Abstract
Cholesterol is an important precursor for numerous biologically active molecules, and it plays a major role in membrane structure and function. Cholesterol can be endogenously synthesized or exogenously taken up via the endocytic vesicle system and subsequently delivered to post-endo/lysosomal sites including the plasma membrane and the endoplasmic reticulum. Niemann–Pick C (NPC) disease results in the accumulation of exogenously-derived cholesterol, as well as other lipids, in late endosomes and lysosomes (LE/LY). Identification of the two genes that underlie NPC disease, NPC1 and NPC2, has focused attention on the mechanisms by which lipids, in particular cholesterol, are transported out of the LE/LY compartment. This review discusses the role of the NPC2 protein in cholesterol transport, and the potential for concerted action of NPC1 and NPC2 in regulating normal intracellular cholesterol homeostasis.
Keywords: Cholesterol, NPC2, NPC1, Late endosome, Lysosome, Lipid transport
1. Introduction
Intracellular unesterified cholesterol is functionally significant in numerous ways. Cholesterol itself is an important regulator of the structural and permeability properties of membrane phospholipid bilayers. As well, it is the precursor of many biologically active molecules including steroid hormones, bile acids, and oxysterols, many of which serve as ligands for nuclear transcription factors. It is not surprising, then, that intracellular cholesterol is carefully regulated, both at the quantitative and subcellular localization levels. In this review, focus will be made on the insights into intracellular cholesterol homeostasis that have come from studies of Niemann–Pick C, a disease in which cholesterol and other lipids accumulate in the late endosomal/lysosomal (LE/LY) compartment of the cell. In particular, the role of NPC2 protein in cholesterol transport will be addressed.
2. Intracellular distribution of cholesterol
It is generally accepted that the majority of cellular cholesterol is found in the plasma membrane, with intracellular membranes typically maintaining much lower levels. While the transmembrane distribution of cholesterol is still debated, recent studies using fluorescent sterols and membrane-impermeant quenchers suggest that most of the plasma membrane cholesterol faces the cytoplasmic compartment [1]. Elevated levels of intracellular cholesterol are dealt with in two ways. Excess free cholesterol is esterified with fatty acids in the endoplasmic reticulum by the action of acyl-coenzyme A cholesterol acyltransferase (ACAT1), and the cholesteryl esters stored primarily in cytoplasmic lipid droplets. In addition, excess cholesterol may be removed from the cell by efflux at the plasma membrane, mediated by the ATP-binding cassette transporter ABCA1 [reviewed in [2]]. The rate of cholesterol movement from the plasma membrane to the endoplasmic reticulum is higher in cells with increased cholesterol levels, leading to an enhancement of the storage process in cells with excess unesterified cholesterol [3]. It has been proposed that this faster transfer, mediated most likely by cytoplasmic proteins, may occur because elevated cholesterol levels in a membrane exceed the capacity of the membrane phospholipids to directly interact with and thereby sequester them, thus the excess cholesterol would have a lower free energy of dissociation from the membrane [4–6].
The distribution of cholesterol in different subcellular compartments is maintained by a combination of vesicle-mediated inter-organelle transport and protein-mediated monomeric transfer through the aqueous cytoplasm. Given the extremely low solubility of cholesterol, quantitatively little is found unbound in cytosol. At present, several intracellular sterol binding proteins have been identified in mammalian cells, including sterol carrier protein 2 [7], the oxysterol binding proteins, of which there are twelve in humans [8,9], as well as steroidogenic acute regulatory protein related lipid transfer (START) domain proteins, of which atleast three are known to bind sterols—STARD1/StAR, STARD3/MLN64, and STARD5 [10]. While a role in cholesterol transport has been proposed for these proteins, whether they truly function as transporters, and their potential mechanisms of action, remain uncertain.
3. Endogenous and exogenous sources of cellular cholesterol
The total cellular pool of cholesterol consists of that which is endogenously synthesized, that which is delivered to cells from exogenous sources, largely by receptor mediated endocytosis of low density lipoproteins (LDL) but also to some extent by bulk phase endocytosis of circulating lipoproteins [11], and that which is released from storage as fatty acid esters by cholesteryl ester hydrolase activity [2]. De novo cholesterol synthesis occurs largely in the endoplasmic reticulum, and transfer from the endoplasmic reticulum (ER) to the plasma membrane (PM) is rapid, with a half time of approximately 15 min [12]. The majority of ER to PM transfer of cholesterol does not appear to be vesicle-mediated and is independent of passage through the Golgi apparatus [13, 14]. The process has been shown to be ATP-dependent, although the underlying mechanism of this energy dependence is not understood [15]. A role for caveolin in accelerating cholesterol transfer to the plasma membrane has been demonstrated [16].
Exogenous cholesterol is delivered to cells primarily by the well-studied LDL receptor pathway [reviewed in [17]], in which clathrin-coated endocytic vesicles lose their coats and fuse to form larger vesicles termed early endosomes. These acidic vesicles sort specific endocytosed molecules to the trans-Golgi network, and receive numerous hydrolase enzymes from the TGN, developing into late endosomes and lysosomes, with luminal acidity increasing directly with endo-lysosomal vesicle maturation [18–21]. LDL cholesterol enters the endocytic pathway in the form of cholesteryl esters as well as free cholesterol. The esters are hydrolyzed by an acid-optimum cholesteryl ester hydrolase activity [22], and unesterified cholesterol is trafficked from the endo/lysosomal compartment to the plasma membrane and the ER. Since the time of arrival of LDL-derived cholesterol at the ER, as monitored by ACAT1 esterification, is two to three times slower than its arrival at the PM, as monitored by extracellular cyclodextrin extraction, and, as mentioned above, since cholesterol movement from PM to ER is quite rapid, it is likely that the major route of trafficking for endo/lysosomal cholesterol is directly to the PM [22,23]. Recent studies have indicated that a substantial fraction of transfer of cholesterol from the endosomal system passes through the trans-Golgi network prior to arrival at the ER, in a process involving vesicle trafficking [24].
4. Endo/lysosomal cholesterol transport: insights from NPC disease
While the precise molecular mechanisms of egress of endo/ lysosomal cholesterol remain largely unknown, important insight into the process has been gained from studying Niemann–Pick C (NPC) disease cells. NPC is an autosomal recessive disorder that exhibits impaired neuronal and visceral cell trafficking of cholesterol and other lipids, including glycolipids. Specifically, disease cells were found to exhibit marked lipid accumulation in the late endosomal and lysosomal compartments, which indicated that the underlying problems were related to malfunctioning of these organelles. Indeed, identification of the two genes responsible for NPC disease has focused attention on lipid trafficking in the endosomal–lysosomal vesicle system [reviewed in [25,26]].
Two complementation groups for NPC are thought to account for all disease cases. The large majority of cases are caused by mutation of the NPC1 gene, identified in 1997 by positional cloning [27]. The gene for the second complementation group, which accounts for approximately 5% of disease cases, was identified as NPC2 from a proteomic screen of mannose-6-phosphorylated proteins [28]. As will be discussed below, both NPC1 and NPC2 gene products are localized primarily in the late endosomal and lysosomal (LE/LY) compartments, and their structural, biochemical, and transport characteristics suggest that both are involved in the normal egress of cholesterol from these organelles.
5. NPC1
The NPC1 gene has been mapped to chromosome 18q11–12, spans more than 47 kb, and contains 25 exons which range in size from 74 to 788 nucleotides, and introns which range from 0.097 to 7 kb in length [29–31]. The human NPC1 protein is a 1278 amino acid transmembrane glycoprotein with 13 putative transmembrane helices, three large hydrophilic domains that project into the lumen of the endo/ lysosomes, as well as four small luminal loops, six small cytoplasmic loops, and a cytoplasmic tail [32]. Over 200 disease-causing mutations have been identified in the NPC1 gene [33,34].
The region between the third and seventh transmembrane helices of NPC1, comprising amino acid residues 615 to 797, is considered a sterol sensing domain (SSD) based upon sequence homology to the SSD of several other membrane proteins, including the morphogen receptor Patched [35,36], the sterol-sensing protein SCAP (Sterol regulatory element binding protein cleavage activating protein), the rate determining enzyme of cholesterol biosynthesis 3-hydroxy-3-methylglutaryl-CoA reductase [32,35,37] and a protein with 51% homology to NPC1, NPC1L1, which has been shown to be the target of the dietary cholesterol absorption inhibitor ezetimibe [38–40]. The SSD in NPC1 has been shown to bind a photoactivatable cholesterol analog in cells, and a known loss-of-function mutation in the SSD markedly inhibited the binding [41,42]. The NPC1 SSD may also be involved in protein stability, as this same loss-of-function mutation, P691S, caused a decrease in NPC1 unbiquitination secondary to cellular cholesterol depletion [41,42]. It has also been found that homologous mutations in the SSD's of SCAP and NPC1 lead to increased activities of both proteins [36]. Finally, it has been suggested that a motif in the SSD is important for NPC1 localization in late endosomes [43]. Thus, while the exact role of the NPC1 SSD is not certain, it appears to be necessary for protein function and normal cholesterol egress from the late endosome/lysosome compartment.
