Summary
The gastrointestinal tract is covered by mucus that has different properties in the stomach, small intestine and colon. The large highly glycosylated gel-forming mucins MUC2 and MUC5AC are the major components of the mucus in the intestine and stomach, respectively. In the small intestine mucus limits the number of bacteria that can reach the epithelium and the Peyer’s patches. In the large intestine the inner mucus layer separates the commensal bacteria from the host epithelium. The outer colonic mucus layer is the natural habitat for the commensal bacteria. The intestinal goblet cells not only secrete the MUC2 mucin, but also a number of typical mucus components: CLCA1, FCGBP, AGR2, ZG16, and TFF3.
The goblet cells have recently been shown to have a novel gate-keeping role for the presentation of oral antigens to the immune system. Goblet cells deliver small intestinal luminal material to the lamina propria dendritic cells of the tolerogenic CD103+-type.
In addition to the gel forming mucins, the transmembrane mucins MUC3, MUC12 and MUC17 form the enterocyte glycocalyx that can reach about a micrometer out from the brush border. The MUC17 mucin can shuttle from a surface to an intracellular vesicle localization suggesting that enterocytes might control and report epithelial microbial challenge.
There is not only communication from the epithelial cells to the immune system, but also in the opposite direction. One example of this is IL10 that can affect and improve the properties of the inner colonic mucus layer.
The mucus and epithelial cells of the gastrointestinal tract are the primary gate keepers and controllers of bacterial interactions with the host immune system, but our understanding of this relationship is still in its infancy.
Introduction
The gastrointestinal tract is the largest surface that the body exposes to the outer world, a world that is not very friendly. It contains everything we eat and swallow as well as an enormous colony of bacteria that resides in the gut. However, the potential danger is not wholly exogenous as we secrete potentially harmful molecules into the gut such as hydrochloric acid, digestive enzymes and bile salts. The concentration of hydrochloric acid in the stomach is high enough to hydrolyze chemical bonds; the digestive proteases are capable of cleaving all types of peptide bonds and bile salts are able to dissolve cell membranes. If this was not enough, most of the immune system is also localized to the gastrointestinal tract and ready to react with all of its content. It is thus quite remarkable that this system is relatively stable and that we do not digest ourselves or trigger fulminant immune responses.
It is important that the immune system develop tolerances, a process that has to be well balanced in order to trigger appropriate responses to threatening pathogens as well as tolerate the commensal bacteria. The gastrointestinal mucus system is important for lowering the exposure of antigens to the immune system, but the mucus system is even more important for protecting from self-digestion. Typical for the gastrointestinal mucus and the corresponding membrane bound glycocalyx is their dense decoration with complex carbohydrates. These oligosaccharides are formed from monosaccharides that are linked to each other by bonds that we cannot cleave as we do not secrete any such glycosidases in the intestine. When these carbohydrates decorate the proteins, the intestinal proteases cannot reach the peptide bonds, rendering the surface coating of the intestine essentially inert to proteolytic degradation by the host. The hydrophilic nature of the mucus and glycocalyx also provides a physical barrier that protects the epithelial cells by acting as a diffusion barrier with its bound water. This is illustrated by the proton gradient from the mucus surface to the epithelium in the stomach inner mucus layer (1). Another example is the structured inner mucus layer of colon that forms a barrier that prevents bacteria from penetrating due to its nature as a size exclusion filter (2).
The focus of this review is the mucus layer and its intimate relation with the enterocytes and goblet cells of the intestine as well as the innate and adaptive immune systems. Mucus is secreted by the goblet cells and typically contains several major components. One of these, the mucins, gives the mucus its gel-like properties. All mucins are characterized by mucin domains which have abundant Ser, Thr and Pro amino acid residues that are heavily O-glycosylated thus giving these domains an extended and stiff conformation that resembles a 'bottle brush'. The mucins produced by the goblet cells are the classical gel-forming mucins MUC2, MUC5AC, MUC6 and MUC5B which are secreted by the intestine, stomach surface, stomach glands and salivary glands, respectively. All of these form extremely large polymers. The enterocytes also express mucins, but of another type. The transmembrane mucins cover the apical surface of these cells and form the glycocalyx. The transmembrane mucins MUC1, MUC3, MUC4, MUC12, MUC13, and MUC17 are all found in the gastrointestinal tract. In addition to a transmembrane single pass domain, they all have a cytoplasmic tail and an enormous extracellular mucin domain densely decorated with glycans.
The gastrointestinal tract is covered by a single layer of epithelial cells. Most of these cells are columnar surface cells involved in different tasks depending on their localization along the tract. The enterocytes of the small intestine are the most abundant as these are responsible for the final digestion and absorption of nutrients. The enterocytes are interspersed by goblet cells which show an increased prevalence in the distal direction. The Paneth cells of the small intestinal crypts provide antibacterial peptides/proteins. Other less abundant cells are the tuft and enteroendocrine cells (3). All of these cell types are interconnected with tight junctions and function to separate the luminal material from the subepithelium. Interestingly, these epithelial cells are also mixed with intraepithelial lymphocytes (IEL) that comprise a mixture of αβ and γδ T-cells (4).
Most of the immune system resides in the subepithelial compartment where it lies ready to take action when the mucus and epithelial cell lining fail. The dendritic cells (DC) are ready to take up and present antigens to T-cells for action or tolerance development. Macrophages (MΦ) screen the intercellular space and keep it clear by phagocytosis. The DC and MΦ have crucially important roles in determining the outcome of any challenge, but this outcome is probably also highly dependent on their interactions with the epithelial cells. All of these components need to cooperate to maintain the intestinal barrier function and the field of mucosal immunology must attempt to study the system as an integrated whole (4;5).
This review will focus on the mucus and epithelial cells and will try to bring together what is currently known about their relationship to the immune system and what might be expected in the near future.
Mucus
Organization
The organization of the mucus along the gastrointestinal canal has only been well characterized over the last decade. At first it might seem unbelievable that this had not been previously addressed in such an important and easily accessible organ. The reason for this is that it has taken some time to realize that normal mucus is totally transparent and that more or less any manipulation causes the mucus gel to collapse. A major observation was made by Atuma, whilst working with Lena Holm in Uppsala, when it was shown that the mucus surface can be visually observed by allowing charcoal to sediment down on top of the mucus (6). Following this procedure a relatively thick mucus layer was observed along the whole rat intestine. Previous fluid flow observations had defined a phenomenon called ‘unstirred water’ that roughly describes one aspect of the mucus layer (7).
We now know that in principle there are two types of mucus organization in the gastrointestinal tract (Figure 1). The glandular stomach and colon have a two-layered system with an inner and an outer mucus layer whereas the small intestine has only a single layer (6;8). This organization and the location of luminal bacteria is shown in Figure 1 with the mucus illustrated in different shades of green depending on mucus density and the bacteria shown in red (8). The number of bacteria in the gastrointestinal tract increases in a proximal-distal direction. The stomach is home to very few microorganisms whereas the human colon harbors more than a kilogram of bacteria. In the small intestine, bacteria do not normally contact the epithelial cells other than at the villi tips. In contrast to what was previously believed, the colonic bacteria do not normally have any direct contact with the epithelial cells as the inner mucus layer of the colon is impermeable to them (2). The permeable outer colonic mucus layer is, on the other hand, the normal habitat for the commensal bacteria. The inner layer of both the colonic and stomach mucus is ‘firmly’ attached to the epithelial cells and cannot be removed with simple aspiration. The outer layer of colon can easily be aspirated off, whereas the outer layer of the stomach is more difficult to aspirate. The mucus of the small intestine is normally non-attached and can easily be removed.
Figure 1.
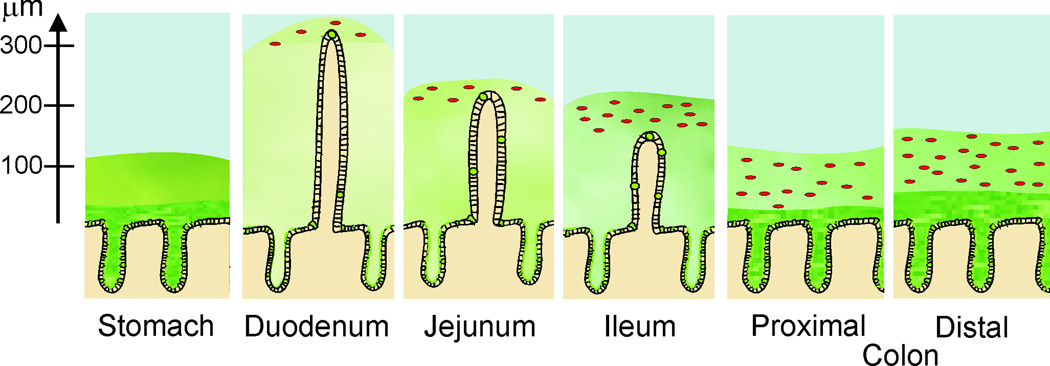
Schematic representation of the gastrointestinal mucus system. The mucus is depicted in green and the bacteria represented as red dots. The axis to the left shows the thickness of the mucus as measured in mice (8).
To summarize, the gastrointestinal tract is covered by mucus that has different properties in the stomach, small intestine and colon.
Composition
Mucus is of course mostly water (normally >98%) and will shrink to a very thin structure when dehydrated. That is why mucus is not normally observed on formaldehyde-fixed tissue sections and can be best preserved using Carnoy fixative based on dry methanol, dry chloroform and glacial acetic acid (Methacarn) (9). Using this fixative, the two mucus layers of colon can be well visualized, although even this method results in some shrinkage and often a gap between the epithelial cells and the inner mucus layer (2).
