Abstract
Anti-inflammatory and anti-oxidant agents can alleviate ischemic cerebral injury. The immunomodulary drug Setarud, which is composed of herbal extracts including Rosa canina, Urtica dioica and Tanacetum vulgare, supplemented with selenium exhibits anti-inflammatory and anti-oxidant properties. Therefore, we hypothesized that Setarud will have a neuroprotective effect against ischemic cerebral injury. To validate this hypothesis, rats were intraperitoneally administered with 0.66 mL/kg Setarud for 30 minutes after middle cerebral artery occlusion. Triphenyltetrazolium chloride staining showed that Setarud could reduce cerebral infarct volume of rats subjected to cerebral ischemia. Transmission electron microscopy and hematoxylin-eosin staining results showed that Setarud could alleviate the degenerative changes in cortical neurons of rats with cerebral ischemia. The inclined plate test and prehensile test showed that Setarud could significantly improve the motor function of rats with cerebral ischemia. These findings suggest that Setarud shows neuroprotective effects against ischemic brain injury.
Keywords: immunomodulatory drug, stroke, prehensile test, antioxidant, inclined plate test, cerebral infarct, cortex, neuroprotection, brain injury, neural regeneration
Research Highlights
-
(1)
The immunomodulatory drug Setarud can reduce cerebral infarct volume in rats subjected to middle cerebral artery occlusion (MCAO).
-
(2)
Setarud can alleviate the degenerative changes in cortical neurons of rats subjected to MCAO.
-
(3)
Setarud can improve muscle strength in rats subjected to MCAO.
-
(4)
Setarud exhibits neuroprotective effects against ischemic brain injury.
Abbreviations
IMOD, immunomodulatory drug; MCAO, middle cerebral artery occlusion; TTC, triphenyltetrazolium
INTRODUCTION
The immunomodulatory drug (IMOD™) Setarud is a mixture of herbal extracts (Rosa canina, Urtica dioica, Tanacetum vulgare) supplemented with selenium. Of the drug constituents, beta-carotene from the fruits of Rosa canina increases serum cholesterol and blood glucose levels, the polysaccharide and lectin fractions of Urtica dioica (nettle) have been shown to have antiviral and anti-inflammatory activities, Tanacetum vulgare (tansy) is a useful remedy for the treatment of a wide range of symptoms due to its anti-inflammatory activity[1], and selenium has antioxidant effects. A previous experimental study has shown that Setarud does not exhibit any mutagenic or genotoxic properties[2]. Recent studies have shown that Setarud has anti-inflammatory, hepatoprotective, antihyperlipidemic and antihyperglycemic effects[3,4,5].
Stroke is a progressive neurodegenerative disease[6]. The prevention and treatment of stroke are the main therapeutic targets of therapies[7]. Stroke is a complicated disease with different pathophysiological mechanisms, including oxidative stress, the intracellular accumulation of calcium ions, excitotoxicity, and inflammation[8]. Anti-oxidant and anti-inflammatory agents protect the brain against ischemic cerebral injury[9,10]. Based on the anti-oxidant and anti-inflammatory properties of Setarud, this study was designed to evaluate the neuroprotective effects of Setarud on ischemic cerebral injury in male rats.
RESULTS
Quantitative analysis of experimental animals
Sixty adult Wistar rats were randomly divided into three groups: sham-operated (n = 6), vehicle (n = 30; treated with ethanol (i.p.) 30 minutes after middle cerebral artery occlusion (MCAO)), and IMOD (n = 24; treated with Setarud (i.p.) 30 minutes after MCAO). The mortality rate of rats was 50%, 60% and 0% in the IMOD, vehicle and sham-operated groups, respectively. Rats did not survive mainly because of subarachnoid hemorrhage. Lost rats were supplemented allowing for 60 rats to be included in the final analysis.
Effects of Setarud on infarct volume in rats with cerebral ischemia/reperfusion
Setarud injection at 30 minutes after MCAO significantly reduced brain infarct volume in rats with cerebral ischemia/ reperfusion (n = 4 rats/group; P < 0.05; Figures 1, 2).
Figure 1.
