Abstract
Recently, elevated peripheral blood monocyte counts at diagnosis have been shown to be an independent marker associated with poor prognosis in patients with both non-Hodgkin and Hodgkin lymphoma. In this study, we retrospectively analyzed the data from a total of 550 patients with diffuse large B-cell lymphoma and evaluated the relationship between central nervous system relapse and absolute monocyte counts at diagnosis. Twenty-six patients developed central nervous system relapse. The central nervous system relapse-free survival rate was significantly lower in patients with the absolute monocyte counts ≥0.51×109/L (87.8% versus 96.4%; P<0.001). This association was independently significant after adjusting for other significant factors, including systemic relapse as a time-dependent covariate by multivariate analysis (hazard ratio 2.46; 95% confidence intervals 1.05–5.75; P=0.039). These results suggest that the absolute monocyte count at diagnosis is an independent significant risk factor for central nervous system relapse in patients with diffuse large B-cell lymphoma.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is characterized as an aggressive lymphoma with heterogeneous clinical behaviors. DLBCL accounts for 25–30% of non-Hodgkin lymphomas among adults in the west, and it is even more prevalent in developing countries.1 In Japan, it was reported that DLBCL is the most frequent subtype and accounts for over 30% of cases of non-Hodgkin lymphomas among adults.2 Central nervous system (CNS) involvement is not common in DLBCL, but several risk factors for CNS involvement have been identified, including high International Prognostic Index score, involvement of more than one extranodal site, elevated lactate dehydrogenase level, poor Eastern Cooperative Oncology Group performance status (PS), advanced stage, and involvement of bone marrow, breast, testis, retroperitoneal lymph nodes, orbit, or epidural space.3,4
Macrophages are an important component of the tumor microenvironment and the immune response to malignancy. Recently, elevated peripheral blood monocyte counts have been shown to be an independent predictor of poor prognosis in patients with both non-Hodgkin and Hodgkin lymphoma.5–10 Here, we investigated the relationship between peripheral blood monocytes at diagnosis and prognosis, focusing on CNS relapse in DLBCL.
Methods
Patients’ characteristics
In the present study, we reviewed the medical records of patients with DLBCL who were treated at the Cancer Institute Hospital of the Japanese Foundation for Cancer Research from February 2005 to May 2014, and retrospectively analyzed the data of a total of 550 consecutive DLBCL patients, excluding seven patients who had CNS involvement at diagnosis. This study was approved by the Institutional Review Board at the Cancer Institute Hospital of the Japanese Foundation for Cancer Research.
The diagnosis of CNS relapse required at least one of the following conditions: the presence of histologically confirmed CNS involvement, neuroimaging findings compatible with CNS relapse with lymphoma associated with consistent clinical presentation and the absence of any other clinically feasible diagnosis, or positive cerebrospinal fluid involvement.
Systemic and central nervous system prophylaxis
Patients in this study were treated with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) or R-CHOP-based regimens with minor modifications. CNS prophylaxis was given to 72 patients with DLBCL having “high-risk” features for CNS involvement including an involvement of breast, testis, orbit, or epidural space, and paravertebral body or high tumor burden. The intrathecal injection for CNS prophylaxis consisted of methotrexate 15 mg, cytarabine 40 mg, and predonisone 20 mg at least once in the first course of chemotherapy. Furthermore, two patients with DLBCL with the MYC translocation received intravenous high-dose methotrexate and cytarabine chemotherapy and one patient received R-DHAP (rituximab, dexamethasone, cytarabine, cisplatin).
Statistical analysis
Receiver operating characteristics (ROC) curve analysis was used to determine the optimal cutoff points of the absolute monocyte count (AMC) or monocyte percentage of the total white blood cell count at diagnosis.10 Monocyte percentages were confirmed by visual examination of blood smears in cases in which the automated counts were abnormal.
CNS relapse-free survival was calculated by cause-specific function, censoring patients who died without CNS relapse, in accordance with previously published studies.11–14 The starting date of the time-to-event analysis was defined at diagnosis. Univariate and multivariate analyses of estimated risk factors for CNS relapse in DLBCL patients were performed using the log-rank test and Cox proportional hazard regression analysis. P values <0.05 were considered statistically significant. Background characteristics of the patients with and without CNS involvement were compared using the Fisher exact test and Student t-test. All statistical analyses were performed with EZR15 (Saitama, Medical Center, Jichi Medical University, Japan).
