Abstract
Ethnopharmacological relevance
To understand the role of khat (Catha edulis) use on the aberrations in appetite and weight which are common comorbidities for khat and other amphetamine users.
Materials and methods
We provide a comprehensive overview and conceptual summary of the historical cultural use of khat as a natural stimulant and describe the similarities and differences between cathinone (the main psychoactive constituent of khat) and amphetamine highlighting the limited literature on the neurophysiology of appetite and subsequent weight effects of khat.
Results
Animal and some human studies indicate that khat produces appetite suppression, although little is known about mechanisms of this effect. Both direct and indirect effects of khat stem from multiple factors including behavioral, chemical and neurophysiological effects on appetite and metabolism. Classic and newly identified appetite hormones have not been explored sufficiently in the study of appetite and khat use. Unique methodological challenges and opportunities are encountered when examining effects of khat and cathinone including khat-specific medical comorbidities, unique route of administration, differential patterns of behavioral effects relative to amphetamines and the nascent state of our understanding of the neurobiology of this drug.
Conclusion
A considerable amount of work remains in the study of the appetite effects of khat chewing and outline a program of research that could inform our understanding of this natural amphetamine’s appetite effects and help prepare health care workers for the unique health effects of this drug.
Keywords: khat, cathinone, amphetamine, appetite, weight loss, health
1. Introduction
Khat is a natural amphetamine-like stimulant which comes from the leaves or young shoots of Catha edulis, a plant cultivated in East Africa and the Arabian Peninsula. Although synthetic amphetamine-class stimulants such as pure amphetamine (AMPH) have well known appetite suppression effects which may help account for, in part, the malnutrition and low body mass index seen with AMPH abuse (Ross et al., 2012), much less is known about their natural counterparts such as khat. This review seeks to describe khat’s social/cultural history and argues that there is value in furthering research on its appetite suppression effect as a negative health outcome of khat use. Parallels between khat and the more extensively studied, and structurally similar, AMPH will be reviewed and used to propose new avenues for the study of the physiological mechanisms of khat’s appetite suppression.
2. Khat as a cultural tradition and natural “amphetamine”
Khat has been widely consumed by residents in East Africa and the Arabian Peninsula for its psychoactive properties and associated social values. Chewing is the most common mode of administration, and fresh leaves are usually used during afternoon sessions that last two to five hours. Khat chewing has a social and cultural tradition, as it usually occurs while in company (Kennedy et al., 1983) and often runs along both family and social ties (Mahfouz et al., 2013). As with many psychoactive plants, khat continues to have a long history of indigenous traditional use, changes in use patterns due to immigration, and governmental attempts to control its use and trade (Anderson and Carrier, 2009; Gebissa, 2010; Sheikh, 2014). Indigenous use has been as persisted for eight centuries as a mild stimulant for enhanced energy during work, maintenance of prayers during long fasts, facilitation of social ties and as a commodity for trade, as dowry, and for dispute resolutions (Anderson and Carrier, 2009; Gebissa, 2010). Khat left to traditional use guided by culture is believed by users to be of little more harm than other stimulants such as coffee or tea but its future is unclear. Some have suggested that the future of khat could move towards either widespread legal commercialization or refinement into an illicit drug (Gebissa, 2010).
In many Middle East and East African cultures khat use motives differ by gender. Men use more frequently than women, though the use by women is increasing (Nakajima et al., 2013). Although both genders may refer to the upkeep of tradition to explain khat use, men reportedly use khat for occupational and recreational purposes while women’s use is often for its perceived health benefits such as relief of headache, weight loss and assisting birth and delivery (Stevenson et al., 1996). Women tend to cite family as a source of motivation for not using khat (Nakajima and al’Absi, 2013). Concurrent tobacco use is quite high and also differs by gender. Two thirds or more of men use tobacco as well as khat, mostly in the form of cigarettes, but only one third of the women use tobacco via waterpipe (majority of use) or cigarettes (less than one quarter) (Nakajima et al., 2013). Despite clinical evidence to the contrary (al’absi et al., 2014; Bongard et al., 2011), users believe that khat decreases depression (Wabe, 2011). Users also acknowledge negative effects of khat use such as insomnia and other sleep dysregulation, irritability and malaise during withdrawal (Gebissa, 2010; Nakajima et al., 2014; Stevenson et al., 1996).
Khat contains many different types of chemical constituents. Cathinone (CATH), the major alkaloid found in khat and a structural analog of AMPH, is responsible for most of khat’s psychoactive properties (1975; Szendrei, 1980). Early work confirmed that tolerance can occur with khat (al’Absi et al., 2013; Nencini and Ahmed, 1989; Nencini et al., 1984; Schechter and McBurney, 1991) which is mediated, in part, through dopaminergic mechanisms (Schechter, 1990). There exists a rich body of literature documenting the subjective effects associated with acute khat use (Brenneisen et al., 1990; Halbach, 1972; Nencini et al., 1986; Pantelis et al., 1989), including euphoria, excited mood, increased wakefulness and alertness, and to most of our interest, suppression of appetite. In a recent study (Murray et al., 2008), khat’s anorectic effect on humans was confirmed in a laboratory setting using a controlled experiment. In addition, rat studies using purified CATH have demonstrated that acute administration induces a significant reduction in food intake (Knoll, 1979; Zelger and Carlini, 1980). In a chronic experimental study, CATH was found to induce a marked reduction in body weight (Zelger and Carlini, 1980).
Although evidence from human and animal studies has confirmed khat’s anorectic effect, limited information is known about its underlying mechanisms. Only one published paper examining the possible physiological mechanisms behind the anorectic effect of khat was found (Murray et al., 2008). Contrary to khat, the anorectic effect of AMPH has been studied extensively for decades. Given the similarities in the chemical structures and psychoactive properties between AMPH and khat’s major constituent CATH, we will begin by examining the relevant knowledge on the anorectic effect of AMPH. From this, we will identify potential neurophysiological processes that might be responsible for the anorectic effect of CATH, and hence, khat. In the subsequent sections, we will then provide the conceptual basis for a potential program of research dedicated to the understanding of the mediators of the anorectic effects of khat use.
3. Brief review of neuroendocrine appetite modulators
The control of appetite and feeding occurs via highly interdependent peripheral and central signaling factors. These mediators can roughly be divided into appetite inducing (orexigenic) and suppressing (anorexigenic) factors, but it is important to note that there are complex interactions between factors which can blur this distinction. In general, there is much more that is known about appetite suppression than induction, due, perhaps, to the intense clinical and research interest in obesity. Among the appetite suppressing factors there are classic neurotransmitters and peptides (somatostatin, proopiomelanocortin (POMC), corticotropin-releasing hormone (CRH), peptide YY, serotonin (SE), cocaine- and amphetamine-related transcript (CART)), hormones (thyrotropin-releasing hormone (TRH), glucagon, amylin, glucagon-like peptide-1) and gut peptides (cholecystokinin (CCK), bombesin, enterostatin). At this time, a complete understanding of the complex signaling that initiates or terminates feeding is lacking despite significant progress over the past decade (Suzuki et al., 2012). As will be outlined in the subsequent sections, our understanding of AMPH’s effects on these central and peripheral appetite modulators is also rapidly developing but remains incomplete. Furthermore, translating what is known about AMPH to CATH is in its infancy.
