Abstract
Apathy, a reduction in goal-directed behavior (GDB), affects 90% of individuals with behavioral variant frontotemporal degeneration, which is a common cause of early onset neurodegenerative disease. The cognitive and neural impairments associated with apathy make it difficult to initiate, plan, and self-motivate activities toward a specific goal, such as dressing or bathing. These impairments are associated with significant decline in functional ability, caregiver burden, and increased cost of care due to early institutionalization. The current article reviews the evidence suggesting that apathy arises from the interruption of one or any combination of three GDB processes: initiation, planning, and motivation. From this perspective, three subtypes of apathy related to dysfunction at the level of GDB and the corresponding neuroanatomy are explored. Further research is required to confirm and measure these subtypes of apathy for use in clinical and research settings. A more precise classification of apathy by subtype will allow implementation of the most appropriate person-centered, individualized therapy.
Apathy, defined as a reduction in self-generated or voluntary behavior (Levy & Dubois, 2006), has profound consequences for morbidity and mortality in patients with neurodegenerative disease and contributes significantly to family caregiver burden (Butterfield, Cimino, Oelke, Hauser, & Sanchez-Ramos, 2010; Chio et al., 2010; Karttunen et al., 2011). Apathy is especially prevalent in behavioral variant frontotemporal degeneration (bvFTD) and is reported in up to 90.5% of patients with mild stage bvFTD (Diehl-Schmid, Pohl, Perneczky, Forstl, & Kurz, 2006).
FTD is the second most common young-onset neurodegenerative disease (Ratnavalli, Brayne, Dawson, & Hodges, 2002; Rosso et al., 2003). Afflicted individuals experience neuronal loss in the frontal and temporal lobes of the brain, which results in difficulty with regulating social behavior (Massimo & Grossman, 2008). In the field of neurodegenerative disease, abnormal social behavior includes a wide range of neuropsychiatric symptoms that are disruptive to social interaction (Massimo, Evans, & Benner, 2013). Abnormal social behavior is the hallmark symptom of bvFTD, with apathy being the most common behavior, evident pervasively throughout the duration of the disease (Le Ber et al., 2006; Mendez, Lauterbach, & Sampson, 2008). Although apathy is a common and significant behavior of bvFTD, the mechanisms contributing to apathy have rarely been studied. To date, no proven effective treatments exist for apathy, in part because the underlying dysfunction is not fully understood (Chase, 2011).
Apathy is a common neuropsychiatric behavior that negatively affects patient and caregiver outcomes (Chio et al., 2010; Karttunen et al., 2011), including increased patient mortality (Vilalta-Franch, Calvo-Perxas, Garre-Olmo, Turro-Garriga, & Lopez-Pousa, 2013). Apathy is associated with various undesirable consequences in patients, such as poor insight and poor cognitive performance (Chase, 2011; Ishii, Weintraub, & Mervis, 2009; Pedersen, Alves, Aarsland, & Larsen, 2009; Pluck & Brown, 2002). The deficits observed in patients with apathy, such as poor planning, poor motivation, and the inability to initiate self-care activities, contribute to functional deterioration (Pedersen et al., 2009). These findings suggest that apathy contributes significantly to global decline and mortality and support the need for its identification and effective management in at-risk patient populations.
Caring for an individual with apathy is challenging. The physical and emotional demands associated with performing many activities for patients with apathy are profound. High levels of caregiver depression, burden, and stress have been reported in caregivers of patients with apathy (Chio et al., 2010; Massimo et al., 2009). Apathetic bvFTD patients lack insight into their social difficulties and are unaware of the consequences of their behavior (Eslinger et al., 2005). Caregivers often misinterpret apathy as a sign of oppositional or volitional behavior, leading to dissatisfaction with caregiving (Landes, Sperry, Strauss, & Geldmacher, 2001; Massimo et al., 2013). A study of spousal caregivers demonstrated that apathetic behavior had the greatest impact on the decline of the marital relationship (de Vugt et al., 2006). This decline has significant implications for caregiver burnout, as it is the bond between caregiver and care recipient that sustains caregiving under adverse conditions (Wrubel & Folkman, 1997). Helping caregivers reframe apathy as brain-based rather than character-based, may strengthen their connection to the affected individual, while also reducing caregiver anger and frustration (Massimo et al., 2013).
