Abstract
The protein palmitoylation cycle has been shown to be important for protein signaling and synaptic plasticity. Data from our lab showed a change in the palmitoylation status of certain proteins with age. A greater percentage of the NMDA receptor subunits GluN2A and GluN2B, along with Fyn and PSD95 proteins, were palmitoylated in the old mice. The higher level of protein palmitoylation was also associated with poorer learning scores. Xanthohumol is a prenylated flavonoid that has been shown to increase beta-oxidation in the livers of rodents, decreasing circulating free fatty acids in the serum. What is not known is whether the application of xanthohumol could influence the palmitoylation status of proteins. In this study, young and old mice were fed a diet supplemented with xanthohumol for 8 weeks. Spatial memory was assessed with the Morris water maze and protein palmitoylation quantified. The young xanthohumol-treated mice showed a significant improvement in cognitive flexibility. However, this appeared to be associated with the young control mice, on a defined, phytoestrogen-deficient diet, performing as poorly as the old mice and xanthohumol reversing this effect. The old mice receiving xanthohumol did not significantly improve their learning scores. Xanthohumol treatment was unable to affect the palmitoylation of NMDA receptor subunits and associated proteins assessed in this study. This evidence suggests that xanthohumol may play a role in improving cognitive flexability in young animals, but it appears to be ineffective in adjusting the palmitoylation status of neuronal proteins in aged individuals.
Keywords: palmitoylation, reversal trials, aging, memory, palmitate
Introduction
Memory is a process that is vulnerable to degradation with the advancement of age. Indeed, many adults start to experience deficits in memory around 50 years of age and some aspects of cognition begin to decline around 40 years of age [1, 2]. Cognitive decline related to aging affects many mammalian species, from humans to primates to rodents, therefore rodents have served as an appropriate model for cognitive aging [3].
Although there is a clear loss of cognitive function with increased age, normal aging is not associated with gross morphological changes in the brain. Studies have demonstrated that there appears to be no significant change in neuronal cell numbers in the prefrontal cortex or hippocampus of humans [4, 5], primates [6, 7], or rodents [8, 9]. Similarly, the extent of dendritic branching shows no significant age-related decline in the hippocampus of humans [10, 11], primates [12], or rodents [13]. Recent evidence suggests that the morphology of hippocampal synaptosomes and synaptic vesicles may not be altered with increased age [14]. Areas of the prefrontal cortex, however, may be vulnerable to dendritic loss with age in humans [15] and rodents [16], suggesting a potential difference in the modes of age-related decline in neuronal function in the prefrontal cortex and hippocampus.
While the morphology of neurons may not be greatly altered with age, the expression of many synaptic proteins is significantly affected by aging [17]. Specifically, the GluN2B subunit of the NMDA receptor and the scaffolding protein PSD-95 are particularly vulnerable to decline with increased age in the hippocampus of rodents [18, 19]. Recent evidence suggests that the relationship between PSD-95 and GluN2B may be altered during aging in the frontal cortices of mice [20]. Data from our lab [21] suggested that age-induced alterations in protein-protein interactions might be due to increased palmitoylation of those proteins, which may have led to the decline in spatial memory.
Protein palmitoylation is a post-translational modification whereby a 16 carbon fatty acid, palmitate, is covalently bound to a free sulfhydryl on the cysteine of a protein via a labile thioester bond. Palmitoyl-CoA, the esterified biologically active form of palmitate, serves as the substrate for palmitoyltransferase enzymes. Protein palmitoylation is an important regulator of synaptic plasticity [22]. The NMDA receptor subunits GluN2A and GluN2B are both palmitoylated under normal conditions [23]. Palmitoylation of these NMDA receptor subunits affects localization of the receptor by enhancing access of the Src kinase, Fyn, to its substrate on the C-terminus of the GluN2B subunit [23]. Fyn and PSD-95 are also palmitoylated, which enriches each protein on synaptic membranes [24, 25]. Interestingly, depalmitoylation of PSD-95 is required for internalization of AMPA receptors, but not NMDA receptors [24]. However, it may be that a global increase in the percentage of neuronal proteins that are palmitoylated could lead to significant perturbations in trafficking and localization of NMDA receptors. A consequence of aging in humans and rodents is a redistribution of fat deposition from adipose tissue to muscle and liver, leading to metabolic syndrome in many elderly [26]. Cellular metabolism undergoes an age-associated increase in lipogenesis [27]. Evidence also indicates that the lipid composition of the brain in rodents and humans changes with age. In humans, there is a small, but significant increase in the levels of palmitate in erythrocytes, which is matched in the cerebral cortex [28]. In mice, palmitate shifts more towards phosphatidylcholine (PC) membranes and the turnover rate decreases during aging [29]. Manipulations of fatty acids can affect memory. Mice fed a high fat diet performed much worse in the Morris water maze than control mice [30]. These studies indicate that aging results in perturbed fatty acid metabolism and the presence of increased fat can lead to poorer spatial memory. This led us to hypothesize that fatty acid changes during aging could contribute to the increase in palmitoylated proteins in aged individuals.
Xanthohumol is a prenylated chalconoid present in hops [31]. The compound has been used to successfully lower body weight and fasting glucose in a rat model of obesity, Zucker fa/fa [32]. Xanthohumol appears to increase beta-oxidation of fatty acids in the liver and reduce overall cellular oxidative stress [33]. What is not known is whether xanthohumol could specifically reduce total plasma palmitate and/or palmitoylation of proteins important to synaptic plasticity in an aged rodent.
We also addressed whether steady-state palmitoyl-CoA pools may be influencing the age-related increase in palmitoylated proteins in the frontal cortex of mice and whether these could be altered by xanthohumol. The goal of this study was to increase beta-oxidation in the livers of old mice with a dietary supplement, xanthohumol. The hypothesis tested was that supplementation would decrease the systemic levels of palmitate, which could lower the steady-state levels of palmitoyl-CoA and palmitoylation in the brain and lead to better spatial memory performance in old mice.
Materials and Methods
Animals
A total of 55 male C57BL/6 mice from two age groups (3 and 24 months of age) were used for the study. The older age group was obtained from National Institute on Aging, NIH. Young mice were purchased from JAX mice (Bar Harbor, MA), which stocks the NIH colony. They were fed ad libitum and housed with a 12/12 h light/dark cycle.
Treatment
A total of 49 mice (24 young, 25 old) were fed a diet with or without xanthohumol for a duration of 8 weeks. Xanthohumol was emulsified by mixing 547 mg of xanthohumol powder (gifted from Hopsteiner Inc. New York, N.Y.) with a solution (OPT) consisting of 12.6 g oleic acid, 14 g propylene glycol, and 14 g Tween 80. A modified diet (Dyets, Inc, Bethlehem, PA) was made by mixing 20 g of either OPT or xanthohumol emulsion with 1 kg of modified AIN-93G defined diet. The AIN-93G formula was made free of soy isoflavones by substituting corn oil for soybean oil. After the behavioral testing, all animals were euthanized by exposure to CO2 and decapitated. Blood was collected in heparin-treated vials (BD Bioscience, San Jose, CA) and stored on ice until further processed. The brains and livers were harvested, frozen in dry ice and stored at −80°C.
Six additional mice (3 young, 3 old), separate from the spatial memory study, were treated with xanthohumol via gavage in order to measure steady state xanthohumol concentrations in the cerebral cortex. Xanthohumol was emulsified in OPT to a final concentration of 1.4 mg xanthohumol/100 μl of OPT for old mice and 1 mg/100 μl OPT for the young mice. The mice were gavaged once daily with 100 μl of xanthohumol/OPT emulsion for a total of 5 days. All animals were euthanized by exposure to CO2 and decapitated, with the brain immediately harvested, frozen on dry ice, and stored at −80°C until further processed.
