Abstract
The organic anion transporter (OAT) subfamily, which constitutes roughly half of the SLC22 (solute carrier 22) transporter family, has received a great deal of attention because of its role in handling of common drugs (antibiotics, antivirals, diuretics, nonsteroidal anti-inflammatory drugs), toxins (mercury, aristolochic acid), and nutrients (vitamins, flavonoids). Oats are expressed in many tissues, including kidney, liver, choroid plexus, olfactory mucosa, brain, retina, and placenta. Recent metabolomics and microarray data from Oat1 [Slc22a6, originally identified as NKT (novel kidney transporter)] and Oat3 (Slc22a8) knockouts, as well as systems biology studies, indicate that this pathway plays a central role in the metabolism and handling of gut microbiome metabolites as well as putative uremic toxins of kidney disease. Nuclear receptors and other transcription factors, such as Hnf4α and Hnf1α, appear to regulate the expression of certain Oats in conjunction with phase I and phase II drug metabolizing enzymes. Some Oats have a strong selectivity for particular signaling molecules, including cyclic nucleotides, conjugated sex steroids, odorants, uric acid, and prostaglandins and/or their metabolites. According to the “Remote Sensing and Signaling Hypothesis,” which is elaborated in detail here, Oats may function in remote interorgan communication by regulating levels of signaling molecules and key metabolites in tissues and body fluids. Oats may also play a major role in interorganismal communication (via movement of small molecules across the intestine, placental barrier, into breast milk, and volatile odorants into the urine). The role of various Oat isoforms in systems physiology appears quite complex, and their ramifications are discussed in the context of remote sensing and signaling.
I. INTRODUCTION
A. The Organic Anion Transport Pathway
The organic anion transporter (OAT) family comprises a group of over 10 transmembrane proteins (Table 1) falling into the SLC22 (solute carrier 22) subfamily of the major facilitator superfamily (MFS); the SLC22 subfamily also includes the organic cation transporters (OCTs) and organic carnitine (zwitterion) transporters (OCTNs) (127). OAT family members are highly similar within this subclass of SLC22 transporters and share many structural characteristics with other MFS proteins. Modeling, mutagenesis, and other studies are consistent with the view that these transmembrane proteins are composed of about 540–560 amino acids comprising 12 transmembrane domains (69, 127, 282, 283) (Figure 1).
Table 1.
List of named organic anion transporters
| OAT | Human | Mouse | Expression (Human and/or Rodent) | Substrates |
|---|---|---|---|---|
| OAT1 | SLC22A6 | Slc22a6 | Kidney | Numerous small molecule xenobiotics |
| Choroid Plexus | PAH | |||
| Cyclic nucleotides | ||||
| Indoxyl sulfate | ||||
| Prostaglandin E2 | ||||
| Mercurials | ||||
| OAT2 | SLC22A7 | Slc22a7 | Liver | Antivirals |
| Kidney | cGMP | |||
| Prostaglandin E2 | ||||
| Salicylate | ||||
| OAT3 | SLC22A8 | Slc22a8 | Kidney | Numerous small molecule xenobiotics |
| Brain endothelium | Conjugated sex steroids | |||
| Choroid plexus | Carnitine | |||
| Retina | Prostaglandin E2 | |||
| Testes | Vitamins | |||
| Plant-derived metabolites | ||||
| OAT4 | SLC22A11 | Placenta | Estrone sulfate | |
| Kidney | Dehydroepiandrosterone sulfate | |||
| Brain | Prostaglandin E2 | |||
| Urate | ||||
| Ochratoxin A | ||||
| Oat5 | Slc22a19 | Kidney | Estrone sulfate | |
| Dehydroepiandrosterone sulfate | ||||
| Ochratoxin A | ||||
| Oat6 | SLC22A20 | Slc22a20 | Nasal mucosa | Estrone sulfate |
| Testes | Odorants | |||
| OAT7 | SLC22A9 | Liver | Estrone sulfate | |
| Dehydroepiandrosterone sulfate | ||||
| Butyrate | ||||
| rOat8 | Slc22a9 (rat) | Kidney | Estrone sulfate | |
| Dehydroepiandrosterone sulfate | ||||
| Ochratoxin A | ||||
| Oat9 | Slc22a27 | Liver | Xenobiotics | |
| Estrone sulfate | ||||
| Carnitine | ||||
| Ochratoxin A | ||||
| OAT10 | SLC22A13 | Slc22a13 | Kidney | Nicotine |
| Brain | Urate | |||
| Small intestine | ||||
| Colon | ||||
| URAT1 | SLC22A12 | Slc22a12 | Kidney | Urate |
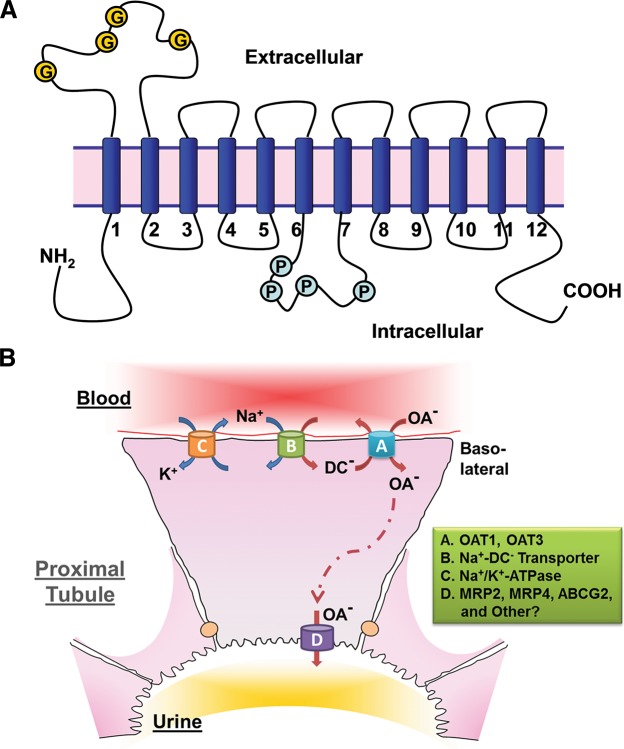
FIGURE 1.
OAT structure and the mechanism of OAT-mediated uptake and transport of organic anions. A: illustration of the predicted topology of organic anion transporters. Two pairs of 6-transmembrane domains are connected by a large intracellular loop and both NH2 and COOH termini are intracellular (G, glycosylation sites; P, PKC phosphorylation sites). B: a renal proximal tubule cell is depicted as a prototypical epithelial cell to illustrate the Oat-mediated uptake and transcellular movement of organic anionic substrates (OA−) from the blood to the urine. Oat1 and Oat3 (A), localized to the basolateral membrane of the proximal tubule cell, transport OA− across the basolateral membrane and into the cell through the exchange of dicarboxylates (DC−). As a secondary active membrane transporter system (76), the Oat-mediated entry of OA− is linked to the transmembrane electrochemical potential of dicarboxylates generated by their movement against a concentration gradient and intracellular accumulation maintained through the action of the Na+/dicarboxylate cotransporter (B). Thus the energy driving this ”tertiary“ mechanism is the ATP consumed by the Na+-K+-ATPase in generating the sodium gradient (C). OA− exit into the urinary luminal space (D) is via transporters found on the apical membrane. [Modified from Eraly et al. (69), with permission from ASPET.]
Although the initial focus in this field was on the kidney, the OATs have been localized to almost all barrier epithelia of the body, as well as endothelium and other cells, and have demonstrated roles in the regulated transcellular movement of numerous small organic anionic molecules across these epithelial barriers and between body fluid compartments (i.e., blood-central nervous system, blood-urine, intestine-blood, blood-bile, blood-placenta, and others). While prototypical members of this transporter family are capable of the bidirectional movement of substrates, most of the Oats are generally viewed as facilitating the movement of organic anions into the epithelial cells (influx transporters). Prototypical Oats such as Oat1 are secondary active transporters; Oat-mediated influx involves the exchange, or countertransport, with another solute (which for the prototypical Oats is believed to be α-ketoglutarate) (194, 217, 235), and these transporters are thought to be part of a so-called “tertiary” transport system involving the organic anion transporter, the Na+-K+-ATPase, and the sodium-dicarboxylate cotransporter (see below; Figure 1).
Some interesting aspects of this family of transport proteins (discussed in more detail later) include the following: range of substrates (drugs, toxins, metabolites, regulatory molecules), substrate overlap, embryonic expression (144, 187, 233), postnatal maturation, evolutionary conservation (71, 230, 283), transcriptional regulation, genomic clustering of family members (70, 149, 283), as well as the association of single nucleotide polymorphisms (SNPs) with metabolic disease (such as disorders of uric acid) and alterations in drug handling (12, 66, 71, 83, 141, 153, 284, 292).
B. History
Prior to the cloning of NKT (Novel Kidney Transporter, now called organic anion transporter 1 or OAT1, and also designated as SLC22A6), the Oat pathway had been the subject of much investigation, particularly from the viewpoint of kidney physiology, over many decades. For example, in the 1940s, Homer Smith suggested that a substituted hippuric acid derivative, p-aminohippuric acid (PAH), might be a suitable tracer for tubule excretion (225). PAH was subsequently recognized as a prototypical organic anion substrate, and it helped to define the classical renal organic anion transporter pathway, since implicated in the handling of a large number of small molecule organic anions including endogenous metabolites, toxins, and drugs.
During World War II, it was realized that penicillin was being rapidly excreted by the kidney through an organic acid transport system (198). As a strategy to slow the excretion of penicillin in the context of limited availability of antibiotics, the uricosuric agent probenecid (benemid) was used to competitively inhibit the excretion of penicillin when the two drugs were administrated together (36). This was also found to affect PAH transport (213). Probenecid eventually became the standard inhibitor of the classical organic anion (PAH) transporter system; indeed, the system was, for many years, operationally defined by the effect of probenecid. With the availability of a prototypical tracer (PAH) and what was perceived as a specific inhibitor (probenecid), the role of the “classical” organic anion transport pathway in the excretion of many drugs became well established in the subsequent decades (18, 49, 260).
After Na+-K+-ATPase activity was localized to the basolateral membrane of the renal proximal tubule cell, a link between its activity and PAH transport was established (221). However, the nature of this link appeared indirect since a sodium gradient did little to facilitate the uptake of PAH in cell membrane vesicle preparations, while glutarate, a dicarboxylate, in the presence of sodium, was able to substantially stimulate the uptake of PAH (194, 217). Hence, an additional intermediary step, involving a sodium gradient that maintains the dicarboxylate gradient, was postulated to exist between the Na+-K+-ATPase and the PAH transporter. Such a “tertiary” transporter system of epithelial cells is therefore envisioned to utilize the sodium gradient generated by the Na+-K+-ATPase to indirectly facilitate the influx of organic anion molecules from the blood (or other body fluids) and into the polarized epithelial cell (Figure 1) (33, 49, 232, 282).
Thus, before the cloning in 1996 of NKT, a great deal of physiology was already done (much of it in the kidney), making it possible to suggest the role of NKT (later Oat1) in the transport of organic anions and/or cations (143–145). As detailed below, both roles were subsequently established in transport assays in which the cloned gene was overexpressed in frog oocytes or transfected cells and, later, in the knockout mice and tissues derived from them. Nevertheless, consistent with its key role in probenecid-sensitive organic anion transport, Oat1 has a much greater preference for organic anions.
It is now clear that the Oat system is important for the transport of an extraordinarily broad range of molecules (including many clinically important drugs, as well as a number of endogenous hormones, nutrients, and metabolites) across multiple tissues (including kidney, liver, brain, eye, and intestine) (270). Among drugs, substrates of the probenecid-sensitive classical PAH pathway (mediated largely by Oat1) include many pharmaceuticals (e.g., antibiotics, non-steroidal anti-inflammatory drugs, diuretics, antivirals) (2, 35, 168, 177, 231, 270, 294) which are small, water-soluble organic anion molecules with an ability to bind albumin (27). Because of their albumin-binding capacity, these molecules are not freely filtered by the glomerulus; instead, they continue into the peri-tubular capillaries, which are adjacent to the basolateral (blood) surface of the proximal tubule cells. By binding the basolaterally localized Oat1 (and/or Oat3-also designated SLC22A8), they gain entry to the proximal tubule cell (the intracellular behavior of the organic anions as they transit the cell is not well defined and may depend on the specific class of molecules). Through a separate apical surface transport step, likely involving multiple ATP-binding cassette (ABC) transporters [e.g., Abcc2, also known as Mrp2 (multidrug resistance-associated protein 2) and Abcc4/Mrp4] and SLC transporters, they achieve egress to the lumen of the proximal tubule (Figure 1). This enables the transcellular movement of small organic anionic drugs, toxins, and endogenous metabolites from the blood to the urinary space. The process is very efficient and is largely a first pass phenomenon.
This transport pathway is also of considerable toxicological importance, since many drugs and other xenobiotics that are toxic in overdose are weak organic anions at physiological pH, and therefore handled by this system (159). In addition, other compounds that are not themselves transported can be detoxified by conjugation to glycine, glucuronide, or sulfate, thus enabling them to be handled by this system (159). Many of these toxins, drugs, and metabolites have been shown to directly compete for the same transport pathway (and therefore potentially inhibit the transport of one another, possibly leading to toxic accumulation in body fluids; this is an area for future investigations). Furthermore, since drugs cleared by this route are concentrated in cells of the transporting epithelia, a specific toxic effect on proximal tubule cells, which are metabolically active and highly sensitive to toxins, can be exacerbated.
C. Scope of This Review
Although we detail biochemical and other data related to individual Oats, there is a heavy emphasis in this review on the systems level physiology and computational biology related to Oats and on highlighting potential areas for future Oat research. While the physiology of the Oats has been extensively studied in the proximal tubule of the kidney, it is now clear that Oats, meaning Oat1 and its many relatives, are likely important to physiological processes in many tissues. These tissues include choroid plexus, liver, brain capillary endothelium, retina, placenta, olfactory mucosa, and others. Indeed, Oats and other multispecific “drug” transporters from the SLC and ABC families are expressed in virtually all barrier epithelia (34) and appear to mediate the movement of drugs and toxins between body fluid compartments and tissues. Examples include movement between blood and urine, blood and the central nervous system (CNS) (i.e., blood-brain barrier), cerebrospinal fluid (CSF) and blood, blood and placenta, blood and vitreous humor, and possibly across the olfactory mucosa (5, 113, 284).
Moreover, knockout and other data indicate that the endogenous Oat substrates include rate-limiting metabolites and signaling molecules. This has led to the view that Oats and other “drug” transporters (SLC and ABC families) may form a “remote communication” system involving the movement of metabolites, nutrients, and signaling molecules into various tissues and body fluid compartments (Figure 2). “The Remote Sensing and Signaling Hypothesis” argues that this SLC and ABC “drug transporter” network throughout the body functions in parallel with, and akin to, the endocrine, growth factor, and autonomic nervous systems to regulate systemic physiology (Figure 3) (5, 113, 284). This is done by regulation, via the expression and/or activity of “drug” transporters, of the movement of key metabolites (e.g., α-ketoglutarate, uric acid, indoxyl sulfate) and signaling molecules (e.g., cyclic nucleotides, prostaglandins, conjugated sex steroids, odorants) into different body fluid compartments and tissues. For example, reduction in the expression of a single organic anion transporter in Drosophila not only reduced the expression of multiple transporters, it also disrupted methotrexate-induced transporter upregulation (39, 40).
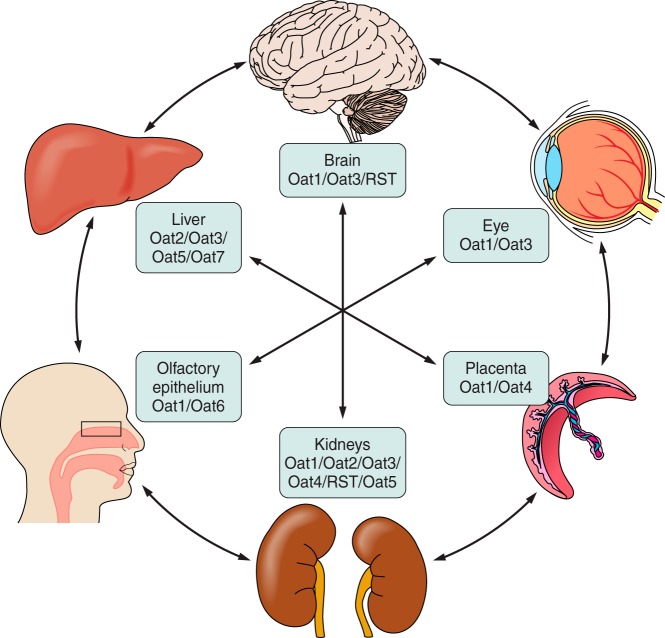
FIGURE 2.
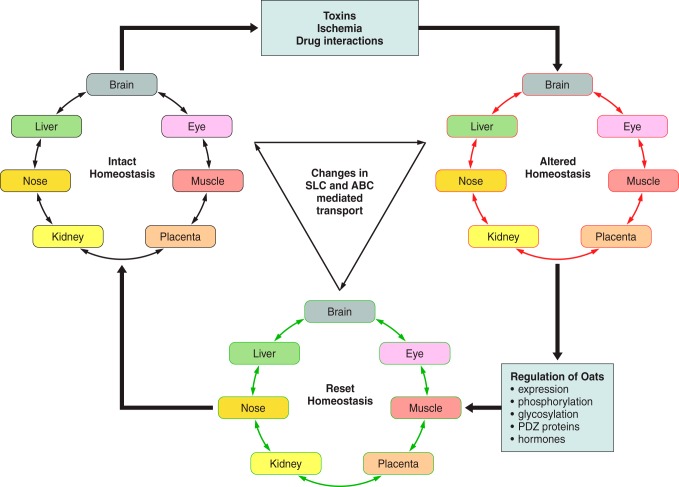
Interorgan communication mediated by organic anion transporters. Organic anion transporters (Oats) have been localized to most barrier epithelia. In these tissues, the Oats represent rate-limiting steps involved in the uptake and transcellular movement of small molecule anionic substrates (including metabolites, toxins, and drugs) between body fluid compartments. These various substrates, including many with informational content (e.g., signaling molecules, hormones, and growth factors, as well as toxins and xenobiotics), are ”sensed" by the other organs via their own set of variably expressed transporters and information is shared between tissues and organs. [Modified from Ahn and Bhatnagar (2), with permission from Wolters Kluwer Health.]
FIGURE 3.