The mucus protein composition of the gastrointestinal tract has recently been studied by modern proteomics (2;10;11). As discussed, the major component of the intestinal mucus is the MUC2 mucin and in the stomach it is the MUC5AC mucin, both of which are produced by goblet cells. The MUC6 mucin, found in the gastric glands, is not a component of the surface mucus and instead passes through transient channels formed in the surface mucus together with the gastric hydrochloric acid (12;13). Other major mucus proteins secreted by goblet cells are CLCA1, FCGBP, ZG16, AGR2 and of course antibodies, especially IgA. The CLCA1 protein was previously believed to be a Ca2+ channel and more lately to be involved in controlling Ca2+ secretion (14;15). However, as the secreted CLCA1 protein is so abundant in the mucus it probably has other as yet not understood functions (Nystöm et al., unpublished). FCGBP (Fc globulin binding protein) is not an immunoglobulin binding protein as its name suggests (16). Instead it can covalently bind and cross-link mucus proteins via FCGBP’s autocatalytically cleaved von Willebrand D domains, as shown by its binding to the MUC2 mucin (10). ZG16 is a small lectin-like protein that binds to Gram+ bacteria and by this move them further away from the epithelium (Bergstrom et al., unpublished). AGR2 is an ER residential protein with an ER retention signaling sequence (17). Even so, this protein is secreted out into the mucus in molar quantities comparable to the amount of MUC2 (Rodríguez-Piñeiro et al., unpublished). This suggests that AGR2 has still unknown extracellular functions in the intestine.
To summarize, the MUC2 mucin forms the bulk of the intestinal mucus whereas the surface mucus of the stomach is mostly comprised of MUC5AC.
Mucus function in the small intestine
The small intestinal mucus fills the luminal space between the villi and usually also covers the villi tips (Figure 1). However, as the mucus is not anchored to the epithelial surface, it moves with the peristaltic waves in a distal direction. Fresh mucus is constantly secreted from the goblet cells, especially from the crypt openings (8). Mucus secreted from the crypt mixes with Paneth cell secretions containing antibacterial peptides, lysozyme, DMBT1 and also MUC2 (18–21). The Paneth cell products will, together with enterocyte produced antibacterial proteins like RegIIIγ, generate an antibacterial gradient in the mucus and keep the bacteria away from the epithelial cell surfaces (22;23). The small intestinal mucus is penetrable to bacteria, but the mucus still provides a diffusion barrier and the bacteria would need to travel against the mucus flow in order to penetrate down to the tissue surface. This is likely to be a major reason why flagellar motility is so often an essential virulence factor for intestinal pathogens (24;25).
Of special immunological interest are the aggregated lymphoid follicles that form the Peyer’s patches which are important for immune surveillance (26). These patches are covered by a single layer of follicle-associated epithelium (FAE), consisting of enterocyte-like cells, M cells and also some goblet cells (27;28). Our recent studies suggest that the Peyer’s patches are also covered by mucus that is largely secreted by goblet cells on adjacent villi (28). The FAE goblet cells do not secrete mucus upon carbachol or PGE2 stimulation as other small intestinal goblet cells do. As the mucus on the Peyer’s patches is not anchored, it can easily be washed away and its antigen retrieving M cells better exposed to luminal material.
The small intestinal mucus is normally not attached to the epithelial cells. However, in the absence of a functional CFTR channel, as in Cystic Fibrosis, the mucus was found to be anchored to the epithelium (29). This was shown to be related to a lack of CFTR-secreted bicarbonate. Extensive analysis of this phenomenon provided the surprising observation that the MUC2 mucin is anchored to the goblet cell after secretion and that a specific enterocyte protease needs to cleave the protein core of MUC2 to release the mucin and the mucus (Schütte et al., unpublished).
To summarize, the combined small intestinal mucus and antibacterial peptides/proteins function to limit the number of intact bacteria that can reach the epithelium and the Peyer’s patches.
Mucus function in colon
As discussed, the large intestine has a different type of mucus organization than the small intestine, a two-layered system as shown in Figure 1. The inner mucus layer is continuously formed by secretion from surface goblet cells (30). Upon secretion the MUC2 mucin unfolds and forms enormous net-like structures that are arranged by interacting with the previously secreted inner mucus layer (31).
The goblet cells in the upper part of the colonic crypts contribute to the inner mucus layer by compound secretion in response to stress stimuli (32). An example of this is manifested in colonic ischemia after which these goblet cells secrete their mucus and quickly wash away any bacteria that may have entered the crypts (33;34). These goblet cells in the upper crypt do not seem to synthesize MUC2 mucin continuously as is the case for the surface goblet cells (30). The replenishment of new goblet cells and the required MUC2 biosynthesis, assembly and accumulation in granulae is slow as the time to replace or refill these mucin filled cells is longer than 4–5 hours (33). This suggests that continuous stress will limit MUC2 mucin availability (35).
The inner mucus layer is ‘firmly’ attached to the epithelium and about 50 µm thick in distal colon of mice and 200–300 µm thick in humans (2;6;36). At the outer side of the inner mucus, a surprisingly sharp border was observed where the mucus is converted from being attached to easily aspirated (2). This conversion is dependent on host protease activities as this conversion process also occurs in the mucus of germ-free mice (2). The nature of the protease(s) and how the activation can be triggered at such a large distance away from the epithelial cells is not known, but protease inhibitors can prevent the conversion process (Schütte et al., unpublished). These proteases appear to act directly on the MUC2 mucin and allow the expansion of the net-like structure, as suggested by the appearance of new MUC2 cleavage products in the outer mucus layer (2).
This inner mucus layer is continuously renewed by secretion from the surface epithelial cells. An estimate of the turnover of the inner mucus layer in live murine distal colonic tissue suggests that the layer is renewed every 1–2 hours (30). This fast turnover is of course important for maintaining this inner mucus layer free from bacteria (2). Another important factor is the staggered layers of the MUC2 mucin that act as a size-exclusion filter and that does not normally allow bacteria to penetrate (2;37). This inner layer is not penetrable to bacteria or beads with sizes down to 0.5 µm (36). However, smaller objects, for example 50 kDa proteins, are able to pass through this layer.
Interestingly, the properties of this inner mucus layer are not static. Initially we noticed that the most commonly used model for colitis in rodents, oral administration of Dextran Sodium Sulfate (DSS), rendered the inner mucus layer immediately penetrable to both bacteria and beads of their size (38). Recently, we have also observed that all mouse models with spontaneous colitis have an inner mucus layer that is penetrable to bacteria (36). It has also been observed that patients with active ulcerative colitis and some with ulcerative colitis in remission have penetrable mucus. Once the inner mucus layer of the colon is lost or becomes penetrable to bacteria, a large number of bacteria will reach the epithelial cells. This mimics mice lacking the Muc2 mucin (2). In these mice, bacteria were found far down into the crypts and inside epithelial cells. This massive amount of bacteria in close contact with host tissues triggers inflammation with all the characteristics of ulcerative colitis, as well as development of colon cancer (39;40). Surprisingly, the properties of the inner mucus layer were also found to be coupled to the immune system. The widely used murine colitis model, IL10−/−, has a normal inner mucus layer in terms of thickness, but produces a mucus structure that is penetrable to bacteria (36). It can thus be suggested that a penetrable inner mucus layer allowing bacteria to reach the epithelial cells in large quantities is a common mechanism that underlies many forms of acute or chronic colonic inflammation (35).
To summarize, the normal inner colonic mucus layer is impenetrable to bacteria. When this inner layer is penetrable to bacteria, they reach the epithelial cells and trigger inflammation.
Commensal bacteria and mucus
The outer colonic mucus layer is the natural habitat for the commensal bacteria (41). A proteolytic increase in the MUC2 mucin pore sizes allows bacteria to penetrate into the mucin net-like structure and gain access to the plentiful mucin-bound carbohydrates that can be utilized by the bacteria as an energy source (42–44). Approximately one third of the commensal microbiome is comprised of genes involved in carbohydrate digestion and many bacteria have specialized operons for different type of complex carbohydrates (45;46). The gut contains complex carbohydrates from ingested food, in the form of undigested polysaccharides, and from the numerous glycans found on the mucins. The latter glycans are probably more important for gut homeostasis as they can contribute to bacterial selection. Furthermore, the complexity of the mucin glycans can regulate bacterial mucin degradation. The normal degradation of mucin glycans is relatively slow as one monosaccharide is removed at a time. If the glycans are shorter, the degradation process will be faster and not only the outer mucus layer, but also the inner can be degraded fast enough to allow bacteria to reach the epithelium (47;48).
The commensal bacteria produce a range of metabolites, some of which are useful to the host. Substantial quantities of short fatty acids are absorbed by the tissue epithelial cells; for example butyrate seems to have a key role to play in colon epithelial protection and metabolism (49;50). Bacterial metabolites can further affect the host immune system, host metabolism, and more recently are implied to affect host brain function (51–53).
To summarize, the commensal bacteria have their habitat in the outer colonic mucus layer.
