Acquired immunodeficiency syndrome (AIDS)-related primary central nervous system lymphoma (AR-PCNSL) is an Epstein-Barr (EBV)-associated malignancy that occurs in severely immunocompromised human immunodeficiency virus (HIV)-infected patients. Lack of EBV-specific CD4+ T cells contributes to its pathogenesis.1 Although AR-PCNSL incidence in the United States (US) decreased with availability of combination antiretroviral therapy (cART), 26 PCNSL cases per 100,000 person-years continue to occur in persons with AIDS, making up over 10% of US HIV-associated lymphoma cases. Prognosis remains poor, with greater than 75% 2-year mortality.2 Neurological co-morbidities, difficulties distinguishing AR-PCNSL from other CNS pathology, undertreatment of AR-PCNSL and health care disparities may contribute to mortality.3
Central nervous system immune reconstitution inflammatory syndrome (CNS-IRIS) is characterized by neurological deterioration despite immune recovery from an immunodeficient state. In AIDS patients with CNS opportunistic infections (OIs) such as Cryptococcus, John Cunningham (JC) virus or cytomegalovirus (CMV),4 CNS-IRIS can complicate cART. CNS-IRIS is described as paradoxical when neurological deterioration occurs despite cART and treatment of a known underlying CNS infection. Risk factors include low CD4+ nadir at time of cART initiation and rate of peripheral HIV viral load (VL) decay. Diagnosis is supported by neuroradiological findings and in some cases, evidence of T-cell infiltration in pathological specimens.5 CNS-IRIS is associated with high morbidity and mortality, largely attributed to exacerbated neuroinflammation associated with antigen specific T cells.6 Changes in antigen-presenting cells, exaggerated innate immune responses, and dynamics of CSF HIV viremia may also contribute to CNS-IRIS pathogenesis.6,7 To our knowledge, paradoxical CNS-IRIS has not been previously described in the treatment of AR-PCNSL.
We describe the case of a 54-year old man with HIV, not on cART, who presented with several months functional decline and more than two weeks progressively worsening altered mental status. Brain magnetic resonance imaging (MRI) showed a ring-enhancing left basal ganglia lesion with edema and mass effect. CSF examination was remarkable for 98 white blood cells (WBC)/mL and elevated protein. Microbiology studies were negative (Table 1).8,9 Peripheral CD4+ lymphocyte count was 2 cells/uL and HIV VL was 569,422 copies/mL. The patient started cART and was administered two weeks of empiric toxoplasmosis therapy. Despite antibiotics and decreasing HIV VL, he had no neurological improvement. Stereotactic needle brain biopsy was performed. Pathology revealed CD20+, EBV+ malignant lymphoid cells diagnostic of AR-PCNSL. He was referred to the National Cancer Institute (NCI) for treatment.
Table 1.
Results of repeated examinations of cerebrospinal fluid.
