Abstract
Arsenic is an environmental carcinogen, its mechanisms of carcinogenesis remain to be investigated. Reactive oxygen species (ROS) are considered to be important. A previous study (Carpenter et al., Biochem. Biophys. Res. Commun. 414:533–538, 2011) has measured ROS level in human lung bronchial epithelial (BEAS-2B) cells and arsenic-transformed BEAS-2B cells and found that ROS levels were higher in transformed cells than that in parent normal cells. Based on these observations, the authors concluded that cell transformation induced by arsenic is mediated by increased cellular levels of ROS. This conclusion is problematic because this study only measured the basal ROS levels in transformed and parent cells and did not investigate the role of ROS in the process of arsenic-induced cell transformation. The levels of ROS in arsenic-transformed cells represent the result and not the cause of cell transformation. Thus question concerning whether ROS are important in arsenic-induced cell transformed remains to be answered. In the present study, we used expressions of catalase (antioxidant against H2O2) and superoxide dismutase 2 (SOD2, antioxidant against O2•−) to decrease ROS level and investigated their role in the process of arsenic-induced cell transformation. Our results show that inhibition of ROS by antioxidant enzymes decreased arsenic-induced cell transformation, demonstrating that ROS are important in this process. Moreover, we have also shown that in arsenic-transformed cells, ROS generation was lower and levels of antioxidants are higher than that in parent cells, in a disagreement with the previous report. The present study has also shown that the arsenic-transformed cells acquired apoptosis resistance. The inhibition of catalase to increase ROS level restored apoptosis capability of arsenic-transformed BEAS-2B cells, further showing that ROS levels are low in these cells. The apoptosis resistance due to the low ROS levels may increase cells proliferation, providing a favorable environment for tumorigenesis of arsenic-transformed cells.
Keywords: Arsenic, Reactive oxygen species, Antioxidant enzymes, Cell transformation
1. Introduction
Epidemiologic studies have shown that long-term exposure to inorganic arsenic induces lung, skin, liver, and bladder cancers [1–6]. Human exposure to arsenic-containing drinking water is a world-wide environmental health concern. In United States, nearly 3.7 million individuals drink water from private wells in which the arsenic contamination in water is higher than that of US EPA standard (10 ppb) [7]. Although the mechanism of arsenic-induced carcinogenesis remains to be investigated, arsenic-induced generation of reactive oxygen species (ROS) is considered to be important [8–21]. ROS refer to a diverse group of reactive, short-lived, oxygen containing species, such as superoxide radical (O2•−), H2O2, and hydroxyl radical (•OH). ROS have been conventionally regarded as having carcinogenic potential and have been associated with tumor initiation and promotion [22]. Cellular systems are protected from ROS-induced cell injuries by an array of defenses composed of various antioxidants with different functions. When the ROS present in the cellular system overpower the defense systems, they will cause oxidative injuries, leading to the development of various diseases, including cancer. Increasing evidences suggest that exposure of arsenic results in the generation of ROS [21]. ROS production has been reported in various cellular systems exposed to arsenite at various concentrations, including in U937 cells [23], human vascular smooth muscle cells [24], human-hamster hybrid cells [25], vascular endothelial cells [26], HEL30 cells [27], NB4 cells [28], CHO-K1 cells [29], and human lung bronchial epithelial BEAS-2B cells [30].
Various studies have suggested that NADPH oxidase (NOX) may be the primary source for the generation of O2•− [31, 32]. Arsenic is not only able to induce expressions of NOX components including p47, p67, p91, and several scaffolding protein for the assembly of this complex [31] but also able to stimulate enzyme activity of NOX by inducing phosphorylation and translocation of p47 [33]. Although it has been generally viewed that ROS are the key mediators for arsenic-induced carcinogenesis through oxidative stress, the role of ROS in arsenic-induced malignant transformation has not been reported. The link between ROS and arsenic-induced cell transformation has not established. With an attempt to establish this linkage, a previous study has measured the ROS levels in BEAS-2B cells and arsenic-transformed ones [34]. This study found that the basal levels of ROS were higher in transformed cells than that in parent cells. Based on these observations, the authors concluded that cell transformation induced by arsenic is mediated by increased cellular levels of ROS. The problem with this conclusion is that the authors only measured the basal ROS levels in transformed and parent cells and did not investigate the role of ROS in the process of arsenic-induced cell transformation. The levels of ROS in arsenic-transformed cells represent the result and not the cause of cell transformation. Thus question concerning whether ROS are important in arsenic-induced cell transformed remains to be answered. In order to answer this important question, we used expressions of catalase (antioxidant enzyme against H2O2) and superoxide dismutase 2 (SOD2, antioxidant enzyme against O2•−) to decrease the levels of ROS and investigated their role in arsenic-induced cell transformation. The results of higher basal levels of ROS in arsenic transformed BEAS-2B cells than that in parent cells reported in the previous study [34] are contradictory to what we obtained in our previous study [30]. The ROS status in transformed cells is very important to understand the mechanism of tumorigenesis of these cells. In the present study, we have also examined the ROS levels in both arsenic transformed cells and their present cells.
