Abstract
In the present study, we constructed a lentivirus, FIV-CMV-GFP-miR-7-3, containing the microRNA-7-3 gene and the green fluorescent protein gene, and used it to transfect human glioma U251 cells. Fluorescence microscopy showed that 80% of U251 cells expressed green fluorescence. Real-time reverse transcription PCR showed that microRNA-7-3 RNA expression in U251 cells was significantly increased. Proliferation was slowed in transfected U251 cells, and most cells were in the G1 phase of the cell cycle. In addition, the expression of the serine/threonine protein kinase 2 was decreased. Results suggested that transfection with a lentivirus carrying microRNA-7-3 can effectively suppress epidermal growth factor receptor pathway activity in U251 cells, arrest cell cycle transition from G1 phase to S phase and inhibit glioma cell growth.
Keywords: microRNA-7-3, lentivirus, serine/threonine protein kinase 2, glioma, proliferation, epidermal growth factor receptor, cell cycle, neural regeneration
Research Highlights
A recombinant lentivirus containing microRNA-7-3 effectively inhibited epidermal growth factor receptor pathway expression and blocked cell cycle transition from G1 phase to S phase, thereby inhibiting glioma growth.
Abbreviations
EGFR, epidermal growth factor receptor; Akt, protein kinase B; miR, microRNA
INTRODUCTION
Recent molecular biology studies suggest that the occurrence and malignant progression of glioma are closely related to gene abnormalities, including oncogene activation and over-expression[1]. Activation of the oncogene epidermal growth factor receptor (EGFR) or its over-expression mainly activates the phosphoinositide 3-kinase/protein kinase B (Akt) pathway, and Akt over-expression is a malignant phenotype[2,3,4,5,6,7,8,9].
MicroRNAs (miRs) are short, endogenous, non-coding RNA molecules that mediate distinct gene regulatory pathways, including some triggered by EGFR and by the activation of the important downstream kinase Akt2, which occurs commonly in gliomas with poor prognosis[10,11,12]. Previously, miR-7 has been reported to suppress EGFR expression and function as a tumor suppressor in glioblastoma[13,14,15].
The promotion of target mRNA degradationor inhibition of target mRNA translation to regulate the expression of target genes plays an important role in cell differentiation, proliferation and apoptosis. Studies have found that miR-7 transfection can inhibit Akt2 expression and block glioma cell proliferation and cell cycle progression. However, these studies used transient transfection or liposome-embedded plasmid transfection. The expression time of genes after such transfection methods is short and it is difficult to maintain sustainable and stable expression.
Lentiviral vectors can be used to transfect exogenous genes into dividing cells and non-dividing cells, and integrate within the genome. Lentiviral vectors provide a potential gene therapy delivery system for difficult in vitro transfections, such as for primary cultured cells, and have been shown to provide effective gene transfer in vivo[16,17]. In the present study, we constructed a lentiviral vector carrying the human miR-7 sequence and used it to transfect the human glioma cell line U251 to investigate the effect of miR-7 on glioma cell proliferation.
RESULTS
Construction of the lentiviral vector FIV-CMV-GFP-miR-7-3
pENTR-miRNA plasmid clones containing miR-7-3 were successfully amplified by PCR. After restriction enzyme digestion the target gene and vector were transformed into DH5α competent cells. PCR of bacterial clones showed that the miR-7-3 sequence in the pFIV-CMV-GFP-miR-7-3 plasmid completely matched the MI0000265 miR-7-3 sequence in GenBank. The four plasmids from the lentiviral expression system were co-transfected into cells from the human embryonic kidney epithelial cell line 293T. Green fluorescent protein expression was positive 12 hours later, as seen under a fluorescence microscope (Figure 1).
Figure 1.
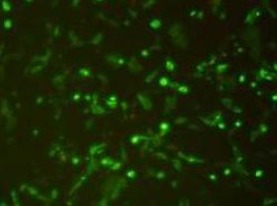
293T cells after 24 hours of lentiviral PFIV-CMV-GFP-miR-7-3 infection (fluorescence microscope, × 200).
