Abstract
Objectives:
Use of alternative medicine to control oral streptococci is a new topic worthy of further investigation. This study aimed to elucidate the dose-dependent anti-bacterial activity of crude aqueous extract of ripe Morinda citrifolia L. (Family: Rubiaceae) fruits against oral streptococci i.e. Streptococcus mutans and Streptococcus mitis, that cause dental caries in humans.
Methods:
Fresh ripe M. citrifolia fruits (750g) were ground in an electronic blender with sterile water (500ml). The crude aqueous extract was lyophilized to yield a brown colored powder. Various concentrations (1000-100μg/ ml) of the extract were tested for its antibacterial activity (Kirby and Bauer method) against whole cells of S. mutans and S. mitis. Minimum Inhibitory Concentration (MIC) was determined by micro-dilution method, using serially diluted (2 folds) fruit extract, according to the National Committee for Clinical Laboratory Standards (NCCLS).
Results:
Crude aqueous extract (1000μg/ ml) of ripe M. citrifolia fruits effectively inhibited the growth of S. mutans (19±0.5 mm) and S. mitis (18.6±0.3 mm) compared to the streptomycin control (21.6±0.3 mm). The growth inhibition was clearly evident with “nil” bacteriostasis, even after 48 hours of incubation at 37°C. The MIC of the extract for S. mutans and S. mitis was 125 μg and 62.5 μg, respectively.
Conclusion:
Our results suggest that phytochemicals naturally synthesized by M. citrifolia have an inhibitory effect on oral streptococci. Furthermore, purification and molecular characterization of the “bioactive principle” would enable us to formulate a sustainable oral hygiene product.
Keywords: Morinda citrifolia L., Streptococcus mutans, Streptococcus, mitis, Antibacterial activity
INTRODUCTION
Infections of the oral cavity result from the loss of equilibrium between the hosts’ immune response and virulence factors of the indigenous microbiota [1, 2]. Irrespective of the advancements in medicine, these infections continue to pose a threat to public health, and put a heavy burden on health care services globally. This is particularly true in developing countries [3, 4].
Despite general advances in the overall health, including oral and dental health of the people living in industrialized countries, the prevalence of dental caries in school aged children is close to 90% and majority of adults are also affected [5]. There is evidence linking poor oral health and systemic diseases, such as cardiovascular diseases, rheumatoid arthritis, and osteoporosis [6], while periodontal diseases may also contribute to the risk of pregnancy complications such as preterm low-birth weight [7]. Furthermore, tooth loss caused by poor periodontal health can lead to significant morbidity and premature death [8]. The link between oral infections and the activities of microbial species that form part of the micro-biota of the oral cavity is well established [9 ]. Around 750 bacterial species colonize the oral cavity, out of which 50% are yet to be identified. Interestingly many of these bacteria are associated with oral diseases. The progression of dental caries is governed by acidogenic and aciduric gram-positive bacteria like S. mutans, lactobacilli and actinomycetes, which convert sucrose to organic acids, particularly lactic acid, that dissolve the calcium phosphate present in teeth and eventually lead to decalcification and tooth decay [10]. Several agents are available that can alter the profile of oral microflora but can cause undesirable contraindications such as vomiting, diarrhea and staining [11, 12]. Since ancient times medicinal plants have been utilized for oral hygiene. Scientific evaluation of several herbs has been undertaken against oral streptococci. Crude aqueous twig extract (50%) of Mangifera indica L. effectively inhibits the growth of S. mutans and S. mitis [13]. Among the plants in Rubiaceae family, Isertia laevis inhibits S. mutans and S. sobrinus growth with a MIC of 2mg/ ml [14]. Similarly, the adherence of S. mutans ATCC 35688 to dental enamel and dentine was greatly reduced after treatment with boiled aqueous extract of Coffea arabica L. [15]. Morinda citrifolia, popularly known as noni, has been an important medicinal plant for many centuries throughout the south pacific and has been used in folk remedies by Polynesians for over 2000 years [16]. It is a small shrub and its potential therapeutic properties remain vastly unknown [17]. It is reported to have anti-microbial, analgesic, hypotensive, anti-inflammatory and immunomodulatory properties [18, 19]. In traditional medicine, M. citrifolia fruit juice has been used to treat various illnesses including arthritis, diabetes, muscle aches, menstrual cramps, cardiac diseases, cancers, gastric ulcers, vascular diseases, and drug addiction. Nevertheless, the inhibitory activity of M. citrifolia fruits against predominant caries causing bacteria, S. mutans and S. mitis, has not been evaluted. Therefore the objective of this study was to evalute the inhibitory effect of M. citrifolia fruits on development of dental caries caused by S. mutans and S. mitis.
