Abstract
Osteosarcoma is an aggressive bone tumor that preferentially develops in adolescents. The tumor is characterized by an abundance of genomic aberrations, which hampers the identification of the driver genes involved in osteosarcoma tumorigenesis. Our study aims to identify these genes by the investigation of focal copy number aberrations (CNAs, <3 Mb). For this purpose, we subjected 26 primary tumors of osteosarcoma patients to high-resolution single nucleotide polymorphism array analyses and identified 139 somatic focal CNAs. Of these, 72 had at least one gene located within or overlapping the focal CNA, with a total of 94 genes. For 84 of these genes, the expression status in 31 osteosarcoma samples was determined by expression microarray analysis. This enabled us to identify the genes of which the over- or underexpression was in more than 35% of cases in accordance to their copy number status (gain or loss). These candidate genes were subsequently validated in an independent set and furthermore corroborated as driver genes by verifying their role in other tumor types. We identified CMTM8 as a new candidate tumor suppressor gene and GPR177 as a new candidate oncogene in osteosarcoma. In osteosarcoma, CMTM8 has been shown to suppress EGFR signaling. In other tumor types, CMTM8 is known to suppress the activity of the oncogenic protein c-Met and GPR177 is known as an overexpressed upstream regulator of the Wnt-pathway. Further studies are needed to determine whether these proteins also exert the latter functions in osteosarcoma tumorigenesis.
Introduction
Osteosarcoma is the most frequent primary bone tumor in children and adults [1]. The metaphyseal regions of long bones, i.e. the regions with high osteoblastic activity, which include the distal femur, proximal tibia and proximal humerus, are the main sites of primary tumor [2]. Tumor cells are thought to be of mesenchymal lineage and poorly differentiated, however they still produce osteoid [3]. With the introduction of chemotherapy the survival rate has risen to 60–75% in the last three decades of the 20th century provided that no metastases are present at the time of diagnosis [4]. Survival rates decrease to less than 30% in metastatic disease [5], with the lungs as the primary site of metastasis [6].
Osteosarcomas have a complex karyotype that contain numerous chromosomal aberrations, which consist of gains, amplifications, deletions, translocations and overall aneuploidy [7]. Frequent copy number gains, suggestive for the presence of oncogenes, have been reported for chromosome regions 1p, 1q, 6p, 8q, and 17p11.2-p12 and copy number losses, suggestive for the presence of tumor suppressor genes, for chromosome regions 3q, 6q, 9, 10, 13, 17p13, and 18q. A number of oncogenes, including MYC and RUNX2, and tumor suppressor genes, notably DOCK5, CCNE1, and LSAMP, have been shown to be present in the affected regions, but other genes remain to be identified (see recent reviews by Martin et al. [8] and Kuijjer et al. [9]).
Each tumor genome harbours a mixture of genetic aberrations affecting genes that are directly responsible for its development (drivers) and random events whereby the affected genes have no biological significance (passengers) [10]. Distinguishing driver from passenger events in the cancer genome is crucial for our understanding of tumor development and will aid to identify novel oncogenes or tumor suppressor genes as potential targets for therapeutical intervention. The identification of driver genes has been a major challenge in earlier copy number studies due to the low resolution and consequently large sizes of the detected aberrations. The increase in resolution of array CGH and SNP platforms in recent years has allowed for the identification of small aberrations that went previously undetected. These sub-microscopic gains and losses, termed focal copy number aberrations (focal CNAs), were reported for different tumor types such as lung, breast, and colon cancer [11], [12]. Alike large CNAs of somatic origins, they are thought to be the result of a Darwinian-like, yet somatic, evolutionary selection process. Hence, a single gene in a focal CNA would give the tumor a selective growth advantage, which concept is instrumental in the fore laying study. The definition of focal CNAs does not have a biological basis. Based on pragmatic considerations, such as a limited number of genes, size definitions in previous studies and the size of CNVs as defined by Feuk et al [13] led us to select 3 Mb as the upper size limit for a focal CNA [14]. To our knowledge, only a few studies in primary human osteosarcoma that use high-resolution arrays have been published. Kuijjer et al (2012) [15] performed an integrative analysis of copy number and expression profiling in 29 osteosarcoma samples. They identified 31 candidate driver genes, mainly located in regions with recurrent chromosomal losses, of which a substantial number proved to be involved in genomic instability. Previously, we employed a comparable methodology to search for driver genes in chromosome region 17p11.2-p12 in osteosarcoma samples using high-resolution, genome-wide SNP array and expression microarray analyses [16]. In the present study, we combined both analytical tools to identify novel driver genes in chromosomal regions other than 17p11.2-p12. This was accomplished by identifying the recurrent focal CNAs in the genome of osteosarcomas and by gene expression analysis of the genes they affected.
Materials and Methods
Ethics statement
Clinical samples were irreversibly anonymised and results of scientific research could not be linked to individual patients. The Committee Medical Ethics of the Academic Medical Center (AMC) specifically waived approval for this study because it falls under paragraph 7∶467 Civil Law Code of The Netherlands.
Patient samples
A total of 37 osteosarcomas, collected in the AMC, were analyzed in this study. The clinical data of the patients are summarized in Table 1. Sections of the tumors were H&E stained and reviewed by an experienced pathologist (J.Bras) to ensure high tumor cell content (>90%). Primary human fetal osteoblasts were cultured in osteoblast basal medium with osteoblast growth supplement (Cell Applications, Inc, San Diego, CA USA).
Table 1. Clinicopathological characteristics of patients and tumors.
| PatientCharacteristics | Number of samples (%) | |
| Age | Mean | 16.8 |
| Median | 16 | |
| Range | 6–58 | |
| Sex | Male | 20 (54.1) |
| Female | 17 (45.9) | |
| Location of Primary Tumor | Femur | 17 (45.9) |
| Tibia/Fibula | 9 (24.3) | |
| Humerus | 5 (13.5) | |
| Axialskeleton | 2 (5.4) | |
| Other | 4 (10.8) | |
| Histological subtype | Osteoblastic | 31 (83.8) |
| Fibroblastic | 3 (8.1) | |
| Telangiectatic | 2 (5.4) | |
| Unknown | 1 (2.7) | |
| Metastasis at diagnosis | No | 34 (91.9) |
| Yes | 3 (8.1) |
Experimental design
DNA and RNA were isolated from alternating sections (10 µm) of fresh frozen tumor samples. A standard proteinase K digestion, followed by a chloroform extraction was used to obtain DNA of high molecular weight. Isolation of RNA was performed using Trizol (Invitrogen, Carlsbad, CA, USA) and a subsequent automated RNeasy protocol (QIAgen, Hilden, Germany). The quality of the RNA was assessed on a BioAnalyzer (Agilent, Santa Clara, CA, USA). Only samples with a RNA Integrity Number (RIN) score higher than 7.5 were included. Human primary fetal osteoblasts were cultured to a confluency of 90% and RNA was isolated to act as a reference in the microarray expression analyses.