Green fluorescence expression was observed in 293T cells.
Green fluorescent protein expression reached its peak after 48 hours, clarifying the timeline for optimal recombinant miR-7-3 lentiviral vector packaging and expression.
Lentivirus transfection efficiency and miR-7-3 RNA expression in transfected U251 cells
Using a multiplicity of infection of 10, 80% of lentivirus-transfected U251 cells stably expressed green fluorescence (Figure 2). Real time reverse transcription PCR showed that the expression level of miR-7-3 RNA in U251 cells was significantly increased compared with non-transfected cells (data not shown; Figure 3).
Figure 2.
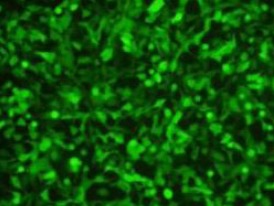
U251 cells transfected with lentivirus for 48 hours (multiplicity of infection = 10): 80% of cells expressed green fluorescence (fluorescence microscope, × 200).
Figure 3.
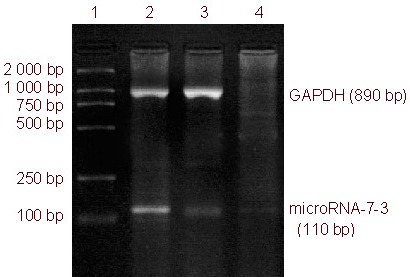
microRNA-7-3 mRNA expression in U251 glioma cells, as determined by reverse transcription-PCR.
microRNA-7-3 expression in the transfected group was significantly higher than in the negative control and PBS control groups.
1: DNA marker; 2 transfected group; 3: negative control group; 4: PBS control group.
Transfection of a lentivirus carrying miR-7-3 inhibited Akt2 expression in U251 cells
A western blot showed a signal for Akt2 in U251 at a molecular weight of 56 kDa, and indicated expression of high levels of Akt2. The β-actin signal in U251 cells was at a molecular weight of 42 kDa, and the expression levels between the samples were approximately equal. Akt2 expression was reduced by 32% in transfected U251 cells, suggesting that Akt2 expression was inhibited by miR-7-3 over-expression. A blank control (PBS) group and a negative control (empty vector) group were used as controls (Figure 4).
Figure 4.
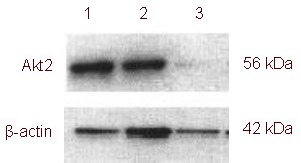
Akt2 expression in U251 cells (western blot).
Glioma cells in 1: negative control group; 2: blank control group; 3: transfection group. The expression of Akt2 in the transfection group was significantly lower than in the other groups.
A lentivirus carrying miR-7-3 inhibits proliferative activity of U251 cells
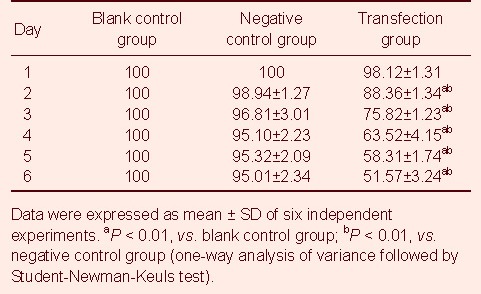
Proliferative activity in miR-7-3 transfected U251 cells was determined by a MTT assay. Results showed that the proliferative activity of the transfection group was significantly inhibited compared with the negative control and blank control groups (P < 0.01; Table 1). There was no significant difference between the survival rates of U251 cells and empty vector-transfected cells in the first 5–6 days (P > 0.05; Table 1).
Table 1.
Cell proliferation (%) as detected by MTT
Cell cycle changes of U251 cells transfected with a lentivirus carrying the miR-7-3 gene
Flow cytometry at 48 hours post-transfection revealed that the number of cells was similar in each phase of the cell cycle between the blank control and negative control groups. The number of cells in S phase was significantly reduced, but the number of cells in G0 + G1 phases was significantly increased after transfection with the lentivirus carrying miR-7-3 (P < 0.05). Thus, U251 cell proliferation was inhibited (Figure 5).
