Abstract
Neutrophils play an important role in the initiation of innate immunity against infection and injury. Although many different types of G-protein coupled receptors are functionally expressed in neutrophils, no reports have demonstrated functional expression of umami taste receptor in these cells. We observed that mouse neutrophils express the umami taste receptor T1R1/T1R3 through RNA sequencing and quantitative RT-PCR analysis. Stimulation of mouse neutrophils with L-alanine or L-serine, which are ligands for the umami taste receptor, elicited not only ERK or p38 MAPK phosphorylation but also chemotactic migration. Moreover, addition of L-alanine or L-serine markedly reduced the production of several cytokines including TNF-α induced by lipopolysaccharide (LPS) through inhibition of NF-κB activity or STAT3 phosphorylation in neutrophils. Our findings demonstrate that neutrophils express the umami taste receptor, through which tastants stimulate neutrophils, resulting in chemotactic migration, and attenuation of LPS-induced inflammatory response. [BMB Reports 2014; 47(11): 649-654]
Keywords: Chemotaxis, Cytokine, Inflammation, Neutrophil, Umami taste receptor T1R1/T1R3
INTRODUCTION
Chemoreception is a process by which organisms or cells respond to their external chemical environment. Detection depends on the specialized chemosensory (odorant and taste) receptors that are a subset of the G protein-coupled receptor (GPCR) family, and were originally reported in the olfactory sensory neurons of olfactory epithelium and taste receptor cells of lingual epithelium (1, 2). For example, taste receptors are divided into sweet receptor (T1R2/T1R3 heterodimer), umami receptor (T1R1/T1R3 heterodimer) and bitter receptors (T2Rs). Sweet and bitter taste receptors have been suggested as novel regulators of innate immunity by regulating anti-microbial peptide production in the respiratory tract (3). However, chemosensory receptors have recently been reported to be expressed in diverse tissues, where they are thought to have additional roles (4, 5).
Among the taste receptors umami taste receptor recognizes amino acids (6). Since amino acids are mainly generated by food digestion, umami taste receptor expression has been extensively studied in the organs involved in food recognition, food intake, and digestion (7-9). However previous reports also demonstrated that injury and infection result in the increased release of amino acids in liver, knee joint, muscle and plasma (10-12). It has been also reported that injury and infection causes the increased whole body flux of amino acids throughout the body (13). Although amino acid release is observed in inflammation, injury and infection, the pathological role of amino acid and the detection of amino acid by inflammatory or immune cells have not been studied.
Neutrophils, the first immune cells recruited into the event area such as infected or injury sites, initiate innate immune response by sensing extracellular stimuli such as N-formyl peptides (14, 15). Binding of N-formyl peptides to their specific cell surface receptors, formyl peptide receptors (FPRs) elicits chemotactic migration of the cells into event area such as an infected site, where they induce immune responses by engulfing and killing pathogens, or secreting several soluble factors (14, 15). The secreted soluble factors such as cytokines, mediate immune responses not only by recruiting additional leukocytes or inflammatory cells into the area, but also by modulating cellular activities (14). FPRs are GPCRs that induce intracellular signaling via heterotrimeric G-protein (15). Neutrophils express so diverse GPCRs including FPRs and several chemokine receptors, and these receptors mediate important functional activity of the cells (14). Although many GPCRs have been reported to regulate neutrophil activity, identification of additional GPCRs in neutrophils is necessary.
In this study, we investigated whether mouse neutrophils express the umami taste receptor, which recognize amino acid, by performing RNA sequencing and quantitative RT-PCR (qRT-PCR) analysis. We also tested whether the receptor is functional in regards to neutrophil activity.