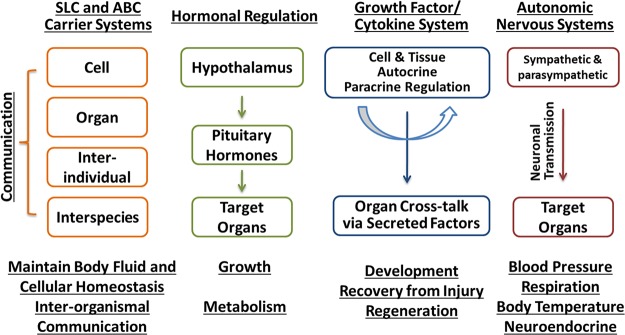
Transporter-mediated remote sensing and signaling. The Oats, members of the SLC family of solute carriers, are believed to function along with members of the ATP-binding cassette (ABC) transport system, to maintain body fluid and cellular homeostasis. The movement of the small molecule substrates handled by these transport systems is postulated to provide a means of communication between cells, as well as between tissues/organs. This intraorganismal process can be viewed as being analogous and working with regulatory mechanisms of the autonomic nervous system, growth factor/cytokine system, and neuroendocrine system. However, through their secretion (e.g., milk) or excretion (e.g., urine), these substrates are also postulated to allow for interorganismal communication between individuals of the same species (e.g., mother/neonate) or of different species (e.g., predator/prey). [Modified from Wu et al. (284), with permission from ASPET.]
These transporters are also advantageously situated for a role in interorganismal communication, regulating the passage of key metabolites and signaling molecules between the body and the gut microbiome, the fetus (maternal-fetal barrier), the neonate (via breast milk), as well as by the elimination of odorants into the urine that may be “sensed” by the SLC (and/or GPCR)-containing olfactory apparatus of another organism of the same or different species (Figures 2 and 3). The central ideas of the “Remote Sensing and Signaling Hypothesis” (5, 284) are reviewed and then discussed in considerable detail toward the end of this article with the goal of furthering research in the systems biology and multiscale physiology of Oats. We begin with a discussion of the basic biology of individual Oats and their widely accepted roles in the handling of many common drugs and toxins.
II. ORGANIC ANION TRANSPORTER FAMILY
A. Discovery of NKT, Later Called Oat1
The prototypical organic anion transporter, Oat1, was originally cloned from mouse in 1996 as novel kidney transporter (NKT) (143, 145). It was originally suggested to act as an organic anion transporter (i.e., the “classical” PAH transporter) or organic cation transporter (144). In subsequent work, it was confirmed that Oat1/NKT was indeed the PAH transporter, responsible for the transport of many small water-soluble organic anion drugs, toxins, metabolites, and signaling molecules (Table 1) (34, 187, 235, 270), but whether it also transported organic cations remained a matter of debate (3). Perhaps because they were termed organic “anion” transporters, these types of molecules were largely tested as substrates. From knockout in vivo and in vitro studies (35, 73, 74, 112, 169, 234, 250, 265), it is now clear that although Oat1, and the closely related Oat3, primarily handle organic anions, they are also capable of transporting a variety of organic cationic drugs, such as cimetidine, as well as metabolites like creatinine, and possibly polyamines and carnitine (3, 4, 133, 264). This points to limitations in the nomenclature of organic anion transporters which, as was originally suggested (144), are able to transport many organic anions and some organic cations. Nevertheless, the name “Oat” has remained and, by and large, is sufficient to describe the general functionality of this class of transporters.
B. Identification of the SLC22 Transporter Subfamily
Together with Oct1 (organic cation transporter 1; Slc22a1) and NLT (novel liver transporter, now Oat2/Slc22a7), NKT (Oat1) was proposed to comprise a new subfamily of transporters (144), now designated as SLC22 consisting of 20–30 members. Although there is only limited functional data on several family members, at this point, it appears that one-third to one-half of the SLC22 family members are Oats with varying substrate specificities and tissue expression patterns, while the remaining family members consist of organic cation transporters (Octs), organic carnitine (zwitterion) transporters (Octns), and so-called Usts (unknown substrate transporters, many of which, based on functional and sequence similarity data, appear to be more similar to the Oat group than the Oct and Octn groups). There is also a group of transporters that is sometimes referred to as the Flipt (fly-like putative transporter) and CT (carnitine transporter); while their main function may be in carnitine transport, these transporters have not been studied in sufficient detail (16, 72).
Although we will be focusing on the Oats in this review, it is important to emphasize again that they are capable of transporting some Oct and Octn substrates such as creatinine, carnitine, and cimetidine (3, 126, 133, 264). The substrate specificity of Octs and Octns may be somewhat more restricted to cationic compounds and metabolites, but this needs to be rigorously analyzed for the entire SLC22 family (in a single species and using the same assay) since it appears that all, or nearly all, family members are identified.
III. OAT NOMENCLATURE
A. Physiological, Pharmaceutical, and Toxicological Importance of Oats 1–10 Based on In Vitro and Knockout Data
Here we will discuss in vitro, in vivo knockout, and human data for each of these Oats. There are several reviews covering individual family members (2, 34, 35, 79, 177, 270). In this review, we emphasize unique characteristics of individual transporters to help present an integrated view of a vast amount of transport data. This will set the stage for the “systems and computational biology” perspective of the latter part of the review, where we will discuss the information in the context of the “Remote Sensing and Signaling Hypothesis.” Although the focus here is exclusively on the Oat family, it is also worth mentioning that many of the ideas are applicable to other multispecific SLC transporters [i.e., Oct, Octn, Oatp (organic anion transporting polypeptides, also SLC21 or SLCO), MATE (multidrug and toxin extrusion proteins, also SLC47)] and ABC transporters [P-glycoprotein/MDR1 (multidrug resistance protein 1), BCRP (breast cancer resistance protein), and Mrps] in the context of this hypothesis. It is important to keep in mind that transepithelial vectorial transport involves transporters at the basolateral and apical surfaces and often this is a combination of SLC (“uptake/influx”) transporters and ABC (“efflux”) transporters. For example, in the kidney it now appears that basolateral Oat1 and Oat3 uptake of organic anions is loosely coupled to apically located transporters including Mrp2 and Mrp4 for efflux.
Organic anion transporters of the SLC22 family play a major role in the handling of common drugs and toxins. Initially thought to be localized largely to the kidney, it is now clear that they are expressed in many other tissues, including choroid plexus (Oat1, Oat3) (170, 234), olfactory mucosa (Oat6) (113, 160, 212), and placenta (OAT4) (38). Recent systems biological analyses indicate that the Oat pathway plays a central role in metabolism (4, 73, 74, 216, 279, 285). Certain Oat family members have a strong selectivity for particular signaling molecules. This is important for understanding the “Remote Sensing and Signaling Hypothesis,” where it is proposed that Oats and other multispecific drug transporters of the SLC and ABC families function in remote communication by regulating levels of rate-limiting metabolites and key signaling molecules in various cell types, tissues, and body fluid compartments (Figures 2 and 3).
Below we describe the named major organic anion transporters of the SLC22 family (Table 1). The nomenclature and numbering of various Oats in humans and rodents can be quite confusing and probably requires revision in light of new sequence data from many species and a greater appreciation of substrate specificities.
B. OAT1 (SLC22A6)
OAT1 was first identified in 1996 as a NKT in a screen for G protein-coupled receptors (GPCRs) (which is possibly relevant to some of the arguments below) (143–145). NKT/Oat1 was almost exclusively expressed in the kidney (144), although to a lesser degree, it can also be found in other rodent tissues. Based on its homology to the two organic ion transporters identified at that time (NLT and Oct1), it was proposed as an organic ion transporter functioning in either organic anion or cation transport (144). It turned out that NKT/Oat1 can function in both (3, 264); for example, a set of seven Oct1-interacting compounds, including verapamil, cimetidine, and nicotine, were found to interact with Oat1 in vitro, albeit at higher concentrations than that seen with the better organic anion substrates (3). Nevertheless, this transporter is generally regarded as the “prototypical” transporter of small molecule organic anionic compounds.
The transcript was initially postulated to encode a ∼550-amino acid polypeptide possessing at least 11 membrane-spanning domains that were characterized by two large interconnecting loops (one extracellular and one intracellular), strikingly similar to other bacterial and mammalian transporters (144). Now it is more generally believed that the protein, though not yet crystallized, consists of 12 transmembrane domains (Figure 1). A number of potential modification sites for protein kinases and other enzymes were found within the interconnecting loops (Figure 1) (144), and in vitro investigations of some of these sites (e.g., glycosylation and protein kinase-mediated phosphorylation) have raised the question of whether they might modulate Oat function by regulating the trafficking and expression of the transporter at the plasma membrane (60). Furthermore, in cells transfected with a tagged human OAT1, the transporter was found to oligomerize (93), which may also be important for its expression at the plasma membrane (59).
Oat1, among the most highly expressed genes in the adult kidney, is localized to the basolateral surface of the proximal tubule; it is also highly expressed in choroid plexus (100, 144, 170, 195, 250). In the kidney, probenecid-inhibitable uptake of a fluorescent tracer molecule [6-carboxyfluorescein (6CF)] in coronal sections of Oat3-deficient kidneys revealed the nonuniform sequestration of Oat1 function in portions of the renal cortex consistent with the proximal tubule (169).
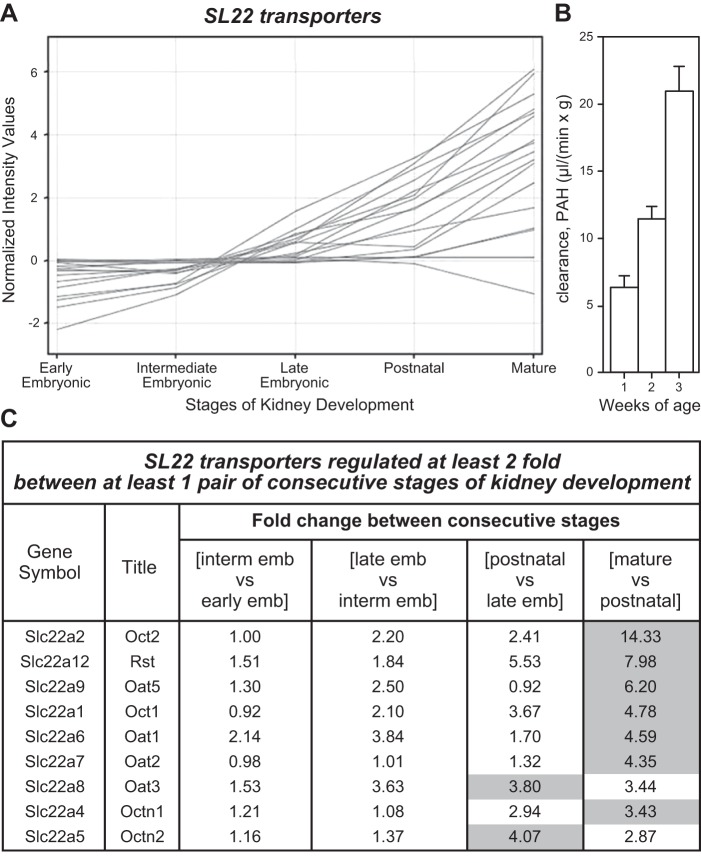
Oat1 is expressed not only in the developing kidney at around embryonic day 14–15, but also in the fetal and adult brain (144, 187). The renal expression of the transporter was found to increase during gestation and after birth, while functional assays in either cultured whole embryonic kidneys or in culture models of nephrogenesis indicated that Oat1 may be functional in embryonic tissue (144, 149, 187, 230, 233, 250). For example, cultured isolated metanephric mesenchymes (embryonic precursor tissues of the nephron) induced to form proximal tubule-like structures are capable of probenecid-inhibitible accumulation of a fluorescent Oat1 substrate (233). This was similar to the accumulation seen in cultured whole embryonic kidney (233) or in kidney-like constructs engineered from embryonic tissue (204).
The range of Oat1 drug, toxin, and metabolite substrates is now well established by in vitro and in vivo studies and has been thoroughly described in several excellent recent reviews on the topic (34, 127, 270). A brief list of its substrates include PAH, antivirals, nonsteroidal anti-inflammatory drugs (NSAIDs), antibiotics, diuretics, folate, α-ketoglutarate, cyclic nucleotides, prostaglandins, gut microbial metabolites, uremic toxins, vitamins, dietary compounds, uric acid, mercury conjugates, and other toxins (Table 1) (4, 112, 279). In the Oat1 knockout, much of the natriuretic response to loop and thiazide diuretics is blunted (266), and the knockout kidneys are substantially protected from mercury toxicity (249). In addition, ex vivo transport assays using knockout kidney and choroid plexus indicate a defect in handling antiviral drugs (169, 170, 250). The Oat1 knockout is also defective in the handling of many important metabolites involved in endogenous metabolism (Table 2) (74, 279). For example, among the multiple endogenous metabolites identified by mass spectrophotometric profiling of the plasma and urine from WT and Oat1-deficient mice (Figure 4) were several physiologically important metabolites, including vitamins and uremic toxins, as well as gut microbiome metabolites (279) (Table 2).
Table 2.
Metabolites with altered levels in Oat1-deficient mice
| Metabolite | Phenotype | Reference Nos. |
|---|---|---|
| 2-Hydroxy-3-methylvalerate | Elevated plasma concentration | 74 |
| 2-Hydroxyisovalerate | Reduced urine concentration | 74 |
| 2-Oxo-3-methylvalerate | Reduced urine concentration | 74 |
| 2-Oxoisocaproate | Reduced urine concentration | 74 |
| 3-Hydroxypropionate | Elevated plasma concentration | 74 |
| 3-Hydroxyvalerate | Reduced urine concentration | 74 |
| 3-Hydroxybutyrate | Elevated plasma concentration | 74 |
| 3-Hydroxyisobutyrate | Elevated plasma concentration | 74 |
| 3-Methylcrotonylglycine | Reduced urine concentration | 74 |
| 4-Hydroxyphenylacetate | Reduced urine concentration | 74 |
| 4-Hydroxyphenyllactate | Elevated plasma conc./reduced urine conc. | 74 |
| 4-Hydroxyphenylpyruvate | Reduced urine concentration | 74 |
| 4-Pyridoxic acid | Elevated plasma concentration | 279 |
| 5-Methyl cytidine | Reduced urine concentration | 279 |
| Amino-cresol sulfate | Reduced urine concentration | 279 |
| Benzoate | Elevated plasma concentration | 74 |
| Creatinine | Reduced renal secretion | 264 |
| Hexanoylglycine | Reduced urine concentration | 74 |
| Indole lactic acid | Elevated plasma concentration | 279 |
| Indoxyl sulfate | Elevated plasma concentration | 279 |
| Kynurenine | Elevated plasma concentration | 279 |
| Mercurials | Protective from mercurial renal toxicity | 249 |
| Methionine | Elevated plasma concentration | 279 |
| N2-N2-dimethyl guanosine | Reduced urine concentration | 279 |
| N-acetylaspartate | Elevated plasma conc./reduced urine conc. | 74 |
| N-acetylglycine | Reduced urine concentration | 279 |
| N-methyl adenosine | Reduced urine concentration | 279 |
| Orotate | Reduced urine concentration | 74 |
| Orotic acid | Reduced urine concentration | 279 |
| Pantothenic acid | Elevated plasma concentration | 279 |
| Phenyl sulfate | Elevated plasma concentration | 279 |
| Phenylacetyl glycine | Elevated plasma concentration | 279 |
| p-Hydroxy phenyllactic acid | Elevated plasma concentration | 279 |
| Propionylglycine | Reduced urine concentration | 74 |
| Thymidine | Reduced urine concentration | 279 |
| Uracil | Reduced urine concentration | 74 |
| Urate | Decreased secretion/reduced urine conc. | 73, 279 |
| Xanthurenic acid | Reduced urine concentration | 279 |
| α-Ketoglutarate | Reduced plasma conc./elevated urine conc. | 74 |
FIGURE 4.
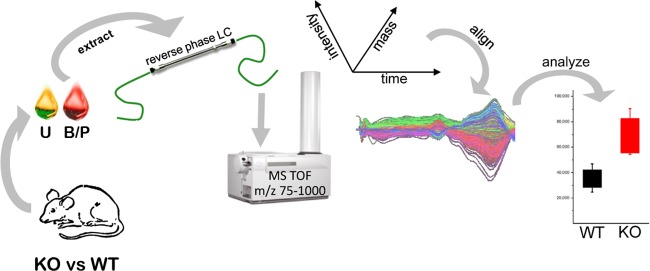
Strategy utilized for the en masse identification of endogenous OAT substrates. An untargeted metabolomics analysis strategy used to identify endogenous substrates of the Oats is depicted (279, 285). Samples of body fluids [e.g., blood/plasma (B/P; red) and urine (U; yellow)] were collected from wild-type (WT) and Oat-deficient (KO) mice, and extracts of these specimens were subjected to reverse-phase liquid chromatography (LC) followed by time-of-flight mass spectrophotometry (MS TOF) (279). LC-MS features were statistically ranked and aligned based on their mass, time of flight, and intensity (224). Metabolites with significant differences between WT and Oat-KO mice were identified by searching available metabolomics databases. The ability of some of the identified metabolites to interact with Oats was then validated in wet-lab functional assays (279, 285). [From Wikoff et al. (279). Copyright 2011 American Chemical Society.]
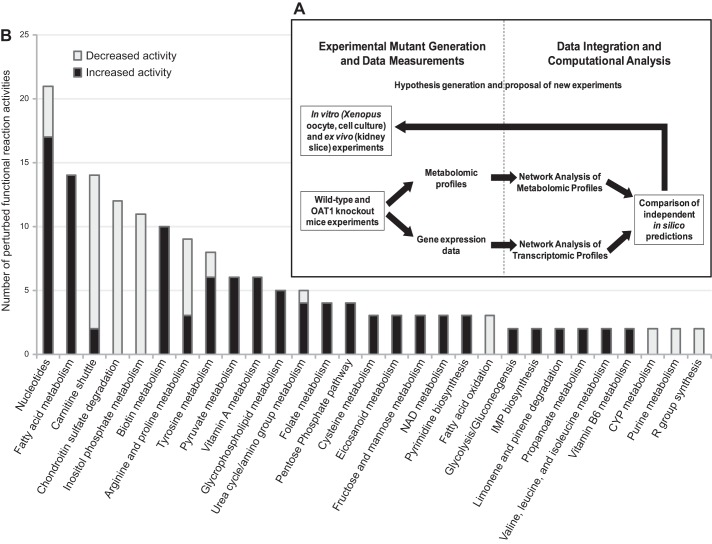
Systems biology analyses combining both transcriptomic and metabolomics data have been used to reconstruct metabolism that directly or indirectly depends on Oat1 (4). For example, computational integration of metabolomic and kidney transcriptomic data from wild-type and Oat1 knock-out animals contextualized changes in the concentration of pathway intermediates with alterations in the expression of pathway components and suggested previously undescribed linkages between the transporter and endogenous metabolic pathways, including the polyamine pathway (4). Wet-lab functional assays of Oat1-mediated transport of some of the pathway intermediates (e.g., arginine, spermidine, spermine) validated the computational findings (4). Among the Oats, Oat1 (as well as Oat3) has been highlighted by regulatory agencies as a key transporter involved in drug excretion and potential drug-drug interactions (DDI) (79, 163). The kinds of computational wet-lab studies described above also suggest a role for Oat1 in less-well understood drug-metabolite interactions (DMI).