2. Material and methods
2.1.Chemicals and reagents
Sodium arsenite (Na2AsO2), apocynin, 5,5-dimethyl-1-pyrroline-1-oxide (DMPO), and Annexin V/Propidium iodide (PI) were purchased from Sigma (St Louis, MO). Both 5-(and -6)-chloromethyl-2, 7-dichlorodihydrofluorescein diacetate, acetyl ester (DCFDA) and dihydroethidium (DHE) were purchased from Molecular Probes (Eugene, OR). Manganese(III) tetrakis(1-methyl-4-pyridyl)porphyrin (MnTMPyP) was purchased from Cayman Chemical (Ann Arbor, MI). Plasmids DNA encoding human catalase and SOD2 and catalase shRNA were purchased from Origene (Rockville, MD). Antibody against SOD2 was purchased from Millipore (Billerica, MA). Antibodies against catalase, C-PARP, C-Caspase-3, and Bcl-2 were from Cell Signaling Tech (Danvers, MA).
2.2. Cell culture and arsenic exposure
Human bronchial epithelial (BEAS-2B) cells (ATCC, Rockville, MD) were cultured in DMEM supplemented with 10% FBS, 2mM L-glutamine, and 5% penicillin/streptomycin at 37°C in a humidified atmosphere with 5% CO2. For short-term exposure of arsenic, BEAS-2B cells were grown to 80–90% confluent, then cell culture medium containing 0.1% FBS was added for overnight followed by arsenic treatment as indicated incubation time and doses. Antioxidants or inhibitors were pretreated to the cells 2 hrs prior to arsenic treatment as designated. BEAS-2B cells with stably expressing catalase and SOD2 were generated by transfection of catalase or SOD2 plasmid to the cells followed by antibiotics G418 selection.
2.3. H2O2 and O2•− generation
H2O2 and O2•− generations were examined using the fluorescent dye DCFDA and DHE, respectively, as described previously [35]. The cells were cultured in 96-well plates with 5×104 cells/well. The cells were treated with 5 µM of arsenic for 6 hrs and then incubated with DCFDA or DHE (final concentration, 10 µM) for 20 min at 37°C. Fluorescence intensity was measured using a Gemini XPS fluorescence microplate spectrofluorometer (Molecular Devices).
2.4. Electron spin resonance (ESR) assay
ESR measurement was conducted using a Bruker EMX spectrometer (Bruker Instruments, Billerica, MA) and a flat cell assembly. The intensity of the ESR signal is used to measure the amount of hydroxyl radical generation. DMPO was used as spin or radical trap. DMPO was charcoal purified and distilled to remove all ESR detectable impurities before use. Hyperfine couplings were measured (to 0.1 G) directly from magnetic field separation using potassium tetraperoxochromate (K3CrO8) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) as reference standard. Reactants were mixed in test tubes to a total final volume of 0.5 mL. The reaction mixture was then transferred to a flat cell for ESR measurement.
2.5. NOX activity assay
NOX activity was measured by the lucigenin enhanced chemiluminescence method as described [35]. Briefly, cultured cells were homogenized in lysis buffer followed by centrifugation to remove the unbroken cells and debris. 100 µL aliquot of homogenates were added to 900 µL of 50mM phosphate buffer. Relative light units of photon emission was measured in Glomax luminometer (Promega).
2.6. Arsenic-induced cell transformation
BEAS-2B cells or BEAS-2B cells with overexpression of catalase or SOD2 were treated with 0.25 µM arsenic. The fresh medium was added for every 3 days. After 24 weeks, 1×104 cells were suspended in 2 mL culture medium containing 0.35% agar and seeded into 6-well plates with 0.5 % agar base layer, and maintained in an incubator for 4 weeks. The cells were stained with 1 mg/mL iodonitrotetrazolium violet, and colonies greater than 0.1 mm in diameter were scored by microscope examination.
The arsenic-transformed cells from anchorage-independent colonies were picked up and continued to grow in DMEM. Passage-matched cells without arsenic treatment were used as control.
2.7. Immunoblotting analysis
Cell lysates were prepared in RIPA buffer. The protein concentration was measured using Bradford Protein Assay Reagent (Bio-Rad) and 30 µg of protein was separated by SDS-PAGE, and incubated with primary antibodies. The blots were then re-probed with second antibodies conjugated to horseradish peroxidase. Immunoreactive bands were detected by the enhanced chemiluminescence reagent (Amersham).
2.8. Apoptosis analysis
Annexin V-fluorescein isothiocyanate (FITC)/PI double staining was used to measure percentile of apoptosis. Briefly, the cells were treated with arsenic at 2.5, 5, 10 µM for 24 hours. The cells were digested with 0.25% trypsin/EDTA followed by re-suspension in binding buffer and addition of Annexin V-FITC/PI. The apoptotic cells were measured using flow cytometry.
