Abstract
The innate immune system has a key role in the mammalian immune response. Recent research has demonstrated that mitochondria participate in a broad range of innate immune pathways, functioning as signalling platforms and contributing to effector responses. In addition to regulating antiviral signalling, mounting evidence suggests that mitochondria facilitate antibacterial immunity by generating reactive oxygen species and contribute to innate immune activation following cellular damage and stress. Therefore, in addition to their well-appreciated roles in cellular metabolism and programmed cell death, mitochondria appear to function as centrally positioned hubs in the innate immune system. Here, we review the emerging knowledge about the roles of mitochondria in innate immunity.
Following infection, microorganisms are initially sensed by pattern-recognition receptors (PRRs) of the innate immune system, which bind conserved molecular patterns that are shared by different classes of microorganisms. These pathogen-associated molecular patterns (PAMPs) include microbial structural components, nucleic acids and proteins. The list of PRRs known to sense PAMPs is extensive, and is comprised most notably of four families: Toll-like receptors (TLRs), nucleotide oligomerization domain (NOD)-like receptors (NLRs), C-type lectin receptors (CLRs) and retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs)1–3 (BOX 1). PRR ligation triggers multiple signalling pathways that culminate in the activation of nuclear factor-κB (NF-κB), mitogen-activated protein kinases (MAPKs) and interferon regulatory factors (IRFs), which control the expression of pro-inflammatory cytokines and chemokines, type I interferons (IFNs) and co-stimulatory molecules1,2,4. The resulting pro-inflammatory state is necessary for the generation of a robust antimicrobial environment and for the proper activation of the adaptive immune response.
Box 1. Pattern recognition by TLRs, NLRs, CLRs and RLRs.
Toll-like receptors (TLRs) are type I transmembrane proteins of the interleukin-1 receptor family that possess an amino-terminal leucine-rich repeat (LRR) domain for ligand binding, a single transmembrane domain and a carboxy-terminal intracellular signalling domain. TLRs are widely expressed by many cell types, although most cells express only a specific subset of these receptors. Haematopoietically derived sentinel cells, such as macrophages, neutrophils and dendritic cells (DCs), however, express the largest complement of TLRs. TLR1, TLR2, TLR4, TLR5 and TLR6 localize to the plasma membrane and mainly recognize bacterial pathogen-associated molecular patterns (PAMPs), such as the cell wall components lipopolysaccharide (LPS) and lipoteichoic acid, whereas TLR3, TLR7, TLR8 and TLR9 are predominately expressed in the endocytic compartment of immune cells and recognize viral and bacterial nucleic acids2,4.
NOD-like receptors (NLRs) are cytosolic receptors that contain N-terminal caspase-recruitment domains (CARDs), a central nucleotide oligomerization domain (NOD) and a C-terminal LRR domain99. NLRs recognize a wide variety of microbial PAMPs and endogenous damage-associated molecular patterns (DAMPs), and several notable examples of this family are NOD1, NOD-, LRR- and pyrin domain-containing 3 (NLRP3) and absent in melanoma 2 (AIM2). Signalling via many NLRs activates inflammasomes for subsequent engagement of caspase 1, the activity of which is crucial for proteolytic processing of the pro-inflammatory cytokines interleukin-1β (IL-1β) and IL-18 (REFS 82,99).
C-type lectin receptors (CLRs) comprise a large family of soluble and transmembrane proteins that possess one or more C-type lectin domain, which was initially characterized as a calcium-dependent carbohydrate-binding domain in mannose-binding lectin (MBL)3,100. CLRs recognize a wide range of carbohydrate structures on pathogens, although many CLRs also recognize self molecules and are therefore suggested to participate in both innate immune responses and cell and tissue homeostasis100. Notable examples of this family include the soluble CLR MBL and the transmembrane proteins dectin 1 (also known as CLEC7A) and DC-specific ICAM3-grabbing non-integrin (DC-SIGN).
The RIG-I-like receptor (RLR) family comprises three members: retinoic acid-inducible gene I (RIG-I), melanoma differentiation-associated gene 5 (MDA5) and laboratory of genetics and physiology 2 (LGP2; also known as DHX58)4,11. RLRs localize to the cytosol, are expressed in both immune and non-immune cells, and are required for type I interferon and pro-inflammatory cytokine production in response to viral infection. RIG-I and MDA5 possess two N-terminal CARDs that are required for signalling, a DExD/H box RNA helicase domain that detects viral RNA in the cytoplasm and a C-terminal repressor domain. LGP2 lacks N-terminal CARDs and has been suggested to function as both a negative and positive regulator of RIG-I and MDA5 signalling4,12.
Mitochondria are dynamic double-membrane-bound organelles that are involved in a wide range of cellular processes, including ATP generation, programmed cell death and calcium homeostasis, as well as the biosynthesis of amino acids, lipids, nucleotides and haem. Although mitochondria possess their own genome (mitochondrial DNA (mtDNA)) that encodes 13 proteins of the oxidative phosphorylation machinery, 2 ribosomal RNAs and 22 transfer RNAs essential for translation in the mitochondria, most of the ~1,500 proteins comprising the mitochondrial proteome are nuclear encoded5. Many of these proteins function in oxidative phosphorylation or other metabolic pathways, but they also include those needed for replication and expression of mtDNA (that is, the mitochondrial transcription and translation machinery). Consequently, coordination between the mitochondrial and nuclear genomes is required for proper assembly and function of the mitochondrial network, and cellular signalling cascades mediate crosstalk between mitochondria and the nucleus6. The mitochondrial network forms a reticular branching structure that, through association with the cytoskeleton, is motile and undergoes regular fusion and division7. Mounting evidence suggests that mitochondrial dynamics regulate many aspects of mitochondrial biology and are influenced by a variety of metabolic and cellular signals7.
Mitochondrial regulation of apoptotic signalling has been appreciated for some time; however, more recent evidence suggests that mitochondria also participate in various additional signalling pathways7,8. For example, research over the past several years has unveiled previously unappreciated roles for mitochondria in the innate immune response, and it is becoming increasingly apparent that mitochondria participate in RLR signalling, antibacterial immunity and sterile inflammation. Although mitochondrial control of apoptosis during infection is an important aspect of the mammalian innate immune response, this topic has been reviewed thoroughly by others9,10 and therefore is not discussed further in this article. Instead, we review and discuss the involvement of mitochondria in innate immune signalling pathways and the mechanisms by which these organelles facilitate effector responses of the innate immune system.
Mitochondria and antiviral immunity
The RLR family includes three members: RIG-I, melanoma differentiation-associated gene 5 (MDA5) and laboratory of genetics and physiology 2 (LGP2; also known as DHX58)4,11. RLRs are expressed in the cytosol, are present in both immune and non-immune cells, and are required for type I IFN and pro-inflammatory cytokine production in response to viral infection. RIG-I and MDA5 each possess two amino-terminal caspase-recruitment domains (CARDs) that are required for signalling, a DExD/H box RNA helicase domain that detects viral double-stranded RNA in the cytoplasm and a carboxy-terminal repressor domain. Although LGP2 was initially suggested to function as a negative regulator of RIG-I and MDA5 signalling, a more recent study indicates that LGP2 positively regulates RIG-I- and MDA5-dependent antiviral responses4,12.
In 2005, four laboratories independently identified a novel RLR adaptor molecule that is essential for RLR signal transduction. This protein was termed mitochondrial antiviral signalling protein (MAVS), IFNβ promoter stimulator 1 (IPS1), CARD adaptor inducing IFNβ (CARDIF) and virus-induced signalling adaptor (VISA)13–16. For convenience, we refer to the protein as MAVS in this article. One of the most intriguing properties of MAVS is its association with the outer mitochondrial membrane (OMM), a location that is required for proper antiviral signalling by MAVS15. Subsequent research has yielded additional insights into MAVS signalling downstream of RLRs, thereby demonstrating that mitochondria are centrally positioned in the innate immune response against viral pathogens.
MAVS
MAVS is comprised of 540 amino acids that form three functional domains: an N-terminal CARD (amino acids 10–77), a proline-rich region (amino acids 107–173) and a C-terminal transmembrane segment (amino acids 514–535)15,17. The CARD of MAVS interacts with the CARDs of RIG-I and MDA5, and this interaction is essential for the activation of downstream NF-κB and IRF signalling pathways for pro-inflammatory cytokine and type I IFN production13–15,18. However, the exact mechanisms by which cytosolic RLRs are recruited to mitochondria to interact with MAVS following viral infection remain unclear, and the interaction between these molecules at the mitochondrial surface has not been demonstrated in vivo13–15. The proline-rich region has also been implicated in downstream signalling via its interaction with TNF receptor-associated factor (TRAF) family members, although a MAVS mutant lacking the proline-rich region retains signalling capacity when overexpressed, suggesting that TRAF proteins may interact with additional regions of MAVS15,16,19. The C-terminal transmembrane domain of MAVS resembles that of other OMM proteins (such as B cell lymphoma 2 (BCL-2), BCL-XL and translocase of the outer membrane 20 (TOM20)), and a mutant MAVS protein lacking this region is mislocalized to the cytosol and fails to signal15. Chimeric MAVS constructs that contain the transmembrane domains of BCL-2 or BCL-XL, and therefore localize to the OMM, signal similarly to the wild-type protein, demonstrating that OMM targeting is required for effective MAVS signalling. The transmembrane domain also facilitates MAVS dimerization, a process necessary for TRAF binding and subsequent downstream signalling20.
MAVS adaptor molecules
It is well appreciated that MAVS activates NF-κB, IRF3 and IRF7 signalling cascades during viral infection, but the mechanisms by which this occurs are not fully understood. Although a thorough characterization of endogenous MAVS signalling complexes has not been conducted, many common innate immune signalling molecules have been shown to be downstream of MAVS17 (FIG. 1a). First, several TRAF family members, including TRAF2, TRAF3 and TRAF6, have been shown to bind TRAF-interaction motifs in the proline-rich region of MAVS to facilitate NF-κB, IRF3 and IRF7 signalling15,16,19. TRAF5 has also been suggested to participate in MAVS signalling by associating with dimerized MAVS CARDs21. Interestingly, the proline-rich region of MAVS is largely dispensable for NF-κB and IRF activation when MAVS is overexpressed but, as mentioned previously, the CARD is essential15. Consistent with this observation, TRAF3 has also been shown to bind dimerized CARDs (similarly to TRAF5), suggesting that the CARD, and not the proline-rich region, mediates interactions between MAVS and TRAF proteins20,21. Although additional research is required to fully understand TRAF-mediated MAVS signalling, it is apparent that the differential use of TRAF family members by MAVS allows for robust NF-κB- and/or IRF3- and IRF7-mediated activation of pro-inflammatory cytokine and/or type I IFN production. This is similar to the differential use of the signalling adaptors myeloid differentiation primary response protein 88 (MYD88) and TIR-domain-containing adaptor protein inducing IFNβ (TRIF) by TLRs.
