Abstract
Hematopoietic cytokines, traditionally known to influence cellular proliferation, differentiation, maturation, and lineage commitment in the bone marrow, include granulocyte colony-stimulating factor (G-CSF), granulocyte–macrophage colony-stimulating factor, stem cell factor, Flt-3 ligand, and erythropoietin among others. Emerging evidence suggests that these cytokines also exert multifarious biological effects on diverse nonhematopoietic organs and tissues. Although the precise mechanisms remain unclear, numerous studies in animal models of myocardial infarction (MI) and heart failure indicate that hematopoietic cytokines confer potent cardiovascular benefits, possibly through mobilization and subsequent homing of bone marrow-derived cells into the infarcted heart with consequent induction of myocardial repair involving multifarious mechanisms. In addition, these cytokines are also known to exert direct cytoprotective effects. However, results from small-scale clinical trials of G-CSF therapy as a single agent after acute MI have been discordant and largely disappointing. It is likely that cardiac repair following cytokine therapy depends on a number of known and unknown variables, and further experimental and clinical studies are certainly warranted to accurately determine the true therapeutic potential of such therapy. In this review, we discuss the biological features of several key hematopoietic cytokines and present the basic and clinical evidence pertaining to cardiac repair with hematopoietic cytokine therapy.
Keywords: Cytokine, Myocardial infarction, Myocardial regeneration, Stem cell, Bone marrow, Mobilization
Introduction
Hematopoietic cytokines, which include granulocyte colony-stimulating factor (G-CSF), granulocyte–macrophage colony-stimulating factor (GM-CSF), stem cell factor (SCF), Flt-3 ligand (FL), and erythropoietin (EPO) among others, have traditionally been known to participate in survival, proliferation, lineage commitment (decision to enter a specific maturation pathway), differentiation (qualitative cellular phenotypic changes due to synthesis of new gene products), and maturation (quantitative cellular phenotypic changes resulting in functional competence) of hematopoietic progenitors, lymphoid and myeloid cells, red blood cells, and platelets [107, 128] (Fig. 1). However, growing evidence indicates that the biological effects of these cytokines extend well beyond the traditional realm of hematopoiesis regulation. In this regard, a number of recent studies have shown that administration of hematopoietic cytokines is associated with improvement in survival, left ventricular (LV) function, and remodeling in animal models of acute myocardial infarction (MI) and cardiomyopathy (Tables 1, 2). Based on this evidence, safety and efficacy of cytokine therapy for cardiac repair in patients with acute MI have been tested in several randomized controlled trials (RCTs) [35, 36, 66, 70, 89, 133, 148, 160, 174]. Although results from these studies have been variable, subsequent meta-analyses of pooled data from RCTs of G-CSF therapy for cardiac repair in patients with acute MI suggested that G-CSF therapy does not improve LV function and structure in unselected patients with acute MI beyond those achieved with conventional therapy [2, 173] (Table 3).
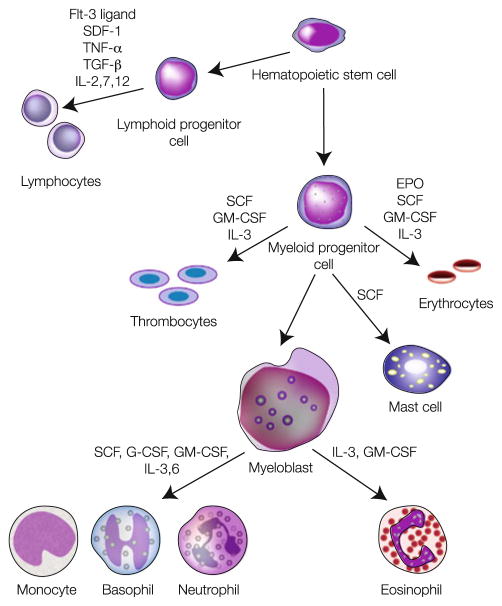
Fig. 1.
Schematic representation of hematopoiesis in which pluripotent stem and progenitor cells in the bone marrow divide and differentiate, passing through intermediate steps to form red blood cells, platelets, and white blood cells. The specific roles of hematopoietic cytokines known to stimulate transitions are indicated
Table 1.
Animal studies of G-CSF therapy for cardiac repair
| Study | Host | Type of ischemia | Duration of therapy | Dose | Follow-up period | Outcomes |
|---|---|---|---|---|---|---|
| Models of acute myocardial infarction | ||||||
| Mouse | ||||||
| Fukuhara et al. [44] | C57BL/6 mouse | Permanent coronary occlusion | 3 days before and 5 days after MI | 200 μg/kg per day, i.p. | 4 weeks | ↓ Mortality ↓ Infarct size |
| Harada et al. [51] | Mouse | Permanent coronary occlusion | Immediately after MI for 5 days | 10–100 μg/kg per day, s.c. | 1 week | ↓ Apoptosis ↓ Infarct size ↑ LVFS Improved hemodynamic parameters |
| Deindl et al. [29] | C57BL/6 mouse | Permanent coronary occlusion | Immediately after MI for 5 days | 100 μg/kg per day, s.c. | 30 days | ↓ Mortality ↑ LVEF Improved hemodynamic parameters ↔ Infarct size Neovascularization + |
| Fujita et al. [43] | C57BL/6 mouse | Permanent coronary occlusion | 24 h after MI for 10 days | 300 μg/kg per day, s.c. | 60 days | ↑ LVEF Improved hemodynamic parameters ↓ Mortality ↓ Infarct size in chronic phase Myocardial regeneration + |
| Rat | ||||||
| Sugano et al. [144] | Wistar rat | Permanent coronary occlusion | 3 h after MI and every 24 h thereafter for 7 days | 20 μg/kg per day, s.c. | 2 weeks | ↓ LVEDD Improved hemodynamic parameters ↔ Infarct size |
| Li et al. [92] | Sprague–Dawley rat | Permanent coronary occlusion | Immediately after MI for 5 days | 100 μg/kg per day, s.c. | 4 weeks | ↓ Infarct size ↑ LVEF Improved remodeling Reendothelialization + |
| Werneck-de-Castro et al. [167] | Wistar rat | Permanent coronary occlusion | 3 h after MI for 7 days | 100 μg/kg per day, s.c. | 19–21 days | ↔ Infarct size ↔ LVEF ↔ Hemodynamics ↔ LV remodeling |
| Cheng et al. [23] | Sprague– Dawley rat | Permanent coronary occlusion | 3 h after MI for 5 days | 50 μg/kg per day, s.c. | 3 months | ↓ LVEF ↑ LV dilation Worse hemodynamic parameters ↑ Infarct size ↑ Cardiac fibrosis ↑ Mortality |
| Ueda et al. [158] | Wistar rat | Ischemia/reperfusion in isolated-perfused hearts | Started at the onset of reperfusion and continued for 2 h | 300 ng/mL | 2 h | ↓ Infarct size ↑ LV diastolic pressure |
| Ieishi et al. [68] | Wistar rat | 2 Groups: permanent coronary occlusion; and ischemia/reperfusion | For 5 days in both the groups | 100 μg/kg per day, s.c. | 4 weeks | Permanent occlusion: ↔ Infarct size ↔ LV wall thickness ↔ LVEF Ischemia/reperfusion: ↓ Infarct size ↑ LV wall thickness ↑ LVEF |
| Rabbit | ||||||
| Minatoguchi et al. [108] | Rabbit | Open chest ischemia/reperfusion injury | From 1 to 5 days after MI | 10 μg/kg per day, s.c. | 3 months | ↓ LV dilation ↓ Infarct size ↑ LVEF ↑ Infarct wall thickness |
| Misao et al. [109] | Rabbit | Ischemia/reperfusion injury | From 3 days to 7 days post-MI | 10 μg/kg per day, s.c. | 4 weeks | ↓ Scar area/LV area ratio ↑ LVEF ↓ LV dimensions |
| Dog | ||||||
| Takahama et al. [146] | Dog | Open chest ischemia/reperfusion injury | For 30 min from the onset of reperfusion | 0.33 μg/kg/min, i.v. | 6 h | ↓ Arrhythmias ↓ Infarct size |
| Pig | ||||||
| Iwanaga et al. [73] | Swine | Permanent coronary occlusion | From 24 h after MI to 7 days | 10 μg/kg per day, s.c. | 4 weeks | ↓ Apoptosis ↓ Infarct size ↑ LVEF Neovascularization + |
| Beohar et al. [10] | Swine | Ischemia/reperfusion injury | Early group: immediately after injury on every other day for 20 days Delayed group: beginning 5 days after injury for 10 days |
10 μg/kg per day, i.m. | 56 days | Early treatment: ↔ LVEF ↓ LVEDV ↓ Capillary density Delayed treatment: ↑ LVEDV ↓ Capillary density |
| Angeli et al. [7] | Swine | Ischemia/reperfusion injury | i.v. bolus at reperfusion, daily s.c. injections 5–9 days post-MI | i.v. bolus: 10 μg/kg per day; s.c. injections: 5 μg/kg per day | 6 weeks | ↑ LVEF ↑ Wall motion score index ↑ Vascular density ↑ Areas of viable myocardium |
| Models of cardiomyopathy | ||||||
| Mouse | ||||||
| Li et al. [93] | C57BL/6 mouse | Permanent coronary occlusion | 12 weeks after MI, on the first 5 days of each week, continued for 4 weeks | 10 μg/kg per day, s.c. | 16 weeks | ↓ Infarct size ↓ Fibrosis ↑ LVEF Improved hemodynamic parameters ↑ Cardiomyocyte size |
| Tomita et al. [153] | C57BL/6 mouse | Doxorubicin-induced cardiomyopathy | Early group: immediately after doxorubicin injection for 8 days Delayed group: 3 weeks after doxorubicin injection for 8 days |
50 μg/kg per day, s.c. | 8 weeks | ↓ Mortality ↓ Cardiac toxicity Myocardial regeneration + |
| Li et al. [91] | C57BL/6 mouse | Doxorubicin-induced cardiomyopathy | Immediately after doxorubicin injection for 5 days | 100 μg/kg per day, s.c. | 10 weeks | ↓ LV dilation ↓ Fibrosis ↓ Inflammation ↑ LVEF Improved hemodynamic parameters |
| Rat | ||||||
| Louzada et al. [96] | Wistar rat | Permanent coronary occlusion | 4 weeks after MI, rats were assigned to two protocols. Protocol I: G-CSF daily for 7 days Protocol II: G-CSF for 4 weeks on first 5 days of each week |
Protocol I: 50 μg/kg per day, s.c. Protocol II: 10 μg/kg per day, s.c. |
10 weeks | ↔ LV fractional shortening ↔ Hemodynamics ↔ Infarct size ↔ Hypertrophy |
| Hou et al. [65] | Wistar rat | Doxorubicin-induced cardiomyopathy | 2 weeks after doxorubicin injection for 8 days | 50 μg/kg per day, s.c. | 4 weeks | ↓ Apoptosis ↑ LVEF |
| Hamster | ||||||
| Miyata et al. [111] | UM-X7.1 hamster | Autosomal recessive cardiomyopathy | 5 days/wk from 15 to 30 weeks of age | 10 μg/kg per day, s.c. | 15 weeks | ↓ Mortality Improved remodeling ↓ Fibrosis ↑ LVEF ↑ Cardiomyocyte size |
| Pig | ||||||
| Hasegawa et al. [54] | Swine | Ameroid-induced chronic coronary occlusion | Immediately after MI for 7 days | 10 μg/kg per day, s.c. | 4 weeks and 8 weeks | ↓ LV dilation ↓ Cardiac fibrosis ↓ Apoptosis ↑ LVEF Improved hemodynamic parameters Neovascularization + |
BMC bone marrow cell, G-CSF granulocyte colony-stimulating factor, I.M. intramuscular, I.P. intraperitoneal, I.V. intravenous, LV left ventricular, LVEDD LV end-diastolic diameter, LVEDV LV end-diastolic volume, LVEF LV ejection fraction, LVFS LV fractional shortening, MI myocardial infarction, S.C. subcutaneous, ↑ increased, ↓ decreased, ↔ no change
Table 2.
Animal studies of cytokine combination therapy for cardiac repair
| Study | Host | Type of ischemia | Duration of therapy | Dose | Follow-up period | Outcomes |
|---|---|---|---|---|---|---|
| Mouse | ||||||
| Orlic et al. [121] | C57BL/6 mouse | Permanent coronary occlusion | G-CSF + SCF for 5 days prior and 3 days after MI | SCF (200 μg/kg per day), s.c. G-CSF (60 μg/kg per day), s.c. |
27 days | ↓ Mortality ↓ Infarct size Improved remodeling ↑ LVEF Myocardial regeneration + |
| Ohtsuka et al. [118] | C57BL/6 mouse | Permanent coronary occlusion | 4 treatment groups: G-CSF + SCF from 5 days before through 3 days after MI; G-CSF + SCF for 5 days after MI; G-CSF for 5 days after MI and SCF for 5 days after MI | SCF (200 μg/kg per day), s.c. G-CSF (100 μg/kg per day), s.c. |
14 days | All cytokine-treated groups: ↓ Mortality Improved remodeling ↓ Apoptosis ↑ LVEF Improved hemodynamic parameters Neovascularization + |
| Deten et al. [31] | Balb/c mouse | Permanent coronary occlusion | Immediately after MI, G-CSF + SCF twice daily for 1 week | SCF (200 μg/kg per day), s.c. G-CSF (50 μg/kg per day), s.c. |
6 weeks | ↔ Infarct size ↔ Mortality ↔ LV and RV function ↔ Myocardial regeneration |
| Kuhlmann et al. [87] | C57BL/6 mouse | Permanent coronary occlusion | G-CSF + SCF for 3 days prior and 3 days after MI | SCF (50 μg/kg per day), s.c.; G-CSF (200 μg/kg per day), s.c. | 35 days | ↓ Arrhythmias ↓ Infarct size ↑ LVEF Neovascularization + ↔ Myocardial regeneration |
| Kanellakis et al. [75] | DBA/2 mouse | Permanent coronary occlusion | Immediately after MI, G-CSF + SCF for 5 days | SCF (5 μg/100 g per day), i.p.; G-CSF (20 μg/100 g per day), i.p. | 28 days | Improved hemodynamic parameters Neovascularization + |
| Yeghiazarians et al. [172] | eGFP mouse | Permanent coronary occlusion | 4 groups: 1) EPO + G-CSF at 4 h post-MI and on days 1 to 4; 2) EPO + GCSF at 4 h post-MI and on days 1 to 4; 3) EPO + G-CSF at 4 h post-MI and on days 1 to 4; 4) EPO + G-CSF at 4 h post-MI and on days 1 to 4 | Group 1: EPO (1 μg/kg, i.p.) and G-CSF (10 μg/kg, i.p.) Group 2: EPO (1 μg/kg, i.p.) and G-CSF (25 μg/kg, i.p.) Group 3: EPO (5 μg/kg, i.p.) and G-CSF (10 μg/kg, i.p.) Group 4: EPO (5 μg/kg, i.p.) and G-CSF (25 μg/kg, i.p.) | 28 days | All treatment groups: ↑ capillaries and arterioles in infarct border zone ↑ LV function ↓ fibrosis ↑ mobilized cells to the infarct border zone ↓ apoptotic cardiomyocytes |
| Dawn et al. [25] | C57BL/6 mouse | Ischemia/reperfusion injury | 4 h after reperfusion, G-CSF, G-CSF + SCF and G-CSF + FL for 5 to 10 days | SCF (200 μg/kg per day), s.c. G-CSF (250 μg/kg per day), s.c. FL (333 μg/kg per day), s.c. |
35 days | ↑ LVEF and improved remodeling in G-CSF + FL and G-CSF + SCF-treated mice ↓ LV dilation in all cytokine-treated hearts Myocardial regeneration in mice treated with G-CSF + FL or G-CSF + SCF |
| Sanganalmath et al. [135] | ICR mouse | Ischemia/reperfusion injury | 4 h after reperfusion, G-CSF, G-CSF + SCF and G-CSF + FL for 5 to 10 days | SCF (200 μg/kg per day), s.c. G-CSF (250 μg/kg per day), s.c. FL (333 μg/kg per day), s.c. |
48 weeks | ↑ LVEF, improved remodeling and ↓ LV hypertrophy was sustained only in G-CSF + FL at 48 weeks |
| Rat | ||||||
| Sesti et al. [138] | Sprague–Dawley rat | Permanent coronary occlusion | 2 h after MI, G-CSF + SCF for 4 days | SCF (25 μg/kg per day), s.c. G-CSF (100 μg/kg per day), s.c. |
8 weeks | ↑ LVEF ↓ LV volumes ↔ Infarct size ↔ Myocardial regeneration |
| Pig | ||||||
| Kawamoto et al. [79] | Swine | Ameroid-induced progressive coronary occlusion | Immediately after occlusion for 7 days | SCF (20 μg/kg per day), s.c. G-CSF (5 μg/kg per day), s.c. |
4 weeks | ↑ LVEF ↓ Infarct size Neovascularization + |
| Angeli et al. [6] | Swine | Ischemia/reperfusion injury | Long-acting EPO at reperfusion, then weekly SC for 4 weeks; G-CSF at reperfusion and from 5 to 9 days post-MI | EPO (0.9 μg/kg per day as i.v. bolus); EPO (0.4 μg/kg per day as s.c. injections); G-CSF (10 μg/kg per day as i.v. bolus); G-CSF (5 μg/kg per day as s.c. injections) | 6 weeks | ↑ LVEF ↓ LVEDV ↓ LVESV ↑ Wall motion score index ↑ Vascular density ↑ Areas of viable myocardium |
EPO erythropoietin, G-CSF granulocyte colony-stimulating factor, FL FLT-3 ligand, I.P. intraperitoneal, I.V. intravenous, LV left ventricular, LVEDD LV end-diastolic diameter, LVEDV LV end-diastolic volume, LVEF LV ejection fraction, LVESV LV end-systolic volume, LVFS LV fractional shortening, RV right ventricular, SCF stem cell factor, S.C. subcutaneous, ↑ increased, ↓ decreased, ↔ no change
Table 3.