4. Amphetamine and cathinone: Structural and neurophysiological similarities
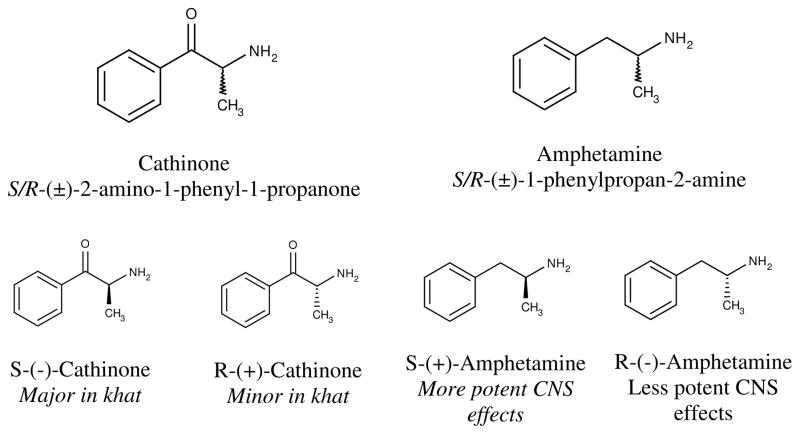
Both AMPH and CATH are comprised of a linear chain of three carbons with a phenyl group attached to carbon #1 and an amino group attached carbon #2. The only structural difference between CATH and AMPH lies at the carbon #1 as it is oxidized to a ketone in CATH but not in AMPH (see Figure 1). Both AMPH and CATH contain two stereoisomers. It was originally reported that there exists only the S-(-)-cathinone in khat (1975; Szendrei, 1980) but a later report suggests a small amount of R-enantiomer is also present in khat (Dawson et al., 1994). Both AMPH and CATH are lipophilic and can readily cross the blood-brain barrier. However, the addition of a ketone makes CATH more polar (hence, less lipophilic) relative to AMPH. This difference manifests physiologically and behaviorally as CATH is found to be relatively weaker than AMPH in its interactions with the CNS to produce various behavioral symptoms (more details to follow). Nonetheless, the high degree of similarities in the structure and neurophysiological effects of CATH and AMPH have led some to refer to CATH as “khatamines” (Islam et al., 1990).
Figure 1.
Cathinone, amphetamine, and their stereoisomers
The stimulatory effects of AMPH and CATH are primarily mediated by their interactions with the catecholamine neurotransmitter systems, in particular, the dopaminergic (DA) and norepinephrinergic (NE) system after acute administration of AMPH (Heikkila et al., 1975; Von Voigtlander and Moore, 1973) and CATH (Cleary and Docherty, 2003; Kalix, 1982, 1983). Dopamine receptor antagonists or DA release inhibitors reverse or inhibit the behavioral stimulatory effects induced by AMPH (Davis et al., 1974; Lemberger et al., 1970) and CATH (Calcagnetti and Schechter, 1992; Kalix, 1986; Schechter, 1986).
The mechanisms behind CATH’s effects on DA and NE systems have not been as extensively investigated, although they appear to be similar to those observed with amphetamines. Both cardiovascular and adrenocortical disruptions have been documented using human cross-sectional analysis, animal stress manipulation, and acute khat administration, all of which support both DA and NE mechanisms (al’Absi et al., 2013; al’absi et al., 2014; Nyongesa et al., 2013). Preliminary work indicates that CATH exhibits a high level of substrate activities on DA and NE transporters, albeit less potent than AMPH (Cleary and Docherty, 2003; Rothman et al., 2003; Simmler et al., 2013). This is consistent with the behavioral findings that CATH is less active than AMPH in inducing various behavioral effects in animals (Valterio and Kalix, 1982; Zelger et al., 1980) and appetite suppression (Zelger and Carlini, 1980). It has also been reported that AMPH and CATH interact with the serotonergic (SE) system. It is worth noting that certain structural modifications of synthetic AMPH can lead to increased SE activity (Rothman et al., 2001). Work on CATH has also demonstrated similar findings (Kalix, 1984; Nielsen, 1985; Rothman et al., 2003), suggesting that CATH’s effect on increasing synaptic SE availability is present but very weak. In addition, there exist a large body of evidence suggesting that activation of the SE system actually dampens the various stimulatory effects of AMPH and its analogs (Baumann et al., 2011; Breese et al., 1974; Hollister et al., 1976).
CATH’s structural similarity to AMPH leads to functional similarities at both neurophysiological and behavioral level (see Figure 1 for a summary). Alterations in the NE and DA systems are believed to mediate the behavioral effects of the two compounds including appetite suppression. Numerous behavioral, neurophysiological and molecular studies have pointed to the NE and DA-dependent mechanisms in hypothalamus to be central to the anorectic effect of AMPH. Therefore, given the high structural and neurophysiological similarities between CATH and AMPH, it is reasonable to infer that the anorectic effect of CATH is most likely being mediated by the same mechanisms as identified in AMPH. In addition to CATH, cathine or d-norpseudoephedrine has been identified as an additional but minor psychoactive ingredient in khat with psychostimulant properties (Pehek and Schechter, 1990) but its appetite effects are unknown.
5. Anorectic effect of amphetamines & neurophysiological mechanisms
AMPH is no longer being used for the clinical management of obesity due to its high potential for abuse and addiction, but the neurophysiological mechanisms behind its anorectic effect are continuously being investigated. AMPH results in increased locomotion, decreased food consumption, and a negative energy balance (Walters et al., 2012) which has been linked to the lateral hypothalamus (LH) (Carlisle, 1964; Russek et al., 1973). Animal studies support the notation that LH-innervating NE and DA fiber systems but not SE fibers are involved in the anorectic effect of AMPH (Ahlskog, 1974; Carey, 1976; Carey and Goodall, 1975; Carlisle and Reynolds, 1961; Samanin et al., 1975). Pharmacological studies using NE and DA synthesis inhibitors (Leibowitz, 1975), NE receptor antagonists (Leibowitz, 1975; Leibowitz and Rossakis, 1978; Sanghvi et al., 1975), and DA receptor antagonists (Chen et al., 2001; Leibowitz, 1975) all showed attenuation or complete blockage of AMPH’s anorexic effects. Combining these pieces of evidence with the knowledge that AMPH increases the synaptic availabilities of NE and DA, it is suggested that NE and DA dependent mechanisms in the LH most likely mediate the anorectic effect of AMPH.
In summary, it is clear that AMPH suppresses appetite by increasing the synaptic availabilities of NE and DA in hypothalamus, and subsequently activates the NE- and DA-dependent mechanisms that attenuate the central nervous system control of food intake. In addition to the catecholamine effects, recent studies have reported that the interaction between hypothalamic DA and neuropeptide Y (NPY), an orexigenic neuropeptide, plays a key role in the anorectic effect of AMPH. Molecular evidence showed that AMPH treatment to hypothalamic NPY neurons reduces the expression of NPY at both transcriptional and posttranslational levels (Hsieh et al., 2006; Hsieh et al., 2005). However, it is likely that NPY is not the sole mediator of these effects. Other central appetite messengers such as proopiomelanocortin (POMC), cocaine and amphetamine regulated transcript (CART), and orexin, as well as peripheral appetite messengers such as leptin, ghrelin and glucose, might also be involved. Many of these factors are sensitive to catecholamine signaling in the paraventricular, arcuate nucleus, ventromedial hypothalamus and suprachiasmic nucleus neurons containing Agouti gene-related protein (AgRP) and CART receptors. Table 2 demonstrates the extensive breadth and sophistication of the current work on the appetite effects of AMPH on a wide variety of appetite modulators. Selected references from this broad body of work span both human and animal observational and experimental studies utilizing behavioral, physiological, neurophysiological, microbiological and genetic techniques. As will be argued in subsequent sections, our understanding of CATH’s appetite suppression is relatively less sophisticated but warrants further investigation.
Table 2.
