Abstract
Objective
Obesity is an important risk factor for colorectal neoplasia; however, little research exists on racial differences in obesity measures (BMI, waist circumference (WC), and waist-hip-ratio (WHR)) associated with adenoma.
Design and Methods
We used data from the Diet and Health Studies, Phases III-V to examine differences in the contribution of obesity measures to adenoma risk by race. The sample consisted of 2,184 patients (1,806 white, 378 African American) undergoing outpatient colonoscopy for average risk screening. Covariates included demographics, health history, and validated measures of diet and physical activity.
Results
Among whites, BMI (Overweight: OR 1.31, 95% CI 1.00—1.71; Obese: OR 1.89, 95% CI 1.41—2.56), WC (OR 1.47, 95% CI 1.09—1.99), and WHR (OR 1.60 95% CI 1.24—2.06) were associated with adenomas. BMI was not associated with adenomas in African Americans. Although the confidence intervals were wide, the point estimates for WHR (OR 1.07, 95% CI 0.51—2.22) and WC (OR 1.04, 95% CI 0.56—1.92) were slightly elevated above the null.
Conclusions
BMI was associated with adenomas only among whites, whereas WHR and WC appeared to be important risk factors among both races. Racial differences in adenoma risk may be due to differences in body shape and weight and/or fat distribution.
Keywords: colorectal neoplasia, colorectal adenoma, obesity, racial differences
Introduction
Obesity has been consistently associated with an increased risk of colorectal cancer (CRC). Several meta-analyses indicate common clinical measures of obesity, including body mass index (BMI), waist circumference (WC), and waist-hip-ratio (WHR), are positively associated with CRC in both men and women (1, 2).
Epidemiologic studies also suggest measures of obesity are associated with an increased risk of colorectal adenomas, the precursor to most CRCs. Results from studies of body size and adenoma risk vary with respect to which obesity measure was used (e.g., BMI, WC, WHR), as well as by sex (3–7). However, a recent meta-analysis (8) reported the highest levels of WC and WHR independently contributed to an increased risk for adenomas. Similarly, a meta-analysis (9) that examined the relationship between BMI and adenomas suggested a 5-unit increase in BMI is associated with an increased risk for adenomas. The findings from both studies were independent of age, sex, and geographic location.
Although obesity has been established as an important risk factor for colorectal adenomas, we lack sufficient data on which clinical measures of obesity are most relevant to the development of adenomas in different racial populations. It is well understood that CRC incidence and mortality differ by race and ethnicity, but few studies have examined potential differences in how obesity measures relate to cancer risk by race. Further, there has been little research on the racial differences in the association of various obesity measures with colorectal adenomas. Many existing studies have utilized predominantly white-American or European samples, which may limit generalizability, and few studies have examined obesity measures in the context of other known adenoma risk factors (e.g., diet, family history, smoking). It is important to use the most relevant measure of obesity in specific racial populations to adequately characterize cancer risk. Therefore, we examined the racial differences in the relationship between obesity measures (BMI, WC, and WHR) and colorectal adenomas in a large, diverse screening population.
Methods
Participants and Procedures
We used data from the University of North Carolina Diet and Health Studies (DHS), Phases III-V to determine the association of different obesity measures with colorectal adenoma by race. The design and methods of the studies have been described in detail elsewhere (10, 11). Briefly, DHS were cross-sectional studies that examined environmental and lifestyle factors associated with presence of colorectal adenomas. Patients undergoing an outpatient colonoscopy in 1998—2010 (DHS III: 1998–2000; DHS IV: 2001–2001; DHS V: 2009–2010) at the University of North Carolina Hospitals (Chapel Hill, NC) were recruited to participate. The most common indication for colonoscopy was average risk CRC screening. Eligible patients were between the ages of 30—80 years, had satisfactory preparation for the colonoscopy with complete examination to the cecum, and gave informed consent. Patients with a previous history of colon resection or diagnosis of polyposis, colitis, or CRC were excluded. For the purposes of this analysis, we further excluded any patient who reported a race/ethnicity other than white or African American (n=274).
