Current strategies for the treatment of acute myeloid leukemia (AML) focus on the induction of cell death by standard chemotherapy or targeted therapy. In elderly patients with chemorefractory disease the prognosis is still poor and allogeneic hematopoietic stem cell transplantation is rarely feasible. Several retrospective studies have assessed the role of hypomethylating agents 5-azacytidine (AZA) and 2-deoxy-5-azacytidine (decitabine) in this patient group, but response rates were disappointing when compared to first-line treatment.1–3 Therefore, novel salvage strategies should be developed focusing on induction of remission and prolongation of survival. The most striking improvement in AML therapy has been achieved in acute promyelocytic leukemia (APL) treated with the retinoic acid receptor (RAR) specific ligand, all-trans retinoic acid (ATRA). RARs belong to the family of ligand-dependent transcription factors that heterodimerize with the retinoid X receptor (RXR). The peroxisome proliferator-activated receptor (PPAR)γ also functions as a heterodimer with RXR and is a transcriptional key regulator of cell growth, differentiation and apoptosis.4 In vitro models have demonstrated that natural and synthetic PPARγ ligands [e.g. pioglitazone (PGZ)] suppressed clonogenic growth of AML and their combination with ATRA synergistically induced myeloid cell differentiation.5,6 We herein report that, in a small cohort of 5 elderly AML patients, a novel biomodulatory therapy with low-dose AZA combined with PGZ, and ATRA (APA) induced complete molecular remissions in primary chemorefractory disease.
Five elderly AML patients were treated after informed consent with AZA, PGZ, and ATRA (APA) on a compassionate-use basis. Treatment was performed in the absence of alternative therapeutic options outside a clinical trial. Four patients were refractory to standard induction and/or high-dose cytarabine treatment (Patients 1–4) and one patient had progressed from chronic myelomonocytic leukemia (CMML)-2 to AML during standard-dose AZA treatment (Patient 5). Patients were not eligible for allogeneic hematopoietic stem-cell transplantation because of age, comorbidities or lack of suitable donors. Patients’ and disease characteristics are summarized in Table 1. The treatment consisted of low-dose AZA 75 mg/day given subcutaneously from day 1–7, PGZ 45 mg/day per os, and ATRA 45 mg/m2/day per os. Subsequent cycles of AZA were administered at an interval of 28 days or later after hematologic recovery. PGZ and ATRA were given continuously from day 1 on. Treatment response was frequently monitored and remission was defined according to standard criteria.7 Data analysis was performed in a retrospective manner.
