Abstract
Objectives:
Some manufacturers claim to have produced new irreversible hydro-colloids that are able to maintain their dimensional stability during storage. The present study evaluated the effect of storage time on dimensional stability of three alginates: Hydrogum 5, Tropicalgin and Alginoplast.
Materials and Methods:
In this experimental in-vitro trial, a total of 90 alginate impressions were made from a Dentoform model using Hydrogum 5, Tropicalgin and Alginoplast alginates. The impressions were stored in a sealed plastic bag without a damp paper towel for 0, 24, 48, 72 and 120 hours and then poured with type III dental stone. Cross-arch (facial of 6 to facial of 6 on the opposite side) and antero-posterior (distal of right first molar to the ipsilateral central incisor) measurements were made with a digital caliper on the casts. Data were analyzed by two-way and one-way ANOVA and Tukey’s post-hoc test (P<0.05).
Results:
Alginate type and the pouring time significantly affected the dimensional stability of alginate impressions (both Ps<0.001). Pouring of Hydrogum 5 impressions can be delayed for up to 120 hours without significant dimensional changes. Alginoplast impressions may be poured after 72 hours, but Tropicalgin should be poured immediately and the storage time should not be more than 24 hours.
Conclusion:
Immediate pouring of alginate impressions provides the highest accuracy in reproducing the teeth and adjacent tissues; however, this study demonstrated that pouring may be delayed for up to five days using extended-pour (Hydrogum 5) alginates.
Keywords: Dimensional Measurement Accuracy, Dental Impression Materials, Alginates, Plaster Cast
INTRODUCTION
Irreversible hydrocolloids (alginates) are among the most commonly used impression materials. Because of their acceptable accuracy, availability, reasonable price and facile handling, this type of impression material is used for many purposes such as preparation of a study cast for diagnosis, fabrication of provisional prosthesis, custom trays, appliances and a definitive cast for fabrication of complete dentures in cases with undercut areas, partial denture and for maxillofacial prostheses [1].
Due to differences in formulations, products of different manufacturing companies have variable characteristics and properties in terms of consistency, setting time, dimensional stability, elasticity and strength [1].
Irreversible hydrocolloids suffer one major drawback, namely the dimensional change of impressions that have set. Dimensions of the impression are affected by several factors including the composition of the irreversible hydrocolloid material, conditions under which the impressions are stored and the duration of storage before pouring [2].
Irreversible hydrocolloid impressions undergo shrinkage if stored exposed to air; whereas, water storage leads to swelling of the impression and distortion as the result of imbibition. However, minor dimensional changes have been reported as the result of storage in 100% relative humidity. Syneresis also leads to considerable shrinkage [2].
Researchers have recommended that in order to maintain dimensional accuracy, hydrocolloid impressions should be poured immediately [1, 3] or maximally within 12 minutes [4]. However, some have claimed that it is possible to store the impression in a humid environment for up to one hour [5, 6].
Numerous studies have evaluated the dimensional accuracy of conventional alginates [2,7–15]. Some studies show that it is possible to store a hydrocolloid impression for up to 1, 2 and even 4 hours [8–10]. Due to clinical limitations, in the majority of cases, the impressions are poured after a time delay longer than the recommended period. Recently, manufactures are trying to increase the storage time of hydrocolloid impression materials. Some impression material manufacturing companies have produced a new generation of alginates (extended-pour) and claim that these materials are capable of maintaining their dimensional stability for up to 5 days. Only a few studies have investigated the dimensional accuracy of extended-pour alginates [16–19]. Considering the lack of adequate evidence based data to use this type of alginate in clinic and controversial results of the available studies, the present study was designed to assess the effect of storage time on the dimensional stability of conventional and extended-pour alginates. The null hypothesis of this study is that the type of alginate and storage time does not affect the dimensional accuracy of the fabricated casts.
MATERIALS AND METHODS
In this experimental study, a total of 90 impressions were made from a maxillary Dentoform model (NISSIN Dental Products Inc., Kyoto, Japan) using Hydrogum 5 (Zhermack, Italy) as the extended-pour (n=30), and Tropicalgin (Zhermack, Italy) (n=30) and Alginoplast (Heraeus Kulzer, Germany) (n=30) as the conventional alginate.
