Abstract
Background
Heart failure (HF) is a heterogeneous condition of both symptoms and hemodynamics.
Objective
The goal of this study was to identify distinct profiles among integrated data on physical and psychological symptoms and hemodynamics, and quantify differences in 180-day event-risk among observed profiles.
Methods
A secondary analysis of data collected during two prospective cohort studies by a single group of investigators was performed. Latent class mixture modeling was used to identify distinct symptom-hemodynamic profiles. Cox proportional hazards modeling was used to quantify difference in event-risk (HF emergency visit, hospitalization or death) among profiles.
Results
The mean age (n=291) was 57±13 years, 38% were female, and 61% had class III/IV HF. Three distinct symptom-hemodynamic profiles were identified. 17.9% of patients had concordant symptoms and hemodynamics (i.e. moderate physical and psychological symptoms matched the comparatively hemodynamic profile), 17.9% had severe symptoms and average hemodynamics, and 64.2% had poor hemodynamics and mild symptoms. Compared to those in the concordant profile, both profiles of symptom-hemodynamic mismatch were associated with a markedly increased event-risk (severe symptoms hazards ratio = 3.38, p=0.033; poor hemodynamics hazards ratio = 3.48, p=0.016).
Conclusions
A minority of adults with HF have concordant symptoms and hemodynamics. Either profile of symptom-hemodynamic mismatch in HF is associated with a greater risk of healthcare utilization for HF or death.
Keywords: heart failure, symptoms, symptom clusters, hemodynamics
Introduction
Heart failure (HF) is a complex and heterogeneous condition and an epidemic among industrialized nations.1, 2 As a clinical syndrome, the diagnosis of HF and the evaluation of treatment effectiveness is largely based on symptoms like dyspnea and fatigue.3 Beyond their importance to clinical care, symptoms are also key determinants of self-care behaviors4, 5 and quality-of-life,6, 7 and principal antecedents to urgent healthcare utilization8, 9 among adults with HF. It is well-known that both hemodynamics (e.g. metrics of contractility, pressure and flow)10–12 and symptoms13, 14 are important and independent predictors of clinical outcomes in HF. Despite their mutual importance in HF, however, we are bereft of insight into the relationship between hemodynamics and what patients experience as physical and psychological symptoms.
Investigations into the link between objective markers of HF severity (e.g. those derived from right heart catheterization, echocardiographic, cardiopulmonary stress test and laboratory parameters) and symptoms have collectively concluded that there is limited-to-no association between the two.15–20 In the absence of a single test for HF or responsiveness to HF therapies, clinicians must integrate objective and subjective data to personalize treatment strategies.21 For example, integrating data on current heart function and physical symptoms may allow clinicians to tailor monitoring strategies and/or promote effective self-care behaviors to enhance the patient’s quality of life and reduce healthcare utilization. But, the general lack of association between hemodynamics and symptoms makes it difficult to integrate this information in planning care, particularly for patients who have symptoms that are markedly worse than anticipated based on their hemodynamics and those who are surprisingly asymptomatic despite severely decompensated hemodynamics. Moreover, the clinical relevance of these types of symptom-hemodynamic mismatch in HF is unknown.
Accordingly, the purpose of this study was to identify common profiles among integrated data on physical and psychological symptoms and hemodynamics among adults with HF. To gain insight into the symptom biology of this heterogeneous syndrome, our main hypothesis was that distinct profiles of symptoms and hemodynamics could be identified. Further, we sought to position the clinical relevance of symptom-hemodynamic mismatch by comparing 180-day HF-related clinical event-risk (i.e. emergency room visits, hospitalizations or death) among the observed profiles; we hypothesized that there would be significant differences in event-risk among observed profiles.
Methods
Study Design
We completed a secondary analysis of data collected on 291 unique participants from two prospective cohort studies conducted by a single team of HF investigators from 2010–2013. The goal of the first study was to quantify the prognostic values of physical and psychological symptoms in HF,22 and the goal of the second study was to describe profiles of HF symptom response behaviors.23 All participants were recruited through a single outpatient HF clinic in the Pacific Northwest that specializes in evaluating patients for advanced HF therapies. Written informed consent was obtained from all interested participants by study staff not directly involved in patient care. Both studies were approved by our institutional review board.
The sampling frame for each study was community-dwelling adults with symptomatic HF (i.e. New York Heart Association (NYHA) class II–IV HF). Formal inclusion criteria (identical for both studies) included; 1) being willing and able to provide informed consent, 2) being 21 years of age or greater, 3) having the ability to read and comprehend 5th grade English, 4) experiencing current HF symptoms (i.e. NYHA class II–IV), 5) being on optimal HF treatment or having HF treatment optimized in the opinion of the treating cardiologist, and 6) receiving health services locally or by a referral practice to facilitate follow-up. Patients were deemed ineligible if they had a diagnosis of major cognitive impairment in the medical record (e.g. Alzheimer’s disease), had received a heart transplant or long-term mechanical circulatory support, or were otherwise unable to complete the study requirements.
Measurement
Socio-demographic and Clinical Data
Socio-demographics were assessed by self-report using a questionnaire asking about gender, age, marital/partnership status, ethnicity/race and employment. NYHA functional classification was assessed on the same day as enrollment by the treating attending cardiologists. Comorbidities were assessed with the Charlson Comorbidity Index24 during a review of the electronic medical record.
Clinical and treatment characteristics, including last known left ventricular ejection fraction (LVEF) and left ventricular internal end-diastolic diameter (LVIDd) from echocardiographic assessments, and pulmonary capillary wedge pressure (PCWP), right atrial pressure (RAP) and cardiac index (calculated by the Fick principle) from right heart catheterization, were collected during an in-depth review of participants’ electronic medical record. As this sample was comprised of adults with NYHA class II–IV HF and was having treatment optimized or being evaluated for advanced therapies, the median time from echocardiographic assessment to symptom measurement was 42 days (interquartile range = 8–90 days prior) and the median time from right heart catheterization to symptom assessment was 9 days (interquartile range = 60 days prior to 11 days afterward).
