Abstract
Human immunodeficiency virus (HIV) infection commonly results in a myriad of comorbid conditions secondary to immune deficiency. Infection also affects broad organ system function. Although current antiretroviral therapy (ART) reduces disease morbidity and mortality through effective control of peripheral viral load, restricted infection in HIV reservoirs including gut, lymphoid and central nervous system tissues, is not eliminated. What underlies these events is, in part, poor ART penetrance into each organ across tissue barriers, viral mutation and the longevity of infected cells. We posit that one means to improve these disease outcomes is through nanotechnology. To this end, this review discusses a broad range of cutting-edge nanomedicines and nanomedicine platforms that are or can be used to improve ART delivery. Discussion points include how polymer-drug conjugates, dendrimers, micelles, liposomes, solid lipid nanoparticles and polymeric nanoparticles can be harnessed to best yield cell-based delivery systems. When completely developed, such nanomedicine platforms have the potential to clear reservoirs of viral infection.
Keywords: Dendrimers, drug delivery systems, gut associated lymphoid tissue, HIV, HIV reservoir targeted ART, liposomes, lymphoid tissues, micelles, nanomedicine, polymeric nanoparticles
1. INTRODUCTION
1.1. Human Immunodeficiency Virus (HIV)
HIV, a lentivirus, is the cause of acquired immunodeficiency syndrome (AIDS). An estimated 35 million people are infected worldwide. Viral infection is transmitted person to person through intimate contact by unprotected sex, needle sharing, accidental blood borne infection or from mother to child. Virus does not survive alone outside the body and is not transmitted through routine daily activities such as food or food utensils, shaking of hands or close non-intimate contact [1]. The virus, ~100 nm in diameter, is a single-stranded, positive-sense enveloped RNA with a coat comprised of two host cellular lipids. The virus’ outer viral envelope is comprised of gp120 and gp41 that govern viral entry through the CD4 and CCR5 or CXCR4 cell receptors [2, 3]. In addition to the viral core HIV has six regulatory genes (tat, rev, nef, vif, vpr and vpu) that affect its abilities to infect, replicate and assemble within the cell [4–9]. Upon entering its target CD4+ T lymphocytes or mononuclear phagocytes (MP; monocytes, macrophages and dendritic cells), genomic viral RNA is reverse transcribed into double-stranded DNA by the viral enzyme reverse transcriptase. Circular double stranded DNA, which motions to the nucleus is integrated into cellular DNA by the viral enzyme integrase. Once integrated into the host genome, viral genes are transcribed into messenger and genomic RNA and translated into viral proteins; viral RNA and proteins undergo processing and assembly before budding from host cell membranes or endosomes to produce progeny virions used for spreading infection [10, 11]. Infection and ultimate destruction of CD4+ T cells heralds secondary immune deficiency and concomitant co-morbid conditions that include opportunistic infections and primary virus-induced metabolic changes that affect also the function of the central nervous system (CNS), gut and lymphoid tissues [12, 13]. Within days after primary infection HIV invades the nervous system through lymphocytes and macrophages that cross the blood brain barrier (BBB) and serve as Trojan Horses for viral dissemination [14].
1.2. Antiretroviral Therapy (ART)
ART has revolutionized the care of HIV infection with significant reductions in disease morbidities and mortalities [15]. Treatment is generally initiated early in the course of infection and continues throughout the life of the patient [16]. Currently there are more than 26 drugs available on the market or in the latter stages of approval by the United States Food and Drug Administration (USFDA), that target a number of viral life cycle components including viral cell entry (entry inhibitors), reverse transcription (nucleoside and non-nucleoside reverse transcriptase inhibitors), integration (integrase inhibitors) and assembly (protease inhibitors) [17]. Combination ART which includes regimens of at least three drugs from at least two categories, readily suppresses plasma viral load to undetectable levels resulting in maintenance or partial recovery of CD4+ T cell numbers and function [18, 19]. However, life-long daily administration of ART is associated with adherence challenges that include treatment fatigue, drug toxicity, drug-drug interactions and viral resistance. Furthermore, the poor pharmacokinetics and biodistribution of current ART to cellular, subcellular and tissue reservoirs affect long-term drug therapeutic efficacy [15].
1.3. Infection of Lymphoid tissues, Lung and Gut
Overwhelming evidence supports the notion that cells and tissues sheltered from the lymphoid system, such as the CNS, lymph nodes, testes, gut, vaginal epithelium and lungs, serve as viral sanctuaries that complicate HIV treatment. This occurs, in part, because many drugs cannot infiltrate these sites in sufficient concentrations to exert optimal therapeutic responses [20–23]. HIV infection and progression involves three phases. The acute phase is characterized by high viremia and extensive viral replication in lymphoid tissues, including alveolar macrophages in the lung [24, 25]. The onset of the second phase is preceded by secondary immune events. During this phase, replication of the virus occurs mainly in the secondary and tertiary lymphoid tissues such as the lymph nodes and mucosa associated lymphoid tissues [26, 27]. The third stage is characterized by an increase in peripheral viral load and significant depletion of CD4+ T lymphocytes [28]. It has been shown that most of viral replication occurs in activated infected CD4+ T cells of the blood, lymph nodes, spleen, gut associated lymphoid tissue (GALT), and the CNS [29, 30]. Infection is greater in the lymphoid tissue because of the numbers of CD4+ T lymphocytes [30]. Intestinal mucosa is a major target for HIV based upon the route of exposure [31, 32] and GALT is a depot for Th-17 cells, which are responsible for spreading infection. Lymphoid tissues and gut are considered the major reservoirs for HIV replication; therefore targeting antiretroviral therapies to these areas is crucial to HIV eradication.
1.4. Infection of the CNS
The CNS is considered another viral reservoir site, where HIV persists in long-lived cells (e.g. blood borne brain macrophages and microglia), resulting in difficulty of viral clearance by ART and the emergence of drug-resistant viral strains [12]. To date, no drugs are able to eradicate viral reservoirs, thus lifelong treatment with sustained and durable viral suppression is the major goal of current therapies. Of particular importance, the low permeability of current ART across the BBB is associated with higher cerebrospinal fluid viral loads, which further limits the possibility of HIV eradication and can contribute to the development of HIV-associated neurocognitive disorders (HAND) [33]. In the CNS, HIV can further infect and sustain HIV infection [34, 35]. On the clinical side, HAND includes neurological disorders of various severities. This includes severe forms of disease or HIV-associated dementia (HAD), HIV encephalopathy, and milder neurocognitive disorders. HIV-associated neurocognitive impairment is most commonly mild and associated with immunosuppression [19]. Nearly half of all people infected with HIV show some form of neurological dysfunction [36]. In all the combination of CNS HIV infection, life-long medications, the presence of other neurological and non-neurological medical conditions (e.g. cerebrovascular disease, hepatitis C co-infection), and aging-associated neurodegenerative disease may exacerbate or be a cause of HAND [37].
1.5. ART Targeting of Viral Reservoirs
Despite the tremendous progress in antiretroviral therapy, HIV continues to replicate in anatomical and intracellular sites where ART has restricted access. Rebound in viral plasma levels commonly occurs following ART withdrawal [38]. Penetration of ART into viral sanctuaries is limited by blood tissue barriers [39–41]. As noted, viral replication occurs predominantly in the peripheral lymphoid organs such as the spleen, lymph nodes and GALT that are rich in CD4+ T-lymphocytes [42]. These tissues are also the major sites for CD4+ T-cell viral destruction [43]. Delivery of ART to these sites will have a great impact on HIV eradication efforts.
As another important barrier of antiretroviral therapy, the BBB is an active, dynamic, and complex interface between blood and CNS that regulates transport of proteins, molecules and cells and as such maintains the homeostasis of the brain microenvironment. Notable, the BBB plays a key role in shielding the CNS against potentially toxic substances. Structurally, it is formed by a complex system of endothelial cells, astroglia, pericytes, perivascular macrophages and a basal lamina [44, 45]. In contrast to the peripheral vasculature, endothelial cells of the BBB are characterized by lack of fenestrations, reduced pinocytic activity and the presence of strong intracellular tight junctions. The tight junctions between endothelial cells of the BBB have low paracellular permeability which significantly impedes the access of many therapeutic agents [46, 47]. Overcoming the BBB is one of the major hurdles for development of efficient CNS drug delivery strategies that avoid the need for direct administration or the use of high doses with increased risks of adverse side effects [46]. Small molecule drugs are transported across the BBB by either passive or active transport. Several transporters are expressed on the BBB endothelial cells that regulate transport of many lipophilic and hydrophilic drugs into the brain and include efflux (P-glycoprotein (Pgp) and multi-drug resistance-associated protein (MRP)) and influx (system L-transporters, organic anion transporter, organic cation transporter) transporters [1, 48]. Macromolecular therapeutic agents can be transported into the brain parenchyma by receptor-mediated transcytosis, nonspecific adsorptive-mediated transcytosis, or cell-mediated transcytosis. In all, these strategies are only applicable for a limited number of agents. To overcome the limitations of CNS drug delivery, various strategies have been developed that include drug modification, disruption of the BBB and novel drug delivery systems such as nanomedicines [48–50].
2. ART NANOMEDICINES
Nanomedicines have been widely studied as drug delivery platforms due to their versatile and tunable properties, such as large surface to volume ratio, surface charge and hydrophobicity, particle shape, and small and controllable size. Moreover, nanomedicines can be biodegradable, biocompatible and functionalized depending on proper material selection and manufacturing. Numerous nanosized pharmaceuticals have been developed, investigated and applied for the treatment and prevention of human disease [46, 51–53]. They exhibit properties useful in improving drug delivery such as increased drug stability, enhanced intestinal absorption and bioavailability, prolonged pharmacokinetics, optimized drug biodistribution, improved toxicity profiles, and selective drug delivery. Nanomedicines can also significantly improve the permeability of different therapeutic agents across anatomical and physiological barriers [54–56]. Many reviews have been published that focus on specific aspects or types of nanomedicines for the management of HIV infection [57, 58]. Nanomedicine platforms that deliver antiretroviral agents to HIV reservoirs, which includes drug polymer conjugates, dendrimers, micelles, liposomes, solid lipid nanoparticles (SLN), nanosuspensions, polymeric nanoparticles and cell-mediated NP drug delivery are a focus for discussion in this review.