3. Results
3.1.Arsenic increases ROS production
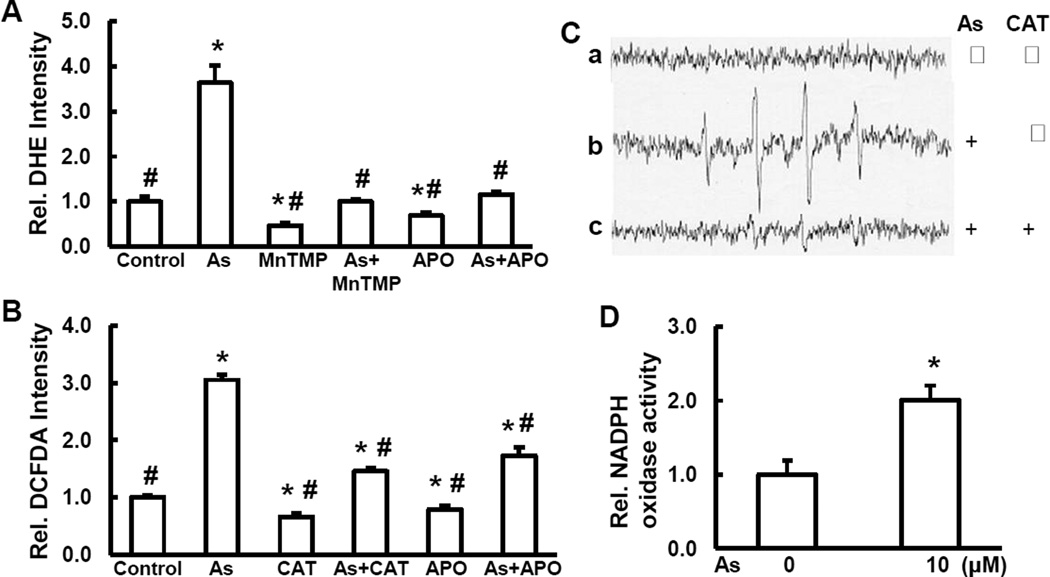
Our previous study has demonstrated that exposure of BEAS-2B cells to arsenic is able to induce actin filaments reorganization, activate Cdc42 and NOX, and generate O2•− [8]. In the present study, we measured ROS generation in the cells treated with 5.0 µM of arsenic for 6 hrs with or without catalase (CAT, H2O2 scavenger), MnTMPyP, cell permeable superoxide dismutase (SOD) mimetic (O2•− scavenger), or apocynin (APO, NOX inhibitor). The results show that arsenic caused generation of O2•− (Fig. 1A). Pretreatment with MnTMPyP decreased O2•− generation induced by arsenic. A similar result is observed when the cells were pretreated with apocynin, an inhibitor of NOX (a major ROS generating enzyme in arsenic-treated cells) [35], indicating the involvement of NOX in arsenic-induced O2•− generation (Fig. 1A). To avoid the possible non-specificity of DCFDA for measurement of H2O2, we used catalase, a specific H2O2 inhibitor. Our results show that catalase decreased DCFDA fluorescence intensity by 50% compared to control without treatment (Fig. 1B), indicating the H2O2 generation. Our results also show that arsenic increased H2O2 generation by 3 fold compared to control and that pretreatment of the cells with either catalase or apocynin decreased arsenic-induced H2O2 generation (Fig. 1B), suggesting that H2O2 was generated and NOX is responsible for arsenic-induced H2O2 generation. We have also measured •OH generation using ESR spin trapping. The results showed that arsenic was able to generate •OH. Addition of catalase blocked •OH generation induced by arsenic (Fig. 1C). NOX activity was increased 2-fold in arsenic treated cells compared to control without arsenic treatment (Fig. 1D). Taking together, the results suggest that activation of NOX is required for ROS generation induced by arsenic.
Fig. 1.
Arsenic induces ROS generation. Generation of O2•− (A) and H2O2 (B) were determined by staining the cells with DHE and DCFDA, respectively, by fluorescence spectrofluorometer analysis. BEAS-2B cells were pretreated with either MnTMPyP, catalase (CAT), or apocynin (APO) for 2 hrs followed by arsenic treatment for 6 hrs. (C) generation of •OH. (a) ESR spectrum was recorded from a mixture containing 100 mM DMPO and BEAS-2B cells. (b) same as (a), but with 1 mM arsenic. (c) same as (b), but with catalase (CAT, 5,000 U/mL). (D) NADPH activity. * and #, p<0.05 compared to control without treatment and arsenic treatment, respectively.
