Abstract
A 48-year old woman with metastatic breast cancer and extensive bone marrow infiltration was admitted with extreme lethargy, jaundice and deranged liver function tests. She had been started on anastrozole in May 2013 for bony metastases, detected on a bone scan. A CT scan performed at that time had shown no evidence of metastatic or nodal disease elsewhere. Over the subsequent 2 months, the patient had become progressively jaundiced. Outpatient abdominal ultrasound and CT liver had shown a fatty liver with no focal lesions. She was admitted in August 2013 with bilirubin 567, alkaline phosphatase 385, alanine aminotransferase 98, albumin 25 and international normalised ratio 1.9. The patient ultimately had a liver biopsy, which demonstrated features of drug-induced steatohepatitis, and anastrozole was found to have been the probable cause. This case explores the differentials of jaundice in a patient with cancer and describes a rare cause of drug-induced liver injury.
Background
Anastrozole is an aromatase inhibitor that blocks the conversion of androgens to oestrogens in peripheral tissues. It is used as adjuvant therapy for the treatment of breast cancer in postmenopausal women with oestrogen-receptor-positive disease. It is known to increase susceptibility to osteoporosis and is associated with a number of side effects, including bone pain and fractures. Effects of anastrozole on the liver, however, are not well documented and, in the Arimidex, Tamoxifen Alone or in Combination (ATAC) trial, this drug was shown to have a good liver safety profile.1 In 2009, the National Institute of Health and Care Excellence (NICE) recommended it be used as first-line adjuvant therapy for early-stage hormone-sensitive breast cancer in postmenopausal women. On performing a literature search, three case reports relating anastrozole use to hepatitis were found.2–4
Breast cancer is the most common cancer affecting women in the UK, with 41 259 new cases diagnosed in 2010.5 Consequently, many women will receive anastrozole treatment and this case represents a very rare and important complication of this drug.
This case also presented a diagnostic dilemma to the medical team. As the liver is a common site of breast cancer metastases, and the patient had received a number of blood transfusions, the ultimate diagnosis was not the one initially deemed most likely.
Case presentation
A 48-year old woman was admitted with profound jaundice. She had been diagnosed with invasive adenocarcinoma of the right breast in 2007. This was an oestrogen receptor positive, 35 mm grade 2 duct cancer with ductal carcinoma in situ and involved 6/15 axillary nodes and 1/2 apical nodes. This was treated with neoadjuvant chemotherapy using 5-fluorouracil, epirubicin and cyclophosphamide (FEC regime) followed by wide local excision and axillary node clearance in May 2008. The patient then received radiotherapy to the right breast and supraclavicular nodes and was started on tamoxifen 20 mg once daily, which she took for 5 years, finishing in 2012.
At the time of diagnosis, the patient had had normal liver function test results; alkaline phosphatase (ALP) 94 IU/L, alanine aminotransferase (ALT) 25 IU/L, bilirubin 6 μmol/L, albumin 45 g/L, and an ultrasound scan of her abdomen was reported as normal. The patient had no other medical history and consumed only very occasional alcohol, amounting to less than five units a week on average.
In April 2013, after the patient reported significant weight loss, profound tiredness, and was found to be anaemic, a bone scan revealed she had extensive neoplastic bone marrow infiltration. Blood tests taken in March 2013 revealed an elevated ALP 254 IU/L, likely related to the bone involvement, with normal ALT, 54 IU/L, bilirubin, 14 μmol/L and albumin, 36 g/L. She was started on anastrozole 1 mg once daily in May 2013. The patient was not taking any concurrent medications such as antibiotics or non-steroidal anti-inflammatory drugs. The oncology team planned for the patient to start second-line chemotherapy with paclitaxel, but she required frequent admissions for blood transfusions and this was postponed. In July 2013, the patient became jaundiced and this progressively worsened throughout the month. The patient received an outpatient abdominal ultrasound scan and CT liver and an elective admission for liver biopsy was organised, but the patient became progressively weak and fatigued and required emergency admission in August 2013.
Investigations
On admission, the patient was haemodynamically stable. She looked profoundly jaundice, with a soft, non-tender abdomen and palpable hepatosplenomegaly. First-line laboratory investigations revealed haemoglobin 54 g/L, white cell count 5.31×109/L, platelets 143×109/L, mean corpuscular volume 118.2 fL, haematocrit 0.137, neutrophils 3.71×109/L, sodium 133 mmol/L, potassium 3.7 mmol/L, urea 4.6 mmol/L, creatinine 41 mmol/L, albumin 25 g/L, bilirubin 567 μmol/L, ALP 385 IU/L, ALT 98 IU/L, lactate dehydrogenase 1711, total protein 72 g/L. Conjugated bilirubin 534, reticulocytes 5.1, international normalised ratio (INR) 1.9.