Table 1.
Patients’ characteristics and response.
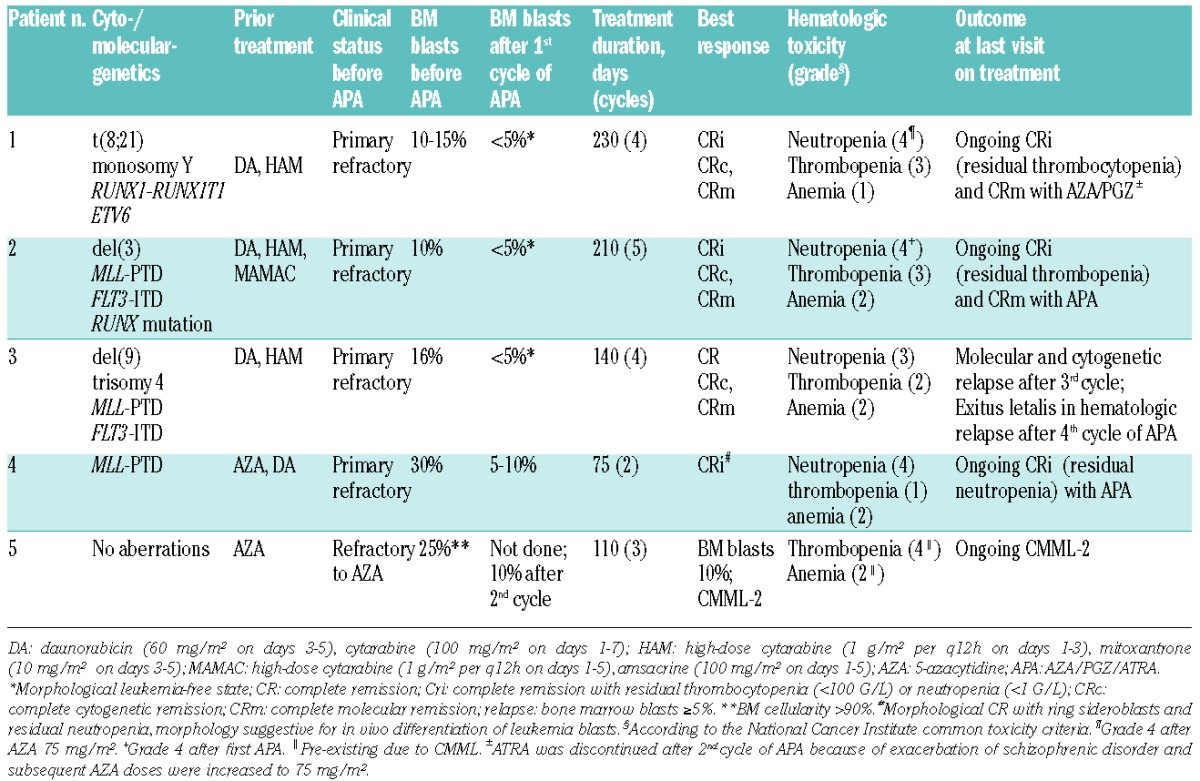
Patient 1 was a 65-year old man who started APA treatment after induction failure to daunorubicin/cytarabine (DA) and high-dose cytarabine/mitoxantrone (HAM). He achieved complete remission (CR) with residual thrombocytopenia (CRi) and cytogenetic CR (CRc) after the first cycle and molecular CR (CRm) after the second cycle of therapy. Because of exacerbation of his pre-existing schizophrenic disorder, ATRA was discontinued on day 43 (after cycle 2) and hence further AZA doses were increased to 75 mg/m2. The patient remained in CRm at last visit under AZA/PGZ treatment for 7.5 months after start of APA (Table 1). Patient 2 was a 64-year old man with secondary AML and resistance to three different induction therapies (Table 1). During APA therapy he achieved a rapid and sustained CRc and CRm (MLL-ratio, 0.320 and FLT3-ITD negative after third APA cycle) (Figure 1A). At last visit, the patient remained in transfusion-independent CRi (minimum platelet count 50 × 109/L) and CRm for seven months after start of APA. White blood cell and neutrophil counts during APA treatment are shown in Figure 1B.
Figure 1.
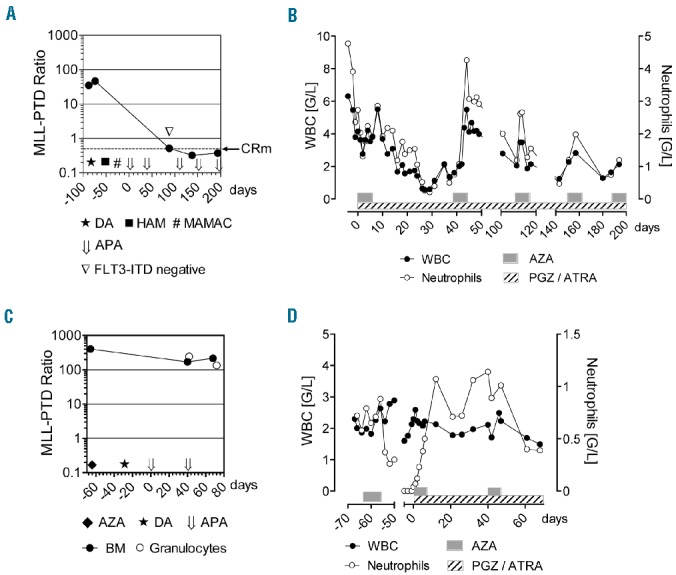
Response to APA treatment. (A) Molecular response of Patient 2 during chemotherapies with daunorubicin/cytarabine (★, DA), high-dose cytarabine/mitoxantrone (■, HAM), high-dose cytarabine/amsacrine (#,MAMAC), and biomodulatory therapy with AZA/PGZ/ATRA (⇓, APA). APA was started in a primary refractory situation with BM blasts of 10%. MLL-PTD ratio from BM (•), was monitored at the indicated time points. In addition, FLT3-ITD transcripts were not detectable in PCR performed from BM on day 88 (▽). The dotted line indicates the threshold of complete molecular remission (CRm; MLL-PTD ratio 0.05). MLL-PTD ratio was calculated as copies MLL-PTD/copies ABL-control. (B) White blood cell (WBC) and neutrophil counts of Patient 2 during 5 cycles of APA therapy. (C) MLL-PTD ratio from BM (•) and purified peripheral blood granulocytes (❍) of Patient 4 during prior therapies with AZA (75 mg/m2) and daunorubicine/cytarabine (DA), and during therapy with APA. (D) WBC and neutrophil counts of Patient 4 during AZA monotherapy (75 mg/m2) and APA combination therapy.
Patient 3 was a 71-year old man refractory to induction treatment with DA and HAM. The patient obtained CRc and CRm (MLL-ratio 0.262) after the first cycle of APA. However, after the fourth course of therapy, the patient had hematologic relapse and died of AML progression.