RESULTS
Mouse neutrophils express taste receptors
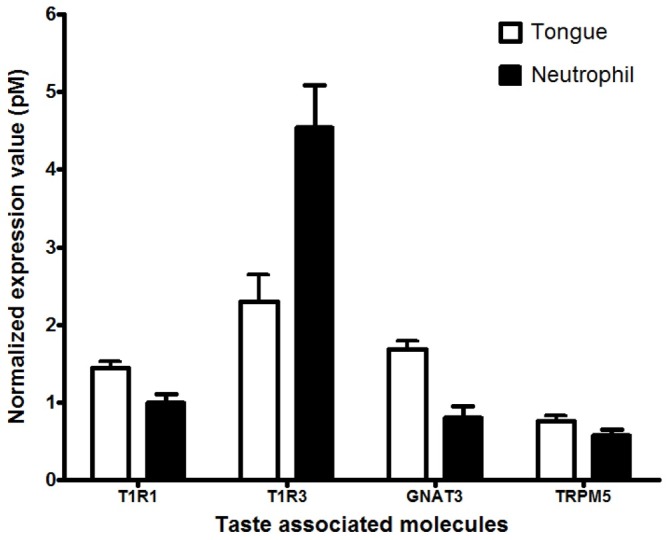
We investigated whether mouse neutrophils express taste receptor by using RNA-seq. Interestingly, heterodimer T1R1/T1R3 umami taste receptor and 6 T2Rs (135, 143, 126, 120, 118, and 117) bitter taste receptor mRNAs were identified (Table 1). In the lingual epithelium, the taste receptor 1 (T1R) family comprises of three members, T1R1, T1R2, and T1R3, and the taste receptor 2 (T2R) family presents ∼30 T2Rs members (2). Heterodimer T1R2/T1R3 and T1R1/T1R3 functions as sweet and umami receptors, respectively and T2Rs mediate bitter taste (2). The mouse umami receptor was specifically characterized as a broad-spectrum L-amino acid sensor (2, 16). Very recently, Lee and colleagues proposed that T1R2/T1R3 and T2Rs were human respiratory innate immunity regulators (3). The T2Rs responded to bitter microbial products and mediated antimicrobial peptide secretion regulation and T1R2/ T1R3 inhibited T2Rs-mediated response. To confirm the result of RNA-seq analysis, we performed qRT-PCR analysis using each specific primer for T1R1, T1R3, and taste receptor signaling cascade-associated genes such as GNAT3 and TRPM5 and the total RNAs isolated from mouse primary neutrophils and tongue as a positive control. Mouse neutrophils were found to express T1R1/T1R3 and all taste signaling genes with different expression levels (Fig. 1). The expression was also confirmed by PCR product sequencing (data not shown). This result suggests that neutrophils may function to detect L-amino acid via T1R1/T1R3 in innate immune response.
Table 1. List of taste receptors expressed in mouse neutrophils.
| Symbol | Reference Sequence Accession no | Expression values (RPKM) | Annotation |
|---|---|---|---|
|
| |||
| TAS1R3 (T1R3) | NM_031872.2 | 0.759 | Umami/sweet receptor |
| TAS2R135 (T2R135) | NM_199159.1 | 0.35 | Bitter receptor |
| TAS1R1 (T1R1) | NM_031867.2 | 0.201 | Umami receptor |
| TAS2R143 (T2R143) | NM_001001452.1 | 0.144 | Bitter receptor |
| TAS2R126 (T2R126) | NM_207028.1 | 0.046 | Bitter receptor |
| TAS2R120 (T2R120) | NM_207023.1 | 0.032 | Bitter receptor |
| TAS2R118 (T2R118) | NM_207022.1 | 0.016 | Bitter receptor |
| TAS2R117 (T2R117) | NM_207021.1 | 0.012 | Bitter receptor |
Expression values are represented in reads/kb/million (RPKM).
Fig. 1. Identification of the T1R1/T1R3 umami taste receptor in mouse neutrophils. Whole mouse tongue tissues, mouse neutrophils were isolated from the bone marrow of 8-week-old C57BL/6 mice femurs and tibias. T1R1/T1R3 and taste signaling-associated components (GNAT3 and TRPM5) mRNA were quantified by qRT-PCR (n=6-9). Values are reported as the mean pM of mRNA±SEM and were quantified in reference to the known standard gene and normalized to eukaryotic Elongation Factor2 RNA.
