Abstract
Bortezomib-dexamethasone is widely used for relapsed myeloma in routine clinical practice, but comparative data versus single-agent bortezomib are lacking. This retrospective analysis compared second-line treatment with bortezomib-dexamethasone and bortezomib using 109 propensity score-matched pairs of patients treated in three clinical trials: MMY-2045, APEX, and DOXIL-MMY-3001. Propensity scores were estimated using logistic regression analyses incorporating 13 clinical variables related to drug exposure or clinical outcome. Patients received intravenous bortezomib 1.3 mg/m2 on days 1, 4, 8, and 11, in 21-day cycles, alone or with oral dexamethasone 20 mg on the days of/after bortezomib dosing. Median bortezomib cumulative dose (27.02 and 28.60 mg/m2) and treatment duration (19.6 and 17.6 weeks) were similar with bortezomib-dexamethasone and bortezomib, respectively. The overall response rate was higher (75% vs. 41%; odds ratio=3.467; P<0.001), and median time-to-progression (13.6 vs. 7.0 months; hazard ratio [HR]=0.394; P=0.003) and progression-free survival (11.9 vs. 6.4 months; HR=0.595; P=0.051) were longer with bortezomib-dexamethasone versus bortezomib, respectively. Rates of any-grade adverse events, most common grade 3 or higher adverse events, and discontinuations due to adverse events appeared similar between the groups. Two patients per group died of treatment-related adverse events. These data indicate the potential benefit of bortezomib-dexamethasone compared with single-agent bortezomib at first relapse in myeloma. The MMY-2045, APEX, and DOXIL-MMY-3001 clinical trials were registered at, respectively, clinicaltrials.gov identifier: 00908232, 00048230, and 00103506.
Introduction
The activity of the proteasome inhibitor bortezomib as a single agent in relapsed and/or refractory multiple myeloma (MM) has been established in the phase III APEX study1,2 and in the phase III DOXIL-MMY-3001 study.3 Overall response rates (ORR) with single-agent bortezomib in patients with MM who had received 1–3 prior lines of therapy were 41–43%, including 2–9% complete responses (CR), the median time-to-progression (TTP) was 6.2–6.5 months, and the median overall survival (OS) was 29.8 months.1–3 Multiple phase II and phase III studies have also demonstrated the activity of bortezomib with or without dexamethasone in the setting of relapsed and/or refractory MM.4–11 Results from several of these studies have suggested that adding dexamethasone to bortezomib can improve response rates in patients with suboptimal response to bortezomib alone.4–6,8,9 Bortezomib in combination with dexamethasone is widely used in routine clinical practice in the relapsed setting, as noted in the 2013 European Society for Medical Oncology Clinical Practice Guidelines.12 The combination of bortezomib and dexamethasone is also listed among the preferred regimens for salvage therapy in the US National Comprehensive Cancer Network Clinical Practice Guidelines for MM.13 However, phase II data reporting on combination treatment with bortezomib-dexamethasone from first relapse in MM have only recently become available,14,15 and prospective, randomized comparative data for bortezomib-dexamethasone versus single-agent bortezomib in relapsed and/or refractory MM are lacking.
In the absence of such data, we adopted an alternative approach for evaluating the benefit of adding dexamethasone to bortezomib from first relapse in MM. Using patient-level data from a phase II study of bortezomib-dexamethasone,14 and from the single-agent bortezomib arms of two phase III studies,1,3 propensity score-matched pairs of patients were identified for a retrospective analysis of the efficacy and safety of second-line treatment with bortezomib-dexamethasone versus single-agent bortezomib. The matched-pair methodology was used to control for differences in base-line characteristics and to allow for meaningful cross-study comparisons.
Methods
Patients, treatment, and assessments
Patient-level data from three industry-sponsored studies of bortezomib and bortezomib-dexamethasone in relapsed and/or refractory MM were used. Data were included from the non-randomized cohort of patients treated with bortezomib-dexamethasone in the phase II MMY-2045 study (clinicaltrials.gov identifier: 00908232)14 and from patients randomized to single-agent bortezomib in the phase III APEX study of bortezomib versus high-dose dexamethasone (clinicaltrials.gov identifier: 00048230)1 and phase III DOXIL-MMY-3001 study of bortezomib plus liposomal doxorubicin versus bortezomib alone (clinicaltrials.gov identifier: 00103506),3 the latter representing all the patient-level data on single-agent bortezomib from randomized studies accessible to the sponsor. Independent ethics committees/institutional review boards approved the trials, which were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed consent.
Except for 2 patients, all patients in the MMY-2045 non-randomized cohort had received one prior line of therapy. Therefore, for this analysis, involving the generation of matched pairs of patients who had been treated with bortezomib-dexamethasone and single-agent bortezomib, only patients with one prior line in MMY-2045, APEX, and DOXIL-MMY-3001 were considered. Thus, 142 patients from MMY-2045 and 242 patients from APEX and DOXIL-MMY-3001 who had received one prior line of therapy were used for identification of matched pairs. All patients were bortezomib-naïve.