Pathogenic bacteria and mucus
The gastrointestinal mucus also plays an important role in pathogenic infections as these pathogens must acquire the ability to overcome the protective mechanisms of the mucus system. Mucins, as exemplified by MUC2, can act as an environmental cue for bacteria to modulate the expression of virulence/colonization related genes, as is the case with Campylobacter jejuni (54). During Citrobacter rodentium infection, a murine model of human enteropathogenic Escherichia coli infection, the bacteria can penetrate and reside beneath the inner mucus layer (55). Despite this, clearance of this pathogen still requires Muc2 as colonization of Muc2−/− mice results in a more severe, and often lethal, infection as the mice are unable to clear the pathogen from the gut (55;56). This severe form of colitis with a lethal outcome was also found in Muc2−/− mice when challenged with Salmonella enterica serovar Typhimurium bacteria (57). Although the mucus has a protective function, there are examples of pathogenic bacteria that can utilize the goblet cells and mucus secretion to increase invasion effectiveness. Listeria monocytogenes invades the host by taking advantage of binding to normally hidden E-cadherin, which is exposed on emptied and shrunken goblet cells (58).
As the inner mucus layer is normally impermeable to bacteria, opportunistic pathogens should in theory not be able to reach the epithelium and invade the host. However, some specialist microorganisms have developed mechanisms for circumventing this system. We have identified the molecular mechanism for a few examples. One is the large parasite Entamoeba histolytica that secretes a specific protease that is able to cleave the human MUC2 mucin at a susceptible site that causes the polymeric network to dissolve (59). More recently the oral bacteria Porphyromonas gingivalis, found also in the colon, was shown to secrete a protease (RgpB) which is able to cleave MUC2 only one amino acid away from the E. histolytica cleavage site (60). More recently, several E. coli family proteases with similar effects have been identified (van der Post et al., unpublished).
The intestinal mucin genes are expressed early before birth (61), but a fully developed and protective secreted mucus barrier is not formed until days after birth (Birchenough et al., unpublished). Poorly developed mucus could be a problem for premature infants that are also known to have an increased risk of infections. Neonatal bacterial meningitis, a severe infection in premature children, is mainly caused by E. coli and group B Streptococci. In an experimental model of E. coli neonatal bacterial meningitis, the poorly developed intestinal mucus provided opportunities for this pathogen to translocate the intestinal tract (62). Necrotizing enterocolitis is another common and devastating disease of premature infants and these infants have been shown to have fewer filled goblet cells and less mucus (63). A poorly developed mucus system and less mature ability to secrete mucus as a response to infection are likely reasons for the predominance of these diseases in premature infants.
To summarize, the inner mucus layer of the large intestine separates the commensal bacteria from the host epithelium thus limiting exposure of the host to bacteria. The outer mucus layer is the natural habitat for the commensal bacteria.
Goblet cell
The goblet cell of the gastrointestinal tract is specialized in producing and secreting mucus. As MUC2 mucin is the major structural molecule of the intestinal mucus, the assembly of this large and complex molecule is a major task for the goblet cell (64;65). The human MUC2 is still not fully sequenced, but recent next generation sequencing suggests that MUC2 is 5,100 amino acids long (Svensson et al., unpublished), a little shorter than previously suggested (66). MUC2 has two centrally localized domains rich in the amino acids proline, threonine and serine (PTS domains) (66). The N- and C-terminal ends of the MUC2 mucin are large and cysteine-rich.
The primary full-length translational product of MUC2 is quickly covalently dimerized by the formation of C-terminal disulfide bonds in the endoplasmic reticulum (ER). The MUC2 mucin contains 215 cysteine amino acids that make up >10% of the total amino acids outside of the PTS mucin domains. As all cysteines need to be interlinked (oxidized) in order to exit the ER, the correct assembly of the MUC2 mucin is a formidable challenge. This is reflected in the recent observation that the chaperone ERN2 (IRE1β) is uniquely required for the goblet cell ER to process the gel forming mucins (67–69). This stress placed on the ER is probably also the reason for the relationship between ER stress, the unfolded protein response and colonic inflammation, something that has been discussed in detail elsewhere (20;70;71).
The small PDI-like protein AGR2 is a goblet cell protein that has a KTEL C-terminal sequence and is thus retained in the ER (17;72;73). AGR2 has been suggested to covalently bind to the MUC2 mucin, but later studies could not confirm this (Bergström et al., unpublished) (17). Mice lacking Agr2 are more susceptible to the development of colitis (17;74), but still have mucus secretion, although they do possess a less developed inner mucus layer (Bergström et al., unpublished). Although it is obvious that AGR2 is involved in the biosynthesis of the MUC2 mucin, its precise function has yet to be identified.
After the ER, the dimers pass into the Golgi apparatus, where O-glycosylastion takes place by adding GalNAc to the serine and threonine residues of the two central PTS domains (75). Additional monosaccharides are added to the GalNAc to form complex oligosaccharides that characterize the mucin domains. These glycopeptides are long (about 0.5 µm) highly glycosylated (>80%) extended domains that are like stiff rods and are visually comparable to a bottle brush. The mass of the MUC2 monomer increases to about 2.5 MDa by glycosylation and as the building block is a dimer it will be around 5 MDa in total. In the trans-Golgi network the MUC2 mucin is sorted to the regulated secretory pathway, the N-terminal MUC2 organized into concatenated rings and new disulfide bonds formed generating N-terminal trimers (31;76). This organization of MUC2 is triggered by the low pH and high calcium concentration in this part of the secretory pathway. Secretion of the MUC2 mucin requires packing that is highly organized to allow a >1,000-fold expansion in volume. This expansion can only take place if the calcium ions are removed and the pH raised, a process that is accomplished by bicarbonate secretion (29;31).
The goblet cell also produces a number of important and abundant proteins that are major components of the intestinal mucus; CLCA1, FCGBP, AGR2, ZG16, and TFF3 (10;11). The goblet cells synthesize and secrete the vast majority of the proteins that comprise the intestinal mucus.
To summarize, the goblet cells biosynthesize, assemble and secrete the MUC2 mucin that forms the skeleton of the intestinal mucus.
Goblet cells as luminal sensors for the immune system
Goblet cells have always been thought to be a purely secretory cell. However, several recent observations have shown that this is not the case. The groups of Newberry and Miller have shown that small intestinal goblet cells can take up luminal material and deliver this material to lamina propria dendritic cells (DC) (77). More recently, Stappenbeck and coworkers could show that goblet cell endocytosis was linked to autophagy and controlled secretion (78). This was in line with earlier observations that autophagy is coupled to the formation of early endosomes (79). Stappenbeck observed that goblet cells in mice lacking one of the autophagy proteins (Atg5) accumulated mucus in their secretory granulae. They could further show that NADPH oxidase driven ROS production was necessary as inhibition of this, inhibition of endocytosis, or inhibition of autophagy all led to the accumulation of mucin granulae causing decreased mucin secretion. Interestingly, these processes were suggested to take place at the rim of the goblet cell theca (78). This is of interest as we have observed that the uptake of luminal material in the small intestinal goblet cells also takes place at the rim of the theca (Gustafsson et al, unpublished). The uptake of luminal material by goblet cells is stimulated by acetyl choline analogues that are effective stimulators of small intestinal mucus secretion (8;77). Together these observations suggest a coupling between goblet cell antigen uptake, autophagy and secretion. One should also remember that another component in the autophagy machinery, ATG16L1, is genetically linked to Crohn’s disease, one of the inflammatory bowel diseases (80;81). Recent studies have actually linked ATG16L1 more to the control of secretion rather than to autophagy, further supporting a connection between endocytosis and secretion in cells with a regulated secretory pathway (82).
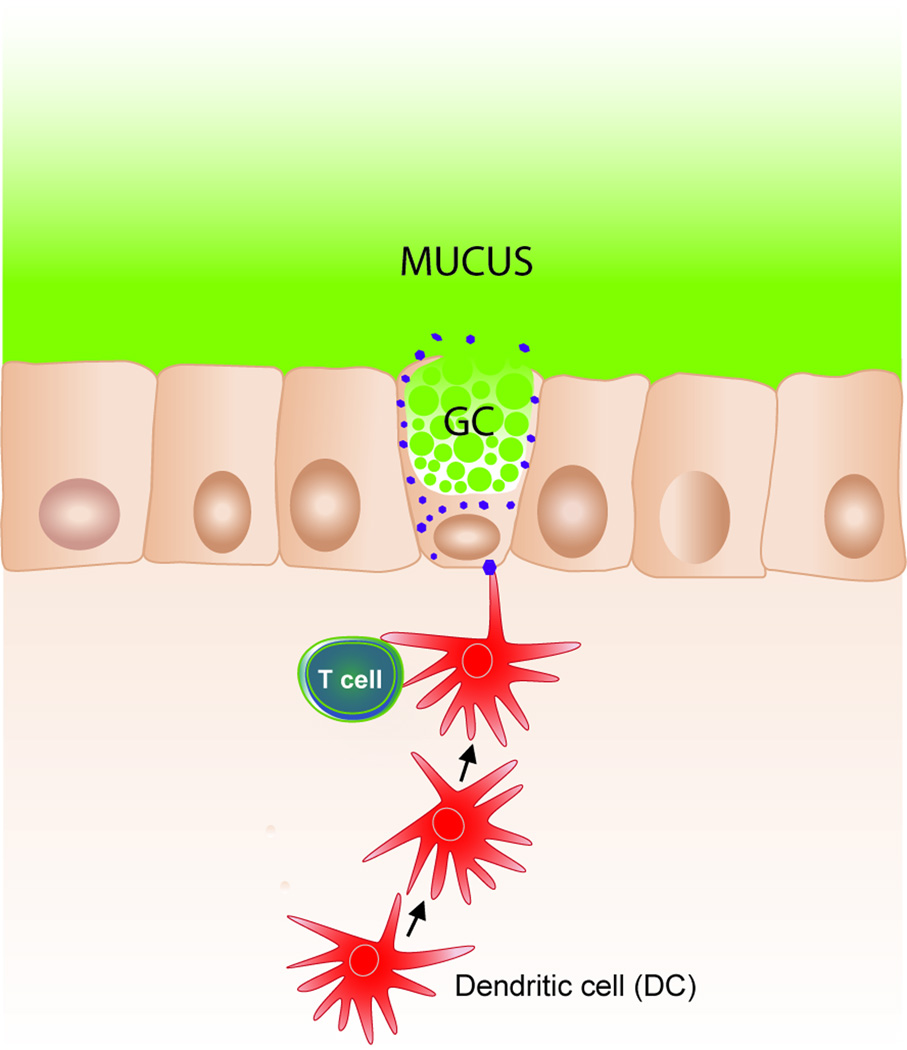
Ever since Rescigno et al. observed that lamina propria DC can extend dendrites between the epithelial cells and sample luminal antigen, this mechanism has been the favored model of intestinal antigen retrieval (83;84). However, using live animal imaging it was evident that DC dendrite extension into the lumen was a very rare event (77). Paracellular leakage could also be excluded as a way to expose DC to luminal antigens. Instead, the two-photon imaging and time-lapse recordings showed the remarkable process of luminal fluorescent dextran filling up ‘gaps’ in the epithelium (77). DCs in the lamina propria then approached these gaps and ‘ate’ the dextran. The authors called these sites GAPs and could indirectly argue that the GAPs were indeed goblet cells. Using mice with fluorescently labeled MUC2 the GAPs have now been positively identified as mucin-secreting goblet cells (Gustafsson, Svensson, Wising et al., unpublished). There is an upper size limit to the molecules capable of passing these GAPs with 10 kDa dextran, OVA and also BSA rapidly reaching lamina propria DCs, whereas larger molecules only transit slowly and micrometer beads do not pass at all (77). This filter effect is might be mediated by the MUC2 mucin and makes it less likely that whole bacteria will be presented this way.