Clinical trials of G-CSF therapy for cardiac repair
| Study/name of the trial |
Trial design | Number of patients |
Duration of therapy | Dose | End-point evaluation method |
Follow-up period |
Outcomes | Side effects in G-CSF-treated patients |
|---|---|---|---|---|---|---|---|---|
| Patients with acute myocardial infarction | ||||||||
| Kang et al. [76] (MAGIC cell trial) | Randomized | Cell infusion = 10 G-CSF = 10, control = 7 | >48 h after AMI for 4 days | 10 μg/kg per day, s.c. | SPECT | 6 months | ↑ Exercise capacity ↑ Myocardial perfusion ↑ LVEF angiogenesis + |
2/3 surviving patients developed in-stent restenosis |
| Suarez de Lezo et al. [142] | Observational | G-CSF = 13 | 5 days after MI for 10 days | 10 μg/kg per day, s.c. | Angiography | 3 months | ↑ LVEF; BMC mobilization + | Spontaneous spleen rupture in 1 patient |
| Ince et al. [70, 71] (FIRSTLINE- AMI) | Randomized | G-CSF = 25, control = 25 | For 6 days | 10 μg/kg per day, s.c. | Angiography, echocardiography | 4 and 12 months | ↑ Regional wall motion in infarct zone ↑ Metabolic activity in infarct zone at 4 months ↑ Resting LVEF Improved remodeling |
Comparable rate of restenosis in G-CSF-treated and control groups |
| Valgimigli et al. [160] | Randomized, placebo- controlled | G-CSF = 10, control = 10 | For 4 days | 5 μg/kg per day, s.c. | SPECT | 6 months | ↑ LVEF ↓ LVEDV EPC mobilization + |
No clinical or angiographic adverse effect |
| Kuethe et al. [86] | Nonrandomized, open-label | G-CSF = 14, control = 9 | 48 h after stent implantation, for mean duration of 7±1 days | 10 μg/kg per day, s.c. | SPECT | 3 months | ↑ Regional wall motion and perfusion ↑ LVEF |
In-stent restenosis in 1 patient |
| Leone et al. [89] (RIGENERA) | Randomized | G-CSF = 14, control = 27 | Starting ≥5 days after AMI for 5 days | 10 μg/kg per day, s.c. | Echocardiography | 4–6 months | ↑ LVEF ↓ LVEDV ↓ LVESV |
No adverse effect |
| Suzuki et al. [145] | Randomized | G-CSF = 12 (AMI), control = 12 (AMI) | For 10 days | 2 μg/kg per day, s.c. titrated to WBC count of 30,000/μl | SPECT, angiography | 1 months | ↑ LVEF ↑ Regional wall motion; CD34 + BMC mobilization + |
No clinical and angiographic adverse effect |
| Ellis et al. [35] | Pilot, dose-escalation randomized | G-CSF = 12, control = 6 | For 5 days | 5-10 μg/kg per day, s.c. | Echocardiography | 1 months | ↔ LV function BMC mobilization + | No serious adverse effect |
| Ripa et al. [133] (STEMMI) | Randomized, double-blind, placebo- controlled | G-CSF = 39, control = 39 | <12 h after MI for 6 days | 10 μg/kg per day, s.c. | MRI | 6 months | ↔ LVEF BMC mobilization + | 8/39 patients developed musculoskeletal pain |
| Zohlnhofer et al. [174] (REVIVAL-2) | Randomized, double-blind, placebo- controlled | G-CSF = 56, control = 58 | For 5 days | 10 μg/kg per day, s.c. | MRI, SPECT, angiography | 4–6 months | ↔ LVEF ↔ Infarct size BMC mobilization + |
7/56 patients developed musculoskeletal pain |
| Engelmann et al. [36, 37] (G-CSF- STEMI) | Randomized, double-blind, placebo-controlled | G-CSF = 23, control = 21 | For 5 days after PCI | 10 μg/kg per day, s.c. | MRI, angiography | 12 months | ↔ LVEF ↔ LV size BMC mobilization + ↑ Myocardial perfusion (early) |
Bone/muscle pain in 2 patients |
| Takano et al. [148] | Randomized, placebo-controlled | G-CSF = 18, control = 22 | Within 24 h after PCI for 5 days | 2.5 μg/kg per day, s.c. | SPECT, angiography | 6 months | ↑ LVEF ↔ LVEDV BMC mobilization + |
In-stent restenosis in 1 patient |
| Achilli et al. (STEM-AMI) [4] | Randomized, placebo-controlled | G-CSF = 24, control = 25 | For 5 days, starting within 12 h after PCI | 5 μg/kg per day b.i.d., s.c. | MRI, echocardiography, SPECT, angiography | 6 months | ↓ Infarct size Improved remodeling ↔ LVEF |
Adverse effects were similar in G-CSF-treated and control groups |
| Patients with chronic myocardial ischemia | ||||||||
| Hill et al. [59] | Non-randomized | G-CSF = 16, control = 15 | For 5 days | 10 μg/kg per day, s.c. | MRI | 3 months | ↔ LVEF ↔ Regional wall motion; BMC mobilization + |
One patient had MI 8 h after the fifth G-CSF dose; one had MI and died 17 days after G-CSF therapy |
| Wang et al. [163] | Non-randomized | G-CSF = 13, control = 16 | For 6 days | 5 μg/kg per day, s.c. | SPECT, MRI and echocardiography | 2 months | Improved clinical symptoms ↓ LVEF ↔ Myocardial perfusion |
No major side effect |
| Ripa et al. [134] | Non-randomized | G-CSF+VEGF = 16 VEGF = 16 Placebo = 16 |
1 week after ischemia for 6 days | 10 μg/kg per day, s.c. | SPECT, MRI and echocardiography | 3 months | ↔ LVEF ↔ Myocardial perfusion ↔ LVEDV |
No major side effect |
| Huttmann et al. [67] | Non-randomized | G-CSF = 16, control = 8 | Four G-CSF treatment periods of 10 days off and 10 days on | 480 μg b.i.d, s.c. (titrated) for 4 × 10 days courses | Echocardiography | 6 months | ↓ NYHA class ↑ 6-min walk distance; BMC mobilization + |
Moderate to severe bone pain |
AMI acute myocardial infarction, BMC bone marrow cell, EPC endothelial progenitor cell, G-CSF granulocyte colony-stimulating factor, LV left ventricular, LVEDD LV end-diastolic diameter, LVEDV LV end-diastolic volume, LVESV LV end-systolic volume, LVEF LV ejection fraction, LVFS LV fractional shortening, MI myocardial infarction, MRI magnetic resonance imaging, NYHA New York Heart Association, RV right ventricular, S.C. subcutaneous, SPECT single photon emission computed tomography, VEGF vascular endothelial growth factor, WBC white blood cell, ↑ increased, ↓ decreased, ↔ no change
Although it is difficult to reconcile these divergent observations from animal studies and clinical trials, it is important to consider that cardiac benefits of cytokine therapy may potentially depend on a number of variables, including the extent of BMC mobilization and homing, characteristics of mobilized cells, timing of therapy, mobilization-independent effects of cytokines, and also on patient characteristics. Indeed, in our meta-analysis [2], G-CSF therapy was beneficial in patients with acute MI with impaired LV ejection fraction (EF) at baseline, and when therapy was initiated early. It is also important to recognize that potential differences in intracellular signal transduction pathways among different species may account for the failure to recapitulate findings from one species in another [140]. Moreover, cytokine combinations with synergistic effects may be more potent compared with G-CSF treatment alone [25]. It is therefore likely that optimization of various therapeutic as well as patient selection variables in future studies may result in superior cardiac repair with cytokines. In this article, we review the basic and clinical evidence supporting a beneficial role of specific hematopoietic cytokines in cardiac repair and the putative mechanisms underlying these benefits.
Granulocyte colony-stimulating factor
G-CSF is a glycoprotein consisting of 4 antiparallel α-helices with a molecular weight of 19 kDa [166]. Produced by BMCs, macrophages, endothelial cells, fibroblasts, mesothelial cells, and astrocytes in response to a variety of stimuli [30, 104], G-CSF primarily acts via activation of the G-CSF receptor (G-CSFR). The G-CSFR has a composite structure consisting of an extracellular domain, a single transmembrane domain, and a cytoplasmic domain, which consists of three conserved amino-acid sequences [30, 45] (Fig. 2). Stimulation of G-CSF receptor initiates maturation, survival, proliferation, and functional activation of granulocytes [30]. Although the signaling details continue to unfold, an involvement of STAT3 has been reported in the differentiation and maturation of various subsets of BMCs in response to G-CSF [26, 105]. G-CSF also plays a crucial role in the mobilization of granulocytes, as well as stem and progenitor cells from BM into the peripheral circulation [84]. Recent reports have documented an increase in the number of circulating progenitor cells [90, 139] as well as in the level of endogenous G-CSF [90] in the peripheral blood of patients with acute MI, underscoring the importance of G-CSF signaling in ischemic heart disease.
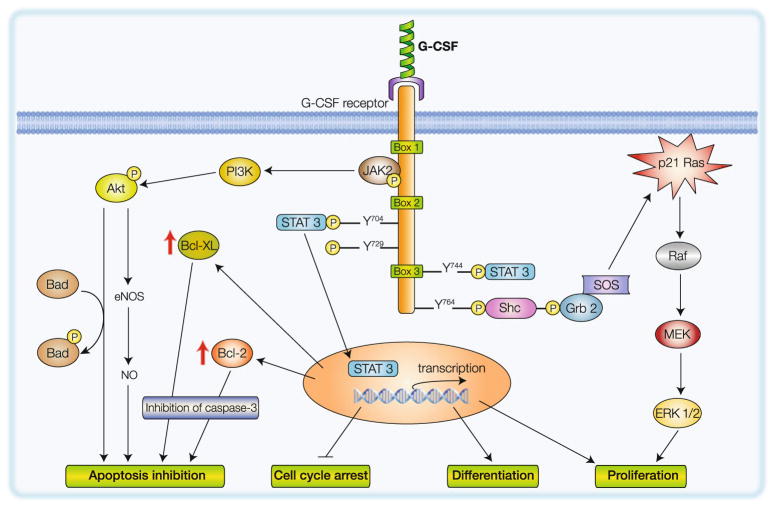
Fig. 2.
Schematic representation of intracellular signaling pathways activated by G-CSF via the activation of G-CSF receptor complex. Activation of JAK/STAT, MAPK, and PI3-K/Akt pathways leads to inhibition of apoptosis, cell survival, and differentiation
Studies of G-CSF therapy in animal models of acute MI
The safety and efficacy of G-CSF therapy for infarct repair in the setting of an acute MI have been evaluated in a large number of studies in mouse, rat, rabbit, pig, dog, and primate models (summarized in Table 1). Orlic et al. [121] were the first to demonstrate that administration of G-CSF and SCF (5 days prior to and 3 days after coronary occlusion) in mice with acute MI improves survival, improves LV performance, and mitigates LV remodeling by inducing formation of new cardiomyocytes and vessels in the infarct region. Subsequently, Minatoguchi et al. [108] reported attenuation of LV remodeling and dysfunction with G-CSF therapy and attributed these observations to regulation of phagocytosis of necrotic tissue, fibroblast proliferation, and angiogenesis by G-CSF-mobilized leukocytes. In mouse and pig models of coronary occlusion/reperfusion, respectively, Ohtsuka et al. [118] and Iwanaga et al. [73] documented the efficacy of G-CSF in improving LV function and remodeling by promoting angiogenesis and decreasing apoptosis. Since cytokine therapy in the study by Orlic et al. [121] was started before coronary occlusion, using an EGFP chimeric mouse model Dawn et al. [25] examined whether such therapy would still be effective when started after the ischemic event, a more clinically relevant scenario. In this study [25], G-CSF and FL administered after a reperfused MI resulted in cardiac and vascular differentiation of bone marrow-derived cells and improved LV function and remodeling. Importantly, G-CSF administered as monotherapy failed to impart significant reparative benefits [25]. Nonetheless, intravenous administration of G-CSF for 30 min from the onset of reperfusion was able to reduce infarct size and the incidence of arrhythmias in a dog model of 90-min coronary occlusion/6 h of reperfusion confirming the benefits of this therapy in large animal models of acute MI [146].
Although G-CSF therapy, alone (Table 1) or in combination with other cytokines (Table 2), was associated with improved cardiovascular outcomes, G-CSF alone has failed to confer cardiac repair in several studies from other laboratories. For example, Norol et al. [116] observed an increase in myocardial blood flow and endothelial cell differentiation in a nonhuman primate model after a single administration of G-CSF and SCF 4 h after coronary occlusion; however, these were not associated with any functional benefit or reduction in infarct size. In another study [167], G-CSF treatment in rats with acute MI neither improved cardiac function nor showed cardiomyocyte proliferation. In view of the above, it is fair to conclude that the efficacy of G-CSF as a single agent for cardiac repair in animal models remains debatable.
Studies of G-CSF therapy in animal models of cardiomyopathy
G-CSF therapy has also been applied in animal models of both ischemic as well as nonischemic cardiomyopathy (Table 1). The ability of G-CSF to improve myocardial function in the setting of ischemic cardiomyopathy was examined by Kawamoto et al. [79] in a swine model of chronic circumflex artery ischemia. A combination of G-CSF and SCF along with intramyocardial VEGF-2 gene transfer improved myocardial vascularity as well as LV performance. However, cytokine therapy alone without VEGF-2 gene transfer was ineffective in improving cardiac function. In the study by Hasegawa et al. [54], pigs with chronic hibernating myocardium were treated with G-CSF for 1 week and followed up for 2 months. G-CSF therapy increased vascular density, decreased fibrosis, and decreased apoptosis within the ischemic zone, thereby improving the global LV function. The reparative efficacy of G-CSF in bona fide ischemic cardiomyopathy was further tested by Li et al. [93], who examined the effects of G-CSF treatment in mice with established postinfarct LV remodeling and dysfunction at 12 weeks after coronary occlusion. A considerably smaller dose of G-CSF (10 μg/kg per day) was administered 5 days/week for a period of 4 weeks (in contrast to 5–10 consecutive days in most studies of acute MI). G-CSF therapy enhanced cardiomy-ocyte G-CSFR expression, decreased fibrosis by increasing the expression of MMP-2 and MMP-9, induced hypertrophy of surviving cardiomyocytes, and improved cardiac function [93]. In contrast, treatment with both low-dose and high-dose G-CSF failed to improve LV function, infarct size, and hypertrophy in a rat model of postinfarct cardiomyopathy in a more recent study [96].
