Abstract
Background
Atherosclerotic calcification is a risk factor for cardiovascular events, independent of other traditional risk factors. Studies of the relation of menopausal hormone therapy to cardiovascular events have had inconsistent results, and often have been confounded by lifestyle behaviors and the “healthy user” effect. The authors evaluated the cross-sectional association of hormone therapy use with the presence and severity of atherosclerosis in postmenopausal women, independent of lifestyle factors, including diet and physical activity levels.
Methods
The authors consecutively enrolled postmenopausal asymptomatic women who were referred for coronary artery calcium scanning to measure cardiovascular risk. After consent was obtained, women were interviewed prior to their cardiac scan about cardiac risk factors, hormone therapy use, menopausal status, diet, and physical activity. Coronary artery calcium prevalence was defined as any calcification present (score >0).
Results
Of the 544 enrolled women aged 50–80 years, 252 (46.3%) were hormone therapy users. Hormone therapy users had a significantly lower prevalence of any coronary artery calcium (defined as coronary artery calcium score >0; 37%), than non-users (50%, p = 0.04), as well as significantly lower mean calcium scores (p = 0.02). Multiple logistic regression models demonstrated a significantly reduced odds of coronary artery calcium in hormone therapy users compared to non-users with an adjusted odds ratio of 0.58 (p = 0.04), adjusting for traditional cardiac risk factors and body mass index. Women who reported consuming a vegetarian or a high-protein diet had almost two-fold odds of coronary artery calcium compared with women who reported regular, mixed, or low-fat, low-salt diets (OR = 1.78, p = 0.02). Severity of coronary artery calcium was less with increasing levels of physical activity, and a significant association was observed between physical activity and hormone therapy use (adjusted OR = 4.05, p = 0.03), independent of coronary artery calcium severity.
Conclusion
This cross-sectional study demonstrated a protective association of hormone therapy with the presence and severity of coronary artery calcium. Although a strong relationship was observed between hormone therapy and physical activity, their complex interplay may affect mechanistic biochemical and physiological processes that have yet to be clearly delineated. Thus, physical activity and diet should be taken into account in prospective studies of the relation of hormone therapy use to coronary artery calcium.
Keywords: hormone therapy, atherosclerosis, coronary calcium, perimenopause, women
INTRODUCTION
Data are still conflicting regarding the use of menopausal hormone therapy (HT) in postmenopausal women relative to the risk-benefit ratio for coronary heart disease (CHD). While some previous studies have demonstrated improvement in CHD risk in women on HT(Mosca et al., 2004; Sourander et al., 1998), large randomized studies of HT among postmenopausal women, given as unopposed estrogen or combined estrogen-progestin therapy, have shown no significant effector an increased cardiovascular risk for women (Hsia et al., 2006; Hulley et al., 1998; Rossouw et al., 2002). The inconsistency of results of the CHD risk-benefit ratio found in randomized trials and those in some observational studies may be at least partially explained by the older age of participants, and delayed start of HT in the Women’s Health Initiative (WHI), which did not correspond to the traditional use of HT, most frequently used clinically perimenopausally (Lemay, 2002). This discrepancy was recently validated in a WHI analysis demonstrating cardiovascular protection for women starting HT closer to menopause compared to those starting therapy late (p = 0.02; Rossouw et al., 2007).
Additionally, an emerging hypothesis about the benefits of HT in younger postmenopausal women is the potential effect of HT on atherosclerotic plaques. A number of studies have shown that coronary artery calcium (CAC), detected on computed tomography, is a sensitive marker for atherosclerotic disease and the presence of significant angiographic stenoses (Haberl et al., 2001; Budoff et al., 2002). As CAC has been strongly independently related to cardiovascular risk (Budoff et al., 2009), various studies have investigated and found lower CAC severity scores in women on HT compared to non-users (Kuller et al., 1999; Manson et al., 2007; Ratti et al., 2007; Barrett–Connor & Laughlin, 2005; Akhrass et al., 2003). Investigations related to cardiac risk factors lend biological plausibility to cardioprotection by HT, suggesting that estrogens improve atherosclerosis by altering plasma lipid profiles (PEPI Trial Writing Group, 1995). Other lifestyle factors, such as physical activity (PA)of postmenopausal women, also have favorable effects on cardiovascular risk factors (Pettee et al., 2007; Hagberg et al., 2003). To date, few studies have assessed the relation of lifestyle factors to atherosclerosis, especially among perimenopausal women. A major potential confounder that may differ between some of the beneficial observational studies and randomized trials has been the “healthy user” effect, whereby users of HT may be more likely to engage in more PA, have better diets, and better weight maintenance, among other factors. Thus, the authors sought to evaluate specifically the associations of these lifestyle factors with the presence and severity of CAC, to assess whether HT was protectively associated with CAC, or if only through lifestyle factors were users benefited. The authors’ hypothesis was that HT use would be associated with a decreased prevalence and severity of subclinical atherosclerosis (as measured by CAC), independent of beneficial lifestyle behaviors, including diet and PA.