To date, treatments for apathy have not been effective. In a systematic review of pharmacological treatments, the use of medications for the improvement of apathy in neurodegenerative disease could not be supported due to insufficient evidence (Drijgers, Dujardin, Reijnders, Defebvre, & Leentjens, 2010). One reason for failures in treatment may be the way in which apathy is currently conceptualized—that apathy is derived simply from a lack of motivation (Marin, 1996). Evidence suggests that several different mechanisms contribute to apathy, including deficits in initiation and planning, as well as motivation (Chow et al., 2009; Eslinger, Moore, Antani, Anderson, & Grossman, 2012; Levy & Dubois, 2006; Massimo et al., 2009). In addition, neuroanatomical evidence supports a multicomponent approach to apathy. Several neuroimaging studies associate apathy with numerous regions in the frontal cortex (Massimo et al., 2009; Rosen et al., 2005; Zamboni, Huey, Krueger, Nichelli, & Grafman, 2008). Mechanisms underlying apathy are qualitatively different; thus, they may require different interventions. Knowledge of distinct subtypes of apathy would help explain treatment failures that may be due, at least in part, to the attempt to treat all apathy with a single approach. In addition, apathy is often ignored by clinicians because of patients’ lack of apparent distress (Butterfield et al., 2010). One of the primary obstacles in furthering the research in this area has been the absence of an empirically based approach that can elucidate the mechanisms contributing to apathy.
In the current article, the authors apply a model of goal-directed behavior (GDB) in individuals with bvFTD in whom apathy is highly prevalent to support personalized approaches to management of apathy in this population, targeting problems with initiation, planning, and motivation. With a better understanding of mechanisms underlying apathy, carefully designed, individualized interventions may become a helpful resource for family caregivers. These tailored interventions are person-centered, as their individual characteristics are integral to treatment.
BACKGROUND
The word apathy derives from the Greek word pathos. It describes a state of indifference or inertia (Robert et al., 2009). Over time, the concept of apathy has undergone changes in meaning and remains vaguely defined and broadly applied (Chase, 2011). Sometimes described as a symptom of other disorders (e.g., depression), apathy can also be associated with medical diagnoses. Marin (1990) clarified the concept of apathy for medical purposes by proposing its definition as a lack of motivation. He suggested that apathy is a syndrome or dimension of behavior that results from psychiatric, neurological, or medical disorders. One problem with Marin’s definition is that a lack of motivation is not the only mechanism that contributes to apathetic behavior. Another issue is that “lack of motivation” is not easily quantifiable. Levy and Dubois (2006) proposed to define apathy as the quantitative reduction of self-generated voluntary and purposeful GDB. Through this perspective, it is possible to observe and measure the various mechanisms contributing to apathy. Furthermore, it may be possible to operationalize these underlying mechanisms and postulate subtypes of apathy based on impaired GDB.
A consensus for the clinical diagnosis of apathy in neurodegenerative conditions has been proposed by an international task force (Robert et al., 2009). Diagnostic criteria require that patients meet the following requirements: (a) the core feature of diminished motivation must be present for at least 4 weeks (domain 1), (b) a reduction in two of three domains (domain 2), and (c) functional impairment attributed to the behavior (domain 3). Domain 1 refers to reduced GDB. It describes the loss of self-initiated behavior (e.g., starting a conversation) and loss of environment-stimulated behavior (e.g., responding to conversation). Domain 2 refers to a reduction in goal-directed cognitive behavior, described as a loss of ideas and curiosity for new routines (e.g., recent news, social opportunities). Domain 3 refers to a reduction in emotion, specifically a loss of spontaneous emotion or emotional responsiveness to positive or negative stimuli (i.e., little reaction to exciting news). A reliable clinical diagnosis of apathy is necessary to identify its presence and distinguish it from other clinical syndromes, such as depression. However, these proposed criteria focus solely on clinical presentation of apathy. In the current article, the authors provide a clinical description of apathy to develop an understanding of the different mechanisms that underlie apathy so that meaningful treatments that target unique patient characteristics can be pursued.
APATHY AS THE PATHOLOGY OF GOAL-DIRECTED BEHAVIOR
Apathy can be explained and examined within the concept of GDB (Figure). GDB is operationalized as a “broad spectrum of purposeful actions and their determinants, from the simplest movement to the most complex patterns of behavior” (Brown & Pluck, 2000, p. 415). This concept is related to the belief that when action a is taken, goal x may be obtained as a result. The integration of the processes that influence an individual to act (i.e., intention) is central to GDB. According to the model, three processes (i.e., initiation, planning, and motivation) influence the intention to act. Although each step is necessary to achieve GDB, clinical observations of patients with neurodegenerative disease suggest that these processes may not be sequential. Within the hypothesized model, apathy arises when any one of these three processes is impaired. For example, patients who have profound impairments in the executive abilities needed to design and carry out plans of action may be motivated to engage in GDB, but their planning impairments make it difficult to engage in GDB. Therefore, it is likely that each process is independent and, when compromised, contributes to apathy.