Mild cognitive dysfunction was assessed using the Montreal Cognitive Assessment (MoCA).25 The MoCA has a sensitivity of 90% and specificity of 87% to detect mild cognitive impairment with the cutoff score of 26 (i.e. below a score of 26 out of 30) in the general population.25 Use of the MoCA in persons with HF has been validated,26 and it was administered in-person immediately following informed consent.
Symptom Measurement
In the absence of a single comprehensive symptom measure in HF, multiple symptom measures with established psychometric properties and frequent use in HF were chosen to capture common physical and psychological domains. Beyond the physical symptoms that are hallmarks of HF,3 depression, anxiety and hostility are common psychological symptoms experience by adults with HF.27–30 Moreover, wake disturbances are a common and distressing symptoms associated with HF.31
Physical symptoms were measured using the 18-item Heart Failure Somatic Perception Scale (HFSPS).32 Participants rated how much they were bothered by 18 common HF symptoms; four response options are provided that range from 0 (not at all) to 5 (extremely). Scores were calculated by summing responses; higher values on the HFSPS (range 0–90) indicate worse physical symptoms. Theta reliability of the original HFSPS was 0.71–0.78.33
Wake disturbances (a.k.a. daytime sleepiness) were measured using the 8-item Epworth Sleepiness Scale (ESS).34 The ESS asks respondents to rate how likely they would be to doze off or fall asleep in 8 soporific situations (e.g. sitting in traffic) by choosing response options that range from 0 (would never doze) to 3 (high chance). The ESS correlates significantly with sleep latency measures, and scores distinguish normal sleep patterns, obstructive sleep apnea syndrome, narcolepsy, idiopathic hypersomnia, and insomnia.34 Scores were calculated by summing responses. Higher ESS scores (range 0–24) indicate worse wake disturbances.
Depression was measured with the 9-Item Patient Health Questionnaire (PHQ9).35 The PHQ9 scores each of the 9 related DSM-IV criteria providing four response options ranging from 0 (not at all) to 3 (nearly every day). The PHQ9 has 70% sensitivity and 92% specificity for major depression among adults and is a valid and reliable measure of depression in HF.36 Higher score (range 0–27) indicate worse depression; scores of ≥10 are indicative of moderate or greater depression.35
Anxiety and hostility were measured using the Brief Symptom Inventory (BSI).37 The BSI asks about feelings during the past seven days and provides five response options ranging from 0 (no) to 4 (extreme). Subscale scores (ranging from 0 to 4) are calculated by adding the ratings and dividing the total by the number of items in the subscale, with higher scores indicating worse anxiety or hostility.37 In adults with HF, Cronbach’s alpha was 0.80 for the BSI hostility score and 0.86 for the BSI anxiety score.22
Clinical Events
We completed a review of the electronic medical record at 180 days looking specifically for emergency room visits or hospitalizations for HF and HF-related mortality. Our sample received care locally and/or was part of an extensively-linked electronic medical record system (i.e. Epic® Care Everywhere Network). Thus, for the vast majority of events data were extracted directly from discharge summaries. We also contacted study participants by phone every 90 days to inquire about events that may have occurred outside of the health system and network. In such instances, we solicited sufficient detail directly from participants whether or not the event was primarily related to their HF or for other reasons.
Statistical Analysis
Descriptive statistics (mean ± standard deviation (SD), proportions, and medians with inter-quartile ranges) were used to describe the sample at large. Latent class mixture modeling (LCMM) was used to identify distinct profiles among symptom (HFSPS score, ESS score, PHQ9 cutoff for moderate or greater depression (dichotomous), and the BSI anxiety and hostility score) and HF hemodynamics (LVEF, LVIDd, cardiac index, PCWP, and RAP). LCMM is a type of clustering that accommodates continuous and categorical data and allows for the quantification of uncertainty in classification. The Lo-Mendell-Rubin adjusted likelihood ratio test (LMRT)38 and parametric bootstrap likelihood ratio test (PBLRT) (p-value for both), model convergence (entropy near 1.0), the size of the observed profiles (not less than 5% of the sample), and posterior probabilities (average posterior probabilities for most likely class near 1.0) were used to assess the performance of alternative models (e.g. 3 vs. 2 profiles).39 Neither the number nor composition of the profiles is predetermined using LCMM; thus, the resulting profiles are distinct, common and naturally-occurring.
Comparative statistics (F-statistics from analysis of variance, and χ2) were used to compare factors among the observed profiles. The profiles were then labeled according to key differentiating characteristics. Cox proportional hazards modeling was used to quantify 180-day HF event-risk (emergency room visit or hospitalization for HF or all-cause death). The proportional hazards assumption was justified based on Schoenfeld residuals. We present three Cox models; a) unadjusted models (just the observed profiles), b) adjusted models (including the observed profiles and any clinical or socio-demographic factor that was different across the three profiles with p<0.20), and c) backward selection (p-values < 0.2) using all baseline clinical and socio-demographic characteristics (e.g. comorbid conditions, cognitive dysfunction, HF medications, demographics). These three approaches to Cox modeling were undertaken to demonstrate that the influence of the profiles on clinical event-risk was not a function of the groups being otherwise imbalanced and was that the relationship robust to other confounding factors that are known to be directly related to HF clinical event-risk. Hazard ratios (HR) and 95% confidence intervals (CI) are presented. LCMM was generated using Mplus v7.11 (Los Angeles, CA); all other analyses were performed using Stata MP v13 (College Station, TX).
Results
Socio-demographic and clinical characteristics of the sample are presented in Table 1. In brief, the majority of participants were male and Caucasian, and the average age of the sample was just under 57 years. A majority (61.2%) of participants had NYHA class III/IV HF, and a majority of the sample was prescribed beta adrenergic blockers (90.4%) and angiotensin converting enzyme-inhibitors (ACE-I) or angiotensin receptor blockers (ARBs) (81.8%). Eighty-one (27.8%) participants reported symptoms indicative of moderate or greater depression, and 31.3% of the sample had mild cognitive dysfunction.
Table 1.