C. OAT2/SLC22A7
As with NKT/Oat1/Slc22a6, what is now called Oat2 was originally identified as a novel liver transporter (NLT) (222). Indeed, as described above, it was the sequence relationships of NKT, NLT, and Oct1 that first enabled the proposal that these transporters were part of a larger family of transport proteins, now called SLC22 (144). Slc22a7 or Oat2 is a multispecific organic anion transporter with high expression in the liver and kidney (124, 129, 187). This transporter displays a much broader pattern of expression in the developing mouse embryo, where it is expressed in the lungs, developing bone/cartilage and kidney, as well as the liver (187). More recent RNA expression analyses of adult tissues have also demonstrated expression for Oat2 in several other tissues, including the lung, brain, small intestine, heart, and corneal epithelium of the eye (46, 48). Differences in Oat2 expression have also been shown to be dependent on age, sex, and species. For example, male rats display higher expression in the liver compared with kidneys, while female rats show higher kidney expression (138). In mice, however, Oat2 is predominantly expressed in the kidney of males, while female mice display similar levels of expression in both organs (125). Substrates for Oat2 include salicylate, acetylsalicylate, prostaglandin E2, dicarboxylates, glutamate, and PAH, as well as some antivirals (Table 1) (34). Importantly, Oat2 can also facilitate the transport of guanine nucleotide-related compounds and cGMP itself, which may be an important endogenous substrate (46). Thus Oat2 is one of several Oats (including Oat1) that are capable of transporting cyclic nucleotides and hence may play a modulatory role in intracellular signaling. This idea remains to be fully explored.
D. OAT3/SLC22A8
As with the case of NKT/Oat1 and with NLT/Oat2, Slc22a8/Oat3 was not initially referred to as an Oat, but was identified by Brady et al. (28) as Roct [reduced in osteosclerosis (oc) transporter] after observing reduced expression of the gene in the kidneys of mice homozygous for the osteosclerosis mutation. Nevertheless, the significance of this gene in bone biology awaits further study. Oat3 in adult animals is very highly expressed in the renal proximal tubule (100); however, unlike Oat1, it is also expressed and functional in the distal tubule (100, 250). Its physiological role in these nonproximal tubule segments requires more study. In addition to a proximal and distal tubule expression of Oat3 in the kidney, this transporter is also more broadly expressed than Oat1, with expression in, apart from the kidney, the choroid plexus, the brain capillary endothelium, and retina (Table 1).
In the retina, Oat3 is believed to be expressed in retinal vascular endothelial cells where it appears to be involved in the efflux of organic anions and drugs from the vitreous humor to the blood (96). Similarly, ex vivo functional assays using choroid plexus isolated from Oat3-deficient mice indicate that it is also involved in the movement of substrates from the CSF to the blood (170, 234, 236). Expression of Oat3 in brain capillary endothelium, where it also presumably functions as an efflux transporter (184, 261), has attracted recent interest, as well.
Although expression of Oat3 in the mouse kidney is largely undetectable before day 16 of gestation, during embryogenesis Oat3 is found in the liver and nervous system as early as day 14 (187). However, this embryonic liver and nervous system expression decreases after day 16, and Oat3 is virtually undetectable in the adult mouse liver (187). In contrast, the kidney-specific expression of Oat3 increases during gestation and after birth, similar to what is seen with Oat1. In the genome, as discussed below, OAT3 exists in tandem with OAT1, and indeed they are part of a large cluster of six human OAT-like genes (8 in mouse) (70, 71, 283).
The substrate specificity of Oat3 overlaps with Oat1; nevertheless, there are some substrates that clearly preferentially interact with either Oat1 or Oat3 (112, 285). Oat3 mediates the uptake of a wide array of small molecule anions including a large number of small molecule xenobiotics, endogenous metabolites such as conjugates of signaling sex steroids, as well as vitamins and other plant-derived metabolites (e.g., flavonoids) (285). In fact, most of the top 10 mass spectrometry features with a minimum of a 5-fold increase in plasma concentration in the Oat3 knockout mouse were associated with metabolites of plant origin, including multiple dietary phyto-phenolic metabolites (Table 3) (285). In addition, Oat3 also transports aristolochic acid and ochratoxin A and is thus thought to be important in the pathogenesis of Balkan Nephropathy (287). While the ability of Oat1 to transport cations is quite restricted, Oat3 can bind and transport a number of cations, some with ∼10-fold greater affinity than that seen with Oat1, even though it, like Oat1, is predominantly an organic anion transporter (3, 264). Presumably the ability of Oat3 to bind organic cations better than Oat1 is reflected in the nature of the ligand binding site, but this awaits three-dimensional structural determination.
Table 3.
Metabolites with altered levels in Oat3-deficient mice
| Metabolite | Phenotype | Reference Nos. |
|---|---|---|
| 2-Oxo-9-methylthionoanoic acid | Elevated plasma concentration | 285 |
| 4-Hydroxyphenylacetate | Reduced urinary concentration | 285 |
| 7-Methylguanosine | Elevated plasma concentration | 285 |
| 9-O-Acetylneuraminic acid | Elevated plasma concentration | 285 |
| Citrate | Reduced urinary concentration | 285 |
| Creatinine | Reduced renal secretion | 264 |
| Dehydroepiandrosterone sulfate | Delayed efflux from brain | 156 |
| Estrone sulfate | Increased plasma levels in female | 269 |
| Estrone sulfate | Delayed efflux from brain | 156 |
| Flavin mononucleotide (FMN) | Elevated plasma concentration | 265 |
| Hydantoin-5-propionic acid | Reduced plasma concentration | 285 |
| Palmitoyl serotonin | Reduced plasma concentration | 285 |
| Taurocholate | Reduced renal slice uptake | 234 |
| Thymidine | Elevated plasma concentration | 265 |
| Urate | Decreased secretion | 73 |
| Valine | Elevated urinary concentration | 285 |
| α-Ketoglutarate | Reduced urinary concentration | 285 |
Knockouts of Oat3 are the only Oat mutants that display a well-defined physiological phenotype, exhibiting lower systolic blood pressure suggesting that this transporter is involved in the uptake and clearance of endogenous blood pressure regulators (265). Metabolomics analyses identified several putative metabolites, including thymidine which was transported by Oat3 (but not Oat1) and reduced blood pressure in wild-type mice (265). The Oat3-deficient mice also display altered uric acid handling (73); poorer handling of antivirals (170, 250), penicillin (269), and methotrexate (271); and, importantly, an attenuated response to diuretics (74, 266). In addition, metabolic reconstruction of transcriptomic data derived from the kidneys of Oat3-null mice combined with untargeted metabolomics data from the blood and urine of these knockout mice also revealed a role for this transporter in several metabolic pathways, including the tricarboxylic acid cycle, nucleotide and amino acid metabolism, phase I and phase II xenobiotic metabolism (i.e., hydroxylation and glucuronidation), prostaglandin and steroid metabolism, as well as the metabolism of dietary flavonoids (285).
E. OAT4/SLC22A11
OAT4/SLC22A11, which was cloned from a human kidney library, is a human multispecific organic anion transporter with strong expression in the placenta and some expression in the kidney (38, 90). Potential substrates of OAT4 include sulfated steroids, NSAIDs, antihypertensives, prostaglandins, and uric acid (Table 1) (38, 82, 119, 241, 288). OAT4 has also been found to mediate the reabsorption of perfluorinated chemicals (along with URAT1) (289), man-made environmental contaminants of considerable current concern as they have been associated with toxic effects on a number of organ systems (303).
Similar to Oat1 (60), OAT4 function appears to be dependent on covalent posttranslational modifications. For example, the trafficking of OAT4 to the plasma membrane in transfected cells was found to be dependent on N-linked glycosylation, as well as interaction with PDZ scaffolding proteins (157, 305). Expression of OAT4 at the plasma membrane was also found to be regulated by progesterone, while protein kinase C (PKC) and the PDZ protein NHERF1 also modulate levels of OAT4 at the plasma membrane by regulating clathrin-mediated endocytosis of the transporter (60). Thus there appear to be a number of potential points of regulation by hormones and intracellular signaling.
In the placenta OAT4 has been localized to the syncytiotrophoblast cells (256) and is believed to mediate the clearance of sulfated steroids, such as dehydroepiandrosterone sulfate, from the fetal blood (197, 258, 259, 275, 305). Thus OAT4 is attracting interest because of its potential role in regulating the transport of hormones, drugs, and toxins across the maternal-fetal barrier (35). OAT4 is also expressed in the apical membrane of renal proximal tubular cells where it is believed to contribute to reabsorption of organic anions, including uric acid, from the urine back into proximal tubular cells (63, 203). Genome-wide association studies (GWAS) have associated SNPs in OAT4 with elevated levels of serum uric acid (130, 268, 291); in at least one case, a common SNP has been associated with gout due to renal under-excretion of uric acid (208). In contrast, transport assays employing a trophoblast-derived cell line (BeWo cells) which expresses OAT4, as well as a number of other transporters found in the syncytiotrophoblast, indicated that paracellular diffusion, rather than transport-mediated uptake, may play a key role in the transplacental movement of urate, suggesting that OAT4-mediated urate handling might be somewhat tissue-specific, although more study is needed (256).
F. Oat5/Slc22a19
As described above, the nomenclature of the Oats can be quite confusing, and this transporter is but one example. There have been two separate transporters given Oat5 as a designation, SLC22A10 and Slc22a19; despite the similar Oat designation, these genes are not orthologs (108, 123, 229). SLC22A10/OAT5 is human-specific and found to be expressed almost exclusively in embryonic and adult liver (72, 229), while Slc22a19/Oat5, a mouse gene, was found in the kidney (13, 295). Much of the investigation of these nonorthologous transporters has been done on mouse Oat5/Slc22a19; therefore, this is discussed here.
Oat5/Slc22a19 is preferentially expressed in the kidney where it is located on the apical surface of the proximal tubule cells, with stronger staining observed in the S3 and S2 segments (135). Similar to other Oats, expression of this transporter appears to be sex dependent, with female rodents displaying higher levels of the transporter apparently due to androgen (testosterone)-dependent downregulation of Oat5 (30). Although its in vivo role remains to be clarified, Oat5 can mediate the uptake of some common organic anion substrates in in vitro assays, including estrone sulfate, dehydroepiandrosterone sulfate, as well as ochratoxin A. However, some classic organic anion molecules do not seem to be good substrates for Oat5; neither PAH nor urate seems to be transported by Oat5 (13, 295).
G. OAT6/SLC22A20
Oat6 was initially identified in the mouse based on sequence homology to Oats (160); the existence of a human homolog has been described, although its functionality remains to be established (108). Expression of Oat6 is restricted, with strong expression observed in nasal epithelia (Figure 5) and weaker expression in testis (160, 212, 246). Similar to Oat1 (23), expression of Oat6 in rat olfactory epithelium can be induced by in vivo exposure to dexamethasone (247). In the testis, significant expression was found in Sertoli cells which comprise the blood-testis barrier, suggesting that Oat6 plays a role in the function of this barrier epithelium (211).
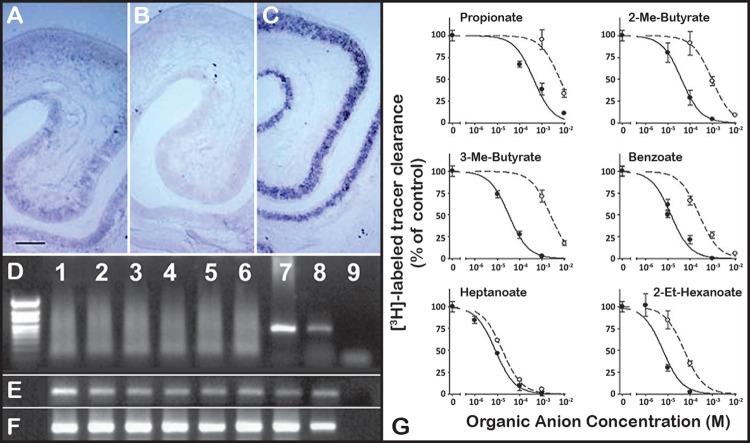
FIGURE 5.
Oat6 is expressed in olfactory epithelium. Oat6, initially identified using an in silico homology-based analysis of the Ensembl mouse genome database (160), was localized to the olfactory mucosa by in situ hybridization in coronal sections of nasal mucosa [A–C; anti-sense Oat6 (A), sense Oat6 (B), olfactory marker protein control (C); scale bar = 100 μm]. D–F: RT-PCR analysis of mouse nasal olfactory organs reveals epithelial expression of Oat6. D: Oat6 is expressed in whole main olfactory epithelium (MOE) (lane 7) and whole vomeronasal organ (VNO) (lane 8), but not in MOE sensory neurons (lanes 1–3) or VNO sensory neurons (lanes 4–6). (Lane 9, no template control.) E: β-tubulin control. F: olfactory marker protein control. G: dose-dependent inhibition of the uptake of labeled estrone sulfate by various odorant molecules in Xenopus oocytes microinjected with either Oat6 (solid line) or Oat1 (dashed line). [Modified from Kaler et al. (113), with permission from Elsevier.]
Oat6 can bind conjugated steroids and some drugs, but perhaps most interestingly, it can interact with volatile odorants (e.g., propionate, butyrate) (Figure 5), some of which were also found to accumulate in the Oat1 knockout (112, 113, 279). Thus certain volatile odorants that are normally eliminated in the urine via the Oat1 pathway have the potential to interact with Oat6. Although its exact role in the olfactory mucosa remains undefined, it has been suggested that Oat6 may somehow participate in olfactory odorant processing by recycling odorants for presentation to GPCRs or perhaps in transepithelial movement of odorants or other compounds either for the clearance of odorant molecules to maintain olfactory sensitivity or for transport into the central nervous system (112, 113, 160, 211, 246, 284). Since some of its odorant substrates accumulate in the Oat1 knockout mouse and are thus excreted in the urine, they could potentially be substrates of Oat6 (or Oat1, also in the olfactory mucosa) in another organism of the same species or another species (Figure 3). These intriguing aspects of Oat6 have been discussed in the context of interorganismal communication and the Remote Sensing and Signaling Hypothesis (see below) (5, 284).
H. OAT7/SLC22A9
OAT7/SLC22A9 is an apparently liver specific organic anion transporter (229); its gene product is located on the sinusoidal membrane of hepatocytes. As with other Oats, there is some nomenclature confusion about this Oat. SLC22A9/OAT7 is found in humans, and its ortholog is found in primates, but not in rodents. Although not well-studied, human OAT7 can mediate the uptake of some classical organic anion substrates such as estrone sulfate and dehydroepiandrosterone sulfate. Remarkably, neither PAH nor probenecid has been shown to effectively interact with OAT7 (34, 218).
i. rOat8/Slc22a9rat
The term rOat8 is used to describe a rat organic anion transporter (293). This transporter is also named as Ust1r/Slc22a9 (293). rOat8 mRNA is detectable in proximal tubules and possibly collecting ducts. By sequence homology, rOat8/Slc22a9rat is a homolog of mouse and rat Slc22a19/Oat5 (293).
J. Oat9/Slc22a27
Oat9/Slc22a27, an organic anion transporter located on mouse chromosome 19, was initially reported as a part of a mouse-specific gene amplification in a cluster of organic anion transporters (283). The gene product of Slc22a27 was later called Oat9, and while limited studies have been performed regarding its substrate specificity, it appears capable of transporting carnitine that can be inhibited by estrone sulfate but not by PAH or probenecid (254).
K. OAT10/SLC22A13
OAT10/SLC22A13 was originally identified as organic cation transporter-like 3 (ORCTL3) of the SLC22 family due to its shared homology with Oct1 and NKT (178). It was later renamed OAT10 to reflect its high-affinity uptake of nicotine as well as low-affinity uptake of uric acid when heterogeneously expressed in Xenopus oocytes and Caco2 cells (10, 22, 34). Transcripts of OAT10/SLC22A13 are broadly distributed, with higher expression observed in kidney, small intestine, and colon. Its gene product is found in the apical membrane of proximal tubule cells. Gender preferential expression of this gene has been noted with higher expression observed in female kidneys (22).
L. URAT1/SLC22A12
What is now called URAT1 in humans was originally identified as Rst (renal specific transporter) in mice (161). This organic anion transporter of the SLC22 gene family is closely related to Oat1, Oat3, and Oat6, and it is paired with OAT4 in the genome (70). Knockouts of Urat1 (Rst), as well as knockouts of Oat1 and Oat3, have alterations in urate handling (73, 97). Genetic variations of URAT1 have been identified as determinants of human urate handling anomalies of hyperuricemia and hypouricemia (67). Although, as mentioned, the Urat1 (Rst) knockout has a defect in urate handling, it is modest, and at the time, it was suggested that other genes must be important (73). Since then, a number of other SLC and ABC transporter genes have been found to be involved in urate handling and implicated in human syndromes affecting uric acid levels. These have been reviewed extensively elsewhere (7, 12, 166, 281, 284), so we limit the discussion here. At present, it is unclear how many transporters regulate uric acid in vivo and which are most important in human syndromes affecting uric acid. In general, there is a growing appreciation of SNPs in transporters other than URAT1 in common human hyperuricemic syndromes (165, 281, 284). While URAT1 clearly does transport uric acid, because it is so closely related to Oat1 and Oat3, it is worth reevaluating its functional similarities to these other Oats.
M. Other Oats in Rodents
Among the currently 10 “named” organic anion transporters (OAT1-OAT10), there are at least 3 transporter genes that only exist in rodents (Table 1). For example, Oat5/Slc22a19 was initially characterized in the rodents, and although it shares a high level of sequence identity with human SLC22A9 and they are homologous to each other, they are not orthologs. On the other hand, Oat6/Slc22a20 has a human ortholog in SLC22A20 (108), but the sequence similarity between them possibly only extends to two-thirds of the 5' coding region, at least as suggested by complementary cDNA clones.
Another named Oat that has no human ortholog is Oat9/Slc22a27 (283), which is part of a large cluster of amplified transporter genes that includes a total of eight Slc22 family members on mouse chromosome 19q (discussed in sect. VIB). These eight Slc22 family genes are, in order from the centromere, Oat5/Slc22a19, Slc22a26/BC014805, Oat9/Slc22a27/AB056442, Slc22a28/EG43674, Slc22a29/D630002G06Rik, Slc22a30/C730048C13Rik, Oat3/Slc22a8, and Oat1/Slc22a6 (Figure 6) (283). The substrates for some of these transporters have not been defined, but based on their sequence similarity, and the fact that each contains a full-length coding region, it would not be surprising if most, if not all, of these transporters can handle small molecule organic anions.
FIGURE 6.