Single nucleotide polymorphism (SNP) and expression arrays
DNA of 26 osteosarcomas was hybridized for whole-genome copy number variation using Illumina HumanCNV370-Quad BeadChips as previously reported in Both et al. (2012) [16]. The array contained probes for 373,397 SNPs. Processing of DNA samples, hybridization, staining, and scanning of the BeadChips, and primary data extraction were all performed according to the Illumina Infinium II protocol at the array facility of ServiceXS (Leiden, the Netherlands).
Gene expression analysis using Illumina HumanHT-12 v3 Expression Beadchips (Illumina, San Diego USA) was performed on RNA of 31 osteosarcoma samples (of which 20 samples were also analyzed on the SNP array) as previously reported in Both et al. (2012) [16]. Each array contained 48,804 probes, spanning the human transcriptome. Labeling of RNA samples, hybridization, staining, and scanning of the Beadchips, and primary data extraction were all performed according to the Illumina Infinium II protocol, and under ISO 17025 certification, at the array facility of ServiceXS (Leiden, the Netherlands). Expression fold changes of a probe were determined by normalizing the intensity of the average signal in the tumor sample against the average signal in the primary human osteoblasts sample.
The SNP data and expression microarray data have been deposited in NCBI's GEO Omnibus and are accessible through GEO Series accession number GSE32964.
SNP and expression array data analysis
Median normalized Log2ratios were segmented with Circular Binary Segmentation (CBS) [17]. After segmentation samples were mode normalized [18]. Chromosomal copy number changes were defined using the RpackageCGHcall [18]. Several criteria were applied to distinguish the tumor acquired and thus somatic copy number aberrations (CNAs) from the inherited and thus germline copy number variations, as reviewed by Feuk et al [13]. Germline copy number variants (CNVs) were removed from the dataset in 2 steps: 1) all DNA copy number changes of 3 Mb and smaller were removed if overlapping CNVs regions as archived in the database of genomic variants (DGV), population size and datasets can be found at: http://dgv.tcag.ca/ [19], 2) all copy number changes of 3 Mb and smaller that showed both recurrent (≥2) gains and losses were marked as CNVs and removed.
For each recurrent focal CNA, the smallest genomic overlap of the focal aberrations and the frequency in the dataset was determined and defined as high frequency region (HFR). Genes were retrieved using biomaRt (R/Bioconductor) and Ensembl (hg18/NCBI 36, ensemble 54).
Expression intensities were transformed to log2ratios with average primary osteoblast intensities as a reference. Array normalization was performed on the log2ratios using LOESS correction on each sample and a quantile normalization to correct for bias between the arrays [20].
For validation purposes the publicly available expression array of 84 osteosarcoma samples and 3 osteoblast samples previously published by Kuijjer et al. (2012) [15] was downloaded from NCBI's GEO Omnibus database (accession number GSE33383). This was normalized and analyzed using the same tools and settings as for our own dataset. All analyses were performed and plots were made using the statistical programming language R version 2.11.1 and Bioconductor packages (http://www.r-project.org).
Selection bias
The 26 samples used for copy number profiling in this study were previously selected for the presence of an amplification event on chromosome arm 17p [16]. To determine whether this selection biased the type and frequency CNAs in our sample set, we compared the frequency of aberrations in our sample set with that of an unselected sample set. For the latter, the copy number data (raw log2ratios) of 20 samples, deposited by Kresse et al [21], was downloaded from http://www.ebi.ac.uk/arrayexpress/; file E-MEXP-1219 and processed with similar settings as for our samples. Matching of the platforms was performed as described by Van Wieringen et al. [22] with the “overlapPlus” function. Statistical differences between the matched series were determined using a Wilcoxon test with ties using the R-package CGHtest [18]. Apart from the expected higher frequency of 17p gain only one other chromosomal difference, a lower frequency of 7p gain, was observed (FDR q<0.05). No other significant differences were observed in the frequency of gains and losses between the two sets.
Enrichment analysis
To test whether the focal aberrations were enriched for cancer related genes as published in the Cancer Census list (http://www.sanger.ac.uk/genetics/CGP/Census/) [23], enrichment analysis was performed as described previously [24].
Results
Focal aberrations
DNA copy number profiling of 26 osteosarcoma samples showed many regions of frequent gains and losses. As shown in Fig. 1 (top panel), most frequent copy number changes were gains of chromosome arms 1p, 5p, 6p, 8q, 17p, 19 and 21 and losses of chromosome arms 3q, 5q, 6q, 8p, 9p, 10 and 13. Focal copy number changes were found in most samples with a median number of 26 (range: 4–61) for losses and 6 (range: 0–19) for gains per sample. We observed a total of 550 recurrent (n≥2) copy number changes. Representative examples of focal copy number changes are shown in Fig. 2. Out of the 550 recurrent copy number changes 266 were disregarded since they overlapped with known CNVs. Of the remaining 284 recurrent copy number changes a further 145 regions were disregarded as CNVs since both recurrent (≥2) gains and losses were detected in the dataset at the same location. This leaves a total of 139 unique genomic locations (Fig. 1, bottom panel) recognized as bonafide focal CNAs. Of these, 72 had at least one gene located within or overlapping the high frequency region (HFR), with a total of 94 genes (Table 2). These focal CNAs were significantly enriched for genes described in the Cancer Gene Census list (p-value = 0.002, http://www.sanger.ac.uk/genetics/CGP/) and include CBFA2T3, EBF1, FHIT, MYC, PTEN and RANBP17. The focal CNAs we identified did not contain known microRNAs.
Figure 1. Genome-wide frequency plot of all copy number aberrations (top) and of focal copy number aberrations (bottom) in 26 osteosarcoma samples.
Frequencies of gains (in red) and losses (in blue) are indicated. Vertical bars in B represent detected focal copy number aberrations (<3 Mb).
