Abstract
Anemia in oncology patients is often considered a side effect of cancer therapy; however, it may occur before any antineoplastic treatment (cancer-related anemia). This study was aimed to evaluate the prevalence of cancer-related anemia in a large cohort of oncology patients and whether inflammation and malnutrition were predictive of its development and severity. The present study included 888 patients with cancer at different sites between May 2011 and January 2014. Patients were assessed at diagnosis before any cancer treatment. The prevalence of anemia according to the main clinical factors (tumor site, stage and performance status) was analyzed. In each patient markers of inflammation, iron metabolism, malnutrition and oxidative stress as well as the modified Glasgow prognostic score, a combined index of malnutrition and inflammation, were assessed and their role in predicting hemoglobin level was evaluated. The percentage of anemic patients was 63% with the lowest hemoglobin levels being found in the patients with most advanced cancer and compromised performance status. Hemoglobin concentration differed by tumor site and was lowest in patients with ovarian cancer. Hemoglobin concentration was inversely correlated with inflammatory markers, hepcidin, ferritin, erythropoietin and reactive oxygen species, and positively correlated with leptin, albumin, cholesterol and antioxidant enzymes. In multivariate analysis, stage, interleukin-6 and leptin were independent predictors of hemoglobin concentration. Furthermore, hemoglobin was inversely dependent on modified Glasgow Prognostic Score. In conclusion, cancer-related anemia is a multifactorial problem with immune, nutritional and metabolic components that affect its severity. Only a detailed assessment of the pathogenesis of cancer-related anemia may enable clinicians to provide safe and effective individualized treatment.
Introduction
Quality of life is the main objective of any management of oncology patients1 and anemia is one of the most frequently reported problems in patients with cancer.2 Anemia is associated with decline in patients’ performance status (PS), cognitive function, energy-activity levels,3 and decreased survival.4 Anemia is often considered a side effect of cancer therapy; however, many patients are already anemic before the start of any treatment. In our view, anemic cancer patients can be divided into two major groups: those with hemoglobin (Hb) concentration in the normal range prior to medical treatment (often receiving adjuvant cancer therapy after surgery, with at most microscopic tumor burden); here, the occurrence of anemia during cancer therapy should be considered a specific side effect of treatment; and those with preceding cancer-related anemia (CRA) (often undergoing cancer therapy for clinically detectable tumors): here, CRA may be a consequence of the chronic inflammation present in patients with advanced cancer.5
Several pieces of evidence attribute a central role in the etiopathogenesis of CRA to inflammatory mediators.5 Indeed, pro-inflammatory cytokines induce changes in the proliferation of erythroid progenitors, erythropoietin (EPO) production, and survival of circulating erythrocytes.6 This inflammatory state is characterized by elevated plasma C-reactive protein (CRP) levels, weight loss with hypoalbuminemia, and erythropoietin-resistant anemia.5 Plasma CRP levels reflect the levels of interleukin (IL)-6,7 which also modulates the concentration and biological activity of hepcidin,8 and other acute-phase proteins9 that may induce serious hematologic, nutritional, and metabolic disorders.10 The identification of hepcidin has enabled a better understanding of the relationship between the immune system, iron homeostasis, and anemia of chronic inflammatory diseases.11 Hepcidin, the synthesis of which by the liver is strongly induced by IL-6,8 inhibits duodenal absorption of iron and blocks iron release from macrophages.12 The release of pro-inflammatory cytokines in cancer patients is often associated with increased production of reactive oxygen species (ROS), either as a component of the immune response or as a consequence of increased metabolism.13 ROS, in turn, may inhibit erythropoiesis.14 Inflammation can also interfere with nutritional status, which in turn may induce anemia.15,16 In cancer patients, it is, therefore, essential to analyze all these factors that cause anemia, particularly before beginning any form of therapy that may worsen anemia.
The aim of this work was to document the prevalence of anemia in a large cohort of patients with solid tumors before any exposure to antineoplastic treatment, and to assess the possible correlation between Hb levels and the commonly used indices of inflammation, malnutrition, and metabolic stress. We hypothesized that inflammation and malnutrition are independent predictors of the development and severity of anemia and that a better knowledge of CRA may enable its more adequate treatment.
Methods
This study was a prospective, observational trial performed in accordance with the Helsinki declaration after approval by the Local Institutional Ethics Committee. Between May 2011 and January 2014, 888 consecutive patients with histologically confirmed solid cancer at different sites referred to the Departments of Obstetrics and Gynecology, Sirai Hospital, Carbonia, Medical Oncology at “N.S. Bonaria” Hospital, San Gavino, “Nuova Casa di Cura”, Decimomannu, and “A. Businco” Hospital, Cagliari, Italy, were enrolled. Table 1 reports the participants’ clinical characteristics. Patients were assessed at diagnosis before receiving any cancer treatment. Exclusion criteria were: evidence of infections, chronic inflammatory disease, active bleeding, hemolysis, renal insufficiency, or hypothyroidism; known history of hematologic disorders (including hemoglobinopathies), family history of thalassemia or hemocromatosis; treatment with EPO, i.v. iron or blood transfusion in the preceding 12 weeks; current iron, vitamin B12 or folate supplementation.
Table 1.
Patients’ clinical characteristics.