Characteristics of the sample (n=291)
| Patient Characteristics: | mean±SD, n (%), or median [IQR] |
|---|---|
| Age (years) | 56.7±13.3 |
| Female | 111 (38.1%) |
| Non-Hispanic Caucasian | 237 (81.6%) |
| Married/Living with Partner | 184 (63.2%) |
| Body Mass Index (kg/m2) | 31.0±7.5 |
| Charlson Comorbidity Index (weighted) | 2.3±1.4 |
| Atrial fibrillation | 111 (38.1%) |
| Stage 3 chronic kidney disease | 42 (14.4%) |
| General Heart Failure Characteristics: | |
| Time with heart failure in months: | 48 [20–96] |
| NYHA Functional Class: | |
| Class II | 113(38.8%) |
| Class III | 164 (56.4%) |
| Class IV | 14 (4.8%) |
| Ischemic Heart Failure | 101 (34.8%) |
| Prescribed a β-blocker | 263 (90.4%) |
| Prescribed an ACE-I or ARB | 238 (81.8%) |
| Prescribed an aldosterone antagonist | 129 (44.3%) |
| Serum sodium (mEq/L) | 137.8±3.3 |
| Serum hematocrit (%) | 39.0±5.7 |
| Serum BUN-to-creatinine ratio (mg/dL:1) | 20.2±9.4 |
| Heart Failure Hemodynamics: | |
| Left ventricular internal end-diastolic diameter (cm) | 6.1±1.1 |
| Left ventricular ejection fraction (%) | 28.3±12.4 |
| Pulmonary capillary wedge pressure (mm/Hg) | 18.8±8.4 |
| Right atrial pressure (mm/Hg) | 9.5±5.5 |
| Cardiac index (L/min/m2 by Fick equation) | 2.1±0.5 |
| Symptoms: | |
| Physical symptoms (HFSPS; 0–90) | 24.6±16.7 |
| Wake disturbances (ESS; 0–24) | 8.2±4.9 |
| Depression (PHQ9; 0–27) | 7.2±6.1, 6 [2–10.5] |
| Anxiety (BSI; 0–4) | 0.33 [0–0.88] |
| Hostility (BSI; 0–4) | 0.20 [0–0.60] |
Abbreviations: ACE-I = Angiotensin Converting Enzyme-Inhibitor, ARB = Angiotensin Receptor Blocker, BSI = Brief Symptom Inventory, ESS = Epworth Sleepiness Scale, HFSPS = Heart Failure Somatic Perception Scale, IQR = interquartile range, NYHA = New York Heart Association, PHQ9 = Patient Health Questionnaire, SD = standard deviation.
Three distinct profiles of symptoms and hemodynamics were identified (entropy = 0.84; LMRT = 131.15, p=0.0218; PBLRT p<0.001; and posterior probabilities exceeded 0.9, all supporting a good model). Significant differences in physical symptoms, depression, anxiety, hostility, wake disturbances, LVIDd, LVEF, and cardiac index were observed across the three profiles; the differences in PCWP and RAP, however, were not significant among the profiles (Table 2).
Table 2.
Mixture model of heart failure symptoms and hemodynamics
| Symptoms and Hemodynamics | Concordant (17.9%) |
Severe Symptoms (17.9%) |
Poor Hemodynamics (64.2%) |
F, p-value |
|---|---|---|---|---|
| Physical HF symptoms (HFSPS) | 22.9±15.6 | 43.3±15.3 | 19.5±13.2 | 57.1, p<0.0001 |
| Wake disturbances (ESS) | 7.5±5.5 | 10.9±5.1 | 7.6±4.3 | 10.7, p<0.0001 |
| Depression (PHQ9) | 5.4±4.6 | 15.6±5.3 | 5.2±4.3 | 109.3, p<0.0001 |
| Anxiety (BSI) | 0.34±0.46 | 1.53±0.67 | 0.30±0.32 | 168.4, p<0.0001 |
| Hostility (BSI) | 0.37±0.47 | 1.07±0.81 | 0.28±0.36 | 52.6, p<0.0001 |
| LV internal diastolic diameter (cm) | 4.8±0.7 | 6.1±1.2 | 6.5±1.0 | 48.7, p<0.0001 |
| LV ejection fraction (%) | 49.8±9.9 | 27.6±8.0 | 22.8±6.4 | 258.2, p<0.0001 |
| Cardiac index (L/min/m2) | 2.4±0.6 | 2.2±0.5 | 2.0±0.5 | 7.8, p=0.0006 |
| Wedge pressure (mm/Hg) | 16.4±8.5 | 19.8±8.4 | 19.0±8.4 | 1.3, p=0.2674 |
| Right atrial pressure (mm/Hg) | 10.2±5.6 | 10.8±8.4 | 9.1±5.1 | 1.6, p=0.1982 |
Abbreviations: BSI = Brief Symptom Inventory, ESS = Epworth Sleepiness Scale, HF = heart failure, HFSPS = Heart Failure Somatic Perception Scale, LV = left ventricle, PHQ9 = 9-item patient health questionnaire.