MATERIALS AND METHODS
Plant Collection and Extraction
Fresh ripe M. citrifolia fruits were collected in December (Temperature: 95°F, Humidity: 35%). Seven hundred fifty grams of the fruit were ground in an electronic blender that was sterilized with 70% ethanol.
Five hundred milliliters of sterile water were added to this pulp and the mixture was soaked for 48 hours in vitro, at room temperature. The slurry was filtered using filter paper (Whatman®, need to insert the location of the manufacturer here) and the extract was condensed in a lyophilizer (Martin Christ-alpha 1–2 LD plus, location of the manufacturer) at −55°C under 0.25 mbar pressure for 72 hours. The powdered extract was stored at −20°C till usage for up to 3 months.
Microorganism Source
The microbial strains used for this study were procured from the Institute of Microbial Technology, Chandigarh (Streptococcus mutans MTCC 497, Streptococcus mitis MTCC 2696).
Preparation of Inoculum
Stock cultures of the bacterial pathogens were maintained at 4°C on nutrient rich agar slants. Inoculum culture for the bioassay was prepared by transferring a loop full of cells from the stock culture to test tubes containing sterile Mueller-Hinton broth (HiMedia®, insert the location of the manufacturer here) that was incubated in an incubator at 37°C for 24 hours. Prior to the assay, the turbidity of the cultures was adjusted as per McFarland standard (0.5), using sterile Mueller-Hinton broth.
Fortification of discs
Sterile Whatman filter paper discs (HiMedia®, insert the location of the manufacturer here) were segregated in a pre-sterilized petri dish. Different concentrations of the extract, including 1000, 500, 250 and 100μg/20μl were prepared with DMSO (Dimethyl sulfoxide) and loaded on the discs and dried in a vertical air draft for three hours to remove residual solvent.
Disc-diffusion Bioassay Method (20)
Sterile Muller Hinton Agar (MHA) medium was prepared and dispensed in sterile petri dishes (30ml/ dish). After solidification, 100 μl of the bacterial inoculum was added to the plate using a micropipette (Finnpipette®, insert the location of the manufacturer), and was evenly spread with the aid of a sterilized glass spreader. Extract-fortified discs were placed on the plate using sterile forceps. The plates were incubated for 24 hours at 37°C. Growth inhibition was determined by measuring the diameter of the zones of inhibition using a meter scale. This procedure was repeated three times for both organisms (S. mutans, S. mitis) and the average values were calculated. Bacteriostasis (emergence of resistant colonies) was observed by continued incubation of the assay plates for another 24 hours at 37°C.
Determination of Minimum Inhibitory Concentration (MIC)
Minimum inhibitory concentation was determined by micro-dilution method using serially diluted (2 folds) extracts of M. citrifolia fruit, according to the NCCLS (National Committee for Clinical Laboratory Standards, 2000). Various concentrations of the extract including 1 mg, 500 μg, 250 μg, 125 μg, 62.5 μg, 31.25 μg, 15.62 μg and 7.81 μg/ml were prepared. One millliter of each dilution was added to a test tube and equal volume of sterile Mueller-Hinton broth was added. Subsequently, 0.1 ml of standardized inoculum (1×107 CFU/ml) was added to each tube.
The tubes were incubated aerobically at 37°C for 24 hours. Antibiotic (positive control) and Organism (negative control) i.e. tube containing the growth medium, saline and the inoculum were maintained in ideal conditions throughout the assay. The least concentration of the extract that exhibited “nil” visible bacterial growth (absence of turbidity) compared with the negative control was regarded as MIC.
