Abstract
Objective
To identify factors associated with bronchodilator administration to infants with bronchopulmonary dysplasia (BPD) and evaluate inter-institutional prescribing patterns.
Study Design
A retrospective cohort study of <29-week-gestation infants with evolving BPD defined at age 28 days within the Pediatric Health Information System database. Controlling for observed confounding with random-effects logistic regression, we determined demographic and clinical variables associated with bronchodilator use and evaluated between-hospital variation.
Result
During the study period, 33% (N=469) of 1429 infants with BPD received bronchodilators. Lengthening mechanical ventilation duration increased the odds of receiving a bronchodilator [OR 19.6(11–34.8) at ≥54 days]. There was profound between-hospital variation in use, ranging from 0–81%.
Conclusion
Bronchodilators are frequently administered to infants with BPD at U.S. children’s hospitals with increasing use during the first hospital month. Increasing positive pressure exposure best predicts bronchodilator use. Frequency and treatment duration vary markedly by institution even after adjustment for confounding variables.
Keywords: bronchopulmonary dysplasia, prematurity, bronchodilators, short acting Beta-2-adrenergic receptor agonist, nonspecific muscarinic receptor antagonist, salbutamol, albuterol, ipratropium bromide, pharmacoepidemiology, drug utilization, comparative effectiveness, practice variation, patient-centered outcomes
INTRODUCTION
Neonates with severe bronchopulmonary dysplasia (BPD) are predisposed to airway smooth muscle hyperreactivity.1, 2 Inhaled bronchodilators, including both beta-agonists and muscarinic antagonists, have been administered to infants with BPD for over 30 years to reduce airway reactivity and subsequently improve respiratory outcomes.3 Short-term benefits, including decreased airway resistance and improved gas exchange, are noted following administration. Bronchodilators have not been shown to prevent BPD, treat BPD, or diminish the severity of BPD.4, 5
Although inhaled bronchodilator use is presumed widespread in preterm infants with BPD, 6, 7 factors associated with bronchodilator administration to these patients have not, to our knowledge, been fully reported. Therefore, the current study was undertaken to: 1) determine demographic and clinical variables associated with bronchodilator administration and 3) to identify variability between-hospitals in frequency and duration of inhaled bronchodilator usage for infants with BPD.
METHODS
We conducted a retrospective cohort study to evaluate inhaled bronchodilator use for infants with BPD. Infants were considered to have evolving BPD if they survived until at least 28 days of age and received respiratory support for the first 28 consecutive days of life via mechanical ventilation, CPAP, and/or supplemental oxygen.8 The cohort, as described previously by Slaughter et al, 8 included infants with evolving BPD born prior to 29 weeks of gestation with a birth weight <1500 grams and admitted to neonatal intensive care units (NICUs) in children’s hospitals at <8 days of age with discharge dates from January 2007–June 2011, as recorded in the Children’s Hospital Association (CHA) Pediatric Health Information System (PHIS) database (Shawnee Mission, Kansas). We chose our gestational age (<29 weeks) and birth weight (<1500 grams) cutoffs to include >97% of infants with BPD2. We excluded infants admitted after seven days of age to minimize exposure to unmeasured inhaled bronchodilator treatment at outside hospitals and only included those who lived ≥28 days, the earliest age at which BPD may be assigned.1 We chose to define BPD at its 28 day onset, prior to severity staging at 36 weeks corrected age, since an infant’s respiratory condition at BPD onset was more likely to influence a clinician’s decision regarding inhaled bronchodilator usage throughout the remainder of their hospitalization than the 36 week outcome measurement. For reference, we also determined the frequency of mild, moderate, and severe BPD at 36 weeks according to the National Institutes of Health (NIH) Consensus Definition,1 as modified by Ehrenkranz et al. 9 Surviving infants with evolving BPD at age 28 days were assigned mild BPD if they were off oxygen at 36 weeks or discharged alive prior to 36 weeks, moderate BPD if on oxygen only at 36 weeks, and severe BPD if they required CPAP or mechanical ventilation at 36 weeks gestation.
Data Source
The PHIS database contains administrative, billing, and record-review data including patient demographics, diagnoses, procedures, and medications labeled by route of administration from member U.S. Children’s Hospitals, which account for 85% of all national freestanding children’s hospitals (Children’s Hospital Association, Shawnee Mission, KS).