Responses were assessed by International Myeloma Working Group uniform criteria16 in MMY-2045 and European Group for Blood and Marrow Transplantation criteria17 in APEX and DOXIL-MMY-3001. Adverse events (AEs) were graded according to either the National Cancer Institute (NCI) Common Toxicity Criteria Version 2.0 or the NCI Common Terminology Criteria for Adverse Events (CTCAE) Version 3.0.
Matched-pairs analysis
For identification of variables to be included in a propensity score model for the matched-pairs analysis, a systematic approach was used following published guidelines.18–20 All base-line parameters consistently collected across the three studies were identified. These parameters were then categorized as being related to study drug exposure, related to clinical outcome, or not related to either, based on the current literature.21–30 Our initial comprehensive approach for the matched-pairs analysis was to include all identified variables, of which 13 were identified as being either exposure- or outcome-related: age; body surface area; Eastern Cooperative Oncology Group (ECOG) score; type of myeloma; percent of plasma cells in the bone marrow; presence of extramedullary plasmacytomas; prior stem cell transplantation; prior exposure to immunomodulatory drugs (IMiDs); prior exposure to dexamethasone; hemoglobin level; platelet count; creatinine clearance; and albumin level. Details of the propensity score model for identifying matched patient pairs are provided in the Online Supplementary Appendix.
A second, confirmatory matched-pairs analysis was conducted using the same methodology, but in which the propensity score model was estimated using only 8 of the 13 identified variables – those mainly associated with clinical outcome (i.e. of prognostic importance in myeloma): age; ECOG score; type of myeloma; percent of plasma cells in the bone marrow; prior exposure to dexamethasone; hemoglobin level; creatinine clearance; and albumin level.
Statistical analysis of response rates and time-to-event outcomes in the treatment groups, and details of the sensitivity analyses performed, are provided in the Online Supplementary Appendix.
Results
Matched pairs of patients
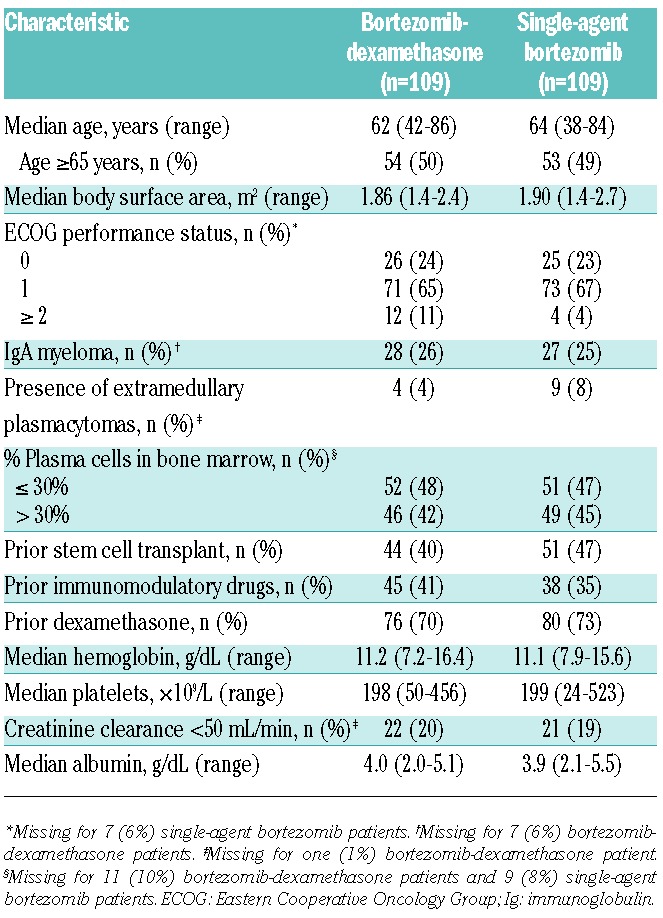
From the 384 patients included in this analysis, a total of 109 matched pairs of patients (n=218) treated with bortezomib-dexamethasone and single-agent bortezomib were identified by the first propensity score matching model. The base-line characteristics of these matched pairs of patients are summarized in Table 1. As expected, the groups were well balanced with respect to these variables, which were used in the propensity score matching model. The median time since initial diagnosis was 2.7 years (range 0.2–11.2 years) in the bortezomib-dexamethasone group and 2.0 years (range 0.4–24.9 years) in the single-agent bortezomib group.
Table 1.
Base-line characteristics of patients in the 109 matched pairs identified by propensity score matching.
Exposure to bortezomib was similar between groups. Median duration of treatment was 19.6 versus 17.6 weeks in the bortezomib-dexamethasone and single-agent bortezomib groups, respectively. The respective median cumulative doses of bortezomib were 27.02 mg/m2 (range 1.3–41.7 mg/m2) and 28.60 mg/m2 (range 2.4–59.1 mg/m2). In the bortezomib-dexamethasone group, patients received a median cumulative dose of dexamethasone of 880 mg (range 20–1280 mg).