RESULTS
Zone of inhibition
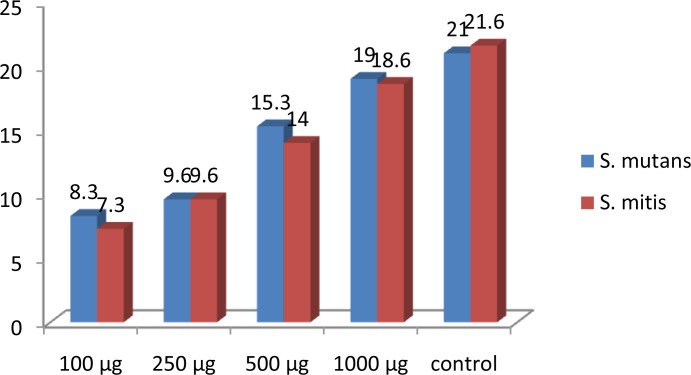
Different concentrations of the M. citrifolia ripe fruit extract including 1000, 500, 250 and 100 μg were analysed for their action against S. mutans and S. mitis and were compared with the standard antibiotic streptomycin. Crude aqueous extract of the ripe M. citrifolia fruits at 1000 μg/ml concentration effectively inhibited the growth of S. mutans (19±0.5 mm) and S. mitis (18.6±0.3 mm) compared to the positive control streptomycin (21.6±0.3 mm). Extracts at 100 μg/ml, 250 μg/ml and 500 μg/ml showed zones of inhibition of 8.3 mm, 9.6 mm, 15.3 mm for S. mutans and 7.3 mm, 9.6 mm, 14 mm for S. mitis, respectively. Thus, the inhibition pattern was found to be dose-dependent and increased with increased concentration of the extract against both organisms (Table 1).
Table 1.
Anti-microbial activity of aqueous fruit (ripe) extract of M. citrifolia L.
| S. No | Microorganism | Concentration of the Extract | Zone of Inhibition (in mm) | Standard Deviation | Standard Error |
|---|---|---|---|---|---|
| 1 | Streptococcus mitis | 1000 μg | 18.6±0.3 | 0.58 | 0.33 |
| 500 μg | 14.0±0.5 | 1.00 | 0.58 | ||
| 250 μg | 9.6±0.3 | 0.58 | 0.33 | ||
| 100 μg | 7.3±0.3 | 0.58 | 0.33 | ||
| Streptomycin (100μg) | 21.6±0.3 | 0.58 | 0.33 | ||
| DMSO Control | - | - | - | ||
| 2 | Streptococcusmutans | 1000 μg | 19.0±0.5 | 1.00 | 0.58 |
| 500 μg | 15.3±0.6 | 1.15 | 0.67 | ||
| 250 μg | 9.6±0.3 | 0.58 | 0.33 | ||
| 100 μg | 8.3±0.3 | 0.58 | 0.33 | ||
| Streptomycin (100μg) | 21.0±0.5 | 1.00 | 0.58 | ||
| DMSO Control | - | - | - |
The average of triplicates is measured for the zone of inhibition
The bar graph depicted for the different concentrations of M. citrifolia extract and the control shows this pattern clearly (Figure 1).
Fig 1.
Average zone of inhibition values for different concentrations of M. citrifolia extract and the control on S. mutans and S. mitis.
Minimum inhibitory concentration
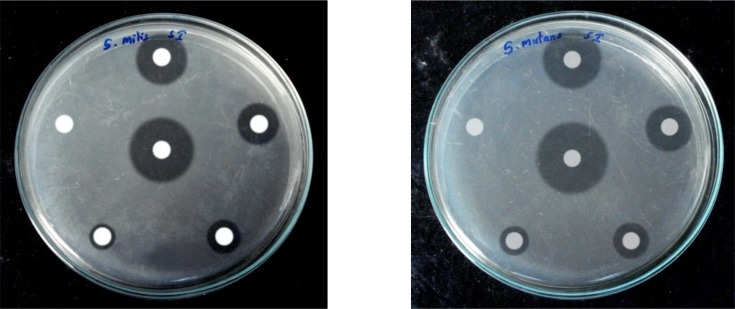
Growth inhibition was clearly evident with “nil” bacteriostasis, even after 48 hours of incubation at 37°C (Figure 2).