The study sample for patient-level analyses consisted of 1429 admissions from 35 hospitals with cases of evolving BPD at 28 days, as defined above. To prevent bias from the overweighting of institutions with smaller BPD sample sizes, between-hospital comparisons based on the mean proportion of bronchodilator usage at each hospital were confined to the 15 hospitals with at least 25 BPD cases representing 86% (N=1226) of the study-sample.
Study Variables
Daily drug-specific inhaled bronchodilator administration, mechanical ventilation, CPAP, oxygen use, as well as length of stay and demographic variables were determined from each hospital’s daily charge records as included in PHIS. Each hospital’s daily charge codes are translated into a common classification system, the Clinical Transaction Classification (CTC) Codes, to ensure comparability of charge-level data between institutions. CTC codes evaluated included: inhaled albuterol (181211.42), inhaled levalbuterol (181231.42), inhaled albuterol/ipratropium combination (181315.42), inhaled ipratropium bromide (181311.42), inhaled pirbuterol (181251.42), inhaled formoterol (181221.42) inhaled salmeterol (181241.42), mechanical ventilation (521166), CPAP (521162), and oxygen delivery by cannula, tent, or mask (521171). We evaluated the use of all inhaled bronchodilators included in the PHIS database within our cohort population and excluded those with <1% frequency of use (pirbuterol, formoterol, salmeterol) from further analysis. We queried the specific dosage form for each drug administration to determine whether delivery was by metered dose inhaler (MDI) or nebulizer. International Statistical Classification of Diseases, Ninth Revision (ICD-9) Codes were used to determine the diagnoses of patent ductus arteriosus (PDA)(747.0), intraventricular hemorrhage (IVH)(772.1), and necrotizing enterocolitis (NEC)(777.5).
Statistical Analysis
All analyses were conducted using Stata 12.1 (StataCorp, College Station, Texas). Unadjusted odds ratios for bivariate associations between bronchodilator use and neonatal demographic/clinical risk factors were determined with simple logistic regression for binary outcome variables and simple ordinary least squares (OLS) regression for continuous outcomes. Multivariable mixed logistic regression modeling with a random intercept for hospitals was used to adjust odds ratios for confounding variables and to evaluate the contribution of within-hospital clustering to variation in bronchodilator administration. The model was created by purposeful selection and included gestational age, gender, duration of mechanical ventilation or CPAP, IVH, PDA, and NEC as variables. A second model was created with birth weight replacing gestational age to obtain adjusted odds ratios (aORs) for birth weight. This was done to avoid multicollinearity between birth weight and gestational age. All statistical testing was two-sided and an alpha-level of 0.05 was considered significant. We chose to report the median measure of central tendency because the distributions for days of use for all bronchodilator formulations were skewed to the right, indicating wide variation in treatment duration.
Ethics Review
The Nationwide Children’s Hospital Institutional Review Board determined that this was not human subjects’ research, because it was an analysis of a pre-existing, de-identified dataset and involved no patient contact.
