The molecular heterogeneity of acute myeloid leukemia (AML) underlies the wide variation in responses to standard therapy. This heterogeneity occurs at multiple regulatory steps affecting cell survival and proliferation.1 We identified overexpression of the eukaryotic translation initiation factor 4E (eIF4E) as a targetable aberrancy in all examined cases of M4 and M5 FAB (French, American and British classification) AML subtypes, as well as in some M0, M1, and M2 subtypes.2 eIF4E is both over-expressed and highly enriched in the nucleus of these specimens. eIF4E acts in nuclear mRNA export and translation of specific transcripts necessary for the promotion of proliferation, survival and metastases.3,4 These eIF4E functions depend on its binding the m7G cap on the 5′ end of mRNAs.3,4 Use of ribavirin, a competitive inhibitor of the m7G cap, impairs the biochemical and oncogenic functions of eIF4E.5,6 The first clinical trial to directly target eIF4E activity used ribavirin in AML patients with elevated eIF4E who were unfit for induction chemotherapy or who had relapsed disease.7 Complete and partial responses were observed, and responding patients demonstrated a reduction in overall levels of eIF4E, loss of its nuclear localization, and impaired production of eIF4E targets.7
In vitro combination studies with ribavirin and cytarabine showed an added impairment in cell growth in primary AML patient samples.8 Given these findings, we combined ribavirin po BID continuous dosing with low-dose cytarabine (LDAC) sc BID for ten days every 28 days in a phase I trial following a “3+3” design. The primary objective was to determine the recommended phase II dose (RP2D) of the combination based on pharmacokinetics and safety. The secondary objectives revealed predicted cellular changes in eIF4E but also a novel mechanism of resistance to ribavirin and cytarabine.9 Twenty-nine patients with elevated eIF4E and who were unsuitable for induction chemotherapy or with relapsed/refractory disease were enrolled. Nine of these had not received induction with “7+3” for their AML (median age 70 years) and 3 had received prior therapy for myelodysplastic syndrome (MDS) (Table 1). The combination was generally well tolerated with no unexpected adverse events. Hemolytic anemia, a known ribavirin toxicity, was seen in 4 patients (14%), and was uncontrolled by dose reduction but resolved after ribavirin was discontinued.
Table 1.
Patients’ characteristics.
In the first dose escalation, four dose levels of ribavirin (1000, 1400, 1800 and 2200 mg BID) were combined with LDAC 20 mg BID. At a ribavirin dose of 2200 mg BID, there were 2 dose-limiting toxicities (DLTs) and no further dose escalation was performed. Importantly, the mean maximum ribavirin plasma levels were below 20 μM for all dose levels (Online Supplementary Figure S1). This was in contrast to levels above 20 μM seen with monotherapy, where patients were treated with ribavirin 1000 mg BID. Interestingly, Patient 1 had a doubling of ribavirin serum levels once LDAC was reduced to 10 mg BID for toxicity during cycle 2 (Figure 1). Thereafter, this patient achieved a complete remission (CR) (with incomplete red cell recovery), and molecular targeting of eIF4E, as seen by re-localization to the cytoplasm. This patient remained on ribavirin therapy for two years with LDAC discontinued on day 179.
Figure 1.
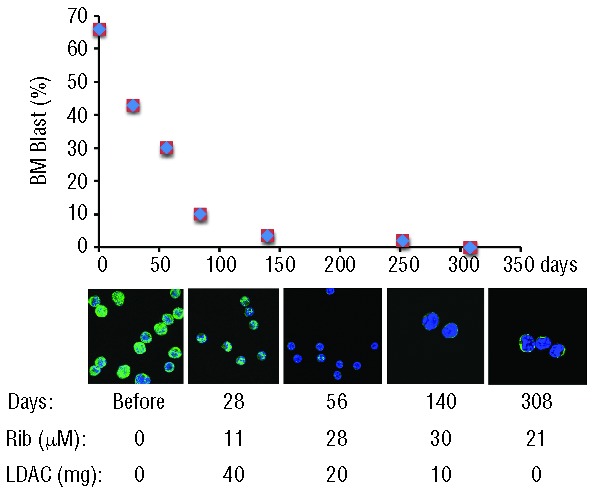
(Top) Patient 1 percentage bone marrow blast counts versus days of treatment. (Middle) Confocal micrographs indicating alterations in eIF4E (green) subcellular distribution as a function of ribavirin treatment indicating molecular response. DAPI, a nuclear marker, is shown in blue. Magnification was 100X with a further 2X zoom for days 140 and 308. Blasts were sorted using flow cytometry gating on CD45 dim and side scatter, as described in the Methods. Note that cells sorted from specimens obtained during CR are likely not leukemic blasts but rather normal primitive progenitors. Note that at lower ribavirin concentrations, 11 μM at 28 days, there was no targeting of eIF4E, but at higher levels of ribavirin, eIF4E re-localization is observed. (Lower) Ribavirin plasma levels (micromolar) were determined by mass spectrometry and total daily dose of LDAC is given below. Patient 1 discontinued LDAC at day 179, as per protocol.
