Abstract
Sulodexide is a mixed glycosaminoglycan composed of heparin and dermatan sulfate. In this study, the anti-angiogenic effect of sulodexide was investigated using an oxygen-induced retinopathy (OIR) mouse model. The retinas of sham-injected OIR mice (P17) had a distinctive central area of nonperfusion, and this area was significantly decreased in sulodexide-injected mice. The number of neovascular tufts measured by SWIFT_NV and mean neovascular lumen number were significantly decreased in sulodexide-injected mice. Hyperbaric oxygen exposure resulted in increased levels of VEGF, MMP-2 and MMP-9, and when mice were treated with sulodexide, a dose-dependent reduction in VEGF, MMP-2 and MMP-9 levels was observed. Our results clearly demonstrate the anti-angiogenic effect of sulodexide and highlight sulodexide as a candidate supplementary substance to be used for the treatment of ocular pathologies that involve neovascularization. [BMB Reports 2014; 47(11): 637-642]
Keywords: Angiogenesis, MMP, Retinopathy, Sulodexide, VEGF
INTRODUCTION
The term “ischemic retinopathy” refers to a group of diseases including retinopathy of prematurity, diabetic retinopathy and retinal vein occlusion, where progressive irreversible visual loss occurs as a consequence of pre-retinal neovascularization (1). Among these diseases, retinopathy of prematurity (ROP) is known as a major cause of blindness in children exposed to high levels of oxygen soon after birth. Exposure to high levels of oxygen at certain stages of retinal vascular development results in retinal neovascularization and retinal detachment. Currently, the mainstay of ROP treatment is an attempt to halt retinal neovascularization by performing indirect laser photocoagulation and/or administering intravitreal anti-vascular endothelial growth factor (anti-VEGF) agents (2). Although these treatment strategies are somewhat effective, each carries a risk of structural and systemic complications (3). Thus, the demand for new and better treatment strategies continues to grow. Substantial effort has been devoted to finding new treatment modalities that confer less toxicity and more effectively improve the microcirculation.
Sulodexide is a highly purified mixed glycosaminoglycan preparation extracted from porcine intestines that has been used in various vasculopathies such as myocardial infarction, transient ischemic attacks, peripheral vascular insufficiency, retinal thrombosis, and diabetic retinopathy (4-8). Sulodexide consists of 80% low-molecular weight heparin and 20% dermatan sulphate (9, 10). This unique composition results in high bioavailability and a long plasma half-life. The compound is generally well tolerated and the low molecular weight of both fractions facilitates oral absorption (5, 10). Recently, sulodexide was shown to be effective in inhibiting neo-angiogenesis in a rat model of peritoneal perfusion (11). Sulodexide is a strong candidate for the treatment of ischemic retinopathy, as it not only possesses high bioavailability but may also possess anti-angiogenic properties to slow the formation of new retinal vessels.
Little is known about the anti-angiogenic effect of sulodexide in ocular tissues. However, sulodexide is currently prescribed widely for patients with various vasculopathies, and many of these patients have comorbid ocular diseases such as diabetic retinopathy or retinal vein occlusion. Notably, many of these comorbid ocular pathologies are related to ocular ischemia and neovascularization. Further research to characterize the compound is therefore essential. Moreover, determining whether sulodexide can attenuate new vessel formation induced by ischemia may be of use in alternative treatments for certain blinding diseases. In this study, in vivo models have been used to investigate the hypothesis that sulodexide can act as an anti-angiogenic agent. We show for the first time, to our knowledge, that the intraperitoneal administration of sulodexide reduced retinal neovascularization in a mouse model of oxygen-induced retinopathy (OIR).