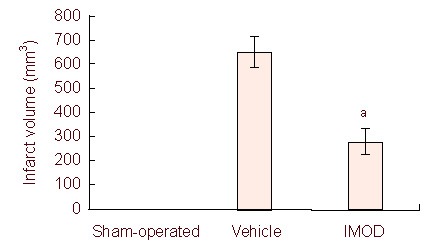
Effect of Setarud on infarct volume of rats subjected to middle cerebral artery occlusion.
Data are expressed as mean ± SEM (n = 4 rats/group, aP < 0.05, vs. vehicle group). The data were analyzed with one-way analysis of variance followed by post-hoc Tukey's test. IMOD: Immunomodulatory drug.
Figure 2.
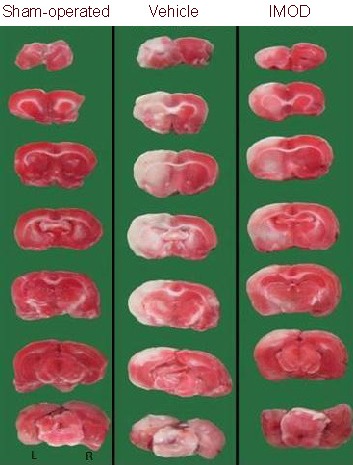
Treatment with Setarud markedly reduced the cortical infarction in rats with cerebral ischemia/reperfusion injury.
Marked infarction (white areas) is observed in the rat left cortex in the vehicle group. IMOD: Immunomodulatory drug; L: left; R: right.
Effects of Setarud on ultrastructure of cortical neurons in rats with cerebral ischemia/reperfusion
Degenerative changes in cortical neurons were induced by 15 minutes of MCAO followed by 24 hours of reperfusion. Changes included organelle swelling, chromatin condensation and nuclear membrane disruption. Setarud treatment protected the ultrastructure of cortical neurons against these degenerative alterations (Figure 3).
Figure 3.
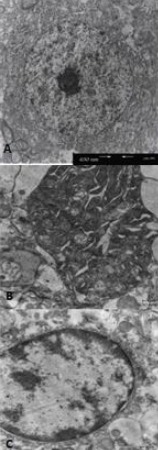
Effect of Setarud treatment on the ultrastructure of cortical neurons in rats with cerebral ischemia/reperfusion (transmission electron microscopy).
Scale bar: 630 nm.
(A) An intact neuron in the sham-operated group.
(B) A degenerated neuron in the vehicle group. Note the dilated organelles and the loss of nucleus.
(C) A protected neuron treated with Setarud.
Effects of Setarud on neuronal damage in the rat cortex following cerebral ischemia/reperfusion
Transient ischemia followed by reperfusion resulted in severe damage to cortical neurons of male rats. Treatment with Setarud protected cortical neurons against MCAO-induced injury (Figure 4).
Figure 4.
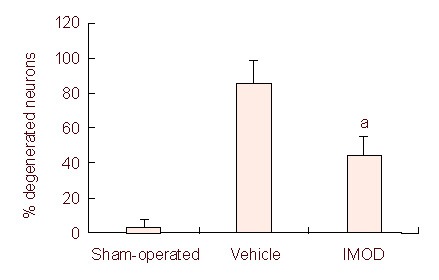
Effect of Setarud treatment on neuronal injury in the rat cortex after cerebral ischemia/reperfusion.
Treatment with Setarud significantly protected cortical neurons against ischemic injury (the percentage of degenerated neurons in comparison to surviving ones was calculated).
All values are expressed as mean ± SEM (n = 4 rats/group, aP < 0.001, vs. vehicle group, Student's t-test). IMOD: Immunomodulatory drug.
Effects of Setarud on motor function in rats with cerebral ischemia/reperfusion
Treatment with Setarud significantly improved motor function, as evaluated by the inclined plate test and prehensile test (n = 8 rats/group; Figures 5, 6).
Figure 5.
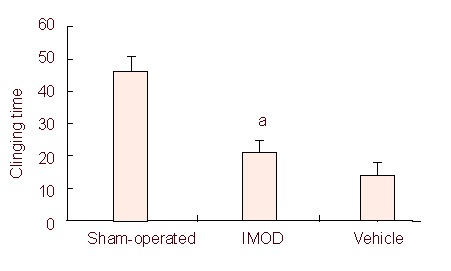
Effect of Setarud treatment on motor function of rats detected by the traction test.