Efficacy
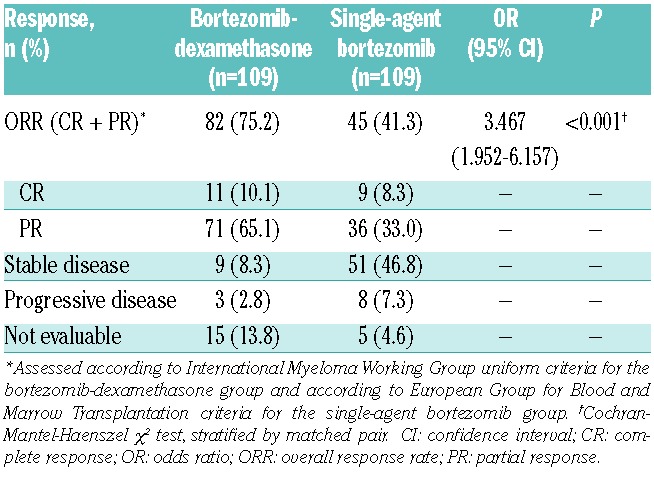
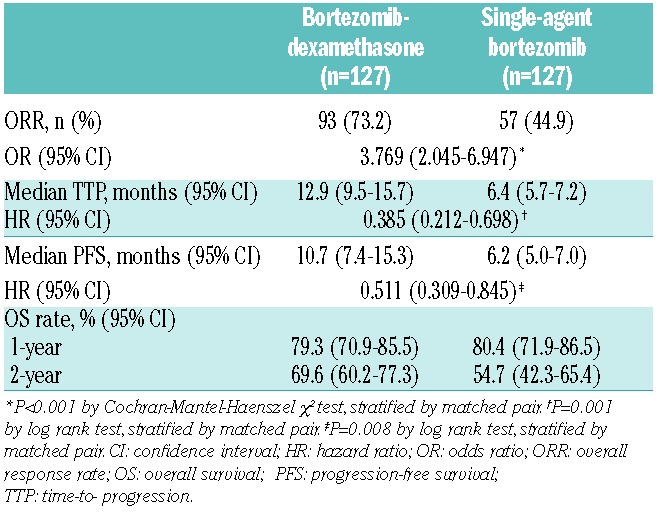
The ORR was significantly higher with bortezomib-dexamethasone versus single-agent bortezomib, at 75% versus 41% (odds ratio [OR] = 3.467; P<0.001) (Table 2). The CR rate appeared similar at 10% versus 8%.
Table 2.
Response rates with bortezomib-dexamethasone and single-agent bortezomib in the matched-pairs analysis.
Time-to-progression was significantly longer, and there was a trend to longer progression-free survival (PFS), with bortezomib-dexamethasone versus single-agent bortezomib (Figure 1A and B). With 47% and 49% of patients having progressed, respectively, the median TTP was 13.6 versus 7.0 months with bortezomib-dexamethasone versus single-agent bortezomib, with a hazard ratio (HR) of 0.394 (P=0.003). With 57% and 53% of patients having progressed or died, respectively, median PFS was 11.9 versus 6.4 months, with an HR of 0.595 (P=0.051) in favor of bortezomib-dexamethasone. In the additional analysis of PFS with stratification by propensity score quintile instead of by matched pair, the HR was 0.539 (95% confidence interval [CI]: 0.365–0.796; P=0.002).
Figure 1.
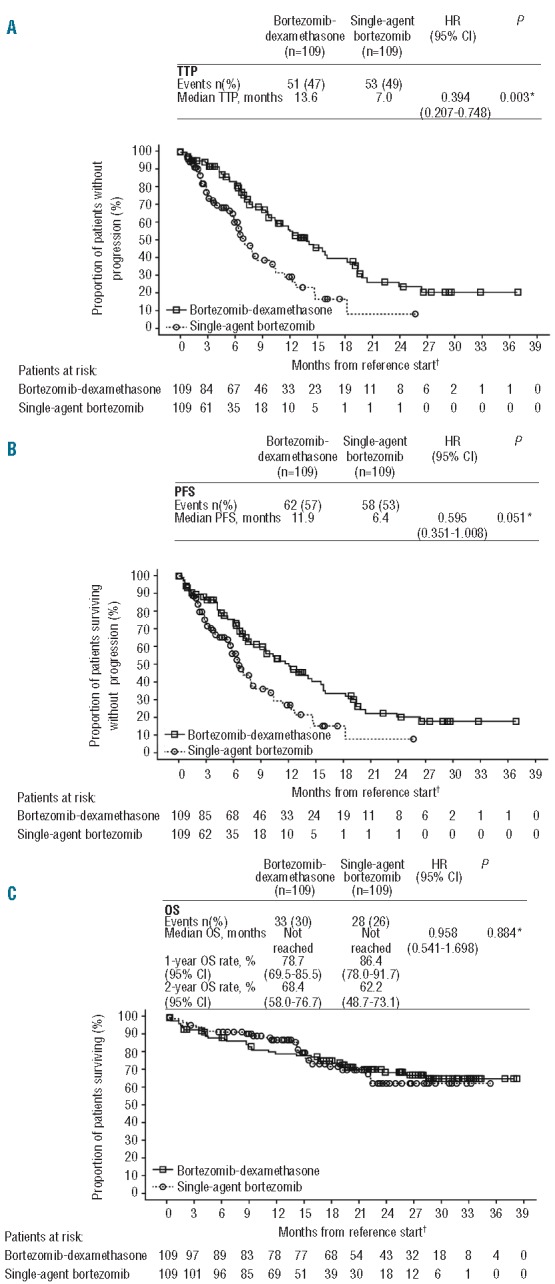
Kaplan-Meier analysis of (A) time-to-progression (TTP), (B) progression-free survival (PFS), and (C) overall survival (OS) among patients in the matched-pairs analysis treated with bortezomib-dexamethasone and single-agent bortezomib. *Log rank test stratified by matched-pair. †Reference start date is the date of randomization for randomized studies; otherwise, the date of first dose received is used. CI: confidence interval; HR: hazard ratio.