METHODS
Study Participants
To evaluate the association of HT and PA with atherosclerosis, the authors evaluated 544 consecutive post-menopausal women undergoing outpatient CAC scanning at their institution. Over 98% of women approached participated in this study. Participants were excluded from the study if they had documented coronary artery disease (CAD) prior to entry into the study (n = 21). All patients were asymptomatic (free of symptoms or known CHD), and referred by their primary physician for risk stratification.
Data Collection
Demographic data, risk factors for CHD (as defined by the Framingham Heart Study; Greenland et al., 2004), HT use, and other medication use were ascertained by interview by a research nurse after informed, written consent was obtained (Akhrass et al., 2003). Weight was measured, and height was obtained by interview to calculate body mass index (BMI) in kg/m2. In addition to the continuous variables in the study, data were collected for a number of categorical variables to describe each participant’s family history of CHD, cholesterol level, hypertension, stress level, diet type, frequency of sweat during exercise, and race/ethnicity.
The presence and number of risk factors for a participant were calculated based on the National Cholesterol Education Program guidelines (NCEP, 1993). Risk factors included: current cigarette smoking, history of premature coronary disease in a first-degree relative, diabetes, hypertension, and hypercholesterolemia. Current cigarette smoking was defined as use of >10 cigarettes per day. Participants currently using insulin or oral hypo-glycemic agents were classified as diabetic. Hypertension was defined by current use of anti-hypertensive medication or known and untreated hypertension, and hypercholesterolemia was similarly defined by use of cholesterol-lowering medication or known but untreated high cholesterol. Each participant filled out a self-administered Baecke questionnaire, a validated instrument, to assess habitual PA; units of PA per week were quantified, ranging from zero to 138 metabolic equivalents (METs) per week of PA (Baecke, Burema, & Frijters, 1982). No woman had suffered a cardiovascular event or developed symptoms consistent with ischemic heart disease.
The study protocol was approved by the Institutional Review Board of the Los Angeles Biomedical Research Institute at Harbor-UCLA.
CAC Methodology
The CAC studies were performed using an Imatron C-150XL Electron Beam tomographic scanner (GE-Imatron, South San Francisco, CA) in the high resolution volume mode, using a 100 ms exposure time. Electrocardiographic triggering was employed, so that each image was obtained at the same point in early diastole, to minimize cardiac motion. Proximal coronary artery visualization was obtained without contrast medium injection, and at least 30 consecutive images were obtained at 3 mm intervals, beginning one centimeter below the carina and progressing caudally to include the entire course of the coronary arteries. Coronary artery calcification was defined as a plaque of at least three contiguous pixels (area = 1.02 mm2) with a density of >130 Hu. Total radiation exposure using this technique was 0.7 milliSieverts per participant (similar to exposure from mammography).
The lesion score was calculated by multiplying the lesion area by a density factor derived from the maximal Hounsfield unit within this area, as described by Agatston et al. (1990). The density factor was assigned in the following manner: one for lesions whose maximal density was 130–199 Hu, two for lesions 200–299 Hu, three for lesions 300–399 Hu, and four for lesions >400 Hu. A total calcium score was determined by summing individual lesion scores from each of four anatomic sites (left main, left anterior descending, circumflex, and right coronary arteries). A single investigator, blinded to the clinical status of the participant and hormone use, interpreted all studies.