3.2. Arsenic induces cell transformation and the role of ROS
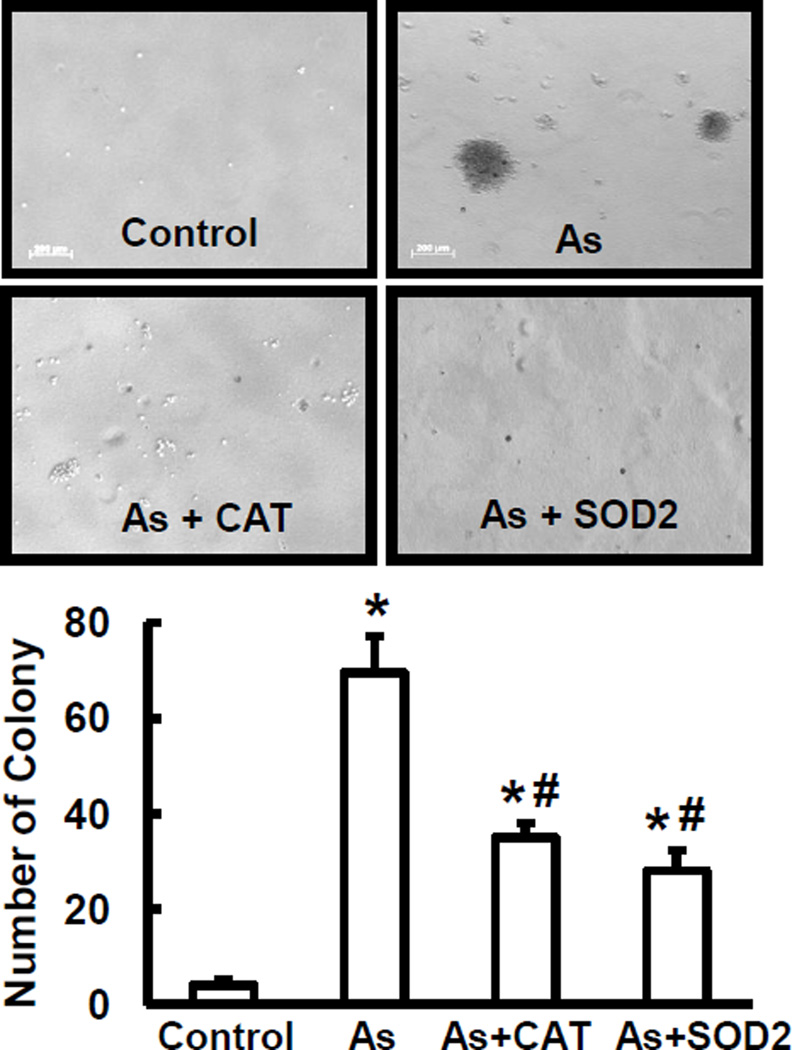
The transformative capability of arsenic has long been established in several types of mammalian cells [36]. To determine whether ROS are responsible for arsenic-induced transformation, BEAS-2B cells and their genetic variety with over-expressing SOD2 or catalase were chronically treated with 0.25 µM of arsenic for 24 weeks. Anchorage-independent cell growth was evaluated for cell transformation. As shown in Fig. 2, chronic exposure to arsenic caused cell transformation. The cells with over-expression of catalase or SOD2 decreased the number of colonies formed. These results indicate that arsenic is able to induce cell transformation, and that ROS are important in this process.
Fig. 2.
Arsenic-induced cell transformation and the role of ROS. BEAS-2B cells were stably transfected with catalase or SOD2 plasmid. BEAS-2B cells and their stable expressing ones were treated with 0.25 µM arsenic for 24 weeks. Cell transformation assay was conducted. Colonies > 0.1 mm in diameter were counted. Control, BEAS-2B cells without arsenic treatment; As, arsenic-treated cells; As+CAT, arsenic-treated cells with over-expression of catalase; and As+SOD2, arsenic- treated cells with overexpression of SOD2. * and #, p < 0.05 compared to control and arsenic treatment, respectively.
3.3. Reduced capability of ROS generation in the arsenic-transformed cells
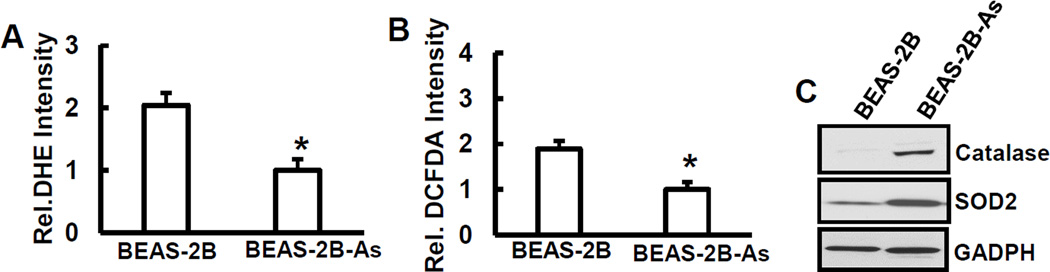
To determine whether ROS generating capacity was altered in arsenic-transformed cells, we measured ROS generation in arsenic-transformed cells and parent cells exposed to 5 µM of arsenic for 6 hrs. O2•− and H2O2 generation were determined by DHE and DCFDA staining described in the legends of Figs. 1A and 1B. Both O2•− and H2O2 generations in normal cells were double compared to that in arsenic-transformed cells (Figs. 3A and 3B). To probe the mechanism of reduced ROS generation in arsenic-transformed cells, we measured cellular levels of catalase and SOD2, the two important key antioxidant enzymes. As shown in Fig. 3C, both catalase and SOD2 were up-regulated in arsenic-transformed cells compared to that of non-transformed ones, indicating that constitutive activation of catalase or SOD2 in arsenic-transformed cells protects cells from exotic oxidative stress.
Fig. 3.