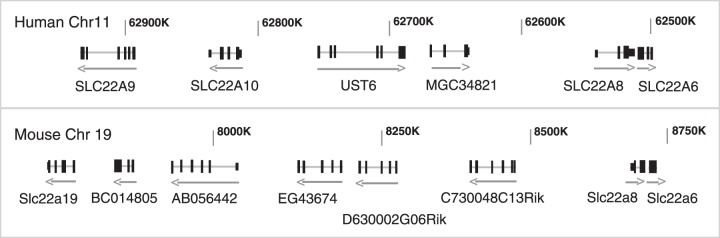
Chromosomal clustering of members of the SLC22 family of genes. The discovery of the organic anion transporters allowed for the chromosomal mapping of their genes, and many of the Oats were found to exist in pairs and/or clusters (70), which was also found to be true for Octs and Oatps (70, 205). For example, OAT1 and OAT3 were found to exist as a tandem repeat with no other genes between them on human chromosome 11, as well as on mouse chromosome 19. Subsequent sequence analysis of adjacent areas of these chromosomes identified additional transporters clustered together with OAT1 (SLC22A6) and OAT3 (SLC22A8) on both the human and mouse chromosomes (283). The figure depicts the organization of this SLC22 organic anion transporter-containing cluster on human chromosome 11 (top) and the corresponding region on mouse chromosome 19 (bottom). The significance of this genomic clustering remains to be clarified. [Modified from Wu et al. (283).]
N. Oat-PG/Slc22a22
Among rodent Oat genes without a clear human ortholog is Oat-PG/Slc22a22 on mouse chromosome 15q. Mouse Oat-PG has high affinity for prostaglandin E2 (PGE2) and is preferentially expressed on the basolateral membrane of renal proximal tubules (219). Similarly, an Oat-PG ortholog has been reported in rat, also with high affinity for PGE2, and with strong expression in renal cortex. In addition, stronger expression of rat Oat-PG is found in the kidney of adult male versus female rats (88), which is thought to be regulated by glucocorticoids in a mechanism distinct from that regulating the male-dominant expression of Oat1 and Oat3, which involves testosterone and BCL6 (87, 139, 278). Oat-PG is one of several Oats that appear capable of transporting prostaglandins (219), and its expression in the kidney appears to be the primary determinant of PGE2 concentration in the renal cortex (88), which plays an important role in a number of renal functions, including maintenance of glomerular filtration rate (GFR) (84). GFR increases during pregnancy, and the concentration of PGE2 was also found to increase in the kidneys of gestating animals, while the renal expression of Oat-PG is reduced (116). Studies suggest that pregnancy-induced increases in GFR are due to increased levels of PGE2 resulting from reduced clearance of this prostaglandin due to downregulation of Oat-PG expression, perhaps because of increased levels of estrogen and progesterone during pregnancy (116).
IV. ORGANIC ANIONS HANDLED BY OATS
As a major purpose of this review is to discuss emerging concepts related to the endogenous function of Oats in signaling and metabolism, here we discuss some areas in which Oats may play important roles independent of their roles in drug and toxin handling with an emphasis on the categories of signaling molecules and key metabolites transported by Oats.
A. Odorants
When the unusual localization of Oat6 was noticed, it was also noted that several volatile organic anion odorants [including those that were later shown to bind Oat6 (Figure 5)] accumulated in the body fluids of the Oat1 knockout, presumably due to the lack of this transporter (74, 279). Oat6 tends to interact with small mono-anions, and its substrates include propionate, benzoate, heptanoate, and other odorants, some of which have up to a 70-fold higher affinity for Oat6 versus Oat1 (112). Nevertheless, these data come mainly from binding studies rather than actual transport in Oat6-expression systems. The location of Oat6 expression as well as the set of molecules it can interact with raised the speculation that Oat6 might mediate substrate interaction “remotely” between organisms (112, 113, 250) (Figures 2 and 3). Thus one envisions that organic anion odorants of importance in interorganismal and interspecies communication might be excreted into the urine by one animal, become volatile, and interact with either odorant G protein-coupled olfactory receptors or be transported by Oat6 (5, 284). Such a mechanism could play a role via the sensing and signaling communication between individuals of the same species (male-female; mother-offspring) as well as different species (predator-prey). It is to be emphasized that this is highly speculative. Estrone sulfate is also an Oat6 substrate, and sex steroids in the urine may be transported from the olfactory apparatus in the CNS (154, 180). It is not clear whether Oat6 is on the apical or basolateral surface of olfactory mucosa, whether it is involved in transepithelial transport or recycling of odorants, or whether it is also expressed in neurons. Nevertheless, based on its localization and putative transport function, it has been suggested that Oat6 could modulate the bioavailability of the odorant stimulus to olfactory neurons (246). Whether it is involved in transport of drugs across the nose-brain barrier has not been determined. It is also worth noting that there are many so-called odorant receptors in non-olfactory tissue, including the kidney (190, 302). Odorants transported by Oats (e.g., Oat1 in the kidney and other tissues as well as Oat6 in the testes) might somehow interact with these non-olfactory odorant receptors, but this remains to be shown. It is also worth emphasizing that Oat1, as well as other SLC22 transporters and ABC transporters, are present in olfactory epithelia (160).
B. Cyclic Nucleotides
Among the best substrates in vitro for Oat2 is cGMP (46). Indeed, it has long been known that Oat1 and Oat3 are able to transport cyclic nucleotides. The extent to which these and other Oats regulate intracellular cyclic nucleotide concentrations, and thereby potentially regulate a myriad of signaling events, is largely unexplored. Given that certain Oats (e.g., Oat3) are expressed in endothelial cells where cyclic nucleotides regulate vascular tone (96, 132), and since the Oat3 knockout has a reduced blood pressure (265), the role of various Oats in modulating the cellular and systemic effects of cyclic nucleotide levels in various tissues and body fluids seems to demand further study.
C. Prostaglandins
Among the best sets of substrates for a number of Oats are prostaglandins and related molecules (284). Indeed, Oat-PG appears to be highly specific for prostaglandin substrates (219). How Oat isoforms that are expressed in various epithelial cells throughout the body regulate local concentrations of prostaglandins, and thereby signaling events in different tissues and body fluid compartments, remains to be addressed at the physiological level. Nevertheless, there is evidence of a role for Oat3 in regulating the concentrations of prostaglandins in the cerebrospinal fluid. For example, Oat3 expressed in the choroid plexus has been proposed to act as a cerebral clearance pathway for both PGE2 (238) and PGD2 (239). This may be interesting in light of the proposed role of these prostaglandins in the regulation of CNS physiology. For example, PGE2 has been demonstrated to play a key role in modulating wakefulness (152, 242), while PGD2 has a role in promoting physiological sleep (99). Thus Oat3-mediated uptake and clearance of these prostaglandins from the CSF could conceivably modulate sleep patterns. Therefore, given the high affinity of many Oat isoforms for prostaglandins and the localization of distinct sets of Oats to particular tissues (e.g., choroid plexus for uptake and clearance from the CSF, kidney for uptake and clearance from the blood), this might prove a fruitful area of future research for understanding how the levels of prostaglandins and prostaglandin-like molecules are modulated in specific tissues and body fluids, thereby playing a role in the regulation of complex physiological processes (50, 85, 209).
D. Conjugated Sex Steroids
Estrone-sulfate has, in vitro and to some extent ex vivo, proven to be a “prototypical” substrate for Oat3, OAT4, and Oat6 (35, 211). This is in distinction to, for instance, Oat1, which seems to have a lesser preference for this sulfated sex steroid. Moreover, other conjugated estrogens, such as estrogen-glucuronides, are excellent Oat substrates (35, 270). The implications for a transporter like OAT4, which is highly expressed in the placenta, could be quite important in the context of maternal-fetal communication. However, it is also possible that certain Oats modulate the entry into and/or the exit from many different cells of various conjugated estrogens and perhaps other steroids. This may be related to the different patterns of expression of certain Oats in males and females. Nevertheless, the extent to which such an Oat-mediated mechanism actually affects nuclear receptors that regulate transcription is unclear. Intracellular enzymatic reactions could conceivably “deconjugate” the imported conjugated steroids, adding another layer of complexity to regulation (196).
E. Gut Microbiome Metabolites, Uremic Toxins, Vitamin-Related Metabolites, Dietary Compounds, and Antioxidants
One of the important findings from untargeted metabolomics studies of the Oat1 knockout was the accumulation of a number of gut microbiome metabolites and metabolites modified by phase I and phase II drug metabolizing enzymes (DMEs) (279, 284). These included many so-called uremic toxins of CKD such as indoxyl sulfate, p-cresol sulfate, kynurenine, hippurate, and others (Tables 2 and 4). Also found to accumulate in the Oat knockouts were compounds in vitamin-related metabolism (e.g., pantothenic acid) and dietary compounds with antioxidant properties (e.g., flavonoids) (Tables 2–5). Other studies have also indicated a role in folate transport (74). Levels of uric acid, thought to function as an antioxidant, were altered in the Oat1, Oat3, and Urat1 (originally Rst) knockout mice (73). Creatinine is an in vitro and in vivo substrate of Oat3 (103, 264). Oat1 may also play some role in creatinine secretion, but the data appear less strong compared with Oat3. The extent to which SNPs in OATs and other SLC22 transporters modulate creatinine levels in humans is currently unclear.
Table 4.
List of Oat1 metabolites with kinetic data
| Oat1 Metabolite | Metabolic Subsystem | Km, μM | Ki, μM | IC50, μM (Substrate) | Reference Nos. |
|---|---|---|---|---|---|
| 1,3,7-Trimethyluric acid | Purine, caffeine | 3.9 | 3.9 (0.24 PAH) | 227 | |
| 1,3-Dimethyluric acid | Purine, caffeine | 9.2 | 9.2 (0.24 PAH) | 227 | |
| 1,7-Dimethyluric acid | Purine, caffeine | 15 | 15.0 (0.24 PAH) | 227 | |
| 1,7-Dimethylxanthine | Purine, caffeine | 8.3 | 8.4 (0.24 PAH) | 227 | |
| 17β-Estradiol-d-17β-glucuronide | Steroid | >300 | 228 | ||
| 1-Methyluric acid | Purine, caffeine | 77 | 79.4 (0.24 PAH) | 227 | |
| 1-Methylxanthine | Purine, caffeine | 10 | 10.3 (0.24 PAH) | 227 | |
| 2-Methylbutyrate | Amino acid | 909 | 920 (0.238 PAH) | 113 | |
| 3,4-Dihydroxymandelic acid | Tyrosine | 872 | 1,090 (5 PAH) | 8 | |
| 3,4-Dihydroxyphenylacetic acid | Tyrosine | 560 (5 PAH) | 8 | ||
| 3-Carboxy-4-methyl-5-propyl-2-furanpropionate | Furan fatty acids | 85 | 240 | ||
| 3-Hydroxybutyrate | Ketone body | 3220 | 112 | ||
| 3-Hydroxyglutarate | Lysine, tryptophan | 98 | 81 | ||
| 3-Methylxanthine | Purine, caffeine | 178.6 (0.238 PAH) | 227 | ||
| 4-Hydroxyphenyllactate | Tyrosine | 223 | 112 | ||
| 4-Hydroxyphenylpyruvate | Tyrosine | 73 | 112 | ||
| 5-Hydroxyindole −3-acetate | Tryptophan | 110 (5 PAH) | 8 | ||
| 5-Methoxyindole-3-acetic acid | Tryptophan | 30 (5 PAH) | 8 | ||
| 5-Methoxytryptamine | Tryptophan | 1,038 (5 PAH) | 8 | ||
| 5-Methoxytryptophol | Tryptophan | <1600 | <2,000 (5 PAH) | 8 | |
| 7-Methylxanthine | Purine, caffeine | 122 (0.24 PAH) | 227 | ||
| β-Hydroxybutyrate | Butanoate | 1,023 | 8,700 (30 6-CF) | 4 | |
| Butyrate | Butanoate | 3,500 | 112 | ||
| Citrulline | Amino acid | 238 | 171 | ||
| d-2-Hydroxyglutarate | Butanoate | 369 | 81 | ||
| Dehydroepiandrosterone sulfate | Steroid | 80.9 | 86 | ||
| Edaravone sulfate | Xenobiotic | 10.8 | 158 | ||
| Estradiol disulfate | Steroid | 220 | 112 | ||
| Estrone sulfate | Steroid | 50.1 | 203 (0.238 PAH) | 86 | |
| Fumarate | TCA cycle | 610 | 112 | ||
| Glutarate | Pentose phosphate | 4.9 | 10.7 (4 6-CF) | 44 | |
| Hcy-s-Hg-s-Hcy | Xenobiotic | 128 | 299 | ||
| Hippurate | Xenobiotic | 23.5 | 18.8 | 52 | |
| Homovanillic acid | Tyrosine | 65 (5 PAH) | 8 | ||
| Iindoleacetate | Xenobiotic, tryptophan | 14 | 21 | 52 | |
| Indoxyl sulfate | Xenobiotic, tryptophan | 20.5 | 13.2 | 52 | |
| Kynurenate | Tryptophan | 34 (5 6-CF) | 24 | ||
| Kynurenine | Tryptophan | 1.4 | 12 (30 6-CF) | 279 | |
| l-2-Hydroxyglutarate | Butanoate | 748 | 81 | ||
| Loxoprofen trans-OH metabolite | Xenobiotic | 12.2 (0.5 MTX) | 263 | ||
| MeHg-2,3-dimercapto-1-propanesulfonic acid | Xenobiotic | 9 | 128 | ||
| MeHg-N-acetyl-l-cysteine | Xenobiotic | 31 | 128 | ||
| Methylmercury | Xenobiotic | 39.1 | 298 | ||
| Mycophenolic acid glucuronide | Xenobiotic | 512.3 (5 PAH) | 262 | ||
| N-acetyl-5-hydroxytryptamine | Neurotransmitter, tryptophan | 440 (5 PAH) | 8 | ||
| N-acetyl-aspartate | Amino acid | 840 | 112 | ||
| N-acetyl-l-cysteine-Hg2 | Xenobiotic | 44 | 20 | ||
| N-acetyl-leukotriene E4 | Arachidonic acid | 9 | 192 | ||
| Octanoate | Fatty acid | 5.41 | 111 | ||
| o-Hydroxyhippuric acid | Xenobiotic | 27 (10 PAH) | 164 | ||
| Phenyl-pyruvate | Amino acid | 79 | 112 | ||
| Propionate | Propanoate | 8,083 | 8180 (0.238 PAH) | 113 | |
| Prostaglandin E2 | Arachidonic acid | 0.97 | 119 | ||
| Pyruvate | Energy | 1,720 | 4,300 (5 6-CF) | 4 | |
| Salicylurate | Xenobiotic | 11 | 17 | ||
| Spermidine | Amino acid | 235 | 2,000 (30 6-CF) | 4 | |
| Spermine | Amino acid | 188 | 1,600 (30 6-CF) | 4 | |
| Urate | Purine | 304 | 312.5 (0.24 PAH) | 227 | |
| Vanilmandelic acid | Tyrosine | 70 (5 PAH) | 8 | ||
| Xanthine | Purine, caffeine | 238 | 243.9 (0.24 PAH) | 227 | |
| Xanthurenate | Tryptophan | 15 (5 6-CF) | 24 | ||
| Xanthurenate | Tryptophan | 6 | 50 (30 6CF) | 279 |
Table 5.
List of Oat3 metabolites with kinetic data
| Oat3 Metabolite | Metabolic Subsystem | Km, μM | Ki, μM | IC50, μM (Substrate) | Reference Nos. |
|---|---|---|---|---|---|
| 3,4-Dihydroxyphenylacetic acid | Tyrosine | 980 | 990 (0.050 ES | 8 | |
| 3-Carboxy-4-methyl-5-propyl-2-furanpropionate | Furan fatty acids | 6.43 | 27.9 | 52 | |
| 5-Hydroxyindole-3-acetic acid | Tryptophan | 901 | 910 (0.050 ES) | 8 | |
| 5-Methoxyindole-3-acetic acid | Tryptophan | 69 | 70 (0.050 ES) | 8 | |
| 5-Methoxytryptamine | Tryptophan | 604 | 610 (0.050 ES) | 8 | |
| 5-Methoxytryptophol | Tryptophan | 485 | 490 (0.050 ES) | 8 | |
| Chenodeoxycholic acid | Bile acid | 33.5 | 41 | ||
| Cholic acid | Bile acid | 230 | 41 | ||
| Cortisol | Steroid | 2.4 | 19 | ||
| Deoxycholic acid | Bile acid | 72.7 | 41 | ||
| Dehydroepiandrosterone sulfate | Steroid | 12.9 | 182 | ||
| Estrone sulfate | Steroid | 6.3 | 257 | ||
| Glycochenodeoxycholic acid | Bile acid | 54.1 | 41 | ||
| Glycocholic acid | Bile acid | 203 | 41 | ||
| Hippurate | Xenobiotic, phenylalanine | 18 | 11.9 (2 IS) | 8 | |
| Homovanillic acid | Tyrosine | 274 | 162 | ||
| Indoleacetate | Xenobiotic, tryptophan | 582 | 509 (2 IS) | 52 | |
| N-acetyl-5-hydroxytryptamine | Neurotransmitter, tryptophan | 485 | 490 (0.050 ES) | 8 | |
| Octanoate | Fatty acid | 8.6 | 111 | ||
| Prostaglandin E2 | Arachidonic acid | 0.345 | 119 | ||
| Prostaglandin F2α | Arachidonic acid | 1.092 | 119 | ||
| Taurochenodeoxycholic acid | Bile acid | 207 | 41 | ||
| Taurocholate | Bile acid | 882 | 41 | ||
| Urate | Purine | 287 | 290 (0.050 ES) | 270 | |
| Vanillylmandelic acid | Catecholamines | 1,228 | 1,240 (0.050 ES) | 8 | |
| Xanthurenate | Tryptophan | 8 | 11.5 (5 6-CF) | 270 |
V. PHYSIOLOGICAL ROLES OF OATS
A. Connections Between the Oat Pathway and Phase I and Phase II DMEs
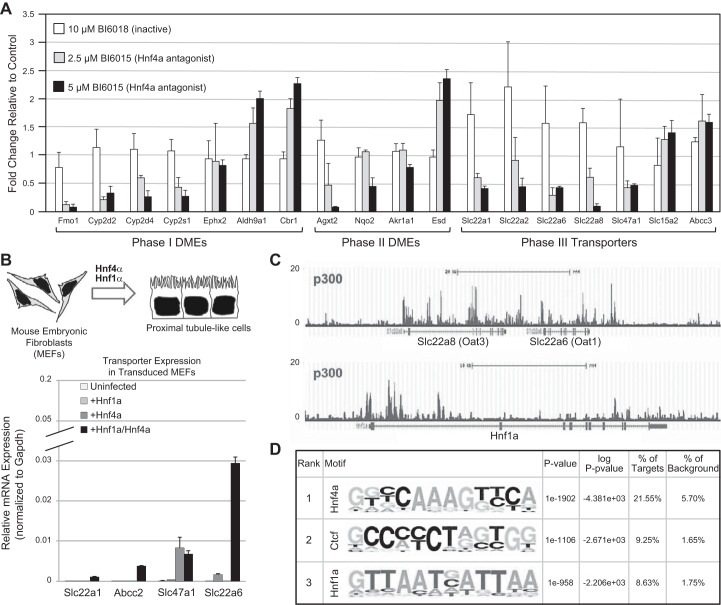
Metabolomics studies in the knockouts also provide support for the connections of Oats with phase I (e.g., introduction of polar groups) and phase II (e.g., sulfation, glucuronidation) DME pathways. While there had already been in vitro evidence for the role of Oats, particularly Oat3 and Oat1, in the transport of sulfated and glucuronidated substrates, these were also among the major metabolites (among many others) found in the Oat1 and Oat3 knockouts (265, 279, 285). The combined in vitro and in vivo data demonstrated that these transporters are intimately connected to phase I and phase II metabolism and, indeed, are a major mechanism for the distribution and elimination of metabolites altered by phase I and phase II processes (285). This area requires further exploration. In this regard, it is interesting to note that Oats are regulated by some of the same transcription factors (e.g., Hnf4α) as other DMEs (149). For example, treatment of whole embryonic kidney cultures with an Hnf4 antagonist not only perturbed the expression of a number of DMEs, but it also altered the expression of several SLC transporters, including Oat1 and Oat3 (Figure 7) (149). Furthermore, overexpression of both Hnf1α and Hnf4α in primary embryonic mouse fibroblasts not only induced the expression of phase I and phase II DMEs as well as transporters (Figure 7), but it also induced the probenecid-inhibitable uptake of organic anions (149). Systems biology analysis also implicated, in addition to Hnf4α and Hnf1α, other transcription factors in the regulation of phase I, phase II, and phase III (transporters) DMEs in the proximal tubule (149).
