Abstract
Background
Chronic inflammation has been linked to cancers, and use of non-steroidal anti-inflammatory drugs (NSAIDs) has been associated with reduced risk of several cancers. To further refine the magnitude of NSAID-related associations, in particular for cancers related to inflammation, such as alcohol-, infection-, obesity-, and smoking-related cancers, as well as for less common cancers, we evaluated the use of NSAIDs and cancer risk in a very large cohort. We used propensity scores to account for potential selection bias and hypothesized that NSAID use is associated with decreased cancer incidence.
Methods
We conducted a prospective study among 314,522 participants in the NIH-AARP Diet and Health Study. Individuals who completed the lifestyle questionnaire, which included NSAID use, in 1996–1997 were followed through 2006. Information on cancer incidence was ascertained by linking to cancer registries and vital status databases.
Findings
During 2,715,994 person-years of follow-up (median 10.1 person-years), there were 51,894 incident cancers. Compared with non-users of NSAIDs, individuals who reported use in the 12 months prior to interview had a significantly lower risk of all inflammation-related cancer, alcohol-related, infection-related, obesity-related, and smoking-related cancers [hazard ratio (HR) (95% CI)) 0.90 (0.87–0.93), 0.80 (0.74–0.85), 0.82 (0.78–0.87), 0.88 (0.84–0.92), and 0.88 (0.85–0.92) respectively)].
Conclusions
After accounting for potential selection bias, our data showed an inverse association between NSAID use and alcohol-related, infection-related, obesity-related, and smoking-related cancers and support the hypothesis that inflammation is related to an increased risk of certain cancers.
Introduction
Inflammation has been linked to several cancers, including cancers of the colon, liver, stomach, and gallbladder. Inflammation is involved in cancer initiation, progression, angiogenesis, invasion, and metastasis and can be prompted by several factors, including infection, smoking, alcohol use, and obesity [1]–[4]. Therefore, it is plausible that NSAIDs could attenuate the risks of cancers for which these causes of inflammation are risk factors.
Non-steroidal anti-inflammatory drug (NSAID) use, especially of aspirin, has been linked to reduced risk of cancers in several, [5]–[8] but not all [5]–[11] observational studies. Data from clinical trials of NSAIDs have shown that NSAID use can lower ovarian and colorectal cancer risk [12]–[15]. However, the role of NSAID use in less common cancers is unclear due to the small numbers of these cancers in previous studies. In addition, cancers that have inflammation-related causes in common have not been jointly evaluated. Evaluating these cancers as a group could help eliminate some of the uncertainty from previous studies and elucidate the role of NSAIDs in inflammation-related cancers.
Our objective is to investigate the role of NSAIDs use in less common cancer and cancers with related inflammatory causes (i.e., obesity, infection, alcohol, and smoking). Therefore, we examined the association between incident cancer and the self-reported aspirin and non-aspirin NSAIDs in a large (>300,000 subjects), well-designed study: the National Institute of Health-AARP (NIH-AARP) Diet and Health Study cohort [16]. Given the large size of the NIH-AARP cohort, we were well-powered to investigate the role of NSAID in several less common and inflammation-related cancers.
Subjects and Methods
Study population
The NIH-AARP cohort was established in 1995–1996 as described elsewhere [16]. In brief, questionnaires were mailed to 50–71-year-old AARP members in two metropolitan areas (Detroit, Michigan, and Atlanta, Georgia) and six U.S. states (Pennsylvania, New Jersey, North Carolina, Louisiana, Florida, and California); 18% returned the baseline questionnaire. A subsequent questionnaire requesting additional risk factor data, including NSAID use, was mailed six months later to participants without self-reported colon, breast or prostate cancer in the baseline questionnaire.
Ethical approval
Ethical approval of the NIH-AARP Diet and Health Study was granted by the National Cancer Institute's Special Studies Institutional Review Board. Participants mailed the written consent and the study materials to the study team.
For the current analysis, we excluded proxies (n = 10,383), individuals with cancer ascertained in the death report only (n = 1,786), individuals with missing NSAID use data (n = 3,634), and those with no follow-up (n = 40). Our analytic NIH-AARP cohort included 314,522 persons (132,462 men and 182,060 women) with data from the baseline and risk factor questionnaires.
Cohort follow-up and cancer ascertainment
Identification of cancer cases
The study linked cohort members to state cancer registry databases in the original eight states and three additional states (AZ, NV, and TX) where participants tended to move during follow-up. The case ascertainment method identified approximately 90% of all cancer cases in our cohort [17]. In order to update participants' vital status, annual linkage of the Social Security Administration Death Master and the cohort were attained. Further confirmation of participants' vital status was verified by other methods including; matching the Social Security Administration Death Master File with the National Death Index Plus, mailings, and questionnaire responses.
NSAID use assessment
Participants were asked about their use of aspirin (e.g. Anacin, Bayer, Bufferin, Ecotrin, Excedrin, or generic aspirin) and non-aspirin (e.g. Advil, Aleve, Anaprox, Clinoril, Feldene, Fenoprofen, generic ibuprofen, Indocin, Indomethacin, Ketoprofen, Motrin, Naprosyn, Nalfon, Nambumetone, Nuprin, Orudis, Piroxicam, Relafen, or Sulindac) pain relievers during the past 12 months. When asked about non-aspirin NSAIDs, participants were directed not to include acetaminophen, Tylenol, or any other non-NSAID pain relievers. For individuals reporting NSAID use in the year prior to the risk-factor questionnaire, we defined the frequency of aspirin and non-aspirin NSAID use as follows: monthly (≤3 times/month), weekly (≥1 times/week) and daily (≥1 times/day). In the analysis, use of NSAIDs, aspirin, or non-aspirin was defined as yes/no while frequency of use was defined as never, monthly, weekly or daily. In addition, we created a 4-category variable defining type of NSAID use (no NSAID, aspirin-only, non-aspirin-only, both aspirin and non-aspirin).
Statistical analysis
All analyses were conducted using SAS/STAT software, Version 9.2 (SAS Institute Inc., Cary, NC, USA). We assessed the association between incident inflammation-related cancers and the use of any NSAIDs, aspirin, and non-aspirin NSAIDs. We used non-users of any NSAIDs as the reference group in all analyses.
We assessed overall inflammation-related cancer risk, and risk for cancers with specific inflammation-related causes, explicitly: obesity-related cancers (esophageal, gallbladder, colorectal, pancreatic, post-menopausal breast, endometrial, kidney, and thyroid); infection-related cancers (head and neck, stomach, liver, colorectal, lymphoma, anal, and female genital) [18], alcohol-related cancers (head and neck, esophageal, colorectal, liver and breast) [19], [20], and smoking-related cancers (lung, head and neck, esophageal, pancreatic, and urinary bladder) [21]. To avoid reverse causality, we excluded cancer cases that were reported during the first year of follow-up and conducted sensitivity analysis excluding cancers observed in the first five years. Although we lacked information on the indication for NSAID use, we assessed the potential for indication bias by examining NSAID usage among non-diabetics and individuals without cardiovascular disease history.