The first molars of both quadrants and the central incisor of the right quadrant of the model were indexed using a milling machine and 0.8 mm carbide fissure bur [17] (Figures 1 and 2). In order to allow 4–5 mm space for adequate uniform thickness of the alginate, two layers of spacer wax were placed on teeth surfaces and the palatal surface of the model [16]. The tray was placed over the wax and three stops were made for stable seating of the tray using autopolymerizing acrylic resin. Two stops were fabricated at canine areas and the third stop was made at the end of the palatal midline of the model (Figure 3).
Fig 1.
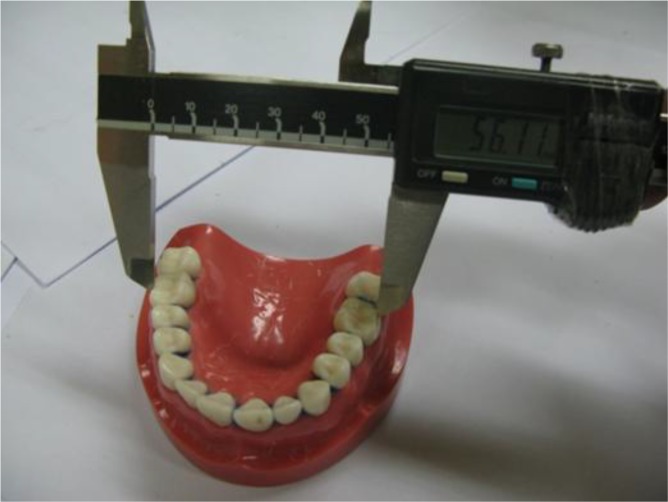
Cross-arch width (facial surface of the right first molar to the facial surface of the left first molar)
Fig 2.
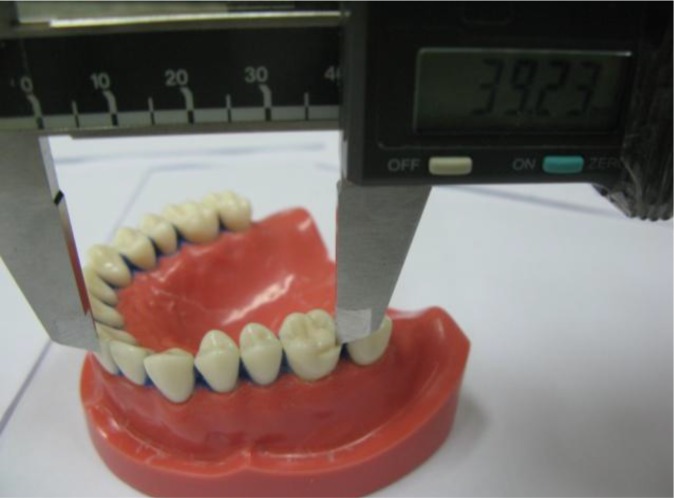
Antero-posterior measurement (distal surface of the right first molar to the labial surface of the central incisor on the same side)
Fig 3.
Three stops were made for stable seating of the tray using autopolymerizing acrylic resin
These stops are required to achieve reproducible positioning of trays during impression making. In the next step, the spacer wax was eliminated and the model was prepared for impression making.
To simulate the oral environment, the impression along with the model were immersed in a water bath at 35°C±1°C and pressed with a 1-kg weight until completion of the setting time [19]. Water temperature was adjusted after every 5-impressions taken, using a mercury thermometer.
The casts were generated immediately and at four additional times of 24 h, 48 h, 72 h and 120 h. The impressions were stored in sealed plastic bags (100% relative humidity) at room temperature for the specified storage time periods according to the manufacturers instructions.