FIGURE 7.
Hnf4α and Hnf1α, regulate drug transporter expression in the developing kidney. A: bar graph demonstrating the changes in the expression of phase I and phase II DMEs, as well as phase III transporters in whole embryonic rat kidneys cultured in the presence of a small molecule antagonist of Hnf4α (120). B, top: viral transduction of mouse embryonic fibroblasts (MEFs) with both Hnf1α and Hnf4α leads to the formation of cells with a proximal tubule-like character. B, bottom: qPCR analysis of MEFs virally transduced with Hnf1α, Hnf4α, or both revealed highest expression of transporter genes when both Hnf1α and Hnf4α are present. C: screenshots of p300 ChIP-seq in adult kidney cortex. P300 binding sites are highly enriched in Oat1 and Oat3, as well as in in the Hnf1α locus. D: two of the most highly enriched transcription factor binding motifs were Hnf4α and Hnf1α. [Modified from Martovetsky et al. (149), with permission from ASPET.]
B. Maternal-Fetal and Maternal-Neonatal Communication
Relatively little is known about transport via Oats across the maternal-fetal barrier. Of particular interest is the high expression of OAT4, which can transport conjugated sex steroids, drugs, and toxins (35). One of the important underexplored questions is whether the embryonically expressed Oats, such as Oat1 and Oat3, can transport drugs, toxins, metabolites, and signaling molecules that cross the maternal-fetal barrier by OAT4 or other placental transporters (176, 187, 283). Transporter-mediated small molecule communication may occur in both directions across the placenta. This would have potentially important clinical applications and is of obvious relevance in the context of the Remote Sensing and Signaling Hypothesis (5, 283). Even less is known about the role of Oats in maternal-neonate communication via breast milk, which is the neonate's primary source of carnitine, necessary for beta oxidation of fatty acids. This appears primarily mediated by carnitine transporters, including Octn1, Octn2, and possibly other carnitine transporters (284). Organic cation transporters are also expressed in mammary gland (106), but Oat expression appears to be comparatively low. Thus it is not clear to what extent Oats, as opposed to other transporters of organic anions and zwitterions, are involved in the transport of metabolites, drugs, and toxins into breast milk.
VI. RECENT ADVANCES IN OAT RESEARCH
A. Substrate Modeling and Transporter Modeling
Several computational chemistry approaches have been used to study Oats and their substrates. In general, there are two basic approaches, the transporter protein-based approach and the ligand-based approach (3, 57, 112, 131, 250, 251, 253, 279).
The protein-based approach attempts to recapitulate the three-dimensional structure of the transporters themselves. Unfortunately, there is very little detailed structural information on mammalian SLC22 transporters. Nevertheless, homology-based modeling using the crystal structure of glycerol-3-phosphate transporter (GlpT) as a template has been used to construct a human OAT1 structure model (Figure 8A) (251). With this model, a putative active site, positioned on a central cavity and created by an angled juxtaposition of two human OAT1 hemidomains spanning the plasma membrane (such that the extracellular aspects of the hemidomains are in close approximation), was proposed to be the main site for the substrate-transporter interaction (253). A 100-ns in silico simulation suggested that opening of the central cavity to the extracellular milieu was accomplished by a tilting of the two hemidomains of human OAT1 such that the intracellular aspects moved towards each other causing the extracellular portions of the hemidomains to move apart so that substrates could enter the central cavity of the transporter (253). Other static models have helped reconcile site-directed mutagenesis data with the putative Oat1 structure (188).
FIGURE 8.
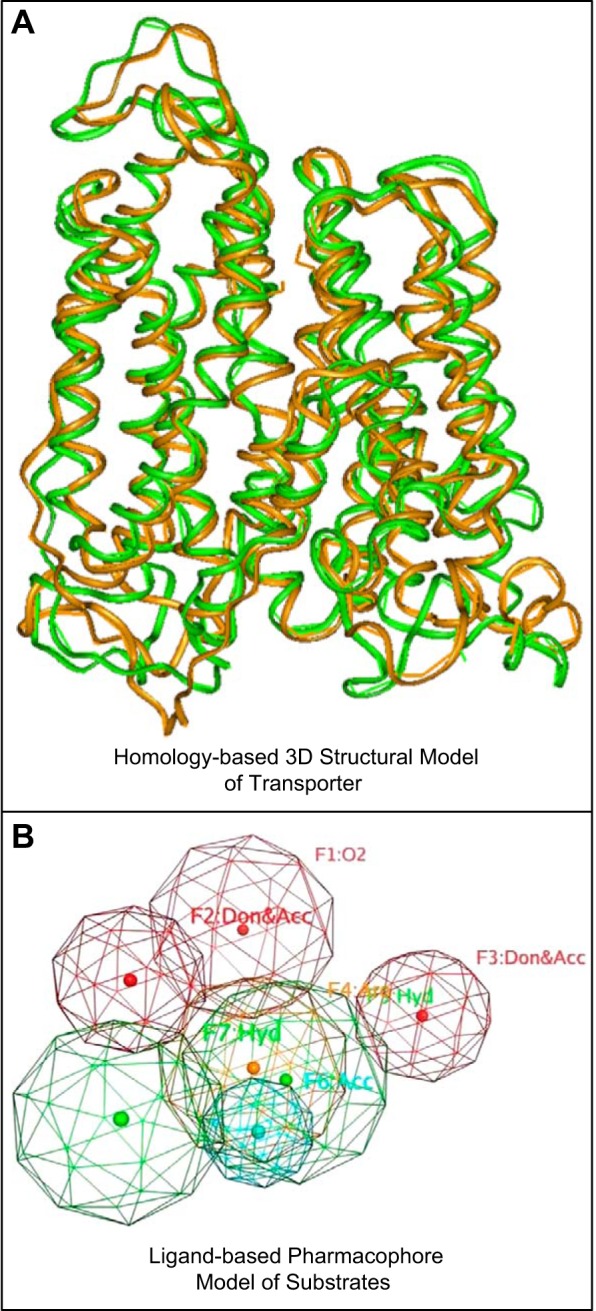
A: molecular dynamic simulation of Oat1-mediated transport. Ribbon diagram of superimposed Oat1 structural conformers obtained at 40 ns (brown) and 94 ns (green) of a ∼100-ns molecular dynamic transport simulation (253). The transport simulation was performed on a homology-based computational model of Oat1, and the superimposition of the structural conformers allowed for visualization of Oat1 movements during the intial stages of substrate transport. Alterations in the distances between amino acid residues suggested that the early stages of Oat1-mediated transport were characterized by opening of the extracellular portion of the transporter allowing substrates access to a transporter channel. [Modified from Tsigelny et al. (253), with permission from Springer Science and Business Media.] B: pharmacophore modeling of Oat1 substrates. A pharmacophore model based on the 3-dimensional chemical structures common to certain Oat1 substrates. Colored spheres represent various structural features of the pharmacophore [e.g., hydrophobic (green), aromatic (orange), and hydrogen-bond acceptor (red)]. Such a model can be used to virtually screen chemical libraries for potential novel substrates which can then be validated in transport assays. [Modified from Wikoff et al. (279) Copyright 2011 American Chemical Society.]
Ligand-based modeling, on the other hand, is aimed at identification of chemical features common to transporter substrates; these are then used to generate pharmacophore models (Figure 8B) (3, 57, 112, 131, 250, 279). Employing this type of approach, Oat1- and Oat3-selective pharmacophore models have been created and used to virtually screen chemical libraries (57, 112, 131).
Here we discuss an interesting example related to Oat3 substrates. The possibility that Oats could bind anions or cations was suggested with the original discovery of Oat1 as NKT (144). To investigate this, an Oat3-specific pharmacophore model was built using chemical features common to several organic cationic drugs found to be capable of high-affinity binding to Oat3, and included hydrogen bond acceptor features, a hydrophobic core feature and a positive ionizable feature (3). Finally, this pharmacophore was used in a virtual screen of a chemical database which identified novel cationic molecules with the potential to interact with Oat3. Some of these molecules inhibited substrate-Oat3 interactions in wet-lab assays (3). These studies support the view that, even though Oats share many structural similarities, their binding domains are likely to enable differential binding of substrates (3).
Other studies of substrate characteristics have employed quantitative structure-activity relationship (QSAR)-based approaches to analyze Oat1, Oat3, and Oat6 substrate specificity (112, 250). Instead of analyzing the structure of ligands as a whole group, QSAR analysis focuses on finding individual physiochemical properties of ligands one by one and discovering the correlations between these molecular, atomic properties, and the substrate affinity.
One of the current questions relates to understanding how the various modeling approaches and platforms relate to each other.
B. Evolution and Clustering in the Genome
Organic anion transporters belong to a SLC22 subfamily that is a part of a large solute carrier family of transmembrane proteins (70, 144). Similar to other SLC transporter subfamilies, many members of the SLC22 family, such as OAT1 and OAT3, are highly conserved, and their orthologs can often be found in most vertebrate species as well as fly and worm (70, 283). A typical organic anion transporter gene is usually transcribed to a gene product of ∼550 amino acids for a full-length transporter with 12-transmembrane helixes (Figure 1). Sequence and phylogenetic tree analyses suggest that the 12-transmembrane domains of MFS transporters are usually comprised of 2 halves of 6-transmembrane segments, each of which are thought to have originated from a multiplication of a two-transmembrane core structure (199).
Some of the Slc22 family organic anion transporters have an extremely high percentage of sequence identity. For example, as described earlier, there are four mouse Slc22 family transporters on mouse chromosome 19, Oat9/Slc22a27/AB056442, Slc22a28/EG43674, Slc22a29/D630002G06Rik, and Slc22a30/C730048C13Rik (Figure 6). When their coding regions were compared, at least 81% of the amino acids were conserved among all four transporters (283). Furthermore, there is 95% amino acid sequence identity between AB056442 and D630002G06Rik, and 97% amino acid sequence identity between EG43674 and C730048C13Rik. This high percentage of sequence identity suggests that these four genes are likely to have been generated from recent sequential gene duplication events (Figure 9) (283). Thus, at least in the mouse genome, Oats may still be under active selection. If so, it will be very important to try to attempt to understand the driving forces.
FIGURE 9.
Phylogeny of human and mouse Oats and Usts. Slc22a6 (Oat1) and Slc22a8 (Oat3), both of which exist in mouse and human, appear to have arisen from tandem duplication of an Oat ancestral gene. Multiple rounds of duplication of the Ust (unknown substrate transporter) ancestral gene are proposed to have led to several Ust-like genes by the time of human and mouse divergence. After this, certain mouse Usts are proposed to have undergone further duplication to generate other mouse-specific Ust genes. Some Usts exist in human while others exist in mouse, but no orthologous Usts exist in both species. [Modified from Wu et al. (283).]
These four novel genes are part of a larger cluster of genes that are all members of the Slc22 family. Flanking these four novel transporter genes are Oat1/Slc22a6 and Oat3/Slc22a8 on one side, and Oat5/Slc22a19 on the other. As described above, these three transporters share some substrate specificity, and sequence analysis indicates that all of the transporter genes in the cluster (spanning about 1 Mb) share a high degree of sequence similarity (283), raising the possibility that these novel transporters may also be able to handle small molecule organic anions similar to that of Oat1, Oat3, and Oat5 (70, 283).
A similar cluster of genes related to organic anion transporters by sequence comparisons on (human) chromosome 11 not only includes the prototypical OATs of OAT1/SLC22A6 and OAT3/SLC22A8, it also includes SLC22A9, SLC22A10, UST6/SLC22A25 (unknown substrate transporter 6), and MGC34821/SLC22A24. MGC34821 is a SLC22 family gene based on sequence similarity and encodes a 322-amino acid COOH-terminal truncated gene product. Similar to its mouse cluster counterpart, these human genes in the cluster are highly similar.
However, it appears that only the OAT1 and OAT3 genes have clear orthologs between human and mouse: the other genes in the two clusters are homologous but do not appear to be orthologous by usual sequence comparisons. It has thus been suggested that the non-orthologous transporter genes in the clusters evolved independently, after mouse and human speciation (283).
C. Epigenetic Regulation
Epigenetic regulatory mechanisms are dynamic, potentially inheritable, processes which alter transcriptional activity without affecting DNA sequence and represent a key mechanism for the response to environmental and other changes (109). These processes are broadly construed to include covalent modifications of DNA and histones, chromatin folding, and regulatory noncoding RNAs (microRNAs). There is a growing amount of data supporting epigenetic regulation of transporter expression levels with potential functional consequences. For example, DNA methylation of the OAT3 promoter has been found to play a role in determining the accessibility of HNF1 resulting in the negative regulation hOAT3 expression in transfected cells (118). Similarly, the kidney cortex-specific expression of human and mouse urate transporter 1 (URAT1) was found to be dependent on DNA methylation (117). Altered methylation patterns of the Oct1 gene (Slc22a1), which encodes an organic cation transporter that is very closely related to Oat1 (Slc22a6) and Oat3 (Slc22a8) and has some overlapping substrate specificity, occur in liver tumors (89, 210). Furthermore, histone acetylation is thought to be a major determinant of tissue-specific expression (e.g., liver versus kidney expression) of other SLC drug transporters (Oatp subfamily members, which has isoforms often largely expressed in either liver or kidney). There are now data that suggest that the changes in transporter expression (including the Oat-related SLC22 transporter gene, Oct3) can at least be partly reversed by clinical drugs targeting epigenetic modifications (42, 290). Analysis of the growing amount of data available from the ENCODE project (61) should set the stage for a great deal of experimentation in this area.
D. Transcriptional and Posttranslational Regulation
Transcription factors play a critical role by binding cis-regulatory genomic elements and recruiting transcriptional machinery, chromatin modifying complexes, or proteins that repress transcription (61). In addition to defining gene expression programs during development, transcription factors can respond to numerous extracellular and intracellular signals, potentially acting as “sensors” of molecules elaborated from remote tissues or of molecules entering the body exogenously (e.g., nutrients, gut microbiome metabolites, toxins, drugs). Studies have demonstrated that Oat1 and Oat3 mRNA levels are regulated not only during development but also in response to various physiological and pathophysiological stimuli (toxins, growth factors, and various hormones). For example, administration of Simiao pill, a traditional Chinese medicine used to treat gout and hyperuricemia, which presumably blocks URAT1 and possibly other uric acid transporters, resulted in the upregulation of Oat1 expression in mice (98). In addition, ochratoxin A and mercury conjugates are substrates of Oats (249, 307) that have been shown to regulate Oat1 and Oat3, at the mRNA and protein levels (54, 249, 307). Some of the earliest examples focused on induction by PAH and penicillin, both Oat1 substrates (91). In addition, early studies were also performed in both pregnant animals and neonatal animals, which indicated that during these critical stages of development a “inducibility window” is likely to exist (95, 176).
It is important to note that Oat1, Oat3, and most likely the remaining Oat family members are regulated posttranslationally. Protein kinase C (PKC) sites were noted when Oat1 was cloned as NKT (144). While the role of phosphorylation of these particular sites is unclear, Oat1 trafficking, protein turnover, and specificity has been shown to be modulated at various phosphorylation sites by PKC and likely other signal transduction pathways (300). In addition, there is an association with PDZ domains (14). Thus consideration of transcriptionally driven responses to internal or external stimuli should include not only direct regulation of Oat mRNA levels, but also transcriptional regulation of upstream transcriptional networks and downstream posttranslational regulatory pathways (207).
Studying the proximal promoter sequences of Oat1 and Oat3 implicated Hnf1α and Hnf4α (118, 183). However, these experiments were carried out in artificial systems and did not address the endogenous regulation of these transporters. While few transcription factors have been definitively linked to the Oats, the deletion of Hnf1α has clearly shown that it is connected to Oat1 and Oat3 regulation (147). Deletion of Hnf1α in mice has been shown to cause Fanconi syndrome, insulin secretion defects, and other metabolic disorders (193).
While Hnf1α has been the only transcription factor confirmed with a knockout model, multiple lines of evidence have indicated a major role for Hnf4α in the transcriptional regulation of Oat1 and Oat3. In the kidney, Oat1 and Oat3 are highly expressed in the proximal tubule. Of the factors identified by in silico analysis, Hnf1α and Hnf4α expression is limited to the proximal tubule in the kidney after nephrogenesis has ceased (245). While the importance of Hnf1α was confirmed with a mouse model, deletion of Hnf4α leads to embryonic lethality, and a model with proximal tubule-specific deletion of Hnf4α has not yet been reported. However, it was recently shown that Hnf4α was bound at Oat1 and Oat3 promoters in vivo in 2-wk-old rats, when Oat1 and Oat3 expression is rapidly increasing (78). ChIP-seq studies led to the identification of binding sites (149). It was also shown that Hnf4α and Hnf1α, when virally transduced into mouse embryonic fibroblasts, regulate the expression of Oat1 and other drug transporters (Figure 7) (149). This further supports an important role for Hnf4α in Oat regulation, as well as the relevance of in vitro findings in the developmental in vivo context. In addition, Bcl6 has also been shown to transactivate the Oat1 promoter (278).
