them further during the printing process could improve the mechanobiological characteristics of the vascular conduits considerably. In this regard, the fabricated vascular conduits can be tested in vivo safely for long-term monitoring of the performance of the conduits.
5. Conclusion
In this paper, we demonstrated a new practical technique for vasculature fabrication, where micro vascular conduits were directly printed using a coaxial nozzle configuration. Vascular conduits in this work were reinforced with CNTs to improve the mechanical properties that are essential for further applications such as generating blood vessels or producing supplement vasculatures for engineered tissues. Mechanical and perfusion testing was conducted for CNT-reinforced vascular conduits, and biological characterization was performed in the short and long term to understand cellular viability and extracellular matrix formation. Although short-term results were acceptable, CNT-induced toxicity reduced the biological performance of the conduits.
In sum, this work provides a foundation for direct printing of mechanically reinforced vascular conduits, where CNTs could be replaced with natural polymer nanofibers for further applications such as scale-up tissue fabrication or blood vessel generation. Effectiveness of natural polymer fibers has already been demonstrated in the literature [25]. For future studies, we will thus reinforce electro-spun collagen type-1 nanofibers along with lower percentages of polymer solution and generate an endothelial lining inside the lumen to biomimetically fabricate a vasculature network. The network will be integrated with tissue printing process using the Multi-Arm Bioprinter published in our recent work [26]. It will promote oxygenation and media transport, be a part of the hybrid tissue in short period of time and degrade eventually.
Acknowledgments
This study was supported by the National Institutes of Health (NIH) and the Institute for Clinical and Translational Science (ICTS) grant number ULIRR024979, and by a U.S. Department of Education Graduate Assistance in Areas of National Need Fellowship (GAANN P200A120071). The authors would like to thank Prof. David Cwiertny from The University of Iowa’s Civil and Environmental Engineering Department for providing MWCNTs.
References
- 1.L’Heureux N, Dusserre N, Konig G, Victor B, Keire P, Wight TN, McAllister TN. Human tissue-engineered blood vessels for adult arterial revascularization. Nature medicine. 2006;12(3):361–365. doi: 10.1038/nm1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yow KH, Ingram J, Korossis SA, Ingham E, Homer-Vanniasinkam S. Tissue engineering of vascular conduits. Br J Surg. 2006;93:652–661. doi: 10.1002/bjs.5343. [DOI] [PubMed] [Google Scholar]
- 3.Yildirim E, Yin X, Guceri S, Sun W. A Preliminary Study on Using Multi-Nozzle Polymer Deposition System to Fabricate Composite Alginate/Carbon Nanotube Tissue Scaffolds. Proceedings of 17th Solid Freeform Fabrication Symposium; pp. 84–95. [Google Scholar]
- 4.Kai D, Prabhakaran MP, Stahl B, Eblenkamp M, Wintermantel E, Ramakrishna S. Mechanical properties and in vitro behavior of nanofiber–hydrogel composites for tissue engineering applications. Nanotechnology. 2012;23(9):095705. doi: 10.1088/0957-4484/23/9/095705. [DOI] [PubMed] [Google Scholar]
- 5.Bu H, Kjøniksen AL, Knudsen KD, Nyström B. Rheological and structural properties of aqueous alginate during gelation via the Ugi multicomponent condensation reaction. Biomacromolecules. 2004;5(4):1470–1479. doi: 10.1021/bm049947+. [DOI] [PubMed] [Google Scholar]
- 6.Coleman JN, Khan U, Blau WJ, Gun’ko YK. Small but strong: a review of the mechanical properties of carbon nanotube–polymer composites. Carbon. 2006;44(9):1624–1652. [Google Scholar]
- 7.Augst AD, Kong HJ, Mooney DJ. Alginate hydrogels as biomaterials. Macromolecular Bioscience. 2006;6(8):623–633. doi: 10.1002/mabi.200600069. [DOI] [PubMed] [Google Scholar]
- 8.Spitalsky Z, Tasis D, Papagelis K, Galiotis C. Carbon nanotube–polymer composites: chemistry, processing, mechanical and electrical properties. Progress in Polymer Science. 2010;35(3):357–401. [Google Scholar]