The ability of G-CSF to improve LV function in the setting of nonischemic cardiomyopathy was demonstrated in a study by Miyata et al. [111], who used a hamster model of autophagic dilated cardiomyopathy. G-CSF therapy attenuated LV remodeling and improved LV function as well as survival. Furthermore, in a model of doxorubicin-induced LV dysfunction, an 8-day course of G-CSF initiated 2 weeks after the cessation of doxorubicin treatment improved LV function and normalized LV pressures by decreasing cardiomyocyte apoptosis and the expression of apoptotic mediators such as Fas [65].
Mechanisms of G-CSF-induced cardiac repair
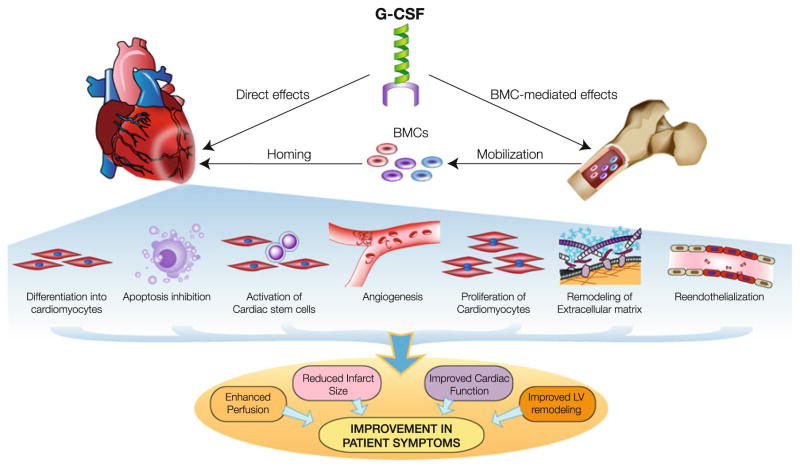
Although myocardial homing of mobilized BMCs is thought to play a critical role in G-CSF-induced cardiac repair, a number of additional mechanisms have emerged from recent studies. These include apoptosis inhibition, promotion of angiogenesis and reendothelialization, proliferation and differentiation of cardiomyocytes, direct cardioprotective effects, modulation of extracellular matrix, and other paracrine mechanisms, all of which can potentially contribute to the observed improvement in cardiac structure and function (Fig. 3).
Fig. 3.
Potential mechanisms underlying the cardioprotective actions of G-CSF. G-CSF stimulates mobilization of BMCs, which can home to the heart to initiate myocardial repair via several mechanisms: a differentiation into cells of cardiac lineages; b activation of antiapoptotic signaling; c promote angiogenesis and reendothelialization; d paracrine effects on resident progenitors and myocytes leading to cellular proliferation; e and favorable paracrine effects on the extracellular matrix. Apart from these BMC-mediated effects, G-CSF can also exert direct cytoprotective and angiogenic effects on the infarcted myocardium
Mobilization and myocardial homing of BMCs
In studies of cardiac repair, although cytokine-induced mobilization and myocardial homing of BMCs with subsequent differentiation into cells of cardiac lineages have been reported [25, 121], these effects were noted primarily with combination (G-CSF + SCF and G-CSF + FL) cytokine therapy, while G-CSF monotherapy proved relatively ineffective [25]. Additional studies have generated conflicting evidence regarding the relative contribution of cardiomyocytic differentiation of G-CSF-mobilized BMCs toward the functional and structural improvement after MI [31, 44]. In the study by Askari et al. [8], transplantation of SDF-1-transfected syngeneic cardiac fibroblasts into the periinfarct zone along with G-CSF therapy induced homing of CD117+ BMCs to the heart and resulted in improved cardiac function, although G-CSF alone was unable to induce BMC homing. In the study by Kawada et al. [78], G-CSF administration after MI resulted in myocardial homing and cardiomyocytic differentiation of mobilized cardiomyogenic cells. Similarly, increased infiltration of the infarcted myocardium by BM-derived side-population cells following G-CSF therapy has also been reported [5]. The importance of BMC homing in infarct repair is further supported by findings from a study in which administration of AMD3100, a specific inhibitor of CXCR4, reduced myocardial homing of CXCR4+ BMCs and abolished the reparative effects of G-CSF therapy [109]. Collectively, these results suggest that myocardial recruitment of BMCs via the SDF-1/CXCR4 axis plays an important role in mediating the reparative effects of G-CSF.
Antiapoptotic, proliferative, and direct cytoprotective effects of G-CSF
Convincing data from recent studies indicate that various cell types in the heart, including cardiomyocytes, endothelial cells, and interstitial cells, express G-CSFR [51, 87]. Since G-CSFR can activate prosurvival signaling via the JAK/STAT pathway [14], it is conceivable that G-CSF can potentially exert direct cytoprotective and proliferative effects in the myocardium (Fig. 3). In the study by Harada et al. [51], G-CSF treatment inhibited H2O2-induced reduction in Bcl-2 and inhibited myocyte apoptosis in vitro. In transgenic mice overexpressing a dominant negative mutant STAT3 (dnSTAT3-Tg), G-CSF therapy failed to improve cardiac function despite increased number of c-kit+/Sca-1+ BMCs in the peripheral blood. Furthermore, the post-MI beneficial effects of G-CSF could be recapitulated in a Langendorff model of myocardial ischemia/reperfusion injury. These results suggest that the beneficial effects of G-CSF may be attributed, at least in part, to its antiapoptotic actions via activation of the JAK/STAT pathway [51].
Growing evidence indicates that G-CSF influences cell cycle-regulating molecules, including the cyclin-dependent kinase inhibitor p27, thereby shortening the G0/G1 phase and promoting cellular proliferation and survival [34, 154, 164]. Consistently, G-CSF has been shown to induce proliferation of mouse cardiomyocytes and human troponin I-positive cells from cardiomyopathic heart [50]. Furthermore, in a mouse model of myocardial infarction, G-CSF therapy increased the number of resident cardiac Sca-1+ cells, indicating that G-CSF actions may also involve activation of certain cardiac progenitors [17].
A direct cardioprotective effect of G-CSF was also reported in a hamster model of dilated cardiomyopathy [111]. Although protection against apoptosis was not observed, G-CSF therapy protected cardiomyocytes against autophagic cellular degradation via JAK/STAT activation thereby attenuating progression to heart failure [111]. In addition to the antiapoptotic and antiautophagic effects, G-CSF may also exert an acute “postconditioning-like” effect [158]. In isolated-perfused rat hearts (wherein the contribution of BMCs was excluded completely), G-CSF administration at the onset of reperfusion led to the activation of Akt/eNOS signaling cascade leading to increased NO production and reduction in infarct size [57, 158]. Furthermore, administration of L-NAME, a specific eNOS inhibitor, blunted the G-CSF-induced reduction in infarct size, thereby suggesting the involvement of endothelial cells in G-CSF-mediated cardioprotection. Together, the above studies indicate that by activating several prosurvival signaling pathways, G-CSF is able to exert multifarious beneficial effects on the postinfarct myocardium independent of BMC mobilization.
Induction of angiogenesis and arteriogenesis
In addition to the above cytoprotective actions on cardiomyocytes, a number of additional beneficial actions of G-CSF on ischemic tissues have been identified in recent studies. G-CSF administration shortly after an acute MI has been associated with increased neovascularization in the periinfarct area and decreased endothelial cell apoptosis in both small animal [29, 51, 75, 87, 109, 118] as well as large animal [73] models. In the study by Ohtsuka et al. [118], G-CSF therapy for 5 days after coronary occlusion in mice improved cardiac function, attenuated adverse remodeling and increased the number of capillaries in the infarct borderzone without significant regeneration of cardiomyocytes. Although the precise mechanism remains unclear, recent reports by Ohki et al. [117] and Capoccia et al. [22] suggest that G-CSF-stimulated neutrophils and monocytes are capable of promoting neovascularization in ischemic tissues via paracrine mechanisms. G-CSF also mobilizes EPCs [62] that may contribute to angiogenesis as well as reduce atherosclerosis progression [136]. Since G-CSF can increase the expression of intracellular adhesion molecule-1 [29] and SDF-1 [99, 109], greater accumulation of circulating leukocytes, EPCs, and CXCR4+ cells [42, 109] may be responsible for the observed increase in vascularity in the periinfarct areas.
In addition to angiogenesis, the vasculogenic benefits of G-CSF therapy may also involve arteriogenesis (formation of mature and functional arteries from pre-existing arterio-arteriolar connections after arterial occlusion). Although the impact of G-CSF and GM-CSF in arteriogenesis has been examined largely in the context of cerebral vasculature [63], G-CSF administration has also been shown to enhance myocardial arteriogenesis after MI [29, 87]. Arteriogenesis is a complex process that involves leukocytes, cytokines, and adhesion molecules such as ICAM-1, which mediates leukocyte adhesion [28]. In this regard, administration of G-CSF following MI was associated with increased expression of ICAM-1 in arterioles at the infarct borderzone, accumulation of leukocytes, and proliferation of arteriolar endothelial cells and smooth muscle cells [29].
Antiinflammatory effects of G-CSF
Acute myocardial ischemic injury is characterized by inflammation and oxidative stress, which influence the outcomes both in the acute setting and chronically during remodeling. After an acute MI, a number of inflammatory cytokines [tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-1β, interferon (IFN)-γ, and such] released by the inflammatory cells as well as the myocytes impact myocyte survival, apoptosis, angiogenesis, alteration in matrix, and cardiac function and remodeling [82, 115]. While the production of G-CSF by human monocytes is stimulated by inflammatory mediators [97], G-CSF has been shown to exert antiinflammatory effects by modulating the release of cytokines that are known to play important roles in post-infarct myocardial inflammation and remodeling [52]. Indeed, pretreatment with G-CSF in vivo has been shown to reduce the release of TNF-α by macrophages [49] and IL-2 and IFN-γ by T cells [124] following lipopolysaccharide (LPS) challenge. Consistently, G-CSF pretreatment in humans reduced TNF-α release from monocytes, and increased the whole blood levels of IL-1 receptor antagonist (IL-1ra) and soluble TNF-α receptors I and II following LPS stimulation ex vivo [53]. Subsequent studies have verified similar effects in vivo in humans [123] and identified the involvement of functional G-CSF receptors on monocytes in this process [15]. Although the molecular specifics in the context of myocardial inflammation remain relatively unexplored, these antiinflammatory effects of G-CSF may potentially improve LV remodeling following MI.
A role of G-CSF in protection against oxidative stress was documented in a recent study by Hou et al. [64], in which treatment with G-CSF decreased myocardial malondialdehyde levels and increased glutathione levels in rats with doxorubicin-induced cardiotoxicity. These antioxidant effects of G-CSF may also contribute toward improving cardiac function and structure following MI. Future investigations of markers and molecular determinants of myocardial oxidative stress may elucidate additional mechanisms underlying the benefits of G-CSF therapy in the setting of an acute MI.
Other beneficial effects of G-CSF
Modulation of various constituents of myocardial extra-cellular matrix (ECM) has been advanced as an additional mechanism of G-CSF-induced benefits. In the post-MI period, G-CSF therapy has been shown to increase the levels of MMP-1 and MMP-9 with accelerated resorption of necrotic tissue and reduction in infarct scar size [108, 109]. G-CSF-induced increase in TGF-β and procollagen type-I and type-III mRNA expression in the infarcted area may also result in infarct size reduction [144]. Further evidence of ECM alteration came from the study by Fujita et al. [43], in which G-CSF therapy after MI in EGFP chimeric mice led to increased homing of EGFP + BMCs into the infarct region with subsequent differentiation into vimentin+ and α-SMA+ myofibroblasts. Although regeneration of bona fide cardiomyocytes was not noted, cardiac function improved in G-CSF-treated mice, indicating that population of the infarcted myocardium with BMC-derived myofibroblasts can also effectively improve LV function. Additional favorable effects of G-CSF on the ECM include increase in connexin-43 expression in the peri-infarct zone and modulation of function of gap junctions in cardiomyocytes, which have been shown to reduce the incidence of ventricular arrhythmias [87].
Clinical trials of G-CSF therapy in acute MI
Although the results from animal studies of cardiac repair with G-CSF as monotherapy have been mixed, the safety and efficacy of G-CSF in patients with acute MI have been evaluated in several clinical trials (Table 3). In the MAGIC randomized trial [76], patients underwent percutaneous coronary intervention (PCI) of the infarct-related coronary artery and received either G-CSF alone (10 μg/kg for 4 days before PCI) or a combination of G-CSF and intracoronary infusion of peripheral blood stem cells harvested by plasmapheresis. Despite improvement in LVEF, exercise capacity, LV end-systolic volume (LVESV) and myocardial perfusion, the study was prematurely terminated after 6 months of follow-up because of a high incidence of in-stent restenosis in G-CSF-treated patients [76].
Improvement in various parameters of LV function and anatomy was reported in several subsequent trials. In the randomized phase I FIRSTLINE-AMI trial, patients with ST-elevation MI (STEMI) received a 6-day course of 10 μg/kg G-CSF (starting at 90 min after reperfusion) and were followed up for 1 year. Treatment with G-CSF improved LV function and enhanced systolic infarct wall thickening with no increase in the incidence of restenosis [70, 71]. In the study by Kuethe et al. [86], patients with acute MI who received G-CSF (10 μg/kg) for 7 days starting 2 days after PCI showed improvement in regional wall motion, myocardial perfusion, and LVEF after 3 months with no significant adverse effect. In the GLEAM trial, a lower dose of G-CSF (2.5 μg/kg for 5 days) was administered in acute MI patients with total occlusion of left anterior descending coronary artery. After 6 months, greater LVEF and smaller LVESV were noted in G-CSF-treated patients without any increase in restenosis compared with controls [148].
However, several other RCTs have failed to confirm the above beneficial effects of G-CSF therapy in patients with acute MI. The STEMMI [133] and the REVIVAL-2 [174] trials included patients with ST-elevation MI treated with PCI within 12 h after symptom onset. In STEMMI, although G-CSF injection was safe and well tolerated, it did not lead to greater improvement in LV function or infarct size compared with placebo-treated patients [133]. In REVIVAL-2, while G-CSF therapy successfully mobilized BMCs, there was no significant improvement in LV function or infarct size after 6 months. In the G-CSF-STEMI trial [36] and the dose-escalation study by Ellis et al. [35], G-CSF therapy was unable to improve cardiac function after MI. Furthermore, in G-CSF-STEMI trial, changes in global and regional cardiac function during 12 months of follow-up were monitored by magnetic resonance imaging, which showed no improvement in myocardial function with G-CSF therapy [37].
Because of the above diversity in outcomes in small clinical trials, the efficacy of G-CSF therapy for infarct repair has been evaluated in several meta-analyses [2, 173]. In the meta-analysis by Zohlnhofer et al., which included data from 10 RCTs, G-CSF therapy was not associated with any significant benefit in terms of LV function or anatomy compared with controls. In our meta-analysis [2], although G-CSF therapy was ineffective in unselected patients with acute MI, subgroup analyses suggested that G-CSF therapy might be beneficial in patients with reduced LV function at baseline. Our results also indicated that such therapy might be more beneficial when G-CSF was administered early after an acute MI [2]. Indeed, studies in which G-CSF was started early after acute MI has shown improvement in LV function [71, 148] in comparison with studies in which G-CSF treatment was started later [36, 133, 174]. Moreover, as discussed earlier, the benefits of G-CSF therapy may also involve direct cytoprotective actions, which are likely to be more beneficial during the early phase of reperfusion after an acute MI. However, these inferences remain to be validated in future animal experiments and clinical trials. Importantly, results from these meta-analyses [2, 72, 173] indicate that G-CSF therapy does not lead to increased risk of in-stent restenosis or other major adverse effects.