RESULTS
Quantification of the central area of non-perfusion and retinal tufts in flat mounts
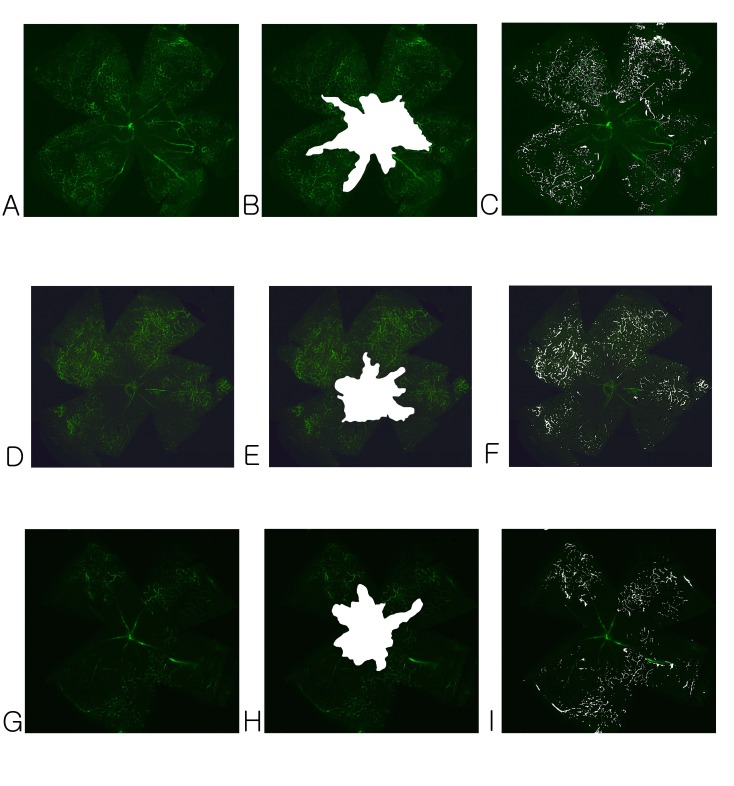
Retinal flat mount fluorescein staining was performed using FITC-dextran to quantify retinal neovascularization. Retinal flat mounts of samples obtained from the OIR group at P17 demonstrated a clear central area of non-perfusion (Fig. 1A). Retinas from the sham-injected group showed that 47.87 ± 4.51% of the total retinal area was ischemic (Fig. 1B, Table 1), whereas the retinas of low- (5 mg/kg) or high-dose (15 mg/kg) sulodexide-injected animals showed 37.75 ± 3.61% and 36.25 ± 2.96% ischemia, respectively. The central area of non-perfusion was significantly reduced in size (P < 0.01). Neovascular tufts on superficial vascular plexuses were also prominent in the sham-injected group (Fig. 1C). In contrast, in the pups treated with low- (5 mg/kg) or high-dose (15 mg/kg) sulodexide, the number of neovascular tufts was significantly reduced (Fig. 1F and 1I, respectively). No statistical significance was found when comparing the avascular area or the number of neovascular tufts between the low- (5 mg/kg) and high-dose (15 mg/kg) groups.
Fig. 1. FITC-dextran-perfused retinal flat mounts showing retinal vascularization (left column), avascular area (middle column), and neovascular tufts (right column). In the sham-injected group, a distinctive central ischemic area (A, B) and many neovascular tufts were observed (C). However, the area of ischemia was clearly reduced in the low- (D, E) and high-dose (G, H) sulodexide groups. Neovascular tufts were also significantly reduced in the low- (F) and high-dose (I) sulodexide groups.
Table 1. Quantification of avascular area and neovascular tufts in each group.
| Avascular Area (percentage of total retinal area) ± S.D. | Neovascular Tufts (percentage of total retinal area) ± S.D. | |
|---|---|---|
|
| ||
| Sham-treated control | 47.87 ± 4.51 | 3.98 ±0.67 |
| Sulodexide 5 mg/kg | 37.75 ± 3.61 | 2.56 ± 0.22 |
| Sulodexide 15 mg/kg | 36.25 ± 2.96 | 2.37 ± 0.19 |
| P value* | <0.01 | <0.01 |
*Statistical significance was tested by one-way ANOVA (Kruskal-Wallis test).
