Abstract
Several lines of evidence implicate dysfunction of the serotonin (5-HT) system in autism spectrum disorder (ASD). Specifically, the serotonin transporter (5-HTT, SERT) has been scrutinized as an ASD candidate risk gene. SERT plays key roles in the development of circuits that underlie sensory function, particularly in the somatosensory system. One previous study in ASD found association of a rare, hyperfunctional SERT variant with sensory aversion, but studies of common SERT variants have never examined sensory symptoms in ASD. Using standardized caregiver assessments of sensory function in children, we evaluated patterns of sensory responsiveness in 47 children with ASD and 38 typically developing (TD) children. Study participants were genotyped for the functional SERT promoter polymorphisms, 5-HTTLPR and rs25531, to test the hypothesis that the higher expressing genotypes would be associated with hyperresponsiveness to touch, a common sensory aversion in ASD. All measures of sensory hypo- and hyperresponsiveness were increased in children with ASD, with hyporesponsive sensory patterns negatively correlated to age and hyperresponsive sensory patterns positively correlated to repetitive behavior. Strikingly, high-expressing SERT genotypes were associated with increased tactile hyperresponsiveness in the ASD group. Our findings indicate genetic variation that increases SERT function may specifically impact somatosensory processing in ASD.
Keywords: SERT, tactile hyperresponsiveness, sensory processing, autism spectrum disorder
1. Introduction
Although autism spectrum disorder (ASD) has been traditionally defined by social communication impairments and restricted, repetitive behavior, there is a growing appreciation that a significant proportion of individuals with ASD also exhibit sensory dysfunction (Crane, Goddard, & Pring, 2009; Leekam, Nieto, Libby, Wing, & Gould, 2007). Studies of sensory function in ASD have primarily focused on broad patterns of sensory behavior, which have been categorized as hyper- and hyporesponsiveness. While several reports indicate that hyporesponsiveness to sensory stimuli is highly prevalent in children with ASD and correlates strongly with clinical features (Foss-Feig, Heacock, & Cascio, 2012), it remains unclear whether patterns of hyperresponsive sensory behaviors are a distinctive ASD characteristic (Baranek, David, Poe, Stone, & Watson, 2006; Ben-Sasson et al., 2009; Rogers & Ozonoff, 2005; Tavassoli, Hoekstra, & Baron-Cohen, 2014; Watson et al., 2011). To clarify these findings, recent investigations of sensory processing have also analyzed patterns of behavior across multiple sensory modalities (Kern et al., 2006; Little et al., 2011; O’Riordan & Passetti, 2006; Tomchek & Dunn, 2007; L. D. Wiggins, Robins, Bakeman, & Adamson, 2009). These studies report distinct differences in auditory, visual, and tactile processing in ASD, but the general patterns of sensory dysfunction are heterogeneous, with each modality exhibiting varying degrees of impairment. While variation in sensory behavior documented in ASD may reflect differences in study methodology and demographics, heterogeneity in sensory processing could also indicate phenotypically and genetically distinct subpopulations of affected individuals.
One of the most replicated findings in ASD is elevated whole blood serotonin (5-HT) levels, termed hyperserotonemia, in approximately 30% of children (Gabriele, Sacco, & Persico, 2014; Mulder et al., 2004). Due to its central role as a regulator of 5-HT homeostasis in the platelet as well as at the serotonergic synapse in the brain, the serotonin transporter (5-HTT, SERT) has been extensively studied as a candidate ASD risk gene. In particular, the impact of SERT function on human behavior has been scrutinized in the context of the SLC6A4 promoter polymorphism, 5-HTTLPR, a variable tandem repeat polymorphism consisting of two primary alleles, the short allele and higher-expressing long allele (Heils et al., 1996; Lesch et al., 1996). More recent 5-HTTLPR studies have also taken into account the functional effects of a single nucleotide polymorphism (SNP), rs25531, which modulates long allele SERT expression (Hu et al., 2005). While consistent 5-HTTLPR/rs25531 associations with anxiety and affective behavior have been reported in the general population (Canli & Lesch, 2007), there have been inconsistent relationships found with the core behavioral features in ASD (Brune et al., 2006; Cook & Leventhal, 1996; Devlin et al., 2005; Mulder et al., 2004; Tordjman et al., 2001).