To summarize, the goblet cells have a new type of gate keeping role for the presentation of oral antigen to the immune system. This is illustrated in Figure 2.
Figure 2.
Schematic presentation of goblet cells passing luminal antigen to lamina propria dendritic cells (77).
Enterocytes
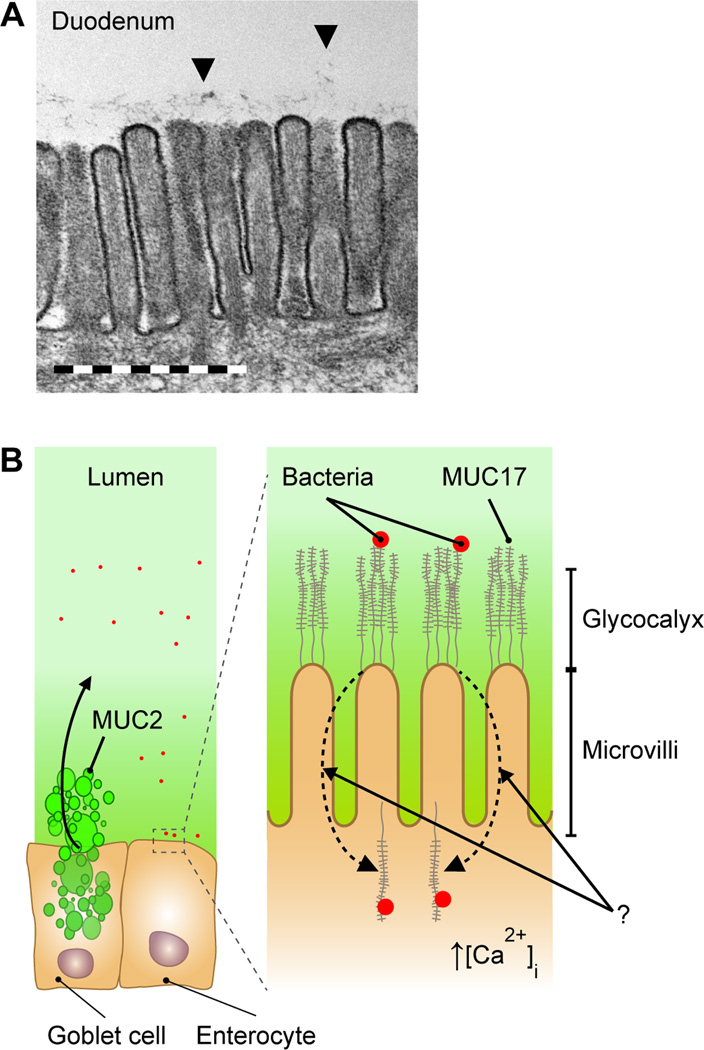
The major intestinal cell is the enterocyte. All enterocytes have a columnar shape with a highly specialized apical membrane domain. This membrane domain mediates uptake of nutrients and ions by special transporters driven by the small intestinal sodium gradient from the lumen to the enterocyte cytoplasm. Certain cells also have specialized functions like the uptake of bile acids. The surface of the apical membrane is expanded by microvilli and a well-developed carbohydrate rich micrometer thick glycocalyx (Figure 3). This was easily observed by electron microscopy during the 1960’s, but the molecular nature of this glycocalyx has been less well explored (85–87).
Figure 3.
Electron micrograph of duodenal microvilli with surface glycocalyx (A) and schematic presentation of a potential function of the MUC17 enterocyte mucin binding and internalizing of bacteria at the same time as mucus is secreted from the goblet cell after carbachol stimulation (B).
More recently it has become clear that the glycocalyx of the intestine is built and composed of the transmembrane mucins (88–91). That the transmembrane mucins and the gel-forming mucins belong to the same gene name nomenclature group (MUC) causes confusion, as their functions are very different. All transmembrane mucins have a single domain passing the plasma membrane and a large highly glycosylated extracellular mucin domain with a similar structure to the PTS domains of the gel-forming mucins. All these mucins also have a cytoplasmic tail that interacts with the cytoskeleton. There are two groups of transmembrane mucins; the SEA-type with an approximately 100-amino acid long SEA domain close to the membrane on the luminal side (MUC1, MUC3, MUC12, MUC13, MUC16, MUC17) or the NIDO-AMOP-VWD-type with a more complex domain structure at a similar location (MUC4) (92–99). Both of these domains are cleaved by autocatalytic mechanisms during mucin biosynthesis (100;101). The SEA domain is autocatalytically cleaved in the ER using folding energy and after this cleavage the large outer fragment is still anchored to the transmembrane fragment by strong interactions between four β-pleated sheets of the SEA domain (100). However, the SEA domain can be torn apart by mechanical forces, a mechanism that probably protects the apical membrane from mechanical stress (102). It is likely that the unfolding of the SEA domain and the separation of the two parts can be sensed by the cell (100).
The transmembrane MUC3, 12 and 17 mucins are the major components of the enterocyte glycocalyx. The MUC3, 12 and 17 genes are located at the same genomic locus and encode proteins that are about 5,000 amino acids long with extended mucin domains that extend out about one µm from the cell membrane (Figure 3). Only small amounts of MUC1 are found in the normal intestine, but it is more abundant in the stomach (103). Under pathological conditions a special splice variant of MUC1 is found in the small intestine (104).
The MUC13 mucin is also an SEA-type mucin and a major enterocyte component (95), but is short with only 400 amino acid residues extending outside of the membrane. It is thus more like a normal glycoprotein, suggesting that it will not contribute significantly to the extended glycocalyx. An alternative to this assumption is that it might be a second line of defense or sensor once, for example, bacteria have bypassed the long transmembrane mucins. Studies on the Muc13−/− mice suggest that MUC13 has an anti-inflammatory function and anti-apoptotic effect in the epithelial cells (105;106).
The cytoplasmic tails of the transmembrane mucins are relatively divergent. MUC1 has a longer tail with several tyrosine phosphorylation sites (107). On the other hand the MUC3, 12 and 17 mucins all have PDZ-binding motifs in their far C-terminal region (108). The MUC17 mucin has been shown to interact with three of the four PDZ domains in PDZK1, an apical scaffolding protein known to also bind several ion channels such as NHE3 and CFTR (109;110). Mice lacking Pdzk1 protein have their Muc17 mucin dislocated from the apical membrane to intracellular vesicles (108). Interestingly, PDZK1 is also a major regulator of CFTR localization to the apical membrane (110;111).
The PDZ binding motif of the MUC3 mucin has been shown to bind GOPC (Golgi-associated PDZ and coiled-coil motif containing) protein that only has a single PDZ domain (112). When CFTR binds to GOPC it is targeted for lysosomal degradation (113). Interestingly, overexpression of GOPC lowered the total cellular levels of MUC3, something that was reversed by introducing CFTR (112). This suggests that CFTR and MUC3 compete for binding to the single PDZ domain on GOPC, which in turn regulates the relative levels of these two proteins. Thus there is functional coupling between MUC3 and the CFTR channel.
The paracellular route for traversing the epithelial sheet has for a long time been regarded as the major path for entering the submucosa. The tightness and other properties of this seal are controlled by the tight junction proteins occludin and claudin and the adherens junction protein cadherin which all residing in the plasma membrane (114). These form a ring around the cell and are coupled to the cell cytoskeleton. The tightness of the tight junctions varies between different tissues and is also believed to vary under certain conditions, for example inflammation (114). The relative importance of the transcellular or paracellular route is often debated in relation to luminal antigens and bacteria uptake, but the paracellular path is obviously more relevant after epithelial cell damage than under basal conditions.