The first luminal domain of NPC1 (amino acids 25–264), called the N-terminal domain (NTD) and also the NPC1 domain, contains several conserved cysteine residues and a leucine zipper motif. Interestingly, when this domain was expressed as a soluble protein, high affinity binding to cholesterol and side-chain oxysterols was found [44], suggesting the NTD as a possible sterol binding site for NPC1. Yet the full length NPC1 protein with a Q79A mutation, which interrupts the sterol binding by the NTD, can still rescue the cholesterol-accumulation phenotype of NPC1 deficient cells [45], suggesting that a cholesterol binding site(s) other than the NTD may be present in the NPC1 protein, possibly in the SSD. Reconstitution of the full length NPC1 protein in detergent micelles was recently reported, and results using fluorescent cholesterol analogues demonstrated a 1:1 stoichiometry for sterol binding, with ligand buried within the protein and inaccessible to aqueous phase quenchers [46]. While a much lower ligand: protein ratio was reported in an independent study of purified full length NPC1 [44], both studies found that cholesterol and side-chain oxysterol bound with sub-micromolar affinities. It remains to be seen whether both the soluble NTD and membrane-localized sites, e.g. the SSD, participate in sterol binding by full length NPC1.
The third luminal domain of NPC1 is a loop between amino acids 855 and 1098, located between transmembrane helices 8 and 9 [32]. It is rich in conserved cysteine residues and contains a ring-finger motif.This domain contains approximately 50% of described NPC1 missense mutations including I1061T, the most frequent mutation, accounting for about 20% of cases in Western Europeans and resulting in a juvenile onset neurological disease [47]. Mutations that give rise to the so-called variant phenotype, which presents with mild alterations in cellular cholesterol trafficking, are also primarily located in the cysteine-rich domain [48–51].
6. NPC2
The other gene responsible for NPC disease, NPC2, maps to chromosome 14q24.3, is 13.5-kb long, and contains five exons ranging in size from 78 to 342 bp [28]. The NPC2 protein had been previously studied as HE1, a major secretory protein of the human epididymus shown to bind cholesterol with micromolar affinity [52]. In defining the proteome of lysosomally targeted mannose-6-phosphorylated proteins from human brain, HE1 was identified; subsequent studies definitively demonstrated it to be the second NPC gene, in that it was absent or mutated in fibroblasts from NPC2 patients, and since exogenous addition of purified protein was able to correct the cholesterol-accumulation phenotype of NPC2−/− cells [28]. Thus, the NPC2 gene encodes a small soluble glycoprotein found inlysosomes and secretory fluids such as milk, epididymal fluid, bile, and plasma [28,53–55]. The mature human NPC2 has 132 amino acids, with an additional 19 amino acid signal peptide (Fig. 1). The crystal structure of unliganded bovine NPC2 shows an immunoglobulin-like β-sandwich fold consisting of seven β-strands arranged in two β-sheets [56]. The topology of NPC2 was found to be identical to that of three other members of the MD-2-related lipid recognition domain family, GM2 activator protein [57] and the dust mite allergens Der f2 and Der p2 [58,59]. The loosely packed hydrophobic core of apo NPC2 was suggested to be an incipient, internal ligand binding pocket [56]. The recent report of holo bovine NPC2 with cholesterol sulfate bound indeed demonstrated that the incipient binding pocket, present as several adjacent small cavities, expands to accommodate a closely sequestered steroid nucleus, with the protein β-strands separating slightly and with substantial side chain reorientation, to accommodate the bound ligand [60]. In the holo NPC2 structure, the 3-position sulfate moiety is the only portion of the ligand in contact with bulk solvent, and the cholesterol iso-octyl side chain is buried deep in the protein interior (Fig. 2) [60].
Fig. 1.
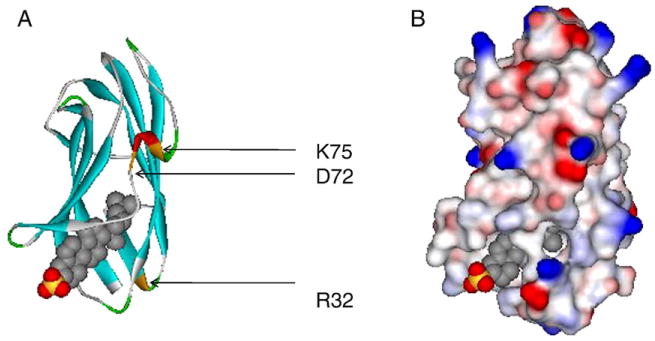
Crystal structure of bovine NPC2 (bNPC2)-cholesterol sulfate complex (PDB ID: 2HKA). Structures generated with Accelrys DS Visualizer (www.accelrys.com). (A) Solid ribbon style, colored by secondary structure type. R32, D72 and K75 are highlighted; these residues in bNPC2 are identical or homologous to those identified in murine NPC2 as being able to bind cholesterol normally, but as unable to clear the cholesterol accumulation in NPC2-deficient cells [61]. (B) Solvent surface style, with probe radius of 1.4 Å, colored by electrostatic potential – red indicates positively charged, blue indicates negatively charged, and white indicates neutral residues.
Fig. 2.
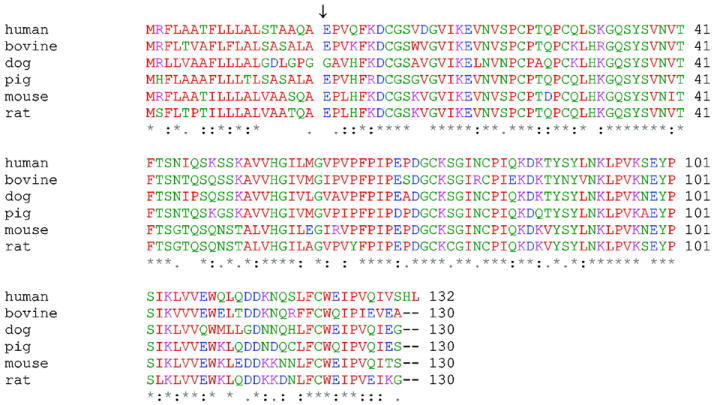
Alignment of mammalian NPC2 sequences. The 19 amino acid signal sequences are shown. NPC2 sequences are numbered starting with the first amino acid after the signal peptide, marked with an arrow. Residues with hydrophobic side chains are colored in red, and residues with hydrophilic side chains are colored in blue (acidic), magenta (basic) and green (neutral). * indicates identical residues, : indicates conserved residues, and . indicates semi-conserved residues.
Both the NPC2 crystallographic structure and biochemical studies show that NPC2 binds cholesterol with a 1:1 stoichiometry [60]. Binding affinities ranging from 30 nM to 2uM have been reported using different methods [45,52,61,62]. A variety of cholesterol-related molecules can also bind to NPC2, including precursors of cholesterol biosynthesis such as desmosterol, plant sterols such as β-sitosterol and the fluorescent dehydroergosterol, some 3-position cholesteryl esters (cholesterol acetate and cholesterol sulfate but not cholesterol esterified with long chain fatty acid), and some but not other side-chain oxysterols, the latter binding more weakly than cholesterol [45,52,56,62]. Depending upon the position on the sterol molecule, addition of a hydrophilic moiety on the cholesterol side chain, such as a hydroxyl group at position 25, may inhibit the binding to NPC2, whereas additions at the 3-hydroxyl position either inhibits binding entirely, or may induce higher affinity binding, such as found with cholesterol sulfate. The discrimination is likely due to the solubility and self-association properties of the particular ligand rather than an inherent property of the NPC2 binding site, since the 3 position of the cholesterol A ring is exposed to solvent and does not interact with NPC2 [60,62]. One report indicates that NPC2 may function in dimeric form to transfer specific glycolipids on and off of CD1d in natural killer (NKT) cells [63], however others have not found that NPC2 forms dimers, and it has been reported not to bind glycolipids [62].