Motor function was significantly improved in animals treated with Setarud (n = 8 rats/group, aP < 0.05, vs. vehicle group).
All values are expressed as mean ± SEM. The data were analyzed with one-way analysis of variance followed by post-hoc Tukey's test. IMOD: Immunomodulatory drug.
Figure 6.
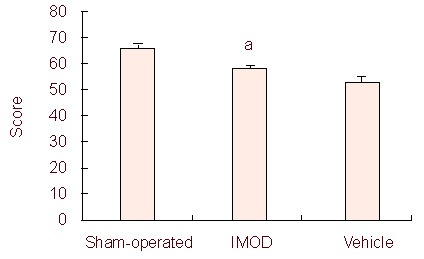
Effect of Setarud treatment on rat motor function determined by the angle board test.
Treatment with Setarud significantly enhanced the motor function score in the angle board test (n = 8 rats/group, aP < 0.05, vs. vehicle group).
All values are expressed as mean ± SEM. Statistical analysis was performed using the Kruskal-Wallis test. IMOD: Immunomodulatory drug.
DISCUSSION
Our histological and ultrastructural findings showed that Setarud protected cortical neurons against ischemia reperfusion-induced insults. According to our data, 15 minutes of MCAO followed by 24 hours of reperfusion resulted in severe damage to cortical neurons in the vehicle group. This finding is in agreement with the results of other studies[11,12]; thus, Setarud treatment can protect neurons against ischemic cerebral injury. In line with the above findings, light and electron microscopic examination showed degenerative changes in cortical neurons after cerebral ischemia/reperfusion, whereas Setarud showed protective effects against these changes.
Motor disorders are one of the most destructive outcomes of cerebral ischemia due to MCAO because most of the pyramidal tract and motor cortex lie inside the territory supplied by the middle cerebral artery[13]. Motor disorders can result from a failure of cortical excitability and/or the inhibition of electrical impulses in the subcortical area. Axonal conduction readily recovers after ischemia and reperfusion. However, a constant failure at the cortical synapses leads to motor dysfunction[14]. Some studies have shown neurological and locomotor deficits after ischemia in rats[15,16]. The behavioural scores in the current study were significantly lower in the vehicle group than in the Setarud group, verifying deficits in muscle co-ordination and grip strength. A decrease in muscle co-ordination and grip strength was also shown by various studies on the MCAO model of cerebral ischemia[17,18]. Treatment with Setarud in the current study was found to attenuate muscle weakness, supporting its protective effect against ischemia -reperfusion damage.
The brain is almost completely dependent on a continuous flow of glucose and oxygen in order to undergo oxidative phosphorylation for energy production. The first effect of reduced cerebral blood flow is a decrease in available substrates, mainly glucose and oxygen, which causes an accumulation of lactate via anaerobic glycolysis. Acidosis can increase free radical formation, interfering with intracellular protein synthesis and worsening ischemic brain damage[19].
A cascade of complex biochemical events occur seconds to minutes after cerebral ischemia. Cerebral ischemia is caused by reduced blood supply to the microcirculation. Ischemia causes impairment of brain energy metabolism, loss of aerobic glycolysis, intracellular accumulation of sodium and calcium ions, and release of excitotoxic neurotransmitters. Lactate levels are elevated with local acidosis, free radical production, cell swelling, over activation of lipases and proteases, and cell death. Many neurons undergo apoptosis after focal brain ischemia. Ischemic cerebral injury is exacerbated by leukocyte infiltration and brain edema development. Exciting new treatments for stroke target these biochemical changes[20].
In addition, re-perfusion in the brain after ischemia induces an inflammatory response that can exacerbate the initial levels of tissue damage. There are a number of possible mechanisms by which post-ischemic inflammation can contribute to brain damage, including the production of toxic mediators such as nitric oxide by activated inflammatory cells and vascular occlusion by neutrophils[8]. Reactive oxygen species can be released by injured tissues to trigger activation of the immune response. Besides the vascular endothelium and activated microglia/macrophages, neutrophils are also an important source of reactive oxygen species[21]. It has also been reported that the inhibition of reactive oxygen species generation, inflammatory cell activation, pro-inflammatory cytokine production and apoptotic gene induction provides neuroprotective effects against ischemic cerebral injury[8].