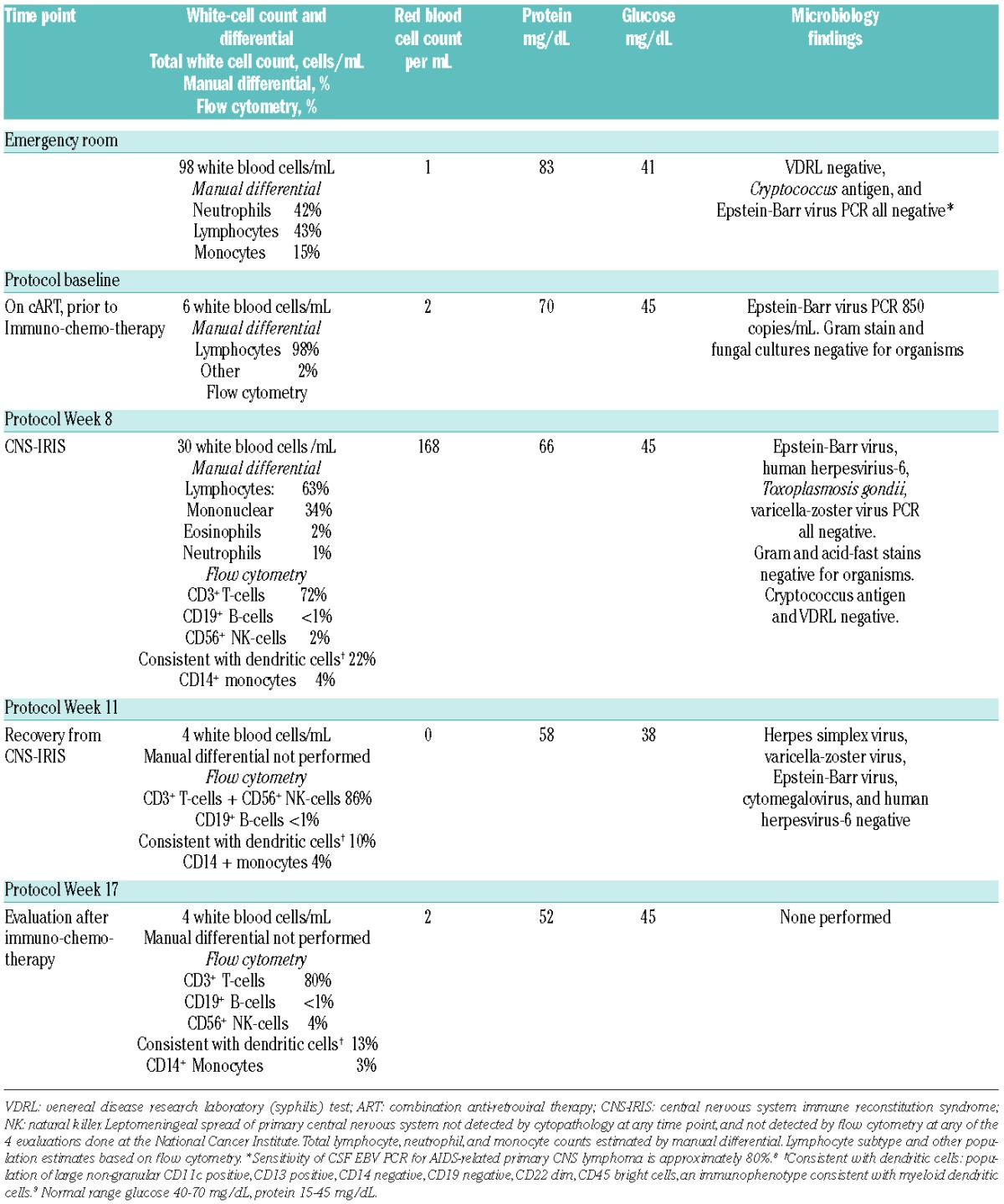
At the NCI, his Eastern Cooperative Oncology Group (ECOG) performance status was 3 due to neurological compromise. Neurological exam demonstrated mixed aphasia, with limited speech production (anomia) and reception (difficulty with complex commands), but relatively preserved repetition. Right arm strength was decreased (4/5 deltoids, biceps and triceps, 2/5 finger extensors). CD4+ count was 52 cells/uL (CD4+/CD8+ ratio 0.02) and HIV VL 397 copies/mL. Anti-toxoplasmosis antibodies (IgG and IgM) were undetectable. Repeat CSF examination (Table 1, Protocol Baseline) showed 6 WBC/mL, elevated protein and EBV viral load of 850 copies/mL. Cytopathology and flow cytometry revealed no leptomeningeal lymphoma. Computerized tomography (CT) showed no systemic adenopathy. Brain 18fluorodeoxyglucose positron emission tomography (18FDG-PET) demonstrated pathological left basal ganglia uptake, ipsilateral diffuse cortical metabolic suppression and crossed cerebellar diaschisis. Ophthalmological exam revealed no intraorbital lymphoma or CMV retinitis.