Measures
Outcome Variable
Presence of any adenoma on the colonoscopy report was assessed as the binary dependent variable.
Obesity Measures
A trained research assistant measured height, weight, and waist and hip circumference at the time of colonoscopy. BMI was measured as kg/m2 and categorized according to standard cutpoints: normal (18.5–25), overweight (≥25–30), and obese (>30). Waist circumference was measured at the narrowest part of the torso, and hip circumference was measured at the level of greatest lateral extension of the hips (both in cm). WHR was calculated as the ratio of waist and hip circumference. In accordance with WHO standards, high WHR was defined as greater than 0.9 and 0.8 for men and women, respectively. Waist circumference above 102 cm in men and 88 cm in women was considered high (12).
Covariates
Age, sex, race, health history, and lifestyle factors were examined as covariates. Race was self-reported by study participants.
Within 12 weeks of colonoscopy, participants were contacted by telephone to complete a questionnaire on lifestyle behaviors (e.g., diet, physical activity) and health history. Interviewers were blind to colonoscopy results. Dietary information was measured with the Block Diet History Questionnaire (DHS III) (13) and the National Cancer Institute Diet History Questionnaire (DHS IV-V) (14). Participants reported usual dietary habits (e.g., fiber, total fat, and red meat consumption) during the 1-year period preceding colonoscopy. Dietary measures were adjusted for total energy intake (15). Physical activity was assessed using a modified version of the Stanford 7-day physical activity recall (16) for participants in DHS III and IV. Physical activity in the DHS V was measured with the International Physical Activity Questionnaire (17). Because different physical activity measures were used across phases of DHS, raw metabolic equivalents (MET) were categorized into quartiles to allow comparison across all study participants (18).
Interviewers also collected information on participants’ family history of CRC (first-degree relative) and personal history of risk factors for CRC, including NSAID use, smoking, and alcohol use.
Data Analysis
Logistic regression models were used to examine the association between each obesity measure (BMI, WC, and WHR) and presence of colorectal adenomas. We examined differences in effect estimates across race/ethnicity by including a cross-product term of race/ethnicity and each obesity measure and calculating a p-value for heterogeneity using the -2-log likelihood statistic. Because the overall results suggested effect estimates varied across the two strata of race (p-value from likelihood ratio test: 0.003 (BMI), 0.02 (WC), 0.16 (WHR)), subsequent analyses were stratified (white vs. African American).
We evaluated the potential for confounding by age, sex, smoking, alcohol use, NSAID use, diet (total energy, fat, and fiber intake, and red meat consumption), physical activity, and family history of CRC using a directed acyclic graph (DAG) to identify a minimally sufficient adjustment set of covariates (19–21). In addition to race/ethnicity, DAG analyses suggested the model be adjusted for sex, age, total fiber intake, total fat intake, red meat consumption, smoking, and physical activity. Unadjusted and adjusted associations between each measure of obesity and colorectal adenomas are reported as prevalence odds ratios and 95% confidence intervals. Participants with missing data on obesity measures and/or confounders included in the adjusted analysis were excluded.
To further examine the reasons for potential differences in adenoma risk by various obesity measures, we plotted BMI and WHR among whites and African Americans. We also examined the linear association between BMI and WHR in each racial group by calculating Pearson’s correlation coefficient.
Statistical analyses were conducted using Stata/1C, Version 13.0 (College Station, TX).
Results
The analysis included 2,184 participants. The mean age of study participants at the time of colonoscopy was 55.9 (SD 9.26). The majority were female (57.8%), and 17.3% (n=378) reported their race as African American. In total, 629 (29%) had one or more adenomas noted in their colonoscopy report and 1,555 (71%) had no adenomas. Characteristics of the study population by race and presence of adenomas are shown in Table 1.
Table 1.