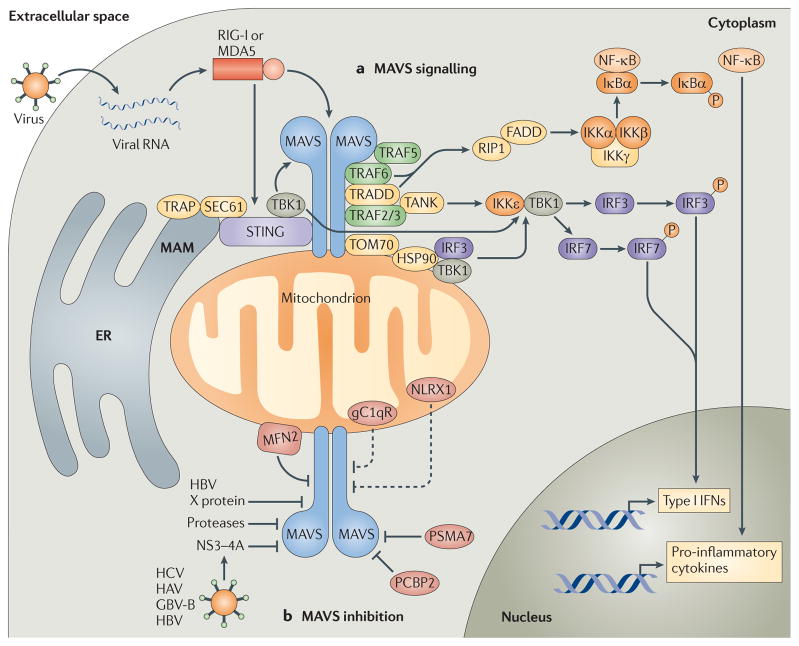
Figure 1. Mitochondrial antiviral signalling pathways.
a | Cytosolic viral RNA is recognized by the RIG-I-like receptors (RLRs) retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated gene 5 (MDA5), which activate mitochondrial antiviral signalling protein (MAVS) through caspase-recruitment domain (CARD)–CARD interactions. MAVS then recruits various signalling molecules to transduce downstream signalling, such as TNF receptor-associated factor 6 (TRAF6) and TRAF5. TRAF6, along with TNFR1-associated death domain protein (TRADD), activates canonical nuclear factor-κB (NF-κB) signalling via receptor-interacting protein 1 (RIP1) and FAS-associated death domain protein (FADD). Canonical NF-κB signalling occurs as the IκB kinase (IKK) complex — consisting of IKKα, IKKβ and IKKγ — phosphorylates NF-κB inhibitor-α (IκBα), resulting in the proteasomal degradation of IκBα and thus liberating NF-κB to translocate into the nucleus and initiate pro-inflammatory cytokine gene expression. MAVS also interacts with various molecules that activate interferon regulatory factor (IRF) signalling (such as stimulator of interferon genes (STING)). These molecules, together with the translocon-associated protein (TRAP) complex and the SEC61 translocon, mediate the activation of TANK-binding kinase 1 (TBK1), which phosphorylates IRF3 and IRF7. In addition, MAVS interacts with translocase of the outer membrane 70 (TOM70), which also interacts with heat shock protein 90 (HSP90) and thereby localizes TBK1 and IRF3 in proximity to the MAVS signalosome. Finally, MAVS binds TRAF2 and TRAF3 and, through TRADD and TRAF family member-associated NF-κB activator (TANK) interactions, promotes IKKε- and/or TBK1-mediated phosphorylation of IRF3. This promotes IRF3 nuclear translocation, leading to the expression of type I interferon (IFN) genes. b | MAVS signalling can also be inhibited by various molecules. For example, hepatitis C virus (HCV) encodes a serine protease, termed NS3–4A, that inhibits MAVS by cleaving it from the outer mitochondrial membrane and preventing the formation of MAVS–IKKε signalling complexes. Hepatitis A virus (HAV) and GB virus B (GBV-B) also encode proteases that disrupt the mitochondrial targeting of MAVS, and hepatitis B virus (HBV) X protein was shown to promote polyubiquitin conjugation to MAVS, leading to its degradation. Endogenous molecules such as poly(rC)-binding protein 2 (PCBP2) and the 20S proteasomal subunit PSMA7 can negatively regulate MAVS signalling during viral infection by promoting its degradation. Other molecules, such as mitofusin 2 (MFN2), receptor for globular head domain of complement component 1q (gC1qR) and NLR family member X1 (NLRX1) are also thought to inhibit MAVS signalling by direct interaction, although gC1qR and NLRX1 are thought to localize predominately to the mitochondrial matrix. Therefore, the mechanisms by which these molecules inhibit MAVS signalling remain under investigation (dashed lines). ER, endoplasmic reticulum; MAM, mitochondria-associated membrane.
The adaptor TNFR1-associated death domain protein (TRADD) also interacts with MAVS and recruits TRAF3 and TRAF family member-associated NF-κB activator (TANK), which then activate IκB kinase-ε (IKKε) and/or TANK-binding kinase 1 (TBK1), resulting in IRF3 and IRF7 activation22. In addition, MAVS–TRADD signalling leads to the recruitment of FAS-associated death domain protein (FADD) and receptor-interacting protein 1 (RIP1; also known as RIPK1), which induce canonical NF-κB signalling13,22. Furthermore, IKKα and IKKβ, and IKKε, which are kinases responsible for NF-κB and IRF activation, respectively, have also been identified as MAVS signalling partners, and IKKε colocalizes with mitochondria following viral infection14,23. Finally, WDR5 (WD repeat-containing protein 5) was recently shown to regulate MAVS signalling during viral infection by facilitating the assembly of active MAVS signalling complexes on the mitochondrial surface24.
Despite the identification of many cytosolic signalling molecules linking MAVS to the NF-κB, IRF3 and IRF7 pathways, the regulation of MAVS signalling during viral infection is not well understood. Furthermore, how cytosolic signalling molecules are recruited to mitochondrial membrane-bound MAVS complexes during viral infection remains undocumented. As the OMM contains numerous proteins that regulate many aspects of mitochondrial biology — including protein import, nutrient and ion exchange, organelle dynamics and apoptosis regulation — it is likely that mitochondrial proteins directly influence MAVS signalling. Recently, several bona fide mitochondrial proteins have been identified as MAVS binding partners, yielding new insight into the regulation of MAVS signalling at the mitochondrion.
Mitochondrial cofactors for MAVS signalling
Nuclear DNA-encoded mitochondrial proteins are imported across the OMM via the TOM machinery. The multi-protein TOM complex forms the translocation pore that recognizes and localizes pre-proteins for import into the intermembrane space of the mitochondrion. TOM20 and TOM70 serve as the primary pre-protein receptors and are anchored to the OMM via N-terminal transmembrane segments, whereas the hydrophilic domains of these proteins face the cytosol and bind mitochondrial targeting sequences (MTSs)25. TOM20 mainly binds pre-proteins containing a classical N-terminal MTS, whereas TOM70 interacts with proteins that possess internal hydrophobic targeting sequences, as well as chaperones such as heat shock protein 90 (HSP90) that are required for the proper localization of some pre-proteins to the TOM complex25.
Interestingly, HSP90 forms a complex with TBK1 and IRF3, and this complex is necessary for the robust activation of IRF3 during viral infection; however, as discussed above, TBK1 and IRF3 do not directly interact with MAVS26. Analysis of immunoprecipitated MAVS-containing complexes revealed that TOM70 directly interacts with MAVS and that the association between these molecules via their transmembrane domains increases following Sendai virus infection27. TOM70 also binds TRAF6, TRADD, TBK1 and IRF3, and the interaction between TOM70 and TBK1–IRF3 is mediated by HSP90 (REF. 27). Consistent with a role for TOM70 in RLR signalling, overexpression of TOM70 enhances the production of antiviral molecules such as IFNβ in response to viral infection or transfection with polyinosinic–polycytidylic acid (polyI: C; a mimic of viral double-stranded RNA), whereas small interfering RNA (siRNA)-mediated knockdown of TOM70 decreases antiviral innate immune responses27. Finally, overexpression of TOM70 reduces replication of vesicular stomatitis virus (VSV) and Newcastle disease virus in human embryonic kidney 293 cells, and TOM70-deficient cells display increased viral replication and susceptibility to infection27. This study does not clarify exactly how TOM70 transduces signals from MAVS to TBK1 and IRF3, and it remains unclear whether MAVS interacts with additional members of the TOM complex. However, the discovery that TOM70 functions as a mitochondrial adaptor in MAVS signalling is an important step towards characterizing the MAVS signalosome at the OMM.
Four recent reports have described a new modulator of MAVS signalling, which was independently termed stimulator of interferon genes (STING), mediator of IRF3 activation (MITA), endoplasmic reticulum interferon stimulator (ERIS) and MPYS28–31. For simplicity, we refer to this protein as STING throughout this article. STING localizes to the OMM and the outer endoplasmic reticulum (ER) membrane via four transmembrane domains28–30,32. Although the data more strongly support the notion that STING preferentially localizes to the ER membrane, the ER is tightly juxtaposed to mitochondria in areas termed mitochondria-associated membranes (MAMs), thereby making it difficult to unequivocally assign its site of subcellular localization33,34.
Nonetheless, overexpression of STING induces NF-κB and IRF3 signalling, as well as type I IFN production and IFN-dependent gene transcription, and this results in potent restriction of VSV replication28,29. Conversely, knockdown or knockout of Sting decreases IRF3 activation and type I IFN production in response to VSV or herpes simplex virus type 1 infection (or cytosolic B form DNA) and consequently increases susceptibility to these viruses. Interestingly, STING does not appear to be necessary for polyI:C-mediated type I IFN production via MDA5, suggesting that STING functions to selectively enhance RIG-I-mediated antiviral responses28. Indeed, STING directly interacts with RIG-I, MAVS and TBK1, leading to the phosphorylation and activation of IRF3 (REF. 29). In addition, interactions with components of the ER translocon-associated protein (TRAP) complex and the SEC61 translocon are crucial for STING-dependent antiviral responses28. Thus, it is likely that STING functions at MAMs, and this discovery highlights the importance of the ER–mitochondrion interface as a critical new domain for RLR–MAVS signalling32,33.
