Abstract
Cerebral ischemia occurs when blood flow to the brain is insufficient to meet metabolic demand. This can result from cerebral artery occlusion that interrupts blood flow, limits CNS supply of oxygen and glucose, and causes an infarction/ischemic stroke. Ischemia initiates a cascade of molecular events inneurons and cerebrovascular endothelial cells including energy depletion, dissipation of ion gradients, calcium overload, excitotoxicity, oxidative stress, and accumulation of ions and fluid. Blood-brain barrier (BBB) disruption is associated with cerebral ischemia and leads to vasogenic edema, a primary cause of stroke-associated mortality. To date, only a single drug has received US Food and Drug Administration (FDA) approval for acute ischemic stroke treatment, recombinant tissue plasminogen activator (rt-PA). While rt-PA therapy restores perfusion to ischemic brain, considerable tissue damage occurs when cerebral blood flow is re-established. Therefore, there is a critical need for novel therapeutic approaches that can “rescue” salvageable brain tissue and/or protect BBB integrity during ischemic stroke. One class of drugs that may enable neural cell rescue following cerebral ischemia/reperfusion injury is the HMG-CoA reductase inhibitors (i.e., statins). Understanding potential CNS drug delivery pathways for statins is critical to their utility in ischemic stroke. Here, we review molecular pathways associated with cerebral ischemia and novel approaches for delivering drugs to treat ischemic disease. Specifically, we discuss utility of endogenous BBB drug uptake transporters such as organic anion transporting polypeptides (OATPs/Oatps) and nanotechnology-based carriers for optimization of CNS drug delivery. Overall, this chapter highlights state-of-the-art technologies that may improve pharmacotherapy of cerebral ischemia.
Keywords: blood-brain barrier, drug delivery, drug transporters, ischemic stroke, ischemia/reperfusion, nanoparticles, organic anion transporting polypeptide, reactive oxygen species, statins
1. Introduction
Pharmacological treatment of cerebral ischemia requires a detailed understanding of pathophysiological changes that occur in the brain and in the cerebral microvasculature following ischemia/reperfusion injury. Cerebral hypoxia and subsequent reoxygenation is a central component of several diseases, including traumatic brain injury, acute respiratory distress syndrome, obstructive sleep apnea, high-altitude cerebral edema and acute mountain sickness, cardiac arrest, and ischemic stroke (Ronaldson & Davis, 2013). Stroke is the fourth leading cause of death and is a major cause of long-term morbidity in the United States (Feng & Belagaje, 2013). Of all stroke cases, 87% are ischemic (Roger et al., 2011). Ischemic stroke results from restricted blood flow to a portion of the brain that causes an irreversibly damaged ischemic core and a surrounding region of potentially viable, yet functionally impaired brain tissue known as the penumbra (Astrup, Siesjo, & Symon, 1981; Liu, Levine, & Winn, 2010). A complex cascade of molecular events initiated by cerebral ischemia is responsible for the widespread necrosis observed in the ischemic core and apoptosis detected in the penumbra. Theoretically, the penumbra can be salvaged if reperfusion therapy and/or pharmacotherapy are administered early during the course of disease (Shah & Abbruscato, 2013). This therapeutic objective is underscored by challenges in delivering drugs to the ischemic brain. Here, we discuss pathological mechanisms associated with cerebral ischemia and associated hypoxia and how detailed knowledge of such processes can lead to cutting-edge approaches to deliver drugs to ischemic brain. In particular, we focus on targeting of endogenous blood-brain barrier (BBB) uptake transporters and utilization of nanoparticle delivery systems, two non-invasive chemical-based approaches that are highly promising for effective CNS drug delivery.
2. Pathophysiology of Ischemia
Physiologically, energy requirements of the CNS are met by brain uptake of glucose and oxygen, which are incorporated into metabolic pathways to enable phosphorylation of ADP to ATP. Cerebral ischemia results in reduction of molecular oxygen delivery to all CNS cell types within the core of the infarct zone. Lack of oxygen availability halts molecular shuttling of electrons in oxidative phosphorylation, which is essential for ATP generation. Most ATP generated within the brain is used for maintenance of intracellular homeostasis and transmembrane gradients for monovalent and divalent ions (i.e., Na+, K+, Ca2+) (Adibhatla & Hatcher, 2008). Energy depletion in neuronal cells causes ion gradient failure via cessation of ATP-dependent Na+/K+-ATPase and Ca2+-ATPase activity. When energy dependent ion extrusion is impeded, cations in extracellular fluid (i.e., Na+) follow a strong inwardly directed electrochemical gradient and accumulate within the cell. Uptake of Na+ is accompanied by influx of monovalent anions (i.e., Cl̄). Extracellular fluid then follows this net movement of ions resulting in cytotoxic edema. Additionally, Na+ ion uptake causes extensive plasma membrane depolarization, leading to opening of voltage-gated cation channels and reverses the direction of the Na+/Ca2+ exchanger, bringing additional Ca2+ into the cell (Kiedrowski, 2007; Luo et al., 2008). The widespread depolarization seen in ischemic neurons thwarts plasma membrane hyperpolarization, which is required to close and reactivate these cation channels.
Influx of Ca2+ prompts intracellular vesicles, containing glutamate or dopamine, to fuse with the neuronal presynaptic bout on membrane, releasing the neurotransmitters into the synapse. This uncontrolled increase in glutamate and dopamine concentrations is neurotoxic and leads to neuronal cell death and development of an infarction (i.e., ischemic stroke) (Adibhatla et al., 2008). Glutamate excitotoxicity coupled with cellular depolarization is particularly deleterious to the CNS due to overstimulation of metabotropic glutamate receptors as well as extensive activation of AMPA and NMDA receptors, resulting in disruption of CNS calcium homeostasis (Adibhatla, Hatcher, & Dempsey, 2006; Adibhatla, Hatcher, Larsen, et al., 2006; Arai et al., 2011). Energy reserves are quickly depleted in an effort to sequester increasing intracellular Ca2+ concentrations (Pundik, Xu, & Sundararajan, 2012). Inadvertent activation of inositol trisphosphate and ryanodine receptors, a process linked to mitochondrial reactive oxygen species (ROS) generation, can liberate intracellular Ca2+ stores (Camello-Almaraz, Gomez-Pinilla, Pozo, & Camello, 2006). Calcium overload also causes excessive stimulation of Ca2+/calmodulin-dependent enzymes such as nitric oxide synthase (i.e., eNOS, nNOS and mtNOS), as well as a host of Ca2+-dependent enzymes such as proteases, phospholipases, and endonucleases (Fellman & Raivio, 1997). Over activation of such catalytic enzymes can cause protein degradation, phospholipid hydrolysis, and DNA damage as well as a disruption of cellular signaling and enzymatic reactions. ROS generation increases dramatically during ischemia due to high Ca2+-induced mitochondria dysfunction and impairment of ROS defense enzymes, and superoxide anion is released into the cytosol in increasing amounts. Neurons in the ischemic core that have died via necrotic processes release cytotoxic elements into the interstitial space which then penetrate adjacent neurons through damaged plasma membranes caused by lipid peroxidation and the activity of phospholipases. In addition to ROS generation, cerebral ischemia is accompanied by widespread inflammation demarcated by cytokines, adhesion molecules, and other inflammatory mediators (Iadecola & Alexander, 2001).
2.1. Reactive Oxygen Species Generation
Oxidative stress is observed in the central nervous system (CNS) at early time points following ischemic injury and is well known to contribute to neuronal injury and cell death in the ischemic core (Candelario-Jalil, 2009). The CNS is especially sensitive to oxidative stress because it consumes substantial amounts of oxygen, contains large amounts of polyunsaturated fatty acids, accrues redox metal ions, and possesses relative low levels of endogenous antioxidants (Aksenova, Aksenov, Mactutus, & Booze, 2005). ROS have been recognized as central mediators of neuroinflammation and cytotoxicity in ischemia/reperfusion injury (Singhal, Morris, Labhasetwar, & Ghorpade, 2013). Furthermore, evidence of improved stroke outcome following clinical trials of antioxidant therapy underscores the critical role of ROS generation and oxidative stress in CNS pathology following cerebral ischemia/hypoxia (Lutsep & Clark, 2001).
Superoxide anion is the principal ROS generated when molecular oxygen is reduced by only one electron. This reaction occurs spontaneously and non-enzymatically through activity of electron transport systems in mitochondria. Briefly, electrons are donated by NADH, the reduced form of the coenzyme essential to all living cells, initiating a shuttling of electrons involving NADH-ubiquinone oxidoreductase or complex I, succinate dehydrogenase or complex II, ubiquinol-cytochrome c oxidoreductase or complex III, and cytochrome c oxidase or complex IV, ending with electron acceptance by molecular oxygen. Small amounts of superoxide (i.e., 1-4%) are regularly generated during this process, as the majority of reactions within the electron transport chain (ETC) involve single electron transfers (Turrens, 2003). In particular, complex I as well as both sides of complex III (i.e., Qi and Qo sites) are the most common sources of mitrochondrial superoxide (Murphy, 2009). Superoxide generated within the intermembrane space of mitochondria can reach the cytosol through voltage-dependent mitochondrial anion channels (Zhang & Gutterman, 2007). Additionally, superoxide is produced by NADPH oxidases in endothelial cells, macrophages, microglia, and granular leukocytes, as well as by cytochrome P450-dependent oxygenases and cyclo oxygenases (i.e., COX-2) (Pacher, Beckman, & Liaudet, 2007; Turrens, 2003). Furthermore, epithelial and neuronal nitric oxide synthase (eNOS/nNOS) directly produce superoxide when required cofactors, such as arginine or tetrahydrobiopterin, are deficient (i.e., uncoupled NOS) (Fang, Yang, & Wu, 2002), as occurs during cerebral ischemia.
Under normal physiological conditions, superoxide is scavenged by the cellular ROS defense system. However, due to activation of degradative enzymes and proteases, ROS defense enzymes (i.e., superoxide dismutases) can become compromised and overwhelmed by high ROS concentrations. ROS induce mutations in mitochondrial DNA (mtDNA) and damage enzymes and cytochrome complexes involved in the ETC. This leads to dysfunction of oxidative phosphorylation and further generation of ROS (Schild & Reiser, 2005). Superoxide levels steadily rise during ischemia in both microvascular endothelial cells and neurons (Fabian, DeWitt, & Kent, 1995; Pacher et al., 2007). This paradoxical increase of superoxide despite low oxygen concentrations has been well-described (Guzy & Schumacker, 2006; Murphy, 2009). It is possible that physiological levels of nitric oxide (NO) can out compete oxygen for binding to cytochrome oxidase in the setting of cerebral ischemia/hypoxia. NO binding can cause these cytochrome complexes to facilitate production of superoxide as well as increase the apparent Km of this enzyme for NO, events that further interfere with oxidative phosphorylation (Murphy, 2009).
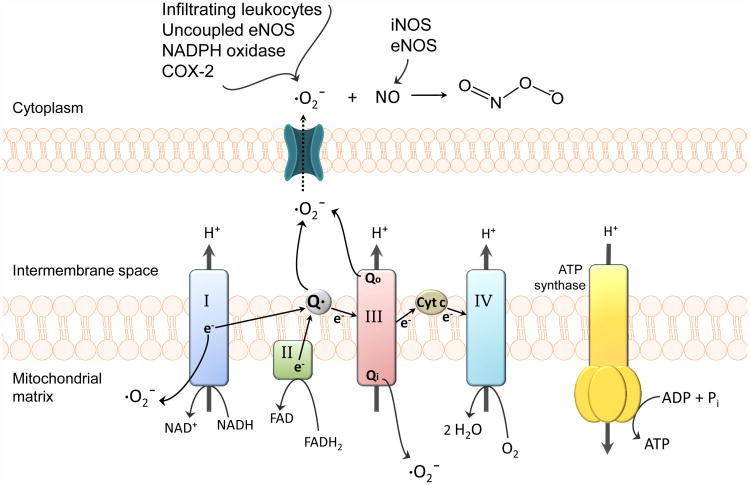
Peroxynitrite (ONOO-), a potent cytotoxic and proinflammatory molecule, is formed rapidly and non-enzymatically from the combination of nitric oxide with superoxide and causes extensive damage to neurons and cerebral microvessels through lipid peroxidation, consumption of endogenous antioxidants (i.e., reduced glutathione), DNA fragmentation, and induction of mitochondrial failure (Pacher et al., 2007). Figure 1 illustrates ROS generation in cerebro vascular endothelial cells during ischemia/reperfusion, including peroxynitrite formation. [Insert Figure 1 here] Peroxynitrite causes cellular damage via its ability to nitrosylate tyrosine residues, leading to functional modifications of critical proteins (Salvemini, Doyle, & Cuzzocrea, 2006). Breakdown of peroxynitrite into nitrogen dioxide and hydroxyl radicals also leads to endothelial cell dysfunction and BBB disruption during cerebral ischemia (Heo, Han, & Lee, 2005). Administration of peroxynitrite decomposition catalysts 5,10,15,20-tetrakis (N-methyl-4′-pyridyl) porphyrinato iron III (FeTMPyP) and 5,10,15,20-tetrakis (4-sulphonatophenyl) porphyrinato iron (FeTPPS) during reperfusion, or even up to six hours following reperfusion, have shown effectiveness in mitigating neuronal damage and reducing infarct size in rats subjected to transient middle cerebral artery occlusion (MCAO), an in vivo model of ischemic stroke. Additionally, brain edema was drastically reduced (i.e., up to 70%) in these studies, implying that peroxynitrite is a major contributor to BBB breakdown in ischemic brain (Pacher et al., 2007). Peroxynitrite formation in BBB endothelial cells and neurons becomes more likely with activation of epithelial NOS (eNOS)and inducible NOS (iNOS) because NO diffuses easily through membranes and readily reacts with superoxide anion (Pacher et al., 2007).
Figure 1. Generation of reactive oxygen species (ROS) in cerebrovascular endothelial cells.