Behavioral testing
Spatial reference memory, cognitive flexibility and associative memory (cued control task) were tested with the use of the Morris water maze as previously described [34]. Briefly, for the first two days, mice were acclimated to the water maze, followed by 4 days of testing for spatial reference memory, 1 day of reversal training to test cognitive flexibility and 1 day of associative memory testing (cued control task). Reference memory consisted of 8 place trials per day and probe trials at the end of each day. A naive probe trial was performed at the beginning of the first day of memory testing. The platform was kept in the same quadrant for each place trial. Place trials consisted of a maximum of 60 seconds in the water searching for the platform, 30 seconds on the platform and 2 minutes of cage rest. If a mouse failed to find the platform within the designated 60 second swim time, it was led to the platform by the experimenter. Probe trials were performed to assess the animal’s ability to show a bias for the platform location. During the probe trial, the platform was removed and the mouse was allowed to search in the water for 30 seconds. After 4 days of place trials, a reversal task was performed in order to assess cognitive flexibility. The platform was placed in the opposite quadrant in the tank, but there were 8 place and 1 probe trial, similar to the reference memory task. Cued trials were designed to test motivation, visual acuity, and physical ability for the task. The mice performed 6 cued trials. The positions of entry and the platform positions varied between trials. The platform was kept submerged, but was marked by a 20.3 cm support with a flag. The mice were allowed to search for the platform for 60 seconds. The animal’s movements in the water maze were tracked and analyzed with the SMART tracking system (San Diego Instruments, San Diego, CA).
Tissue processing
The rostral 4 mm of the frontal cortex and the hippocampi from each brain were dissected. The remaining caudal cortex was reserved for xanthohumol assays and protein standards. The frontal cortices and hippocampi were homogenized on ice in a Dounce homogenizer with 500 μl of homogenization buffer. The homogenization buffer consisted of 10mM ammonium acetate (pH 5.3) with 1.0 nM 17:0 CoA internal standard (Avanti Polar Lipids, Alabaster, AL) and 10 mM N-ethylmaleimide (NEM). Homogenization involved 12 strokes from each of 2 pestles of increasing sizes in homogenization buffer, followed by addition of 1.0 ml of chloroform:methanol, (1:2, v/v) and 10 more strokes from the larger pestle. An additional 1.5 ml of chloroform:methanol (1:2, v/v) was added to the homogenate. The mixture was vortexed and centrifuged at 4500g for 15 minutes in a hanging bucket Beckman J2-HC centrifuge. The top layer, containing acyl-CoA; lower organic layer, and protein interface were all separated. The acyl-CoA and organic layers were purged with nitrogen and stored at −80°C until further processed. The protein pellet was resuspended in 4SB buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM ethylenediaminetetraacetic acid (EDTA), 4% sodium dodecyl sulfate (SDS), 10 mM NEM, and protease inhibitor cocktail (Sigma, Saint Louis, MO)) and aliquots were saved and stored at −80°C until further processed.
Blood samples were centrifuged at 1000g for 10 minutes in a tabletop Eppendorf 5415C centrifuge. The plasma was removed and stored at −80°C until further processed. Approximately 200 mg of liver tissue was weighed from each mouse and homogenized in 1.0 ml of ice cold methanol:water (90:10, v/v). Whole cortices (450–500 mg) from gavaged mice were homogenized in 2.0 ml of ice cold methanol:water (90:10, v/v). Samples were centrifuged at 4000g for 10 minutes in an Eppendorf 5415C centrifuge. The supernatant was removed and stored at −80°C until further processed.
Acyl-biotinyl exchange
In order to quantify levels of protein palmitoylation, proteins were subjected to the acyl-biotinyl exchange method as previously described [35]. Briefly, protein lysates from the separation described above were thawed and mixed with rotation at 4°C overnight. Excess NEM was stripped and proteins were precipitated with three sequential chloroform:methanol (1:3, v/v) precipitations. Precipitated proteins were solubilized in 300 μl of 4SB and diluted with 1.2 ml of +HA buffer (0.7 M hydroxylamine, pH 7.4, 0.4 mM N-[6-(Biotinamido)hexyl]-3′-(2′-pyridyldithio)propionamide (HPDP-biotin) (Pierce, Rockford, IL), 0.2% Triton X-100, 150 mM NaCl, protease inhibitor cocktail) or 1.2 ml of –HA buffer (50 mM Tris-HCl, pH 7.4, 0.4 mM HPDP-biotin, 150 mM NaCl, 0.2% Triton X-100). The mixtures were incubated with rotation at room temperature for 2 hours, followed by 3 sequential chloroform:methanol (1:3, v/v) precipitations. Precipitated proteins were solubilized in 150 μl of 2SB buffer (50 mM Tris-HCl, pH 7.4, 2% SDS, 5 mM EDTA, 150 mM NaCl, protease inhibitor cocktail) and diluted in 2.8 ml of buffer LB (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, protease inhibitor cocktail, 0.2% Triton X-100). Proteins were precipitated from the mixture by incubation with 60 μl of Streptavidin-agarose (Pierce) for 2 hours at room temperature with rotation. Beads were pelleted, washed 3 times in LB, and proteins were eluted by boiling the beads in 150 μl of LB + 10% β-mercaptoethanol.
Xanthohumol analysis
Aliquots of plasma, liver, and brain extractions from each mouse were subjected to enzymatic hydrolysis as previously described [36, 37]. Briefly, 20 μl of extract was mixed with 380 μl 0.1M sodium acetate, pH 4.7; 10 μl internal standard 4,2 dihydroxychalcone, and 600 U of Helix pomatia sulfatase/glucuronidase. Samples were incubated at 37°C for 2 hours, followed by vortexing, and centrifugation (Eppendorf 5415C) at 13000 g for 2 minutes. Samples were extracted with Whatman filter paper 8×45 mm and the paper was dried overnight in a vacuum desiccator over Drierite. Dry paper strips were extracted with 500 μl of acidified methanol (0.1% formic acid in methanol) and shaken 30 minutes at room temperature. Paper strips were removed and samples were centrifuged (Eppendorf 5415C, Eppendorf, Hauppauge, NY) at 13000 g for 2 minutes. Supernatant was utilized for determining xanthohumol concentrations by liquid chromatography with tandem mass spectrometry (LC-MS/MS) analysis. LC-MS/MS assessment was conducted using a hybrid linear ion trap-triple quadrupole instrument (Applied Biosystems 4000 QTRAP, AB Sciex, Framingham, MA). Instrument and chromatography conditions are fully described in previous works [36–38]. Concentrations were calculated using an internal calibration method with matrix based calibration curves and Analyst Software (Analyst 1.5, AB Sciex, Foster City, CA).
Fatty acid analysis
Aliquots of plasma were treated as previously described [38]. Briefly, 20 μl of plasma was mixed with 2 μl of internal free fatty acid standard 19:1 (final conc, 2 μM; NuChek, Waterville, MN). The plasma/standard mixtures were added to 100 μL of a solvent mixture (methanol: n-hexane: phosphoric acid (2M), (40:10:1, v/v)) and vortexed. After 5 minutes, 140 μl of n-hexane and 60 μl of water were added, vortexed and centrifuged for 5 minutes at 12000g in a tabletop centrifuge. The organic supernatant was transferred to another vial, dried under a nitrogen stream, and reconstituted in 100 μl of acetonitrile.
The organic layers extracted from the frontal cortices and hippocampi were dried under nitrogen and reconstituted in 1 ml of chloroform:methanol (2:1, v/v). A 500 μl aliquot of reconstituted organic extracts was combined with 10 μl of internal free fatty acid standard 19:1 (final concentration 10 μM; NuChek) and subjected to base hydrolysis by adding 1 ml of 1M KOH in methanol:water (7:3, v/v) and incubated for 5 minutes at 50°C. The mixture was vigorously shaken, and then 1 ml of hexane (pH adjusted to 3 with 5M HCl) was added and vortexed again. The upper hexane layer was transferred to a 1 ml vial, dried under nitrogen, and reconstituted in 1 ml of acetonitrile.