2.1. Drug polymer conjugates
There has been remarkable growth in the use of drug polymer conjugate nanomedicine platforms to improve the delivery of therapeutic agents across physiological barriers. The use of water-soluble polymers for drug, protein and gene delivery offers the advantage of smaller size (around 10 nm), improved drug bioavailability, long circulation time and increased stability compared to that of free therapeutic agents. Attachment of enzymes or biological response modifiers to polymers further improves their stability and pharmacokinetic properties. The main feature of drug polymer conjugates involves the use of a biologically responsive linker to covalently attach the drugs onto biodegradable polymeric materials. These are synthesized with the aim of improving the physicochemical, biopharmaceutic and pharmacokinetic properties of the therapeutic agents [59, 60]. Drug polymer conjugates of nucleoside reverse transcriptase inhibitors have attracted considerable attention to overcome drawbacks associated with limited stability and systemic toxicity of these drugs. Yang et al. [61] investigated improvement of antiretroviral efficacy of nucleoside reverse transcriptase inhibitors by developing a nanosized monophosphate-polymer conjugate delivery system using stavudine (d4T) as a model prodrug (Scheme 1). Conjugation of d4T to chitosan was achieved through a phosphoramide linkage between glucosamine and the nucleoside’s monophosphate. The synthesized chitosan-O-isopropyl-5′-O-d4T monophosphate conjugate exhibited enhanced antiretroviral activity and low cytotoxicity when compared to the native nucleoside d4T.
Scheme 1.
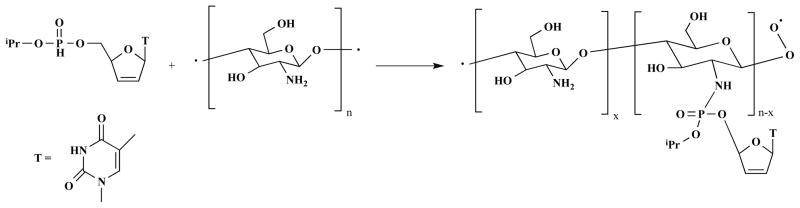
Synthetic scheme for chitosan-O-isopropyl-5′-O-d4T monophosphate conjugate.
Modification of drugs with polyethylene glycol (PEG) materials has led to improved pharmacokinetic and pharmacodynamic profiles of therapeutic agents. Li et al. [62] developed a series of methoxy poly(ethylene glycol)-succinyl-5′-O-zidovudine conjugates (mPEG-succinyl-zidovudine (AZT), Scheme 2), and evaluated their pharmacokinetic and antiretroviral activities in rats and MT-4 cells. The polymeric drug conjugates displayed enhanced pharmacokinetic profiles and antiretroviral activity when compared to native drug.
Scheme 2.
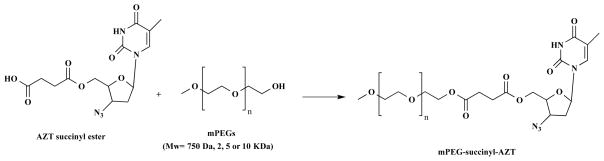
Synthetic route for mPEG-succinyl-AZT conjugates.
Neeraj et al. [63] synthesized a 2-hydroxyethyl methacrylate (HEM) AZT polymeric conjugate and drug release kinetic studies. Their studies demonstrated that the drug-polymer conjugate significantly increased drug uptake and was characterized by a sustained release profile. Indeed, polymeric drug conjugates have shown considerable promise in the delivery of antiretroviral therapies across physiological barriers. However, inadequate linker chemistry, insufficient drug loading and polymer toxicity concerns have constrained their clinical applications.
2.2. Dendrimers
Dendrimers are macromolecules that are comprised of hydrophobic cores and highly branched surface functional groups that make them ideal for transport of drugs across biological barriers. The end groups of these molecules can be functionalized to generate dendrimers that can be used as drug carriers and targeting moieties can be attached that influence biodistribution and toxicity of the dendrimers [64]. Even though no dendrimer-based delivery systems have been approved for HIV treatment, numerous studies are exploring their application in the delivery of antiretroviral drugs to viral reservoirs. Huang et al. [65] evaluated nanoscopic polyamidoamine dendrimers (PMAM) as vectors for gene transfer. For their study, PMAM was converted to PAMAM-PEG-Tf through surface modification with transferrin targeting ligand. Transferrin receptor is expressed at the brain capillaries, thereby forming a solid basis of ligand choice. The authors observed a 2-fold increase in the accumulation of PAMAM-PEG-Tf/DNA complex in the brain when compared side by side with PAMAM/DNA and PMAM-PEG/DNA untargeted complexes. The targeted system therefore holds great promise for efficient delivery of therapeutic agents across barriers. Elsewhere, Dutta et al. [66] investigated the targeted delivery of lamuvidine (3TC) using mannosylated poly(propyleneimine) dendrimers (MPPI). The 3TC loaded MPPI formulation showed a 21-fold increase in drug uptake by MT2 cells when compared to native drug. Similarly, the antiretroviral activity of the 3TC loaded MPPI dendrimers was found to be 2.6-fold higher than the native drug. In an earlier study Dutta et al. [67] developed poly(propyleneimine) (PPI) dendrimer nanocontainers for targeting efavirenz to monocytes/macrophages. These dendrimer based nanocontainers enhanced cell uptake of efavirenz.
In another study by Vinogradov et al. [68], nanogel carriers consisting of dendritic networks decorated with apolipoprotien E (ApOE) peptide molecules were synthesized for delivery of nucleoside reverse transcriptase inhibitors. Antiviral efficacy of the nanocarriers was evaluated in HIV-1 infected monocyte derived macrophages (MDM). It was found that decorating the dendrimers with vector peptides greatly improved antiviral efficacy. These investigators also observed less mitochondrial toxicity associated with targeted nanocarriers as compared to native drugs. Kumar et al. [69] developed PEGylated (EDA)-PAMAM dendrimer-based carriers encapsulating lamivudine. The PEGylated dendrimers were found to improve drug entrapment efficiency and released drug over a prolonged period of time. In addition, hemolytic toxicity studies demonstrated that the dendrimers were less toxic compared to non-PEGylated PAMAM carriers. This report also noted that the formulation could be safely administered. The use of PAMAM dendrimers as carriers for efavirenz was demonstrated by Pyreddy et al. [70] Ethylenediamine PAMAM dendrimers were synthesized and coated with PEG 600 using epichlorohydrin as a cross linker. This system exhibited better therapeutic efficacy due to prolonged and targeted release of the drug payload. Overall, dendrimer carriers have great potential for drug delivery across barriers because of their unique small size and ease of surface functionalization to facilitate drug trafficking. However, inherent toxicity associated with many dendrimers has limited their application.
2.3. Micelles
Micelles are self-assembled colloidal systems consisting of amphiphilic molecules that spontaneously aggregate into particles at a concentration beyond the critical micelle concentration (CMC). A typical micelle has hydrophilic heads forming a shell structure, and the inner core structure serves as a reservoir for poorly water-soluble drugs. Given their small size (10–100 nm), ease of preparation, and prolonged circulation time in vivo, micelles have attracted attention as potential drug carriers across physiological barriers [71]. Indeed, polymeric micelles have been investigated for diverse pharmaceutical applications, including oral delivery, sustained release and site-specific targeting.
Pluronic® block copolymers consist of one hydrophobic poly (propylene oxide) (PPO) at the core, and two hydrophilic poly (ethylene oxide) (PEO) termini (Scheme 3). Previous studies aimed at understanding mechanisms of drug resistance have shown that Pgp and MRP expressed on brain microvascular endothelial cells (BMVEC) regulate passage of certain molecules into the CNS. These transporters limit translocation of systemic drugs [72–74]. The use of Pluronic® copolymers inhibits efflux transporters, including Pgp and MRP, consequently facilitating drug delivery of substrates. Using polarized monolayers of bovine endothelial cells, Batrakova et al. [75] examined the influence of Pluronic® block copolymers on the permeability of drugs, including Pgp substrates, organic anion transporter substrates, and compounds with less specificity for efflux transporters. The results indicated a 1.3- to 20-fold enhancement in the permeability of all the compounds evaluated. Since many antiretroviral drugs are substrates for efflux transporters, antiretroviral agent-loaded Pluronic® micelles were developed for ART penetrance across BMVEC. It was found that Pluronic® P85 could enhance drug tissue permeability of ritonavir [75]. Spitzenberger et al. [76] demonstrated that P85 could facilitate antiretroviral drug efficacy in a severe combined immunodeficiency (SCID) mouse model of viral encephalitis. Interestingly, 0.2% P85 itself also exhibited antiretroviral effects (13.4% HIV-1p24 positive) compared with control group (68.5% HIV-1p24 positive) after 2 weeks treatment. What underlies the inhibition of HIV replication by P85 might be due to direct interaction with Pgp or the glycolipid membrane of HIV, resulting in virus membrane disruption [76]. Another interesting micelle delivery system has been described by Chiappetta et al. [77]. Efavirenz was loaded into poly (ethylene oxide)–poly(propylene oxide) (PEO-PPO) micelles, and anatomically targeted to brain by intranasal administration.
Scheme 3.
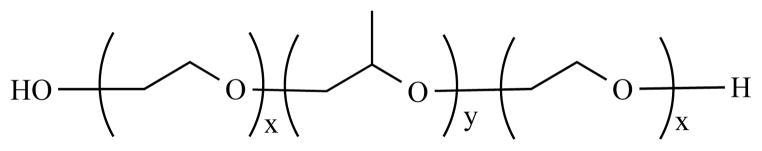
Chemical structure of poloxamer block copolymer.
2.4. Liposomes
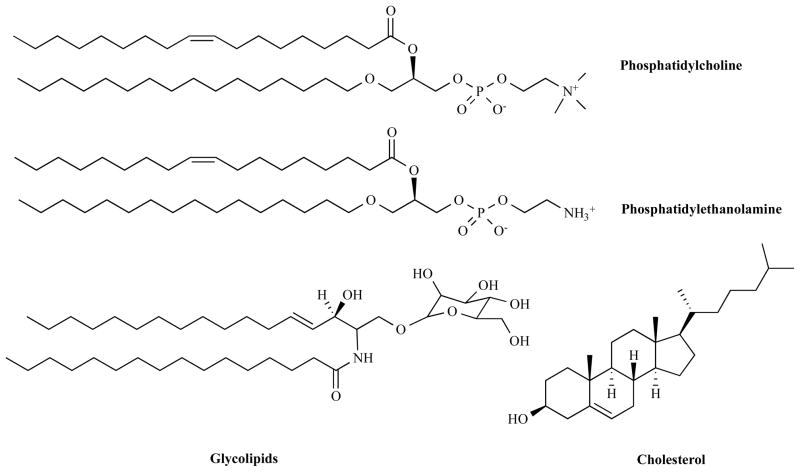
Liposomes are artificially constructed vesicles that consist of an aqueous core separated from the continuous aqueous solvent by one or more spherical, bilayer membranes of surfactant molecules. Liposomes are composed of phospholipids (eg. phosphatidylcholine and phosphatidylethanolamine), and may contain small amounts of other molecules, such as glycolipids that serve as cellular recognition markers and cholesterol that regulates membrane fluidity and stability (Scheme 4). As drug delivery systems, liposomes have the capability of encapsulating both hydrophobic as well as hydrophilic payloads [78]. Liposomes have several other benefits, such as solubilization improvement, protection of cargoes from enzymatic degradation and enhancement of intracellular uptake.