Vascular lumen measurement in hematoxylin and eosin (H&E)-stained cross-sections
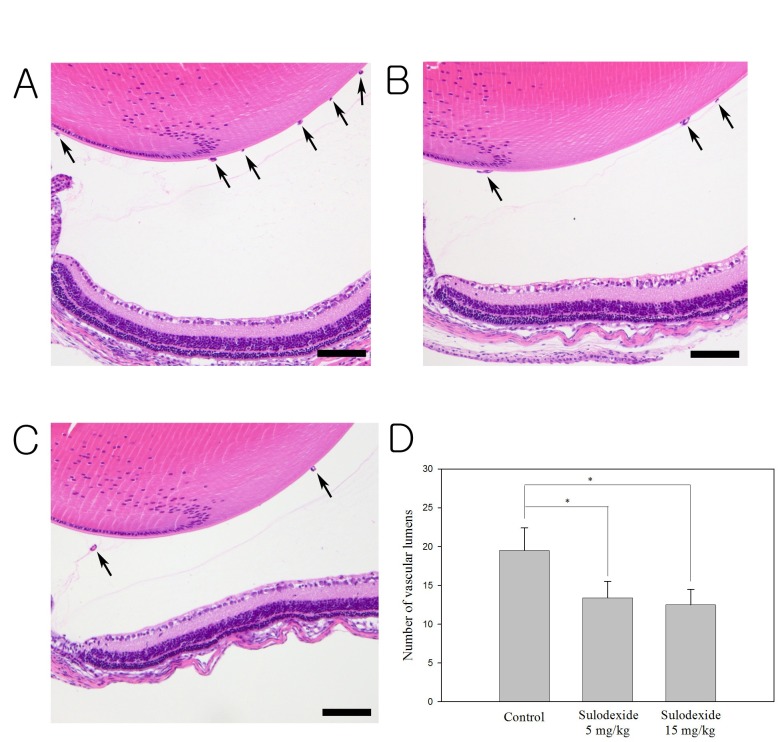
H&E staining revealed many neovascular lumens in the sham-treated group (Fig. 2A). In the sham-treated group, the average number of vascular lumens was 19.72 ± 3.25, whereas numbers in the low- (5 mg/kg, Fig. 2B) and high-dose (15 mg/kg, Fig. 2C) groups were 13.18 ± 1.32 and 11.90 ± 2.07, respectively. Sulodexide significantly reduced the number of vascular lumens in comparison to sham treatment (P < 0.05, Fig. 2D). There was no significant difference in the number of vascular lumens when the low- (5 mg/kg) and high-dose (15 mg/kg) groups were compared.
Fig. 2. H&E staining of the retina for the counting of neovascular lumens in each group. Numerous neovascular lumens were observed in the sham-treated group (A). There was a significant reduction in the number of neovascular lumens in both the low- (B) and high-dose (C) sulodexide groups compared to the control group (P < 0.05). Sulodexide significantly reduced the number of vascular lumens in comparison to sham treatment (D). Arrows indicate the vascular lumens of new vessels growing into the vitreous. Data shown are the mean ± SD; lumens were counted at ×40 magnification. Scale bars: 50 μm.
Effect of sulodexide on the expression of vascular endothelial growth factor (VEGF), matrix metalloproteinase (MMP)-2, MMP-9, platelet-derived growth factor (PDGF)-1, and PDGF-2
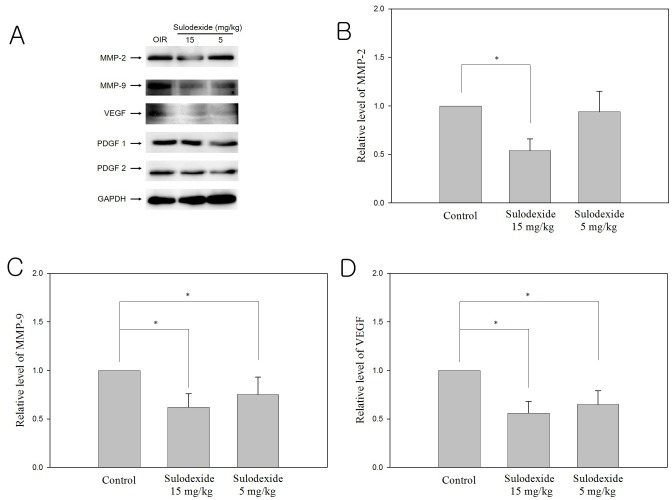
Changes in the expression of pro-angiogenic proteins such as VEGF, MMP-2, MMP-9, PDGF-1, and PDGF-2 in the sham and sulodexide groups were evaluated via western blot analysis (Fig. 3A). In the sham-treated group, exposure to hyperbaric conditions from P12 to P17 significantly increased the levels of pro-angiogenic factors VEGF, MMP-2 and MMP-9. In the highdose treatment group, sulodexide significantly downregulated the expression of MMP-2, although this effect was not observed in the low-dose group (Fig. 3B). Both dosages of sulodexide (5 mg/kg, 15 mg/kg) significantly inhibited the expression of MMP-9 and VEGF (Fig. 3C and 3D). However, at both investigated concentrations, sulodexide did not affect the expression of either PDGF-1 or PDGF-2.