STATISTICAL ANALYSES
The continuous independent variables in the study were years of HT use, PA (METS/week), age, and BMI. In addition, the outcome variable, CAC score was also a continuous variable. The continuous years of HT use variable was dichotomized into groups of users and non-users, to assess relationships with PA, diet, CAC scores, and BMI, with all traditional cardiovascular risk factors in the multivariable model. Pearson’s chi-square statistics, using a continuity correction factor for 2×2 contingency tables, were computed to test for differences in the proportions of non-HT users for each of the dichotomously coded independent variables (Rosner, 1995). When CAC was used as a dichotomous outcome, to generate odds ratios (ORs), the presence of CAC was defined as any calcium (CAC score >0), and logistic regression was used. When CAC was used as a continuous variable, to assess relationships of factors to increasing severity of calcification, linear regression was used.
Independent sample Student’s t-tests were conducted to determine if the mean values of continuous variables differed by HT use status. Univariate and multivariable linear regression analyses were conducted to assess the relation to CAC score for each of the independent variables: age, race, BMI, elevated total cholesterol, hypertension, current cigarette smoking, and family history of premature heart disease. Variables that were significantly related to CAC in univariate analyses at p < 0.05 were then entered into a multivariable model using multiple linear regression, with increasing CAC score as the outcome variable. Model fit was assessed using the Akaike information criterion (AIC), with the best fit indicated by the lowest AIC. The statistical analyses were conducted using the STATA V8 statistical package (Stata Corp., College Station, Texas).
RESULTS
The participants had a mean age of 60.1 (±7.0) years, ranging from 50 to 80 years (Table 1). The HT users had a mean age of 59.5 (±6.5) years, while HT non-users had a mean age of 60.7 (±7.4) years. A significantly greater proportion of HT users than non-users were Caucasian (p = 0.011), and mean BMI was significantly higher in HT users (Table 1). No other traditional cardiovascular risk factors differed significantly between HT users and non-users. The mean age at which HT use was initiated was 54 (±6) years. The mean number of years of HT use among HT users was 2.2 years (±4.8), ranging from zero to 33 years. A strong positive relationship was found between age and the presence of CAC, with an OR of 1.79 for every additional 10 years of age (p = 0.01). The mean amount of PA was 22.7 (±19.6) MET per week for all study participants, ranging from zero to 138 METs/week. HT users had a significantly lower prevalence of CAC (score >0) than non-users (37% vs. 50%, p = 0.04) (Table 1). The continuous outcome CAC score had a mean of 77.6 (±222.9) Agatstonunits for the entire sample. Among HT users, the mean CAC score was significantly lower (56.8 [±173.9]) than among HT non-users (96.4 [±257.6]) (p < 0.001). On average, the HT users had a 39.6-unit lower CAC score than HT non-users (p < 0.001). No significant differences were detected between HT users and non-use or by duration of HT use in the mean PA score. No significant difference was detected between women with and without a family history of CHD in HT use (p = 0.410). Similarly, no significant differences in the proportions of HT use were detected between women with or without high cholesterol (p = 0.410), with or without hypertension (p = 0.630), and with or without a stressful lifestyle (p = 0.147).
TABLE 1.
Characteristics of Study Participants by HT Use
| HT use (n = 252) | No HT use (n = 292) | p-Value | |
|---|---|---|---|
| Age in years (SD) | 59.5 (6.5) | 60.7 (7.4) | n.s. |
| Race/ethnicity | 0.011 | ||
| Caucasian | 172 (68%) | 175 (60%) | |
| Chinese | 11 (4%) | 15 (5%) | |
| Black | 36 (14%) | 52 (18%) | |
| Hispanic | 33 (13%) | 50 (17%) | |
| Diabetes | 43 (16%) | 53 (18%) | n.s. |
| Hypertension | 154 (61%) | 187 (64%) | n.s. |
| Smoking status | n.s. | ||
| Never | 161 (64%) | 187 (64%) | |
| Former | 68 (27%) | 70 (24%) | |
| Current | 23 (9%) | 35 (12%) | |
| BMI (kg/m2), mean (SD) | 26.0 (± 5.0) | 25.5 (± 4.6) | 0.03 |
| PA score, mean (SD) | 23.9 (± 20.9) | 21.7 (± 18.4) | 0.110 |
| Stressful lifestyle | 91 (36%) | 114 (39%) | 0.147 |
| High cholesterol | 111 (44%) | 123 (42%) | n.s. |
| CAC prevalence | 37% | 50% | 0.04 |
| Mean (SD) CAC score | 56.8 (± 173.9) | 96.4 (± 257.6) | <0.001 |
Note. Categorical variables = n (%). SD = standard deviation; n.s. = not significant.