He was treated with rituximab, high-dose methotrexate with leucovorin rescue and continued cART on a research protocol. In brief, patients receive cART, rituximab 375 mg/m2 and methotrexate 6 grams/m2 with leucovorin rescue (high-dose MTX) every two weeks for 6 cycles, followed by 2 cycles of high-dose MTX alone (clinicaltrials.gov identifier: 00267865). Response criteria are based on a modification of International Primary CNS Lymphoma Collaborative Group Criteria.10 This protocol was approved by the NCI’s Center for Cancer Research Institutional Review Board, and written informed consent was obtained.
Over six weeks on protocol immunochemotherapy, he made steady gains in speech and use of his right hand. After 2 cycles, brain MRI showed decreased edema and resolution of ring enhancement (Figure 1). HIV VL was 174 copies/mL and CD4+ count had increased to 69 cells/uL (CD4+/CD8+ ratio 0.04).
Figure 1.
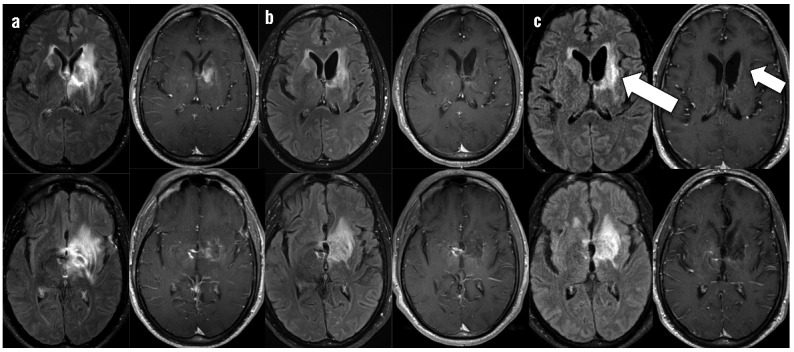
Brain MRI post-contrast fluid attenuated inversion recovery (FLAIR) and post-contrast T1 sequences throughout clinical course. For time points a–c, left column shows FLAIR post-gadolinium axial images and right column shows T1 post-gadolinium axial images at the same level. a) Base-line contrast ring-enhancing mass in the left basal ganglia with surrounding edema prior to initiation of immunochemotherapy, b) Improvement in edema and enhancement prior to initiating the 3rd cycle of immunochemotherapy, T1 post-contrast images shows no residual ring enhancement. c) New progression of enhancement (left arrow) seen at cycle 3 day 24 when the patient became obtunded, T1 post-contrast image shows no recurrence of primary tumor despite new enhancement (right arrow).
During his third cycle of therapy, approximately four months after initiating cART and six weeks after starting immunochemotherapy, he began to decline neurologically. Over ten days, he developed somnolence, worsening aphasia and right arm weakness, new right extrapyramidal symptoms, and confusion. At clinical nadir he was obtunded, requiring intubation for airway protection, with intact cranial reflexes but minimal response to painful stimuli. He was afebrile. Electroencephalogram showed generalized slowing. Serum C-reactive protein (CRP) was 96.8 mg/L (<3.0 mg/L). Brain MRI showed increased enhancement associated with the left basal ganglia lesion (Figure 1) but no other new pathology. CSF evaluation (Table 1, Protocol Week 8) showed 30 WBC/mL and elevated protein. All microbiology studies were negative. No lymphoma was identified by cytopathology.