Differential diagnosis
At this point, the differential diagnoses for jaundice in this patient were as follows:
Liver metastases: The liver is a common site of metastases from a breast cancer primary and should be considered early on in this case. Lesions may obstruct the porta hepatis causing an obstructive picture but tumour infiltration may lead to a cholestatic picture without ductal dilation.
Choledocholithiasis: Other common causes of jaundice unrelated to the history of breast cancer must also be ruled out. Gall stones are often a cause in women of this age group.
Primary biliary cirrhosis: This condition causes progressive bile duct destruction, eventually leading to liver cirrhosis. 90% cases affect women in their fourth decade.
Autoimmune hepatitis: Again, this condition primarily affects women and often this age group. It can present with an acute hepatitis and jaundice.
Viral hepatitis:This was considered less likely as the blood tests pointed more towards an obstructive picture.
Outcome and follow-up
Second-line investigations included further investigation of anaemia, a liver autoimmune and viral screen as well as blood film for malaria and α-1 antitrypsin levels, results of which are given in table 1. An abdominal ultrasound scan had shown the liver to be enlarged with marked fatty change. There was no intrahepatic or common bile duct dilation and no focal liver lesions. A CT liver had confirmed hepatomegaly, with generalised low attenuation throughout. It also identified splenomegaly but no cause could be identified. The patient then proceeded to an inpatient liver biopsy, which was also sent for a second opinion from a tertiary centre. The biopsy revealed extensive macrovesicular steatosis with moderate fibrosis. Radiological investigation results are summarised in table 2.
Table 1.
Blood test investigations for patient with marked liver dysfunction
| Liver autoimmune screen | Anti-Smith | Negative |
| ANA ELISA | Negative | |
| Antimitochondrial | Negative | |
| IgG | 28.2 (7–16) | |
| IgA | 5.09 (0.7–4.0) | |
| IgM | 2.82 (0.4–2.3) | |
| Anaemia investigation | Antigastric parietal cell | Negative |
| Iron | ||
| Transferrin | 3.3 | |
| Transferrin saturation | 15% | |
| Direct antiglobulin test | 3+ | |
| Blood film | leukoerythroblastic features and spherocytes | |
| Viral Screen | Hepatitis A IgM | Negative |
| Hepatitis B sAg | Negative | |
| Hepatitis C | Negative | |
| HIV (1+2) | Negative | |
| CMV IgM | Negative | |
| Infectious mononucleosis screen | Negative | |
| EBNA IgG | Positive (demonstrating previous, not recent, EBV infection) | |
| Other | Malaria screen | Blood film×3 negative |
| α-1 antitrypsin | Normal |
ANA, antinuclear antibody; CMV, cytomegalovirus; EBNA, Epstein-Barr nuclear antigen.
Table 2.
Results of radiological investigation and subsequent liver biopsy in patient with marked liver dysfunction
| Abdominal ultrasound scan | Enlarged liver, with marked fatty change No intrahepatic or common bile duct dilatation No focal liver lesions |
| CT liver | Hepatomegaly, with generalised low attenuation throughout. Splenomegaly, no cause identified |
| Liver biopsy | Severe extensive macrovesicular steatosis with marked lobulitis (Ishak grade 4/4) Some of the portal tracts show mild fibrous expansion (Ishak stage 1/6). Marked mixed inflammation in most of the portal tracts (Ishak grade 2/4) including numerous neutrophils, lymphocytes, plasma cells and eosinophils No bile duct loss or periductal fibrosis Features supporting a diagnosis of steatohepatitis Moderate fibrosis, not yet amounting to cirrhosis Well-formed Mallory-Denk bodies not seen. (Excluding a chronic picture) |
Following second-line investigations, the most common causes for jaundice were ruled out and the differential diagnoses were redefined as below
Autoimmune haemolytic anaemia: Blood tests revealed a positive direct antiglobulin test with raised IgG and complement, suggesting a possible haemolytic anaemia contributing to the presentation. In fact, these results were noted prior to the patient starting anastrozole treatment. Blood films demonstrated leukoerythroblastic features and spherocytes, consistent with bone marrow infiltration and haemolytic anaemia. However, the significantly elevated conjugated bilirubin, (534), confirmed this was not the predominant cause for this patient's jaundice, and pointed towards coexistent parenchymal or obstructive jaundice.
Micrometastases to liver: Radiological investigation did not detect any focal liver lesions so metastatic liver involvement was unlikely to explain the patient's profound jaundice. The liver biopsy ruled out metastases undetectable on imaging.