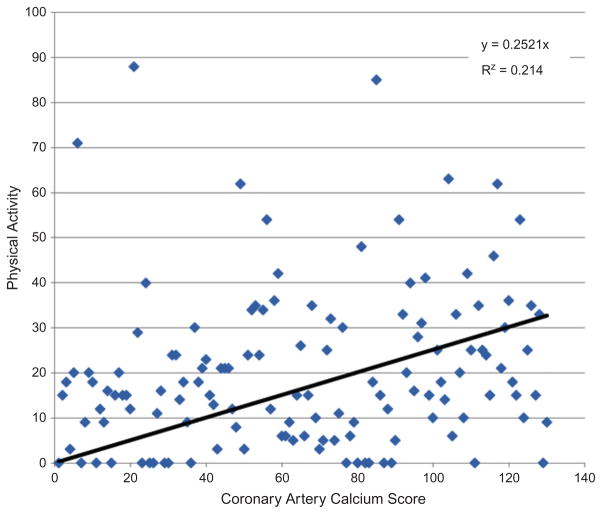
In bivariate analyses of the presence of any CAC, a two-fold odds of CAC was observed with every 10-year increase in age (OR = 2.14, p < 0.01). In addition, unexpectedly women who reported having a vegetarian or high-protein diet had an almost two-fold increased odds of CAC, compared with women who reported regular, mixed, or low-fat, low-salt diets (OR = 1.78, p = 0.02). Age, HT use, PA, and diet type “other” were significantly related to CAC scores (Figures 1 and 2). When examined alone, HT use was significantly negatively related to presence of any CAC in unadjusted analyses (OR = 0.67, p = 0.02) (Table 2).
FIGURE 1.
Relationship of PA and calcium score in women taking HT (color figure available online).
FIGURE 2.
Relationship of PA and calcium score in women not taking HT (color figure available online).
TABLE 2.
Univariate and Multivariable Probability of Coronary Calcification
| Variable | Univariate OR | p-Value | Multivariable OR | p-Value |
|---|---|---|---|---|
| PA | 1.0 | 0.99 | 0.44 | 0.09 |
| HT (95% CI) | 0.67 (0.38, 0.88) | 0.02 | 0.08 (0.01, 0.14) | 0.006 |
| Interaction PA x HT | 4.00 (1.92, 8.82) | 0.03 | 2.07 (1.12, 4.58) | 0.04 |
| Age (per 10 years) | 1.79 (1.46, 3.12) | 0.005 | 1.84 (1.47, 2.99) | 0.004 |
| Diet* | 1.88 (1.29, 2.96) | 0.01 | 1.78 (1.08, 2.87) | 0.02 |
| BMI | 1.02 (0.66, 2.82) | 0.52 | — | — |
| Cholesterol | 0.74 (0.33, 4.41) | 0.41 | — | — |
| Hypertension | 0.97 (0.44, 5.12) | 0.63 | — | — |
| Family history | 1.41 (0.52, 5.83) | 0.41 | — | — |
| Stressful lifestyle | 0.71 (0.44, 1.22) | 0.147 | — | — |
| Race/ethnicity | 0.67 (0.37, 1.96) | 0.24 | — | — |
Vegetarian or high-protein diet as compared with women who reported regular, mixed, or low-fat, low-salt diets. CI = confidence intervals.
Only age, HT use, diet, and PA were retained in the multiple logistic regression model (Kleinbaum,1994). After controlling for age, the odds of CAC among HT users was over two-fold compared to non-HT users in the presence of PA (OR = 2.4, p = 0.02). The association between PA and CAC score was modified by HT use (p for interaction = 0.04, n = 252) (Table 2). Thus, stratifying on HT use, among HT non-users, as expected, the authors observed a significant inverse relationship between increasing PA and CAC, such that women in the top quartile of PA had a 56% reduced prevalence of CAC (p < 0.01). However, among HT users, increased PA was associated with an unexpected increased mean CAC score (Figure 1), and women in the top quartile of PA had a 22% increased prevalence of CAC (p = 0.03).