Given our observations of Patient 1, and the failure to achieve higher plasma levels of ribavirin in the higher dose cohorts, we carried out a second ribavirin dose escalation in the presence of LDAC 10 mg BID. This led to increased ribavirin plasma levels overall (Online Supplementary Figure S1). The median maximum level of ribavirin when given with LDAC 10 mg BID was 23 μM (range 6–37 μM, n=9), more than double that observed at LDAC 20 mg BID, which was 11.5 μM (range 2–33 μM, n=10). Increasing doses of ribavirin beyond 1400 mg BID did not result in increased serum steady state levels (Online Supplementary Figure S1). No DLTs were observed in this dose escalation. Thus, the recommended phase II dose (RP2D) was determined to be ribavirin 1400 mg BID and LDAC 10 mg BID.
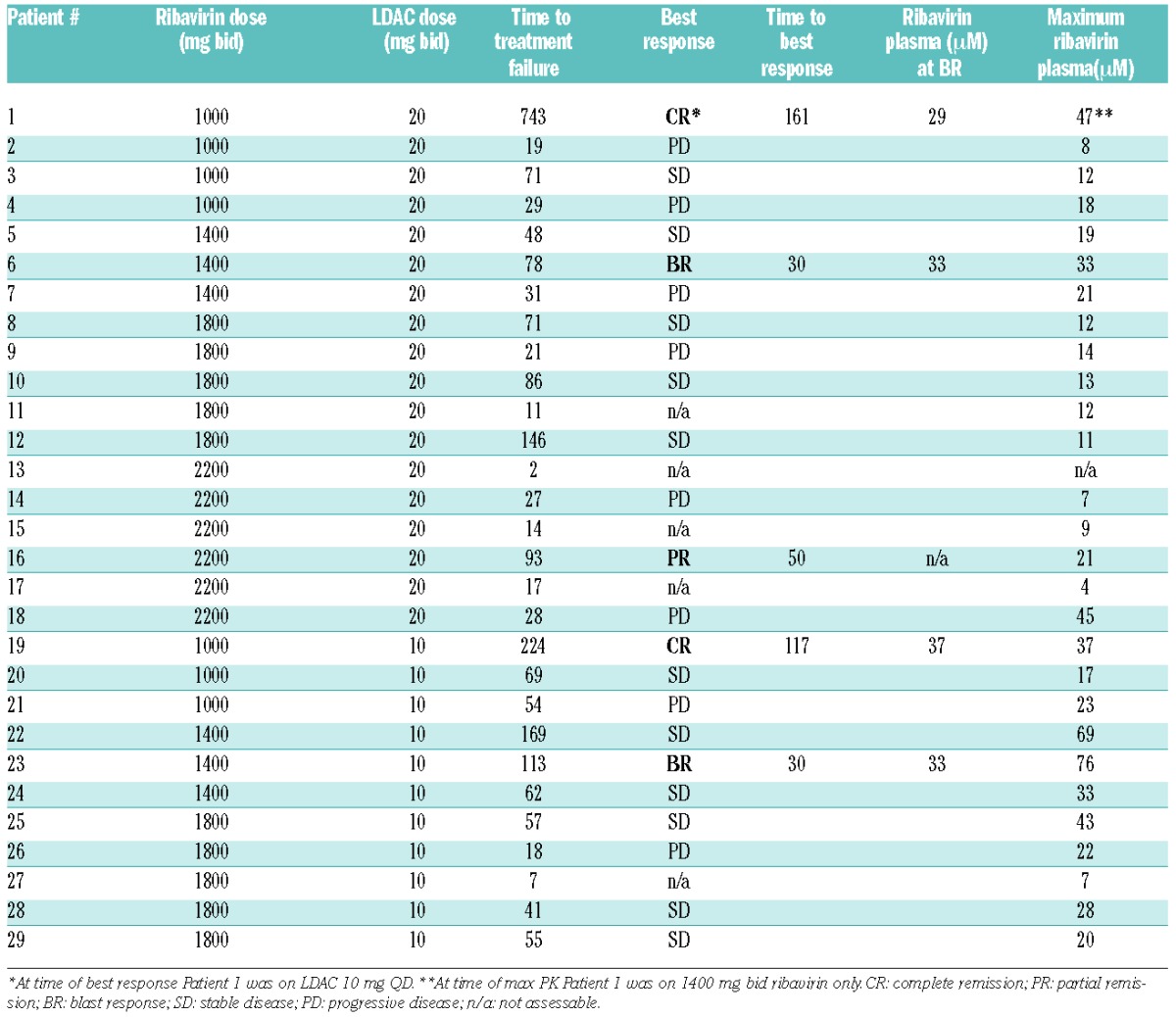
Twenty-one patients treated for 28 or more days were evaluable for response. There were 2 CRs, one partial remission (PR), and 2 blast responses (BR) (Table 2). Responding patients had a median ribavirin plasma level of 33 μM at best response whereas non-responders had a median maximum ribavirin plasma level of 19 μM. In total, 14 patients had a maximum plasma level of ribavirin more than 20 μM, and all 5 responding patients were in this group. The addition of LDAC to ribavirin tended to increase the median time to treatment failure from 104 days for the monotherapy trial (range 93–263 days, n=3) to 225 days (range 96–743 days, n=3), although the number of patients included in both trials is small. Patients with adverse cytogenetics were less likely to respond. No difference in FLT3 ITD/mutation or NPM1 mutation among responders and non-responders was observed.
Table 2.
Response and ribavirin plasma levels.
Clinical response correlated with molecular targeting of eIF4E. Targeting was determined by changes in eIF4E mRNA levels and eIF4E protein re-localization to the cytoplasm. Protein levels of eIF4E and its downstream targets were assessed when sufficient material was available. No targeting of eIF4E was observed among patients with progressive disease (PD). Six patients had a full molecular response with both lower eIF4E levels and eIF4E re-localization (Online Supplementary Table S1) including the patients who achieved CR, PR, BR (Table 2). All had 20+ μM maximum plasma levels of ribavirin. Patients 3 and 10 had a partial molecular