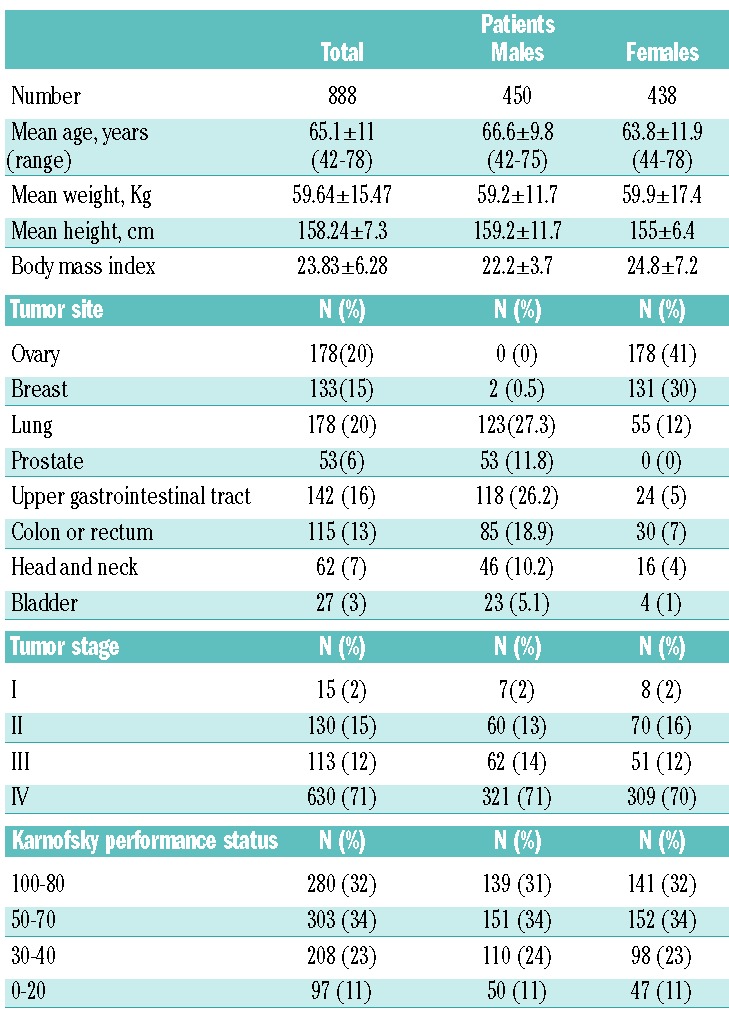
Anemia was defined according to our laboratory population-based normal ranges as Hb <13.0 g/dL for males and <12.0 g/dL for females. Karnofsky PS was categorized into four prognostic classes.17 Blood samples were obtained at 8 a.m. after overnight fasting since serum hepcidin and iron levels show similar circadian changes. In accordance with Ganz et al.,18 the 8 a.m. fasting hepcidin concentrations were more consistent than those at other times of the day. After centrifugation of the blood samples, serum was stored at −80°C until analysis.
In all patients Hb levels and parameters of chronic inflammation [CRP, fibrinogen, IL-6, IL-1β, tumor necrosis factor-α (TNFα)], iron metabolism (iron, ferritin, transferrin, hepcidin), EPO, nutritional status (albumin, leptin, cholesterol, HDL, LDL, triglycerides) and oxidative stress [ROS, glutathione peroxidase (GPx), superoxide dismutase (SOD)] were measured. The formula for expected EPO was: 2.5 × (140-Hb g/L).19 Since leptin is highly dependent on body mass index (BMI), the leptin/BMI ratio was reported. The modified Glasgow prognostic score (mGPS) was calculated as follows: 2, both elevated CRP (≥10 mg/L) and low albumin (<3.5 g/dL); 1, elevated CRP only; 0, normal CRP (<10 mg/L).20
Laboratory assays
Routine analyses of Hb, CRP, fibrinogen, serum iron, transferrin, ferritin, triglycerides, cholesterol, HDL, and LDL were performed. Pro-inflammatory cytokines, EPO and leptin were detected by commercially available enzyme-linked immunosorbent assays (ELISA) (DRG Instruments GmbH, Marburg, Germany, for IL-6, TNFα, EPO; Immunotech SA, Marseille, France for IL-1β; DSL Inc., Webster, Texas, USA for leptin). The coefficient of variation was <5%. Hepcidin was measured by a competitive ELISA using a commercial kit (DRG Instruments GmbH, Marburg, Germany),21 recently considered a “well-performing method” in a “Round Robin” study.22 Intra- and inter-assay variations were 4.4% and 9.7%, respectively. ROS were determined by the FORT test (Callegari, Parma, Italy). GPx and SOD were measured using a commercial kit (Ransod; Randox Lab, Crumlin, UK). More details of these assays are provided elsewhere.23
Statistical analysis
Differences between two groups were compared by the t-test. Multiple groups were compared by analysis of variance with the Tukey post-hoc test and polynomial contrast for trend where indicated. Pearson (or Spearman) correlation analysis was performed using the Bonferroni correction for multiple comparisons. Significant relationships were included in multivariate linear regression analysis. Results were considered statistically significant when the P value was <0.05. Computations were performed using SPSS version 17.0 (SPSS, Chicago, IL, USA).
Results
Evaluation of hemoglobin levels and incidence of anemia
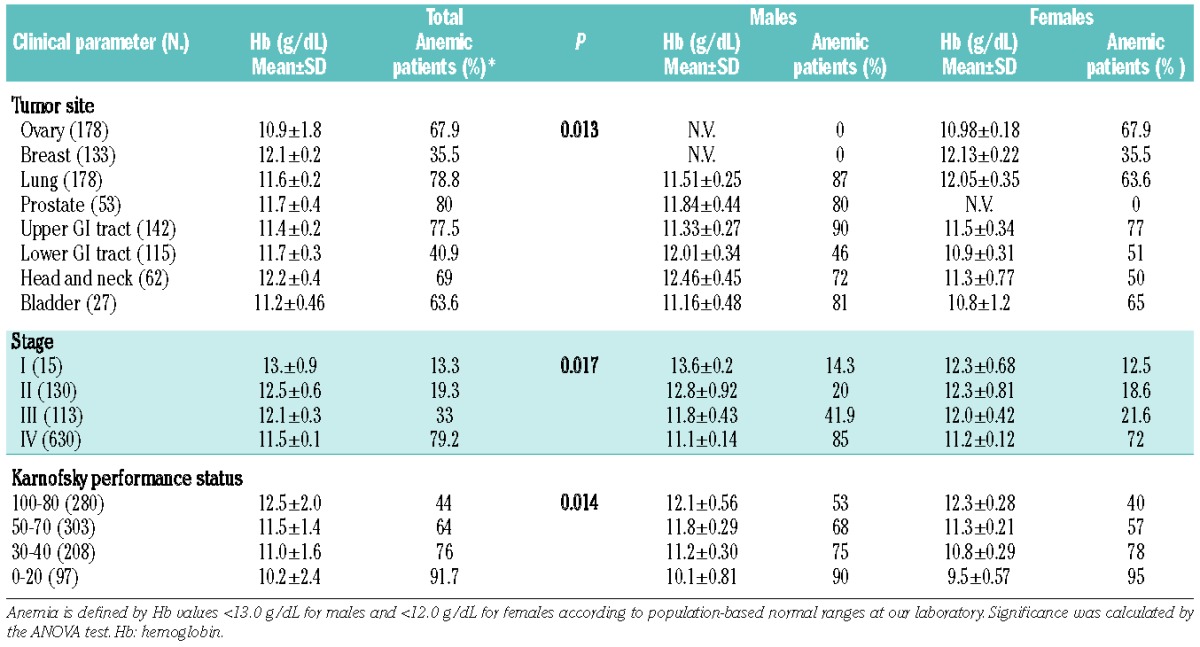
At diagnosis, the mean Hb was 11.6 g/dL (11.8 g/dL in males and 11.4 g/dL in females) and 63.4% of the patients were anemic (Table 2). The mean Hb differed significantly depending on the site of the cancer. Specifically, patients with ovarian cancer had the lowest Hb, while breast cancer patients were the least anemic, accounting for gender (Table 3). The Hb also differed significantly according to disease stage and PS. The percentage of anemic patients was significantly higher among those with the most advanced stage of disease and compromised PS (Table 3). As anemia seems to be a characteristic of advanced stages, we compared stage III-IV patients with stage I-II patients. We found that advanced stage patients had significantly lower Hb concentrations compared with early stage patients (P<0.05). Moreover, the percentage of anemic subjects was higher among the stage III-IV patients than among the stage I-II patients (P<0.05).