The air inside the plastic bags was removed as much as possible [17, 20]. The impressions were poured with dental stone type III (Tara 250, Kheyzaran, Isfahan, Iran) at the determined time points. Forty-five minutes later, the model was removed from the impression and allowed to dry for 24 hours [17]. Measurements were made with a CD-15b digital caliper (Mitutoyo Ltd., UK) with 0.01 mm readability [16, 17]. The cross-arch (facial surface of the right first molar to the facial surface of the left first molar) and antero-posterior (distal surface of the right first molar to the labial surface of the central incisor on the same side) measurements were made by measuring the distances between the indices on the stone models three times [18]. Then, these measurements were compared with the measurements on the master model. Data were statistically analyzed using SPSS version 20.0 software (IBM Corp., Armonk, NY), one-way and two-way ANOVA and Tukey’s post hoc tests (P<0.05).
RESULTS
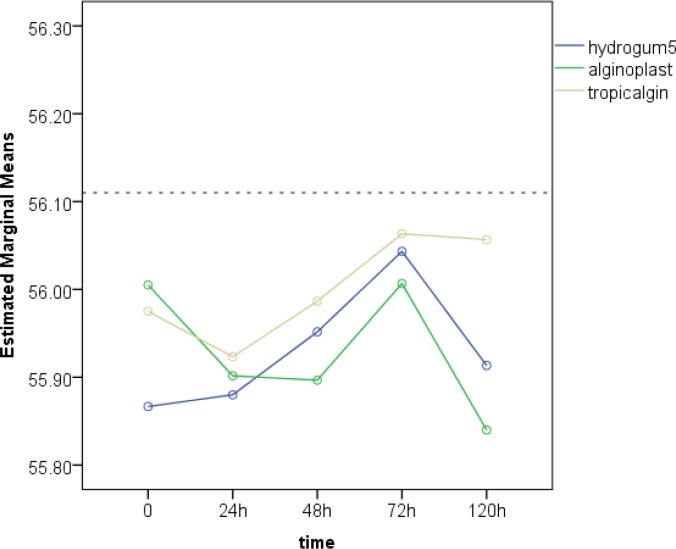
The cross-arch width on the master model was 56.11 mm. According to the results of two-way ANOVA, the type of alginate (P=0.12), storage time (P=0.09) and their interaction (P=0.57) had no significant effect on cross-arch measurements. Considering the insignificant effect of the type of alginate and storage time on cross-arch measurements, no other statistical analyses were performed on the results.
The percentage of cross-arch changes in impression materials following different storage times is demonstrated in Table 1.
Table 1.
Cross-Arch Measurements in Casts Produced from the Understudy Alginates Following Different Storage Times
| Type of Material | Storage Time | Mean (±SD) | Percentage of Dimensional Changes | Error Terms | Intraclass Correlation Coefficient | Mean (±SD) |
|---|---|---|---|---|---|---|
| Hydrogum 5 | 0 | 55.87 (±0.16) | −0.48 | 0.24 | 55.93 (± 0.03) | |
| 24 | 55.88 (±0.23) | −0.41 | 0.23 | 0.13 | ||
| 48 | 55.95 (±0.06) | −00.29 | 0.16 | |||
| 72 | 56.04 (±0.17) | −0.12 | 0.07 | |||
| 120 | 55.91 (±0.20) | −0.36 | 0.20 | |||
|
| ||||||