Figure 5.
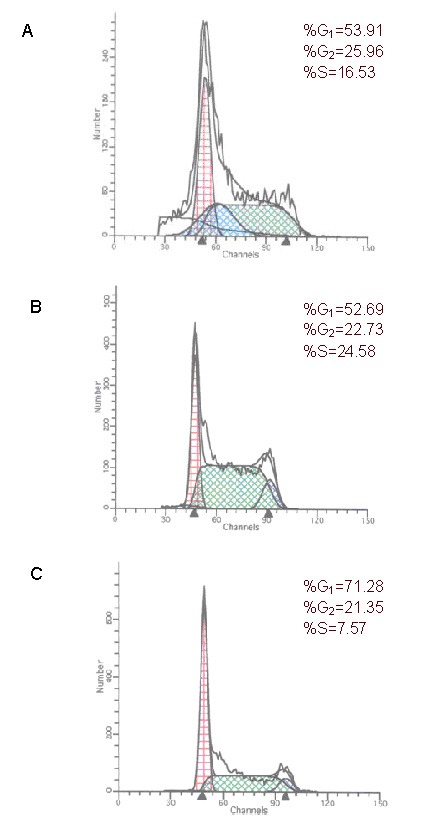
Cell cycle changes as detected by flow cytometry.
(A) Blank control group; (B) negative control group; (C) transfection group.
The number of cells in S phase is reduced in the transfection group.
DISCUSSION
The selection of target genes for gene therapy in malignant gliomas has been explored. Gene therapy is expected to become a comprehensive treatment for glioma. The EGFR pathway is an important molecular pathway in malignant glioma[13,14,15], acting mainly by activating the phosphoinositide 3-kinase-serine/ threonine protein kinase pathway[13,14,15]. Akt is an important kinase downstream of phosphoinositide 3-kinase, and promotes cell growth, inhibits apoptosis, maintains important cell signaling functions, and influences the forward progression of the cell cycle, telomerase activity, tumor angiogenesis and tumor invasion[18,19,20,21,22].
Non-viral and viral delivery of a miR to cancer models and cell lines has been shown to reduce malignancy or tumors in xenograft models[23,24,25,26]. Lentiviral vectors are advantageous in the treatment of chronic lymphocytic leukemia, because they transduce non-dividing cells and hematopoietic cells[27,28]. Integrase-defective lentiviral vectors are thought to be less hazardous as they integrate into the host genome but are not able to use the host cells to produce new virus particles[29]. Currently, most of the lentiviral vectors are constructed using a four-plasmid system, which enhances the safety of viral vectors. The expression vectors for pRsv-REV, pMDlg-pRRE and pMD2G, are placed in three separate plasmids, and the fourth one is the plasmid containing the target gene, miR-7-3. Lentiviral vectors infect actively dividing cells, and highly efficiently infect slow-dividing or non-mitotic cells, are able to carry larger genes for transfection, exhibit strong resistance to transcriptional silencing, and provide long-term stable expression of target genes.
Many miRNAs can inhibit cell proliferation and survival, acting as oncogenes or tumor suppressor genes[30]. Increasing evidence has indicated that miR-7 is a potential tumor suppressor in several human cancers. Its variants include miR-7-1, miR-7-2, and miR-7-3, which have different functions[31,32]. miR-7-1 has been shown to belong to a subset of miRNAs that are down-regulated in schizophrenia[33]. miR-7-1 was reported to negatively regulate EGFR and Akt2 expression after a miR-7 precursor construct was transfected into human glioma cell lines. It also induced apoptosis in neuroblastoma cells[13]. By instantaneous transfection, over-expression of miR-7 inhibited the invasion and migration of U87 and U251 cells, and up-regulation of miR-7-1 reduced focal adhesion kinase protein expression[34]. miR-7 was also found to regulate cell growth and apoptosis of cervical cancer cells[35], neuroblastoma cells[36] and lung cancer cells[37]. In the present study, we successfully constructed and packaged a recombinant lentiviral vector expressing miR-7-3. Results demonstrated that the transfected U251 cells effectively expressed miR-7-3, which effectively inhibited EGFR pathway expression, blocked cell cycle transition from G1 phase to S phase, and inhibited cell proliferation.