Table 2.
Evaluation of Hb and laboratory parameters of chronic inflammation, iron metabolism, nutritional status and oxidative stress in 888 cancer patients.
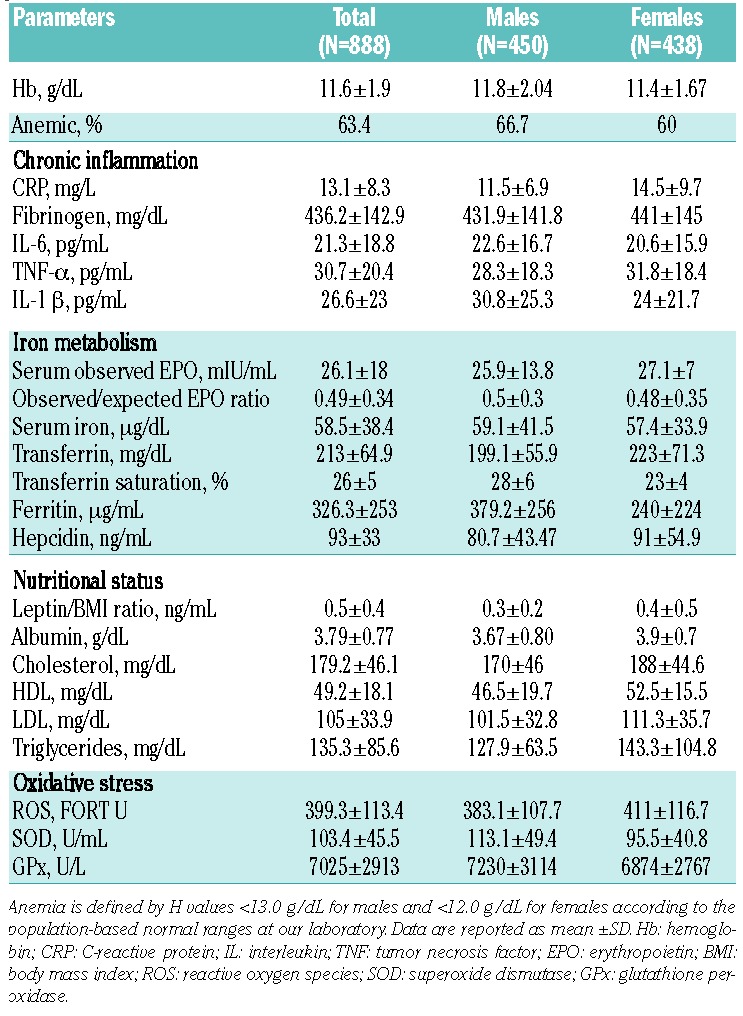
Table 3.
Mean Hb levels and percentage of anemic patients according to tumor site, stage of disease and Karnofsky performance status.
Laboratory parameters of chronic inflammation, iron metabolism, nutritional status, and oxidative stress
Levels of inflammatory, iron metabolism, nutritional and oxidative stress parameters are reported in Table 2. Comparing the non-anemic with the anemic cancer patients, we found significant differences in inflammatory, iron metabolism, nutritional and oxidative stress parameters between the two groups (Online Supplementary Table S1).
Comparing the parameters of chronic inflammation, iron metabolism, nutritional status, and oxidative stress according to stage, we found that CRP, fibrinogen, IL-6, TNFα, IL-1β, ferritin, hepcidin, EPO and ROS were significantly higher in the stage III-IV patients than in the stage I-II patients. In contrast, iron, leptin, triglyceride, and GPx were significantly lower in stage III-IV patients than in early stage patients (Online Supplementary Table S2).
Additionally, comparing the patients with the same stage disease with and without anemia we found that: patients at stage I did not show any difference in laboratory parameters on the basis of the presence of anemia; patients at stage II showed a significant difference in serum levels of serum iron, ferritin, leptin, albumin and cholesterol (total cholesterol and HDL) according to the anemic status, thus suggesting a potential role for nutritional-related factors; patients at stage III showed a significant difference of serum levels of CRP, IL-6, EPO, iron, hepcidin and leptin between anemic and non-anemic patients; patients at stage IV had a significant difference in all inflammatory parameters as well as nutritional, iron and oxidative stress parameters in relation to the anemic status (Online Supplementary Table S3).
In order to verify whether the mediators of chronic inflammation, iron metabolism, nutritional status, and oxidative stress may differ according to tumor sites, we performed a detailed comparison of all laboratory parameters between patients with different types of cancer. The results showed that: inflammatory parameters CRP and IL-6 were significantly different depending on cancer types, with ovarian cancer patients having the highest values and breast cancer patients the lowest ones; serum iron, ferritin and hepcidin levels were significantly different between patients, with lower gastrointestinal tract cancer patients showing the lowest levels of these parameters in comparison to patients with cancer in other sites. This suggests a major role for iron deficiency in the pathogenesis of CRA in patients with colorectal cancer (Online Supplementary Table S4).