Increased antioxidant expression and reduced capability of ROS generation in the arsenic-transformed cells. Generations of O2•− (A) and H2O2 (B) were determined in arsenic-transformed cells (BEAS-2B-As) and their passage-matched non-transformed cells (BEAS-2B) by staining with DHE and DCFDA as described by Fig. 1, followed by fluorescence spectrofluorometer measurement. C, BEAS-2B-As and BEAS-2B cells were seeded in 10-cm cell culture dishes. The whole cell lysates were collected for immunoblotting. Expressions of catalase and SOD2 were examined.
3.4.Resistance to apoptosis of arsenic-transformed cells and restoration of apoptosis by inhibition of catalase
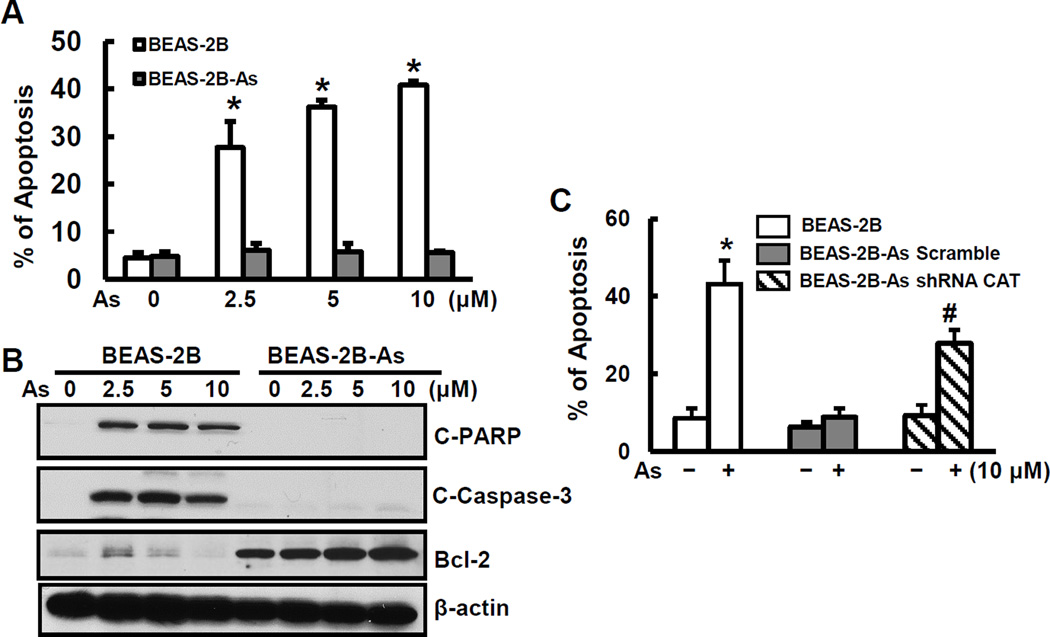
Previous studies have shown that ROS are inducers for apoptosis [37–39]. We hypothesize that the reduced capability of arsenic-transformed cells to generate ROS may contribute to development of resistance to apoptosis of these calls. Resistance to apoptotic cell death and increased cell survival in response to genotoxic insults are key characteristics of cancer cells. To test whether arsenic-transformed cells possess these properties, we analyzed apoptosis in response to further arsenic treatment. The results show a decreased apoptotic response to arsenic in arsenic-transformed BEAS-2B cells compared to non-transformed parent cells (Fig. 4A). Further investigation demonstrates that arsenic-transformed cells exhibited reduced levels of apoptotic proteins, cleaved poly(ADP-ribose) polymerase (C-PARP) and cleaved caspase 3 (C-Caspase 3), and elevated expression of anti-apoptotic protein Bcl-2 (Fig. 4B).
Fig. 4.
Resistance to apoptosis of arsenic-transformed cells and restoration of apoptosis by inhibition of catalase expression. (A) and (B) BEAS-2B-As and BEAS-2B cells were seeded into 6-well culture plates. Cells were treated with different concentrations of arsenic for 24 hrs. (A) The percentage of apoptotic cells was measured using flow cytometry. Data are mean±SD (n=6). * p< 0.05 compared to non-transformed cells. (B) Whole cell lysates were collected for immunoblotting analysis. Expression levels of C-PARP, C-caspase 3, and Bcl-2 were measured. (C) BEAS-2B-As were transfected with either scramble or catalase shRNA for 24 hrs. BEAS-2B, scramble arsenic-transformed (BEAS-2B-As Scramble), and shRNA catalase arsenic-transformed (BEAS-2B-As-shRNA CAT) cells were treated with 10 µM arsenic for 24 hrs followed by apoptosis analysis. Data are mean±SD (n=6). * and #, p < 0.05 compared to the BEAS-2B cells and BEAS-2B-As-shRNA CAT without arsenic treatment, respectively.
ROS are apoptosis-inducing agents [37–39]. The observed apoptosis resistance of arsenic-transformed cells is likely due to decreased ROS generation or/and increased antioxidant expression. We used catalase shRNA to decrease the activity of catalase and increase H2O2. The results show that inhibition of catalase restored the capability of apoptosis of arsenic-transformed cells (Fig. 4C). These results also support the conclusion in the previous section (Section 3.3.) that the catalase level is high and H2O2 is low in arsenic-transformed BEAS-2B cells.