L-alanine or L-serine stimulates neutrophil chemotactic migration
After we determined that mouse neutrophils express the umami taste receptor, T1R1/T1R3 (Table 1 and Fig. 1), we next investigated whether the umami receptor is functional in mouse neutrophils. Activation of cell surface receptors induces diverse intracellular signaling molecules including intracellular calcium increase and mitogen-activated protein kinase (MAPK) activation (17). The activation of the T1R1/T1R3 taste receptor also induces intracellular calcium increase (6). Therefore, we tested the effects of L-alanine or L-serine on intracellular calcium levels in mouse neutrophils. Although the human T1R1/T1R3 receptor is stimulated with L-glutamate, mouse T1R1/T1R3 exhibits increases in the L-alanine and L-serine activity instead of with L-glutamate (16). Neither L-alanine nor L-serine induced intracellular calcium increase in mouse neutrophils in this study (Fig. 2A). As a positive control, a formyl peptide receptor agonist WKYMVm (18), strongly induced intracellular calcium increases in the cells (Fig. 2A). However, stimulation of mouse neutrophils with L-alanine elicited ERK phosphorylation in mouse neutrophils (Fig. 2B). The amino acid-induced ERK phosphorylation was apparent 2-30 min after stimulation (Fig. 2B). Unlike L-alanine, L-serine stimulated p38 MAPK phosphorylation transiently, showing apparent effects at 2-5 min after stimulation in mouse neutrophils (Fig. 2B).
Fig. 2. L-alanine or L-serine stimulates chemotactic migration in mouse neutrophils. (A) Fura-2 loaded neutrophils were stimulated with L-alanine (100 mM), L-serine (100 mM), or WKYMVm (1 μM). The relative intracellular calcium concentrations are expressed as fluorescence ratios. (B) Neutrophils were stimulated with L-alanine (100 mM) or L-serine (100 mM) for 0, 2, 5, 10, and 30 min. The levels of phosphorylated ERK or p38 MAPK were measured by Western blot analysis. The data represents three independent experiments (A and B). (C) Various concentrations (0, 0.1, 1, 10, or 100 mM) of L-alanine or L-serine were used for the chemotaxis assay (C). Vehicle or PTX (1 μg/ml) pretreated cells were subjected to the chemotaxis assay with 100 mM of L-alanine, 100 mM of L-serine, or 100 nM of WKYMVm (D). The number of migrated cells was determined by counting in a high-power field (400×). Data are presented as the mean±SEM of triplicate experiments. *P<0.05, **P<0.01, ***P<0.001, compared with the vehicle control (C and D).
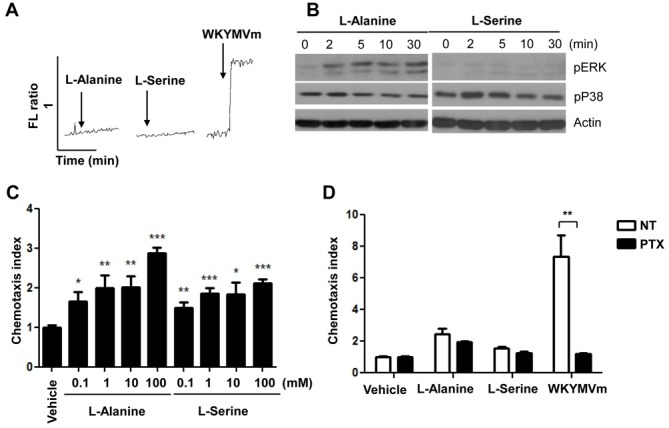
In this study, we examined the effects of L-alanine or L-serine on the chemotactic migration of neutrophils. Stimulation of mouse neutrophils with several different concentrations of L-alanine caused chemotactic migration (Fig. 2C). We determined that 100 mM of L-alanine elicited approximately a 3-fold neutrophil migration response (Fig. 2C). L-serine also significantly increased neutrophil migration, showing concentration-dependency (Fig. 2C). Several previous reports demonstrated that neutrophil chemotaxis is mediated by pertussis toxin (PTX)-sensitive G-protein(s) (15, 19). We also tested the effect of PTX on neutrophil migration induced by L-alanine or L-serine. As shown in Fig. 2D, neutrophil migration induced by L-alanine or L-serine was not inhibited by PTX. However WKYMVm-induced neutrophil migration was almost completely inhibited by PTX (Fig. 2D). The results indicate that L-alanine or L-serine-induced neutrophil migration is mediated independently of PTX-sensitive G-protein(s).