Correlation of hemoglobin levels with clinical and laboratory parameters
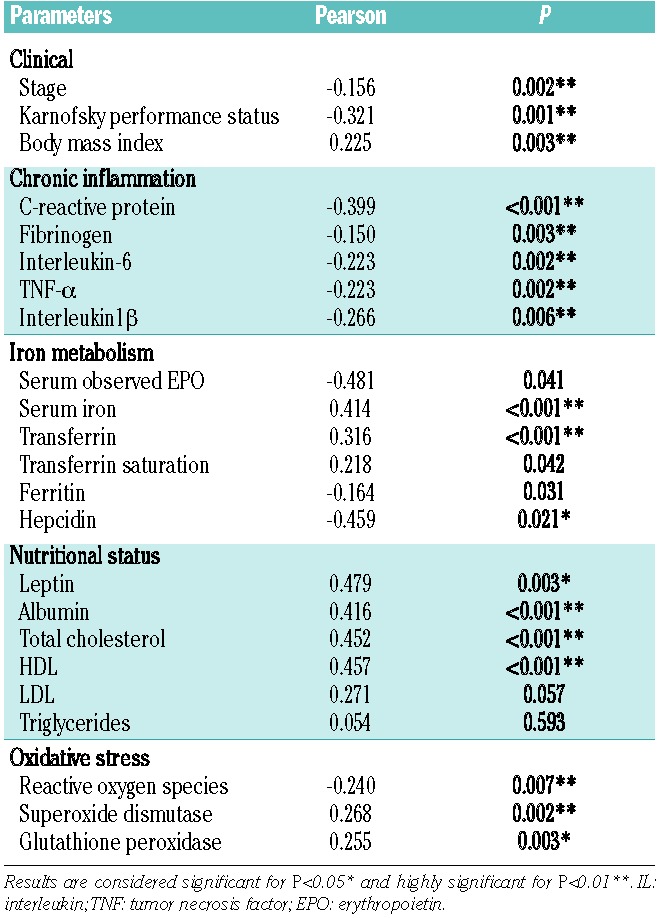
Hemoglobin was inversely correlated with stage and PS. A significant positive relationship was found between Hb and BMI. Among markers of chronic inflammation, iron metabolism, nutritional status, oxidative stress, Hb was significantly inversely correlated with CRP, fibrinogen, IL-6, TNFα, IL-1β, ferritin, hepcidin, EPO and ROS, whereas it was positively correlated with serum iron, transferrin, leptin, albumin, total cholesterol, HDL, GPx, and SOD (Table 4). We also assessed the correlation between Hb and laboratory parameters of chronic inflammation, iron metabolism, nutritional status, and oxidative stress in the different categories of stage, PS and tumor sites. This analysis showed that patients at advanced stage (III and IV) had the same significant correlations found in the overall population, whereas in the early stage patients (stage I and II), Hb was positively, significantly correlated with serum levels of EPO, iron, transferrin saturation, ferritin as well as leptin, albumin, total cholesterol and HDL (Online Supplementary Table S5). The correlation between Hb and laboratory parameters in the different categories of PS was superimposable to that found in the overall population (Online Supplementary Table S6). The correlations between Hb and other laboratory parameters in patients with cancer in different sites are reported in Online Supplementary Table S7: the main results emerging from this analysis are the positive significant correlation between Hb and serum ferritin in patients with gastrointestinal cancers, differently from patients with cancer in other sites in whom Hb was significantly, inversely related with serum ferritin. These data suggest a role for iron-deficiency in the pathogenesis of CRA in patients with gastrointestinal cancer.
Table 4.
Correlation of Hb levels with clinical parameters (stage, performance status and body mass index) and markers of chronic inflammation, iron metabolism, nutritional status and oxidative stress in patients with cancer at different sites.
Since it is known that in conditions of chronic inflammation and oxidative stress, as observed in our patients, the synthesis of EPO can be blunted by pro-inflammatory cytokines,24 we also evaluated the expected EPO value in comparison to Hb levels.25 Indeed, in different anemic states EPO levels increase proportionally to the severity of anemia (decrease of Hb levels).26 The observed/predicted EPO ratio has been correlated with the main indices of inflammation and oxidative stress. In the present study we observed “inappropriately” low EPO levels in comparison to the expected values for the degree of anemia in accordance with findings reported in the literature regarding patients with anemia of chronic disease.5 The observed/expected EPO ratio was inversely related with serum levels of CRP, fibrinogen, IL-6, and ROS and was positively related to GPx and SOD (Online Supplementary Table S8).
Multivariate regression analysis showed that stage (β coefficient= −0.537; 95% CI: −0.971 to −0.071; P=0.004), IL-6 (β coefficient= −0.831; 95% CI: −0.146 to −0.029; P =0.023), and leptin (β coefficient= 0.745; 95% CI: 1.694 to 0.050; P=0.015) were independent predictors of Hb. We, therefore, tested whether both IL-6 and leptin correlated with each other and with the other laboratory markers of inflammation, iron metabolism, nutritional status, and oxidative stress. Single regression analysis showed that: IL-6 and leptin were inversely related to each other (P<0.001); IL-6 was positively correlated with CRP (P<0.001), fibrinogen (P=0.009), IL-1 (P<0.001), hepcidin (P=0.003), and ROS (P=0.034), while it was inversely related to EPO ratio (P=0.035), total cholesterol (P=0.017), albumin (P=0.020), and GPx (P<0.001); leptin ratio was inversely correlated with CRP (P=0.036), IL-1 (P=0.027), and ROS (P=0.036), while it was positively related to EPO ratio (P=0.029), serum iron (P=0.006), albumin (P=0.045), total cholesterol (P=0.015), and GPx (P=0.009).
Evaluation of laboratory parameters according to hemoglobin levels
Anemic patients were also divided on the basis of whether the Hb values were ≤ or >10 g/dL, which is the threshold for starting erythropoiesis-stimulating agent treatment for chemotherapy-induced anemia according to international guidelines.27 Patients with Hb≤10.0 g/dL had significantly higher levels of CRP, fibrinogen, IL-6, ferritin, hepcidin, EPO and ROS than patients with Hb>10 g/dL. Conversely, the levels of leptin, albumin, total cholesterol, HDL, and GPx were significantly lower in patients with Hb≤10.0 g/dL compared to the levels in patients with Hb>10 g/dL (Online Supplementary Table S9).