The precise reasons for this apparent failure of G-CSF monotherapy in cardiac repair remain to be fully understood. Although G-CSF therapy mobilizes BMCs [84], different cytokines preferentially mobilize different subsets of BMCs [56, 114], and the efficacy of mobilization of primitive progenitors is relatively lower with G-CSF compared with other cytokine combinations [25]. Moreover, the cytokine regimens also influence the expression of surface markers responsible for homing on mobilized cells [25]. Thus, the lack of efficacy in clinical trials of G-CSF monotherapy may be due, at least in part, to mobilization and myocardial homing of a relatively small number of primitive stem/progenitor cells.
Clinical trials of G-CSF therapy in chronic myocardial ischemia
Several clinical trials have evaluated the safety and clinical benefits of G-CSF therapy in patients with chronic myocardial ischemia due to severe occlusive coronary artery disease (CAD) [58, 67, 134, 163] (Table 3). In patients with chronic myocardial ischemia, G-CSF therapy increased the number of circulating CD34+ cells without improving LVEF or perfusion [58, 163]. The improvement in angina score and nitroglycerin intake [163] supported the importance of mobilized stem cells toward the observed clinical benefits. Although data from animal studies demonstrated vasculogenic benefits with combined VEGF-A gene transfer and G-CSF treatment for the differentiation of stem cells into endothelial cells [77, 79], in the study by Ripa et al. [134], VEGF-A165 gene transfer followed by subcutaneous injection of G-CSF in patients with severe occlusive CAD neither induced vasculogenesis nor improved patient symptoms. In another study involving patients with ischemic or dilated cardiomyopathy, treatment with G-CSF improved NYHA class and 6-min walking distance [67].
In summary, although results from animal studies have been promising, clinical trials evaluating safety and efficacy of G-CSF therapy in patients with chronic myocardial ischemia have failed to show convincing and consistent benefits. Whether further modifications in cytokine combinations, dose, and patient selection will improve outcomes remains to be tested in future clinical trials.
Granulocyte–macrophage colony-stimulating factor
In addition to its actions on bone marrow hematopoietic progenitors, GM-CSF can effectively modulate proliferation, migration, and other biological properties of a number of nonhematopoietic cells, including bone marrow fibroblasts, tumor cell lines, and endothelial cells [19, 27, 141]. GM-CSF-induced upregulation of adhesion molecules enhances the recruitment of circulating leukocytes, especially monocytes, to endothelial cells [32, 46]. GM-CSF also increases the number of circulating monocytes, which have been implicated both in angiogenesis and LV remodeling [61, 101, 165], and the level of circulating GM-CSF is elevated in patients with ischemic dilated cardiomyopathy [126]. Moreover, in models of hindlimb ischemia and ocular micropocket assay, mobilization of endothelial progenitor cells by GM-CSF has been shown to enhance angiogenesis [147].
Studies of GM-CSF therapy for cardiac repair in animal models
Because of its ability to mobilize BMCs and in view of the above considerations, the efficacy of GM-CSF therapy for cardiac repair has been tested in animal models of acute MI. In the study by Terrovitis et al. [150], GM-CSF administration for 3 weeks after a reperfused MI in pigs failed to reduce infarct size or improve LV function. In the study by Maekawa et al. [102], upregulation of GM-CSF by romurtide reduced expression of TGF-β1 and collagen types I and III in the infarcted myocardium and promoted tissue infiltration by ED-1+ monocyte-derived macrophages, which were associated with marked infarct expansion after acute MI [102]. Similar worsening of outcomes after MI with GM-CSF therapy has been reported by Naito et al. [113], who noted increased infiltration of the myocardium by dendritic cells with GM-CSF therapy. Therefore, although GM-CSF may confer cardiac reparative benefits when administered with other growth factors [170], current evidence suggests that GM-CSF-induced cellular infiltration and molecular changes may cause more harm than good in the post-infarction period.
Clinical trials of GM-CSF therapy
In humans, short-term administration of GM-CSF in patients with chronic myocardial ischemia has been shown to improve collateral blood flow [137]. Despite this beneficial effect, plasma levels of GM-CSF in patients with heart failure correlates with neurohormonal activation, hemodynamic deterioration, and LV dysfunction [125, 126]. In addition, administration of GM-CSF in cancer patients has been shown to transiently increase LV end-systolic dimensions and decrease cardiac contractility [83]. Future studies in animal models will be necessary to evaluate whether the angiogenic potential of GM-CSF can be safely utilized for myocardial repair.
Stem cell factor
SCF, also known as kit ligand or steel factor, binds to c-kit (a type III tyrosine kinase receptor) and exerts its activity at the early stages of hematopoiesis acting synergistically with colony-stimulating factors [16]. The activation of c-kit is triggered by the binding of dimeric SCF leading to homodimerization of two c-kit molecules. Phosphorylated tyrosine residues on c-kit have binding sites for many signaling proteins such as members of the PI3-K/Akt, RAS/MAPK and JAK/STAT signaling pathways. Subsequent activation of these signaling proteins and their related pathways leads to cell proliferation, differentiation, survival, and chemotaxis [16, 85, 100]. Administration of SCF, especially in combination with G-CSF, results in mobilization of hematopoietic progenitors into the peripheral blood [40, 171].
In addition, SCF (also known as ‘mast cell growth factor’) not only elicits differentiation, maturation and adhesion of mast cells [119], but also regulates their proliferation [156] and migration [106], and promotes survival by inhibiting apoptosis [69]. Mast cells arise from multi-potent hematopoietic stem cells [81], and participate in host defense, innate immunity, and inflammation [3]. SCF has been shown to promote mast cell development from bone marrow, umbilical cord blood and peripheral blood progenitors [80, 110, 159]. In the heart, SCF expressed in macrophages in the infarcted myocardium plays a key role in promoting chemotaxis and growth of mast cell precursors [41]. However, although SCF is expressed abundantly in the myocardium, its expression decreases after MI [169], and the accumulation of mast cells after MI varies depending on the species [33].
Studies of SCF therapy for cardiac repair in animal models
Since Lin-/c-kit+ cells were shown to induce robust infarct repair [120] and because administration of SCF along with G-CSF could induce a 250-fold increase in Lin-/c-kit+ cells in the circulation [13], in a seminal study [121], Orlic et al. tested whether G-CSF + SCF therapy would induce cardiac repair after MI. Mice received SCF and G-CSF injections for 5 days, underwent coronary ligation, and received cytokines for an additional 3 days. At 27 days after MI, SCF + G-CSF therapy reduced mortality and improved cardiac function and remodeling by promoting formation of new myocytes and vessels from mobilized BMCs that homed into the myocardium. Expanding upon these observations, in a subsequent study [25], we examined whether cytokine combination therapy would confer reparative benefits when initiated after an acute MI. After 35 days of follow-up, G-CSF and SCF therapy was associated with improved LV function indicating that such therapy is efficacious in a clinically relevant scenario after a reperfused MI. Although improvement in LV function has been reported in other studies in rats [88, 138] and mice [75, 87, 118], in the study by Ohtsuka et al. [118], administration of SCF alone after MI was unable to improve post-MI outcomes. In addition, in the study by Deten et al. [31], no cardiac functional benefit was observed with G-CSF + SCF therapy in a mouse model of coronary ligation. Although regeneration of infarcted myocardium has been shown by G-CSF + SCF therapy [25, 121], the mechanisms remain controversial [138], and the efficacy of this therapy remains to be proven in humans.
FLT-3 ligand
As a ligand for the flt-3/flk-2 tyrosine kinase receptors, FL plays a critical role in proliferation, survival, and differentiation of early hematopoietic precursors [98]. Binding of FL to flt-3 activates PI3-K/Akt and RAS signaling cascades, leading to inhibition of apoptosis and increased cell proliferation, respectively. PI3-K activation stimulates several downstream molecules such as PDK-1, Akt and mTOR, which in turn activate p70 S6 kinase (S6K) and inhibit eukaryotic initiation factor 4E-binding protein (4E-BP1) leading to gene transcription. Additionally, activation of PI3K inhibits apoptotic cell death through phosphorylation of proapoptotic Bcl-2 family protein Bad. The p21RAS pathway is activated when FL associates with GRB2 through SHC. The activation of RAS stimulates downstream effectors such as Raf, MAPK, and ERK1/2, which activates cAMP-response element binding protein (CREB), ELK and STATs and leads to transcription of genes involved in cell proliferation.
Studies of FL therapy for cardiac repair in animal models
Studies in mice have shown a striking synergistic effect on mobilization and engraftment of hematopoietic progenitors when G-CSF and FL are used in combination [114, 143]. Because of this synergy, we examined the comparative efficacy of G-CSF + FL therapy versus G-CSF + SCF and G-CSF alone for cardiac repair after a reperfused MI in mice [25]. Among these combinations, G-CSF + FL therapy was associated with the greatest extent of cardiac differentiation of EGFP + BMCs, and was most effective in improving LV function and remodeling. Interestingly, G-CSF + FL therapy was also associated with increased expression of CD62L and CD11a on mobilized BMCs, suggesting that improved homing of BMCs to the infarcted myocardium contributed to the reparative benefits with this therapy [25]. Increased mobilization and myocardial homing of Lin-/Sca-1+/c-kit+ BMCs may also explain the superiority of G-CSF + FL in comparison with other cytokine combinations. Although our results from a recent study [135] indicate that the beneficial effects of G-CSF + FL therapy are also sustained during long-term follow-up (up to 48 weeks), the efficacy of FL in cardiac repair remains to be examined in the clinical setting.
Erythropoietin
Produced primarily in kidneys in response to hypoxia, EPO acts synergistically with other growth factors, such as GM-CSF, SCF, and IL-3, to promote maturation as well as proliferation of erythroid progenitor cells [39]. The actions of EPO in the BM are mediated by activation of specific membrane-bound EPO receptor (belongs to a cytokine type-1 receptor superfamily), which is expressed primarily by erythroid progenitor cells [74]. Interaction of EPO with its membrane-bound receptor leads to dimerization of receptor and activation of receptor associated JAK2, leading to the activation of numerous intracellular signaling cascades, including PI3-K/Akt, MAPK, and JAK/STAT pathways [39, 131]. Activation of these pathways by EPO results in upregulation of antiapoptotic protein Bcl-XL, inhibition of caspase activation, and increase in eNOS expression, which contribute collectively to EPO-induced inhibition of apoptosis in various tissues [18, 103].
Studies of EPO therapy for cardioprotection in animal models
Although EPO-induced improvement in cardiac parameters in patients with CHF was noted many years ago, these benefits were attributed primarily to correction of anemia [48]. Subsequent studies revealed that EPO can favorably modulate the activity of cardiac Na+/K+/ATPase, thereby improving cardiac contractility [162]. Consistent with its ability to mobilize cellular antiapoptotic machinery [47, 155], a direct cardioprotective role of EPO in the setting of myocardial ischemic injury was documented in more recent studies, in which administration of recombinant EPO was associated with reduced infarct size, reduced apoptosis, and improved LV function [20, 21, 112, 127].
Apart from its direct cytoprotective effects, EPO also exerts a potent angiogenic effect [11, 132], which can contribute to improved cardiac structure and function after MI. In the infarcted heart, EPO therapy has been shown to increase capillary density [60, 129, 152, 161, 168], which was associated with decreased periinfarct fibrosis [152], attenuation of LV hypertrophy [161], and improvement in LV function [60, 129, 152, 161, 168]. Consistent with its effects on hematopoietic cells in the bone marrow, EPO has been shown to increase the number of circulating endothelial progenitor cells (EPCs), which might account for ischemia-induced neovascularization [55, 129, 157, 168]. Recent studies indicate that upregulation of myocardial expression of VEGF also contributes toward EPO-induced improvement in myocardial vascularity [168].
Clinical trials of erythropoietin therapy after acute MI
With the above evidence supporting a beneficial role of EPO, several clinical trials have examined the safety and efficacy of EPO for myocardial salvage in the setting of an acute MI. In the first single-center prospective safety and feasibility study, one bolus dose (300 μg iv) of the long-acting EPO analogue darbepoietin alfa administered before primary PCI in patients with acute MI failed to improve LV EF significantly [95]. However, darbepoietin therapy increased the number of CD34+/CD45− cells in the peripheral blood, and was well tolerated without any major adverse effect. Similar results were obtained in a randomized placebo-controlled safety study in patients with non-ST-elevation acute coronary syndromes, in which a single dose of EPO (40,000 IU iv) failed to limit myocardial damage assessed by enzyme levels [94]. EPO administration was associated with increased systolic blood pressure, although no other adverse effect was noted. This failure to reduce enzymatically determined infarct size was mirrored in a larger study with 236 patients with acute ST-elevation MI who received EPO (30,000 IU iv) immediately before thrombolytic therapy [12].
In contrast to these earlier findings, improvement in LV function with EPO treatment has been reported in more recent randomized controlled trials [38, 122, 149]. In the study by Ferrario et al. [38], administration of EPO (33,000 IU) before PCI and at 24 and 48 h after admission reduced CK-MB release by 30% and improved remodeling in patients with first uncomplicated MI. In the EPO/AMI-1 study [122], EPO (12,000 IU iv) administered after PCI in patients with acute MI improved LVEF in treated patients. In the EPOC-AMI study [149], therapy with low-dose EPO (6,000 IU iv) during PCI, and at 24 and 48 h later improved LVEF, reduced LVESV, and decreased infarct size compared with baseline in the treated group, although the outcomes in treated and control groups were not significantly different. However, these studies enrolled relatively few patients (30–36 patients) and the follow-up duration was rather short (6 months). The results from the prospective randomized HEBE III trial [9], in which 466 patients with fist acute MI will be enrolled and a bolus of EPO (60,000 IU iv) will be administered within 3 h of PCI, will provide more definitive information in this regard.
Future perspectives
Although a number of cytokines have been tested in animal models, the clinical evidence regarding the utility of such therapy in cardiac repair comes largely from studies with G-CSF; and recent meta-analyses of pooled data have shown the lack of efficacy of G-CSF in inducing cardiac repair in unselected patients [2, 173]. This is somewhat paradoxical, because G-CSF mobilizes BMCs, and substantial clinical evidence supports the efficacy of BMC therapy in patients with ischemic heart disease [1, 24]. However, stem cell-mediated cardiac repair is far more complex than once perceived, and a number of seemingly minor variables may influence this process. Indeed, the dose of G-CSF, duration of therapy, the interval between acute MI and therapy initiation, the LV function at baseline, and other patient characteristics may all influence the outcomes. In this regard, the final verdict on G-CSF therapy will emerge not from meta-analyses, but from adequately powered randomized controlled trials with optimized study parameters, i.e., dose, duration, timing, patient population, and outcome parameters, to name a few.
However, the results from G-CSF monotherapy may not be extrapolated to cytokines in general or cytokine combination therapies, which may induce more vigorous mobilization of BMCs compared with G-CSF alone. Indeed, although animal studies have shown that addition of other factors to G-CSF such as SDF-1 [8], SCF [25, 121] and FL [25, 135] could enhance myocardial homing of BMCs, clinical data from such combinatorial therapy are not available yet. At present two clinical trials using combination therapy are underway. The effect of sitagliptin (a dipeptidyl peptidase-IV inhibitor) in combination with G-CSF on myocardial function in patients undergoing PCI for acute MI is being tested in a phase III randomized placebo-controlled trial (SITAGRAMI, NCT00650143) [151]; and the efficacy and safety of combination cytokine treatment with short-term G-CSF administration and intracoronary infusion of mobilized peripheral stem cell with darbepoetin infusion are being evaluated in MAGIC Cell-5-Combicytokine Trial (NCT00501917). However, the results with combination therapy in animal studies have also been mixed [6, 25, 130, 135], and the success with such therapy will need to be eventually validated in real-world patients.