RESULTS
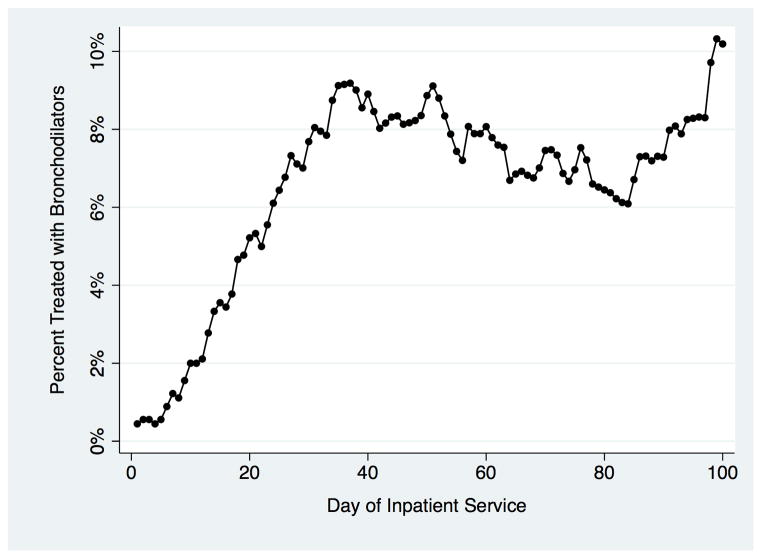
A total of 1429 infants met the criteria for BPD at 28 days, of which 469 (33%) were treated with at least one inhaled bronchodilator dose. Among those treated with nebulized bronchodilators in the first 100 days post-admission, the percentage treated per hospital day is demonstrated in Figure 1. Usage steadily increased during the first month of hospitalization until day 37 when 9.7% received nebulized albuterol treatment. The proportion of bronchodilator-treated infants increased further during the third month of hospitalization, ultimately peaking at 10.3% on day 99. In unadjusted analysis, decreasing birth weight, decreasing gestational age, male gender, increasing exposure to CPAP or mechanical ventilation, intraventricular hemorrhage, and necrotizing enterocolitis were all associated with significantly increased odds of bronchodilator treatment. When all variables were included in a multivariable logistic regression model with a random intercept to adjust for confounding and within-hospital clustering (Table 1), the odds of an infant with BPD ever receiving inhaled bronchodilators were significantly associated with birth weight between 500 and 749 grams, birth at 25–26 weeks gestation, and increasing duration of airway exposure to positive-pressure via CPAP or mechanical ventilation. There was no significant association between inhaled bronchodilator administration and birth at <24 weeks gestation or birth weight <500-grams, which could be related to the small sample size (n = 40) of <500-gram infants and increased inpatient mortality risk (risk ratio: 2.93; P =0.004) relative to the other infants in the cohort.
Figure 1.
Percentage of infants with evolving BPD that received nebulized bronchodilator treatments by day of hospitalization.
Table 1.
Multivariable Adjusted Odds of Receiving Bronchodilators
| Variable | Ever Received Bronchodilators (n = 469, 33%) | |
|---|---|---|
| n (%) | Adjusted Odds Ratio, 95% CI | |
| Gestation | ||
| 27–28 weeks (n = 531, 37%) (Reference) | 122 (23) | - |
| 25–26 weeks (n = 599, 42%) | 209 (35) | 1.41(1.01–1.98)* |
| ≤24 weeks (n = 299, 21%) | 138 (46) | 1.49 (0.99–2.26) |
| Birth Weight (grams) | ||
| 1000–1500 gms (n = 347, 24%) (Reference) | 74 (21) | - |
| 750–999 gms (n = 588, 41%) | 182 (31) | 1.20 (0.81–1.77) |
| 500–749 gms (n = 454, 32%) | 193 (43) | 1.54 (1.003–2.38)* |
| <500 gms (n = 40, 3%) | 20 (50) | 1.91 (0.82–4.44) |
| Gender | ||
| Male (n = 786, 55%) (Reference) | 280 (36) | - |
| Female (n = 643, 45%) | 189 (29) | 0.76 (0.57–1.0) |
| Days on CPAP or Mechanical Ventilation | ||
| Vent ≤20 days (n = 371, 26%) (Reference) | 99 (27) | - |
| Vent 21–35 days (n = 349, 24%) | 79 (23) | 3.02 (1.82–5.03)* |
| Vent 36–53 days (n = 361, 25%) | 112 (31) | 5.98 (3.52–10.2)* |
| Vent ≥54 days (n = 348, 24%) | 179 (51) | 19.6 (11–34.8)* |
| Major Comorbidities | ||
| IVH (n = 549, 38%) | 209 (38) | 1.31 (0.98–1.76) |
| NEC (n = 293, 21%) | 125 (43) | 1.05 (0.74–1.49) |
| PDA (n = 868, 61%) | 297 (34) | 1.12 (0.83–1.51) |
All regression odds ratios (95% C.I.), except those for Major Comorbidities are reported relative to the reference group indicated by (−). Variable column percentiles were calculated from the entire BPD cohort (N = 1429). All other column percentiles were calculated by using row n as the denominator. Adjusted odds ratios (aORs) were determined using a mixed-effects logistic regression model with a random intercept for hospital, in which all variables in the table were fit in the model except for gestational age. Gestational age aORs were determined by a second model, which included all variables except for birth weight, in order to avoid multicollinearity between birth weight and gestational age.