4. Discussion
The present study shows that BEAS-2B cells stimulated by arsenic generate ROS, which were identified as O2•−, H2O2, and •OH using antioxidant inhibition and ESR spin trapping. These results are in agreement with those reported previously [34]. Although ROS are considered important in arsenic-induced carcinogenesis, there is no convinced evidence to demonstrate it. Cell transformation assay is a widely used approach to identify carcinogenetic properties of a particular carcinogen. Arsenic has been shown to cause transformation of BEAS-2B cells [30, 34]. These arsenic-transformed cells are able to cause tumorigenesis [30]. Using arsenic-transformed cells, a recent study has shown that arsenic transformed cells have increased basal levels of ROS, which in turn activate AKT, ERK1/2, and p70S6K1[34]. Forced expression of catalase reduced activations of AKT, ERK1/2, and p70S6K1 and inhibited proliferation and anchorage-independent growth of arsenic transformed cells. Based on these observations using arsenic-transformed cells, this study [34] has concluded that arsenic-induced cell transformation is mediated via ROS and ROS-activated signaling pathways. As discussed below, this conclusion is problematic. The study did not examine the role of ROS nor their target proteins, AKT, ERK1/2, and p70S6K1 in the process of arsenic induced cell transformation. The investigators did not alter the cellular levels of ROS to examine their effect on arsenic-induced cell transformation. Instead, the authors measured the basal levels of ROS and their target signaling proteins, AKT, ERK1/2, and p70S6K1 in the transformed cells. The basal levels of ROS in arsenic-transformed cells, regardless low or high, only represent the results rather than the cause of arsenic-induced cell transformation. In other word, the question concerning whether ROS or their down-stream proteins, AKT, ERK1/2, and p70S6K1 play a role in the mechanism of arsenic-induced cell transformation was not answered by this previous study [34]. In our present study, we used BEAS-2B cells with expressing SOD2 or catalase to decrease the levels of ROS during the chronic arsenic treatments of the cells (24 weeks). The cell transformation as indicated by anchorage-independent cell growth was evaluated for the deference between chronic arsenic treated cells with antioxidant expressions and these without antioxidant expressions. The decreased cell transformation of the cells with antioxidant expressions or decrease levels of ROS shows that ROS are important in the mechanism of arsenic-induced BEAS-2B cell transformation.
The higher basal levels of ROS in arsenic transformed BEAS-2B cells than that in parent non-transformed cells reported in this previous study [34] are contradictory to what we obtained in our previous [30] and the present studies. In our previous study, we provided evidence that the capacity of ROS generation is severely compromised in the arsenic-transformed cells [30]. In present study, we have shown that basal levels of ROS in arsenic-transformed BEAS-2B cells are lower than that in parent cells. In consistence, we have shown that the levels of two major antioxidant enzymes, catalase and SOD2, were elevated in arsenic-transformed BEAS-2B cells. We have also found that Nrf2, a maser antioxidant regulative protein, was constitutively activated in arsenic-transformed cells (data not shown), indicating that the higher levels of catalase and SOD are likely due to the constitutive activation of Nrf2 in arsenic-transformed cells. In agreement with increased levels of antioxidant and decreased levels of ROS, we have observed the development of apoptosis resistance in arsenic-transformed BEAS-2B cells. Because ROS are inducers for apoptosis [37–39], the observed apoptosis resistance of arsenic-transformed cells is in agreement with the low levels of ROS in these cells. Additional support of low levels of ROS in arsenic-transformed cells in our present study is that inhibition of catalase restored the apoptosis capability of these transformed cells. Although we are unable to figure out exact reason for the reported elevation ROS levels [34], we believe that the arsenic-transformed BEAS-2B cells used in the study are likely not fully transformed.
In summary, we used expressions of catalase and SOD as approaches to decrease levels of ROS and investigated their role in the process of arsenic-induced cell transformation. Inhibition of ROS by antioxidant enzymes decreased arsenic-induced cell transformation, demonstrating that ROS are important in this process. In arsenic-transformed BEAS-2B cells, the levels of ROS are sharply reduced and the levels of antioxidants are much higher than that in parent cells. Due to the decrease of ROS levels, the arsenic-transformed cells acquired apoptosis resistance. The inhibition of catalase to increase ROS levels restored apoptosis capability of arsenic-transformed BEAS-2B cells, further showing that the ROS levels are low in these cells. The apoptosis resistance due to the low ROS levels may increase cells proliferation, providing a favorable environment for tumorigenesis of arsenic-transformed cells.
Highlights.
Short term exposure of BEAS-2B cells to arsenic caused ROS generation.
Chronical exposure of BEAS-2B cells to arsenic caused malignant cell transformation.
Inhibition of ROS generation by genetic expressions of antioxidant enzyme reduced cell transformation by arsenic exposure.
Arsenic-transformed cells exhibited reduced capacity of generating ROS and increased levels of antioxidant.
Arsenic-transformed cells have a property of apoptosis resistance and inhibition of catalase (antioxidant) restored apoptosis in arsenic-transformed cells.