During ischemia, mitochondrial superoxide levels increase via NO-inhibition of cytochrome complexes and oxidation of reducing equivalents in the electron transport chain (ETC). Complex I as well as both sides of complex III (i.e., Qi and Qo sites) are the most common sources of mitochondrial superoxide. Superoxide generated within the intermembrane space of mitochondria can reach the cytosol through voltage-dependent mitochondrial anion channels (Zhang et al., 2007). Superoxide levels further increase via cyclooxygenase-2, NADPH oxidase, uncoupled eNOS, and infiltrating leukocytes. The resulting high levels of superoxide coupled with the activation of NO-producing eNOS/iNOS, increases the likelihood of peroxynitrite formation. Peroxynitrite-induced cellular damage includes protein oxidation, tyrosine nitration, DNA damage and poly (ADP-ribose) polymerase (PARP) activation, lipid peroxidation, and mitochondrial dysfunction.
2.2. Poly (ADP-ribose) Polymerase
Recent evidence suggests that deleterious effects of peroxynitrite involve direct DNA damage and subsequent activation of poly (ADP-ribose) polymerase (PARP). PARP is a family of nuclear enzymes involved in DNA repair, programmed cell death, and necrotic tissue damage. PARP-1 is the dominant member of the PARP family and is critical in detection and repair of damaged DNA. PARP-1 binding to specific DNA motifs such as single- and double-strand breaks, supercoils, cruciforms, and crossovers activates its catalytic domain. Activated PARP then utilizes NAD+ to poly (ADP-ribosyl)ate itself, as well as other transcription-related factors (i.e., p53, nuclear factor-κB, activator protein 1, E2F-1) and DNA repair machinery (Chaitanya, Steven, & Babu, 2010; M. Y. Kim, Zhang, & Kraus, 2005). Neurons with extensive DNA damage, such as is observed during cerebral ischemia, will experience depletion of nuclear and cytosolic pools of NAD+ due to PARP-1 over activation (M. Y. Kim et al., 2005). To prevent energy-failure-induced necrosis, activated caspases-3 and -7 will cleave PARP between aspartic acid 214 and glycine 215, yielding protein fragments of 24 kDa and 89 kDa (Chaitanya et al., 2010). This cleavage effectively terminates PARP's ability to initiate DNA repair, an event that leads to DNA fragmentation and subsequent apoptosis. Therefore, pharmacological interventions that decrease PARP activation and cleavage in the brain are indicative of a potentially protective therapy that can attenuate neural apoptosis. Pharmacological compounds that inhibit PARP activation (i.e., 3-aminobenzamide, INO-1001, PJ-34) bestow neuroprotection against ischemia/reperfusion injury, even if administered several hours after hypoxic insult and, therefore, are potential candidates for clinical use (Pacher et al., 2007). Indeed, genetic deletion of PARP has protected animal subjects against DNA-damage associated with pathophysiological conditions such as ischemia-reperfusion injury, neuroinflammatory stress, and glutamate excitotoxicity (M. Y. Kim et al., 2005). Significant reductions (i.e., up to 80%) in infarct volume and brain tissue damage have also been observed in PARP knockout mice following transient MCAO (Pacher et al., 2007).
In vivo, hypoxic-ischemic insult and/or H/R stress results in increased PARP cleavage in the brain (Martinez-Romero et al., 2009; Thompson et al., 2014; Tu, Lu, Huang, Ho, & Chou, 2012). The ratio of cleaved-to-uncleaved PARP is an established early indicator of end-stage cell death (Chaitanya et al., 2010; Thompson et al., 2014). Moreover, poly (ADP-ribose), the negatively charged polymer that results from PARP activity, has been shown to accumulate in neural tissue following global cerebral hypoxia (Pacher et al., 2007). Consistent with previous findings (A. Ghosh, Sarkar, Mandal, & Das, 2013), our laboratory found elevated cleaved-to-uncleaved PARP ratios in whole brain lysates prepared from animals subjected to hypoxic insult (1 h, 6% O2) and re oxygenated for 10 min, 30 min and 1 h as compared to these same ratios in brain lysates from normoxic animals (Thompson et al., 2014). We showed that a single dose of the 3-hydroxy-3- methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor atorvastatin administered prior to hypoxic insult attenuated the H/R-induced increase in the cleaved-to-uncleaved PARP ratio at these early reoxygenation time points (Thompson et al., 2014). Recent evidence, including our own data, suggests that PARP cleavage is a critical factor in post-ischemic brain injury and represents a useful biomarker for neuroprotective drug efficacy in pharmacotherapy of diseases with an H/R component. Therefore, our data with atorvastatin suggests that pretreatment with statins may limit hypoxic injury and reduce the incidence of associated neurological deficits, as can occur following coronary artery bypass grafting (Kuhn et al., 2013; Kulik & Ruel, 2009).
2.3. Reperfusion & Immune Response
Reperfusion is known to cause large increases in ROS generation within the penumbra (Fabian et al., 1995; Zhao, Patzer, Herdegen, Gohlke, & Culman, 2006). Specifically, superoxide levels increase in both cerebrovascular endothelial cells and neurons during the early reoxygenation phase (Pacher et al., 2007). NO-inhibited cytochrome oxidases coupled with free radical-induced damage to the ETC machinery can further enhance production of superoxide, when molecular oxygen is reintroduced to the ischemic brain. Inhibition of ubiquinol-cytochrome c oxidoreductase or complex III of the ETC has been shown to reverse these reoxygenation-induced increases in superoxide in vitro (Therade-Matharan et al., 2005; Zhang et al., 2007). Ceramide, which has been found to increase upon the onset of hypoxia, is also positively correlated with ROS generation during reoxygenation (Therade-Matharan et al., 2005).
A major contributor to reperfusion injury is the inflammatory response initiated by the uncontrolled release of cytotoxic chemicals and cellular debris into the interstitial space within damaged brain tissue. H/R is associated with activation of hypoxia-sensitive transcription factors such as nuclear factor-κB (NFκB), hypoxia-inducible factor-1 (HIF-1), and signal transducer and activator of transcription 3 (STAT3) (Lochhead et al., 2010; Pacher et al., 2007; Witt, Mark, Huber, & Davis, 2005). Consequently, proinflammatory molecules and cytokines such as tumor necrosis factor-α (TNFα), interferon-γ, interleukin-1β (IL-1β), IL-6, and IL-18 are produced and secreted (Pacher et al., 2007). Endothelial cell adhesion molecules like P-selectins and E-selectins and intercellular adhesion molecule-1 (ICAM-1) are activated (Pacher et al., 2007), which allow leukocytes and macrophages to flood the infarcted area upon reperfusion. TNFα has also been linked to excitotoxicity and both TNFα and interferon-γ have been shown to increase expression of inducible nitric oxide synthase (iNOS) (Pundik et al., 2012). Enzymes activated by the inflammatory response include iNOS and cyclooxygenase-2 which produce substantial quantities of NO and ·O2- respectively (Pacher et al., 2007). Additionally, reactive nitrogen species (RNS) production in ischemic brain is enhanced due to activated macrophages and neutrophils releasing copious amounts of NO and ·O2- in the penumbra. Nitric oxide and superoxide rapidly form peroxynitrite, an effect that escalates peroxynitrite-induced cellular damage. As reperfusion proceeds, neuroinflammation and apoptosis become more prevalent and dramatically affect viability of salvageable brain tissue within the penumbra (Candelario-Jalil, 2009).
2.4. ROS and Changes to the BBB
Cerebral ischemia is a complex insult that not only involves deprivation of oxygen and essential nutrient delivery (del Zoppo & Hallenbeck, 2000), but is also associated with increased microvascular permeability (Kempski, 2001; Petty & Wettstein, 2001). The BBB has developed as both a physical and metabolic barrier that is critical for survival. It is well established that disruption of the BBB during ischemia/reperfusion leads to vasogenic brain edema, a primary cause of stroke-associated mortality (Vibbert & Mayer, 2010); however, the majority of edema formation, occurs across an intact BBB. Detectable BBB breakdown is not observed until approximately five hours after onset of ischemia in experimental stroke models (O'Donnell, Lam, Tran, Foroutan, & Anderson, 2006; Shah et al., 2013). Increased blood-to-brain net movement of Na+ mediated by BBB Na+ transporters appears to have a critical role in edema formation (Shah et al., 2013; Wallace, Foroutan, & O'Donnell, 2011). For example, O'Donnell and colleagues have demonstrated that increased activity of the luminal Na+-K+-2Cl-cotransporter in BBB endothelial cells contributes to development of cerebral edema following ischemia (O'Donnell, Tran, Lam, Liu, & Anderson, 2004). Furthermore, Na+-K+-Cl- cotransporter inhibition reduces edema and infarct volume in a rat permanent MCAO model (O'Donnell et al., 2004; Wallace et al., 2011). Additionally, estradiol reduces both activity of the Na+-K+-Cl- cotransporter and edema formation, suggesting that estrogens play a prominent neuroprotective role during stroke (O'Donnell et al., 2006). Integrity of BBB transport pathways during and after ischemic stroke is crucial, as perturbations in these processes can have significant effects on BBB permeability and therefore can exacerbate vasogenic edema.
BBB permeability is controlled by tight junction protein complexes localized between endothelial cells, which act to limit paracellular diffusion. Tight junctions are dynamic complexes of multiple protein constituents including junctional adhesion molecules (JAMs), occludin, claudins (i.e. claudin-1, -3, and -5), and membrane-associated guanylate kinase (MAGUK)-like proteins (i.e. ZO-1, -2 and -3) (Sanchez-Covarrubias, Slosky, Thompson, Davis, & Ronaldson, 2013). Production of ROS and subsequent oxidative stress alters expression and molecular organization of critical tight junction proteins claudin-5 and occludin at the BBB, leading to increased paracellular solute leak (Lochhead et al., 2010; Schreibelt et al., 2007). Reorganization of tight junction complexes and associated leak across the BBB following focal ischemia enables considerable movement of vascular fluid across the microvascular endothelium and development of vasogenic edema (Heo et al., 2005; Pillai et al., 2009; Sandoval & Witt, 2008). Reductions in post-ischemic edema and injury have been shown in vivo by vascular endothelial growth factor antagonism (van Bruggen et al., 1999), which has been identified as a possible mechanism that controls tight junction integrity (Fischer et al., 2007); these data indicate that tight junction disruption is involved in progression of ischemic brain injury.
Decreased expression of occludin is directly associated with increased BBB permeability as shown by an in vivo rodent model of H/R (Witt, Mark, Hom, & Davis, 2003; Witt et al., 2005). Additionally, H/R causes trafficking of occludin away from BBB tight junction protein complexes (Lochhead et al., 2010; McCaffrey et al., 2009). This occludin re-localization can be prevented in vivo by 4-hydoxy-2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPOL) treatment, a superoxide scavenging antioxidant that readily crosses the BBB (Cuzzocrea et al., 2000; Deng-Bryant, Singh, Carrico, & Hall, 2008; Kwon et al., 2003; Lochhead et al., 2012; Rak et al., 2000; Saito, Takeshita, Ueda, & Ozawa, 2003; Zhelev et al., 2009). Specifically, TEMPOL prevents breakage of disulfide bonds on occludin monomers there by blocking disruption of occludin oligomeric assemblies and subsequent blood-to-brain leak of circulating solutes (Lochhead et al., 2010). Similarly, SOD-mimetics such as metalloporphyrin catalytic antioxidants and ceria nanoparticles have also been successful in protecting against ischemic damage to the BBB in in vivo rodent model systems (C. K. Kim et al., 2012; Pacher et al., 2007).
The increase in BBB permeability observed during ischemic stroke involves changes to transcellular transport pathways in addition to tight junction modifications. For example, Yeh et al. demonstrated in immortalized rat brain endothelial cells that hypoxia up regulates glucose transporters (GLUT1) (Yeh, Lin, & Fu, 2008). Functional expression of the sodium glucose cotransporter-1 (SGLT1) was also increased after ischemia/reperfusion (Elfeber et al., 2004). Vemula et al. showed that SGLT plays a significant role along with GLUT1 in glucose uptake across the BBB and cerebral edema formation during ischemia (Vemula et al., 2009). Recently, our own laboratory discovered that the endogenous transporter, Oatp1a4, also increases at the rodent BBB and is capable of promoting transcellular xenobiotic transport (Thompson et al., 2014). Specifically, Oatp1a4 contributes to blood-to brain flux of therapeutic drugs following H/R, some of which show considerable potential as neuroprotectants (Thompson et al., 2014). Increases in non-specific vesicular transport and pinocytosis within BBB endothelial cells have also been reported (Cipolla, Crete, Vitullo, & Rix, 2004; Plateel, Teissier, & Cecchelli, 1997).
Ischemic stroke is an amalgamation of a vascular disorder and a neuronal disease. Central to the pathogenesis of ischemic damage is the neurovascular unit, a cohesive organization of endothelial cells, pericytes, neurons, and astrocytes as well as extracellular matrix. Cell-to-cell interactions and signaling occur in a co-coordinated manner between these multiple cell types and matrix constituents, events required for physiological and pathological functioning of the BBB. For example, chemical destruction of perivascular astrocytes has been shown to cause increased BBB permeability that is characterized by decreased occludin protein expression (Willis, Leach, Clarke, Nolan, & Ray, 2004). Focal loss of astrocytes both in the necrotic core and in the apoptotic penumbra contributes to increased BBB permeability during cerebral ischemia. Similarly, pericyte association with the microvasculature endothelium is critical to vascular integrity and loss of this relationship may lead to vascular leakage and induction of edema (Bonkowski, Katyshev, Balabanov, Borisov, & Dore-Duffy, 2011). Perturbation of the neurovascular unit generally leads to compromised BBB integrity and increased permeability. Unquestionably, BBB permeabilization enables blood-borne substances that are normally restricted, such as excitatory amino acids, kinins, prostaglandins, metals, and proteins to enter the brain (Plateel et al., 1997). Pharmacological interventions aimed at preservation of the neurovascular unit and protection of neurons within the penumbra would clearly prevent exacerbation of brain tissue damage during cerebral ischemia/reperfusion.