Ultra high-pressure liquid chromatography was performed on a Shimadzu Nexera system (Shimadzu, Kyoto, Japan) coupled to a hybrid quadruple-time of flight (TOF) mass spectrometer (MS), AB SCIEX Triple TOF 5600. Chromatographic separations were carried out on an ACQUITY UPLC HSS T3 column (100 × 2.1 mm, 1.8 μm, Waters, Milford, MA). Mobile phases consisted of water (A) and acetonitrile (B), both with 0.1% acetic acid. The elution gradient was as follows: 0 minute, 60% B; 3.5 minute, 90% B with a 0.3 ml/minute flow rate; 10 minute, 100% B; 13.5 minute, 60% B with a 0.6 ml/minute flow rate. The column temperature was held at 45°C, and the injection volume was 10 μL. The instrument was operated at a source temperature of 500°C and IonSpray voltage of −4500 kV. The instrument was set in negative ion mode and the information-dependent MS/MS acquisition mode, with the collision energy set at −45 V and a collision energy spread of 10 V. TOF MS acquisition time was 0.25 seconds, and MS/MS acquisition time was 0.1 seconds. The scan range was 70–1200 m/z for TOF MS and 50–1200m/z for MS/MS. Ion source gas 1 and 2 and curtain gas (all nitrogen) were set at 45, 50, and 35, respectively. Quantitation of analytes was carried out with PeakView® software (AB Sciex)
Acyl-CoA analysis
Acyl-CoA molecules were extracted from frontal cortices and hippocampi separately as previously described [39]. Briefly, acyl-CoA samples were washed twice with 2 ml of 10 mM ammonium acetate (pH 5.3): chloroform (1:1, v/v). The top aqueous layers from each wash were removed, combined, and dried under nitrogen. The acyl-CoA extracts were reconstituted in 100 μl of 10 mM ammonium acetate (pH 5.3).
LC-MS/MS was carried out on an Applied Biosystems 4000 QTRAP hybrid linear ion trap-triple quadrupole instrument (AB Sciex) operated at a source temperature of 550°C with a needle voltage of 5200 kV. Chromatographic separations were carried out on XTerra MS C8 column (2.1 × 50 mm, 2.5 μm, Waters) at a temperature of 40°C. The mobile phases consisted of (A)10 mM ammonium formate (pH 5.5) and (B) acetonitrile. The elution gradient was as follows: 0 minute, 20% B; 2.8 minute, 45% B; 3 minute, 25% B; 4 minute, 65% B; 9 minute, 90% B; 9.1 minute, 100% B; 12.1 minute, 20% B with a flow rate of 0.3 ml/minute. Multiple reaction monitoring was carried out in the positive ion mode. Concentrations were calculated using the internal calibration method and Analyst Software (Analyst 1.5, AB Sciex).
Western blot
Sodium dodecyl sulfate–poly acrylamide gel electrophoresis (10%) was used for Western blotting as described previously [40]. Each gel contained 4 different μg loads (2, 4, 8 and 16 μg/well) of standards, obtained from homogenate prepared by combining caudal cortices from all young control animals. Protein samples from representatives of each different age/treatment group were loaded on each gel and analyzed in triplicate. Proteins were transferred to PVDF membranes, blocked in Odyssey blocking buffer (LiCor, Lincoln, NE): Tris-buffered saline (TBS) (1:1, v/v) and incubated at 4°C in one of the following primary antibodies (diluted in blocking buffer): GluN2B (Millipore, Billerica, MA; 1:1000 dilution), GluN2A (Santa Cruz, Santa Cruz, CA; 1:250), Fyn (Santa Cruz; 1:250), PSD-95 (ThermoScientific, Waltham, MA; 1:1000), Flotillin (Santa Cruz; 1:250) or GAPDH (Calbiochem, Millipore, 1:10000). Membranes were rinsed three times with TBS-T and incubated in fluorescence-based secondary antibody (Rockland Immunochemicals, Gibertsville, PA; 1:5000) diluted in blocking buffer. Bands were visualized by scanning in the LI-COR Odyssey imager.
Data analysis
Data for behavioral testing were analyzed as previously described [34]. Cumulative proximity measures, which reflect search distance from the platform, were used for the place, reversal and cued trials and average proximity measures were used for probe trials [41]. Protein blots were analyzed using Li-Cor Odyssey software. Integrated intensity measures were obtained using median background subtraction method. A standard curve was obtained using a linear fit with Excel (Microsoft) from integrated intensity values for known loads of caudal cortex. Sample values were interpolated from the standard curve as caudal cortex equivalents. Each protein was normalized to GAPDH within each sample of total protein. Because the relative percentage of Flotillin molecules that are palmitoylated does not change with age [42], all proteins precipitated through ABE were normalized to Flotillin. Statistical analyses for behavioral trials, protein expression, xanthohumol, acyl Co-A and fatty acids using data from individual animals, were done with analysis of variance (ANOVA) followed by Fisher’s protected least significant difference (PLSD) using Statview software (SAS Institute). Based on previous findings from our lab that showed differential associations of frontal and hippocampal NMDA receptors with different phases of learning in place trials [34, 43], we also examined each day of reference memory testing separately.
Results
Xanthohumol distribution and effects
The average daily food intake for an adult male C57BL/6 mouse has previously been documented to be around 4 g/day [44]. Accordingly, the amount of xanthohumol administered in the diet was calculated to be 1 mg xanthohumol/4 g of diet or approximately 30 mg xanthohumol/kg body weight/day. Previous data had shown that fatty acid metabolism, body weight, and glucose were significantly altered in Zucker fa/fa rats when fed 16.9 mg xanthohumol/kg body weight [32]. Therefore, based on the FDA guideline on interspecies scaling factors for dosage equivalents between mice and rats, a daily dose approximating 30 mg/kg body weight was expected to significantly alter the fatty acid metabolism in the test mice. Dietary consumption was monitored biweekly in all groups. The average dietary intake was approximately 2.7 g of diet/day for each of the 4 groups (Fig. 1A). Dietary intake was not significantly affected by age (F(1,43)=.004, p=.95) or treatment (F(1,43)=.08, p=.78). However, the lower than expected intake reduced the estimated xanthohumol dosage to about 20 mg/kg/day. The mice were also weighed biweekly and the total change in body weight over the 8 weeks of treatment was documented for each animal (Fig. 1B). There was no significant effect of treatment (F(1,43)=.35, p =.56), however there was a significant difference between ages (F(1,43)=8.6, p=.005) with respect to total weight gain (Fig. 1B), with young gaining more weight than old.
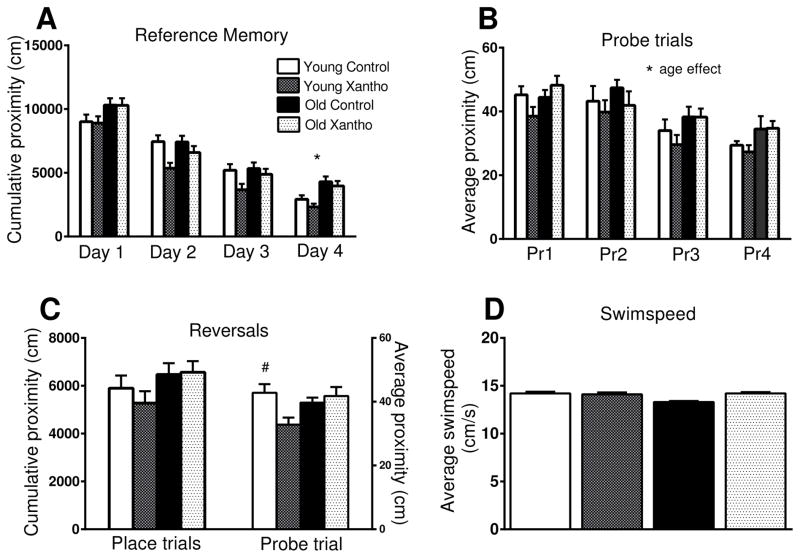
Figure 1. Dietary intake and body weight change of xanthohumol-treated mice.
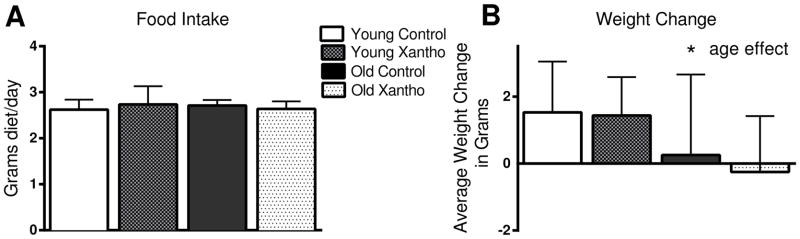
The average daily food intake did not vary by age or treatment (A). There was an aging effect, but not a treatment effect in weight change during the study (ANOVA and Fisher’s PLSD). (B). Data = mean +/− SEM. N=12–13.