Results
The incidence of central nervous system relapse and the impact of monocyte count at diagnosis
The clinical features of all 550 DLBCL patients, including 26 patients who eventually developed CNS relapse and those who did not, are summarized in Table 1. The median age, AMC, and the monocyte percentage were 64.5 years, 567.8×109/L, and 8.8%, respectively, for patients who developed CNS relapse and 65 years, 378.0×109/L, and 6.0%, respectively, for those who did not. The CNS relapse-free survival was 95.6% at 3 years and 94.2% at 5 years. The median duration of follow-up was 44.5 months (range, 1–111 months) for the whole cohort and 52 months (range, 2–111) for surviving patients.
Table 1.
Patient’s characteristics.
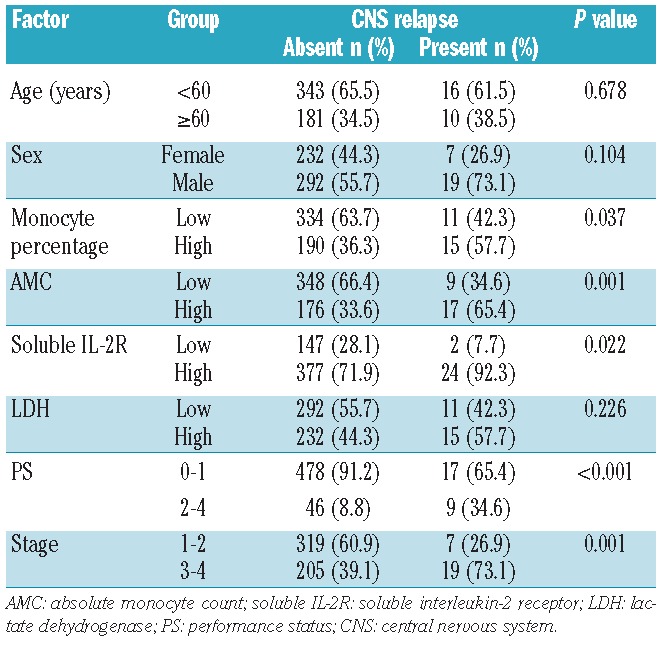
Both AMC and monocyte percentage at diagnosis as continuous variables were significantly associated with CNS relapse-free survival (P=0.0054 and P=0.0040, respectively). ROC curve analysis revealed that optimal cutoff values, defined as those with the highest sum of sensitivity and specificity, were 0.51×109/L) for AMC and 8% for monocyte percentage. The areas under the ROC curves were 0.66 [95% confidence interval (CI) 0.57–0.76] for AMC and 0.64 (95% CI 0.53–0.75) for monocyte percentage. The 5-year CNS relapse-free survival was 96.4% and 90.0% for patients with low and high AMC, respectively, at diagnosis (P<0.001, Figure 1) and 96.1% and 90.8% for patients with low and high monocyte percentage (P=0.0177), respectively.
Figure 1.
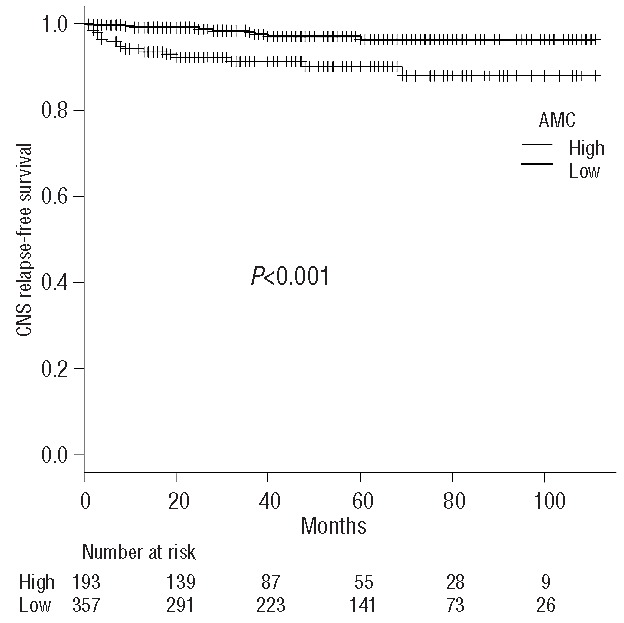
The CNS relapse-free survival curves grouped according to the absolute monocyte count (AMC) at diagnosis. CNS: central nervous system.