The elucidation of TOM70 and STING as mitochondrial cofactors for MAVS further expands our understanding of RLR signalling, and the search for additional mitochondrial proteins that influence MAVS will probably remain an active area of research in future years.
Negative regulators of MAVS signalling
Two negative regulators of MAVS signalling have recently been identified and are proposed to modulate the expression levels of MAVS during viral infection (FIG. 1b). Poly(rC)-binding protein 2 (PCBP2) is induced following viral infection and attenuates MAVS-dependent NF-κB and IRF3 signalling by recruiting the E3 ubiquitin ligase atrophin-1-interacting protein 4 (AIP4; also known as ITCH)35. PCBP2 serves as an adaptor molecule that bridges MAVS and AIP4 and, once recruited, AIP4 conjugates lysine 48 (K48)-linked polyubiquitin chains to MAVS, leading to its degradation by the proteasome. The 20S proteasomal subunit PSMA7 also inhibits MAVS signalling by inducing degradation, although the mechanistic details of this process remain unclear36.
Some RNA viruses use mitochondrial membranes for genome replication, and therefore the mitochondrial localization of MAVS might have evolved to concentrate innate immune receptors and signalling cascades in proximity to the sites of viral PAMP generation37. However, many viruses possess the ability to evade detection and/ or suppress host innate antiviral immune responses, and several reports have uncovered viral evasion strategies involving suppression of MAVS signalling10,38. For example, hepatitis C virus encodes a serine protease, termed NS3–4A, that inhibits RLR signalling and IFNβ production by cleaving MAVS from the OMM and preventing the formation of MAVS–IKKε signalling complexes14,23,39. Hepatitis A virus and GB virus B also encode proteases that disrupt the mitochondrial targeting of MAVS, although these two proteases target different residues of MAVS40,41. More recently, hepatitis B virus X protein was shown to inhibit MAVS signalling and IFNβ production by promoting polyubiquitin conjugation to residue K136 of MAVS, an event that induces MAVS degradation42.
Recent research has identified two negative regulators of MAVS that constitutively localize to mitochondria. Receptor for globular head domain of complement component 1q (gC1qR; also known as C1QBP, p33 and p32) is a 33 kDa protein that was initially identified as a receptor for the globular heads of C1q, although subsequent research has shown that it interacts with many additional intracellular and extracellular proteins43. gC1qR localizes to many cellular compartments, including the ER, nucleus, cell surface and mitochondria, although more recent research suggests that gC1qR is predominantly found in mitochondria and functions to regulate oxidative phosphorylation43–45.
A recent report suggests that gC1qR inhibits RLR-dependent NF-κB and IRF3 signalling by interacting with MAVS at the OMM46. This is suggested to occur as a result of the interaction between gC1qR and the cytosolic domain of MAVS, which disrupts RIG-I–MAVS interactions. gC1qR-deficient cells produce substantially more RLR-induced IFNβ in response to virus, and consequently are less susceptible to VSV infection, indicating that gC1qR suppresses antiviral immune responses46. However, gC1qR contains a classical N-terminal MTS and is imported into the mitochondrial matrix, which is seemingly inconsistent with OMM localization44,47. Therefore, the mechanisms governing gC1qR inhibition of MAVS signalling at the OMM during viral infection remain ambiguous, and more investigation is required to determine the molecular details governing gC1qR regulation of RLR signalling.
In 2008, two groups independently characterized a novel NLR family member, NLRX1 (also known as NOD9), and demonstrated that it is predominantly expressed in mitochondria and is targeted there by a putative N-terminal MTS48,49. Moore et al. showed that NLRX1 localizes to the OMM and interacts with the CARD of MAVS via its NOD-binding domain48. Overexpression of NLRX1 inhibits RLR- and MAVS-mediated NF-κB and IRF3 activation, whereas knockdown of NLRX1 augments MAVS-dependent antiviral immune responses. Furthermore, NLRX1 overexpression decreases the binding of RIG-I and MAVS during Sendai virus infection, suggesting that NLRX1 negatively regulates RLR signalling by masking the CARD of MAVS48. The second report demonstrated that NLRX1 overexpression augments reactive oxygen species (ROS) production, which potentiates NF-κB and JUN N-terminal kinase (JNK) signalling in response to tumour necrosis factor (TNF) stimulation and Shigella flexneri infection49. Although this study did not examine whether NLRX1 regulates MAVS signalling, a follow-up study from the same laboratory demonstrated that NLRX1 constitutively localizes to the mitochondrial matrix and is not present on the OMM, questioning the likelihood that NLRX1 negatively regulates MAVS at the OMM by blocking CARD signalling50.
Purification of NLRX1 complexes yielded a novel interaction partner, ubiquinol–cytochrome c reductase complex subunit 2 (UQCRC2), which is an integral component of complex III of the oxidative phosphorylation machinery50. UQCRC2 is anchored to the inner mitochondrial membrane (IMM), facing the mitochondrial matrix, which further supports the notion that NLRX1 localizes to the IMM. At present, it is unclear exactly how NLRX1 regulates MAVS signalling, which occurs at the OMM, although it is possible that NLRX1 localizes to both mitochondrial membranes during viral infection.
Mitochondrial dynamics govern antiviral signalling
The mitochondrial network in eukaryotic cells is highly dynamic, with both fusion and fission events occurring regularly to regulate morphology and activity. Mitochondrial dynamics determine the integrity of the mitochondrial network and maintain respiratory capacity, but also participate in several other processes, including mammalian development, neurodegeneration and apoptosis51,52. Mitofusin (MFN) proteins are the best-characterized fusion regulators, whereas mitochondrial fission protein 1 (FIS1) and dynamin-related protein 1 (DRP1) control fission51. MFN1 and MFN2 are dynamin-related GTPases expressed on the OMM that tether mitochondria together during fusion, although MFN2 is also expressed on MAMs and regulates mitochondrial–ER tethering53.
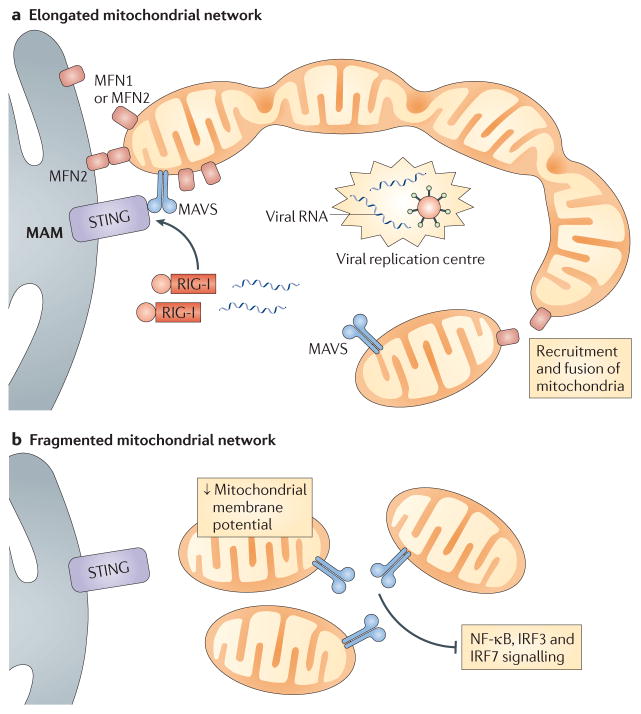
Recently, several studies have implicated mitochondrial dynamics in the regulation of RLR signalling, introducing a new layer of complexity to mitochondrial antiviral immune responses. First, MFN2 was shown to constitutively interact with MAVS in high molecular weight complexes and to inhibit RLR signalling54. Overexpression of MFN2 blocked NF-κB and IRF3 activation downstream of RIG-I, MDA5 and MAVS, whereas MFN2-depleted cells displayed heightened MAVS signalling and generated considerably more IFNβ following viral infection54. Interestingly, manipulating MFN1 expression levels did not yield similar phenotypes, suggesting that MFN2 has a unique role in regulating MAVS signalling independent of its function in mitochondrial fusion.
However, two additional reports directly implicate MFN1 in RLR signalling and demonstrate a role for mitochondrial dynamics in antiviral responses. Castanier et al. showed that MAVS interacts with MFN1 and that mitochondrial fusion is required for efficient RLR signalling, as inhibition of fusion by knockdown of either MFN1 or optic atrophy 1 (OPA1) decreased virus-induced NF-κB and IRF3 activation32. Conversely, cells depleted of one of the fission-inducing proteins DRP1 and FIS1 displayed elongated mitochondrial networks and increased RLR signalling. The study further demonstrated that elongated mitochondria promote ER–mitochondria interactions during viral infection, thus enhancing the association of MAVS with STING to augment RLR signalling32 (FIG. 2). In agreement with these findings, Onoguchi et al. also observed interactions between MAVS and MFN1, and showed that MFN1 is required for efficient RLR signalling55. They discovered that RIG-I localizes around centres of viral replication that contain nucleocapsid proteins and RNA, and that this leads to the recruitment of MAVS-enriched mitochondria and subsequent downstream signalling. This process requires MFN1, further implicating mitochondrial fusion as a requisite event in MAVS-dependent RLR signalling.
Figure 2. Mitochondrial dynamics regulate MAVS signalling.
a | During infection, retinoic acid-inducible gene I (RIG-I) and mitochondrial antiviral signalling protein (MAVS)-enriched mitochondria are recruited around centres of viral replication to promote MAVS signalling. This occurs as mitofusin 1 (MFN1) and MFN2 induce fusion of the mitochondrial network, which also serves to increase MAVS interactions with downstream signalling molecules. Mitochondrial MFN1 and MFN2 also interact with endoplasmic reticulum-localized MFN2, which promotes interactions between MAVS and stimulator of interferon genes (STING) at mitochondria-associated membranes (MAMs). b | Fragmentation of the mitochondrial network — which is induced by viral infection, mitofusin deficiency or overexpression of fission-promoting molecules — results in decreased mitochondrial membrane potential and blocks interactions between MAVS and signalling molecules such as STING. This leads to reduced signalling by nuclear factor-κB (NF-κB), interferon regulatory factor 3 (IRF3) and IRF7.