Since individuals were not randomized to NSAID use, NSAID users might be systematically different from non-users, leading to selection bias. To minimize selection bias and mimic experimental design, we used the propensity score stratification method [22]. The propensity score is the likelihood of NSAID use, which was calculated using logistic regression such that NSAID use was regressed on baseline characteristics (Table 1). Accordingly, data were divided into five approximately equal strata using the calculated propensity scores. Because the propensity score should act as a balancing score, we used the standardized difference method [23] to examine whether propensity score stratification balanced the distribution of variables used in calculating propensity score among NSAID users and non-users in the five strata. The standardized difference was calculated as the difference in means/proportion of the variables in NSAID and non-NSAID users divided by the standard deviation of the variable. To estimate the effect of NSAID use on cancer risk, we used propensity score stratified proportional hazards Cox regression models for calculating hazard ratios (HRs) and 95% confidence intervals (CIs). Such approach led to weighted HRs, and allowed the baseline hazards to differ among propensity score strata. Since, sex, race, age, education, marital status, family history of cancer, diabetes, alcohol use, smoking, self-reported history of cardiovascular disease, and body mass index (BMI) were used in propensity score calculation, these variables were not included in the Cox models except if a variable was not balanced after propensity score stratification. By using propensity score stratification, we minimize potential bias due to different distribution of these variables among NSAID and non-NSAID users. Additional covariates that were specific to individual types of cancer were added to the Cox models as appropriate for that cancer. For example, prostate cancer was adjusted for prostate-specific antigen (PSA) testing, and breast cancer was adjusted for parity, age at first birth, age at menopause, hormonal replacement therapy (HRT) use, HRT duration, hysterectomies, and oophorectomies. To adjust for multiple comparisons we used false discovery rate (FDR) method. FDR adjusted p values were calculated allowing not more than 0.2 true null hypothesis [24]. Trend was assessed by including the frequency of NSAID use as a continuous variable that ranges from 0 to 3, where 0 is never used, 1 is monthly use, 2 is weekly use and 3 is daily use.
Table 1. Baseline characteristics of participants in NIH-AARP Diet and Health Study.
| No cancer | Cancer | No NSAIDs use | NSAIDs use | ||
| N | 262,628 | 51,894 | 272288 | 42234 | |
| Age (mean, SD) | 62.6 (5.4) | 64.1 (4.9) | 63.7 (5.1) | 62.7 (5.3) | |
| Person years (mean, SD) | 9.4 (2.1) | 5.0 (2.9) | 8.5 (2.8) | 8.7 (2.7) | |
| Sex (n, %) | Female | 147,564 (56.2) | 33,496 (64.5) | 22,267 (52.7) | 159,784 (58.7) |
| Male | 115,064 (43.8) | 17,398 (33.5) | 19,958 (47.3) | 112,504 (41.3) | |
| Race/ethnicity (n, %) | White, non-Hispanic | 242,680 (92.4) | 48,532 (93.5) | 37642 (90.3) | 253570 (94.2) |
| Black, non-Hispanic | 8,684 (3.3) | 1,628 (3.1) | 2,248 (5.4) | 8,064 (3.00) | |
| Others | 8,284 (3.2) | 1,150 (2.2) | 1,785 (4.3) | 7,649 (2.8) | |
| Marital status (n, %) | Married | 176,559 (67.2) | 36,809 (70.9) | 26,466 (63.2) | 186,902 (69.1) |
| Education (n, %) | High school or less | 61,500 (23.4) | 12,181 (23.5) | 12,052 (29.4) | 61,629 (23.2) |
| Post high school training | 259,94(9.9) | 4,950 (9.5) | 4,283 (10.4) | 26,661 (10.0) | |
| Some college | 61,365 (23.4) | 12,000 (23.1) | 9,477 (23.1) | 63,888 (24.1) | |
| College graduate | 107,279 (40.8) | 21,377 (41.2) | 15,199 (37.1) | 113,457 (42.7) | |
| Family history of cancer (n, %) | Yes | 129,722 (49.4) | 27,057 (52.1) | 20,765 (51.5) | 136,014 (52.3) |
| History of disease (n, %) | Diabetes | 22,245 (8.5) | 4,555 (8.8) | 4,031 (9.5) | 22,769 (8.4) |
| Heart disease | 35,469 (13.5) | 8,083 (15.6) | 5,269 (12.5) | 38,283 (14.1) | |
| Body mass index (n, %) | <25 | 95,987 (36.5) | 17,979 (34.6) | 16,730 (40.7) | 97,236 (37.4) |
| 25-<30 | 106,892 (40.7) | 22,236 (42.8) | 16,059 (39.1) | 113,069 (42.3) | |
| 30-<35 | 38,771 (14.8) | 7,739 (14.9) | 5,725 (13.9) | 40,785 (15.3) | |
| 35+ | 15,627 (6.0) | 2,907 (5.6) | 2,570 (6.3) | 15,964 (6.0) | |
| Smoking (n, %) | Never | 96,873 (36.9) | 15,478 (29.8) | 16,336 (40.0) | 96,015 (36.5) |
| Former | 74,136 (28.2) | 14,143 (27.3) | 19,634 (48.1) | 137,306 (52.2) | |
| Current | 55,462 (21.1) | 13,199 (25.4) | 4,850 (11.9) | 29,902 (11.3) | |
| Alcohol (n, %) | None | 19,495 (7.4) | 3,448 (6.6) | 4,742 (11.2) | 18,201 (6.7) |
| <5 g/day | 147,447 (56.1) | 27,196 (52.4) | 24,882 (58.9) | 149,761 (55.0) | |
| 5-<15 g/day | 40,967 (15.6) | 8,277 (15.9) | 5,124 (12.1) | 44,120 (16.2) | |
| 15-<30 g/day | 28,374 (10.8) | 6,228 (12.0) | 3,652 (8.7) | 30,950 (11.4) | |
| 30+ g/day | 26,345 (10.0) | 6,745 (13.0) | 3,834 (9.1) | 29,256 (10.7) |
Results
Population characteristics
During 2,715,994 person-years of follow-up (median 10.1, inter-quartile range 8.7–10.1 years), 51,894 individuals answered the NSAID use questions in the lifestyle questionnaire developed cancer, and 262,628 did not. The baseline mean age of study participants was 62.9 (SD 5.3) years; 57.9% were male, and 93.7% were white. Diabetes, heart disease, stroke, hypertension, and ever smoking were reported by 8.5%, 13.9%, 1.9%, 43.4% and, 63.0%, respectively (Table 1). Approximately 86.5% of the subjects reported using NSAIDs; 30% used only aspirin, 13.4% used only non-aspirin NSAIDs, and 43.0% used both aspirin and non-aspirin NSAIDs.
Use of any NSAID, any aspirin, and any non-aspirin NSAID
Risk of all inflammation-related cancers was reduced in association with the use of any NSAID (HR 0.90, 95% CI 0.87–0.93), aspirin (HR 0.94, 95% CI 0.92–0.97), and non-aspirin (HR 0.93, 95% CI 0.91–0.95) (Table 2). Risks of alcohol-related, infection-related, obesity-related, and smoking-related cancers were also reduced with NSAID use. Aspirin (regardless of non-aspirin NSAID use) was associated with reduced risk of infection-related and obesity-related cancers. Non-aspirin NSAIDs (regardless of aspirin use) were significantly associated with a lower risk of alcohol-related, infection-related, obesity-related, and smoking-related cancers (Table 2).