2.5. Therapeutic Approaches
Mechanisms of cell injury and/or death in the ischemic core occur extremely rapidly (i.e., within minutes), thereby rendering this region difficult to protect using traditional pharmacological approaches. In contrast, cells within the penumbra die more slowly by active cell death mechanisms (Arai et al., 2011). Residual and collateral blood flow to neurons within the penumbra allow preservation of brain tissue for up to six hours following ischemic stroke, thus rendering therapeutic interventions theoretically possible (Arai et al., 2011; Lutsep et al., 2001). The primary goal of drug therapy for acute ischemic stroke is to salvage the penumbra as much as possible and as early as possible to prevent continued growth of the ischemic core and progressively worsening neurological outcomes (Liu et al., 2010). Throughout ischemia/reperfusion brain injury, the biophysical ramifications from changes in cerebral blood flow create unique challenges to drug delivery. Differences in these changes may occur between ischemic core and penumbra, or between ipsilateral and contralateral ischemic hemispheres. For example, decreased blood flow to the penumbra will decrease drug CNS bioavailability, reducing the ability of a drug to attain efficacious concentrations at its target site. Additionally, increased BBB permeability, a key determinant of blood-to-brain drug uptake, is not a static phenomenon during cerebral ischemia/reperfusion injury. Following transient focal ischemia in experimental stroke models, enhanced BBB permeability has been observed at approximately 5 hours after ischemic insult, with a secondary increase at 72 hours (Ronaldson & Davis, 2012; Shah et al., 2013). However, in the clinic, stroke patients have been reported to experience BBB opening only during early reperfusion (Barr et al., 2010; Henning, Latour, & Warach, 2008; Kastrup et al., 2008). Focal ischemic stroke, which causes lesions in discrete brain regions, results in regional BBB permeability differences between the ipsilateral and contralateral hemispheres (Cui et al., 2010; Hatashita & Hoff, 1990). However, within the affected hemisphere, recent studies have found no significant difference in BBB permeability between the ischemic core and the penumbra in human patients with acute hemispheric stroke as assessed by first-pass perfusion computed tomography (Dankbaar et al., 2008; Nguyen et al., 2013). Given the similarities in pathophysiological damage between the core and the penumbra, BBB permeability and therefore blood-to-brain movement of drugs and fluid is likely comparable between these two brain regions during ischemia/reperfusion injury.
Currently, there is only one therapeutic agent that has been approved by the US Food and Drug Administration for acute ischemic stroke treatment, recombinant tissue plasminogen activator (rt-PA) (Jahan & Vinuela, 2009). The objective of rt-PA therapy is to restore blood flow and oxygen supply to ischemic brain tissue. However, considerable brain cellular damage occurs when cerebral perfusion is re-established (i.e., reoxygenation). Additionally, rt-PA therapy has a narrow therapeutic window, high risk of intracerebral bleeding, and other adverse effects (Messe et al., 2012; Shah et al., 2013). Among hospitals participating in the Get With The Guidelines (GWTG)-Stroke program, only 24.7% of ischemic stroke patients that presented themselves within 3 hours were even eligible to receive rt-PA (Messe et al., 2012). Additionally, aspirin is included in the clinical standard of care for ischemic stroke because its anticoagulant properties may prevent against recurrent strokes during the high-risk period immediately after the initial ischemic insult (Chen et al., 2000). Aspirin treatment is exclusively preventative and does not confer any protection and/or rescue of ischemic brain tissue. Therefore, there is a critical need in stroke therapy for neuroprotective and/or antioxidant drugs that can be effectively delivered to the brain for “rescue” of salvageable tissue from further damage.
Currently, there is considerable interest in neuroprotective/antioxidant properties of HMG-CoA reductase inhibitors (i.e., statins). Recent evidence suggests that statins can act as free-radical scavengers independent of their well-documented effects on cholesterol biosynthesis (Barone et al., 2011; Butterfield et al., 2012; Kassan, Montero, & Sevilla, 2010). For example, in vivo studies in dogs demonstrated that high-dose atorvastatin reduced markers of oxidative and nitrosative stress (i.e., protein carbonyls, 4-hydroxy-2-nonenal, 3-nitrotyrosine) and increased the ratio of GSH to reduced GSH in the brain but not in the periphery, suggesting that this drug has efficacy as a neuroprotectant and CNS antioxidant (Barone et al., 2011). Studies in an in vivo rodent model of subarachnoid hemorrhage showed that atorvastatin reduced brain caspase-3 activity and DNA fragmentation, implying an ability to attenuate neuronal apoptosis (Cheng, Wei, Zhi-Dan, Shi-Guang, & Xiang-Zhen, 2009; Pan et al., 2010). In addition, rosuvastatin has been shown to protect neurons from stress induced by oxygen-glucose deprivation in rat cerebrocortical neuronal cultures, perhaps by decreasing ROS levels (Domoki et al., 2009). Similarly, simvastatin, a lipophilic HMG-CoA reductase inhibitor, has shown neuroprotective effects against oxygen-glucose deprivation and subsequent reoxygenation by inhibiting production of 4-hydroxy-2E-nonenal (HNE), a cytotoxic product of lipid peroxidation, and directly reducing HNE toxicity (Lim et al., 2006). In addition to these preclinical studies, statin treatment has reduced cerebral expression of oxidative stress markers (i.e., nitrotyrosine and F2-isoprostanes) in clinical investigations (Davignon, Jacob, & Mason, 2004; Shishehbor et al., 2003).
To date, the exact mechanism for statin-induced neuroprotection has not been elucidated. It has been proposed that neuroprotective effects of atorvastatin may be due to targeting and subsequent upregulation of biliverdin reductase-A, a pleiotropic enzyme known to be involved in cellular stress responses (Barone et al., 2012). Of particular note, Barone et al. (2012) reported that increased activity of biliverdin reductase-A induced by atorvastatin was inversely correlated with indices of oxidative stress, which points towards an antioxidant mechanism for statins. It has also been suggested that statins canexert neuroprotective effects through enhancement of eNOS expression in the CNS (Endres et al., 1998; Sironi et al., 2003), thereby improving collateral blood flow to the ischemic penumbra.
3. Drug Delivery to the Hypoxic/Ischemic Brain
The ability of drugs such as statins to be effective neurotherapeutics following cerebral hypoxia/ischemia requires efficient and precise CNS delivery. A recent comparative in vitro study that evaluated efficacy of statins as neuroprotectants by assessing their chemical structure, theoretical lipophilicity, and ability to protect against neuronal cell death induced by okadaic acid, concluded that both atorvastatin and rosuvastatin were effective in mitigating neuronal cell death (Sierra et al., 2011). However, both of these drugs had CNS permeability values close to zero and BBB penetration estimates of less than 5% (Sierra et al., 2011). This study illustrates the critical importance of identifying and characterizing endogenous transport mechanisms that can be utilized to facilitate CNS statin delivery. Here, we describe two strategies for optimizing CNS statin delivery: targeting endogenous BBB uptake transport systems (i.e., organic anion transporting polypeptides (OATP in humans; Oatp in rodents)) and nanoparticle-based drug delivery technologies.
3.1. Organic Anion Transporting Polypeptide (OATP/Oatp)
Brain uptake and distribution of currently marketed drugs such as statins are governed by transport systems that are endogenously expressed at the microvascular endothelium. Of these transport systems, some are unidirectional (i.e., facilitate either blood-to-brain or brain-to-blood peptide transport) and others are bidirectional. One family of transporters that may have utility in brain delivery (i.e., blood-to-brain transport) of statins is the OATPs/Oatps. OATPs/Oatps are a group of sodium-independent transporters classified within the larger solute carrier (SLC) super family (Ronaldson et al., 2013). The concept of focusing on influx processes (i.e., OATP) at the BBB as opposed to inhibition of efflux processes (i.e., P-gp) represents a highly promising approach to optimizing CNS drug delivery.
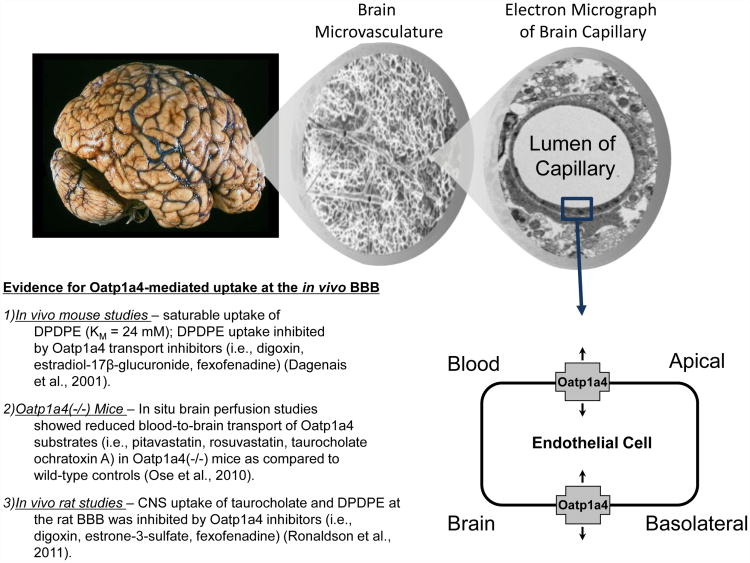
In rodent brain, expression of Oatp1a4, Oatp1c1, and Oatp2a1 have been reported in capillary enriched fractions, capillary endothelial cells, and brain microvessels (Kis et al., 2006; Ronaldson & Davis, 2011; Westholm, Stenehjem, Rumbley, Drewes, & Anderson, 2009). Oatp1c1 primarily transports thyroxine and conjugated sterols (Westholm et al., 2009) while Oatp2a1 regulates BBB transport of prostaglandins (Kis et al., 2006). In contrast, Oatp1a4 is the primary drug transporting Oatp isoform expressed at the rat BBB (Ronaldson et al., 2013). As shown in Figure 2, studies in Oatp1a4 (-/-) mice have demonstrated reduced blood-to-brain transport of pitavastatin and rosuvastatin as compared to wild-type controls, which suggests involvement of Oatp1a4 in statin transport across the BBB (Ose et al., 2010). [Insert Figure 2 here] The human orthologue of Oatp1a4 is OATP1A2, which exhibits an enrichment of mRNA expression in the brain as compared with other tissues including liver, kidney, and gastrointestinal tract (Kullak-Ublick et al., 1995; Steckelbroeck et al., 2004). Immunofluorescence staining of human brain frontal cortex demonstrated OATP1A2 localization at both the apical and basolateral sides of the microvascular endothelium (Gao et al., 2000). Although not directly studied at the BBB, OATP1A2 has been shown to transport rosuvastatin in isolated human hepatocytes and atorvastatin in human embryonic kidney cells (HEK293) stably transfected with OATP1A2 (Ho et al., 2006; Mandery et al., 2011). Localization and substrate profiles of OATP/Oatp isoforms known to be present at BBB are summarized in Table 1. [Insert Table 1 here] Recently, we reported for the first time increased functional expression of Oatp1a4 at the BBB in rats subjected to H/R (Thompson et al., 2014). Evidence for increased Oatp1a4 transport at the BBB included increased brain accumulation of both taurocholate and atorvastatin, two known Oatp substrates, and attenuation of taurocholate and atorvastatin uptake by Oatp transport inhibitors (i.e., estrone-3-sulfate, fexofenadine). Oatp1a4-mediated delivery of atorvastatin across the brain microvascular endothelium was demonstrated by our laboratory under normoxic conditions, hypoxic conditions, and following H/R stress (Thompson et al., 2014).
Figure 2. Evidence for blood-to-brain drug transport mediated by Oatp1a4 at the blood-brain barrier (BBB).
Previous in vivo studies have shown that CNS uptake of drugs such as opioid peptide analgesics (i.e., DPDPE) and HMG-CoA reductase inhibitors (i.e., pitavastatin, rosuvastatin) is determined by functional expression of Oatp1a4 at the luminal and abluminal plasma membrane of the brain microvascular endothelium. Adapted from: Ronaldson & Davis, (2013). Pharmacol Rev. 65:291-314.
Table 1. Localization and substrate profiles of OATP/Oatp isoforms known to be present at the blood-brain barrier (BBB).
| Human OATP Isoform | BBB expression | Rodent Ortholog | BBB expression | Potential Substrate Drugs |
|---|---|---|---|---|
| OATP1A2 | apical & basolateral | Oatp1a4 | apical & basolateral | HMG-CoA reductase inhibitors (e.g., atorvastatin, cerivastatin, fluvastatin, pitavastatin, pravastatin, rosuvastatin, simvastatin acid); opioid analgesic peptides (e.g., DPDPE) |
| OATP1C1 | localization not confirmed | Oatp1c1 | apical | thyroid hormones and conjugated sterols |
| OATP2A1 | localization not confirmed | Oatp2a1 | apical | prostaglandins |
OATP/Oatp isoforms are also known to be involved in blood-to-brain transport of opioid analgesic peptides such as deltorphin II and DPDPE (Gao et al., 2000; Ose et al., 2010; Ronaldson, Finch, Demarco, Quigley, & Davis, 2011). This is highly significant due to preclinical evidence that such opioid peptides may have efficacy in treatment of ischemic stroke, particularly in the context of cerebral edema. For example, opioid peptides that selectively bind to the μ-opioid receptor (e.g., [Tyr-D-Ala, N-CH, -Phe4, Glyol]-enkephalin [DAMGO]), δ-opioid receptor (e.g., DPDPE), and κ-opioid receptor (e.g., U50, 488) reduced water uptake in rat hippocampal slices in situ (Yang, Shah, Wang, Karamyan, & Abbruscato, 2011; Yang, Wang, Shah, Karamyan, & Abbruscato, 2011). However, the utility of these drugs as stroke therapeutics is dependent on their effective CNS delivery. Our group (Ronaldson, Finch, et al., 2011) and others (Dagenais, Graff, & Pollack, 2004; Ose et al., 2010) have shown that opioid peptides (i.e., DPDPE) are Oatp1a4 substrates, further emphasizing the need to evaluate this SLC transporter as a facilitator of CNS drug delivery. Such an understanding of Oatp-mediated transport mechanisms at the BBB involved in CNS delivery of peptides will undoubtedly aid in development of these compounds as potential therapeutics.
