Abstract
In this study, we investigated non-captive four-striped mice (Rhabdomys pumilio) for evidence that adult neurogenesis occurs in the adult brain of animal models in natural environment. Ki-67 (a marker for cell proliferation) and doublecortin (a marker for immature neurons) immunostaining confirmed that adult neurogenesis occurs in the active sites of subventricular zone of the lateral ventricle with the migratory stream to the olfactory bulb, and the subgranular zone of the dentate gyrus of the hippocampus. No Ki-67 proliferating cells were observed in the striatum substantia nigra, amygdala, cerebral cortex or dorsal vagal complex. Doublecortin-immunoreactive cells were observed in the striatum, third ventricle, cerebral cortex, amygdala, olfactory bulb and along the rostral migratory stream but absent in the substantia nigra and dorsal vagal complex. The potential neurogenic sites in the four-striped mouse species could invariably lead to increased neural plasticity.
Keywords: nerve regeneration, adult neurogenesis, four-striped mouse, Ki-67, doublecortin, subventricular zone, dentate gyrus, olfactory bulb, potential neurogenic site, neural plasticity, neural regeneration
Introduction
Neurogenesis has been defined as the ability of brain cells to regenerate themselves (Gage, 2000). This process has been known to occur in the adult mammalian brain for over 50 years (Altman, 1962; Altman and Das, 1965). Neural stem cells are self-renewing multipotent cells that normally generate the main phenotypic cells of the nervous system namely, neurons, astrocytes and oligodendrocytes (Taupin, 2006). Neurogenesis has been reported in the mammalian brain of rats (Kuhn, et al., 1996), mice (Kempermann, et al., 1997), tree shrews (Gould, et al., 1997), guinea pigs (Altman and Das, 1967), rabbits (Gueneau, et al., 1982), cats (Wyss and Sripanidkulchai, 1985), monkeys (Rakic and Nowakowski, 1981; Gould, et al., 1998, 1999), humans (Eriksson et al., 1998) and the hippocampi of several other species (Patzke et al., 2013).
New neurons are continually produced in the adult mammalian brain from progenitor cells located in specific regions, including the subgranular zone of the dentate gyrus of the hippocampus (Siwak-Tapp, 2007). The mammalian brain consists of two types of neurogenic sites, active and potential neurogenic sites (Ihunwo and Pillay, 2007). The two active neurogenic regions of the brain are the rostral subventricular zone of the lateral ventricle and the subgranular zone of the dentate gyrus of the hippocampus (Kaplan and Hinds, 1977; Kaplan and Bell, 1984). The subventricular zone is considered the largest active neurogenic site in the brain (Schauwecker, 2006) and it has been shown to be a source for cortical and subcortical neurons (Alvarez-Buylla and Garcia-Verdugo, 2002; Watts et al., 2005). Other areas of the brain where adult neurogenesis has been reported include the septum, substantia nigra, ependymal wall of the third ventricle, cerebral cortex, olfactory bulb and dorsal vagal complex (Reynolds and Weiss, 1992; Ihunwo and Pillay, 2007). Evidence by Bernier et al. (2002) indicated that neurogenesis is present in the amygdala and surrounding cortex of adult monkeys with the occurrence of a temporal migratory stream, which is similar to rostral migratory stream.
Animals in their natural environment experience a complex interactions compared to the laboratory animals that are limited in their exposures. The four-striped mouse is widespread and found in habitats such as grasslands, deserts and forests of Southern Africa and parts of the northern Africa (Schradin and Pillay, 2004) and usually in colonies with very few population living as a group. They demonstrate bi-parental care and cover long distances in foraging for food. It has a body mass that ranges from 40 to 80 g (Maini, 2003; Schradin and Pillay, 2004) and a small brain with an average mass of about 0.64 g (Schradin et al., 2014). Exploring effects of environmental influences, animal behavior and social interactions on adult neurogenesis is becoming a major initiative in the field and collectively, these factors constitute a number of combined forces acting on brain plasticity, and ultimately, the rate and location of neurogenesis in adulthood (Boonstra et al., 2001). The aim of this study therefore is to explore adult neurogenesis occurrence in the active neurogenic sites: subventricular zone and dentate gyrus, as well as other reported potential sites in the four-striped mice using immunohistochemistry.