| Alginoplast | 0 | 56.01 (±0.09) | −0.18 | 0.11 | 55.93 (±0.03) | |
| 24 | 55.90 (±0.15) | −0.37 | 0.21 | −0.03 | ||
| 48 | 55.90 (±0.14) | −0.37 | 0.21 | |||
| 72 | 56.01 (±0.11) | −0.18 | 0.10 | |||
| 120 | 55.84 (±0.25) | −0.48 | 0.27 | |||
|
| ||||||
| Tropicalgin | 0 | 55.98 (±0.07) | −0.23 | 0.14 | 56.0 (±0.03) | |
| 24 | 55.92 (±0.14) | −0.39 | 0.19 | −0.04 | ||
| 48 | 55.99 (±0.14) | −0.21 | 0.12 | |||
| 72 | 56.06 (±0.10) | −0.10 | 0.05 | |||
| 120 | 56.06 (±0.10) | −0.10 | 0.05 | |||
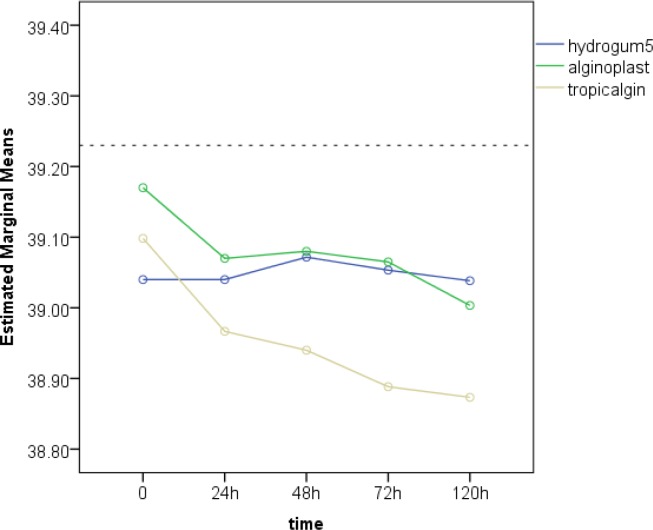
The antero-posterior dimension on the master model was 39.23 mm. Two-way ANOVA revealed that the type of impression material (P<0.001), storage time (P<0.001) and their interaction (P=0.05) had significant effects on antero-posterior measurements.
The percentage of change in antero-posterior measurements in understudy materials following different storage times is demonstrated in Table 2. In addition, error terms of the measurements and intraclass correlation coefficient were calculated in two dimensions for each material (Tables 1 and 2) and each time (Table 3).
Table 2.
Antero-Posterior Measurements in Casts Produced from the Understudy Alginates at Different Storage Times
| Type of Material | Storage Time | Mean (±SD) | Percentage of Dimensional Changes | Error Terms | Intraclass Correlation Coefficient | Mean (±SD) |
|---|---|---|---|---|---|---|
| Hydrogum 5 | 0 | 39.04 (±0.06) | 0.48 | 0.19 | −0.17 | 39.05 (± 0.02) |
| 24 | 39.04 (±0.13) | −0.48 | 0.19 | |||
| 48 | 39.07 (±0.04) | −0.41 | 0.16 | |||
| 72 | 39.05 (±0.10) | −.46 | 0.18 | |||
| 120 | 39.04 (±0.11) | 0.48 | 0.19 | |||
|
| ||||||
| Alginoplast | 0 | 39.17 (±0.06) | 0.15 | 0.06 | −.19 | 39.08 (±0.02) |
| 24 | 39.07 (±0.02) | −.41 | 0.16 | |||
| 48 | 39.08 (±0.03) | −.38 | 0.15 | |||
| 72 | 39.07 (±0.05) | −.41 | 0.16 | |||
| 120 | 39.01 (±0.11) | −.59 | 0.23 | |||
|
| ||||||
| Tropicalgin | 0 | 39.10 (±0.12) | 0.33 | 0.13 | 0.02. | 38.95 (±0.02) |
| 24 | 38.97 (±0.07) | 0.66 | 0.26 | |||
| 48 | 38.94 (±0.08) | −0.74 | 0.29 | |||
| 72 | 38.89 (±0.08) | −.87 | 0.34 | |||
| 120 | 38.87 (±0.07) | −.92 | 0.36 | |||
Table 3.