Conclusions
Accumulating evidence from animal models of MI as well as heart failure indicates that therapy with hematopoietic cytokines improves cardiac structure and function. Although myocardial homing and subsequent cardiac differentiation of cytokine-mobilized BMCs plays a role in this reparative process, recent reports have identified various other salubrious effects of cytokines, including inhibition of myocyte apoptosis and induction of neovascularization. However, clinical trials of G-CSF therapy in humans have yielded discordant results, and no significant cardiac benefit was observed with G-CSF monotherapy in unselected patients with acute MI. Given the extensive evidence in support of a beneficial role of cytokine therapy in cardiac repair and myocardial protection, further experimental and clinical studies are warranted to investigate the potential of other hematopoietic cytokines as well as cytokine combinations in cardiac repair in patients with ischemic heart disease.
Acknowledgments
This publication was supported in part by NIH grants R01 HL-89939, and R21 HL-89737. We gratefully acknowledge Ms. Heather L. Jones for expert assistance with graphics design.
Footnotes
Conflict of interest None.
Contributor Information
Santosh K. Sanganalmath, Division of Cardiovascular Diseases, Cardiovascular Research Institute, University of Kansas Medical Center, 3901 Rainbow Blvd, Rm. 1001 Eaton, MS 3006, Kansas City, KS 66160, USA
Ahmed Abdel-Latif, Division of Cardiovascular Medicine, University of Kentucky, Lexington, KY 40536, USA.
Roberto Bolli, Institute of Molecular Cardiology, University of Louisville, Louisville, KY 40292, USA.
Yu-Ting Xuan, Division of Cardiovascular Diseases, Cardiovascular Research Institute, University of Kansas Medical Center, 3901 Rainbow Blvd, Rm. 1001 Eaton, MS 3006, Kansas City, KS 66160, USA.
Buddhadeb Dawn, Email: bdawn@kumc.edu, Division of Cardiovascular Diseases, Cardiovascular Research Institute, University of Kansas Medical Center, 3901 Rainbow Blvd, Rm. 1001 Eaton, MS 3006, Kansas City, KS 66160, USA.
References
- 1.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 2.Abdel-Latif A, Bolli R, Zuba-Surma EK, Tleyjeh IM, Hornung CA, Dawn B. Granulocyte colony-stimulating factor therapy for cardiac repair after acute myocardial infarction: a systematic review and meta-analysis of randomized controlled trials. Am Heart J. 2008;156:216–226. e219. doi: 10.1016/j.ahj.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abraham SN, Malaviya R. Mast cells in infection and immunity. Infect Immun. 1997;65:3501–3508. doi: 10.1128/iai.65.9.3501-3508.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Achilli F, Malafronte C, Lenatti L, Gentile F, Dadone V, Gibelli G, Maggiolini S, Squadroni L, Di Leo C, Burba I, Pesce M, Mircoli L, Capogrossi MC, Di Lelio A, Camisasca P, Morabito A, Colombo G, Pompilio G. Granulocyte colony-stimulating factor attenuates left ventricular remodelling after acute anterior STEMI: results of the single-blind, randomized, placebo-controlled multicentre STem cEll Mobilization in Acute Myocardial Infarction (STEM-AMI) Trial. Eur J Heart Fail. 2010;12:1111–1121. doi: 10.1093/eurjhf/hfq150. [DOI] [PubMed] [Google Scholar]
- 5.Adachi Y, Imagawa J, Suzuki Y, Yogo K, Fukazawa M, Kuromaru O, Saito Y. G-CSF treatment increases side population cell infiltration after myocardial infarction in mice. J Mol Cell Cardiol. 2004;36:707–710. doi: 10.1016/j.yjmcc.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Angeli FS, Amabile N, Shapiro M, Mirsky R, Bartlett L, Zhang Y, Virmani R, Chatterjee K, Boyle A, Grossman W, Yeghiazarians Y. Cytokine combination therapy with erythropoietin and granulocyte colony stimulating factor in a porcine model of acute myocardial infarction. Cardiovasc Drugs Ther. 2010 doi: 10.1007/s10557-010-6263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angeli FS, Smith C, Amabile N, Shapiro M, Bartlett L, Virmani R, Chatterjee K, Boyle A, Grossman W, Yeghiazarians Y. Granulocyte colony stimulating factor in myocardial infarction with low ejection fraction. Cytokine. 2010;51:278–285. doi: 10.1016/j.cyto.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 9.Belonje AM, Voors AA, van Gilst WH, Anker SD, Slart RH, Tio RA, Zijlstra F, van Veldhuisen DJ. Effects of erythropoietin after an acute myocardial infarction: rationale and study design of a prospective, randomized, clinical trial (HEBE III) Am Heart J. 2008;155:817–822. doi: 10.1016/j.ahj.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 10.Beohar N, Flaherty JD, Davidson CJ, Vidovich M, Singhal S, Rapp JA, Erdogan A, Lee DC, Rammohan C, Brodsky A, Wu E, Pieper K, Virmani R, Bonow RO, Mehta J. Granulocyte-colony stimulating factor administration after myocardial infarction in a porcine ischemia-reperfusion model: functional and pathological effects of dose timing. Catheter Cardiovasc Interv. 2007;69:257–266. doi: 10.1002/ccd.20925. [DOI] [PubMed] [Google Scholar]
- 11.Bikfalvi A, Han ZC. Angiogenic factors are hematopoietic growth factors and vice versa. Leukemia. 1994;8:523–529. [PubMed] [Google Scholar]
- 12.Binbrek AS, Rao NS, Al Khaja N, Assaqqaf J, Sobel BE. Erythropoietin to augment myocardial salvage induced by coronary thrombolysis in patients with ST segment elevation acute myocardial infarction. Am J Cardiol. 2009;104:1035–1040. doi: 10.1016/j.amjcard.2009.05.050. [DOI] [PubMed] [Google Scholar]
- 13.Bodine DM, Seidel NE, Gale MS, Nienhuis AW, Orlic D. Efficient retrovirus transduction of mouse pluripotent hematopoietic stem cells mobilized into the peripheral blood by treatment with granulocyte colony-stimulating factor and stem cell factor. Blood. 1994;84:1482–1491. [PubMed] [Google Scholar]
- 14.Boengler K, Hilfiker-Kleiner D, Drexler H, Heusch G, Schulz R. The myocardial JAK/STAT pathway: from protection to failure. Pharmacol Ther. 2008;120:172–185. doi: 10.1016/j.pharmthera.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Boneberg EM, Hareng L, Gantner F, Wendel A, Hartung T. Human monocytes express functional receptors for granulocyte colony-stimulating factor that mediate suppression of monokines and interferon-gamma. Blood. 2000;95:270–276. [PubMed] [Google Scholar]
- 16.Broudy VC. Stem cell factor and hematopoiesis. Blood. 1997;90:1345–1364. [PubMed] [Google Scholar]
- 17.Brunner S, Huber BC, Fischer R, Groebner M, Hacker M, David R, Zaruba MM, Vallaster M, Rischpler C, Wilke A, Gerbitz A, Franz WM. G-CSF treatment after myocardial infarction: impact on bone marrow-derived vs cardiac progenitor cells. Exp Hematol. 2008;36:695–702. doi: 10.1016/j.exphem.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Burger D, Lei M, Geoghegan-Morphet N, Lu X, Xenocostas A, Feng Q. Erythropoietin protects cardiomyocytes from apoptosis via up-regulation of endothelial nitric oxide synthase. Cardiovasc Res. 2006;72:51–59. doi: 10.1016/j.cardiores.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 19.Bussolino F, Ziche M, Wang JM, Alessi D, Morbidelli L, Cremona O, Bosia A, Marchisio PC, Mantovani A. In vitro and in vivo activation of endothelial cells by colony-stimulating factors. J Clin Invest. 1991;87:986–995. doi: 10.1172/JCI115107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai Z, Semenza GL. Phosphatidylinositol-3-kinase signaling is required for erythropoietin-mediated acute protection against myocardial ischemia/reperfusion injury. Circulation. 2004;109:2050–2053. doi: 10.1161/01.CIR.0000127954.98131.23. [DOI] [PubMed] [Google Scholar]
- 21.Calvillo L, Latini R, Kajstura J, Leri A, Anversa P, Ghezzi P, Salio M, Cerami A, Brines M. Recombinant human erythropoietin protects the myocardium from ischemia-reperfusion injury and promotes beneficial remodeling. Proc Natl Acad Sci USA. 2003;100:4802–4806. doi: 10.1073/pnas.06304441000630444100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capoccia BJ, Shepherd RM, Link DC. G-CSF and AMD3100 mobilize monocytes into the blood that stimulate angiogenesis in vivo through a paracrine mechanism. Blood. 2006;108:2438–2445. doi: 10.1182/blood-2006-04-013755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng Z, Ou L, Liu Y, Liu X, Li F, Sun B, Che Y, Kong D, Yu Y, Steinhoff G. Granulocyte colony-stimulating factor exacerbates cardiac fibrosis after myocardial infarction in a rat model of permanent occlusion. Cardiovasc Res. 2008;80:425–434. doi: 10.1093/cvr/cvn202. [DOI] [PubMed] [Google Scholar]
- 24.Dawn B, Abdel-Latif A, Sanganalmath SK, Flaherty MP, Zuba-Surma EK. Cardiac repair with adult bone marrow-derived cells: the clinical evidence. Antioxid Redox Signal. 2009;11:1865–1882. doi: 10.1089/ARS.2009.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dawn B, Guo Y, Rezazadeh A, Huang Y, Stein AB, Hunt G, Tiwari S, Varma J, Gu Y, Prabhu SD, Kajstura J, Anversa P, Ildstad ST, Bolli R. Postinfarct cytokine therapy regenerates cardiac tissue and improves left ventricular function. Circ Res. 2006;98:1098–1105. doi: 10.1161/01.RES.0000218454.76784.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Koning JP, Soede-Bobok AA, Ward AC, Schelen AM, Antonissen C, van Leeuwen D, Lowenberg B, Touw IP. STAT3-mediated differentiation and survival and of myeloid cells in response to granulocyte colony-stimulating factor: role for the cyclin-dependent kinase inhibitor p27(Kip1) Oncogene. 2000;19:3290–3298. doi: 10.1038/sj.onc.1203627. [DOI] [PubMed] [Google Scholar]
- 27.Dedhar S, Gaboury L, Galloway P, Eaves C. Human granulocyte-macrophage colony-stimulating factor is a growth factor active on a variety of cell types of nonhemopoietic origin. Proc Natl Acad Sci USA. 1988;85:9253–9257. doi: 10.1073/pnas.85.23.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deindl E, Schaper W. The art of arteriogenesis. Cell Biochem Biophys. 2005;43:1–15. doi: 10.1385/CBB:43:1:001. [DOI] [PubMed] [Google Scholar]
- 29.Deindl E, Zaruba MM, Brunner S, Huber B, Mehl U, Assmann G, Hoefer IE, Mueller-Hoecker J, Franz WM. G-CSF administration after myocardial infarction in mice attenuates late ischemic cardiomyopathy by enhanced arteriogenesis. FASEB J. 2006;20:956–958. doi: 10.1096/fj.05-4763fje. [DOI] [PubMed] [Google Scholar]
- 30.Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor. Blood. 1991;78:2791–2808. [PubMed] [Google Scholar]
- 31.Deten A, Volz HC, Clamors S, Leiblein S, Briest W, Marx G, Zimmer HG. Hematopoietic stem cells do not repair the infarcted mouse heart. Cardiovasc Res. 2005;65:52–63. doi: 10.1016/j.cardiores.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Devereux S, Bull HA, Campos-Costa D, Saib R, Linch DC. Granulocyte macrophage colony stimulating factor induced changes in cellular adhesion molecule expression and adhesion to endothelium: in vitro and in vivo studies in man. Br J Haematol. 1989;71:323–330. doi: 10.1111/j.1365-2141.1989.tb04287.x. [DOI] [PubMed] [Google Scholar]
- 33.Dewald O, Ren G, Duerr GD, Zoerlein M, Klemm C, Gersch C, Tincey S, Michael LH, Entman ML, Frangogiannis NG. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol. 2004;164:665–677. doi: 10.1016/S0002-9440(10)63154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duarte RF, Frank DA. SCF and G-CSF lead to the synergistic induction of proliferation and gene expression through complementary signaling pathways. Blood. 2000;96:3422–3430. [PubMed] [Google Scholar]
- 35.Ellis SG, Penn MS, Bolwell B, Garcia M, Chacko M, Wang T, Brezina KJ, McConnell G, Topol EJ. Granulocyte colony stimulating factor in patients with large acute myocardial infarction: results of a pilot dose-escalation randomized trial. Am Heart J. 2006;152:1051 e1059–1014. doi: 10.1016/j.ahj.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Engelmann MG, Theiss HD, Hennig-Theiss C, Huber A, Win-tersperger BJ, Werle-Ruedinger AE, Schoenberg SO, Steinbeck G, Franz WM. Autologous bone marrow stem cell mobilization induced by granulocyte colony-stimulating factor after subacute ST-segment elevation myocardial infarction undergoing late revascularization: final results from the G-CSF-STEMI (granulocyte colony-stimulating factor ST-segment elevation myocardial infarction) trial. J Am Coll Cardiol. 2006;48:1712–1721. doi: 10.1016/j.jacc.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 37.Engelmann MG, Theiss HD, Theiss C, Henschel V, Huber A, Wintersperger BJ, Schoenberg SO, Steinbeck G, Franz WM. G-CSF in patients suffering from late revascularised ST elevation myocardial infarction: final 1-year-results of the G-CSF-STEMI Trial. Int J Cardiol. 2010;144:399–404. doi: 10.1016/j.ijcard.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 38.Ferrario M, Arbustini E, Massa M, Rosti V, Marziliano N, Raineri C, Campanelli R, Bertoletti A, De Ferrari GM, Klersy C, Angoli L, Bramucci E, Marinoni B, Ferlini M, Moretti E, Raisaro A, Repetto A, Schwartz PJ, Tavazzi L. High-dose erythropoietin in patients with acute myocardial infarction: A pilot, randomised, placebo-controlled study. Int J Cardiol. 2009 doi: 10.1016/j.ijcard.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 39.Fisher JW. Erythropoietin: physiology and pharmacology update. Exp Biol Med (Maywood) 2003;228:1–14. doi: 10.1177/153537020322800101. [DOI] [PubMed] [Google Scholar]
- 40.Fleming WH, Alpern EJ, Uchida N, Ikuta K, Weissman IL. Steel factor influences the distribution and activity of murine hematopoietic stem cells in vivo. Proc Natl Acad Sci USA. 1993;90:3760–3764. doi: 10.1073/pnas.90.8.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frangogiannis NG, Perrard JL, Mendoza LH, Burns AR, Lindsey ML, Ballantyne CM, Michael LH, Smith CW, Entman ML. Stem cell factor induction is associated with mast cell accumulation after canine myocardial ischemia and reperfusion. Circulation. 1998;98:687–698. doi: 10.1161/01.cir.98.7.687. [DOI] [PubMed] [Google Scholar]