Materials and Methods
Experimental animals
Four adult male four-striped mice were used with an average age of 100 days. The animals were captive-reared at the Central Animal Service Unit of the University of the Witwatersrand, South Africa. Their ancestors were of a defined colony typical of the description under introduction and captured with cage traps. The animals were treated and used according to the guidelines of the University of the Witwatersrand Animal Ethics and Screening Committee, which parallel those set down by the National Institute of Health (NIH) for use of animals in scientific experiments. The four-striped mice are widely distributed in Southern Africa occurring in different habitats, such as grassland, marsh, forests, semi-deserts and deserts (Skinner and Chimimba, 2005). Four-striped mice are small diurnal murid rodents that are solitary in their living. They demonstrate bi-parental care with a very flexible social organization and mating system which is controlled mainly by resource availability and population density (Schradin and Pillay, 2005). They are very unique by the four distinct dark longitudinal stripes running the length of their back.
Tissue processing
The animals were euthanized intraperitoneally with sodium pentobarbital (80 mg/kg) and transcardially perfused with 0.9% cold saline followed by 4% paraformaldehyde in 0.1 mol/L phosphate buffer. Brains were carefully removed, weighed and post-fixed in 4% paraformaldehyde in 0.1 mol/L phosphate buffer, then allowed to equilibrate in 30% sucrose in 0.1 mol/L PBS. Brains were then kept frozen in dry ice and sectioned using a sliding microtome in the sagittal plane at 50 μm section thickness covering the entire brain. One in five series of sections was stained for a cell proliferation marker Ki-67 and an immature neuronal marker doublecortin (DCX).
Immunohistochemistry for Ki-67
The Ki-67 is a chromosome-associated protein present during division (G1, S, G2, and M, but absent from cells at rest, G0). Free floating sections were incubated for epitope retrieval in citrate buffer, pH 6.0, at 90˚C for 40 minutes, followed by incubation in endogenous peroxidase blocking reagent, 0.6% H2O2 in Tris-buffered saline (TBS)-Triton (0.05% Triton X-100 in TBS, pH 7.4) for 30 minutes at room temperature. Thereafter, sections were pre-incubated in 2% serum (normal goat serum) + 0.1% bovine serum albumin (BSA) + 0.25% Triton in TBS, for 60 minutes at room temperature. Afterwards, sections were incubated with polyclonal rabbit-anti-lyophilized-Ki-67p antibody (Novocastra, Newcastle, UK; 1:5,000 in preincubation solution) overnight at 4˚C. Incubation with biotinylated goat anti-rabbit IgG (1:1,000 + 2% normal goat serum + 0.1% BSA in TBS; Vector lab, CA, USA; 1:250) was performed for 2 hours at room temperature followed by incubation with streptavidin-biotin complex (Vectastain Elite ABC kit) and stained with 3,3′-diaminobenzidine (DAB) as chromogen. Until incubation with primary antibody, all rinses in between incubations were made with TBS-Triton, afterwards with TBS alone.
Immunohistochemistry for DCX
DCX is a microtubule associated protein expressed specifically in newly generated neuronal precursors, immature & migrating neurons. Sections were washed three times for 10 minutes in PBS and then rinsed with Tris-buffered saline and Tween 20 (TBST) once for 5 minutes under gentle shaking at room temperature. Thereafter, sections were then treated with blocking solution, 5 % normal rabbit serum in TBST for 30 minutes. Tissues were transferred into primary antibody goat anti-mouse DCX antibody (Santa Cruz Biotechnology, CA, USA; 1:400) in TBST supplemented with 2 % BSA and 2 % normal rabbit serum overnight at 4˚C under gentle shaking. Incubation with secondary antibodies (rabbit anti-goat IgG (1:250) + 2 % normal rabbit serum + 0.1% BSA in TBS) was performed for 2 hours followed by incubation with streptavidin-biotin complex (Vectastain Elite ABC kit) and stained with DAB as chromogen.
Microscopic analysis
Sections of the brains were analyzed and photomicrographs were taken with the aid of Zeiss Axioskop light microscope (Carl Zeiss Microscopy GmbH, Jena, Germany) using variable objective lenses and Axiovision software (Carl Zeiss Microscopy GmbH).
Results
Ki-67-immunoreactive cells
Ki-67-immunoreactive cells appeared darkly stained and were easily seen in various sites of the brain (Figure 1A–C). The Ki-67-immunoreactive cells appeared more numerous in the subventricular layer of the dentate gyrus. These cells were present in the two established neurogenic sites; subventricular zone and the dentate gyrus (Figure 1A, B). Also, Ki-67-immunoreactive neurons were visible in the rostral migratory stream and olfactory bulb (Figure 1C). No Ki-67-immunoreactive cells were observed in the striatum, amygdala, cerebral cortex or dorsal vagal complex.