Figure.
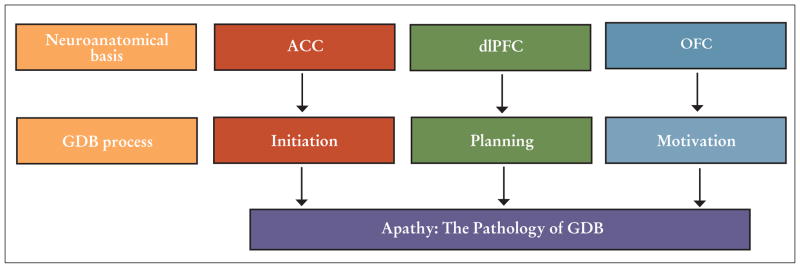
Model of apathy as the pathology of goal-directed behavior (GDB) in behavioral variant frontotemporal degeneration. Adapted from Levy and Dubois (2006).
Note. ACC = anterior cingulate cortex; dlPFC = dorsolateral prefrontal cortex; OFC = orbitofrontal cortex.
COMPONENTS OF GOAL-DIRECTED BEHAVIOR
Initiation Component
The failure to execute behavior leads to apathy when processing is unable to generate a signal significant enough to initiate a response. Difficulty with initiation has been reported in patients with focal lesions in the anterior cingulate cortex (ACC). For example, the akinetic mute state is a medical term describing patients who tend to sit quietly in the same position all day without speaking or talking. The akinetic mute state has been specifically related to ACC damage (Mega & Cohenour, 1997).
The ACC has been well studied in neurodegenerative disease, and neuroimaging evaluations have linked the ACC region to apathy in various dementia groups. Low grey matter density in the cingulate gyrus was associated with increased severity measures of apathy in Parkinson’s disease (Reijnders et al., 2010). Other researchers have implicated this region in apathetic bvFTD patients (Massimo et al., 2009; Zamboni et al., 2008). Although disease in the ACC contributes to apathy in patients, few evaluations have described the relationship as it pertains to initiation of GDB.
Planning Component
The ability to execute an action is highly dependent on the cognitive processes needed to formulate and carry out goals. Apathy related to “cognitive inertia” results from impairments in executive functions, such as planning, working memory, and task switching (Levy & Dubois, 2006, p. 919). These cognitive processes are needed to organize and structure GDB. The loss of these abilities will quantitatively reduce behavior.
Planning is an important aspect of executive function. The cognitive demand of planning includes selecting, organizing, and executing numerous tasks within a given time period (Burgess, 2000). The anatomical basis of executive dysfunction has been linked to dorsolateral portions of the prefrontal cortex (Miller & Cohen, 2001). This region has been shown to play a critical role in planning (Newman, Carpenter, Varma, & Just, 2003). Studies suggest an association between apathy and poor executive function in bvFTD (Zamboni et al., 2008). Imaging studies of patients with neurodegenerative disease have linked apathy to tissue loss in dorsolateral portions of the prefrontal cortex (Massimo et al., 2009; Zamboni et al., 2008). Patients who experience dysfunction in these circuits fail to elaborate, manipulate, and integrate important information needed for behavior that is goal directed.
Motivation Component
Apathy may also result from a lack of responsiveness to either reward or negative feedback, thereby making goal selection difficult (Levy & Dubois, 2006; Rosen et al., 2002). Because rewards and avoidance of negative consequences constitute basic goals of behavior, motivational functions are based partly on the processing of reward information (Schultz, Tremblay, & Hollerman, 2000).
Evidence from magnetic resonance imaging studies of healthy individuals suggests that the orbitofrontal cortex is important for determining information regarding interpretation of reward (Hare, Camerer, Knoepfle, & Rangel, 2010; Kable & Glimcher, 2007). Individuals with bvFTD have been examined extensively in reward processing because they have an early degeneration of the associated frontal circuit in comparison to other neurodegenerative conditions (Rabinovici et al., 2007). Grossman et al. (2010) examined the interpretation of positive and negative situations in individuals with bvFTD and found that these individuals were particularly impaired in interpreting negative consequences of a social situation (e.g., “rolling through a red light at 2 a.m. when there is a police car at the intersection,” p. 5). Given these patients’ insensitivity to negative consequences, it is hypothesized that this insensitivity may underlie reduced motivation.