MATERIALS AND METHODS
Design
A non-randomized, controlled, in vitro study.
Time and setting
The experiment was performed at the Central Laboratory, Clinical Medical College of Yangzhou University, China from July 2008 to April 2010.
Materials
293T human embryonic kidney epithelial cells (293T), and human U251 glioblastoma cells were purchased from the Institute of Biochemistry and Cell Biology, Chinese Academy of Science, China.
Methods
Lentiviral vector system construction
We used the NCBI/Primer-BLAST program to design forward and reverse primers to introduce XhoI and EcoRI restriction sites, respectively (Shanghai Telebio Biomedical Co., Ltd., China). The plasmid pENTR-miRNA vector containing the miR-7-3 gene (Wuhan Genesil Biotechnology Co., Ltd., Wuhan, China) was used as a template to amplify the target gene by PCR. The PCR was performed following the manufacturer's instructions (Takara Biotechnology (Dalian) Co., Ltd., Dalian, China). Reaction conditions were as follows: a cycle of 95°C for 10 minutes, 95°C for 10 seconds, 60°C for 20 seconds, 72°C for 20 seconds for a total of 40 cycles and 72°C for 5 minutes. The vector Lenti-GFP-RNAi (Wuhan Genesil Biotechnology Co., Ltd.) and the PCR products were digested with XhoI and EcoRI enzymes (Takara Biotechnology (Dalian) Co., Ltd., Dalian, China). The digested PCR products were then ligated into the expression vector by T4 DNA ligase (Takara Biotechnology (Dalian) Co., Ltd.) and transformed into DH5α competent cells (Invitrogen, Carlsbad, CA, USA). The bacterial clones containing miR-7-3 were identified by PCR, and the plasmids were sent to Takara (Takara Biotechnology (Dalian) Co., Ltd.) for gene sequencing. miR-7-3 DNA was extracted with a plasmid DNA extraction kit (Zhongshan Goldenbridge Biotechnology, Beijing, China). miR-7-3 was transfected into 293T cells by lipofectamine liposome-mediated transfection. After transfection for 36–48 hours, GFP expression was observed under a fluorescence microscope (Olympus, Tokyo, Japan). Oligonucleotide sequences for the miR-7-3 gene:
Forward primer:
5’-gc ctc gag gag gag ctg ggc agg ggt ct-3’
Reverse primer:
5’-acg aat tcg gat cct ggc cag ccc att gaa ggc g-3’
Packaging and virus titer of recombinant lentivirus carrying miR-7-3
The virus packaging system was the four-plasmid system, composed of pRsv-REV, pMDlg-pRRE, pMD2G, and Lenti-GFP-RNAi. The lenti-GFP-miR-7-3 gene expression plasmid expressed GFP (Wuhan Genesil Biotechnology Co., Ltd.). The lentivirus was packaged by transfecting 293T cells in the logarithmic phase. Cells at a density of 0.5 × 108/L were re-inoculated in 25 mL of Dulbecco's modified Eagle's medium (Invitrogen) in a 15-cm cell culture dish at 37°C with 5% CO2. The DNA solution of the four plasmids in the lentiviral packaging system was prepared using 10 μg pRsv-REV, 15 μg pMDlg-pRRE, 7.5 μg pMD2G (Hai Biang Biomedical Technology Co., Ltd., Shanghai, China), and 20 μg Lenti-GFP-miR-7-3 plasmid. The volume was brought to 1 800 μL by adding sterile water, and then 200 μL CaCl2 (2.5 M) solution and 2 000 μL 2 × BBS buffer saline solution were added. The mixture was incubated at room temperature for 20–30 minutes. When the cells reached 80% transfection, the DNA and calcium phosphate mixture was transferred to culture medium containing monolayer cells. After 12 hours, the culture medium containing the transfection mixture was discarded and replaced with complete medium. After 48 hours, cell supernatant containing lentiviral particles was collected, and virus titer was determined. To determine the lentiviral titer, the virus stock solution harvested above was diluted to a concentration of 10-1–10-12 and 100 μL were added to each well of a 96-well plate containing 80%-confluent 293T cells. After 4 days in culture at 37°C with 5% CO2, the lentiviral titer was calculated according to the following formula: titer = (F × Co/V) × D. F is the frequency of GFP-positive cells determined by flow cytometry; Co is the total number of target cells infected; V is the volume of the inoculum; D is the virus dilution factor.