Acknowledgements
The study is supported by NIH/NIEHS 1R01ES020870 to Dr. Xianglin Shi.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Subcommittee on Arsenic in Drinking Water. Arsenic in Drinking Water. Washington, D.C.: National Academy of Sciences Press; 1999. [Google Scholar]
- 2.Morales KH, Ryan L, Kuo TL, Wu MM, et al. Risk of internal cancers from arsenic in drinking water. Environ. Health Persp. 2000;108:655–661. doi: 10.1289/ehp.00108655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinmaus C, Moore L, Hopenhayn-Rich C, Biggs ML, et al. Arsenic in Drinking Water and Bladder Cancer. Cancer Investigation. 2000;18:174–182. doi: 10.3109/07357900009038249. [DOI] [PubMed] [Google Scholar]
- 4.Tseng CH, Chong CK, Tseng CP, et al. Long term arsenic exposure and ischemic heart disease in arseniasis-hyperendemic villages in Taiwan. Toxicol. Lett. 2003;137:15–21. doi: 10.1016/s0378-4274(02)00377-6. [DOI] [PubMed] [Google Scholar]
- 5.NRC, National Research Council Report. Arsenic in the drinking water. Washington, DC: National Acadamy Press; 2003. [Google Scholar]
- 6.Martinez VD, Vucic EA, Becker-Santos DD, et al. Arsenic exposure and the induction of human cancers. J. Toxicol. 2011:431287. doi: 10.1155/2011/431287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. http://pubs.usgs.gov/fs/2000/fs063-00/Arsenic in ground-water resources of the United States. U.S. Geological Survey Fact Sheet 063-00
- 8.Qian Y, Liu KJ, Chen Y, et al. Cdc42 regulates arsenic-induced NADPH oxidase activation and cell migration through actin filament reorganization. J. Biol. Chem. 2005;280:3875–3884. doi: 10.1074/jbc.M403788200. [DOI] [PubMed] [Google Scholar]
- 9.Fry RC, Navasumrit P, Valiathan C, et al. Activation of inflammation/NF-kB signaling in infants born to arsenic exposed mothers. PLOS Genetics. 2007;3:2180–2189. doi: 10.1371/journal.pgen.0030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan PC, Huff J. Arsenic carcinogenesis in animals and in humans: mechanistic, experimental, and epidemiological evidence. Environ. Carcinog. Ecotoxicol. Revs. 1997;C15:83–122. [Google Scholar]
- 11.Simeonova PP, Wang S, Toriuma W, et al. Arsenic mediates cell proliferation and Gene Expression in the Bladder Epithelium: Association with Activating Protein-1 Transactivation. Cancer Res. 2000;60:3445–3453. [PubMed] [Google Scholar]
- 12.Chen CJ, Wang CJ. Ecological correlation between arsenic level in well water and age-adjusted mortality from malignant neoplasms. Cancer Res. 1990;50:5470–5474. [PubMed] [Google Scholar]
- 13.Germolec DR, Spalding J, Yu H-S, et al. Arsenic enhancement of skin neoplasia by chronic stimulation of growth factors. Am. J. Pathol. 1988;153:1775–1785. doi: 10.1016/S0002-9440(10)65692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee TC, Tanaka N, Lamb PW, et al. Induction of gene amplification by arsenic. Science. 1988;241:79–81. doi: 10.1126/science.3388020. [DOI] [PubMed] [Google Scholar]
- 15.Kasprzak KS. Oxidative DNA and protein damage in metal-induced toxicity and carcinogenesis. Free Rad. Biol. Med. 2002;32:958–967. doi: 10.1016/s0891-5849(02)00809-2. [DOI] [PubMed] [Google Scholar]
- 16.Buzard GS, Kasprzak KS. Possible roles of nitric oxide and redox cell signaling in metal-induced toxicity and carcinogenesis: a review. J. Environ. Pathol. Toxicol. Oncol. 2000;19:179–199. [PubMed] [Google Scholar]
- 17.Harris GK, Shi X. Signaling by carcinogenic metals and metal-induced reactive oxygen species. Mutation Res. 2003;533:183–200. doi: 10.1016/j.mrfmmm.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 18.Leonard SS, Harris GK, Shi X. Metal-induced oxidative stress and signal transduction. Free Rad. Biol. Med. 2004;37:1921–1942. doi: 10.1016/j.freeradbiomed.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Huang C, Ke Q, Costa M, et al. Molecular mechanism of arsenic carcinogenesis. Mol. Cell. Biochem. 2004;255:57–66. doi: 10.1023/b:mcbi.0000007261.04684.78. [DOI] [PubMed] [Google Scholar]
- 20.Gao N, Shen L, Zhang Z, et al. Arsenite induces HIF-1α and VEGF through PI3K, Akt and reactive oxygen species in DU145 human prostate carcinoma cells. Mol. Cell. Biochem. 2004;255:33–45. doi: 10.1023/b:mcbi.0000007259.65742.16. [DOI] [PubMed] [Google Scholar]
- 21.Shi H, Shi X, Liu KJ. Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol. Cell. Biochem. 2003;255:67–78. doi: 10.1023/b:mcbi.0000007262.26044.e8. [DOI] [PubMed] [Google Scholar]