A comparison between amphetamines and cathinone on known neuroendocrine appetite modulating factors.
| Amphetamines | Khat/Cathinone | |
|---|---|---|
| Appetite Inducing | ||
| Neuropeptide Y (NPY) | AE1–4 | -- |
| Norepinephrine | AE5–9 | AE10,11 |
| Aguoti related peptide (AgRP) | -- | -- |
| Orexin A & B | AE-NA12–14 | -- |
| Melanin concentrating hormone (MCH) | -- | -- |
| Ghrelin | AE-NA15,16 | HE17 |
| Appetite Suppressing | ||
| Proopiomelanocortin (POMC) | AE18,19 | -- |
| Cocaine and amphetamine regulated transcript (CART) | AE20,21 | -- |
| Leptin | AE-NA22 | AE23 HE24 |
| Thyrotropin releasing hormone (TRH) | AE25,26 | -- |
| Melanin | AE-NA27 | -- |
| Corticotrophin releasing hormone (CRH) | AE-NA27 | -- |
| Bombesin | AE28 | -- |
| Glucagon | AE-NA29 | -- |
| Glucagon-like peptide-1 (GLP-1) | AE-NA30 | -- |
| Peptide YY (PYY) | AE28 | HE17 |
| Apolipoprotein A-IV | -- | -- |
| Amylin | -- | -- |
| Somatostatin | AE-NA31 | -- |
| Enterostatin | -- | -- |
| Ciliary neurotrophic factor (CNF) | -- | -- |
| Serotonin | AE32–35 HE38 HO39 |
AE36,37 |
NOTE: Abbreviations include AE = Animal Experimental, AE-NA = Animal Experimental Not Appetite specific, HE = Human Experimental, HO = Human observational studies. Select references are shown but are not meant to be comprehensive.
Cleary and Docherty, 2003; Harris and Baldessarini, 1973; Heikkila et al., 1975; Holmes and Rutledge, 1976; Kalix, 1983;
Hao et al., 2004;
6. Anorectic effects of khat
Early in the experimental investigation of CATH it was suggested that CATH’s inhibition of food intake was greater than equivalent AMPH doses (Knoll, 1979), though others have challenged this (Zelger and Carlini, 1980). In addition to catecholamines, other metabolic hormones that are responsive to AMPH are also responsive to CATH. Both triiodothyronine and thyroxine are elevated in a similar manner by high doses of AMPH and CATH, though CATH appears to elevate thyroxine earlier than AMPH (Islam et al., 1990). This limited literature on metabolic hormones supports the hypothesis that CATH and AMPH may have similar effects on both direct and indirect appetite modulators and metabolism.
As indicated above, several gut peptides are important appetite modulators. These include orexigenic factors such as orexin and ghrelin and the anorexigenic factors such as leptin, peptide YY (PYY), bombesin and glucagon-like peptide-1 (GLP-1). In one of the few studies of appetite hormones, khat chewing, at least by habitual users, decreased subjective feelings of hunger and increased subjective feelings of fullness despite no change in ghrelin or PYY secretion (Murray et al., 2008). This is in contrast to AMPH where leptin has been called into question as a mediator of food restriction sensitization of d-amphetamine’s reward effects (Hao et al., 2006). Whether other critical distinctions exist between appetite hormones and the effects of AMPH and CATH remains to be seen.
7. Health and behavioral properties of khat, CATH & AMPH
The previous discussion outlined early evidence of khat’s resemblance to AMPH in basic structure, neurophysiology and appetite effects. It may also be instructive to consider the ways in which khat is subtly distinct from AMPH, particularly in relation to diseases or conditions that could indirectly affect appetite or feeding behaviors. Mounting evidence indicates that chronic khat use can result in unique health effects less frequently seen in AMPH users. These health effects may indirectly impact appetite through the presence of sickness behavior, oral cavity lesions or changes in macronutrient selection. Any study of appetite in experimental animals or khat using human samples will need to consider these secondary effects in the design of experimental or quasi-experimental studies. Each of these will be discussed in turn with the purpose of identifying additional avenues of research in a program of study specific to the appetite effects of khat.
7.1. Health effects as secondary factors
The impact of hepatotoxicity and renal dysfunction on appetite and weight could potentially be quite important to the study of the anorectic effect of CATH. Sickness behaviors such as fatigue, nausea and changes in both appetite and thirst are common symptoms of liver and kidney disease (Strid et al., 2002). Experimental evidence in Sprague-Dawley rats indicates that minor use of khat (subchronic) can lead to impaired liver and kidney functioning (Alsalahi et al., 2012). Changes in weight and leptin levels were seen after 4 weeks of use in male but not female rats but impaired renal function was evident in female but not male rats in response to subchronic use (Alsalahi et al., 2012). Hepatotoxicity in this study was evident in both male and female rats. A case report of similar hepatotoxicity (acute hepatitis) in a human khat user has been published which was interpreted by that author to be more similar to amphetamine derivatives with a higher SE profile such as 3, 4-methylenedioxy-N-methylamphetamine (MDMA) (Chapman et al., 2010). MDMA has long been linked to serotonergic toxicity and dehydration, which may be important secondary appetite modulating effects (Parrott, 2001). Renal dysfunction has also been documented with other drugs such as heroin, cocaine and polysubstance abuse (Milroy and Parai, 2011) but relatively less often with AMPH abuse. Chronic kidney disease has also been linked to changes in ghrelin, leptin and cytokines leading to decreased appetite, weight loss and muscle wasting (Gunta and Mak, 2013; Suzuki et al., 2013). Thus, any study of these appetite regulators in human khat users should consider verifying adequate hepatic and renal functioning. Some investigators have noted that such renal and hepatic health effects are found in immigrants but not khat users living in the regions where the khat is grown, possibly due to suspected contamination at export (Coton et al., 2011; Douglas et al., 2011).
Khat-induced changes in activity levels, feeding and macronutrient selection due to the nature of drug delivery (chewing large boluses of fresh leaves) may also indirectly affect appetite. Cathinone’s similarity to AMPH has been proposed as a justification for similar effects on appetite and weight, it is important to note that not all investigators find a negative correlation with khat use and BMI. For example, Yemeni medical students were shown to demonstrate a positive correlation with khat use and BMI, possibly due to a relative sedentary lifestyle related to khat use (Laswar and Darwish, 2009). Others have noted that khat’s bitter flavor leads users to excessively consume sweets (candy, sugar cubes, etc.) to counteract the taste which, in turn, elevates blood sugar, weight and risk of dental caries (Douglas et al., 2011). Taken together these factors may be a behavioral source of increased diabetes risk seen in some khat users (Hassan et al., 2007).
Finally, khat may indirectly affect appetite and feeding behavior through oral cavity lesions. A population survey of 1,500 immigrant showed that khat users demonstrated increased abnormal oral pigmentation, tooth staining, xerostomia (dry mouth) and risk of dental caries compared with nonusers (Yarom et al., 2010). Khat users who immigrated to the United Kingdom showed a 14% higher rate of symptomatic dental and medical service use (Kassim and Croucher, 2012). Xerostoma and increased cavities have not been documented with the more pure and traditional AMPHs (d-amphetamine, l-amphetamine), but synthetic drugs such as methamphetamine are well known to result in high rate of caries, xerostomia and excessive tooth wear (Hamamoto and Rhodus, 2009; Ravenel et al., 2012). Although polysubstance abuse limits the ability of dental researchers to directly implicate methamphetamine in the presentation of “meth mouth” (Leserman Robbins et al., 2012), the effects of excessive cavities, xerostomia and tooth loss common with khat use strongly suggests an indirect effect on feeding behavior and nutrient content (e.g. increased sweet consumption to counteract xerostomia) due to the use of some, but not all, AMPH-based substances. Likewise, increased sweet consumption may lead to subsequent dental caries (Douglas et al., 2011) despite the fact that khat use in humans may shift the bacterial profile of the mouth towards beneficial species (Al-Hebshi and Skaug, 2005; Yarom et al., 2010).
7.2. Motor and thermoregulation effects as secondary factors
In addition to changes in diet, khat’s effects on motor behavior have been likened to those of AMPH (Kalix, 1980). A direct comparison of khat to AMPHs using catecholamine antagonists supports that the motor patterns demonstrated in experimental animals treated with CATH is likely secondary to dopaminergic and serotonergic effects (Connor et al., 2002). In this study, motor patterns were more circumspect at lower khat doses (head twitch) and at higher khat doses (stereotypies) than AMPH which had a broader range of spontaneous gross motor behaviors. Although still a limited literature, this suggests that a simultaneous comparison of motor effects induced by CATH and AMPH as a behavioral mechanism to weight loss will be important.