The concentration of xanthohumol in wet tissue extracts of liver, plasma, and brain were measured with HPLC-MS/MS. There was a non-significant increase in xanthohumol concentration in the liver (F(1,19)= 2.18, p=.17; Fig. 2A). Old and young mice differed little in plasma concentration (F(1,14)=.12, p = .73; Fig. 2B) in the long-term feeding study. The initial attempts to quantify xanthohumol in the brains of behaviorally-tested mice were limited to caudal cortical regions without the frontal cortex and hippocampus that were used for the rest of the analyses. The limited tissue, coupled with a lower than expected dosage of xanthohumol, provided a signal that was too low to quantify. In order to determine if xanthohumol was able to cross the blood-brain barrier, 3 young and 3 old mice were gavaged with xanthohumol emulsion (40 mg/kg) for five days. Xanthohumol was extracted from the whole cortex, including hippocampus, of each mouse. There was no significant effect of age on xanthohumol concentration in the cortex (F(1,5)=.685, p =.45; Fig 2C). Although only a small percentage of the plasma xanthohumol concentration was found in the cortices of the mice (Fig. 2B,C), the compound was able to cross the blood-brain barrier in quantifiable amounts.
Figure 2. Xanthohumol tissue concentrations.
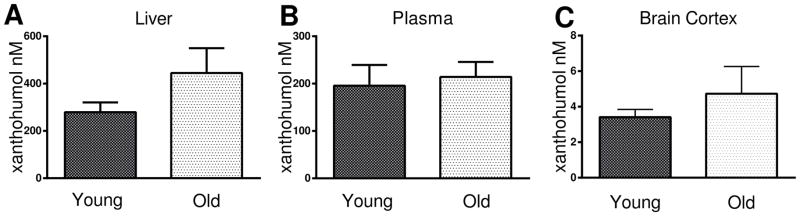
Concentrations of xanthohumol were examined by HPLC-MS/MS in the (A) liver, (B) plasma, and (C) cortex of young and old treated mice. There were no significant differences between ages (ANOVA and Fisher’s PLSD). Data = mean +/− SEM. N =3–8.
Behavioral testing
The spatial memory and cognitive flexibility of the mice was assessed over 5 days in the Morris water maze. The first four days, the mice searched for the hidden platform in the same quadrant of the maze and then were tasked with finding the platform in the opposite quadrant on the fifth day. There was a near significant overall effect of age (F(1,45)=3.66, p=.06), but no effect of treatment (F(1,45))=1.9, p=.18) across four days of reference memory. Analysis of each day of platform trials for reference memory showed a significant effect of age during the fourth (F(1,45)=6.27, p=.016) day of testing and a near-significant effect of age on the first day (F(1,45)=3.44 p=.07), with old mice having higher cumulative proximity to the platform, indicating that they spent more time searching further from the platform than the young mice (Fig. 3A). There was a near significant effect of treatment (F(1,45)=3.62 p=.064) on reference memory trials on Day 2, with xanthohumol-treated mice having lower cumulative proximities than controls (Fig. 3A) There was a significant overall effect of age on probe trials (F((1,45)=6.4, p=.015), with old mice having higher average proximities than young, but no effect of treatment (F(1,45)=1.3, p=.26; Fig. 3B). The reversal platform trials did not show any significant effect of treatment (F(1,44)=.03, p =.86) or age (F(1,44)=2.23, p=.14). There was no significant main effect of age (F(1,45)=1.5, p=.22) or treatment (F(1,45)=2.72, p=.11) during the reversal probe trial, but there was a significant interaction between age and treatment (F(1,45)=6.22, p=.016). A closer examination revealed a significant treatment effect in the young mice (p=.01), but not the old (p =.54), with the xanthohumol-treated young mice having a significantly lower average proximity during the reversal probe trial than the young controls (Fig. 3C). There was no significant effect of age (F(1,45)=.18, p =.67) or treatment (F(1,45)=.01, p=.92) on cumulative proximity in the cued trials (young control (mean±SEM; 853±142 cm), young treatment (1085±166 cm), old control (1170±161 cm), old treatment (905±139 cm).
Figure 3. Behavioral testing.
Behavioral performances shown for place (A) and probe (B) trials for reference memory and reversal trials for cognitive flexibility (C). Xanthohumol treatment was effective in enhancing performance in young mice in reversal probe trials (C). Old mice performed worse than young in probe trials (B) and on Day4 in place trials (A). A significant treatment effect was evident in young mice during reversal probe trials (C). There was no effect of age or treatment on swim speed in place trials (D). * p<.05 for difference between young and old. # p<.0001 for young control vs. young treated. (ANOVA and Fisher’s PLSD). N=12–13. Data = mean +/− SEM. Pr1–4, probe trial number.
Xanthohumol is known to increase beta-oxidation, leading to increased mitochondrial respiration [33]. The average swim speed of each group was compared in order to account for the possibility of a treatment-induced increase in mobility. There was no significant main effect of age (F(1,45)=1.32, p=.26) or treatment (F(1,45)=1.49, p=.23) on average swim speed in place trials (Fig. 3D)
Palmitate and palmitoyl-CoA concentrations
Palmitate is the precursor to palmitoyl-CoA, which is the substrate needed for protein palmitoylation. Reducing the concentration of palmitate may, theoretically, lead to a reduction in protein palmitoylation. Xanthohumol is effective in increasing beta-oxidation in the livers of rodents, thereby lowering plasma fatty acids [32]. In order to assess the ability of xanthohumol to reduce palmitate in this study, plasma and brain samples were evaluated by LC-MS/MS. There was a significant effect of age (F(1,28)=10.93, p=.002; Fig. 4A) and treatment (F(1,28)=6.14, p=.01) on plasma palmitate. Old individuals had less plasma palmitate than young and xanthohumol-treated mice showed lower plasma palmitate than controls (Fig. 4A).
Figure 4. Relative amounts of palmitate in plasma and brain.
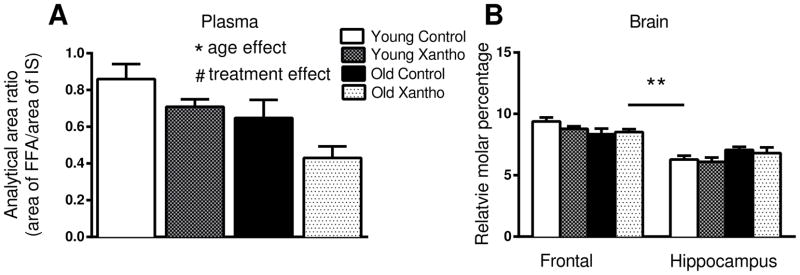
A) Xanthohumol-treated mice had significantly lower plasma palmitate across ages and old mice had lower plasma palmitate than young across treatments. B) The molar percentage of palmitate was unaffected by treatment in the frontal cortices and the hippocampi of young and old mice. ** denotes p < .0001 for difference between frontal cortices and hippocampi.* denotes p<.01 for difference between ages. # denotes p<.05 for difference between treatments. Data = mean +/− SEM. N =8. FFA=free fatty acids; IS=internal standard.
Examination of the relative percentage of palmitate in total fatty acids in the frontal cortex and hippocampus of mice yielded no significant main effect of age (F(1,56)=.04, p=.84) or treatment (F(1,56)=.84; p=.36), but there was a significant effect of brain region (F(1,56)=83, p<.0001) and a significant interaction between age and brain region (F(1,56)=8.38, p=.005; Fig. 4B). The relative percentage of palmitate was significantly higher overall in the frontal cortex, as compared to the hippocampus, and within the old hippocampus, as compared to young (p=.04; Fig. 4B). However, palmitate was nearly significantly lower in the frontal cortex of old mice, as compared to young (p=.058; Fig. 4B).