To the best of our knowledge, this is the first report to provide evidence of the effectiveness of Setarud in an experimental model of cerebral ischemia. The Setarud used in the present study is a new drug containing herbal extracts with known beneficial effects. Some of its components are urea, selenium and extracts of Rosa canina, Urtica dioica and Tanacetum vulgarer. Previous studies have shown that urea can improve cerebral blood flow and oxygenation and it may work in the same way as mannitol, a known neuroprotective agent[22]. According to the results of different studies, selenium is a potent antioxidant agent that may have neuroprotective activities[23,24]. The anti-inflammatory activities of the polysaccharide and lectin fractions of Urtica dioica (nettle) and Tanacetum vulgare (tansy) have also been shown in recent studies. Thus, based on the different effects of these agents, we can conclude that most if not all beneficial effects of Setarud are due to the anti-inflammatory and anti-oxidant properties of these agents, which improve cerebral blood flow. Other studies have shown that Setarud significantly decreases the levels of the pro-inflammatory cytokines tumor necrosis factor α and interleukin 1β in an experimental model of inflammatory bowel disease[3].
The administration of Setarud in rats with ischemic stroke protects the brain from ischemic cerebral injury. If confirmed by clinical studies, this finding may open up new avenues for treatment of brain ischemia.
MATERIALS AND METHODS
Design
A randomized, controlled animal study.
Time and setting
All experiments were performed at the general laboratory of Neuroscience Research Center, Kerman Medical University, Kerman, Iran from June 2010 to March 2011.
Materials
Animals
Wistar male rats, aged 4 months, weighing 220–290 g, were provided by the Quality Supervision Neuroscience Research Center, Kerman, Iran and were included in this study. The rats had free access to food and water and were kept at 12-hour light/dark conditions at 20 ± 3°C.
Drugs and reagents
The Setarud used in this study was provided by Pars Roos Company, Tehran, Iran. Triphenyltetrazolium chloride (TTC) and chloral hydrate were purchased from Sigma, St. Louis, MO, USA.
Methods
Establishment of cerebral ischemia/reperfusion animal model
Left middle cerebral artery occlusion was induced using the intraluminal filament model and the method described by Longa et al[25]. In brief, animals were anesthetized with chloral hydrate (360 mg/kg, i.p.). In the supine position, a midline ventral incision was made to expose the left common carotid artery, which was carefully separated from the vagus nerve. All of the branches of the external carotid artery and the extracranial internal carotid artery were blocked. Then, a 3-0 nylon suture was introduced into the internal carotid artery and advanced intracranially to block the blood flow into the middle cerebral artery. After 15 minutes of ischemia, the suture was withdrawn to restore blood flow for 24 hours (reperfusion). Rectal temperature was maintained at 37 ± 0.5°C using a thermistor coupled to a heating blanket during surgery. The animals were returned to their cages after recovering from the anesthesia. The neurologic status of each rat was evaluated carefully after full recovery. The rat was held in the air by the tail to observe symmetry in the movement of the four limbs. A score of 3 indicated that all four limbs extend symmetrically; a score of 2 indicated that limbs on the right side extended less or more slowly than those on the left side; a score of 1 indicated that limbs on the right side showed minimal movement; and a score of 0 indicated that the forelimb on the right side did not move at all[26]. Neurologic examination was performed in 3 to 5 minutes. Rats given a score of 1 were considered to be a successful MCAO model. In the sham-operated group, surgical procedure was performed without ischemia induction.
Setarud treatment
Rats in the vehicle group (15 minutes of MCAO followed by 24 hours of reperfusion) were treated with a single dose of ethanol (0.86 mL/kg, i.p.) 30 minutes after MCAO, and rats in the IMOD group (15 minutes of MCAO followed by 24 hours of reperfusion) were treated with a single dose of Setarud (0.66 mL/kg, i.p.) 30 minutes after MCAO. The dose of Setarud was selected based on a pilot study.