After a median follow up of 26.1 months and 18.4 months, respectively, 30% and 26% of patients in the bortezomib-dexamethasone and single-agent bortezomib groups had died. The median OS was not reached in either group and the HR was 0.958. In the bortezomib-dexamethasone and single-agent bortezomib groups, respectively, 1- and 2-year OS rates were 78.7% and 68.4%, and 86.4% and 62.2% (Figure 1C). In an additional time-dependent analysis of OS, adjusting for onset of subsequent therapy, there was no difference in OS between the bortezomib-dexamethasone and single-agent bortezomib groups (HR 1.012; 95%CI: 0.547–1.873).
Sensitivity analyses of ORR and the time-to-event end points, using covariate-adjusted and propensity score-weighted regression analyses of all 384 patients, showed findings consistent with those from the matched-pairs analysis (Table 3). ORs for ORR were 3.774 and 3.628, HRs for PFS were 0.497 and 0.433, HRs for TTP were 0.387 and 0.353, and HRs for OS were 1.067 and 0.903 in these two analyses, respectively. In addition, efficacy results from the second, confirmatory matched-pairs analysis (n=127 pairs) employing only the eight variables associated with clinical outcome in the propensity score matching methodology (Table 4) were consistent with the findings of the 13-variable matched-pairs analysis.
Table 3.
Efficacy results for the bortezomib-dexamethasone versus single-agent bortezomib matched-pairs analysis and the two sensitivity analyses.
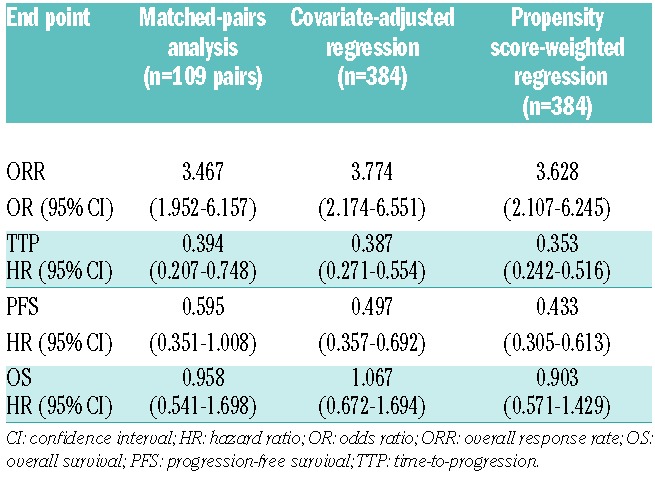
Table 4.
Efficacy results for bortezomib-dexamethasone versus single-agent bortezomib in a confirmatory matched-pairs analysis (n=127).
Safety
The safety profiles of bortezomib-dexamethasone and single-agent bortezomib in the matched-pairs analysis are summarized in Table 5. Overall rates of any-grade AEs, grade 4 or higher AEs, and discontinuations due to AEs appeared similar between the groups. There were numerically lower rates of grade 3 or higher AEs and serious AEs in the bortezomib-dexamethasone group. Common grade 3 or higher AEs, with rates for bortezomib-dexamethasone versus single-agent bortezomib, were thrombocytopenia (21% vs. 25%), infections (17% vs. 16%), peripheral neuropathy (18% vs. 13%), and neutropenia (4% vs. 17%).
Table 5.
Summary of safety data for bortezomib-dexamethasone and single-agent bortezomib in the matched-pairs analysis.