3.1.1. Regulation of Oatp1a4
Although pathophysiological stressors can modulate endogenous BBB transporters, such changes must be effectively controlled in order to provide optimal CNS drug delivery. For example, studies in our in vivo inflammatory pain model demonstrated increased BBB functional expression of Oatp1a4 only between 1 h and 6 h after induction of pain/inflammation (Ronaldson, Finch, et al., 2011). Therefore, if Oatps are to be utilized for effective delivery of therapeutics (i.e., statins, opioid peptides) for treatment of diseases with an H/R component, its functional expression must be controlled over a more desirable time course than is possible by only relying on pathophysiological processes. This objective can be accomplished by pharmacological targeting of signaling pathways that regulate Oatp functional expression such as the transforming growth factor-β (TGF-β) system (Ronaldson, Finch, et al., 2011). TGF-β signaling is well known to regulate multiple cellular processes including vascular remodeling (Pepper, 1997). The TGF-βs are a family of pleiotropic cytokines that signal by binding to a heterotetrameric complex of type I and type II serine/threonine kinase receptors (Derynck & Zhang, 2003). The type I receptors, also known as activin receptor-like kinases (ALKs), propagate intracellular signals through phosphorylation of receptor-specific Smad proteins (i.e., receptor-regulated (R)-Smads). Phosphorylated (R)-Smads form complexes with the common Smad (i.e., Smad4), enabling nuclear translocation and subsequent changes in target gene transcription (Derynck et al., 2003). At the BBB, only two ALK receptors (i.e., ALK1, ALK5) have been identified (Ronaldson, Demarco, Sanchez-Covarrubias, Solinsky, & Davis, 2009). TGF-β regulates the endothelial cell activation state and angiogenesis through a precise balance between ALK1 and ALK5 signaling processes (Goumans et al., 2002; Wu, Ma, Han, Wang, & Chen, 2006). Whereas the ALK1 pathway leads to endothelial activation characterized by increased permeability, ALK5-mediated signaling promotes vascular resolution that is demarcated by decreased permeability (Lebrin, Deckers, Bertolino, & Ten Dijke, 2005; Wu et al., 2006). Such effects on vascular permeability have been attributed to the ability of TGF-β signaling to alter expression of tight junction constituent proteins (Ishihara et al., 2008; Ronaldson et al., 2009; Watabe et al., 2003).
Recently, our laboratory demonstrated that pharmacological inhibition of TGF-β signaling led to increased microvascular expression and activity of Oatp1a4 at the BBB (Ronaldson, Finch, et al., 2011; Thompson et al., 2014). Of particular interest was the observation that this blockade of TGF-β/ALK5 signaling using the specific ALK5 antagonist, SB431542, enhanced Oatp1a4 transport activity in saline-treated control animals as indicated by increased delivery to the brain of Oatp substrates such as taurocholate and atorvastatin (Ronaldson, Finch, et al., 2011; Thompson et al., 2014). Since TGF-β1 expression (i.e., the natural ligand for ALK5) is increased in the brain and in the periphery following cerebral hypoxia (Doyle, Cekanaviciute, Mamer, & Buckwalter, 2010), pharmacological blockade of TGF-β/ALK5 signaling may be critical in targeting Oatps for CNS drug delivery. A crucial consideration in interpretation of our data is the contribution of paracellular diffusion to brain uptake of Oatp substrate drugs. Our laboratory has previously reported that inhibition of TGF-β/ALK5 signaling with SB431542 increased paracellular BBB permeability for solutes such as sucrose by altering tight junction integrity (Ronaldson et al., 2009). The molecular weight of taurocholate (537.7 Da) and atorvastatin (558.6 Da) are greater than that of sucrose (342 Da), suggesting a lesser degree of paracellular diffusion. As our data showed no statistical difference in taurocholate or atorvastatin uptake in the presence of Oatp1a4 inhibitors in animals administered SB431542, we conclude that paracellular diffusion was not a significant factor in CNS uptake of taurocholate or atorvastatin. Nonetheless, it is critical to correct for paracellular transport in any study examining the effect of targeting TGF-β signaling for optimization of CNS drug delivery. Our work on TGF-β/ALK5 signaling is highly novel and significant because we showed that this pathway can regulate permeability at the BBB by increasing functional expression of an influx transporter. Furthermore, these studies highlight the potential of the TGF-β/ALK5 pathway as a pharmacological target that can be used for optimization of drug delivery to the CNS, particularly for treatment of cerebral ischemia.
3.1.2. Transporter/Substrate Interactions at the BBB
The ability of a pharmacological agent to cross the BBB endothelium and achieve efficacious concentrations within the CNS is dependent on multiple mechanisms of transport. Such mechanisms include uptake into the brain via an influx transporter and/or extrusion from the CNS mediated by an efflux transporter. For many drugs, it is this discrete balance between influx and efflux that determines whether a pharmacological agent will accumulate within the brain extracellular milieu and, therefore, elicits a therapeutic effect. The complexity of drug transporter biology at the BBB is further underscored by the observation that functional expression of such transport proteins may be dramatically altered by pathophysiological stressors (Hayashi et al., 2006; Ronaldson, Finch, et al., 2011; Seelbach, Brooks, Egleton, & Davis, 2007; Yeh et al., 2008). A thorough understanding of regulation and functional expression of endogenous BBB transporters in both health and disease is critical for optimization of pharmacotherapy. Furthermore, such information will enable more effective targeting of transporters and/or transporter regulatory mechanisms, thus allowing endogenous BBB transport systems to be specifically exploited for improvement of CNS drug delivery.
OATP/Oatp family members are multispecific transporters capable of transporting a vast array of structurally diverse drugs, metabolites, and physiologic substrates. However, a full comprehension of how such transporters can be targeted to promote CNS delivery of therapeutics requires an appreciation that substrates transported by OATP/Oatp family members may also be transport substrates for organic anion transporters (OATs), P-gp, MRP/Mrp isoforms, and breast cancer resistance protein (BCRP in humans: Bcrp in rodents). At the BBB, organic anion transporter 3 (OAT3) (Miyajima, Kusuhara, Fujishima, Adachi, & Sugiyama, 2011; Ohtsuki et al., 2005), P-gp (Bendayan, Ronaldson, Gingras, & Bendayan, 2006; Hawkins, Sykes, & Miller, 2010; Seelbach et al., 2007), and several MRP/Mrp isoforms (Dallas, Miller, & Bendayan, 2006; Hawkins, Ocheltree, Norwood, & Egleton, 2007) are expressed and contribute to brain-to-blood (i.e., efflux) substrate transport. Many drug substrates of OATP/Oatp substrates are also transported by at least one additional transporter such as OAT1, OAT3, P-gp, MRP/Mrp isoforms, or BCRP/Bcrp. For example, DPDPE is a P-gp substrate as well as an Oatp1a4 substrate (Ose et al., 2010; Ronaldson, Finch, et al., 2011). Additionally, rosuvastatin is a substrate for OAT3 (Windass, Lowes, Wang, & Brown, 2007), Bcrp (Huang, Wang, & Grimm, 2006), and Mrp2 (Abe, Bridges, Yue, & Brouwer, 2008). Therefore, it is highly possible that drugs that enter the brain microvascular endothelium or choroid plexus epithelium via one class of transporter may exit by another. Understanding how changes in expression of a specific transporter might affect brain uptake of a given drug will depend upon a thorough assessment of all competing transporters.
Previous research has attempted to overcome drug efflux transport by pharmacological targeting of transporters such as P-gp. P-gp is a major obstacle to CNS delivery of therapeutics, having almost an inexhaustible substrate profile of small, lipophilic drugs and a strong presence at the BBB. Reducing P-gp activity to allow enhanced passage of therapeutics across the BBB is an attractive prospect. However, translation of direct P-gp inhibition from animal models to the clinic has been unsuccessful, due mostly to systemic toxicity of the P-gp inhibitors themselves (Cannon, Peart, Hawkins, Campos, & Miller, 2012). As an alternative, identification and targeting of molecular signaling pathways that control basal P-gp activity have recently been done by Miller et al. Sphingosine-1-phosphate (S1P), a bioactive lipid metabolite, acting through its receptor (S1PR1) was shown to rapidly and reversibly reduce P-gp transport activity in rats, thereby enhancing brain uptake of drugs such as verapamil, loperamide and paclitaxel (Cannon et al., 2012). S1P signaling was found to be a downstream link to TNFα signaling through TNFR1, end othelin, iNOS and PKCβ1, a pathway previously elucidated by Cannon and colleagues (Cannon et al., 2012). Speculation as to the mechanism of this P-gp manipulation includes covalent modification or changes to the microenvironment of the transporter. Further investigation into such a signaling pathway may lead to control of BBB efflux transport without sacrificing the neuroprotection afforded by P-gp. Theoretically, such control of P-gp-mediated transport at the molecular level may enhance the ability of influx transporters (i.e., OATPs/Oatps) to optimally deliver drugs to the brain.
3.2. Nanoparticles
Development of new drug delivery technologies is an area of intense research and scientific interest. Common flaws that plague conventional drug treatments include problems with accurate dosing, rapid drug metabolism or degradation, and unwanted distribution profiles. Nanotechnology-based delivery vehicles (i.e., nanoparticles) have emerged as a promising solution to such drug delivery issues. Nanocarriers can give new hope to existing therapeutics that suffer from inefficient delivery or problems with drug-tissue distribution. For example, cytidine 5′ diphosphocholine, found to be neuroprotective against cerebral ischemia/reperfusion, is rapidly metabolized by the liver, rendering it incapable of reaching the brain if administered via the systemic circulation (S. Ghosh, Das, Mandal, Dungdung, & Sarkar, 2010). Encapsulation of cytidine 5′ diphosphocholine into nanoparticle liposomes has been shown to reduce hepatic hydrolysis and enabled this drug to attain therapeutic concentrations in brain parenchyma (S. Ghosh et al., 2010; Pinzon-Daza et al., 2013).
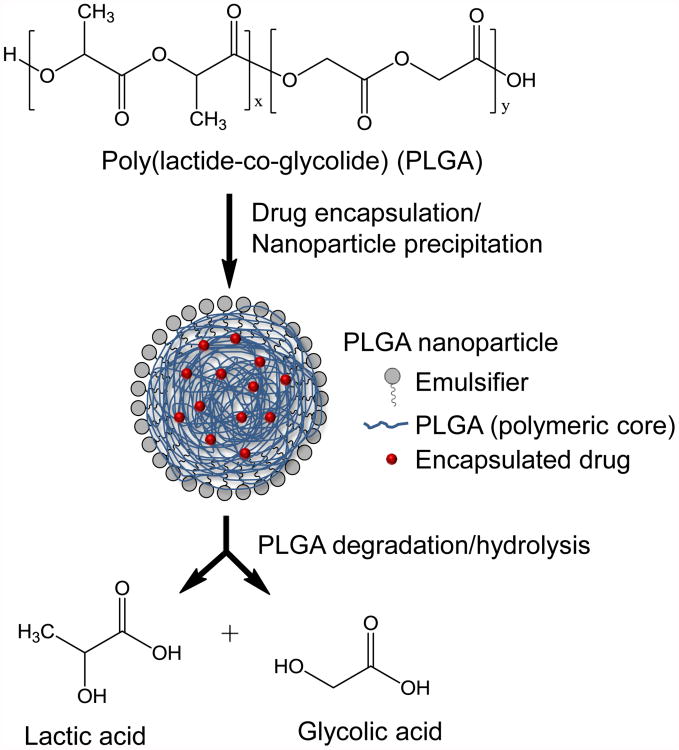
The term “nanoparticle” is used broadly to describe various nanosystems including liposomes, polymeric particles, hydrogels, micelles, inorganic/solid particles, dendrimers, nanotubes, and quantum dots (Marrache et al., 2013; Singh & Lillard, 2009). Therapeutic agents are typically encapsulated, entrapped, adsorbed or chemically attached to the nanoparticle surface (Denora, Trapani, Laquintana, Lopedota, & Trapani, 2009). The most widely used and successful nanoparticle systems for delivery of bioactive compounds have been liposomes and polymeric nanoparticles or polymer-drug conjugates (Marrache et al., 2013; Singh et al., 2009). The composition of biodegradable polymeric nanoparticles along with their degradation to biocompatible components can be seen in Figure 3. [Insert Figure 3 here] In order for nanoparticles to be therapeutically effective, they must exhibit i) prolonged circulation in the bloodstream (i.e., not immediately filtered or metabolized), ii) specificity for adequate accumulation in a target tissue, iii) selective cellular uptake by target endothelial cells, and iv) controlled release of medicinal drugs. Nanoparticles are generally defined as ranging from 10 to 1000 nm in diameter. Typically, nanoparticles > 200 nm are not commonly synthesized because smaller nanoparticles are more readily taken up into cells and/or tissues (Singh et al., 2009). In comparison with microparticles of 1 μm in size, 100 nm particles showed a 2.5 fold greater cellular uptake in Caco-2 cells (Desai, Labhasetwar, Walter, Levy, & Amidon, 1997). Due to their small size and mobility, nanoparticles are able to access a wide variety of “druggable” targets, both extracellular and intracellular. As the smallest capillaries in the body have a diameter of approximately 5-6 μm (Hans & Lowman, 2002), administration of nanoparticles through the microcirculation is a viable approach for facilitation of CNS drug delivery.
Figure 3. Schematic representation of the synthesis and degradation of poly (lactide-co-glycolide) (PLGA) nanoparticles.