Table 2. Hazard ratios and 95% confidence intervals for cancer risk in relation to NSAID, aspirin and non-aspirin use in the past 12 months from the NIH-AARP Diet and Health Study*.
| Use of NSAIDs | Use of aspirin | Use of non-aspirin | |
| All inflammation related cancers | 0.90 (0.87–0.93) | 0.94 (0.92–0.97) | 0.93 (0.91–0.95) |
| Alcohol-related cancer& | 0.80 (0.74–0.85) | 0.95 (0.90–1.01) | 0.78 (0.74–0.82) |
| Infection-related cancer$ | 0.82 (0.78–0.87) | 0.92 (0.88–0.96) | 0.84 (0.81–0.88) |
| Obesity-related cancer# | 0.88 (0.84–0.92) | 0.89 (0.83–0.97) | 0.95 (0.92–0.98) |
| Smoking-related cancer∧ | 0.88 (0.85–0.92) | 0.98 (0.91–1.00) | 0.88 (0.86–0.90) |
* Three models each for one of the variables including use of NSAID, use of aspirin, or use of non-aspirin.
& Head and neck, esophagus, colorectal, liver and breast cancer for women.
$ Head and neck, stomach, liver, colorectal, lymphoma, and female genital cancers.
# Esophagus, gallbladder, colorectum, pancreas, breast (after menopause), endometrium, kidney, and thyroid.
∧ Lung, head and neck, esophagus, pancreas, and urinary bladder cancers.
For individual cancers, we also observed that overall NSAIDs were associated with significantly reduced risk of esophageal (HR 0.74, 95% CI 0.58–0.95), stomach (HR 0.73, 95% CI 0.58–0.93), liver (HR 0.59, 95% CI 0.44–0.78), colorectal (HR 0.79, 95% CI 0.73–0.86), prostate (HR 0.94, 95% CI 0.89–0.99), endometrial (HR 0.77, 95% CI 0.65–0.92), and lung (HR 0.89, 95% CI 0.83–0.96) cancers (data not in tables). Aspirin was associated with reduced risk of cancers of the liver (HR 0.62, 95% CI 0.49–0.79), and endometrium (HR 0.86, 95% CI 0.75–0.99) but the excess risk of urinary bladder cancers (HR 1.16, 95% CI 1.05–1.27). Non-aspirin was associated with reduced risk of cancers of the esophagus (HR 0.74, 95% CI 0.62–0.89), stomach (HR 0.70, 95% CI 0.58–0.84), pancreas (HR 0.87, 95% CI 0.77–0.98), colorectum (HR 0.75, 95% CI 0.71–0.80), head and neck (HR 0.87, 95% CI 0.77–0.97), lung (HR 0.94, 95% CI 0.89–0.99), urinary bladder (HR 0.88, 95% CI 0.81–0.95), and myeloid monocytic leukemia (HR 0.77, 95% CI 0.63–0.94), prostate (HR 0.94, 95% CI 0.91–0.97), and endometrium (HR 0.87, 95% CI 0.76–1.00) (data not in tables).
Independent and combined effects of aspirin and non-aspirin NSAID use
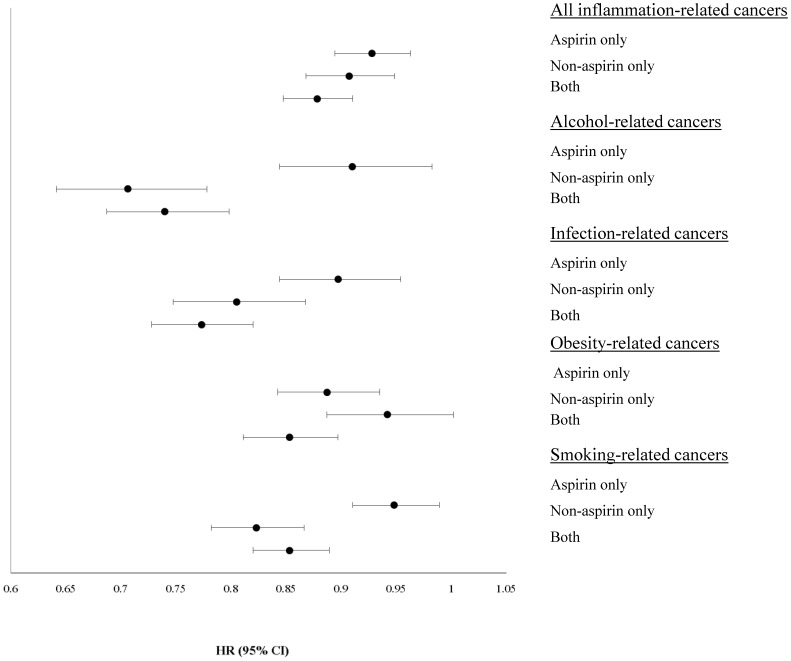
In addition to evaluating the use of any NSAID, aspirin, and non-aspirin, we examined the use of aspirin alone, use of non-aspirin NSAIDs alone, and use of both aspirin and non-aspirin NSAIDs (Fig. 1). Compared with non-users, aspirin-only, non-aspirin-only, and both aspirin and non-aspirin NSAID users had lower risks of all inflammation-related, alcohol-related, infection-related, obesity-related and smoking-related cancers.
Figure 1. Hazard ratios and 95% confidence interval for cancer risk in relation to exclusive use of Aspirin or non-aspirin NSAID, or use of both NSAISDs in the past 12 months from the NIH-AARP Diet and Health Study*.
*NSAID use is a four-level variable, of NSAID non-use, use of aspirin alone, use of non-aspirin NSAIDs alone, or use of both aspirin and non-aspirin NSAIDs. NSAID non-use is the reference. # Esophagus, gallbladder, colorectum, pancreas, breast (after menopause), endometrium, kidney, and thyroid. $ Head and neck, stomach, liver, colorectal, lymphoma, and female genital cancers. & Head and neck, esophagus, colorectal, liver and breast cancer for women. ∧Lung, head and neck, esophagus, pancreas, and urinary bladder cancers. HR: hazard ratio. 95% CI: 95% confidence interval.
Models for specific cancer sites showed that aspirin-only users had reduced risks of liver (HR 0.52, 95% CI 0.37–0.73), lung (HR 0.91, 95% CI 0.84–0.99), and skin (HR 0.88, 95% CI 0.78–0.98) cancers, but excess risk of urinary bladder (HR 1.18, 95% CI 1.04–1.35) cancers (data not in tables). In addition, non-aspirin NSAID-only users had reduced risks of esophageal (HR 0.70, 95% CI 0.50–0.99), stomach (HR 0.66, 95% CI 0.47–0.93), colorectal (HR 0.68, 95% CI 0.60–0.76), endometrial (HR 0.76, 95% CI 0.61–0.95), lung (HR 0.81, 95% CI 0.73–0.90), and any skin (HR 0.79, 95% CI 0.69–0.91) cancers, including melanoma (HR 0.80, 95% CI 0.69–0.93). Users of both aspirin and non-aspirin had reduced risk of esophageal (HR 0.66, 95% CI 0.50–0.86), stomach (HR 0.65, 95% CI 0.50–0.84), liver (HR 0.58, 95% CI 0.42–0.79), colorectal (HR 0.73, 95% CI 0.66–0.79), lung (HR 0.90, 95% CI 0.83–0.98), myeloid monocytic leukemia (HR 0.70, 95% CI 0.51–0.94), and endometrial cancers (HR 0.73, 95% CI 0.60–0.88) (data not in tables).