- 42.Friedrich EB, Werner C, Walenta K, Bohm M, Scheller B. Role of extracellular signal-regulated kinase for endothelial progenitor cell dysfunction in coronary artery disease. Basic Res Cardiol. 2009;104:613–620. doi: 10.1007/s00395-009-0022-6. [DOI] [PubMed] [Google Scholar]
- 43.Fujita J, Mori M, Kawada H, Ieda Y, Tsuma M, Matsuzaki Y, Kawaguchi H, Yagi T, Yuasa S, Endo J, Hotta T, Ogawa S, Okano H, Yozu R, Ando K, Fukuda K. Administration of granulocyte colony-stimulating factor after myocardial infarction enhances the recruitment of hematopoietic stem cell-derived myofibroblasts and contributes to cardiac repair. Stem Cells. 2007;25:2750–2759. doi: 10.1634/stemcells.2007-0275. [DOI] [PubMed] [Google Scholar]
- 44.Fukuhara S, Tomita S, Nakatani T, Ohtsu Y, Ishida M, Yutani C, Kitamura S. G-CSF promotes bone marrow cells to migrate into infarcted mice heart, and differentiate into cardiomyocytes. Cell Transplant. 2004;13:741–748. doi: 10.3727/000000004783983486. [DOI] [PubMed] [Google Scholar]
- 45.Fukunaga R, Ishizaka-Ikeda E, Pan CX, Seto Y, Nagata S. Functional domains of the granulocyte colony-stimulating factor receptor. EMBO J. 1991;10:2855–2865. doi: 10.1002/j.1460-2075.1991.tb07835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gamble JR, Elliott MJ, Jaipargas E, Lopez AF, Vadas MA. Regulation of human monocyte adherence by granulocyte-macrophage colony-stimulating factor. Proc Natl Acad Sci USA. 1989;86:7169–7173. doi: 10.1073/pnas.86.18.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghaboura N, Tamareille S, Ducluzeau PH, Grimaud L, Loufrani L, Croue A, Tourmen Y, Henrion D, Furber A, Prunier F. Diabetes mellitus abrogates erythropoietin-induced cardioprotection against ischemic-reperfusion injury by alteration of the RISK/GSK-3beta signaling. Basic Res Cardiol. 2011;106:147–162. doi: 10.1007/s00395-010-0130-3. [DOI] [PubMed] [Google Scholar]
- 48.Goldberg N, Lundin AP, Delano B, Friedman EA, Stein RA. Changes in left ventricular size, wall thickness, and function in anemic patients treated with recombinant human erythropoietin. Am Heart J. 1992;124:424–427. doi: 10.1016/0002-8703(92)90608-x. [DOI] [PubMed] [Google Scholar]
- 49.Gorgen I, Hartung T, Leist M, Niehorster M, Tiegs G, Uhlig S, Weitzel F, Wendel A. Granulocyte colony-stimulating factor treatment protects rodents against lipopolysaccharide-induced toxicity via suppression of systemic tumor necrosis factor-alpha. J Immunol. 1992;149:918–924. [PubMed] [Google Scholar]
- 50.Hamamoto M, Tomita S, Nakatani T, Yutani C, Yamashiro S, Sueda T, Yagihara T, Kitamura S. Granulocyte-colony stimulating factor directly enhances proliferation of human troponin I-positive cells derived from idiopathic dilated cardiomyopathy through specific receptors. J Heart Lung Transplant. 2004;23:1430–1437. doi: 10.1016/j.healun.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 51.Harada M, Qin Y, Takano H, Minamino T, Zou Y, Toko H, Ohtsuka M, Matsuura K, Sano M, Nishi J, Iwanaga K, Akazawa H, Kunieda T, Zhu W, Hasegawa H, Kunisada K, Nagai T, Nakaya H, Yamauchi-Takihara K, Komuro I. G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nat Med. 2005;11:305–311. doi: 10.1038/nm1199. [DOI] [PubMed] [Google Scholar]
- 52.Hartung T. Anti-inflammatory effects of granulocyte colony-stimulating factor. Curr Opin Hematol. 1998;5:221–225. doi: 10.1097/00062752-199805000-00013. [DOI] [PubMed] [Google Scholar]
- 53.Hartung T, Docke WD, Gantner F, Krieger G, Sauer A, Stevens P, Volk HD, Wendel A. Effect of granulocyte colony-stimulating factor treatment on ex vivo blood cytokine response in human volunteers. Blood. 1995;85:2482–2489. [PubMed] [Google Scholar]
- 54.Hasegawa H, Takano H, Iwanaga K, Ohtsuka M, Qin Y, Niitsuma Y, Ueda K, Toyoda T, Tadokoro H, Komuro I. Cardioprotective effects of granulocyte colony-stimulating factor in swine with chronic myocardial ischemia. J Am Coll Cardiol. 2006;47:842–849. doi: 10.1016/j.jacc.2005.09.048. [DOI] [PubMed] [Google Scholar]
- 55.Heeschen C, Aicher A, Lehmann R, Fichtlscherer S, Vasa M, Urbich C, Mildner-Rihm C, Martin H, Zeiher AM, Dimmeler S. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood. 2003;102:1340–1346. doi: 10.1182/blood-2003-01-0223. [DOI] [PubMed] [Google Scholar]
- 56.Hess DA, Levac KD, Karanu FN, Rosu-Myles M, White MJ, Gallacher L, Murdoch B, Keeney M, Ottowski P, Foley R, Chin-Yee I, Bhatia M. Functional analysis of human hematopoietic repopulating cells mobilized with granulocyte colony-stimulating factor alone versus granulocyte colony-stimulating factor in combination with stem cell factor. Blood. 2002;100:869–878. doi: 10.1182/blood.v100.3.869. [DOI] [PubMed] [Google Scholar]
- 57.Heusch G, Boengler K, Schulz R. Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation. 2008;118:1915–1919. doi: 10.1161/CIRCULATIONAHA.108.805242. [DOI] [PubMed] [Google Scholar]
- 58.Hill JM, Bartunek J. The end of granulocyte colony-stimulating factor in acute myocardial infarction? Reaping the benefits beyond cytokine mobilization. Circulation. 2006;113:1926–1928. doi: 10.1161/CIRCULATIONAHA.106.623777. [DOI] [PubMed] [Google Scholar]
- 59.Hill JM, Syed MA, Arai AE, Powell TM, Paul JD, Zalos G, Read EJ, Khuu HM, Leitman SF, Horne M, Csako G, Dunbar CE, Waclawiw MA, Cannon RO., 3rd Outcomes and risks of granulocyte colony-stimulating factor in patients with coronary artery disease. J Am Coll Cardiol. 2005;46:1643–1648. doi: 10.1016/j.jacc.2005.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hirata A, Minamino T, Asanuma H, Fujita M, Wakeno M, Myoishi M, Tsukamoto O, Okada K, Koyama H, Komamura K, Takashima S, Shinozaki Y, Mori H, Shiraga M, Kitakaze M, Hori M. Erythropoietin enhances neovascularization of ischemic myocardium and improves left ventricular dysfunction after myocardial infarction in dogs. J Am Coll Cardiol. 2006;48:176–184. doi: 10.1016/j.jacc.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 61.Hockel M, Jung W, Vaupel P, Rabes H, Khaledpour C, Wissler JH. Purified monocyte-derived angiogenic substance (angiotropin) induces controlled angiogenesis associated with regulated tissue proliferation in rabbit skin. J Clin Invest. 1988;82:1075–1090. doi: 10.1172/JCI113664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Honold J, Lehmann R, Heeschen C, Walter DH, Assmus B, Sasaki K, Martin H, Haendeler J, Zeiher AM, Dimmeler S. Effects of granulocyte colony simulating factor on functional activities of endothelial progenitor cells in patients with chronic ischemic heart disease. Arterioscler Thromb Vasc Biol. 2006;26:2238–2243. doi: 10.1161/01.ATV.0000240248.55172.dd. [DOI] [PubMed] [Google Scholar]
- 63.Hossmann KA, Buschmann IR. Granulocyte-macrophage colony-stimulating factor as an arteriogenic factor in the treatment of ischaemic stroke. Expert Opin Biol Ther. 2005;5:1547–1556. doi: 10.1517/14712598.5.12.1547. [DOI] [PubMed] [Google Scholar]
- 64.Hou XW, Jiang Y, Wang LF, Xu HY, Lin HM, He XY, He JJ, Zhang S. Protective role of granulocyte colony-stimulating factor against adriamycin induced cardiac, renal and hepatic toxicities. Toxicol Lett. 2009;187:40–44. doi: 10.1016/j.toxlet.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 65.Hou XW, Son J, Wang Y, Ru YX, Lian Q, Majiti W, Amazouzi A, Zhou YL, Wang PX, Han ZC. Granulocyte colony-stimulating factor reduces cardiomyocyte apoptosis and improves cardiac function in adriamycin-induced cardiomyopathy in rats. Cardiovasc Drugs Ther. 2006;20:85–91. doi: 10.1007/s10557-006-7652-9. [DOI] [PubMed] [Google Scholar]
- 66.Hristov M, Weber C. The therapeutic potential of progenitor cells in ischemic heart disease—past, present and future. Basic Res Cardiol. 2006;101:1–7. doi: 10.1007/s00395-005-0573-0. [DOI] [PubMed] [Google Scholar]
- 67.Huttmann A, Duhrsen U, Stypmann J, Noppeney R, Nuckel H, Neumann T, Gutersohn A, Nikol S, Erbel R. Granulocyte colony-stimulating factor-induced blood stem cell mobilisation in patients with chronic heart failure—feasibility, safety and effects on exercise tolerance and cardiac function. Basic Res Cardiol. 2006;101:78–86. doi: 10.1007/s00395-005-0556-1. [DOI] [PubMed] [Google Scholar]
- 68.Ieishi K, Nomura M, Kawano T, Fujimoto S, Ikefuji H, Noda Y, Nishikado A, Ito S. The effect of G-CSF in a myocardial ischemia reperfusion model rat. J Med Invest. 2007;54:177–183. doi: 10.2152/jmi.54.177. [DOI] [PubMed] [Google Scholar]
- 69.Iemura A, Tsai M, Ando A, Wershil BK, Galli SJ. The c-kit ligand, stem cell factor, promotes mast cell survival by suppressing apoptosis. Am J Pathol. 1994;144:321–328. [PMC free article] [PubMed] [Google Scholar]
- 70.Ince H, Petzsch M, Kleine HD, Eckard H, Rehders T, Burska D, Kische S, Freund M, Nienaber CA. Prevention of left ventricular remodeling with granulocyte colony-stimulating factor after acute myocardial infarction: final 1-year results of the Front-Integrated Revascularization and Stem Cell Liberation in Evolving Acute Myocardial Infarction by Granulocyte Colony-Stimulating Factor (FIRSTLINE-AMI) Trial. Circulation. 2005;112:I73–I80. doi: 10.1161/CIRCULATIONAHA.104.524827. [DOI] [PubMed] [Google Scholar]
- 71.Ince H, Petzsch M, Kleine HD, Schmidt H, Rehders T, Korber T, Schumichen C, Freund M, Nienaber CA. Preservation from left ventricular remodeling by front-integrated revascularization and stem cell liberation in evolving acute myocardial infarction by use of granulocyte-colony-stimulating factor (FIRSTLINE-AMI) Circulation. 2005;112:3097–3106. doi: 10.1161/CIRCULATIONAHA.105.541433. [DOI] [PubMed] [Google Scholar]
- 72.Ince H, Valgimigli M, Petzsch M, de Lezo JS, Kuethe F, Dunkelmann S, Biondi-Zoccai G, Nienaber CA. Cardiovascular events and restenosis following administration of G-CSF in acute myocardial infarction: systematic review and meta-analysis. Heart. 2008;94:610–616. doi: 10.1136/hrt.2006.111385. [DOI] [PubMed] [Google Scholar]
- 73.Iwanaga K, Takano H, Ohtsuka M, Hasegawa H, Zou Y, Qin Y, Odaka K, Hiroshima K, Tadokoro H, Komuro I. Effects of G-CSF on cardiac remodeling after acute myocardial infarction in swine. Biochem Biophys Res Commun. 2004;325:1353–1359. doi: 10.1016/j.bbrc.2004.10.149. [DOI] [PubMed] [Google Scholar]
- 74.Jelkmann W, Wagner K. Beneficial and ominous aspects of the pleiotropic action of erythropoietin. Ann Hematol. 2004;83:673–686. doi: 10.1007/s00277-004-0911-6. [DOI] [PubMed] [Google Scholar]
- 75.Kanellakis P, Slater NJ, Du XJ, Bobik A, Curtis DJ. Granulocyte colony-stimulating factor and stem cell factor improve endogenous repair after myocardial infarction. Cardiovasc Res. 2006;70:117–125. doi: 10.1016/j.cardiores.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 76.Kang HJ, Kim HS, Zhang SY, Park KW, Cho HJ, Koo BK, Kim YJ, Soo Lee D, Sohn DW, Han KS, Oh BH, Lee MM, Park YB. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomised clinical trial. Lancet. 2004;363:751–756. doi: 10.1016/S0140-6736(04)15689-4. [DOI] [PubMed] [Google Scholar]
- 77.Kastrup J. Therapeutic angiogenesis in ischemic heart disease: gene or recombinant vascular growth factor protein therapy? Curr Gene Ther. 2003;3:197–206. doi: 10.2174/1566523034578366. [DOI] [PubMed] [Google Scholar]
- 78.Kawada H, Fujita J, Kinjo K, Matsuzaki Y, Tsuma M, Miyatake H, Muguruma Y, Tsuboi K, Itabashi Y, Ikeda Y, Ogawa S, Okano H, Hotta T, Ando K, Fukuda K. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood. 2004;104:3581–3587. doi: 10.1182/blood-2004-04-1488. [DOI] [PubMed] [Google Scholar]
- 79.Kawamoto A, Murayama T, Kusano K, Ii M, Tkebuchava T, Shintani S, Iwakura A, Johnson I, von Samson P, Hanley A, Gavin M, Curry C, Silver M, Ma H, Kearney M, Losordo DW. Synergistic effect of bone marrow mobilization and vascular endothelial growth factor-2 gene therapy in myocardial ischemia. Circulation. 2004;110:1398–1405. doi: 10.1161/01.CIR.0000141563.71410.64. [DOI] [PubMed] [Google Scholar]
- 80.Kirshenbaum AS, Goff JP, Kessler SW, Mican JM, Zsebo KM, Metcalfe DD. Effect of IL-3 and stem cell factor on the appearance of human basophils and mast cells from CD34+ pluripotent progenitor cells. J Immunol. 1992;148:772–777. [PubMed] [Google Scholar]
- 81.Kitamura Y, Yokoyama M, Matsuda H, Ohno T, Mori KJ. Spleen colony-forming cell as common precursor for tissue mast cells and granulocytes. Nature. 1981;291:159–160. doi: 10.1038/291159a0. [DOI] [PubMed] [Google Scholar]
- 82.Kleinbongard P, Heusch G, Schulz R. TNFalpha in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol Ther. 2010;127:295–314. doi: 10.1016/j.pharmthera.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 83.Knoops S, Groeneveld AB, Kamp O, Lagrand WK, Hoekman K. Granulocyte-macrophage colony-stimulating factor (GM-CSF) decreases left ventricular function. An echocardiographic study in cancer patients. Cytokine. 2001;14:184–187. doi: 10.1006/cyto.2001.0869. [DOI] [PubMed] [Google Scholar]
- 84.Kronenwett R, Martin S, Haas R. The role of cytokines and adhesion molecules for mobilization of peripheral blood stem cells. Stem Cells. 2000;18:320–330. doi: 10.1634/stemcells.18-5-320. [DOI] [PubMed] [Google Scholar]
- 85.Kuang D, Zhao X, Xiao G, Ni J, Feng Y, Wu R, Wang G. Stem cell factor/c-kit signaling mediated cardiac stem cell migration via activation of p38 MAPK. Basic Res Cardiol. 2008;103:265–273. doi: 10.1007/s00395-007-0690-z. [DOI] [PubMed] [Google Scholar]
- 86.Kuethe F, Figulla HR, Herzau M, Voth M, Fritzenwanger M, Opfermann T, Pachmann K, Krack A, Sayer HG, Gottschild D, Werner GS. Treatment with granulocyte colony-stimulating factor for mobilization of bone marrow cells in patients with acute myocardial infarction. Am Heart J. 2005;150:115. doi: 10.1016/j.ahj.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 87.Kuhlmann MT, Kirchhof P, Klocke R, Hasib L, Stypmann J, Fabritz L, Stelljes M, Tian W, Zwiener M, Mueller M, Kienast J, Breithardt G, Nikol S. G-CSF/SCF reduces inducible arrhythmias in the infarcted heart potentially via increased connexin43 expression and arteriogenesis. J Exp Med. 2006;203:87–97. doi: 10.1084/jem.20051151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lehrke S, Mazhari R, Durand DJ, Zheng M, Bedja D, Zimmet JM, Schuleri KH, Chi AS, Gabrielson KL, Hare JM. Aging impairs the beneficial effect of granulocyte colony-stimulating factor and stem cell factor on post-myocardial infarction remodeling. Circ Res. 2006;99:553–560. doi: 10.1161/01.RES.0000238375.88582.d8. [DOI] [PubMed] [Google Scholar]