Scheme 4.
Chemical structures of liposomal components.
Liposome formulations have been used to deliver therapeutic agents that cross tissue barriers through vessel fenestrations [79, 80]. PEG coated liposomes have benefits as long circulation carriers for brain and other tissue delivery [81, 82]. Targeted PEG-liposomes coated with brain specific ligands, such as transferrin and insulin whose receptors are highly expressed on endothelial cells are under investigation. In this strategy liposomes are designed to cross tissue barriers through receptor-mediated transcytosis [83, 84]. Alternative approaches to enhance HIV reservoir penetration has been achieved through cell-penetrating peptides (CPP) and antibody conjugation such as OX26 antibody linkage to the liposome surface (immunoliposome) to block transferrin activity [85, 86].
In addition to the above liposomal delivery mechanisms, another mechanism for crossing tissue barriers is through mononuclear phagocytic system targeting by conventional liposomes (not sheltered by PEG) [87]. Kim et al. [88] directly injected zalcitabine loaded multivesicular liposomes intraventricularly into a Sprague-Dawley rat model. The drug half-life in the CNS was significantly increased from 1.1 to 23 hours for the liposomes when compared to native zalcitabine. In a different study, Jin et al. [89] investigated the pharmacokinetics and tissue distribution of zidovudine in rats following intravenous administration of AZT myristate (a prodrug of zidovudine) loaded liposomes. Compared to free AZT, liposome treated rats had a two-fold increase of zidovudine in the brain and other organs of the reticuloendothelial system; therefore liposomes are potential vehicles for improving the therapeutic index of ART. Elsewhere, Saiyed et al. [90] developed magnetic azidothymidine 5′-triphosphate (AZTTP) liposomal nanoformulations. Under the influence of an external magnetic field, AZTTP can cross the BBB and deliver a higher concentration of drug when compared to controls in the absence of a magnetic field.
To target the lymphoid virus reservoir, surface-engineered liposomes have been developed [91]. Kaur et al. modified the surface of zidovudine-loaded liposomes by incorporating surface charge or site-specific targeting ligands. Here negatively charged liposomes had higher accumulation in lymph nodes and spleen compared to positively charged formulations. Most importantly, when coated with mannose, whose receptors are highly expressed on immune cells, liposomes showed much higher accumulation in the lymphoid tissues, suggesting active lymphatic targeting [91]. Another strategy for lymphatic targeting is the utilization of immunoliposomes (liposomes incorporated with antibodies). Human HLA-DR determinant of the major histocompatibility complex class II is expressed on activated CD4+ T lymphocytes and antigen presenting cells including macrophages and dendritic cells, which are reservoirs of HIV-1. By conjugating Fab’ fragments of anti-HLA-DR antibody onto the liposome surface, Bergeron et al. developed indinavir-loaded immunoliposomes, which showed 21- to 126-fold enhanced drug accumulation in the liver, spleen, and lymph nodes when compared to free indinavir [92, 93].
2.5. Solid Lipid Nanoparticles (SLNs)
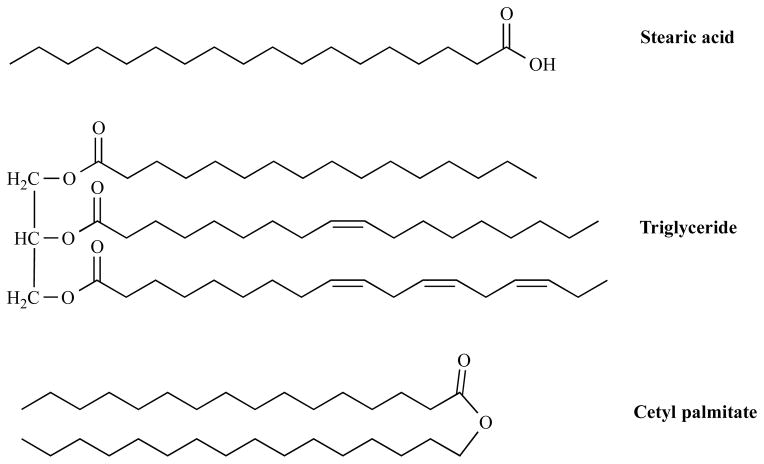
SLNs are solid particles consisting of one or more biocompatible solid lipids, which are stabilized by emulsifiers and/or coemulsifiers (Scheme 5). SLNs have gained increased attention as drug delivery systems because they combine the advantages of polymeric nanoparticles, fat emulsions and liposomes, such as: long retention of encapsulated drugs for several weeks, ease of introduction of targeting ligands to specific tissues and/or cells, superior biocompatibility and biodegradability of lipid excipients, good stability of SLNs for years, excellent loading capability of both hydrophilic and hydrophobic payloads, and avoid recognition and internalization by reticuloendothelial cells [94–99].
Scheme 5.
Representative solid lipids (fatty acids, glycerides, and waxes) for the preparation of SLNs.
In 1999, two independent groups investigated on the use of SLNs for brain and other tissue targeting [97, 100]. The studies showed accumulation of camptothecin and doxorubicin in the brain, lung, heart and spleen following both oral and intravenous administration [97, 100]. The nanoparticles were taken up in the gastrointestinal tract and translocated to reticuloendothelial cell containing organs. Since these organs are HIV targets, SLNs have potentials to be applicable to the targeted treatment of HIV. Similarly, other studies using rat models have demonstrated that both stealth and non-stealth stearic acid labeled SLNs could access the liver, lung and brain tissues [101], with stealth SLNs exhibiting significantly improved drug accumulation when compared to non-stealth SLNs [98]. In general, many excipients for SLN, including poloxamer 188 stabilized stearic acid [102], emulsifying wax/Brij® 78, and Brij® 2/Tween 80 SLNs [103] and targeting ligands have been used to achieve brain and other tissue specific targeting [104].
For CNS delivery of ART, Kuo and Su [105] tested the permeability of stavudine, delavirdine, and saquinavir encapsulated in different nanocarriers, including polymer nanoparticles (NP) and SLN, across an artificial BBB. There was increased permeability of SLN encapsulated drugs compared to free drugs. In addition, delavirdine and saquinavir loaded SLN showed better permeability than those loaded into polymer nanoparticles [105]. The same group also demonstrated that electromagnetic force (EMF) could enhance permeability of saquinavir loaded SLNs, implying that combination of SLN and EMF might benefit a clinical application [106]. Chattopadhyay et al. [107] suggested that SLN could bypass cell efflux pumps. Using a human brain microvessel endothelial cell line (hCMEC/D3), they found a significant improvement of atazanavir uptake when loaded on SLNs in comparison with aqueous solution. Similarly, higher cellular concentration of rhodamine-123, a substrate of Pgp, was also observed [107]. Kuo and Ko [108] used 83-14 MAb, an insulin-like peptidomimetic MAb, as a targeting moiety to further enhance the BBB permeability of saquinavir loaded SLN. However, despite these promising in vitro data, there is no supportive in vivo data so far. Therefore, in vivo studies are necessary to further confirm the authenticity of SLN for the delivery of ART. For lymphatic targeting, SLN also showed promising results. Alex et al. developed lopinavir loaded SLN by a hot homogenization process followed by sonication. The SLN are stable for as long as three months. According to their intestinal lymphatic transport study, the SLN exhibited a 4.9-fold increase of cumulative percentage dose of lopinavir secreted into the lymphoid tissue when compared to the native drugs [109].
2.6. Nanosuspensions
Over the past decade, our laboratory has focused on the development of nanoformulated antiretroviral therapeutics (called nanoART) using high-pressure homogenization and wet milling for targeted long-acting antiretroviral therapy. NanoART are polymer excipient coated drug nanosuspensions, which demonstrate high drug loading capacity, controllable size and charge, and tunable surface conjugation. Our group has developed mononuclear phagocyte (MP; monocyte, macrophage and dendritic cell) targeted nanoART for delivery to HIV reservoirs including lymphoid tissues [110–114]. Using this strategy, drug loaded nanoART can be taken up efficiently by MP in vivo and by translocation deliver high drug concentrations to lymph nodes, spleen and other tissues that harbor the virus with high drug concentration. The drugs are then released into the target sites at levels above the effective therapeutic concentration over prolonged periods. Studies in our group have also shown that immune responses against the virus facilitate movement of the drug carrying MP to these areas of inflammation [115, 116].
To improve pharmacokinetics and pharmacodynamics (PK/PD) of antiretroviral therapy, we further developed nanoART targeted to HIV reservoirs. Previous studies have shown that folate receptor (FOLR) is overexpressed on activated macrophages. Based on these findings, folate-decorated drug delivery systems have been developed to target macrophages for the treatment of inflammatory diseases with improved therapeutic efficacy [117–120]. Using this same strategy we conjugated folic acid onto the coating excipient poloxamer 407 (P407) to generate folate-decorated nanoART (FA-nanoART) for macrophage targeting [121] In vitro studies using a human MDM system, FA-nanoART showed a 2-fold increase in drug uptake, longer drug retention, and superior antiretroviral efficacy over non-targeted nanoART. Similarly, in vivo PK/PD studies showed that FA-nanoART increased the plasma levels of ATV approximately 5-fold over that observed with non-targeted nanoART. This increase was reflected in the tissue levels of ATV. We also noted that FA-nanoART resulted in a 4-fold increase in ATV in spleen compared to non-targeted nanoART. It is noteworthy that ATV levels in lymph nodes were detected weeks after a single administration in only the FA-nanoART group. These results demonstrated that FA-nanoART has potential to cross tissue barriers for efficient HIV suppression.
Other investigators have also utilized nanosuspensions for viral reservoir targeting. Shegokar and Singh [122] prepared surface modified nanosuspensions to improve the intracellular targeting of nevirapine. The surface of the particles was modified with serum albumin, polysaccharide and PEG. Both in vitro and in vivo studies revealed enhanced uptake of the nanosuspensions when compared to free drugs. Pharmacokinetic and tissue distribution studies in rats revealed improved drug accumulation in the spleen, liver and thymus tissues without toxicity [122].
2.7. Polymeric Nanoparticles
There is interest in the use of polymeric nanoparticles to improve outcomes in HIV therapy. Polymers are versatile materials since they can be customized to allow for encapsulation and controlled release of therapeutic agents from the nanoparticles. To facilitate reservoir targeting, polymeric nanoparticles have been decorated with ligands that bind to receptors on the target site. Targeting ligands such as peptides, proteins and antibodies have been shown to promote binding and uptake of nanoparticles encapsulating the drugs [123, 124].