Next, the relative amount of palmitoyl-CoA was measured in the frontal cortex and hippocampus from each mouse. There was no significant main effect of age (F(1,56)=.66, p =.42) or treatment (F(1,56)=.45, p=.51), but there was a significant effect of brain region (F(1,56)=64, p<.0001) in palmitoyl-CoA (Fig. 5). When the regional differences in brain palmitoyl-CoA were examined, the frontal cortex was found to have a significantly higher level of palmitoyl-CoA than the hippocampus (Fig. 5).
Figure 5. Palmitoyl-CoA levels in frontal cortex and hippocampus.
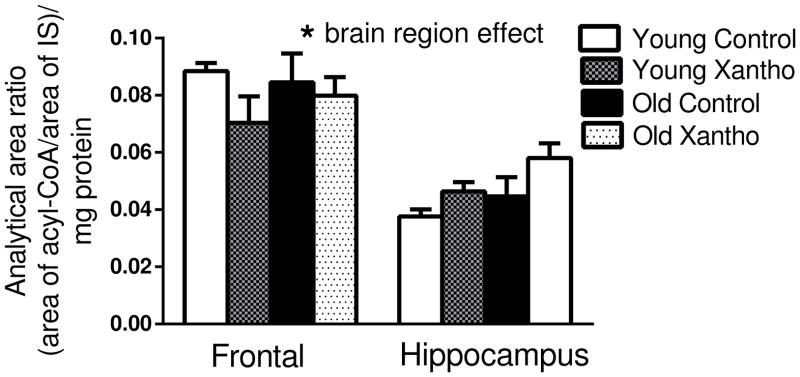
Xanthohumol treatment did not affect palmitoyl-CoA levels in the frontal cortex or hippocampus of mice. Frontal cortex had higher levels of palmitoyl-CoA than hippocampus. (ANOVA and Fisher’s PLSD). FFA= free fatty acids; IS=internal standard. Data = mean +/− SEM. N =8.
Protein palmitoylation
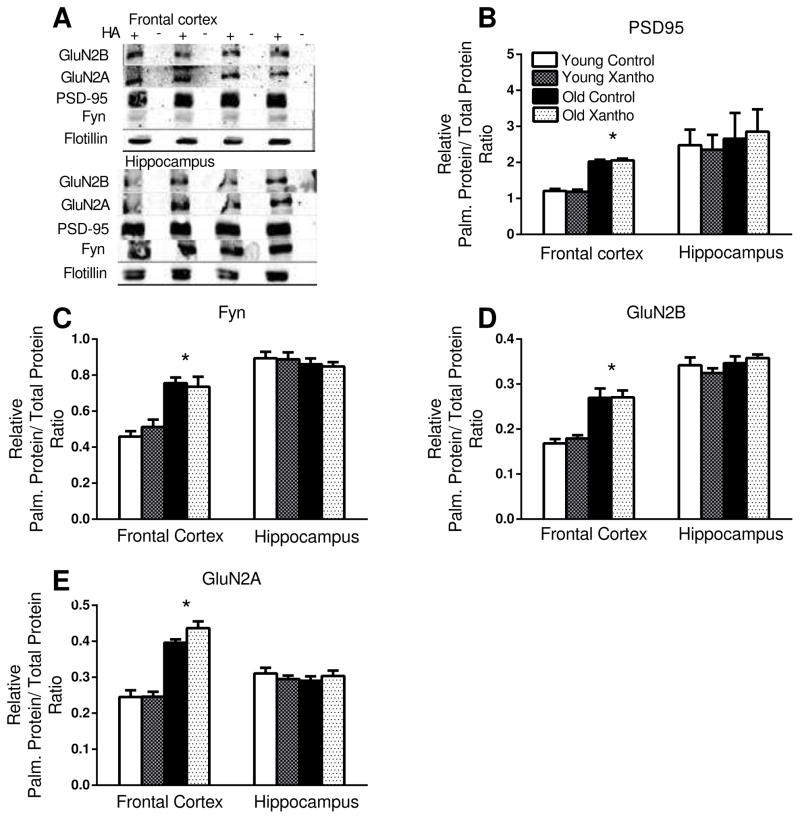
Previous evidence from our lab [21] demonstrated that protein palmitoylation was disturbed in the frontal cortices of old mice. Specifically, the percentage of GluN2B, GluN2A, Fyn, and PSD95 that were palmitoylated was greater in the frontal cortices, but not the hippocampi, of old mice. Xanthohumol treatment was applied in order to determine if it could modify the palmitoylation status of proteins in the frontal cortices of old mice. There were significant main effects of age on the percentage of palmitoylated proteins for PSD-95 (F(1,22)=105, p<.0001; Fig. 6A), Fyn (F(1,18)=40, p<.0001; Fig. 6B), GluN2B (F(1,28)=63, p<.0001; Fig. 6C), and GluN2A (F(1,28)=106, p<.0001; Fig. 6D) in the frontal cortex. For each protein, there were significantly more palmitoylated proteins in the frontal cortex of old mice than the young (Fig. 6). There was a significant effect of treatment in the percentage of palmitoylated GluN2A in the frontal cortex, with xanthohumol treatment being associated with an increase in palmitoylation of GluN2A across ages (F(1,28)=6.4, p<.017; Fig. 6D). There were no significant effects of treatment on the other proteins in the frontal cortex (p =.26–.89; Fig. 6). There were no significant effects of age (p=.14–.65) or treatment (p=.15–.72) on the palmitoylation status of proteins in the hippocampus (Fig. 6).
Figure 6. Protein palmitoylation.
A significant age-related increase in the relative percentage of (B) PSD95, (C) Fyn, (D) GluN2B and (E) GluN2A that were palmitoylated in the frontal cortices, but not the hippocampi of mice. A representative blot (A) of palmitoylated proteins. Blots were stripped and reprobed for GluN2B after being probed for GluN2A. * denotes p < .0001 for difference between young and old mice (ANOVA and Fisher’s PLSD). Data = mean +/− SEM. N =6–8. HA=hydroxylamine.
Discussion
In this study, young and old mice were treated with xanthohumol for 8 weeks. Xanthohumol improved cognitive flexibility in the young treated mice, as compared to age-matched controls. Xanthohumol was able to lower palmitate in the plasma in both young and old mice. Regional comparisons of palmitate and palmitoyl-CoA in the brain revealed significantly higher levels of each molecule in the frontal cortex versus the hippocampus. Relative protein palmitoylation levels were also increased significantly in the frontal cortex with increasing age, but there were no aging changes in the hippocampus. Xanthohumol treatment was unable to reduce palmitoylation levels in any brain region or age of mouse.
There was a significant decline with increased age in spatial reference memory and cognitive flexibility, when data was averaged across treatments, similar to previous findings in C57BL/6 mice [20, 43] and other species [45]. Xanthohumol was not effective in significantly reversing these effects in the aged mice. Xanthohumol did improve cognitive flexibility in the young mice and showed a trend for enhancing spatial learning in young and old mice. However, this appeared to be mostly due to the young mice on the control diet performing similar to old controls and worse than young controls previously studied our lab [20, 43] and the xanthohumol reversing this effect.
The primary difference among the studies, in which the behavioral results were compared, was the diet. The diets for our previous studies mentioned above [20, 43] were standard chow diets based on the NIH-31 formulation [46], while the base formula for this study was AIN-93G. The NIH-31 formulation contains whole foods from animal protein and grains that are rich in phytoestrogens. The AIN-93G-based diet used in this study was formulated from synthesized compounds and used corn oil instead of soy oil, rendering the diet free of most phytoestrogens. While the effects of soy phytoestrogens on improving spatial memory have been the most prominent in female ovariectomized rodents [47], male mice treated with the phytoestrogen daidzein were less susceptible to anxiety than control mice [48]. It is possible that the stress of swimming in the water maze may have been exacerbated by the lack of phytoestrogens in the diet of the young control mice. The fact that xanthohumol could reverse this deficiency with respect to cognitive flexibility for spatial memory confirms the importance of phytochemicals in the diet for cognitive function [49]. The effects of stress on spatial memory are much greater in young male mice than in aged male mice [50]. It may be that the poor basal performances already seen in aged mice in the Morris water maze were less affected by an additional mild stressor like a lack of phytoestrogens in the diet.