DISCUSSION
This cross-sectional study demonstrated that menopausal HT use, PA, age, and diet type were significantly related to CAC. CAC is pathognomic of atherosclerosis, and a strong predictor of future cardiovascular events (Budoff et al., 2006). CAC is a process of vascular changes from the initial fatty streak to the development of a complex atherosclerotic plaque. Overall, a significant 41% reduction of CAC score was observed in HT users compared with non-users, independent of PA. The reduction in CAC score is consistent with findings from Manson et al. (2007), examining the relationship between estrogen and calcific plaques in a sub-study of the WHI, which demonstrated that the mean CAC score for women who received conjugated equine estrogen (CEE) was 83.1, while those women who received placebo had a higher score of 123.1 (p = 0.02), an approximate 33% significant reduction of CAC score in HT users. The reduction in coronary calcium score was even greater at 60% with those with at least 80% adherence to estrogen treatment over a five-year period. Furthermore, HT users had a higher probability of having lower CAC scores (<100) and lower probability of high CAC scores (>400) (OR 0.5, p = 0.02) compared with non-users (Akhrass et al., 2003). Yet, in another study, no independent association was observed between HT and coronary calcium score (Schisterman et al., 2002).
The authors also observed an inverse correlation between PA and CAC in non-HT users in the current study. The protective association of PA on subclinical atherosclerosis in non-HT users may have been due to its association with significantly lower prevalence of high BMI, high blood pressure, low HDL cholesterol, and diabetes, as well as reduced inflammation (Appt et al., 2006). An inverse association has been observed in recent studies between vigorous PA and the prevalence and degree of subclinical atherosclerosis in an asymptomatic population with multiple metabolic risk factors (Desai et al., 2004). Advanced CAC was observed in 26% of sedentary patients and 16% of patients who engaged in long-duration PA (p < 0.05). In multiple regression analysis, long-duration PA had an independent inverse association with advanced CAC. This is consistent with other studies showing favorable plasma lipid profiles in physically active women independent of HT use (Pettee et al., 2007; Hagberg et al., 2003).
The results of the current study were consistent with early initiation of HT seen in multiple trials. The combined trials of the WHI study (CEE trial and CEE with medroxyprogesterone acetate [MPA]) demonstrated increasing numbers of CHD cases with increasing years since menopause, with a hazard ratio (HR) of 0.76 for <10 years, HR of 1.10 between 10–19 years, and HR of 1.28 for ≥ 20 years, with an overall p = 0.02 for trend (Rossouw et al., 2007). The suggestion of favorable outcomes of clinical coronary events in younger menopausal women is also supported by the CEE trial arm of the WHI, in which women aged 50–59, 60–69, and 70–79 years had a HR of 0.56, 0.92, and 1.04, respectively, though the findings were not significant with an overall p = 0.14 (Schisterman et al., 2002).
Direct evidence indicates that in surgically postmenopausal cynomolgus monkeys, athero-protective effects of estrogen are limited to the early stages of atherogenesis (Appt et al., 2006). These primates, which develop atheromatous lesions indistinguishable from those in humans when fed an atherogenic diet, have consistently shown HT with CEE to reduce coronary atherosclerosis by as much as 50–70% if treatment is begun immediately after ovariectomy. However, no beneficial effect has been seen when CEE treatment was delayed for two years, leading the investigators to conclude that, in the delayed treatment model, HT did not improve pre-existing atherosclerosis (Jayo, Register, & Carlson, 2008). These findings are consistent with the above-noted failure to observe secondary prevention by HT in human trials (Hulley et al., 1998; Rossouw et al., 2002; Rossouw et al., 2007), and may explain the benefit seen in the current study, as the current authors evaluated younger, perimenopausal women, and they generally had no or early atherosclerosis.