Fig. 3. Effect of sulodexide on pro-angiogenic proteins following oxygen- induced retinopathy (western blot analysis). The protein levels (normalized to GAPDH) of MMP-2 (B), MMP-9 (C), and VEGF (D) in eyes injected with sham or sulodexide (5 mg/kg, 15 mg/kg) were compared. Sulodexide clearly inhibited the expression of MMP-2, MMP-9, and VEGF in a dose-dependent manner. Expression levels of PDGF-1 and PDGF-2 were not affected by sulodexide at high or low concentration.
DISCUSSION
Our results show that sulodexide can significantly attenuate retinal neovascularization in vivo. The anti-angiogenic properties of sulodexide were investigated using a mouse model of oxygen-induced retinopathy. The results presented here may provide insight into the potential mechanisms by which this compound exerts its anti-angiogenic effects. The inhibition of angiogenesis mediated by sulodexide is not unlike that described in a rat model of peritoneal perfusion (11). In that model, sulodexide was effective in reducing the thickness of the submesothelial layer of the parietal peritoneum but did not reduce VEGF levels in the dialysate. Remarkably, in our study, sulodexide not only effectively attenuated retinal neovascularization in a mouse model of OIR but also significantly reduced the expression of various pro-angiogenic proteins such as VEGF, MMP-2, and MMP-9. Although the reason for these discrepancies remains unclear at present, potential explanation include the specific characteristics of the organ itself or a particular microenvironment induced by different angiogenesis models.
Despite the clear demonstration of sulodexide’s efficacy as an anti-angiogenic compound, the precise mechanism by which this occurs is a matter for speculation. In our study, sulodexide significantly and dose-dependently inhibited the expression of VEGF, MMP-2, and MMP-9. VEGF is well known to stimulate endothelial cell activation and proliferation (12). It also alters endothelial cell gene expression, which results in the increased production of several proteases such as interstitial collagenase and matrix metalloproteinases (13-15). Previous research has also demonstrated positive feedback regulation between MMP-9 and VEGF under pathologic conditions (13). Thus, it is generally thought that the expression of MMP is closely linked to that of VEGF. Our results suggest that sulodexide inhibits neo-angiogenesis by blocking VEGF activity and thus downregulating the expression of MMPs. Note, while the levels of VEGF, MMP-2 and MMP-9 were significantly decreased by sulodexide, of the expression of PDGF-1 and PDGF-2 was not affected by low- or high-dose sulodexide. The link between VEGF and PDGF, especially in ocular tissue, remains to be elucidated. However, recent studies have suggested that VEGF may competitively block PDGF receptors, affecting cell signaling (16), and treatment with an anti-angiogenic compound inhibited the expression of PDGF-2 in a mouse model of OIR (17). Our results suggest that although sulodexide, similarly to other anti-angiogenic compounds, is effective in inhibiting VEGF, the precise mechanism of its anti-angiogenic activity may differ from those underlying the effects of other compounds.
In the Diabetic Nephropathy and Albuminuria Sulodexide (Di.N.A.S.) study, various dosages (50-200 mg/d) of sulodexide were administered to human subjects (18). No severe adverse effect was observed. Another study that evaluated the long-term efficacy and safety of sulodexide after acute myocardial infarction showed that this compound is well tolerated and is unassociated with significant adverse events (4). Although our in vivo experiments were performed in a mouse model, sulodexide was used at concentrations as high as 15 mg/kg without inducing any biochemical or morphological changes in the mouse retina. This finding is consistent with previous observations that sulodexide is a relatively non-toxic compound that could be used as a supplement in various vasculopathies.
Although originally thought to increase the risk of vitreous and retinal hemorrhage in diabetic retinopathy, sulodexide is now widely used in patients with diabetic retinopathy. In patients with type 2 diabetes, oral sulodexide administration restored vascular permeability (19). A large clinical study is now underway to evaluate the effects of sulodexide in the treatment of hard retinal exudates. Nonetheless, it was recently shown that sulodexide has no therapeutic benefit in type 2 diabetic nephropathy (20). The somewhat confusing nature of these reports may be due to the organ-specific cellular distribution of VEGF expression. Following ischemia, VEGF expression in the ocular tissue is markedly elevated. For example, the vitreous concentration of VEGF was significantly higher in patients with diabetic retinopathy (812 ± 1,108 pg/ml) compared to controls (1.7 ± 4.4 pg/ml) (21). However, both the plasma and urinary concentrations of VEGF in patients with diabetic nephropathy with proteinuria were relatively low (median 14.2 pg/ml, 245 pg/mg creatinine, respectively) (22). Thus, the effect of inhibiting VEGF and other pro-angiogenic factors may vary depending on the target tissue or organ.