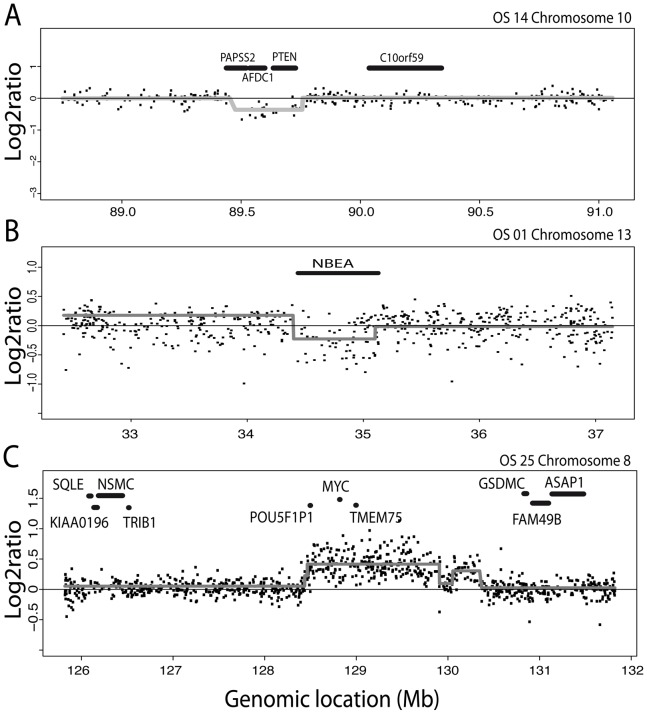
Figure 2. Representative examples of focal copy number aberrations.
Log2ratio for each SNP is plotted against its genomic location. Gray lines indicate segment values as derived using circular binary segmentation (CBS). A, Focal deletion encompassingPAPSS2, AFDC1, and PTEN on chromosome 10; B, Focal deletion restricted to NBEA on chromosome 13; C, Focal gain including POU5F1P1, MYC, and TMEM75 on chromosome 8.
Table 2. High frequency regions (HFRs) of focal aberrations and gene(s) within or overlapping these HFRs.
| HFR1 Focal abberration | Focal2 | Total3 | ||||||
| Chrom. | Start (bp) | End (bp) | Size (kb) | Gain | Loss | Gain | Loss | Genes |
| 1 | 68394382 | 68461186 | 67 | 2 | 0 | 10 | 0 | GPR177 |
| 1 | 166259174 | 166263398 | 4 | 2 | 0 | 8 | 0 | IQWD1 |
| 1 | 145961855 | 146262362 | 301 | 2 | 1 | 8 | 1 | FAM108A2, NBPF11 |
| 2 | 51022554 | 51022614 | 0.06 | 2 | 0 | 3 | 1 | NRXN1 |
| 2 | 153790092 | 154905667 | 1116 | 2 | 0 | 3 | 1 | RPRM, GALNT13 |
| 2 | 212844992 | 212863839 | 19 | 1 | 3 | 1 | 5 | ERBB4 |
| 2 | 237017756 | 237067914 | 50 | 0 | 3 | 1 | 8 | IQCA1 |
| 3 | 38177003 | 38246945 | 70 | 1 | 2 | 1 | 6 | OXSR1 |
| 3 | 57426028 | 57504090 | 78 | 0 | 2 | 0 | 8 | DNAH12L |
| 3 | 59855182 | 59891414 | 36 | 0 | 2 | 0 | 9 | FHIT |
| 3 | 60208127 | 60420353 | 212 | 0 | 2 | 0 | 9 | FHIT |
| 3 | 24260772 | 24290280 | 30 | 0 | 3 | 0 | 5 | THRB |
| 3 | 32341229 | 32364439 | 23 | 0 | 3 | 0 | 6 | CMTM8 |
| 4 | 90072621 | 90081252 | 9 | 0 | 2 | 1 | 3 | FAM13A1 |
| 4 | 183476755 | 184028352 | 552 | 0 | 2 | 1 | 7 | ODZ3 |
| 4 | 73484965 | 73485361 | 0.396 | 0 | 15 | 0 | 17 | ADAMTS3 |
| 5 | 170214294 | 170645196 | 431 | 1 | 2 | 1 | 5 | RANBP17 |
| 5 | 55470977 | 55502030 | 31 | 0 | 2 | 1 | 10 | ANKRD55 |
| 5 | 110611609 | 110683366 | 72 | 0 | 2 | 0 | 8 | CAMK4 |
| 5 | 135294758 | 135330960 | 36 | 0 | 2 | 0 | 8 | FBXL21, LECT2 |
| 5 | 158336707 | 158375568 | 39 | 0 | 4 | 0 | 7 | EBF1 |
| 5 | 58765648 | 58766102 | 0.454 | 0 | 5 | 0 | 12 | PDE4D |
| 5 | 80023854 | 80024518 | 0.664 | 0 | 11 | 0 | 14 | MSH3 |
| 6 | 44998738 | 45119343 | 121 | 5 | 0 | 11 | 0 | SUPT3H |
| 6 | 13965760 | 14255124 | 289 | 2 | 1 | 4 | 1 | CD83, RNF182 |
| 6 | 14051934 | 14103151 | 51 | 2 | 1 | 4 | 1 | RNF182 |
| 6 | 86262042 | 86335470 | 73 | 0 | 3 | 0 | 11 | SNX14, NT5E |
| 6 | 110510873 | 110539046 | 28 | 0 | 4 | 0 | 12 | WASF1 |
| 7 | 57480106 | 57659360 | 179 | 2 | 0 | 3 | 1 | ZNF716 |
| 7 | 63149422 | 63486197 | 337 | 2 | 0 | 3 | 1 | ZNF679, ZNF735 |
| 7 | 943615 | 954205 | 11 | 1 | 2 | 1 | 3 | ADAP1 |
| 7 | 15536047 | 15536441 | 0.394 | 0 | 6 | 0 | 7 | TMEM195 |
| 8 | 128437695 | 130578879 | 2141 | 4 | 1 | 14 | 1 | TMEM75, MYC, POU5F1P1 |
| 8 | 124855221 | 124894704 | 39 | 3 | 0 | 17 | 0 | FAM91A1 |
| 8 | 132030642 | 132421393 | 391 | 2 | 0 | 16 | 0 | ADCY8 |
| 8 | 64257961 | 64390486 | 133 | 2 | 1 | 12 | 1 | YTHDF3 |
| 8 | 145064850 | 145118710 | 54 | 2 | 1 | 11 | 1 | PLEC1 |
| 8 | 25130171 | 25130291 | 0.12 | 1 | 14 | 1 | 15 | DOCK5 |
| 9 | 3339487 | 3417226 | 78 | 1 | 2 | 1 | 8 | RFX3 |
| 9 | 71289871 | 71308842 | 19 | 0 | 2 | 0 | 5 | APBA1 |
| 9 | 87357661 | 87432833 | 75 | 0 | 3 | 0 | 5 | AGTPBP1 |
| 9 | 33230225 | 33242111 | 12 | 0 | 8 | 0 | 12 | SPINK4 |
| 10 | 115580402 | 115675237 | 95 | 1 | 2 | 1 | 11 | NHLRC2, DCLRE1A |
| 10 | 25604754 | 25659341 | 55 | 1 | 3 | 1 | 15 | GPR158 |