In the current investigation, PA among HT-users was associated with a two-fold increase in CAC. This finding was similar to results from a previous study, which indicated a four-fold increased odds of CAC reflected in the interaction term between PA and HT (Hunter et al., 2001). Similar relationships have been shown with marathon runners, who exhibited increased CAC compared to more sedentary patients. Further, it is not clear if estrogen, in the presence of “external stressors” (PA), would enhance calcium progression. Together, the complex interplay of estrogen and “external stressors” hinges upon the underlying immunocytochemical effects on vascular smooth muscle integrity and function (Hunter et al., 2001; Jayo et al., 2008). Additional research in this area would foster a better understanding of the mechanistic biochemical and physiological processes of HT and PA. Finally, reasons for women initiating higher levels of PA, such as significant family history of premature heart disease, prior severe hyperlipidemia, or prior obesity, cannot be elucidated from the current cross-sectional study. A randomized trial of PA among HT users would likely be challenging.
Interestingly, the current authors found that a vegetarian or high-protein diet associated with more calcification. This may have been related to underlying reasons for increased use of vegetarian or high-protein diet (baseline obesity or prior weight issues), and was significant despite including BMI in the multivariable model. Clearly, randomized trials evaluating these diets need to be performed to evaluate the cardiovascular benefit (or harm) of these diets on the vasculature.
Limitations
The authors relied heavily on risk factors derived from interviews with a medical health professional, rather than measured risk factors, which may have resulted in misclassification of these factors for some participants. Further, the cross-sectional study design did not permit assessment of temporal relations of variables with CAC. Additionally, potential selection and participation biases could have led to inaccuracy of findings and to lack of generalizability of the findings. Most notably, the authors were also unable to assess the exact timing of the start of HT use relative to the menopause. While over 90% of women reported “perimenopausal” initiation of HT, the authors could not further elucidate the exact time of initiation or duration of therapy. The timing of initiation of menopausal HT and its relation to risk of heart disease has been identified as a very significant factor, which the authors were not able to examine fully in this study, and which thus requires additional rigorous study (Harman et al., 2005; Hsia et al., 2006). The current authors also did not measure lipid subclasses, and these were significantly related to outcomes in the Women’s Health Initiative (Hsia et al., 2008).
The results of this study are consistent with current guidelines on the use of estrogen and progestin in postmenopausal women (North American Menopause Society, 2010), which states, “The benefit-risk ratio for menopausal HT is favorable for women who initiate HT close to menopause but decreases in older women and with time since menopause in previously untreated women.” Results of this study demonstrated that HT may be associated with lower levels of atherosclerotic plaque burden, lending greater evidence to the existing body of research of the dynamic effects of HT on coronary calcium. The underlying complexity of the association between HT with other factors, such as PA and diet, may be rooted in biochemical and physiological changes that have yet to be clearly delineated. The authors have previously demonstrated that unopposed estrogen inhibits progression of atherosclerosis (Budoff et al., 2005), and the current study further supports the potential for cardioprotection with estrogen if started early. Further randomized trials of menopausal HT in a target population of young postmenopausal women are necessary to elucidate further the risk-benefit ratio of such HT in atherosclerosis. Ongoing studies, such as KEEPS: The Kronos Early Estrogen Prevention Study (Harman et al., 2005) and ELITE: Early vs. Late Intervention Trial with Estradiol (Hodis &Mack, 2008) are prospectively testing the timing hypothesis related to early initiation of estrogen in perimenopausal women. Both PA levels and diet type must be taken into account when evaluating HT and atherosclerosis. This study provides additional evidence of the potential protective link between HT and atherosclerosis. The interaction between HT and CAC is interesting and raises important questions for further research.
Footnotes
There are no conflicts of interest with any author.