Frequency of NSAID use
As shown in Table 3, inverse dose response trends were observed for infection-related and obesity-related cancers with frequency of aspirin use compared to non-use (ptrend<0.001). Interestingly, smoking-related cancers exhibited a positive dose response relationship with aspirin use compared to non-use (ptrend<0.001). For the frequency of non-aspirin NSAID use, an inverse trend was observed for all inflammation-related (ptrend<0.001), alcohol-related (ptrend<0.001), infection-related (ptrend<0.0001), and smoking-related (ptrend<0.001) cancers. None of the examined cancers showed a significant inverse dose response association with aspirin use compared to non-use (data not in tables). However, cancers of the esophagus, stomach, pancreas, colorectum, endometrium, head and neck, urinary bladder, leukemia, and NHL all had significant inverse dose response trend with non-aspirin NSAD use compared to non-use.
Table 3. Hazard ratios and 95% confidence intervals for cancer risk in relation to frequency of aspirin and non-aspirin use and cancer in the NIH-AARP Diet and Health Study.
| Monthly | Weekly | Daily | P for trend* | |
| Aspirin use | ||||
| All inflammation related cancers | 0.92 (0.89–0.95) | 0.92 (0.89–0.96) | 0.99 (0.96–1.02) | 0.64 |
| Alcohol-related cancer& | 0.95 (0.89–1.01) | 0.89 (0.82–0.96) | 1.01 (0.94–1.08) | 0.87 |
| Infection-related cancer$ | 0.92 (0.87–0.97) | 0.89 (0.83–0.94) | 0.93 (0.88–0.98) | <0.01 |
| Obesity-related cancer# | 0.92 (0.88–0.96) | 0.89 (0.84–0.93) | 0.86 (0.82–0.90) | <0.001 |
| Smoking-related cancer∧ | 0.93 (0.90–0.96) | 0.94 (0.90–0.98) | 1.08 (1.04–1.12) | <0.001 |
| Non-aspirin use | ||||
| All inflammation related cancers | 0.92 (0.90–0.95) | 0.93 (0.89–0.96) | 0.96 (0.92–1.00) | <0.001 |
| Alcohol-related cancer& | 0.83 (0.78–0.88) | 0.74 (0.68–0.81) | 0.70 (0.64–0.77) | <0.001 |
| Infection-related cancer$ | 0.86 (0.82–0.90) | 0.82 (0.77–0.87) | 0.81 (0.75–0.87) | <0.001 |
| Obesity-related cancer# | 0.94 (0.90–0.99) | 0.97 (0.92–1.02) | 0.96 (0.90–1.01) | 0.05 |
| Smoking-related cancer∧ | 0.89 (0.86–0.92) | 0.85 (0.81–0.88) | 0.88 (0.84–0.93) | <0.001 |
*FDR adjusted p values did not appreciably differ from the non-FDR adjusted p values.
& Head and neck, esophagus, colorectal, liver and breast cancer for women.
$ Head and neck, stomach, liver, colorectal, lymphoma, and female genital cancers.
# Esophagus, gallbladder, colorectum, pancreas, breast (after menopause), endometrium, kidney, and thyroid.
∧ Lung, head and neck, esophagus, pancreas, and urinary bladder cancers.
Prior to examining the association between NSAIDs use and cancer adjusting for confounders, we examined whether gender modified the risk. Because no effect modification was detected except for the infection-related cancer and ever use of aspirin, and obesity-related cancer with ever use of ibuprofen, we did not present all analyses stratified by gender. Of note, the association between infection-related cancer and ever use of aspirin was significant among women (HR 0.87, 95% CI 0.82–0.93), but not among men (HR 0.96, 95% CI 0.90–1.02). On the other hand, while obesity-related cancer was significantly reduced among women and men who reported ever use of ibuprofen, however the effect was more profound among women (HR 0.85, 95% CI 0.80–0.90), compared to men (HR 0.91, 95% CI 0.88–0.95).
Sensitivity analyses
The results among non-diabetics and individuals with no cardiovascular disease history were similar to those for the overall analysis. Among non-diabetics, use of any NSAID was associated with a reduced risk of inflammation-related (HR 0.89, 95% CI 0.86–0.93), alcohol-related (HR 0.79, 95% CI 0.73–0.85), infection-related (HR 0.81, 95% CI 0.77–0.86), obesity-related (HR 0.88, 95% CI 0.84–0.92), and smoking-related cancers (HR 0.87, 95% CI 0.84–0.92). Among individuals without history of cardiovascular diseases, use of any NSAID was associated with a reduced risk of inflammation-related (HR 0.92, 95% CI 0.88–0.96), alcohol-related (HR 0.81, 95% CI 0.73–0.89), infection-related (HR 0.85, 95% CI 0.79–0.82), obesity-related (HR 0.93, 95% CI 0.87–0.99) and smoking-related cancers (HR 0.88, 95% CI 0.84–0.93).
Since some anti-cholesterol medication might affect cancer risk, we conducted a sensitivity analysis restricted to individuals who had information on the frequency of anti-cholesterol medication within last year. Our results revealed that the overall risk of cancer (HR 0.92, 95% CI 0.89–0.96), inflammation-related (HR 0.90, 95% CI 0.86–0.94), alcohol-related (HR 0.77, 95% CI 0.70–0.84), infection-related (HR 0.80, 95% CI 0.75–0.86), obesity-related (HR 0.88, 95% CI 0.83–0.93) and smoking-related cancers (HR 0.87, 95% CI 0.82–0.91) remained significantly reduced after adjusting for anti-cholesterol medication. Interestingly, the frequency of use of anti-cholesterol medication was significantly associated with excess overall cancer risk (HR 1.028, 95% CI 1.024–1.033), inflammation-related (HR 1.027, 95% CI 1.020–1.033), alcohol-related (HR 1.032, 95% CI 1.018–1.046), and smoking-related cancers (HR 1.055, 95% CI 1.047–1.063).
We further conducted a sensitivity analysis to assess whether excluding the first five years of follow up would change our findings. The risk of inflammation-related cancer in association with any NSAID (HR 0.90, 95% CI 0.86–0.95), aspirin (HR 0.94, 95% CI 0.91–0.98), and non-aspirin use (HR 0.93, 95% CI 0.90–0.96) did not appreciably change.
Discussion
In this large prospective study, we conducted extensive analyses of NSAID use and cancer that expanded on previous studies by 1) evaluating groups of cancers with common inflammation-related causes and 2) accounting for self-selection bias through propensity scores. By combining cancers with similar causes, we could better evaluate non-aspirin NSAID use, which has a lower prevalence than aspirin use. The use of propensity scores provides increased confidence in these results given concerns that previously observed inverse associations may be largely due to selection bias [25].
We showed that NSAID use was associated with a reduced risk of cancers that usually develop in the presence of inflammatory conditions such as obesity, smoking, infection, and excessive alcohol consumption. Aspirin use was associated with reduced risk of infection-related, obesity-related, liver, and endometrial cancers. Non-aspirin NSAID use was associated with lower risk of inflammation-related, alcohol-related, infection-related, obesity-related, smoking-related, head and neck, esophageal, stomach, pancreas, colorectal, lung, urinary bladder, endometrial and prostate cancers, and myeloid monocytic leukemia. More specifically, aspirin-only users had reduced risks of liver, lung, skin, and endometrial cancers but the excess risk of urinary bladder cancers. Non-aspirin NSAID-only users had a reduced risk of esophageal, stomach, colorectal, prostate, skin, melanoma, and lung. Both aspirin and non-aspirin NSAID use were associated with lower risk of all inflammation-related, alcohol-related, infection-related, obesity-related, and smoking-related cancers, and individual cancers including esophageal, stomach, liver, colorectal, endometrial, and lung cancers.