TTC staining for determination of cerebral infarct volume
At 1 day after MCAO, rats were sacrificed and intracardially perfused with saline. Whole brain tissue was carefully removed, immersed in cold saline for 10 minutes, and sliced into 2.00 mm-thick sections using a brain matrix apparatus. The slices were incubated in 2% (w/v) TTC (Sigma), and immersed in distilled water for 15 minutes at room temperature. The area of infarction on each brain slice was measured (blinded) using a digital scanner and an image tool program (UTHSCSA Image Tool for Windows, version 3.00, Department of Dental Diagnostic Science at the University of Texas Health Science Center, Texas, USA). In order to minimize any artifacts induced by post-ischemic swelling in the infarcted tissue, the infarct area on the left cortex was directly measured by subtracting the non-infarcted area in the left cortex from the total cortical area of the right hemisphere. The total infarct volume was obtained from the product of the average slice thickness and the sum of the area of infarction in all brain slices. The infarct volume was calculated according to the following formula:
(infarct volume – left hemisphere volume) – right hemisphere volume = corrected infarct volume.
Electron microscopy for cortical ultrastructures
Rats anesthetized with chloral hydrate were transcardially perfused with 4% (w/v) paraformaldehyde in 0.1 M PBS (pH 7.4). Whole brains were removed and immersed in 2% (v/v) buffered glutaraldehyde overnight. A small block of the cortical area was dissected and fixed in the same fixative (glutaraldehyde) for an additional 24 hours. Specimens were post-fixed in 1% (w/v) OsO4/1% (v/v) phosphate buffer. After dehydration in ethanol, the slices were embedded in Epon 812 resin and a section (300 nm) was stained with toluidine blue to identify the areas of interest. Subsequently, 70-80 nm sections were cut and stained with uranyl acetate (K & K laboratories, Inc., Jamaica, USA) and lead citrate. The sections were examined under a Philips transmission electron microscope (EM 300, Eindhoven, The Netherlands).
Hematoxylin-eosin staining for determination of neuronal damage
Under deep anesthesia with chloral hydrate (360 mg/kg), rats were sacrificed by transcardial perfusion with 10% (v/v) formaldehyde. The brains were removed and immersed in the same fixative at 4°C overnight. The brains were then processed using the standard method for light microscopy studies. Coronal sections, 1.6– 2.8 mm posterior to the bregma, were cut at a thickness of 5 µm using a microtome. Hematoxylin-eosin staining was performed according to the standard protocol[27]. Degenerated neurons that appeared shrunken or necrotic[28] were counted by sampling at least three consecutive fields using a magnification of × 400 in three sections per animal. The number of degenerated neurons was expressed as a percentage of the total number of neurons.
Motor performance tests
Inclined plate test: The inclined plane was comprised of a 28 cm × 30 cm floor covered with a grooved, 1 mm-thick rubber surface that was 20 cm × 30 cm, and 10 cm high on three sides. This task evaluates an animal's ability to maintain its body position on a board that is incrementally raised at increasing angles. The rat was placed on the inclined plane with its head down or up and to the right or left. Alternatively, it can be placed in such a position that its body was perpendicular to the axis of the plane. Usually, testing in two upright directions, called bidirectional inclined plane testing, was sufficient. The angle of inclination was then gradually increased towards the vertical position until the rat could no longer remain in place at the starting position. The largest angle at which the rat could maintain a stable position for 5 seconds was recorded. This test could be used as an index of animal function[29].
Prehensile test: A piece of rope 2 mm in diameter and 40 cm long was stretched horizontally, 50 cm above the base. The rat was forced to grasp the rope with its two forepaws and the time (seconds) that the rat held onto the rope was measured for up to 60 seconds. The trial was conducted three times for each rat, and the mean hanging time was used as the mean result for the test. When the duration of clinging was more than 60 seconds, the rat was released from the wire and the clinging time was recorded as 60 seconds[30].
Statistical analysis
Statistical analysis was performed using SPSS software 13.5. All values were expressed as mean ± SEM. One-way analysis of variance followed by post-hoc Tukey's test, Student's t-test, and the Kruskal-Wallis tests were used for statistical comparisons. A statistical difference was determined at P < 0.05.
Acknowledgments:
We thank all of the staff at the Kerman Neuroscience Research Center (KNRC) for providing instruments and Technical assistance.
Footnotes
Funding: This work was financially supported by a grant from the Vice Chancellor of Research at Kerman Medical University.
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Ethical Committee of the Vice Chancellor of Research at Kerman Medical University (K/90/58).