Statistically significant at alpha= 0.05
Utilization of Specific Inhaled Bronchodilators
We determined the frequencies of use for the three inhaled bronchodilators that were ever prescribed to >1% of infants within the cohort of BPD patients. Inhaled albuterol was administered to 484 (34%) infants, levalbuterol to 94 (7%), and ipratropium bromide to 32 (2%). Fourteen (0.98%) infants received a combined albuterol/ipratropium medication. Some infants were treated with more than one of these medications at some point during their hospitalization. Albuterol and levalbuterol were administered to 37 (3%) infants, 39 (3%) received albuterol and ipratropium, 6 (0.4%) received levalbuterol and ipratropium, and 5 (0.4%) infants received all three medications prior to discharge.
Between-Hospital Variation in Bronchodilator Treatment
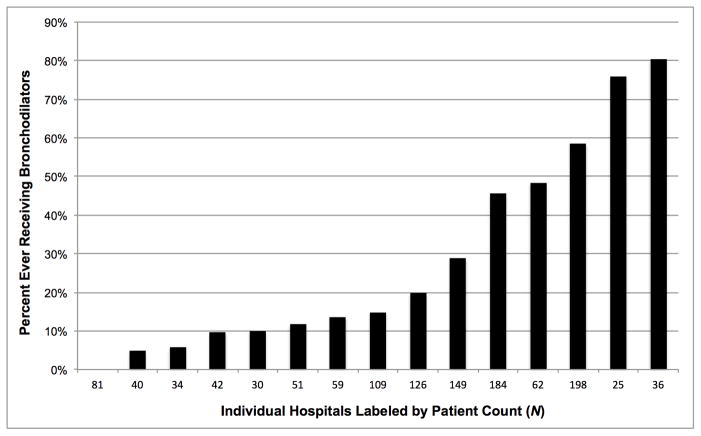
Inhaled bronchodilator use for infants with BPD varied between children’s hospitals. By hospital (N=15), the percentage that ever received these medications ranged from 0–81% (median: 15; IQR: 10%–48%) (Figure 2).
Figure 2.
Percentage of infants that ever received inhaled bronchodilators (nebulized or MDI) during their hospital stay, by hospital. Range: 0 to 81% (median: 15%). Hospitals were included if at least 25 patients developed BPD during the study period (N=15).
Variation in bronchodilator use between hospitals persisted even after controlling for length of exposure to positive pressure via mechanical ventilation or CPAP, birth weight, gestational age, IVH, PDA, and NEC in our multivariable logistic regression models with random intercepts (Table 1). The intraclass correlation coefficient (ICC), a measure of the proportion of total variance in bronchodilator use due to variation between hospitals, indicated that clustering by hospital was a significant component of the overall variation in the frequency of both short-and longer-courses prescribed (ICC = 0.51; 95% CI: 0.36–0.66). There was no significant correlation between the number of patients with evolving BPD at 28 days hospitalized at each center during the study period and bronchodilator use (R = 0.17, p = 0.54).
Median Duration of Nebulized Albuterol Therapy
Since MDI treatments are billed per inhaler, we could only determine cumulative days for nebulized treatments, which are billed per administration. A total of 362 infants (25%) ever received nebulization versus 221 (15%) by MDI. Overall, treated patients (n=320) received a median of 7 (25th–75th percentiles: 2–27) (range 1–412) days of nebulized albuterol. Those receiving albuterol via MDI (n=208) were billed for a median of 2 (25th–75th percentiles: 1–3) (range 1–26) inhalers. Nebulized levalbuterol (n=60) was administered for a median of 26 (25th–75th percentiles: 9–54) (range 1–162) days and infants received one median MDI levalbuterol dose (n=12) (25th–75th percentiles: 1–3) (range 1–4). Although median duration of use for levalbuterol exceeded that of albuterol, the majority of nebulized levalbuterol use was clustered at only two hospitals (21% and 39% of hospitalized days on nebulized levalbuterol respectively). Ipratropium was rarely administered, with a 10-day median for nebulized therapy (n=17) and one median MDI per patient (n=11).