In conclusion, sulodexide was shown to be effective, at least in part, in inhibiting retinal neovascularization in a mouse model of retinopathy. Sulodexide acts by inhibiting pro-angiogenic proteins including VEGF, MMP-1, and MMP-9. Because pathologic angiogenesis following retinal ischemia is one of the leading causes of blindness, drugs with low toxicity and potent anti-angiogenic activity are urgently needed. Our results show that sulodexide fits these criteria and can be suggested as a supplementary compound in the treatment of ocular pathologic angiogenesis. Detailed information on the pharmacokinetics of this substance in the eye will require further investigation.
MATERIALS AND METHODS
Materials
Animals
Breeding pairs of ICR mice were originally purchased from Daehan Biolink (Seoul, Korea). All mice were provided with food and water ad libitum and kept on a 12-h light/dark cycle. Experiments were designed and performed in accordance with the guidelines of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. All experimental protocols were approved by the Institutional Animal Care and Use Committee of Incheon St. Mary’s Hospital.
Oxygen-induced retinopathy in mice
ICR pups were randomly divided into three groups: a normoxia group (control group), an oxygen-exposed group (OIR group), and a sulodexide group; each group had one nursing mother and 5-7 pups. Oxygen-induced retinopathy was induced in ICR pups, as described previously (23). For the OIR model, the newborn pups were transferred at post-natal day (P) 7 along with their mother to a chamber supplied with 75 ± 2% oxygen, under continual monitoring with a ProOx 110 oxygen controller (Biospherix, Ltd., Lacona, NY, USA) for 120 h. On P12, the mice were returned to the room air and given daily intraperitoneal (IP) injections of vehicle (saline) or 5-15 mg/kg of sulodexide dissolved in the vehicle. The mice in the normoxia group were maintained in room air from birth until P17.
Whole flat mount fluorescent staining
Retinal flat mount fluorescein staining was performed as previously described (24). Before injection, FITC-dextran (FD2000S; Sigma-Aldrich, St. Louis, MO, USA) was dissolved in ultrapure water at a concentration of 50 mg/mL, centrifuged at 10,000 × g for 5 min, and collected as the supernatant. The FITC-dextran was protected from light during the preparation. In small animals such as pups, the intravenous injection of FITC-dextran is technically challenging. Moreover, direct injection of the dye into the heart can directly damage the heart of this frail animal. We therefore administered retro-orbital injections of FITC-dextran according to a previously published protocol (25). Mice were anesthetized on P17 with IP injections of ketamine (1%), xylazine (0.1%), and sodium chloride (0.9%) at a concentration of 0.1 ml/10 g mouse body weight. A 27-gauge needle with a 1-ml syringe attached was gently pierced 3 mm into the mouse’s left orbital venous sinus with the bevel on the needle facing forward at a 45° angle, allowing for the injection of 100 ml FITC-dextran. Eyes were carefully harvested and fixed in 4% paraformaldehyde for 40 min at room temperature. The cornea, iris, lens, and vitreous were gently removed under a stereomicroscope (Leica, Wetzlar, Germany). After making 4-6 radial incisions in the dissected retina, it was flattened with a coverslip. The vessels of the retina in each P17 OIR mouse were viewed under a confocal microscope (Carl Zeiss, Jena, Germany). The retinal segments were merged to generate an image of the total retina (Photoshop CS3; Adobe Systems Inc., San Jose, CA, USA).
Quantification of retinal neovascularization
Retinal neovascularization and vaso-obliteration were quantified as reported earlier (26). Both neovascularization and vasoobliteration are represented as percentages of total retinal area. Total retinal area and vasoobliteration were quantified using Adobe Photoshop CS3 as previously described (26). Neovascularization was quantified using a semi-automated computer program, SWIFT_NV, which is a set of macros run on NIH’s Image J software. Artifacts such as hyper fluorescent retinal ends, hyaloid vasculature, and cellular debris were excluded from the analysis. SWIFT_NV divides the retinal image into four quadrants, and a threshold was set to highlight the neovascular tufts while excluding normal vasculature from the quantification. The program thus calculates the area of the neovascular tufts to ultimately generate a stitched image highlighting the neovascular tufts on the original retinal image.