The primary structure of NPC2 contains three potential N-glycosylation sites at positions 19, 39, and 116. Purified NPC2 protein is composed of different glycosylated isoforms [62,64]. It has been found that Asn-19 is never glycosylated, while Asn-39 is always linked to an endo H-sensitive oligosaccharide, and Asn-116 may be unglycosylated or modified with either endo H-sensitive or endo H-resistant oligosaccharides [62]. The Asn-39 modification is suggested to be necessary for proper NPC2 targeting to lysosomes while the Asn-116 is non-essential [62,64,65]. Importantly, all the glycoforms are able to bind cholesterol, and all can effectively deplete the cholesterol accumulation of NPC2-deficient fibroblasts [62]. While one report indicated an aberrant glycosylation pattern for NPC2 in NPC1-deficient mouse liver and suggested that the cholesterol transport deficiency in NPC1 disease may arise from defects in the NPC2 protein [65], there is considerable heterogeneity in glycosylation of NPC2 in different tissues [66]. Moreover, the glycosylation pattern of the lysosomal protease tripeptidyl-peptidase 1 also changes in both NPC1-and NPC2-deficient mouse liver, suggesting that alterations in glycosylation are a secondary effect of disease (P. Lobel, personal communication).
7. Cholesterol binding and NPC2 function
There is some debate as to whether the cellular phenotype of NPC disease is due to aberrant trafficking of cholesterol or whether defects in other lipids which are also known to accumulate in the LE/LY compartment in affected cells, in particular glycolipids, are the primary cause of cellular and whole body symptoms. It has been proposed that malfunction of glycolipid metabolism or transport may cause a secondary defect in lysosomal cholesterol egress [67–69]. Recently, it was suggested that NPC disease is initiated by sphingosine accumulation which, in turn, depletes organelle calcium stores, thereby leading to accumulation of cholesterol, sphingolipids, and glycolipids [70]. As both of the identified NPC gene products have been shown to directly bind cholesterol and other sterols, rather than such other lipids, an argument for defective cholesterol transport can be made on that basis. The fact that sterol binding is required for function makes the case more compelling. For NPC1, marked reduction of sterol binding was demonstrated for the SSD point mutants P692S and Y635S, which are known to be unable to correct the cholesterol accumulation of deficient cells [41].
For NPC2, the case is stronger still. It has been clearly demonstrated that cholesterol binding is necessary for the protein to function normally in cellular cholesterol homeostasis. In an important study of NPC2 structure-function relationships, it was found that in order for NPC2 to restore normal cholesterol levels in human NPC2−/− fibroblasts, it was necessary that it bind cholesterol with high affinity. Thus, in contrast to wild type NPC2, point mutations that disrupt cholesterol binding were unable to clear the elevated cholesterol levels when added to NPC2−/− cells [61]. It was of great interest, in addition, that three point mutations were identified in which cholesterol binding was essentially normal, but the modified proteins remained unable to correct the cholesterol accumulation phenotype of diseased cells [61]. This suggested that cholesterol binding by NPC2 was necessary but not sufficient for normal functioning of the protein, and indicated that NPC2 may have not only a sterol binding function, but perhaps also a cholesterol transport function that is distinct from sterol binding at the protein structure level.
8. The cholesterol transport function of NPC2
A potential cholesterol transport function for NPC2 has been demonstrated with in vitro methods. By using the endogenous tryptophan fluorescence of NPC2, the rate of transfer of cholesterol from purified human NPC2 to model membrane vesicles has been directly determined. When cholesterol binds to NPC2, its tryptophan signal is quenched, with stoichiometric binding resulting in a 20 to 30% reduction in tryptophan fluorescence [71]. Upon subsequent addition of acceptor phospholipid vesicles, the rate of transfer of cholesterol from the NPC2 protein to the membranes can be monitored directly by the increase in tryptophan fluorescence as a function of time. In order to distinguish between an aqueous diffusion-mediated cholesterol transfer mechanism, and one in which cholesterol transfer occurs during NPC2-membrane interactions, the rate of cholesterol transfer from NPC2 to membranes was assessed as a function of membrane phospholipid concentration and composition. In an aqueous diffusion transfer mechanism, alterations in neither acceptor concentration nor its chemical composition would alter the rate of ligand transfer, since the rate determining step would be dissociation of the ligand from the protein. In a collisional mechanism, by contrast, the ligand transfer rate would be expected to increase in direct proportion to protein-membrane ‘collisions,’ and would likely be affected by changes in the acceptor membrane composition as well [71-73].
To test whether NPC2 can transfer cholesterol to membranes by a protein-membrane interaction mechanism, the theoretical number of collisions between small unilamellar egg phosphatidylcholine membrane vesicles and NPC2 was modified by varying the concentration of SUV phospholipid. The rate of cholesterol transfer from NPC2 increased in proportion to the frequency of NPC2-membrane interactions, suggesting a collisional mechanism of transfer. Moreover, incorporation of several anionic phospholipids including phosphatidylserine, phosphatidylinositol, or lyso-bis phosphatidic acid (LBPA, also known as bis(monoacylglycerol)phosphate, BMP), into the acceptor membranes resulted in increased cholesterol transfer rates relative to the zwitterionic phosphatidylcholine vesicles, particularly with membranes containing LBPA; inclusion of sphingomyelin resulted in a decreased rate of cholesterol transfer. It was also found that cholesterol transfer from NPC2 was most rapid at acidic pH, in keeping with its localization in the acidic LE/LY compartment [71]. The results imply that NPC2 can not only bind cholesterol but can also transfer it to membranes very rapidly. Indeed, the absolute cholesterol transfer rates were orders of magnitude faster than off-rates of cholesterol from NPC2 into aqueous buffer [61]. The transfer of cholesterol is likely to involve NPC2-membrane contacts, with electrostatic interactions important in formation of the putative protein-membrane complex [71].
The late endosomal-lysosomal compartment contains not only a limiting outer membrane that borders the cytosol but also numerous internal membranes [74] (Fig. 3). It has been shown that LBPA is particularly rich in these internal membranes [75,76]. Recent studies have examined the process of cholesterol transport from membranes to the NPC2 protein, as well as the effect of NPC2 on inter-membrane sterol transport, so as to model the transfer of cholesterol from internal LE/LY membranes to the limiting organellar membrane. The results indicate that NPC2 can markedly accelerate the rates of cholesterol transport from and between membranes, as well as the extent of cholesterol transfer [77,78]. Further, it was shown that transfer occurs by direct NPC2-membrane interactions [78]. The proposed collisional mechanism of NPC2 action was supported by results of FTIR and fluorescence spectral shift studies, which indicated that NPC2 is likely binding at the membrane surface without penetration into the bilayer hydrophobic core [78]. Additionally, the rate and extent of cholesterol transfer by NPC2 is significantly increased by LBPA [77,78], and reduced by an anti-LBPA antibody [78], further supporting a critical role for this unique phospholipid in endo/lysosomal cholesterol trafficking [78].
Fig. 3.
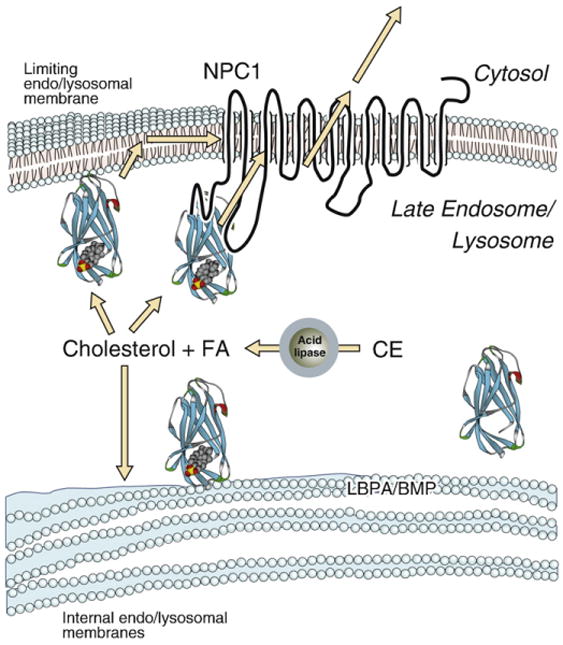
Potential role of NPC2 in the egress of cholesterol from the late endosomal/ lysosomal compartment. CE, cholesteryl ester; FA, unesterified fatty acid; LBPA/BMP, lyso-bis phosphatidic acid, also known as bis(monoacylglycerol)phosphate.