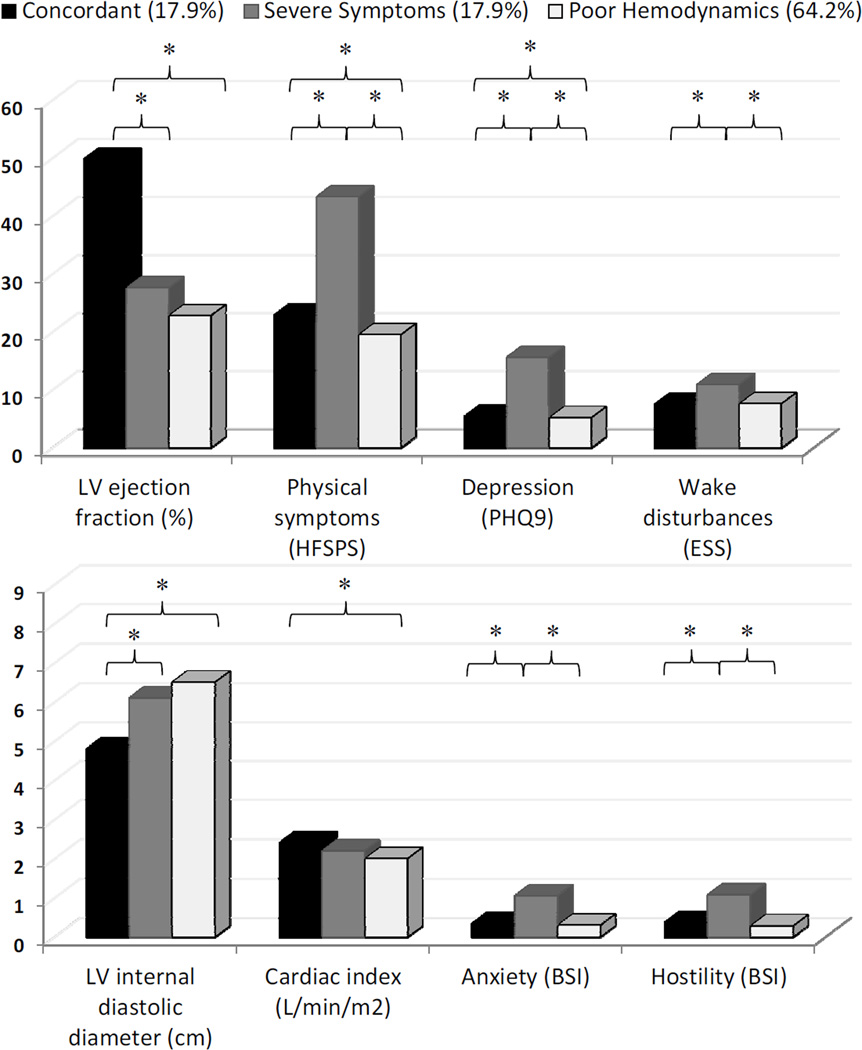
Approximately 18% of the sample (n=52) had what we labeled as ‘concordant’ symptoms and hemodynamics. That is, the moderate physical and psychological symptoms matched the comparatively good hemodynamic profile; 50% of those whose characteristics fit the concordant profile had HF with preserved ejection fraction (i.e. LVEF >40). We also observed two profiles wherein there was a mismatch in symptoms and hemodynamics. First, there was a mismatch profile (n=52 (17.9%)) that was characterized by ‘severe symptoms’ compared with the relatively moderate hemodynamic profile (i.e. they had the worst symptoms across all measures, and hemodynamic values close to sample averages). Second, there was a mismatch profile (n=187 (64.2%)) that had ‘poor hemodynamics’ and a flat symptom profile (i.e. they had the worst hemodynamics but lowest symptom burden across all measures) (Figure 1).
Figure 1.
Bar graphs present significant differences in four hemodynamic values and four symptom measures among the three symptom-hemodynamic profiles. All measures were significantly different across the three groups based on overall -tests with p-values <0.0001.
* = statistically significant between-group tests with p<0.01 corrected for multiple comparisons.
Abbreviations: BSI = Brief Symptom Inventory, ESS = Epworth Sleepiness Scale, HFSPS = Heart Failure Somatic Perception Scale, LV left ventricle, PHQ9 = Patient Health Questionnaire.
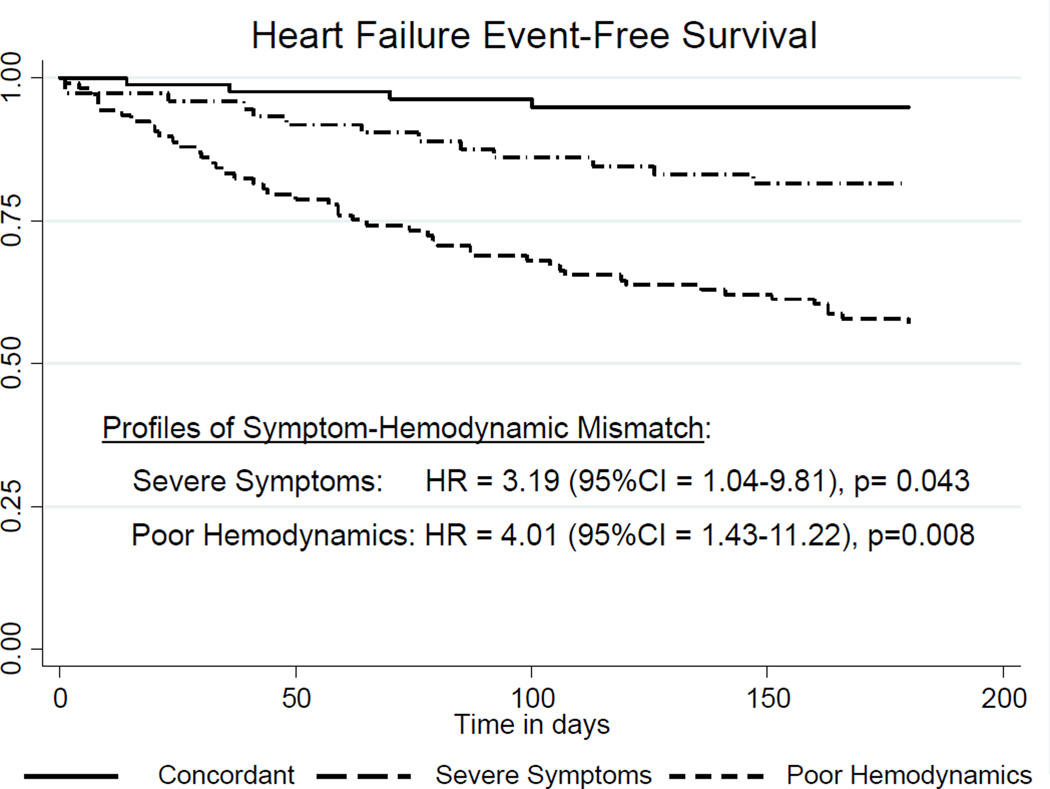
There were a few differences in socio-demographic and clinical characteristics among the three symptom and hemodynamic profiles (Table 3). Namely, there were statistically significant or numerical differences with p-values <0.20 in age, gender, level of education and prescribed HF therapies (i.e. ACE or ARB, and aldosterone antagonists). The HF event rate at 180 days was 22.3% (65 events in 44,492 at-risk days of observation). Compared with patients in the concordant profile and adjusting for observed difference among profiles, patients classified in the severe symptom profile were 3.3 times as likely to have a clinical HF event, and those in the poor hemodynamic profile were 3.9 times as likely to have a clinical HF event (Table 4). Adjusted HRs derived from backward covariate selection were similar (Figure 2).