| 10 | 15898437 | 15929438 | 31 | 0 | 2 | 0 | 16 | C10orf97 |
| 10 | 38082301 | 38147982 | 66 | 0 | 2 | 1 | 11 | ZNF248 |
| 10 | 71648150 | 71656573 | 8 | 0 | 2 | 0 | 12 | PPA1 |
| 10 | 123566469 | 123752493 | 186 | 0 | 2 | 1 | 13 | TACC2, NSMCE4A, ATE1 |
| 10 | 89716166 | 89756083 | 40 | 0 | 3 | 0 | 14 | PTEN |
| 10 | 72166137 | 72172267 | 6 | 0 | 4 | 0 | 12 | ADAMTS14 |
| 11 | 20922810 | 20971401 | 49 | 0 | 2 | 0 | 3 | NELL1 |
| 11 | 92517181 | 92571937 | 55 | 0 | 2 | 0 | 5 | SLC36A4 |
| 11 | 104441220 | 104484303 | 43 | 0 | 2 | 0 | 4 | CARD17 |
| 11 | 47391562 | 47453463 | 62 | 0 | 3 | 0 | 3 | CUGBP1, RAPSN, PSMC3, SLC39A13 |
| 11 | 102486510 | 102663548 | 177 | 0 | 4 | 0 | 5 | DYNC2H1 |
| 12 | 51710169 | 51719305 | 9 | 0 | 2 | 0 | 5 | EIF4B |
| 12 | 91645948 | 91679726 | 34 | 0 | 2 | 1 | 5 | PLEKHG7 |
| 12 | 18413696 | 18414167 | 0.471 | 0 | 4 | 1 | 7 | PIK3C2G |
| 13 | 92502207 | 93946439 | 1444 | 1 | 2 | 1 | 12 | DCT, GPC6 |
| 13 | 34399863 | 34517051 | 117 | 0 | 5 | 0 | 14 | NBEA |
| 14 | 59138607 | 59161895 | 23 | 2 | 1 | 5 | 1 | RTN1 |
| 14 | 69961223 | 69998909 | 38 | 0 | 6 | 0 | 6 | ADAM21, ADAM21P |
| 15 | 59943982 | 60046989 | 103 | 0 | 2 | 1 | 3 | VPS13C |
| 16 | 87557619 | 87557679 | 0.06 | 1 | 2 | 1 | 8 | CBFA2T3 |
| 16 | 87527215 | 87549395 | 22 | 1 | 3 | 1 | 9 | CBFA2T3 |
| 16 | 71734726 | 71798103 | 63 | 0 | 2 | 0 | 6 | C16orf47 |
| 16 | 45728834 | 45887805 | 159 | 0 | 4 | 0 | 6 | ITFG1, NETO2 |
| 17 | 37018570 | 37057586 | 39 | 0 | 4 | 1 | 7 | KRT42P, KRT17, KRT16 |
| 19 | 33996261 | 34984966 | 989 | 4 | 1 | 12 | 1 | C19orf12, PLEKHF1, POP4, VSTM2B, UQCRFS1, UQCRFSL1 |
| 19 | 532070 | 556653 | 25 | 0 | 4 | 1 | 4 | HCN2, BSG |
| 19 | 60711906 | 60737394 | 25 | 0 | 4 | 1 | 6 | SBK2 |
| X | 33147645 | 33243866 | 96 | 1 | 6 | 1 | 15 | DMD |
HFR: common region of focal aberration (gain or loss);
Focal: number of samples with focal aberration;
Total: total number of samples containing the HFR of the focal aberration. Genes in bold have been reported in the Cancer Gene Census list.
Expression of genes in focal CNAs
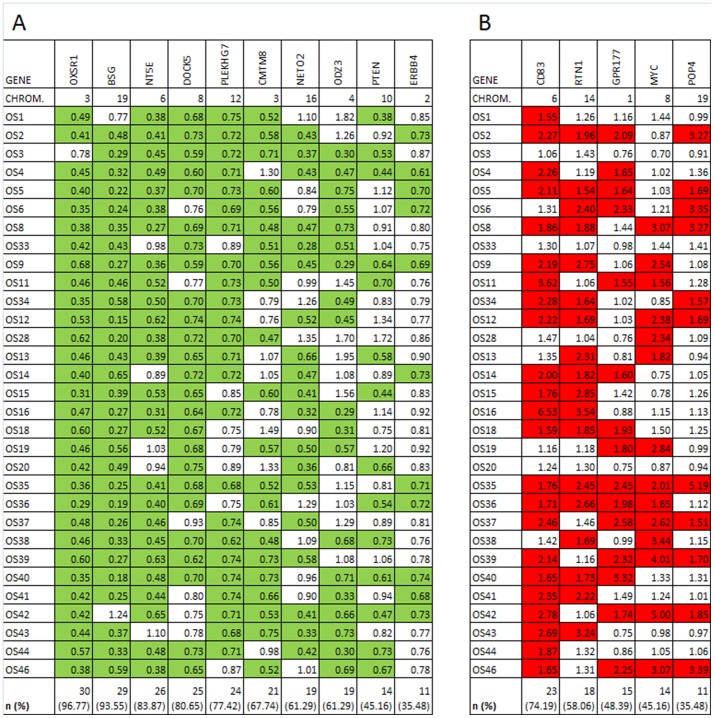
Whole genome expression data was available for 31 osteosarcoma samples, including 20 samples from the SNP-array analysis set. These data were used for further analysis of our candidate list of potential driver genes (Table 2). For 85 of the 94 genes at least 1 probe was available on the expression array. Expression fold changes for these genes were calculated by normalizing the intensity of the average signal in the tumor sample against the average signal in the reference osteoblast sample. The genes in the focal copy number losses and in the focal copy number gains were separately assayed for underexpression (expression fold change ≤0.75) and overexpression (expression fold change ≥1.5), respectively. All data are shown in S1 Table. From this table a top candidate gene list was extracted with aberrant expression (underexpression in case of focal loss, overexpression in case of focal gain) in ≥35% of the tumors (Fig. 3). Besides the known tumor suppressor genes DOCK5 and PTEN, genes OXSR1, BSG, NT5E, PLEKHG7,CMTM8, NETO2, ODZ3, and ERBB4 adhered to our criteria and were qualified as novel candidate tumor suppressor genes. Besides the known oncogene MYC, genes CD83, RTN1, GRP177, and POP4 adhered to our criteria and were qualified as novel candidate oncogenes.