A specific role for LBPA in cholesterol transport by NPC2 is further suggested by studies which showed that treatment of cells with an anti-LBPA antibody resulted in an NPC-like phenotype characterized by substantial cholesterol accumulation [79]. LBPA is known to accumulate along with cholesterol in NPC cells [80], perhaps as a compensatory attempt to increase cholesterol clearance. Further, recent studies have shown that LBPA may be involved in regulating the level of late endosomal cholesterol via its interaction with the protein Alix, which is involved in intralumenal multivesicular membrane fusion and sorting events [81]. Interestingly, addition of LBPA to NPC1−/− fibroblasts by liposome incubation was shown to partially relieve the intracellular cholesterol accumulation [81]. It is possible that LBPA may serve a general role in facilitating the normal lipid transport and enzymatic functions of the LE/LY compartment, as in vitro studies of glycosphingolipid hydrolysis showed that LBPA works in concert with sphingolipid activator proteins to increase the extent of substrate hydrolysis [82,83].
The in vitro studies of sterol transfer by NPC2 strongly suggest that it can function as a cholesterol transporter. A key finding is that the absolute rates of cholesterol transfer found in vitro for NPC2, up to 10 s−1, are very rapid, and likely sufficient to account for the rapid egress of cholesterol from NPC2−/− cells upon addition of wild type protein to the media [61,71,78]. Studies that examined the transfer of cholesterol between NPC2 and the soluble N-terminal domain (NTD) of NPC1, and between each of the proteins and membrane vesicles, showed that only NPC2 and not the NPC1 NTD was able to effectively transfer cholesterol to or from an acceptor, and that transfer was sufficiently rapid as to preclude quantification [84]. In the cell, it is possible that the delivery of NPC2-bound cholesterol can occur either following extrinsic interactions with the phospholipid bilayer, upon which cholesterol exits the protein binding pocket; subsequently the sterol could reach a putative transmembrane transporter via lateral diffusion in the plane of the membrane, or it could flip across the limiting LE/LY membrane and become accessible for transfer to the plasma membrane or other destinations [5]. Alternatively, liganded NPC2 could interact directly with NPC1 or another lysosomal membrane protein, resulting in the movement of cholesterol from NPC2 to the membrane protein, which then directs the egress of cholesterol out of the lysosome (Fig. 3). Either of these scenarios is consistent with the rapid clearance by NPC2 of accumulated cholesterol in NPC2−/− fibroblasts [61].
Overall, it is very likely that for NPC2 to function properly in situ, it must not only bind cholesterol, but also transfer/transport it—from, perhaps, a particular membrane domain in the inner LE/LY membranes—to, perhaps, a specific domain or protein in the limiting membrane. Preliminary studies have examined the in vitro sterol transfer properties of the three NPC2 mutant forms that were previously shown to bind cholesterol but were unable to function at the cellular level to alleviate the cholesterol accumulation caused by NPC2 deficiency, Lys 75, Asp 72, and Arg 32 (Fig. 2) [61]. They are all charged residues and the NPC2 tertiary structure shows them to be on the surface of the protein, which is consistent with the formation of electrostatic interactions with target membranes and/or proteins. All three point mutants had markedly slower rates of cholesterol transfer to membranes compared with the native NPC2 (unpublished observations). The protein structure shows that while two of these residues are located in close proximity to each other and far from the opening of the ligand binding pocket, the third residue is situated closer to the opening. This suggests that there may be more than one interactive domain on the surface of NPC2.
9. (How) do NPC2 and NPC1 interact?
This remains a crucial question for the understanding of intracellular cholesterol transport. That both proteins are operative in the trafficking of LDL-derived cholesterol in the endo/lysosomal vesicle system is generally agreed upon, but whether they work in concert, interacting at the functional and potentially at the structural levels, is not yet certain. It is worth noting at the outset that direct protein– protein interactions for NPC2 with NPC1 have not been reported as of now.
It was known for some time that there were two complementation groups for the assemblage of pathophysiological symptoms manifested in NPC patients. Since discovery of the two NPC genes, the seemingly inseparable phenotypes of patients with NPC1 disease and NPC2 disease has been further documented, although this is complicated by the multiple types of mutations identified in each of the genes, which lead to a range of disease onsets, symptoms, and severity [34,85–87]. A direct comparison of mice null for Npc1, an Npc2 hypomorph expressing 0 to 4% residual protein depending on the tissue, and an Npc1/Npc2 double mutant strain showed that all three murine models were phenotypically similar in disease progression, neurological and visceral pathology, and biochemistry of cholesterol and lipid accumulation, providing important evidence for functional cooperativity between the two proteins [66]. Functional interactions are also suggested by the compensatory increases in Npc2 expression observed in the Npc1 null mouse liver and brain, and in human NPC1−/− fibroblasts [55,88,89]. Conversely, cells deficient in mannose-6-phosphate receptors, which were found to secrete almost all their NPC2 and have very little intracellular NPC2, were found to have increased NPC1 expression [90]. In addition, both NPC2 and NPC1 genes are coordinately upregulated by LXR agonists in human macrophages [91].
Whether the apparent functional interaction between NPC1 and NPC2 is based on a physical/biochemical interaction between the two proteins would seem to depend, at minimum, on their residing in the same subcellular compartment. At present, more information is available about NPC1 localization than about NPC2. Many studies indicate a late endosomal site for NPC1 in various cell types using a variety of localization methods [64,92–97]. This might be expected since NPC1 has a C-terminal di-leucine motif, which has been shown to be necessary and, for other proteins, sufficient for targeting membrane proteins to the endosomal compartment. For NPC1, however, it was found that this motif was not sufficient for late endosomal targeting, and a site in transmembrane domain 3 in the SSD was reported to act as an endosomal targeting signal [43]. NPC1 has also been variously reported to be partially localized to other subcellular sites including endoplasmic reticulum, caveolin-1 rafts, and the trans-Golgi network [92–94]. Examination of an NPC1-fluorescent protein fusion in live Chinese hamster ovary cells showed that a fraction of NPC1-containing organelles moved rapidly between perinuclear regions and the periphery of the cell, sometimes in association with strands of ER [98].
The soluble protein NPC2 is targeted to a mannose-6-phosphate receptor-positive lysosome/late endosome compartment [28,90], and localized to the lysosome by subcellular fractionation [28]. Several investigations have addressed the localization of both proteins simultaneously. NPC2 is consistently found in the lysosomal compartment, and although a suggestion of separate localization from NPC1has been considered, several studies report substantial or partial overlap of the two proteins [24,64,97,99]. In one study, NPC1 and an NPC2-fluorescent protein fusion were observed in live cells, and it was shown that in the regions of colocalization, NPC2 was observed in the core while NPC1 was at the periphery of the organelle [97]. This is consistent with NPC1 being present in the limiting membrane and NPC2 in the lumen of the lysosome, available for interaction with internal LE/LY membranes.
Overall, the highly dynamic nature of the endocytic recycling system, coupled with inherent limitations of antibody specificity, cell fixation, ectopic fusion protein localization, and subcellular fractionation approaches, coupled with the potential for intrinsic cell-type differences, makes the question of colocalization of the integral membrane protein, NPC1, and the soluble protein, NPC2, surprisingly difficult to answer definitively. Nevertheless, the localization data to date suggest that NPC2 and NPC1 are likely to have at least partial temporal overlap in the same vesicular compartment(s).
A comparison of the binding specificities for NPC2 and NPC1 also affords insight into their potential interactions. The simplest model of interaction is one in which the proteins bind the same ligand sequentially, effecting vectorial transport out of the LE/LY compartment. This sequential binding could involve protein–protein interaction, but does not require it. NPC2 is clearly a cholesterol binding protein, as demonstrated consistently by a variety of methods [52,56,61,62,71,84]. The tertiary structure of the cholesterol sulfate-NPC2 complex provides further evidence that unesterified cholesterol binds in an interior pocket [60]. For NPC1, it is also likely that cholesterol is a ligand for the protein. As mentioned earlier, labeling with a photoactivatable cholesterol analogue was demonstrated, and two recent reports of NPC1 purification also demonstrate cholesterol binding [41,44,46]. Both NPC1 and NPC2 also bind the fluorescent sterols dehydroergosterol and cholestatrienol [46,56,78]. Nevertheless, a seemingly more robust binding of the oxysterol 25-hydroxy-cholesterol (25-OH-C) was shown by the displacement of cholesterol binding to NPC1 by 25-OH-C, but not of 25-OH-C by cholesterol [44]. Indeed, although binding data for both NPC2 and NPC1 are relatively limited, with only one paper extensively examining substrate specificities for NPC2 [62] and only one comparing both NPC2 and the NPC1-NTD in a single study [45], there appear to be distinct differences between the two proteins. In particular, both full length reconstituted NPC1 and the NPC1-NTD bind side-chain oxysterols but not ring-modified oxysterols [44–46]. In contrast, NPC2 was found not to bind 25-OH-C, and to exhibit only weak if any binding of 24-OH-C and 27-OH-C[44,62]. Further, while cholesterol sulfate was shown to bind even more avidly to NPC2 than cholesterol (likely because of its greater aqueous solubility rather than because of inherent NPC2-dependent differences), it was reported not to bind to NPC1-NTD [45,62]. Taken together, the substrate specificities of NPC1 and NPC2 show some overlap, perhaps most importantly in cholesterol binding, indicating that they could interact to efficiently traffic LDL-derived cholesterol and maintain the normally low cholesterol levels found in late endosome/lysosome compartment. Nevertheless, apparent differences in ligand binding profiles, particularly with regard to the regulatory side-chain oxysterols, could also point to functional divergences.