Nanoparticle size, solubility, lipophilicity, and surface charge are all critical parameters to consider for efficient CNS drug delivery. Nanoparticle surface modifications are necessary to increase biocompatibility and usually involve a coating or specific attachment of hydrophilic or amphiphilic polymers/surfactants (Pinzon-Daza et al., 2013; Singh et al., 2009). Without such surface modifications, nanoparticles introduced into systemic circulation are quickly cleared from the blood by the reticuloendothelial system (RES) at organs such as the liver, spleen, and lungs. Additionally, blood components (i.e., opsonins) bind to and mark the particle for destruction by phagocytic cells via a process known as opsonization. The degree of opsonization in vivo has been shown to correlate with the hydrophobicity of the nanoparticle (Singh et al., 2009). For nanoparticles to be used effectively to deliver therapeutics to the CNS, they must be retained in the systemic circulation long enough to distribute to the desired target. Polysorbate, polyethylene glycol (PEG), polyethylene oxide (PEO), poloxamine, poloxamer, and pluronic polymers are commonly used to coat the surface of nanoparticles for this purpose (Pinzon-Daza et al., 2013; Singh et al., 2009). PEGylation of drug-loaded liposomes has been particularly effective in enhancing the longevity of the particles in systemic circulation. The chemical nature of PEG allows liposomes to circulate in the bloodstream longer, avoiding collection by the reticuloendothelial system (RES) in the liver and spleen (Hans et al., 2002; Pinzon-Daza et al., 2013; Xie et al., 2012).
3.2.1. Targeting and Delivery across the BBB
In addition to increasing the half-life of the encapsulated drug, nanoparticle surface coatings and covalent modifications also present an opportunity to enhance specificity, allowing nanoparticles to cross biological membranes, such as the BBB endothelium. For many nanoparticle delivery systems, polyethylene glycol (PEG) is used for this purpose because it has minimal effects on drug-matrix interactions (Singh et al., 2009). PEG is often used as a linker, providing the chemical moieties or functional groups necessary for conjugation. Once incorporated onto the surface of the nanoparticle, the PEG “arms” of a specified length could then be conjugated with antibodies, peptide sequences, or ligands for targeting specific tissues. The adaptability of nanoparticle drug delivery systems suggests that specific transcellular transport routes can be targeted (i.e., adsorptive-mediated transcytosis, receptor-mediated transcytosis, carrier-mediated transcytosis) for CNS drug delivery (Alyautdin, Khalin, Nafeeza, Haron, & Kuznetsov, 2014). Paracellular diffusion of nanoparticles has also been achieved after administration of hyperosmotic mannitol (Avgoustakis et al., 2002), which causes “shrinkage” of microvascular endothelial cells, thereby mechanically stretching tight junction protein complexes (Denora et al., 2009; Ikeda, Bhattacharjee, Kondoh, Nagashima, & Tamaki, 2002). However, such a non-selective increase in paracellular diffusion also allows potentially neurotoxic blood-borne substances to accumulate in the brain. A more efficient approach is targeting of receptor-mediated endocytosis for delivery of drug loaded nanoparticles through the BBB (Pinzon-Daza et al., 2013). Insulin, transferrin, lactoferrin, glutathione, peptides and apolipoproteins are among the ligands successfully utilized to achieve receptor-mediated transcytosis of nanoparticles and drug delivery to the CNS (Alyautdin et al., 2014; Pinzon-Daza et al., 2013).
The biopolymer polysorbate has been especially successful in delivering therapeutics to brain parenchyma. Polysorbate coated nanoparticles are thought to permeate the BBB through receptor-mediated endocytosis by binding to low density lipoprotein (LDL) receptors (Hans et al., 2002). Apolipoprotein E (ApoE), found in blood plasma, has been shown to adsorb on the surface of the polysorbate 20, 40, 60, or 80-coated nanoparticles and likely mimics LDL, allowing receptor binding (Kreuter, 2001). Polysorbate 80 in particular has been shown to successfully cross the BBB as demonstrated by Kreuter et al. using polybutylcyanoacrylate (PBCA) nanoparticles (Beletsi, Leontiadis, Klepetsanis, Ithakissios, & Avgoustakis, 1999; Kreuter et al., 2003; Ramge et al., 2000). PLGA-b-PEG nanoparticles loaded with atorvastatin and coated in polysorbate 80 have been characterized and shown to accumulate in the rat brain in vivo (Simsek, Eroglu, Kurum, & Ulubayram, 2013). Similar to polysorbate-coated particles, poly (methoxy-PEG-cyanoacrylate-co-hexadecyl cyanoacrylate) (PEG-PHDCA) nanoparticles incubated in rat serum also adsorbed ApoE and ApoB100 on their surface and were delivered across the BBB into the CNS with greater success than apolipoprotein-lacking nanoparticles (H. R. Kim et al., 2007). However, dependence on whole protein adsorption on nanoparticle surfaces may not be the most efficient method for delivery of therapeutics across the BBB. Only a fraction of the nanoparticles will have adsorbed whole protein in the correct conformation to bind to and initiate endocytosis. Additionally, maximal efficacy of receptor-mediated endocytosis has been observed when smaller, targeting peptides for a specific endogenous receptor, such as transferrin, were employed in place of the whole protein as shown by Prades et al. using gold nanoparticles (Prades et al., 2012). To date, utility of nanoparticle-mediated delivery of neuroprotective drugs (i.e., statins) has not been studied in the context of cerebral hypoxia and/or ischemia.
Specific endogenous BBB transporters have also been targeted as means of nanoparticle drug release into the CNS. For example, Xie and colleagues synthesized liposomes modified by PEGs of varying lengths, covalently linking cholesterol with glucose. The glucose molecule was then recognized by the GLUT1 transporter in BBB endothelial cells and the liposomes were successful in delivering their drug load into the CNS. The length of the PEG chain was also found to affect BBB crossing as PEG-modified liposomes with a relative long chain length (i.e., PEG1000) maximized drug transport across the BBB barrier and subsequent uptake into brain parenchyma. However, the PEG linker between the ligand (i.e., glucose molecule for GLUT1) and liposome can be too long (i.e., PEG > 2000) and may result in self-folding and insufficient exposure of the covalently attached ligand (Xie et al., 2012).
3.2.2. Drug Release
Nanoparticles composed of biodegradable polymers such as poly (lactic acid-co-glycolic acid) (PLGA) are perhaps the most accepted and widely used nanoparticle material. PLGA and related polymers PGA and PLA have been approved by the FDA as biocompatible and biodegradable drug delivery systems (Marrache et al., 2013; Singhal et al., 2013). The success of these polymeric nanoparticles is largely due to their aptitude for controlled drug release, which is perhaps the most critical parameter in nanoparticle formulation. The rate of drug release from nanoparticles is dependent on interactions between drug molecules and nanoparticle materials, the solubility of the drug, the diffusion rate of the drug through the nanoparticle matrix, and the degradation rate of the nanoparticle (Singh et al., 2009). Nanoparticle drug delivery systems tend to exhibit an initial burst of drug release followed by a slower, sustained release. For example, Suh et al. demonstrated in vitro that 40% of loaded drug (i.e., paclitaxel) was released from PEO-PLGA nanoparticles within the first three days (Suh, Jeong, Rathi, & Kim, 1998). Afterwards, drug release abated but continued, liberating a total of 85% of paclitaxel in four weeks. This initial burst observed is ascribed to drug molecules being weakly adsorbed to the nanoparticle matrix (Singh et al., 2009), leading to rapid dissociation. Particle size has a significant effect on drug loading and unloading. Specifically, particles that are larger in size have a reduced surface area-to-volume ratio compared to smaller particles, and therefore exhibit a smaller initial burst of drug release followed by a longer sustained release. Smaller particles, conversely, have smaller cores and therefore the majority of the drug molecules are either at or near the nanoparticle surface. This leads to an enhanced initial burst of drug release (Hans et al., 2002; Singh et al., 2009). Regardless, the utility of nanoparticle carriers as drug delivery mechanisms has been well-demonstrated and shows considerable potential for targeted drug delivery to the brain (Hans et al., 2002; Marrache et al., 2013; Singh et al., 2009).
4. Conclusion
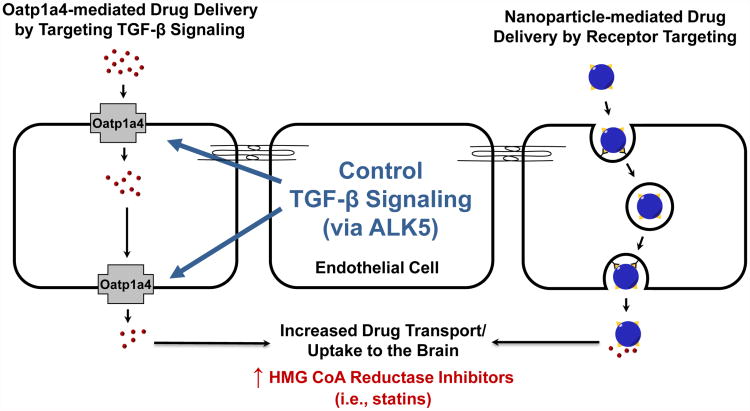
Cerebral ischemia involves a dynamic pathophysiology that comprises a multiplicity of processes, including excitotoxicity, disruption of Ca2+ homeostasis, ROS generation, cerebral inflammation, and neuronal apoptosis. A detailed understanding of such pathways offers immense opportunities to design novel treatment strategies for cerebral ischemia, a condition with few viable therapeutic options. Successful pharmacotherapy of cerebral ischemia requires that drugs achieve effective concentrations in the CNS, a therapeutic goal that is hindered by the BBB. It was originally believed that microvascular endothelial cells in the brain represented a static barrier to administered therapeutics and that drug delivery across the BBB largely depended on physiochemical properties of the drug (i.e., lipophilicity, charge, size) and passive diffusion. However, many lipophilic drugs have limited or no efficacy in treatment of neurological diseases due to a clear inability to cross the BBB. This observation emphasizes the involvement of ATP-binding cassette (ABC) drug efflux transporters, which are key determinants of the ability of a therapeutic agent to accumulate within the brain. Such efflux transporters are a formidable obstacle; however, recent studies have shown that small-molecule drug delivery can be facilitated by targeting endogenous uptake transporters (i.e., OATPs/Oatps) at the BBB. Furthermore, molecular mechanisms that regulate the activity of OATPs/Oatps (i.e., TGF-β/ALK5 signaling) have been uncovered, offering an opportunity to “control” OATP/Oatp functional expression in order to provide optimal drug delivery to the CNS. [Insert Figure 4 here] Additionally, drug-loaded nanoparticles represent a highly adaptable delivery system that offers significant advantages in drug stability and controlled release to the CNS. A better understanding of mechanisms that regulate endocytosis-mediated uptake at the BBB will allow greater control of drug permeation and/or transport across the BBB via nanoparticles. Perhaps such drug delivery approaches will prove “paradigm-shifting” for treatment of cerebral ischemia by enabling precise delivery of therapeutics with neuroprotective properties such as statins. Future work will continue to provide more insight on therapeutic targeting of the BBB via endogenous uptake transport systems and drug-loaded nanoparticles. Ultimately, data derived from these studies will allow achievement of more precise and more effective drug concentrations within the brain, thereby improving treatment of cerebral ischemia.
Figure 4. Summary.
Data from our laboratory shows that organic anion transporting polypeptide 1a4 (Oatp1a4) facilitates brain delivery of drugs that may exhibit neuroprotective efficacy in treatment of cerebral hypoxia/ischemia. The transforming growth factor-β (TGF-β) signaling pathway regulates Oatp1a4 functional expression and may offer an opportunity to “control” Oatp1a4 expression/activity via small-molecule inhibition of activin receptor-like kinase 5 (ALK5). We also propose that nanotechnology-based delivery vehicles may offer unique advantages in the CNS penetration of therapeutics. Blood-brain barrier (BBB) endothelial cell receptors can be targeted for transcytosis of nanoparticles via attachment of specific ligands (i.e., transferrin).
Abbreviations
- ALK
activin receptor-like kinase
- BBB
blood-brain barrier
- CNS
central nervous system
- DPDPE
[D-penicillamine 2,5]-enkephalin
- ETC
electron transport chain
- H/R
hypoxia/reoxygenation
- LDL
low density lipoprotein
- NCX
sodium/calcium exchanger
- NO
nitric oxide
- NOS
nitric oxide synthase
- OATP/Oatp
organic anion transporting polypeptide
- PARP
poly (ADP-ribose) polymerase
- PEG
polyethylene glycol
- PLGA
poly (lactic acid-co-glycolic acid)
- RES
reticuloendothelial system
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- rt-PA
recombinant tissue plasminogen activator
- SOD
superoxide dismutase
- TEMPOL
4-hydoxy-2,2,6,6-tetramethylpiperidine-N-oxyl
- TGF-β
transforming growth factor-β
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest to declare.