Figure 3. Genes in focal losses with underexpression (FC<0.75, A) and in focal gains with overexpression (FC>1.5, B) in more than 35% of osteosarcomas.
Green: expression fold change (FC) <0.75 of the gene in the tumor relative to osteoblasts (underexpression). Red: expression fold change (FC) >1.50 of the gene in the tumor relative to osteoblasts (overexpression). For each gene, the number (n) and fraction (%) of osteosarcomas with the indicated fold change (FC) are given.
Validation analysis
To confirm our findings of the proposed candidate genes in an independent dataset we used expression data for 84 osteosarcoma samples and 3 osteoblast samples, which were generated by Kuijjer et al [15]. Expression fold changes for our candidate genes were calculated by normalizing their expression level in the osteosarcoma samples against the mean expression level in the osteoblast reference samples. Next, they were assayed for their frequency of underexpression (expression fold change ≤0.75, in case of a candidate tumor suppressor gene) or overexpression (expression fold change ≥1.5, in case of a candidate oncogene) in the osteosarcoma samples (see S1 Fig.). In Table 3, the frequencies of involvement of our candidate driver genes in this validation set are summarized and compared with those in our own osteosarcoma set. Considering a frequency of involvement for a driver gene of at least 35% as a convincing event, we conclude that the validation set confirms our identification of OXSR1, BSG, NT5E, CMTM8, and NETO2 as novel candidate tumor suppressor genes and of CD83, RTN1, and GPR177 as novel oncogenes.
Table 3. Validation of identified candidate tumor suppressor genes and oncogenes in an independent dataset.
| Gene | Our set | Validation set |
| Candidate tumor suppressor gene | ||
| OXSR1 | 97 | 89 |
| BSG | 94 | 70 |
| NT5E | 84 | 93 |
| (DOCK5 | 81 | 90) |
| PLEKHG7 | 77 | 0 |
| CMTM8 | 68 | 65 |
| NETO2 | 61 | 60 |
| ODZ3 | 61 | NI1 |
| (PTEN | 45 | NI2) |
| ERBB4 | 35 | 0 |
| Candidate oncogene | ||
| CD83 | 74 | 51 |
| RTN1 | 58 | 74 |
| GPR177 | 48 | 43 |
| (MYC | 45 | 4) |
| POP4 | 35 | 18 |
Frequency of involvement (percentage underexpression of a candidate tumorsuppressor gene and percentage overexpression of a candidate oncogene) in the original and validation set
Known tumor suppressor genes (DOCK5, PTEN) and oncogene (MYC) between brackets
No information. Applied probe does not recognize ODZ3 in 4q35.1
No information. Applied probe recognizes not only PTEN on 10q23.31, but also the expressed pseudogene (PTENP1) in 9p13.3.
Discussion
Due to the complex landscape of genomic rearrangements in osteosarcomas the identification of driver genes remains a challenging task. Nonetheless, by analysis of focal aberrations, we identified potential new and known genes driving osteosarcoma tumorigenesis. In our set of 26 osteosarcoma samples, 94 genes were identified within recurrent focal aberrations. Some were found in 2 samples only, others in a large portion of the samples, such as DOCK5 (14/26) and ADAMTS3 (15/26) (Table 2). It should be noted that the previously published amplifications in the 17p11.2-p12 region and the potential oncogenes therein [16] were not identified in this study since these amplifications were generally large (>3 Mb), and hence not recognized as focal CNAs in this study.
For most of the genes in the focal aberrations (85 of 94) expression data in a set of 31 osteosarcoma samples were available. This allowed us to identify the genes of which the expression status (over- or underexpression) in these tumors was frequently (>35%) in accordance to their copy number status (gain or loss), as deduced from the focal aberration analyses. These were, in addition to the known tumor suppressor genes PTEN and DOCK5 and the known oncogene MYC, in total 8 candidate tumor suppressor genes and 4 candidate oncogenes as potential new driver genes in osteosarcoma tumorigenesis (Table 3). As discussed below for the individual genes, validation using an independent dataset, confirmed the candidacy of most of the driver genes that we identified.
Known tumor suppressor genes and oncogenes found in our analysis
The strength of the applied approach of focal aberration analysis might be concluded from the enrichment of the focal CNAs we identified in our osteosarcoma dataset with driver genes from the Cancer Census list [23] and in particular the identification of PTEN, DOCK5, and MYC as known driver genes in osteosarcoma. For instance, PTEN has been implicated in osteosarcoma tumorigenesis in a multitude of studies. Loss of the PTEN gene has been reported in patient samples [25] and lower PTEN expression levels in cell lines [26]. However, because the available probe in the validation set proved to be not specific to PTEN, we could not confirm its frequent involvement as a tumor suppressor gene in this set. We found frequent underexpression of DOCK5 in our set as well as in the validation set. Expression of DOCK5 is essential for bone differentiation in osteoclasts [27]. Recently, DOCK5 expression was shown to be down-regulated in osteosarcoma [28]. The MYC oncogene is involved in many tumor types, including carcinomas of cervix, lung, breast, colon, and stomach, and also in osteosarcoma [29]. MYC acts as a cell proliferation controller and overexpression, as found in a multitude of tumors as well as in our dataset, drives higher proliferation and blocks cell differentiation. Furthermore, a secondary effect of MYC overexpression is the induction of chromosomal instability by faulty control of the G1-S checkpoint [30]. For unknown reasons, we could not confirm the frequent overexpression of MYC, as noted in our osteosarcoma set, in the validation set. Unfortunately, no expression data for another MYC probe in the latter set was available to substantiate this unexpected result.
Newly identified candidate tumor suppressor genes
In our analysis eight new candidate tumor suppressor genes were identified, OXSR1 (3p22.2), BSG(19p13.3), NT5E(6q14.3), PLEKHG7(12q22), CMTM8(3p22.3), NETO2(16q12.1), ODZ3(4q35.1) and ERBB4(2q34). However, since the involvement of PLEKHG7 and ERBB4 could not be confirmed in the validation set and conflicting data have been reported for OXSR1, BSG, NT5E, and NETO2 in other tumor types (summarized in Table 4), we consider these as less probable candidate tumor suppressor genes in osteosarcoma tumorigenesis.