Characteristics of the study population by race and presence of adenoma (n=2,184)
| Characteristic | Whites
|
African-Americans
|
||
|---|---|---|---|---|
| Adenoma (n=513) | No Adenoma (n=1,293) | Adenoma (n=116) | No Adenoma (n=262) | |
|
|
|
|||
| Mean ± SD or n (%) | Mean ± SD or n (%) | |||
| Age, y | 58 ± 9.1 | 55 ± 9.1 | 59 ± 9.9 | 55 ± 9.2 |
| Sex | ||||
| Male | 281 (36.5) | 490 (63.6) | 55 (36.4) | 96 (63.6) |
| Female | 232 (22.5) | 801 (77.5) | 61 (26.9) | 166 (73.1) |
| BMI, kg/m2 | 28 ± 5.6 | 26 ± 5.4 | 31 ± 7.7 | 29 ± 6.7 |
| Normal (18.5–25) | 169 (23.0) | 567 (77.0) | 28 (35.4) | 51 (64.6) |
| Overweight (≥25–30) | 180 (30.7) | 407 (69.3) | 33 (34.7) | 62 (65.3) |
| Obese (>30) | 139 (36.4) | 243 (63.6) | 45 (26.5) | 125 (73.5) |
| WHR (continuous) | 0.94 ± 0.087 | 0.91 ± 0.092 | 0.96 ± 0.087 | 0.93 ± 0.083 |
| Normal | 80 (19.5) | 330 (80.5) | 16 (29.1) | 39 (70.9) |
| Higha | 352 (32.3) | 739 (67.7) | 80 (32.7) | 165 (67.4) |
| WC, cm (continuous) | 100 (14.8) | 94 (14.2) | 104 (16.6) | 103 (14.8) |
| Normal | 195 (24.9) | 588 (75.1) | 34 (34.7) | 64 (65.3) |
| Higha | 237 (33.0) | 482 (67.0) | 62 (30.7) | 140 (69.3) |
| Tobacco Useb, pack y | 44 ± 40.3 | 35 ± 34.8 | 41 ± 33.6 | 40 ± 32.5 |
| Smoking Status | ||||
| Never | 251 (27.1) | 675 (72.9) | 60 (29.4) | 144 (70.6) |
| Former | 196 (28.7) | 486 (71.3) | 31 (34.8) | 58 (65.2) |
| Current | 63 (32.6) | 130 (67.4) | 24 (29.3) | 59 (70.7) |
| Alcohol Usec, g/day | 16 ± 43.3 | 12 ± 23.9 | 5 ± 7.1 | 6 ± 14.9 |
| Regular NSAID Use (≥15/month) | 148 (27.8) | 385 (72.2) | 25 (26.0) | 69 (73.4) |
| Total Energy Intake, kcal/day | 1907 ± 821.4 | 1821 ± 728.2 | 1666 ± 966.5 | 1792 ± 1037.3 |
| Fat, g/day | 73 ± 34.9 | 69 ± 32.1 | 67 ± 45.4 | 70 ± 42.8 |
| Total Fiber, g/day | 19 ± 8.9 | 20 ± 9.2 | 14 ± 10.0 | 16 ± 10.1 |
| Red Meat, oz/day | 3 ± 2.3 | 2 ± 2.0 | 3 ± 2.5 | 3 ± 2.3 |
| Physical Activity, MET | ||||
| Quartile 1 | 134 (31.6) | 290 (68.4) | 45 (37.5) | 75 (62.5) |
| Quartile 2 | 131 (31.1) | 290 (68.8) | 18 (22.8) | 61 (77.2) |
| Quartile 3 | 92 (22.6) | 315 (77.4) | 14 (26.9) | 38 (73.1) |
| Quartile 4 | 110 (26.7) | 302 (73.3) | 26 (32.5) | 54 (67.5) |
| Family History of CRC | ||||
| No | 428 (28.6) | 1067 (71.4) | 93 (31.1) | 206 (68.9) |
| Yes | 61 (27.1) | 174 (72.9) | 14 (29.2) | 34 (70.8) |
Abbreviations: y, years; BMI, body mass index; WHR, waist-hip ratio; WC, waist circumference; NSAID, nonsteroidal anti-inflammatory drug; MET, metabolic equivalent task; CRC, colorectal cancer
NOTE: Raw METs were not equivalent between the two measures used to assess physical activity (see text for details). METs were categorized into quartiles based on the distribution in the control group. Missing values range from 0 (age) to 383 (WHR).