The study of reward processing and resultant apathetic behavior in the bvFTD population offers essential insights into the functions of the orbitofrontal cortex. Experimental evidence using imaging techniques in patients with bvFTD has emphasized the link between orbitofrontal regions and apathetic behaviors. Comparison of brain activity between apathetic and nonapathetic bvFTD participants using positron emission tomography data revealed decreased activity in the orbitofrontal cortex in apathetic participants (Peters et al., 2006). Rosen et al. (2005) examined apathy, as measured by the Neuropsychiatric Inventory (NPI; Cummings et al., 1994), and found apathy scores to be independently associated with atrophy in the ventromedial frontal gyrus. The conclusions of these imaging studies suggest that the orbitofrontal cortex has a relationship to apathy, although distinct areas of this region may have specific roles.
THE PHILADELPHIA APATHY COMPUTERIZED TEST
One of the primary obstacles to advancing knowledge about apathy has been the absence of a quantitative method that directly measures specific mechanisms contributing to apathy. Although several global apathy assessment tools exist for the cognitively impaired population, a lack of agreement exists regarding the interpretability of the data from these measures (Clarke et al., 2011). This lack of agreement may be due, in part, to the fact that traditional instruments used to ascertain apathy commonly use proxy report from caregivers. Unfortunately, this approach is subject to caregiver confounds (e.g., burden, strain), which may impact the evaluation. Furthermore, beyond confirming the presence of apathy, current instruments, such as the NPI, are ineffective in identifying different subtypes of apathy (Chow et al., 2009). Thus, the Philadelphia Apathy Computerized Task (PACT; Massimo, 2014) was designed to quantify components of GDB in apathetic participants in an objective manner that is minimally confounded by proxy report. Experimental computer tests examining the basis for a social behavior are useful in studying the mechanisms contributing to the behavior. Moreover, they are quantitatively rigorous. The PACT was intended to measure three components of GDB: initiation, planning, and motivation. The PACT was developed based on a review of experimental paradigms in the scientific literature and clinical observations (Elliott, Agnew, & Deakin, 2010; Jenkins, Jahanshahi, Jueptner, Passingham, & Brooks, 2000; Ruh, Cooper, & Mareschal, 2010). In all experimental conditions, a trial begins when a participant depresses a computer “start” key with one finger. Reaction time (RT) to lift this finger from the start key in response to a signal (RT1), as well as reaction time to depress the target key once lifted from the start key (RT2) are measured. A practice block, in which participants receive instructions about task performance and 12 practice trials, precedes each of the three experimental conditions described below.
Initiation refers to an individual’s ability to self-generate or activate actions (Levy & Dubois, 2006). Initiation is measured in the simplest condition, wherein the participant begins a trial by depressing the start key. A central stimulus appears on the computer screen, and a fixed central target key is depressed in response to this stimulus. Over 48 trials, the signal was found to occur on average 1,250 msec (range = 500 to 2,000 msec) after depressing the start key. Initiation is assessed by RT1 in this condition.
Planning refers to the ability to elaborate plans of action (Levy & Dubois, 2006). Therefore, assessing the planning component requires a resource-demanding task that depends on the integration of strategies to meet the challenges of the condition (Sorel & Pennequin, 2008; Toglia & Berg, 2013). In the second condition, which is designed to assess the planning component of GDB, two levels of task difficulty are assessed. For level one (i.e., simple planning), participants depress the start key and are signaled by randomly ordered lateralized visual stimuli to press a left or right target key (i.e., stimulus appears on left→go left; stimulus appears on right→go right). In the second, more complex level, one of two lateralized keys is pressed contingent on the combination of patterns in a central visual stimulus (i.e., blue and horizontal stripes→go left; orange and vertical stripes→go right). To assure that planning can be isolated and assessed specifically, the influence of working memory confounds are minimized by making the patterns visually available to participants during performance. Two measures of planning are assessed: (a) total latency in the complex planning condition and (b) the difference in response times between the two levels of difficulty.
Motivation refers to the ability to associate affective signals (positive or negative) with value to perform actions (Levy & Dubois, 2006). In the third condition, designed to assess motivation, the simple condition (i.e., triangle appears centrally) is repeated with an explicit monetary incentive using a point system (i.e., monetary units) to reward participants for responding correctly and more rapidly. Participants receive feedback on the computer screen about their response speed after each trial. Sensitivity to negative consequence is assessed by having a “penalty” condition. In this penalty condition, participants are given a number of monetary units at the beginning of each task, and monetary units are taken away if they do not respond correctly and more rapidly. The authors of the current study elected to use the penalty condition measure to assess motivation because previous work has shown that bvFTD patients are particularly insensitive to negative feedback (Grossman et al., 2010). A point system involving monetary units that are converted to actual money allowed all participants to receive the same total payment at the end of the study regardless of performance.
Latency means and standard deviations are used to derive individualized apathy profiles that have been developed according to predetermined criteria (Table) that correspond to each of the three conditions of the PACT. These profiles allow the subtypes of apathy to be differentiated.