The NIH-31 chow diet and xanthohumol AIN-93G-based diet differ in the base ingredients. The AIN-93G diet is a synthetic diet that uses casein as a protein source and cellulose as filler for bulk. The NIH-31 chow diet is composed of whole grains and fishmeal. One of the effects of altering the diet in a significant way is a change in the composition of the gut microbiota. A comparison of a synthetic versus a whole food diet in mice found significant differences in the population of the gut flora [51]. While the study of the relationships between microbiota and memory is a burgeoning field, one study found that supplementing a normal chow diet with probiotics improved spatial memory in rats [52]. Xanthohumol, however, does not affect the gut microbiota in rats [53], indicating that if the brain-gut axis were disturbed, xanthohumol would likely have no effect. This study revealed that several different problems may arise when mice are switched from their normal chow diet to a synthetic defined diet. In addition, the mice in this study did not receive the full-intended dosage of xanthohumol. The xanthohumol daily dosage was calculated based on the average daily intake for the C57BL/6 mouse on a normal chow diet [44]. Mice in this study consumed roughly 67% of what was expected, thereby exposing them to a lower daily intake of xanthohumol. Xanthohumol has been shown to be safe at up to 1000 mg/kg body weight/day, so increasing the dosage is quite feasible [54]. A larger dosage of xanthohumol in a whole food chow diet may be able to have a significant effect on spatial memory in aged mice.
The significant effect of treatment on plasma palmitate in the old mice was not matched in the brains of old or young mice. There was no treatment effect in either the frontal cortex or hippocampus of either age group. There was, however, a significant difference between brain regions in palmitate as a percentage of total fatty acids, with palmitate constituting a higher percentage in the frontal cortex than in the hippocampus. Because there is little de novo fatty acid synthesis in the brain, lipids must be imported across the blood-brain barrier [55]. The first step after crossing the membrane is esterification to acyl-CoA. In this study, we showed that there was a significant regional, but not age or treatment, difference in the steady-state levels of palmitoyl-CoA, with a greater amount present in the frontal cortex. The resting pool of palmitoyl-CoA in either brain region may not be related to efficiency of palmitate crossing the blood-brain barrier. It may be that downstream metabolism varies between the two brain regions. Too much acyl-CoA has a detergent-like effect so there may be a limit to how much acyl-CoA a cell can hold [56]. An analysis into the rate of fatty acid acylation in the brain showed that there is an age-related increase in arachidonyl-CoA formation in both the frontal cortex and hippocampus [57]. Although the authors were unable to note any age-related differences in palmitoyl-CoA formation [57], it may be that revisiting the experiment with modern equipment could reveal some differences. It is important to note that we also found no age-related differences in the pools of arachidonyl-CoA in either the frontal cortex or the hippocampus (data not shown). It is also important to note that both arachidonyl-CoA and palmitoyl-CoA are synthesized by the same enzyme acyl-CoA synthetase 6 (ACSL6) [58]. Two isoforms of ACSL6 exist, with one having a lower affinity for ATP. Because the aging brain is deficient in ATP [59], it may be that the isoform that has a lower affinity for ATP is upregulated in old mice, compensating for the possibility of a loss of activity due to lower levels of ATP. This may explain why we were unable to see any aging differences in palmitoyl-CoA pools in either the frontal cortex or hippocampus. There was a significant increase in the levels of protein palmitoylation in the frontal cortex, but not the hippocampus, of old mice. Treatment with xanthohumol was unable to affect protein palmitoylation levels in either the young or old mice. Protein palmitoylation is integral to neuronal development and plasticity [22], but depalmitoylation is equally as important as it allows proteins to cycle off of membranes thereby modulating a signal [60]. Palmitoylation of PSD-95 and the N-methyl-D-aspartate receptor (NMDAR) subunits GluN2A and 2B will increase when activation of the NMDAR is diminished allowing the proteins to remain on the synaptic membrane [23, 61]. Palmitoylation of the GluR1 subunit of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) will also increase when the NMDAR is blocked, but unlike the NMDAR GluN2 subunits, palmitoylation interferes with AMPAR insertion into the synaptic membrane decreasing LTP [62]. Synaptic vesicle fusion dysfunction occurs when synaptic proteins are not properly depalmitoylated [63]. It may be that decreased synaptic activity in the aged frontal cortex is associated with persistent anchoring of some synaptic proteins to plasma membranes leading to a loss of LTP and pruning of dendritic arbors. Although the spatial memory of the aged mice in this study was not significantly improved, there was a significant reduction in plasma palmitate in the treatment group. There was, however, no treatment-induced change in the palmitoylation status of GluN2A, GluN2B, PSD-95 or Fyn. Treating pancreatic β-cells with palmitate can induce greater protein palmitoylation, leading to cell death [64]. The concentration of palmitate used to treat the β-cells was far greater than normal physiological levels. The approximately 30% reduction of plasma palmitate in the old mice was not enough to affect protein palmitoylation. It may be physiologically impossible to safely lower palmitate enough to affect protein palmitoylation.
Previous work has indicated that xanthohumol may be effective in treating metabolic syndrome [33]. Metabolic syndrome has been associated with age-related deficits in memory [65]. There was no significant effect of xanthohumol on swim speed or spatial memory in the old mice. This is suggestive, but not definitive, that xanthohumol had little effect on metabolic rate. Flavanoids are important for cognition. They have been shown to be neuroprotective [66] and good for spatial memory in young and old mice [49, 67]. Soy isoflavones are also beneficial to memory [68]. Xanthohumol is a prenylated calconoid that is a part of the flavonoid family. Treatment with xanthohumol has been shown to be neuroprotective in stroke-induced rats [69]. Recent evidence found that derivatives of xanthohumol can induce neurite growth in mouse neuronal cells [70]. In this study we were able to demonstrate that dietary intake of xanthohumol improved cognitive flexibility in young mice on a flavanoid-deficient diet and lowered plasma palmitate in young and old mice. A combination of a diet rich in soy isoflavones supplemented with a higher concentration of xanthohumol could still prove to be beneficial to spatial learning in aged mice.
Highlights.
Young and old mice treated with xanthohumol
Cognitive flexibility improved in young mice
Plasma palmitate significantly reduced in old mice
Lower plasma palmitate did not affect brain palmitate or palmitoyl-CoA
Lower plasma palmitate did not affect protein palmitoylation in brain
Acknowledgments
This work was supported by NIH grant AG16322 to KRM.