Additionally, stratifying patients into quartiles on the basis of their Hb concentration, polynomial contrast for trend showed that: CRP, IL-6, EPO, hepcidin, and ROS were progressively higher with decreasing quartiles of Hb, and that serum iron, leptin, total cholesterol, HDL, and GPx were progressively lower with decreasing quartiles of Hb (Table 5). In an alternative analysis comparing Hb levels according to quartiles of laboratory parameters, we found similarly that Hb was progressively lower with decreasing quartiles of serum iron, transferrin saturation, leptin, total cholesterol, HDL, and GPx, whereas it was progressively lower with increasing quartiles of CRP, IL-6, IL-1β, EPO, ferritin, hepcidin, and ROS (Online Supplementary Table S10).
Table 5.
Evaluation of laboratory parameters of chronic inflammation, iron metabolism, nutritional status and oxidative stress in the population of anemic cancer patients according to quartiles of Hb levels.
Evaluation of hemoglobin levels according to modified Glasgow prognostic score
Considering these results, we assessed whether in the clinical practice a simple tool, such as the mGPS, which evaluates the correlation between inflammation (CRP) and nutritional status (albumin levels), correlates with Hb and is able to predict it. The concentration of Hb differed significantly between patients in different mGPS categories (P<0.001). In detail, a post-hoc test showed that Hb was significantly lower in patients in the highest mGPS category compared to those in the lower categories. Moreover, in a linear regression model mGPS category was a significant negative predictor of Hb (β coefficient=−1.176; P<0.001) (Figure 1).
Figure 1.
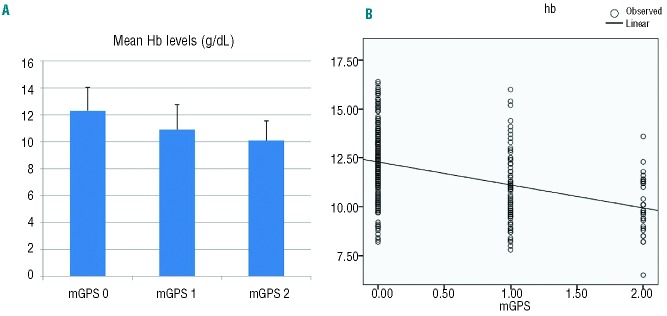
Evaluation of Hb levels according to mGPS categories. (A) Bars represent mean ±SD. Mean Hb levels were significantly different between mGPS categories (ANOVA test; P<0.001). The post-hoc test showed that Hb level was lower in patients in the highest mGPS category vs. the other mGPS categories (mGPS 0 vs. mGPS 1 category, P<0.001; mGPS 0 vs. mGPS 2, P<0.001; mGPS 1 vs. mGPS 2, P=0.045). (B) Scatter plots with linear regression line (black line) showing that mGPS category is a significant negative predictor of Hb level (dependent variable) (β coefficient=−1.176; P<0.001). mGPS: modified Glasgow prognostic score; Hb: hemoglobin.
Moreover, mGPS was positively correlated with fibrinogen (P<0.001), IL-6 (P<0.001), TNF-α (P<0.001), IL-1 (P=0.001), hepcidin (P=0.016), EPO (P=0.034), ROS (P=0.047), and inversely related with serum iron (P<0.001), leptin (P=0.024), total cholesterol (P<0.001), HDL (P<0.001), and GPx (P=0.006).
Discussion
More than 30% of cancer patients have CRA at the time of diagnosis28 and thus before starting any antineoplastic treatment. CRA has been associated with more advanced stages28 and has biological and hematologic features similar to those observed in chronic inflammatory disease-related anemia.26 However, only rare studies have correlated CRA with markers of inflammation.23,29 Moreover, if we consider that in cancer patients the state of chronic inflammation is associated with serious eating disorders and with profound changes in energy metabolism, which by themselves are capable of inducing anemia, the literature on this subject is even more inadequate.
In the present study, we confirmed in 888 cancer patients at diagnosis that the percentage of anemic patients was high, and that Hb was significantly lower in the patients in most advanced stages and with the most compromised PS. Mean Hb was significantly different according to tumor site: in particular, patients with ovarian cancer had the lowest levels, as previously observed.28 Furthermore, we confirmed that the lowest Hb levels were associated with the highest values of inflammatory markers. The multivariate analysis demonstrated that IL-6, in particular, was a strong predictor of Hb. Of note, IL-6 was in turn positively correlated with other markers of inflammation (CRP, fibrinogen, IL-1), hepcidin, ferritin, and oxidative stress (ROS), and inversely related to EPO, nutritional parameters (cholesterol, leptin, albumin) and antioxidants (GPx). It is known that pro-inflammatory cytokines impair EPO production, the proliferation and differentiation of erythroid progenitors and shorten the survival of circulating erythrocytes.5 In our study, EPO production was not optimal for the level of anemia as previously demonstrated.6 Importantly, high cytokine levels are also associated with “functional iron deficiency”.30 This condition is mediated by hepcidin, a liver-derived peptide regulated by IL-6 and included among the inflammatory (type II) acute-phase proteins.31 Increased hepcidin levels have been detected in patients with anemia associated with chronic inflammatory diseases, such as inflammatory bowel disease, chronic kidney disease, and hematologic cancers.32–35 Our data showed that hepcidin was inversely correlated with Hb, particularly in patients with advanced cancer. High hepcidin levels were associated with higher ferritin, lower serum iron, and lower transferrin saturation levels. Accordingly, Shu et al. recently showed that in cancer patients with anemia of chronic disease, hepcidin was positively correlated with IL-6 and inversely with serum iron, differently from cancer patients with iron-deficiency anemia, in whom hepcidin decreased with decreasing quartiles of serum iron.36 In contrast, in a population of patients with early breast cancer undergoing adjuvant chemotherapy, Durigova et al. demonstrated a positive correlation between hepcidin and Hb levels concluding that baseline low hepcidin levels were predictive of the onset of severe anemia.37
The profile of iron metabolism seems to be quite different depending on the various tumor sites assessed by us: in the presence of comparable inflammatory status, patients with colorectal cancer had lower hepcidin and ferritin levels than patients with tumors in other sites thus suggesting a role of iron deficiency in the pathogenesis of CRA in this subgroup, as already reported in literature.38