Understanding the appetite and weight effects of AMPH-class stimulants, either natural or synthetic, also requires an examination of thermoregulation as well as behavioral neuromodulation. Hyperthermia is a major adverse side effect of acute use of several AMPH-class stimulants and may be one mechanism behind the weight loss associated with AMPH (Parrott, 2001). It has been known for some time that both AMPH and CATH increase brown adipose tissue and rectal temperature in rats, though AMPH shows a threefold higher temperature response than CATH (Tariq et al., 1989). Acute administration of a high dose of CATH (10 mg kg −1) resulted in elevations to rectal temperature but not tail temperature in rats while MDMA decreased temperature at all doses and time points (Shortall et al., 2013). This work also indicated that housing (single versus three per cage) also had a significant modulating effect on thermogenic and catecholaminergic changes. How this finding relates to the highly social nature of human khat and other AMPH-class stimulant use is not clear.
8. Conclusions and future directions
The above review highlights the chemical, neurophysiological, health and behavioral similarities and differences between AMPH and CATH. These similarities have prompted some to coin the term “khatamines” to reflect the broad range of similarities between khat’s CATH and AMPH. Both khat and AMPH have been linked to appetite changes and weight loss. As discussed above, numerous studies of critical neuroendocrine appetite modulators have been conducted related to AMPH, however, to our knowledge, many of these modulators such as NPY, CART and POMC have not been studied specific to khat (natural) or pure CATH. POMC’s role in the analgesic effect of khat (Nencini et al., 1986) has been investigated but not for its possible role in khat’s anorectic effect.
Given the extensive and meaningful similarities between these molecules, further research is needed. While advances in molecular biology, neuroimaging and chemistry will undoubtedly be invaluable to the further study of appetite and chronic khat use, advancing the psychometric validation of behavioral and psychological tools in the native language of local users will be important (Nakajima et al., 2012; Nakajima et al., 2014). In addition, a great deal of research is needed such as direct comparisons of CATH to multiple types of AMPH compound’s cellular and molecular neurophysiology, studies of health-related effects on feeding behavior, analyses of thermogenesis, observation of drug-induced motor behavior changes in both animal and human subjects, study of gender differences in drug response at all levels of analysis (molecular to whole animal), and toxicological studies of the purity of natural khat. A program of study on the direct and indirect effects of khat on feeding behavior would include studies of human and experimental animals that consider the potential indirect effects of plant bitterness-induced behavioral changes on macronutrient selection. In such a program of research, the potential for sickness behaviors such as malaise, nausea and appetite loss induced by sub-clinical hepatic and renal abnormalities in khat chewers are also important considerations in need of controlled experimental or quasi-experimental studies. Studies of the effects of khat on gut associated neuromodulators such as NPY, leptin, and ghrelin are also needed. We have shown that chronic tobacco use, which is common to khat chewers, disrupts leptin, that PYY and leptin correlate with nicotine craving, and that leptin and ghrelin predict relapse with attempted smoking cessation (al’Absi et al., 2011; al’Absi et al., 2014; Potretzke et al., 2014). Although khat use is associated with heightened leptin levels (Al-Dubai et al., 2006), the same breadth and sophistication in understanding khat or CATH’s appetite regulation is lacking. Additionally, research is needed examining the purity of the khat product due to contaminants at export when considering behavioral, chemical, and neurophysiological effects on appetite and metabolism. The relatively less severe withdrawal symptoms of khat (Thomas and Williams, 2013) may also affect free feeding behavior in the positive direction (potentially less prolonged anorexia), which may account for the inconsistent correlations between khat use and BMI.
This review suggests that the neuroendocrine evaluation of khat’s anorectic effects should start with several hypotheses. One hypothesis is that CATH’s anorectic effect is secondary to NE and DA-dependent mechanisms within the hypothalamus. It is possible that, as with AMPH, the anorectic effect of CATH is mediated by NPY and requires co-stimulation of both D1 and D2 receptors. Further, pharmacologic studies of the impact of the additional ketone in CATH may be critical to understanding CATH’s seemingly weaker appetite suppressant effect. It can also be hypothesized that cathine and d-norpseudoephedrine independently or in combination may contribute to different facets of the appetite suppressant effect of khat and clarifying their pharmacological effect on all direct and indirect feeding behaviors could be fruitful. It is important to not lose sight of the weak, but significant, SE effect of CATH.
All of these hypotheses remain hypothetical until confirmed by a thoughtful, systematic program of research. Drug use on an international scale causes a critical drain on national economies. While khat is currently a relatively smaller proportion of substance abuse in Europe and the U.S., it is gaining increased political attention (Odenwald et al., 2010). It is primarily consumed in localized East African and Middle Eastern immigrant communities across the U.S. and Europe. Although initially it was thought to be a substance that was only used by adult immigrants with experience with khat in their home country (Klein, 2012), anecdotal evidence suggests that khat is used by both first generation immigrants and UK born young adults for its unique, “mellow” high and presumed safety (Holligen, 2009). It is possible that the same factors leading to its adoption by new users in the UK could also hold true in the U.S. A careful program of research to understand critical health consequences such as appetite changes and weight loss from the use of khat is warranted.
Table 1.
A comparison of the physical, behavioral, and neurophysiological properties of amphetamine and cathinone.
| Amphetamine | Cathinone | ||
|---|---|---|---|
| Chemical | A linear chain of three carbons A phenyl group attached to carbon #1 An amino group attached to carbon #2. Lipophilic Readily cross the blood-brain barrier Pulse rate and blood pressure increase |
Same, except Carbon #1 is oxidized to a ketone (see Figure 1) May delay gut motility |
|
| Behavioral | Human | Euphoria Excited mood Increased wakefulness Increased alertness Reduction of appetite Decrease response inhibition Toxic psychosis |
Mostly the same, but less potent Increase response inhibition |
| Animal | Increased spontaneous locomotion Stereotypical behaviors Reduction of food intake |
Similar, but more circumspect | |
| Neurophysiology | DA | Increase synaptic DA availability, via Transporter-mediated reuptake inhibition and DA release | Same, but less potent |
| NE | Increase synaptic NE availability, via Transporter-mediated reuptake inhibition and NE release | Same, but less potent | |
| SE | Minimal interaction | Same | |
Acknowledgments
Andrine Lemieux’s effort on this project was supported by the Essentia Institute of Rural Health and the Khat Research Program (KRP). Mustafa al’Absi was supported by the KRP via a FIRCA grant from the National Institutes of Health/Fogarty International Center (R03TW007219), an R21 National Institute for Drug Abuse grant (DA024626), and a grant from the Office of International Programs at the University of Minnesota. Bingshuo Li’s effort was supported by the University of Tuebingen, Tuebingen, Germany.