(Edited by Yalcin A, Shi XQ, Zhan DS/Song LP)
REFERENCES
- [1].Khairandish P, Mohraz M, Farzamfar B, et al. Preclinical and phase 1 clinical safety of Setarud (IMOD™), a novel immunomodulator. DARU. 2009;17(3):148–156. [Google Scholar]
- [2].Khorram-Khorshid HR, Abdollahi M, Novitsky YA, et al. Studies on potential mutagenic and genotoxic activity of Setarud. DARU. 2008;16(4):223–228. [Google Scholar]
- [3].Baghaei A, Esmaily H, Bdolghaffari AH, et al. Efficacy of Setarud (IMOD), a novel drug with potent anti-toxic stress potential in rat inflammatory bowel disease and comparison with dexamethasone and infliximab. Indian J Biochem Biophys. 2010;47:219–226. [PubMed] [Google Scholar]
- [4].Khorram-Khorshid HR, Azonov JA, Novitsky YA, et al. Hepatoprotective effects of setarud against carbon tetrachloride-induced liver injury in rats. Indian J Gastroenterol. 2008;27:110–112. [PubMed] [Google Scholar]
- [5].Khorram-Khorshid HR, Azonov JA, Novitsky YA, et al. Protective effects of setarud (IMODTM) on development of diet-induced hypercholesterolemia in rabbits. DARU. 2008;16(4):218–222. [Google Scholar]
- [6].Gemma C, Mesches MH, Sepesi B, et al. Diets enriched in foods with high antioxidant activity reverse age-induced decreases in cerebellar-adrenergic function and increases in proinflammatory cytokines. J Neurosci. 2002;22:6114–6120. doi: 10.1523/JNEUROSCI.22-14-06114.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Caplan LR. Treatment of acute stroke: still struggling. JAMA. 2004;292:1883–1885. doi: 10.1001/jama.292.15.1883. [DOI] [PubMed] [Google Scholar]
- [8].Durukan A, Tatlisumak T. Acute ischemic stroke: Overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behave. 2007;87:179–197. doi: 10.1016/j.pbb.2007.04.015. [DOI] [PubMed] [Google Scholar]
- [9].Sweeney MI, Kalt W, MacKinnon SL, et al. Feeding rats diets enriched in lowbush bluberries for six weeks decreases ischemia-induced brain damage. Nutr Neurosci. 2002;5:427–431. doi: 10.1080/1028415021000055970. [DOI] [PubMed] [Google Scholar]
- [10].Panahi M, Asadi-Shekaari M, Kalantaripour TP, et al. Aqueous extract of date fruit protects CA1 neurons against oxidative injury: an ultrastructural Study. Curr Topics Nutraceutical Res. 2008;6(3):125–130. [Google Scholar]
- [11].Zhao J, Zhao Y, Zheng W, et al. Neuroprotective effect of curcumin on transient focal cerebral ischemia in rats. Brain Res. 2008;6:969–985. doi: 10.1016/j.brainres.2008.06.117. [DOI] [PubMed] [Google Scholar]
- [12].Masada T, Hua Y, Xi G, et al. Attenuation of ischemic brain edema and cerebrovascular injury after ischemic preconditioning in the rat. J Cerebral Blood Flow Metabol. 2001;21:22–33. doi: 10.1097/00004647-200101000-00004. [DOI] [PubMed] [Google Scholar]
- [13].Caplan L, Babikian V, Helgason C, et al. Occlusive disease of the middle cerebral artery. Neurology. 1985;35:975–982. doi: 10.1212/wnl.35.7.975. [DOI] [PubMed] [Google Scholar]
- [14].Bolay H, Dalkara T. Mechanisms of motor dysfunction after transient MCA occlusion: persistent transmission failure in cortical synapses is a major determinant. Stroke. 1998;29:1988–1993. doi: 10.1161/01.str.29.9.1988. [DOI] [PubMed] [Google Scholar]
- [15].Aggarwal A, Gaur V, Kumar A. Nitric oxide mechanism in the protective effect of naringin against post-stroke depression (PSD) in mice. Life Sci. 2010;86:928–935. doi: 10.1016/j.lfs.2010.04.011. [DOI] [PubMed] [Google Scholar]