In addition to its role as a neurotransmitter, 5-HT is an important signaling molecule during neurodevelopment. Whereas SERT expression in the adult brain is limited to midbrain serotonergic neurons, the transporter is transiently expressed in a number of brain regions during neurodevelopment, including multiple areas involved in sensory processing (Gaspar, Cases, & Maroteaux, 2003). Perinatal SERT function plays a key role in the topographical organization of cortical sensory maps, most notably rodent barrel field architecture in primary somatosensory cortex (Lebrand et al., 1996; Salichon et al., 2001). Changes in the somatotopic organization of primary somatosensory cortex have been described in individuals with ASD, but their relationship with altered 5-HT signaling is unknown (Coskun et al., 2009). In families with evidence for genetic linkage of autism to SLC6A4, the rare SERT Ala56 variant, which leads to increased and dysregulated SERT function, was associated with sensory aversion (Sutcliffe et al., 2005). While sensory aversion commonly comprises tactile hyperresponsiveness (Rogers, Hepburn, & Wehner, 2003; Tomchek & Dunn, 2007), specific sensory modalities were not evaluated in affected carriers of the Ala56 allele. Collectively, these findings suggest that enhanced SERT function could play a specific role in tactile hyperresponsiveness in ASD.
To test the hypothesis that higher-expressing SERT alleles are associated with tactile hypersensitivity, we evaluated sensory behavior in children with ASD and age-matched controls that were also genotyped for the 5-HTTLPR and rs25531 polymorphisms. Our findings provide further evidence of heterogeneous patterns of sensory disturbances in ASD, which exhibit previously noted relationships with age and repetitive behavior. In addition, we report that measures of tactile hyperresponsiveness are uniquely associated with high-expressing SERT genotypes in affected individuals.
2. Methods and materials
2.1 Participants
Participants were 47 (40 male) children with ASD and 38 (33 male) typically developing controls ages 4–10. Diagnosis of ASD was confirmed by research-reliable administration of the Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 2000) and the Autism Diagnostic Interview-Revised (ADI-R) (Lord, Rutter, & Le Couteur, 1994), as well as clinical diagnosis by a licensed clinical psychologist specializing in ASD. Children in the control group did not have any psychiatric, learning or behavioral diagnoses, did not have a first degree relative with ASD, and did not have elevated scores on an ASD symptom screening measure, the Social Communication Questionnaire (SCQ) (Rutter, Bailey, & Lord, 2003). IQ was assessed for all participants with either the Kaufman Brief Intelligence Test, Second Edition (KBIT-2) (Kaufman & Kaufman, 2004) or the Weschler Abbreviated Scales of Intelligence (WASI) (Weschsler, 1999). When participants were unable to complete these measures due to poor language skills, the Mullen Scales of Early Learning (MSEL) (Mullen, 1995) was used to assess cognitive ability; however IQ was not able to be calculated because those individuals did not fall within the normed age range for this measure. Thus, IQ information was missing for four participants with ASD tested using the MSEL. Parents gave informed consent and participants gave informed assent, when able. Procedures were approved by Vanderbilt Institutional Review Board. See Table 1 for participant characteristics.
Table 1.
Participant Characteristicsa
| n | Age (yrs) Mean(SD) |
% Male | % Caucasian | FSIQ Mean (SD) |
|
|---|---|---|---|---|---|
|
ASD
| |||||
| High SERT | 17 | 7.00 (1.8) | 76.5 | 88.2 | 105.86 (19.6) |
| Low SERT | 30 | 7.60 (1.5) | 90 | 96.7 | 98.93 (18.0) |
|
TD
| |||||
| High SERT | 9 | 6.89 (1.6) | 100 | 88.9 | 115.22 (12.0) |
| Low SERT | 29 | 7.45 (1.7) | 82.8 | 79.3 | 110.59 (16.74) |
Age, gender, race and IQ for participants in each diagnostic group and genotype group. ASD participants did not differ from TD participants on age, gender, or race. ASD participants had significantly lower IQ scores than TD participants. Within each diagnostic group, participants in the high and low SERT expressing genotypes group did not differ on any of these variables. (ASD = autism spectrum disorder; TD = typically developing; FSIQ = full scale IQ)
2.2 5-HTTLPR/rs25531 genotyping
5-HTTLPR and rs25531 SNP genotyping were performed as previously described with the following modifications (Dickel et al., 2007). Saliva samples were collected from each study participant using the Oragene DNA Collection Kit (DNA Genotek Inc., Kanata, Ontario, Canada). Forward primer, 5′-GGCGTTGCCGCTCTGAATGC-3′, and reverse primer, 5′-AGGGACTGAGCTGGACAACCAC-3′, were used for PCR amplification. The rs25531 SNP was genotyped by digesting 5-HTTLPR PCR product with HpaII, a restriction enzyme that cuts at the SNP site only if the G allele is present. To determine genotypes, 5-HTTLPR amplicons and HpaII digest products were analyzed by agarose gel electrophoresis. The resulting genotypes were combined to create composite 5-HTTLPR alleles (i.e. LA, LG, SA, SG). Individuals were categorized into two genotype comparison groups for statistical analysis: high SERT expressing genotypes (LA/LA) and low SERT expressing genotypes (LA/LG, LA/SA, LG/SA, and SA/SA).