Motivated by the evidence of iron-restricted erythropoiesis, multiple clinical trials demonstrated that, in patients with CRA, i.v. iron improves the response rate to erythropoiesis-stimulating agents, reduces the time to target Hb levels, decreases erythropoiesis-stimulating agent requirements, and is more effective than oral iron.39 However, to date long-term data on the role of iron supplementation in managing CRA are not available and it has been reported that iron supplementation may act very differently in various cancers.39,40 In particular, the use of i.v. iron, in the presence of functional iron deficiency, has been recently debated.41,42 Accordingly, in a previous randomized clinical trial we suggested the need to choose the route of iron administration as a function of the patients’ clinical characteristics, and showed that oral lactoferrin, in anemic patients with advanced cancer undergoing chemotherapy, reduces ferritin levels, and supports the efficacy of erythropoiesis-stimulating agents, similarly to i.v. iron.43
Cancer growth and associated inflammation also induce changes in energy metabolism and food intake (cancer-anorexia), potentially contributing to anemia. These conditions lead to severely altered glycemia, albumin, and cholesterol levels, with consequent reduced circulating leptin levels.44 Generally, in patients with advanced cancer leptin levels are inversely correlated with pro-inflammatory cytokines and stage, independently of BMI.45 We found that leptin, albumin, and cholesterol, along with BMI, were positively correlated with Hb. Of relevance, here we found that leptin, besides IL-6 and stage of disease, was an independent predictor of Hb levels. Unfortunately, to date, the close correlation between nutritional status and anemia in cancer patients has been studied very little, in contrast to that in patients with chronic renal failure, in whom this condition has been widely investigated and, as a result, in whom the need for proper nutritional support, especially in patients candidates for the use of erythropoiesis-stimulating agents,46 is well established.
It is also known that any inflammatory condition characterized by high cytokines levels is associated with high ROS levels, the synthesis of which is considered an integral part of the inflammatory response, and also evidence of metabolic deregulation induced by cytokines.47 In the present study, ROS were negatively correlated, while GPx and SOD were positively correlated with Hb. ROS are able to inhibit the synthesis of EPO.16 Moreover, oxidative stress per se increases the fragility of red blood cells, decreases the rate of erythroid maturation and shortens erythrocyte lifespan. A recent study demonstrated in vitro that sustained levels of H2O2, similar to inflammatory conditions, are sufficient to activate hepcidin transcription in hepatocytes via increased STAT-3 phosphorylation.48 The authors further found that H2O2 acts synergistically with IL-6 in inducing hepcidin, thus suggesting another mechanism through which oxidative stress contributes to the anemia of chronic disease.48
To strengthen our results we compared anemic and non-anemic patients, confirming that anemia was significantly associated with the highest levels of CRP, inflammatory cytokines, EPO, ferritin, hepcidin and ROS levels, and with the lowest levels of serum iron, transferrin, leptin, albumin, lipid profile parameters and antioxidant enzymes. Moreover, to increase the power of analysis, we divided anemic patients according to Hb quartiles and further confirmed that increasing severity of anemia was associated with progressively increased CRP, cytokines, ferritin, EPO and ROS and decreased leptin, cholesterol, and GPx levels. These results highlight that CRA is a multifactorial problem with immune, nutritional, and metabolic components, all of which can affect the onset and severity of anemia.
In the attempt to give to practicing physicians a simple tool to evaluate the correlation between Hb levels and the immune-metabolic status, we used the mGPS, which evaluates the correlation between inflammation (CRP) and nutritional status (albumin levels), correlating it with Hb. Our results showed that the highest mGPS score was predictive of lower Hb levels, in agreement with data obtained from patients with chronic kidney failure, in whom the “malnutrition-inflammation score” was associated with anemia.49,50 In turn, mGPS in our study was positively correlated with IL-6, hepcidin, EPO and ROS and inversely related to leptin and GPx levels, thus demonstrating its ability to reflect the complex inflammatory, nutritional/metabolic, and oxidative status of cancer patients.
The main limitations of our study included the difficulty in assessing so many different parameters in patients as well as the fact that the patients were enrolled from a small number of centers in a limited geographical area and were heterogeneous with respect to tumor site and stage of disease.
The present findings suggest that a careful evaluation of cancer patients should be performed before starting treatment for anemia: in addition to including erythropoiesis-stimulating agents, the treatment of CRA should include the correction of nutritional deficiencies, personalized iron supplementation42,43,51 and, in some cases, antioxidant treatment.52 Moreover, our results suggest that patients with different cancer types and at various stages may have different factors influencing the pathogenesis and severity of anemia and, therefore, need specific individualized mechanism-based treatment of CRA. For example, patients with gastrointestinal tumors showing, as observed in our study, lower levels of serum iron, ferritin and hepcidin may have more benefit from iron supplementation in comparison with patients with tumors in other sites. Additionally, recent findings that anti-IL6 monoclonal antibody, administered to patients with different types of advanced cancer,53,54 produced significant increases in Hb together with decreases in CRP, suggests that this or other anti-cytokine or anti-inflammatory treatments may prove useful in the treatment of CRA.
On the basis of our data, we recommend that the evaluation of anemia in cancer patients should be extended over and above the current standard testing for blood loss, iron deficiency, and vitamin B12 or folate nutritional deficiency, and should include the assessment of CRP, albumin (ideally associated as in the mGPS) and, where available, analysis of oxidative stress status, which is feasible and simple with currently available methodologies. In conclusion, a detailed understanding of the pathogenesis of CRA should enable clinicians to provide effective individualized treatment thus promoting a much better quality of life for patients.