- 9.Yakobson BI, Avouris P. Carbon Nanotubes. Springer; Berlin Heidelberg: 2001. Mechanical properties of carbon nanotubes; pp. 287–327. [Google Scholar]
- 10.Salvetat JP, Bonard JM, Thomson NH, Kulik AJ, Forro L, Benoit W, Zuppiroli L. Mechanical properties of carbon nanotubes. Applied Physics A. 1999;69(3):255–260. [Google Scholar]
- 11.Andrews R, Weisenberger MC. Carbon nanotube polymer composites. Current Opinion in Solid State and Materials Science. 2004;8(1):31–37. [Google Scholar]
- 12.Yildirim ED, Yin X, Nair K, Sun W. Fabrication, characterization, and biocompatibility of single-walled carbon nanotube-reinforced alginate composite scaffolds manufactured using freeform fabrication technique. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2008;87(2):406–414. doi: 10.1002/jbm.b.31118. [DOI] [PubMed] [Google Scholar]
- 13.Mattson MP, Haddon RC, Rao AM. Molecular functionalization of carbon nanotubes and use as substrates for neuronal growth. Journal of Molecular Neuroscience. 2000;14(3):175–182. doi: 10.1385/JMN:14:3:175. [DOI] [PubMed] [Google Scholar]
- 14.Hu H, Ni Y, Montana V, Haddon RC, Parpura V. Chemically functionalized carbon nanotubes as substrates for neuronal growth. Nano letters. 2004;4(3):507–511. doi: 10.1021/nl035193d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lobo AO, Antunes EF, Machado AHA, Pacheco-Soares C, Trava-Airoldi VJ, Corat EJ. Cell viability and adhesion on as grown multi-wall carbon nanotube films. Materials Science and Engineering: C. 2008;28(2):264–269. [Google Scholar]
- 16.Zhang Y, Yu Y, Chen H, Ozbolat IT. Characterization of printable cellular micro-fluidic channels for tissue engineering. Biofabrication. 2013;5(2):025004. doi: 10.1088/1758-5082/5/2/025004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith B, Wepasnick K, Schrote KE, Cho HH, Ball WP, Fairbrother DH. Influence of surface oxides on the colloidal stability of multi-walled carbon nanotubes: A structure– property relationship. Langmuir. 2009;25(17):9767–9776. doi: 10.1021/la901128k. [DOI] [PubMed] [Google Scholar]
- 18.Khalil SED. Doctoral dissertation. Drexel University; 2005. Deposition and structural formation of 3D alginate tissue scaffolds. [Google Scholar]
- 19.Ozbolat IT, Koc B. 3D hybrid wound devices for spatiotemporally controlled release kinetics. Computer methods and programs in biomedicine. 2012;108(3):922–931. doi: 10.1016/j.cmpb.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Chan AW, Neufeld RJ. Modeling the controllable pH-responsive swelling and pore size of networked alginate based biomaterials. Biomaterials. 2009;30(30):6119–6129. doi: 10.1016/j.biomaterials.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 21.Magrez A, Kasas S, Salicio V, Pasquier N, Seo JW, Celio M, Forró L. Cellular toxicity of carbon-based nanomaterials. Nano letters. 2006;6(6):1121–1125. doi: 10.1021/nl060162e. [DOI] [PubMed] [Google Scholar]
- 22.Thostenson ET, Chou TW. Aligned multi-walled carbon nanotube-reinforced composites: processing and mechanical characterization. Journal of physics D: Applied physics. 2002;35(16):L77. [Google Scholar]
- 23.Boccaccini AR, Gerhardt LC. Carbon nanotube composite scaffolds and coatings for tissue engineering applications. Key Engineering Materials. 2010;441:31–52. [Google Scholar]
- 24.Rim NG, Shin CS, Shin H. Current approaches to electrospun nanofibers for tissue engineering. Biomedical Materials. 2013;8:014102. doi: 10.1088/1748-6041/8/1/014102. [DOI] [PubMed] [Google Scholar]
- 25.Gentleman E, Nauman EA, Dee KC, Livesay GA. Short Collagen Fibers Provide Control of Contraction and Permeability in Fibroblast-Seeded Collagen Gels. Tissue Engineering. 2004;10:421–427. doi: 10.1089/107632704323061780. [DOI] [PubMed] [Google Scholar]
- 26.Ozbolat IT, Chen H, Ozbolat IT. Development of ‘Multi-arm Bioprinter’ for Hybrid Biofabrication of Tissue Engineering Constructs. Robotics and Computer-Integrated Manufacturing. 2014;30(3):295–304. [Google Scholar]