Fig 2.
Anti-microbial activity of aqueous extract of M. citrifolia L. fruit against oral streptococci causing dental caries (representative sample of the three tests)
*Clockwise from Top: 1000, 500, 250 and 100μg/ disc and solvent (DMSO) control Center: Streptomycin (100 μg/ disc) control
The MIC was checked with different concentrations of the extract including 1 mg, 500 μg, 250 μg, 125 μg, 62.5 μg, 31.25 μg, 15.62 μg and 7.81 μg/ml. The MIC values of the extract for S. mutans and S. mitis were found to be 125 μg and 62.5 μg, respectively. An independent t-test was done to compare the action of M. citrifolia extract and streptomycin against S. mutans.
The P-value was found to be 0.64, proving that there was no significant difference in mean value between M. citrifolia extracts and streptomycin against S. mutans.
DISCUSSION
Dental caries are a major health concern throughout the world due to common issues such as socioeconomic factors, immigration, lack of preventive efforts and dietary changes. Therefore, we are in need of new and renewed efforts to cut down the drastic increases in dental caries [21].
Our aim was to find an herbal anti-caries agent which would effectively replace the commercially available agents. M. citrifolia was chosen because of its wellknown antimicrobial and therapeutic properties.
It is said to be an underutilised miracle plant that grows naturally in most geographical conditions, even without proper care and it is now being cultivated by the farmers as a crop in different parts of India [22].
Recent studies have shown that M. citrifolia has a wide array of biologically active compounds.
Around 160 phytochemical compounds have been isolated from the M. citrifolia plant, the majority of which are organic acids, phenolic compounds, and alkaloids.
Among the phenolic compounds, the most important ones are anthraquinones, aucubin, asperuloside, and scopoletin [23]. M. citrifolia is reported to have antibacterial, antiviral, anti-fungal, antitumor, antihelminthic, analgesic, hypotensive, anti-inflammatory, and immune enhancing properties [23–25].
Therefore, M. citrifolia can be regarded as a valuable medicinal plant and a possible source for modern drug development.
Morinda citrifolia has also been demonstrated to be effective in removing smear layers in endodontically treated teeth. In a trial by Murray, M. citrifolia was more effective than chlorhexidine in removing the smear layer.
The efficacy of M. citrifolia was similar to sodium hypochloride (NaOCl) in conjunction with EDTA as an intracanal irrigant. Morinda citrifolia appears to be one of the first fruits to be identified as a possible alternative to NaOCl as an intracanal irrigant [26]. A recent small trial in eleven patients has shown that the combination of good oral hygiene and administration of M. citrifolia juice is a promising treatment for reducing bleeding caused by probing. Similarly, additional treatment with M. citrifolia juice significantly alleviated the gingival inflammation [27]. Morinda citrifolia has also been shown to have in vitro antibacterial activity against different strains of several oral bacterial pathogens isolated from different sources [28]. However, scientific studies on the anti-cariogenic properties of M. citrifolia remain scarce.