- 89.Leone AM, Galiuto L, Garramone B, Rutella S, Giannico MB, Brugaletta S, Perfetti M, Liuzzo G, Porto I, Burzotta F, Niccoli G, Biasucci LM, Leone G, Rebuzzi AG, Crea F. Usefulness of granulocyte colony-stimulating factor in patients with a large anterior wall acute myocardial infarction to prevent left ventricular remodeling (the rigenera study) Am J Cardiol. 2007;100:397–403. doi: 10.1016/j.amjcard.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 90.Leone AM, Rutella S, Bonanno G, Contemi AM, de Ritis DG, Giannico MB, Rebuzzi AG, Leone G, Crea F. Endogenous G-CSF and CD34+ cell mobilization after acute myocardial infarction. Int J Cardiol. 2006;111:202–208. doi: 10.1016/j.ijcard.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 91.Li L, Takemura G, Li Y, Miyata S, Esaki M, Okada H, Kanamori H, Ogino A, Maruyama R, Nakagawa M, Minatoguchi S, Fujiwara T, Fujiwara H. Granulocyte colony-stimulating factor improves left ventricular function of doxorubicin-induced cardiomyopathy. Lab Invest. 2007;87:440–455. doi: 10.1038/labinvest.3700530. [DOI] [PubMed] [Google Scholar]
- 92.Li Y, Fukuda N, Yokoyama S, Kusumi Y, Hagikura K, Kawano T, Takayama T, Matsumoto T, Satomi A, Honye J, Mugishima H, Mitsumata M, Saito S. Effects of G-CSF on cardiac remodeling and arterial hyperplasia in rats. Eur J Pharmacol. 2006;549:98–106. doi: 10.1016/j.ejphar.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 93.Li Y, Takemura G, Okada H, Miyata S, Esaki M, Maruyama R, Kanamori H, Li L, Ogino A, Misao Y, Khai NC, Mikami A, Minatoguchi S, Fujiwara T, Fujiwara H. Treatment with granulocyte colony-stimulating factor ameliorates chronic heart failure. Lab Invest. 2006;86:32–44. doi: 10.1038/labinvest.3700367. [DOI] [PubMed] [Google Scholar]
- 94.Liem A, van de Woestijne AP, Bruijns E, Roeters van Lennep HW, de Boo JA, van Halteren HK, van Es TP, Jukema JW, van der Laarse A, Zwinderman AH, van Veldhuisen DJ. Effect of EPO administration on myocardial infarct size in patients with non-STE acute coronary syndromes; results from a pilot study. Int J Cardiol. 2009;131:285–287. doi: 10.1016/j.ijcard.2007.07.076. [DOI] [PubMed] [Google Scholar]
- 95.Lipsic E, van der Meer P, Voors AA, Westenbrink BD, van den Heuvel AF, de Boer HC, van Zonneveld AJ, Schoemaker RG, van Gilst WH, Zijlstra F, van Veldhuisen DJ. A single bolus of a long-acting erythropoietin analogue darbepoetin alfa in patients with acute myocardial infarction: a randomized feasibility and safety study. Cardiovasc Drugs Ther. 2006;20:135–141. doi: 10.1007/s10557-006-7680-5. [DOI] [PubMed] [Google Scholar]
- 96.Louzada RA, Oliveira PF, Cavalcanti-de-Albuquerque JP, Cunha-Carvalho L, Baldanza MR, Kasai-Brunswick TH, Goldenberg RC, Campos-de-Carvalho AC, Werneck-de-Castro JP. Granulocyte-colony stimulating factor treatment of chronic myocardial infarction. Cardiovasc Drugs Ther. 2010;24:121–130. doi: 10.1007/s10557-010-6215-2. [DOI] [PubMed] [Google Scholar]
- 97.Lu L, Walker D, Graham CD, Waheed A, Shadduck RK, Broxmeyer HE. Enhancement of release from MHC class II antigen-positive monocytes of hematopoietic colony stimulating factors CSF-1 and G-CSF by recombinant human tumor necrosis factor-alpha: synergism with recombinant human interferon-gamma. Blood. 1988;72:34–41. [PubMed] [Google Scholar]
- 98.Lyman SD, James L, Vanden Bos T, de Vries P, Brasel K, Gliniak B, Hollingsworth LT, Picha KS, McKenna HJ, Splett RR, et al. Molecular cloning of a ligand for the flt3/flk-2 tyrosine kinase receptor: a proliferative factor for primitive hematopoietic cells. Cell. 1993;75:1157–1167. doi: 10.1016/0092-8674(93)90325-k. [DOI] [PubMed] [Google Scholar]
- 99.Ma J, Ge J, Zhang S, Sun A, Shen J, Chen L, Wang K, Zou Y. Time course of myocardial stromal cell-derived factor 1 expression and beneficial effects of intravenously administered bone marrow stem cells in rats with experimental myocardial infarction. Basic Res Cardiol. 2005;100:217–223. doi: 10.1007/s00395-005-0521-z. [DOI] [PubMed] [Google Scholar]
- 100.Madonna R, Rokosh G, De Caterina R, Bolli R. Hepatocyte growth factor/Met gene transfer in cardiac stem cells—potential for cardiac repair. Basic Res Cardiol. 2010;105:443–452. doi: 10.1007/s00395-010-0102-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Maekawa Y, Anzai T, Yoshikawa T, Asakura Y, Takahashi T, Ishikawa S, Mitamura H, Ogawa S. Prognostic significance of peripheral monocytosis after reperfused acute myocardial infarction: a possible role for left ventricular remodeling. J Am Coll Cardiol. 2002;39:241–246. doi: 10.1016/s0735-1097(01)01721-1. [DOI] [PubMed] [Google Scholar]
- 102.Maekawa Y, Anzai T, Yoshikawa T, Sugano Y, Mahara K, Kohno T, Takahashi T, Ogawa S. Effect of granulocyte-macrophage colony-stimulating factor inducer on left ventricular remodeling after acute myocardial infarction. J Am Coll Cardiol. 2004;44:1510–1520. doi: 10.1016/j.jacc.2004.05.083. [DOI] [PubMed] [Google Scholar]
- 103.Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005;293:90–95. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Malipiero UV, Frei K, Fontana A. Production of hemopoietic colony-stimulating factors by astrocytes. J Immunol. 1990;144:3816–3821. [PubMed] [Google Scholar]
- 105.McLemore ML, Grewal S, Liu F, Archambault A, Poursine-Laurent J, Haug J, Link DC. STAT-3 activation is required for normal G-CSF-dependent proliferation and granulocytic differentiation. Immunity. 2001;14:193–204. doi: 10.1016/s1074-7613(01)00101-7. pii:S1074-7613(01)00101-7. [DOI] [PubMed] [Google Scholar]
- 106.Meininger CJ, Yano H, Rottapel R, Bernstein A, Zsebo KM, Zetter BR. The c-kit receptor ligand functions as a mast cell chemoattractant. Blood. 1992;79:958–963. [PubMed] [Google Scholar]
- 107.Metcalf D. Hematopoietic cytokines. Blood. 2008;111:485–491. doi: 10.1182/blood-2007-03-079681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Minatoguchi S, Takemura G, Chen XH, Wang N, Uno Y, Koda M, Arai M, Misao Y, Lu C, Suzuki K, Goto K, Komada A, Takahashi T, Kosai K, Fujiwara T, Fujiwara H. Acceleration of the healing process and myocardial regeneration may be important as a mechanism of improvement of cardiac function and remodeling by postinfarction granulocyte colony-stimulating factor treatment. Circulation. 2004;109:2572–2580. doi: 10.1161/01.CIR.0000129770.93985.3E. [DOI] [PubMed] [Google Scholar]
- 109.Misao Y, Takemura G, Arai M, Ohno T, Onogi H, Takahashi T, Minatoguchi S, Fujiwara T, Fujiwara H. Importance of recruitment of bone marrow-derived CXCR4+ cells in post-infarct cardiac repair mediated by G-CSF. Cardiovasc Res. 2006;71:455–465. doi: 10.1016/j.cardiores.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 110.Mitsui H, Furitsu T, Dvorak AM, Irani AM, Schwartz LB, Inagaki N, Takei M, Ishizaka K, Zsebo KM, Gillis S, et al. Development of human mast cells from umbilical cord blood cells by recombinant human and murine c-kit ligand. Proc Natl Acad Sci USA. 1993;90:735–739. doi: 10.1073/pnas.90.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Miyata S, Takemura G, Kawase Y, Li Y, Okada H, Maruyama R, Ushikoshi H, Esaki M, Kanamori H, Li L, Misao Y, Tezuka A, Toyo-Oka T, Minatoguchi S, Fujiwara T, Fujiwara H. Autophagic cardiomyocyte death in cardiomyopathic hamsters and its prevention by granulocyte colony-stimulating factor. Am J Pathol. 2006;168:386–397. doi: 10.2353/ajpath.2006.050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moon C, Krawczyk M, Ahn D, Ahmet I, Paik D, Lakatta EG, Talan MI. Erythropoietin reduces myocardial infarction and left ventricular functional decline after coronary artery ligation in rats. Proc Natl Acad Sci USA. 2003;100:11612–11617. doi: 10.1073/pnas.1930406100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Naito K, Anzai T, Sugano Y, Maekawa Y, Kohno T, Yoshikawa T, Matsuno K, Ogawa S. Differential effects of GM-CSF and G-CSF on infiltration of dendritic cells during early left ventricular remodeling after myocardial infarction. J Immunol. 2008;181:5691–5701. doi: 10.4049/jimmunol.181.8.5691. pii:181/8/5691. [DOI] [PubMed] [Google Scholar]
- 114.Neipp M, Zorina T, Domenick MA, Exner BG, Ildstad ST. Effect of FLT3 ligand and granulocyte colony-stimulating factor on expansion and mobilization of facilitating cells and hematopoietic stem cells in mice: kinetics and repopulating potential. Blood. 1998;92:3177–3188. [PubMed] [Google Scholar]
- 115.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94:1543–1553. doi: 10.1161/01.RES.0000130526.20854. [DOI] [PubMed] [Google Scholar]
- 116.Norol F, Merlet P, Isnard R, Sebillon P, Bonnet N, Cailliot C, Carrion C, Ribeiro M, Charlotte F, Pradeau P, Mayol JF, Peinnequin A, Drouet M, Safsafi K, Vernant JP, Herodin F. Influence of mobilized stem cells on myocardial infarct repair in a nonhuman primate model. Blood. 2003;102:4361–4368. doi: 10.1182/blood-2003-03-0685. [DOI] [PubMed] [Google Scholar]
- 117.Ohki Y, Heissig B, Sato Y, Akiyama H, Zhu Z, Hicklin DJ, Shimada K, Ogawa H, Daida H, Hattori K, Ohsaka A. Granulocyte colony-stimulating factor promotes neovascularization by releasing vascular endothelial growth factor from neutrophils. FASEB J. 2005;19:2005–2007. doi: 10.1096/fj.04-3496fje. [DOI] [PubMed] [Google Scholar]
- 118.Ohtsuka M, Takano H, Zou Y, Toko H, Akazawa H, Qin Y, Suzuki M, Hasegawa H, Nakaya H, Komuro I. Cytokine therapy prevents left ventricular remodeling and dysfunction after myocardial infarction through neovascularization. FASEB J. 2004;18:851–853. doi: 10.1096/fj.03-0637fje. [DOI] [PubMed] [Google Scholar]
- 119.Okayama Y, Kawakami T. Development, migration, and survival of mast cells. Immunol Res. 2006;34:97–115. doi: 10.1385/IR:34:2:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 121.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ozawa T, Toba K, Suzuki H, Kato K, Iso Y, Akutsu Y, Kobayashi Y, Takeyama Y, Kobayashi N, Yoshimura N, Akazawa K, Aizawa Y. Single-dose intravenous administration of recombinant human erythropoietin is a promising treatment for patients with acute myocardial infarction—randomized controlled pilot trial of EPO/AMI-1 study. Circ J. 2010;74:1415–1423. doi: 10.1253/circj.cj-10-0109. pii:JST.JSTAGE/circj/CJ-10-0109. [DOI] [PubMed] [Google Scholar]
- 123.Pajkrt D, Manten A, van der Poll T, Tielvan Buul MM, Jansen J, Wouter ten Cate J, van Deventer SJ. Modulation of cytokine release and neutrophil function by granulocyte colony-stimulating factor during endotoxemia in humans. Blood. 1997;90:1415–1424. [PubMed] [Google Scholar]
- 124.Pan L, Delmonte J, Jr, Jalonen CK, Ferrara JL. Pretreatment of donor mice with granulocyte colony-stimulating factor polarizes donor T lymphocytes toward type-2 cytokine production and reduces severity of experimental graft-versus-host disease. Blood. 1995;86:4422–4429. [PubMed] [Google Scholar]
- 125.Parissis JT, Adamopoulos S, Venetsanou K, Kostakis G, Rigas A, Karas SM, Kremastinos D. Plasma profiles of circulating granulocyte-macrophage colony-stimulating factor and soluble cellular adhesion molecules in acute myocardial infarction. Contribution to post-infarction left ventricular dysfunction. Eur Cytokine Netw. 2004;15:139–144. [PubMed] [Google Scholar]
- 126.Parissis JT, Adamopoulos S, Venetsanou KF, Mentzikof DG, Karas SM, Kremastinos DT. Clinical and neurohormonal correlates of circulating granulocyte-macrophage colony-stimulating factor in severe heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2000;86:707–710. A709–A710. doi: 10.1016/s0002-9149(00)01062-6. [DOI] [PubMed] [Google Scholar]
- 127.Parsa CJ, Matsumoto A, Kim J, Riel RU, Pascal LS, Walton GB, Thompson RB, Petrofski JA, Annex BH, Stamler JS, Koch WJ. A novel protective effect of erythropoietin in the infarcted heart. J Clin Invest. 2003;112:999–1007. doi: 10.1172/JCI18200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pimentel E. Colony-stimulating factors. Ann Clin Lab Sci. 1990;20:36–55. [PubMed] [Google Scholar]
- 129.Prunier F, Pfister O, Hadri L, Liang L, Del Monte F, Liao R, Hajjar RJ. Delayed erythropoietin therapy reduces post-MI cardiac remodeling only at a dose that mobilizes endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2007;292:H522–H529. doi: 10.1152/ajpheart.00357.2006. [DOI] [PubMed] [Google Scholar]
- 130.Quinn CM, Dawn B. G-CSF and erythropoietin combination therapy for infarct repair: two plus two equals two? Editorial to: “Cytokine combination therapy with erythropoietin and granulocyte colony stimulating factor in a porcine model of acute myocardial infarction” by F.S. Angeli et al. Cardiovasc Drugs Ther. 2010 doi: 10.1007/s10557-010-6268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ratajczak J, Majka M, Kijowski J, Baj M, Pan ZK, Marquez LA, Janowska-Wieczorek A, Ratajczak MZ. Biological significance of MAPK, AKT and JAK-STAT protein activation by various erythropoietic factors in normal human early erythroid cells. Br J Haematol. 2001;115:195–204. doi: 10.1046/j.1365-2141.2001.03058.x. [DOI] [PubMed] [Google Scholar]
- 132.Ribatti D, Presta M, Vacca A, Ria R, Giuliani R, Dell’Era P, Nico B, Roncali L, Dammacco F. Human erythropoietin induces a pro-angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Blood. 1999;93:2627–2636. [PubMed] [Google Scholar]
- 133.Ripa RS, Jorgensen E, Wang Y, Thune JJ, Nilsson JC, Sondergaard L, Johnsen HE, Kober L, Grande P, Kastrup J. Stem cell mobilization induced by subcutaneous granulocyte-colony stimulating factor to improve cardiac regeneration after acute ST-elevation myocardial infarction: result of the double-blind, randomized, placebo-controlled stem cells in myocardial infarction (STEMMI) trial. Circulation. 2006;113:1983–1992. doi: 10.1161/CIRCULATIONAHA.105.610469. [DOI] [PubMed] [Google Scholar]