Patient 4 was a 70-year old woman with therapy-related AML after long-term azathioprine treatment because of active lupus erythematosus. Primary diagnosis was myelodysplastic syndrome (MDS) [i.e. refractory anemia with excess blasts (RAEB-II)] and treatment was started with AZA (75 mg/m2). After a first course of AZA, the RAEB-II progressed to AML. Standard chemotherapy with DA was initiated which resulted in a slight reduction in bone marrow (BM) blasts from 45% to 30% (Table 1). After two cycles of APA, morphological CR with detection of ring sideroblasts was achieved. However, MLL-PTD ratio in BM did not decline significantly (Figure 1C). During APA treatment, morphological features of granulocytes in peripheral blood were suggestive of in vivo differentiation of AML blasts and, therefore, we quantified MLL-PTD ratio from isolated granulocytes during APA. Granulocytes were isolated from peripheral blood by autofluorescence-based fluorescence-activated cell sorting (FACS) and MLL-PTD transcripts were subsequently quantified by real-time polymerase chain reaction (PCR) from purified RNA. Granulocytic MLL-PTD ratio (243.5) was comparable to the ratios in peripheral blood (183.3) and BM (169.9) collected after the first cycle of APA and did not decline significantly after the second cycle of APA (134.6) (Figure 1C). This result suggested that the early increase in numbers of granulocytes (Figure 1B and D) observed during APA treatment included a considerable fraction of differentiation-induced leukemia blasts, which might be similar to the differentiation effect described in APL during ATRA therapy. Interestingly, in Patient 4, clinical symptoms of lupus erythematosus improved during APA therapy.8
Patient 5 was a 55-year old man with CMML-2 who started standard-dose AZA (75 mg/m2, day 1–7) therapy when BM blasts increased to 15%–20%. After the third course of AZA monotherapy, the CMML-2 transformed to AML with leukocyte counts in peripheral blood up to 60 × 109/L (30% blasts in differential). The patient also developed intracerebral bleeding, so that initially only cytoreductive therapy with hydroxyurea was feasible. Hydroxyurea treatment was stopped when leukocyte counts had decreased to below 5 × 109/L. Because of the recent bleeding history, we started APA therapy instead of standard induction chemotherapy with daunorubicin/cytarabine. At start of APA treatment, BM histology showed a very high cellularity (>90%) and blasts of 25%. After the second cycle of APA, the patient went back into CMML-2 with BM blasts of 10% and remained in stable disease at last visit (Table 1).
Compared with conventional myelosuppressive chemotherapy regimens, APA treatment was associated with much lower toxicity and could be easily administered in an outpatient setting. The most common APA-related adverse events were hematologic toxicities grade 1–4 (Table 1) without the need for platelets or erythrocyte transfusions or administration of growth factors. In cases of therapy-related neutropenia (grade 3/4) or thrombocytopenia (grade 3), the next cycle of AZA was postponed until recovery (median 42 days; range 28–71). Severe adverse events included an urinary tract infection with Klebsiella pneumoniae in Patient 3 (at time of hematologic relapse) and rectal ulceration in Patient 4, which recovered during acyclovir treatment.
Combined transcriptional targeting by PPARγ and RAR ligands as well as AZA treatment have been shown to induce myeloid differentiation and to attenuate leukemia cell growth.4,9–11 The induction of molecular CR, strong myeloid differentiation in leukemia blasts (Figure 1C) and in residual hematopoiesis (Figure 1B and data not shown) during APA treatment emphasizes our hypothesis that combined biomodulation with APA might act synergistically on leukemic differentiation and growth control. Previous data had already suggested that metronomic biomodulatory therapy approaches differentially target hallmarks of cancer, i.e. angiogenesis, inflammation and anticancer immune response.12,13 Despite the heterogeneity of AML characteristics in our cohort, APA therapy induced ongoing morphological CR in 3 of 5 patients, including 2 molecular CRs. In one patient with secondary AML transformed from CMML-2, AML blasts decreased during APA treatment. Four of the 5 patients were pre-treated with intensive standard chemotherapy and in all patients the proportion of leukemia blasts in BM did not exceed 30% at start of APA therapy. As tumor burden and adjacent stroma cells14 might be important factors for clinically relevant APA activity, we will investigate in a proposed clinical trial if APA treatment is also effective in patients with subtotal leukemia cell infiltration of BM. As recently shown, biomodulatory combination therapies are widely applicable in metastatic tumor disease and have the capacity to control metastatic lesions and to induce CRs in single patients.13 By targeting the physical constituents (rationalizations) of the hallmarks of cancer with “top-down” approaches, we may bypass (molecular-)genetic heterogeneity in chemorefractory AML. To our knowledge, this is the first report describing the combined use of low-dose AZA, PGZ and ATRA to successfully overcome resistance to induction chemotherapy. The rapid decrease in BM blasts even after the first APA cycle, as well as clinical effectiveness, in AZA refractory Patients 4 and 5 argues against a mere anti-leukemic effect mediated by low-dose AZA.15 These promising data with APA combination therapy warrant further investigation in clinical trials including patients with chemorefractory AML or patients who do not qualify for allogeneic transplantation.