Glossary
- AgRP
Aguoti gene-related peptide
- AMPH
Amphetamine
- BMI
Body mass index
- CART
Cocaine- and amphetamine-related transcript
- CATH
Cathinone
- CCK
Cholecystokinin
- CRH
Corticotropin-Releasing Hormone
- DA
Dopamine
- GLP-1
Glucagon-like peptide 1
- LH
Lateral hypothalamus
- MDMA
3,4-methylenedioxy-N-methylamphetamine
- NE
Norepinephrine
- NPY
Neuropeptide Y
- POMC
Proopiomelanocortin
- PYY
Peptide YY
- SE
Serotonin
- TRH
Thyrotropin-releasing hormone
Footnotes
Declaration of interest: The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlskog JE. Food intake and amphetamine anorexia after selective forebrain norepinephrine loss. Brain Res. 1974;82:211–240. doi: 10.1016/0006-8993(74)90600-3. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Hooker S, Fujiwara K, Kiefer F, von der Goltz C, Cragin T, Wittmers LE. Circulating leptin levels are associated with increased craving to smoke in abstinent smokers. Pharmacol Biochem Behav. 2011;97:509–513. doi: 10.1016/j.pbb.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al’Absi M, Khalil NS, Al Habori M, Hoffman R, Fujiwara K, Wittmers L. Effects of chronic khat use on cardiovascular, adrenocortical, and psychological responses to stress in men and women. Am J Addict. 2013;22:99–107. doi: 10.1111/j.1521-0391.2013.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al’Absi M, Lemieux A, Nakajima M. Peptide YY and ghrelin predict craving and risk for relapse in abstinent smokers. Psychoneuroendocrinology. 2014;49c:253–259. doi: 10.1016/j.psyneuen.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al’absi M, Nakajima M, Dokam A, Sameai A, Alsoofi M, Saem Khalil N, Al Habori M. Concurrent tobacco and khat use is associated with blunted cardiovascular stress response and enhanced negative mood: a cross-sectional investigation. Hum Psychopharmacol. 2014 doi: 10.1002/hup.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Dubai W, Al-Habori M, Al-Geiry A. Human khat (Catha edulis) chewers have elevated plasma leptin and nonesterified fatty acids. Nutrition Research. 2006;26:632–636. [Google Scholar]
- Al-Hebshi NN, Skaug N. Effect of khat chewing on 14 selected periodontal bacteria in sub- and supragingival plaque of a young male population. Oral Microbiol Immunol. 2005;20:141–146. doi: 10.1111/j.1399-302X.2004.00195.x. [DOI] [PubMed] [Google Scholar]
- Alsalahi A, Abdulla MA, Al-Mamary M, Noordin MI, Abdelwahab SI, Alabsi AM, Shwter A, Alshawsh MA. Toxicological Features of Catha edulis (Khat) on Livers and Kidneys of Male and Female Sprague-Dawley Rats: A Subchronic Study. Evid Based Complement Alternat Med. 2012;2012:829401. doi: 10.1155/2012/829401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D, Carrier N. Khat in colonial Kenya: A history of prohibition and control. Journal of African History. 2009;50:377–397. [Google Scholar]
- Baumann MH, Clark RD, Woolverton WL, Wee S, Blough BE, Rothman RB. In vivo effects of amphetamine analogs reveal evidence for serotonergic inhibition of mesolimbic dopamine transmission in the rat. J Pharmacol Exp Ther. 2011;337:218–225. doi: 10.1124/jpet.110.176271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendotti C, Borsini F, Cotecchia S, De Blasi A, Mennini T, Samanin R. d- Amphetamine-induced anorexia and motor behavior after chronic treatment in rats: relationship with changes in the number of catecholamine receptor sites in the brain. Arch Int Pharmacodyn Ther. 1982;260:36–49. [PubMed] [Google Scholar]
- Bongard S, al’Absi M, Khalil NS, Al Habori M. Khat use and trait anger: effects on affect regulation during an acute stressful challenge. Eur Addict Res. 2011;17:285–291. doi: 10.1159/000330317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray GA. Use and abuse of appetite-suppressant drugs in the treatment of obesity. Ann Intern Med. 1993;119:707–713. doi: 10.7326/0003-4819-119-7_part_2-199310011-00016. [DOI] [PubMed] [Google Scholar]
- Breese GR, Cooper BR, Mueller RA. Evidence for involvement of 5- hydroxytryptamine in the actions of amphetamine. Br J Pharmacol. 1974;52:307–314. doi: 10.1111/j.1476-5381.1974.tb09714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneisen R, Fisch HU, Koelbing U, Geisshüsler S, Kalix P. Amphetamine-like effects in humans of the khat alkaloid cathinone. Br J Clin Pharmacol. 1990;30:825–828. doi: 10.1111/j.1365-2125.1990.tb05447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagnetti DJ, Schechter MD. Psychostimulant-induced activity is attenuated by two putative dopamine release inhibitors. Pharmacol Biochem Behav. 1992;43:1023–1031. doi: 10.1016/0091-3057(92)90476-v. [DOI] [PubMed] [Google Scholar]
- Carey RJ. Effects of selective forebrain depletions of norepinephrine and serotonin on the activity and food intake effects of amphetamine and fenfluramine. Pharmacol Biochem Behav. 1976;5:519–523. doi: 10.1016/0091-3057(76)90262-8. [DOI] [PubMed] [Google Scholar]
- Carey RJ, Goodall EB. Attenuation of amphetamine anorexia by unilateral nigral striatal lesions. Neuropharmacology. 1975;14:827–834. doi: 10.1016/0028-3908(75)90110-0. [DOI] [PubMed] [Google Scholar]
- Carlisle HJ. Differential effects of amphetamine on food and water intake in rats with lateral hypothalamic lesions. J Comp Physiol Psychol. 1964;58:47–54. doi: 10.1037/h0048203. [DOI] [PubMed] [Google Scholar]
- Carlisle HJ, Reynolds RW. Effect of amphetamine on food intake in rats with brain- stem lesions. American Journal of Physiology -- Legacy Content. 1961;201:965–967. doi: 10.1152/ajplegacy.1961.201.5.965. [DOI] [PubMed] [Google Scholar]
- Chapman MH, Kajihara M, Borges G, O’Beirne J, Patch D, Dhillon AP, Crozier A, Morgan MY. Severe, acute liver injury and khat leaves. N Engl J Med. 2010;362:1642–1644. doi: 10.1056/NEJMc0908038. [DOI] [PubMed] [Google Scholar]
- Chen TY, Duh SL, Huang CC, Lin TB, Kuo DY. Evidence for the involvement of dopamine D(1) and D(2) receptors in mediating the decrease of food intake during repeated treatment with amphetamine. J Biomed Sci. 2001;8:462–466. doi: 10.1007/BF02256608. [DOI] [PubMed] [Google Scholar]
- Cleary L, Docherty JR. Actions of amphetamine derivatives and cathinone at the noradrenaline transporter. Eur J Pharmacol. 2003;476:31–34. doi: 10.1016/s0014-2999(03)02173-3. [DOI] [PubMed] [Google Scholar]
- Connor JD, Rostom A, Makonnen E. Comparison of effects of khat extract and amphetamine on motor behaviors in mice. J Ethnopharmacol. 2002;81:65–71. doi: 10.1016/s0378-8741(02)00035-1. [DOI] [PubMed] [Google Scholar]
- Coton T, Simon F, Oliver M, Kraemer P. Hepatotoxicity of khat chewing. Liver Int. 2011;31:434. doi: 10.1111/j.1478-3231.2010.02338.x. [DOI] [PubMed] [Google Scholar]
- Davis WM, Logston DG, Hickenbottom JP. Antagonism of acute amphetamine intoxication by haloperidol and propranolol. Toxicol Appl Pharmacol. 1974;29:397–403. doi: 10.1016/0041-008x(74)90112-4. [DOI] [PubMed] [Google Scholar]
- Dawson BA, Black DB, Lavoie A, Lebelle MJ. Nuclear-magnetic-resonance identification of the phenylalkylamine alkaloids of khat using a chiral solvating agent. Journal of Forensic Sciences. 1994;39:1026–1038. [Google Scholar]
- Douglas H, Boyle M, Lintzeris N. The health impacts of khat: a qualitative study among Somali-Australians. Med J Aust. 2011;195:666–669. doi: 10.5694/mja11.10166. [DOI] [PubMed] [Google Scholar]
- Egecioglu E, Engel JA, Jerlhag E. The glucagon-like peptide 1 analogue, exendin-4, attenuates the rewarding properties of psychostimulant drugs in mice. PLoS One. 2013;8:e69010. doi: 10.1371/journal.pone.0069010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Gebissa E. Khat in the Horn of Africa: Historical perspectives and current trends. Journal of Ethnopharmacology. 2010;132:607–614. doi: 10.1016/j.jep.2010.01.063. [DOI] [PubMed] [Google Scholar]