Real-time reverse transcription PCR determination of miR-7-3 mRNA expression in U251 cells after recombinant lentiviral transfection
The lentiviral supernatant was obtained and U251 cells were transfected at a multiplicity of infection of 10. Cells were observed by fluorescence microscopy (Bio-Rad, Hercules, CA, USA). When fluorescence expression was stable in the U251 cells, the cells were collected to extract total RNA. Total RNA was prepared using Trizol (Gibco, CA, USA) 48 hours after transfection. After reverse transcription with AMV reverse transcriptase (Takara Biotechnology (Dalian), Co., Ltd.), the cDNA was used as the template to amplify the target gene, and miR-7-3 expression in transfected cells was observed. The miR-7-3 amplicon was 110 bp. Reaction conditions were as follows: a cycle of 95°C for 10 minutes, 95°C for 10 seconds, 60°C for 20 seconds, 72°C for 20 seconds for a total of 40 cycles for 5 minutes. Human GAPDH (890 bp) was used as an internal reference. Primer design was as follows: Oligonucleotide sequences for miR-7-3: forward 5’-gac ccc ttc att gac ctc aac tac a-3’, reverse: 5’-cat gtg ggc cat gag gtc cac cac-3’. Reaction conditions were the same as miR-7-3. Reaction products were identified by 1% agarose gel electrophoresis, and photographed. Empty vector transfection served as the negative control group and PBS as the blank control group.
Transfection efficiency of U251 cells with recombinant lentivirus carrying miR-7-3
The human U251 glioblastoma cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (Invitrogen), 2 mM glutamine (Sigma, St. Louis, MO, USA), 100 units of penicillin/mL (Sigma), and 100 μg of streptomycin/mL (Sigma), at 37°C with 5% CO2. Cells were plated the day before the cell transfection experiments in a 6-well culture dish (35 mm), with 2 mL medium per well containing 3 × 105 cells. The transfected cell density in Petri dishes was generally 80%. On the day of transfection, the medium was replaced using 1 mL of fresh serum or serum-free medium a short time before the lentiviral vector was added at a multiplicity of infection of 10. After transfection, the U251 cells were incubated in 5% CO2 at 37°C. Five hours later, 2–3 mL fresh growth medium was added. On the next day, the medium was replaced with fresh 10% calf serum-containing culture medium and cultured for 48 hours. Cells were amplified in culture flasks. The expression of green fluorescent protein was observed by fluorescence microscopy at a 488 nm excitation wavelength to assess transfection efficiency. Blank transfection served as the negative control group.
Western blot analysis for Akt2 expression in U251 cells before and after transfection
Transfected cells were washed three times with pre-chilled PBS and lysed in 1% Nonidet P-40 lysis buffer (20 mM Tris, pH 8.0, 137 mM NaCl, 1% Nonidet P-40, 10% glycerol, 1 mM CaCl2, 1 mM MgCl2, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium fluoride, 1 mM sodium orthovanadate, and a protease inhibitor mixture (20 mM sodium phosphate, pH 6.5; 0.15 M NaCl, 10 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 2 μg/mL pepstatin, 2 μg/mL aprotinin, 2 μg/mL leupeptin, and 10 mM 2-mercaptoethanol). A total of 40 μg of lysates were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis on an 8% sodium dodecyl sulfate-acrylamide gel. Proteins were transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA) and incubated with a polyclonal rabbit anti-rat Akt-2 (Santa Cruz, Santa Cruz, CA, USA) antibody (1:200) overnight at 4°C, followed by incubation with a horseradish peroxidase-conjugated secondary goat anti-rabbit antibody (1:5 000) in blocking buffer for 30 minutes at room temperature. Specific proteins were detected using a SuperSignal protein detection kit (Pierce, Rockford, IL, USA). The membrane was stripped and re-probed with a primary antibody against β-actin (Santa Cruz) antibody (1:1 000), followed by incubation with a horseradish peroxidase-conjugated secondary goat anti-mouse antibody (1:5 000).