Acknowledgments
This work was supported by the “Associazione Sarda per la ricerca nell’Oncologia Ginecologica-ONLUS”.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.The Lancet Oncology. Keeping patients front and centre in the era of negative results. Lancet Oncol. 2013;14(10):909. [DOI] [PubMed] [Google Scholar]
- 2.Spivak JL, Gascón P, Ludwig H. Anemia management in oncology and hematology. Oncologist. 2009;14(Suppl 1):43–56. [DOI] [PubMed] [Google Scholar]
- 3.Steinmetz T, Totzke U, Schweigert M, Mittermüller J, Nawka S, Tesch H, et al. A prospective observational study of anaemia management in cancer patients - results from the German Cancer Anaemia Registry. Eur J Cancer Care. 2011;20(4):493–502. [DOI] [PubMed] [Google Scholar]
- 4.Waters JS, O’Brien ME, Ashley S. Management of anemia in patients receiving chemotherapy. J Clin Oncol. 2002;20(2):601–3. [DOI] [PubMed] [Google Scholar]
- 5.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352(10): 1011–23. [DOI] [PubMed] [Google Scholar]
- 6.Means RT, Jr, Krantz SB. Progress in understanding the pathogenesis of the anemia of chronic disease. Blood. 1992;80(7):1639–47. [PubMed] [Google Scholar]
- 7.Streetz KL, Wüstefeld T, Klein C, Manns MP, Trautwein C. Mediators of inflammation and acute phase response in the liver. Cell Mol Biol (Noisy-le-grand). 2001;47(4):661–73. [PubMed] [Google Scholar]
- 8.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113(9):1271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bode JG, Albrecht U, Häussinger D, Heinrich PC, Schaper F. Hepatic acute phase proteins–regulation by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk with NF-κB-dependent signaling. Eur J Cell Biol. 2012;91(6–7):496–505. [DOI] [PubMed] [Google Scholar]
- 10.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–54. [DOI] [PubMed] [Google Scholar]
- 11.Ganz T, Nemeth E. Hepcidin and disorders of iron metabolism. Annu Rev Med. 2011;62:347–60. [DOI] [PubMed] [Google Scholar]
- 12.Rivera S, Nemeth E, Gabayan V, Lopez MA, Farshidi D, Ganz T. Synthetic hepcidin causes rapid dose-dependent hypoferremia and is concentrated in ferroportin-containing organs. Blood. 2005;106(6):2196–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kundu JK, Surh YJ. Emerging avenues linking inflammation and cancer. Free Radic Biol Med. 2012;52(9):2013–37. [DOI] [PubMed] [Google Scholar]
- 14.Neumcke I, Schneider B, Fandrey J, Pagel H. Effects of pro- and antioxidative compounds on renal production of erythropoietin. Endocrinology. 1999;140(2):641–5. [DOI] [PubMed] [Google Scholar]
- 15.Takeda A, Toda T, Shinohara S, Mogi Y, Matsui N. Factors contributing to higher hematocrit levels in hemodialysis patients not receiving recombinant human erythropoietin. Am J Kidney Dis. 2002;40(1):104–9. [DOI] [PubMed] [Google Scholar]
- 16.Stenvinkel P, Heimbürger O, Lönnqvist F, Bárány P. Does the ob gene product leptin stimulate erythropoiesis in patients with chronic renal failure? Kidney Int. 1998;53(5):1430–1. [DOI] [PubMed] [Google Scholar]
- 17.Barbot AC, Mussault P, Ingrand P, Tournai JM. Assessing 2-month clinical prognosis in hospidalized patients with advanced solid tumors. J Clin Oncol. 2008;26(15):2538–43. [DOI] [PubMed] [Google Scholar]
- 18.Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood. 2008;112(10):4292–7. [DOI] [PubMed] [Google Scholar]
- 19.Fehr T, Ammann P, Garzoni D, Korte W, Fierz W, Rickli H, et al. Interpretation of erythropoietin levels in patients with various degrees of renal insufficiency and anemia. Kidney Int. 2004;66(3):1206–11. [DOI] [PubMed] [Google Scholar]
- 20.Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O’Reilly DS, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer. 2011;47(17):2633–41. [DOI] [PubMed] [Google Scholar]
- 21.Zipperer E1, Post JG, Herkert M, Kündgen A, Fox F, Haas R, et al. Serum hepcidin measured with an improved ELISA correlates with parameters of iron metabolism in patients with myelodysplastic syndrome. Ann Hematol. 2013;92(12):1617–23. [DOI] [PubMed] [Google Scholar]
- 22.Kroot JJ, van Herwaarden AE, Tjalsma H, Jansen RT, Hendriks JC, Swinkels DW. Second round robin for plasma hepcidin methods: first steps toward harmonization. Am J Hematol. 2012;87(10):977–83. [DOI] [PubMed] [Google Scholar]
- 23.Macciò A, Madeddu C, Massa D, Mudu MC, Lusso MR, Gramignano G, et al. Hemoglobin levels correlate with interleukin-6 levels in patients with advanced untreated epithelial ovarian cancer: role of inflammation in cancer-related anemia. Blood. 2005;106(1):362–7. [DOI] [PubMed] [Google Scholar]
- 24.Jelkmann W. Proinflammatory cytokines lowering erythropoietin production. J Interferon Cytokine Res. 1998;18(8):555–9. [DOI] [PubMed] [Google Scholar]
- 25.Barosi G. Inadequate erythropoietin response to anemia: definition and clinical relevance. Ann Hematol. 1994;68(5):215–23. [DOI] [PubMed] [Google Scholar]
- 26.Davis SL, Littlewood TJ. The investigation and treatment of secondary anaemia. Blood Rev. 2012;26(2):65–71. [DOI] [PubMed] [Google Scholar]
- 27.Schrijvers D, De Samblanx H, Roila F; ESMO Guidelines Working Group: Erythropoiesis-stimulating agents in the treatment of anaemia in cancer patients: ESMO Clinical Practice Guidelines for use. Ann Oncol. 2010;21(Suppl 5):v244–7. [DOI] [PubMed] [Google Scholar]
- 28.Ludwig H, Van Belle S, Barrett-Lee P, Birgegård G, Bokemeyer C, Gascón P, et al. The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer. 2004;40(15):2293–306. [DOI] [PubMed] [Google Scholar]
- 29.Falkensammer CE, Thurnher M, Leonhartsberger N, Ramoner R. C-reactive protein is a strong predictor for anaemia in renal cell carcinoma: role of IL-6 in overall survival. BJU Int. 2011;107(12):1893–8. [DOI] [PubMed] [Google Scholar]