Crude mixtures of phytochemicals from natural products have been routinely evaluated against oral streptococci, specifically S. mutans and S. mitis. Ethanol and diethyl ether extracts (30mg/ml) of Nigella sativa L. (Black seed) inhibited the growth of S. mutans (12.7 ±2.1 and 6.3±0.6 mm) and S. mitis (10.4 ±0.9 and 5.1±0.6 mm), respectively [29]. Chava et al. proved that crude aqueous extract of Azadirachta indica L. twigs at 50% concentration inhibited the growth of S. mutans (4.6mm), S. mitis (3.1mm), S. salivarius (2.3mm) and S. sanguis (3.2mm) at 48 hours respectively [30]. In the present study, 1000 μg (1mg/disc) of M. citrifolia fruit extract demonstrated superior inhibitory activity against S. mutans that is five times (19.0±0.5mm) as effective as that of A. indica extract. It has been reported that M. citrifolia L. inhibits pathogenic bacteria such as S. aureus, Pseudomonas aeruginosa, Proteus morgaii, Bacillus subtilis, E. coli, Helicobacter pylori, Salmonella species and Shigella species. This might be attributed to the presence of secondary metabolic phenolic compounds such as acubin, L-asperuloside, alizarin and anthraquinones including scopoletin [31]. Another study demonstrated that crude acetonitrile extract of the dried fruit was bactericidal against P. aeruginosa, Bacillus subtilis, E.coli, and S. pyogenes [32]. Ethanol and hexane extracts of M. citrifolia L. inhibit 89–95% of Mycobacterium tuberculosis species [33]. Other studies have reported a significant antimicrobial effect on various strains of Salmonella, Shigella, and E. coli [34]. Nevertheless, the inhibitory activity against cariogenic S. mutans and S. mitis has not been proven previously. Furthermore, reports have shown that this anti-microbial activity is highly dependent on the stage of ripeness of the fruit and its processing, showing greater activity when the fruit is ripe and undried. This is in agreement with our observation in oral streptococci.
Base on our results, further purification and formulation of the extract would certainly pave the way for an antibacterial mouth wash/rinse that is easily available and could be used safely. Chloride, chlorhexidine, fluoride, and fluoride-containing agents can cause tooth staining, and ethanol, the main ingredient of the common mouthwash, can cause oral cancer [35]. Hence, the search for alternative products still continues and phytochemicals used in traditional medicine should be considered as viable alternatives to synthetic products.
REFERENCES
- 1.Faveri M, Mayer MP, Feres M, de Figuei-redo LC, Dewhirst FE, Paster BJ. Microbiological diversity of generalized aggressive periodontitis by 16S rRNA clonal analysis. Oral Microbiol Immunol. 2008 Apr;23(2):112–8. doi: 10.1111/j.1399-302X.2007.00397.x. doi: 10.1111/j.1399-302X.2007.00397.x. [DOI] [PubMed] [Google Scholar]
- 2.Feng Z, Weinberg A. Role of bacteria in health and disease of periodontal tissues. Periodontol. 2000;40:50–76. doi: 10.1111/j.1600-0757.2005.00148.x. , 2006. [DOI] [PubMed] [Google Scholar]
- 3.Singh J, Kumar A, Budhiraja S, Hooda A. Ethnomedicine: use in dental caries. Braz. J. Oral Sci. 2007;6:1308–1312. [Google Scholar]
- 4.Poole K. Overcoming antimicrobial resistance by targeting resistance mechanisms. J Pharm Pharmacol. 2001 Mar;53(3):283–94. doi: 10.1211/0022357011775514. [DOI] [PubMed] [Google Scholar]