References
- Abe K, Bridges AS, Yue W, Brouwer KL. In vitro biliary clearance of angiotensin II receptor blockers and 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors in sandwich-cultured rat hepatocytes: comparison with in vivo biliary clearance. J Pharmacol Exp Ther. 2008;326(3):983–990. doi: 10.1124/jpet.108.138073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibhatla RM, Hatcher JF. Tissue plasminogen activator (tPA) and matrix metalloproteinases in the pathogenesis of stroke: therapeutic strategies. CNS Neurol Disord Drug Targets. 2008;7(3):243–253. doi: 10.2174/187152708784936608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibhatla RM, Hatcher JF, Dempsey RJ. Lipids and lipidomics in brain injury and diseases. AAPS J. 2006;8(2):E314–321. doi: 10.1208/aapsj080236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibhatla RM, Hatcher JF, Larsen EC, Chen X, Sun D, Tsao FH. CDP-choline significantly restores phosphatidylcholine levels by differentially affecting phospholipase A2 and CTP: phosphocholine cytidylyltransferase after stroke. J Biol Chem. 2006;281(10):6718–6725. doi: 10.1074/jbc.M512112200. [DOI] [PubMed] [Google Scholar]
- Aksenova MV, Aksenov MY, Mactutus CF, Booze RM. Cell culture models of oxidative stress and injury in the central nervous system. Curr Neurovasc Res. 2005;2(1):73–89. doi: 10.2174/1567202052773463. [DOI] [PubMed] [Google Scholar]
- Alyautdin R, Khalin I, Nafeeza MI, Haron MH, Kuznetsov D. Nanoscale drug delivery systems and the blood-brain barrier. Int J Nanomedicine. 2014;9:795–811. doi: 10.2147/ijn.s52236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K, Lok J, Guo S, Hayakawa K, Xing C, Lo EH. Cellular mechanisms of neurovascular damage and repair after stroke. J Child Neurol. 2011;26(9):1193–1198. doi: 10.1177/0883073811408610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke. 1981;12(6):723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- Avgoustakis K, Beletsi A, Panagi Z, Klepetsanis P, Karydas AG, Ithakissios DS. PLGA-mPEG nanoparticles of cisplatin: in vitro nanoparticle degradation, in vitro drug release and in vivo drug residence in blood properties. J Control Release. 2002;79(1-3):123–135. doi: 10.1016/s0168-3659(01)00530-2. [DOI] [PubMed] [Google Scholar]
- Barone E, Cenini G, Di Domenico F, Martin S, Sultana R, Mancuso C, et al. Butterfield DA. Long-term high-dose atorvastatin decreases brain oxidative and nitrosative stress in a preclinical model of Alzheimer disease: a novel mechanism of action. Pharmacol Res. 2011;63(3):172–180. doi: 10.1016/j.phrs.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone E, Mancuso C, Di Domenico F, Sultana R, Murphy MP, Head E, Butterfield DA. Biliverdin reductase-A: a novel drug target for atorvastatin in a dog pre-clinical model of Alzheimer disease. J Neurochem. 2012;120(1):135–146. doi: 10.1111/j.1471-4159.2011.07538.x. [DOI] [PubMed] [Google Scholar]
- Barr TL, Latour LL, Lee KY, Schaewe TJ, Luby M, Chang GS, et al. Warach S. Blood-brain barrier disruption in humans is independently associated with increased matrix metalloproteinase-9. Stroke. 2010;41(3):e123–128. doi: 10.1161/STROKEAHA.109.570515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beletsi A, Leontiadis L, Klepetsanis P, Ithakissios DS, Avgoustakis K. Effect of preparative variables on the properties of poly (dl-lactide-co-glycolide)-methoxypoly (ethyleneglycol) copolymers related to their application in controlled drug delivery. Int J Pharm. 1999;182(2):187–197. doi: 10.1016/s0378-5173(99)00058-7. [DOI] [PubMed] [Google Scholar]
- Bendayan R, Ronaldson PT, Gingras D, Bendayan M. In situ localization of P-glycoprotein (ABCB1) in human and rat brain. J Histochem Cytochem. 2006;54(10):1159–1167. doi: 10.1369/jhc.5A6870.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkowski D, Katyshev V, Balabanov RD, Borisov A, Dore-Duffy P. The CNS microvascular pericyte: pericyte-astrocyte crosstalk in the regulation of tissue survival. Fluids Barriers CNS. 2011;8(1):8. doi: 10.1186/2045-8118-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Barone E, Di Domenico F, Cenini G, Sultana R, Murphy MP, et al. Head E. Atorvastatin treatment in a dog preclinical model of Alzheimer's disease leads to up-regulation of haem oxygenase-1 and is associated with reduced oxidative stress in brain. Int J Neuropsychopharmacol. 2012;15(7):981–987. doi: 10.1017/S1461145711001118. [DOI] [PubMed] [Google Scholar]
- Camello-Almaraz C, Gomez-Pinilla PJ, Pozo MJ, Camello PJ. Mitochondrial reactive oxygen species and Ca2+ signaling. Am J Physiol Cell Physiol. 2006;291(5):C1082–1088. doi: 10.1152/ajpcell.00217.2006. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E. Injury and repair mechanisms in ischemic stroke: considerations for the development of novel neurotherapeutics. Curr Opin Investig Drugs. 2009;10(7):644–654. [PubMed] [Google Scholar]
- Cannon RE, Peart JC, Hawkins BT, Campos CR, Miller DS. Targeting blood-brain barrier sphingolipid signaling reduces basal P-glycoprotein activity and improves drug delivery to the brain. Proc Natl Acad Sci U S A. 2012;109(39):15930–15935. doi: 10.1073/pnas.1203534109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaitanya GV, Steven AJ, Babu PP. PARP-1 cleavage fragments: signatures of cell-death proteases in neurodegeneration. Cell Commun Signal. 2010;8:31. doi: 10.1186/1478-811x-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZM, Sandercock P, Pan HC, Counsell C, Collins R, Liu LS, et al. Peto R. Indications for early aspirin use in acute ischemic stroke : A combined analysis of 40 000 randomized patients from the chinese acute stroke trial and the international stroke trial. On behalf of the CAST and IST collaborative groups. Stroke. 2000;31(6):1240–1249. doi: 10.1161/01.str.31.6.1240. [DOI] [PubMed] [Google Scholar]
- Cheng G, Wei L, Zhi-Dan S, Shi-Guang Z, Xiang-Zhen L. Atorvastatin ameliorates cerebral vasospasm and early brain injury after subarachnoid hemorrhage and inhibits caspase-dependent apoptosis pathway. BMC Neurosci. 2009;10:7. doi: 10.1186/1471-2202-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla MJ, Crete R, Vitullo L, Rix RD. Transcellular transport as a mechanism of blood-brain barrier disruption during stroke. Front Biosci. 2004;9:777–785. doi: 10.2741/1282. [DOI] [PubMed] [Google Scholar]
- Cui L, Zhang X, Yang R, Wang L, Liu L, Li M, Du W. Neuroprotection of early and short-time applying atorvastatin in the acute phase of cerebral ischemia: down-regulated 12/15-LOX, p38MAPK and cPLA2 expression, ameliorated BBB permeability. Brain Res. 2010;1325:164–173. doi: 10.1016/j.brainres.2010.02.036. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, McDonald MC, Mota-Filipe H, Mazzon E, Costantino G, Britti D, et al. Thiemermann C. Beneficial effects of tempol, a membrane-permeable radical scavenger, in a rodent model of collagen-induced arthritis. Arthritis Rheum. 2000;43(2):320–328. doi: 10.1002/1529-0131(200002)43:2<320∷AID-ANR11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Dagenais C, Graff CL, Pollack GM. Variable modulation of opioid brain uptake by P-glycoprotein in mice. Biochem Pharmacol. 2004;67(2):269–276. doi: 10.1016/j.bcp.2003.08.027. [DOI] [PubMed] [Google Scholar]
- Dallas S, Miller DS, Bendayan R. Multidrug resistance-associated proteins: expression and function in the central nervous system. Pharmacol Rev. 2006;58(2):140–161. doi: 10.1124/pr.58.2.3. [DOI] [PubMed] [Google Scholar]
- Dankbaar JW, Hom J, Schneider T, Cheng SC, Lau BC, van der Schaaf I, et al. Wintermark M. Dynamic perfusion CT assessment of the blood-brain barrier permeability: first pass versus delayed acquisition. AJNR Am J Neuroradiol. 2008;29(9):1671–1676. doi: 10.3174/ajnr.A1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davignon J, Jacob RF, Mason RP. The antioxidant effects of statins. Coron Artery Dis. 2004;15(5):251–258. doi: 10.1097/01.mca.0000131573.31966.34. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ, Hallenbeck JM. Advances in the vascular pathophysiology of ischemic stroke. Thromb Res. 2000;98(3):73–81. doi: 10.1016/s0049-3848(00)00218-8. [DOI] [PubMed] [Google Scholar]
- Deng-Bryant Y, Singh IN, Carrico KM, Hall ED. Neuroprotective effects of tempol, a catalytic scavenger of peroxynitrite-derived free radicals, in a mouse traumatic brain injury model. J Cereb Blood Flow Metab. 2008;28(6):1114–1126. doi: 10.1038/jcbfm.2008.10. [DOI] [PubMed] [Google Scholar]
- Denora N, Trapani A, Laquintana V, Lopedota A, Trapani G. Recent advances in medicinal chemistry and pharmaceutical technology--strategies for drug delivery to the brain. Curr Top Med Chem. 2009;9(2):182–196. doi: 10.2174/156802609787521571. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425(6958):577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Desai MP, Labhasetwar V, Walter E, Levy RJ, Amidon GL. The mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependent. Pharm Res. 1997;14(11):1568–1573. doi: 10.1023/a:1012126301290. [DOI] [PubMed] [Google Scholar]
- Domoki F, Kis B, Gaspar T, Snipes JA, Parks JS, Bari F, Busija DW. Rosuvastatin induces delayed preconditioning against oxygen-glucose deprivation in cultured cortical neurons. Am J Physiol Cell Physiol. 2009;296(1):C97–105. doi: 10.1152/ajpcell.00366.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle KP, Cekanaviciute E, Mamer LE, Buckwalter MS. TGFbeta signaling in the brain increases with aging and signals to astrocytes and innate immune cells in the weeks after stroke. J Neuroinflammation. 2010;7:62. doi: 10.1186/1742-2094-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfeber K, Kohler A, Lutzenburg M, Osswald C, Galla HJ, Witte OW, Koepsell H. Localization of the Na+-D-glucose cotransporter SGLT1 in the blood-brain barrier. Histochem Cell Biol. 2004;121(3):201–207. doi: 10.1007/s00418-004-0633-9. [DOI] [PubMed] [Google Scholar]
- Endres M, Laufs U, Huang Z, Nakamura T, Huang P, Moskowitz MA, Liao JK. Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1998;95(15):8880–8885. doi: 10.1073/pnas.95.15.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian RH, DeWitt DS, Kent TA. In vivo detection of superoxide anion production by the brain using a cytochrome c electrode. J Cereb Blood Flow Metab. 1995;15(2):242–247. doi: 10.1038/jcbfm.1995.30. [DOI] [PubMed] [Google Scholar]
- Fang YZ, Yang S, Wu G. Free radicals, antioxidants, and nutrition. Nutrition. 2002;18(10):872–879. doi: 10.1016/s0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- Fellman V, Raivio KO. Reperfusion injury as the mechanism of brain damage after perinatal asphyxia. Pediatr Res. 1997;41(5):599–606. doi: 10.1203/00006450-199705000-00001. [DOI] [PubMed] [Google Scholar]
- Feng W, Belagaje SR. Recent advances in stroke recovery and rehabilitation. Semin Neurol. 2013;33(5):498–506. doi: 10.1055/s-0033-1364215. [DOI] [PubMed] [Google Scholar]
- Fischer S, Gerriets T, Wessels C, Walberer M, Kostin S, Stolz E, et al. Preissner KT. Extracellular RNA mediates endothelial-cell permeability via vascular endothelial growth factor. Blood. 2007;110(7):2457–2465. doi: 10.1182/blood-2006-08-040691. [DOI] [PubMed] [Google Scholar]
- Gao B, Hagenbuch B, Kullak-Ublick GA, Benke D, Aguzzi A, Meier PJ. Organic anion-transporting polypeptides mediate transport of opioid peptides across blood-brain barrier. J Pharmacol Exp Ther. 2000;294(1):73–79. [PubMed] [Google Scholar]
- Ghosh A, Sarkar S, Mandal AK, Das N. Neuroprotective role of nanoencapsulated quercetin in combating ischemia-reperfusion induced neuronal damage in young and aged rats. PLoS One. 2013;8(4):e57735. doi: 10.1371/journal.pone.0057735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Das N, Mandal AK, Dungdung SR, Sarkar S. Mannosylated liposomal cytidine 5′ diphosphocholine prevent age related global moderate cerebral ischemia reperfusion induced mitochondrial cytochrome c release in aged rat brain. Neuroscience. 2010;171(4):1287–1299. doi: 10.1016/j.neuroscience.2010.09.049. [DOI] [PubMed] [Google Scholar]
- Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 2002;21(7):1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol. 2006;91(5):807–819. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- Hans ML, Lowman AM. Biodegradable nanoparticles for drug delivery and targeting. Current Opinion in Solid State and Materials Science. 2002;6(4):319–327. doi:dx.doi.org/10.1016/S1359-0286(02)00117-1. [Google Scholar]
- Hatashita S, Hoff JT. Role of blood-brain barrier permeability in focal ischemic brain edema. Adv Neurol. 1990;52:327–333. [PubMed] [Google Scholar]