Table 3.
Characteristics by observed profiles of symptoms and hemodynamics
| Concordant (17.9%) |
Severe Symptoms (17.9%) |
Poor Hemodynamics (64.2%) |
F or χ2, p-value |
|
|---|---|---|---|---|
| Age (years) | 62.6±15.1 | 52.8±12.9 | 56.5±12.6 | 5.9, p=0.003 |
| Female | 51.1% | 32.7% | 37.7% | 3.9, p=0.145 |
| Married/Living with Partner | 61.7% | 53.9% | 66.3% | 2.7, p=0.257 |
| Non-Hispanic Caucasian | 84.0% | 84.6% | 80.1% | 12.0, p=0.445 |
| Body Mass Index (kg/m2) | 31.4±7.4 | 31.5±9.1 | 30.8±7.1 | 0.3, p=0.764 |
| Charlson Comorbidity Index | 2.2±1.3 | 2.3±1.2 | 2.3±1.4 | 0.1, p=0.943 |
| College Education | 39.2% | 13.5% | 27.7% | 13.8, p=0.008 |
| Mild Cognitive Dysfunction * | 29.4% | 34.6% | 30.6% | 0.9, p=0.627 |
| Heart failure duration (months) | 57.9±57.8 | 70.2±82.0 | 71.7±67.8 | 0.79, p=0.454 |
| Ischemic Etiology | 28.0% | 36.5% | 36.2% | 1.2, p=0.537 |
| Prescribed a β-blocker | 92.2% | 86.5% | 91.0% | 1.1, p=0.566 |
| Prescribed an ACE-I or ARB | 74.5% | 76.9% | 85.1% | 4.03, p=0.133 |
| Prescribed an aldosterone antagonist | 29.4% | 50.0% | 46.8% | 5.74, p=0.057 |
Abbreviations: ACE-I = angiotensin converting enzyme inhibitor, ARB = angiotensin receptor blocker
= assessed with the Montreal Cognitive Assessment
Table 4.
180-day heart failure event-risk by profile of symptoms and hemodynamics
| Characteristic | HR (95%CI), p-value | HR† (95%CI), p-value | HR‡ (95%CI), p-value |
|---|---|---|---|
| Symptom and Hemodynamic Profile | |||
| Severe Symptoms* | 3.38 (1.10–10.37), p=0.033 3.27 (1.07–10.08), p=0.039 3.19 (1.04–9.81), p=0.043 | ||
| Poor Hemodynamics* | 3.48 (1.26–9.66), p=0.016 3.85 (1.38–10.77), p=0.010 4.01 (1.43–11.22), p=0.008 | ||
| Other Factors | |||
| Age | - | 0.99 (0.98–1.02), p=0.987 | - |
| Male | - | 1.23 (0.73–2.06), p=0.442 | - |
| College Education | - | 0.64 (0.34–1.21), p=0.167 | - |
| ACE/ARB | - | 0.46 (0.26–0.83), p=0.009 | 0.47 (0.27–0.83), p=0.010 |
| Aldosterone antagonist | - | 1.03 (0.62–1.69), p=0.918 | - |
| Cognitive dysfunction ¶ | - | - | 1.68 (1.02–2.75), p=0.040 |
| Β-adrenergic blocker | - | - | 0.55 (0.28–1.10), p=0.090 |
= relative to participants in the concordant profile.
= adjusted for among-group differences in age, gender, education, angiotensin converting enzyme inhibitor/angiotensin receptor blocker, and aldosterone antagonist.
= retained factors from backward selection with removal of factors with a p-value >0.20. Factors entered into the model were age, gender, married/living with partner, race, body mass index, Charlson comorbidity index, education, mild cognitive dysfunction, heart failure duration, ischemic etiology, prescribed a β-adrenergic blocker, prescribed an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, and prescribed an aldosterone antagonist.
= assessed with the Montreal Cognitive Assessment
Abbreviations: CI = confidence interval, HR = hazards ratio, ACE/ARB= angiotensin converting enzyme inhibitor/ angiotensin receptor blocker
Figure 2.
Heart failure event-free survival by observed profiles of symptoms and hemodynamic mismatch. Hazards ratios are relative to patients with concordant symptoms and hemodynamics and adjusted for mild cognitive dysfunction, and treatment with a β-adrenergic blocker, and treatment with an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker.
Abbreviations: CI = confidence interval, HR = adjusted hazards ratio
Discussion
In this sample of 291 adult patients with moderate to advanced HF, we identified three profiles among integrated data on physical and psychological symptoms and hemodynamic parameters that are commonly used to help guide HF management and evaluate treatment effectiveness. A ‘concordant’ profile was characterized by moderate symptoms and comparatively good hemodynamics that were largely comparable. We also identified two profiles of symptom-hemodynamic mismatch; a ‘severe symptom’ profile identified participants who experienced the worst symptoms and average hemodynamics, and a ‘poor hemodynamic’ profile identified those with the lowest symptom burden but very poor hemodynamics. Compared to those with concordant symptoms and hemodynamics, patients with the severe symptom profile and those with the poor hemodynamic profile had markedly increased 180-day HF event-risk. To the best of our knowledge, ours is the first study to identify distinct profiles of symptom-hemodynamic mismatch in HF and quantify significant and clinically-meaningful associations with clinical event-risk.
We know very little about how symptoms relate to the underlying pathogenesis of HF. For example, Shah15 found that invasive right heart catheterization parameters are not associated significantly with dyspnea. Rector16 observed that several common objective measures of HF severity, including LVEF, blood pressure, and serum creatinine and hemoglobin, were not independently associated with HF symptoms. Meyers17 identified that symptoms of HF correlate poorly with objective measures of exercise capacity (i.e. peak oxygen uptake). Finally, Guglin20 recently concluded that there was no association between HF symptoms and multiple clinical factors that predict morbidity and mortality, including peak oxygen uptake, LVEF, Nt-proBNP, right heart catheterization parameters, and echocardiographic parameters. Our approach was different from prior research in that our goal was to identify distinct and common profiles among integrated data on symptoms and hemodynamics. In doing so, we identified three profiles that capture differences in the relationship among symptoms and hemodynamic, with one congruent profile and two profiles of symptom-hemodynamic mismatch. Thus, clustering combined data on symptoms and hemodynamics may be an avenue to gain new insights into relationships among symptoms and their biological and non-biological underpinnings.