L-alanine or L-serine blocks LPS-stimulated cytokine production in neutrophils
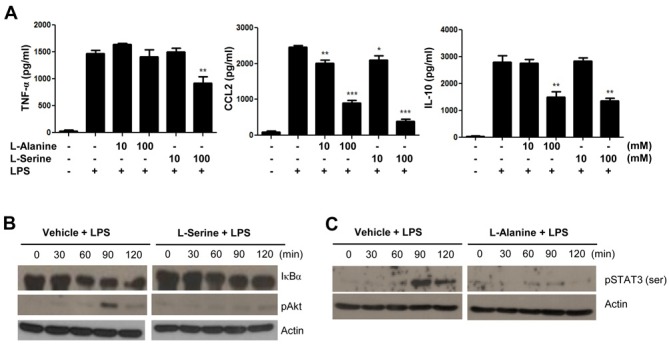
We tested the effects of L-alanine or L-serine on the production of several cytokines in mouse neutrophils. Stimulation of mouse neutrophils with L-alanine or L-serine did not induce the production of several cytokines such as TNF-α, CCL2, and IL-10 (data not shown). To observe the effects of L-alanine or L-serine on the production of several LPS-induced cytokines, we added the amino acid prior to LPS stimulation. Interestingly, the addition of L-alanine or L-serine dramatically inhibited LPS-stimulated CCL2 and IL-10 production from mouse neutrophils (Fig. 3A). In case of L-serine, it also significantly inhibited LPS-stimulated TNF-α production in the cells (Fig. 3A).
Fig. 3. L-alanine or L-serine strongly blocks LPS-induced signaling in mouse neutrophils. (A) Neutrophils were stimulated with vehicle (PBS), L-alanine (10 mM, 100 mM), or L-serine (10 mM, 100 mM) for 30 min, and were stimulated with PBS or LPS (1 μg/ml) for 24 h. TNF-α, CCL-2, and IL-10 levels were measured by ELISA. Data are expressed as the mean±SEM (n=3). *P<0.05, **P<0.01, ***P<0.001, compared with the vehicle control (A). Neutrophils were stimulated with vehicle or L-serine (100 mM, for B) or L-alanine (100 mM, for C) for 30 min, and were stimulated with PBS or LPS (1 μg/ml) for 0, 30, 60, 90, or 120 min. The levels of I-κB, phosphorylated Akt or phosphorylated STAT3 were measured by Western blot analysis. The data represents three independent experiments (B and C).
To test whether L-alanine or L-serine decreased LPS-stimulated cytokine production via nonspecific cell death, we examined the effect of L-serine or L-alanine on the cell viability. Stimulation of 10 or 100 mM of L-serine or L-alanine did not induce a significant reduction in cell viability, which was measured by tryphan blue staining (data not shown). The results indicate that the inhibitory effects of L-serine or L-alanine on LPS-induced cytokine production are not mediated by amino acid cytotoxicity.
For several cytokines to be expressed, including TNF-α, CCL2, and IL-10 by LPS, NF-κB activity is required downstream of the TLR4-induced signaling (20). NF-κB activation is accompanied by I-κB degradation (21). We also found that stimulation of mouse neutrophils with LPS elicited I-κB degradation, indicating NF-κB activation (Fig. 3B). Addition of L-serine markedly attenuated the LPS-induced I-κB degradation at 60-120 min after LPS stimulation (Fig. 3B). LPS-stimulated Akt phosphorylation was also markedly inhibited by L-serine in mouse neutrophils (Fig. 3B).
Activation of TLR4, downstream of LPS-induced signaling, induces STAT3 phosphorylation, which is a prerequisite for IL-1 receptor antagonist gene expression (22). In this study, we also found that stimulation of mouse neutrophils with LPS elicited STAT3 phosphorylation, which is strongly inhibited by L-alanine (Fig. 3C).