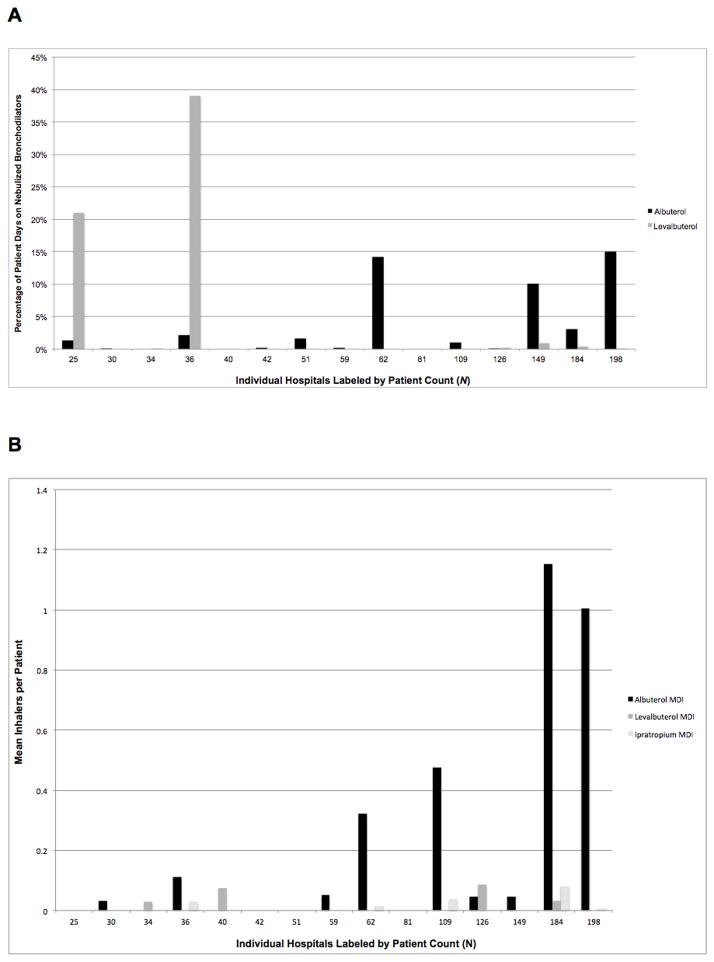
Figure 3A shows that the proportion of nebulized bronchodilator treatment days, and the predominant bronchodilator used, varied markedly by institution. All but two hospitals (87%) administered nebulized bronchodilators to infants with evolving BPD and all but three (80%) administered albuterol. The proportion of days on nebulized albuterol ranged from 0–15%. Only 7 hospitals (47%) administered nebulized levalbuterol (range 0–39% of days), with almost all use clustered at two institutions. Ipratropium was only administered at 5 hospitals (33%) and therefore excluded from Figure 3A given its low frequency of use (range 0.1–0.5% of days). Figure 3B displays similar variation in mean bronchodilator MDIs prescribed per institution. Four hospitals prescribed no MDIs. Albuterol MDIs were prescribed at 9 centers (60%) at a range of 0–1.2 inhalers per infant (median 0.05 inhalers).
Figure 3.
A. Mean proportion of days that infants with evolving BPD received nebulized bronchodilators during their NICU stay, by hospital. Patient days= (mean patient days on any inhaled bronchodilator/mean length of stay). Mean length of stay= 96 days (SD= 47 days); median= 92 days. B. Mean bronchodilator inhalers charged per patient, by hospital. Hospitals were included if at least 25 patients developed BPD during the study period (N=15).
Cohort at 36 Weeks Postmenstrual Age
At 36 weeks postmenstrual age, 96% (n=1376) of the cohort survived with 313 discharges occurring prior to 36 weeks. Oxygen requirement (moderate BPD as defined by Ehrenkranz et al 9) was noted in 34% (n=473) and 22% (n=316) required CPAP or mechanical ventilation (severe BPD 9). Bronchodilators were administered to 19 (36%) of the 53 infants who died prior to 36 weeks. Increasing severity of lung disease was associated with bronchodilator treatment (p< 0.0001). Among surviving infants, 93 (16%) with mild BPD (off oxygen or discharged prior to 36 weeks gestation; N=587), 120 (25%) with moderate BPD, and 93 (29%) with severe BPD received at least one inhaled bronchodilator course during their stay. Death occurred in 24 hospitalized infants after 36 weeks.