Xia et al. [125] functionalized PEG-PLA nanoparticles with penetratin peptide to facilitate drug targeting. In vivo pharmacokinetic and biodistribution studies showed enhanced cellular and brain uptake of the particles, underscoring its application in the delivery of antiretroviral therapies to viral reservoirs. Polymethylmethacrylate (PMMA) nanoparticles have been applied in the delivery of therapies to HIV reservoirs. Kuo et al. [126] synthesized methylmethacrylate-sulfopropylmethacrylate (MMA-SPM) nanoparticles coated with a synthetic pseudopeptide of bradykinin (RMP-7). Stavudine, delavirdine and saquinavir antiretroviral therapies were encapsulated into the particles and evaluated for individual drug permeability and uptake in co-cultures of human BMVEC and astrocytes. Smaller particle sizes were found to improve drug loading efficiency and uptake. Surfactant coated chitosan based nanoparticles have also attracted attention for drug delivery. Dhanya et al. [127] demonstrated that chitosan nanospheres coated with Tween 80 could be used as alternative carriers for targeted drug delivery. Different strategies have also been used to improve polymeric nanoparticle mediated delivery of antiretroviral agents to viral reservoirs. Modification of the polymers with ligands that target receptors expressed at the surface of endothelial cells has greatly improved this effort. For instance, mannan and mannose coated nanoparticles have been studied as a means to enhance intracellular targeting of antiretroviral therapies [128, 129]. In another study, Destache et al. demonstrated the in vivo feasibility of poly (lactic-co-glycolic acid) nanoparticles to deliver multiple antiretroviral drugs. A single injection of the nanoformulation in mice improved serum and tissue concentrations of ritonavir, lopinavir and efavirenz [130]. In another effort to improve intracellular delivery, Shah and Amiji prepared poly(ethylene oxide)-modified poly(epsilon-caprolactone) (PEO-PCL) nanocarriers for saquinavir. This delivery system enhanced drug encapsulation and subsequent intracellular delivery in a macrophage cell line [131]. Overall, polymeric surface modified nanoparticles provide a versatile platform for targeting HIV sanctuaries.
2.8. Cell-Based NP Drug Delivery
Immunocytes including MP, neutrophils, and lymphocytes are highly mobile towards zones of inflammation, are able to migrate across impermeable barriers and can be exploited for drug delivery [112]. Although the use of immunocytes as drug carriers is still at a preliminary stage, it offers several advantages over use of traditional drug carriers. Immunocytes are spontaneously targeted to sites of injury, inflammation, and tumors; they can also serve as Trojan horses, carrying concealed payloads while migrating across impermeable barriers; in addition this can diminish the immunogenicity and non-specific cytotoxicity of the drug cargoes. Drug loaded nanomedicines, such as liposomes, magnetoliposomes, and nanoparticles can be efficiently taken up by the immunocytes, and then delivered to specific sites of injury for targeted therapy (Fig. 1) [132]. Nanomedicine entry into these cells is also mediated by cell surface receptors, such as mannose, complement, and Fc receptors. Surface conjugation of the nanomedicines with specific targeting ligands significantly enhances the loading capacity of the cells [112].
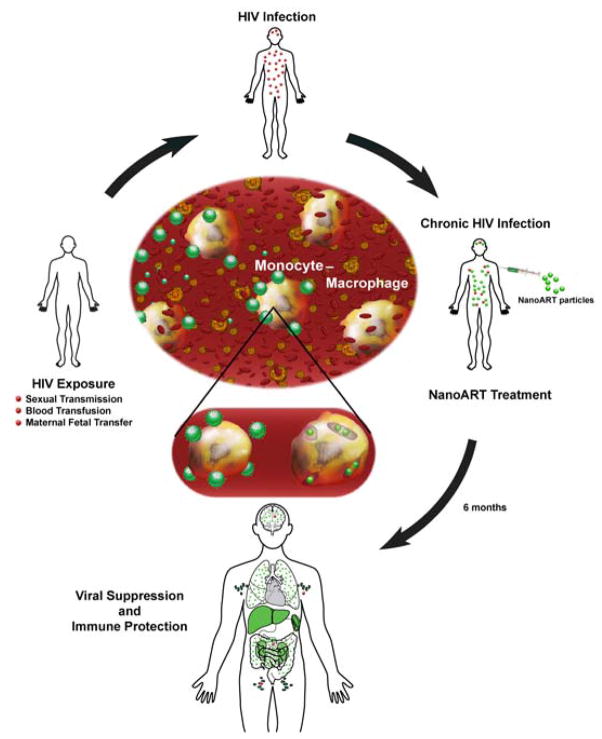
Figure 1. Schematic model illustrating the cell-mediated drug delivery for nanoformulated antiretroviral therapy (nanoART).
This schematic reflects the potential of long-acting antiretrovirals to reduce viral load and protect both CD4+ T cells and viral sanctuaries against HIV-1-associated injuries. Human HIV exposure through sexual transmission, blood transfusions, or maternal/fetal transfer results in chronic HIV infection (red virions). Infected patients are treated with nanoART (green particles), which can gain entry into monocyte-macrophages (center of picture), captured by blood-filtrating liver/splenic tissue macrophages and serve as a long-term drug depot leading to the suppression of viral replication and protection of CD4+ T cell numbers. The nanoformulations may also facilitate ART delivery to the CNS, spleen, lymph nodes and gut associated lymphoid tissue to reduce viral replication (green organs).
Cell-mediated delivery of nanoformulations has gained increasing attention for the treatment of various brain diseases, including brain cancer [133], neurodegenerative disorders [134], and HAND [111, 135]. Parkinson’s disease has been one of the first targets for cell-based therapy [136]. In our group, macrophages have been exploited for targeted ART delivery [135, 137–140]. NanoART with high loading capacity and a size range from 200 to 350 nm was developed with poloxamer and/or phospholipid coatings. An HIV-encephalitis mouse model was used to determine the brain targeting of indinavir nanoART loaded mouse bone marrow macrophages after ex vivo cultivation and loading. NanoART loaded BMM were administered intravenously to mice resulting in continuous indinavir release for up to 14 days. Notably, there was robust indinavir nanoART present in HIVE brain regions, where significant inhibition of HIV replication was observed [141]. To determine intracellular transfer of nanoART Kanmogne et al. [142] loaded MDM with a combination of atazanavir, ritonavir, indinavir, and efavirenz nanoART. The nanoART-loaded MDM were then cocultivated with endothelial cells. NanoART transfer from MDM to endothelial cells was observed in up to 52% of cells, and folate coating of nanoART further increased MDM to BMVEC particle transfer by up to 77% [142]. The results demonstrated that nanoART could transfer NP through cell-to-cell contacts and thus facilitate the penetration of nanoART across the BBB. Another study by Saiyed et al. [90] investigated the potential of azidothymidine 5′-triphosphate magnetoliposomes for cell-based delivery. Magnetoliposomes resulted in an up to 90% monocytes magnetization level. Under an external magnetic field, magnetic monocytes exhibited 3 times greater tissue permeability compared to their non-magnetic counterparts [90, 143].
3. FUTURE PERSPECTIVE
Significant progress has been made to combat HIV/AIDS and improve the quality of life of HIV infected people over the last few decades. The use of ART has successfully shifted HIV infection from a rapidly progressive to a chronic disease and many patients have little to no detectable viral load for a prolonged period of time. However, HIV eradication or cure is still elusive, in part due to the occurrence of drug resistance and viral reservoirs. A long-term goal is to develop nanotechnology-based drug delivery systems that can improve antiretroviral therapy by more precisely controlling drug concentrations in target cells and tissues. To achieve this goal novel innovative and efficient therapies are required. Herein, we have reviewed a broad range of nanomedicine platforms that are being developed for delivery of ART to CNS and other HIV reservoirs. These systems can significantly enhance uptake of antiretroviral agents into HIV-infected cell and tissue reservoirs and improve the pharmacokinetics, pharmacodynamics and biodistribution of antiretroviral agents by using targeting ligands and improving drug delivery across tissue barriers.
It is noteworthy that the current complexity of viral reservoirs of HIV remains significant; as such, a better understanding of these reservoirs holds the potential for translating novel nanomedicine platforms into successful and sustainable effective HIV therapies. However, most current nanomedicine platforms for HIV treatment focus on CNS delivery and improving pharmacokinetic profiles. Minimal effort has been directed at developing drug delivery systems that would target other major HIV reservoirs, such as GALT, lymphoid tissues and lung. Our laboratory and others have shown that targeted delivery to macrophages and other HIV susceptible cells will potentially improve antiretroviral therapy by accessing HIV sanctuary sites. We believe the goal of HIV eradication can be achieved through developing different nanomedicine platforms and decorating nanomedicines with specific ligands to interact with receptors expressed on HIV infected cells. Therefore, identification and characterization of HIV receptor targets will lead to development of targeted delivery systems that will allow for treatment of various HIV variants. To further achieve the goal of HIV eradication, it will be important to devise therapeutic approaches that target both CNS and other viral reservoirs (GALT, lymphoid tissues) since either alone will not be successful. Indeed, the concept of lymphoid targeting has successfully been applied in cancer treatment using ultra-small nanoparticles or decorating ligands onto the nanoparticle surface [144–148]. Simply transferring these nanomedicine platforms into the HIV field holds the promise of targeting ART to GALT and other viral reservoirs. There is no doubt that nanomedicines will play a decisive role in engineering efficacious regimens over the next decade for HIV eradication.
In summary, the nanomedicines described here are still at the pre-clinical stage, and advancement to clinical trials will not guarantee the future clinical success. To advance these platforms beyond the pre-clinical stage, many challenges need to be faced, and the combined efforts of both basic science and translational research will be needed to further nanomedicine research and development. First, nanomedicines should exert superior antiretroviral efficacy. Second, nanomedicines should be able to efficiently overcome biological barriers to achieve the goal of HIV eradication from viral reservoirs. Third, nanomedicines should have an acceptable safety profile. Furthermore, most nanomedicines use only a single antiretroviral agent and one targeting ligand for proof-of-concept studies, thus, further research is needed to develop combinational therapies and multi-targeted nanomedicine platforms for antiretroviral optimization. Future nanomedicines that addresses these challenges may pave the way for HIV eradication and significantly contribute to an improved quality of life and life span for HIV-infected patients.
Acknowledgments
This work was supported in part by the Carol Swarts MD Neuroscience Research Laboratory and National Institutes of Health grants: 1P01 DA028555, 2R01 NS034239, 2R37 NS36126, P01 NS31492, P20 RR15635, P01MH64570, and P01 NS43985 (to H.E.G.).