10. Summary and perspectives
In summary, from the evidence to date it appears that NPC2 has the properties of a protein that could traffic cholesterol rapidly in an acidic milieu enriched in endo/lysosome-specific phospholipids. The NPC2 and NPC1 proteins reside in the same subcellular compartment at least partially and at least some of the time, and cholesterol binding by both proteins is likely. Are they functioning together, in a direct manner, to transport cholesterol? As mentioned earlier, recent studies examined cholesterol transfer between NPC2 and the soluble NPC1-NTD, from each of these proteins to phosphatidylcholine vesicles, and from vesicles to each of the proteins in the presence or absence of the other. The results showed that in these systems, only NPC2 was effective at stimulating rapid cholesterol transfer, and that the NPC1-NTD showed little effect in the absence of NPC2. NPC2 appeared to be equally effective at transferring cholesterol to membranes or to NPC1-NTD [84]. It will be of interest to determine whether the full length NPC1 provides evidence for more direct interactions with NPC2, however from a functional perspective it remains possible that the two proteins act in a common pathway but do not require direct interactions, rather organellar membrane lipids provide the requisite intermediate in cholesterol trafficking. In addition, the identification of NPC1 as a 25-hydroxycholesterol binding protein indicates it may have a more specific role in oxysterol metabolism, one that is functionally supported by NPC2 by promoting the egress of cholesterol from the LE/LY to sites of oxysterol biogenesis [100].
Acknowledgments
The authors would like to thank Dr. Peter Lobel for helpful discussions. Funding from the Ara Parseghian Medical Research Foundation (J.S.) and the American Heart Association (Z.X.) is gratefully acknowledged.
Abbreviations
- ER
endoplasmic reticulum
- LDL
low density lipoprotein
- LE/LY
late endosome/lysosome compartment
- NPC1
Niemann–Pick C1 protein
- NPC2
Niemann–Pick C2 protein
- PM
plasma membrane
- SCAP
sterol regulatory element binding protein cleavage activating protein
Contributor Information
Judith Storch, Email: USA.storch@aesop.rutgers.edu.
Zhi Xu, Email: xz@aesop.rutgers.edu.
References
- 1.Mondal M, Mesmin B, Mukherjee S, Maxfield FR. Sterols are mainly in the cytoplasmic leaflet of the plasma membrane and the endocytic recycling compartment in CHO cells. Mol Biol Cell. 2008;20:581–588. doi: 10.1091/mbc.E08-07-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang TY, Chang CC, Ohgami N, Yamauchi Y. Cholesterol sensing, trafficking, and esterification. Annu Rev Cell Dev Biol. 2006;22:129–157. doi: 10.1146/annurev.cellbio.22.010305.104656. [DOI] [PubMed] [Google Scholar]
- 3.Wustner D, Mondal M, Tabas I, Maxfield FR. Direct observation of rapid internalization and intracellular transport of sterol by macrophage foam cells. Traffic. 2005;6:396–412. doi: 10.1111/j.1600-0854.2005.00285.x. [DOI] [PubMed] [Google Scholar]
- 4.Lange Y, Ye J, Steck TL. Activation of membrane cholesterol by displacement from phospholipids. J Biol Chem. 2005;280:36126–36131. doi: 10.1074/jbc.M507149200. [DOI] [PubMed] [Google Scholar]
- 5.Maxfield FR, Menon AK. Intracellular sterol transport and distribution. Curr Opin Cell Biol. 2006;18:379–385. doi: 10.1016/j.ceb.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Radhakrishnan A, McConnell H. Condensed complexes in vesicles containing cholesterol and phospholipids. Proc Natl Acad Sci U S A. 2005;102:12662–12666. doi: 10.1073/pnas.0506043102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seedorf U, Ellinghaus P, Roch Nofer J. Sterol carrier protein-2. Biochim Biophys Acta. 2000;1486:45–54. doi: 10.1016/s1388-1981(00)00047-0. [DOI] [PubMed] [Google Scholar]
- 8.Yang H. Nonvesicular sterol transport: two protein families and a sterol sensor? Trends Cell Biol. 2006;16:427–432. doi: 10.1016/j.tcb.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Fairn GD, McMaster CR. Emerging roles of the oxysterol-binding protein family in metabolism, transport, and signaling. Cell Mol Life Sci. 2008;65:228–236. doi: 10.1007/s00018-007-7325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alpy F, Tomasetto C. Give lipids a START: the StAR-related lipid transfer (START) domain in mammals. J Cell Sci. 2005;118:2791–2801. doi: 10.1242/jcs.02485. [DOI] [PubMed] [Google Scholar]
- 11.Liu B, Xie C, Richardson JA, Turley SD, Dietschy JM. Receptor-mediated and bulk-phase endocytosis cause macrophage and cholesterol accumulation in Niemann-Pick C disease. J Lipid Res. 2007;48:1710–1723. doi: 10.1194/jlr.M700125-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.DeGrella RF, Simoni RD. Intracellular transport of cholesterol to the plasma membrane. J Biol Chem. 1982;257:14256–14262. [PubMed] [Google Scholar]
- 13.Kaplan MR, Simoni RD. Transport of cholesterol from the endoplasmic reticulum to the plasma membrane. J Cell Biol. 1985;101:446–453. doi: 10.1083/jcb.101.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heino S, Lusa S, Somerharju P, Ehnholm C, Olkkonen VM, Ikonen E. Dissecting the role of the Golgi complex and lipid rafts in biosynthetic transport of cholesterol to the cell surface. Proc Natl Acad Sci U S A. 2000;97:8375–8380. doi: 10.1073/pnas.140218797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liscum L, Munn NJ. Intracellular cholesterol transport. Biochim Biophys Acta. 1999;1438:19–37. doi: 10.1016/s1388-1981(99)00043-8. [DOI] [PubMed] [Google Scholar]
- 16.Smart EJ, Ying Y, Donzell WC, Anderson RG. A role for caveolin in transport of cholesterol from endoplasmic reticulum to plasma membrane. J Biol Chem. 1996;271:29427–29435. doi: 10.1074/jbc.271.46.29427. [DOI] [PubMed] [Google Scholar]
- 17.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 18.Lin SX, Mallet WG, Huang AY, Maxfield FR. Endocytosed cation-independent mannose 6-phosphate receptor traffics via the endocytic recycling compartment en route to the trans-Golgi network and a subpopulation of late endosomes. Mol Biol Cell. 2004;15:721–733. doi: 10.1091/mbc.E03-07-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 20.van der Goot FG, Gruenberg J. Intra-endosomal membrane traffic. Trends Cell Biol. 2006;16:514–521. doi: 10.1016/j.tcb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat Rev Mol Cell Biol. 2007;8:622–632. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- 22.Sugii S, Reid PC, Ohgami N, Du H, Chang TY. Distinct endosomal compartments in early trafficking of low density lipoprotein-derived cholesterol. J Biol Chem. 2003;278:27180–27189. doi: 10.1074/jbc.M300542200. [DOI] [PubMed] [Google Scholar]
- 23.Cruz JC, Chang TY. Fate of endogenously synthesized cholesterol in Niemann-Pick type C1 cells. J Biol Chem. 2000;275:41309–41316. doi: 10.1074/jbc.M008272200. [DOI] [PubMed] [Google Scholar]