2.3 Parent Questionnaires – Sensory Behaviors
Caregivers of study participants completed two sensory questionnaires about their child’s current sensory behaviors, the Sensory Profile (SP) (Dunn, 1999) and Sensory Experience Questionnaire (SEQ) (Baranek et al., 2006). The SP is a 125-item questionnaire measuring a child’s sensory processing abilities across a variety of sensory modalities. The SEQ consists of 41 questions and was designed specifically to evaluate patterns of sensory processing in children with ASD. Both questionnaires ask caregivers to rate their child’s behavior on a 5-point Likert scale.
To achieve the most comprehensive representation of each participant’s sensory behavior, items from both questionnaires were used to calculate composite hypo- and hyper-responsiveness variables for tactile, visual, and auditory processing. Items used for the hyperresponsiveness variables were the low threshold items from the SP and the hyperresponsive items from the SEQ. Composite hyporesponsiveness variables were created from the high threshold items from the SP and the hyporesponsive items from the SEQ. Items from the SP were reversed scored such that higher values represent greater sensory impairment, consistent with scoring on the SEQ. Within each composite variable, items that were identical in content (n=2) or were not significantly correlated (n=5) with other included items were dropped. Each composite variable had, at most, two items excluded. The composite variable score was then averaged from the remaining items. Composite scores contained 4 to 14 items, with the auditory hyperresponsiveness score having the fewest and the tactile hyperrepsonsiveness score having the most items. Item-to-total composite score Spearman Rank correlation coefficient ranged from .38–.88 (see supplemental table).
2.4 Parent Questionnaire – Repetitive Behaviors
Parents in the ASD group completed the Repetitive Behavior Scale – Revised (RBS-R) (Lam & Aman, 2007), a questionnaire about their child’s repetitive behaviors. The RBS-R contains 43 items that comprise six subscales corresponding to common repetitive behavior patterns in ASD. Parents rate each item on a scale from 0–3 based on how problematic each behavior is for their child. A total score, including all 43 items, was also calculated.
2.5 Analysis
Univariate ANOVAs were conducted to examine the effects of diagnostic group on the composite sensory variables. A t-test was used to test the primary hypothesis, that the high-expressing genotype would be associated with somatosensory hyperresponsiveness within children with ASD. Exploratory two-way ANOVAs were conducted to examine possible interactions between diagnostic group and genotype. A correlation analysis with age and the six composite sensory variables was also performed to examine how age uniquely affects these sensory behaviors. Finally, relationships between the sensory variables and the total score from the RBS-R were explored using Pearson correlations. To further explore significant effects, additional correlations between the sensory variable and the six subscales of the RBS-R were conducted in order to determine the specific kind of repetitive behavior that was related to the sensory behavior. All statistical analysis was performed using SPSS Version 21.
3. Results
3.1 Participant Characterization
Participants with ASD did not differ from control participants on age (t(83) = .188, p > .1), gender (χ2 = .05, p > .1), or race (χ2 = 4.69, p > .1). Participants with ASD had significantly lower full scale IQ (FSIQ) scores than controls (t(79) = 2.7, p < .01). 5-HTTLPR (χ2 = .099, df = 2, p = .95) and rs25531 (χ2 = 0, df = 2, p = 1) were in Hardy-Weinberg equilibrium in our total sample population. Within each diagnostic group, individuals in the high expressing versus low expressing SERT groups did not differ in age, gender, race, or FSIQ, all p’s > .1.
3.2 Sensory variables and genetic analysis
All six univariate ANOVAs with each composite sensory variable as the dependent variable revealed a main effect of diagnostic group, all p’s < .001, confirming more aberrant sensory behaviors across modalities and processing patterns in children with ASD. The primary analysis revealed a significant difference in tactile hyperresponsiveness by genotype within the ASD group (t(45) = 2.07, p < .05). These analyses were repeated including only the Caucasian participants. All ANOVA results remained highly significant, all p’s < .001, and the effect of genotype for tactile hyperresponsiveness in the ASD group remained (t(42) = 2.05, p < .05), suggesting these effects are not due to race. No other sensory domain showed a significant association with SERT genotype in the ASD group. Two-way ANOVA revealed a trend for an interaction between diagnostic and genotype group that was unique to tactile hyperresponsiveness, F(1,81) = 2.87, p = .094. There were no main effects of genotype group on any of the composite sensory variables across both diagnostic groups, all p’s > .1. Visual inspection of group means suggests that children with ASD exhibit more tactile hyperresponsive behavior patterns; however, those with ASD and high expressing SERT show these behaviors to a greater degree (see Figure 1).
Figure 1. Tactile hyperresponsiveness in children with and without ASD, with high and low expressing SERT genotypes.