To summarize, the enterocyte glycocalyx is built and composed of the transmembrane mucins MUC3, MUC12 and MUC17 that reach about a micrometer out from the brush border.
Enterocytes as luminal sensors for the immune system
As discussed, a dense layer of transmembrane mucins that act as a diffusion barrier covers the apical surface of the enterocytes. The porosity and penetrability of this glycocalyx has not been studied, but most likely bacteria will have difficulties in passing this barrier whereas partly digested food products will pass more easily. As this glycocalyx is extending out into the intestinal lumen it is important to understand if it only acts as a passive barrier or has other more dynamic functions. The observation that the transmembrane mucins MUC3, 12 and 17 have typical PDZ-binding motifs could suggest more active functions (108). As carbachol stimulation is known to alter the localization of the ion channels CFTR and NHE3, both of which have PDZ-binding motifs, the possibility that transmembrane mucins were also relocalized was tested on both cultured cells and tissues (88). As expected, the CFTR channel was relocated from an intracellular vesicle pool to the surface membrane and the NHE3 exchanger was moved from the apical membrane to an intracellular location. Interestingly the MUC17 mucin was also relocated from the apical membrane to an intracellular vesicular pool distinct from classical endosomes (88). This was not the case for the MUC3 and 12 transmembrane mucins, suggesting specific roles for each of these closely related molecules. Preliminary studies suggest that bacterial adhesins and whole bacteria can be transported into the epithelial cell by this process (Pelaseyed et al, unpublished). The observation of intracellular bacteria in enterocytes of mice lacking the Muc2 mucin and thus having a massive exposure of bacteria to the apical membrane could speak for such a specific mechanism for bacterial uptake (2). The further fate of the material taken up this way is currently not known, but it can be suggested that enterocytes will communicate with the immune system.
The enterocytes are known to have a number of innate immune signaling molecules that are localized to both the surface and intracellular regions of the cell (5;115). These include the intracellular peptidoglycan receptors NOD1 and NOD2 as well as surface and intracellular Toll-like receptors (TLR) which recognize a range of conserved bacterial, fungal and viral structures (116;117). These are all coupled by signaling pathways to the master regulator of immune responses, NFκB (115).
The intraepithelial lymphocytes (IEL) are found between the epithelial cells of the intestine and thus form part of the first line of cells that encounter luminal threats. The IELs are a mixture of conventional CD8+ αβ T-cells and non-MHC restricted αβ or γδ T-cells (115;118;119). Their location requires an intimate relationship with the epithelial cells and it is likely that their relationship with enterocytes is crucial to their function. It is also probable that this relationship involves soluble cytokines, but is more likely controlled by direct cell-cell interactions (119). The nature of these interactions is still only partly understood. Butyrophilins (Btns) and butyrophilin-like (Btnl) proteins are a novel family of B7-related proteins with the expression of Btnl1, Btnl4, and Btnl6 limited to enterocytes (120;121). These molecules appear to be localized in both the intracellular and cell surface regions and seem to be involved in modulating intraepithelial T-cell interactions and by this mechanism inhibit and regulate inflammation of the intestine.
To summarize, it is now increasingly evident that the epithelial cells and enterocytes have a clear role in determining the immunological events subsequent to microbial challenge. This system of primary immune regulation has been suggested to be called the ‘epimmunome’ (115).
The subepithelial immune cells
The immune system of the lamina propria is part of an integrated mucosal immune system (5). Although this review is focusing on the mucus and first line of single cells protecting the gastrointestinal tract, the lamina propria immune system has to be shortly addressed although there are numerous reviews covering this system including their T-cells, B-cells, MΩ, and DC-cells (122). Of special importance are of course the B-cells which secrete IgA that is transported through the enterocytes into the lumen where it helps to control intestinal bacteria (123). Resident intestinal macrophages (MΩ) are important for removing bacteria that penetrate the epithelial cell barrier without triggering a stronger immune response (124). Other MΩ cells are recruited upon inflammation.
The interactions of epithelial cells with DC are of special importance. As discussed above, the goblet cells in the small intestine deliver luminal antigens to the DC of the lamina propria (77). However, not all types of DC were sampling from the GAPs as it turned out that it was only the CD11c+/CD103+ DC subpopulation that were capable of doing this (77). These cells are known to cross-present antigens to T-cells and induce tolerance (77;125). The CD103+ DC interaction and retinoic acid in the lamina propria promote Foxp3 induction and formation of T-reg cells (126). The mechanism of why and how the goblet cells and CD103+DC cooperate to promote tolerance is far from understood, but recently it was suggested that the MUC2 mucin has a special role in this process (127).
Currently we do not know if there is any direct interaction between the enterocytes and the lamina propria immune cells other than cyto- and chemokine mediated processes. However, it is very likely that there are more specific mechanisms for the enterocytes to regulate the lamina propria immune system.
There is of course also interactions in the other direction, from the immune system to the epithelial cells. An important molecule in this sense is IL10 that is produced by T cells, certain macrophages and other cells in the lamina propria (115;124). IL10 acts in an anti-inflammatory way on other immune cells, but our recent observations also show that IL10 can directly affect the mucus produced by the goblet cells and the mucus properties (36). This altered mucus must be due to IL10 affecting goblet cells and enterocytes. This emphasizes that it is not only the epithelial cells that can be affected by the lamina propria immune system, but also that the mucus system can be modulated by the immune cells.
To summarize, the lamina propria DCs that sample luminal material via the goblet cells are of the tolerogenic CD103+-type. There is also communication in the other direction as IL10 from the lamina propria immune cells can affect mucus properties.
Conclusions and perspective
The mucus that covers and protects the intestine is a dynamic system that is coupled to the immune system via the goblet cells that are the main producers of the mucus and at the same time sampling luminal antigen and presenting these to the dendritic cells. The enterocyte surface glycocalyx reaches out into the intestinal lumen and is likely also involved in sensing luminal phenomena.
The epithelial goblet cells and enterocytes are positioned to take a leading role in controlling the function of the intestinal immune system. Our current understanding of this role is limited, but what we have already uncovered of the goblet cell’s secrets has shown that this field is promising for future research.
Acknowledgments
This work was supported by the Swedish Research Council, The Swedish Cancer Foundation, The Knut and Alice Wallenberg Foundation, IngaBritt and Arne Lundberg Foundation, Sahlgren's University Hospital (LUA-ALF), Wilhelm and Martina Lundgren’s Foundation, Torsten och Ragnar Söderbergs Stiftelser, The Sahlgrenska Academy, the National Institute of Allergy and Infectious Diseases (U01AI095473, the content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH), and The Swedish Foundation for Strategic Research - The Mucus-Bacteria-Colitis Center (MBC) of the Innate Immunity Program.