Figure 1.
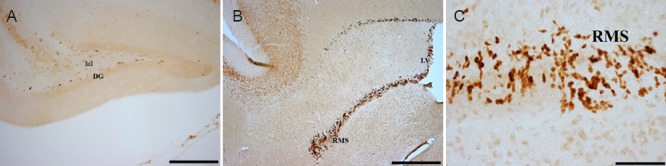
Representative photomicrograph of Ki-67 immunohistochemical staining in the various brain regions of four-striped mice.
(A) DG; (B) subventricular zone and RMS. (C) A magnified image of the RMS showing the darkly stained neurons. Scale bars: 20 μm in A, 10 μm in B and 1 μm in C. DG: Dentate gyrus; RMS: rostral migratory stream; hil: hilum; LV: lateral ventricle.
DCX-immunoreactive cells
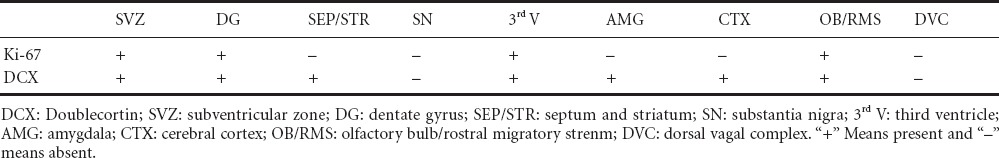
DCX-immunoreactive cells presented visible bipolar neurons with ovoid cell bodies and axonal and dendritic processes, an evidence of immature neurons. The dendrites extended towards the outer molecular layer of the dentate gyrus. In addition to the two active neurogenic sites, DCX-immunoreactive cells were observed in the striatum, third ventricle, cerebral cortex, amygdala, olfactory bulb and along the rostral migratory stream. They were however absent in the substantia nigra and dorsal vagal complex. The staining intensity of the DCX in the dentate gyrus of the hippocampus and the rostral migratory stream were quiet conspicuous (Figure 2). A summary of sites where adult neurogenesis was either present or absent in the four-striped mice using immunohistochemistry for Ki-67 and DCX are shown in Table 1.
Figure 2.
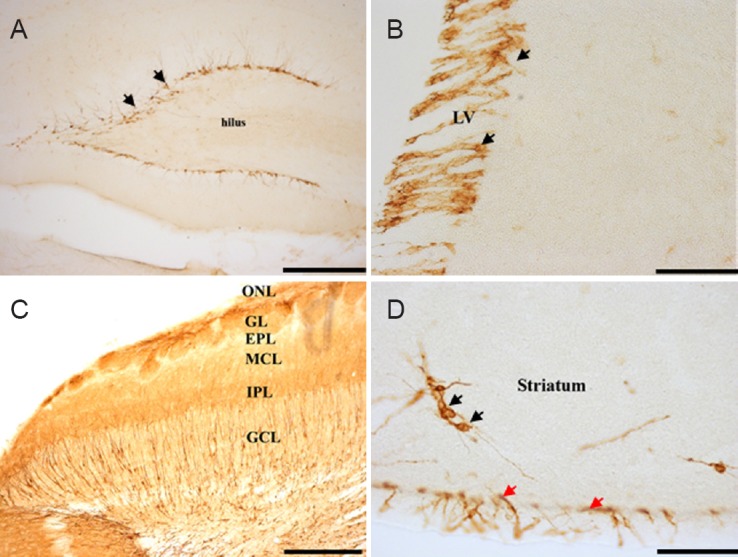
Representative photomicrograph of doublecortin (DCX) immunohistochemical staining in the brain of four-striped mice.
(A) Dentate gyrus with the immunoreactive cells lining the whole length. (B) DCX-immunoreactive cells on the subventricular zone of the lateral ventricle. (C) Six different layers in the olfactory bulb region with the immature neurons. (D) The striatum and isolated neurons (black arrows) and the subventricular zone (red arrows). Scale bars: A, C, 20 μm; B, D, 2.5 μm. LV: Lateral ventricle; ONL: olfactory nerve layer; GL: glomerular layer; EPL: external plexiform layer; MCL: mitral cell layer; IPL: internal plexiform layer; GCL: granule cell layer.