Flow cytometry was also performed to evaluate for lymphoma and other lymphocyte subsets. CSF was immediately stabilized in RPMI with 10% fetal calf serum and processed within four hours of collection per consensus guidelines.11 Cells were stained for 30 min at room temperature with an antibody panel assessing CD3, CD5, CD10, CD11c, CD13,9 CD14, CD16, CD19, CD20, CD22, CD45, CD56, surface kappa and surface lambda.12 Cells were fixed in 1.0% paraformaldehyde and stored at 4°C for up to 12 hours before acquisition. Specimens were acquired using an 8-color multiparametric approach on a 3-laser FACS Canto II (BD Biosciences, San Jose, CA, USA) with DiVa 6.1.1 software and analyzed by FCS Express 3 software (DeNovo Software, Los Angeles, CA, USA). At least 5000 lymphocytes were acquired. Cell populations were analyzed by gating on forward scatter, side scatter, and CD45. (Table 1)
Differential diagnosis included progressive AR-PCNSL, methotrexate-induced leukoencephalopathy, other CNS infections not tested in the CSF, hyponatremia (intermittent hyponatremia over the first 3 cycles, sodium range, 124–134 mmol/L; at time of neurological deterioration, sodium 128 mmol/L, urine osm 420 mOsm/kg) and CNS-IRIS. Paradoxical CNS-IRIS was considered the most likely diagnosis. Solumedrol 1 gram intravenously daily was given for three days followed by a 10-day taper. On day 4 of steroids, he was extubated. One week later he returned to his prior neurological baseline; he was again able to play piano although with reduced right-sided dexterity. Hyponatremia, which was attributed to syndrome of inappropriate antidiuretic hormone secretion (SIADH) resolved. Repeat CSF evaluation three weeks later (Table 1, Protocol Week 11) showed a dramatic decrease in the inflammatory CSF cells that was sustained two months after solumedrol (Table 1, Protocol Week 17). The patient completed immunochemotherapy. Per protocol, methotrexate dosing was adjusted for renal function, then discontinued after the fourth cycle due to slow elimination leading to excessive treatment delays. He completed the planned 6 cycles of rituximab. After immunochemotherapy, he obtained a complete response. Eleven months since treatment for CNS-IRIS and twelve months since completing immunochemotherapy he has continued on cART, with his CD4+ count rising to 271cells/uL. He continues to improve neurologically, as evidenced by his most recent exam, which shows 5/5 strength in the left arm, 4+/5 in the right deltoid and 5/5 otherwise in the right arm. Speech has also improved with only mild word-finding difficulty during conversation.
IRIS is usually a consequence of an exaggerated activation of the immune system against a persistent antigen or viable pathogens.4 Antigen-specific immune reconstitution is hypothesized to be beneficial in the treatment of AR-PCNSL, however paradoxical CNS-IRIS has not previously been described in the setting of treatment for AR-PCNSL. In this case, timing of symptoms in relation to therapy, HIV suppression, demonstration of CD4+ immune reconstitution on cART, tumor response prior to symptoms, MRI findings, elevated CRP13 and pathological CSF lymphocytosis, along with no other evidence of an ongoing OI process; made paradoxical CNS-IRIS the most likely diagnosis. While peripheral CD4+ count only increased modestly, this may have been in part due to methotrexate. Furthermore, tumor-associated IRIS may also occur without CD4+ increases in the blood. However, CSF flow cytometry showed a new pathological mononuclear infiltrate consisting largely of CD3+ T cells and an unusually high number of large non-granular CD11c+/CD13+/CD14− consistent with myeloid dendritic cells.9,14 Rapid response to steroids, decreased CSF inflammatory cells; lack of further lesions on repeat MRI, and maintained clinical improvement over 13 months after discontinuing steroids further support this diagnosis. Steroid responsiveness is unlikely due to treatment of refractory CNS lymphoma that by all other measures was responding to therapy. Indeed, he obtained a complete response with the protocol-defined immunochemotherapy, and has experienced no relapse during follow up. Interestingly, life-threatening CNS-IRIS in this case appears to be due to immune response in the CNS to the EBV+ tumor itself, although CNS-IRIS to occult pathogens cannot be fully excluded.
Severe CNS-IRIS represents a neurological emergency and steroids are indicated to mitigate additional CNS injury due to exaggerated immune response.15 Duration of steroids depends on the specific antigenic stimuli, the antigenic burden and ability to treat the underlying CNS pathology. Benefits must be weighed against risk for other infectious complications in immunosuppressed patients. This case is the first report of treatment for EBV-associated AR-PCNSL complicated by paradoxical CNS-IRIS and demonstrates the importance of prompt recognition of this syndrome and potential reversibility.