- Hawkins BT, Ocheltree SM, Norwood KM, Egleton RD. Decreased blood-brain barrier permeability to fluorescein in streptozotocin-treated rats. Neurosci Lett. 2007;411(1):1–5. doi: 10.1016/j.neulet.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BT, Sykes DB, Miller DS. Rapid, reversible modulation of blood-brain barrier P-glycoprotein transport activity by vascular endothelial growth factor. J Neurosci. 2010;30(4):1417–1425. doi: 10.1523/JNEUROSCI.5103-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Pu H, Andras IE, Eum SY, Yamauchi A, Hennig B, Toborek M. HIV-TAT protein upregulates expression of multidrug resistance protein 1 in the blood-brain barrier. J Cereb Blood Flow Metab. 2006;26(8):1052–1065. doi: 10.1038/sj.jcbfm.9600254. [DOI] [PubMed] [Google Scholar]
- Henning EC, Latour LL, Warach S. Verification of enhancement of the CSF space, not parenchyma, in acute stroke patients with early blood-brain barrier disruption. J Cereb Blood Flow Metab. 2008;28(5):882–886. doi: 10.1038/sj.jcbfm.9600598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo JH, Han SW, Lee SK. Free radicals as triggers of brain edema formation after stroke. Free Radic Biol Med. 2005;39(1):51–70. doi: 10.1016/j.freeradbiomed.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Ho RH, Tirona RG, Leake BF, Glaeser H, Lee W, Lemke CJ, et al. Kim RB. Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics. Gastroenterology. 2006;130(6):1793–1806. doi: 10.1053/j.gastro.2006.02.034. [DOI] [PubMed] [Google Scholar]
- Huang L, Wang Y, Grimm S. ATP-dependent transport of rosuvastatin in membrane vesicles expressing breast cancer resistance protein. Drug Metab Dispos. 2006;34(5):738–742. doi: 10.1124/dmd.105.007534. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Alexander M. Cerebral ischemia and inflammation. Curr Opin Neurol. 2001;14(1):89–94. doi: 10.1097/00019052-200102000-00014. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Bhattacharjee AK, Kondoh T, Nagashima T, Tamaki N. Synergistic effect of cold mannitol and Na(+)/Ca(2+) exchange blocker on blood-brain barrier opening. Biochem Biophys Res Commun. 2002;291(3):669–674. doi: 10.1006/bbrc.2002.6495. [DOI] [PubMed] [Google Scholar]
- Ishihara H, Kubota H, Lindberg RL, Leppert D, Gloor SM, Errede M, et al. Frei K. Endothelial cell barrier impairment induced by glioblastomas and transforming growth factor beta2 involves matrix metalloproteinases and tight junction proteins. J Neuropathol Exp Neurol. 2008;67(5):435–448. doi: 10.1097/NEN.0b013e31816fd622. [DOI] [PubMed] [Google Scholar]
- Jahan R, Vinuela F. Treatment of acute ischemic stroke: intravenous and endovascular therapies. Expert Rev Cardiovasc Ther. 2009;7(4):375–387. doi: 10.1586/erc.09.13. [DOI] [PubMed] [Google Scholar]
- Kassan M, Montero MJ, Sevilla MA. In vitro antioxidant activity of pravastatin provides vascular protection. Eur J Pharmacol. 2010;630(1-3):107–111. doi: 10.1016/j.ejphar.2009.12.037. [DOI] [PubMed] [Google Scholar]
- Kastrup A, Groschel K, Ringer TM, Redecker C, Cordesmeyer R, Witte OW, Terborg C. Early disruption of the blood-brain barrier after thrombolytic therapy predicts hemorrhage in patients with acute stroke. Stroke. 2008;39(8):2385–2387. doi: 10.1161/STROKEAHA.107.505420. [DOI] [PubMed] [Google Scholar]
- Kempski O. Cerebral edema. Semin Nephrol. 2001;21(3):303–307. doi: 10.1053/snep.2001.21665. [DOI] [PubMed] [Google Scholar]
- Kiedrowski L. NCX and NCKX operation in ischemic neurons. Ann N Y Acad Sci. 2007;1099:383–395. doi: 10.1196/annals.1387.035. [DOI] [PubMed] [Google Scholar]
- Kim CK, Kim T, Choi IY, Soh M, Kim D, Kim YJ, et al. Hyeon T. Ceria nanoparticles that can protect against ischemic stroke. Angew Chem Int Ed Engl. 2012;51(44):11039–11043. doi: 10.1002/anie.201203780. [DOI] [PubMed] [Google Scholar]
- Kim HR, Andrieux K, Gil S, Taverna M, Chacun H, Desmaele D, et al. Couvreur P. Translocation of poly (ethylene glycol-co-hexadecyl) cyanoacrylate nanoparticles into rat brain endothelial cells: role of apolipoproteins in receptor-mediated endocytosis. Biomacromolecules. 2007;8(3):793–799. doi: 10.1021/bm060711a. [DOI] [PubMed] [Google Scholar]
- Kim MY, Zhang T, Kraus WL. Poly (ADP-ribosyl)ation by PARP-1: ‘PAR-laying’ NAD+ into a nuclear signal. Genes Dev. 2005;19(17):1951–1967. doi: 10.1101/gad.1331805. [DOI] [PubMed] [Google Scholar]
- Kis B, Isse T, Snipes JA, Chen L, Yamashita H, Ueta Y, Busija DW. Effects of LPS stimulation on the expression of prostaglandin carriers in the cells of the blood-brain and blood-cerebrospinal fluid barriers. J Appl Physiol (1985) 2006;100(4):1392–1399. doi: 10.1152/japplphysiol.01259.2005. [DOI] [PubMed] [Google Scholar]
- Kreuter J. Nanoparticulate systems for brain delivery of drugs. Adv Drug Deliv Rev. 2001;47(1):65–81. doi: 10.1016/s0169-409x(00)00122-8. [DOI] [PubMed] [Google Scholar]
- Kreuter J, Ramge P, Petrov V, Hamm S, Gelperina SE, Engelhardt B, et al. Begley DJ. Direct evidence that polysorbate-80-coated poly (butylcyanoacrylate) nanoparticles deliver drugs to the CNS via specific mechanisms requiring prior binding of drug to the nanoparticles. Pharm Res. 2003;20(3):409–416. doi: 10.1023/a:1022604120952. [DOI] [PubMed] [Google Scholar]
- Kuhn EW, Liakopoulos OJ, Stange S, Deppe AC, Slottosch I, Scherner M, et al. Wahlers T. Meta-analysis of patients taking statins before revascularization and aortic valve surgery. Ann Thorac Surg. 2013;96(4):1508–1516. doi: 10.1016/j.athoracsur.2013.04.096. [DOI] [PubMed] [Google Scholar]
- Kulik A, Ruel M. Statins and coronary artery bypass graft surgery: preoperative and postoperative efficacy and safety. Expert Opin Drug Saf. 2009;8(5):559–571. doi: 10.1517/14740330903188413. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Hagenbuch B, Stieger B, Schteingart CD, Hofmann AF, Wolkoff AW, Meier PJ. Molecular and functional characterization of an organic anion transporting polypeptide cloned from human liver. Gastroenterology. 1995;109(4):1274–1282. doi: 10.1016/0016-5085(95)90588-x. [DOI] [PubMed] [Google Scholar]
- Kwon TH, Chao DL, Malloy K, Sun D, Alessandri B, Bullock MR. Tempol, a novel stable nitroxide, reduces brain damage and free radical production, after acute subdural hematoma in the rat. J Neurotrauma. 2003;20(4):337–345. doi: 10.1089/089771503765172291. [DOI] [PubMed] [Google Scholar]
- Lebrin F, Deckers M, Bertolino P, Ten Dijke P. TGF-beta receptor function in the endothelium. Cardiovasc Res. 2005;65(3):599–608. doi: 10.1016/j.cardiores.2004.10.036. [DOI] [PubMed] [Google Scholar]
- Lim JH, Lee JC, Lee YH, Choi IY, Oh YK, Kim HS, et al. Kim WK. Simvastatin prevents oxygen and glucose deprivation/reoxygenation-induced death of cortical neurons by reducing the production and toxicity of 4-hydroxy-2E-nonenal. J Neurochem. 2006;97(1):140–150. doi: 10.1111/j.1471-4159.2006.03715.x. [DOI] [PubMed] [Google Scholar]
- Liu S, Levine SR, Winn HR. Targeting ischemic penumbra: part I - from pathophysiology to therapeutic strategy. J Exp Stroke Transl Med. 2010;3(1):47–55. doi: 10.6030/1939-067x-3.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead JJ, McCaffrey G, Quigley CE, Finch J, DeMarco KM, Nametz N, Davis TP. Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation. J Cereb Blood Flow Metab. 2010;30(9):1625–1636. doi: 10.1038/jcbfm.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead JJ, McCaffrey G, Sanchez-Covarrubias L, Finch JD, Demarco KM, Quigley CE, et al. Ronaldson PT. Tempol modulates changes in xenobiotic permeability and occludin oligomeric assemblies at the blood-brain barrier during inflammatory pain. Am J Physiol Heart Circ Physiol. 2012;302(3):H582–593. doi: 10.1152/ajpheart.00889.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Wang Y, Chen H, Kintner DB, Cramer SW, Gerdts JK, et al. Sun D. A concerted role of Na+ -K+ -Cl- cotransporter and Na+/Ca2+ exchanger in ischemic damage. J Cereb Blood Flow Metab. 2008;28(4):737–746. doi: 10.1038/sj.jcbfm.9600561. [DOI] [PubMed] [Google Scholar]
- Lutsep H, Clark W. An update of neuroprotectants in clinical development for acute stroke. Curr Opin Investig Drugs. 2001;2(12):1732–1736. [PubMed] [Google Scholar]
- Mandery K, Sticht H, Bujok K, Schmidt I, Fahrmayr C, Balk B, et al. Glaeser H. Functional and structural relevance of conserved positively charged lysine residues in organic anion transporting polypeptide 1B3. Mol Pharmacol. 2011;80(3):400–406. doi: 10.1124/mol.111.071282. [DOI] [PubMed] [Google Scholar]
- Marrache S, Pathak RK, Darley KL, Choi JH, Zaver D, Kolishetti N, Dhar S. Nanocarriers for tracking and treating diseases. Curr Med Chem. 2013;20(28):3500–3514. doi: 10.2174/0929867311320280007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Romero R, Canuelo A, Martinez-Lara E, Javier Oliver F, Cardenas S, Siles E. Poly (ADP-ribose) polymerase-1 modulation of in vivo response of brain hypoxia-inducible factor-1 to hypoxia/reoxygenation is mediated by nitric oxide and factor inhibiting HIF. J Neurochem. 2009;111(1):150–159. doi: 10.1111/j.1471-4159.2009.06307.x. [DOI] [PubMed] [Google Scholar]
- McCaffrey G, Willis CL, Staatz WD, Nametz N, Quigley CA, Hom S, et al. Davis TP. Occludin oligomeric assemblies at tight junctions of the blood-brain barrier are altered by hypoxia and reoxygenation stress. J Neurochem. 2009;110(1):58–71. doi: 10.1111/j.1471-4159.2009.06113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messe SR, Fonarow GC, Smith EE, Kaltenbach L, Olson DM, Kasner SE, Schwamm LH. Use of tissue-type plasminogen activator before and after publication of the European Cooperative Acute Stroke Study III in Get With The Guidelines-Stroke. Circ Cardiovasc Qual Outcomes. 2012;5(3):321–326. doi: 10.1161/circoutcomes.111.964064. [DOI] [PubMed] [Google Scholar]
- Miyajima M, Kusuhara H, Fujishima M, Adachi Y, Sugiyama Y. Organic anion transporter 3 mediates the efflux transport of an amphipathic organic anion, dehydroepiandrosterone sulfate, across the blood-brain barrier in mice. Drug Metab Dispos. 2011;39(5):814–819. doi: 10.1124/dmd.110.036863. [DOI] [PubMed] [Google Scholar]
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen GT, Coulthard A, Wong A, Sheikh N, Henderson R, O'Sullivan JD, Reutens DC. Measurement of blood-brain barrier permeability in acute ischemic stroke using standard first-pass perfusion CT data. Neuroimage Clin. 2013;2:658–662. doi: 10.1016/j.nicl.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell ME, Lam TI, Tran LQ, Foroutan S, Anderson SE. Estradiol reduces activity of the blood-brain barrier Na-K-Cl cotransporter and decreases edema formation in permanent middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2006;26(10):1234–1249. doi: 10.1038/sj.jcbfm.9600278. [DOI] [PubMed] [Google Scholar]
- O'Donnell ME, Tran L, Lam TI, Liu XB, Anderson SE. Bumetanide inhibition of the blood-brain barrier Na-K-Cl cotransporter reduces edema formation in the rat middle cerebral artery occlusion model of stroke. J Cereb Blood Flow Metab. 2004;24(9):1046–1056. doi: 10.1097/01.WCB.0000130867.32663.90. [DOI] [PubMed] [Google Scholar]
- Ohtsuki S, Tomi M, Hata T, Nagai Y, Hori S, Mori S, et al. Terasaki T. Dominant expression of androgen receptors and their functional regulation of organic anion transporter 3 in rat brain capillary endothelial cells; comparison of gene expression between the blood-brain and -retinal barriers. J Cell Physiol. 2005;204(3):896–900. doi: 10.1002/jcp.20352. [DOI] [PubMed] [Google Scholar]
- Ose A, Kusuhara H, Endo C, Tohyama K, Miyajima M, Kitamura S, Sugiyama Y. Functional characterization of mouse organic anion transporting peptide 1a4 in the uptake and efflux of drugs across the blood-brain barrier. Drug Metab Dispos. 2010;38(1):168–176. doi: 10.1124/dmd.109.029454. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87(1):315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan HC, Yang DY, Ou YC, Ho SP, Cheng FC, Chen CJ. Neuroprotective effect of atorvastatin in an experimental model of nerve crush injury. Neurosurgery. 2010;67(2):376–388. doi: 10.1227/01.neu.0000371729.47895.a0. discussion 388-379. [DOI] [PubMed] [Google Scholar]
- Pepper MS. Transforming growth factor-beta: vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev. 1997;8(1):21–43. doi: 10.1016/s1359-6101(96)00048-2. [DOI] [PubMed] [Google Scholar]
- Petty MA, Wettstein JG. Elements of cerebral microvascular ischaemia. Brain Res Brain Res Rev. 2001;36(1):23–34. doi: 10.1016/s0165-0173(01)00062-5. [DOI] [PubMed] [Google Scholar]
- Pillai DR, Dittmar MS, Baldaranov D, Heidemann RM, Henning EC, Schuierer G, et al. Schlachetzki F. Cerebral ischemia-reperfusion injury in rats--a 3 T MRI study on biphasic blood-brain barrier opening and the dynamics of edema formation. J Cereb Blood Flow Metab. 2009;29(11):1846–1855. doi: 10.1038/jcbfm.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzon-Daza ML, Campia I, Kopecka J, Garzon R, Ghigo D, Riganti C. Nanoparticle- and liposome-carried drugs: new strategies for active targeting and drug delivery across blood-brain barrier. Curr Drug Metab. 2013;14(6):625–640. doi: 10.2174/1389200211314060001. [DOI] [PubMed] [Google Scholar]