TABLE.
PACT CRITERIA FOR APATHY SUBTYPES
| Subtype Profile | Criteria |
|---|---|
| Initiation | Significantly slow RT1 in simple condition |
| Does not have slowed latencies for complex condition | |
| Able to improve performance in response to penalty | |
| Planning | Significantly slowed on complex planning condition |
| Does not have slowed initiation for simple condition | |
| Able to improve performance in response to penalty | |
| Motivation | Significantly slowed on simple penalty condition and fails to improve performance with penalizing motivators |
| Does not have slowed initiation for simple condition | |
| Does not have slowed latencies for complex condition |
Note. PACT = Philadelphia Apathy Computerized Test; RT1 = reaction time to lift finger from start key in response to a signal.
IMPLICATIONS FOR PERSON-CENTERED CARE
A pathophysiological model of GDB, which revealed three distinct mechanisms likely contributing to apathy—impairments in initiation, planning, and motivation—was previously examined (Massimo, 2014). Although the initial work is biomedical in nature, a future goal is to optimize interventions for apathy subtypes so that families and other direct care providers can implement effective care strategies that are focused on individual characteristics of the patient.
Nurses are encouraged to assess for the presence of apathy subtypes. Without this crucial step, interventions may be ineffective. The assessment of the efficacy of treatments for apathy has been hindered because of methodological failures in trials where apathetic patients are viewed homogeneously (e.g., solely as displaying a lack of motivation). The authors find that lack of motivation is not the only process that contributes to apathy (Massimo, 2014). Based on the authors’ work, future treatments for apathy would more appropriately be tailored to the specific characteristics of GDB that are compromised in an individual. For example, when apathy emerges in response to planning difficulties, benefit is likely to be gained from restructuring a complex activity into simple components for the patient. For patients with impaired goal selection, modifications, such as amplified lighting in a room or onto a specific activity or object, may increase the reward potential of the environment, thereby increasing motivation (Ishii et al., 2009). Rewards, such as positive verbal feedback and reinforcements with food, can also be used to motivate individuals with FTD (Merrilees, Klapper, Murphy, Lomen-Hoerth, & Miller, 2010). Lastly, multisensory stimulation—a therapeutic approach that provides visual, auditory, tactile, and olfactory stimulation—may be helpful for patients with initiation difficulty (Baker et al., 2001). However, the use of multisensory stimulation in a patient with planning difficulty may worsen rather than improve apathy because it can cause distractibility. To facilitate research, a systematic evaluation of existing interventions for apathy is warranted, followed by the categorization and testing of interventions designed for specific subtypes. These studies are important to improve patient and family outcomes.
Recognizing and making a reliable diagnosis of apathy by subtype is essential to initiate treatment. Patients with apathy are often overlooked because of their lack of apparent distress (Butterfield et al., 2010). Although several apathy assessment tools exist for the cognitively impaired population, a lack of agreement exists regarding the interpretability of the data from these measures (Clarke et al., 2011). Traditional instruments to ascertain the presence of apathy commonly rely on proxy report. One goal of the PACT was to identify subtypes of apathy in an objective manner that is minimally confounded by proxy report. The current work is the first step in the development of an instrument that would be based on objective, empirical measurements of impairments in each of the components of GDB that contribute to apathy. Such an instrument would improve the current instruments because of the objective basis and would increase the likelihood of detection and targeted treatment of specific subtypes of apathy.
Empirical data on apathy in FTD are critical to facilitating a person-centered approach in individuals with dementia and are consistent with work by Downs (2013), which emphasized the need for recognizing the unique needs of the individual with dementia. The current study helps families and direct care staff characterize difficulties with initiation, planning, and motivation to better understand apathy. Eventually, this will lead to improved individualization of activities and interventions for apathy in FTD.
CONCLUSION
GDB is a multicomponent process that involves initiation, planning, and motivation. These three GDB processes map onto three distinct brain regions that work together in a large-scale neural network. This network captures the information from internal and external environments needed for GDBs. A specific GDB process suffers when one of these frontal areas is compromised and is associated with behavior currently referred to as apathy. Presently, apathy is viewed as a unitary concept. The authors’ view is that apathy is a multicomponent phenomenon—a complex behavioral syndrome that emerges when dysfunction exists in any GDB component. Thus, it is likely that the pathophysiology is not a single mechanism, but is rather multifaceted. It is possible to identify single impairments in GDB that may contribute to different characteristics or subtypes of apathy. This initial work will change the paradigm for assessing and treating apathy, thus leading to improved diagnostic accuracy and effective personalized interventions to improve the ability of families, nurses, and other health care professionals to manage a pervasive feature of neurodegenerative disease.