ABBREVIATIONS
- 3TC
lamuvidine
- AIDS
acquired immunodeficiency syndrome
- ART
antiretroviral therapy
- AZTTP
azidothymidine 5′-triphosphate
- BBB
blood brain barrier
- BMEC
brain microvessel endothelial cells
- BMM
bone-marrow-derived macrophage
- cART
combination antiretroviral therapy
- CMC
critical micelle concentration
- CNS
central nervous system
- CPPs
cell-penetrating peptides
- CSF
higher cerebrospinal fluid
- EMF
electromagnetic force
- EPR
enhanced permeability and retention
- FOLR
folate receptor
- GALT
gut associated lymphoid tissue
- HAART
highly active antiretroviral therapy
- HAD
HIV-associated dementia
- HAND
HIV-associated neurocognitive disorder
- HEM
2-hydroxyethyl methacrylate
- HIV
human immunodeficiency virus
- HIVE
HIV-1 encephalitis
- MDM
monocyte-derived macrophages
- MMA-SPM
methylmethacrylate-sulfopropylmethacrylate
- MND
mild neurocognitive disorder
- MP
mononuclear phagocytes
- MPPI
mannosylated poly (propyleneimine)
- MRP
multi-drug resistance-associated protein
- nanoART
nanoformulated antiretroviral therapeutics
- PEG
polyethylene glycol
- PEI-PEG
polyethyleneimine-poly (ethylene glycol)
- PEO
poly (ethylene oxide)
- Pgp
P-glycoprotein
- PMAM
polyamidoamine dendrimers
- PMMA
polymethylmethacrylate
- PPI
poly (propyleneimine)
- SLNs
solid lipid nanoparticles
- TAT
transcactivating protein of HIV-1
Footnotes
CONFLICT OF INTEREST
The authors confirm no conflict of interest
References
- 1.Gomes MJ, Neves JD, Sarmento B. Nanoparticle-based drug delivery to improve the efficacy of antiretroviral therapy in the central nervous system. International journal of nanomedicine. 2014;9:1757–1769. doi: 10.2147/IJN.S45886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature (London) 1996;381(6584):661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 3.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science (Washington, D C) 1996;272(5263):872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 4.Ruben S, Perkins A, Purcell R, Joung K, Sia R, Burghoff R, Haseltine WA, Rosen CA. Structural and functional characterization of human immunodeficiency virus tat protein. J Virol. 1989;63(1):1–8. doi: 10.1128/jvi.63.1.1-8.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zapp ML, Green MR. Sequence-specific RNA binding by the HIV-1 Rev protein. Nature (London) 1989;342(6250):714–716. doi: 10.1038/342714a0. [DOI] [PubMed] [Google Scholar]
- 6.Kim S, Byrn R, Groopman J, Baltimore D. Temporal aspects of DNA and RNA synthesis during human immunodeficiency virus infection: evidence for differential gene expression. J Virol. 1989;63(9):3708–3713. doi: 10.1128/jvi.63.9.3708-3713.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen EA, Dehni G, Sodroski JG, Haseltine WA. Human immunodeficiency virus vpr product is a virion-associated regulatory protein. J Virol. 1990;64(6):3097–3099. doi: 10.1128/jvi.64.6.3097-3099.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz S, Felber BK, Fenyoe EM, Pavlakis GN. Env and Vpu proteins of human immunodeficiency virus type 1 are produced from multiple bicistronic mRNAs. J Virol. 1990;64(11):5448–5456. doi: 10.1128/jvi.64.11.5448-5456.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strebel K, Daugherty D, Clouse K, Cohen D, Folks T, Martin MA. The HIV ‘A’ (sor) gene product is essential for virus infectivity. Nature (London) 1987;328(6132):728–730. doi: 10.1038/328728a0. [DOI] [PubMed] [Google Scholar]
- 10.Freed EO. HIV-1 replication. Somat Cell Mol Genet. 2001;26(1–6):13–33. doi: 10.1023/a:1021070512287. [DOI] [PubMed] [Google Scholar]
- 11.Perelson AS, Neumann AU, Markowitz M, Leonard JM, Ho DD. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science (New York, NY) 1996;271(5255):1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nature reviews Immunology. 2005;5(1):69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 13.Boisse L, Gill MJ, Power C. HIV infection of the central nervous system: clinical features and neuropathogenesis. Neurol Clin. 2008;26(3):799–819. x. doi: 10.1016/j.ncl.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Meltzer MS, Gendelman HE. Mononuclear phagocytes as targets, tissue reservoirs, and immunoregulatory cells in human immunodeficiency virus disease. Current topics in microbiology and immunology. 1992;181:239–263. doi: 10.1007/978-3-642-77377-8_9. [DOI] [PubMed] [Google Scholar]
- 15.Volberding PA, Deeks SG. Antiretroviral therapy and management of HIV infection. Lancet. 2010;376(9734):49–62. doi: 10.1016/S0140-6736(10)60676-9. [DOI] [PubMed] [Google Scholar]
- 16.Thompson MA, Aberg JA, Hoy JF, Telenti A, Benson C, Cahn P, Eron JJ, Jr, Gunthard HF, Hammer SM, Reiss P, Richman DD, Rizzardini G, Thomas DL, Jacobsen DM, Volberding PA. Antiretroviral treatment of adult HIV infection. 2012 Recommendations of the International Antiviral Society-USA Panel. JAMA J Am Med Assoc. 2012;308(4):387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 17.Michaels SH, Clark R, Kissinger P. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;339(6):405–406. doi: 10.1056/NEJM199808063390612. [DOI] [PubMed] [Google Scholar]
- 18.Brew BJ. Evidence for a change in AIDS dementia complex in the era of highly active antiretroviral therapy and the possibility of new forms of AIDS dementia complex. AIDS. 2004;18(Suppl 1):S75–78. [PubMed] [Google Scholar]
- 19.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. Journal of NeuroVirology. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amiji MM, Vyas TK, Shah LK. Role of nanotechnology in HIV/AIDS treatment: potential to overcome the viral reservoir challenge. Discov Med. 2006;6(34):157–162. [PubMed] [Google Scholar]
- 21.Vyas TK, Shah L, Amiji MM. Nanoparticulate drug carriers for delivery of HIV/AIDS therapy to viral reservoir sites. Expert Opin Drug Delivery. 2006;3(5):613–628. doi: 10.1517/17425247.3.5.613. [DOI] [PubMed] [Google Scholar]
- 22.Moyer MP, Gendelman HE. HIV replication and persistence in human gastrointestinal cells cultured in vitro. Journal of leukocyte biology. 1991;49(5):499–504. doi: 10.1002/jlb.49.5.499. [DOI] [PubMed] [Google Scholar]
- 23.Kalter DC, Gendelman HE, Meltzer MS. Monocytes, dendritic cells, and Langerhans cells in human immunodeficiency virus infection. Dermatologic clinics. 1991;9(3):415–428. [PubMed] [Google Scholar]
- 24.Quinn TC. Acute primary HIV infection. JAMA. 1997;278(1):58–62. [PubMed] [Google Scholar]
- 25.Schacker T, Little S, Connick E, Gebhard-Mitchell K, Zhang ZQ, Krieger J, Pryor J, Havlir D, Wong JK, Richman D, Corey L, Haase AT. Rapid accumulation of human immunodeficiency virus (HIV) in lymphatic tissue reservoirs during acute and early HIV infection: implications for timing of antiretroviral therapy. J Infect Dis. 2000;181(1):354–357. doi: 10.1086/315178. [DOI] [PubMed] [Google Scholar]
- 26.Clark SJ, Saag MS, Decker WD, Campbell-Hill S, Roberson JL, Veldkamp PJ, Kappes JC, Hahn BH, Shaw GM. High titers of cytopathic virus in plasma of patients with symptomatic primary HIV-1 infection. N Engl J Med. 1991;324(14):954–960. doi: 10.1056/NEJM199104043241404. [DOI] [PubMed] [Google Scholar]
- 27.Heise C, Dandekar S, Kumar P, Duplantier R, Donovan RM, Halsted CH. Human immunodeficiency virus infection of enterocytes and mononuclear cells in human jejunal mucosa. Gastroenterology. 1991;100(6):1521–1527. doi: 10.1016/0016-5085(91)90648-5. [DOI] [PubMed] [Google Scholar]
- 28.Donaldson YK, Bell JE, Ironside JW, Brettle RP, Robertson JR, Busuttil A, Simmonds P. Redistribution of HIV outside the lymphoid system with onset of AIDS. Lancet. 1994;343(8894):383–385. doi: 10.1016/s0140-6736(94)91222-x. [DOI] [PubMed] [Google Scholar]
- 29.Pomerantz RJ. Reservoirs of human immunodeficiency virus type 1: The main obstacles to viral eradication. Clin Infect Dis. 2002;34(1):91–97. doi: 10.1086/338256. [DOI] [PubMed] [Google Scholar]
- 30.Blankson JN, Persaud D, Siliciano RF. The challenge of viral reservoirs in HIV-1 infection. Annu Rev Med. 2002;53:557–593. doi: 10.1146/annurev.med.53.082901.104024. [DOI] [PubMed] [Google Scholar]
- 31.Kotler DP. HIV infection and the gastrointestinal tract. AIDS. 2005;19(2):107–117. doi: 10.1097/00002030-200501280-00002. [DOI] [PubMed] [Google Scholar]
- 32.Adachi A, Koenig S, Gendelman HE, Daugherty D, Gattoni-Celli S, Fauci AS, Martin MA. Productive, persistent infection of human colorectal cell lines with human immunodeficiency virus. J Virol. 1987;61(1):209–213. doi: 10.1128/jvi.61.1.209-213.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Morgello S, Simpson D, Grant I, Ellis RJ. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Archives of neurology. 2008;65(1):65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grant I. Neurocognitive disturbances in HIV. International review of psychiatry. 2008;20(1):33–47. doi: 10.1080/09540260701877894. [DOI] [PubMed] [Google Scholar]
- 35.Letendre S, McCutchan JA, Ellis RJ. Highlights of the 15th Conference on Retroviruses and Opportunistic Infections. Neurologic complications of HIV disease and their treatment. Topics in HIV medicine: a publication of the International AIDS Society, USA. 2008;16(1):15–22. [PubMed] [Google Scholar]
- 36.McArthur JC. HIV dementia: an evolving disease. Journal of neuroimmunology. 2004;157(1–2):3–10. doi: 10.1016/j.jneuroim.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 37.Valcour V, Sithinamsuwan P, Letendre S, Ances B. Pathogenesis of HIV in the central nervous system. Current HIV/AIDS reports. 2011;8(1):54–61. doi: 10.1007/s11904-010-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saksena NK, Haddad DeN. Viral reservoirs an impediment to HAART: New strategies to eliminate HIV-1. Curr Drug Targets: Infect Disord. 2003;3(2):179–206. doi: 10.2174/1568005033481187. [DOI] [PubMed] [Google Scholar]