Several symptom clusters have been identified in prior clinical HF research. For example, Song and colleagues14 identified a symptom cluster centered on dyspnea and another centered on lack of energy and difficulty sleeping. Hertzog and group40 identified three physical symptom profiles. Finally, Jurgens et al.,41 and Lee and colleagues13 both identified clusters among physical and psychological symptoms in HF. Our findings build upon prior work in HF symptom clustering by integrating hemodynamics data with both physical and psychological symptoms. Importantly, symptom clusters have been shown by others to independently predict clinical event-risk. Specifically, Lee and colleagues13 identified an emotional cluster that predicted event-free survival (HR=1.18), and Song and colleagues14 recognized a weary symptom cluster that predicted hospitalization (HR=1.45) and a dyspnea cluster that predicted mortality (HR=2.00). Thus, our research is a logical extension of prior work on symptom clusters and survival by linking symptom-hemodynamic mismatch profiles to significant differences in HF event risk. It is worth noting that these prior studies exemplify the two main approaches to symptom clustering in HF (i.e. clustering symptoms themselves and clustering patients based on symptoms). Hence, the aims and analyses of these prior studies varied according to the overall approach to symptom clustering. The patient, not the symptom, was the unit of analysis in this study.
Our most important finding is that symptom-hemodynamic mismatch of either kind was associated with a greater 180-day HF event-risk. Simply put, the clinical relevance of the symptom-hemodynamic mismatch we observed is centered on future urgent healthcare resource utilization. We have learned from large HF registries that the vast majority (89–93%) of patients who are hospitalized for HF present with symptoms like dyspnea, are hypertensive, and have preserved ejection fraction.42, 43 We also know that poor hemodynamics, when not assessed along with symptoms, are predictive of poor clinical outcomes in HF.12, 44 The novel findings of our research are a) the majority of adults with HF do not have concordant symptoms and hemodynamics, and b) that either severe symptoms or poor hemodynamics is associated with greater event-risk.
Implications and Directions for Future Research
Our findings don’t change the fact that a detailed history and examination remain the cornerstones of evaluation of HF and the responsiveness to therapy.3 Further, it would be well beyond the scope of this paper to provide specific treatment recommendations that differ from those detailed in international guidelines. There are, however, clear implications based on what we have observed about the nature of the relationship between hemodynamics and symptoms. First, a minority of patients in our sample had symptoms and hemodynamics that were on par with one another. These patients were also significantly older and had more education than those in the mismatched profiles and half had HF with preserved ejection fraction. Thus, the relative symptom-hemodynamic concordance we observed may be a function of these or other factors. Second, patients with severe symptom and average hemodynamics have a markedly elevated event-risk compared to those with symptom-hemodynamic concordance. Thus, what patients have to tell us about their experience in living with this condition, even if that does not match what we can objectively measure, is important in predicting future healthcare resource utilization. Interventions that could be particularly helpful with this type of symptom-hemodynamic mismatch may be centered on assessing and removing barriers to effective symptom management strategies to prevent unnecessary hospitalization. Third, patients with mild-to-no symptoms and poor hemodynamics also had a markedly elevated event-risk compared with those who had concordant symptoms and hemodynamics. Hence, minimal symptoms, particularly in the context of poor hemodynamics, should not be interpreted as a sign that all is well. Instead, patients with this type of symptom-hemodynamic mismatch may require more intensive monitoring of hemodynamics or even advanced therapies to prevent hospitalization and/or death. Fourth, the only right heart catheterization parameter that was helpful in differentiating among the three types of symptom-hemodynamic congruence or mismatch was cardiac index. That is, PCWP and RAP were not different among the three profiles, and there were minimal differences in cardiac index. Thus, profiles of symptom-hemodynamic mismatch can be observed in the general cardiology population with a good history and physical examination and echocardiogram. Finally, our results once again highlight how little we know about HF in general, and about HF symptom biology in particular.
Accordingly, future research is needed to: a) gain insight into the biological and non-biological underpinning of physical and psychological symptoms in HF, b) validate and/or find new profiles of symptom-hemodynamic mismatch that are associated with significant differences in clinical and patient-oriented outcomes, and c) determine how symptom-hemodynamic concordance and mismatch may change over the course of HF progression and in response to HF therapies. Additional research is needed to test tailored disease management and self-care strategies according to profiles of symptom-hemodynamic mismatch.
Strengths and Limitations
Our approach to this study has several strengths. First, we used a robust method to identify unique patterns of similarity among a population of patients that is notoriously heterogeneous with respect to symptoms and underlying hemodynamics. Second, we integrated data on symptoms and hemodynamics to gain insight into how, and in whom, these subjective and objective data are related. Third, we used two approaches to adjust our estimates of event hazard associated with the profiles of symptom-hemodynamic mismatch, one that results in balancing among groups based on observed differences, and the other that helps choose covariates that are also strongly related to the risk of clinical events.
There are several potential limitations to our work that must also be acknowledged and taken into consideration when interpreting our findings. First, our study is a non-experimental study and therefore prone to bias that is associated with all observational research. Second, we make no claim about the temporal relationship between symptoms and hemodynamics and present our findings as associations not causal mechanisms. Third, we sought to identify difference in clinical and socio-demographic determinants among the observed profiles to effectively adjust for those differences in our hazards modeling. There may be other determinants of the types of symptom-hemodynamic concordance or mismatch that we did not test. Finally, this research was on relatively young adults with symptomatic HF. Thus, these results may not be generalizable to all cohorts of adults with HF.