KEYPOINTS.
Apathy is a common behavior in neurodegenerative conditions, especially frontotemporal degeneration.
The concept of goal-directed behavior (GDB) provides a useful model for examining the mechanisms underlying apathy.
Treatments for apathy should be tailored to the specific characteristics of GDB that are compromised in an individual.
Footnotes
The authors have disclosed no potential conflicts of interest, financial or otherwise.
References
- Baker R, Bell S, Baker E, Gibson S, Holloway J, Pearce R, Wareing LA. A randomized controlled trial of the effects of multi-sensory stimulation (MSS) for people with dementia. British Journal of Clinical Psychology. 2001;40:81–96. doi: 10.1348/014466501163508. [DOI] [PubMed] [Google Scholar]
- Brown RG, Pluck G. Negative symptoms: The ‘pathology’ of motivation and goal-directed behavior. Trends in Neuroscience. 2000;23:412–417. doi: 10.1016/s0166-2236(00)01626-x. [DOI] [PubMed] [Google Scholar]
- Burgess PW. Strategy application disorder: The role of the frontal lobes in human multitasking. Psychological Research. 2000;63:279–288. doi: 10.1007/s004269900006. [DOI] [PubMed] [Google Scholar]
- Butterfield LC, Cimino CR, Oelke LE, Hauser RA, Sanchez-Ramos J. The independent influence of apathy and depression on cognitive functioning in Parkinson’s disease. Neuropsychology. 2010;24:721–730. doi: 10.1037/a0019650. [DOI] [PubMed] [Google Scholar]
- Chase TN. Apathy in neuropsychiatric disease: Diagnosis, pathophysiology, and treatment. Neurotoxicity Research. 2011;19:266–278. doi: 10.1007/s12640-010-9196-9. [DOI] [PubMed] [Google Scholar]
- Chio A, Vignola A, Mastro E, Giudici AD, Iazzolino B, Calvo A, Montuschi A. Neurobehavioral symptoms in ALS are negatively related to caregivers’ burden and quality of life. European Journal of Neurology. 2010;17:1298–1303. doi: 10.1111/j.1468-1331.2010.03016.x. [DOI] [PubMed] [Google Scholar]
- Chow TW, Binns MA, Cummings JL, Lam I, Black SE, Miller BL, van Reekum R. Apathy symptom profile and behavioral associations in frontotemporal dementia vs dementia of Alzheimer type. Archives of Neurology. 2009;66:888–893. doi: 10.1001/archneurol.2009.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke DE, Ko JY, Kuhl EA, van Reekum R, Salvador R, Marin RS. Are the available apathy measures reliable and valid? A review of the psychometric evidence. Journal of Psychosomatic Research. 2011;70:73–97. doi: 10.1016/j.jpsychores.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- de Vugt ME, Riedijk SR, Aalten P, Tibben A, van Swieten JC, Verhey FR. Impact of behavioural problems on spousal caregivers: A comparison between Alzheimer’s disease and frontotemporal dementia. Dementia and Geriatric Cognitive Disorders. 2006;22(1):35–41. doi: 10.1159/000093102. [DOI] [PubMed] [Google Scholar]
- Diehl-Schmid J, Pohl C, Perneczky R, Forstl H, Kurz A. Behavioral disturbances in the course of frontotemporal dementia. Dementia and Geriatric Cognitive Disorders. 2006;22:352–357. doi: 10.1159/000095625. [DOI] [PubMed] [Google Scholar]
- Downs M. Embodiment: The implications for living well with dementia. Dementia. 2013;12:368–374. doi: 10.1177/1471301213487465. [DOI] [PubMed] [Google Scholar]
- Drijgers RL, Dujardin K, Reijnders JS, Defebvre L, Leentjens AF. Validation of diagnostic criteria for apathy in Parkinson’s disease. Parkinsonism & Related Disorders. 2010;16:656–660. doi: 10.1016/j.parkreldis.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Elliott R, Agnew Z, Deakin JF. Hedonic and informational functions of the human orbitofrontal cortex. Cerebral Cortex. 2010;20:198–204. doi: 10.1093/cercor/bhp092. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Dennis K, Moore P, Antani S, Hauck R, Grossman M. Metacognitive deficits in frontotemporal dementia. Journal of Neurology, Neurosurgery, and Psychiatry. 2005;76:1630–1635. doi: 10.1136/jnnp.2004.053157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslinger PJ, Moore P, Antani S, Anderson C, Grossman M. Apathy in frontotemporal dementia: Behavioral and neuroimaging correlates. Behavioural Neurology. 2012;25:127–136. doi: 10.3233/BEN-2011-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Eslinger PJ, Troiani V, Anderson C, Avants B, Gee JC, Antani S. The role of ventral medial prefrontal cortex in social decisions: Converging evidence from fMRI and frontotemporal lobar degeneration. Neuropsychologia. 