DISCUSSION
Our key findings were that: 1) 33% of the overall cohort received an inhaled bronchodilator with increasing usage throughout the first month of hospitalization and 2) the duration of mechanical ventilation or CPAP exposure, a marker of respiratory disease severity, was the greatest predictor of inhaled bronchodilator exposure. Our investigation also indicates that patterns of inhaled bronchodilator use for infants with BPD at US children’s hospitals vary markedly between institutions. We found that some institutions did not routinely prescribe bronchodilators to infants with evolving BPD, while others greatly exceeded the cohort average. Among hospitals with ≥25 BPD patients during the study period, the percentage of patients receiving an inhaled bronchodilator ranged from 0% to 81%. Furthermore, the specific bronchodilator(s) prescribed to neonates with BPD varied by institution and frequency and duration of use were unrelated to the number of admitted infants with a BPD diagnosis. Variation among centers in the percentage of infants receiving inhaled bronchodilators persisted even after controlling for the confounding variables associated with bronchodilator use. Thus, there seems to be no standardized approach to the management of BPD with bronchodilators. Our finding of bronchodilator use in the first postnatal month was unexpected, since reduced compliance and atelectasis play the major pathologic role in newly admitted preterm infants with Respiratory Distress Syndrome (RDS). Nevertheless, even extremely premature infants have been shown to have adequate bronchial smooth muscle to respond to inhaled bronchodilators.10
Aerosolized beta-adrenergic agonists such as albuterol (known in many countries as salbutamol), levalbuterol (also known as levosalbutamol), isoproterenol, metaproterenol, and terbutaline function through direct action on beta2-receptors to relax smooth muscle and therefore cause bronchodilation. These drugs have been shown to cause short-term improvements in lung function in infants with developing or established BPD.11, 12, 13, 14, 15 However, these studies included only a small number of patients and may have been underpowered. Many of these reports occurred before surfactant use and the era of routine antenatal steroid administration and may not be applicable to patients with “new BPD”.1, 16 To date, only one prospective, randomized, double blind, placebo controlled, multicenter study has evaluated long term clinical outcomes including mortality or risk of developing BPD. Denjean et al randomized 173 infants < 31 weeks gestation who remained on mechanical ventilation at the 10th postnatal day to receive inhaled salbutamol, inhaled salbutamol plus beclomethasone or placebo via metered-dose inhaler (MDI) for a period of 28 days.17 The authors found no significant effect of treatment on survival, diagnosis and severity of BPD, duration of ventilator support, or duration of oxygen. To our knowledge, no studies focusing on long-term clinical outcomes for patients with established BPD receiving bronchodilators have been published.
Ipratropium bromide, a muscarinic antagonist, functions by antagonizing the actions of acetylcholine at parasympathetic effector cells, thus inhibiting parasympathetic mediated bronchoconstriction and also decreasing mucosal secretions.18 Studies evaluating the use of ipratropium bromide in the treatment of infants with BPD are limited to case reports following short-term use.19, 20
Given the retrospective and observational nature of our investigation, we were unable to establish the causal effects of inhaled bronchodilators on important clinical outcomes such as duration of ventilation, duration of oxygen use, severity of BPD at 36 weeks, and length of stay. Thus, the relationship between the use of bronchodilators and important clinical outcomes in intubated and non-intubated infants needs further scrutiny.
Our diagnoses, including BPD, were based on hospital records, and potential recording errors might reduce the accuracy of our diagnoses. Although we used hospital charge data for specific date of service to determine days receiving oxygen, days on mechanical ventilation and CPAP, and dates of inhaled bronchodilator administration, we had to rely on less specific ICD-9 codes for the diagnosis of IVH, PDA, and NEC. Even though PHIS data are rigorously screened for errors and rejected if quality thresholds for inclusion are not met, they were initially collected for hospital administrative purposes instead of specifically for research.