Tissue section preparation and HE staining
Pups from all groups were sacrificed on P17. Eyes of 4 mice from each group were analyzed (each experiment was performed minimum thrice, with 12 animal per group). Eyes were removed and fixed in 4% paraformaldehyde in PBS for 24 h and then embedded in paraffin. Sagittal sections of 8-μm thickness, each 32 μm apart, were cut parallel to the optic nerve. The sections were stained with H&E to assess the vascular lumens of new vessels growing into the vitreous via light microscopy (Carl Zeiss, Chester, VA, USA). Vascular lumens between the lens and the inner limiting membrane were counted in at least 5 sections from each eye by two independent observers following a masked protocol. The mean number of neovascular lumens per section was calculated for each group. Color photos were taken of histological sections obtained from the posterior margin of the mouse ciliary body to the retina (Fig. 2).
Western blot analysis
For immunoblot analysis, the entire retina was lysed in RIPA buffer [150 mM NaCl, 1.0% IGEPAL CA-630, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris, pH 8.0, and 1× protease inhibitors] and incubated for 30 min at 4℃. These samples were then sonicated and centrifuged at 14,000 × g for 10 min at 4℃, and the supernatant was transferred to a new microcentrifuge tube. The protein concentration in the entire retina was determined using a Bio-Rad Protein Assay kit (Life science, Hercules, CA, USA). Next, an equal volume of 4 × sample buffer [200 mM Tris, pH 6.8, 8% SDS, 0.4% bromophenol blue, 40% glycine, and 400 mM DTT] was added to the samples, followed by boiling for 3-5 min at 100℃. The protein samples were subsequently separated via SDS-gel electrophoresis on a 10-12% polyacrylamide gel and then transferred to a polyvinylidenedifluoride (PVDF) membrane (Hybond-P; GE Healthcare, Amersham, UK). After blocking with 5% skim milk in PBST buffer [8 g/l NaCl, 0.2 g/l KCl, 1.44 g/l Na2HPO4, 0.24 g/l NaH2HPO4, and 0.1% Tween-20] for 30 min, the membranes were incubated overnight at 4℃ with the indicated primary antibody diluted in PBST containing 1% skim milk. Mouse monoclonal antibodies against VEGF (1:1000) and PDGF (1:1000) were purchased from Abcam (Cambridge, MA, USA). Rabbit monoclonal antibodies against MMP-2 (1:1000), MMP-9 (1:1000) and GAPDH (1:1,000) were purchased from Cell Signaling Technology (Beverly, MA, USA). Subsequently, the membranes were incubated with the appropriate secondary antibody (horseradish peroxidase-conjugated goat anti-rabbit or horse anti-mouse secondary antibody [1:3,000, Santa Cruz Biotechnology, CA, USA]). The immunoreactive bands were visualized using enhanced chemiluminescence reagents (GE Healthcare, Amersham, UK) and then quantified via densitometry using an LAS-4000 image reader and Multi Gauge 3.1 software (Fuji Photo Film, Tokyo, Japan).
Statistical analysis
Each experiment was performed at least three times, and the data were expressed as the mean ± standard deviation (SD). The central area of non-perfusion and the number of neovascular tufts were compared between groups using one-way analysis of variance (ANOVA, Kruskal-Wallis test). Statistical analysis was performed using STATA/IC (version 11.2; Stata-Corp LP, TX, USA). P-values less than 0.05 were considered statistically significant.
Acknowledgments
The authors wish to acknowledge the financial support from the Catholic Medical Center Research Foundation provided for the program year of 2013. This study was supported by a generous grant from Aju Pharmacy. We thank Dr. Andreas Stahl for his generous gift of the SWIFT_NV software.