High WHR was defined as greater than 0.9 and 0.85 for men and women, respectively. Waist circumference above 102 cm in men and 88 cm in women was considered high (12).
Excludes participants who reported no history of tobacco use (n=1,130)
Excludes participants who reported no alcohol use (n=404)
The distribution of BMI, WHR, and WC was fairly similar among whites and African Americans (Table 2). Mean values in each strata of all obesity measures were generally comparable between the two races (p-value from test of equal means: normal BMI (0.14), overweight BMI (0.11), obese BMI (<0.01), normal WC (<0.01), high WC (<0.01), normal WHR (0.71), high WHR (0.09)), although there were some differences in WC. A greater proportion of African Americans had higher levels of BMI (49.4 vs. 22.2%, p <0.01), WHR (81.7 vs. 72.7%, p <0.01), and WC (70.0 vs. 47.9%, p<0.01).
Table 2.
Distribution of obesity measures by race/ethnicity (n=2,184)
| Whites (n=1,806) | African-Americans (n=378) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Measure | n (%) | Mean | SD | n (%) | Mean | SD |
| BMI, kg/m2 (continuous) | 1705 | 25.9 | 5.50 | 344 | 30.7 | 7.49 |
| Normal (18.5–25) | 736 (43.2) | 22.3 | 1.92 | 79 (23.0) | 22.0 | 2.52 |
| Overweight (≥25–30) | 587 (34.4) | 27.3 | 1.41 | 95 (27.6) | 27.5 | 1.48 |
| Obese (>30) | 382 (22.4) | 34.8 | 4.63 | 170 (49.4) | 36.6 | 5.82 |
| WHR (continuous) | 1501 | 0.92 | 0.09 | 300 | 0.94 | 0.08 |
| Normal | 410 (27.3) | 0.81 | 0.05 | 55 (18.3) | 0.81 | 0.05 |
| Higha | 1091 (72.7) | 0.96 | 0.06 | 245 (81.7) | 0.97 | 0.06 |
| WC, cm (continuous) | 1502 | 95.3 | 14.61 | 300 | 103.3 | 15.36 |
| Normal | 783 (52.1) | 85.7 | 9.36 | 98 (32.7) | 88.8 | 8.80 |
| Higha | 719 (47.9) | 105.7 | 11.90 | 202 (67.3) | 110.3 | 12.72 |
Abbreviations: BMI, body mass index; WHR, waist-hip ratio; WC, waist circumference; SD, standard deviation
High WHR was defined as greater than 0.9 and 0.85 for men and women, respectively. WC above 102 cm in men and 88 cm in women was considered high (12).
Table 3 shows prevalence odds ratios for the association of obesity measures with colorectal adenomas. BMI, WHR, and WC elevated the odds of adenoma among whites (n=1,806). Within levels of BMI, we estimated crude odds ratios of 1.48 (95% CI 1.16—1.90) and 1.92 (95% CI 1.46—2.51) for overweight and obese, respectively. The odds of adenoma were elevated among whites with high WHR (OR 1.96, 95% CI 1.49—2.59) and WC (OR 1.48, 95% CI 1.18—1.86). Odds ratios from the model accounting for potential confounders were similar. Participants who were overweight (OR 1.31, 95% CI 1.00—1.71), obese (OR 1.89, 95% CI 1.41—2.56), or had high WHR (OR 1.47, 95% CI 1.09—1.99) or WC (OR 1.60, 95% CI 1.24—2.06) had higher odds of adenoma compared to those classified as normal. BMI and WHR were also correlated (r = 0.26) (Figure 1).
Table 3.