- 5.Petersen PE, Bourgeois D, Ogawa H, Estupinan-Day S, Ndiaye C. The global burden of oral diseases and risks to oral health. Bull World Health Organ. 2005 Sep;83(9):661–9. Epub 2005 Sep 30. [PMC free article] [PubMed] [Google Scholar]
- 6.Rautemaa R, Lauhio A, Cullinan MP, Seymour GJ. Oral infections and systemic disease—an emerging problem in medicine. Clin Microbiol Infect. 2007 Nov;13(11):1041–7. doi: 10.1111/j.1469-0691.2007.01802.x. Epub 2007 Aug 21. [DOI] [PubMed] [Google Scholar]
- 7.Yeo BK, Lim LP, Paquette DW, Williams RC. Periodontal disease—the emergence of a risk for systemic conditions: pre-term low birth weight. Ann Acad Med Singapore. 2005 Jan;34(1):111–6. [PubMed] [Google Scholar]
- 8.Petersen PE. The World Oral Health Report 2003: continuous improvement of oral health in the 21st century- the approach of the WHO Global Oral Health Programme. Community Dent Oral Epidemiol. 2003 Dec;31(Suppl 1):3–23. doi: 10.1046/j..2003.com122.x. [DOI] [PubMed] [Google Scholar]
- 9.Jenkinson HF, 1, Lamont RJ. Oral microbial communities in sickness and in health. Trends Microbiol. 2005 Dec;13(12):589–95. doi: 10.1016/j.tim.2005.09.006. Epub 2005 Oct 7. [DOI] [PubMed] [Google Scholar]
- 10.Loesche W. Dental caries and Periodontitis: contrasting two infections that have medical implications. Infect Dis Clin North Am. 2007 Jun;21(2):471–502. doi: 10.1016/j.idc.2007.03.006. , vii. [DOI] [PubMed] [Google Scholar]
- 11.Park KM, You JS, Lee HY, Baek NI, Hwang JK. An antibacterial agent from the root bark of Morus alba against oral pathogens. J Ethnopharmacol. 2003 Feb;84(2–3):181–5. doi: 10.1016/s0378-8741(02)00318-5. [DOI] [PubMed] [Google Scholar]
- 12.Chung JY, Choo JH, Lee MH, Hwang JK. Anticariogenic activity of macelignan isolated from Myristica fragrans (nutmeg) against Streptococcus mutans. Phytomedicine. 2006 Mar;13(4):261–6. doi: 10.1016/j.phymed.2004.04.007. Epub 2005 Jun 28. [DOI] [PubMed] [Google Scholar]
- 13.Prashanth GM, Chandu GN, Murulikrishna KS, Shafiulla MD. The effect of mango and neem extract on four organisms causing dental caries: Streptococcus mutans, Streptococcus salivavius, Streptococcus mitis, and Streptococcus sanguis: an in vitro study. Indian J Dent Res . 2007 Oct-Dec;18(4):148–51. doi: 10.4103/0970-9290.35822. [DOI] [PubMed] [Google Scholar]
- 14.Téllez N, Téllez M, Perdomo M, Alvarado A, Gamboa F. Anticariogenic activity of the active fraction from Isertia laevis against S. mutans and S. sobrinus: comparison of two extraction methods. Acta Odontol Latinoam. 2010;23(3):188–95. [PubMed] [Google Scholar]
- 15.de Oliveira LD, da Silva Brandão EH, Landucci LF, Koga-Ito CY, Jorge AOC. Effects of Coffea arabica on Streptococcus mutans adherence to dental enamel and dentine. Braz. J. Oral Sci. 2007;6(23):1438–1441. [Google Scholar]
- 16.Whistler WA. Traditional and herbal medicine in the Cook Islands. J Ethnopharmacol. 1985 Jul;13(3):239–80. doi: 10.1016/0378-8741(85)90072-8. [DOI] [PubMed] [Google Scholar]
- 17.McClatchey W. From Polynesian healers to health food stores: changing perspectives of Morinda citrifolia (Rubiaceae) Integr Cancer Ther. 2002 Jun;1(2):110–20. doi: 10.1177/1534735402001002002. ; discussion 120. [DOI] [PubMed] [Google Scholar]
- 18.Liu G, Bode A, Ma WY, Sang S, Ho CT, Dong Z. Two novel glycoside from the fruit of Morinda citrifolia fruit inhibit AP-1 transactivation and cell transformation in the mouse epidermal JB6 cell line. Cancer Res. 2001 Aug 1;61(15):5749–56. [PubMed] [Google Scholar]
- 19.Duke J, Bogenschutz M, Duke . Handbook of medicinal plants. 2nd. Boca Raton, FL: CRC Press; 2002. p. 529. [Google Scholar]