References
- 1.Aiello L. P., Pierce E. A., Foley E. D., Takagi H., Chen H., Riddle L., Ferrara N., King G. L., Smith L. E. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc. Natl. Acad. Sci. U. S. A. (1995);92:10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim J, Kim S. J., Chang Y. S., Park W. S. Combined intravitreal bevacizumab injection and zone I sparing laser photocoagulation in patients with zone I retinopathy of prematurity. Retina. (2014);34:77–82. doi: 10.1097/IAE.0b013e318296e26d. [DOI] [PubMed] [Google Scholar]
- 3.Simpson J. L., Melia M., Yang M. B., Buffenn A. N., Chiang M. F., Lambert S. R. Current role of cryotherapy in retinopathy of prematurity: a report by the American Academy of Ophthalmology. Ophthalmology. (2012);119:873–877. doi: 10.1016/j.ophtha.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Condorelli M., Chiariello M., Dagianti A., Penco M., Dalla Volta S., Pengo V., Schivazappa L., Mattioli G., Mattioli A., Brusoni B. IPO-V2: a prospective, multicenter, randomized, comparative clinical investigation of the effects of sulodexide in preventing cardiovascular accidents in the first year after acute myocardial infarction. J. Am. Coll. Cardiol. (1994);23:27–34. doi: 10.1016/0735-1097(94)90498-7. [DOI] [PubMed] [Google Scholar]
- 5.Lasierra-Cirujeda J., Coronel P., Aza M., Gimeno M. Use of sulodexide in patients with peripheral vascular disease. J. Blood Med. (2010);1:105–115. doi: 10.2147/JBM.S10558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kristova V., Liskova S., Sotnikova R., Vojtko R., Kurtansky A. Kurtansky Sulodexide improves endothelial dysfunction in streptozotocin-induced diabetes in rats. Physiol. Res. (2008);57:491–494. doi: 10.33549/physiolres.931506. [DOI] [PubMed] [Google Scholar]
- 7.Corbu C., Predoi D., Goicea D. Sulodexide treatment in retinal vein obstructions. Oftalmologia. (1996);40:393–397. [PubMed] [Google Scholar]
- 8.Weiss R., Niecestro R., Raz I. The role of sulodexide in the treatment of diabetic nephropathy. Drugs. (2007);67:2681–2696. doi: 10.2165/00003495-200767180-00004. [DOI] [PubMed] [Google Scholar]
- 9.Harenberg J. Review of pharmacodynamics, pharmacokinetics, and therapeutic properties of sulodexide. Med. Res. Rev. (1998);18:1–20. doi: 10.1002/(SICI)1098-1128(199801)18:1<1::AID-MED1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Lauver D. A., Lucchesi B. R. Sulodexide: a renewed interest in this glycosaminoglycan. Cardiovasc. Drug Rev. (2006);24:214–226. doi: 10.1111/j.1527-3466.2006.00214.x. [DOI] [PubMed] [Google Scholar]
- 11.Pletinck A., Van Landschoot M., Steppan S., Laukens D., Passlick-Deetjen J., Vanholder R., Van Biesen W. Oral supplementation with sulodexide inhibits neo-angiogenesis in a rat model of peritoneal perfusion. Nephrol. Dial. Transplant. (2012);27:548–556. doi: 10.1093/ndt/gfr370. [DOI] [PubMed] [Google Scholar]
- 12.Simonetti O., Lucarini G., Brancorsini D., Nita P., Bernardini M. L., Biagini G., Offidani A. Immunohistochemical expression of vascular endothelial growth factor, matrix metalloproteinase 2, and matrix metalloproteinase 9 in cutaneous melanocytic lesions. Cancer. (2002);95:1963–1970. doi: 10.1002/cncr.10888. [DOI] [PubMed] [Google Scholar]
- 13.Hollborn M., Stathopoulos C., Steffen A., Wiedemann P., Kohen L., Bringmann A. Positive feedback regulation between MMP-9 and VEGF in human RPE cells. Invest. Ophthalmol. Vis. Sci. (2007);48:4360–4367. doi: 10.1167/iovs.06-1234. [DOI] [PubMed] [Google Scholar]
- 14.Oshima Y., Oshima S., Nambu H., Kachi S., Hackett S. F., Melia M., Kaleko M., Connelly S., Esumi N., Zack D. J., Campochiaro P. A. Increased expression of VEGF in retinal pigmented epithelial cells is not sufficient to cause choroidal neovascularization. J. Cell Physiol. (2004);201:393–400. doi: 10.1002/jcp.20110. [DOI] [PubMed] [Google Scholar]