Crude and adjusted prevalence odds ratios for colorectal adenomas among whites (n=1,806) and African Americans (n=378)
| Whites (n=1,806)
|
African Americans (n=378)
|
|||
|---|---|---|---|---|
| Crude OR (95% CI) | Adjusteda OR (95% CI) | Crude OR (95% CI) | Adjusteda OR (95% CI) | |
| BMI, kg/m2 | ||||
| Normal (18.5–25) | 1.00 (REF) | 1.00 (REF) | 1.00 (REF) | 1.00 (REF) |
| Overweight (25–30) | 1.48 (1.16—1.90) | 1.31 (1.00—1.71) | 0.97 (0.52—1.81) | 0.98 (0.48—1.99) |
| Obese (>30) | 1.92 (1.46—2.51) | 1.89 (1.41—2.56) | 0.66 (0.37—1.16) | 0.67 (0.35—1.30) |
| WHR | ||||
| Normal | 1.00 (REF) | 1.00 (REF) | 1.00 (REF) | 1.00 (REF) |
| Highb | 1.96 (1.49—2.59) | 1.47 (1.09—1.99) | 1.18 (0.62—2.24) | 1.07 (0.51—2.22) |
| WC | ||||
| Normal | 1.00 (REF) | 1.00 (REF) | 1.00 (REF) | 1.00 (REF) |
| Highb | 1.48 (1.18—1.86) | 1.60 (1.24—2.06) | 0.83 (0.50—1.39) | 1.04 (0.56—1.92) |
Abbreviations: OR, prevalence odds ratio; CI, confidence interval; BMI, body mass index; WHR, waist-hip ratio; WC, waist circumference; REF, referent
NOTE: Missing values ranged from 0 (age) to 383 (WHR). The adjusted model consists of 1,600 (BMI), 1,414 (WHR) 1,415 (WC) whites and 308 (BMI), 269 (WHR), and 269 (WC) African Americans.
Adjusted for sex, smoking, age, physical activity, red meat consumption, total fat intake, and total fiber intake
High WHR was defined as greater than 0.9 and 0.85 for men and women, respectively. WC above 102 cm in men and 88 cm in women was considered high (12).
Figure 1.
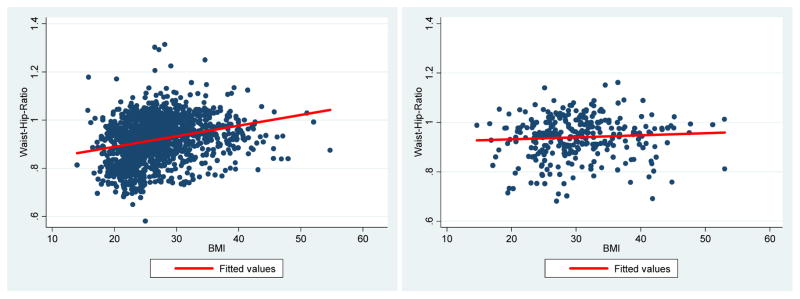
Linear association between BMI and WHR among whites (n=1,806) and African Americans (n=374)
Abbreviations: BMI, body mass index; WHR, waist-hip-ratio
NOTE: Pearson’s correlation coefficient: 0.2587 (whites, p<0.05) and 0.0658 (African Americans, p not significant)
Among African Americans (n=378), the crude and adjusted odds ratios suggest BMI was not associated with adenomas (Table 3) (overweight: adjusted OR 0.98, 95% CI 0.48—1.99; obese: adjusted OR 0.67, 95% CI 0.35—1.30) Although the confidence intervals were wide, the WHR point estimates were elevated in the expected direction above the null (Crude: OR 1.18, 95% CI 0.62—2.24; Adjusted: OR 1.07, 95% CI 0.51—2.22). Similarly, the adjusted odds ratio for the association between WC and adenoma was slightly elevated (OR 1.04, 95% CI 0.56—1.92) but with a wider confidence interval (confidence limit ratio: 3.43). BMI and WHR were not correlated among African Americans (r = 0.07) (Figure 1).