The project described was supported, in part, by Award Number P30ES000210 from the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIEHS or NIH. The authors acknowledge the Biomolecular Mass Spectrometry Core of the Environmental Health Sciences Core Center at Oregon State University
Footnotes
There is no conflict associated with this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Scherr PA, Albert MS, Funkenstein HH, Cook NR, Hennekens CH, Branch LG, et al. Correlates of cognitive function in an elderly community population. Am J Epidemiol. 1988;128:1084–101. doi: 10.1093/oxfordjournals.aje.a115051. [DOI] [PubMed] [Google Scholar]
- 2.Singh-Manoux A, Kivimaki M, Glymour MM, Elbaz A, Berr C, Ebmeier KP, et al. Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. The BMJ. 2012;344:d7622. doi: 10.1136/bmj.d7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallagher M, Stocker AM, Koh MT. Mindspan: lessons from rat models of neurocognitive aging. ILAR J. 2011;52:32–40. doi: 10.1093/ilar.52.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet. 1994;344:769–72. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- 5.Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: effect of sex and age. J Comp Neurol. 1997;384:312–20. [PubMed] [Google Scholar]
- 6.Keuker JI, Luiten PG, Fuchs E. Preservation of hippocampal neuron numbers in aged rhesus monkeys. Neurobiol Aging. 2003;24:157–65. doi: 10.1016/s0197-4580(02)00062-3. [DOI] [PubMed] [Google Scholar]
- 7.Gazzaley AH, Thakker MM, Hof PR, Morrison JH. Preserved number of entorhinal cortex layer II neurons in aged macaque monkeys. Neurobiol Aging. 1997;18:549–53. doi: 10.1016/s0197-4580(97)00112-7. [DOI] [PubMed] [Google Scholar]
- 8.Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc Natl Acad Sci U S A. 1996;93:9926–30. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merrill DA, Chiba AA, Tuszynski MH. Conservation of neuronal number and size in the entorhinal cortex of behaviorally characterized aged rats. J Comp Neurol. 2001;438:445–56. doi: 10.1002/cne.1327. [DOI] [PubMed] [Google Scholar]
- 10.Hanks SD, Flood DG. Region-specific stability of dendritic extent in normal human aging and regression in Alzheimer’s disease. I. CA1 of hippocampus. Brain Res. 1991;540:63–82. doi: 10.1016/0006-8993(91)90493-f. [DOI] [PubMed] [Google Scholar]
- 11.Flood DG, Buell SJ, Horwitz GJ, Coleman PD. Dendritic extent in human dentate gyrus granule cells in normal aging and senile dementia. Brain Res. 1987;402:205–16. doi: 10.1016/0006-8993(87)90027-8. [DOI] [PubMed] [Google Scholar]
- 12.Flood DG. Critical issues in the analysis of dendritic extent in aging humans, primates, and rodents. Neurobiol Aging. 1993;14:649–54. doi: 10.1016/0197-4580(93)90058-j. [DOI] [PubMed] [Google Scholar]
- 13.Turner DA, Deupree DL. Functional elongation of CA1 hippocampal neurons with aging in Fischer 344 rats. Neurobiol Aging. 1991;12:201–10. doi: 10.1016/0197-4580(91)90098-5. [DOI] [PubMed] [Google Scholar]
- 14.VanGuilder HD, Yan H, Farley JA, Sonntag WE, Freeman WM. Aging alters the expression of neurotransmission-regulating proteins in the hippocampal synaptoproteome. J Neurochem. 2010;113:1577–88. doi: 10.1111/j.1471-4159.2010.06719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Brabander JM, Kramers RJ, Uylings HB. Layer-specific dendritic regression of pyramidal cells with ageing in the human prefrontal cortex. Eur J Neurosci. 1998;10:1261–9. doi: 10.1046/j.1460-9568.1998.00137.x. [DOI] [PubMed] [Google Scholar]
- 16.Markham JA, Juraska JM. Aging and sex influence the anatomy of the rat anterior cingulate cortex. Neurobiol Aging. 2002;23:579–88. doi: 10.1016/s0197-4580(02)00004-0. [DOI] [PubMed] [Google Scholar]
- 17.Xiong XD, Chen GH. Research progress on the age-related changes in proteins of the synaptic active zone. Physiol Behav. 2010;101:1–12. doi: 10.1016/j.physbeh.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 18.Magnusson KR. Aging of the NMDA receptor: from a mouse’s point of view. Future Neurol. 2012;7:627–37. doi: 10.2217/fnl.12.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.VanGuilder HD, Farley JA, Yan H, Van Kirk CA, Mitschelen M, Sonntag WE, et al. Hippocampal dysregulation of synaptic plasticity-associated proteins with age-related cognitive decline. Neurobiol Dis. 2011;43:201–12. doi: 10.1016/j.nbd.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zamzow DR, Elias V, Shumaker M, Larson C, Magnusson KR. An increase in the association of GluN2B containing NMDA receptors with membrane scaffolding proteins was related to memory declines during aging. J Neurosci. 2013;33:12300–5. doi: 10.1523/JNEUROSCI.0312-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zamzow DR, CMD, DBJ, KRM The palmitoylation state of NMDA receptor-associated proteins in the aged mouse brain. Society for Neuroscience Meeting Abstracts Online 2012. :#105.23. [Google Scholar]
- 22.Fukata Y, Fukata M. Protein palmitoylation in neuronal development and synaptic plasticity. Nat Rev Neurosci. 2010;11:161–75. doi: 10.1038/nrn2788. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi T, Thomas GM, Huganir RL. Dual palmitoylation of NR2 subunits regulates NMDA receptor trafficking. Neuron. 2009;64:213–26. doi: 10.1016/j.neuron.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.el El-Husseini AD, Schnell E, Dakoji S, Sweeney N, Zhou Q, Prange O, et al. Synaptic strength regulated by palmitate cycling on PSD-95. Cell. 2002;108:849–63. doi: 10.1016/s0092-8674(02)00683-9. [DOI] [PubMed] [Google Scholar]
- 25.Sato I, Obata Y, Kasahara K, Nakayama Y, Fukumoto Y, Yamasaki T, et al. Differential trafficking of Src, Lyn, Yes and Fyn is specified by the state of palmitoylation in the SH4 domain. J Cell Sci. 2009;122:965–75. doi: 10.1242/jcs.034843. [DOI] [PubMed] [Google Scholar]
- 26.Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–84. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuhla A, Blei T, Jaster R, Vollmar B. Aging is associated with a shift of fatty metabolism toward lipogenesis. J Gerontol A Biol Sci Med Sci. 2011;66:1192–200. doi: 10.1093/gerona/glr124. [DOI] [PubMed] [Google Scholar]
- 28.Carver JD, Benford VJ, Han B, Cantor AB. The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain Res Bull. 2001;56:79–85. doi: 10.1016/s0361-9230(01)00551-2. [DOI] [PubMed] [Google Scholar]
- 29.Ando S, Tanaka Y, Toyoda nee Ono Y, Kon K, Kawashima S. Turnover of synaptic membranes: age-related changes and modulation by dietary restriction. J Neurosci Res. 2002;70:290–7. doi: 10.1002/jnr.10352. [DOI] [PubMed] [Google Scholar]
- 30.Pancani T, Anderson KL, Brewer LD, Kadish I, DeMoll C, Landfield PW, et al. Effect of high-fat diet on metabolic indices, cognition, and neuronal physiology in aging F344 rats. Neurobiol Aging. 2013;34:1977–87. doi: 10.1016/j.neurobiolaging.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevens JF, Page JE. Xanthohumol and related prenylflavonoids from hops and beer: to your good health! Phytochemistry. 2004;65:1317–30. doi: 10.1016/j.phytochem.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 32.Legette LL, Luna AY, Reed RL, Miranda CL, Bobe G, Proteau RR, et al. Xanthohumol lowers body weight and fasting plasma glucose in obese male Zucker fa/fa rats. Phytochemistry. 2013;91:236–41. doi: 10.1016/j.phytochem.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 33.Kirkwood JS, Legette LL, Miranda CL, Jiang Y, Stevens JF. A metabolomics-driven elucidation of the anti-obesity mechanisms of xanthohumol. J Biol Chem. 2013;288:19000–13. doi: 10.1074/jbc.M112.445452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Das SR, Jensen R, Kelsay R, Shumaker M, Bochart R, Brim B, et al. Reducing expression of GluN1(0XX) subunit splice variants of the NMDA receptor interferes with spatial reference memory. Behav Brain Res. 2012;230:317–24. doi: 10.1016/j.bbr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wan J, Roth AF, Bailey AO, Davis NG. Palmitoylated proteins: purification and identification. Nat Protoc. 2007;2:1573–84. doi: 10.1038/nprot.2007.225. [DOI] [PubMed] [Google Scholar]