In the following analyses we used AMC rather than monocyte percentage, because AMC better discriminated high-risk patients for CNS relapse. The 5-year cumulative incidence of CNS relapse was 3.6% and 10.0% for patients with low and high AMC at diagnosis, respectively. Treating death without relapse as a competing risk, the 5-year cumulative incidence of CNS relapse was 3.3% and 9.3% for patients with low and high AMC at diagnosis, respectively.
Multivariate analysis to adjust for other significant factors
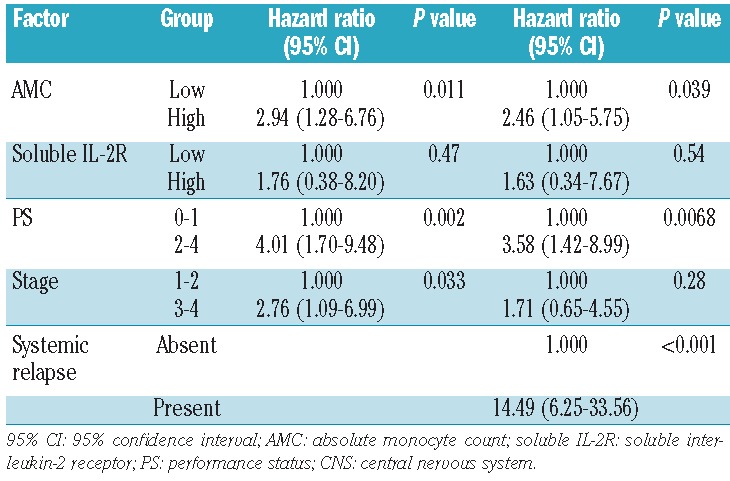
To adjust the impact of AMC at diagnosis for other significant factors for CNS relapse, we identified the following risk factors by univariate analysis (Table 2): clinical stage III–IV, PS ≥2, and elevated soluble interluekin-2 receptor levels. We then performed multivariate analyses using all these factors in the Cox proportional hazard model. As shown in Table 3, high AMC [hazard ratio (HR) 2.94, 95% CI 1.28–6.76; P=0.011), poor PS (HR 4.01, 95% CI 1.70–9.48; P=0.0016), and stage III-IV disease (HR 2.76, 95% CI 1.09–6.99; P=0.033) were identified as independent significant prognostic factors for CNS relapse. In multivariate analyses including extra-nodal involvement or the presence of intrathecal treatment as an independent variable, the impact of AMC did not change by adding these variables into the model (HR 2.85, 95% CI 1.24–6.54; P=0.013 and HR 2.83, 95% CI 1.23–6.50; P=0.014, respectively). The presence of intrathecal treatment was associated with a non-significant increase in CNS relapse (HR 1.92, 95% CI 0.79–4.65; P=0.15).
Table 2.
Univariate analysis for CNS relapse.
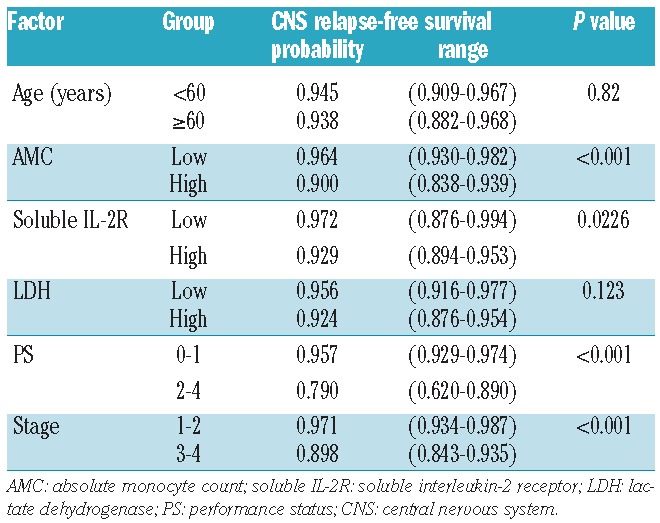
Table 3.
Multivariate analysis for CNS relapse. Systemic relapse was treated as a time-dependent covariate and was included in the model on the right.