As further discussed below, one of the most likely mechanisms involved in remote sensing and signaling is regulation through the nuclear receptor family of transcription factors. Nuclear receptors act as sensors of various internal and external cues (e.g., steroids, xenobiotics, lipids), poised to respond to the appropriate stimulus by inducing the corresponding transcriptional response. While some nuclear receptors do have constitutive activity in the absence of their ligand, a major mode of the known nuclear receptors' transcriptional activity is dependent on stimulation, thus providing a convenient mechanism for dynamic transcriptional response to signaling molecules from remote tissues and for substrate inducibility. Moreover, hormones acting on nuclear receptors that regulate Na+-K+-ATPase activity potentially ultimately alter Oat transport due to the tertiary transport system. Male and female sex hormones are known to result in increased or decreased organic anion transport expression (139), and glucocorticoid hormone has an effect on sodium transport (296). In addition, activation of nuclear receptors, including Vdr (277), Rar, Gr, Fxr, and Lxr (121), is known to be involved in integrating signals in proximal tubules. Furthermore, as described earlier, another nuclear receptor that has been suggested to play an important role in direct Oat transcriptional regulation is Hnf4α (149). In addition to having a ligand-binding domain, Hnf4α has multiple phosphorylation sites, protein coregulators, and isoforms based on alternate splicing and promoter usage in different tissues. For example, the P1 promoter of Hnfa4a is primarily used in the kidney, while the more distal P2 promoter is primarily used in the pancreas (51). This likely holds true to some extent for the other implicated nuclear receptors and transcription factors, creating many different potential combinations that alter specificity and, through protein-protein interactions, the potential for sensing.
E. Human SNPs and Handling of Drugs and Metabolites
Although known to be important drug and toxin transporters, the clinical effects of variation in SLC22A6 and SLC22A8, which are located in tandem on human chromosome 11, have not been completely characterized. A low-frequency polymorphism in OAT3 (Ile305Phe) affects cephalosporin (cefotaxime) handling in Asians, and this is supported by lower transport activity in vitro in transfected cells (292). A SNP at R454 in OAT1 has been associated with altered transport of the antiviral drug adefovir in vitro (77). An intergenic polymorphism located between these genes was found to be associated with the blood-pressure-lowering effects of thiazide diuretics (83). An interesting study examining the effects of 18 SNPs in mercury transporter genes was carried out in individuals with varying degrees of exposure to gold mines in Indonesia, the Philippines, Tanzania, and Zimbabwe. Individual SNPs in both OAT1 and OAT3 were associated with urinary concentrations of mercury in Tanzanians (66). In addition, the correlation between methotrexate (MTX) plasma levels and variation in 12 genes involved in MTX transport was examined in 151 pediatric acute lymphoplastic leukemia patients. SLC22A6-SLC22A8 haplotypes were associated with MTX levels, but this association was no longer deemed significant after controlling for multiple comparison testing (141).
It is also worth noting that transepithelial transport of organic anions involves entry into and exit out of the cell, not to mention movement between cellular compartments. Not all the genes involved are unambiguously identified. Nevertheless, to the extent possible, it seems reasonable in future studies to evaluate SNPs in transporters (and other genes) involved in all these steps (26, 286).
A number of SLC and ABC transporters have been implicated in the modulation of uric acid levels (12, 284). Among these are several Oat family members. SLC22A12 (URAT1) and SLC22A11 (OAT4) have primarily been associated with uric acid levels (110, 114, 130, 237, 243, 248, 268, 291) and prevalence of gout (153, 268). While Oat1 and Oat3 clearly are involved in urate handling in vitro and in Oat knockouts (73), the role of these genes in humans remains to be clarified. One study found the effect of genotype was modified by the presence of certain SLC22A11 variants and use of thiazide or loop diuretics (153). These drugs are primarily handled by Oat1 (Slc22a6) and Oat3 (Slc22a8) as has been confirmed in knockout animals (74, 266). However, SLC22A12 is also involved in drug handling. For example, the chemotherapeutic response of acute myeloid leukemia patients was found to be SLC22A12 genotype-dependent (101).
F. Expression and Function in the Developing Kidney and During Regeneration
As kidney function matures from the fetal to the postnatal period, changes in the filtration, excretion, and reabsorption profiles of metabolites, toxins, and xenobiotics occur. These alterations presumably account for the difference in drug pharmacokinetics observed in neonates and infants compared with adults (11, 167, 267). Underlying the developmental changes in renal metabolite and drug clearance are alterations in expression and function of various transporters including those of the SLC and ABC families. Recent studies have described the differential expression of the SLC family of transporters, including the organic anion (Oats), organic cation (Octs), and others, during kidney development (Figure 10) (144, 169, 230, 233, 245). Gender is an important factor in their expression patterns (32). Moreover, environmental stressors such as ischemia and nephrotoxic drugs may alter the expression of the tubular transporters in the developing kidney, leading to significant changes in drug metabolism and renal clearance by premature infants and neonates (176). Recent studies have provided some insight into the developmental changes in the expression and function of the Oats in the maturing kidney (230, 233). The embryonic kidney in organ culture is able to transport organic anions (Figure 11) (149, 169, 204, 233, 250). Oat expression generally appears to increase with postnatal kidney development, which also correlates with an increase in transport of PAH (Figure 10) (230). As discussed elsewhere, Oat expression and function have been shown to be under the control of many developmental regulatory mechanisms; however, further investigations are required to elucidate the precise manner by which these regulatory mechanisms come into play during the different developmental stages of the kidney such as those defined by global transcriptomics data (226, 252). Recent studies investigating Oat expression and regulation in the developing kidney suggest a key developmental role for Hnf4α and Hnf1α (149).
FIGURE 10.
The expression of the SLC22 family of transporters during kidney development. A: microarray analyses of developing embryonic [early embryonic (e13, e14, e15, e16 days of gestation), intermediate embryonic (e17, e18), late embryonic (e19, e20, e21,e22)], postnatal (birth and 1 wk), and mature (week 4 and adult) rodent kidneys demonstrate major increases in the expression of Slc22 transporters during kidney development and postnatal maturation. The grouping of the different stages of kidney development was based on a cluster analysis of gene expression data across the time course of kidney development (252). B: bar graph showing the clearance of the prototypical Oat substrate, PAH, in postnatal mice at 1, 2, and 3 wk of age. The rate of clearance increases during maturation of the postnatal kidney. C: comparison of Slc22 gene expression between consecutive stages of kidney development indicates that major changes in expression occur between the late embryo (late emb)/postnatal and postnatal/mature. [Modified from Sweeney et al. (230), with permission from ASPET.]
FIGURE 11.
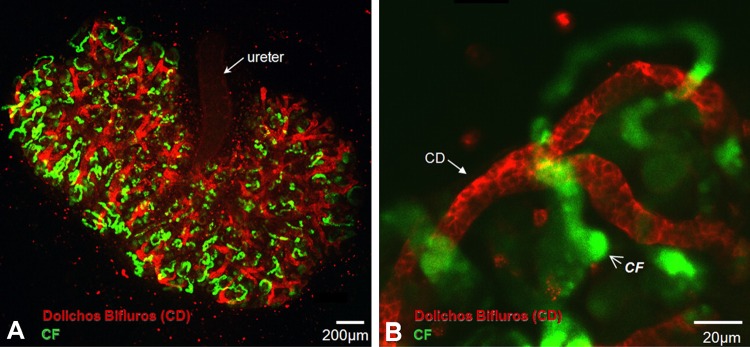
Oat-mediated transport in kidney organ cultures. A and B: uptake of a fluorescent Oat substrate (6-carboxyfluorscein, 6CF; green) in cultured embryonic rodent kidneys. A: low-magnification examination of Oat-mediated transport of 6CF (green) in an embryonic rodent kidney cultured for 4 days. Red staining indicates the collecting ducts labeled specifically with fluorescently labeled Dolichos biflorus lectin. 6CF accumulates in tubular structures distinct from the collecting duct. B: higher magnification view of kidney from A. The green fluorescent probe accumulates in nascent proximal tubular structures; the green staining does not overlap with the developing collecting ducts which are indicated by red staining. CD, collecting duct; CF, carboxyfluorescein. [Modified from Truong et al. (250). Copyright the American Society for Biochemistry and Molecular Biology.]
G. Regulation of Metabolism
Oat1 has been directly and indirectly implicated in a broad group of metabolic pathways. From in vitro assays, it is known that Oat1, as well as other Oats bind and/or transport a wide range of metabolites. This concept has been supported in vitro by targeted and untargeted metabolomics of the Oat1 and Oat3 knockout (Tables 2–5) (74, 279, 285). Global gene expression alterations in the Oat1 knockout, together with metabolic changes, have enabled reconstructions of Oat1-dependent regulation of metabolic pathways (Figure 12) (4). The changes in the metabolic pathways may be due to altered transport of Oat1 substrates in the pathway or indirectly affected, for instance, because Oat1 transports a cofactor necessary for the pathway to be active. Similar reconstructions have been performed for the Oat3 knockout (285). These metabolic reconstructions suggest that Oats play a much broader role in metabolism than generally appreciated.
FIGURE 12.
Network-based approach for linking Oat1-mediated transport to metabolism. A, inset: an integrative computational approach was used to analyze both gene expression data from the kidneys of Oat1 knockout mouse and metabolomics data derived from the plasma and urine of Oat1 knockout animals. Certain predictions based on the comparison of these two separate network analyses are supported by wet-lab data. B: the integrative network-based analyses of a comparison of the wild-type and Oat1-knockout mouse suggested alterations in many metabolic reactions in the Oat1 knockout. Implicated pathways included those involved in nucleotide, fatty acid, carnitine, chondroitin sulfate, inositol phosphate, and biotin metabolism. [From Ahn et al. (4). Copyright the American Society for Biochemistry and Molecular Biology.]
VII. THE REMOTE SENSING AND SIGNALING HYPOTHESIS
A. Overview
SLC22 is medically, pharmacologically, and physiologically an interesting transporter family because substrates of various family members span small molecules that include drugs, toxins, and metabolites; mutations in some family members cause serious human metabolic disease (127). The remote sensing and signaling hypothesis, first proposed around 2006–2007 (5, 113, 160, 284), was developed as it became increasingly clear that 1) many of these endogenous substrates were also important rate-limiting metabolites and signaling molecules and 2) many of the isoforms of the more newly described Oats were not significantly expressed in the kidney (e.g., Oat6) or were not exclusively expressed in the kidney as originally seemed to be the case (e.g., Oat1, Oat3). Instead, they are expressed in many tissues, some of which are less well studied in the context of drug transporters. These include the selectively expressed SLC22 isoforms in the olfactory mucosa (Oat6), the placenta (OAT4), the choroid plexus (SLC22A17) (170), and elsewhere in adult and in developing tissue. In the developmental case, the expression is sometimes transient such as in the developing brain/neuroectoderm (Oat1 and Oat3) and the aortic arch (Oct1) (187). As already described above for individual Oats, over the years, novel substrates discovered through in vitro and in knockout analyses have included isoform specific transport of cyclic nucleotides, prostaglandins, odorants, antioxidants, and conjugated sex steroids. These small molecules have potential roles in intracellular signaling, morphogenesis, organ maturation, repair, and communication across the placenta, as well as between organisms (5, 203, 207, 284).
Thus it appears that transporters of the SLC22 family, and perhaps SLC and ABC drug transporters in general, might by virtue of both their expression in multiple tissues and their capacity to transport diverse signaling molecules, mediate cross-talk between different organs, tissues, and cells (4, 5, 64, 207, 284). This general notion seems to be supported by the fact that various transporters in different tissues appear to be regulated by injury to the same or another tissue (29, 174, 207). While the mechanisms are far from clear, there is now a body of evidence for substrate regulation possibly mediated by “sensing” via transcription factors (62, 149) and the potential specific interaction of molecules transported by Oats involved in chemosensation by non-olfactory odorant GPCRs (190, 302). There is also evidence for regulation at multiple levels by growth factors and hormones produced elsewhere in the body (9, 58, 62, 211, 284). Much of this has been already described in the first half of this review on Oats, their substrates, and regulation at multiple levels.
In addition, there is circumstantial support for the possibility that metabolites and signaling molecules produced by one organism such as bacteria or an animal might be directly accessible to another organism, whereupon they could affect metabolism or signaling events in that other organism (279, 284). Possibilities include fetal-maternal communication via the placenta (151, 220), maternal-neonate communication via breast-feeding (80, 136, 284), effects of the gut microbiome on host systemic physiology (273, 279), and communication between animals by excretion of signaling molecules (e.g., odorants) into the urine (5, 6, 25, 107, 113, 160, 175, 212, 284).
Taken together, these many types of data suggest that the SLC22 transporters and possibly other “drug” transporters need to be considered from a broader systems biology perspective rather than as isolated transporters involved in specific transport of drugs and toxins (5, 112, 160, 284). The Remote Sensing and Signaling Hypothesis, in its several elaborations over the years, is an effort to build a broader systems physiology perspective on the role of Oats interorgan crosstalk in the normal homeostatic setting as well as after acute and chronic perturbation (5, 113, 160, 284).
This is in contrast to the frequent focus on an individual transporter or a set of transporters in a single epithelial tissue. It is argued that remote-sensing and signaling mediated through Oats and other drug transporters in various tissues regulating small molecule access to cells and body fluid compartments could function in parallel with the neuroendocrine system and growth factor regulatory systems to regulate homeostasis and restore the system after stress (Figures 2 and 3) (5, 284).
Connections to so-called phase I and phase II DMEs in tissues are emphasized from the perspective of generating and inactivating key metabolites and signaling molecules involved in remote communication between organs, epithelial and nonepithelial (including the nervous system and blood cells), body fluids, as well as other organisms (149, 279, 285). Oat3, for example, transports (in addition to a set of unmodified organic anions and some cations) many glucuronidated and sulfated substrates, including dietary flavonoids, conjugated drugs, and conjugated sex steroids.
An important feature of the system is the ability to adapt itself to prenatal and postnatal developmental needs or upon perturbation through transcriptional and posttranscriptional alterations in Oats and other transporters so that necessary small molecule communication is maintained or reset systemically and/or locally (4, 187, 284). The hypothesis is relevant to the understanding of drug-metabolite interactions, metabolite-metabolite interactions, and systemic and local metabolic abnormalities seen in such syndromes as diabetes, metabolic syndrome, hyperuricemia, acute organ injury (e.g., acute kidney injury or AKI), liver disease, and the uremia of chronic kidney disease (284).
B. Underlying Ideas and Potential Implications of the “Remote Sensing and Signaling Hypothesis”
The hypothesis may facilitate building a new framework for novel avenues of molecular, cellular, and physiological experimentation and analysis of large data sets in the field, as well as reevaluation of older studies in a systems biology context. What has been described so far in this overview is essentially a summary of the original hypothesis and its elaboration between 2006 and 2011 (5, 112, 113, 160, 284). Given that it is a broad multilevel perspective to establish guidelines for future Oat research, it is deemed important to break down the global picture into basic concepts, important to the hypothesis, that can be further evaluated. One goal here is to suggest new experimentation in specific areas that may help with further refinement or restructuring of the hypothesis. Highlighted below are what might be considered some key underlying ideas and implications of the “Remote Sensing and Signaling Hypothesis.” Although they may be yet to be proven at the whole organismal or interorganismal physiological level, they are supported by varying degrees of in vitro, ex vivo, and in vivo data for certain Oats. The concepts are an attempt to bridge the gap between the older physiological studies and the newer molecular and cell biological work, as well as systems biology interpretations that have occurred since the cloning of Oats and other drug transporters nearly two decades ago. What follows is also an explicit attempt to set the stage for computational modeling of a remote sensing and signaling system that may have complex nonlinear properties. We believe it is crucial for the field that these types of questions be more systematically addressed. Both because of the exclusive focus so far on Oat biology, and because it is easier to explain data and examples as well as future research avenues in the context of a single transporter subfamily, the remote sensing and signaling hypothesis is discussed almost entirely from the viewpoint of Oats.
1. Role of Oats and other multispecific transporters in communication between different tissues, organs and body fluid compartments
Oats and other multispecific transporters may have evolved in part to handle exogenous toxins, but their physiological role seems to be regulation of metabolite and signaling pathways (see sect. IV). Under basal physiological circumstances, levels are presumed to be regulated by particular sets of Oats and other transporters maintaining the influx into or efflux out of cells, tissues, and body fluid compartments of particular types of metabolites and signaling molecules necessary for local and systemic physiology. Although we discuss below a more “active” role in regulation (and reestablishment) of homeostasis that is analogous to the neuroendocrine and growth factor systems with respect to interorgan communication, it is worth emphasizing the importance of the role of the Oats and other multispecific transporters in the straightforward movement of small molecules with informational content (e.g., key metabolites, antioxidants, signaling molecules) (74, 279, 285) between body compartments: remote communication. Some of these compounds, such as cyclic nucleotides, steroids, and prostaglandins, have central roles in classical intercellular or cell surface signaling, whereas others such as flavonoids can at least indirectly affect signaling pathways (e.g., those mediated by mitogen-activated protein kinase and phosphatidylinositol 3-kinase) (223).
As already discussed (sect. IV), this notion is supported by the fact that Oats interact with numerous endogenous metabolites and signaling molecules, many of which accumulate in single Oat knockouts (4, 74, 279). As discussed below, it is presumed that more such molecules would accumulate if the system is acutely stressed, especially if more than one Oat isoform is inactivated. Moreover, by virtue of their expression in most barrier epithelia in the body, Oats are presumed to help regulate the net levels of these molecules within cells, tissues, and body fluid compartments (such as CSF, blood, urine, and bile) (160).
2. Restoring homeostasis after perturbation or injury
Remote sensing and signaling via multispecific “drug” transporters, although important for basal state physiology, may be even more critical for resetting the perturbed system to normal. In this regard, it may function akin to endocrine, autonomic, and growth factor signaling systems in responding to injury and acute, subacute, and chronic perturbations.
While certain Oats may be functionally important for basal physiology, others may become important when the system is perturbed and help to coordinate the restoration toward the original state (Figure 13). In other words, cells in organs, as well as multicellular structures responsible for physiological functions (e.g., kidney proximal tubule), not to mention the organ itself, are specialized for the handling of metabolites, signaling molecules, and nutrients as well as endogenous and exogenous toxins via a particular set of transporters. Upon injury to the same or another organ (e.g., ischemic, toxic, partial resection), the expression of the functionally relevant sets of transporters (or key covalent modifications) changes, not only in the injured organ but in other organs as well. For example, after renal injury, not only are there expected changes in the expression of Oats and other transporters, but there are also changes in the expression of drug transporters, such as P-glycoprotein (P-gp) and Mrp2, in the liver and intestine (172, 173, 181). While the factors inducing these changes remain to be unambiguously identified, it has been recently shown that treatment of Caco-2 (an intestinal cell line) or Hep3B (an hepatic cell line) cells with either deproteinized uremic serum or with representative uremic toxins (i.e., CMPF, hippuric acid, indole-3-acetic acid, 3-indoxyl sulfate, p-cresol) altered the expression of Mrp2 and Oatp1b1 and Oatp2b1 (255). The importance of covalent modifications of Oats has been studied (300), but is not well understood in the setting of cell and organ perturbation/recovery (5, 207). Taken together, the data support the notion that small molecules, many of which have been identified as key substrates of Oats and other multispecific transporters, are able to signal between different organs and cell types, which in the setting of injury leads to alterations in the functional set of transporters.