- 39.Gartner S, Markovits P, Markovitz DM, Kaplan MH, Gallo RC, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science (New York, NY) 1986;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 40.Nicholson JK, Cross GD, Callaway CS, McDougal JS. In vitro infection of human monocytes with human T lymphotropic virus type III/lymphadenopathy-associated virus (HTLV-III/LAV) Journal of immunology (Baltimore, Md: 1950) 1986;137(1):323–329. [PubMed] [Google Scholar]
- 41.Levy JA. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993;57(1):183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geeraert L, Kraus G, Pomerantz RJ. Hide-and-seek: the challenge of viral persistence in HIV-1 infection. Annual review of medicine. 2008;59:487–501. doi: 10.1146/annurev.med.59.062806.123001. [DOI] [PubMed] [Google Scholar]
- 43.Nelson JA, Wiley CA, Reynolds-Kohler C, Reese CE, Margaretten W, Levy JA. Human immunodeficiency virus detected in bowel epithelium from patients with gastrointestinal symptoms. Lancet. 1988;1(8580):259–262. doi: 10.1016/s0140-6736(88)90348-0. [DOI] [PubMed] [Google Scholar]
- 44.Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. The Journal of cell biology. 1969;40(3):648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rubin LL, Staddon JM. The cell biology of the blood-brain barrier. Annual review of neuroscience. 1999;22:11–28. doi: 10.1146/annurev.neuro.22.1.11. [DOI] [PubMed] [Google Scholar]
- 46.Nowacek A, Gendelman HE. NanoART, neuroAIDS and CNS drug delivery. Nanomedicine (London, U K) 2009;4(5):557–574. doi: 10.2217/nnm.09.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu X, Chen C, Smith BJ. Progress in brain penetration evaluation in drug discovery and development. The Journal of pharmacology and experimental therapeutics. 2008;325(2):349–356. doi: 10.1124/jpet.107.130294. [DOI] [PubMed] [Google Scholar]
- 48.Misra A, Ganesh S, Shahiwala A, Shah SP. Drug delivery to the central nervous system: a review. Journal of pharmacy & pharmaceutical sciences: a publication of the Canadian Society for Pharmaceutical Sciences, Societe canadienne des sciences pharmaceutiques. 2003;6(2):252–273. [PubMed] [Google Scholar]
- 49.Pathan SA, Iqbal Z, Zaidi SM, Talegaonkar S, Vohra D, Jain GK, Azeem A, Jain N, Lalani JR, Khar RK, Ahmad FJ. CNS drug delivery systems: novel approaches. Recent patents on drug delivery & formulation. 2009;3(1):71–89. doi: 10.2174/187221109787158355. [DOI] [PubMed] [Google Scholar]
- 50.Biddlestone-Thorpe L, Marchi N, Guo K, Ghosh C, Janigro D, Valerie K, Yang H. Nanomaterial-mediated CNS delivery of diagnostic and therapeutic agents. Advanced drug delivery reviews. 2012;64(7):605–613. doi: 10.1016/j.addr.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bawarski WE, Chidlowsky E, Bharali DJ, Mousa SA. Emerging nanopharmaceuticals. Nanomedicine (N Y, NY, U S) 2008;4(4):273–282. doi: 10.1016/j.nano.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 52.Gunaseelan S, Gunaseelan K, Deshmukh M, Zhang X, Sinko PJ. Surface modifications of nanocarriers for effective intracellular delivery of anti-HIV drugs. Adv Drug Delivery Rev. 2010;62(4–5):518–531. doi: 10.1016/j.addr.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Govender T, Ojewole E, Naidoo P, Mackraj I. Polymeric Nanoparticles for Enhancing Antiretroviral Drug Therapy. Drug Delivery. 2008;15(8):493–501. doi: 10.1080/10717540802321776. [DOI] [PubMed] [Google Scholar]
- 54.Destache CJ. Chapter 12- Brain as an HIV sequestered site: Use of nanoparticles as a therapeutic option. Progress in brain research. 2009;180:225–233. doi: 10.1016/S0079-6123(08)80012-X. [DOI] [PubMed] [Google Scholar]
- 55.Nowacek A, Gendelman HE. NanoART, neuroAIDS and CNS drug delivery. Nanomedicine: nanotechnology, biology, and medicine. 2009;4(5):557–574. doi: 10.2217/nnm.09.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong HL, Chattopadhyay N, Wu XY, Bendayan R. Nanotechnology applications for improved delivery of antiretroviral drugs to the brain. Advanced Drug Delivery Reviews. 2010;62(4–5):503–517. doi: 10.1016/j.addr.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 57.das Neves J, Amiji MM, Bahia MF, Sarmento B. Nanotechnology-based systems for the treatment and prevention of HIV/AIDS. Advanced Drug Delivery Reviews. 2010;62(4–5):458–477. doi: 10.1016/j.addr.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 58.Mahajan SD, Aalinkeel R, Law WC, Reynolds JL, Nair BB, Sykes DE, Yong KT, Roy I, Prasad PN, Schwartz SA. Anti-HIV-1 nanotherapeutics: promises and challenges for the future. International journal of nanomedicine. 2012;7:5301–5314. doi: 10.2147/IJN.S25871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haag R, Kratz F. Polymer therapeutics: concepts and applications. Angew Chem, Int Ed. 2006;45(8):1198–1215. doi: 10.1002/anie.200502113. [DOI] [PubMed] [Google Scholar]
- 60.Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discovery. 2003;2(5):347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 61.Yang L, Chen L, Zeng R, Li C, Qiao R, Hu L, Li Z. Synthesis, nanosizing and in vitro drug release of a novel anti-HIV polymeric prodrug: Chitosan-O-isopropyl-5′-O-d4T monophosphate conjugate. Bioorg Med Chem. 2010;18(1):117–123. doi: 10.1016/j.bmc.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 62.Li W, Wu J, Zhan P, Chang Y, Pannecouque C, De Clercq E, Liu X. Synthesis, drug release and anti-HIV activity of a series of PEGylated zidovudine conjugates. Int J Biol Macromol. 2012;50(4):974–980. doi: 10.1016/j.ijbiomac.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 63.Neeraj A, Chandrasekar MJN, Sara UVS, Rohini A. Poly(HEMA-Zidovudine) conjugate: A macromolecular pro-drug for improvement in the biopharmaceutical properties of the drug. Drug Delivery. 2011;18(4):272–280. doi: 10.3109/10717544.2010.536272. [DOI] [PubMed] [Google Scholar]
- 64.Nanjwade BK, Bechra HM, Derkar GK, Manvi FV, Nanjwade VK. Dendrimers: Emerging polymers for drug-delivery systems. Eur J Pharm Sci. 2009;38(3):185–196. doi: 10.1016/j.ejps.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 65.Huang RQ, Qu YH, Ke WL, Zhu JH, Pei YY, Chen J. Efficient gene delivery targeted to the brain using a transferrin-conjugated polyethyleneglycol-modified polyamidoamine dendrimer. FASEB J. 2007;21(4):1117–1125. doi: 10.1096/fj.06-7380com. doi:1110.1096/fj.1106-7380com. [DOI] [PubMed] [Google Scholar]
- 66.Dutta T, Jain NK. Targeting potential and anti-HIV activity of lamivudine loaded mannosylated poly (propyleneimine) dendrimer. Biochim Biophys Acta, Gen Subj. 2007;1770(4):681–686. doi: 10.1016/j.bbagen.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 67.Dutta T, Agashe HB, Garg M, Balasubramanium P, Kabra M, Jain NK. Poly (propyleneimine) dendrimer based nanocontainers for targeting of efavirenz to human monocytes/macrophages in vitro. J Drug Targeting. 2007;15(1):89–98. doi: 10.1080/10611860600965914. [DOI] [PubMed] [Google Scholar]
- 68.Vinogradov SV, Poluektova LY, Makarov E, Gerson T, Senanayake MT. Nano-NRTIs: efficient inhibitors of HIV type-1 in macrophages with a reduced mitochondrial toxicity. Antiviral Chem Chemother. 2010;21(1):1–14. doi: 10.3851/IMP1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dinesh Kumar P, Vijayaraj Kumar P, Panneer Selvam T, Sambasiva Rao KRS. Prolonged drug delivery system of PEGylated PAMAM dendrimers with a anti-HIV drug. Res Pharm. 2013;3(2):08–17. [Google Scholar]
- 70.Pyreddy S, Kumar PD, Kumar PV. Polyethylene glycolated PAMAM dendrimers-Efavirenz conjugates. Int J Pharm Investig. 2014;4(1):15–18. doi: 10.4103/2230-973X.127735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trivedi R, Kompella UB. Nanomicellar formulations for sustained drug delivery: strategies and underlying principles. Nanomedicine (London, England) 2010;5(3):485–505. doi: 10.2217/nnm.10.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cordon-Cardo C, O’Brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, Bertino JR. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proceedings of the National Academy of Sciences. 1989;86(2):695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Y, Han H, Elmquist WF, Miller DW. Expression of various multidrug resistance-associated protein (MRP) homologues in brain microvessel endothelial cells. Brain Research. 2000;876(1–2):148–153. doi: 10.1016/s0006-8993(00)02628-7. [DOI] [PubMed] [Google Scholar]
- 74.Fontaine M, Elmquist WF, Miller DW. Use of rhodamine 123 to examine the functional activity of P-glycoprotein in primary cultured brain microvessel endothelial cell monolayers. Life Sciences. 1996;59(18):1521–1531. doi: 10.1016/0024-3205(96)00483-3. [DOI] [PubMed] [Google Scholar]
- 75.Batrakova E, Li S, Miller D, Kabanov A. Pluronic P85 Increases Permeability of a Broad Spectrum of Drugs in Polarized BBMEC and Caco-2 Cell Monolayers. Pharmaceutical research. 1999;16(9):1366–1372. doi: 10.1023/a:1018990706838. [DOI] [PubMed] [Google Scholar]
- 76.Spitzenberger TJ, Heilman D, Diekmann C, Batrakova EV, Kabanov AV, Gendelman HE, Elmquist WF, Persidsky Y. Novel delivery system enhances efficacy of antiretroviral therapy in animal model for HIV-1 encephalitis. J Cereb Blood Flow Metab. 2007;27(5):1033–1042. doi: 10.1038/sj.jcbfm.9600414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chiappetta DA, Hocht C, Opezzo JAW, Sosnik A. Intranasal administration of antiretroviral-loaded micelles for anatomical targeting to the brain in HIV. Nanomedicine: nanotechnology, biology, and medicine. 2012;8(2):223–237. doi: 10.2217/nnm.12.104. [DOI] [PubMed] [Google Scholar]