- Gillard ER, Dang DQ, Stanley BG. Evidence that neuropeptide Y and dopamine in the perifornical hypothalamus interact antagonistically in the control of food intake. Brain Res. 1993;628:128–136. doi: 10.1016/0006-8993(93)90947-l. [DOI] [PubMed] [Google Scholar]
- Gunta SS, Mak RH. Ghrelin and leptin pathophysiology in chronic kidney disease. Pediatr Nephrol. 2013;28:611–616. doi: 10.1007/s00467-012-2380-9. [DOI] [PubMed] [Google Scholar]
- Halbach H. Medical aspects of the chewing of khat leaves. Bull World Health Organ. 1972;47:21–29. [PMC free article] [PubMed] [Google Scholar]
- Hamamoto DT, Rhodus NL. Methamphetamine abuse and dentistry. Oral Dis. 2009;15:27–37. doi: 10.1111/j.1601-0825.2008.01459.x. [DOI] [PubMed] [Google Scholar]
- Hao J, Cabeza de Vaca S, Pan Y, Carr KD. Effects of central leptin infusion on the reward-potentiating effect of D-amphetamine. Brain Res. 2006;1087:123–133. doi: 10.1016/j.brainres.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Harris JE, Baldessarini RJ. The uptake of (3H)dopamine by homogenates of rat corpus striatum: effects of cations. Life Sci. 1973;13:303–312. doi: 10.1016/0024-3205(73)90221-x. [DOI] [PubMed] [Google Scholar]
- Hassan NA, Gunaid AA, Murray-Lyon IM. Khat (Catha edulis): health aspects of khat chewing. East Mediterr Health J. 2007;13:706–718. [PubMed] [Google Scholar]
- Heal DJ, Cheetham SC, Prow MR, Martin KF, Buckett WR. A comparison of the effects on central 5-HT function of sibutramine hydrochloride and other weight-modifying agents. Br J Pharmacol. 1998;125:301–308. doi: 10.1038/sj.bjp.0702067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila RE, Orlansky H, Mytilineou C, Cohen G. Amphetamine: evaluation of d- and l-isomers as releasing agents and uptake inhibitors for 3H-dopamine and 3H-norepinephrine in slices of rat neostriatum and cerebral cortex. J Pharmacol Exp Ther. 1975;194:47–56. [PubMed] [Google Scholar]
- Holligen A. BBC World Service. BBC News; London: 2009. Khat use spreads to British youth. [Google Scholar]
- Hollister AS, Breese GR, Kuhn CM, Cooper BR, Schanberg SM. An inhibitory role for brain serotonin-containing systems in the locomotor effects of d-amphetamine. J Pharmacol Exp Ther. 1976;198:12–22. [PMC free article] [PubMed] [Google Scholar]
- Holmes JC, Rutledge CO. Effects of the d- and l-isomers of amphetamine on uptake, release and catabolism of norepinephrine, dopamine and 5-hydroxytryptamine in several regions of rat brain. Biochem Pharmacol. 1976;25:447–451. doi: 10.1016/0006-2952(76)90348-8. [DOI] [PubMed] [Google Scholar]
- Hsieh YS, Yang SF, Chiou HL, Kuo DY. Activations of c-fos/c-jun signaling are involved in the modulation of hypothalamic superoxide dismutase (SOD) and neuropeptide Y (NPY) gene expression in amphetamine-mediated appetite suppression. Toxicol Appl Pharmacol. 2006;212:99–109. doi: 10.1016/j.taap.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Hsieh YS, Yang SF, Kuo DY. Amphetamine, an appetite suppressant, decreases neuropeptide Y immunoreactivity in rat hypothalamic paraventriculum. Regul Pept. 2005;127:169–176. doi: 10.1016/j.regpep.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Islam MW, Tariq M, el-Feraly FS, al-Meshal IA. Effect of khatamines and their enantiomers on plasma triiodothyronine and thyroxine levels in normal Wistar rats. Am J Chin Med. 1990;18:71–76. doi: 10.1142/S0192415X90000101. [DOI] [PubMed] [Google Scholar]
- Jaworska L, Budziszewska B, Lasoń W. The effect of repeated amphetamine administration on the proopiomelanocortin mRNA level in the rat pituitary: an in situ hybridization study. Drug Alcohol Depend. 1994;36:123–127. doi: 10.1016/0376-8716(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Kalix P. Hypermotility of the amphetamine type induced by a constituent of khat leaves. Br J Pharmacol. 1980;68:11–13. doi: 10.1111/j.1476-5381.1980.tb10690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalix P. The amphetamine-like releasing effect of the alkaloid (−)cathinone on rat nucleus accumbens and rabbit caudate nucleus. Prog Neuropsychopharmacol Biol Psychiatry. 1982;6:43–49. doi: 10.1016/s0364-7722(82)80106-9. [DOI] [PubMed] [Google Scholar]
- Kalix P. Effect of the alkaloid (−) cathinone on the release of radioactivity from rabbit atria prelabelled with 3H-norepinephrine. Life Sci. 1983;32:801–807. doi: 10.1016/0024-3205(83)90316-8. [DOI] [PubMed] [Google Scholar]
- Kalix P. Effect of the alkaloid (−)-cathinone on the release of radioactivity from rat striatal tissue prelabelled with 3H-serotonin. Neuropsychobiology. 1984;12:127–129. doi: 10.1159/000118124. [DOI] [PubMed] [Google Scholar]
- Kalix P. The releasing effect of the isomers of the alkaloid cathinone at central and peripheral catecholamine storage sites. Neuropharmacology. 1986;25:499–501. doi: 10.1016/0028-3908(86)90174-7. [DOI] [PubMed] [Google Scholar]
- Kassim S, Croucher R. Factors associated with dental and medical care attendance in UK resident Yemeni khat chewers: a cross sectional study. BMC Public Health. 2012;12:486. doi: 10.1186/1471-2458-12-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy JG, Teague J, Rokaw W, Cooney E. A medical evaluation of the use of qat in North Yemen. Soc Sci Med. 1983;17:783–793. doi: 10.1016/0277-9536(83)90029-1. [DOI] [PubMed] [Google Scholar]
- Klein A. Khat and the informal globalisation of a psychoactive commodity. In: Storti CC, De Grauwe P, editors. Illicit trade and the global economy. MIT Press; Boston, Massachusetts: 2012. pp. 179–203. [Google Scholar]
- Knoll J. Studies on the central effects of (−)cathinone. NIDA Res Monogr. 1979;27:322–323. [PubMed] [Google Scholar]
- Kuo DY, Chen PN, Kuo MH, Chen CH, Hsieh YS, Chu SC. NF-κB knockdown can modulate amphetamine-mediated feeding response. Neuropharmacology. 2012;62:1684–1694. doi: 10.1016/j.neuropharm.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Laswar AK, Darwish H. Prevalence of cigarette smoking and khat chewing among Aden university medical students and their relationship to BP and body mass index. Saudi J Kidney Dis Transpl. 2009;20:862–866. [PubMed] [Google Scholar]
- Leibowitz SF. Catecholaminergic mechanisms of the lateral hypothalamus: their role in the mediation of amphetamine anorexia. Brain Res. 1975;98:529–545. doi: 10.1016/0006-8993(75)90371-6. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Rossakis C. Analysis of feeding suppression produced by perifornical hypothalamic injection of catecholamines, amphetamines and mazindol. Eur J Pharmacol. 1978;53:69–81. doi: 10.1016/0014-2999(78)90269-8. [DOI] [PubMed] [Google Scholar]
- Lemberger L, Witt ED, Davis JM, Kopin IJ. The effects of haloperidol and chlorpromazine on amphetamine metabolism and amphetamine stereotype behavior in the rat. J Pharmacol Exp Ther. 1970;174:428–433. [PubMed] [Google Scholar]
- Leserman Robbins J, Lorvick J, Lutnick A, Wenger L, Kral AH. Self-reported oral health needs and dental-care seeking behavior among women who use methamphetamine. Subst Use Misuse. 2012;47:1208–1213. doi: 10.3109/10826084.2012.696228. [DOI] [PubMed] [Google Scholar]