Next, we evaluated whether the impact of AMC on CNS relapse is independent of its impact on systemic relapse, as systemic relapse is a strong predictor of CNS relapse, and high monocyte count at diagnosis has been shown to be a significant risk factor for systemic relapse.5 In fact, in this cohort, systemic progression-free survival was significantly associated with AMC at diagnosis (78.4% versus 67.4% at 5 years; P=0.0019) (Figure 2).
Figure 2.
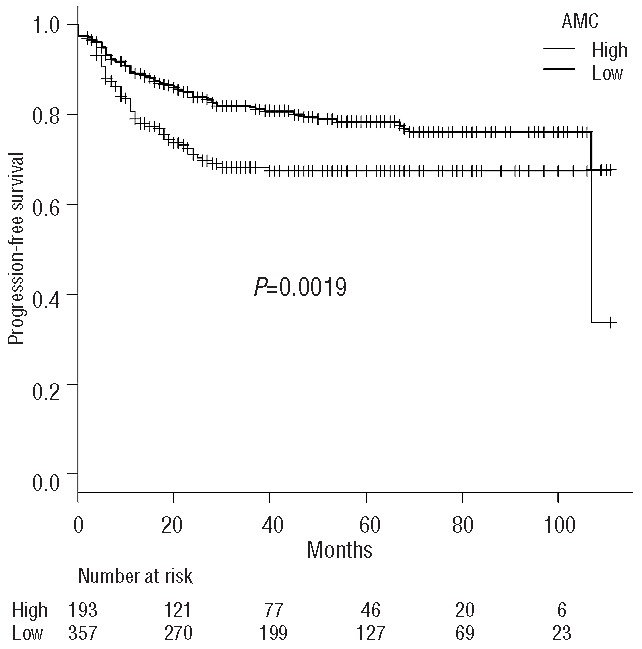
Progression-free survival curves grouped according to the absolute monocyte count (AMC) at diagnosis.
We, therefore, added systemic relapse as a time-dependent covariate into the Cox proportional hazard model. The impact of AMC was still significant even after adjusting for systemic relapse (HR 2.46, 95% CI 1.04–5.75; P=0.039) (Table 3). In addition, among the 130 patients who developed systemic relapse, the incidence of subsequent CNS relapse was higher in patients with high AMC at diagnosis (78.0% versus 65.9% at 5 years after systemic relapse; P=0.030) (Figure 3). These findings suggest that the impact of AMC on CNS relapse is independent of its impact on systemic relapse.
Figure 3.
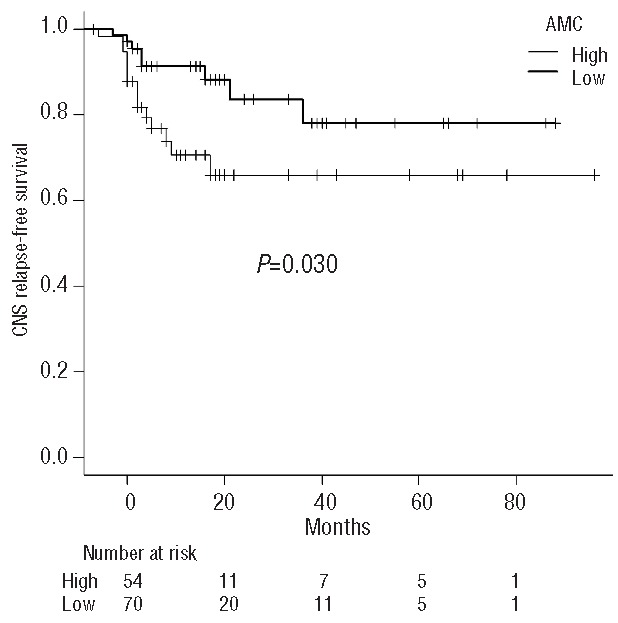
The CNS relapse-free survival curves after systemic relapse, grouped according to the absolute monocyte count (AMC) at diagnosis. CNS, central nervous system.