Discussion
Our study provides new evidence that the most relevant measures of obesity with respect to colonic neoplasia may be different among races. The results highlight the importance of considering multiple obesity measures to assess adenoma and/or CRC risk in patients of different racial/ethnic backgrounds. We found that BMI was associated with adenomas only among whites, whereas WHR and WC appeared to be important risk factors among both whites and African Americans. The difference in the BMI-adenoma association between whites and African Americans may reflect differences in body composition. Researchers have hypothesized the variation in risk among obese individuals can be explained by variations in body composition and weight and/or fat distribution (22). In our study, the linear relationship between BMI and WHR (Figure 1) among whites could indicate greater levels of abdominal obesity in this subgroup. Our data suggest that higher-BMI whites tend to have more abdominally and/or centrally distributed fat (i.e., “apple” body shape), which may confer a greater risk for adenomas. BMI may be an important risk factor among whites because it is closely correlated with both WHR and WC.
Conversely, we did not observe a correlation between BMI and WHR in African Americans (Figure 1). Although a greater proportion of African Americans had a high WHR or WC, there was no relationship between BMI and WHR. This finding provides evidence that African Americans may have less abdominal fat and more fat around the hips (i.e., “pear” body shape). It could also explain why BMI was not associated with adenomas in the crude or adjusted analyses. Our data suggest BMI is not a useful measure of risk in African Americans because it does not adequately capture relevant patterns of abdominal obesity. Rather, measures of central obesity (e.g., WC and WHR) may be stronger indicators of risk. African Americans who have a body shape similar to whites (i.e., more abdominally distributed fat) may be at a higher risk for colorectal adenomas. In the future, researchers should examine the underlying differences in body composition and how these differences affect adenoma risk across racial/ethnic groups.
The differences we observed in adenoma risk may also be due to variations in visceral adipose tissue (VAT) among whites and African Americans. VAT is widely considered an important marker of health risk, with greater specificity than total fat mass or generalized obesity (23). Limited research suggests African Americans have consistently lower VAT compared to whites and/or Hispanics within similar WC and BMI strata (24). Others have reported whites exhibit higher levels of VAT than African Americans at higher levels of BMI, WC, and total body fat for both men and women (25, 26). Collectively, this research suggests the health hazards associated with obesity are differentially expressed through abdominal VAT. Our data support this view: African Americans had a lower risk of colorectal adenomas associated with BMI, which may reflect their lower abdominal VAT amounts. Further research is needed to determine whether different BMI cutoff points are necessary to adequately assess risk in different racial groups.
Our results also indicate that WHR and WC are better measures of central obesity than BMI, which is often poorly correlated with true body composition. A recent meta-analysis (27) of studies that assess the performance of BMI to detect body adiposity suggests BMI values used to diagnose obesity have high specificity, but low sensitivity to identify adiposity, as they fail to identify the majority of individuals with excess body fat. This is consistent with our finding that WC and WHR were stronger measures of adenoma risk in both whites and African Americans. Alternative obesity measures, such as bioelectrical impedance analysis (BIA), may not be the best predictor of adenoma risk because it measures the total amount of fat but not the distribution (28). Other researchers have proposed the body adiposity index (BAI), calculated from hip circumference and height, as a better direct estimate of adiposity that can be easily implemented in clinical settings (29). While this measure may be encouraging, it has not been widely validated.
A strength of our study is the large, diverse screening population that comprised our sample. To date, the majority of studies of adenoma risk have been of predominantly white-American or European populations. Few have included large samples of racial/ethnic minorities. Another strength is our use of reliable data on other established adenoma risk factors and validated measures of diet and physical activity (13, 14, 16, 17), which combine to limit the possibility of confounding bias or spurious results due to other important markers of adenoma risk.
Although our study is the largest to examine differences in obesity measures related to adenoma risk, the relatively small number of African Americans (compared to whites) may have contributed to the imprecision around the point estimates in this subgroup. Another potential limitation is measurement error from food frequency questionnaires and/or the different instruments used across DHS phases. However, we would not expect that diet would be differentially reported with respect to adenoma status. Finally, study participants with missing data were excluded from the analysis. Chi-square tests (all p<0.05) indicated that respondents with incomplete data (n=536, 25%) were more likely to be African American, male, and have higher BMI; however, there was no difference in the presence of adenoma on colonoscopy between participants with complete versus incomplete data. It is therefore unlikely that our results are overestimated associations.