Acknowledgments
The authors would like to thank Drs. Stefania Pittaluga, Armando Filie, and Katherine Calvo for review of cytopathology of the cerebrospinal fluid in this case.
Footnotes
Funding: This manuscript was supported by the Intramural Research Program of the National Institutes of Health.
Trial registration: clinicaltrials.gov identifier: NCT00267865
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Gasser O, Bihl FK, Wolbers M, Loggi E, Steffen I, Hirsch HH, et al. HIV patients developing primary CNS lymphoma lack EBV-specific CD4+ T cell function irrespective of absolute CD4+ T cell counts. PLoS Med. 2007;4(3):e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gopal S, Patel MR, Yanik EL, Cole SR, Achenbach CJ, Napravnik S, et al. Temporal trends in presentation and survival for HIV-associated lymphoma in the antiretroviral therapy era. J Natl Cancer Inst. 2013; 105(16):1221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uldrick TS, Pipkin S, Scheer S, Hessol NA. Factors associated with survival among patients with AIDS-related primary central nervous system lymphoma. AIDS. 2014;28(3):397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M, et al. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(4):251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manabe YC, Campbell JD, Sydnor E, Moore RD. Immune reconstitution inflammatory syndrome: risk factors and treatment implications. J Acquir Immune Defic Syndr. 2007;46(4):456–62. [DOI] [PubMed] [Google Scholar]
- 6.Mutnal MB, Schachtele SJ, Hu S, Lokensgard JR. T-cell reconstitution during murine acquired immunodeficiency syndrome (MAIDS) produces neuroinflammation and mortality in animals harboring opportunistic viral brain infection. J Neuroinflammation. 2013;10(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falcone EL, Adegbulugbe AA, Sheikh V, Imamichi H, Dewar RL, Hammoud DA, et al. Cerebrospinal fluid HIV-1 compartmentalization in a patient with AIDS and acute varicella-zoster virus meningomyeloradiculitis. Clin Infect Dis. 2013;57(5):e135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cingolani A, De Luca A, Larocca LM, Ammassari A, Scerrati M, Antinori A, et al. Minimally invasive diagnosis of acquired immunodeficiency syndrome-related primary central nervous system lymphoma. J Natl Cancer Inst. 1998;90(5):364–9. [DOI] [PubMed] [Google Scholar]
- 9.Bueno C, Almeida J, Lucio P, Marco J, Garcia R, de Pablos JM, et al. Incidence and characteristics of CD4(+)/HLA DRhi dendritic cell malignancies. Haematologica. 2004;89(1):58–69. [PubMed] [Google Scholar]
- 10.Abrey LE, Batchelor TT, Ferreri AJ, Gospodarowicz M, Pulczynski EJ, Zucca E, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23(22):5034–43. [DOI] [PubMed] [Google Scholar]
- 11.Kraan J, Gratama JW, Haioun C, Orfao A, Plonquet A, Porwit A, et al. Flow cytometric immunophenotyping of cerebrospinal fluid. Curr Protoc Cytom. 2008;Chapter 6:Unit 6 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jasper GA, Arun I, Venzon D, Kreitman RJ, Wayne AS, Yuan CM, et al. Variables affecting the quantitation of CD22 in neoplastic B cells. Cytometry B Clin Cytom. 2011;80(2):83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porter BO, Ouedraogo GL, Hodge JN, Smith MA, Pau A, Roby G, et al. d-Dimer and CRP levels are elevated prior to antiretroviral treatment in patients who develop IRIS. Clin Immunol. 2010; 136(1):42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pashenkov M, Huang YM, Kostulas V, Haglund M, Soderstrom M, Link H. Two subsets of dendritic cells are present in human cerebrospinal fluid. Brain. 2001;124(Pt 3):480–92. [DOI] [PubMed] [Google Scholar]
- 15.Lesho E. Evidence base for using corticosteroids to treat HIV-associated immune reconstitution syndrome. Expert Rev Anti Infect Ther. 2006;4(3):469–78. [DOI] [PubMed] [Google Scholar]