- 30.Goodnough LT. Erythropoietin and iron-restricted erythropoiesis. Exp Hematol. 2007;35(4 Suppl 1):167–72. [DOI] [PubMed] [Google Scholar]
- 31.Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101(7): 2461–3. [DOI] [PubMed] [Google Scholar]
- 32.Basseri RJ, Nemeth E, Vassilaki ME, Basseri B, Enayati P, Shaye O, et al. Hepcidin is a key mediator of anemia of inflammation in Crohn’s disease. J Crohns Colitis. 2012;7(8):e286–91. [DOI] [PubMed] [Google Scholar]
- 33.Zaritsky J, Young B, Wang HJ, Westerman M, Olbina G, Nemeth E, et al. Hepcidin-a potential novel biomarker for iron status in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(6):1051–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma S, Nemeth E, Chen YH, Goodnough J, Huston A, Roodman GD, et al. Involvement of hepcidin in the anemia of multiple myeloma. Clin Cancer Res. 2008;14(11):3262–7. [DOI] [PubMed] [Google Scholar]
- 35.Hohaus S, Massini G, Giachelia M, Vannata B, Bozzoli V, Cuccaro A, et al. Anemia in Hodgkin’s lymphoma: the role of interleukin-6 and hepcidin. J Clin Oncol. 2010;28(15):2538–43. [DOI] [PubMed] [Google Scholar]
- 36.Shu T, Jing C, Lv Z, Xie Y, Xu J, Wu J. Hepcidin in tumor-related iron deficiency anemia and tumor-related anemia of chronic disease: pathogenic mechanisms and diagnosis. Eur J Haematol. 2014. June 21 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 37.Durigova A, Lamy PJ, Thezenas S, Pouderoux S, Montels F, Romieu G, et al. Anemia and iron biomarkers in patients with early breast cancer. Diagnostic value of hepcidin and soluble transferrin receptor quantification. Clin Chem Lab Med. 2013;51(9):1833–41. [DOI] [PubMed] [Google Scholar]
- 38.Ludwig H, Muldur E, Endler G, Hubl W. Prevealence of iron deficiency across different tumors and its association with poor performance status, disease status and anemia. Ann Oncol. 2013;24(7):1886–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aapro M, Osterborg A, Gascon P, Ludwig H, Beguin Y. Prevalence and management of cancer related anaemia, iron deficiency and the specific role of i.v. iron. Ann Oncol. 2012;23(8):1954–62. [DOI] [PubMed] [Google Scholar]
- 40.Beguin Y, Aapro M, Ludwig H, Mizzen L, Osterborg A. Epidemiological and nonclinical studies investigating effects of iron in carcinogenesis–a critical review. Crit Rev Oncol Hematol. 2014;89(1):1–15. [DOI] [PubMed] [Google Scholar]
- 41.Spivak JL. Iron and the anemia of chronic disease: vindication for the non-essential role of iron supplementation. Oncology. 2011;25(5): 421–3. [PubMed] [Google Scholar]
- 42.Steensma DP, Sloan JA, Dakhil SR, Dalton R, Kahanic SP, Prager DJ, et al. Phase III, randomized study of the effects of parenteral iron, oral iron, or no iron supplementation on the erythropoietic response to darbepoetin alfa for patients with chemotherapy-associated anemia. J Clin Oncol. 2011;29(1):97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macciò A, Madeddu C, Gramignano G, Mulas C, Sanna E, Mantovani G. Efficacy and safety of oral lactoferrin supplementation in combination with rHuEPO-beta for the treatment of anemia in advanced cancer patients undergoing chemotherapy: open-label, randomized controlled study. Oncologist. 2010;15(8):894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee MJ, Fried SK. Integration of hormonal and nutrient signals that regulate leptin synthesis and secretion. Am J Physiol Endocrinol Metab. 2009;296(6): E1230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Macciò A, Madeddu C, Massa D, Astara G, Farci D, Melis GB, et al. Interleukin-6 and leptin as markers of energy metabolic changes in advanced ovarian cancer patients. J Cell Mol Med. 2009;13(9B):3951–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hung SC, Tung TY, Yang CS, Tarng DC. High-calorie supplementation increases serum leptin levels and improves response to rHuEPO in long-term hemodialysis patients. Am J Kidney Dis. 2005;45(6):1073–83. [DOI] [PubMed] [Google Scholar]
- 47.Naviaux RK. Oxidative shielding or oxidative stress? J Pharmacol Exp Ther. 2012; 342(3):608–18. [DOI] [PubMed] [Google Scholar]
- 48.Millonig G, Ganzleben I, Peccerella T, Casanovas G, Brodziak-Jarosz L, Breitkopf-Heinlein K, et al. Sustained submicromolar H2O2 levels induce hepcidin via signal transducer and activator of transcription 3 (STAT3). J Biol Chem. 2012;287(44):37472–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2001;38(6):1251–63. [DOI] [PubMed] [Google Scholar]
- 50.Rattanasompattikul M, Molnar MZ, Zaritsky JJ, Hatamizadeh P, Jing J, Norris KC, et al. Association of malnutrition-inflammation complex and responsiveness to erythropoiesis-stimulating agents in long-term hemodialysis patients. Nephrol Dial Transplant. 2013; 28(7):1936–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steinmetz HT, Tsamaloukas A, Schmitz S, Wiegand J, Rohrberg R, Eggert J, et al. A new concept for the differential diagnosis and therapy of anaemia in cancer patients. Support Care Cancer. 2010;19(2):261–9. [DOI] [PubMed] [Google Scholar]
- 52.Hsu SP, Chiang CK, Yang SY, Chien CT. N-acetylcysteine for the management of anemia and oxidative stress in hemodialysis patients. Nephron Clin Pract. 2010;116(3): c207–16. [DOI] [PubMed] [Google Scholar]
- 53.Stone RL, Nick AM, McNeish IA, Balkwill F, Han HD, Bottsford-Miller J, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366(7):610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bayliss TJ, Smith JT, Schuster M, Dragnev KH, Rigas JR. A humanized anti-IL-6 antibody (ALD518) in non-small cell lung cancer. Expert Opin Biol Ther. 2011;11(12):1663–8. [DOI] [PubMed] [Google Scholar]