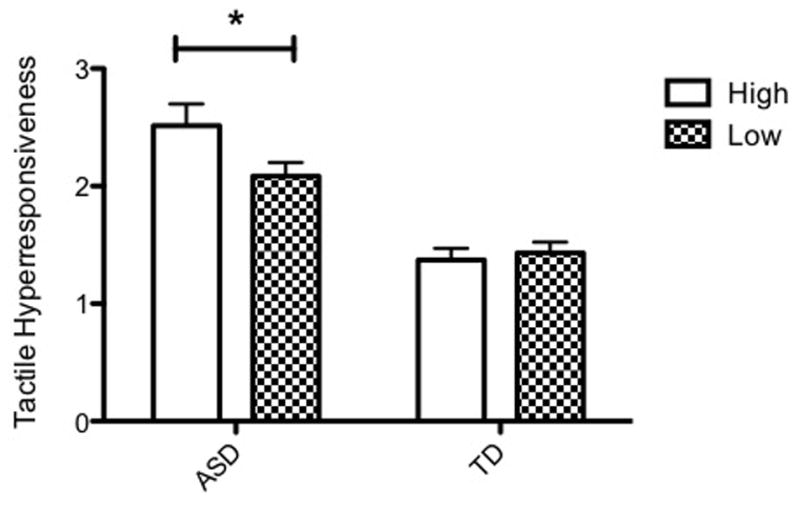
In ASD group, children with high-expressing SERT genotypes display increased tactile hyperresponsiveness compared to children with low-expressing SERT genotypes (t(45) = 2.07, p < .05). SERT genotype effects on tactile hyperresponsiveness were absent in TD children.
3.3 Sensory variables and age/IQ analysis
The six composite sensory variables were correlated with age and FSIQ separately in each group. There were no significant correlations in either group with any of the sensory variables and FSIQ. There were no significant relationships between age and any of the sensory variables in the TD control group. In the ASD group, age was negatively correlated with sensory hyporesponsiveness variables, with older individuals displaying fewer sensory behaviors (Table 2).
Table 2.
Correlations between sensory variables and age.b
| ASD | TD | ||
|---|---|---|---|
|
| |||
| Age | |||
|
|
|||
| Auditory | Hyper- | −0.134 | 0.058 |
| Hypo- | −0.298* | −0.305 | |
|
|
|||
| Visual | Hyper- | −0.198 | −0.075 |
| Hypo- | −0.308* | −0.087 | |
|
|
|||
| Tactile | Hyper- | −0.126 | −0.211 |
| Hypo- | −0.385** | −0.233 | |
|
|
|||
| Overall | Hyper- | −0.179 | −0.082 |
| Hypo- | −0.409** | −0.232 | |
|
|
|||
Age was significantly correlated with hyporesponsive patterns in all sensory modalities, in which older individuals showed fewer hyporesponsive sensory behaviors.
p < .05;
p < .01
3.4 Sensory variables and repetitive behaviors
Visual inspection of the data revealed one extreme outlier (greater than 3 SD above the mean) on the RBS-R. After excluding this outlier, the six composite sensory variables were correlated with the overall score from the RBS-R in the ASD group only. Repetitive behaviors were positively related to all sensory scores. This relationship was significant for tactile (r = .58, p < .001) and auditory (r = .51, p < .01) hyperresponsiveness, with the tactile domain showing the strongest correlation to repetitive behaviors (Table 3). Additional correlations between tactile and auditory hyperresponsiveness and the subscales of the RBS-R revealed strongest relationships with the sameness and restricted behaviors subscales. See Table 3 for all correlation coefficients. Analyses including the single outlier revealed similar results; however, correlations with the repetitive behavior score and both visual hyper- and hypo- responsiveness also became significant. Effects of genotype on repetitive behaviors were also explored revealing no significant effects.
Table 3.
Correlations between sensory variables and RBS-R overall/subscale scores.c
| A | ||
|---|---|---|
| RBS-R Overall Score | ||
|
|
||
| Auditory | Hyper- | 0.392* |
| Hypo- | 0.078 | |
|
|
||
| Visual | Hyper- | 0.236 |
| Hypo- | 0.295 | |
|
|
||
| Tactile | Hyper- | 0.582*** |
| Hypo- | 0.266 | |
|
|
||
| Overall | Hyper- | 0.512** |
| Hypo- | 0.261 | |
|
|
||
| B | ||
|---|---|---|
| Auditory Hyper- | Tactile Hyper- | |
| RBS-R Subscale | ||
| Stereotyped | 0.14 | 0.40* |
| Self-Injurious | 0.18 | 0.29 |
| Compulsive | 0.27 | 0.40* |
| Ritualistic | 0.21 | 0.33 |
| Sameness | 0.44** | 0.65*** |
| Restricted | 0.51** | 0.52** |
A) Overall repetitive behavior score as measured by the RBS-R was significantly positively correlated with hyperresponsive patterns in the auditory and tactile modalities. B) Auditory and tactile hyperresponsive behaviors were positively related to all subscales of the RBS-R, with the sameness and restricted behaviors scales showing the strongest relationships. (RBS-R = Repetitive Behavior Scale – Revised)
p < .05;
p < .01;
p < .001
4. Discussion
To our knowledge, this is the first study to examine the influence of SERT genetic variation on specific patterns of sensory behavior. While all measures of auditory, visual, and tactile processing impairment were significantly increased in children with ASD compared to TD participants, only patterns of tactile hyperresponsive behavior in ASD exhibited a relationship with SERT genotype. Specifically, we found that tactile hyperresponsiveness scores were significantly increased in affected individuals with high SERT expressing genotypes, matching our primary hypothesis.