References
- Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- Akhrass F, Evans AR, Wang Y, Rich S, Kannan CR, Fogefeld L, Mazzone T. Hormone replacement therapy is associated with less coronary atherosclerosis in postmenopausal women. J Clin Endo & Metab. 2003;88(12):5611–4. doi: 10.1210/jc.2003-031008. [DOI] [PubMed] [Google Scholar]
- Appt SE, Clarkson TB, Lees CJ, Anthony MS. Low dose estrogens inhibit coronary artery atherosclerosis in postmenopausal monkeys. Maturitas. 2006;55(2):187–94. doi: 10.1016/j.maturitas.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–42. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- Barrett–Connor E, Laughlin GA. Hormone therapy and coronary artery calcification in asymptomatic postmenopausal women: The Rancho Bernardo study. Menopause. 2005;12(1):40–8. doi: 10.1097/00042192-200512010-00009. [DOI] [PubMed] [Google Scholar]
- Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, et al. Assessment of coronary artery disease by cardiac computed tomography: A scientific statementf rom the American Heart Association committee on cardiovascular imaging and intervention, council on cardiovascular radiology and intervention, and committee on cardiac imaging, council on clinical cardiology. Circulation. 2006;114(16):1761–91. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]
- Budoff MJ, Chen GPW, Hunter CJ, Takasu J, Agrawal N, Sorochinsky B, Mao SS. Effects of hormone replacement on progression of coronary calcium as measured by electron beam tomography. J Women’s Health (Larchmt) 2005;14(5):410–7. doi: 10.1089/jwh.2005.14.410. [DOI] [PubMed] [Google Scholar]
- Budoff MJ, Nasir K, McClelland RL, Detrano R, Wong N, Blumenthal RS, et al. Coronary calcium predicts events better with absolute calcium scores than age-gender-race percentiles—The Multi-Ethnic Study of Atherosclerosis (MESA) J Am Coll Cardiol. 2009;53:345–52. doi: 10.1016/j.jacc.2008.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budoff MJ, Raggi P, Berman D, Arad Y, Guerci AD, Callister TQ, Diamond GA. Continuous probabilistic prediction of angiographically significant coronary artery disease using electron beam tomography. Circulation. 2002;105(15):1791–6. doi: 10.1161/01.cir.0000014483.43921.8c. [DOI] [PubMed] [Google Scholar]
- Desai MY, Nasir K, Rumberger JA, Braunstein JB, Post WS, Budoff MJ, Blumenthal RS. Relation of degree of physical activity to coronary artery calcium score in asymptomatic individuals with multiple metabolic risk factors. Am J Cardiol. 2004;94(6):729–32. doi: 10.1016/j.amjcard.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA–J Am Med Assoc. 2004;291:210–5. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- Haberl R, Becker A, Leber A, Knez A, Becker C, Lang C, et al. Correlation of coronary calcification and angiographically documented stenoses in patients with suspected coronary artery disease: Results of 1,764 patients. J Am Coll Cardiol. 2001;37:451. doi: 10.1016/s0735-1097(00)01119-0. [DOI] [PubMed] [Google Scholar]
- Hagberg JM, McCole SD, Ferrel RE, Smuda JM, Rodgers KS, Wilund KR, Moore GE. Physical activity, hormone replacement therapy and plasma lipoprotein-lipid levels in postmenopausal women. Int J Sports Med. 2003;24(1):22–9. doi: 10.1055/s-2003-37198. [DOI] [PubMed] [Google Scholar]
- Harman SM, Brinton EA, Cedars M, Lobo R, Manson JE, Merria GR, et al. KEEPS: The Kronos Early Estrogen Prevention Study. Climacteric. 2005;8(1):3–12. doi: 10.1080/13697130500042417. [DOI] [PubMed] [Google Scholar]
- Hodis HN, Mack WJ. Postmenopausal hormone therapy and cardiovascular disease in perspective. Clin Obstet Gynecol. 2008;51:564–80. doi: 10.1097/GRF.0b013e318181de86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia J, Langer RD, Manson JE, Kuller L, Johnson KC, Hendrix SL, et al. Conjugated equine estrogens and coronary heart disease. The women’s health initiative. Arch Intern Med. 2006;166:357–65. doi: 10.1001/archinte.166.3.357. [DOI] [PubMed] [Google Scholar]