- Plateel M, Teissier E, Cecchelli R. Hypoxia dramatically increases the nonspecific transport of blood-borne proteins to the brain. J Neurochem. 1997;68(2):874–877. doi: 10.1046/j.1471-4159.1997.68020874.x. [DOI] [PubMed] [Google Scholar]
- Prades R, Guerrero S, Araya E, Molina C, Salas E, Zurita E, et al. Giralt E. Delivery of gold nanoparticles to the brain by conjugation with a peptide that recognizes the transferrin receptor. Biomaterials. 2012;33(29):7194–7205. doi: 10.1016/j.biomaterials.2012.06.063. [DOI] [PubMed] [Google Scholar]
- Pundik S, Xu K, Sundararajan S. Reperfusion brain injury: focus on cellular bioenergetics. Neurology. 2012;79(13 Suppl 1):S44–51. doi: 10.1212/WNL.0b013e3182695a14. [DOI] [PubMed] [Google Scholar]
- Rak R, Chao DL, Pluta RM, Mitchell JB, Oldfield EH, Watson JC. Neuroprotection by the stable nitroxide Tempol during reperfusion in a rat model of transient focal ischemia. J Neurosurg. 2000;92(4):646–651. doi: 10.3171/jns.2000.92.4.0646. [DOI] [PubMed] [Google Scholar]
- Ramge P, Unger RE, Oltrogge JB, Zenker D, Begley D, Kreuter J, Von Briesen H. Polysorbate-80 coating enhances uptake of polybutylcyanoacrylate (PBCA)-nanoparticles by human and bovine primary brain capillary endothelial cells. Eur J Neurosci. 2000;12(6):1931–1940. doi: 10.1046/j.1460-9568.2000.00078.x. [DOI] [PubMed] [Google Scholar]
- Ronaldson PT, Davis TP. Targeting blood-brain barrier changes during inflammatory pain: an opportunity for optimizing CNS drug delivery. Ther Deliv. 2011;2(8):1015–1041. doi: 10.4155/tde.11.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson PT, Davis TP. Blood-brain barrier integrity and glial support: mechanisms that can be targeted for novel therapeutic approaches in stroke. Curr Pharm Des. 2012;18(25):3624–3644. doi: 10.2174/138161212802002625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson PT, Davis TP. Targeted drug delivery to treat pain and cerebral hypoxia. Pharmacol Rev. 2013;65(1):291–314. doi: 10.1124/pr.112.005991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson PT, Demarco KM, Sanchez-Covarrubias L, Solinsky CM, Davis TP. Transforming growth factor-beta signaling alters substrate permeability and tight junction protein expression at the blood-brain barrier during inflammatory pain. J Cereb Blood Flow Metab. 2009;29(6):1084–1098. doi: 10.1038/jcbfm.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson PT, Finch JD, Demarco KM, Quigley CE, Davis TP. Inflammatory pain signals an increase in functional expression of organic anion transporting polypeptide 1a4 at the blood-brain barrier. J Pharmacol Exp Ther. 2011;336(3):827–839. doi: 10.1124/jpet.110.174151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Takeshita K, Ueda J, Ozawa T. Two reaction sites of a spin label, TEMPOL (4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl), with hydroxyl radical. J Pharm Sci. 2003;92(2):275–280. doi: 10.1002/jps.10304. [DOI] [PubMed] [Google Scholar]
- Salvemini D, Doyle TM, Cuzzocrea S. Superoxide, peroxynitrite and oxidative/nitrative stress in inflammation. Biochem Soc Trans. 2006;34(Pt 5):965–970. doi: 10.1042/BST0340965. [DOI] [PubMed] [Google Scholar]
- Sanchez-Covarrubias L, Slosky LM, Thompson BJ, Davis TP, Ronaldson PT. Transporters at CNS Barrier Sites: Obstacles or Opportunities for Drug Delivery? Curr Pharm Des. 2013 doi: 10.2174/13816128113199990463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32(2):200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Schild L, Reiser G. Oxidative stress is involved in the permeabilization of the inner membrane of brain mitochondria exposed to hypoxia/reoxygenation and low micromolar Ca2+ FEBS J. 2005;272(14):3593–3601. doi: 10.1111/j.1742-4658.2005.04781.x. [DOI] [PubMed] [Google Scholar]
- Schreibelt G, Kooij G, Reijerkerk A, van Doorn R, Gringhuis SI, van der Pol S, et al. de Vries HE. Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J. 2007;21(13):3666–3676. doi: 10.1096/fj.07-8329com. [DOI] [PubMed] [Google Scholar]
- Seelbach MJ, Brooks TA, Egleton RD, Davis TP. Peripheral inflammatory hyperalgesia modulates morphine delivery to the brain: a role for P-glycoprotein. J Neurochem. 2007;102(5):1677–1690. doi: 10.1111/j.1471-4159.2007.04644.x. [DOI] [PubMed] [Google Scholar]
- Shah K, Abbruscato T. The Role of Blood-Brain Barrier Transporters in Pathophysiology and Pharmacotherapy of Stroke. Curr Pharm Des. 2013 doi: 10.2174/13816128113199990465. [DOI] [PubMed] [Google Scholar]
- Shishehbor MH, Aviles RJ, Brennan ML, Fu X, Goormastic M, Pearce GL, et al. Hazen SL. Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. JAMA. 2003;289(13):1675–1680. doi: 10.1001/jama.289.13.1675. [DOI] [PubMed] [Google Scholar]
- Sierra S, Ramos MC, Molina P, Esteo C, Vazquez JA, Burgos JS. Statins as neuroprotectants: a comparative in vitro study of lipophilicity, blood-brain-barrier penetration, lowering of brain cholesterol, and decrease of neuron cell death. J Alzheimers Dis. 2011;23(2):307–318. doi: 10.3233/JAD-2010-101179. [DOI] [PubMed] [Google Scholar]
- Simsek S, Eroglu H, Kurum B, Ulubayram K. Brain targeting of Atorvastatin loaded amphiphilic PLGA-b-PEG nanoparticles. J Microencapsul. 2013;30(1):10–20. doi: 10.3109/02652048.2012.692400. [DOI] [PubMed] [Google Scholar]
- Singh R, Lillard JW., Jr Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 2009;86(3):215–223. doi: 10.1016/j.yexmp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal A, Morris VB, Labhasetwar V, Ghorpade A. Nanoparticle-mediated catalase delivery protects human neurons from oxidative stress. Cell Death Dis. 2013;4:e903. doi: 10.1038/cddis.2013.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sironi L, Cimino M, Guerrini U, Calvio AM, Lodetti B, Asdente M, et al. Tremoli E. Treatment with statins after induction of focal ischemia in rats reduces the extent of brain damage. Arterioscler Thromb Vasc Biol. 2003;23(2):322–327. doi: 10.1161/01.atv.0000044458.23905.3b. [DOI] [PubMed] [Google Scholar]
- Steckelbroeck S, Nassen A, Ugele B, Ludwig M, Watzka M, Reissinger A, et al. Hans VH. Steroid sulfatase (STS) expression in the human temporal lobe: enzyme activity, mRNA expression and immunohistochemistry study. J Neurochem. 2004;89(2):403–417. doi: 10.1046/j.1471-4159.2004.02336.x. [DOI] [PubMed] [Google Scholar]
- Suh H, Jeong B, Rathi R, Kim SW. Regulation of smooth muscle cell proliferation using paclitaxel-loaded poly (ethylene oxide)-poly (lactide/glycolide) nanospheres. J Biomed Mater Res. 1998;42(2):331–338. doi: 10.1002/(sici)1097-4636(199811)42:2<331::aid-jbm19>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Therade-Matharan S, Laemmel E, Carpentier S, Obata Y, Levade T, Duranteau J, Vicaut E. Reactive oxygen species production by mitochondria in endothelial cells exposed to reoxygenation after hypoxia and glucose depletion is mediated by ceramide. Am J Physiol Regul Integr Comp Physiol. 2005;289(6):R1756–1762. doi: 10.1152/ajpregu.00480.2004. [DOI] [PubMed] [Google Scholar]
- Thompson BJ, Sanchez-Covarrubias L, Slosky LM, Zhang Y, Laracuente ML, Ronaldson PT. Hypoxia/reoxygenation stress signals an increase in organic anion transporting polypeptide 1a4(Oatp1a4) at the blood-brain barrier: relevance to CNS drug delivery. J Cereb Blood Flow Metab. 2014 doi: 10.1038/jcbfm.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu YF, Lu PJ, Huang CC, Ho CJ, Chou YP. Moderate dietary restriction reduces p53-mediated neurovascular damage and microglia activation after hypoxic ischemia in neonatal brain. Stroke. 2012;43(2):491–498. doi: 10.1161/STROKEAHA.111.629931. [DOI] [PubMed] [Google Scholar]
- Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552(Pt 2):335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bruggen N, Thibodeaux H, Palmer JT, Lee WP, Fu L, Cairns B, et al. Ferrara N. VEGF antagonism reduces edema formation and tissue damage after ischemia/reperfusion injury in the mouse brain. J Clin Invest. 1999;104(11):1613–1620. doi: 10.1172/JCI8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemula S, Roder KE, Yang T, Bhat GJ, Thekkumkara TJ, Abbruscato TJ. A functional role for sodium-dependent glucose transport across the blood-brain barrier during oxygen glucose deprivation. J Pharmacol Exp Ther. 2009;328(2):487–495. doi: 10.1124/jpet.108.146589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibbert M, Mayer SA. Early decompressive hemicraniectomy following malignant ischemic stroke: the crucial role of timing. Curr Neurol Neurosci Rep. 2010;10(1):1–3. doi: 10.1007/s11910-009-0081-y. [DOI] [PubMed] [Google Scholar]
- Wallace BK, Foroutan S, O'Donnell ME. Ischemia-induced stimulation of Na-K-Cl cotransport in cerebral microvascular endothelial cells involves AMP kinase. Am J Physiol Cell Physiol. 2011;301(2):C316–326. doi: 10.1152/ajpcell.00517.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe T, Nishihara A, Mishima K, Yamashita J, Shimizu K, Miyazawa K, et al. Miyazono K. TGF-beta receptor kinase inhibitor enhances growth and integrity of embryonic stem cell-derived endothelial cells. J Cell Biol. 2003;163(6):1303–1311. doi: 10.1083/jcb.200305147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westholm DE, Stenehjem DD, Rumbley JN, Drewes LR, Anderson GW. Competitive inhibition of organic anion transporting polypeptide 1c1-mediated thyroxine transport by the fenamate class of nonsteroidal antiinflammatory drugs. Endocrinology. 2009;150(2):1025–1032. doi: 10.1210/en.2008-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis CL, Leach L, Clarke GJ, Nolan CC, Ray DE. Reversible disruption of tight junction complexes in the rat blood-brain barrier, following transitory focal astrocyte loss. Glia. 2004;48(1):1–13. doi: 10.1002/glia.20049. [DOI] [PubMed] [Google Scholar]
- Windass AS, Lowes S, Wang Y, Brown CD. The contribution of organic anion transporters OAT1 and OAT3 to the renal uptake of rosuvastatin. J Pharmacol Exp Ther. 2007;322(3):1221–1227. doi: 10.1124/jpet.107.125831. [DOI] [PubMed] [Google Scholar]
- Witt KA, Mark KS, Hom S, Davis TP. Effects of hypoxia-reoxygenation on rat blood-brain barrier permeability and tight junctional protein expression. Am J Physiol Heart Circ Physiol. 2003;285(6):H2820–2831. doi: 10.1152/ajpheart.00589.2003. [DOI] [PubMed] [Google Scholar]
- Witt KA, Mark KS, Huber J, Davis TP. Hypoxia-inducible factor and nuclear factor kappa-B activation in blood-brain barrier endothelium under hypoxic/reoxygenation stress. J Neurochem. 2005;92(1):203–214. doi: 10.1111/j.1471-4159.2004.02871.x. [DOI] [PubMed] [Google Scholar]
- Wu X, Ma J, Han JD, Wang N, Chen YG. Distinct regulation of gene expression in human endothelial cells by TGF-beta and its receptors. Microvasc Res. 2006;71(1):12–19. doi: 10.1016/j.mvr.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Xie F, Yao N, Qin Y, Zhang Q, Chen H, Yuan M, et al. He Q. Investigation of glucose-modified liposomes using polyethylene glycols with different chain lengths as the linkers for brain targeting. Int J Nanomedicine. 2012;7:163–175. doi: 10.2147/IJN.S23771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Shah K, Wang H, Karamyan VT, Abbruscato TJ. Characterization of neuroprotective effects of biphalin, an opioid receptor agonist, in a model of focal brain ischemia. J Pharmacol Exp Ther. 2011;339(2):499–508. doi: 10.1124/jpet.111.184127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Wang H, Shah K, Karamyan VT, Abbruscato TJ. Opioid receptor agonists reduce brain edema in stroke. Brain Res. 2011;1383:307–316. doi: 10.1016/j.brainres.2011.01.083. [DOI] [PubMed] [Google Scholar]
- Yeh WL, Lin CJ, Fu WM. Enhancement of glucose transporter expression of brain endothelial cells by vascular endothelial growth factor derived from glioma exposed to hypoxia. Mol Pharmacol. 2008;73(1):170–177. doi: 10.1124/mol.107.038851. [DOI] [PubMed] [Google Scholar]
- Zhang DX, Gutterman DD. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am J Physiol Heart Circ Physiol. 2007;292(5):H2023–2031. doi: 10.1152/ajpheart.01283.2006. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Patzer A, Herdegen T, Gohlke P, Culman J. Activation of cerebral peroxisome proliferator-activated receptors gamma promotes neuroprotection by attenuation of neuronal cyclooxygenase-2 overexpression after focal cerebral ischemia in rats. FASEB J. 2006;20(8):1162–1175. doi: 10.1096/fj.05-5007com. [DOI] [PubMed] [Google Scholar]
- Zhelev Z, Bakalova R, Aoki I, Matsumoto K, Gadjeva V, Anzai K, Kanno I. Nitroxyl radicals for labeling of conventional therapeutics and noninvasive magnetic resonance imaging of their permeability for blood-brain barrier: relationship between structure, blood clearance, and MRI signal dynamic in the brain. Mol Pharm. 2009;6(2):504–512. doi: 10.1021/mp800175k. [DOI] [PubMed] [Google Scholar]