response, whereby they did not have full relocalization of eIF4E. For Patient 3, determination of ribavirin plasma levels was inaccurate because of frequent dose interruptions due to hemolytic anemia. Of the 3 patients (Patients 3, 10 and 22) with partial or full molecular response who did not achieve PR, CR or BR, all showed a decrease in blast count and/or hematologic improvement. Levels above 20 μM ribavirin were also observed in some non-responders, indicating that ribavirin level alone did not predict response. At relapse, all 6 patients with complete eIF4E targeting showed eIF4E re-localization back to the nucleus and elevated eIF4E levels consistent with the loss of clinical activity (Online Supplementary Table S1). Similarly, the partial molecular response was lost by Patients 3 and 10 at progression.
In our previous monotherapy trial, we noted upon clinical relapse an increase in the levels of the sonic hedgehog transcription factor Gli1, which led to glucuronidation of ribavirin, loss of the eIF4E-ribavirin interaction, and ultimately drug resistance.9 Additionally, primary refractory and a few relapsed patients had markers of impaired drug uptake with low levels of the ribavirin transporter (ENT1) and/or an enzyme required for the pro-drug metabolism of ribavirin, adenosine kinase (ADK).9 We examined these resistance markers here (Online Supplementary Table S1). For 5 of 27 patients, we observed lowered ADK and/or ENT1 mRNA and/or protein levels at baseline relative to healthy volunteers, suggesting that ribavirin pro-drug metabolism and drug uptake were impaired. None of these 5 patients responded to treatment. An additional 3 of 18 and 6 of 18 patients had reduced ADK or ENT1 levels at relapse/EOT relative to before treatment, respectively. For 14 of 18 patients, we observed an elevation in Gli1 and/or UDP-glucunosyltransferase 1A (UGT1A) at the end of treatment, relative to before treatment. In our resistance studies, elevated Gli1 correlated with increased UGT1A protein levels, ribavirin and cytarabine glucuronidation and drug resistance.9 Finally, as in our monotherapy trial, many non-responding patients had elevated Gli1 or low ADK/ENT1 levels underlying their primary resistance. Thus, these patients likely did not respond due to inactive drug. Some patients had markers of both Gli1/UGT1A and ADK/ENT1 mediated resistance.