Drug-induced steatohepatitis secondary to anastrozole: This diagnosis was confirmed on liver biopsy, (see report in table 2). The findings did not correlate with a typical autoimmune picture and drug-related toxicity was deemed the most likely explanation. This was also confirmed following a significant clinical improvement and a dramatic reduction in bilirubin following withdrawal of anastrozole.
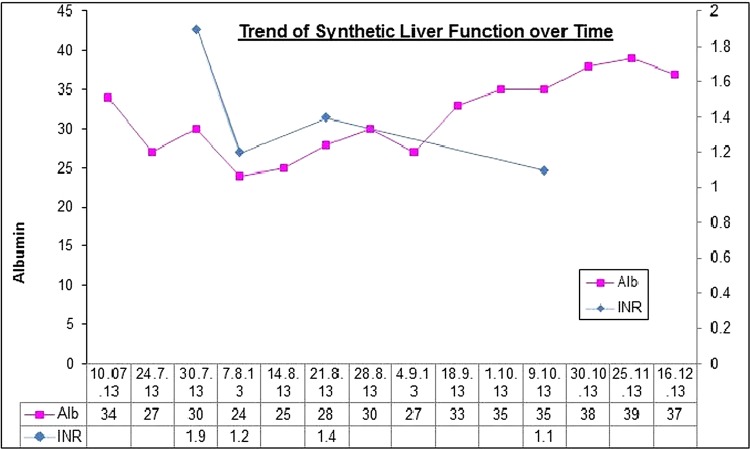
Anastrozole was stopped and the patient was started on prednisolone 40 mg once daily, which was reduced to 20 mg after 2 weeks and ultimately stopped after 2 months. This was largely to cover any possible concurrent haemolytic anaemia. Figure 1 demonstrates the trend of the patient's liver function tests over a 6-month period July–December 2013; immediately preceding, during and following her admission in August. It is clear that her liver function has stabilised although the ALT initially continued to rise suggesting an element of ongoing inflammation. The Roussel-Uclaf Causality Assessment method (RUCAM) tool defines a decrease in total bilirubin of greater than or equal to 50% within 180 days of stopping the medication as suggestive of a drug-induced liver injury.6 In this case, the bilirubin went from 586 μmol/L at its peak to 63 μmol/L precisely 1 month later and following discontinuation of the offending drug. In this case, the ALP, although reduced, remained elevated likely as a result of the neoplastic bone marrow involvement. The trend of synthetic liver function over time is demonstrated in figure 2. The INR reduced following withdrawal of anastrozole. On admission, the patient was given a one-off dose 5 mg vitamin K prior to biopsy. INR at time of biopsy was 1.2.
Figure 1 .
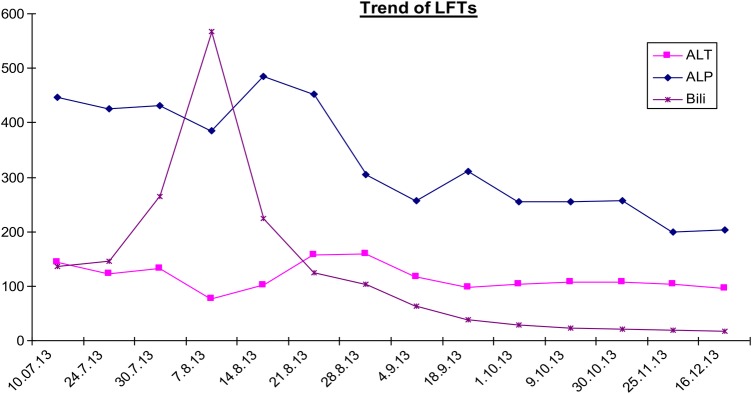
Trend of liver function tests over time.
Figure 2 .
Trend of synthetic function.
Clinically, the patient is markedly improved and has gained weight appropriately since discontinuation of anastrozole. She has required no further blood transfusions since the steroids were started and, interestingly, her blood count has improved despite the steroids being stopped.
The time interval between initiation of anastrozole treatment and onset of symptoms, the dramatic reduction in total bilirubin following withdrawal of the drug and the exclusion of concomitant drug, viral or other causes for the liver injury, render the possibility of anastrozole being culpable high. Using the RUCAM, this case scores 6; suggesting a probable causal effect of anastrozole to the liver injury. A score of greater than 8 is required to deem this ‘highly probable’.
Discussion
This case describes an unusual cause of jaundice in a patient with breast cancer. Following first-line investigations, common causes were ruled out. This led to the patient receiving a liver biopsy which showed steatohepatitis and, combined with the improving clinical and biochemical picture following drug cessation, ultimately led to a diagnosis of anastrozole-induced hepatitis. However, a score of greater than 8 on the scoring system used is required to deem a causal relationship between anastrozole and the liver injury ‘highly probable’, as opposed to ‘probable’ as in this case.