DISCUSSION
The umami T1R1/T1R3 heterodimer identified as a mechanism for detecting umami taste in the lingual epithelium taste cells (6) and has recently been suggested to be expressed in the intestine, pancreatic β-cells, and heart (23-26). The umami receptor has also been proposed to as control mechanisms in the secretion of hormones, such as cholecystokinin, insulin, and duodenal HCO3- as an internal amino acid sensor through nutrient availability. The receptor was also characterized as an early sensor of amino acid availability, which regulates the mammalian rapamycin complex 1 (mTORC1) target through T1R1/T1R3 for protein synthesis and cell growth (27). The expression and function of T1R1/T1R3 have been increasingly reported outside of taste buds and limited to the gastrointestinal tract. Recently, two members of the taste receptor family, the T1R2/T1R3 heterodimeric sweet receptor and T2Rs bitter receptors were reported to regulate innate immunity by regulating antimicrobial peptide production (3). In addition to food dietary source, amino acids (ligands for umami taste receptor) can be released under infectious or injury condition (10-12), which can act as alarm signal to the host. The alarm signal will be useful to recruit neighboring leukocytic cells into the event area to control the injury or infection. Because neutrophils are first innate immune cells responding infection and injury, we investigated whether mouse neutrophils express amino acid (umami) taste receptor. In this study, we found that mouse neutrophils express the functional umami taste receptor T1R1/T1R3. Stimulation of mouse neutrophils with L-alanine or L-serine, the umami receptor ligand elicited neutrophil migration (Fig. 2). The results suggest that amino acid-driven neutrophil recruitment will be useful response to control infection, and umami receptor may mediate this process. Additionally, we also observed that L-alanine or L-serine blocked the production of proinflammatory cytokines such as CCL2 induced by LPS in mouse neutrophils (Fig. 3). The results also suggest that umami receptor may have putative role in the regulation of infection or injury-induced inflammatory response.
Based on a previous report, T1R1/T1R3 activation induces the activation of PLCβ and the subsequent release of intracellular calcium which is potentiated by inosine-5'-monophosphate stimulation (6). However, in this study we failed to identify an intracellular calcium increase using L-alanine or L-serine stimulation in the absence or presence of inosine-5'-monophosphate (Fig. 2A and data not shown). Unlike calcium signaling, L-alanine or L-serine markedly inhibited LPS-induced signaling and cellular responses (Fig. 3). Based on the results of our analysis and previous reports, we propose that L-alanine or L-serine may stimulate T1R1/T1R3, which demonstrates a unique signaling pathway independent of PLCβ and intracellular calcium increases in mouse neutrophils.
We also found that the addition of L-serine attenuated I-κB degradation induced by LPS in mouse neutrophils (Fig. 3B) which is affected by LPS-induced cytokine production molecular mechanisms. Because I-κB degradation is associated with NF-κB activation downstream of TLR4, the results indicate that L-serine blocks LPS-induced NF-κB activation in the cells. The addition of L-serine or L-alanine markedly blocks LPS-stimulated Akt phosphorylation or STAT3 phosphorylation in mouse neutrophils. These results suggest that the activation of T1R1/T1R3 by L-alanine or L-serine may interrupt LPS-induced TLR4 signaling in neutrophils.
In conclusion, we observed that mouse neutrophils express mRNAs for T1R1 and T1R3, and L-alanine or L-serine (ligand for T1R1/T1R3) induces at least two responses; one for chemotactic migration activation and the other to inhibit the LPS-induced inflammatory response. This finding suggests a novel insight into the functional role of the umami taste receptor in mouse neutrophils.
MATERIALS AND METHODS
Mouse neutrophil isolation
All animal procedures were approved by the Daegu Gyeongbuk Institute of Science & Technology Institutional Animal Care and Use Committee (DGIST-IACUC). Mouse neutrophils were isolated according to a previous report (28).