A more recent study demonstrated that fibroblasts deficient in both MFN1 and MFN2 (but not cells lacking only one of these proteins) display markedly decreased RLR-dependent antiviral responses. These double-knockout fibroblasts exhibit heterogeneous mitochondrial membrane potential owing to a total absence of fusion, which the authors suggest may be responsible for the reduced MAVS signalling observed in these cells56. In addition, treatment of cells with chemical uncoupling compounds that decrease mitochondrial membrane potential reduces RLR signalling to NF-κB and IRF3 and lowers type I IFN production, further implicating robust mitochondrial membrane potential as a necessary component of MAVS signalling.
There are some discrepancies between the findings discussed above, but it is quite apparent that MFNs interact with MAVS during RLR signalling, and additional research should clarify the exact roles of MFN1 and MFN2 in this process. Taken together, these studies detail a scenario in which mitochondrial fusion serves to enhance mitochondria–MAMs interactions and RLR–MAVS signalosome formation around intracellular sites of viral infection.
Mitochondrial ROS and antiviral signalling
A consequence of electron transport through mitochondrial oxidative phosphorylation complexes is the generation of ROS (BOX 2). Although ROS can damage cellular proteins, lipids and nucleic acids via oxidation, they are also crucial second messengers in various redox-sensitive signalling pathways. As mitochondria are a significant source of ROS in many eukaryotic cells, mitochondrial ROS (mROS) have been suggested to modulate several signalling pathways8. Interestingly, ROS have been implicated as both positive and negative modulators of RLR signalling57–59.
Box 2. Mitochondrial oxidative phosphorylation and ROS generation.
The mitochondrial respiratory chain (which carries out oxidative phosphorylation) comprises five multisubunit protein complexes localized on the inner mitochondrial membrane (see the figure). Electrons (e−) are donated to complex I from NADH or to complex II from FADH2 and are passed to coenzyme Q (CoQ; also known as ubiquinone), which then carries them to complex III. Complex III then passes electrons to cytochromec (Cyt c), which relays them to complex IV, where the electrons mediate the combination of hydrogen ions (H+) and molecular oxygen (O2) to form water (H2O). The movement of electrons across complex I, complex III and complex IV is also coupled to H+ pumping across the inner mitochondrial membrane from the matrix to the intermembrane space. This generates an electrochemical gradient that is used by complex V (also known as ATP synthase) to generate ATP from ADP and inorganic phosphate (Pi).
Mitochondrial oxidative phosphorylation is a major cellular source of reactive oxygen species (ROS), as approximately 1–2% of oxygen consumed during physiological respiration is converted into superoxide (O2•−) when electrons prematurely leak from the electron transport chain and are aberrantly transferred to molecular oxygen101. However, under specific metabolic or stress conditions, more electrons can prematurely exit the respiratory chain to further augment mitochondrial superoxide generation. Leakage occurs at complex I, complex II or complex III, although complex I and complex III are the major sites of superoxide generation within mitochondria61,101. Superoxide from complex I and complex II is released into the matrix, whereas superoxide from complex III can be produced on either side of the inner membrane62. Superoxide can then cross the outer mitochondria membrane via a voltage-dependent anion-selective channel (VDAC) or can be converted into hydrogen peroxide (H2O2) in the matrix by superoxide dismutase 2 (SOD2) or in the intermembrane space by SOD1 (REF. 102). H2O2 can then freely cross mitochondrial membranes (dashed arrows) or can be further detoxified by additional mitochondrial antioxidant enzymes, such as glutathione peroxidase (GPX). In the cytosol, superoxide is converted by SOD1 into H2O2, which is further detoxified by the peroxisomal enzyme catalase.

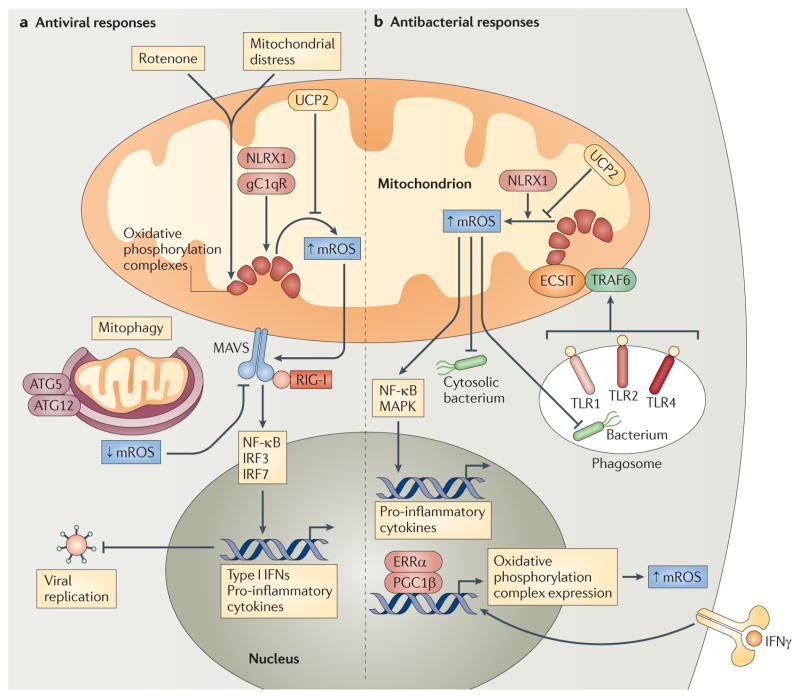
Autophagy-related gene 5 (ATG5) and ATG12 — which are crucial regulators of autophagy and mitophagy (the specific removal of damaged mitochondria) — can inhibit RLR signalling, and Tal et al. demonstrated that Atg5−/− cells accumulate dysfunctional mitochondria and exhibit increased mROS levels and increased type I IFN production in response to polyI:C stimulation59,60. Treatment of Atg5−/− fibroblasts and primary macrophages with antioxidants reduces the production of type I IFNs to wild-type levels, suggesting that aberrant ROS generation potentiates RLR signalling in these cells (FIG. 3a). Furthermore, augmentation of mROS via exposure of cells to the oxidative phosphorylation complex I inhibitor rotenone (which induces the production of mROS) increases polyI:C-induced IFNβ generation in both wild-type and Atg5−/− cells59. Therefore, increased RLR signalling and subsequent antiviral responses in the absence of ATG5 are possibly the result of heightened mROS generation from accumulated abnormal or damaged mitochondria in these cells. Furthermore, mROS induction also potentiates RLR signalling in wild-type cells, thus implicating mROS more broadly as important second messengers in RLR–MAVS signalling59.
Figure 3. Mitochondrial ROS and innate immune responses.
a | Mitochondrial distress and/or rotenone treatment augment mitochondrial reactive oxygen species (mROS) generation from oxidative phosphorylation complexes, and this potentiates RIG-I-like receptor (RLR)–mitochondrial antiviral signalling protein (MAVS) signalling to nuclear factor-κB (NF-κB) and interferon regulatory factor 3 (IRF3) and IRF7. This increases the production of type I interferons (IFNs) and pro-inflammatory cytokines, which limit viral replication. NLR family member X1 (NLRX1) and receptor for globular head domain of complement component 1q (gC1qR) interact with oxidative phosphorylation complexes and may increase mROS generation, whereas uncoupling protein 2 (UCP2) decreases mROS production. Mitophagy mediated by autophagy-related gene 5 (ATG5) and ATG12 also decreases mROS production and subsequent RLR–MAVS signalling by removing dysfunctional, mROS-generating mitochondria. b | Phagocytized bacteria activate Toll-like receptor 1 (TLR1), TLR2 and TLR4, and this promotes the translocation of TNF receptor-associated factor 6 (TRAF6) to mitochondria, where it engages ECSIT (evolutionary conserved signalling intermediate in Toll pathways) to potentiate mROS generation from oxidative phosphorylation complexes. This leads to increased ROS-dependent bactericidal responses and/or activation of NF-κB and mitogen-activated protein kinase (MAPK) signalling to augment pro-inflammatory cytokine production. IFNγ signalling can also promote mROS generation and increased antibacterial innate immunity by engaging oestrogen-related receptor-α (ERRα) and the co-activator peroxisome proliferator-activated receptor-γ co-activator 1β (PGC1β), which upregulate mitochondrial biogenesis and the expression of nuclear-encoded oxidative phosphorylation genes. UCP2 is a negative regulator of mROS generation, whereas NLRX1 can enhance mROS production under certain circumstances. RIG-I, retinoic acid-inducible gene I.
In addition to the above findings, several other lines of evidence support the notion that mROS participate in RLR signalling. First, the negative regulator of MAVS signalling gC1qR localizes to the mitochondrial matrix and regulates the activity of oxidative phosphorylation complex I, complex III, complex IV and complex V45. Knockdown of gC1qR results in the dysregulation of these complexes, a process that can lead to heightened mROS generation61,62. Therefore, it is possible that depletion of gC1qR potentiates MAVS signalling by altering oxidative phosphorylation protein abundance and increasing mROS generation46. Some viruses can directly modulate oxidative phosphorylation complexes to potentially reduce mROS generation, and it is possible that viral infection might upregulate gC1qR to limit mROS generation, and thus MAVS signalling46,63.
In addition, NLRX1 regulates ROS generation and binds a core component of oxidative phosphorylation complex III, a major site of mROS generation49,50. Hence, it is tempting to speculate that NLRX1 may modulate RLR signalling by altering mROS production, in addition to interacting with MAVS at the OMM48,50. Finally, Koshiba et al. showed that mitochondrial uncoupling, either pharmacologically or by overexpression of uncoupling protein 2 (UCP2), potently decreases RLR–MAVS signalling56. Mild uncoupling decreases mROS generation and, as such, the reduced MAVS signalling and RLR-dependent antiviral responses induced by uncoupling may be due in part to lowered mROS generation64,65.
Collectively, these data indicate that mROS may have a central role in the regulation of RLR–MAVS signalling during viral infection, although additional research is needed to definitively delineate the mechanisms by which this occurs. It remains unclear which aspects of the RLR pathway are sensitive to mROS, and whether viruses directly manipulate mROS levels during infection to control RLR signalling is unknown. As such, this area of research will probably expand significantly in the future.
Mitochondrial ROS and antibacterial responses
The phagocytic response of the innate immune system is crucial for the effective clearance of microbial pathogens and is indispensable for host defence. This response is initiated following microbial contact with host phagocytes (mainly macrophages and neutrophils) and results in the engulfment and killing of microorganisms within phagosomes. Phagocytosis is associated with the production of ROS via the respiratory burst, a necessary effector response for the destruction of intracellular microorganisms66,67. Although ROS are primarily produced by the NADPH oxidase system in phagocytes, mitochondrial oxidative metabolism is also a major source of cellular ROS. The production of mROS has traditionally been considered a deleterious consequence of electron transport, but mounting evidence indicates that mROS also facilitate antibacterial innate immune signalling and phagocyte bactericidal activity (FIG. 3b). Below we summarize the most convincing evidence in support of this hypothesis.