- 24.Urano Y, Watanabe H, Murphy SR, Shibuya Y, Geng Y, Peden AA, Chang CC, Chang TY. Transport of LDL-derived cholesterol from the NPC1 compartment to the ER involves the trans-Golgi network and the SNARE protein complex. Proc Natl Acad Sci U S A. 2008;105:16513–16518. doi: 10.1073/pnas.0807450105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sturley SL, Patterson MC, Balch W, Liscum L. The pathophysiology and mechanisms of NP-C disease. Biochim Biophys Acta. 2004;1685:83–87. doi: 10.1016/j.bbalip.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Ory DS. The Niemann-Pick disease genes; regulators of cellular cholesterol homeostasis. Trends Cardiovasc Med. 2004;14:66–72. doi: 10.1016/j.tcm.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA, Pavan WJ, Krizman DB, Nagle J, Polymeropoulos MH, Sturley SL, Ioannou YA, Higgins ME, Comly M, Cooney A, Brown A, Kaneski CR, Blanchette-Mackie EJ, Dwyer NK, Neufeld EB, Chang TY, Liscum L, Strauss JF, III, Ohno K, Zeigler M, Carmi R, Sokol J, Markie D, O'Neill RR, vanDiggelen OP, Elleder M, Patterson MC, Brady RO, Vanier MT, Pentchev PG, Tagle DA. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 1997;277:228–231. doi: 10.1126/science.277.5323.228. [DOI] [PubMed] [Google Scholar]
- 28.Naureckiene S, Sleat DE, Lackland H, Fensom A, Vanier MT, Wattiaux R, Jadot M, Lobel P. Identification of HE1 as the second gene of Niemann-Pick C disease. Science. 2000;290:2298–2301. doi: 10.1126/science.290.5500.2298. [DOI] [PubMed] [Google Scholar]
- 29.Pentchev PG, Blanchette-Mackie EJ, Liscum L. Biological implications of the Niemann-Pick C mutation. Subcell Biochem. 1997;28:437–451. doi: 10.1007/978-1-4615-5901-6_14. [DOI] [PubMed] [Google Scholar]
- 30.Bauer P, Knoblich R, Bauer C, Finckh U, Hufen A, Kropp J, Braun S, Kustermann-Kuhn B, Schmidt D, Harzer K, Rolfs A. NPC1: complete genomic sequence, mutation analysis, and characterization of haplotypes. Hum Mutat. 2002;19:30–38. doi: 10.1002/humu.10016. [DOI] [PubMed] [Google Scholar]
- 31.Morris JA, Zhang D, Coleman KG, Nagle J, Pentchev PG, Carstea ED. The genomic organization and polymorphism analysis of the human Niemann-Pick C1 gene. Biochem Biophys Res Commun. 1999;261:493–498. doi: 10.1006/bbrc.1999.1070. [DOI] [PubMed] [Google Scholar]
- 32.Davies JP, Ioannou YA. Topological analysis of Niemann-Pick C1 protein reveals that the membrane orientation of the putative sterol-sensing domain is identical to those of 3-hydroxy-3-methylglutaryl-CoA reductase and sterol regulatory element binding protein cleavage-activating protein. J Biol Chem. 2000;275:24367–24374. doi: 10.1074/jbc.M002184200. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez-Valero EM, Ballart A, Iturriaga C, Lluch M, Macias J, Vanier MT, Pineda M, Coll MJ. Identification of 25 new mutations in 40 unrelated Spanish Niemann-Pick type C patients: genotype-phenotype correlations. Clin Genet. 2005;68:245–254. doi: 10.1111/j.1399-0004.2005.00490.x. [DOI] [PubMed] [Google Scholar]
- 34.Runz H, Dolle D, Schlitter AM, Zschocke J. NPC-db, a Niemann-Pick type C disease gene variation database. Hum Mutat. 2008;29:345–350. doi: 10.1002/humu.20636. [DOI] [PubMed] [Google Scholar]
- 35.Nohturfft A, Brown MS, Goldstein JL. Topology of SREBP cleavage-activating protein, a polytopic membrane protein with a sterol-sensing. J Biol Chem. 1998;273:17243–17250. doi: 10.1074/jbc.273.27.17243. [DOI] [PubMed] [Google Scholar]
- 36.Millard EE, Gale SE, Dudley N, Zhang J, Schaffer JE, Ory DS. The sterol-sensing domain of the Niemann-Pick C1 (NPC1) protein regulates trafficking of low density lipoprotein cholesterol. J Biol Chem. 2005;280:28581–28590. doi: 10.1074/jbc.M414024200. [DOI] [PubMed] [Google Scholar]
- 37.Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 38.Altmann SW, Davis HR, Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, Zeng M, Wang L, Murgolo N, Graziano MP. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 39.Davies JP, Levy B, Ioannou YA. Evidence for a Niemann-Pick C (NPC) gene family: identification and characterization of NPC1L1. Genomics. 2000;65:137–145. doi: 10.1006/geno.2000.6151. [DOI] [PubMed] [Google Scholar]
- 40.Temel RE, Tang W, Ma Y, Rudel LL, Willingham MC, Ioannou YA, Davies JP, Nilsson LM, Yu L. Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J Clin Invest. 2007;117:1968–1978. doi: 10.1172/JCI30060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohgami N, Ko DC, Thomas M, Scott MP, Chang CC, Chang TY. Binding between the Niemann-Pick C1 protein and a photoactivatable cholesterol analog requires a functional sterol-sensing domain. Proc Natl Acad Sci U S A. 2004;101:12473–12478. doi: 10.1073/pnas.0405255101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohsaki Y, Sugimoto Y, Suzuki M, Hosokawa H, Yoshimori T, Davies JP, Ioannou YA, Vanier MT, Ohno K, Ninomiya H. Cholesterol depletion facilitates ubiquitylation of NPC1 and its association with SKD1/Vps4. J Cell Sci. 2006;119:2643–2653. doi: 10.1242/jcs.02993. [DOI] [PubMed] [Google Scholar]
- 43.Scott C, Higgins ME, Davies JP, Ioannou YA. Targeting of NPC1 to late endosomes involves multiple signals, including one residing within the putative sterol-sensing domain. J Biol Chem. 2004;279:48214–48223. doi: 10.1074/jbc.M406090200. [DOI] [PubMed] [Google Scholar]
- 44.Infante RE, Abi-Mosleh L, Radhakrishnan A, Dale JD, Brown MS, Goldstein JL. Purified NPC1 protein. I. Binding of cholesterol and oxysterols to a 1278-amino acid membrane protein. J Biol Chem. 2008;283:1052–1063. doi: 10.1074/jbc.M707943200. [DOI] [PubMed] [Google Scholar]
- 45.Infante RE, Radhakrishnan A, Abi-Mosleh L, Kinch LN, Wang ML, Grishin NV, Goldstein JL, Brown MS. Purified NPC1 protein: II. Localization of sterol binding to a 240-amino acid soluble luminal loop. J Biol Chem. 2008;283:1064–1075. doi: 10.1074/jbc.M707944200. [DOI] [PubMed] [Google Scholar]
- 46.Liu R, Lu P, Chu JW, Sharom FJ. Characterization of fluorescent sterol binding to purified human NPC1. J Biol Chem. 2009;284:1840–1852. doi: 10.1074/jbc.M803741200. [DOI] [PubMed] [Google Scholar]
- 47.Millat G, Marcais C, Rafi MA, Yamamoto T, Morris JA, Pentchev PG, Ohno K, Wenger DA, Vanier MT. Niemann-Pick C1 disease: the I1061T substitution is a frequent mutant allele in patients of Western European descent and correlates with a classic juvenile phenotype. Am J Hum Genet. 1999;65:1321–1329. doi: 10.1086/302626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Millat G, Marcais C, Tomasetto C, Chikh K, Fensom AH, Harzer K, Wenger DA, Ohno K, Vanier MT. Niemann-Pick C1 disease: correlations between NPC1 mutations, levels of NPC1 protein, and phenotypes emphasize the functional significance of the putative sterol-sensing domain and of the cysteine-rich luminal loop. Am J Hum Genet. 2001;68:1373–1385. doi: 10.1086/320606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greer WL, Dobson MJ, Girouard GS, Byers DM, Riddell DC, Neumann PE. Mutations in NPC1 highlight a conserved NPC1-specific cysteine-rich domain. Am J Hum Genet. 1999;65:1252–1260. doi: 10.1086/302620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tarugi P, Ballarini G, Bembi B, Battisti C, Palmeri S, Panzani F, Di Leo E, Martini C, Federico A, Calandra S. Niemann-Pick type C disease: mutations of NPC1 gene and evidence of abnormal expression of some mutant alleles in fibroblasts. J Lipid Res. 2002;43:1908–1919. doi: 10.1194/jlr.m200203-jlr200. [DOI] [PubMed] [Google Scholar]