Despite these limitations, our cohort study has multiple strengths. It benefits from a large sample size and nationally representative sample of children’s hospitals included in the PHIS database, as well as measures taken by the Children’s Hospital Association to ensure data quality. Our findings are likely generalizable to most NICUs within large US children’s hospital systems. These baseline findings will serve to inform the design of prospective, comparative effectiveness investigations to determine whether long-term use of inhaled bronchodilators in BPD patients is beneficial and, in turn, allow for the creation of standardized guidelines regarding bronchodilator treatment of BPD.
Although full comprehension of the practice choices surrounding bronchodilator treatment for BPD, especially at the individual patient physician level, are beyond the reach of our investigation, the profound variation in prescribing practices between institutions clearly indicates a lack of objective data regarding when to use these medications, for how long, and the indications for cessation of therapy. High variability in provider’s practice attitudes are likely attributable to a dearth of knowledge regarding, first of all, whether bronchodilators should be used for infants with BPD at all, and if so, under what specific indications. Performing pulmonary function tests in infants with BPD may be helpful, as objectivity is high and personalized approaches are then possible. However, such programs require skilled personnel and are labor intensive. Simplified tests in the ICU setting are needed. Some infants may develop tachyphylaxis to bronchodilator treatment requiring increasing doses to obtain a response, while others may develop un-anticipated adverse effects such as air hunger, hypoxemia and tachycardia. Thus, indications for stopping these agents in infants with BPD are also needed. An objective, evidence-based strategy for bronchodilator treatment of BPD is overdue.
CONCLUSIONS
The frequency of inhaled bronchodilator administration, relatively common for infants with BPD at US children’s hospitals, increases throughout the first month of hospitalization and is related to duration of positive pressure exposure via ventilation or CPAP. However, practice patterns vary markedly by institution even after adjustment for confounding variables indicating a need for further research to determine the effectiveness and safety of long-term inhaled bronchodilator therapy for BPD patients, in order to develop evidence-based recommendations.
Acknowledgments
Grant support: Supported in part by National Heart, Lung, and Blood Institute grant K08HL121182 (Slaughter).
Abbreviations
- aOR
adjusted odds ratio
- BPD
bronchopulmonary dysplasia
- CHA
Children’s Hospital Association
- CI
confidence interval
- CPAP
continuous positive airway pressure
- FiO2
fraction of inspired oxygen
- ICD-9
International Classification of Diseases, Ninth Revision
- IVH
intraventricular hemorrhage
- NEC
necrotizing enterocolitis
- NICU
neonatal intensive care unit
- OR
odds ratio
- PDA
patent ductus arteriosus
- PHIS
Pediatric Health Information System
- SD
standard deviation
Footnotes
The authors have no conflicts of interest relevant to this article to disclose.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CONFLICTS OF INTEREST DISCLOSURE
The authors have no conflicts of interest or financial interests relevant to this article to disclose.
References
- 1.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. American journal of respiratory and critical care medicine. 2001;163(7):1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 2.Walsh MC, Szefler S, Davis J, Allen M, Van Marter L, Abman S, et al. Summary proceedings from the bronchopulmonary dysplasia group. Pediatrics. 2006;117(3 Pt 2):S52–56. doi: 10.1542/peds.2005-0620I. [DOI] [PubMed] [Google Scholar]
- 3.Smyth JA, Tabachnik E, Duncan WJ, Reilly BJ, Levison H. Pulmonary function and bronchial hyperreactivity in long-term survivors of bronchopulmonary dysplasia. Pediatrics. 1981;68(3):336–340. [PubMed] [Google Scholar]