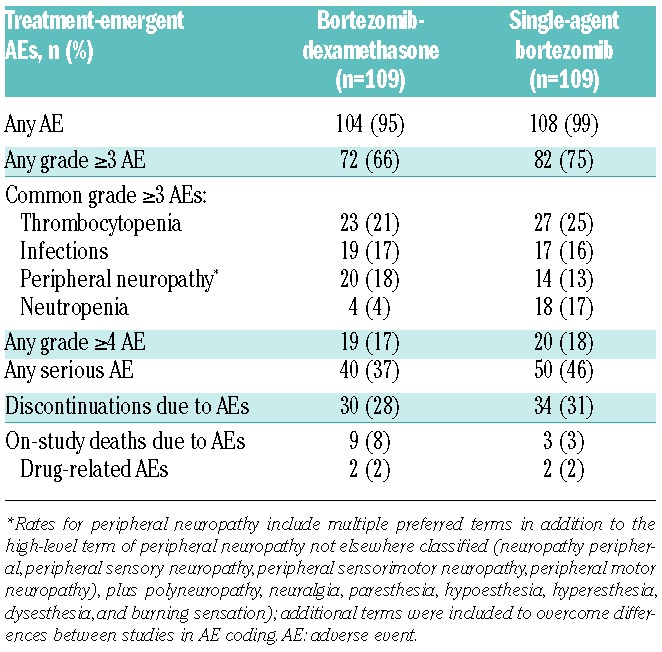
There was a numerically higher rate of on-treatment deaths due to AEs in the bortezomib-dexamethasone group (8%) compared with the single-agent bortezomib group (3%). Of the 9 patients who died on-study due to AEs in the bortezomib-dexamethasone group, median age was 72 years and 7 (78%) were aged 65 years or over (whereas the median age was 62 years and 50% were aged 65 years or over in the overall population in the bortezomib-dexamethasone group), 7 (78%) had creatinine clearance of 60 mL/min (compared with 31% in the overall population), and 5 (56%) had serum albumin 3.5 g/dL (compared with 21% in the overall population); other base-line characteristics were similar.
Adverse events resulting in on-study deaths that were not considered related to treatment in the bortezomib-dexamethasone group included: septic shock in a patient with a high disease burden and a history of thrombocytopenia and anemia; aortic aneurysm in a patient aged 70 years with base-line serum creatinine 3.1 mg/dL; myocardial infarction in a patient with a history of hypertension; small intestinal obstruction in a patient with a high disease burden, extensive lymph node enlargement, and amyloidosis of the duodenum; cardiorespiratory arrest that was a post-operative (bone fracture) complication in a patient with a history of diabetes mellitus, hypertension, and myocardial infarction; acute respiratory failure; and viral infection in a patient with a history of chronic obstructive pulmonary disease, chronic urinary tract infection, sick sinus syndrome, and pacemaker implantation. In the single-agent bortezomib group, one on-study death not considered related to treatment occurred; this was due to a road traffic accident.
Two patients (2%) in each group died due to treatment-related AEs. In the bortezomib-dexamethasone group, these were sepsis in one patient with a history of diverticulitis, anterior resection of the large bowel, and a fistula repair of the bowel and bladder (considered to be possibly related to dexamethasone), and oliguria, hypotension, and acute respiratory distress syndrome, all considered to be possibly related to bortezomib, in one patient with a history of arterial hypertension, anemia, diabetes mellitus, and decreased glomerular filtration rate. In the single-agent bortezomib group, these were both AEs of pneumonia.
Discussion
This matched-pairs analysis comparing bortezomib-dexamethasone with single-agent bortezomib as second-line treatment for MM showed that the ORR was significantly improved and TTP and PFS were prolonged with the addition of dexamethasone to bortezomib. Our results also appear to suggest that the addition of dexamethasone, per the dose and schedule received by patients in the MMY-2045 study, to bortezomib is not associated with increased toxicity. Together, these data indicate the potential benefit of using bortezomib-dexamethasone from first relapse in MM, compared with single-agent bortezomib. There was no difference in OS between the two groups. OS did not appear to be influenced by the receipt of subsequent salvage therapy; however, a comprehensive analysis of time to next therapy and the nature of subsequent therapy received by patients after second-line bortezomib-based treatment was not possible due to inconsistencies in the methods of data collection between the APEX, DOXIL-MMY-3001, and MMY-2045 studies. As there was a gap of 5–6 years between the conduct of the APEX/DOXIL-MMY-3001 studies and the MMY-2045 study, it is possible that the front-line and subsequent treatment options available to patients in these different trials may have contributed to a lack of difference in OS between the two groups in the present analysis; however, subsequent therapy data were not consistently collected in MMY-2045 in order to test this hypothesis. Alternatively, relapse/progression due to the outgrowth of corticosteroid-insensitive MM clones, which may occur in the presence or absence of prior steroid therapy, might have contributed to the observed lack of difference in OS between bortezomib-dexamethasone and single-agent bortezomib in this study.