- 20.Bauer AW, Perry DM, Kirby WM. Single disc antibiotic sensitivity testing of Staphylococci. AMA Arch Intern Med. 1959 Aug;104(2):208–16. doi: 10.1001/archinte.1959.00270080034004. [DOI] [PubMed] [Google Scholar]
- 21.Bagramian RA, Garcia-Godoy F, Volpe AR. The Global Increase in Dental Caries. A Pending Public Health Crisis. Am J Dent. 2009 Feb;22(1):3–8. [PubMed] [Google Scholar]
- 22.Rethinam P, Sivaraman K. Noni (Morinda citrifolia L) the Miracle Fruit - A Holistic Review. Intl J Noni Res. 2007;2:1–2. [Google Scholar]
- 23.Wang MY, Su C. Cancer preventive effect of Morinda citrifolia (Noni) Ann N Y Acad Sci. 2001 Dec;952:161–8. doi: 10.1111/j.1749-6632.2001.tb02737.x. [DOI] [PubMed] [Google Scholar]
- 24.Deivanayagam Kandaswamy. Nagendrababu Venkateshbabu Root canal irrigants. J Conserv Dent . 2010 Oct-Dec;13(4):256–264. doi: 10.4103/0972-0707.73378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang MY, West BJ, Jensen CJ, Nowicki D, Su C, Palu AK, et al. Morinda citrifolia (Noni): a literature review and recent advances in Noni research. Acta Pharmacol Sin. 2002 Dec;23(12):1127–41. [PubMed] [Google Scholar]
- 26.Murray PE, Farber RM, Namerow KN, Kuttler S, Garcia-Godoy F. Evaluation of Morinda citrifolia as an Endodontic Irrigant. J Endod. 2008 Jan;34(1):66–70. doi: 10.1016/j.joen.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 27.Glang J, Falk W, Westendorf J. Effect of Morinda citrifolia L. fruit juice on gingivitis/periodontitis. MRI. 2013;2(2):21–27. [Google Scholar]
- 28.Chan-Blanco Y, Vaillant F, Mercedes Perez A, Reynes M, Brillouet JM, Brat P. The noni fruit (Morinda citrifolia L.): A review of agricultural research, nutritional and therapeutic properties. J Food Compost Anal. 2006;19:645–54. [Google Scholar]
- 29.Mohammed ND. Effect of Nigella sativa L. extracts against Streptococcus mutans and Streptococcus mitis in vitro. J. Bagh. Coll. Dent. 2012;24(3):154–157. [Google Scholar]
- 30.Chava VR, Manjunath SM, Rajanikanth AV, Sridevi N. The efficacy of Neem Extract on four organisms causing dental caries: Streptococcus mutans, S. salivarius, S. mitis & S. sanguis. An in vitro study. J Contemp Dent Pract. 2012 Nov 1;13(6):769–72. doi: 10.5005/jp-journals-10024-1227. [DOI] [PubMed] [Google Scholar]
- 31.Atkinson N. Antibacterial substances from flowering plants. 3. Antibacterial activity of dried Australian Plants by rapid direct plate test. Aust J Exp Biol Med Sci. 1956 Feb;34(1):17–26. [PubMed] [Google Scholar]
- 32.Locher CP, 1, Burch MT, Mower HF, Berestecky J, Davis H, Van Poel B, et al. Antimicrobial activity and anti-complement activity of extracts obtained from selected Hawaiian medicinal plants. J Ethnopharmacol. 1995 Nov 17;49(1):23–32. doi: 10.1016/0378-8741(95)01299-0. [DOI] [PubMed] [Google Scholar]
- 33.Saludes JP, Garson MJ, Franzblau SG, Aguinaldo AM. Antitubercular constituents from the hexane fraction of Morinda citrifolia Linn (Rubiaceae). Phytother Res. 2002 Nov;16(7):683–5. doi: 10.1002/ptr.1003. [DOI] [PubMed] [Google Scholar]
- 34.Dittmar A. Morinda citrifolia L.-use in indigenous Samoan medicine. J. of Herbs, Spices and Med. Plants. 1993;1:77–92. [Google Scholar]
- 35.McCullough MJ, 1, Farah CS. The role of alcohol in oral carcinogenesis with particular reference to alcohol containing mouthwashes. Aust Dent J. 2008 Dec;53(4):302–5. doi: 10.1111/j.1834-7819.2008.00070.x. [DOI] [PubMed] [Google Scholar]