- 4.Ng G, da Silva O, Ohlsson A. Bronchodilators for the prevention and treatment of chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2012;6:CD003214. doi: 10.1002/14651858.CD003214.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Tin W, Wiswell TE. Drug therapies in bronchopulmonary dysplasia: debunking the myths. Seminars in fetal & neonatal medicine. 2009;14(6):383–390. doi: 10.1016/j.siny.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics. 2006;117(6):1979–1987. doi: 10.1542/peds.2005-1707. [DOI] [PubMed] [Google Scholar]
- 7.Allen J, Zwerdling R, Ehrenkranz R, Gaultier C, Geggel R, Greenough A, et al. Statement on the care of the child with chronic lung disease of infancy and childhood. American journal of respiratory and critical care medicine. 2003;168(3):356–396. doi: 10.1164/rccm.168.3.356. [DOI] [PubMed] [Google Scholar]
- 8.Slaughter JL, Stenger MR, Reagan PB. Variation in the use of diuretic therapy for infants with bronchopulmonary dysplasia. Pediatrics. 2013;131(4):716–723. doi: 10.1542/peds.2012-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116(6):1353–1360. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 10.Motoyama EK, Fort MD, Klesh KW, Mutich RL, Guthrie RD. Early onset of airway reactivity in premature infants with bronchopulmonary dysplasia. The American review of respiratory disease. 1987;136(1):50–57. doi: 10.1164/ajrccm/136.1.50. [DOI] [PubMed] [Google Scholar]
- 11.Cabal LA, Larrazabal C, Ramanathan R, Durand M, Lewis D, Siassi B, et al. Effects of metaproterenol on pulmonary mechanics, oxygenation, and ventilation in infants with chronic lung disease. The Journal of pediatrics. 1987;110(1):116–119. doi: 10.1016/s0022-3476(87)80302-5. [DOI] [PubMed] [Google Scholar]
- 12.Kao LC, Durand DJ, Nickerson BG. Effects of inhaled metaproterenol and atropine on the pulmonary mechanics of infants with bronchopulmonary dysplasia. Pediatric pulmonology. 1989;6(2):74–80. doi: 10.1002/ppul.1950060204. [DOI] [PubMed] [Google Scholar]
- 13.Rotschild A, Solimano A, Puterman M, Smyth J, Sharma A, Albersheim S. Increased compliance in response to salbutamol in premature infants with developing bronchopulmonary dysplasia. The Journal of pediatrics. 1989;115(6):984–991. doi: 10.1016/s0022-3476(89)80755-3. [DOI] [PubMed] [Google Scholar]
- 14.Wilkie RA, Bryan MH. Effect of bronchodilators on airway resistance in ventilator-dependent neonates with chronic lung disease. The Journal of pediatrics. 1987;111(2):278–282. doi: 10.1016/s0022-3476(87)80087-2. [DOI] [PubMed] [Google Scholar]
- 15.Fayon M, Tayara N, Germain C, Choukroun ML, De La Roque ED, Chene G, et al. Efficacy and tolerance of high-dose inhaled ipratropium bromide vs. terbutaline in intubated premature human neonates. Neonatology. 2007;91(3):167–173. doi: 10.1159/000097448. [DOI] [PubMed] [Google Scholar]
- 16.Baraldi E, Filippone M. Chronic lung disease after premature birth. The New England journal of medicine. 2007;357(19):1946–1955. doi: 10.1056/NEJMra067279. [DOI] [PubMed] [Google Scholar]
- 17.Denjean A, Paris-Llado J, Zupan V, Debillon T, Kieffer F, Magny JF, et al. Inhaled salbutamol and beclomethasone for preventing broncho-pulmonary dysplasia: a randomised double-blind study. European journal of pediatrics. 1998;157(11):926–931. doi: 10.1007/s004310050969. [DOI] [PubMed] [Google Scholar]
- 18.Gross NJ, Skorodin MS. Anticholinergic, antimuscarinic bronchodilators. The American review of respiratory disease. 1984;129(5):856–870. doi: 10.1164/arrd.1984.129.5.856. [DOI] [PubMed] [Google Scholar]
- 19.Fisher JT, Froese AB, Brundage KL. Physiological basis for the use of muscarine antagonists in bronchopulmonary dysplasia. Archives de pediatrie: organe officiel de la Societe francaise de pediatrie. 1995;2 (Suppl 2):163S–171S. doi: 10.1016/0929-693x(96)89886-1. [DOI] [PubMed] [Google Scholar]
- 20.Brundage KL, Mohsini KG, Froese AB, Fisher JT. Bronchodilator response to ipratropium bromide in infants with bronchopulmonary dysplasia. The American review of respiratory disease. 1990;142(5):1137–1142. doi: 10.1164/ajrccm/142.5.1137. [DOI] [PubMed] [Google Scholar]