RNA isolation, RNA sequencing, and qRT-PCR analysis
Total RNA was extracted using a MagNA lyser (Roche Molecular Diagnostics GmbH, Penzburg, Germany) with TRIzol reagent (Invitrogen, Carlsbad, CA, USA). For RNA sequencing (RNA-seq), sequencing library was prepared using NEBNext RNA prep kit (NEB, USA). Sequencing was performed using HiSeq 2500 (Illumina, USA) with rapid run 150 bp PE mode. Total 39 million reads were produced and mapped to the house mouse (Mus musculus) genome. Reads with quality score >20 were 90% and reads with quality score >30 were 80%. Final transcript levels of all known neutrophil genes were calculated in RPKM (Reads per kilo base per million). qRT-PCR analysis was performed according to standard procedure using a PrimeScriptTM 1st strand cDNA Synthesis kit (Takara Bio Inc. Shiga, Japan). The forward and reverse primer sequences were 5’-CACGGGAAGAACAATCAGGT-3’ and 5’-AAATGTCCCAG CTTCACAG-3’ for mouse T1R1; 5’-CCAGTGAGTCTTGGCTG ACA-3’ and 5’-TTCAGTGAGGCACAGAATGC-3’ for T1R3; 5’-TCTACATTCCCGGGTGAAAA-3’ and 5’-GCAGGTGACTCCTT CAAAGC-3’ for GNAT3; 5’-GCAAATCCCTCTGGATGAAA-3’ and 5’-TAGCTGAACATGGCGATCAG-3’ for TRPM5. The relative difference in expression for each sample in individual experiments was determined by normalizing the Ct value for each gene against the Ct value of eEF-2 and was calculated using the relative expression value to an equation using the standard concentration curve of the pCI::Rho-olfr544 construct.
Intracellular calcium concentration measurement
The intracellular calcium level was measured by Grynkiewicz's method using Fura-2/AM as described previously (29, 30). Fura-2/AM loaded neutrophils were stimulated with L-alanine (100 mM), L-serine (100 mM) or WKYMVm-NH2 (1 μM). Fluorescence changes were monitored at 500 nm at excitation wavelengths of 340 nm and 380 nm using a RF-5301PC spectrofluorophotometer (Shimadzu Instruments Inc., Kyoto, Japan).
Western blot analysis
Neutrophils were stimulated with L-alanine or L-serine for various time periods. To test the effects of amino acids on the LPS-stimulated signaling, mouse neutrophils were preincubated with L-alanine or L-serine for 30 min prior to LPS (1 μg/ml) stimulation for various time periods. Western blot analysis was conducted according to standard procedure (31).
Chemotaxis assay
Chemotaxis assays were performed according to a previous report using a multiwall chamber (Neuroprobe Inc., Gaithersburg, MD, USA) (18). Briefly, mouse neutrophils were applied to polycarbonate filters (3-μm pore size) for 1.5 h at 37℃. Migrated cells were stained with hematoxylin (Sigma-Aldrich, St. Louis, MO, USA), and counted under a light microscope as previously described (18).
Cytokine measurements
Several cytokine levels were measured according to a previous report (32). Neutrophils (5×105 cells/300 μl) were stimulated by LPS in the absence or presence of L-alanine or L-serine for 24 h. Culture supernatants were collected and analyzed by ELISA to measure TNF-α, CCL2, or IL-10 according to a previous report (32).
Data analysis
Results are expressed as the mean±SEM. The Student’s t-test was used to compare individual treatments with their respective control values. Statistical significance was set at P<0.05.
Acknowledgments
We thank Dr. Mee-Ra Rhyu for valuable discussions. This work was supported by the National Research Foundation of Korea [No. 2012R1A2A2A01007751 (YB) and 2013R1A1A 2009145 (JK)], by the DGIST R&D Program of the Ministry of Science, ICT & Future Planning (14-BD-06), and by the support of "Cooperative Research Program for Agriculture Science & Technology Development (Project title: National Agricultural Genome Program, Project No. PJ010338)" Rural Development Administration, Republic of Korea.