FIGURE 13.
Potential role of Oats and other transporters in normal and perturbed homeostasis. Members of the SLC and ABC transporter families are involved in the handling of a wide variety of endogenous and exogenous substrates, including nutrients, metabolites, signaling molecules, toxins, and drugs. As described in the text, it is hypothesized that these transporters are essential for maintaining normal homeostasis and play key roles in resetting the system after homeostasis is altered. Transporter-mediated interorgan communication is disrupted after insults to the system (e.g., toxins, ischemia, or competitive inhibition by other substrates), leading to perturbed substrate clearance. Resetting and eventual reestablishment of intact homeostatsis likely occurs through changes in expression and/or function of the transporters (e.g., transcriptional, translational, or posttranslational modifications) in either the injured tissues or in other tissues participating in the larger remote-sensing network involving SLC and ABC transporters. [Modified from Ahn and Nigam (5), with permission from ASPET.]
Altering (different) functional sets of Oats and/or other transporters at the cell surface is presumed to enable “sensing” of newly accumulating substrates or changing levels of existing substrates (such as endogenous toxins). There may be additional mechanisms to sense biochemical alterations associated with injury such as altered redox potential or pH; these could affect transporter expression, protein folding, or movement in the secretory pathway or bioassembly of plasma membrane complexes containing drug transporters, among other mechanisms (134). Once substrates (e.g., steroids, cyclic nucleotides, lipids, xenobiotics) gain entry to the cell, there can be alterations in transporter expression via sensing of the substrate by transcriptional machinery, including nuclear receptors. Similarly, a sensing mechanism that is not necessarily within the nucleus may regulate recycling of membrane compartments (holding the transporter), cytoskeletal association, or phosphorylation (which may either directly affect transporter function or affect recycling/cytoskeletal association). Together, these mechanisms could result in different net handling of metabolites, antioxidants, nutrients, and signaling molecules, until recovery occurs, whereupon the system eventually reverts to its earlier state with respect to transporter expression, recycling, cytoskeletal association, phosphorylation, and so on (5, 37, 58, 60, 219, 284, 300). Such autoregulatory mechanisms may not be limited to recovery from severe perturbations but may also be important to normal cellular and organ homeostasis in the basal state. Through similar mechanisms, Oats and other multispecific transporters could also help regulate cell fate during development and regeneration (5, 187, 231, 233, 284).
Much of the targeted and untargeted metabolomics data in knockouts come from plasma samples. This does not indicate the origin of the metabolite or signaling molecule or its site of uptake/action. Nevertheless, coordination, or the lack of it in pathological states, between efflux (generally ABC) and influx (generally SLC) transporters in the same and different tissues may be important. For instance, metabolites accumulating in a diseased liver or muscle may rely on ABC transporters for release into the blood; these, in turn, may be eliminated by SLC transporters, notably Oat1 and Oat3, in the kidney or be remotely “sensed” via uptake by Oats or other SLC transporters in pancreatic endocrine cells or nervous system, thereby altering homeostasis actively (5, 29, 284). Understanding the interorgan coordination of Oat (and other transporter) expression and function in normal and diseased states may not only lead to new pathophysiological insights but also provide important information for the tissue distribution of drugs and toxins in the setting of injury (Figures 12 and 13).
3. Epigenetic “memory” of prior conditions
As described earlier, evidence supports a role for epigenetic mechanisms in the expression of drug transporters (15, 102, 117, 118). Nevertheless, the role of epigenetic changes on transporter gene regulation in tissue injury and recovery remains largely unexplored. After recovery from injury, epigenetic modifications to regulatory elements of drug transporters and phase I and II enzymes linked to their function may remain (104). We discuss here the possibility of this resulting in a “memory” for future insult, which may enhance or otherwise alter the capacity to respond transcriptionally by increasing expression of the same or different SLC/ABC transporter (or, potentially, diminish it). This would change the “overall transport function” of affected cells and tissues. This could be important during the early recovery phase from injury, for example, elimination of deleterious endogenous compounds (e.g., molecules causing uremia or hepatic encephalopathy) that have accumulated (140, 206, 272).
It is known that there is a developmental “window” for substrate induction of Oat-mediated transport in the postnatal kidney (95); it is unclear whether a similar phenomenon occurs in the regenerating kidney with respect to the drug transporters and DMEs. There are, however, striking parallels between kidney development and kidney recovery after injury.
Although it remains unclear whether injury can lead to epigenetic changes affecting subsequent transcriptional regulation of drug transporters, it is worth noting that some substrates of these transporters have been found to induce epigenetic alterations. For example, Oats can take up butyrate and its derivatives (74, 270). Butyrate, as well as a number of other dietary components, has been shown to affect histone acetylation by inhibiting histone deacetylase (HDAC) (189). In addition, epigallocatechin-3-gallate (EGCG), epicatechin, quercetin, catechin, and other flavonoid-like compounds are transported by Oats and other “drug” transporters (276, 280, 285); they can potentially affect epigenetic profiles through their ability to alter the function of histone acetyltransferase (HAT) (189), as well as perturb DNA methylation patterns by inhibiting DNA methyltransferase (DNMT) activity. These effects could conceivably even extend transgenerationally. If such affected regulation of transporter expression does occur, this general mechanism could also affect the ability of a “recovered” organ to handle metabolites, signaling molecules, drugs, and toxins in a way that is distinct from the previous “uninjured” state. By this mechanism, the experience of injury and resultant altered expression profile of drug transporters may be “remembered” despite recovery.
4. Remote sensing and signaling via Oats is closely linked to DMEs
In higher organisms, a complex highly regulated, somewhat hierarchical network of so-called phase I, phase II, and phase III (drug transporters) DMEs exists. Although in the literature this is largely considered in the context of drug or toxin metabolism and elimination, another way of looking at this is as follows: in mammals, in the basal state, this network of DMEs and transporters has been set up for maintenance of homeostasis via remote sensing and signaling, and drugs and toxic substances may usurp this system. In metabolism associated with normal physiology (in the absence of drugs), there may be a stronger interdependence of phase I and II processes with phase III than currently emphasized in much of the literature; this interdependence may even be greater in the settings of postnatal development and organ injury repair. Thus, these dependencies between phase I/phase II DMEs and phase III transporters may differ according to the stage of life and pathophysiology. The activity of phase I and phase II reactions (e.g., hydroxylation, sulfation, and glucuronidation), while creating new metabolically active compounds (e.g., indoxyl sulfate, p-cresol sulfate, flavonoid-glucuronides, estrogen-sulfate) that can affect nuclear receptor or kinase signaling, likely also play a role in targeting these molecules to tissue compartments or body fluids. In fact, it is often the modified form that is best transported by various drug transporters expressed in tissues. For example, Oat3 has a different pattern of expression than Oat1, and it appears to be a better transporter of glucuronidated molecules. Deconjugation reactions may then occur intracellularly, leading to a more active molecule in the cell. For example, if in the future this were found to be the case for steroid hormone conjugates, which are among the best substrates of Oat3, Oat4, and Oat6, regulation of the expression of multispecific transporter genes, or the transport process itself, may begin to be viewed as a key aspect from the perspective of endocrine and developmental physiology.
5. Oats and other multispecific drug transporter families are linked to classical signaling pathways
Molecules handled by this system include signaling molecules potentially capable of activating GPCRs in the body, including odorant molecules acting upon renal and nonrenal odorant receptors, prostaglandins, and gut microbial metabolites (190, 302). In addition, certain drug transporters and certain GPCRs bind similar sets of signaling molecules (e.g., prostaglandins, kynurenine, odorants), and it will be interesting to compare binding in solved three-dimensional structures. Some drug transporters are also efficient cyclic nucleotide transporters and may regulate cellular cGMP and cAMP levels, thereby potentially playing a more direct role in affecting cell signaling and tissue fate by modulating the many different cellular processes known to be regulated by cyclic nucleotides (43, 200). Interestingly, some of the transporters are associated with PDZ domains, a “hot spot” for signaling and intercellular communication as well as for the assembly and regulation of intercellular junctions (14, 53, 115, 306).
In addition, among SLC transporters within the genome, there appear to be some “hemi-transporters” with six transmembrane domains (199); it remains to be determined whether these have similarities to GPCRs. This will be important to explore in future work. In this regard, it is worth noting that the original identification of NKT (now called Oat1) in 1996 was through a screen for GPCR-like sequences in the kidney using codon-optimized differential display (CODD) (142, 144).
6. Oats and other multispecific transporters need to be considered in the context of classical transcriptional regulatory mechanisms
Oats and other multispecific transporters regulate uptake and egress of many signaling and other molecules that are nuclear receptor ligands (e.g., sex steroids, fatty acids, indoxyl sulfate) or activate other transcription factors (cAMP) (279). In turn, their regulation (transcriptional and posttranscriptional) may be critical for nuclear receptor-mediated gene regulation. Indeed, these nuclear receptors may be central to certain types of “remote sensing” mechanisms in that molecules transported by SLC and/or ABC multispecific transporters from one tissue or body fluid compartment may be taken up into cells of a remote tissue (via SLC and/or ABC transporters with overlapping specificities in the remote tissue) whereupon they are bound by nuclear receptors or other transcription factors and thereby modulate the remote tissue transcriptional program. For example, estrone sulfate, the sulfated metabolite of estrogen, is an Oat substrate (e.g., Oat3) and enters cells via these drug transporters. Upon entering cells, estrone sulfate (possibly also the desulfated form) potentially binds to estrogen receptors found in the cytosol or nucleus inducing conformational changes in the receptor followed by its dimerization and assembly on target gene promoters leading to alterations in gene expression (31, 196, 215). Even if this is a very small effect, given circulating levels of steroid hormone conjugate, it may be of physiological significance, for example, in the setting of SNPs that alter intracellular levels. This may include subtly altering the expression of a similar or different set of SLC uptake and/or ABC efflux transporters in that tissue, and thereby, affecting the net movement of substrates. Although the combinatorial possibilities are many, the key point is that the intracellular levels of certain transported substrates that are also ligands for nuclear receptors, or which affect other transcriptional events, may thereby be altered or modulated by such a mechanism. This requires quantitative data from different cell types and perhaps real time measurements of small molecules.
7. Coordination of inter-organ communication during development and regeneration
Transporter and DME activity must be somewhat coordinated at some level between organs during the postnatal period to maintain levels of signaling molecules and key metabolites. The same would seem to apply to the situation after acute injury to one organ (e.g., liver) vis a vis the uninjured organs (e.g., kidney). Little is known about this type of coordination between organs, and this will be an important area for future work. Interorgan communication of this type might be viewed as an emergent property of the transcriptional regulation of phase I, phase II, and phase III DMEs by exogenous and endogenous substrates as well as the modulation by growth factors and neuroendocrine influences, which are known to play critical roles during development and injury repair. The application of methods used to study complex adaptive systems and network science may be fruitful here. As already emphasized, interorgan communication through remote sensing and signaling via SLC and ABC multispecific drug transporters is presumed to be important for the basal physiological state. This notion may be harder to demonstrate due to “redundancy” of transport mechanisms, but it seems to be supported by the significant metabolic alterations observed, for example, in the Oat1 (74, 279) and Oat3 (265, 285) knockouts as well as the lower blood pressure detected in the Oat3 knockout (265). Computational reconstructions of metabolic alterations in the Oat1 and Oat3 knockouts suggest that these transporters play global roles in metabolism extending well beyond the direct transport of a small set of substrate metabolites (Figure 12) (4, 285). This may be generally the case for other drug transporters. The integration of many types of “omics” data (from different tissues and body fluids) in time series analyses of development and injury-repair-regeneration, especially using wild-type and multiple tissue-specific conditionally knocked-out Oats, is a key task for the field.
8. Oats, intracellular concentrations of metabolites and signaling molecules, and possible connections to organellar small molecule transporters
Drugs and toxins enter the proximal tubule cell by Oat1 and Oat3 and, in some cases, cause (such as the instances of cephaloridine and mercury) toxicity to the proximal tubule cell (21, 297). This also appears true of microbiome-derived metabolites like indoxyl sulfate (122, 179). Fluorescent Oat substrates are known to accumulate in intracellular vesicular compartments (55, 155). Key metabolites like α-ketoglutarate and signaling molecules like prostaglandins, cyclic nucleotides, and conjugated steroids also enter the cell through this pathway (33, 270). But there are also small molecule transporters in the mitochondria and elsewhere which may transport Oat substrates (150, 185, 201, 202). If so, this may be another mechanism for regulation of cell metabolism and function: thus Oats could not only mediate interorgan and interorganismal communication, but they could also mediate communication with cellular subcompartments. The hierarchical architecture, or tiering, of these extracellular, cellular, and intracellular transport processes may be important for function and resilience of a remote sensing and signaling system.
C. Some Clinical Implications of the “Remote Sensing and Signaling Hypothesis”
1. The remote sensing and signaling system may be perturbed in disease settings (e.g., uremia, liver injury, toxin exposure, diabetes)
Uremia, or the uremic syndrome, associated with CKD may be a disorder of remote sensing and signaling, at least in part. Certain compounds considered uremic toxins, such as indoxyl sulfate, p-cresol sulfate, and kynurenine, are ultimately derived from the gut bacteria. Many polyamines, such as spermine and spermidine, also accumulate in CKD. Such small molecules, which are bound to plasma proteins, are generally thought to be among those partly removed by peritoneal dialysis and/or hemodialysis to treat the symptoms of renal failure. These molecules also accumulate in the Oat1 and Oat3 knockouts and/or are known Oat substrates (4, 74, 279, 285).
Indoxyl sulfate and other uremic toxins (distributed through the body and eliminated by Oats and other SLC and ABC transporters in different tissues) appear to affect transcriptional regulation (e.g., indoxyl sulfate and the aryl hydrocarbon receptor) (214). Other toxins like kynurenine affect GPCR signaling (274). Furthermore, uremia is a disorder of metabolism, and since uremic toxins are high-affinity substrates of Oats and other drug and metabolite transporters, they likely compete for elimination and distribution with other metabolites and drugs to disrupt metabolic pathways or alter the half-lives and toxicity of drugs. This can create a vicious cycle, since the failed or failing kidney is unable to adapt by the “memory” mechanisms of the recovering organ after injury discussed above. Other organs like liver and muscle may indeed adapt to some extent by differential upregulation of other sets of SLC and ABC transporters to handle the organic anion (uremic toxins, drugs, metabolites) load. But since uptake of these molecules (uremic toxins, certain drugs) may cause, in addition to substrate induction, cellular toxicity (e.g., uremic myopathy, statin myopathy), this compensatory mechanism could be maladaptive in the long run. Thinking about uremia as altered remote sensing and signaling could lead to new approaches to treating the uremic syndrome of chronic kidney disease. Furthermore, many metabolites accumulating in diabetes, ketotic states, and liver disease are substrates for Oats and other drug transporters; it is possible that drugs, or SNPs in the transporters, modulate the severity of these diseases by altering the levels of key metabolites.
2. Metabolic abnormalities caused by certain drugs may be due to altered remote sensing and signaling
Metabolic syndrome is associated with certain drugs or toxins (75). This could be a reflection of disordered remote sensing and signaling due to interaction/competition of key metabolites, antioxidants, and/or signaling molecules with drugs or toxins at the level of the transporter. This could secondarily reset the physiological state because the levels of key metabolites, signaling molecules, and/or antioxidants in tissues and body fluids may be altered. Moreover, systems biology studies and metabolic reconstructions from metabolomics and microarray data in Oat knockouts raise the possibility that such competition between drugs and metabolites can have major effects on metabolites not transported by Oats and perhaps alter entire metabolic pathways (5, 284). In other words, certain aspects of drug-induced metabolic syndrome could be due to altered remote sensing and signaling. This dysregulation of metabolism could be particularly complex in diseased states (e.g., liver or kidney) in which multiple Oat-transported drugs are administered. As metabolomics and genomic data for large numbers of patients becomes available, it should be possible to further evaluate these ideas.
3. Interorganismal communication via Oats and other drug transporters
Propionate, kynurenine, and indole are produced by gut microbial flora and transported via drug and/or nutrient transporters across the intestinal mucosa (146, 273). Propionate, kynurenine, and other such molecules can activate GPCRs (94, 105, 191, 244). Indole metabolites, the result of the action of liver phase I and phase II DME reactions, affect cell function in many ways and can activate transcription of drug transporters (56, 214). Some of these gut microbiome-derived metabolites or their derivatives can be altered in disease states such as uremia or CKD (1, 279), which itself is associated with altered expression of OATs and other drug transporters in humans (e.g., diabetic nephropathy) (216, 304).
Several SLC and ABC drug transporters expressed in a variety of cells have the ability to transport cAMP and cGMP and thus alter the intracellular levels of cyclic nucleotides and potentially kinase activity. Thus this raises the possibility of direct drug transporter modulation of intracellular signaling pathways already activated by cell surface binding of ligands that are themselves absorbed, and excreted by a variety of drug transporters. Volatile odorants excreted into the urine can potentially be transported or sensed in the olfactory mucosa of another animal. This could be via GPCRs acting as odorant receptors in the olfactory epithelium (94, 113, 160) or by the Oat6 transporter which has the ability to bind odorants (113).
There are also nonolfactory odorant receptors in many tissues (190, 302). In the kidney, one such GPCR appears to regulate blood pressure. This may be interesting in light of the low blood pressure observed in the Oat3 knockout (265), which also accumulates many metabolites, including those derived from the gut microbiome (285).
D. Developing a Physiologically Inspired Artificial Remote Sensing and Signaling System
It may be some time before enough data are in to determine to what extent the Remote Sensing and Signaling Hypothesis, in its current form, is consistent with experimental and clinical data. To the extent that it is validated experimentally and clinically, modeling such a system may be useful for understanding, and possibly predicting, metabolite and toxin handling and, with respect to drugs, their absorption, distribution, metabolism, and excretion (ADME). This may be helpful for physiologically-based pharmacokinetic (PBPK) modeling. Modeling this complex adaptive system (92) may be useful for understanding the systemic metabolic consequences of DMI, the uremic syndrome due to CKD, and drug/toxin handling at the extremes of life and in setting of injury and recovery.
Nevertheless, it is also worth pointing out that an “artificial remote sensing and signaling system” may be of considerable interest in its own right and have potential implications in the realm of “biology-inspired” artificial intelligence (AI) similar to work on artificial immune systems (45, 301) and artificial endocrine systems (47, 65).
Such an artificial system, consisting of sets of interactions between multiple organs and/or organisms, could model interorgan and interorganismal communication via small (<1,000 Da) molecules with signaling capacity (or other key informational content) and the ability to regulate key metabolic reactions; these molecules, owing to the multispecificity of SLC and ABC “drug”, have tremendous diversity. Information could be encoded and/or transmitted by chemical fingerprint notation.