The relationships of age and repetitive behaviors with sensory processing patterns in our population were supported by previous literature. Although ASD is associated with sensory dysfunction throughout life (Crane et al., 2009), the degree of sensory impairment appears to decrease with age (Ben-Sasson et al., 2009; Kern et al., 2006). Our data matches this trend; however, only hyporesponsive, and not hyperresponsive, behaviors decrease within the 4–10 year age range in our study, suggesting these behaviors may be more malleable early in life.
Whereas age was more related to hyporesponsive behavior patterns, repetitive behaviors were related to hyperresponsive patterns, consistent with a prior study in a similar age demographic (Boyd et al., 2010). Furthermore, we found that repetitive behaviors, specifically sameness and restricted behavior subscales, were most strongly associated with tactile hyperresponsiveness in ASD. However, in contrast to tactile hyperresponsiveness, RBS-R scores were not associated with SERT genotype. While a link between common SERT polymorphisms and repetitive behavior in ASD has remained elusive, the rare gain-of-function SERT Ala56 variant is associated with ADI-R measures of rigid-compulsive behavior in addition to sensory aversion (Sutcliffe et al., 2005). Interestingly, due to the relationship between sensory hyperresponsiveness and repetitive behavior, it has been proposed that these behaviors may share common neurobiological mechanisms (Baranek, Foster, & Berkson, 1997; Boyd et al., 2010; Chen, Rodgers, & McConachie, 2009). Consequently, it is possible that enhanced SERT function may impact shared brain circuits that underlie tactile hyperresponsiveness and repetitive behavior in ASD.
While our work is the first to implicate SERT genetic variation in tactile function in ASD, there are some notable limitations in our study design. First, our sample size gave us limited statistical power to detect genotype/phenotype relationships. In addition, children with ASD in our study were generally high-functioning, which prohibits us from generalizing our findings to more impaired individuals. Altered patterns of sensory behavior are reported to be more prevalent in low-functioning cases of ASD (Patten, Ausderau, Watson, & Baranek, 2013; Watson et al., 2011), potentially further limiting our power. Furthermore, the SERT promoter polymorphisms are, at best, an indirect assessment of SERT expression and function, hindering our ability to directly test the effects of SERT on sensory functioning. Beyond genotype analyses, it would be of great interest to examine the relationship between sensory behavior and whole blood 5-HT levels in ASD, which are partially influenced by SERT genetic variation (Anderson et al., 2002; Coutinho et al., 2004; Cross et al., 2008). Finally, whereas common variation in a single gene is unlikely to significantly impact subjective measures of human behavior, gene variants of modest effect size may have stronger relationships with endophenotypes: heritable, biological measures associated with a disease state. Using this approach, some ASD neuroimaging studies have had success relating changes in brain structure and activity to SERT function (Wassink et al., 2007; J. L. Wiggins et al., 2012). To date, there have been few empirical studies of tactile processing in ASD (Blakemore et al., 2006; Cascio et al., 2008; Cascio et al., 2012; Coskun et al., 2009; Marco et al., 2012; O’Riordan & Passetti, 2006; Tommerdahl, Tannan, Cascio, Baranek, & Whitsel, 2007), with a limited understanding of the biological mechanisms underlying sensory dysfunction.
In conclusion, the current study highlights a unique association in ASD between high-expressing SERT genotypes and tactile hyperresponsiveness, as measured by parent report. Supplementary analyses with age and repetitive behaviors, factors known to influence sensory behaviors, replicated previous findings in the literature and specified that age is more related to hyporesponsive patterns and repetitive behaviors are more related to hyperresponsive patterns, particularly in the tactile domain. These findings provide an initial foundation to explore the biological mechanisms underlying abnormal sensory behavior in ASD. Previous attempts to relate 5-HT system dysfunction to specific ASD behavioral impairments, such as repetitive behaviors, have produced inconsistent results, but evaluating a potential relationship between 5-HT and sensory processing may be able to connect variation in this key system and its neurodevelopmental sequelae. While our present findings point to a relationship between SERT and tactile processing deficits, future studies will be needed to connect more direct measures of SERT expression and function with aberrant sensory behavior and somatosensory neural circuitry.