References
- 1.Buck L., Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. (1991);65:175–187. doi: 10.1016/0092-8674(91)90418-X. [DOI] [PubMed] [Google Scholar]
- 2.Zhao G. Q., Zhang Y., Hoon M. A., Chandrashekar J., Erlenbach I., Ryba N. J., Zuker C. S. The receptors for mammalian sweet and umami taste. Cell. (2003);115:255–266. doi: 10.1016/S0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- 3.Lee R. J., Kofonow J. M., Rosen P. L., Siebert A. P., Chen B., Doghramji L., Xiong G., Adappa N. D., Palmer J. N., Kennedy D. W., Kreindler J. L., Margolskee R. F., Cohen N. A. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J. Clin. Invest. (2014);124:1393–1405. doi: 10.1172/JCI72094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deshpande D. A., Wang W. C., McIlmoyle E. L., Robinett K. S., Schillinger R. M., An S. S., Sham J. S., Liggett S. B. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat. Med. (2010);16:1299–1304. doi: 10.1038/nm.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang N., Koo J. Olfactory receptors in non-chemosensory tissues. BMB Rep. (2012);45:612–622. doi: 10.5483/BMBRep.2012.45.11.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson G., Chandrashekar J., Hoon M. A., Feng L., Zhao G., Ryba N. J., Zuker C. S. An amino-acid taste receptor. Nature. (2002);416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- 7.Palmer R. K. The pharmacology and signaling of bitter, sweet, and umami taste sensing. Mol. Interv. (2007);7:87–98. doi: 10.1124/mi.7.2.9. [DOI] [PubMed] [Google Scholar]
- 8.Tizzano M., Cristofoletti M., Sbarbati A., Finger T. E. Expression of taste receptors in solitary chemosensory cells of rodent airways. BMC Pulm. Med. (2011);11:3. doi: 10.1186/1471-2466-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwatsuki K., Ichikawa R., Uematsu A., Kitamura A., Uneyama H., Torii K. Detecting sweet and umami tastes in the gastrointestinal tract. Acta Physiol. (Oxf) (2012);204:169–177. doi: 10.1111/j.1748-1716.2011.02353.x. [DOI] [PubMed] [Google Scholar]
- 10.Vary T. C., Siegel J. H., Placko R., Tall B. D., Morris J. G. Effect of dichloroacetate on plasma and hepatic amino acids in sterile inflammation and sepsis. Arch. Surg. (1989);124:1071–1077. doi: 10.1001/archsurg.1989.01410090081018. [DOI] [PubMed] [Google Scholar]
- 11.Lawand N. B., McNearney T., Westlund K. N. Amino acid release into the knee joint: key role in nociception and inflammation. Pain. (2000);86:69–74. doi: 10.1016/S0304-3959(99)00311-5. [DOI] [PubMed] [Google Scholar]
- 12.Askanazi J., Carpentier Y. A., Michelsen C. B., Elwyn D. H., Furst P., Kantrowitz L. R., Gump F. E., Kinney J. M. Muscle and plasma amino acids following injury. Influence of intercurrent infection. Ann. Surg. (1980);192:78–85. doi: 10.1097/00000658-198007000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilmore W. D. Proteins and amino acids: The role of protein and amino acids in sustaining and enhancing performance. 1999 The National Academy of Sciences; Washington DC.: (1999). pp. 155–167. [Google Scholar]
- 14.Mayadas T. N., Cullere X., Lowell C. A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. (2014);9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye R. D., Boulay F., Wang J. M., Dahlgren C., Gerard C., Parmentier M., Serhan C. N., Murphy P. M. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol. Rev. (2009);61:119–161. doi: 10.1124/pr.109.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toda Y., Nakagita T., Hayakawa T., Okada S., Narukawa M., Imai H., Ishimaru Y., Misaka T. Two distinct determinants of ligand specificity in T1R1/T1R3 (the umami taste receptor). J. Biol. Chem. (2013);288:36863–36887. doi: 10.1074/jbc.M113.494443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Y., Oppenheim J. J., Wang J. M. Pleiotropic roles of formyl peptide receptors. Cytokine Growth Factor Rev. (2001);12:91–105. doi: 10.1016/S1359-6101(01)00003-X. [DOI] [PubMed] [Google Scholar]
- 18.Bae Y. S., Yi H. J., Lee H. Y., Jo E. J., Kim J. I., Lee T. G., Ye R. D., Kwak J. Y., Ryu S. H. Differential activation of formyl peptide receptor-like 1 by peptide ligands. J. Immunol. (2003);171:6807–6813. doi: 10.4049/jimmunol.171.12.6807. [DOI] [PubMed] [Google Scholar]