- Mahfouz MS, Alsanosy RM, Gaffar AM. The role of family background on adolescent khat chewing behavior in Jazan Region. Ann Gen Psychiatry. 2013;12:16. doi: 10.1186/1744-859X-12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood SA, Lindequist U. A pilot study on the effect of Catha edulis Frosk., (Celastraceae) on metabolic syndrome in WOKW rats. Afr J Tradit Complement Altern Med. 2008;5:271–277. doi: 10.4314/ajtcam.v5i3.31283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manberg PJ, Nemeroff CB, Prange AJ. Thyrotropin-releasing hormone and amphetamine: a comparison of pharmacological profiles in animals. Prog Neuropsychopharmacol. 1979;3:303–314. doi: 10.1016/0364-7722(79)90042-0. [DOI] [PubMed] [Google Scholar]
- McPherson CS, Featherby T, Krstew E, Lawrence AJ. Quantification of phosphorylated cAMP-response element-binding protein expression throughout the brain of amphetamine-sensitized rats: activation of hypothalamic orexin A-containing neurons. J Pharmacol Exp Ther. 2007;323:805–812. doi: 10.1124/jpet.107.125732. [DOI] [PubMed] [Google Scholar]
- Milroy CM, Parai JL. The histopathology of drugs of abuse. Histopathology. 2011;59:579–593. doi: 10.1111/j.1365-2559.2010.03728.x. [DOI] [PubMed] [Google Scholar]
- Morawska D, Sieklucka-Dziuba M, Kleinrok Z. Central action of glucagon. Pol J Pharmacol. 1998;50:125–133. [PubMed] [Google Scholar]
- Morley JE, Flood JF. An investigation of tolerance to the actions of leptogenic and anorexigenic drugs in mice. Life Sci. 1987;41:2157–2165. doi: 10.1016/0024-3205(87)90534-0. [DOI] [PubMed] [Google Scholar]
- Murray CD, Le Roux CW, Emmanuel AV, Halket JM, Przyborowska AM, Kamm MA, Murray-Lyon IM. The effect of Khat (Catha edulis) as an appetite suppressant is independent of ghrelin and PYY secretion. Appetite. 2008;51:747–750. doi: 10.1016/j.appet.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Nakajima M, al’Absi M. Psychosocial deterrents of tobacco and khat use among men and women. Public Health. 2013;127:684–686. doi: 10.1016/j.puhe.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Nakajima M, al’Absi M, Dokam A, Alsoofi M, Khalil NS. An examination of the Fagerström Test for Nicotine Dependence among concurrent tobacco and khat users. J Psychoactive Drugs. 2012;44:437–441. doi: 10.1080/02791072.2012.737224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, al’Absi M, Dokam A, Alsoofi M, Khalil NS, Al Habori M. Gender differences in patterns and correlates of khat and tobacco use. Nicotine Tob Res. 2013;15:1130–1135. doi: 10.1093/ntr/nts257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, Dokam A, Kasim AN, Alsoofi M, Khalil NS, al’Absi M. Habitual khat and concurrent khat and tobacco use are associated with subjective sleep quality. Prev Chronic Dis. 2014;11:E86. doi: 10.5888/pcd11.130234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nencini P, Ahmed AM. Khat consumption: a pharmacological review. Drug Alcohol Depend. 1989;23:19–29. doi: 10.1016/0376-8716(89)90029-x. [DOI] [PubMed] [Google Scholar]
- Nencini P, Ahmed AM, Amiconi G, Elmi AS. Tolerance develops to sympathetic effects of khat in humans. Pharmacology. 1984;28:150–154. doi: 10.1159/000137956. [DOI] [PubMed] [Google Scholar]
- Nencini P, Ahmed AM, Elmi AS. Subjective effects of khat chewing in humans. Drug Alcohol Depend. 1986;18:97–105. doi: 10.1016/0376-8716(86)90118-3. [DOI] [PubMed] [Google Scholar]
- Nielsen JA. Cathinone affects dopamine and 5-hydroxytryptamine neurons in vivo as measured by changes in metabolites and synthesis in four forebrain regions in the rat. Neuropharmacology. 1985;24:845–852. doi: 10.1016/0028-3908(85)90035-8. [DOI] [PubMed] [Google Scholar]
- Nyongesa AW, Oduma JA, Nakajima M, Odongo HO, Adoyo PA, Al’absi M. Dose-response inhibitory effects of purified cathinone from khat (Catha edulis) on cortisol and prolactin release in vervet monkeys (Chlorocebus aethiops) Metab Brain Dis. 2013 doi: 10.1007/s11011-013-9445-8. [DOI] [PubMed] [Google Scholar]
- Odenwald M, Warfa N, Bhui K, Elbert T. The stimulant khat--another door in the wall? A call for overcoming the barriers. J Ethnopharmacol. 2010;132:615–619. doi: 10.1016/j.jep.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Hindler CG, Taylor JC. Use and abuse of khat (Catha edulis): a review of the distribution, pharmacology, side effects and a description of psychosis attributed to khat chewing. Psychol Med. 1989;19:657–668. doi: 10.1017/s0033291700024259. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Human psychopharmacology of Ecstasy (MDMA): a review of 15 years of empirical research. Hum Psychopharmacol. 2001;16:557–577. doi: 10.1002/hup.351. [DOI] [PubMed] [Google Scholar]
- Pehek EA, Schechter MD. Discriminative stimulus properties of (+)cathine, an alkaloid of the khat plant. Pharmacol Biochem Behav. 1990;36:267–271. doi: 10.1016/0091-3057(90)90402-4. [DOI] [PubMed] [Google Scholar]
- Potretzke S, Nakajima M, Cragin T, al’Absi M. Changes in circulating leptin levels during acute stress and associations with craving in abstinent smokers: A preliminary investigation. Psychoneuroendocrinology. 2014;47:232–240. doi: 10.1016/j.psyneuen.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravenel MC, Salinas CF, Marlow NM, Slate EH, Evans ZP, Miller PM. Methamphetamine abuse and oral health: a pilot study of “meth mouth”. Quintessence Int. 2012;43:229–237. [PubMed] [Google Scholar]
- Rech RH, Borsini F, Samanin R. Effects of d-amphetamine and d-fenfluramine on performance of rats in a food maze. Pharmacol Biochem Behav. 1984;20:489–493. doi: 10.1016/0091-3057(84)90293-4. [DOI] [PubMed] [Google Scholar]
- Rogge G, Jones D, Hubert GW, Lin Y, Kuhar MJ. CART peptides: regulators of body weight, reward and other functions. Nat Rev Neurosci. 2008;9:747–758. doi: 10.1038/nrn2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross LJ, Wilson M, Banks M, Rezannah F, Daglish M. Prevalence of malnutrition and nutritional risk factors in patients undergoing alcohol and drug treatment. Nutrition. 2012;28:738–743. doi: 10.1016/j.nut.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Vu N, Partilla JS, Roth BL, Hufeisen SJ, Compton-Toth BA, Birkes J, Young R, Glennon RA. In vitro characterization of ephedrine-related stereoisomers at biogenic amine transporters and the receptorome reveals selective actions as norepinephrine transporter substrates. J Pharmacol Exp Ther. 2003;307:138–145. doi: 10.1124/jpet.103.053975. [DOI] [PubMed] [Google Scholar]
- Russek M, Rodríguez-Zendejas AM, Teitelbaum P. The action of adrenergic anorexigenic substances on rats recovered from lateral hypothalamic lesions. Physiol Behav. 1973;10:329–333. doi: 10.1016/0031-9384(73)90317-x. [DOI] [PubMed] [Google Scholar]
- Samanin R, Bernasconi S, Garattini S. The effect of selective lesioning of brain catecholamine-containing neurons on the activity of various anorectics in thr rat. Eur J Pharmacol. 1975;34:373–375. doi: 10.1016/0014-2999(75)90265-4. [DOI] [PubMed] [Google Scholar]
- Sanghvi IS, Singer G, Friedman E, Gershon S. Anorexigenic effects of d- amphetamine and l-DOPA in the rat. Pharmacol Biochem Behav. 1975;3:81–86. doi: 10.1016/0091-3057(75)90084-2. [DOI] [PubMed] [Google Scholar]
- Schechter MD. Dopaminergic mediation of a behavioral effect of l-cathinone. Pharmacol Biochem Behav. 1986;25:337–340. doi: 10.1016/0091-3057(86)90006-7. [DOI] [PubMed] [Google Scholar]
- Schechter MD. Dopaminergic nature of acute cathine tolerance. Pharmacol Biochem Behav. 1990;36:817–820. doi: 10.1016/0091-3057(90)90083-t. [DOI] [PubMed] [Google Scholar]
- Schechter MD, McBurney D. Effect of repeated administrations upon cathinone discrimination and conditioned place preference. Gen Pharmacol. 1991;22:779–782. doi: 10.1016/0306-3623(91)90204-j. [DOI] [PubMed] [Google Scholar]
- Sheikh A. Somali ’paradise flower’ chewers savor low-price bliss after UK ban. Reuters. 2014 Reuters.com.