- 134.Ripa RS, Wang Y, Jorgensen E, Johnsen HE, Hesse B, Kastrup J. Intramyocardial injection of vascular endothelial growth factor-A165 plasmid followed by granulocyte-colony stimulating factor to induce angiogenesis in patients with severe chronic ischaemic heart disease. Eur Heart J. 2006;27:1785–1792. doi: 10.1093/eurheartj/ehl117. [DOI] [PubMed] [Google Scholar]
- 135.Sanganalmath SK, Stein AB, Guo Y, Tiwari S, Hunt G, Vincent RJ, Huang Y, Rezazadeh A, Ildstad ST, Dawn B, Bolli R. The beneficial effects of postinfarct cytokine combination therapy are sustained during long-term follow-up. J Mol Cell Cardiol. 2009;47:528–535. doi: 10.1016/j.yjmcc.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Seeger FH, Sedding D, Langheinrich AC, Haendeler J, Zeiher AM, Dimmeler S. Inhibition of the p38 MAP kinase in vivo improves number and functional activity of vasculogenic cells and reduces atherosclerotic disease progression. Basic Res Cardiol. 2010;105:389–397. doi: 10.1007/s00395-009-0072-9. [DOI] [PubMed] [Google Scholar]
- 137.Seiler C, Pohl T, Wustmann K, Hutter D, Nicolet PA, Windecker S, Eberli FR, Meier B. Promotion of collateral growth by granulocyte-macrophage colony-stimulating factor in patients with coronary artery disease: a randomized, double-blind, placebo-controlled study. Circulation. 2001;104:2012–2017. doi: 10.1161/hc4201.097835. [DOI] [PubMed] [Google Scholar]
- 138.Sesti C, Hale SL, Lutzko C, Kloner RA. Granulocyte colony-stimulating factor and stem cell factor improve contractile reserve of the infarcted left ventricle independent of restoring muscle mass. J Am Coll Cardiol. 2005;46:1662–1669. doi: 10.1016/j.jacc.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 139.Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K, Shimada T, Oike Y, Imaizumi T. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776–2779. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- 140.Skyschally A, van Caster P, Boengler K, Gres P, Musiolik J, Schilawa D, Schulz R, Heusch G. Ischemic postconditioning in pigs: no causal role for RISK activation. Circ Res. 2009;104:15–18. doi: 10.1161/CIRCRESAHA.108.186429. [DOI] [PubMed] [Google Scholar]
- 141.Soldi R, Primo L, Brizzi MF, Sanavio F, Aglietta M, Polentarutti N, Pegoraro L, Mantovani A, Bussolino F. Activation of JAK2 in human vascular endothelial cells by granulocyte-macrophage colony-stimulating factor. Blood. 1997;89:863–872. [PubMed] [Google Scholar]
- 142.Suarez de Lezo J, Torres A, Herrera I, Pan M, Romero M, Pavlovic D, Segura J, Ojeda S, Sanchez J, Lopez Rubio F, Medina A. Effects of stem-cell mobilization with recombinant human granulocyte colony stimulating factor in patients with percutaneously revascularized acute anterior myocardial infarction. Rev Esp Cardiol. 2005;58:253–261. [PubMed] [Google Scholar]
- 143.Sudo Y, Shimazaki C, Ashihara E, Kikuta T, Hirai H, Sumikuma T, Yamagata N, Goto H, Inaba T, Fujita N, Nakagawa M. Synergistic effect of FLT-3 ligand on the granulocyte colony-stimulating factor-induced mobilization of hematopoietic stem cells and progenitor cells into blood in mice. Blood. 1997;89:3186–3191. [PubMed] [Google Scholar]
- 144.Sugano Y, Anzai T, Yoshikawa T, Maekawa Y, Kohno T, Mahara K, Naito K, Ogawa S. Granulocyte colony-stimulating factor attenuates early ventricular expansion after experimental myocardial infarction. Cardiovasc Res. 2005;65:446–456. doi: 10.1016/j.cardiores.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 145.Suzuki K, Nagashima K, Arai M, Uno Y, Misao Y, Takemura G, Nishigaki K, Minatoguchi S, Watanabe S, Tei C, Fujiwara H. Effect of granulocyte colony-stimulating factor treatment at a low dose but for a long duration in patients with coronary heart disease. Circ J. 2006;70:430–437. doi: 10.1253/circj.70.430. [DOI] [PubMed] [Google Scholar]
- 146.Takahama H, Minamino T, Hirata A, Ogai A, Asanuma H, Fujita M, Wakeno M, Tsukamoto O, Okada K, Komamura K, Takashima S, Shinozaki Y, Mori H, Mochizuki N, Kitakaze M. Granulocyte colony-stimulating factor mediates cardio-protection against ischemia/reperfusion injury via phosphatidy-linositol-3-kinase/Akt pathway in canine hearts. Cardiovasc Drugs Ther. 2006;20:159–165. doi: 10.1007/s10557-006-8285-8. [DOI] [PubMed] [Google Scholar]
- 147.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 148.Takano H, Hasegawa H, Kuwabara Y, Nakayama T, Matsuno K, Miyazaki Y, Yamamoto M, Fujimoto Y, Okada H, Okubo S, Fujita M, Shindo S, Kobayashi Y, Komiyama N, Takekoshi N, Imai K, Himi T, Ishibashi I, Komuro I. Feasibility and safety of granulocyte colony-stimulating factor treatment in patients with acute myocardial infarction. Int J Cardiol. 2007;122:41–47. doi: 10.1016/j.ijcard.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 149.Taniguchi N, Nakamura T, Sawada T, Matsubara K, Furukawa K, Hadase M, Nakahara Y, Matsubara H. Erythropoietin prevention trial of coronary restenosis and cardiac remodeling after ST-elevated acute myocardial infarction (EPOC-AMI): a pilot, randomized, placebo-controlled study. Circ J. 2010;74:2365–2371. doi: 10.1253/circj.cj-10-0267. pii:JST.JSTAGE/circj/CJ-10-0267. [DOI] [PubMed] [Google Scholar]
- 150.Terrovitis J, Charitos C, Dolou P, Papalois A, Eleftheriou A, Tsolakis E, Charitos E, Mponios M, Karanastasis G, Koudoumas D, Agapitos E, Nanas JN. No effect of stem cell mobilization with GM-CSF on infarct size and left ventricular function in experimental acute myocardial infarction. Basic Res Cardiol. 2004;99:241–246. doi: 10.1007/s00395-004-0473-8. [DOI] [PubMed] [Google Scholar]
- 151.Theiss HD, Brenner C, Engelmann MG, Zaruba MM, Huber B, Henschel V, Mansmann U, Wintersperger B, Reiser M, Steinbeck G, Franz WM. Safety and efficacy of SITAgliptin plus GRanulocyte-colony-stimulating factor in patients suffering from Acute Myocardial Infarction (SITAGRAMI-Trial)—rationale, design and first interim analysis. Int J Cardiol. 2010;145:282–284. doi: 10.1016/j.ijcard.2009.09.555. [DOI] [PubMed] [Google Scholar]
- 152.Toma C, Letts DP, Tanabe M, Gorcsan J, 3rd, Counihan PJ. Positive effect of darbepoetin on peri-infarction remodeling in a porcine model of myocardial ischemia-reperfusion. J Mol Cell Cardiol. 2007;43:130–136. doi: 10.1016/j.yjmcc.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 153.Tomita S, Ishida M, Nakatani T, Fukuhara S, Hisashi Y, Ohtsu Y, Suga M, Yutani C, Yagihara T, Yamada K, Kitamura S. Bone marrow is a source of regenerated cardiomyocytes in doxorubicin-induced cardiomyopathy and granulocyte colony-stimulating factor enhances migration of bone marrow cells and attenuates cardiotoxicity of doxorubicin under electron microscopy. J Heart Lung Transplant. 2004;23:577–584. doi: 10.1016/j.healun.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 154.Tossidou I, Dangers M, Koch A, Brandt DT, Schiffer M, Kardinal C. Tyrosine phosphatase SHP-2 is a regulator of p27(Kip1) tyrosine phosphorylation. Cell Cycle. 2008;7:3858–3868. doi: 10.4161/cc.7.24.7260. pii:7260. [DOI] [PubMed] [Google Scholar]
- 155.Tramontano AF, Muniyappa R, Black AD, Blendea MC, Cohen I, Deng L, Sowers JR, Cutaia MV, El-Sherif N. Erythropoietin protects cardiac myocytes from hypoxia-induced apoptosis through an Akt-dependent pathway. Biochem Biophys Res Commun. 2003;308:990–994. doi: 10.1016/s0006-291x(03)01503-1. pii:S0006291X03015031. [DOI] [PubMed] [Google Scholar]
- 156.Tsai M, Takeishi T, Thompson H, Langley KE, Zsebo KM, Metcalfe DD, Geissler EN, Galli SJ. Induction of mast cell proliferation, maturation, and heparin synthesis by the rat c-kit ligand, stem cell factor. Proc Natl Acad Sci USA. 1991;88:6382–6386. doi: 10.1073/pnas.88.14.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tsuzuki M. Bone marrow-derived cells are not involved in reendothelialized endothelium as endothelial cells after simple endothelial denudation in mice. Basic Res Cardiol. 2009;104:601–611. doi: 10.1007/s00395-009-0021-7. [DOI] [PubMed] [Google Scholar]
- 158.Ueda K, Takano H, Hasegawa H, Niitsuma Y, Qin Y, Ohtsuka M, Komuro I. Granulocyte colony stimulating factor directly inhibits myocardial ischemia-reperfusion injury through Akt-endothelial NO synthase pathway. Arterioscler Thromb Vasc Biol. 2006;26:e108–e113. doi: 10.1161/01.ATV.0000219697.99134.10. [DOI] [PubMed] [Google Scholar]
- 159.Valent P, Spanblochl E, Sperr WR, Sillaber C, Zsebo KM, Agis H, Strobl H, Geissler K, Bettelheim P, Lechner K. Induction of differentiation of human mast cells from bone marrow and peripheral blood mononuclear cells by recombinant human stem cell factor/kit-ligand in long-term culture. Blood. 1992;80:2237–2245. [PubMed] [Google Scholar]
- 160.Valgimigli M, Rigolin GM, Cittanti C, Malagutti P, Curello S, Percoco G, Bugli AM, Della Porta M, Bragotti LZ, Ansani L, Mauro E, Lanfranchi A, Giganti M, Feggi L, Castoldi G, Ferrari R. Use of granulocyte-colony stimulating factor during acute myocardial infarction to enhance bone marrow stem cell mobilization in humans: clinical and angiographic safety profile. Eur Heart J. 2005;26:1838–1845. doi: 10.1093/eurheartj/ehi289. [DOI] [PubMed] [Google Scholar]
- 161.van der Meer P, Lipsic E, Henning RH, Boddeus K, van der Velden J, Voors AA, van Veldhuisen DJ, van Gilst WH, Schoemaker RG. Erythropoietin induces neovascularization and improves cardiac function in rats with heart failure after myocardial infarction. J Am Coll Cardiol. 2005;46:125–133. doi: 10.1016/j.jacc.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 162.Wald M, Gutnisky A, Borda E, Sterin-Borda L. Erythropoietin modified the cardiac action of ouabain in chronically anaemic–uraemic rats. Nephron. 1995;71:190–196. doi: 10.1159/000188711. [DOI] [PubMed] [Google Scholar]
- 163.Wang Y, Tagil K, Ripa RS, Nilsson JC, Carstensen S, Jorgensen E, Sondergaard L, Hesse B, Johnsen HE, Kastrup J. Effect of mobilization of bone marrow stem cells by granulocyte colony stimulating factor on clinical symptoms, left ventricular perfusion and function in patients with severe chronic ischemic heart disease. Int J Cardiol. 2005;100:477–483. doi: 10.1016/j.ijcard.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 164.Ward AC, Csar XF, Hoffmann BW, Hamilton JA. Cyclic AMP inhibits expression of D-type cyclins and cdk4 and induces p27Kip1 in G-CSF-treated NFS-60 cells. Biochem Biophys Res Commun. 1996;224:10–16. doi: 10.1006/bbrc.1996.0976. [DOI] [PubMed] [Google Scholar]
- 165.Weihrauch D, Arras M, Zimmermann R, Schaper J. Importance of monocytes/macrophages and fibroblasts for healing of micronecroses in porcine myocardium. Mol Cell Biochem. 1995;147:13–19. doi: 10.1007/BF00944778. [DOI] [PubMed] [Google Scholar]
- 166.Wells JA, de Vos AM. Hematopoietic receptor complexes. Annu Rev Biochem. 1996;65:609–634. doi: 10.1146/annurev.bi.65.070196.003141. [DOI] [PubMed] [Google Scholar]
- 167.Werneck-de-Castro JP, Costa ESRH, de Oliveira PF, Pinho-Ribeiro V, Mello DB, Pecanha R, Mattos E, Olivares EL, Maia AC, Mill JG, Dos Santos Goldenberg RC, Campos-de-Carvalho AC. G-CSF does not improve systolic function in a rat model of acute myocardial infarction. Basic Res Cardiol. 2006;101:494–501. doi: 10.1007/s00395-006-0605-4. [DOI] [PubMed] [Google Scholar]
- 168.Westenbrink BD, Lipsic E, van der Meer P, van der Harst P, Oeseburg H, Du Marchie Sarvaas GJ, Koster J, Voors AA, van Veldhuisen DJ, van Gilst WH, Schoemaker RG. Eryth-ropoietin improves cardiac function through endothelial progenitor cell and vascular endothelial growth factor mediated neovascularization. Eur Heart J. 2007;28:2018–2027. doi: 10.1093/eurheartj/ehm177. [DOI] [PubMed] [Google Scholar]
- 169.Woldbaek PR, Hoen IB, Christensen G, Tonnessen T. Gene expression of colony-stimulating factors and stem cell factor after myocardial infarction in the mouse. Acta Physiol Scand. 2002;175:173–181. doi: 10.1046/j.1365-201X.2002.00989.x. [DOI] [PubMed] [Google Scholar]
- 170.Woo YJ, Grand TJ, Berry MF, Atluri P, Moise MA, Hsu VM, Cohen J, Fisher O, Burdick J, Taylor M, Zentko S, Liao G, Smith M, Kolakowski S, Jayasankar V, Gardner TJ, Sweeney HL. Stromal cell-derived factor and granulocyte-monocyte colony-stimulating factor form a combined neovasculogenic therapy for ischemic cardiomyopathy. J Thorac Cardiovasc Surg. 2005;130:321–329. doi: 10.1016/j.jtcvs.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 171.Yan XQ, Briddell R, Hartley C, Stoney G, Samal B, McNiece I. Mobilization of long-term hematopoietic reconstituting cells in mice by the combination of stem cell factor plus granulocyte colony-stimulating factor. Blood. 1994;84:795–799. [PubMed] [Google Scholar]
- 172.Yeghiazarians Y, Khan M, Angeli FS, Zhang Y, Jahn S, Prasad M, Mirsky R, Shih H, Minasi P, Boyle A, Grossman W. Cytokine combination therapy with long-acting erythropoietin and granulocyte colony stimulating factor improves cardiac function but is not superior than monotherapy in a mouse model of acute myocardial infarction. J Card Fail. 2010;16:669–678. doi: 10.1016/j.cardfail.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 173.Zohlnhofer D, Dibra A, Koppara T, de Waha A, Ripa RS, Kastrup J, Valgimigli M, Schomig A, Kastrati A. Stem cell mobilization by granulocyte colony-stimulating factor for myocardial recovery after acute myocardial infarction: a meta-analysis. J Am Coll Cardiol. 2008;51:1429–1437. doi: 10.1016/j.jacc.2007.11.073. [DOI] [PubMed] [Google Scholar]
- 174.Zohlnhofer D, Ott I, Mehilli J, Schomig K, Michalk F, Ibrahim T, Meisetschlager G, von Wedel J, Bollwein H, Seyfarth M, Dirschinger J, Schmitt C, Schwaiger M, Kastrati A, Schomig A. Stem cell mobilization by granulocyte colony-stimulating factor in patients with acute myocardial infarction: a randomized controlled trial. Jama. 2006;295:1003–1010. doi: 10.1681/01.asn.0000926832.82033.c5. [DOI] [PubMed] [Google Scholar]