Discussion
In this study, we retrospectively analyzed the data from a total of 550 DLBCL patients and evaluated the relationship between CNS relapse and AMC at diagnosis. The CNS relapse-free survival was significantly lower in patients with AMC ≥0.51×109/L at diagnosis. This association could be explained by the previous findings that systemic relapse is a strong predictor of CNS relapse, and high monocyte count at diagnosis has been shown to be a significant risk factor for systemic relapse.5 In fact, in this cohort, systemic progression-free survival was significantly associated with AMC at diagnosis. Tumor-associated macrophages and monocytes can foster cancer progression by promoting proliferation and angiogenesis.16 It has been reported that an elevated ratio of CD14+ monocytes without HLA expression was significantly related to aggressive clinical behavior in DLBCL.17 These peripheral blood monocytes suppress host immunity by inhibiting the proliferative ability of interferon-γ, impairing the differentiation ability of dendritic cells.1
In this study, however, the impact of AMC was still significant even after adjusting for systemic relapse as a time-dependent covariate. In addition, among patients who developed systemic relapse, the incidence of subsequent CNS relapse was significantly higher in patients with high AMC at diagnosis. AMC at diagnosis does, therefore, have a significant adverse effect on CNS relapse, independently of systemic relapse. Peripheral monocytes, which migrate to cancer tissue and differentiate into tumor-associated macrophages, are increased in a variety of malignancies, including lymphoma.18 The M2c subtype of monocytes can produce CXCL13 and interleukin-10,19,20 which are known to be associated with the development of CNS lymphoma.19 Accordingly, AMC at diagnosis in DLBCL patients may be related to CNS relapse.
Patients with high AMC may require intensified prophylaxis for CNS relapse. Intrathecal prophylaxis has been investigated to determine whether it prevents CNS relapse in high-risk patients, but its efficacy was not proven in studies employing modern regimens.21 In fact, in the current study, intrathecal prophylaxis was added to patients at high-risk of CNS relapse, but these patients still had a higher incidence of CNS relapse.22 Therefore, high-dose intravenous methotrexate and/or cytarabine chemotherapy as dose-intensive systemic antimetabolite-containing chemotherapy may be required in high-risk patients.22
In conclusion, our findings suggest that the AMC at diagnosis has an independent impact on CNS relapse in patients with DLBCL. Novel strategies to prevent CNS relapse should be explored for patients with high AMC.
Footnotes
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Li ZM, Huang JJ, Xia Y, Sun J, Huang Y, Wang, et al. Blood lymphocyte-to- monocyte ratio identifies high-risk patients in diffuse large B-cell lymphoma treated with R-CHOP. PLoS One. 2012;7(7):e41658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lymphoma Study Group of Japanese Pathologists. The World Health Organization classification of malignant lymphomas in Japan: incidence of recently recognized entities. Pathol Int. 2000;50(9): 696–702. [DOI] [PubMed] [Google Scholar]
- 3.Guirguis HR, Cheung MC, Mahrous M, Piliotis E, Berinstein N, Imrie KR, et al. Impact of central nervous system (CNS) prophylaxis on the incidence and risk factors for CNS relapse in patients with diffuse large B-cell lymphoma treated in the rituximab era: a single centre experience and review of the literature. Br J Haematol. 2012;159(1):39–49. [DOI] [PubMed] [Google Scholar]
- 4.Siegal T, Goldschmidt N. CNS prophylaxis in diffuse large B-cell lymphoma: if, when, how and for whom? Blood Rev. 2012; 26(3):97–106. [DOI] [PubMed] [Google Scholar]
- 5.Wilcox RA, Ristow K, Habermann TM, Inwards DJ, Micallef IN, Johnston PB, et al. The absolute monocyte and lymphocyte prognostic score predicts survival and identifies high-risk patients in diffuse large-B-cell lymphoma. Leukemia. 2011;25(9):1502–9. [DOI] [PubMed] [Google Scholar]
- 6.Wilcox RA, Ristow K, Habermann TM, Inwards DJ, Micallef IN, Johnston PB, et al. The absolute monocyte count is associated with overall survival in patients newly diagnosed with follicular lymphoma. Leuk Lymphoma. 2012;53(4):575–80. [DOI] [PubMed] [Google Scholar]