- 22.Ames BN. Measuring oxidative damage in humans: relation to cancer and ageing. IARC. Sci. Publ. 1988;89:407–416. [PubMed] [Google Scholar]
- 23.Iwama K. Apoptosis induced by arsenic trioxide in leukemia U937 cells is dependent on activation of p38, inactivation of ERK and the Ca2+-dependent production of superoxide. Intl. J. Cancer. 2001;92:518–526. doi: 10.1002/ijc.1220. [DOI] [PubMed] [Google Scholar]
- 24.Lynn S, Gurr JR, Lai HT, et al. NADPH oxidase activation is involved in arsenite induced oxidative DNA damage in human vascular smooth muscle cells. Circ. Res. 2000;86:514–519. doi: 10.1161/01.res.86.5.514. [DOI] [PubMed] [Google Scholar]
- 25.Liu S, Athar M, Lippai I, et al. Induction of oxyradicals by arsenic: implication for mechanism of genotoxicity. Proc. Natl. Acad. Sci. USA. 2001;98:1643–1648. doi: 10.1073/pnas.031482998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barchowsky A, Kiel LR, Dudek EJ, et al. Stimulation of reactive oxygen, but not reactive nitrogen species, in vascular endothelial cells exposed to low levels of arsenite. Free Rad. Biol. Med. 1999;27:1405–1412. doi: 10.1016/s0891-5849(99)00186-0. [DOI] [PubMed] [Google Scholar]
- 27.Corsini E. Sodium arsenate induces overproduction of interleukin-lalpha inmurine keratinocytes: role of mitochondria. J. Invest. Dermatol. 1999;113:760–765. doi: 10.1046/j.1523-1747.1999.00748.x. [DOI] [PubMed] [Google Scholar]
- 28.Jing Y. Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood. 1999;94:2102–2111. [PubMed] [Google Scholar]
- 29.Wang TS. Arsenite induces apoptosis in Chinese hamster ovary cells by generation of reactive oxygen species. J. Cell. Physiol. 1996;169:256–268. doi: 10.1002/(SICI)1097-4652(199611)169:2<256::AID-JCP5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 30.Chang Q, Pan J, Wang X, et al. Reduced capacity of reactive oxygen species generation contributes to an enhanced cell growth of the arsenic-transformed epithelial cells. Cancer Res. 2010;70:5127–5135. doi: 10.1158/0008-5472.CAN-10-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chou WC, Jie C, Kenedy AA, et al. Role of NADPH oxidase in arsenic-induced reactive oxygen species formation and cytotoxicity in myeloid leukemia cells. Proc. Natl. Acad. Sci. U S A. 2004;10:4578–4583. doi: 10.1073/pnas.0306687101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Straub AC, Clark KA, Ross MA, et al. Arsenic-stimulated liver sinusoidal capillarization in mice requires NADPH oxidase-generated superoxide. J. Clin. Invest. 2008;118:3980–3989. doi: 10.1172/JCI35092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lemarie A, Bourdonnay E, Morzadec C, et al. Inorganic arsenic activates reduced NADPH oxidase in human primary macrophages through a Rho kinase/p38 kinase pathway. J. Immunol. 2008;180:6010–6017. doi: 10.4049/jimmunol.180.9.6010. [DOI] [PubMed] [Google Scholar]
- 34.Carpenter RC, Jiang Y, Jing Y, et al. Arsenite induces cell transformation by reactive oxygen species, AKT, ERK1/2, and p70S6K1. Biochem. Biophys. Res. Commun. 2011;414:533–538. doi: 10.1016/j.bbrc.2011.09.102. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Son YO, Chang Q, et al. NADPH oxidase activation is required in reactive oxygen species generation and cell transformation induced by hexavalent chromium. Toxic. Sci. 2011;123:399–410. doi: 10.1093/toxsci/kfr180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biedermann KA, Landolph JR. Induction of anchorage independence in human diploid foreskin fibroblasts by carcinogenic metal salts. Cancer Res. 1987;47:3815–3823. [PubMed] [Google Scholar]
- 37.Veljkovic E, Jiricny J, Menigatti M, et al. Chronic exposure to cigarette smoke condensate in vitro induces epithelial to mesenchymal transition-like changes in human bronchial epithelial cells. BEAS-2B. Toxico. In Vitro. 2011;25:446–453. doi: 10.1016/j.tiv.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 38.Gañán-Gómez I, Wei Y, Yang H, et al. Oncogenic functions of the transcription factor Nrf2. Free Radic. Biol. Med. 2013;65:750–764. doi: 10.1016/j.freeradbiomed.2013.06.041. [DOI] [PubMed] [Google Scholar]
- 39.Ye J, Wang S, Leonard SS, et al. Role of reactive oxygen species and p53 in chromium(VI)-induced apoptosis. J. Biol. Chem. 1999;274:34974–34980. doi: 10.1074/jbc.274.49.34974. [DOI] [PubMed] [Google Scholar]