MTT cell proliferation assay
U251 cells were seeded into 96-well plates at 4 000 cells per well. After transfection as described above, 20 μL of MTT solution (5 g/L; Amresco, Solon, OH, USA) was added into each well each day for 6 consecutive days after treatment and the cells were incubated for an additional 4 hours. The supernatant was then discarded. Finally, 200 μL of dimethyl sulfoxide were added to each well to dissolve the precipitate. Absorbance (A) values were measured at a wavelength of 570 nm, with background subtraction at 650 nm. Cell proliferation rate (%) = A value (experimental group) / A value (control group) × 100[9].
Flow cytometry for cell cycle
For cell-cycle analysis using flow cytometry, transfected and control cells in the log phase of growth were harvested, washed with PBS, fixed with 90% ethanol overnight at 4°C and then incubated with RNAse (MP Biomedicals China (Shanghai), Shanghai, China) at 37°C for 30 minutes. The nuclei were stained with propidium iodide (5 mg/mL; Shanghai Suolaibao bio-Technology Co., Ltd., Shanghai, China) for an additional 30 minutes. A total of 104 nuclei were analyzed using FACSCalibur flow cytometry (Bio-rad, Hercules, CA, USA), and turtle blood served as the internal control. Experiments were performed in triplicate.
Statistical analysis
SPSS 10.0 (SPSS, Chicago, IL, USA), a commercially available software package, was used for statistical analysis. Data were expressed as mean ± SD. Mean comparison between two groups was performed using a two-sample t-test, and mean comparison among groups was performed using a completely randomized one-way analysis of variance; intergroup mean differences between two groups were compared using the Student-Newman-Keuls test. A P value < 0.05 was considered statistically significant.
Acknowledgments:
The authors thank Chunsheng Kang, Department of Neurosurgery, Laboratory of Neuro-Oncology, Tianjin Medical University General Hospital, China, for his technical assistance.
Footnotes
Funding: This project was financially supported by the Science and Technology Foundation Program of Jiangsu Province (Tumorigenic nucleostemin genes and adenovirus-based RNA interference targeting to brain tumor stem cell therapy), No. BK2007072.
Conflicts of interest: None declared.
(Edited by You YP, Song JN/Su LL/Wang L)
REFERENCES
- [1].Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- [2].Wechsler-Reya R, Scott MP. The developmental biology of brain tumors. Annu Rev Neurosci. 2001;24:385–428. doi: 10.1146/annurev.neuro.24.1.385. [DOI] [PubMed] [Google Scholar]
- [3].Pu P, Kang C, Zhang Z, et al. Downregulation of PIK3CB by siRNA suppresses malignant glioma cell growth in vitro and in vivo. Technol Cancer Res Treat. 2006;5(3):271–280. doi: 10.1177/153303460600500308. [DOI] [PubMed] [Google Scholar]
- [4].Stockhausen MT, Broholm H, Villingshøj M, et al. Maintenance of EGFR and EGFRvIII expressions in an in vivo and in vitro model of human glioblastoma multiforme. Exp Cell Res. 2011;317(11):1513–1526. doi: 10.1016/j.yexcr.2011.04.001. [DOI] [PubMed] [Google Scholar]
- [5].Han L, Yang Y, Yue X, et al. Inactivation of PI3K/AKT signaling inhibits glioma cell growth through modulation of β-catenin-mediated transcription. Brain Res. 2010;1366:9–17. doi: 10.1016/j.brainres.2010.09.097. [DOI] [PubMed] [Google Scholar]