- [16].Gupta YK, Briyal S, Sharma U, et al. Effect of endothelin antagonist (TAK-044) on cerebral ischemic volume, oxidative stress markers and neurobehavioral parameters in the middle cerebral artery occlusion model of stroke in rats. Life Sci. 2005;77:15–27. doi: 10.1016/j.lfs.2004.11.025. [DOI] [PubMed] [Google Scholar]
- [17].Seyed Jafari SS, Ali Aghaei A, Asadi-Shekaari M, et al. Investigating the effects of adult neural stem cell transplantation by lumbar puncture in transient cerebral ischemia. Neurosci Lett. 2011;495:1–5. doi: 10.1016/j.neulet.2011.02.025. [DOI] [PubMed] [Google Scholar]
- [18].Maheshwari A, Badgujar L, Phukan B, et al. Protective effect of Etoricoxib against middle cerebral artery occlusion induced transient focal cerebral ischemia in rats. Eur J Pharmacol. 2011;667(1-3):230–237. doi: 10.1016/j.ejphar.2011.05.030. [DOI] [PubMed] [Google Scholar]
- [19].Huang Y, McNamara JO. Ischemic stroke: “acidotoxicity” is a perpetrator. Cell. 2004;118:665–666. doi: 10.1016/j.cell.2004.09.004. [DOI] [PubMed] [Google Scholar]
- [20].Biller J, Betsy B, Love B, et al. Ischemic cerebrovascular disease. In: Bradley WG, Darrof RB, Fenichel GM, Jakovic J, editors. Neurology in Clinical Practice. 5th ed. Philadelphia, Pa: Butterworth-Heinemann; 2008. [Google Scholar]
- [21].Wang J, Tsirka E. Neuroprotection by inhibition of matrix metalloproteinases in a mouse model of intracerebral haemorrhage. Brain. 2005;128:1622–1633. doi: 10.1093/brain/awh489. [DOI] [PubMed] [Google Scholar]
- [22].Golubouff B, Shenkin HA, Haft H. The effects of Manitol and Urea on cerebral hemodynamics and cerebrospinal fluid pressure. Neurology. 1964;14:891–899. doi: 10.1212/wnl.14.10.891. [DOI] [PubMed] [Google Scholar]
- [23].Yousuf S, Atif F, Ahmad M, et al. Selenium plays a modulatory role against cerebral ischemia-induced neuronal damage in rat hippocampus. Brain Res. 2007;1174:218–225. doi: 10.1016/j.brainres.2007.01.143. [DOI] [PubMed] [Google Scholar]
- [24].Atif F, Yousuf S, Agrwal SK. Restraint stress-induced oxidative damage and its amelioration with selenium. Eur J Pharmacol. 2008;600:59–63. doi: 10.1016/j.ejphar.2008.09.029. [DOI] [PubMed] [Google Scholar]
- [25].Longa EZ, Weinstein PR, Carlson S, et al. Reversible middle cerebral artery occlusion without craniotomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- [26].Garcia JH, Wagner S, Liu KF, et al. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats statistical validation. Stroke. 1995;26:627–635. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- [27].Longo UG, Franceschi F, Ruzzini L, et al. Characteristics at haematoxylin and eosin staining of ruptures of the long head of the biceps tendon. Br J Sports Med. 2009;43:603–607. doi: 10.1136/bjsm.2007.039016. [DOI] [PubMed] [Google Scholar]
- [28].Yamashita K, Kataoka Y, Nakashima MN, et al. Neuroprotective effect of TTC-909, an isocarbacyclin methyl ester incorporated in lipid microspheres, on hippocampal delayed neuronal death of stroke-prone spontaneously hypertensive rats. Jpn J Pharmacol. 1996;71(4):351–355. doi: 10.1254/jjp.71.351. [DOI] [PubMed] [Google Scholar]
- [29].Kazushige M, Ryuji I, Hirotaka S, et al. Suppression of glucocorticoid secretion induces a behaviorally depressive state in rotarod performance in rat. Pharmacol Biochem Behav. 2008;90:730–734. doi: 10.1016/j.pbb.2008.05.021. [DOI] [PubMed] [Google Scholar]
- [30].Petullo D, Masonic K, Lincoln C, et al. Model development and behavioural assessment of focal cerebral ischemia in rats. Life Sci. 1999;64(13):1099–1198. doi: 10.1016/s0024-3205(99)00038-7. [DOI] [PubMed] [Google Scholar]