2010;48:3505–3512. doi: 10.1016/j.neuropsychologia.2010.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Knoepfle DT, Rangel A. Value computations in ventral medial prefrontal cortex during charitable decision making incorporate input from regions involved in social cognition. Journal of Neuroscience. 2010;30:583–590. doi: 10.1523/JNEUROSCI.4089-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S, Weintraub N, Mervis JR. Apathy: A common psychiatric syndrome in the elderly. Journal of the American Medical Directors Association. 2009;10:381–393. doi: 10.1016/j.jamda.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Jahanshahi M, Jueptner M, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. II. The effect of movement predictability on regional cerebral blood flow. Brain. 2000;123:1216–1228. doi: 10.1093/brain/123.6.1216. [DOI] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nature Neuroscience. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karttunen K, Karppi P, Hiltunen A, Vanhanen M, Valimaki T, Martikainen J, Pirttila T. Neuropsychiatric symptoms and quality of life in patients with very mild and mild Alzheimer’s disease. International Journal of Geriatric Psychiatry. 2011;26:473–482. doi: 10.1002/gps.2550. [DOI] [PubMed] [Google Scholar]
- Landes AM, Sperry SD, Strauss ME, Geldmacher DS. Apathy in Alzheimer’s disease. Journal of the American Geriatrics Society. 2001;49:1700–1707. doi: 10.1046/j.1532-5415.2001.49282.x. [DOI] [PubMed] [Google Scholar]
- Le Ber I, Guedj E, Gabelle A, Verpillat P, Volteau M, Thomas-Anterion C, Dubois B. Demographic, neurological and behavioural characteristics and brain perfusion SPECT in frontal variant of frontotemporal dementia. Brain. 2006;129:3051–3065. doi: 10.1093/brain/awl288. [DOI] [PubMed] [Google Scholar]
- Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cerebral Cortex. 2006;16:916–928. doi: 10.1093/cercor/bhj043. [DOI] [PubMed] [Google Scholar]
- Marin RS. Differential diagnosis and classification of apathy. American Journal of Psychiatry. 1990;147:22–30. doi: 10.1176/ajp.147.1.22. [DOI] [PubMed] [Google Scholar]
- Marin RS. Apathy and related disorders of diminished motivation. In: Dickstein LJ, Riba MB, Oldham JM, editors. Review of psychiatry. Vol. 15. Washington, DC: American Psychiatric Publishing; 1996. pp. 205–242. [Google Scholar]
- Massimo L, Evans LK, Benner P. Caring for loved ones with frontotemporal degeneration: The lived experiences of spouses. Geriatric Nursing. 2013;34:302–306. doi: 10.1016/j.gerinurse.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimo L, Grossman M. Patient care and management of frontotemporal lobar degeneration. American Journal of Alzheimer’s Disease and Other Dementias. 2008;23:125–131. doi: 10.1177/1533317507307961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimo L, Powers C, Moore P, Vesely L, Avants B, Gee J, Grossman M. Neuroanatomy of apathy and disinhibition in frontotemporal lobar degeneration. Dementia & Geriatric Cognitive Disorders. 2009;27:96–104. doi: 10.1159/000194658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimo LM. Unpublished doctoral dissertation. University of Pennsylvania; Philadelphia, PA: 2014. The cognitive and neural basis for apathy in behavioral variant frontotemporal degeneration. [Google Scholar]
- Mega MS, Cohenour RC. Akinetic mutism: Disconnection of frontal-subcortical circuits. Neuropsychiatry, Neuropsychology and Behavioral Neurology. 1997;10:254–259. [PubMed] [Google Scholar]
- Mendez MF, Lauterbach EC, Sampson SM. An evidence-based review of the psychopathology of frontotemporal dementia: A report of the ANPA Committee on Research. Journal of Neuropsychiatry and Clinical Neurosciences. 2008;20:130–149. doi: 10.1176/appi.neuropsych.20.2.130. [DOI] [PubMed] [Google Scholar]
- Merrilees J, Klapper J, Murphy J, Lomen-Hoerth C, Miller BL. Cognitive and behavioral challenges in caring for patients with frontotemporal dementia and amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis. 2010;11:298–302. doi: 10.3109/17482961003605788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.16724/1/167. [DOI] [PubMed] [Google Scholar]