The efficacy and safety findings of this matched-pairs analysis are supported by previously published data on bortezomib ± dexamethasone from prospective clinical trials,4,5,7–11,14,15,31 as well as by data from retrospective,32–34 observational,35,36 and compassionate use37 studies in relapsed and/or refractory MM, and by data from previous prospective clinical trials of single-agent bortezomib in this setting.1–3,38 The improved ORR and prolonged TTP and PFS observed in patients treated with bortezomib-dexamethasone may be due, in part, to synergism between the two drugs, supported by pre-clinical data indicating that bortezomib enhances the anti-myeloma effects of dexamethasone,39 or to an additive rather than synergistic effect of dexamethasone,40 particularly in first relapse when patients may remain sensitive to glucocorticoids. Our data reflect previous clinical experience demonstrating the activity of the combination therapy and the additional activity seen when dexamethasone has been added due to suboptimal response to single-agent bortezomib.4–6,8,9 The findings of this matched-pairs analysis are also supported by the results of the BoMeR study, which compared prospective data on bortezomib-dexamethasone treatment with data from patients treated with single-agent bortezomib in APEX.15 However, it is worthy of note that patients included in the BoMeR study additionally received maintenance therapy with bortezomib-dexamethasone; therefore, long-term outcomes data are not comparable with the present analysis. It should be noted that the 109 matched pairs of patients contributing to the data in this analysis only represent a subset of the overall study populations receiving bortezomib-dexamethasone in MMY-2045 and single-agent bortezomib in APEX and DOXIL-MMY-3001, which may explain the differences between the ORR, PFS, TTP, and OS data in this analysis and in the overall study results.1–3,14
The rationale for performing a matched-pairs analysis to evaluate the benefit/risk profile of bortezomib-dexamethasone in comparison with bortezomib monotherapy was that this methodology can reduce selection bias due to differences in base-line prognostic factors and improve comparability of the patient populations between treatment groups, leading to reliable conclusions for cross-study comparisons. Further to the primary matched-pairs analysis, the additional data analyses and sensitivity analyses conducted were supportive of the primary findings. The alternative analysis of PFS, which employed stratification by propensity score quintile instead of by matched pair, resulted in improved power for the comparison between groups and, consequently, a narrower 95% CI. Extending the primary findings, this analysis demonstrated a statistically significant improvement in PFS with bortezomib-dexamethasone versus single-agent bortezomib. The findings of the sensitivity analyses, using alternative methodologies for comparing ORR and time-to-event end points between groups, and incorporating all 384 patients who received bortezomib-dexamethasone or single-agent bortezomib as second-line therapy, appeared consistent or highly consistent with the findings of the primary matched-pairs analysis, supporting the validity of these findings. In addition, the second, confirmatory matched-pairs analysis, using only eight variables primarily associated with treatment outcome, also provided consistent findings, further supporting these results. The results of this confirmatory matched-pairs analysis have led to regulatory approval in the European Union of the use of bortezomib in combination with dexamethasone for adult patients with progressive MM who have received at least one prior therapy and who have already undergone or who are unsuitable for hematopoietic stem cell transplantation.41 They also provide a solid justification to consider bortezomib-dexamethasone as the backbone regimen upon which more intensive rationally informed triplet therapies [such as bortezomib-thalidomide-dexamethasone (VTD), lenalidomide-bortezomib-dexamethasone (RVD), and bortezomib-cyclophosphamide-dexamethasone (VCD)] have been built.42,43
This matched-pairs analysis had some limitations. An inherent limitation was that this was not a prospective, randomized, controlled comparison between bortezomib-dexamethasone and single-agent bortezomib, the gold-standard method for establishing comparative data. In addition, although the matched-pairs analysis was thorough and included 13 variables associated with treatment duration and outcomes, it was nevertheless limited by the information that was collected consistently across all three clinical studies. Some relevant variables, such as fluorescence in situ hybridization/cytogenetics data, response to prior therapy, and base-line β2-microglobulin level, were not collected consistently and, therefore, could not be included in this matching exercise; thus, we cannot exclude the possibility that there was an imbalance between the two groups for certain prognostic factors. Furthermore, the MMY-2045 study was conducted more recently than the APEX and DOXIL-MMY-3001 studies, and management of bortezomib may thus have improved due to increased experience with this agent, contributing towards lower peripheral neuropathy and serious AE rates in the bortezomib-dexamethasone group. However, bortezomib exposure and treatment duration appeared similar between the bortezomib-dexamethasone and single-agent bortezomib groups, and thus did not appear to be associated with the efficacy differences.
In conclusion, bortezomib-dexamethasone appears an active and generally well tolerated treatment option for second-line therapy in MM. In the absence of randomized study data, the findings of these thorough matched-pairs analyses indicate that, in this setting, the doublet offers greater efficacy in terms of response and delayed progression compared with single-agent bortezomib, without an appreciable increase in the toxicity profile.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
Funding
This work was supported by Janssen Research & Development.