In summary, our study provides further support for a relationship between obesity and risk of colorectal adenomas. In a large, diverse screening population, we found higher BMI, WHR, and WC were all important predictors of adenomas among whites, and a high WHR and WC elevated the likelihood of adenomas among African Americans. These findings are consistent with previous meta-analyses (8, 9) that suggest higher levels of WC and WHR, as well as increases in BMI, independently contribute to an increased risk for adenomas. The group-specific differences we observed in adenoma risk may be due to differences in body shape and/or distribution of fat. Our findings highlight the importance of using multiple obesity measures when assessing adenoma risk in patients of different racial/ethnic backgrounds. There has been some interest in developing risk prediction models based on adenoma risk factors that could be used to determine CRC screening intervals (30). Researchers have examined the utility of risk prediction models for advanced adenomas (31), and our results suggest race-specific risk factors may be necessary when developing future risk stratification tools. Using the most relevant measure of obesity is critical to adequately characterizing risk of adenomas and CRC in specific racial populations.
Acknowledgments
Grant support: This work was supported by grants from the National Institutes of Health (T32 DK07634, P30 DK034987, and R01 CA44684)
References
- 1.Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr. 2007;86:556–65. doi: 10.1093/ajcn/86.3.556. [DOI] [PubMed] [Google Scholar]
- 2.Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev. 2007;16:2533–47. doi: 10.1158/1055-9965.EPI-07-0708. [DOI] [PubMed] [Google Scholar]
- 3.Sass DA, Schoen RE, Weissfeld JL, Weissfeld L, Thaete FL, Kuller LH, et al. Relationship of visceral adipose tissue to recurrence of adenomatous polyps. Am J Gastroenterol. 2004;99:687–93. doi: 10.1111/j.1572-0241.2004.04136.x. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs ET, Martinez ME, Alberts DS, Jiang R, Lance P, Lowe KA, et al. Association between body size and colorectal adenoma recurrence. Clin Gastroenterol Hepataol. 2007;5:982–90. doi: 10.1016/j.cgh.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wise LA, Rosenberg L, Palmer JR, Adams-Campbell LL. Anthropometric risk factors for colorectal polyps in African-American women. Obesity (Silver Spring) 2008;16:859–68. doi: 10.1038/oby.2007.139. [DOI] [PubMed] [Google Scholar]
- 6.Hermann S, Rohrmann S, Linseisen J. Lifestyle factors, obesity and the risk of colorectal adenomas in EPIC-Heidelberg. Cancer Causes Control. 2009;20:1397–408. doi: 10.1007/s10552-009-9366-3. [DOI] [PubMed] [Google Scholar]
- 7.Nam SY, Kim BC, Han KS, Ryu KH, Park BJ, Kim HB, et al. Abdominal visceral adipose tissue predicts risk of colorectal adenoma in both sexes. Clin Gastroenterol Hepatol. 2010;8:443–50. e1–2. doi: 10.1016/j.cgh.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Hong S, Cai Q, Chen D, Zhu W, Huang W, Li Z. Abdominal obesity and the risk of colorectal adenoma: a meta-analysis of observational studies. Eur J Cancer Prev. 2012;21:523–31. doi: 10.1097/CEJ.0b013e328351c775. [DOI] [PubMed] [Google Scholar]
- 9.Ben Q, An W, Jiang Y, Zhan X, Du Y, Cai QC, et al. Body mass index increases risk for colorectal adenomas based on meta-analysis. Gastroenterol. 2012;142:762–72. doi: 10.1053/j.gastro.2011.12.050. [DOI] [PubMed] [Google Scholar]
- 10.Vinikoor LC, Schroeder JC, Millikan RC, Satia JA, Martin CF, Ibrahim J, et al. Consumption of trans-fatty acid and its association with colorectal adenomas. Am J Epidemiol. 2008;168:289–97. doi: 10.1093/aje/kwn134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guilera M, Connelly-Frost A, Keku TO, Martin CF, Galanko J, Sandler RS. Does physical activity modify the association between body mass index and colorectal adenomas? Nutr Cancer. 2005;51:140–5. doi: 10.1207/s15327914nc5102_3. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Waist circumference and waist-hip ratio: report of a WHO expert consultation. Geneva: Dec, 2008. [Google Scholar]