Supplementary Material
Highlights.
Genotypic variation in SERT was associated with tactile hyperresponsiveness in ASD
Age was negatively correlated with hyporesponsive sensory behaviors
Repetitive behaviors were related to hyperresponsive sensory behaviors
Acknowledgments
This work was supported by NIH grants MH094604 and K01MH090232, Landreth Family Discovery Grant, and UL1 TR000445 from NCATS/NIH..
Glossary
- 5-HT
serotonin
- ASD
autism spectrum disorders
- SERT
serotonin transporter
- SEQ
Sensory Experience Questionnaire
- SP
Sensory Profile
- TD
typically developing
- SNP
single nucleotide polymorphism
- ADOS
Autism Diagnostic Observation Schedule
- ADI-R
Autism Diagnostic Interview-Revised
- SCQ
Social Communication Questionnaire
- WASI
Weschler Abbreviated Scale of Intelligence
- KBIT-2
Kaufman Brief Intelligence Test, 2nd edition
- MSEL
Mullen Scales of Early Learning
- RBS-R
Repetitive Behavior Scale – Revised
- FSIQ
full scale IQ
Footnotes
Competing Interests:
JV has served on advisory boards or consulted with Roche Pharmaceuticals, Novartis, and SynapDx. He has received research funding from Roche Pharmaceuticals, Novartis, SynapDx, Seaside Therapeutics, Forest, and Sunovion.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kimberly B. Schauder, Email: kimberly.schauder@rochester.edu.
Christopher L. Muller, Email: christopher.l.muller@vanderbilt.edu.
Jeremy Veenstra-VanderWeele, Email: veenstr@nyspi.columbia.edu.
Carissa J. Cascio, Email: carissa.cascio@vanderbilt.edu.
References
- Anderson GM, Gutknecht L, Cohen DJ, Brailly-Tabard S, Cohen JH, Ferrari P, Tordjman S. Serotonin transporter promoter variants in autism: functional effects and relationship to platelet hyperserotonemia. Mol Psychiatry. 2002;7(8):831–836. doi: 10.1038/sj.mp.4001099. [DOI] [PubMed] [Google Scholar]
- Baranek GT, David FJ, Poe MD, Stone WL, Watson LR. Sensory Experiences Questionnaire: discriminating sensory features in young children with autism, developmental delays, and typical development. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Journal of Child Psychology and Psychiatry. 2006;47(6):591–601. doi: 10.1111/j.1469-7610.2005.01546.x. [DOI] [PubMed] [Google Scholar]
- Baranek GT, Foster LG, Berkson G. Tactile defensiveness and stereotyped behaviors. Am J Occup Ther. 1997;51(2):91–95. doi: 10.5014/ajot.51.2.91. [DOI] [PubMed] [Google Scholar]
- Ben-Sasson A, Hen L, Fluss R, Cermak SA, Engel-Yeger B, Gal E. A meta-analysis of sensory modulation symptoms in individuals with autism spectrum disorders. [Meta-Analysis Research Support, Non-U.S. Gov’t] Journal of Autism and Developmental Disorders. 2009;39(1):1–11. doi: 10.1007/s10803-008-0593-3. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Tavassoli T, Calo S, Thomas RM, Catmur C, Frith U, Haggard P. Tactile sensitivity in Asperger syndrome. Brain and Cognition. 2006;61(1):5–13. doi: 10.1016/j.bandc.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Boyd BA, Baranek GT, Sideris J, Poe MD, Watson LR, Patten E, Miller H. Sensory features and repetitive behaviors in children with autism and developmental delays. [Research Support, N.I.H., Extramural] Autism Research. 2010;3(2):78–87. doi: 10.1002/aur.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune CW, Kim SJ, Salt J, Leventhal BL, Lord C, Cook EH., Jr 5-HTTLPR Genotype-Specific Phenotype in Children and Adolescents With Autism. American Journal of Psychiatry. 2006;163(12):2148–2156. doi: 10.1176/ajp.2006.163.12.2148. [DOI] [PubMed] [Google Scholar]
- Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nature Neuroscience. 2007;10(9):1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Cascio CJ, McGlone F, Folger S, Tannan V, Baranek G, Pelphrey KA, Essick G. Tactile perception in adults with autism: a multidimensional psychophysical study. Journal of Autism and Developmental Disorders. 2008;38(1):127–137. doi: 10.1007/s10803-007-0370-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio CJ, Moana-Filho EJ, Guest S, Nebel MB, Weisner J, Baranek GT, Essick GK. Perceptual and neural response to affective tactile texture stimulation in adults with autism spectrum disorders. Autism Research. 2012;5(4):231–244. doi: 10.1002/aur.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Rodgers J, McConachie H. Restricted and repetitive behaviours, sensory processing and cognitive style in children with autism spectrum disorders. J Autism Dev Disord. 2009;39(4):635–642. doi: 10.1007/s10803-008-0663-6. [DOI] [PubMed] [Google Scholar]