References
- 1.Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352(24):2487–98. [DOI] [PubMed] [Google Scholar]
- 2.Richardson PG, Sonneveld P, Schuster M, Irwin D, Stadtmauer E, Facon T, et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood. 2007; 110(10):3557–60. [DOI] [PubMed] [Google Scholar]
- 3.Orlowski RZ, Nagler A, Sonneveld P, Blade J, Hajek R, Spencer A, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25(25):3892–901. [DOI] [PubMed] [Google Scholar]
- 4.Arnulf B, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, van de Velde H, et al. Updated survival analysis of a randomized phase III study of subcutaneous versus intravenous bortezomib in patients with relapsed multiple myeloma. Haematologica. 2012;97(12):1925–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jagannath S, Barlogie B, Berenson J, Siegel D, Irwin D, Richardson PG, et al. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol. 2004;127(2):165–72. [DOI] [PubMed] [Google Scholar]
- 6.Jagannath S, Richardson PG, Barlogie B, Berenson JR, Singhal S, Irwin D, et al. Bortezomib in combination with dexamethasone for the treatment of patients with relapsed and/or refractory multiple myeloma with less than optimal response to bortezomib alone. Haematologica. 2006; 91(7):929–34. [PubMed] [Google Scholar]
- 7.Jagannath S, Barlogie B, Berenson JR, Siegel DS, Irwin D, Richardson PG, et al. Updated survival analyses after prolonged follow-up of the phase 2, multicenter CREST study of bortezomib in relapsed or refractory multiple myeloma. Br J Haematol. 2008;143(4):537–40. [DOI] [PubMed] [Google Scholar]
- 8.Mikhael JR, Belch AR, Prince HM, Lucio MN, Maiolino A, Corso A, et al. High response rate to bortezomib with or without dexamethasone in patients with relapsed or refractory multiple myeloma: results of a global phase 3b expanded access program. Br J Haematol. 2009; 144(2):169–75. [DOI] [PubMed] [Google Scholar]
- 9.Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Grishunina M, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12(5):431–40. [DOI] [PubMed] [Google Scholar]
- 10.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348(26):2609–17. [DOI] [PubMed] [Google Scholar]
- 11.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin DH, et al. Extended follow-up of a phase II trial in relapsed, refractory multiple myeloma: final time-to-event results from the SUMMIT trial. Cancer. 2006;106(6):1316–9. [DOI] [PubMed] [Google Scholar]
- 12.Moreau P, San Miguel JF, Ludwig H, Schouten H, Mohty M, Dimopoulos M, et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl6):vi133–7. [DOI] [PubMed] [Google Scholar]
- 13.Anderson KC, Alsina M, Bensinger W, Biermann JS, Cohen AD, Devine S, et al. Multiple myeloma, version 1.2013. J Natl Compr Canc Netw. 2013;11(1):11–7. [DOI] [PubMed] [Google Scholar]
- 14.Dimopoulos MA, Beksac M, Benboubker L, Roddie H, Allietta N, Broer E, et al. Phase II study of bortezomib-dexamethasone alone or with added cyclophosphamide or lenalidomide for sub-optimal response as second-line treatment for patients with multiple myeloma. Haematologica. 2013;98(8):1264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrison S, Quach H, Link E, Feng H, Dean J, Copeman M, et al. Bortezomib and dexamethasone from cycle 1 as treatment and maintenance for multiple myeloma relapse (the BoMeR trial) significantly improves response and time to progression: a matched analysis of BoMeR vs APEX. Haematologica. 2011;96(S1): Abstract P-236. [Google Scholar]
- 16.Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9): 1467–73. [DOI] [PubMed] [Google Scholar]
- 17.Blade J, Samson D, Reece D, Apperley J, Bjorkstrand B, Gahrton G, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102(5): 1115–23. [DOI] [PubMed] [Google Scholar]
- 18.Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med. 2007;26(4):734–53. [DOI] [PubMed] [Google Scholar]
- 19.Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med. 2008; 27(12):2037–49. [DOI] [PubMed] [Google Scholar]
- 20.Austin PC. A tutorial and case study in propensity score analysis: an application to estimating the effect of in-hospital smoking cessation counseling on mortality. Multivariate Behav Res. 2011;46(1):119–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barlogie B, Bolejack V, Schell M, Crowley J. Prognostic factor analyses of myeloma survival with intergroup trial S9321 (INT 0141): examining whether different variables govern different time segments of survival. Ann Hematol. 2011;90(4):423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bringhen S, Mateos MV, Zweegman S, Larocca A, Falcone AP, Oriol A, et al. Age and organ damage correlate with poor survival in myeloma patients: meta-analysis of 1435 individual patient data from 4 randomized trials. Haematologica. 2013;98(6): 980–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen HF, Wu TQ, Li ZY, Shen HS, Tang JQ, Fu WJ, et al. Extramedullary plasmacytoma in the presence of multiple myeloma: clinical correlates and prognostic relevance. Onco Targets Ther. 2012;5329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greipp PR, San Miguel JF, Durie BG, Crowley JJ, Barlogie B, Blade J, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15): 3412–20. [DOI] [PubMed] [Google Scholar]