- Hsia J, Otvos JD, Rossouw JE, Wu L, Wassertheil–Smoller S, Hendrix SL, et al. Lipoprotein particle concentrations may explain the absence of coronary protection in the women’s health initiative hormone trials. Arterioscler Thromb Vasc Biol. 2008;28:1666–71. doi: 10.1161/ATVBAHA.108.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) research group. JAMA– J Am Med Assoc. 1998;280:605–13. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- Hunter CJ, Kim HT, Sorochinsky B, Budoff MJ. Physical activity, hormone replacement therapy and atherosclerosis in midlife women. Circulation. 2001;104:535–6. [Google Scholar]
- Jayo MJ, Register TC, Carlson CS. Effects on bone of oral hormone replacement therapy initiated 2 years after ovariectomy in young adult monkeys. Bone. 1998;23(4):361–6. doi: 10.1016/s8756-3282(98)00106-9. [DOI] [PubMed] [Google Scholar]
- Kleinbaum DG. Logistic regression: A self-learning text. New York: Springer–Verlag; 1994. [Google Scholar]
- Kuller LH, Matthews KA, Sutton–Tyrrell K, Edmundowicz D, Bunker CH. Coronary and aortic calcification among women 8 years after menopause and their premenopausal risk factors: The Healthy Women Study. Arterioscler Thromb Vasc Biol. 1999;19:2189–98. doi: 10.1161/01.atv.19.9.2189. [DOI] [PubMed] [Google Scholar]
- Lemay A. The relevance of the women’s health initiative results on combined hormone replacement therapy in clinical practice. J Obstet Gynecol Can. 2002;24(9):711–5. doi: 10.1016/s1701-2163(16)30326-7. [DOI] [PubMed] [Google Scholar]
- Manson JE, Allison MA, Rossouw JE, Carr JJ, Langer RD, Hsia J, et al. Estrogen therapy and coronary-artery calcification. The Women’s Health Initiative. NEJM. 2007;365(25):2591–602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- Mosca L, Appel LJ, Benjamin EJ, Berra K, Chandra–Strobos N, Fabunmi RP, et al. Evidence-based guidelines for cardiovascular disease prevention in women: Expert panel/writing group. Circulation. 2004;109:672–93. doi: 10.1161/01.CIR.0000114834.85476.81. [DOI] [PubMed] [Google Scholar]
- National Cholesterol Education Program (NCEP) Second report of the expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. Bethesda, MD: U. S. Department of Health and Human Services; 1993. (Publication No. HIH 93–3095) [Google Scholar]
- North American Menopause Society. Estrogen and progestogen use in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause. 2010;17(2):242–55. doi: 10.1097/gme.0b013e3181d0f6b9. [DOI] [PubMed] [Google Scholar]
- PEPI Trial Writing Group. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women: The postmenopausal estrogen/progestin interventions (PEPI) trial. JAMA–J Am Med Assoc. 1995;273:199–208. [PubMed] [Google Scholar]
- Pettee KK, Kriska AM, Johnson BD, Conroy MB, Mackey RH, Orchard TJ, Kuller LH. The relationship between physical activity and lipoprotein subclasses in postmenopausal women: The influence of hormone therapy. Menopause. 2007;14(1):115–22. doi: 10.1097/01.gme.0000229573.29258.d5. [DOI] [PubMed] [Google Scholar]
- Ratti C, Grassi L, Chiurlia E, Bursi F, Bompani B, Ferramosca E, Modena MG. Coronary calcifications in a subgroup of post-menopausal women with metabolic syndrome. G Ital Cardiol (Rome) 2007;8(9):574–9. [PubMed] [Google Scholar]
- Rosner B. Fundamentals of biostatistics. 4. Belmont, CA: Duxbury Press; 1995. [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the women’s health initiative randomized controlled trial. JAMA–J Am Med Assoc. 2002;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA–J Am Med Soc. 2007;297:1465–77. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- Schisterman EF, Gallagher AM, BaireyMerz CN, Whitcomb BW, Faraggi D, Moysich KB, Lewin H. The association of hormone replacement therapy and coronary calcium as determined by electron beam tomography. J Women Health Gen-B. 2002;11(7):631–8. doi: 10.1089/152460902760360577. [DOI] [PubMed] [Google Scholar]
- Sourander L, Rajala T, Räihä I, Mäkinen J, Erkkola R, Helenius H. Cardiovascular and cancer morbidity and mortality and sudden cardiac death in postmenopausal women on estrogen replacement therapy. Lancet. 1998;352:1965–9. doi: 10.1016/S0140-6736(98)05066-1. [DOI] [PubMed] [Google Scholar]