Table 4. Excluded newly identified driver genes.
| Gene | Reason for exclusion |
| Candidate tumor suppressor gene(s) | |
| PLEKHG7, ERBB4 | Underexpression in our set, but no confirmation in validation set |
| OXSR1, NT5E, NETO2 | Underexpression in our set and validation set, but conflicting expression data in other tumor types [46]–[51] |
| BSG | Underexpression in our set and validation set, conflicting with oncogenic role in other tumor types [52]–[55] |
| Candidate oncogene | |
| CD83 | Overexpression in our set and validation set of this cell surface marker most probably related to altered tumor microenvironment, but not to tumor formation [56] |
| RTN1 | Overexpression in our set and validation set conflicts with tumor suppressor role in other tumor types [57]–[59] |
In several tumor types, indications have been found for a tumor suppressor function of the CMTM8 gene product. In osteosarcoma, it was demonstrated that CMTM8, also known as CKLFSF8, suppresses the EGFR signaling pathway [31]. CMTM8 underexpression may therefore result in upregulation of EGFR signaling. The latter was recently shown to suppress osteoblast differentiation and to inhibit expression of the osteoblastic transcription factors Runx2 and Osterix, which may lead to the development of immature osteoblastic-like cells characteristic of osteosarcoma [32]. In other tumor types, overexpression of CMTM8 has been shown to result in tumor cell apoptosis [33], [34]. In addition, in hepatocellular carcinoma cells and immortalized breast epithelial cells (MCF-10A), downregulation of CMTM8 was found to result in a transition from an epithelial to a mesenchymal phenotype. This transition results from the loss of c-Met inhibition by CMTM8, which in turn activates migration, invasive growth and cancer malignancy [35], [36]. Interestingly, c-Met is known to be upregulated in human sarcomas [37].We found CMTM8 underexpression in a considerable number of cases in our original set and in the validation set. This gene may therefore have a tumor suppressor gene function in osteosarcoma tumorigenesis.
We found ODZ3 to be underexpressed in a considerable fraction of the investigated osteosarcomas but, because of lack of a specific probe, were unable to validate the expression data for this gene in the independent set. Deletion of ODZ3 and low mRNA expression has been noted to occur in neuroblastoma [38]. In addition, in the latter study the low expression level of this gene proved to be associated with a poor prognosis. Whether ODZ3 has a similar tumor suppressor gene function in osteosarcoma remains to be established.
Newly identified candidate oncogenes
We identified three genes located in focal gains and overexpressed in a significant fraction of both osteosarcoma sets: CD83 (6p23), RTN1 (14q23.1), and GPR177 (WLS/EVI) (1p31.3). However, as briefly explained in Table 4, we consider CD83 and RTN1 as less probable candidate oncogenes in osteosarcoma tumorigenesis.
No information is available about the expression of GPr177(WLS/EVI) in osteosarcomas. It is known that physiologically normal GPR177 protein levels are essential for proper osteoblast differentiation and mineralization [39]. The GPR177 gene encodes an upstream regulator of the Wnt signaling pathway. However, the status of this pathway in osteosarcoma is unclear. Some papers report active Wnt signaling [40], [41], while others find that the Wnt signaling pathway is inactivated in this tumor [42], [43]. GPR177 has been found to be overexpressed in glioma [44]. Moreover, GPR177 proved to be indispensable for Wnt-induced breast tumor formation [45]. In accordance with the latter observations, we found frequent overexpression of GPR177 in both osteosarcoma sets. Taken together, these data suggest a potential oncogenic role for GPR177 in osteosarcoma tumorigenesis.
Conclusions
Based on their frequent aberrant copy number in our osteosarcoma set, the frequent under-, respectively, overexpression in our osteosarcoma set as well as in a validation set, and their documented involvement in other tumor types, we identified CMTM8, and possibly also ODZ3, as a new candidate tumor suppressor gene, and GPR177 as a new candidate oncogene involved in osteosarcoma tumorigenesis.
Supporting Information
Genes in focal losses with underexpression (FC<0.75, A) and in focal gains with overexpression (FC>1.5, B) in more than 35% of osteosarcomas in the validation dataset. Green: expression fold change (FC) <0.75 of the gene in the tumor relative to osteoblasts (underexpression). Red: expression fold change (FC) >1.50 of the gene in the tumor relative to osteoblasts (overexpression). For each gene, the number (n) and fraction (%) of osteosarcomas with the indicated fold change (FC) are given.
(XLSX)
Expression fold change for all genes in focal losses (upper panel) or focal gains (lower panel) in 31 osteosarcoma samples. Green: expression fold change (FC) <0.75 of the gene in the tumor relative to osteoblasts (underexpression). Red: expression fold change (FC) >1.50 of the gene in the tumor relative to osteoblasts (overexpression). For each gene, the number (n) and fraction (%) of osteosarcomas with the indicated fold change (FC) are given.
(XLSX)
Acknowledgments
We thank Dr. J. Bramer (Department of Orthopedic Surgery, AcademicMedical Center, Amsterdam) for osteosarcoma samples and support.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
OK is supported by VUMC-CCA (Cancer Centre Amsterdam, VU medical centre). J. Both is supported by the Dutch Cancer society, grant no. UVA 2006-3525. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ritter J, Bielack SS (2010) Osteosarcoma. Ann Oncol 21 Suppl 7 vii320–vii325. [DOI] [PubMed] [Google Scholar]
- 2. Ottaviani G, Jaffe N (2009) The etiology of osteosarcoma. Cancer Treat Res 152:15–32. [DOI] [PubMed] [Google Scholar]
- 3. Benayahu D, Shur I, Marom R, Meller I, Issakov J (2001) Cellular and molecular properties associated with osteosarcoma cells. J Cell Biochem 84:108–114. [DOI] [PubMed] [Google Scholar]
- 4. Bacci G, Briccoli A, Ferrari S, Longhi A, Mercuri M, et al. (2001) Neoadjuvant chemotherapy for osteosarcoma of the extremity: long-term results of the Rizzoli's 4th protocol. Eur J Cancer 37:2030–2039. [DOI] [PubMed] [Google Scholar]
- 5. Ferguson WS, Goorin AM (2001) Current treatment of osteosarcoma. Cancer Invest 19:292–315. [DOI] [PubMed] [Google Scholar]