Acknowledgments
we thank Lucia Schwarzfischer (University Hospital of Regensburg, Germany) for assistance concerning granulocyte sorting and RNA extraction.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Al-Ali HK, Jaekel N, Junghanss C, Maschmeyer G, Krahl R, Cross M, et al. Azacitidine in patients with acute myeloid leukemia medically unfit for or resistant to chemotherapy: a multicenter phase I/II study. Leuk Lymphoma. 2012;53(1):110–7. [DOI] [PubMed] [Google Scholar]
- 2.Maurillo L, Venditti A, Spagnoli A, Gaidano G, Ferrero D, Oliva E, et al. Azacitidine for the treatment of patients with acute myeloid leukemia: report of 82 patients enrolled in an Italian Compassionate Program. Cancer. 2012;118(4):1014–22. [DOI] [PubMed] [Google Scholar]
- 3.Ivanoff S, Gruson B, Chantepie SP, Lemasle E, Merlusca L, Harrivel V, et al. 5-Azacytidine treatment for relapsed or refractory acute myeloid leukemia after intensive chemotherapy. Am J Hematol. 2013;88(7):601–5. [DOI] [PubMed] [Google Scholar]
- 4.Tabe Y, Konopleva M, Andreeff M, Ohsaka A. Effects of PPARgamma Ligands on Leukemia. PPAR Res. 2012;2012:483656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yasugi E, Horiuchi A, Uemura I, Okuma E, Nakatsu M, Saeki K, et al. Peroxisome proliferator-activated receptor gamma ligands stimulate myeloid differentiation and lipogenensis in human leukemia NB4 cells. Dev Growth Differ. 2006;48(3):177–88. [DOI] [PubMed] [Google Scholar]
- 6.Hui H, Chen Y, Yang H, Zhao K, Wang Q, Zhao L, et al. Oroxylin A has therapeutic potential in acute myelogenous leukemia by dual effects targeting PPARγ and RXRα. Int J Cancer. 2014; 134(5):1195–206. [DOI] [PubMed] [Google Scholar]
- 7.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–9. [DOI] [PubMed] [Google Scholar]
- 8.Vogt T, Coras B, Hafner C, Landthaler M, Reichle A. Antiangiogenic therapy in metastatic prostate carcinoma complicated by cutaneous lupus erythematodes. Lancet Oncol. 2006;7(8):695–7. [DOI] [PubMed] [Google Scholar]
- 9.Curik N, Burda P, Vargova K, Pospisil V, Belickova M, Vlckova P, et al. 5-azacitidine in aggressive myelodysplastic syndromes regulates chromatin structure at PU.1 gene and cell differentiation capacity. Leukemia. 2012;26(8):1804–11. [DOI] [PubMed] [Google Scholar]
- 10.Fujiki A, Imamura T, Sakamoto K, Kawashima S, Yoshida H, Hirashima Y, et al. All-trans retinoic acid combined with 5-Aza-2′-deoxycitidine induces C/EBPα expression and growth inhibition in MLL-AF9-positive leukemic cells. Biochem Biophys Res Commun. 2012;428(2):216–23. [DOI] [PubMed] [Google Scholar]
- 11.Faber K, Bullinger L, Ragu C, Garding A, Mertens D, Miller C, et al. CDX2-driven leukemogenesis involves KLF4 repression and deregulated PPARgamma signaling. J Clin Invest. 2013;123(1):299–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheng Sow H, Mattarollo SR. Combining low-dose or metronomic chemotherapy with anticancer vaccines: A therapeutic opportunity for lymphomas. Oncoimmunology. 2013;(12):e27058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reichle A, Vogt T. Systems biology: a therapeutic target for tumor therapy. Cancer Microenviron. 2008;1(1):159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker PS. Dependence of acute myeloid leukemia on adhesion within the bone marrow microenvironment. Scientific World Journal. 2012;2012:856467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Craddock C, Quek L, Goardon N, Freeman S, Siddique S, Raghavan M, et al. Azacitidine fails to eradicate leukemic stem/progenitor cell populations in patients with acute myeloid leukemia and myelodysplasia. Leukemia. 2013;27(5):1028–36. [DOI] [PubMed] [Google Scholar]