- 19.Bae G. H., Lee H. Y., Jung Y. S., Shim J. W., Kim S. D., Baek S. H., Kwon J. Y., Park J. S., Bae Y. S. Identification of novel peptides that stimulate human neutrophils. Exp. Mol. Med. (2012);44:130–137. doi: 10.3858/emm.2012.44.2.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chow J. C., Young D. W., Golenbock D. T., Christ W. J., Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. (1999);274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 21.Kretz-Remy C., Mehlen P., Mirault M. E., Arrigo A. P. Inhibition of I kappa B-alpha phosphorylation and degradation and subsequent NF-kappa B activation by glutathione peroxidase overexpression. J. Cell. Biol. (1996);133:1083–1093. doi: 10.1083/jcb.133.5.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carl V. S., Gautam J. K., Comeau L. D., Smith M. F., Jr. Role of endogenous IL-10 in LPS-induced STAT3 activation and IL-1 receptor antagonist gene expression. J. Leukoc. Biol. (2004);76:735–742. doi: 10.1189/jlb.1003526. [DOI] [PubMed] [Google Scholar]
- 23.Wang J. H., Inoue T., Higashiyama M., Guth P. H., Engel E., Kaunitz J. D., Akiba Y. Umami receptor activation increases duodenal bicarbonate secretion via glucagon-like peptide-2 release in rats. J. Pharmacol. Exp. Ther. (2011);339:464–473. doi: 10.1124/jpet.111.184788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oya M., Suzuki H., Watanabe Y., Sato M., Tsuboi T. Amino acid taste receptor regulates insulin secretion in pancreatic beta-cell line MIN6 cells. Genes Cells. (2011);16:608–616. doi: 10.1111/j.1365-2443.2011.01509.x. [DOI] [PubMed] [Google Scholar]
- 25.Daly K., Al-Rammahi M., Moran A., Marcello M., Ninomiya Y., Shirazi-Beechey S. P. Sensing of amino acids by the gut-expressed taste receptor T1R1-T1R3 stimulates CCK secretion. Am. J. Physiol. Gastrointest. Liver Physiol. (2013);304:G271–282. doi: 10.1152/ajpgi.00074.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foster S. R., Porrello E. R., Purdue B., Chan H. W., Voigt A., Frenzel S., Hannan R. D., Moritz K. M., Simmons D. G., Molenaar P., Roura E., Boehm U., Meyerhof W., Thomas W. G. Expression, regulation and putative nutrient-sensing function of taste GPCRs in the heart. PLoS One. (2013);8:e64579. doi: 10.1371/journal.pone.0064579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wauson E. M., Zaganjor E., Lee A. Y., Guerra M. L., Ghosh A. B., Bookout A. L., Chambers C. P., Jivan A., McGlynn K., Hutchison M. R., Deberardinis R. J., Cobb M. H. The G protein-coupled taste receptor T1R1/T1R3 regulates mTORC1 and autophagy. Mol. Cell. (2012);47:851–862. doi: 10.1016/j.molcel.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boxio R., Bossenmeyer-Pourie C., Steinckwich N., Dournon C., Nusse O. Mouse bone marrow contains large numbers of functionally competent neutrophils. J. Leukoc. Biol. (2004);75:604–611. doi: 10.1189/jlb.0703340. [DOI] [PubMed] [Google Scholar]
- 29.Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. (1985);260:3440–3450. [PubMed] [Google Scholar]
- 30.Park K. S., Lee H. Y., Kim M. K., Shin E. H., Jo S. H., Kim S. D., Im D. S., Bae Y. S. Lysophosphatidylserine stimulates L2071 mouse fibroblast chemotactic migration via a process involving pertussis toxin-sensitive trimeric G-proteins. Mol. Pharmacol. (2006);69:1066–1073. doi: 10.1124/mol.105.018960. [DOI] [PubMed] [Google Scholar]
- 31.Park K. S., Kim M. K., Lee H. Y., Kim S. D., Lee S. Y., Kim J. M., Ryu S. H., Bae Y. S. S1P stimulates chemotactic migration and invasion in OVCAR3 ovarian cancer cells. Biochem. Biophys. Res. Commun. (2007);356:239–244. doi: 10.1016/j.bbrc.2007.02.112. [DOI] [PubMed] [Google Scholar]
- 32.Kim S. D., Lee H. Y., Shim J. W., Kim H. J., Yoo Y. H., Park J. S., Baek S. H., Zabel B. A., Bae Y. S. Activation of CXCR2 by extracellular matrix degradation product acetylated Pro-Gly-Pro has therapeutic effects against sepsis. Am. J. Respir. Crit. Care Med. (2011);184:243–251. doi: 10.1164/rccm.201101-0004OC. [DOI] [PubMed] [Google Scholar]