Mitochondrial UCPs and biogenesis factors in mROS generation
UCP2 is a member of a family of mitochondrial uncoupling proteins that are homologous to UCP1 (REF. 65). Although UCP2 shares 60% sequence identity with UCP1 and both proteins localize to the IMM, UCP2 exhibits a broad tissue distribution and is abundantly expressed in monocytes and macrophages68, whereas UCP1 expression is restricted to brown adipose tissue. UCP2 has been shown to induce mild mitochondrial uncoupling, which increases the rate of respiration and is thought to reduce electron leak from oxidative phosphorylation complexes, thereby decreasing mitochondrial superoxide generation65.
To investigate the role of UCP2 in immune responses, Arsenijevic and colleagues64 generated Ucp2−/− mice and challenged them with the intracellular pathogen Toxoplasma gondii. Strikingly, Ucp2−/− mice are significantly more resistant to T. gondii-induced lethality and display decreased brain cyst formation compared with wild-type animals. In addition, Ucp2−/− macrophages exhibit heightened toxoplasmacidal activity, increased killing of intracellular Salmonella enterica subsp. enterica serovar Typhimurium and higher basal and T. gondii-induced ROS levels64. A subsequent study detailed similar phenotypes, demonstrating that Ucp2−/− mice are more resistant to Listeria monocytogenes and display higher splenic ROS levels than wild-type mice69.
Additional studies support the notion that UCP2 regulates antibacterial innate immune responses by suppressing mROS production through mitochondrial uncoupling. For example, macrophages overexpressing UCP2 produce substantially lower levels of cellular ROS than wild-type macrophages when stimulated with the bacterially derived TLR4 agonist lipopolysaccharide (LPS), suggesting that mROS contribute to the total oxidative burst of activated macrophages70. LPS stimulation also appears to decrease macrophage UCP2 expression, indicating that the abundance of UCP2 in the cell regulates mROS generation during TLR4 signalling71. Furthermore, the increased mROS levels in macrophages from Ucp2−/− mice result in heightened activation of ROS-sensitive NF-κB and MAPK signalling pathways, and consequently augment pro-inflammatory cytokine production71–73. These reports not only indicate that UCP2 participates in macrophage mROS regulation, but more broadly implicate mROS as important components in innate immune responses against intracellular bacteria and parasites.
Oestrogen-related receptor-α (ERRα; also known as ERR1) is a nuclear orphan receptor that acts coordinately with the transcriptional co-activators peroxisome proliferator-activated receptor-γ co-activator 1α (PGC1β) and PGC1β to control numerous aspects of mitochondrial biology and oxidative metabolism, including mitochondrial biogenesis, fatty acid oxidation and oxidative phosphorylation74. Recently, it was demonstrated that IFNγ induces an ERRα- and PGC1β-dependent transcriptional programme in macrophages that promotes mitochondrial function and mROS production during bacterial infection75. Exposure of macrophages to IFNγ triggers increased ERRα–PGC1β transcriptional activity, leading to heightened respiration, increased mitochondrial membrane potential and augmented mROS generation. Consequently, ERRα- or PGC1β-deficient mice, and macrophages from these mice, produce less ROS following challenge with L. monocytogenes and display a reduced ability to clear these bacteria. The heightened susceptibility of these knockout mice to bacterial challenge occurs in the presence of normal NADPH oxidase-dependent ROS generation and is not due to impairments in mitochondrial function or ATP generation75. Collectively, these results indicate that ERRα and PGC1β act coordinately downstream of IFNγ signalling to control mROS production and macrophage bactericidal activity, further bolstering the notion that mROS are integral participants in innate immune responses.
TLR signalling and mROS production
Although the studies described above strongly implicate mROS as necessary components of the innate immune response to intracellular pathogens, the possibility that PRR signalling directly regulates mROS production has remained unexplored. In addition, these reports did not address whether perturbations in mROS levels alone are sufficient to alter bactericidal activity. A recent report from our laboratory provides new insight into these outstanding questions76. We show that signalling via of a subset of TLRs (TLR1, TLR2 and TLR4) directly augments mROS generation in macrophages. This response involves the translocation of the TLR signalling adaptor TRAF6 to mitochondria, where it engages ECSIT (evolutionarily conserved signalling intermediate in Toll pathways), a previously characterized TRAF6-interacting protein implicated in oxidative phosphorylation complex I assembly77,78. Interaction between these proteins at the mitochondrial surface leads to the TRAF6-dependent ubiquitylation of ECSIT, which is necessary for concomitant increases in mitochondrial and cellular ROS generation following TLR1, TLR2 or TLR4 ligation. Consistent with a role for mROS in bactericidal responses, ECSIT- or TRAF6-deficient macrophages exhibit decreased levels of TLR-induced ROS and are impaired in their ability to kill S. Typhimurium. In addition, the reduced macrophage mROS levels in MCAT mice (which transgenically overexpress the antioxidant enzyme catalase in mitochondria) result in inhibited bacterial killing, similarly to ECSIT depletion. Consequently, ECSIT-deficient and MCAT mice display increased bacterial burdens in the liver and spleen when challenged intraperitoneally with S. Typhimurium. Our results have therefore revealed a novel pathway by which macrophages generate ROS in response to bacteria by coupling TLR1, TLR2 and TLR4 signalling to complex I via TRAF6 and ECSIT. Although future research is needed to determine the exact mechanism by which ECSIT controls mROS generation from oxidative phosphorylation complexes, our study bolsters the conclusion that, in addition to NADPH oxidase-derived ROS, mROS have an important role in macrophage-associated antibacterial immune responses.
Mitochondria and cellular damage responses
In addition to recognizing a diverse array of microbial PAMPs, it is now well appreciated that PRRs of the innate immune system sense cellular damage and stress in the absence of microbial infection79–81. Sterile tissue injury and cellular necrosis, for example, elicit robust responses characterized by pro-inflammatory cytokine production and leukocyte recruitment, and are triggered by PRR-dependent sensing of damage-associated molecular patterns (DAMPs). DAMPs are endogenous molecules that are usually sequestered within intracellular compartments of healthy cells but are released during pathological insult. This so-called ‘hidden-self’ recognition serves to alert the host to cell and tissue dysfunction, leading to containment of the injury and promotion of tissue repair80,81. TLRs and NLRs both sense DAMPs in various contexts, although many DAMP receptors remain uncharacterized79–82. Recently, mitochondrial constituents have been implicated as DAMPs, triggering responses to necrosis and cellular stress (TABLE 1). In addition, two independent reports suggest that mROS activate the NOD-, LRR- and pyrin domain-containing 3 (NLRP3) inflammasome, a cytosolic signalling complex that responds to various cellular stresses. In the following section, we discuss the emerging roles of mitochondria in the activation of the cellular damage response.
Table 1.
Mitochondrial DAMPs
| DAMP | Location of action | Receptor | Inflammatory activity | Refs |
|---|---|---|---|---|
| mtDNA | Endosomal | TLR9 |
|
83–85 |
| Intracellular | AIM2 and other uncharacterized receptor(s) | Caspase 1 activation and cytokine secretion | 94 | |
| N-formyl peptides | Extracellular | FPR1 |
|
85, 87–91 |
| Mitochondrial TFAM | Extracellular (functions in combination with N-formyl peptides) | Uncharacterized | Monocyte activation and cytokine secretion | 91 |
| Mitochondrial ATP | Extracellular | P2RX7 and NLRP3 inflammasome |
|
93 |
| mROS | Intracellular | NLRP3 inflammasome | Caspase 1 activation and cytokine secretion | 94,97 |
AIM2, absent in melanoma 2; DAMP, damage-associated molecular pattern; FPR1, formyl-peptide receptor 1; mtDNA, mitochondrial DNA; mROS, mitochondrial reactive oxygen species; NLRP3, NOD−, LRR− and pyrin domain-containing 3; P2RX7, P2X purinoceptor 7; TFAM, mitochondrial transcription factor A; TLR9, Toll-like receptor 9.
Mitochondrial DAMPS
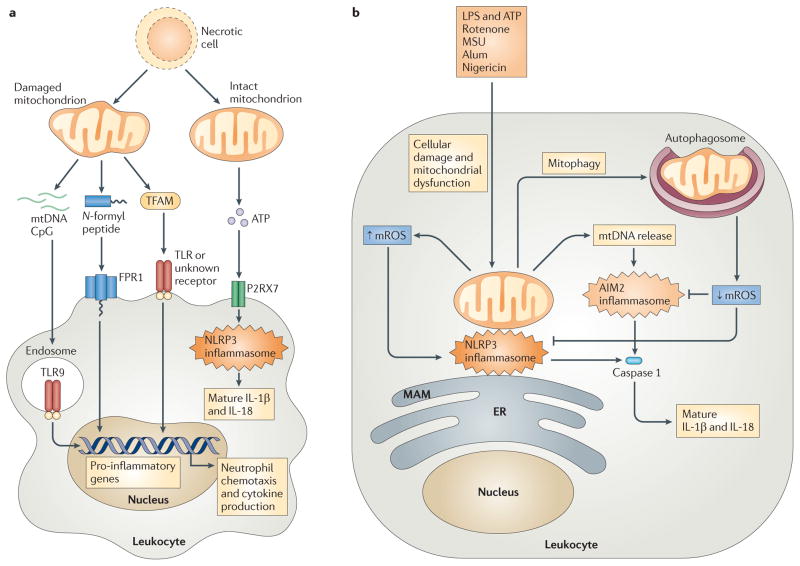
Because of their bacterial origin, eukaryotic mitochondria share several features with bacteria, including a double-membrane structure, a circular genome that replicates independently of nuclear DNA and N-formylated proteins. The innate immune system evolved to recognize conserved bacterial molecules that are largely foreign to host cells. However, owing to the many similarities between mitochondrial and bacterial constituents, it has been hypothesized that mitochondria might contain sequestered DAMPs that could trigger innate immune responses during pathological insult (FIG. 4a). For example, mtDNA contains hypomethylated CpG motifs that resemble bacterial CpG DNA, which is a potent activator of TLR9 (REF. 2). In support of the mitochondrial DAMP hypothesis, Collins et al. demonstrated that injection of purified mtDNA into the joints of mice induces inflammation and arthritis, and that exposure of splenocytes to mtDNA, but not nuclear DNA, augments pro-inflammatory cytokine secretion83. In addition, mtDNA was shown to directly activate neutrophils via TLR9 signalling84,85. Furthermore, mtDNA is released into circulation during trauma and haemorrhagic shock, and in vivo administration of mitochondrial extracts containing mtDNA causes systemic lung and liver inflammation84,85.