- 15.Yafai Y., Iandiev I., Wiedemann P., Reichenbach A., Eichler W. Retinal endothelial angiogenic activity: effects of hypoxia and glial (Muller) cells. Microcirculation. (2004);22:577–586. doi: 10.1080/10739680490503375. [DOI] [PubMed] [Google Scholar]
- 16.Pennock S., Kazlauskas A. Vascular endothelial growth factor A competitively inhibits platelet-derived growth factor (PDGF)-dependent activation of PDGF receptor and subsequent signaling events and cellular responses. Mol. Cell Biol. (2012);32:1955–1966. doi: 10.1128/MCB.06668-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeNiro M., Al-Mohanna F. H., Al-Mohanna F. A. Inhibition of reactive gliosis prevents neovascular growth in the mouse model of oxygen-induced retinopathy. PLoS One. (2011);6:e22244. doi: 10.1371/journal.pone.0022244. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Gambaro G., Kinalska I., Oksa A., Pont P., Hertlova M., Olsovsky J., Manitius J., Fedele D., Czekalski S., Perusicova J., Skrha J., Taton J., Grzeszczak W., Crepaldi G. Oral sulodexide reduces albuminuria in microalbuminuric and macroalbuminuric type 1 and type 2 diabetic patients: the Di.N.A.S. randomized trial. J. Am. Soc. Nephrol. (2002);13:1615–1625. doi: 10.1097/01.ASN.0000014254.87188.E5. [DOI] [PubMed] [Google Scholar]
- 19.Broekhuizen L. N., Lemkes B. A., Mooij H. L., Meuwese M. C., Verberne H., Holleman F., Schlingemann R. O., Nieuwdorp M., Stroes E. S., Vink H. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia. (2010);53:2646–2655. doi: 10.1007/s00125-010-1910-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Packham D. K., Wolfe R., Reutens A. T., Berl T., Heerspink H. L., Rohde R., Ivory S., Lewis J., Raz I., Wiegmann T. B., Chan J. C., de Zeeuw D., Lewis E. J., Atkins R. C. Sulodexide fails to demonstrate renoprotection in overt type 2 diabetic nephropathy. J. Am. Soc. Nephrol. (2012);23:123–130. doi: 10.1681/ASN.2011040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe D., Suzuma K., Suzuma I., Ohashi H., Ojima T., Kurimoto M., Murakami T., Kimura T., Takagi H. Vitreous levels of angiopoietin 2 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Am. J. Ophthalmol. (2005);139:476–481. doi: 10.1016/j.ajo.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Kim N. H., Oh J. H., Seo J. A., Lee K. W., Kim S. G., Choi K. M., Baik S. H., Choi D. S., Kang Y. S., Han S. Y., Han K. H., Ji Y. H., Cha D. R. Vascular endothelial growth factor (VEGF) and soluble VEGF receptor FLT-1 in diabetic nephropathy. Kidney Int. (2005);67:167–177. doi: 10.1111/j.1523-1755.2005.00067.x. [DOI] [PubMed] [Google Scholar]
- 23.Smith L. E., Wesolowski E., McLellan A., Kostyk S. K., D’Amato R., Sullivan R., D’Amore P. A. Oxygen-induced retinopathy in the mouse. Invest. Ophthalmol. Vis. Sci. (1994);35:101–111. [PubMed] [Google Scholar]
- 24.Li S., Li T., Luo Y., Yu H., Sun Y., Zhou H., Liang X., Huang J., Tang S. Retro-orbital injection of FITC-dextran is an effective and economical method for observing mouse retinal vessels. Mol. Vis. (2011);17:3566–3573. [PMC free article] [PubMed] [Google Scholar]
- 25.Nanni C., Pettinato C., Ambrosini V., Spinelli A., Trespidi S., Rubello D., Al-Nahhas A., Franchi R., Alavi A., Fanti S. Retro-orbital injection is an effective route for radiopharmaceutical administration in mice during small-animal PET studies. Nucl. Med. Commun. (2007);28:547–553. doi: 10.1097/MNM.0b013e3281fbd42b. [DOI] [PubMed] [Google Scholar]
- 26.Connor K. M., Krah N. M., Dennison R. J., Aderman C. M., Chen J., Guerin K. I., Sapieha P., Stahl A., Willett K. L., Smith L. E. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat. Protoc. (2009);4:1565–1573. doi: 10.1038/nprot.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]