Overall, our results support the role of NSAIDs in reducing inflammatory conditions and thus diminishing risk of inflammation-related cancers [3]. This role is plausible, given the known mechanisms of action of NSAIDs. The main mechanism involves inhibition of COX enzymes leading to suppression of prostaglandin synthesis, which subsequently modulates cellular proliferation and apoptosis, hindering tumor growth [26]–[28]. Alternative COX-independent anti-neoplastic mechanisms include MAGI1, NF-κB, TGF-β, RAS, β-catenin, and cyclin-D1 pathways [29]–[32].
Our findings are further supported by studies of individual cancers. The observed reduced risk of alcohol-related cancers in association with NSAID use is supported by studies of individual alcohol-related cancers, such as head and neck, esophagus, colorectal, and breast [33]–[37]. Similarly, NSAID use has been associated with reduced risk of obesity-related cancers, including esophageal, gallbladder, colorectal, pancreatic, post-menopause breast, endometrial, kidney, and thyroid cancers [35], [38], [39]. NSAID use is also associated with decreased risk of individual infection-related cancers, specifically head and neck, stomach, liver, colorectal, lymphoma, and female genital cancers [33], [35], [40]. Finally, studies of NSAIDs and; lung, head and neck, esophagus, pancreas, and urinary bladder cancers [5], [41]–[45] support our finding of reduced risk of smoking-related cancer.
Our findings of lower risks at specific cancer sites are also consistent with results from previous studies [5]–[8], [46], [47] and several meta-analyses [6], [35], [41], [43], [48]–[52] demonstrating lower risks of including esophageal, gastric, colorectal, and lung cancers associated with aspirin use. Similarly, the observed reduced risk of stomach, colorectal cancers in association with non-aspirin NSAIDs are supported by epidemiologic and in vitro studies [53]–[55]. For gastric cancer risk a study from Taiwan found that NSAID use was protective against gastric cancer risk even among individuals with Helicobacter pylori infection [56]. A study of the effect of aspirin in the presence of Helicobacter pylori revealed that aspirin increases Helicobacter pylori-induced apoptosis and diminish Helicobacter pylori-induced hyperplasia [57].
Reduced esophageal cancer risk has been associated with both aspirin and non-aspirin NSAID use [34], [43], [58], [59]. Similarly, the lower risk of colorectal cancer among NSAID users has been observed in previous studies [46], [53], [60]–[62], even among individuals with previously treated colorectal cancer [15].
The association of NSAID use with reduced liver cancer in human is a fairly novel finding, as described in a separate AARP publication [25]. Liver cancer risk factors include chronic hepatitis virus infection, alcohol, and obesity, all of which involve chronic inflammation mediated by several molecular pathways [63]. Recently, statin use was reported to diminish HCC risk, a further support of the role of anti-inflammatory agents in reducing HCC risk [64]. In addition, in a recent study of a nude mouse xenograft model, aspirin repressed growth of hepatocellular carcinoma cells and induced apoptosis in vitro [65]. Also, aspirin was found to increase reactive oxygen species production and induce cell cycle arrest and apoptosis in HepG2 cells [25], again supporting the hypothesis that NSAID use may lower the risk of liver cancer by reducing inflammation.
The association between lung cancer and NSAID use has been inconsistent in previous studies. Some studies supported the diminished risk, while other studies failed to detect reduced risk, or found an effect among men, but not women, or an effect in specific histologic subtypes [19], [23], [64], [66]–[68]. However, two large studies found an inverse association between NSAID use and lung cancer [23], [67], as did a recent pooled analysis from the International Lung Cancer Consortium (ILCCO), which reported a 26% risk reduction in men [6]. These studies support our results. Conflicting results from other studies might be attributed to lack of power due to small sample size, residual confounding due to incomplete smoking information, or inadequate NSAID dose and duration information.
In concordance with our findings, a lower risk of myeloid leukemia in association with non-aspirin use has been reported [69], [70]. This association is further supported by in vitro studies of the effect of NSAIDs on inducing apoptosis in acute myeloid leukemia cells [71].
Studies of NSAID use and melanoma have conflicting results. Our finding of reduced risk with non-aspirin-only use, but not with aspirin is interesting. A few studies reported reduced risk of melanoma [72]–[74] including a recent large population-based study from Northern Denmark [75]. On the other hand, some studies reported excess, although non-significant, risk of melanoma with non-aspirin use [76], [77]. A large Dutch population-based study reported a non-significant excess risk with non-aspirin NSAIDs but lower risk with continuous use of low-dose aspirin among women only [74]. Although we found a lower risk of endometrial cancer with non-aspirin NSAID use and use of aspirin and non-aspirin NSAIDs together, we did not detect any dose response effect. Previous reports have conflicting results. While some studies reported lower endometrial cancer risk among obese women using NSAIDs [78], [79] other studies failed to detect any association [80], [81]. Our inability to find significant associations between any NSAID use and some cancers might be due to the lack of information on duration of long-term NSAID use [53], [82], [83]. For instance, a cohort study of 70,144 men demonstrated that the current aspirin or non-aspirin use was not associated with lower prostate cancer risk. However, consistent, long-duration use of non-aspirin NSAIDs (≥30 pills/month for ≥5 years) was found to be related to a reduced risk of prostate cancer (relative risk 0.82, 95% CI (0.71 to 0.94). In addition, three large UK trials found that a daily use of aspirin only led to observable reductions in deaths due to several cancers after five years of follow-up, and benefit seemed to increase with duration of treatment [84]. These data suggest that the duration of use may be important, and information on duration and consistency of use was limited in this study. That said, inflammation may contribute more to some cancers/categories of inflammation-related cancers and less to other cancers/categories of inflammation-related cancers, which could explain why we see an association for some cancers/category of inflammation-related cancers and not others.
Evaluation of non-aspirin NSAID use alone was further complicated by the general lower frequency of non-aspirin NSAID use in this population: merely 13.4% used non-aspirin-only. Because aspirin is the most commonly used drug for cardiovascular disease prevention, this study might lack the power to detect diminished risks associated with non-aspirin NSAID use. However, we did improve the power by combining cancers with like inflammation-related causes.
Our study had several strengths. The NIH-AARP Diet and Health Study is a large cohort with detailed information on NSAID use, which allowed us to evaluate less common cancers. The detailed collection of epidemiologic information enabled us to examine the effect of multiple confounders, such as diabetes, smoking, obesity, and cardiovascular diseases. However, certain limitations have to be noted. We did not have information on the indication for NSAID use. However, our results were similar when restricted to individuals without a history of cardiovascular disease and non-diabetics, suggesting that use of NSAIDs for cardiovascular disease does not modify the results. Finally, cumulative exposure or dosage could not be evaluated.
In summary, our results indicate that NSAID use might reduce the risk of several cancers. The null results for some cancers might indicate that NSAIDs need to be used for a prolonged duration to exert a measurable effect. Taken together, these results warrant further studies on the dosage and duration of NSAID use for chemoprevention of inflammation-related cancer. Such studies will pave the way to a well-designed chemoprevention clinical trial to establish the lowest safest dose and duration required for chemoprevention of different cancer subsites.
Acknowledgments
In memory of Dr. Arthur Schatzkin, visionary investigator who founded the NIH-AARP Diet and Health Study.