Conclusion
A minority of adults with HF had concordant symptoms and hemodynamics. Two profiles of symptom-hemodynamic mismatch were identified. Adults with severe symptoms and average hemodynamics had three times the risk of having HF events within 180 days compared to those with concordant symptoms and hemodynamics. Those with poor hemodynamics and mild symptoms had nearly four times the risk of having HF events within 180 days compared to those with concordant symptoms and hemodynamics. These results have implications for researchers and clinicians alike who are interested in improving clinical outcomes of HF.
Acknowledgment
This work was supported by the Office of Research on Women’s Health and the National Institute of Child Health and Human Development (HD043488-08) and by an award from the American Heart Association (11BGIA7840062). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or American Heart Association.
Contributor Information
Christopher S. Lee, Oregon Health & Science University School of Nursing and Knight Cardiovascular Institute, Portland, OR.
Shirin O. Hiatt, Oregon Health & Science University School of Nursing, Portland, OR.
Quin E. Denfeld, Oregon Health & Science University School of Nursing, Portland, OR, USA.
James O. Mudd, Oregon Health & Science University, Knight Cardiovascular Institute, Portland, OR.
Christopher Chien, Oregon Health & Science University, Knight Cardiovascular Institute, Portland, OR.
Jill M. Gelow, Oregon Health & Science University, Knight Cardiovascular Institute, Portland, OR.
References
- 1.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P. Esc guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the european society of cardiology. Developed in collaboration with the heart failure association (hfa) of the esc. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 2.Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK, Rogers JG, Starling RC, Stevenson WG, Tang WH, Teerlink JR, Walsh MN. Hfsa 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16:e1–e194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 accf/aha guideline for the management of heart failure: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2013 doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 4.Lee CS, Moser DK, Lennie TA, Tkacs NC, Margulies KB, Riegel B. Biomarkers of myocardial stress and systemic inflammation in patients who engage in heart failure self-care management. J Cardiovasc Nurs. 2011;26:321–328. doi: 10.1097/JCN.0b013e31820344be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riegel B, Lee CS, Albert N, Lennie T, Chung M, Song EK, Bentley B, Heo S, Worrall-Carter L, Moser DK. From novice to expert: Confidence and activity status determine heart failure self-care performance. Nurs Res. 2011;60:132–138. doi: 10.1097/NNR.0b013e31820978ec. [DOI] [PubMed] [Google Scholar]
- 6.Bekelman DB, Havranek EP, Becker DM, Kutner JS, Peterson PN, Wittstein IS, Gottlieb SH, Yamashita TE, Fairclough DL, Dy SM. Symptoms, depression, and quality of life in patients with heart failure. J Card Fail. 2007;13:643–648. doi: 10.1016/j.cardfail.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Zambroski CH, Moser DK, Bhat G, Ziegler C. Impact of symptom prevalence and symptom burden on quality of life in patients with heart failure. Eur J Cardiovasc Nurs. 2005;4:198–206. doi: 10.1016/j.ejcnurse.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Gheorghiade M, Zannad F, Sopko G, Klein L, Pina IL, Konstam MA, Massie BM, Roland E, Targum S, Collins SP, Filippatos G, Tavazzi L. Acute heart failure syndromes: Current state and framework for future research. Circulation. 2005;112:3958–3968. doi: 10.1161/CIRCULATIONAHA.105.590091. [DOI] [PubMed] [Google Scholar]
- 9.De Luca L, Fonarow GC, Adams KF, Jr, Mebazaa A, Tavazzi L, Swedberg K, Gheorghiade M. Acute heart failure syndromes: Clinical scenarios and pathophysiologic targets for therapy. Heart Fail Rev. 2007;12:97–104. doi: 10.1007/s10741-007-9011-8. [DOI] [PubMed] [Google Scholar]
- 10.Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Kober L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, Doughty RN. Predicting survival in heart failure: A risk score based on 39 372 patients from 30 studies. Eur Heart J. 2012 doi: 10.1093/eurheartj/ehs337. [DOI] [PubMed] [Google Scholar]
- 11.Zugck C, Kruger C, Kell R, Korber S, Schellberg D, Kubler W, Haass M. Risk stratification in middle-aged patients with congestive heart failure: Prospective comparison of the heart failure survival score (hfss) and a simplified two-variable model. European Journal of Heart Failure. 2001;3:577–585. doi: 10.1016/s1388-9842(01)00167-2. [DOI] [PubMed] [Google Scholar]
- 12.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL, Packer M. The seattle heart failure model: Prediction of survival in heart failure. Circulation. 2006;113:1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 13.Lee KS, Song EK, Lennie TA, Frazier SK, Chung ML, Heo S, Wu JR, Rayens MK, Riegel B, Moser DK. Symptom clusters in men and women with heart failure and their impact on cardiac event-free survival. J Cardiovasc Nurs. 2010;25:263–272. doi: 10.1097/JCN.0b013e3181cfbb88. [DOI] [PubMed] [Google Scholar]
- 14.Song EK, Moser DK, Rayens MK, Lennie TA. Symptom clusters predict event-free survival in patients with heart failure. J Cardiovasc Nurs. 2010;25:284–291. doi: 10.1097/JCN.0b013e3181cfbcbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah MR, Hasselblad V, Stinnett SS, Kramer JM, Grossman S, Gheorghiade M, Adams KF, Jr, Swedberg K, Califf RM, O'Connor CM. Dissociation between hemodynamic changes and symptom improvement in patients with advanced congestive heart failure. Eur J Heart Fail. 2002;4:297–304. doi: 10.1016/s1388-9842(01)00202-1. [DOI] [PubMed] [Google Scholar]