- 7.Tadmor T, Bari A, Sacchi S, Marcheselli L, Liardo EV, Avivi I, et al. Monocyte count at diagnosis is a prognostic parameter in diffuse large B-cell lymphoma: a large multicenter study involving 1191 patients, in the pre and post rituximab era. Haematologica 2013;99(1):125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porrata LF, Ristow K, Habermann TM, Witzig TE, Colgan JP, Inwards DJ, et al. Peripheral blood lymphocyte/monocyte ratio at diagnosis and survival in nodular lymphocyte-predominant Hodgkin lymphoma. Br J Haematol. 2012;157(3):321–30. [DOI] [PubMed] [Google Scholar]
- 9.Rambaldi A, Boschini C, Delaini F, Oldani E, Rossi A, Barbui AM, et al. The peripheral blood lymphocyte to monocyte ratio at diagnosis is a potent outcome predictor in diffuse large B-cell lymphoma treated with R-CHOP: a long-term analysis on 973 patients receiving chemotherapy with or without rituximab. Blood. 2012;120: Abstract 155. [Google Scholar]
- 10.Koh YW, Kang HJ, Park C, Yoon DH, Kim S, Suh C, et al. The ratio of the absolute lymphocyte count to the absolute monocyte count is associated with prognosis in Hodgkin lymphoma: correlation with tumor-associated macrophages. Oncologist. 2012;17(6):871–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheah CY, George A, Giné E, Chiappella A, Kluin-Nelemans HC, Jurczak W, et al. ; European Mantle Cell Lymphoma Network. Central nervous system involvement in mantle cell lymphoma: clinical features, prognostic factors and outcomes from the European Mantle Cell Lymphoma Network. Ann Oncol. 2013;24(8):2119–23. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Angulo AM, Cristofanilli M, Strom EA, Buzdar AU, Kau SW, Broglio KR, et al. Central nervous system metastases in patients with high-risk breast carcinoma after multimodality treatment. Cancer. 2004;101(8):1760–6. [DOI] [PubMed] [Google Scholar]
- 13.Harms DO, Janka-Schaub GE. Co-operative Study Group for Childhood Acute Lymphoblastic Leukemia (COALL): long-term follow-up of trials 82, 85, 89 and 92. Leukemia. 2000;14(12):2234–9. [DOI] [PubMed] [Google Scholar]
- 14.Tsurusawa M, Katano N, Yamamoto Y, Hirota T, Koizumi S, Watanabe A, et al. Improvement in CNS protective treatment in non-high-risk childhood acute lymphoblastic leukemia: report from the Japanese Children’s Cancer and Leukemia Study Group. Med Pediatr Oncol. 1999;32(4):259–66. [DOI] [PubMed] [Google Scholar]
- 15.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allavena P, Mantovani A. Immunology in the clinic review series; focus on cancer: tumor-associated macrophages: undisputed stars of the inflammatory tumour microenviroment. Clin Exp Immunol. 2012;167(2): 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin Y, Gustafson MP, Bulur PA, Gastineau DA, Witzig TE, Dietz AB. Immunosuppressive CD14+HLA-DR(low)/-monocytes in B-cell non-Hodgkin lymphoma. Blood. 2011;117(3):872–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen C, Zhu YB, Shen Y, Zhu YH, Zhang XG, Huang JA. Increase of circulating B7-H4-expressing CD68+ macrophage correlated with clinical stage of lung carcinomas. J Immunother. 2012;35(4):354–8. [DOI] [PubMed] [Google Scholar]
- 19.Rubenstein JL, Wong VS, Kadoch C, Gao HX, Barajas R, Chen L, et al. CXCL13 plus interleukin 10 is highly specific for the diagnosis of CNS lymphoma. Blood. 2013;121(23):4740–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi M, Jeschke MG, Shigematsu K, Asai A, Yoshida S, Herndon DN, et al. M2b monocytes predominated in peripheral blood of severely burned patients. J Immunol. 2010;185(12):7174–9. [DOI] [PubMed] [Google Scholar]
- 21.Boehme V, Schmitz N, Zeynalova S, Loeffler M, Pfreundschuh M. CNS events in elderly patients with aggressive lymphoma treated with modern chemotherapy (CHOP-14) with or without rituximab: an analysis of patients treated in the RICOVER-60 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Blood. 2009;113(17):3896–902. [DOI] [PubMed] [Google Scholar]
- 22.Cheah CY, Herbert KE, O’Rourke K, Kennedy GA, George A, Fedele PL, et al. A multicentre retrospective comparison of central nervous system prophylaxis strategies among patients with high-risk diffuse large B-cell lymphoma. Br J Cancer. 2014;111(6):1072–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