Figure 4. Mitochondrial involvement in cellular damage responses.
a | Cellular injury and necrosis release damaged mitochondria into the extracellular space, where they leak mitochondrial damage-associated molecular patterns (DAMPs) such as mitochondrial DNA (mtDNA), N-formyl peptides and mitochondrial transcription factor A (TFAM). Leukocytes, such as macrophages and neutrophils, detect mtDNA through Toll-like receptor 9 (TLR9), N-formyl peptides via formyl peptide receptor 1 (FPR1), and TFAM through TLRs or an uncharacterized receptor, leading to leukocyte activation and transcription of pro-inflammatory cytokine genes. Necrotic cells can also release intact mitochondria that secrete ATP. This ATP engages the leukocyte purinergic receptor P2RX7, which promotes NOD-, LRR- and pyrin domain-containing 3 (NLRP3) inflammasome activation and the subsequent processing and secretion of the cytokines interleukin-1β (IL-1β) and IL-18. b | Exposure of leukocytes to inflammasome activators — such as lipopolysaccharide (LPS) and ATP, rotenone, monosodium urate (MSU), alum and nigericin — leads to mitochondrial dysfunction and mitochondrial ROS (mROS) generation. mROS promote inflammasome activation at mitochondria-associated membranes (MAMs) of the endoplasmic reticulum (ER), by nucleating and activating a complex of NLRP3, ASC and caspase 1. This induces the proteolytic processing of pro-IL-1β and pro-IL-18 into mature and secreted forms. mROS also promote the release of mtDNA into the cytosol, and this DNA engages the absent in melanoma 2 (AIM2) inflammasome or other receptors to further augment IL-1β and IL-18 processing and secretion. Mitophagy serves as a brake on inflammasome activation by mediating the clearance of damaged, mROS-generating mitochondria.
Bacterial protein synthesis initiates at the N-formyl-methionine residue, and bacterial protein-derived N-formyl peptides (such as N-formyl-methionyl-leucyl-phenylalanine) activate the innate immune response86. N-formyl peptides bind to G protein-coupled formyl peptide receptors (FPRs) that are expressed by neutrophils, leading to intracellular calcium mobilization, MAPK activation and cell migration86. Translation of mtDNA-encoded proteins also initiates at the N-formyl-methionine residue and evidence suggests that mitochondrial N-formyl peptides possess immunostimulatory activity. N-formyl peptides derived from mitochondrially encoded oxidative phosphorylation proteins promote FPR1-mediated neutrophil calcium flux, chemotaxis, oxidative burst and cytokine secretion85,87–90. In addition, release of mitochondrial transcription factor A (TFAM) — a homologue of the DAMP high-mobility group protein B1 (HMGB1) — by necrotic cells potentiates mitochondrial N-formyl peptide-induced secretion of CXC-chemokine ligand 8 (CXCL8; also known as IL-8) from monocytes91. These results collectively demonstrate that mitochondrial constituents can serve as DAMPs, and moreover illustrate that cell or tissue trauma and necrosis elicit the release of these molecules to activate PRR-dependent sterile inflammatory responses.
Mitochondria and inflammasome activation
NLRP3 is a well-characterized member of the cytosolic NLR family that functions coordinately with the adaptor molecule apoptosis-associated speck-like protein containing a CARD (ASC) and the cysteine protease caspase 1 (REFS 82,92). These molecules comprise the NLPR3 inflammasome, which, when assembled, promotes the activation of caspase 1 and its subsequent processing of the cytokines pro-IL-1β and pro-IL-18 into mature and secreted forms82. The NLRP3 inflammasome is activated by a variety of endogenous and exogenous stimuli, such as microbial infection, bacterial poreforming toxins, monosodium urate, extracellular ATP, asbestos and the adjuvant alum92. Although it is appreciated that the NLRP3 inflammasome responds to many agents of cellular stress, the exact mechanisms leading to its activation by such divergent stimuli remain poorly understood. Recent data suggest that many NLRP3 stimuli lead to ROS production, and mounting evidence points to a crucial role for ROS in NLRP3 inflammasome activation92.
A recent study demonstrates a role for mitochondria in NLRP3 inflammasome activation93. Exposure of primed macrophages to the mitochondrial fraction of necrotic cells leads to caspase 1 activation and IL-1β secretion in an NLRP3- and ASC-dependent manner. In addition, intraperitoneal injection of mitochondria from pressure-disrupted cells promotes NLRP3-dependent neutrophil recruitment into the peritoneal cavity. Extracellular ATP is known to contribute to NLRP3 activation via the purinergic receptor P2RX7, and the authors of this study demonstrated that NLRP3 activation in response to mitochondria from necrotic cells is partially P2RX7 dependent93. The presence of additional DAMPs in mitochondrial preparations could account for the P2RX7-independent activity observed, as mtDNA has been recently shown to potentiate inflammasome-regulated IL-1β and IL-18 secretion94. Nonetheless, this study suggests that intact extracellular mitochondria that produce ATP can serve as DAMPs to promote innate inflammatory responses via the NLRP3 inflammasome (FIG. 4a).
As mentioned above, ROS production is common to many activators of the NLRP3 inflammasome, and pharmacological blockade of ROS can inhibit inflammasome activation by several stimuli82,92. The cellular sources of the ROS responsible for NLRP3 inflammasome activation have remained unclear, although several reports have convincingly excluded the NADPH oxidase isoforms NOX1, NOX2 and NOX4 (REFS 95,96). Two recent studies suggest that mROS are a crucial activator of the NLRP3 inflammasome (FIG. 4b). Treatment of macrophages with inhibitors of oxidative phosphorylation that augment mROS generation results in increased NLRP3-dependent caspase 1 activation and heightened IL-1β and IL-18 secretion94,97. Furthermore, oxidative phosphorylation-deficient cells and those treated with mitochondrial antioxidants display decreased responses to NLRP3 stimuli94. NLRP3 and ASC translocate to mitochondria and MAMs when exposed to inflammasome activators, a process that probably localizes inflammasome components proximal to sources of mROS, thus facilitating signalling97. Finally, both reports show that mitophagy serves as a brake on inflammasome signalling by removing dysfunctional, mROS-generating mitochondria, a finding that parallels previous observations regarding mitophagy and control of RLR signalling by mROS reduction94,97,98. These studies do not define the mechanisms by which mROS specifically signal to the inflammasome, although it is possible that NLRP3 or ASC undergo redox-dependent alterations, in a similar manner to caspase 1 (REF. 95). Nonetheless, these studies demonstrate that mROS are potent activators of the NLRP3 inflammasome downstream of several diverse stimuli, implicating mitochondria as important modulators of the inflammatory response to cellular stress.
Conclusions and future perspectives
In the years since the discovery of MAVS as a mitochondria-anchored adaptor molecule involved in RLR signalling, additional research has shown that mitochondria are also intertwined in the innate immune response to bacterial pathogens and cellular damage. Thus, in addition to their well-established roles in cellular metabolism and apoptosis regulation, mitochondria appear to function as centrally positioned hubs for innate immune signalling and the subsequent generation of effector responses. As innate immune activation requires increased cellular energy output and significant metabolic reprogramming, the incorporation of mitochondria into this arm of immunity may have evolved to enhance crosstalk between metabolic and innate immune pathways. Furthermore, many intracellular pathogens directly modulate mitochondrial programmed cell death responses to enhance their survival and replication. Thus, the intersection of mitochondria and innate immune signalling may have arisen to augment the host’s ability to sense this major microbial virulence mechanism.
Future research should reveal additional details surrounding the nucleation of RLR signalling at the interface between mitochondria and MAMs and will probably identify new mitochondrial molecules that regulate MAVS signalling. In addition, further investigation into the regulation of mROS during microbial infection and cellular stress is warranted, as these molecules appear to influence several important aspects of the innate immune response. A more detailed understanding of the mechanisms by which mROS function as both signalling molecules and antimicrobial effectors will promote a clearer characterization of the interplay between mitochondria and the innate immune system.
Acknowledgments
We regret that several important studies could only be cited indirectly through comprehensive reviews, owing to space and reference number limitations. We thank L. Ciaccia for assistance with the figures. This work was supported by grants from the US National Institutes of Health to S.G. (R37-AI33443) and G.S. (ES-011, 163).