Mean (±SD) of Measurements of All Fabricated Casts According to the Pouring Time
| Pouring Time | Cross-arch Width | Antero-Posterior Distance | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean(±SD) | Intraclass Correlation Coefficient | Error Terms | Mean(±SD) | Intraclass Correlation Coefficient | Error Terms | |
| 0h | 55.95 (±0.04) | 0.05 | 0.16 | 39.1 (±0.02) | −.02 | 0.13 |
| 24h | 55.9 (±0.04) | −.20 | 0.21 | 39.03 (±0.02) | 0.17 | 0.20 |
| 48h | 55.95 (±0.04) | 0.35 | 0.17 | 39.03 (±0.02) | −.18 | 0.20 |
| 72h | 56.04 (± 0.04) | −.12 | 0.07 | 39.0 (±0.02) | −.01 | 0.23 |
| 120h | 55.94 (±0.04) | 0.24 | 0.17 | 38.97 (±0.02) | 0.12 | 0.26 |
Furthermore, one-way ANOVA showed that the mean antero-posterior measurement at different storage times was equal in Hydrogum 5 (P=0.97). However, in the other two impression materials, these values were found to be significantly different (P=0.003 for Alginoplast and P<0.001 for Tropicalgin).
For Alginoplast, the difference between the mean antero-posterior distance at 0 and 120 h storage times was statistically significant (Tukey’s post-hoc test, P<0.001). In Tropicalgin, the difference in the mean antero-posterior distance measured at 0 and 72 h storage times (Tukey’s post-hoc test, P=0.002), 0 and 48 h (P=0.03) and 0 and 120 h storage times (P<0.001) was statistically significant. For antero-posterior distances, one-way ANOVA failed to find a significant difference between the three impression materials at 0 (P=0.06) and 24 h (P=0.13) storage times. However, the difference between the three materials in the mean antero-posterior distance measured at 48 h (P<0.001), 72 h (P=0.003) and 120 h (P=0.03) was statistically significant.
The mean (±SD) of measurements of all fabricated casts according to the pouring time is shown in Table 3.
Diagrams 1 and 2 show the mean arch-width and antero-posterior measurements made on casts generated from the understudy impression materials following different storage times.
Diagram 1.
The cross-arch measurements (distance between the facial surface of the first molar of the right quadrant and the facial surface of the first molar of the left quadrant) in casts produced from the understudy impression materials at different storage times
Diagram 2.
The mean antero-posterior measurements (distance between the labial surface of the maxillary right central incisor and the distal of the first molar on the same side) in casts produced from the understudy impression materials at different storage times
DISCUSSION
According to the result of this study, the null hypothesis was rejected because the antero-posterior dimension was directly affected by the type of alginate and storage time. Difference in dimensional stability of the understudy alginates was due to difference in their chemical composition (polymer: filler ratio can affect shrinkage) [2].
In Hydrogum 5, the mean antero-posterior distance at different time points was almost the same. However, in the other two impression materials, these values were significantly different and it seems that the dimensional stability of the two mentioned alginates was compromised in this dimension after various storage periods [21]. It appears that the water content within the mass of impression materials has a greater effect on their dimensional stability than the amount of water present in each impression [22].
Furthermore, water may evaporate from the surface of alginate impressions (syneresis) within the time interval between impression making and generation of plaster cast and it may cause contraction of hydrocolloid materials [23].
If pouring the impression with dental stone is delayed for a long time period, the alginate impression undergoes antero-posterior and cross-arch expansion and shortens [8]. In our study, changes in cross-arch measurements were insignificant; while, changes that occurred in antero-posterior measurements were in the form of shrinkage. Based on our obtained results, the cross-arch distances measured in all three-impression materials were the same at all storage times.
Sedda et al. evaluated the effect of storage time on the accuracy of the casts produced from irreversible hydrocolloid impressions and showed that when impressions were poured immediately, all alginates could reproduce the original model with no significant difference. After 24 h of storage, only Alginoplast and Hydrogum 5 could reproduce the original model with no significant difference in any of the measurements. Furthermore, only casts produced from Hydrogum 5 impressions could comply with the control models at 72 and 120 h of storage time [22]. These findings were observed for Hydrogum 5 and Alginoplast in our study as well.
Reddy et al., reported that Tropicalgin could be used without causing significant dimensional changes after 2 hours of storage in sealed plastic bags. The percentage of dimensional change for Tropicalgin was 0.258% at 2 and 0.565% at 24 h. The latter value was similar to our obtained value at 24 h [18].