We are grateful to the participants in the NIH-AARP Diet and Health Study for their cooperation.
For study outcomes ascertainment and management, we thank Kerry Grace Morrissey and Sigurd Hermansen Westat, in addition, we thank Leslie Carroll at Information Management Services for data support.
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute.
The Georgia Center for Cancer Statistics, Department of Epidemiology, Rollins School of Public Health, Emory University collected cancer incidence data from the Atlanta metropolitan area. Cancer incidence data from California were collected by the California Department of Health Services, Cancer Surveillance Section. The Michigan Cancer Surveillance Program, Community Health Administration, State of Michigan collected cancer incidence data from the Detroit metropolitan area. The Florida cancer incidence data used in this report were collected by the Florida Cancer Data System (FCDC) under contract with the Florida Department of Health (FDOH). Cancer incidence data from Louisiana were collected by the Louisiana Tumor Registry, Louisiana State University Medical Center in New Orleans. Cancer incidence data from New Jersey were collected by the New Jersey State Cancer Registry, Cancer Epidemiology Services, New Jersey State Department of Health and Senior Services. Cancer incidence data from North Carolina were collected by the North Carolina Central Cancer Registry. Cancer incidence data from Pennsylvania were supplied by the Division of Health Statistics and Research, Pennsylvania Department of Health, Harrisburg, Pennsylvania. Cancer incidence data from Arizona were collected by the Arizona Cancer Registry, Division of Public Health Services, Arizona Department of Health Services. Cancer incidence data from Texas were collected by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services. Cancer incidence data from Nevada were collected by the Nevada Central Cancer Registry, Center for Health Data and Research, Bureau of Health Planning and Statistics, State Health Division, State of Nevada Department of Health and Human Services.
The views expressed herein are solely those of the authors and do not necessarily reflect those of the NIH-AARP.
Data Availability
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. Data will be made available upon request. This study involved human subjects and to protect the privacy of study participants, data requests will be reviewed by the NIH-AARP study team. Requests for data related to this PLoS publication should be directed to dietandhealth@mail.nih.gov. Interested researchers may also visit the study website for information about the cohort and available study resources. The study website is: http://dietandhealth.cancer.gov/index.html.
Funding Statement
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Haas M, Buttner M, Rau TT, Fietkau R, Grabenbauer GG, et al. (2011) Inflammation in gastric adenocarcinoma of the cardia: how do EBV infection, Her2 amplification and cancer progression influence tumor-infiltrating lymphocytes? Virchows Arch 458:403–411. [DOI] [PubMed] [Google Scholar]
- 2. Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454:436–444. [DOI] [PubMed] [Google Scholar]
- 3. Allavena P, Garlanda C, Borrello MG, Sica A, Mantovani A (2008) Pathways connecting inflammation and cancer. Curr Opin Genet Dev 18:3–10. [DOI] [PubMed] [Google Scholar]
- 4. Mantovani A, Pierotti MA (2008) Cancer and inflammation: a complex relationship. Cancer Lett 267:180–181. [DOI] [PubMed] [Google Scholar]
- 5. Daugherty SE, Pfeiffer RM, Sigurdson AJ, Hayes RB, Leitzmann M, et al. (2011) Nonsteroidal antiinflammatory drugs and bladder cancer: a pooled analysis. Am J Epidemiol 173:721–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McCormack VA, Hung RJ, Brenner DR, Bickeboller H, Rosenberger A, et al. (2011) Aspirin and NSAID use and lung cancer risk: a pooled analysis in the International Lung Cancer Consortium (ILCCO). Cancer Causes Control 22:1709–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruder EH, Laiyemo AO, Graubard BI, Hollenbeck AR, Schatzkin A, et al. (2011) Non-steroidal anti-inflammatory drugs and colorectal cancer risk in a large, prospective cohort. Am J Gastroenterol 106:1340–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wernli KJ, Newcomb PA, Hampton JM, Trentham-Dietz A, Egan KM (2008) Inverse association of NSAID use and ovarian cancer in relation to oral contraceptive use and parity. Br J Cancer 98:1781–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coogan PF, Kelly JP, Strom BL, Rosenberg L (2010) Statin and NSAID use and prostate cancer risk. Pharmacoepidemiol Drug Saf 19:752–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Danforth KN, Gierach GL, Brinton LA, Hollenbeck AR, Katki HA, et al. (2009) Nonsteroidal anti-inflammatory drug use and endometrial cancer risk in the NIH-AARP Diet and Health Study. Cancer Prev Res (Phila) 2:466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Daugherty SE, Moore SC, Pfeiffer RM, Inskip PD, Park Y, et al. (2011) Nonsteroidal Anti-inflammatory Drugs and Glioma in the NIH-AARP Diet and Health Study Cohort. Cancer Prev Res (Phila) 4:2027–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Legge F, Paglia A, D'Asta M, Fuoco G, Scambia G, et al. (2011) Phase II study of the combination carboplatin plus celecoxib in heavily pre-treated recurrent ovarian cancer patients. BMC Cancer 11:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan AT, Hsu M, Zauber AG, Hawk ET, Bertagnolli MM (2011) The Influence of UGT1A6 Variants and Aspirin Use in a Randomized Trial of Celecoxib for Prevention of Colorectal Adenoma. Cancer Prev Res (Phila). [DOI] [PMC free article] [PubMed]
- 14. Burn J, Bishop DT, Chapman PD, Elliott F, Bertario L, et al. (2011) A randomized placebo-controlled prevention trial of aspirin and/or resistant starch in young people with familial adenomatous polyposis. Cancer Prev Res (Phila) 4:655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, et al. (2003) A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med 348:883–890. [DOI] [PubMed] [Google Scholar]
- 16. Schatzkin A, Subar AF, Thompson FE, Harlan LC, Tangrea J, et al. (2001) Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol 154:1119–1125. [DOI] [PubMed] [Google Scholar]
- 17. Michaud DS, Midthune D, Hermansen S, Leitzmann M, Harlan LC, et al. (2005) Comparison of cancer registry case ascertainment with SEER estimates and self-reporting in a subset of the NIHAARP Diet and Health Study. J Registry Manage 32:70–75. [Google Scholar]
- 18. de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, et al. (2012) Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 13:607–615. [DOI] [PubMed] [Google Scholar]
- 19.IARC Monographs on the Evaluation of Carcinogenic Risks to Humans (2010) Alcohol Consumption and Ethyl Carbamate. Vol 96 Lyon, France: International Agency for Research on Cancer. [PMC free article] [PubMed]
- 20.National Cancer Institute (2013) Fact Sheet Alcohol and Cancer Risk.
- 21.U.S. Department of Health and Human Services.(2004) The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health.