Table 1.
A summary of the neurogenic sites observed in four-striped mice
Discussion
We assessed adult neurogenesis using two different cell markers, the Ki-67 and DCX for cell proliferation and immature neurons respectively. Adult neurogenesis remains a complex process involving the proliferation, survival, differentiation, and functional integration of new cells in the brain. The cellular and molecular mechanisms that regulate adult neurogenesis remain unclear (Schauwecker, 2006) but can be modulated by a variety of factors including glutamate receptor activation (Cameron et al., 1995, 1998; Gould et al., 1997; Bernabeu and Sharp, 2000), dietary restriction (Lee et al., 2002), growth factors (Palmer et al., 1995; Scharfman et al., 2005), stress (Brunson et al., 2005; Nichols et al., 2005) and neuronal injury (Parent, 2003; Cooper-Kuhn et al., 2004). Enriched environments (Kempermann et al., 1997), running wheel exercise (van Praag et al., 1999; Hauser et al., 2009), hippocampal-dependent learning (Gould et al., 1999), and dietary restriction (Lee, et al., 2000, 2002), all these increase neurogenesis in the adult hippocampus. Stress (Gouldet al., 1997, 1998) and social isolation (Lu et al., 2003; Chetty et al., 2009) reduces hippocampal neurogenesis. However, Kannangara et al. (2009) reported that social isolation does not affect adult neurogenesis. It was reported that estradiol alter hippocampal neurogenesis in the adult female rodents (Galea et al., 2006) even though a decrease in hippocampal neurogenesis does not always correlate with the development of learned helplessness in male rats (Vollmayr et al., 2003). According to Boonstra et al. (2001), both the daily behaviour and the immediate environment of species are intimately related with addition of new neurons throughout adulthood which provides a basis for investigating animals in the wild for increased neural plasticity and possible factors for this mechanism. In all the immunostaining of the brain of four-striped mice, new neurogenic cells were observed either in clustered or isolated form. These differences were evident in relation to the neurogenic regions, active or potential sites. In the hippocampus, more Ki-67 and DCX-immunoreactive cells were observed. DCX-immunoreactive cells were also found in the striatum of the brain but sparsely distributed compared to the subventricular zone and dentate gyrus.
The predominant DCX-immunoreactive cells appeared in clusters but varied in shape according to Plumpe et al. (2006). The somas of DCX-immunoreactive cells were primarily located in the subgranular layer and were bipolar with an ovoid soma. Their dendrites reach as far as the molecular layer of the dentate gyrus while some dendrites only end in the granular layer.
The subventricular zone has two precise layers of cells: the first is a monolayer of multi-ciliated cells lining the lateral ventricle called the ependymal layer; and the second layer is a 2–3 cell layer thick area adjacent to the ependymal layer called the subependymal layer. The neuroblasts from the subventricular zone travel a long distance to the olfactory bulb through a network of interconnecting pathways that become confluent at the rostral margin of the lateral ventricle wall to form the rostral migratory stream (Watts et al., 2005). In mammals, the generation of new neurons is mostcommonly observed in the subgranular zone of the hippocampal dentate gyrus and the subventricular zone from where cells migrate along the rostral migratory stream to the olfactory bulb (Ming and Song, 2011). This is true in the four-striped mice. This study therefore adds the four-striped mice to the growing list of wild-caught mammalian species with persistent adult neurogenesis. In conclusion, a combination of Ki-67 and DCX immunohistochemistry has provided evidence of adult neurogenesis in the active and some potential sites in four-striped mice.
Acknowledgments:
We expressed our thanks to Professor Pillay N, School of Animal, Plant and Environmental Sciences Wits University, South Africa for providing four-striped mice, to the Wits Central Animal Services, South Africa for excellent care of animals, and to Ali H and Meskenaite V for assistance with laboratory techniques.
Footnotes
Funding: This project was supported by Individual Faculty Research Grant and Swiss-South Africa Joint Research Progamme (SSAJRP).
Conflicts of interest: None declared.