- 51.Yamamoto T, Nanba E, Ninomiya H, Higaki K, Taniguchi M, Zhang H, Akaboshi S, Watanabe Y, Takeshima T, Inui K, Okada S, Tanaka A, Sakuragawa N, Millat G, Vanier MT, Morris JA, Pentchev PG, Ohno K. NPC1 gene mutations in Japanese patients with Niemann-Pick disease type C. Hum Genet. 1999;105:10–16. doi: 10.1007/s004399900059. [DOI] [PubMed] [Google Scholar]
- 52.Okamura N, Kiuchi S, Tamba M, Kashima T, Hiramoto S, Baba T, Dacheux F, Dacheux JL, Sugita Y, Jin YZ. A porcine homolog of the major secretory protein of human epididymis, HE1, specifically binds cholesterol. Biochim Biophys Acta. 1999;1438:377–387. doi: 10.1016/s1388-1981(99)00070-0. [DOI] [PubMed] [Google Scholar]
- 53.Larsen LB, Ravn P, Boisen A, Berglund L, Petersen TE. Primary structure of EPV20, a secretory glycoprotein containing a previously uncharacterized type of domain. Eur J Biochem. 1997;243:437–441. doi: 10.1111/j.1432-1033.1997.0437a.x. [DOI] [PubMed] [Google Scholar]
- 54.Kirchhoff C, Osterhoff C, Young L. Molecular cloning and characterization of HE1, a major secretory protein of the human epididymis. Biol Reprod. 1996;54:847–856. doi: 10.1095/biolreprod54.4.847. [DOI] [PubMed] [Google Scholar]
- 55.Klein A, Amigo L, Retamal MJ, Morales MG, Miquel JF, Rigotti A, Zanlungo S. NPC2 is expressed in human and murine liver and secreted into bile: potential implications for body cholesterol homeostasis. Hepatology. 2006;43:126–133. doi: 10.1002/hep.20985. [DOI] [PubMed] [Google Scholar]
- 56.Friedland N, Liou HL, Lobel P, Stock AM. Structure of a cholesterol-binding protein deficient in Niemann-Pick type C2 disease. Proc Natl Acad Sci U S A. 2003;100:2512–2517. doi: 10.1073/pnas.0437840100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wright CS, Li SC, Rastinejad F. Crystal structure of human GM2-activator protein with a novel beta-cup topology. J Mol Biol. 2000;304:411–422. doi: 10.1006/jmbi.2000.4225. [DOI] [PubMed] [Google Scholar]
- 58.Ichikawa S, Hatanaka H, Yuuki T, Iwamoto N, Kojima S, Nishiyama C, Ogura K, Okumura Y, Inagaki F. Solution structure of Der f 2, the major mite allergen for atopic diseases. J Biol Chem. 1998;273:356–360. doi: 10.1074/jbc.273.1.356. [DOI] [PubMed] [Google Scholar]
- 59.Derewenda U, Li J, Derewenda Z, Dauter Z, Mueller GA, Rule GS, Benjamin DC. The crystal structure of a major dust mite allergen Der p 2, and its biological implications. J Mol Biol. 2002;318:189–197. doi: 10.1016/S0022-2836(02)00027-X. [DOI] [PubMed] [Google Scholar]
- 60.Xu S, Benoff B, Liou HL, Lobel P, Stock AM. Structural basis of sterol binding byNPC2, a lysosomal protein deficient in Niemann-Pick type C2 disease. J Biol Chem. 2007;282:23525–23531. doi: 10.1074/jbc.M703848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ko DC, Binkley J, Sidow A, Scott MP. The integrity of a cholesterol-binding pocket in Niemann-Pick C2 protein is necessary to control lysosome cholesterol levels. Proc Natl Acad Sci U S A. 2003;100:2518–2525. doi: 10.1073/pnas.0530027100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liou HL, Dixit SS, Xu S, Tint GS, Stock AM, Lobel P. NPC2 the protein deficient in Niemann-Pick C2 disease, consists of multiple glycoforms that bind a variety of sterols. J Biol Chem. 2006;281:36710–36723. doi: 10.1074/jbc.M608743200. [DOI] [PubMed] [Google Scholar]
- 63.Schrantz N, Sagiv Y, Liu Y, Savage PB, Bendelac A, Teyton L. The Niemann-Pick type C2 protein loads isoglobotrihexosylceramide onto CD1d molecules and contributes to the thymic selection of NKT cells. J Exp Med. 2007;204:841–852. doi: 10.1084/jem.20061562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chikh K, Vey S, Simonot C, Vanier MT, Millat G. Niemann-Pick type C disease: importance of N-glycosylation sites for function and cellular location of the NPC2 protein. Mol Genet Metab. 2004;83:220–230. doi: 10.1016/j.ymgme.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 65.Chen FW, Gordon RE, Ioannou YA. NPC1 late endosomes contain elevated levels of non-esterified (‘free’) fatty acids and an abnormally glycosylated form of the NPC2 protein. Biochem J. 2005;390:549–561. doi: 10.1042/BJ20050236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sleat DE, Wiseman JA, El-Banna M, Price SM, Verot L, Shen MM, Tint GS, Vanier MT, Walkley SU, Lobel P. Genetic evidence for nonredundant functional cooperativity between NPC1 and NPC2 in lipid transport. Proc Natl Acad Sci U S A. 2004;101:5886–5891. doi: 10.1073/pnas.0308456101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.te Vruchte D, Lloyd-Evans E, Veldman RJ, Neville DC, Dwek RA, Platt FM, van Blitterswijk WJ, Sillence DJ. Accumulation of glycosphingolipids in Niemann-Pick C disease disrupts endosomal transport. J Biol Chem. 2004;279:26167–26175. doi: 10.1074/jbc.M311591200. [DOI] [PubMed] [Google Scholar]
- 68.Zervas M, Somers KL, Thrall MA, Walkley SU. Critical role for glycosphingolipids in Niemann-Pick disease type C. Curr Biol. 2001;11:1283–1287. doi: 10.1016/s0960-9822(01)00396-7. [DOI] [PubMed] [Google Scholar]
- 69.Gondre-Lewis MC, McGlynn R, Walkley SU. Cholesterol accumulation in NPC1-deficient neurons is ganglioside dependent. Curr Biol. 2003;13:1324–1329. doi: 10.1016/s0960-9822(03)00531-1. [DOI] [PubMed] [Google Scholar]
- 70.Lloyd-Evans E, Morgan AJ, He X, Smith DA, Elliot-Smith E, Sillence DJ, CChurchill G, Schuchman EH, Galione A, Platt FM. Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat Med. 2008;14:1247–1255. doi: 10.1038/nm.1876. [DOI] [PubMed] [Google Scholar]
- 71.Cheruku SR, Xu Z, Dutia R, Lobel P, Storch J. Mechanism of cholesterol transfer from the Niemann-Pick type C2 protein to model membranes supports a role in lysosomal cholesterol transport. J Biol Chem. 2006;281:31594–31604. doi: 10.1074/jbc.M602765200. [DOI] [PubMed] [Google Scholar]
- 72.Roseman MA, Thompson TE. Mechanism of the spontaneous transfer of phospholipids between bilayers. Biochemistry. 1980;19:439–444. doi: 10.1021/bi00544a006. [DOI] [PubMed] [Google Scholar]
- 73.Storch J, Thumser AE. The fatty acid transport function of fatty acid-binding proteins. Biochim Biophys Acta. 2000;1486:28–44. doi: 10.1016/s1388-1981(00)00046-9. [DOI] [PubMed] [Google Scholar]
- 74.van Meel E, Klumperman J. Imaging and imagination: understanding the endo-lysosomal system. Histochem Cell Biol. 2008;129:253–266. doi: 10.1007/s00418-008-0384-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kobayashi T, Startchev K, Whitney AJ, Gruenber J. Localization of lysobisphosphatidic acid-rich membrane domains in late endosomes. Biol Chem. 2001;382:483–485. doi: 10.1515/BC.2001.059. [DOI] [PubMed] [Google Scholar]