- 22. Rosenbaum PR, Rubin DB (1984) Reducing Bias in Observational Studies Using Subclassification on the Propensity Score. Journal of the American Statistical Association 79:516–524. [Google Scholar]
- 23. Austin PC (2009) Using the Standardized Difference to Compare the Prevalence of a Binary Variable Between Two Groups in Observational Research. Communications in Statistics-Simulation and Computation 38:1228–1234. [Google Scholar]
- 24. Benjamini Y, Hochberg Y (1995) Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B 57:289–300. [Google Scholar]
- 25. Sahasrabuddhe VV, Gunja MZ, Graubard BI, Trabert B, Schwartz LM, et al. (2012) Nonsteroidal anti-inflammatory drug use, chronic liver disease, and hepatocellular carcinoma. J Natl Cancer Inst 104:1808–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grosch S, Maier TJ, Schiffmann S, Geisslinger G (2006) Cyclooxygenase-2 (COX-2)-independent anticarcinogenic effects of selective COX-2 inhibitors. J Natl Cancer Inst 98:736–747. [DOI] [PubMed] [Google Scholar]
- 27. Janssen A, Maier TJ, Schiffmann S, Coste O, Seegel M, et al. (2006) Evidence of COX-2 independent induction of apoptosis and cell cycle block in human colon carcinoma cells after S- or R-ibuprofen treatment. Eur J Pharmacol 540:24–33. [DOI] [PubMed] [Google Scholar]
- 28. Khan Z, Khan N, Tiwari RP, Sah NK, Prasad GB, et al. (2011) Biology of Cox-2: an application in cancer therapeutics. Curr Drug Targets 12:1082–1093. [DOI] [PubMed] [Google Scholar]
- 29. Schror K (2011) Pharmacology and cellular/molecular mechanisms of action of aspirin and Non-aspirin NSAIDs in colorectal cancer. Best Pract Res Clin Gastroenterol 25:473–484. [DOI] [PubMed] [Google Scholar]
- 30. Suh N, Reddy BS, Decastro A, Paul S, Lee HJ, et al. (2011) Combination of Atorvastatin with Sulindac or Naproxen Profoundly Inhibits Colonic Adenocarcinomas by Suppressing the p65/beta-Catenin/Cyclin D1 Signaling Pathway in Rats. Cancer Prev Res (Phila) 4:1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaric J, Joseph JM, Tercier S, Sengstag T, Ponsonnet L, et al. (2011) Identification of MAGI1 as a tumor-suppressor protein induced by cyclooxygenase-2 inhibitors in colorectal cancer cells. Oncogene. [DOI] [PubMed]
- 32. Greenspan EJ, Madigan JP, Boardman LA, Rosenberg DW (2011) Ibuprofen inhibits activation of nuclear {beta}-catenin in human colon adenomas and induces the phosphorylation of GSK-3{beta}. Cancer Prev Res (Phila) 4:161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jayaprakash V, Rigual NR, Moysich KB, Loree TR, Nasca MA, et al. (2006) Chemoprevention of head and neck cancer with aspirin: a case-control study. Arch Otolaryngol Head Neck Surg 132:1231–1236. [DOI] [PubMed] [Google Scholar]
- 34. Funkhouser EM, Sharp GB (1995) Aspirin and reduced risk of esophageal carcinoma. Cancer 76:1116–1119. [DOI] [PubMed] [Google Scholar]
- 35. Bosetti C, Gallus S, La Vecchia C (2009) Aspirin and cancer risk: a summary review to 2007. Recent Results Cancer Res 181:231–251. [DOI] [PubMed] [Google Scholar]
- 36. Macfarlane TV, Macfarlane GJ, Thakker NS, Benhamou S, Bouchardy C, et al. (2012) Role of medical history and medication use in the aetiology of upper aerodigestive tract cancers in Europe: the ARCAGE study. Ann Oncol 23:1053–1060. [DOI] [PubMed] [Google Scholar]
- 37. Bosetti C, Talamini R, Franceschi S, Negri E, Garavello W, et al. (2003) Aspirin use and cancers of the upper aerodigestive tract. Br J Cancer 88:672–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo T, Yan HM, He P, Luo Y, Yang YF, et al. (2011) Aspirin use and breast cancer risk: a meta-analysis. Breast Cancer Res Treat. [DOI] [PubMed]
- 39. Gonzalez-Perez A, Garcia Rodriguez LA, Lopez-Ridaura R (2003) Effects of non-steroidal anti-inflammatory drugs on cancer sites other than the colon and rectum: a meta-analysis. BMC Cancer 3:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chang ET, Cronin-Fenton DP, Friis S, Hjalgrim H, Sorensen HT, et al. (2010) Aspirin and other nonsteroidal anti-inflammatory drugs in relation to Hodgkin lymphoma risk in northern Denmark. Cancer Epidemiol Biomarkers Prev 19:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu J, Yin Z, Gao W, Liu L, Wang R, et al. (2011) Meta-Analysis on the Association Between Nonsteroidal Anti-Inflammatory Drug Use and Lung Cancer Risk. Clin Lung Cancer. [DOI] [PubMed]
- 42. Sudbo J, Lee JJ, Lippman SM, Mork J, Sagen S, et al. (2005) Non-steroidal anti-inflammatory drugs and the risk of oral cancer: a nested case-control study. Lancet 366:1359–1366. [DOI] [PubMed] [Google Scholar]
- 43. Corley DA, Kerlikowske K, Verma R, Buffler P (2003) Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology 124:47–56. [DOI] [PubMed] [Google Scholar]
- 44. Anderson KE, Johnson TW, Lazovich D, Folsom AR (2002) Association between nonsteroidal anti-inflammatory drug use and the incidence of pancreatic cancer. J Natl Cancer Inst 94:1168–1171. [DOI] [PubMed] [Google Scholar]
- 45. Wilson JC, Anderson LA, Murray LJ, Hughes CM (2011) Non-steroidal anti-inflammatory drug and aspirin use and the risk of head and neck cancer: a systematic review. Cancer Causes Control 22:803–810. [DOI] [PubMed] [Google Scholar]
- 46. Schreinemachers DM, Everson RB (1994) Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiology 5:138–146. [DOI] [PubMed] [Google Scholar]
- 47. Jacobs EJ, Thun MJ, Bain EB, Rodriguez C, Henley SJ, et al. (2007) A large cohort study of long-term daily use of adult-strength aspirin and cancer incidence. J Natl Cancer Inst 99:608–615. [DOI] [PubMed] [Google Scholar]
- 48. Cole BF, Logan RF, Halabi S, Benamouzig R, Sandler RS, et al. (2009) Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. J Natl Cancer Inst 101:256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Flossmann E, Rothwell PM (2007) Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet 369:1603–1613. [DOI] [PubMed] [Google Scholar]
- 50. Bosetti C, Gallus S, La Vecchia C (2006) Aspirin and cancer risk: an updated quantitative review to 2005. Cancer Causes Control 17:871–888. [DOI] [PubMed] [Google Scholar]
- 51. Yang P, Zhou Y, Chen B, Wan HW, Jia GQ, et al. (2010) Aspirin use and the risk of gastric cancer: a meta-analysis. Dig Dis Sci 55:1533–1539. [DOI] [PubMed] [Google Scholar]
- 52. Sun L, Yu S (2011) Meta-analysis: non-steroidal anti-inflammatory drug use and the risk of esophageal squamous cell carcinoma. Dis Esophagus 24:544–549. [DOI] [PubMed] [Google Scholar]
- 53. Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Curhan GC, et al. (2005) Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. JAMA 294:914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang WH, Huang JQ, Zheng GF, Lam SK, Karlberg J, et al. (2003) Non-steroidal anti-inflammatory drug use and the risk of gastric cancer: a systematic review and meta-analysis. J Natl Cancer Inst 95:1784–1791. [DOI] [PubMed] [Google Scholar]