In summary, we identified the RP2D of ribavirin and LDAC, 1400 mg po BID continuous dosing and 10 mg sc BID for ten days of a 28-day cycle, respectively. This combination is well tolerated, with some patients achieving marked clinical responses. Our results indicate that ribavirin plasma levels are reduced in the presence of LDAC, which impairs the absorption of ribavirin, as was shown with other drugs.11 In addition, LDAC may interfere with ribavirin activity, as patients in the prior monotherapy trial responded molecularly and clinically to ribavirin at lower serum levels.7 Nonetheless, once higher levels of ribavirin were achieved the combination with LDAC may have yielded longer time on study compared with ribavirin alone for patients who achieved remission. A phase II study is needed to determine the rate and duration of response for this combination. Importantly, cellular changes in eIF4E required plasma levels of ribavirin above 20 μM in the presence of LDAC, supporting our clinical observation that this level is associated with response.
Tracking eIF4E targeting, ENT1, ADK, Gli1, UGT1A as well as ribavirin and cytarabine uptake likely predict response and relapse, respectively. We will test the efficacy of overcoming Gli1 inducible drug glucuronidation in an AML trial combining a Gli1 inhibitor with ribavirin (clinicaltrials.gov identifier:02073838). The development of additional drug combinations is also important. Our previous ex vivo AML studies suggest that combinations of eIF4E with azacytidine could be useful.8 Furthermore, augmenting the inhibitory effects of ribavirin on eIF4E using Mnk kinase inhibitors or rapalogs could be effective. In summary, our second clinical trial targeting eIF4E with ribavirin led to clinical responses and highlighted the importance of monitoring resistance markers in AML.
Acknowledgments
we are grateful to Morris and David Goodman and Pharmascience Inc. for producing and providing ribavirin. We thank nursing and supporting staff at all the clinical sites and IRIC.
Footnotes
Funding: KLBB is supported by funds from the NIH (RO1 80728 and 98571), IRICoR and Translational Research Program grants from the Leukemia and Lymphoma Society USA. SA holds an FRSQ Clinician Scientist Award. KLBB holds a Canada Research Chair and HAZ holds a Cole Foundation Fellowship and a CNRS Lebanon Fellowship.
The online version of this article has a Supplementary Appendix.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Cancer Genome Atlas Research N. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013; 368(22):2059–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carroll M, Borden KL. The oncogene eIF4E: using biochemical insights to target cancer. J Interferon Cytokine Res. 2013;33(5):227–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Culjkovic B, Tan K, Orolicki S, Amri A, Meloche S, Borden KL. The eIF4E RNA regulon promotes the Akt signaling pathway. J Cell Biol. 2008;181(1):51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Culjkovic B, Topisirovic I, Skrabanek L, Ruiz-Gutierrez M, Borden KL. eIF4E promotes nuclear export of cyclin D1 mRNAs via an element in the 3′UTR. J Cell Biol. 2005;169(2):245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kentsis A, Topisirovic I, Culjkovic B, Shao L, Borden KL. Ribavirin suppresses eIF4E-mediated oncogenic transformation by physical mimicry of the 7-methyl guanosine mRNA cap. Proc Natl Acad Sci USA. 2004;101(52):18105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kentsis A, Volpon L, Topisirovic I, Soll CE, Culjkovic B, Shao L, et al. Further evidence that ribavirin interacts with eIF4E. RNA. 2005; 11(12):1762–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Assouline S, Culjkovic B, Cocolakis E, Rousseau C, Beslu N, Amri A, et al. Molecular targeting of the oncogene eIF4E in acute myeloid leukemia (AML): a proof-of-principle clinical trial with ribavirin. Blood. 2009;114(2):257–60. [DOI] [PubMed] [Google Scholar]
- 8.Kraljacic BC, Arguello M, Amri A, Cormack G, Borden K. Inhibition of eIF4E with ribavirin cooperates with common chemotherapies in primary acute myeloid leukemia specimens. Leukemia. 2011; 25(7):1197–200. [DOI] [PubMed] [Google Scholar]
- 9.Zahreddine HA, Culjkovic-Kraljacic B, Assouline S, Gendron P, Romeo AA, Morris SJ, et al. The sonic hedgehog factor GLI1 imparts drug resistance through inducible glucuronidation. Nature. 2014; 511(7507):90–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116(3):354–65. [DOI] [PubMed] [Google Scholar]
- 11.Kuhlmann J. Inhibition of digoxin absorption but not of digitoxin during cytostatic drug therapy. Arzneimittel-Forschung. 1982; 32(6):698–704. [PubMed] [Google Scholar]