- 6. Briccoli A, Rocca M, Salone M, Guzzardella GA, Balladelli A, et al. (2010) High grade osteosarcoma of the extremities metastatic to the lung: long-term results in 323 patients treated combining surgery and chemotherapy, 1985–2005. Surg Oncol 19:193–199. [DOI] [PubMed] [Google Scholar]
- 7. Sandberg AA, Bridge JA (2003) Updates on the cytogenetics and molecular genetics of bone and soft tissue tumors: osteosarcoma and related tumors. Cancer Genet Cytogenet 145:1–30. [PubMed] [Google Scholar]
- 8. Martin JW, Squire JA, Zielenska M (2012) The genetics of osteosarcoma. Sarcoma 2012:627254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuijjer ML, Hogendoorn PC, Cleton-Jansen AM (2013) Genome-wide analyses on high-grade osteosarcoma: Making sense of a genomically most unstable tumor. Int J Cancer 133:2512–2521. [DOI] [PubMed] [Google Scholar]
- 10. Futreal PA, Kasprzyk A, Birney E, Mullikin JC, Wooster R, et al. (2001) Cancer and genomics. Nature 409:850–852. [DOI] [PubMed] [Google Scholar]
- 11. Weir BA, Woo MS, Getz G, Perner S, Ding L, et al. (2007) Characterizing the cancer genome in lung adenocarcinoma. Nature 450:893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leary RJ, Lin JC, Cummins J, Boca S, Wood LD, et al. (2008) Integrated analysis of homozygous deletions, focal amplifications, and sequence alterations in breast and colorectal cancers. Proc Natl Acad Sci U S A 105:16224–16229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Feuk L, Carson AR, Scherer SW (2006) Structural variation in the human genome. Nat Rev Genet 7:85–97. [DOI] [PubMed] [Google Scholar]
- 14. Krijgsman O, Carvalho B, Meijer GA, Steenbergen RD, Ylstra B (2014) Focal chromosomal copy number aberrations in cancer-Needles in a genome haystack. Biochim Biophys Acta 1843:2698–2704. [DOI] [PubMed] [Google Scholar]
- 15. Kuijjer ML, Rydbeck H, Kresse SH, Buddingh EP, Lid AB, et al. (2012) Identification of osteosarcoma driver genes by integrative analysis of copy number and gene expression data. Genes Chromosomes Cancer 51:696–706. [DOI] [PubMed] [Google Scholar]
- 16. Both J, Wu T, Bras J, Schaap GR, Baas F, et al. (2012) Identification of novel candidate oncogenes in chromosome region 17p11.2-p12 in human osteosarcoma. PLoS One 7:e30907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Venkatraman ES, Olshen AB (2007) A faster circular binary segmentation algorithm for the analysis of array CGH data. Bioinformatics 23:657–663. [DOI] [PubMed] [Google Scholar]
- 18. van de Wiel MA, Picard F, van Wieringen WN, Ylstra B (2011) Preprocessing and downstream analysis of microarray DNA copy number profiles. Brief Bioinform 12:10–21. [DOI] [PubMed] [Google Scholar]
- 19. Conrad DF, Pinto D, Redon R, Feuk L, Gokcumen O, et al. (2010) Origins and functional impact of copy number variation in the human genome. Nature 464:704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smyth GK, Michaud J, Scott HS (2005) Use of within-array replicate spots for assessing differential expression in microarray experiments. Bioinformatics 21:2067–2075. [DOI] [PubMed] [Google Scholar]
- 21. Kresse SH, Ohnstad HO, Paulsen EB, Bjerkehagen B, Szuhai K, et al. (2009) LSAMP, a novel candidate tumor suppressor gene in human osteosarcomas, identified by array comparative genomic hybridization. Genes Chromosomes Cancer 48:679–693. [DOI] [PubMed] [Google Scholar]
- 22. van Wieringen WN, Unger K, Leday GG, Krijgsman O, de Menezes RX, et al. (2012) Matching of array CGH and gene expression microarray features for the purpose of integrative genomic analyses. BMC Bioinformatics 13:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Futreal PA, Coin L, Marshall M, Down T, Hubbard T, et al. (2004) A census of human cancer genes. Nat Rev Cancer 4:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brosens RP, Haan JC, Carvalho B, Rustenburg F, Grabsch H, et al. (2010) Candidate driver genes in focal chromosomal aberrations of stage II colon cancer. J Pathol 221:411–424. [DOI] [PubMed] [Google Scholar]
- 25. Freeman SS, Allen SW, Ganti R, Wu J, Ma J, et al. (2008) Copy number gains in EGFR and copy number losses in PTEN are common events in osteosarcoma tumors. Cancer 113:1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee JA, Ko Y, Kim DH, Lim JS, Kong CB, et al. (2012) Epidermal growth factor receptor: is it a feasible target for the treatment of osteosarcoma? Cancer Res Treat 44:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brazier H, Stephens S, Ory S, Fort P, Morrison N, et al. (2006) Expression profile of RhoGTPases and RhoGEFs during RANKL-stimulated osteoclastogenesis: identification of essential genes in osteoclasts. J Bone Miner Res 21:1387–1398. [DOI] [PubMed] [Google Scholar]
- 28. Sadikovic B, Thorner P, Chilton-Macneill S, Martin JW, Cervigne NK, et al. (2010) Expression analysis of genes associated with human osteosarcoma tumors shows correlation of RUNX2 overexpression with poor response to chemotherapy. BMC Cancer 10:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Radig K, Schneider-Stock R, Mittler U, Neumann HW, Roessner A (1998) Genetic instability in osteoblastic tumors of the skeletal system. Pathol Res Pract 194:669–677. [DOI] [PubMed] [Google Scholar]
- 30. Li Q, Dang CV (1999) c-Myc overexpression uncouples DNA replication from mitosis. Mol Cell Biol 19:5339–5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jin C, Ding P, Wang Y, Ma D (2005) Regulation of EGF receptor signaling by the MARVEL domain-containing protein CKLFSF8. FEBS Lett 579:6375–6382. [DOI] [PubMed] [Google Scholar]
- 32. Zhu J, Shimizu E, Zhang X, Partridge NC, Qin L (2011) EGFR signaling suppresses osteoblast differentiation and inhibits expression of master osteoblastic transcription factors Runx2 and Osterix. J Cell Biochem 112:1749–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jin C, Wang Y, Han W, Zhang Y, He Q, et al. (2007) CMTM8 induces caspase-dependent and -independent apoptosis through a mitochondria-mediated pathway. J Cell Physiol 211:112–120. [DOI] [PubMed] [Google Scholar]
- 34. Li D, Jin C, Yin C, Zhang Y, Pang B, et al. 2007) An alternative splice form of CMTM8 induces apoptosis. Int J Biochem Cell Biol 39:2107–2119. [DOI] [PubMed] [Google Scholar]