- 76.Kobayashi T, Beuchat MH, Chevallier J, Makino A, Mayran N, Escola JM, Lebrand C, Cosson P, Gruenberg J. Separation and characterization of late endosomal membrane domains. J Biol Chem. 2002;277:32157–32164. doi: 10.1074/jbc.M202838200. [DOI] [PubMed] [Google Scholar]
- 77.Babalola JO, Wendeler M, Breiden B, Arenz C, Schwarzmann G, Locatelli-Hoops S, Sandhoff K. Development of an assay for the intermembrane transfer of cholesterol by Niemann-Pick C2 protein. Biol Chem. 2007;388:617–626. doi: 10.1515/BC.2007.063. [DOI] [PubMed] [Google Scholar]
- 78.Xu Z, Farver W, Kodukula S, Storch J. Regulation of sterol transport between membranes and NPC2. Biochemistry. 2008;47:11134–11143. doi: 10.1021/bi801328u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kobayashi T, Beuchat MH, Lindsay M, Frias S, Palmiter RD, Sakuraba H, Parton RG, Gruenberg J. Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat Cell Biol. 1999;1:113–118. doi: 10.1038/10084. [DOI] [PubMed] [Google Scholar]
- 80.Holtta-Vuori M, Maatta J, Ullrich O, Kuismanen E, Ikonen E. Mobilization of late-endosomal cholesterol is inhibited by Rab guanine nucleotide dissociation inhibitor. Curr Biol. 2000;10:95–98. [PubMed] [Google Scholar]
- 81.Chevallier J, Chamoun Z, Jiang G, Prestwich G, Sakai N, Matile S, Parton RG, Gruenberg J. Lysobisphosphatidic acid controls endosomal cholesterol levels. J Biol Chem. 2008;283:27871–27880. doi: 10.1074/jbc.M801463200. [DOI] [PubMed] [Google Scholar]
- 82.Linke T, Wilkening G, Sadeghlar F, Mozcall H, Bernardo K, Schuchman E, Sandhoff K. Interfacial regulation of acid ceramidase activity. Stimulation of ceramide degradation by lysosomal lipids and sphingolipid activator proteins. J Biol Chem. 2001;276:5760–5768. doi: 10.1074/jbc.M006846200. [DOI] [PubMed] [Google Scholar]
- 83.Wilkening G, Linke T, Uhlhorn-Dierks G, Sandhoff K. Degradation of membrane-bound ganglioside GM1. Stimulation by bis(monoacylglycero)phos-phate and the activator proteins SAP-B and GM2-AP. J Biol Chem. 2000;275:35814–35819. doi: 10.1074/jbc.M006568200. [DOI] [PubMed] [Google Scholar]
- 84.Infante RE, Wang ML, Radhakrishnan A, Kwon HJ, Brown MS, Goldstein JL. NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes. Proc Natl Acad Sci U S A. 2008;105:15287–15292. doi: 10.1073/pnas.0807328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Imrie J, Dasgupta S, Besley GT, Harris C, Heptinstall L, Knight S, Vanier MT, Fensom AH, Ward C, Jacklin E, Whitehouse C, Wraith JE. The natural history of Niemann-Pick disease type C in the UK. J Inherit Metab Dis. 2007;30:51–59. doi: 10.1007/s10545-006-0384-7. [DOI] [PubMed] [Google Scholar]
- 86.Chikh K, Rodriguez C, Vey S, Vanier MT, Millat G. Niemann-Pick type C disease: subcellular location and functional characterization of NPC2 proteins with naturally occurring missense mutations. Hum Mutat. 2005;26:20–28. doi: 10.1002/humu.20173. [DOI] [PubMed] [Google Scholar]
- 87.Verot L, Chikh K, Freydiere E, Honore R, Vanier MT, Millat G. Niemann–Pick C disease: functional characterization of three NPC2 mutations and clinical and molecular update on patients with NPC2. Clin Genet. 2007;71:320–330. doi: 10.1111/j.1399-0004.2007.00782.x. [DOI] [PubMed] [Google Scholar]
- 88.Li H, Repa JJ, Valasek MA, Beltroy EP, Turley SD, German DC, Dietschy JM. Molecular, anatomical, and biochemical events associated with neurodegeneration in mice with Niemann-Pick type C disease. J Neuropathol Exp Neurol. 2005;64:323–333. doi: 10.1093/jnen/64.4.323. [DOI] [PubMed] [Google Scholar]
- 89.Blom TS, Linder MD, Snow K, Pihko H, Hess MW, Jokitalo E, Veckman V, Syvanen AC, Ikonen E. Defective endocytic trafficking of NPC1 and NPC2 underlying infantile Niemann–Pick type C disease. Hum Mol Genet. 2003;12:257–272. doi: 10.1093/hmg/ddg025. [DOI] [PubMed] [Google Scholar]
- 90.Willenborg M, Schmidt CK, Braun P, Landgrebe J, von Figura K, Saftig P, Eskelinen EL. Mannose 6-phosphate receptors, Niemann-Pick C2 protein, and lysosomal cholesterol accumulation. J Lipid Res. 2005;46:2559–2569. doi: 10.1194/jlr.M500131-JLR200. [DOI] [PubMed] [Google Scholar]
- 91.Rigamonti E, Helin L, Lestavel S, Mutka AL, Lepore M, Fontaine C, Bouhlel MA, Bultel S, Fruchart JC, Ikonen E, Clavey V, Staels B, Chinetti-Gbaguidi G. Liver X receptor activation controls intracellular cholesterol trafficking and esterification in human macrophages. Circ Res. 2005;97:682–689. doi: 10.1161/01.RES.0000184678.43488.9f. [DOI] [PubMed] [Google Scholar]
- 92.Garver WS, Jelinek D, Francis GA, Murphy BD. The Niemann-Pick C1 gene is downregulated by feedback inhibition of the SREBP pathway in human fibroblasts. J Lipid Res. 2008;49:1090–1102. doi: 10.1194/jlr.M700555-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Garver WS, Heidenreich RA, Erickson RP, Thomas MA, Wilson JM. Localization of the murine Niemann-Pick C1 protein to two distinct intracellular compartments. J Lipid Res. 2000;41:673–687. [PubMed] [Google Scholar]
- 94.Higgins ME, Davies JP, Chen FW, Ioannou YA. Niemann-Pick C1 is a late endosome-resident protein that transiently associates with lysosomes and the trans-Golgi network. Mol Genet Metab. 1999;68:1–13. doi: 10.1006/mgme.1999.2882. [DOI] [PubMed] [Google Scholar]
- 95.Karten B, Campenot RB, Vance DE, Vance JE. The Niemann-Pick C1 protein in recycling endosomes of presynaptic nerve terminals. J Lipid Res. 2006;47:504–514. doi: 10.1194/jlr.M500482-JLR200. [DOI] [PubMed] [Google Scholar]
- 96.Neufeld EB, Wastney M, Patel S, Suresh S, Cooney AM, Dwyer NK, Roff CF, Ohno K, Morris JA, Carstea ED, Incardona JP, Strauss JF, III, Vanier MT, Patterson MC, Brady RO, Pentchev PG, Blanchette-Mackie EJ. The Niemann-Pick C1 protein resides in a vesicular compartment linked to retrograde transport of multiple lysosomal cargo. J Biol Chem. 1999;274:9627–9635. doi: 10.1074/jbc.274.14.9627. [DOI] [PubMed] [Google Scholar]
- 97.Zhang M, Sun M, Dwyer NK, Comly ME, Patel SC, Sundaram R, Hanover JA, Blanchette-Mackie EJ. Differential trafficking of the Niemann-Pick C1 and 2 proteins highlights distinct roles in late endocytic lipid trafficking. Acta Paediatr Suppl. 2003;92:63–73. doi: 10.1111/j.1651-2227.2003.tb00224.x. discussion 45. [DOI] [PubMed] [Google Scholar]
- 98.Ko DC, Gordon MD, Jin JY, Scott MP. Dynamic movements of organelles containing Niemann-Pick C1 protein: NPC1 involvement in late endocytic events. Mol Biol Cell. 2001;12:601–614. doi: 10.1091/mbc.12.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Berger AC, Salazar G, Styers ML, Newell-Litwa KA, Werner E, Maue RA, Corbett AH, Faundez V. The subcellular localization of the Niemann-Pick Type C proteins depends on the adaptor complex AP-3. J Cell Sci. 2007;120:3640–3652. doi: 10.1242/jcs.03487. [DOI] [PubMed] [Google Scholar]
- 100.Frolov A, Zielinski SE, Crowley JR, Dudley-Rucker N, Schaffer JE, Ory DS. NPC1 and NPC2 regulate cellular cholesterol homeostasis through generation of low density lipoprotein cholesterol-derived oxysterols. J Biol Chem. 2003;278:25517–25525. doi: 10.1074/jbc.M302588200. [DOI] [PubMed] [Google Scholar]