- 55. Gao J, Niwa K, Sun W, Takemura M, Lian Z, et al. (2004) Non-steroidal anti-inflammatory drugs inhibit cellular proliferation and upregulate cyclooxygenase-2 protein expression in endometrial cancer cells. Cancer Sci 95:901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wu CY, Wu MS, Kuo KN, Wang CB, Chen YJ, et al. (2010) Effective reduction of gastric cancer risk with regular use of nonsteroidal anti-inflammatory drugs in Helicobacter pylori-infected patients. J Clin Oncol 28:2952–2957. [DOI] [PubMed] [Google Scholar]
- 57. Li GQ, Xia HH, Chen MH, Tsukamoto T, Tatematsu M, et al. (2008) Effects of aspirin on the development of Helicobacter pylori-induced gastric inflammation and heterotopic proliferative glands in Mongolian gerbils. Helicobacter 13:20–29. [DOI] [PubMed] [Google Scholar]
- 58. Bardou M, Barkun AN, Ghosn J, Hudson M, Rahme E (2004) Effect of chronic intake of NSAIDs and cyclooxygenase 2-selective inhibitors on esophageal cancer incidence. Clin Gastroenterol Hepatol 2:880–887. [DOI] [PubMed] [Google Scholar]
- 59. Vaughan TL, Dong LM, Blount PL, Ayub K, Odze RD, et al. (2005) Non-steroidal anti-inflammatory drugs and risk of neoplastic progression in Barrett's oesophagus: a prospective study. Lancet Oncol 6:945–952. [DOI] [PubMed] [Google Scholar]
- 60. Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Wu K, et al. (2008) Aspirin dose and duration of use and risk of colorectal cancer in men. Gastroenterology 134:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, et al. (2003) A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med 348:891–899. [DOI] [PubMed] [Google Scholar]
- 62. Sorensen HT, Friis S, Norgard B, Mellemkjaer L, Blot WJ, et al. (2003) Risk of cancer in a large cohort of nonaspirin NSAID users: a population-based study. Br J Cancer 88:1687–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Berasain C, Castillo J, Perugorria MJ, Latasa MU, Prieto J, et al. (2009) Inflammation and liver cancer: new molecular links. Ann N Y Acad Sci 1155:206–221. [DOI] [PubMed] [Google Scholar]
- 64.Tsan YT, Lee CH, Ho WC, Lin MH, Wang JD, et al. (2013) Statins and the Risk of Hepatocellular Carcinoma in Patients With Hepatitis C Virus Infection. J Clin Oncol. [DOI] [PubMed]
- 65. Hossain MA, Kim DH, Jang JY, Kang YJ, Yoon JH, et al. (2012) Aspirin induces apoptosis in vitro and inhibits tumor growth of human hepatocellular carcinoma cells in a nude mouse xenograft model. Int J Oncol 40:1298–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, et al. (2005) Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA 294:47–55. [DOI] [PubMed] [Google Scholar]
- 67. Hernandez-Diaz S, Garcia Rodriguez LA (2007) Nonsteroidal anti-inflammatory drugs and risk of lung cancer. Int J Cancer 120:1565–1572. [DOI] [PubMed] [Google Scholar]
- 68. Van Dyke AL, Cote ML, Prysak G, Claeys GB, Wenzlaff AS, et al. (2008) Regular adult aspirin use decreases the risk of non-small cell lung cancer among women. Cancer Epidemiol Biomarkers Prev 17:148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ross JA, Blair CK, Cerhan JR, Soler JT, Hirsch BA, et al. (2011) Nonsteroidal anti-inflammatory drug and acetaminophen use and risk of adult myeloid leukemia. Cancer Epidemiol Biomarkers Prev 20:1741–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kasum CM, Blair CK, Folsom AR, Ross JA (2003) Non-steroidal anti-inflammatory drug use and risk of adult leukemia. Cancer Epidemiol Biomarkers Prev 12:534–537. [PubMed] [Google Scholar]
- 71. Singh R, Cadeddu RP, Frobel J, Wilk CM, Bruns I, et al. (2011) The non-steroidal anti-inflammatory drugs Sulindac sulfide and Diclofenac induce apoptosis and differentiation in human acute myeloid leukemia cells through an AP-1 dependent pathway. Apoptosis 16:889–901. [DOI] [PubMed] [Google Scholar]
- 72. Harris RE, Beebe-Donk J, Namboodiri KK (2001) Inverse association of non-steroidal anti-inflammatory drugs and malignant melanoma among women. Oncol Rep 8:655–657. [DOI] [PubMed] [Google Scholar]
- 73. Curiel-Lewandrowski C, Nijsten T, Gomez ML, Hollestein LM, Atkins MB, et al. (2011) Long-term use of nonsteroidal anti-inflammatory drugs decreases the risk of cutaneous melanoma: results of a United States case-control study. J Invest Dermatol 131:1460–1468. [DOI] [PubMed] [Google Scholar]
- 74. Joosse A, Koomen ER, Casparie MK, Herings RM, Guchelaar HJ, et al. (2009) Non-steroidal anti-inflammatory drugs and melanoma risk: large Dutch population-based case-control study. J Invest Dermatol 129:2620–2627. [DOI] [PubMed] [Google Scholar]
- 75.Johannesdottir SA, Chang ET, Mehnert F, Schmidt M, Olesen AB, et al. (2012) Nonsteroidal anti-inflammatory drugs and the risk of skin cancer: A population-based case-control study. Cancer. [DOI] [PubMed]
- 76. Asgari MM, Maruti SS, White E (2008) A large cohort study of nonsteroidal anti-inflammatory drug use and melanoma incidence. J Natl Cancer Inst 100:967–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jeter JM, Bonner JD, Johnson TM, Gruber SB (2011) Nonsteroidal anti-inflammatory drugs and risk of melanoma. J Skin Cancer 2011:598571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Viswanathan AN, Feskanich D, Schernhammer ES, Hankinson SE (2008) Aspirin, NSAID, and acetaminophen use and the risk of endometrial cancer. Cancer Res 68:2507–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Moysich KB, Baker JA, Rodabaugh KJ, Villella JA (2005) Regular analgesic use and risk of endometrial cancer. Cancer Epidemiol Biomarkers Prev 14:2923–2928. [DOI] [PubMed] [Google Scholar]
- 80. Prizment AE, Folsom AR, Anderson KE (2010) Nonsteroidal anti-inflammatory drugs and risk for ovarian and endometrial cancers in the Iowa Women's Health Study. Cancer Epidemiol Biomarkers Prev 19:435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bodelon C, Doherty JA, Chen C, Rossing MA, Weiss NS (2009) Use of nonsteroidal antiinflammatory drugs and risk of endometrial cancer. Am J Epidemiol 170:1512–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gallicchio L, Visvanathan K, Burke A, Hoffman SC, Helzlsouer KJ (2007) Nonsteroidal anti-inflammatory drugs and the risk of developing breast cancer in a population-based prospective cohort study in Washington County, MD. Int J Cancer 121:211–215. [DOI] [PubMed] [Google Scholar]
- 83. Jacobs EJ, Rodriguez C, Mondul AM, Connell CJ, Henley SJ, et al. (2005) A large cohort study of aspirin and other nonsteroidal anti-inflammatory drugs and prostate cancer incidence. J Natl Cancer Inst 97:975–980. [DOI] [PubMed] [Google Scholar]
- 84. Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, et al. (2011) Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet 377:31–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. Data will be made available upon request. This study involved human subjects and to protect the privacy of study participants, data requests will be reviewed by the NIH-AARP study team. Requests for data related to this PLoS publication should be directed to dietandhealth@mail.nih.gov. Interested researchers may also visit the study website for information about the cohort and available study resources. The study website is: http://dietandhealth.cancer.gov/index.html.