- 13.Block G. Dietary guidelines and the results of food consumption surveys. Am J Clin Nutr. 1991;53:356s–7s. doi: 10.1093/ajcn/53.1.356S. [DOI] [PubMed] [Google Scholar]
- 14.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires : the Eating at America’s Table Study. Am J Epidemiol. 2001;154:1089–99. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 15.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149:531–40. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 16.Richardson MT, Ainsworth BE, Jacobs DR, Leon AS. Validation of the Stanford 7-day recall to assess habitual physical activity. Ann Epidemiol. 2001;11:145–53. doi: 10.1016/s1047-2797(00)00190-3. [DOI] [PubMed] [Google Scholar]
- 17.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 18.Peery AF, Barrett PR, Park D, Rogers AJ, Galanko JA, Martin CF, et al. A high-fiber diet does not protect against asymptomatic diverticulosis. Gastroenterol. 2012;142:266–72. e1. doi: 10.1053/j.gastro.2011.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 20.Textor J, Hardt J, Knuppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiol. 2011;22:745. doi: 10.1097/EDE.0b013e318225c2be. [DOI] [PubMed] [Google Scholar]
- 21.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol. 2008;8:70. doi: 10.1186/1471-2288-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson CL, Berger NA, Chak A, Li L. Racial differences in measures of obesity and risk of colon adenoma. Obesity (Silver Spring) 2012;20:673–7. doi: 10.1038/oby.2011.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–7. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 24.Carroll JF, Chiapa AL, Rodriquez M, Phelps DR, Cardarelli KM, Vishwanatha JK, et al. Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity (Silver Spring) 2008;16:600–7. doi: 10.1038/oby.2007.92. [DOI] [PubMed] [Google Scholar]
- 25.Camhi SM, Bray GA, Bouchard C, Greenway FL, Johnson WD, Newton RL, et al. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: sex and race differences. Obesity (Silver Spring) 2011;19:402–8. doi: 10.1038/oby.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katzmarzyk PT, Bray GA, Greenway FL, Johnson WD, Newton RL, Jr, Ravussin E, et al. Racial differences in abdominal depot-specific adiposity in white and African American adults. Am J Clin Nutr. 2010;91:7–15. doi: 10.3945/ajcn.2009.28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okorodudu DO, Jumean MF, Montori VM, Romero-Corral A, Somers VK, Erwin PJ, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obesity. 2010;34:791–9. doi: 10.1038/ijo.2010.5. [DOI] [PubMed] [Google Scholar]
- 28.Frantz DJ, Crockett SD, Galanko JA, Sandler RS. Percent body fat measured by bioelectrical impedance is not associated with colorectal adenoma status. J Gastroenterol Hepatol Res. 2013;2(3):445–8. doi: 10.6051/j.issn.2224-3992.2013.02.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bergman RN. A better index of body adiposity. Obesity (Silver Spring) 2012;20:1135. doi: 10.1038/oby.2012.99. [DOI] [PubMed] [Google Scholar]
- 30.Lieberman D. Screening for colorectal cancer in individuals at average risk: current methods and emerging issues. JAMA Intern Med. 2014;174(1):10–11. doi: 10.1001/jamainternmed.2013.11499. [DOI] [PubMed] [Google Scholar]
- 31.Schroy PC, 3rd, Coe AM, Mylvaganam SR, Ahn LB, Lydotes MA, Robinson PA, et al. The Your Disease Risk Index for colorectal cancer is an inaccurate risk stratification tool for advanced colorectal neoplasia at screening colonoscopy. Cancer Prev Res. 2012;5(8):1044–52. doi: 10.1158/1940-6207.CAPR-12-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]