- 16.Rector TS, Anand IS, Cohn JN. Relationships between clinical assessments and patients' perceptions of the effects of heart failure on their quality of life. J Card Fail. 2006;12:87–92. doi: 10.1016/j.cardfail.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Myers J, Zaheer N, Quaglietti S, Madhavan R, Froelicher V, Heidenreich P. Association of functional and health status measures in heart failure. J Card Fail. 2006;12:439–445. doi: 10.1016/j.cardfail.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Lewis EF, Lamas GA, O'Meara E, Granger CB, Dunlap ME, McKelvie RS, Probstfield JL, Young JB, Michelson EL, Halling K, Carlsson J, Olofsson B, McMurray JJV, Yusuf S, Swedberg K, Pfeffer MA. Characterization of health-related quality of life in heart failure patients with preserved versus low ejection fraction in charm. European Journal of Heart Failure. 2007;9:83–91. doi: 10.1016/j.ejheart.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Bhardwaj A, Rehman SU, Mohammed AA, Gaggin HK, Barajas L, Barajas J, Moore SA, Sullivan D, Januzzi JL. Quality of life and chronic heart failure therapy guided by natriuretic peptides: Results from the probnp outpatient tailored chronic heart failure therapy (protect) study. Am Heart J. 2012;164:793–799. doi: 10.1016/j.ahj.2012.08.015. e791. [DOI] [PubMed] [Google Scholar]
- 20.Guglin M, Patel T, Darbinyan N. Symptoms in heart failure correlate poorly with objective haemodynamic parameters. Int J Clin Pract. 2012;66:1224–1229. doi: 10.1111/j.1742-1241.2012.03003.x. [DOI] [PubMed] [Google Scholar]
- 21.Packer M. Should b-type natriuretic peptide be measured routinely to guide the diagnosis and management of chronic heart failure? Circulation. 2003;108:2950–2953. doi: 10.1161/01.CIR.0000109205.35813.8E. [DOI] [PubMed] [Google Scholar]
- 22.Lee CS, Gelow JM, Denfeld QE, Mudd JO, Burgess D, Green JK, Hiatt SO, Jurgens CY. Physical and psychological symptom profiling and event-free survival in adults with moderate to advanced heart failure. J Cardiovasc Nurs. 2013 doi: 10.1097/JCN.0b013e318285968a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee CS, Gelow JM, Mudd JO, Green JK, Hiatt SO, Chien C, Riegel B. Profiles of self-care management versus consulting behaviors in adults with heart failure. Eur J Cardiovasc Nurs. 2013 doi: 10.1177/1474515113519188. [DOI] [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 25.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The montreal cognitive assessment, moca: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 26.Cameron J, Worrall-Carter L, Page K, Riegel B, Lo SK, Stewart S. Does cognitive impairment predict poor self-care in patients with heart failure? Eur J Heart Fail. 2010;12:508–515. doi: 10.1093/eurjhf/hfq042. [DOI] [PubMed] [Google Scholar]
- 27.Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48:1527–1537. doi: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 28.Konstam V, Moser DK, De Jong MJ. Depression and anxiety in heart failure. J Card Fail. 2005;11:455–463. doi: 10.1016/j.cardfail.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Moser DK, Dracup K, Evangelista LS, Zambroski CH, Lennie TA, Chung ML, Doering LV, Westlake C, Heo S. Comparison of prevalence of symptoms of depression, anxiety, and hostility in elderly patients with heart failure, myocardial infarction, and a coronary artery bypass graft. Heart Lung. 39:378–385. doi: 10.1016/j.hrtlng.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Jong MJ, Chung ML, Wu JR, Riegel B, Rayens MK, Moser DK. Linkages between anxiety and outcomes in heart failure. Heart Lung. 2011;40:393–404. doi: 10.1016/j.hrtlng.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riegel B, Weaver TE. Poor sleep and impaired self-care: Towards a comprehensive model linking sleep, cognition, and heart failure outcomes. Eur J Cardiovasc Nurs. 2009;8:337–344. doi: 10.1016/j.ejcnurse.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jurgens CY. Somatic awareness, uncertainty, and delay in care-seeking in acute heart failure. Res Nurs Health. 2006;29:74–86. doi: 10.1002/nur.20118. [DOI] [PubMed] [Google Scholar]
- 33.Jurgens CY, Fain JA, Riegel B. Psychometric testing of the heart failure somatic awareness scale. J Cardiovasc Nurs. 2006;21:95–102. doi: 10.1097/00005082-200603000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Johns MW. A new method for measuring daytime sleepiness: The epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 35.Kroenke K, Spitzer RL, Williams JB. The phq-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hammash MH, Hall LA, Lennie TA, Heo S, Chung ML, Lee KS, Moser DK. Psychometrics of the phq-9 as a measure of depressive symptoms in patients with heart failure. Eur J Cardiovasc Nurs. 2013;12:446–453. doi: 10.1177/1474515112468068. [DOI] [PubMed] [Google Scholar]
- 37.Derogatis LR, Melisaratos N. The brief symptom inventory: An introductory report. Psychological Medicine. 1983;13:595–605. [PubMed] [Google Scholar]
- 38.Lo Y, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika. 2001;88:767–778. [Google Scholar]
- 39.Ram N, Grimm KJ. Methods and measures: Growth mixture modeling: A method for identifying differences in longitudinal change among unobserved groups. International Journal of Behavioral Development. 2009;33:565–576. doi: 10.1177/0165025409343765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hertzog MA, Pozehl B, Duncan K. Cluster analysis of symptom occurrence to identify subgroups of heart failure patients: A pilot study. J Cardiovasc Nurs. 2010;25:273–283. doi: 10.1097/JCN.0b013e3181cfbb6c. [DOI] [PubMed] [Google Scholar]
- 41.Jurgens CY, Moser DK, Armola R, Carlson B, Sethares K, Riegel B. Symptom clusters of heart failure. Res Nurs Health. 2009;32:551–560. doi: 10.1002/nur.20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP. Characteristics and outcomes of patients hospitalized for heart failure in the united states: Rationale, design, and preliminary observations from the first 100,000 cases in the acute decompensated heart failure national registry (adhere) Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 43.Goldberg RJ, Spencer FA, Szklo-Coxe M, Tisminetzky M, Yarzebski J, Lessard D, Gore JM, Gaasch W. Symptom presentation in patients hospitalized with acute heart failure. Clin Cardiol. 2010;33:E73–E80. doi: 10.1002/clc.20627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Felker GM, Leimberger JD, Califf RM, Cuffe MS, Massie BM, Adams KF, Jr, Gheorghiade M, O'Connor CM. Risk stratification after hospitalization for decompensated heart failure. J Card Fail. 2004;10:460–466. doi: 10.1016/j.cardfail.2004.02.011. [DOI] [PubMed] [Google Scholar]