- Newman SD, Carpenter PA, Varma S, Just MA. Frontal and parietal participation in problem solving in the Tower of London: fMRI and computational modeling of planning and high-level perception. Neuropsychologia. 2003;41:1668–1682. doi: 10.1016/s0028-3932(03)00091-5. [DOI] [PubMed] [Google Scholar]
- Pedersen KF, Alves G, Aarsland D, Larsen JP. Occurrence and risk factors for apathy in Parkinson disease: A 4-year prospective longitudinal study. Journal of Neurology, Neurosurgery, and Psychiatry. 2009;80:1279–1282. doi: 10.1136/jnnp.2008.170043. [DOI] [PubMed] [Google Scholar]
- Peters F, Perani D, Herholz K, Holthoff V, Beuthien-Baumann B, Sorbi S, Salmon E. Orbitofrontal dysfunction related to both apathy and disinhibition in frontotemporal dementia. Dementia & Geriatric Cognitive Disorders. 2006;21:373–379. doi: 10.1159/000091898. [DOI] [PubMed] [Google Scholar]
- Pluck GC, Brown RG. Apathy in Parkinson’s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 2002;73:636–642. doi: 10.1136/jnnp.73.6.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovici GD, Seeley WW, Kim EJ, Gorno-Tempini ML, Rascovsky K, Pagliaro TA, Rosen HJ. Distinct MRI atrophy patterns in autopsy-proven Alzheimer’s disease and frontotemporal lobar degeneration. American Journal of Alzheimer’s Disease and Other Dementias. 2007;22:474–488. doi: 10.1177/1533317507308779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology. 2002;58:1615–1621. doi: 10.1212/wnl.58.11.1615. [DOI] [PubMed] [Google Scholar]
- Reijnders JS, Scholtissen B, Weber WE, Aalten P, Verhey FR, Leentjens AF. Neuroanatomical correlates of apathy in Parkinson’s disease: A magnetic resonance imaging study using voxel-based morphometry. Movement Disorders. 2010;25:2318–2325. doi: 10.1002/mds.23268. [DOI] [PubMed] [Google Scholar]
- Robert P, Onyike CU, Leentjens AF, Dujardin K, Aalten P, Starkstein S, Byrne J. Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. European Psychiatry. 2009;24:98–104. doi: 10.1016/j.eurpsy.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128:2612–2625. doi: 10.1093/brain/awh628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Perry RJ, Murphy J, Kramer JH, Mychack P, Schuff N, Miller BL. Emotion comprehension in the temporal variant of frontotemporal dementia. Brain. 2002;125:2286–2295. doi: 10.1093/brain/awf225. [DOI] [PubMed] [Google Scholar]
- Rosso SM, Donker Kaat L, Baks T, Joosse M, de Koning I, Pijnenburg Y, van Swieten JC. Frontotemporal dementia in The Netherlands: Patient characteristics and prevalence estimates from a population-based study. Brain. 2003;126:2016–2022. doi: 10.1093/brain/awg204. [DOI] [PubMed] [Google Scholar]
- Ruh N, Cooper RP, Mareschal D. Action selection in complex routinized sequential behaviors. Journal of Experimental Psychology Human Perception and Performance. 2010;36:955–975. doi: 10.1037/a0017608. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cerebral Cortex. 2000;10:272–284. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Sorel O, Pennequin V. Aging of the planning process: The role of executive functioning. Brain and Cognition. 2008;66:196–201. doi: 10.1016/j.bandc.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Toglia J, Berg C. Performance-based measure of executive function: Comparison of community and at-risk youth. American Journal of Occupational Therapy. 2013;67:515–523. doi: 10.5014/ajot.2013.008482. [DOI] [PubMed] [Google Scholar]
- Vilalta-Franch J, Calvo-Perxas L, Garre-Olmo J, Turro-Garriga O, Lopez-Pousa S. Apathy syndrome in Alzheimer’s disease epidemiology: Prevalence, incidence, persistence, and risk and mortality factors. Journal of Alzheimer’s Disease. 2013;33:535–543. doi: 10.3233/JAD-2012-120913. [DOI] [PubMed] [Google Scholar]
- Wrubel J, Folkman S. What informal caregivers actually do: The caregiving skills of partners of men with AIDS. AIDS Care. 1997;9:691–706. doi: 10.1080/713613223. [DOI] [PubMed] [Google Scholar]
- Zamboni G, Huey ED, Krueger F, Nichelli PF, Grafman J. Apathy and disinhibition in frontotemporal dementia: Insights into their neural correlates. Neurology. 2008;71:736–742. doi: 10.1212/01.wnl.0000324920.96835.95. [DOI] [PMC free article] [PubMed] [Google Scholar]