Glossary
- Programmed cell death
A common form of cell death that is also referred to as apoptosis. Many physiological and developmental stimuli cause apoptosis, and this mechanism is frequently used to delete unwanted, superfluous or potentially harmful cells, such as those undergoing transformation
- Oxidative phosphorylation
The metabolic pathway that occurs at the inner mitochondrial membrane and uses an electrochemical gradient created by the oxidation of electron carriers to generate ATP
- Mitochondrial dynamics
Mitochondrial dynamics refers to the movement of mitochondria along the cytoskeleton and the regulation of mitochondrial morphology and distribution mediated by tethering, and fusion and fission events
- Sterile inflammation
Inflammation that results from trauma, ischaemia–reperfusion injury or chemically induced injury that typically occurs in the absence of any microorganisms
- Canonical NF-κB signalling
A typical pathway of NF-κB activation that involves phosphorylation and degradation of the prototypical NF-κB inhibitor, IκBα
- TOM complex
The translocase of the outer membrane (TOM) is a complex of proteins localized to the outer mitochondrial membrane that recognizes and imports nuclear-encoded mitochondrial proteins into the intermembrane space
- Heat shock protein
(HSP). A member of a class of functionally related proteins that function as molecular chaperones and have crucial roles in protein folding and intracellular trafficking
- Mitochondria-associated membranes
(MAMs). Regions of the endoplasmic reticulum that are closely juxtaposed to mitochondria and support communication between the organelles via calcium and phospholipid exchange
- E3 ubiquitin ligase
An enzyme that is required to attach the molecular tag ubiquitin to proteins. Depending on the number of ubiquitin molecules that are attached and the positioning of the links between them, the ubiquitin tag can target proteins for degradation in the proteasomal complex, sort them to specific subcellular compartments or modify their biological activity
- Mitofusin
An outer mitochondrial membrane protein that regulates mitochondrial fusion and ER–mitochondrial interactions by tethering adjacent organelles
- Mitophagy
A term referring to the selective removal of mitochondria by macroautophagy under conditions of nutrient starvation or mitochondrial stress
- Mitochondrial uncoupling
A process involving the disassociation of mitochondrial respiration from ATP generation that is characterized by increased permeability of the inner mitochondrial membrane to protons and subsequent dissipation of mitochondrial membrane potential
- Respiratory burst
A large increase in oxygen consumption and reactive oxygen species generation that accompanies the exposure of neutrophils to microorganisms and/or inflammatory mediators
- NADPH oxidase
A plasma membrane- and phagosomal membrane-bound enzyme complex that transfers electrons from NADPH to molecular oxygen, promoting the generation of the reactive oxygen species superoxide
- Necrosis
The premature death of living cells or tissue, resulting in the release of cellular constituents that promote inflammatory responses
- Haemorrhagic shock
A condition often caused by traumatic injury that results in reduced tissue perfusion and leads to inadequate delivery of oxygen and nutrients to cells and tissues
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- 1.Hayden MS, West AP, Ghosh S. NF-κB and the immune response. Oncogene. 2006;25:6758–6780. doi: 10.1038/sj.onc.1209943. [DOI] [PubMed] [Google Scholar]
- 2.West AP, Koblansky AA, Ghosh S. Recognition and signaling by Toll-like receptors. Annu Rev Cell Dev Biol. 2006;22:409–437. doi: 10.1146/annurev.cellbio.21.122303.115827. [DOI] [PubMed] [Google Scholar]
- 3.Kerrigan AM, Brown GD. Syk-coupled C-type lectin receptors that mediate cellular activation via single tyrosine based activation motifs. Immunol Rev. 2010;234:335–352. doi: 10.1111/j.0105-2896.2009.00882.x. [DOI] [PubMed] [Google Scholar]
- 4.Takeuchi O, Akira S. Innate immunity to virus infection. Immunol Rev. 2009;227:75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonawitz ND, Clayton DA, Shadel GS. Initiation and beyond: multiple functions of the human mitochondrial transcription machinery. Mol Cell. 2006;24:813–825. doi: 10.1016/j.molcel.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 6.Ryan MT, Hoogenraad NJ. Mitochondrial–nuclear communications. Annu Rev Biochem. 2007;76:701–722. doi: 10.1146/annurev.biochem.76.052305.091720. [DOI] [PubMed] [Google Scholar]
- 7.Soubannier V, McBride HM. Positioning mitochondrial plasticity within cellular signaling cascades. Biochim Biophys Acta. 2009;1793:154–170. doi: 10.1016/j.bbamcr.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Hamanaka RB, Chandel NS. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci. 2010;35:505–513. doi: 10.1016/j.tibs.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnoult D, Carneiro L, Tattoli I, Girardin SE. The role of mitochondria in cellular defense against microbial infection. Semin Immunol. 2009;21:223–232. doi: 10.1016/j.smim.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Ohta A, Nishiyama Y. Mitochondria and viruses. Mitochondrion. 2011;11:1–12. doi: 10.1016/j.mito.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brennan K, Bowie AG. Activation of host pattern recognition receptors by viruses. Curr Opin Microbiol. 2010;13:503–507. doi: 10.1016/j.mib.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Satoh T, et al. LGP2 is a positive regulator of RIG-I- and MDA5-mediated antiviral responses. Proc Natl Acad Sci USA. 2010;107:1512–1517. doi: 10.1073/pnas.0912986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawai T, et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nature Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 14.Meylan E, et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 15.Seth RB, Sun L, Ea C-K, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. This paper demonstrates that MAVS signalling originates at the OMM. [DOI] [PubMed] [Google Scholar]
- 16.Xu L-G, et al. VISA is an adapter protein required for virus-triggered IFN-β signaling. Mol Cell. 2005;19:727–740. doi: 10.1016/j.molcel.2005.08.014. References 13–16 were the first to describe MAVS (also termed IPS1, CARDIF and VISA) as an RLR signalling adaptor molecule. [DOI] [PubMed] [Google Scholar]
- 17.Seth RB, Sun L, Chen ZJ. Antiviral innate immunity pathways. Cell Res. 2006;16:141–147. doi: 10.1038/sj.cr.7310019. [DOI] [PubMed] [Google Scholar]
- 18.Potter JA, Randall RE, Taylor GL. Crystal structure of human IPS-1/MAVS/VISA/Cardif caspase activation recruitment domain. BMC Struct Biol. 2008;8:11. doi: 10.1186/1472-6807-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saha SK, et al. Regulation of antiviral responses by a direct and specific interaction between TRAF3 and Cardif. EMBO J. 2006;25:3257–3263. doi: 10.1038/sj.emboj.7601220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang ED, Wang CY. MAVS self-association mediates antiviral innate immune signaling. J Virol. 2009;83:3420–3428. doi: 10.1128/JVI.02623-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang ED, Wang CY. TRAF5 is a downstream target of MAVS in antiviral innate immune signaling. PLoS ONE. 2010;5:e9172. doi: 10.1371/journal.pone.0009172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michallet MC, et al. TRADD protein is an essential component of the RIG-like helicase antiviral pathway. Immunity. 2008;28:651–661. doi: 10.1016/j.immuni.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 23.Lin R, et al. Dissociation of a MAVS/IPS-1/VISA/ Cardif-IKKε molecular complex from the mitochondrial outer membrane by hepatitis C virus NS3-4A proteolytic cleavage. J Virol. 2006;80:6072–6083. doi: 10.1128/JVI.02495-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang YY, et al. WDR5 is essential for assembly of the VISA-associated signaling complex and virus-triggered IRF3 and NF-κB activation. Proc Natl Acad Sci USA. 2010;107:815–820. doi: 10.1073/pnas.0908967107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neupert W, Herrmann JM. Translocation of proteins into mitochondria. Annu Rev Biochem. 2007;76:723–749. doi: 10.1146/annurev.biochem.76.052705.163409. [DOI] [PubMed] [Google Scholar]
- 26.Yang K, et al. Hsp90 regulates activation of interferon regulatory factor 3 and TBK-1 stabilization in Sendai virus-infected cells. Mol Biol Cell. 2006;17:1461–1471. doi: 10.1091/mbc.E05-09-0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu XY, Wei B, Shi HX, Shan YF, Wang C. Tom70 mediates activation of interferon regulatory factor 3 on mitochondria. Cell Res. 2010;20:994–1011. doi: 10.1038/cr.2010.103. [DOI] [PubMed] [Google Scholar]
- 28.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhong B, et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29:538–550. doi: 10.1016/j.immuni.2008.09.003. References 28 and 29 characterize STING (also termed MITA) as a crucial component of the RLR signalling machinery. [DOI] [PubMed] [Google Scholar]
- 30.Sun W, et al. ERIS, an endoplasmic reticulum IFN stimulator, activates innate immune signaling through dimerization. Proc Natl Acad Sci USA. 2009;106:8653–8658. doi: 10.1073/pnas.0900850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin L, et al. MPYS, a novel membrane tetraspanner, is associated with major histocompatibility complex class II and mediates transduction of apoptotic signals. Mol Cell Biol. 2008;28:5014–5026. doi: 10.1128/MCB.00640-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castanier C, Garcin D, Vazquez A, Arnoult D. Mitochondrial dynamics regulate the RIG-I-like receptor antiviral pathway. EMBO Rep. 2010;11:133–138. doi: 10.1038/embor.2009.258. An important mechanistic paper detailing the role of mitochondrial fusion in RLR signalling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giorgi C, De Stefani D, Bononi A, Rizzuto R, Pinton P. Structural and functional link between the mitochondrial network and the endoplasmic reticulum. Int J Biochem Cell Biol. 2009;41:1817–1827. doi: 10.1016/j.biocel.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vance JE. Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem. 1990;265:7248–7256. [PubMed] [Google Scholar]
- 35.You F, et al. PCBP2 mediates degradation of the adaptor MAVS via the HECT ubiquitin ligase AIP4. Nature Immunol. 2009;10:1300–1308. doi: 10.1038/ni.1815. [DOI] [PubMed] [Google Scholar]
- 36.Jia Y, et al. Negative regulation of MAVS-mediated innate immune response by PSMA7. J Immunol. 2009;183:4241–4248. doi: 10.4049/jimmunol.0901646. [DOI] [PubMed] [Google Scholar]
- 37.Salonen A, Ahola T, Kääriäinen L. Viral RNA replication in association with cellular membranes. Curr Top Microbiol Immunol. 2005;285:139–173. doi: 10.1007/3-540-26764-6_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bowie AG, Unterholzner L. Viral evasion and subversion of pattern-recognition receptor signalling. Nature Rev Immunol. 2008;8:911–922. doi: 10.1038/nri2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li XD, Sun L, Seth RB, Pineda G, Chen ZJ. Hepatitis C virus protease NS3/4A cleaves mitochondrial antiviral signaling protein off the mitochondria to evade innate immunity. Proc Natl Acad Sci USA. 2005;102:17717–17722. doi: 10.1073/pnas.0508531102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Z, et al. GB virus B disrupts RIG-I signaling by NS3/4A-mediated cleavage of the adaptor protein MAVS. J Virol. 2007;81:964–976. doi: 10.1128/JVI.02076-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Y, et al. Disruption of innate immunity due to mitochondrial targeting of a picornaviral protease precursor. Proc Natl Acad Sci USA. 2007;104:7253–7258. doi: 10.1073/pnas.0611506104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei C, et al. The hepatitis B virus X protein disrupts innate immunity by downregulating mitochondrial antiviral signaling protein. J Immunol. 2010;185:1158–1168. doi: 10.4049/jimmunol.0903874. [DOI] [PubMed] [Google Scholar]