In a study conducted by Imbery et al. [17], casts produced from conventional and extended-pour alginate immediately and at day 5, had no statistically significant difference with the standard model. These results are different from our obtained results of conventional alginates.
Todd et al., [19] demonstrated in their study that all the tested alginates showed significant dimensional changes at 24 and 100 hours. This finding regarding the extended-pour alginate is different from our obtained result.
Mosharraf and Mokhtari in 2006 also revealed that casts produced from Alginoplast alginate impressions stored for up to 3 hours in a humid environment did not have significant dimensional changes. This storage time is shorter than the 72 h storage time in our study. The percentage of dimensional changes at zero time point in the mentioned study was 0.142 for Alginoplast, which is in accord with our finding [15].
Mosharraf et al., in 2011 reported that Alginmax, Elastic Cromo and Hydrogum alginate impressions did not have significant dimensional changes before 120 h of storage, but were not reliable after 5 days of storage [16]. In the mentioned study, fewer significant differences were found between the casts produced from Hydrogum 5 and the master model, which in this respect, is in agreement with our study results. The difference between our study and the study performed by Mosharraf et al. is that they used damp paper towels and the changes were in the form of expansion of impressions.
Erbe et al. in their study on the effects of storage under humid and wet conditions on dimensional stability of contemporary irreversible hydrocolloids reported the lowest dimensional changes in impressions stored in a humid environment (humidor). According to this study, irreversible hydrocolloid impressions stored in humidor must be poured within 4 hours. Irreversible hydrocolloid impressions stored in bag/tissue storage must be poured within 2 hours [2].
Controversial results reported by different studies may be due to the difference in the master model, tray design and conditions at which the impressions were stored in different studies.
In 1998, Eriksson et al. made an impression of a semi-circular plate containing six dies almost similar to the dental arch using an aluminum tray [9]. In 2001, in a study carried out by Schleier et al., a plate containing four partial cones was used to simulate abutments and an impression was made using a metal tray [8]. In 2008, Sedda et al. used a rectangular plate containing four cylindrical dies with 6° taper. Impressions were taken with acrylic trays [22]. In 1985, Johnson and Craig used a metal model containing two dies. A V-shaped notch was incorporated adjacent to the base of one of these dies. Impressions were made using a prefabricated metal tray [24]. In 2006, Mosharraf and Mokhtari used the same model, but made an impression with an acrylic custom tray [15]. We used a Dentoform model of the maxillary arch in the present study. The first molars of both quadrants and the central incisor of the right quadrant were indexed. Impressions were made using a perforated plastic tray. Imbery et al. [17] used a Dentoform model for dental and arch width measurements. In the time interval between impression making and pouring the impressions with dental stone, the impressions should be stored in a storage medium. In 1998, Eriksson et al. used a humid container for the storage of impressions for up to 2 hours and sealed plastic bags along with damp paper towels for longer storage (24 and 96 h) [9]. In the study conducted by Mosharraf et al. in 2011, alginate impressions were stored in a humid environment in a sealed plastic bag [16]. In our study, impressions were stored in sealed plastic bags without a damp paper towel at room temperature according to the manufacturer’s instruction for the mentioned time periods.
Controversy exists regarding the threshold of dimensional accuracy in order to be clinically unacceptable and this range varies from 0.1% to 0.8% [2]. In addition, another study reported 0 to 0.3 mm distortion after a 20 mm mandibular opening. In terms of change in arch width during opening and protrusion, it has been reported that protrusive movements decrease mandibular arch width from 0.1 to 0.5 mm. The amount of distortion in the mandible during opening and impression making ranges from 0.1 to 0.5 mm [25].
Thus, similar to the study performed by Imbery et al. [17], we considered the mean values (0.5%) as the standard safe threshold for maximum permissible dimensional changes. According to this index, in our study, Hydrogum 5 in both dimensions and at all pouring times had clinically acceptable dimensional changes. Alginoplast changes in both dimensions and at all pouring times were clinically acceptable with the exception of the antero-posterior dimension at 120 h pouring time. Tropicalgin had clinically acceptable dimensional changes in both dimensions only at 0 h pouring time.