- Shortall SE, Green AR, Swift KM, Fone KC, King MV. Differential effects of cathinone compounds and MDMA on body temperature in the rat, and pharmacological characterization of mephedrone-induced hypothermia. Br J Pharmacol. 2013;168:966–977. doi: 10.1111/j.1476-5381.2012.02236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone PH, Oldman D, Johnson B, Cowen PJ. Ondansetron, a 5-HT3 receptor antagonist, partially attenuates the effects of amphetamine: a pilot study in healthy volunteers. Int Clin Psychopharmacol. 1992;7:37–43. doi: 10.1097/00004850-199200710-00005. [DOI] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013;168:458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DG, Qi H, Svenningsson P, Wade M, Davis RJ, Gehlert DR, Nomikos GG. Behavioral and biochemical responses to d-amphetamine in MCH1 receptor knockout mice. Synapse. 2008;62:128–136. doi: 10.1002/syn.20473. [DOI] [PubMed] [Google Scholar]
- Stevenson M, Fitzgerald J, Banwell C. Chewing as a social act: Cultural displacement and khat consumption in the East African communities of Melbourne. Drug and Alcohol Review. 1996;15:73–82. doi: 10.1080/09595239600185691. [DOI] [PubMed] [Google Scholar]
- Strid H, Simrén M, Johansson A, Svedlund J, Samuelsson O, Björnsson ES. The prevalence of gastrointestinal symptoms in patients with chronic renal failure is increased and associated with impaired psychological general well - being. Nephrology Dialysis Transplantation. 2002;17:1434–1439. doi: 10.1093/ndt/17.8.1434. [DOI] [PubMed] [Google Scholar]
- Suchankova P, Jerlhag E, Jayaram-Lindström N, Nilsson S, Toren K, Rosengren A, Engel JA, Franck J. Genetic variation of the ghrelin signalling system in individuals with amphetamine dependence. PLoS One. 2013;8:e61242. doi: 10.1371/journal.pone.0061242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Asakawa A, Amitani H, Nakamura N, Inui A. Ghrelin and cachexia in chronic kidney disease. Pediatr Nephrol. 2013;28:521–526. doi: 10.1007/s00467-012-2241-6. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Jayasena CN, Bloom SR. Obesity and appetite control. Exp Diabetes Res. 2012;2012:824305. doi: 10.1155/2012/824305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szendrei K. The chemistry of khat. Bull Narc. 1980;32:5–35. [PubMed] [Google Scholar]
- Tariq M, Islam MW, al-Meshal IA, el-Feraly FS, Ageel AM. Comparative study of cathinone and amphetamine on brown adipose thermogenesis. Life Sci. 1989;44:951–955. doi: 10.1016/0024-3205(89)90494-3. [DOI] [PubMed] [Google Scholar]
- Tatsuoka Y, Riskind PN, Beal MF, Martin JB. The effect of amphetamine on the in vivo release of dopamine, somatostatin and neuropeptide Y from rat caudate nucleus. Brain Res. 1987;411:200–203. doi: 10.1016/0006-8993(87)90702-5. [DOI] [PubMed] [Google Scholar]
- Thomas S, Williams T. Khat (Catha edulis): A systematic review of evidence and literature pertaining to its harms to UK users and society. Drug Science, Policy and Law. 2013;1:1–25. [Google Scholar]
- United Nations; Nations, U, editor Etude sur la composition chimique du khat: Recherche sur la fraction phenylalkylamine. 1975. [Google Scholar]
- Valterio C, Kalix P. The effect of the alkaloid (−)cathinone on the motor activity in mice. Arch Int Pharmacodyn Ther. 1982;255:196–203. [PubMed] [Google Scholar]
- Vicentic A, Jones DC. The CART (cocaine- and amphetamine-regulated transcript) system in appetite and drug addiction. J Pharmacol Exp Ther. 2007;320:499–506. doi: 10.1124/jpet.105.091512. [DOI] [PubMed] [Google Scholar]
- Volkoff H. The effects of amphetamine injections on feeding behavior and the brain expression of orexin, CART, tyrosine hydroxylase (TH) and thyrotropin releasing hormone (TRH) in goldfish (Carassius auratus) Fish Physiol Biochem. 2013;39:979–991. doi: 10.1007/s10695-012-9756-4. [DOI] [PubMed] [Google Scholar]
- Von Voigtlander PF, Moore KE. Involvement of nigro-striatal neurons in the in vivo release of dopamine by amphetamine, amantadine and tyramine. J Pharmacol Exp Ther. 1973;184:542–552. [PubMed] [Google Scholar]
- Wabe NT. Chemistry, pharmacology, and toxicology of khat (catha edulis forsk): a review. Addict Health. 2011;3:137–149. [PMC free article] [PubMed] [Google Scholar]
- Walters KR, Rupassara SI, Markelz RJ, Leakey AD, Muir WM, Pittendrigh BR. Methamphetamine causes anorexia in Drosophila melanogaster, exhausting metabolic reserves and contributing to mortality. J Toxicol Sci. 2012;37:773–790. doi: 10.2131/jts.37.773. [DOI] [PubMed] [Google Scholar]
- Wellman PJ, Clifford PS, Rodriguez JA. Ghrelin and ghrelin receptor modulation of psychostimulant action. Front Neurosci. 2013;7:171. doi: 10.3389/fnins.2013.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarom N, Epstein J, Levi H, Porat D, Kaufman E, Gorsky M. Oral manifestations of habitual khat chewing: a case-control study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:e60–66. doi: 10.1016/j.tripleo.2010.02.022. [DOI] [PubMed] [Google Scholar]
- Zelger JL, Carlini EA. Anorexigenic effects of two amines obtained from Catha edulis Forsk. (Khat) in rats. Pharmacol Biochem Behav. 1980;12:701–705. doi: 10.1016/0091-3057(80)90152-5. [DOI] [PubMed] [Google Scholar]
- Zelger JL, Schorno HX, Carlini EA. Behavioural effects of cathinone, an amine obtained from Catha edulis Forsk.: comparisons with amphetamine, norpseudoephedrine, apomorphine and nomifensine. Bull Narc. 1980;32:67–81. [PubMed] [Google Scholar]