- Cook EH, Leventhal BL. The serotonin system in autism. Current Opinion in Pediatrics. 1996;8(4):348–354. doi: 10.1097/00008480-199608000-00008. [DOI] [PubMed] [Google Scholar]
- Coskun MA, Varghese L, Reddoch S, Castillo EM, Pearson DA, Loveland KA, Sheth BR. How somatic cortical maps differ in autistic and typical brains. Neuroreport. 2009;20(2):175–179. doi: 10.1097/WNR.0b013e32831f47d1. [DOI] [PubMed] [Google Scholar]
- Coutinho AM, Oliveira G, Morgadinho T, Fesel C, Macedo TR, Bento C, Vicente AM. Variants of the serotonin transporter gene (SLC6A4) significantly contribute to hyperserotonemia in autism. Mol Psychiatry. 2004;9(3):264–271. doi: 10.1038/sj.mp.4001409. [DOI] [PubMed] [Google Scholar]
- Crane L, Goddard L, Pring L. Sensory processing in adults with autism spectrum disorders. [Comparative Study Research Support, Non-U.S. Gov’t] Autism. 2009;13(3):215–228. doi: 10.1177/1362361309103794. [DOI] [PubMed] [Google Scholar]
- Cross S, Kim SJ, Weiss LA, Delahanty RJ, Sutcliffe JS, Leventhal BL, Veenstra-Vanderweele J. Molecular genetics of the platelet serotonin system in first-degree relatives of patients with autism. Neuropsychopharmacology. 2008;33(2):353–360. doi: 10.1038/sj.npp.1301406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin B, Cook EH, Jr, Coon H, Dawson G, Grigorenko EL, McMahon W, Network CG. Autism and the serotonin transporter: the long and short of it. Mol Psychiatry. 2005;10(12):1110–1116. doi: 10.1038/sj.mp.4001724. [DOI] [PubMed] [Google Scholar]
- Dickel DE, Veenstra-VanderWeele J, Bivens NC, Wu X, Fischer DJ, Van Etten-Lee M, Hanna GL. Association studies of serotonin system candidate genes in early-onset obsessive-compulsive disorder. Biological Psychiatry. 2007;61(3):322–329. doi: 10.1016/j.biopsych.2006.09.030. [DOI] [PubMed] [Google Scholar]
- Dunn W. The Sensory Profile Manual. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Foss-Feig JH, Heacock JL, Cascio CJ. Tactile Responsiveness Patterns and Their Association with Core Features in Autism Spectrum Disorders. Research in Autism Spectrum Disorders. 2012;6(1):337–344. doi: 10.1016/j.rasd.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriele S, Sacco R, Persico AM. Blood serotonin levels in autism spectrum disorder: a systematic review and meta-analysis. European Neuropsychopharmacology. 2014;24(6):919–929. doi: 10.1016/j.euroneuro.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nature Reviews Neuroscience. 2003;4(12):1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66(6):2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcoholism: Clinical and Experimental Research. 2005;29(1):8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. KBIT2: Kaufman Brief Intelligence Test. 2. Circle Pines MN: AGS Publishing; 2004. [Google Scholar]
- Kern JK, Trivedi MH, Garver CR, Grannemann BD, Andrews AA, Savla JS, Schroeder JL. The pattern of sensory processing abnormalities in autism. [Research Support, Non-U.S. Gov’t] Autism. 2006;10(5):480–494. doi: 10.1177/1362361306066564. [DOI] [PubMed] [Google Scholar]
- Lam KS, Aman MG. The Repetitive Behavior Scale-Revised: independent validation in individuals with autism spectrum disorders. [Validation Studies] Journal of Autism and Developmental Disorders. 2007;37(5):855–866. doi: 10.1007/s10803-006-0213-z. [DOI] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Adelbrecht C, Doye A, Alvarez C, El Mestikawy S, Gaspar P. Transient uptake and storage of serotonin in developing thalamic neurons. Neuron. 1996;17(5):823–835. doi: 10.1016/s0896-6273(00)80215-9. [DOI] [PubMed] [Google Scholar]
- Leekam SR, Nieto C, Libby SJ, Wing L, Gould J. Describing the sensory abnormalities of children and adults with autism. Journal of Autism and Developmental Disorders. 2007;37(5):894–910. doi: 10.1007/s10803-006-0218-7. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Little LM, Freuler AC, Houser MB, Guckian L, Carbine K, David FJ, Baranek GT. Psychometric validation of the Sensory Experiences Questionnaire. American Journal of Occupational Therapy. 2011;65(2):207–210. doi: 10.5014/ajot.2011.000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Marco EJ, Khatibi K, Hill SS, Siegel B, Arroyo MS, Dowling AF, Nagarajan SS. Children with autism show reduced somatosensory response: an MEG study. Autism Research. 2012;5(5):340–351. doi: 10.1002/aur.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder EJ, Anderson GM, Kema IP, de Bildt A, van Lang ND, den Boer JA, Minderaa RB. Platelet serotonin levels in pervasive developmental disorders and mental retardation: diagnostic group differences, within-group distribution, and behavioral correlates. Journal of American Acadamy of Child and Adolescent Psychiatry. 2004;43(4):491–499. doi: 10.1097/00004583-200404000-00016. [DOI] [PubMed] [Google Scholar]