- 43.Peerschke EIB, Ghebrehiwet B. The contribution of gC1qR/p33 in infection and inflammation. Immunobiology. 2007;212:333–342. doi: 10.1016/j.imbio.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang J, Zhang Y, Krainer AR, Xu RM. Crystal structure of human p32, a doughnut-shaped acidic mitochondrial matrix protein. Proc Natl Acad Sci USA. 1999;96:3572–3577. doi: 10.1073/pnas.96.7.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fogal V, et al. Mitochondrial p32 protein is a critical regulator of tumor metabolism via maintenance of oxidative phosphorylation. Mol Cell Biol. 2010;30:1303–1318. doi: 10.1128/MCB.01101-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu L, Xiao N, Liu F, Ren H, Gu J. Inhibition of RIG-I and MDA5-dependent antiviral response by gC1qR at mitochondria. Proc Natl Acad Sci USA. 2009;106:1530–1535. doi: 10.1073/pnas.0811029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dedio J, Jahnen-Dechent W, Bachmann M, Müller-Esterl W. The multiligand-binding protein gC1qR, putative C1q receptor, is a mitochondrial protein. J Immunol. 1998;160:3534–3542. [PubMed] [Google Scholar]
- 48.Moore CB, et al. NLRX1 is a regulator of mitochondrial antiviral immunity. Nature. 2008;451:573–577. doi: 10.1038/nature06501. In this paper, NLRX1 is shown to localize to mitochondria and inhibit MAVS signalling. [DOI] [PubMed] [Google Scholar]
- 49.Tattoli I, et al. NLRX1 is a mitochondrial NOD-like receptor that amplifies NF-κB and JNK pathways by inducing reactive oxygen species production. EMBO Rep. 2008;9:293–300. doi: 10.1038/sj.embor.7401161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arnoult D, et al. An N-terminal addressing sequence targets NLRX1 to the mitochondrial matrix. J Cell Sci. 2009;122:3161–3168. doi: 10.1242/jcs.051193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- 52.Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nature Rev Mol Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- 53.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 54.Yasukawa K, et al. Mitofusin 2 inhibits mitochondrial antiviral signaling. Sci Signal. 2009;2:ra47. doi: 10.1126/scisignal.2000287. [DOI] [PubMed] [Google Scholar]
- 55.Onoguchi K, et al. Virus-infection or 5′ppp-RNA activates antiviral signal through redistribution of IPS-1 mediated by MFN1. PLoS Pathog. 2010;6:e1001012. doi: 10.1371/journal.ppat.1001012. This study demonstrates that RIG-I, mitochondria and MAVS localize around cytoplasmic centres of viral replication, and that this is regulated by MFN1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koshiba T, Yasukawa K, Yanagi Y, Kawabata S. Mitochondrial membrane potential is required for MAVS-mediated antiviral signaling. Sci Signal. 2011;4:ra7. doi: 10.1126/scisignal.2001147. This paper details the roles of MFN1 and MFN2 in RLR signalling; these roles include preserving mitochondrial fusion and membrane potential. [DOI] [PubMed] [Google Scholar]
- 57.Jin L, Lenz L, Cambier J. Cellular reactive oxygen species inhibit MPYS induction of IFNβ. PLoS ONE. 2010;5:e15142. doi: 10.1371/journal.pone.0015142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soucy-Faulkner A, et al. Requirement of NOX2 and reactive oxygen species for efficient RIG-I-mediated antiviral response through regulation of MAVS expression. PLoS Pathog. 2010;6:e1000930. doi: 10.1371/journal.ppat.1000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tal MC, et al. Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling. Proc Natl Acad Sci USA. 2009;106:2770–2775. doi: 10.1073/pnas.0807694106. An important study showing that mROS can augment RLR signalling and that autophagy and mitophagy regulate antiviral responses by limiting mROS production. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jounai N, et al. The Atg5–Atg12 conjugate associates with innate antiviral immune responses. Proc Natl Acad Sci USA. 2007;104:14050–14055. doi: 10.1073/pnas.0704014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koopman WJ, et al. Mammalian mitochondrial complex I: biogenesis, regulation and reactive oxygen species generation. Antioxid Redox Signal. 2010;12:1431–1470. doi: 10.1089/ars.2009.2743. [DOI] [PubMed] [Google Scholar]
- 62.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reeves MB, Davies AA, McSharry BP, Wilkinson GW, Sinclair JH. Complex I binding by a virally encoded RNA regulates mitochondria-induced cell death. Science. 2007;316:1345–1348. doi: 10.1126/science.1142984. [DOI] [PubMed] [Google Scholar]
- 64.Arsenijevic D, et al. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nature Genet. 2000;26:435–439. doi: 10.1038/82565. The first study to demonstrate that macrophages with increased mROS display heightened resistance to microbial infection. [DOI] [PubMed] [Google Scholar]
- 65.Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2:85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 66.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nature Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 67.Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol. 2002;20:825–852. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- 68.Fleury C, et al. Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nature Genet. 1997;15:269–272. doi: 10.1038/ng0397-269. [DOI] [PubMed] [Google Scholar]
- 69.Rousset S, et al. The uncoupling protein 2 modulates the cytokine balance in innate immunity. Cytokine. 2006;35:135–142. doi: 10.1016/j.cyto.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 70.Kizaki T, et al. Uncoupling protein 2 plays an important role in nitric oxide production of lipopolysaccharide-stimulated macrophages. Proc Natl Acad Sci USA. 2002;99:9392–9397. doi: 10.1073/pnas.142206299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Emre Y, et al. Mitochondria contribute to LPS-induced MAPK activation via uncoupling protein UCP2 in macrophages. Biochem J. 2007;402:271–278. doi: 10.1042/BJ20061430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bai Y, et al. Persistent nuclear factor-κB activation in Ucp2−/− mice leads to enhanced nitric oxide and inflammatory cytokine production. J Biol Chem. 2005;280:19062–19069. doi: 10.1074/jbc.M500566200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nishio K, Qiao S, Yamashita H. Characterization of the differential expression of uncoupling protein 2 and ROS production in differentiated mouse macrophage-cells (Mm1) and the progenitor cells (M1) J Mol Histol. 2005;36:35–44. doi: 10.1007/s10735-004-2915-x. [DOI] [PubMed] [Google Scholar]
- 74.Giguère V. Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr Rev. 2008;29:677–696. doi: 10.1210/er.2008-0017. [DOI] [PubMed] [Google Scholar]
- 75.Sonoda J, et al. Nuclear receptor ERRα and coactivator PGC-1β are effectors of IFN-γ-induced host defense. Genes Dev. 2007;21:1909–1920. doi: 10.1101/gad.1553007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.West AP, et al. TLR signaling augments macrophage bactericidal activity through mitochondrial reactive oxygen species. Nature. 2011;472:476–480. doi: 10.1038/nature09973. This study demonstrates that TLR signalling directly induces mROS, leading to enhanced antibacterial activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kopp E, et al. ECSIT is an evolutionarily conserved intermediate in the Toll/IL-1 signal transduction pathway. Genes Dev. 1999;13:2059–2071. doi: 10.1101/gad.13.16.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vogel RO, et al. Cytosolic signaling protein Ecsit also localizes to mitochondria where it interacts with chaperone NDUFAF1 and functions in complex I assembly. Genes Dev. 2007;21:615–624. doi: 10.1101/gad.408407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kono H, Rock KL. How dying cells alert the immune system to danger. Nature Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rock KL, Kono H. The inflammatory response to cell death. Annu Rev Pathol. 2008;3:99–126. doi: 10.1146/annurev.pathmechdis.3.121806.151456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nature Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cassel SL, Joly S, Sutterwala FS. The NLRP3 inflammasome: a sensor of immune danger signals. Semin Immunol. 2009;21:194–198. doi: 10.1016/j.smim.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Collins LV, Hajizadeh S, Holme E, Jonsson IM, Tarkowski A. Endogenously oxidized mitochondrial DNA induces in vivo and in vitro inflammatory responses. J Leukoc Biol. 2004;75:995–1000. doi: 10.1189/jlb.0703328. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Q, Itagaki K, Hauser CJ. Mitochondrial DNA is released by shock and activates neutrophils via p38 MAP kinase. Shock. 2010;34:55–59. doi: 10.1097/SHK.0b013e3181cd8c08. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Q, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. In this study, mitochondrial N-formylated peptides and mtDNA are demonstrated to function as DAMPs by signalling via PRRs and inducing innate immune responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rabiet MJ, Huet E, Boulay F. The N-formyl peptide receptors and the anaphylatoxin C5a receptors: an overview. Biochimie. 2007;89:1089–1106. doi: 10.1016/j.biochi.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carp H. Mitochondrial N-formylmethionyl proteins as chemoattractants for neutrophils. J Exp Med. 1982;155:264–275. doi: 10.1084/jem.155.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rabiet MJ, Huet E, Boulay F. Human mitochondria-derived N-formylated peptides are novel agonists equally active on FPR and FPRL1, while Listeria monocytogenes-derived peptides preferentially activate FPR. Eur J Immunol. 2005;35:2486–2495. doi: 10.1002/eji.200526338. [DOI] [PubMed] [Google Scholar]
- 89.Raoof M, Zhang Q, Itagaki K, Hauser CJ. Mitochondrial peptides are potent immune activators that activate human neutrophils via FPR-1. J Trauma. 2010;68:1328–1332. doi: 10.1097/TA.0b013e3181dcd28d. [DOI] [PubMed] [Google Scholar]
- 90.McDonald B, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 91.Crouser ED, et al. Monocyte activation by necrotic cells is promoted by mitochondrial proteins and formyl peptide receptors. Crit Care Med. 2009;37:2000–2009. doi: 10.1097/CCM.0b013e3181a001ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nature Rev Immunol. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 93.Iyer SS, et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci USA. 2009;106:20388–20393. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nakahira K, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nature Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. This paper demonstrates that mROS and mtDNA contribute to macrophage inflammasome activation by LPS and ATP, and that this is supressed by mitophagy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Meissner F, Molawi K, Zychlinsky A. Superoxide dismutase 1 regulates caspase-1 and endotoxic shock. Nature Immunol. 2008;9:866–872. doi: 10.1038/ni.1633. [DOI] [PubMed] [Google Scholar]
- 96.van Bruggen R, et al. Human NLRP3 inflammasome activation is Nox1–4 independent. Blood. 2010;115:5398–5400. doi: 10.1182/blood-2009-10-250803. [DOI] [PubMed] [Google Scholar]
- 97.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. This study shows that NLRP3 and ASC localize to mitochondria and MAMs upon exposure of macrophages to DAMPs and that mROS activate the NLRP3 inflammasome. [DOI] [PubMed] [Google Scholar]
- 98.Tal MC, Iwasaki A. Autophagic control of RLR signaling. Autophagy. 2009;5:749–750. doi: 10.4161/auto.5.5.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yu HB, Finlay BB. The caspase-1 inflammasome: a pilot of innate immune responses. Cell Host Microbe. 2008;4:198–208. doi: 10.1016/j.chom.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 100.Robinson MJ, Sancho D, Slack EC, LeibundGut-Landmann S, Reis e Sousa C. Myeloid C-type lectins in innate immunity. Nature Immunol. 2006;7:1258–1265. doi: 10.1038/ni1417. [DOI] [PubMed] [Google Scholar]
- 101.Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- 102.Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem. 2003;278:5557–5563. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]