- 36.Legette L, Ma L, Reed RL, Miranda CL, Christensen JM, Rodriguez-Proteau R, et al. Pharmacokinetics of xanthohumol and metabolites in rats after oral and intravenous administration. Mol Nutr Food Res. 2012;56:466–74. doi: 10.1002/mnfr.201100554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Legette L, Karnpracha C, Reed RL, Choi J, Bobe G, Christensen JM, et al. Human pharmacokinetics of xanthohumol, an antihyperglycemic flavonoid from hops. Mol Nutr Food Res. 2014;58:248–55. doi: 10.1002/mnfr.201300333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zehethofer N, Pinto DM, Volmer DA. Plasma free fatty acid profiling in a fish oil human intervention study using ultra-performance liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2008;22:2125–33. doi: 10.1002/rcm.3597. [DOI] [PubMed] [Google Scholar]
- 39.Kasuya F, Kazumi M, Tatsuki T, Suzuki R. Effect of salicylic acid and diclofenac on the medium-chain and long-chain acyl-CoA formation in the liver and brain of mouse. J Appl Toxicol. 2009;29:435–45. doi: 10.1002/jat.1431. [DOI] [PubMed] [Google Scholar]
- 40.Zhao X, Rosenke R, Kronemann D, Brim B, Das SR, Dunah AW, et al. The effects of aging on N-methyl-D-aspartate receptor subunits in the synaptic membrane and relationships to long-term spatial memory. Neuroscience. 2009;162:933–45. doi: 10.1016/j.neuroscience.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–26. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- 42.Bhattacharyya R, Barren C, Kovacs DM. Palmitoylation of amyloid precursor protein regulates amyloidogenic processing in lipid rafts. J Neurosci. 2013;33:11169–83. doi: 10.1523/JNEUROSCI.4704-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brim BL, Haskell R, Awedikian R, Ellinwood NM, Jin L, Kumar A, et al. Memory in aged mice is rescued by enhanced expression of the GluN2B subunit of the NMDA receptor. Behav Brain Res. 2013;238:211–26. doi: 10.1016/j.bbr.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet. 2002;32:435–43. doi: 10.1023/a:1020884312053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Magnusson KR, Brim BL, Das SR. Selective Vulnerabilities of N-methyl-D-aspartate (NMDA) Receptors During Brain Aging. Front Aging Neurosci. 2010;2:11. doi: 10.3389/fnagi.2010.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barnard DE, Lewis SM, Teter BB, Thigpen JE. Open- and closed-formula laboratory animal diets and their importance to research. J Am Assoc Lab Anim Sci. 2009;48:709–13. [PMC free article] [PubMed] [Google Scholar]
- 47.Luine V, Attalla S, Mohan G, Costa A, Frankfurt M. Dietary phytoestrogens enhance spatial memory and spine density in the hippocampus and prefrontal cortex of ovariectomized rats. Brain Res. 2006;1126:183–7. doi: 10.1016/j.brainres.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 48.Zeng S, Tai F, Zhai P, Yuan A, Jia R, Zhang X. Effect of daidzein on anxiety, social behavior and spatial learning in male Balb/cJ mice. Pharmacol Biochem Behav. 2010;96:16–23. doi: 10.1016/j.pbb.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 49.Rendeiro C, Spencer JP, Vauzour D, Butler LT, Ellis JA, Williams CM. The impact of flavonoids on spatial memory in rodents: from behaviour to underlying hippocampal mechanisms. Genes Nutr. 2009;4:251–70. doi: 10.1007/s12263-009-0137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bowman RE, Maclusky NJ, Diaz SE, Zrull MC, Luine VN. Aged rats: sex differences and responses to chronic stress. Brain Res. 2006;1126:156–66. doi: 10.1016/j.brainres.2006.07.047. [DOI] [PubMed] [Google Scholar]
- 51.McCracken VJ, Simpson JM, Mackie RI, Gaskins HR. Molecular ecological analysis of dietary and antibiotic-induced alterations of the mouse intestinal microbiota. J Nutr. 2001;131:1862–70. doi: 10.1093/jn/131.6.1862. [DOI] [PubMed] [Google Scholar]
- 52.Davari S, Talaei SA, Alaei H, Salami M. Probiotics treatment improves diabetes-induced impairment of synaptic activity and cognitive function: behavioral and electrophysiological proofs for microbiome-gut-brain axis. Neuroscience. 2013;240:287–96. doi: 10.1016/j.neuroscience.2013.02.055. [DOI] [PubMed] [Google Scholar]
- 53.Hanske L, Hussong R, Frank N, Gerhauser C, Blaut M, Braune A. Xanthohumol does not affect the composition of rat intestinal microbiota. Mol Nutr Food Res. 2005;49:868–73. doi: 10.1002/mnfr.200500048. [DOI] [PubMed] [Google Scholar]
- 54.Dorn C, Bataille F, Gaebele E, Heilmann J, Hellerbrand C. Xanthohumol feeding does not impair organ function and homoeostasis in mice. Food Chem Toxicol. 2010;48:1890–7. doi: 10.1016/j.fct.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 55.Hamilton JA, Brunaldi K. A Model for Fatty Acid Transport into the Brain. J Mol Neurosci. 2007;33:12–7. doi: 10.1007/s12031-007-0050-3. [DOI] [PubMed] [Google Scholar]
- 56.Brecher P. The interaction of long-chain acyl CoA with membranes. Mol Cell Biochem. 1983;57:3–15. doi: 10.1007/BF00223520. [DOI] [PubMed] [Google Scholar]
- 57.Terracina L, Brunetti M, Avellini L, De Medio GE, Trovarelli G, Gaiti A. Arachidonic and palmitic acid utilization in aged rat brain areas. Mol Cell Biochem. 1992;115:35–42. doi: 10.1007/BF00229093. [DOI] [PubMed] [Google Scholar]
- 58.Van Horn CG, Caviglia JM, Li LO, Wang S, Granger DA, Coleman RA. Characterization of recombinant long-chain rat acyl-CoA synthetase isoforms 3 and 6: identification of a novel variant of isoform 6. Biochemistry. 2005;44:1635–42. doi: 10.1021/bi047721l. [DOI] [PubMed] [Google Scholar]
- 59.Dorszewska J. Cell biology of normal brain aging: synaptic plasticity-cell death. Aging Clin Exp Res. 2013;25:25–34. doi: 10.1007/s40520-013-0004-2. [DOI] [PubMed] [Google Scholar]
- 60.Conibear E, Davis NG. Palmitoylation and depalmitoylation dynamics at a glance. J Cell Sci. 2010;123:4007–10. doi: 10.1242/jcs.059287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Noritake J, Fukata Y, Iwanaga T, Hosomi N, Tsutsumi R, Matsuda N, et al. Mobile DHHC palmitoylating enzyme mediates activity-sensitive synaptic targeting of PSD-95. J Cell Biol. 2009;186:147–60. doi: 10.1083/jcb.200903101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin DT, Makino Y, Sharma K, Hayashi T, Neve R, Takamiya K, et al. Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nat Neurosci. 2009;12:879–87. doi: 10.1038/nn.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim SJ, Zhang Z, Sarkar C, Tsai PC, Lee YC, Dye L, et al. Palmitoyl protein thioesterase-1 deficiency impairs synaptic vesicle recycling at nerve terminals, contributing to neuropathology in humans and mice. J Clin Invest. 2008;118:3075–86. doi: 10.1172/JCI33482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baldwin AC, Green CD, Olson LK, Moxley MA, Corbett JA. A role for aberrant protein palmitoylation in FFA-induced ER stress and beta-cell death. Am J Physiol Endocrinol Metab. 2012;302:E1390–8. doi: 10.1152/ajpendo.00519.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watts AS, Loskutova N, Burns JM, Johnson DK. Metabolic syndrome and cognitive decline in early Alzheimer’s disease and healthy older adults. J Alzheimers Dis. 2013;35:253–65. doi: 10.3233/JAD-121168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dajas F, Andres AC, Florencia A, Carolina E, Felicia RM. Neuroprotective actions of flavones and flavonols: mechanisms and relationship to flavonoid structural features. Cent Nerv Syst Agents Med Chem. 2013;13:30–5. doi: 10.2174/1871524911313010005. [DOI] [PubMed] [Google Scholar]
- 67.Spencer JP. The impact of fruit flavonoids on memory and cognition. Br J Nutr. 2010;104(Suppl 3):S40–7. doi: 10.1017/S0007114510003934. [DOI] [PubMed] [Google Scholar]
- 68.Lee YB, Lee HJ, Sohn HS. Soy isoflavones and cognitive function. J Nutr Biochem. 2005;16:641–9. doi: 10.1016/j.jnutbio.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 69.Yen TL, Hsu CK, Lu WJ, Hsieh CY, Hsiao G, Chou DS, et al. Neuroprotective effects of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus), in ischemic stroke of rats. J Agric Food Chem. 2012;60:1937–44. doi: 10.1021/jf204909p. [DOI] [PubMed] [Google Scholar]
- 70.Oberbauer E, Urmann C, Steffenhagen C, Bieler L, Brunner D, Furtner T, et al. Chroman-like cyclic prenylflavonoids promote neuronal differentiation and neurite outgrowth and are neuroprotective. J Nutr Biochem. 2013;24:1953–62. doi: 10.1016/j.jnutbio.2013.06.005. [DOI] [PubMed] [Google Scholar]