Since this was an in-vitro study, we could not evaluate the effect of factors such as blood, saliva or oral cavity temperature and other invivo conditions on the accuracy of impression materials; which is a limitation of the present study. Another limitation of our study was not evaluating the effect of different storage temperatures.
CONCLUSION
Within the limitations of this study, the following conclusions were drawn:
Dimensional stability of alginate impressions was directly influenced by the type of alginate and the time the impressions were poured (storage time).
Hydrogum 5 and Alginoplast impressions could be poured after 120 and 72 h of storage, respectively with no significant dimensional changes.
Impressions made with Tropicalgin must be poured as soon as possible and their storage time should be less than 24 h.
In general, when alginates are used, immediate pouring of the impressions is still the best method for precise reproduction of the teeth and adjacent tissues. However, considering the obtained results by using Hydrogum 5, acceptable results can still be obtained by pouring the impressions up to 5 days after impression taking.
REFERENCES
- 1.Shillingburg HT, Sather DA, Wilson EL, Cain JR, Mitchell DL, Blanco LJ, Kessler JC, editors. Fundamentals of fixed prosthodontics. 4th ed. Chicago: Quintessence Publishing Co; 2012. Impressions; pp. 291–306. [Google Scholar]
- 2.Erbe C, Ruf S, Wöstmann B, Balkenhol M. Dimensional stability of contemporary irreversible hydrocolloids: humidor versus wet tissue storage. J Prosthet Dent. 2012 Aug;108(2):114–22. doi: 10.1016/S0022-3913(12)60117-6. [DOI] [PubMed] [Google Scholar]
- 3.Zarb G, Jacob RF. The potentially edentulous patient to be treated with immediatedentures. In: Zarb G, Hobkirk JA, Eckert S, Jacob RF, editors. Prosthodontic treatment for edentulous patients. 13th ed. St Louis: Mosby; 2013. pp. 112–18. [Google Scholar]
- 4.Phoenix RD, Cagna DR, DeFreest CF, editors. Stewart's clinical removable partial prosthodontics. 4th ed. Chicago: Quintessence Publishing Co.; 2008. The first diagnostic appointment; pp. 147–8. [Google Scholar]
- 5.Sakaguchi RL, Powers JM, editors. Craig’s Restorative dental materials. 13th ed. Philadelphia: Mosby; 2012. Replicating materials-impression and casting; pp. 277–325. [Google Scholar]
- 6.VanNoort R, editor. Introduction to dental materials. 3rd ed. Edinburgh: Mosby; 2007. Impression Materials; pp. 186–208. [Google Scholar]
- 7.Khaknegar B, Ettinger RL. Removal time: a factor in the accuracy of irreversible hydrocolloid impressions. J Oral Rehabil. 1977 Oct;4(4):369–76. doi: 10.1111/j.1365-2842.1977.tb01002.x. [DOI] [PubMed] [Google Scholar]
- 8.Schleier PE, Gardner FM, Nelson SK, Pashley DH. The effect of storage time on the accuracy and dimensional stability of reversible hydrocolloid impression material. J Prosthet Dent. 2001 Sep;86(3):244–50. doi: 10.1067/mpr.2001.117758. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson A, Ockert-Eriksson G, Lockowandt P. Accuracy of irreversible hydrocolloids (alginates) for fixed prosthodontics. A comparison between irreversible hydrocolloid, reversible hydrocolloid, and addition silicone for use in the syringe-tray technique. Eur J Oral Sci. 1998 Apr;106(2 Pt 1):651–60. doi: 10.1046/j.0909-8836.1998.eos106207.x. [DOI] [PubMed] [Google Scholar]
- 10.Peters MC, Tieleman A. Accuracy and dimensional stability of a combined hydrocolloid impression system. J Prosthet Dent. 1992 Jun;67(6):873–8. doi: 10.1016/0022-3913(92)90605-a. [DOI] [PubMed] [Google Scholar]