- [6].Fortin SP, Ennis MJ, Savitch BA, et al. Tumor necrosis factor-like weak inducer of apoptosis stimulation of glioma cell survival is dependent on Akt2 function. Mol Cancer Res. 2009;7(11):1871–1881. doi: 10.1158/1541-7786.MCR-09-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].López-Ornelas A, Mejía-Castillo T, Vergara P, et al. Lentiviral transfer of an inducible transgene expressing a soluble form of Gas1 causes glioma cell arrest, apoptosis and inhibits tumor growth. Cancer Gene Ther. 2011;18(2):87–99. doi: 10.1038/cgt.2010.54. [DOI] [PubMed] [Google Scholar]
- [8].Gessler F, Voss V, Dützmann S, et al. Inhibition of tissue factor/protease-activated receptor-2 signaling limits proliferation, migration and invasion of malignant glioma cells. Neuroscience. 2010;165(4):1312–1322. doi: 10.1016/j.neuroscience.2009.11.049. [DOI] [PubMed] [Google Scholar]
- [9].Zhang AL, Kang CS, Han L, et al. The effect of silencing Dicer by small interference RNA on the biological characteristics of human glioma cells. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2009;26(5):521–524. doi: 10.3760/cma.j.issn.1003-9406.2009.05.009. [DOI] [PubMed] [Google Scholar]
- [10].Bartel DP, Chen CZ. Micromanagers of gene expression: the potentially widespread influence of metazoan microRNAs. Nat Rev Genet. 2004;5(5):396–400. doi: 10.1038/nrg1328. [DOI] [PubMed] [Google Scholar]
- [11].Mattick JS, Makunin IV. Small regulatory RNAs in mammals. Hum Mol Genet. 2005;15(1):R121–132. doi: 10.1093/hmg/ddi101. [DOI] [PubMed] [Google Scholar]
- [12].Humphreys DT, Westman BJ, Martin DI, et al. MicroRNAs control translation initiation by inhibiting eukaryotic initiation factor 4E/cap and poly(A) tail function. Proc Natl Acad Sci U S A. 2005;102(47):16961–16966. doi: 10.1073/pnas.0506482102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Webster RJ, Giles KM, Price KJ, et al. Regulation of epidermal growth factor receptor signaling in human cancer cells by microRNA-7. J Biol Chem. 2009;284(9):5731–5741. doi: 10.1074/jbc.M804280200. [DOI] [PubMed] [Google Scholar]
- [14].Kefas B, Godlewski J, Comeau L, et al. microRNA-7 inhibits the epidermal growth factor receptor and the Akt pathway and is down-regulated in glioblastoma. Cancer Res. 2008;68:3566–3572. doi: 10.1158/0008-5472.CAN-07-6639. [DOI] [PubMed] [Google Scholar]
- [15].Lee KM, Choi EJ, Kim IA. microRNA-7 increases radiosensitivity of human cancer cells with activated EGFR-associated signaling. Radiother Oncol. 2011;101(1):171–176. doi: 10.1016/j.radonc.2011.05.050. [DOI] [PubMed] [Google Scholar]
- [16].D’Costa J, Mansfield SG, Humeau LM. Lentiviral vectors in clinical trials: Current status. Curr Opin Mol Ther. 2009;11(5):554–564. [PubMed] [Google Scholar]
- [17].Beaudet MJ, Rueda N, Kobinger GP, et al. Construction of a ganciclovir-sensitive lentiviral vector to assess the influence of angiopoietin-3 and soluble Tie2 on glioma growth. J Neurooncol. 2010;99(1):1–11. doi: 10.1007/s11060-009-0095-y. [DOI] [PubMed] [Google Scholar]
- [18].Fu Y, Zhang Q, Kang C, et al. Inhibitory effects of adenovirus mediated Akt1 and PIK3R1 shRNA on the growth of malignant tumor cells in vitro and in vivo. Cancer Biol Ther. 2009;8(11):1002–1009. doi: 10.4161/cbt.8.11.8285. [DOI] [PubMed] [Google Scholar]
- [19].Mulligan RC. The basic science of gene therapy. Science. 1993;260(5110):926–932. doi: 10.1126/science.8493530. [DOI] [PubMed] [Google Scholar]
- [20].Cully M, You H, Levine AJ, et al. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6(3):184–192. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- [21].Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2(7):489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- [22].Wang X, McCullough KD, Franke TF, et al. Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J Biol Chem. 2000;275(19):14624–14631. doi: 10.1074/jbc.275.19.14624. [DOI] [PubMed] [Google Scholar]