- 35. Salvi A, Arici B, Portolani N, Giulini SM, De PG (2007) In vitro c-met inhibition by antisense RNA and plasmid-based RNAi down-modulates migration and invasion of hepatocellular carcinoma cells. Int J Oncol 31:451–460. [PubMed] [Google Scholar]
- 36. Zhang W, Mendoza MC, Pei X, Ilter D, Mahoney SJ, et al. (2012) Down-regulation of CMTM8 induces epithelial-to-mesenchymal transition-like changes via c-MET/extracellular signal-regulated kinase (ERK) signaling. J Biol Chem 287:11850–11858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Teicher BA (2012) Searching for molecular targets in sarcoma. Biochem Pharmacol 84:1–10. [DOI] [PubMed] [Google Scholar]
- 38. Molenaar JJ, Koster J, Zwijnenburg DA, van Sluis P, Valentijn LJ, et al. (2012) Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature 483:589–593. [DOI] [PubMed] [Google Scholar]
- 39. Zhong Z, Zylstra-Diegel CR, Schumacher CA, Baker JJ, Carpenter AC, et al. (2012) Wntless functions in mature osteoblasts to regulate bone mass. Proc Natl Acad Sci U S A 109:E2197–E2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Iwaya K, Ogawa H, Kuroda M, Izumi M, Ishida T, et al. (2003) Cytoplasmic and/or nuclear staining of beta-catenin is associated with lung metastasis. Clin Exp Metastasis 20:525–529. [DOI] [PubMed] [Google Scholar]
- 41. Kansara M, Tsang M, Kodjabachian L, Sims NA, Trivett MK, et al. (2009) Wnt inhibitory factor 1 is epigenetically silenced in human osteosarcoma, and targeted disruption accelerates osteosarcomagenesis in mice. J Clin Invest 119:837–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cai Y, Mohseny AB, Karperien M, Hogendoorn PC, Zhou G, et al. (2010) Inactive Wnt/beta-catenin pathway in conventional high-grade osteosarcoma. J Pathol 220:24–33. [DOI] [PubMed] [Google Scholar]
- 43. Du X, Yang J, Yang D, Tian W, Zhu Z (2014) The genetic basis for inactivation of Wnt pathway in human osteosarcoma. BMC Cancer 14:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Augustin I, Goidts V, Bongers A, Kerr G, Vollert G, et al. (2012) The Wnt secretion protein Evi/Gpr177 promotes glioma tumourigenesis. EMBO Mol Med 4:38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maruyama EO, Yu HM, Jiang M, Fu J, Hsu W (2013) Gpr177 deficiency impairs mammary development and prohibits Wnt-induced tumorigenesis. PLoS One 8:e56644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Calicchio ML, Collins T, Kozakewich HP (2009) Identification of signaling systems in proliferating and involuting phase infantile hemangiomas by genome-wide transcriptional profiling. Am J Pathol 174:1638–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cappellari AR, Vasques GJ, Bavaresco L, Braganhol E, Battastini AM (2012) Involvement of ecto-5'-nucleotidase/CD73 in U138MG glioma cell adhesion. Mol Cell Biochem 359:315–322. [DOI] [PubMed] [Google Scholar]
- 48. Horak CE, Lee JH, Elkahloun AG, Boissan M, Dumont S, et al. (2007) Nm23-H1 suppresses tumor cell motility by down-regulating the lysophosphatidic acid receptor EDG2. Cancer Res 67:7238–7246. [DOI] [PubMed] [Google Scholar]
- 49. Katoh M (2002) Molecular cloning and characterization of OSR1 on human chromosome 2p24. Int J Mol Med 10:221–225. [PubMed] [Google Scholar]
- 50. Rauch TA, Wang Z, Wu X, Kernstine KH, Riggs AD, et al. (2012) DNA methylation biomarkers for lung cancer. Tumour Biol 33:287–296. [DOI] [PubMed] [Google Scholar]
- 51. Wang H, Lee S, Nigro CL, Lattanzio L, Merlano M, et al. (2012) NT5E (CD73) is epigenetically regulated in malignant melanoma and associated with metastatic site specificity. Br J Cancer 106:1446–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nakamura K, Kodama J, Hongo A, Hiramatsu Y (2012) Role of emmprin in endometrial cancer. BMC Cancer 12:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Omi Y, Shibata N, Okamoto T, Obara T, Kobayashi M (2012) The role of CD147 in the invasiveness of follicular thyroid carcinoma cells. Thyroid 22:383–394. [DOI] [PubMed] [Google Scholar]
- 54. Pan Y, He B, Song G, Bao Q, Tang Z, et al. (2012) CD147 silencing via RNA interference reduces tumor cell invasion, metastasis and increases chemosensitivity in pancreatic cancer cells. Oncol Rep 27:2003–2009. [DOI] [PubMed] [Google Scholar]
- 55. Sweeny L, Dean NR, Frederick JW, Magnuson JS, Carroll WR, et al. (2012) CD147 expression in advanced cutaneous squamous cell carcinoma. J Cutan Pathol 39:603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fujimoto Y, Tedder TF (2006) CD83: a regulatory molecule of the immune system with great potential for therapeutic application. J Med Dent Sci 53:85–91. [PubMed] [Google Scholar]
- 57. Astolfi A, Nannini M, Pantaleo MA, Di BM, Heinrich MC, et al. (2010) A molecular portrait of gastrointestinal stromal tumors: an integrative analysis of gene expression profiling and high-resolution genomic copy number. Lab Invest 90:1285–1294. [DOI] [PubMed] [Google Scholar]
- 58. Di Sano F, Fazi B, Tufi R, Nardacci R, Piacentini M (2007) Reticulon-1C acts as a molecular switch between endoplasmic reticulum stress and genotoxic cell death pathway in human neuroblastoma cells. J Neurochem 102:345–353. [DOI] [PubMed] [Google Scholar]
- 59. Fazi B, Biancolella M, Mehdawy B, Corazzari M, Minella D, et al. (2010) Characterization of gene expression induced by RTN-1C in human neuroblastoma cells and in mouse brain. Neurobiol Dis 40:634–644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Genes in focal losses with underexpression (FC<0.75, A) and in focal gains with overexpression (FC>1.5, B) in more than 35% of osteosarcomas in the validation dataset. Green: expression fold change (FC) <0.75 of the gene in the tumor relative to osteoblasts (underexpression). Red: expression fold change (FC) >1.50 of the gene in the tumor relative to osteoblasts (overexpression). For each gene, the number (n) and fraction (%) of osteosarcomas with the indicated fold change (FC) are given.
(XLSX)
Expression fold change for all genes in focal losses (upper panel) or focal gains (lower panel) in 31 osteosarcoma samples. Green: expression fold change (FC) <0.75 of the gene in the tumor relative to osteoblasts (underexpression). Red: expression fold change (FC) >1.50 of the gene in the tumor relative to osteoblasts (overexpression). For each gene, the number (n) and fraction (%) of osteosarcomas with the indicated fold change (FC) are given.
(XLSX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.