- 11.Cohen BI, Pagnillo M, Deutsch AS, Musikant BL. Dimensional accuracy of three different alginate impression materials. J Prosthodont. 1995 Sep;4(3):195–9. doi: 10.1111/j.1532-849x.1995.tb00340.x. [DOI] [PubMed] [Google Scholar]
- 12.Nassar U, Hussein B, Oko A, Carey JP, Flores-Mir C. Dimensional accuracy of 2 irreversible hydrocolloid alternative impression materials with immediate and delayed pouring. J Can Dent Assoc. 2012;78:c2. [PubMed] [Google Scholar]
- 13.Farzin M, Panahandeh H. Effect of pouring time and storage temperature on dimensional stability of casts made from irreversible hydrocolloid. J Dent (Tehran) 2010 Fall;7(4):179–84. [PMC free article] [PubMed] [Google Scholar]
- 14.Rodrigues SB, Augusto CR, Leitune VC, Samuel SM, Collares FM. Influence of delayed pouring on irreversible hydrocolloid properties. Braz Oral Res. 2012 Sep-Oct;26(5):404–9. doi: 10.1590/s1806-83242012000500005. [DOI] [PubMed] [Google Scholar]
- 15.Mosharraf R, Mokhtari M. The effect of storage time on the accuracy and dimensional stability of two irreversible hydrocolloid impression materials. Majallah-I-Dandanpizishki. 2006;18(2):92–9. [Google Scholar]
- 16.Mosharraf R, Nasouhian S, Salehi M. Effect of storage time on the dimensional stability of Extended-Pour irreversible hydrocolloid materials. J Isfahan Dent Sch. 2011;7(3):246–55. [Google Scholar]
- 17.Imbery TA, Nehring J, Janus C, Moon PC. Accuracy and dimensional stability of extended-pour and conventional alginate impression materials. J Am Dent Assoc. 2010 Jan;141(1):32–9. doi: 10.14219/jada.archive.2010.0018. [DOI] [PubMed] [Google Scholar]
- 18.Reddy CG, Gujjari KA, Shambhu HS. Influence of long term storage on the linear dimensional accuracy of improved alginate impressions-an in vitro study. TPDI. 2010;1:34–7. [Google Scholar]
- 19.Todd JA, Oesterle LJ, Newman SM, Shellhart WC. Dimensional changes of extended-pour alginate impression materials. Am J Orthod Dentofacial Orthop. 2013 Apr;143(4 Suppl):S55–63. doi: 10.1016/j.ajodo.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 20.Alcan T, Ceylanoğlu C, Baysal B. The relationship between digital model accuracy and time-dependent deformation of alginate impressions. Angle Orthod. 2009 Jan;79(1):30–6. doi: 10.2319/100307-475.1. [DOI] [PubMed] [Google Scholar]
- 21.Craig R, Powers J, Wataha J, editors. Dental materials: properties and manipulation. 8th ed. St Louis: Mosby; 2004. Impression Materials; pp. 156–71. [Google Scholar]
- 22.Sedda M, Casarotto A, Raustia A, Borracchini A. Effect of storage time on the accuracy of casts made from different irreversible hydrocolloids. J Contemp Dent Pract. 2008 May 1;9(4):59–66. [PubMed] [Google Scholar]
- 23.Shen C. Impression Materials. In: Anusa-vice KJ, editor. Phillips' science of dental materials. 11th ed. Philadelphia: Saunders; 2003. pp. 206–43. [Google Scholar]
- 24.Johnson GH, Craig RG. Accuracy of four types of rubber impression materials compared with time of pour and a repeat pour of models. J Prosthet Dent. 1985 Apr;53(4):484–90. doi: 10.1016/0022-3913(85)90630-4. [DOI] [PubMed] [Google Scholar]
- 25.Johnson GH, Chellis KD, Gordon GE, Lepe X. Dimensional stability and detail reproduction of irreversible hydrocolloid and elastomeric impressions disinfected by immersion. J Prosthet Dent. 1998 Apr;79(4):446–53. doi: 10.1016/s0022-3913(98)70160-x. [DOI] [PubMed] [Google Scholar]