- 78.Sagar V, Pilakka-Kanthikeel S, Pottathil R, Saxena SK, Nair M. Towards nanomedicines for neuroAIDS. Rev Med Virol. 2014;24(2):103–124. doi: 10.1002/rmv.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fresta M, Wehrli E, Puglisi G. Enhanced Therapeutic Effect of Cytidine-5′-Diphosphate Choline when Associated with GM1 Containing Small Liposomes as Demonstrated in a Rat Ischemia Model. Pharmaceutical research. 1995;12(11):1769–1774. doi: 10.1023/a:1016234226404. [DOI] [PubMed] [Google Scholar]
- 80.Fresta M, Puglisi G. Biological effects of CDP-choline loaded long circulating liposomes on rat cerebral post-ischemic reperfusion. International journal of pharmaceutics. 1996;134(1–2):89–97. [Google Scholar]
- 81.Craparo EF, Bondì ML, Pitarresi G, Cavallaro G. Nanoparticulate Systems for Drug Delivery and Targeting to the Central Nervous System. CNS Neuroscience & Therapeutics. 2011;17(6):670–677. doi: 10.1111/j.1755-5949.2010.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schmidt J, Metselaar JM, Wauben MHM, Toyka KV, Storm G, Gold R. Drug targeting by long-circulating liposomal glucocorticosteroids increases therapeutic efficacy in a model of multiple sclerosis. Brain. 2003;126(8):1895–1904. doi: 10.1093/brain/awg176. [DOI] [PubMed] [Google Scholar]
- 83.Soni V, Kohli DV, Jain SK. Transferrin-conjugated liposomal system for improved delivery of 5-fluorouracil to brain. Journal of Drug Targeting. 2008;16(1):73–78. doi: 10.1080/10611860701725381. [DOI] [PubMed] [Google Scholar]
- 84.Chen H, Qin Y, Zhang Q, Jiang W, Tang L, Liu J, He Q. Lactoferrin modified doxorubicin-loaded procationic liposomes for the treatment of gliomas. European Journal of Pharmaceutical Sciences. 2011;44(1–2):164–173. doi: 10.1016/j.ejps.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 85.Jefferies WA, Williams MRBAF, Hunt SV. Analysis of lymphopoietic stem cells with a monoclonal antibody to the rat transferrin receptor. Immunology. 1985 Feb;54(2):333–341. [PMC free article] [PubMed] [Google Scholar]
- 86.Jefferies WA, Brandon MR, Hunt SV, Williams AF, Gatter KC, Mason DY. Transferrin receptor on endothelium of brain capillaries. Nature. 1984;312(5990):162–163. doi: 10.1038/312162a0. [DOI] [PubMed] [Google Scholar]
- 87.Prior S, Gander B, Blarer N, Merkle HP, Subirá MaL, Irache JM, Gamazo C. In vitro phagocytosis and monocyte-macrophage activation with poly(lactide) and poly(lactide-co-glycolide) microspheres. European Journal of Pharmaceutical Sciences. 2002;15(2):197–207. doi: 10.1016/s0928-0987(01)00218-4. [DOI] [PubMed] [Google Scholar]
- 88.Kim S, Scheerer S, Geyer MA, Howell SB. Direct Cerebrospinal Fluid Delivery of an Antiretroviral Agent Using Multivesicular Liposomes. Journal of Infectious Diseases. 1990;162(3):750–752. doi: 10.1093/infdis/162.3.750. [DOI] [PubMed] [Google Scholar]
- 89.Jin SX, Bi DZ, Wang J, Wang YZ, Hu HG, Deng YH. Pharmacokinetics and tissue distribution of zidovudine in rats following intravenous administration of zidovudine myristate loaded liposomes. Die Pharmazie - An International Journal of Pharmaceutical Sciences. 2005;60(11):840–843. [PubMed] [Google Scholar]
- 90.Saiyed ZM. Magnetic nanoformulation of azidothymidine 5′-triphosphate for targeted delivery across the blood-brain barrier. International journal of nanomedicine. 2010;5:157–166. doi: 10.2147/ijn.s8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kaur CD, Nahar M, Jain NK. Lymphatic targeting of zidovudine using surface-engineered liposomes. J Drug Target. 2008;16(10):798–805. doi: 10.1080/10611860802475688. [DOI] [PubMed] [Google Scholar]
- 92.Bestman-Smith J, Gourde P, Désormeaux A, Tremblay MJ, Bergeron MG. Sterically stabilized liposomes bearing anti-HLA-DR antibodies for targeting the primary cellular reservoirs of HIV-1. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2000;1468(1–2):161–174. doi: 10.1016/s0005-2736(00)00254-6. [DOI] [PubMed] [Google Scholar]
- 93.Gagné JF, Désormeaux A, Perron S, Tremblay MJ, Bergeron MG. Targeted delivery of indinavir to HIV-1 primary reservoirs with immunoliposomes. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2002;1558(2):198–210. doi: 10.1016/s0005-2736(01)00432-1. [DOI] [PubMed] [Google Scholar]
- 94.Freitas C, Müller RH. Effect of light and temperature on zeta potential and physical stability in solid lipid nanoparticle (SLN™) dispersions. International journal of pharmaceutics. 1998;168(2):221–229. [Google Scholar]
- 95.Müller RH. Solid lipid nanoparticles (SLN) for controlled drug delivery - a review of the state of the art. European journal of pharmaceutics and biopharmaceutics. 2000;50(1):161–177. doi: 10.1016/s0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 96.Lockman PR. Brain uptake of thiamine-coated nanoparticles. Journal of Controlled Release. 2003;93(3):271–282. doi: 10.1016/j.jconrel.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 97.Yang S. Body distribution of camptothecin solid lipid nanoparticles after oral administration. Pharmaceutical research. 1999;16(5):751–757. doi: 10.1023/a:1018888927852. [DOI] [PubMed] [Google Scholar]
- 98.Fundaro A, Cavalli R, Bargoni A, Vighetto D, Zara GP, Gasco MR. Non-stealth and stealth solid lipid nanoparticles (SLN) carrying doxorubicin: pharmacokinetics and tissue distribution after i.v. administration to rats. Pharmacol Res. 2000;42(4):337–343. doi: 10.1006/phrs.2000.0695. [DOI] [PubMed] [Google Scholar]
- 99.Chen DB. In vitro and in vivo study of two types of long-circulating solid lipid nanoparticles containing paclitaxel. Chemical & pharmaceutical bulletin. 2001;49(11):1444–1447. doi: 10.1248/cpb.49.1444. [DOI] [PubMed] [Google Scholar]
- 100.Zara GP, Cavalli R, FundarÒ A, Bargoni A, Caputo O, Gasco MR. Pharmacokinetics of doxorubicin incorporated in solid lipid nanospheres (SLN) Pharmacological Research. 1999;40(3):281–286. doi: 10.1006/phrs.1999.0509. [DOI] [PubMed] [Google Scholar]
- 101.Podio V. Biodistribution of stealth and non-stealth solid lipid nanospheres after intravenous administration to rats. Journal of pharmacy and pharmacology. 2000;52(9):1057–1063. doi: 10.1211/0022357001774976. [DOI] [PubMed] [Google Scholar]
- 102.Yang SC. Body distribution in mice of intravenously injected camptothecin solid lipid nanoparticles and targeting effect on brain. Journal of Controlled Release. 1999;59(3):299–307. doi: 10.1016/s0168-3659(99)00007-3. [DOI] [PubMed] [Google Scholar]
- 103.Lockman PR. In vivo and in vitro assessment of baseline blood-brain barrier parameters in the presence of novel nanoparticles. Pharmaceutical research. 2003;20(5):705–713. doi: 10.1023/a:1023492015851. [DOI] [PubMed] [Google Scholar]
- 104.Yusuf M, Khan M, Khan RA, Ahmed B. Preparation, characterization, in vivo and biochemical evaluation of brain targeted Piperine solid lipid nanoparticles in an experimentally induced Alzheimer’s disease model. Journal of Drug Targeting. 2013;21(3):300–311. doi: 10.3109/1061186X.2012.747529. [DOI] [PubMed] [Google Scholar]
- 105.Kuo YC, Su FL. Transport of stavudine, delavirdine, and saquinavir across the blood-brain barrier by polybutylcyanoacrylate, methylmethacrylate-sulfopropylmethacrylate, and solid lipid nanoparticles. International journal of pharmaceutics. 2007;340(1–2):143–152. doi: 10.1016/j.ijpharm.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 106.Kuo YC. Electromagnetic interference in the permeability of saquinavir across the blood-brain barrier using nanoparticulate carriers. International journal of pharmaceutics. 2008;351(1–2):271–281. doi: 10.1016/j.ijpharm.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 107.Chattopadhyay N, Zastre J, Wong HL, Wu X, Bendayan R. Solid Lipid Nanoparticles Enhance the Delivery of the HIV Protease Inhibitor, Atazanavir, by a Human Brain Endothelial Cell Line. Pharmaceutical research. 2008;25(10):2262–2271. doi: 10.1007/s11095-008-9615-2. [DOI] [PubMed] [Google Scholar]
- 108.Kuo YC, Ko HF. Targeting delivery of saquinavir to the brain using 83–14 monoclonal antibody-grafted solid lipid nanoparticles. Biomaterials. 2013;34(20):4818–4830. doi: 10.1016/j.biomaterials.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 109.Aji Alex MR, Chacko AJ, Jose S, Souto EB. Lopinavir loaded solid lipid nanoparticles (SLN) for intestinal lymphatic targeting. European Journal of Pharmaceutical Sciences. 2011;42(1–2):11–18. doi: 10.1016/j.ejps.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 110.Dou H, Destache CJ, Morehead JR, Mosley RL, Boska MD, Kingsley J, Gorantla S, Poluektova L, Nelson JA, Chaubal M, Werling J, Kipp J, Rabinow BE, Gendelman HE. Development of a macrophage-based nanoparticle platform for antiretroviral drug delivery. Blood. 2006;108(8):2827–2835. doi: 10.1182/blood-2006-03-012534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nowacek AS, McMillan J, Miller R, Anderson A, Rabinow B, Gendelman HE. Nanoformulated antiretroviral drug combinations extend drug release and antiretroviral responses in HIV-1-infected macrophages: implications for neuroAIDS therapeutics. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2010;5(4):592–601. doi: 10.1007/s11481-010-9198-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Batrakova EV, Gendelman HE, Kabanov AV. Cell-mediated drug delivery. Expert opinion on drug delivery. 2011;8(4):415–433. doi: 10.1517/17425247.2011.559457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gautam N, Roy U, Balkundi S, Puligujja P, Guo D, Smith N, Liu XM, Lamberty B, Morsey B, Fox HS, McMillan J, Gendelman HE, Alnouti Y. Preclinical pharmacokinetics and tissue distribution of long-acting nanoformulated antiretroviral therapy. Antimicrobial agents and chemotherapy. 2013;57(7):3110–3120. doi: 10.1128/AAC.00267-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dash PK, Gendelman HE, Roy U, Balkundi S, Alnouti Y, Mosley RL, Gelbard HA, McMillan J, Gorantla S, Poluektova LY. Long-acting nanoformulated antiretroviral therapy elicits potent antiretroviral and neuroprotective responses in HIV-1-infected humanized mice. Aids. 2012;26(17):2135–2144. doi: 10.1097/QAD.0b013e328357f5ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Martinez-Skinner AL, Veerubhotla RS, Liu H, Xiong H, Yu F, McMillan JM, Gendelman HE. Functional Proteome of Macrophage Carried Nanoformulated Antiretroviral Therapy Demonstrates Enhanced Particle Carrying Capacity. J Proteome Res. 2013;12(5):2282–2294. doi: 10.1021/pr400185w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Roy U, McMillan J, Alnouti Y, Gautum N, Smith N, Balkundi S, Dash P, Gorantla S, Martinez-Skinner A, Meza J, Kanmogne G, Swindells S, Cohen SM, Mosley RL, Poluektova L, Gendelman HE. Pharmacodynamic and Antiretroviral Activities of Combination Nanoformulated Antiretrovirals in HIV-1-Infected Human Peripheral Blood Lymphocyte-Reconstituted Mice. J Infect Dis. 2012;206(10):1577–1588. doi: 10.1093/infdis/jis395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kularatne SA, Low PS. Targeting of nanoparticles: folate receptor. Methods Mol Biol (Totowa, NJ, U S) 2010;624(Cancer Nanotechnology):249–265. doi: 10.1007/978-1-60761-609-2_17. [DOI] [PubMed] [Google Scholar]