- 25.Hus I, Dmoszynska A, Manko J, Hus M, Jawniak D, Soroka-Wojtaszko M, et al. An evaluation of factors predicting long-term response to thalidomide in 234 patients with relapsed or resistant multiple myeloma. Br J Cancer. 2004;91(11):1873–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pasqualetti P, Collacciani A, Maccarone C, Casale R. Prognostic factors in multiple myeloma: selection using Cox’s proportional hazard model. Biomed Pharmacother. 1996;50(1):29–35. [DOI] [PubMed] [Google Scholar]
- 27.Perosa F, Minoia C, Favoino E, Prete M, Dammacco F. Staging multiple myeloma patients with active disease using serum levels of beta2m-free HLA class I heavy chain together with IgM or platelet count. Blood Cells Mol Dis. 2009;42(1):71–6. [DOI] [PubMed] [Google Scholar]
- 28.Rajkumar SV, Fonseca R, Dispenzieri A, Lacy MQ, Lust JA, Witzig TE, et al. Methods for estimation of bone marrow plasma cell involvement in myeloma: predictive value for response and survival in patients undergoing autologous stem cell transplantation. Am J Hematol. 2001; 68(4):269–75. [DOI] [PubMed] [Google Scholar]
- 29.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, et al. Clinical factors predictive of outcome with bortezomib in patients with relapsed, refractory multiple myeloma. Blood. 2005; 106(9):2977–81. [DOI] [PubMed] [Google Scholar]
- 30.Varettoni M, Corso A, Pica G, Mangiacavalli S, Pascutto C, Lazzarino M. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Ann Oncol. 2010;21(2):325–30. [DOI] [PubMed] [Google Scholar]
- 31.Kropff MH, Bisping G, Wenning D, Volpert S, Tchinda J, Berdel WE, et al. Bortezomib in combination with dexamethasone for relapsed multiple myeloma. Leuk Res. 2005;29(5):587–90. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi T, Kuroda J, Shimura K, Akaogi T, Kawata E, Kiyota M, et al. Bortezomib plus dexamethasone for relapsed or treatment refractory multiple myeloma: the collaborative study at six institutes in Kyoto and Osaka. Int J Hematol. 2010;92(4):579–86. [DOI] [PubMed] [Google Scholar]
- 33.Corso A, Varettoni M, Mangiacavalli S, Zappasodi P, Pica GM, Algarotti A, et al. Bortezomib plus dexamethasone is highly effective in relapsed and refractory myeloma patients but responses are short-lived. Eur J Haematol. 2009;83(5):449–54. [DOI] [PubMed] [Google Scholar]
- 34.Pantani L, Zamagni E, Zannetti BA, Pezzi A, Tacchetti P, Brioli A, et al. Bortezomib and dexamethasone as salvage therapy in patients with relapsed/refractory multiple myeloma: analysis of long-term clinical outcomes. Ann Hematol. 2013;93(1):123–8. [DOI] [PubMed] [Google Scholar]
- 35.Dimopoulos MA, De Samblanx H, Roussou M, Zervas K, Katodritou E, Sargin D, et al. Efficacy of bortezomib plus dexamethasone versus bortezomib monotherapy in patients (pts) with relapsed/refractory multiple myeloma (MM): interim results from an international electronic observational study. Haematologica. 2011;96(S78):Abstract P-166. [Google Scholar]
- 36.Yuan ZG, Jin J, Huang XJ, Li Y, Chen WM, Liu ZG, et al. Different dose combinations of bortezomib and dexamethasone in the treatment of relapsed or refractory myeloma: an open-label, observational, multicenter study in China Chin Med J (Engl). 2011;124(19):2969–74. [PubMed] [Google Scholar]
- 37.Freimann H, Calderoni A, Cornu P, Olie R. Daily practice use of Bortezomib in relapsed/refractory multiple myeloma. Safety/efficacy results of a compassionate use program in Switzerland. Swiss Med Wkly. 2007;137(21–22):317–22. [DOI] [PubMed] [Google Scholar]
- 38.Dimopoulos M, Siegel DS, Lonial S, Qi J, Hajek R, Facon T, et al. Vorinostat or placebo in combination with bortezomib in patients with multiple myeloma (VANTAGE 088): a multicentre, randomised, double-blind study. Lancet Oncol. 2013; 14(11):1129–40. [DOI] [PubMed] [Google Scholar]
- 39.Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61(7):3071–6. [PubMed] [Google Scholar]
- 40.Rosinol L, Oriol A, Mateos MV, Sureda A, Garcia-Sanchez P, Gutierrez N, et al. Phase II PETHEMA trial of alternating bortezomib and dexamethasone as induction regimen before autologous stem-cell transplantation in younger patients with multiple myeloma: efficacy and clinical implications of tumor response kinetics. J Clin Oncol. 2007;25(28):4452–8. [DOI] [PubMed] [Google Scholar]
- 41.European Medicines Agency. VELCADE® (bortezomib). Summary of product characteristics (up-dated February 2014). Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000539/human_med_001130.jsp&mid=WC0b01ac058001d124.
- 42.National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Multiple Myeloma. Latest version published online is now V2.2015. Available from: http://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf
- 43.Lonial S, Mitsiades CS, Richardson PG. Treatment options for relapsed and refractory multiple myeloma. Clin Cancer Res. 2011;17(6):1264–77. [DOI] [PubMed] [Google Scholar]