- [23].Takamizawa J, Konishi H, Yanagisawa K, et al. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64(11):3753–3756. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- [24].Calin GA, Cimmino A, Fabbri M, et al. MiR-15a and miR-16-1 cluster functions in human leukemia. Proc Natl Acad Sci U S A. 2008;105(13):5166–5171. doi: 10.1073/pnas.0800121105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Trang P, Medina PP, Wiggins JF, et al. Regression of murine lung tumors by the let-7 microRNA. Oncogene. 2010;29(11):1580–1587. doi: 10.1038/onc.2009.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kota J, Chivukula RR, O’Donnell KA, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137(6):1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Naldini L, Blömer U, Gallay P, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272(5259):263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- [28].VandenDriessche T, Thorrez L, Naldini L, et al. Lentiviral vectors containing the human immunodeficiency virus type-1 central polypurine tract can efficiently transduce nondividing hepatocytes and antigen-presenting cells in vivo. Blood. 2002;100(3):813–822. doi: 10.1182/blood.v100.3.813. [DOI] [PubMed] [Google Scholar]
- [29].Vargas J, Jr, Gusella GL, Najfeld V, et al. Novel integrase-defective lentiviral episomal vectors for gene transfer. Hum Gene Ther. 2004;15(4):361–372. doi: 10.1089/104303404322959515. [DOI] [PubMed] [Google Scholar]
- [30].Reddy SD, Ohshiro K, Rayala SK, et al. MicroRNA-7, a homeobox D10 target, inhibits p21-activated kinase 1 and regulates its functions. Cancer Res. 2008;68(20):8195–8200. doi: 10.1158/0008-5472.CAN-08-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rai K, Takigawa N, Ito S, et al. Liposomal delivery of MicroRNA-7-expressing plasmid overcomes epidermal growth factor receptor tyrosine kinase inhibitor-resistance in lung cancer cells. Mol Cancer Ther. 2011;10(9):1720–1727. doi: 10.1158/1535-7163.MCT-11-0220. [DOI] [PubMed] [Google Scholar]
- [32].Kong D, Piao YS, Yamashita S, et al. Inflammation-induced repression of tumor suppressor miR-7 in gastric tumor cells. Oncogene. doi: 10.1038/onc.2011.558. in press. [DOI] [PubMed] [Google Scholar]
- [33].Perkins DO, Jeffries CD, Jarskog LF, et al. microRNA expression in the prefrontal cortex of individuals with schizophrenia and schizoaffective disorder. Genome Biol. 2007;8(2):R27. doi: 10.1186/gb-2007-8-2-r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wu DG, Wang YY, Fan LG, et al. MicroRNA-7 regulates glioblastoma cell invasion via targeting focal adhesion kinase expression. Chin Med J (Engl) 2011;124(17):2616–2621. [PubMed] [Google Scholar]
- [35].Cheng AM, Byrom MW, Shelton J, et al. Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucleic Acids Res. 2005;33(4):1290–1297. doi: 10.1093/nar/gki200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Junn E, Lee KW, Jeong BS, et al. Repression of alpha-synuclein expression and toxicity by microRNA-7. Proc Natl Acad Sci U S A. 2009;106(31):13052–13057. doi: 10.1073/pnas.0906277106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Xiong S, Zheng Y, Jiang P, et al. MicroRNA-7 inhibits the growth of human non-small cell lung cancer A549 cells through targeting BCL-2. Int J Biol Sci. 2011;7(6):805–814. doi: 10.7150/ijbs.7.805. [DOI] [PMC free article] [PubMed] [Google Scholar]