The RUCAM method applied to help ascertain the likelihood of the patient's liver injury being attributable to anastrozole use, is widely-used and a well-regarded tool but not perfect. Chalasani et al7 recently published guidelines on diagnosis of idiosyncratic drug-induced liver injury which called into question the reliability of this and similar scoring systems.
This case is perhaps partially complicated by the fact that the patient did have concurrent haemolytic anaemia and was treated with steroids. The liver autoimmune screen and the biopsy findings were not consistent with an autoimmune hepatitis.
Although there are previous documented cases of anastrozole-related hepatitis, the exact mechanism of this liver injury is not clearly understood and reported biochemical and histological findings have varied. Inno et al2 reported a case where the liver biopsy revealed mild steatosis, moderate inflammatory activity and moderate to severe fibrosis. de La Cruz et al3 reported a case in which the liver biopsy showed diffuse liver cell necrosis in acinar zone 3 but no inflammatory changes. A third case, reported by Zupata et al,4 does not include a liver biopsy result. In all three previously documented cases, the patients have a mixed hepatocellular and cholestatic liver injury whereas this case is predominantly cholestatic.
Although rare, drug-induced steatohepatitis has been documented before, caused by amiodarone and perhexiline, which are believed to target mitochondrial ATP production and fatty acid catabolism.8 Anastrozole has been shown to undergo hepatic metabolism and is oxidised mainly by CYP3A4. There is significant pharmacokinetic variability among women receiving this drug, leading to a markedly inconsistent side effect profile. This possibly represents variable CYP3A4 activity due to genetic polymorphisms.9 It would be prudent to avoid administration of this medication in patients with known CYP3A4 dysfunction or who are concurrently taking medications known to also be metabolised via this pathway. It has also been previously noted that CYP3A4 is expressed in human breast cancer,10 perhaps causing polymorphism and increasing this patient's risk of hepatotoxicity directly.
Learning points.
Anastrozole has been identified as a rare cause of acute hepatic injury and this patient's predominantly cholestatic presentation with severe extensive macrovesicular steatosis on the liver biopsy has not been documented before.
This case offered an opportunity to review possible causes of jaundice in a patient with breast cancer.
Although it is important to exclude metastatic disease, common causes of jaundice unrelated to cancer must also be considered in the differentials.
Footnotes
Contributors: The case report was written by RL. AE had an advisory/editorial role prior to final submission.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Buzdar A, Howell A, Cuzick J, et al. ; Arimidex, Tamoxifen, Alone or in Combination Trialists’ Group. Comprehensive side-effects profile of anastrazole and tamoxifen as adjuvant treatment for early-stage breast cancer: long term safety analysis of the ATAC trial. Lancet Oncol 2006;7:633–43. 10.1016/S1470-2045(06)70767-7 [DOI] [PubMed] [Google Scholar]
- 2.Inno A, Basso M, Vecchio F et al. Anastrozole-related acute hepatitis with autoimmune features: a case report. BMC Gastroenterology 2011;11:32 10.1186/1471-230X-11-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de La Cruz L, Romero-Vazquez J, Jimenez-Saenz M et al. Severe acute hepatitis in a patient treated with anastrozole. Lancet 2007;369:23–24. 10.1016/S0140-6736(07)60017-8 [DOI] [PubMed] [Google Scholar]
- 4.Zupata E, Zubiaurre L, Bujanda L et al. Anastrazole-induced hepatotoxicity. Eur J Gastroenterol Hepatol 2006;18:1233–4. 10.1097/01.meg.0000243868.64078.af [DOI] [PubMed] [Google Scholar]
- 5.Office of National Statistics: Breast Cancer in England 2010.
- 6. http://www.livertox.nih.gov/rucam.html.
- 7.Chalasani NP, Hayashi PH, Bonkovsky HL et al. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol 2014;109:950–66. 10.1038/ajg.2014.131 [DOI] [PubMed] [Google Scholar]
- 8.Stravitz R, Sanyal A. Drug-induced steatohepatitis. Clin Liver Dis 2003;7:435–51. 10.1016/S1089-3261(03)00027-8 [DOI] [PubMed] [Google Scholar]
- 9.Kamdem L, Liu Y, Stearns V et al. In vitro and in vivo oxidative metabolism and glucuronidation of anastrazole. Br J Clin Pharmacol 2010;70:854–69. 10.1111/j.1365-2125.2010.03791.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapucuoglu N, Coban T, Raunio H et al. Expression of CYP3A4 in human breast tumour and non-tumour tissues. Cancer Lett 2003;202:17–23. 10.1016/j.canlet.2003.08.015 [DOI] [PubMed] [Google Scholar]