- 118.Lu J, Li Z, Zink JI, Tamanoi F. In vivo tumor suppression efficacy of mesoporous silica nanoparticles-based drug-delivery system: enhanced efficacy by folate modification. Nanomedicine (New York, NY, U S) 2012;8(2):212–220. doi: 10.1016/j.nano.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nukolova NV, Oberoi HS, Cohen SM, Kabanov AV, Bronich TK. Folate-decorated nanogels for targeted therapy of ovarian cancer. Biomaterials. 2011;32(23):5417–5426. doi: 10.1016/j.biomaterials.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xia W, Hilgenbrink AR, Matteson EL, Lockwood MB, Cheng JX, Low PS. A functional folate receptor is induced during macrophage activation and can be used to target drugs to activated macrophages. Blood. 2009;113(2):438–446. doi: 10.1182/blood-2008-04-150789. [DOI] [PubMed] [Google Scholar]
- 121.Puligujja P, McMillan J, Kendrick L, Li T, Balkundi S, Smith N, Veerubhotla RS, Edagwa BJ, Kabanov AV, Bronich T, Gendelman HE, Liu X-M. Macrophage folate receptor-targeted antiretroviral therapy facilitates drug entry, retention, antiretroviral activities and biodistribution for reduction of human immunodeficiency virus infections. Nanomedicine (N Y, NY, U S) 2013;9(8):1263–1273. doi: 10.1016/j.nano.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shegokar R, Singh KK. Surface modified nevirapine nanosuspensions for viral reservoir targeting: In vitro and in vivo evaluation. International journal of pharmaceutics. 2011;421(2):341–352. doi: 10.1016/j.ijpharm.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 123.Sharma P, Garg S. Pure drug and polymer based nanotechnologies for the improved solubility, stability, bioavailability and targeting of anti-HIV drugs. Adv Drug Delivery Rev. 2010;62(4–5):491–502. doi: 10.1016/j.addr.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 124.Wong HL, Chattopadhyay N, Wu XY, Bendayan R. Nanotechnology applications for improved delivery of antiretroviral drugs to the brain. Adv Drug Delivery Rev. 2010;62(4–5):503–517. doi: 10.1016/j.addr.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 125.Xia H, Gao X, Gu G, Liu Z, Hu Q, Tu Y, Song Q, Yao L, Pang Z, Jiang X, Chen J, Chen H. Penetratin-functionalized PEG-PLA nanoparticles for brain drug delivery. Int J Pharm (Amsterdam, Neth) 2012;436(1–2):840–850. doi: 10.1016/j.ijpharm.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 126.Kuo YC, Lee CL. Methylmethacrylate-sulfopropylmethacrylate nanoparticles with surface RMP-7 for targeting delivery of antiretroviral drugs across the blood-brain barrier. Colloids Surf, B. 2012;90:75–82. doi: 10.1016/j.colsurfb.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 127.Dhanya KP, Santhi K, Dhanaraj SA, Sajeeth CI. Formulation and evaluation of chitosan nanospheres as a carrier for the targeted delivery of Lamivudine to the brain. Pharm Globale. 2011;2(5) No pp. given. [Google Scholar]
- 128.Kaur A, Jain S, Tiwary AK. Mannan-coated gelatin nanoparticles for sustained and targeted delivery of didanosine: in vitro and in vivo evaluation. Acta pharmaceutica (Zagreb, Croatia) 2008;58(1):61–74. doi: 10.2478/v10007-007-0045-1. [DOI] [PubMed] [Google Scholar]
- 129.Jain SK, Gupta Y, Jain A, Saxena AR, Khare P, Jain A. Mannosylated gelatin nanoparticles bearing an anti-HIV drug didanosine for site-specific delivery. Nanomedicine: nanotechnology, biology, and medicine. 2008;4(1):41–48. doi: 10.1016/j.nano.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 130.Destache CJ, Belgum T, Goede M, Shibata A, Belshan MA. Antiretroviral release from poly(DL-lactide-co-glycolide) nanoparticles in mice. The Journal of antimicrobial chemotherapy. 2010;65(10):2183–2187. doi: 10.1093/jac/dkq318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shah LK, Amiji MM. Intracellular delivery of saquinavir in biodegradable polymeric nanoparticles for HIV/AIDS. Pharmaceutical research. 2006;23(11):2638–2645. doi: 10.1007/s11095-006-9101-7. [DOI] [PubMed] [Google Scholar]
- 132.Saiyed MPNNaZM. In: The neurology of aids. Gendelman HE, Fox EI, Gelbard HS, Grant HA, Lipton I, Swindells SAS, editors. Oxford University Press; New york: 2011. pp. 999–1004. [Google Scholar]
- 133.Ikehara Y, Niwa T, Biao L, Ikehara SK, Ohashi N, Kobayashi T, Shimizu Y, Kojima N, Nakanishi H. A Carbohydrate Recognition–Based Drug Delivery and Controlled Release System using Intraperitoneal Macrophages as a Cellular Vehicle. Cancer Research. 2006;66(17):8740–8748. doi: 10.1158/0008-5472.CAN-06-0470. [DOI] [PubMed] [Google Scholar]
- 134.Brynskikh AM. Macrophage delivery of therapeutic nanozymes in a murine model of Parkinson’s disease. Nanomedicine (London, England) 2010;5(3):379–396. doi: 10.2217/nnm.10.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dou H. Macrophage delivery of nanoformulated antiretroviral drug to the brain in a murine model of neuroAIDS. The Journal of immunology (1950) 2009;183(1):661–669. doi: 10.4049/jimmunol.0900274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Batrakova EV, Li S, Reynolds AD, Mosley RL, Bronich TK, Kabanov AV, Gendelman HE. A Macrophage Nanozyme Delivery System for Parkinson’s Disease. Bioconjugate chemistry. 2007;18(5):1498–1506. doi: 10.1021/bc700184b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nowacek AS. NanoART synthesis, characterization, uptake, release and toxicology for human monocyte-macrophage drug delivery. Nanomedicine (London, England) 2009;4(8):903–917. doi: 10.2217/nnm.09.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dou H. Development of a macrophage-based nanoparticle platform for antiretroviral drug delivery. Blood. 2006;108(8):2827–2835. doi: 10.1182/blood-2006-03-012534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dou H. Laboratory investigations for the morphologic, pharmacokinetic, and anti-retroviral properties of indinavir nanoparticles in human monocyte-derived macrophages. Virology (New York, NY) 2007;358(1):148–158. doi: 10.1016/j.virol.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 140.Nowacek AS. Nanoformulated antiretroviral drug combinations extend drug release and antiretroviral responses in HIV-1-infected macrophages: implications for neuroAIDS therapeutics. Journal of neuroimmune pharmacology. 2010;5(4):592–601. doi: 10.1007/s11481-010-9198-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dou H, Grotepas CB, McMillan JM, Destache CJ, Chaubal M, Werling J, Kipp J, Rabinow B, Gendelman HE. Macrophage delivery of nanoformulated antiretroviral drug to the brain in a murine model of neuroAIDS. Journal of immunology (Baltimore, Md: 1950) 2009;183(1):661–669. doi: 10.4049/jimmunol.0900274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kanmogne GD, Singh S, Roy U, Liu X, McMillan J, Gorantla S, Balkundi S, Smith N, Alnouti Y, Gautam N, Zhou Y, Poluektova L, Kabanov A, Bronich T, Gendelman HE. Mononuclear phagocyte intercellular crosstalk facilitates transmission of cell-targeted nanoformulated antiretroviral drugs to human brain endothelial cells. International journal of nanomedicine. 2012;7:2373–2388. doi: 10.2147/IJN.S29454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Saiyed Z, Gandhi N, Nair MN. AZT 5′-triphosphate nanoformulation suppresses human immunodeficiency virus type 1 replication in peripheral blood mononuclear cells. Journal of NeuroVirology. 2009;15(4):343–347. doi: 10.1080/13550280903062813. [DOI] [PubMed] [Google Scholar]
- 144.Yoo HS, Park TG. Folate-receptor-targeted delivery of doxorubicin nano-aggregates stabilized by doxorubicin-PEG-folate conjugate. J Controlled Release. 2004;100(2):247–256. doi: 10.1016/j.jconrel.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 145.Lu Y, Low PS. Folate targeting of haptens to cancer cell surfaces mediates immunotherapy of syngeneic murine tumors. Cancer Immunol Immunother. 2002;51(3):153–162. doi: 10.1007/s00262-002-0266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhao X, Li H, Lee RJ. Targeted drug delivery via folate receptors. Expert Opin Drug Delivery. 2008;5(3):309–319. doi: 10.1517/17425247.5.3.309. [DOI] [PubMed] [Google Scholar]
- 147.Majoros IJ, Thomas TP, Mehta CB, Baker JR., Jr Poly(amidoamine) dendrimer-based multifunctional engineered nanodevice for cancer therapy. J Med Chem. 2005;48(19):5892–5899. doi: 10.1021/jm0401863. [DOI] [PubMed] [Google Scholar]
- 148.Yoshizawa T, Hattori Y, Hakoshima M, Koga K, Maitani Y. Folate-linked lipid-based nanoparticles for synthetic siRNA delivery in KB tumor xenografts. Eur J Pharm Biopharm. 2008;70(3):718–725. doi: 10.1016/j.ejpb.2008.06.026. [DOI] [PubMed] [Google Scholar]