Copyedited by J. Gil-Mohapel, B. Lang, Li CH, Song LP, Zhao M
References
- 1.Altman J. Are new neurons formed in the brains of adult mammals? Science. 1962;135:1127–1128. doi: 10.1126/science.135.3509.1127. [DOI] [PubMed] [Google Scholar]
- 2.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 3.Altman J, Das GD. Postnatal neurogenesis in the guinea-pig. Nature. 1967;214:1098–1101. doi: 10.1038/2141098a0. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adultsubventricular zone. J Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernier PJ, Bedard A, Vinet J, Levesque M, Parent A. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc Natl Acad Sci U S A. 2002;99:11464–11469. doi: 10.1073/pnas.172403999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernabeu R, Sharp FR. NMDA and AMPA/kainate glutamate receptors modulate dentate neurogenesis and CA3 synapsin-I in normal and ischemic hippocampus. J Cereb Blood Flow Metab. 2000;20:1669–1680. doi: 10.1097/00004647-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Boonstra R, Galea L, Matthews S, Wojtowicz JM. Adult neurogenesis in natural populations. Can J Physiol Pharmacol. 2001;79:297–302. [PubMed] [Google Scholar]
- 8.Brunson KL, Kramár E, Lin B, Chen Y, Colgin LL, Yanagihara TK, Lynch G, Baram TZ. Mechanisms of late-onset cognitive decline after early-life stress. J Neurosci. 2005;25:9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- 10.Cameron HA, McEwen BS, Gould E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci. 1995;15:4687–4692. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cameron HA, Tanapat P, Gould E. Adrenal steroids and N-methyl-D-aspartate receptor activation regulate neurogenesis in the dentate gyrus of adult rats through a common pathway. Neuroscience. 1998;82:349–354. doi: 10.1016/s0306-4522(97)00303-5. [DOI] [PubMed] [Google Scholar]
- 12.Cooper-Kuhn CM, Winkler J, Kuhn HG. Decreased neurogenesis after cholinergic forebrain lesion in the adult rat. J Neurosci Res. 2004;77:155–165. doi: 10.1002/jnr.20116. [DOI] [PubMed] [Google Scholar]
- 13.Chetty T, Ajao MS, Manger PR, Ihunwo AO. Behavioural patterns and corticosterone levels induced by chronic psychosocial stress in the four-striped mouse (Rhabdomys pumilio) J Animal Vet Adv. 2009;8:459–463. [Google Scholar]
- 14.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 15.Galea LA, Spritzer MD, Barker JM, Pawluski JL. Gonadal hormone modulation of hippocampal neurogenesis in the adult. Hippocampus. 2006;16:225–232. doi: 10.1002/hipo.20154. [DOI] [PubMed] [Google Scholar]
- 16.Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci U S A. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gould E, Reeves AJ, Graziano MS, Gross CG. Neurogenesis in the neocortex of adult primates. Science. 1999;286:548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- 19.Gueneau G, Privat A, Drouet J, Court L. Subgranular zone of the dentate gyrus of young rabbits as a secondary matrix. A high-resolution autoradiographic study. Dev Neurosci. 1982;5:345–358. doi: 10.1159/000112694. [DOI] [PubMed] [Google Scholar]
- 20.Hauser T, Klaus F, Lipp HP, Amrein I. No effect of running and laboratory housing on adult hippocampal neurogenesis in wild caught long-tailed wood mouse. BMC Neurosci. 2009;10:43. doi: 10.1186/1471-2202-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ihunwo AO, Pillay S. Neurogenic sites in the adult mammalian central nervous system. Res J Biol Sci. 2007;2:170–177. [Google Scholar]
- 22.Kannangara TS, Webber A, Gil-Mohapel J, Christie BR. Stress differentially regulates the effects of voluntary exercise on cell proliferation in the dentate gyrus of mice. Hippocampus. 2009;19:889–897. doi: 10.1002/hipo.20514. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan MS, Bell DH. Mitotic neuroblasts in the 9-day-old and 11-month-old rodent hippocampus. J Neurosci. 1984;4:1429–1441. doi: 10.1523/JNEUROSCI.04-06-01429.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- 25.Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci U S A. 1997;94:10409–10414. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J, Duan W, Long JM, Ingram DK, Mattson MP. Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. J Mol Neurosci. 2000;15:99–108. doi: 10.1385/JMN:15:2:99. [DOI] [PubMed] [Google Scholar]