References
- 1.Schade C, Flemstrom G, Holm L. Hydrogen ion concentration in the mucus layer on top of acid-stimulated and -inhibited rat gastric mucosa. Gastroenterology. 107:180–188. doi: 10.1016/0016-5085(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 2.Johansson MEV, Phillipson M, Petersson J, Holm L, Velcich A, Hansson GC. The inner of the two Muc2 mucin dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerbe F, van Es JH, Makrini L, Brulin B, Mellitzer G, Robine S, et al. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol. 192:767–780. doi: 10.1083/jcb.201010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vantourout P, Hayday A. Six-of-the-best: unique contributions of [gamma][delta] T cells to immunology. Nat Rev Immunol. 13:88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gill N, Wlodarska M, Finlay BB. The future of mucosal immunology: studying an integrated system-wide organ. Nat Immunol. 11:558–560. doi: 10.1038/ni0710-558. [DOI] [PubMed] [Google Scholar]
- 6.Atuma C, Strugula V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol. 280:G922–G929. doi: 10.1152/ajpgi.2001.280.5.G922. [DOI] [PubMed] [Google Scholar]
- 7.Wilson FA, Dietschy JM. Characterization of Bile Acid Absorption across the Unstirred Water Layer and Brush Border of the Rat Jejunum. J Clin Invest. 51:3015–3025. doi: 10.1172/JCI107129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ermund A, Schutte A, Johansson MEV, Gustafsson JK, Hansson GC. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer's patches. Am J Physiol Gastroint Liver Physiol. 305:G341–G347. doi: 10.1152/ajpgi.00046.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puchtler H, Waldrop FS, Meloan SN, Terry MS, Conner HM. Methacarn (methanol-Carnoy) fixation. Practical and theoretical considerations. Histochemie. 21:97–116. doi: 10.1007/BF00306176. [DOI] [PubMed] [Google Scholar]
- 10.Johansson MEV, Thomsson KA, Hansson GC. Proteomic Analyses of the Two Mucus Layers of the Colon Barrier Reveal That Their Main Component, the Muc2 Mucin, Is Strongly Bound to the Fcgbp Protein. J Proteome Res. 8:3549–3557. doi: 10.1021/pr9002504. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Pineiro AM, Bergstrom JH, Ermund A, Gustafsson JK, Schutte A, Johansson MEV, et al. Studies of mucus in mouse stomach, small intestine, and colon. II. Gastrointestinal mucus proteome reveals Muc2 and Muc5ac accompanied by a set of core proteins. Am J Physiol Gastroint Liver Physiol. 305:G348–G356. doi: 10.1152/ajpgi.00047.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhaskar KR, Garik P, Turner BS, Bradley JD, Bansil R, Stanley HE, et al. Viscous Fingering of HCl Through Gastric Mucin. Nature. 360:458–461. doi: 10.1038/360458a0. [DOI] [PubMed] [Google Scholar]
- 13.Johansson M, Synnerstad I, Holm L. Acid transport through channels in the mucous layer of rat stomach. Gastroenterology. 119:1297–1304. doi: 10.1053/gast.2000.19455. [DOI] [PubMed] [Google Scholar]
- 14.Gruber AD, Elble RC, Ji HL, Schreur KD, Fuller CM, Pauli BU. Genomic Cloning, Molecular Characterization, and Functional Analysis of Human CLCA1, the First Human Member of the Family of Ca2+-Activated Cl-Channel Proteins. Genomics. 54:200–214. doi: 10.1006/geno.1998.5562. [DOI] [PubMed] [Google Scholar]
- 15.Yurtsever Z, Sala-Rabanal M, Randolph DT, Scheaffer SM, Roswit WT, Alevy YG, et al. Self-cleavage of human CLCA1 by a novel internal metalloprotease domain controls calcium-activated chloride channel activation. J Biol Chem. 287:42138–42149. doi: 10.1074/jbc.M112.410282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harada N, Iijima S, Kobayashi K, Yoshida T, Brown WR, Hibi T, et al. Human IgGFc Binding Protein (Fcgamma BP) in Colonic Epithelial Cells Exhibits Mucin-like Structure. J Biol Chem. 272:15232–15241. doi: 10.1074/jbc.272.24.15232. [DOI] [PubMed] [Google Scholar]
- 17.Park SW, Zhen G, Verhaeghe C, Nakagami Y, Nguyenvu LT, Barczak AJ, et al. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc Natl Acad Sci USA. 106:6950–6955. doi: 10.1073/pnas.0808722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bevins CL. Paneth cell defensins: key effector molecules of innate immunity. Biochem Soc Trans. 34:263–266. doi: 10.1042/BST20060263. [DOI] [PubMed] [Google Scholar]
- 19.Ouellette AJ. Paneth cells and innate mucosal immunity. Curr Opin Gastroenterol. 26:547–553. doi: 10.1097/MOG.0b013e32833dccde. [DOI] [PubMed] [Google Scholar]
- 20.Heazlewood CK, Cook MC, Eri R, Price GR, Tauro SB, Taupin D, et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 5:e54. doi: 10.1371/journal.pmed.0050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renner M, Mollenhauer J, et al. DMBT1 Confers Mucosal Protection In Vivo and a Deletion Variant Is Associated With Crohn's Disease. Gastroenterology. 133:1499–1509. doi: 10.1053/j.gastro.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, et al. The Antibacterial Lectin RegIIIg Promotes the Spatial Segregation of Microbiota and Host in the Intestine. Science. 33:255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansson MEV, Hansson GC. Keeping Bacteria at a Distance. Science. 334:182–183. doi: 10.1126/science.1213909. [DOI] [PubMed] [Google Scholar]
- 24.Hornef MW, Wick MJ, Rhen M, Normark S. Bacterial strategies for overcoming host innate and adaptive immune responses. Nature Immunology. 3:1033–1040. doi: 10.1038/ni1102-1033. [DOI] [PubMed] [Google Scholar]
- 25.Stecher B, Hapfelmeier S, Muller C, Kremer M, Stallmach T, Hardt WD. Flagella and Chemotaxis Are Required for Efficient Induction of Salmonella enterica Serovar Typhimurium Colitis in Streptomycin-Pretreated Mice. Infection and Immunity. 72:4138–4150. doi: 10.1128/IAI.72.7.4138-4150.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newberry RD, Lorenz RG. Organizing a mucosal defense. Immunol Rev. 206:6–21. doi: 10.1111/j.0105-2896.2005.00282.x. [DOI] [PubMed] [Google Scholar]
- 27.Neutra MR, Frey A, Kraehenbuhl JP. Epithelial M cells: Gateways for mucosal infection and immunization. Cell. 86:345–348. doi: 10.1016/s0092-8674(00)80106-3. [DOI] [PubMed] [Google Scholar]
- 28.Ermund A, Gustafsson JK, Hansson GC, Keita ÅA. Mucus properties and goblet cell guantification in mouse, rat, and human ileal Peyer's patches. PLoS ONE. 8:e83688. doi: 10.1371/journal.pone.0083688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gustafsson JK, Ermund A, Ambort D, Johansson MEV, Nilsson HE, Thorell K, et al. Bicarbonate and functional CFTR channel is required for proper mucin secretion and link Cystic Fibrosis with its mucus phenotype. J Exp Med. 209:1263–1272. doi: 10.1084/jem.20120562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johansson MEV. Fast Renewal of the Distal Colonic Mucus Layers by the Surface Goblet Cells as Measured by In Vivo Labeling of Mucin Glycoproteins. PLoS ONE. 7:e41009. doi: 10.1371/journal.pone.0041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ambort D, Johansson MEV, Gustafsson JK, Nilsson H, Ermund A, Johansson BR, et al. Calcium and pH-dependent Packing and Release of the Gel-forming MUC2 Mucin. Proc Natl Acad Sci U S A. 109:5645–5650. doi: 10.1073/pnas.1120269109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Specian D, Neutra MR. Mechanism of rapid mucus secretion in goblet cells stimulated by acetylcholine. J Cell Biol. 85:626–640. doi: 10.1083/jcb.85.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grootjans J, Hundscheild IH, Lenaerts K, Boonen B, Renes IB, Verheyen FK, et al. Ischemia-induced mucus barrier loss and bacterial penetration are rapidly counteracted by increased goblet cell secretory activity in human and rat colon. Gut. 62:250–258. doi: 10.1136/gutjnl-2011-301956. [DOI] [PubMed] [Google Scholar]
- 34.Johansson MEV, Hansson GC. The goblet cell: a key player in ischemia-reperfusion injury. Gut. 62:188–189. doi: 10.1136/gutjnl-2012-302582. [DOI] [PubMed] [Google Scholar]
- 35.Johansson MEV, Sjovall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nature Rev Gastroenterol Hepatol. 10:352–361. doi: 10.1038/nrgastro.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johansson MEV, Gustafsson JK, Holmen-Larsson J, Jabbar KS, Xia L, Xu H, et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and in patients with ulcerative colitis. Gut. 213:281–291. doi: 10.1136/gutjnl-2012-303207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Round AN, Rigby NM, Garcia de la Torre A, Macierzanka A, Mills ENC, Mackie AR. Lamellar Structures of MUC2-Rich Mucin: A Potential Role in Governing the Barrier and Lubricating Functions of Intestinal Mucus. Biomacromolecules. 13:3253–3261. doi: 10.1021/bm301024x. [DOI] [PubMed] [Google Scholar]
- 38.Johansson MEV, Gustafsson JK, Sjoberg KE, Pettersson J, Holm L, Sjovall H, et al. Bacteria penetrate the inner mucus layer before inflammation in the Dextran sulfate colitis model. PLoS ONE. 5:e12238. doi: 10.1371/journal.pone.0012238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van der Sluis M, De Koning BAE, De Bruijn ACJM, Velcich A, Meijerink JPP, Van Goudoever JB, et al. Muc2-Deficient Mice Spontaneously Develop Colitis, Indicating That MUC2 Is Critical for Colonic Protection. Gastroenterology. 131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 40.Velcich A, Yang WC, Heyer J, Fragale A, Nicholas C, Viani S, et al. Colorectal Cancer in Mice Genetically Deficient in the Mucin Muc2. Science. 295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 41.Johansson MEV, Holmen Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci USA. 108:4659–4665. doi: 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-Bacterial Mutualism in the Human Intestine. Science. 307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 43.Sonnenburg JL, Xu J, Leip DD, Chen CH, Westover BP, Weatherford J, et al. Glycan Foraging in Vivo by an Intestine-Adapted Bacterial Symbiont. Science. 307:1955–1959. doi: 10.1126/science.1109051. [DOI] [PubMed] [Google Scholar]