- Mullen E. Mullen scales of early learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- O’Riordan M, Passetti F. Discrimination in autism within different sensory modalities. Journal of Autism and Developmental Disorders. 2006;36(5):665–675. doi: 10.1007/s10803-006-0106-1. [DOI] [PubMed] [Google Scholar]
- Patten E, Ausderau KK, Watson LR, Baranek GT. Sensory Response Patterns in Nonverbal Children with ASD. Autism Research and Treatment. 2013;2013:436286. doi: 10.1155/2013/436286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Hepburn S, Wehner E. Parent reports of sensory symptoms in toddlers with autism and those with other developmental disorders. Journal of Autism and Developmental Disorders. 2003;33(6):631–642. doi: 10.1023/b:jadd.0000006000.38991.a7. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Ozonoff S. Annotation: what do we know about sensory dysfunction in autism? A critical review of the empirical evidence. Journal of Child Psychology and Psychiatry. 2005;46(12):1255–1268. doi: 10.1111/j.1469-7610.2005.01431.x. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. The social communication questionnaire. Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- Salichon N, Gaspar P, Upton AL, Picaud S, Hanoun N, Hamon M, Seif I. Excessive activation of serotonin (5-HT) 1B receptors disrupts the formation of sensory maps in monoamine oxidase a and 5-ht transporter knock-out mice. Journal of Neuroscience. 2001;21(3):884–896. doi: 10.1523/JNEUROSCI.21-03-00884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JS, Delahanty RJ, Prasad HC, McCauley JL, Han Q, Jiang L, Blakely RD. Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors. American Journal of Human Genetics. 2005;77(2):265–279. doi: 10.1086/432648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavassoli T, Hoekstra RA, Baron-Cohen S. The Sensory Perception Quotient (SPQ): development and validation of a new sensory questionnaire for adults with and without autism. Molecular Autism. 2014;5:29. doi: 10.1186/2040-2392-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchek SD, Dunn W. Sensory processing in children with and without autism: a comparative study using the short sensory profile. American Journal of Occupational Therapy. 2007;61(2):190–200. doi: 10.5014/ajot.61.2.190. [DOI] [PubMed] [Google Scholar]
- Tommerdahl M, Tannan V, Cascio CJ, Baranek GT, Whitsel BL. Vibrotactile adaptation fails to enhance spatial localization in adults with autism. Brain Research. 2007;1154:116–123. doi: 10.1016/j.brainres.2007.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordjman S, Gutknecht L, Carlier M, Spitz E, Antoine C, Slama F, Anderson GM. Role of the serotonin transporter gene in the behavioral expression of autism. Molecular Psychiatry. 2001;6(4):434–439. doi: 10.1038/sj.mp.4000873. [DOI] [PubMed] [Google Scholar]
- Wassink TH, Hazlett HC, Epping EA, Arndt S, Dager SR, Schellenberg GD, Piven J. Cerebral cortical gray matter overgrowth and functional variation of the serotonin transporter gene in autism. Archives of General Psychiatry. 2007;64(6):709–717. doi: 10.1001/archpsyc.64.6.709. [DOI] [PubMed] [Google Scholar]
- Watson LR, Patten E, Baranek GT, Poe M, Boyd BA, Freuler A, Lorenzi J. Differential associations between sensory response patterns and language, social, and communication measures in children with autism or other developmental disabilities. Journal of Speech, Language, and Hearing Research. 2011;54(6):1562–1576. doi: 10.1044/1092-4388(2011/10-0029). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschsler D. WASI: Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Harcourt Assessment, Inc; 1999. [Google Scholar]
- Wiggins JL, Peltier SJ, Bedoyan JK, Carrasco M, Welsh RC, Martin DM, Monk CS. The impact of serotonin transporter genotype on default network connectivity in children and adolescents with autism spectrum disorders. Neuroimage: Clinical. 2012;2:17–24. doi: 10.1016/j.nicl.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins LD, Robins DL, Bakeman R, Adamson LB. Brief report: sensory abnormalities as distinguishing symptoms of autism spectrum disorders in young children. Journal of Autism and Developmental Disorders. 2009;39(7):1087–1091. doi: 10.1007/s10803-009-0711-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