- 28.Lee J, Seroogy KB, Mattson MP. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J Neurochem. 2002;80:539–547. doi: 10.1046/j.0022-3042.2001.00747.x. [DOI] [PubMed] [Google Scholar]
- 29.Lu L, Bao G, Chen H, Xia P, Fan X, Zhang J, Pei G, Ma L. Modification of hippocampal neurogenesis and neuroplasticity by social environments. Exp Neurol. 2003;183:600–609. doi: 10.1016/s0014-4886(03)00248-6. [DOI] [PubMed] [Google Scholar]
- 30.Maini PK. How the mouse got its stripes. Proc Natl Acad Sci U S A. 2003;100:9656–9657. doi: 10.1073/pnas.1734061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ming G, Song H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nichols NR, Agolley D, Zieba M, Bye N. Glucocorticoid regulation of glial responses during hippocampal neurodegeneration and regeneration. Brain Res Rev. 2005;48:287–301. doi: 10.1016/j.brainresrev.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 33.Palmer TD, Ray J, Gage FH. FGF-2 responsive neural progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol Cell Neurosci. 1995;6:474–486. doi: 10.1006/mcne.1995.1035. [DOI] [PubMed] [Google Scholar]
- 34.Parent JM. Injury-induced neurogenesis in the adult mammalian brain. Neuroscientist. 2003;9:261–272. doi: 10.1177/1073858403252680. [DOI] [PubMed] [Google Scholar]
- 35.Patzke N, Spocter MA, Karlsson KA, Bertelsen MF, Haagensen M, Chawana R, Streicher S, Kaswera C, Gilissen E, Alagaili AN, Mohammed OB, Reep RL, Bennett NC, Siegel JM, Ihunwo AO, Manger PR. In contrast to many other mammals, cetaceans have relatively small hippocampi that appear to lack adult neurogenesis. Brain Struct Funct. 2013 doi: 10.1007/s00429-013-0660-1. DOI: 10.1007/s00429-013-0660-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plumpe T, Ehninger D, Steiner B, Klempin F, Jessberger S, Brandt M, Romer B, Rodriguez GR, Kronenberg G, Kempermann G. Variability of doublecortin-associated dendrite maturation in adult hippocampal neurogenesis is independent of the regulation of precursor cell proliferation. BMC Neurosci. 2006;7:77. doi: 10.1186/1471-2202-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rakic P, Nowakowski RS. The time of origin of neurons in the hippocampal region of the rhesus monkey. J Comp Neurol. 1981;196:99–128. doi: 10.1002/cne.901960109. [DOI] [PubMed] [Google Scholar]
- 38.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1910. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 39.Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 40.Schauwecker PE. Genetic influence on neurogenesis in the dentate gyrus of two strains of adult mice. Brain Res. 2006;1120:83–92. doi: 10.1016/j.brainres.2006.08.086. [DOI] [PubMed] [Google Scholar]
- 41.Schradin C, Pillay N. The influence of the father on offspring development in the striped mouse. Behav Ecol. 2005;16:450–455. [Google Scholar]
- 42.Schradin C, Larke RH, Bales KL. Growing up in the family or growing up alone influences behavior and hormones, but not arginine vasopressin receptor 1a expression in male African striped mice. Physiol Behav. 2014;129:205–213. doi: 10.1016/j.physbeh.2014.02.060. [DOI] [PubMed] [Google Scholar]
- 43.Skinner JD, Chimimba CT. Cambridge: Cambridge University Press; 2005. Mammals of the Southern African Sub-region. [Google Scholar]
- 44.Siwak-Tapp CT, Head E, Muggenburg BA, Milgram NW, Cotman CW. Region specific neuron loss in the aged canine hippocampus is reduced by enrichment. Neurobiol Aging. 2008;29:39–50. doi: 10.1016/j.neurobiolaging.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taupin P. Neurogenesis in the adult central nervous system. CR Biol. 2006;329:465–475. doi: 10.1016/j.crvi.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 47.Vollmayr B, Simonis C, Weber S, Gass P, Henn F. Reduced cell proliferation in the dentate gyrus is not correlated with the development of learned helplessness. Biol Psychiatry. 2003;54:1035–1040. doi: 10.1016/s0006-3223(03)00527-4. [DOI] [PubMed] [Google Scholar]
- 48.Watts C, McConkey H, Anderson L, Caldwell M. Anatomical perspectives on adult neural stem cells. J Anat. 2005;207:197–208. doi: 10.1111/j.1469-7580.2005.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wyss JM, Sripanidkulchai B. The development of Ammon's horn and the fascia dentata in the cat: a [3H]thymidine analysis. Brain Res. 1985;350:185–198. doi: 10.1016/0165-3806(85)90263-9. [DOI] [PubMed] [Google Scholar]