- 44.Kaoutari AE, Armougom F, Gordon JI, Raoult D, Henrissat B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat Rev Micro. 11:497–504. doi: 10.1038/nrmicro3050. [DOI] [PubMed] [Google Scholar]
- 45.Koropatkin NM, Cameron EA, Martens EC. How glycan metabolism shapes the human gut microbiota. Nat Rev Micro. 10:323–335. doi: 10.1038/nrmicro2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larsbrink J, Rogers TE, Hemsworth GR, McKee LS, Tauzin AS, Spadiut O, et al. A discrete genetic locus confers xyloglucan metabolism in select human gut Bacteroidetes. Nature advance online publication. doi: 10.1038/nature12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.An G, Wei B, Xia B, McDaniel JM, Ju T, Cummings RD, et al. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J Exp Med. 204:1417–1429. doi: 10.1084/jem.20061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fu J, Wei B, Wen T, Johansson MEV, Xiaowei Liu X, Bradford E, et al. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis. J Clin Invest. 121:1657–1666. doi: 10.1172/JCI45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 27:104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- 50.Donohoe DR, Garge N, Zhang X, Sun W, O'Connell TM, Bunger MK, et al. The Microbiome and Butyrate Regulate Energy Metabolism and Autophagy in the Mammalian Colon. Cell Metabolism. 13:517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brestoff JR, Artis D. Commensal bacteria at the interface of host metabolism and the immune system. Nat Immunol. 14:676–684. doi: 10.1038/ni.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 53.De VF, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, et al. Microbiota-Generated Metabolites Promote Metabolic Benefits via Gut-Brain Neural Circuits. Cell. 156:84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 54.Tu QV, McGuckin MA, Mendz GL. Campylobacter jejuni response to human mucin MUC2: modulation of colonization and pathogenicity determinants. Journal of Medical Microbiology. 57:795–802. doi: 10.1099/jmm.0.47752-0. [DOI] [PubMed] [Google Scholar]
- 55.Bergstrom KSB, Kissoon-Singh V, Gibson DL, Ma C, Montero M, Sham HP, et al. Muc2 Protects against Lethal Infectious Colitis by Disassociating Pathogenic and Commensal Bacteria from the Colonic Mucosa. PLoS Pathog. 6:e1000902. doi: 10.1371/journal.ppat.1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gustafsson JK, Navabi N, Rodriguez-Pineiro AM, Alomran AHA, Premaratne P, Fernandez HR, et al. Dynamic changes in mucus thickness and ion secretion during Citrobacter rodentium infection and clearance. PLoS ONE. 8:e84430. doi: 10.1371/journal.pone.0084430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zarepour M, Bhullar K, Montero M, Ma C, Huang T, Velcich A, et al. The mucin Muc2 limits pathogen burdens and epithelial barrier dysfunction during Salmonella enterica serovar Typhimurium colitis. Infect Immun. 81:3672–3683. doi: 10.1128/IAI.00854-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nikitas G, Deschamps C, Disson O, Niault To, Cossart P, Lecuit M. Transcytosis of Listeria monocytogenes across the intestinal barrier upon specific targeting of goblet cell accessible E-cadherin. J Exp Med. 208:2263–2277. doi: 10.1084/jem.20110560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lidell ME, Moncada DM, Chadee K, Hansson GC. Entamoeba histolytica cysteine proteases cleave the MUC2 mucin in its C-terminal part and dissolves the protective colonic mucus gel. Proc Nat Acad Sci Usa. 103:9298–9393. doi: 10.1073/pnas.0600623103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van der Post S, Subramani DB, Backstrom M, Johansson MEV, Vester-Christensen MB, Mandel U, et al. Site-specific O-glycosylation on the MUC2 mucin inhibits cleavage by the Porphyromonas gingivalis secreted cysteine protease (RgpB) J Biol Chem. 288:14636–14646. doi: 10.1074/jbc.M113.459479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buisine MP, Devisme L, Savidge TC, Gespach C, Gosselin B, Porchet N, et al. Mucin gene expression in human embryonic and fetal intestine. Gut. 43:519–524. doi: 10.1136/gut.43.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Birchenough GMH, Johansson MEV, Stabler RA, Dalgakiran F, Hansson GC, Wren BW, et al. Altered innate defenses in the neonatal gastrointestinal tract in response to colonization by neuropathogenic Escherichia coli. Infect Immun. 81:3264–3275. doi: 10.1128/IAI.00268-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McElroy SJ, Prince LS, Weitkamp JH, Reese J, Slaughter JC, Polk DB. Tumor necrosis factor receptor 1-dependent depletion of mucus in immature small intestine: a potential role in neonatal necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 301:G656–G666. doi: 10.1152/ajpgi.00550.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karlsson NG, Johansson M, Asker N, Karlsson H, Gendler SJ, Carlstedt I, et al. Molecular characterization of the large heavily glycosylated domain glycopeptide from the rat small intestinal Muc2 mucin. Glycoconj J. 13:823–831. doi: 10.1007/BF00702346. [DOI] [PubMed] [Google Scholar]
- 65.Hansson GC, Baeckstrom D, Carlstedt I, Klinga-Levan K. Molecular Cloning of a cDNA Coding for a Region of an Apoprotein from the Insoluble Mucin Complex of Rat Small Intestine. Biochem Biophys Res Commun. 198:181–190. doi: 10.1006/bbrc.1994.1026. [DOI] [PubMed] [Google Scholar]
- 66.Gum JR, Hicks JW, Toribara NW, Siddiki B, Kim YS. Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J Biol Chem. 269:2440–2446. [PubMed] [Google Scholar]
- 67.Tsuru A, Fujimoto N, Takahashi S, Saito M, Nakamura D, Iwano M, et al. Negative feedback by IRE1β optimizes mucin production in goblet cells. Proc Natl Acad Sci USA. 110:2864–2869. doi: 10.1073/pnas.1212484110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martino MB, Jones L, Brighton B, Ehre C, Abdulah L, Davis CW, et al. The ER stress transducer IRE1[beta] is required for airway epithelial mucin production. Mucosal Immunol on line. doi: 10.1038/mi.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bertolotti A, Wang X, Novoa I, Jungreis R, Schlessinger K, Cho JH, et al. Increased sensitivity to dextran sodium sulfate colitis in IRE1b-deficient mice. Journal of Clinical Investigation. 107:585–593. doi: 10.1172/JCI11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, et al. XBP1 Links ER Stress to Intestinal Inflammation and Confers Genetic Risk for Human Inflammatory Bowel Disease. Cell. 134:743–756. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaser A, Zeissig S, Blumberg RS. Inflammatory Bowel Disease. Annu Rev Immunol. 28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Higa A, Mulot A, Delom F, Bouchecareilh M, Nguyen DT, Boismenu D, et al. Role of pro-oncogenic protein disulfide isomerase (PDI) family member anterior gradient 2 (AGR2) in the control of endoplasmic reticulum homeostasis. J Biol Chem. 286:44855–44868. doi: 10.1074/jbc.M111.275529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gupta A, Dong A, Lowe AW. AGR2 Gene Function Requires a Unique Endoplasmic Reticulum Localization Motif. J Biol Chem. 287:4773–4782. doi: 10.1074/jbc.M111.301531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao F, Edwards R, Dizon D, Mastroianni JR, Geyfman M, Ouellette AJ, et al. Disruption of Paneth and goblet cell homeostasis and increased endoplasmic reticulum stress in Agr2−/− mice. Develop Biol. 338:270–279. doi: 10.1016/j.ydbio.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bennett EP, Mandel U, Clausen H, Gerken TA, Fritz TA, Tabak LA. Control of mucin-type O-glycosylation: A classification of the polypeptide GalNAc-transferase gene family. Glycobiology. 22:736–756. doi: 10.1093/glycob/cwr182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Godl K, Johansson MEV, Karlsson H, Morgelin M, Lidell ME, Olson FJ, et al. The N-termini of the MUC2 mucin form trimers that are held together within a trypsin-resistant core fragment. J Biol Chem. 277:47248–47256. doi: 10.1074/jbc.M208483200. [DOI] [PubMed] [Google Scholar]
- 77.McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 483:345–349. doi: 10.1038/nature10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Patel KK, Miyoshi H, Beatty WL, Head RD, Malvin NP, Cadwell K, et al. Autophagy proteins control goblet cell function by potentiating reactive oxygen species production. Embo J advance online publication. doi: 10.1038/emboj.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Razi M, Chan EY, Tooze SA. Early endosomes and endosomal coatomer are required for autophagy. J Cell Biol. 185:305–321. doi: 10.1083/jcb.200810098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hampe J, Franke A, Rosenstiel P, Till A, Teuber M, Huse K, et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet. 39 doi: 10.1038/ng1954. [DOI] [PubMed] [Google Scholar]
- 81.Cadwell K, Liu JY, Brown SL, Miyoshi H, Loh J, Lennerz JK, et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 456:259–263. doi: 10.1038/nature07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ishibashi K, Uemura T, Waguri S, Fukuda M. Atg16L1, an essential factor for canonical autophagy, participates in hormone secretion from PC12 cells independently of autophagic activity. Molecular Biology of the Cell. 23:3193–3202. doi: 10.1091/mbc.E12-01-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria 9. Nat Immunol. 2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 84.Rescigno M, Rotta G, Valzasina B, Ricciardi-Castagnoli P. Dendritic cells shuttle microbes across gut epithelial monolayers. Immunobiology. 204:572–581. doi: 10.1078/0171-2985-00094. [DOI] [PubMed] [Google Scholar]
- 85.Ito S. Structure and function of the glycocalyx. Fed Proc. 28:12–25. [PubMed] [Google Scholar]
- 86.Maury J, Bernadac A, Rigal A, Maroux S. Expression and glycosylation of the filamentous brush border glycocalyx (FBBG) during rabbit enterocyte differentiation along the crypt-villus axis. Journal of Cell Science. 108:2705–2713. doi: 10.1242/jcs.108.7.2705. [DOI] [PubMed] [Google Scholar]
- 87.Egberts HJA, Koninkx JFJG, van Dijk JE, Mouwen JMVM. Biological and pathobiological aspects of the glycocalyx of the small intestinal epithelium. A review. The Veterinary Quarterly. 6:186–199. doi: 10.1080/01652176.1984.9693936. [DOI] [PubMed] [Google Scholar]
- 88.Pelaseyed T, Gustafsson JK, Gustafsson IJ, Ermund A, Hansson GC. Carbachol-induced MUC17 endocytosis is concomitant with NHE3 internalization and CFTR membrane recruitment in enterocytes. Am J Physiol Cell Physiol. 305:C457–C467. doi: 10.1152/ajpcell.00141.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kesimer M, Ehre C, Burns KA, Davis CW, Sheehan JK, Pickles RJ. Molecular organization of the mucins and glycocalyx underlying mucus transport over mucosal surfaces of the airways. Mucosal Immunol. doi: 10.1038/mi.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Argueso P, Guzman-Aranguez A, Mantelli F, Cao Z, Ricciuto J, Panjwani N. Association of Cell Surface Mucins with Galectin-3 Contributes to the Ocular Surface Epithelial Barrier. J Biol Chem. 284:23037–23045. doi: 10.1074/jbc.M109.033332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Linden SK, Sutton P, Karlsson NG, Korolik V, McGuckin MA. Mucins in the mucosal barrier to infection. Mucosal Immunol. 1:183–197. doi: 10.1038/mi.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gum JR, Hicks JW, Swallow DM, Lagace RL, Byrd JC, Lamport DTA, et al. Molecular cloning of cDNAs derived from a novel human intestinal mucin gene. Biochem Biophys Res Commun. 171:407–415. doi: 10.1016/0006-291x(90)91408-k. [DOI] [PubMed] [Google Scholar]
- 93.Gum JR, Ho JJL, Pratt WS, Hicks W, Hill AS, Vinall LE, et al. MUC3 human intestinal mucin. J Biol Chem. 272:26678–26686. doi: 10.1074/jbc.272.42.26678. [DOI] [PubMed] [Google Scholar]
- 94.Williams SJ, McGuckin MA, Gotley DC, Eyre HJ, Sutherland GR, Antalis TM. Two novel mucin genes down-regulated in colorectal cancer identified by differential display. Cancer Res. 59:4083–4089. [PubMed] [Google Scholar]
- 95.Williams SJ, Wreschner DH, Tran M, Eyre HJ, Sutherland GR, McGuckin MA. MUC13, a novel human cell surface mucin expressed by epithelial and hemopoietic cells. J Biol Chem. 276:18327–18336. doi: 10.1074/jbc.M008850200. [DOI] [PubMed] [Google Scholar]
- 96.Yin BWT, Lloyd K. Molecular cloning of the CA125 ovarian cancer antigen. J Biol Chem. 276:27371–27375. doi: 10.1074/jbc.M103554200. [DOI] [PubMed] [Google Scholar]
- 97.O'Brien TJ, Beard JB, Underwood LJ, Shigemasa K. The CA125 gene: a newly discovered extesion of the glycosylated N-terminal domain doubles the size of this extracellular superstructure. Tumor Biol. 23:154–169. doi: 10.1159/000064032. [DOI] [PubMed] [Google Scholar]
- 98.Gum JR, Crawley SC, Hicks JW, Szymkowski DE, Kim YS. MUC17, a novel membrane-tethered mucin. Biochem Biophys Res Commun. 291:466–475. doi: 10.1006/bbrc.2002.6475. [DOI] [PubMed] [Google Scholar]
- 99.Lang T, Hansson GC, Samuelsson T. Gel-forming mucins appeared early in metazoan evolution. Proc Natl Acad Sci USA. 104:16209–16214. doi: 10.1073/pnas.0705984104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Macao B, Johansson DGA, Hansson GC, Härd T. Auto-proteolysis coupled to protein folding in the SEA domain of the membrane-bound MUC1 mucin. Nature Struct Mol Biol. 13:71–76. doi: 10.1038/nsmb1035. [DOI] [PubMed] [Google Scholar]
- 101.Lidell ME, Johansson MEV, Hansson GC. An autocatalytic cleavage in the C-terminus of the human MUC2 mucin occurs at the low pH of the late secretory pathway. J Biol Chem. 278:13944–13951. doi: 10.1074/jbc.M210069200. [DOI] [PubMed] [Google Scholar]
- 102.Pelaseyed T, Zäch M, Petersson AC, Svensson F, Johansson DG, Hansson GC. Unfolding dynamics of the mucin SEA domain probed by force spectroscopy suggest that it acts as a cell protective device. FEBS J. 280:1491–1501. doi: 10.1111/febs.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Linden SK, Sheng YH, Every AL, Miles KM, Skoog EC, Florin THJ, et al. MUC1 Limits Helicobacter pylori Infection both by Steric Hindrance and by Acting as a Releasable Decoy. PLoS Pathog. 5:e1000617. doi: 10.1371/journal.ppat.1000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hinojosa-Kurtzberg M, Johansson MEV, Madsen CS, Hansson GC, Gendler SJ. Novel MUC1 splice variants contribute to mucin over-expression in CFTR deficient mice. Am J Physiol Gastrointest Liver Physiol. 284:G853–G862. doi: 10.1152/ajpgi.00326.2002. [DOI] [PubMed] [Google Scholar]
- 105.Sheng YH, Triyana S, Wang R, Das I, Gerloff K, Florin TH, et al. MUC1 and MUC13 differentially regulate epithelial inflammation in response to inflammatory and infectious stimuli. Mucosal Immunol. 6:557–568. doi: 10.1038/mi.2012.98. [DOI] [PubMed] [Google Scholar]
- 106.Sheng YH, Lourie R, Linden SK, Jeffery PL, Roche D, Tran TV, et al. The MUC13 cell-surface mucin protects against intestinal inflammation by inhibiting epithelial cell apoptosis. Gut. 60:1661–1670. doi: 10.1136/gut.2011.239194. [DOI] [PubMed] [Google Scholar]
- 107.Hattrup CL, Gendler SJ. Structure and Function of the Cell Surface (Tethered) Mucins. Annual Review of Physiology. 70:431. doi: 10.1146/annurev.physiol.70.113006.100659. [DOI] [PubMed] [Google Scholar]
- 108.Malmberg EK, Pelaseyed T, Petersson ÅC, Seidler U, de Jonge H, Riordan JR, et al. The Transmembrane MUC17 Mucin C-terminus Binds to the Scaffold Protein PDZK1 that Stably Localizes it to the Enterocyte Apical Membrane in the Small Intestine. Biochem J. 410:283–289. doi: 10.1042/BJ20071068. [DOI] [PubMed] [Google Scholar]
- 109.Donowitz M, Cha B, Zachos NC, Brett CL, Sharma A, Tse CM, et al. NHERF family and NHE3 regulation. J Physiol. 567:3–11. doi: 10.1113/jphysiol.2005.090399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lamprecht G, Seidler UE. The emerging role of PDZ adapter proteins for regulation of intestinal ion transport. Am J Physiol Gastrointest Liver Physiol. 291:G766–G777. doi: 10.1152/ajpgi.00135.2006. [DOI] [PubMed] [Google Scholar]
- 111.Wang S, Yue H, Derin RB, Guggino WB, Li M. Accessory protein facilitated CFTR-CFTR interaction, a molecular mechanism to potentiate the chloride channel activity. Cell. 103:169–179. doi: 10.1016/s0092-8674(00)00096-9. [DOI] [PubMed] [Google Scholar]
- 112.Pelaseyed T, Hansson GC. CFTR anion channel modulates expression of human transmembrane mucin MUC3 via the PDZ protein GOPC. J Cell Sci. 124:3074–3083. doi: 10.1242/jcs.076943. [DOI] [PubMed] [Google Scholar]
- 113.Cheng J, Wang H, Guggino WB. Modulation of Mature Cystic Fibrosis Transmembrane Regulator Protein by the PDZ Domain Protein CAL. J Biol Chem. 279:1892–1898. doi: 10.1074/jbc.M308640200. [DOI] [PubMed] [Google Scholar]
- 114.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 115.Swamy M, Jamora C, Havran W, Hayday A. Epithelial decision makers: in search of the 'epimmunome'. Nat Immunol. 11:656–665. doi: 10.1038/ni.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Philpott DJ, Sorbara MT, Robertson SJ, Croitoru K, Girardin SE. NOD proteins: regulators of inflammation in health and disease. Nat Rev Immunol. 14:9–23. doi: 10.1038/nri3565. [DOI] [PubMed] [Google Scholar]
- 117.Song DH, Lee JO. Sensing of microbial molecular patterns by Toll-like receptors. Immunol Rev. 250:216–229. doi: 10.1111/j.1600-065X.2012.01167.x. [DOI] [PubMed] [Google Scholar]
- 118.Cheroutre H. IELs: enforcing law and order in the court of the intestinal epithelium. Immunological Reviews. 206:114–131. doi: 10.1111/j.0105-2896.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- 119.Witherden DA, Havran WL. Cross-talk between intraepithelial γδ T cells and epithelial cells. Journal of Leukocyte Biology. 94:69–76. doi: 10.1189/jlb.0213101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bas A, Swamy M, Abeler-Dorner L, Williams G, Pang DJ, Barbee SD, et al. Butyrophilin-like 1 encodes an enterocyte protein that selectively regulates functional interactions with T lymphocytes. Proc Natl Acad Sci U S A. 108:4376–4381. doi: 10.1073/pnas.1010647108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Abeler-Dörner L, Swamy M, Williams G, Hayday AC, Bas A. Butyrophilins: an emerging family of immune regulators. Trends in immunology. 33:34–41. doi: 10.1016/j.it.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 122.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of Monocytes, Macrophages, and Dendritic Cells. Science. 327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 1:11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- 124.Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 6:498–510. doi: 10.1038/mi.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jaensson E, Uronen-Hansson H, Pabst O, Eksteen B, Tian J, Coombes JL, et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 205:2139–2149. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Burzyn D, Benoist C, Mathis D. Regulatory T cells in nonlymphoid tissues. Nat Immunol. 14:1007–1013. doi: 10.1038/ni.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Chen K, et al. Mucus Enhances Gut Homeostasis and Oral Tolerance by Delivering Immunoregulatory Signals. Science. 342:447–453. doi: 10.1126/science.1237910. [DOI] [PMC free article] [PubMed] [Google Scholar]