In that tissue-specific and organism-specific expression of SLC and ABC “drug” transporters, not to mention phase I and phase II enzymes (which could also be incorporated into such models), are different from tissue to tissue and can be affected by positive or negative feedback described elsewhere in this review (i.e., substrate, hormones, oxidants, toxins, injury-recovery, organ development, growth factors), such a model can “evolve” from normal homeostasis after a perturbation to a new, possibly unstable, state. Depending on further environmental influences, it can “reset” at a new homeostatic state that may or may not be close to the original state. As in actual physiology, this physiologically inspired AI method could be intertwined with growth factor and neuroendocrine influences which themselves have been proposed as AI techniques (47, 186).
It is to be emphasized that these AI methods may be interesting in themselves. Nevertheless, once such physiologically inspired AI systems are sufficiently developed, they could be constrained by accumulating wet lab and clinical data to determine their value for understanding the systemic behavior of metabolites, drugs, and toxins in dynamic settings such as organ injury-recovery and development.
E. What Are the Conditions Necessary and Sufficient for Establishment of a Remote Sensing and Signaling System?
It is not obvious that, apart from coordinated apical and basolateral tissue specific-gene expression of Oats and other drug transporters, the potential regulation by substrate, and the drug transporter-mediated movement of metabolites and signaling molecules between tissues and fluid compartments, anything more is required for a relatively independently functioning remote sensing and signaling system to emerge. It seems that physiological and systems biology modeling [to be distinguished from the biologically (physiologically) inspired AI method discussed above] might be useful here. Such models need to establish the minimum necessary components (e.g., transporters, signaling molecules, regulatory events) and the nature of the relationships (including feed-forward and feedback) between them in order for the functioning of the proposed remote sensing and signaling system. Such models may also be able to make in silico predictions of the effect of perturbation. They may also be useful for identifying “X factors” or missing components or relationships. This might require a search for additional mechanisms, or reconsidering aspects of the hypothesis. Nevertheless, if hormones, growth factors, cytokines, and other homeostatic mechanisms periodically modulate the system's behavior, as seems to be the case for Oat1, Oat3, and OAT4, it appears qualitatively that the proposed system could form a semi-autonomously functioning system, particularly if closely tied to phase I and phase II DMEs in normal physiology, in the same sense as the neuroendocrine, growth factor, and cytokine systems just mentioned.
As is often the case with these other systems, it may be that the importance of the remote sensing and signaling system becomes more evident in the setting of physiological perturbation or injury and, perhaps even then, only when multiple pathways (e.g., two or more transporters with overlapping substrates or a key transcriptional or other regulatory mechanism) become disrupted. Systems biology models thus also need to consider the ”redundancy issue,“ for example, the fact that Oat1 and Oat3 have many common endogenous substrates, in normal and perturbed states. They will also need to consider the relationship between Oats and phase I and phase II DME pathways in cells of the same organ (e.g., proximal tubule cell) and remote organs (e.g., hepatocytes). In general, it will be useful to analyze gene expression in multiple tissues and metabolomics data from multiple body fluid compartments in wild-type and knockout (single, double, triple) mice, as well as other ”omics“ data, after acute and chronic perturbations affecting one organ or another or after small molecule disruption of a key pathway (e.g., one mediated by a nuclear receptor that regulates Oats). Time series data from the time of perturbation to the restoration, or near-restoration, of a stable state might be particularly valuable. While it may take years to obtain high quality data of this sort, the amount of data currently available for Oat1 and Oat3, the result of work by many labs, may be sufficient to make a useful start in this endeavor.
VIII. SUMMARY
Using the lens of the Remote Sensing and Signaling Hypothesis, we have tried to provide a systems biology perspective on organic anion transporters without sacrificing the specifics. We have considered organic anion transporters individually, as subsets expressed in particular epithelia, and in the context of whole organ and organismal physiology as well as interorganismal physiology. Thus, in the process of examining a broad range of in vitro, ex vivo, in vivo, and in silico data related to various members of the Oat subfamily, we have considered the so-called subsystem, the system, and the supersystem (148).
We have also incorporated a temporal perspective, considering what might be termed feedback and feedforward loops in the dynamic contexts of development, injury, and recovery from injury (5, 284). We argue that many small molecule pharmaceuticals are “tourist traffic” on the remote-sensing and signaling system, which likely arose evolutionarily, at least in part, to optimize interorgan and interorganismal communication via metabolites and signaling molecules in dynamic settings such as development and disease. We have emphasized the analogy to the neuroendocrine and growth factor regulatory systems.
In the latter part of this review, we have tried to “open up,” at the risk of being overly speculative, the Remote Sensing and Signaling Hypothesis to potential experimental and modeling approaches as well as explore its possible ramifications. We have tried to clarify various aspects of the hypothesis in the context of recent data on Oat biology. As extensively detailed here, the Remote Sensing and Signaling Hypothesis owes much to the discovery of Oat expression in certain tissues early in fetal development (187, 230, 233), the evolutionary conservation down to flies and worms (71), the discovery of the odorant binding Oat6 in olfactory mucosa (113, 160), the application of metabolomics to the Oat knockouts (74, 265, 279, 285), computational reconstructions of metabolism based on Oat knockout transcriptomic changes (4, 285), the growing list of Oat substrates that are key metabolites and signaling molecules (Tables 1–5), the fact that mutations in related SLC22 family members are associated with metabolic disease (127, 166, 216, 284), the patterns of Oat expression after injury (29, 207), the phenomenon of Oat substrate induction (91), and analysis of transcriptional regulation of Oats by potential “sensors” (149) as well as evidence of regulation by traditional signaling pathways mediated by hormones, growth factors, and intracellular kinases (137).
The roles of organic anion transporters in metabolism, signaling, toxin and nutrient handling appear to be deeply linked, perhaps reflecting an even more interesting reality. Nevertheless, while we have focused on systems biology here, it is also important to reemphasize that there is still much to learn about the molecular, cellular, structural, and organ biology of the individual Oats.
GRANTS
This work was supported by National Institutes of Health Grants R01-GM104098, R01-GM098449, and U54-HD07160 (to S. K. Nigam).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
acknowledgments
We thank members of the Nigam lab past and present as well as all those investigators whose studies have contributed to the essence of this review.
Address for reprint requests and other correspondence: S. K. Nigam, Univ. of California, San Diego, 9500 Gilman Dr., La Jolla, CA 92093 (e-mail: snigam@ucsd.edu).
REFERENCES
- 1.Adelibieke Y, Shimizu H, Muteliefu G, Bolati D, Niwa T. Indoxyl sulfate induces endothelial cell senescence by increasing reactive oxygen species production and p53 activity. J Ren Nutr 22: 86–89, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Ahn SY, Bhatnagar V. Update on the molecular physiology of organic anion transporters. Curr Opin Nephrol Hypertens 17: 499–505, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Ahn SY, Eraly SA, Tsigelny I, Nigam SK. Interaction of organic cations with organic anion transporters. J Biol Chem 284: 31422–31430, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahn SY, Jamshidi N, Mo ML, Wu W, Eraly SA, Dnyanmote A, Bush KT, Gallegos TF, Sweet DH, Palsson BO, Nigam SK. Linkage of organic anion transporter-1 to metabolic pathways through integrated ”omics“-driven network and functional analysis. J Biol Chem 286: 31522–31531, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahn SY, Nigam SK. Toward a systems level understanding of organic anion and other multispecific drug transporters: a remote sensing and signaling hypothesis. Mol Pharmacol 76: 481–490, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ai M, Min S, Grosjean Y, Leblanc C, Bell R, Benton R, Suh GS. Acid sensing by the Drosophila olfactory system. Nature 468: 691–695, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albrecht E, Waldenberger M, Krumsiek J, Evans AM, Jeratsch U, Breier M, Adamski J, Koenig W, Zeilinger S, Fuchs C, Klopp N, Theis FJ, Wichmann HE, Suhre K, Illig T, Strauch K, Peters A, Gieger C, Kastenmuller G, Doering A, Meisinger C. Metabolite profiling reveals new insights into the regulation of serum urate in humans. Metabolomics 10: 141–151, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alebouyeh M, Takeda M, Onozato ML, Tojo A, Noshiro R, Hasannejad H, Inatomi J, Narikawa S, Huang XL, Khamdang S, Anzai N, Endou H. Expression of human organic anion transporters in the choroid plexus and their interactions with neurotransmitter metabolites. J Pharmacol Sci 93: 430–436, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Aleksunes LM, Klaassen CD. Coordinated regulation of hepatic phase I and II drug-metabolizing genes and transporters using AhR-, CAR-, PXR-, PPARalpha-, and Nrf2-null mice. Drug Metab Dispos 40: 1366–1379, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson CM, Thwaites DT. Hijacking solute carriers for proton-coupled drug transport. Physiology 25: 364–377, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Anderson GD. Developmental pharmacokinetics. Semin Pediatr Neurol 17: 208–213, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Anzai N, Jutabha P, Amonpatumrat-Takahashi S, Sakurai H. Recent advances in renal urate transport: characterization of candidate transporters indicated by genome-wide association studies. Clin Exp Nephrol 16: 89–95, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Anzai N, Jutabha P, Enomoto A, Yokoyama H, Nonoguchi H, Hirata T, Shiraya K, He X, Cha SH, Takeda M, Miyazaki H, Sakata T, Tomita K, Igarashi T, Kanai Y, Endou H. Functional characterization of rat organic anion transporter 5 (Slc22a19) at the apical membrane of renal proximal tubules. J Pharmacol Exp Ther 315: 534–544, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Anzai N, Miyazaki H, Noshiro R, Khamdang S, Chairoungdua A, Shin HJ, Enomoto A, Sakamoto S, Hirata T, Tomita K, Kanai Y, Endou H. The multivalent PDZ domain-containing protein PDZK1 regulates transport activity of renal urate-anion exchanger URAT1 via its C terminus. J Biol Chem 279: 45942–45950, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Aoki M, Terada T, Kajiwara M, Ogasawara K, Ikai I, Ogawa O, Katsura T, Inui K. Kidney-specific expression of human organic cation transporter 2 (OCT2/SLC22A2) is regulated by DNA methylation. Am J Physiol Renal Physiol 295: F165–F170, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Aouida M, Poulin R, Ramotar D. The human carnitine transporter SLC22A16 mediates high affinity uptake of the anticancer polyamine analogue bleomycin-A5. J Biol Chem 285: 6275–6284, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Apiwattanakul N, Sekine T, Chairoungdua A, Kanai Y, Nakajima N, Sophasan S, Endou H. Transport properties of nonsteroidal anti-inflammatory drugs by organic anion transporter 1 expressed in Xenopus laevis oocytes. Mol Pharmacol 55: 847–854, 1999. [PubMed] [Google Scholar]
- 18.Aronson PS. The renal proximal tubule: a model for diversity of anion exchangers and stilbene-sensitive anion transporters. Annu Rev Physiol 51: 419–441, 1989. [DOI] [PubMed] [Google Scholar]
- 19.Asif AR, Steffgen J, Metten M, Grunewald RW, Muller GA, Bahn A, Burckhardt G, Hagos Y. Presence of organic anion transporters 3 (OAT3) and 4 (OAT4) in human adrenocortical cells. Pflügers Arch 450: 88–95, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Aslamkhan AG, Han YH, Yang XP, Zalups RK, Pritchard JB. Human renal organic anion transporter 1-dependent uptake and toxicity of mercuric-thiol conjugates in Madin-Darby canine kidney cells. Mol Pharmacol 63: 590–596, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Atkinson RM, Currie JP, Davis B, Pratt DA, Sharpe HM, Tomich EG. Acute toxicity of cephaloridine, an antibiotic derived from cephalosporin C. Toxicol Appl Pharmacol 8: 398–406, 1966. [DOI] [PubMed] [Google Scholar]
- 22.Bahn A, Hagos Y, Reuter S, Balen D, Brzica H, Krick W, Burckhardt BC, Sabolic I, Burckhardt G. Identification of a new urate and high affinity nicotinate transporter, hOAT10 (SLC22A13). J Biol Chem 283: 16332–16341, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Bahn A, Hauss A, Appenroth D, Ebbinghaus D, Hagos Y, Steinmetzer P, Burckhardt G, Fleck C. RT-PCR-based evidence for the in vivo stimulation of renal tubularp-aminohippurate (PAH) transport by triiodothyronine (T3) or dexamethasone (DEXA) in kidney tissue of immature and adult rats. Exp Toxicol Pathol 54: 367–373, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Bahn A, Ljubojevic M, Lorenz H, Schultz C, Ghebremedhin E, Ugele B, Sabolic I, Burckhardt G, Hagos Y. Murine renal organic anion transporters mOAT1 and mOAT3 facilitate the transport of neuroactive tryptophan metabolites. Am J Physiol Cell Physiol 289: C1075–C1084, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Bates LA, Sayialel KN, Njiraini NW, Moss CJ, Poole JH, Byrne RW. Elephants classify human ethnic groups by odor and garment color. Curr Biol 17: 1938–1942, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Bhatnagar V, Xu G, Hamilton BA, Truong DM, Eraly SA, Wu W, Nigam SK. Analyses of 5' regulatory region polymorphisms in human SLC22A6 (OAT1) and SLC22A8 (OAT3). J Hum Genet 51: 575–580, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Bow DA, Perry JL, Simon JD, Pritchard JB. The impact of plasma protein binding on the renal transport of organic anions. J Pharmacol Exp Ther 316: 349–355, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Brady KP, Dushkin H, Fornzler D, Koike T, Magner F, Her H, Gullans S, Segre GV, Green RM, Beier DR. A novel putative transporter maps to the osteosclerosis (oc) mutation and is not expressed in the oc mutant mouse. Genomics 56: 254–261, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Brandoni A, Hazelhoff MH, Bulacio RP, Torres AM. Expression and function of renal and hepatic organic anion transporters in extrahepatic cholestasis. World J Gastroenterol 18: 6387–6397, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breljak D, Ljubojevic M, Balen D, Zlender V, Brzica H, Micek V, Kusan M, Anzai N, Sabolic I. Renal expression of organic anion transporter Oat5 in rats and mice exhibits the female-dominant sex differences. Histol Histopathol 25: 1385–1402, 2010. [DOI] [PubMed] [Google Scholar]
- 31.Brosens JJ, Tullet J, Varshochi R, Lam EW. Steroid receptor action. Best Pract Res Clin Obstet Gynaecol 18: 265–283, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Buist SC, Klaassen CD. Rat and mouse differences in gender-predominant expression of organic anion transporter (Oat1–3; Slc22a6–8) mRNA levels. Drug Metab Dispos 32: 620–625, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Burckhardt BC, Burckhardt G. Transport of organic anions across the basolateral membrane of proximal tubule cells. Rev Physiol Biochem Pharmacol 146: 95–158, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Burckhardt G. Drug transport by Organic Anion Transporters (OATs). Pharmacol Ther 136: 106–130, 2012. [DOI] [PubMed] [Google Scholar]
- 35.Burckhardt G, Burckhardt BC. In vitro and in vivo evidence of the importance of organic anion transporters (OATs) in drug therapy. In: Drug Transporters, edited by Fromm MF, Kim RB. Berlin: Springer, 2011, p. 29–104. [DOI] [PubMed] [Google Scholar]
- 36.Burnell JM, Kirby WM. Effectiveness of a new compound, benemid, in elevating serum penicillin concentrations. J Clin Invest 30: 697–700, 1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bush KT, Keller SH, Nigam SK. Genesis and reversal of the ischemic phenotype in epithelial cells. J Clin Invest 106: 621–626, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cha SH, Sekine T, Kusuhara H, Yu E, Kim JY, Kim DK, Sugiyama Y, Kanai Y, Endou H. Molecular cloning and characterization of multispecific organic anion transporter 4 expressed in the placenta. J Biol Chem 275: 4507–4512, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Chahine S, Campos A, O'Donnell MJ. Genetic knockdown of a single organic anion transporter alters the expression of functionally related genes in Malpighian tubules of Drosophila melanogaster. J Exp Biol 215: 2601–2610, 2012. [DOI] [PubMed] [Google Scholar]
- 40.Chahine S, Seabrooke S, O'Donnell MJ. Effects of genetic knock-down of organic anion transporter genes on secretion of fluorescent organic ions by Malpighian tubules of Drosophila melanogaster. Arch Insect Biochem Physiol 81: 228–240, 2012. [DOI] [PubMed] [Google Scholar]
- 41.Chen J, Terada T, Ogasawara K, Katsura T, Inui K. Adaptive responses of renal organic anion transporter 3 (OAT3) during cholestasis. Am J Physiol Renal Physiol 295: F247–F252, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Chen L, Hong C, Chen EC, Yee SW, Xu L, Almof EU, Wen C, Fujii K, Johns SJ, Stryke D, Ferrin TE, Simko J, Chen X, Costello JF, Giacomini KM. Genetic and epigenetic regulation of the organic cation transporter 3, SLC22A3. Pharmacogenomics J 13: 110–120, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng J, Grande JP. Cyclic nucleotide phosphodiesterase (PDE) inhibitors: novel therapeutic agents for progressive renal disease. Exp Biol Med 232: 38–51, 2007. [PubMed] [Google Scholar]
- 44.Cihlar T, Ho ES. Fluorescence-based assay for the interaction of small molecules with the human renal organic anion transporter 1. Anal Biochem 283: 49–55, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Cohen IR. Real and artificial immune systems: computing the state of the body. Nat Rev Immunol 7: 569–574, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Cropp CD, Komori T, Shima JE, Urban TJ, Yee SW, More SS, Giacomini KM. Organic anion transporter 2 (SLC22A7) is a facilitative transporter of cGMP. Mol Pharmacol 73: 1151–1158, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cui W, Sheng Q, Gao XZ. Artificial endocrine system and applications. In: Proceedings of the 25th Chinese Control Conference. Heilongjiang, China: Harbin, 2006, p. 1433–1437. [Google Scholar]
- 48.Dahlin A, Geier E, Stocker SL, Cropp CD, Grigorenko E, Bloomer M, Siegenthaler J, Xu L, Basile AS, Tang-Liu DD, Giacomini KM. Gene expression profiling of transporters in the solute carrier and ATP-binding cassette superfamilies in human eye substructures. Mol Pharm 10: 650–663, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dantzler WH, Wright SH. The molecular and cellular physiology of basolateral organic anion transport in mammalian renal tubules. Biochim Biophys Acta 1618: 185–193, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Davidson J, Abul HT, Milton AS, Rotondo D. Cytokines and cytokine inducers stimulate prostaglandin E2 entry into the brain. Pflügers Arch 442: 526–533, 2001. [DOI] [PubMed] [Google Scholar]
- 51.Dean S, Tang JI, Seckl JR, Nyirenda MJ. Developmental and tissue-specific regulation of hepatocyte nuclear factor 4-alpha (HNF4-alpha) isoforms in rodents. Gene Expr 14: 337–344, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deguchi T, Kusuhara H, Takadate A, Endou H, Otagiri M, Sugiyama Y. Characterization of uremic toxin transport by organic anion transporters in the kidney. Kidney Int 65: 162–174, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Denker BM, Nigam SK. Molecular structure and assembly of the tight junction. Am J Physiol Renal Physiol 274: F1–F9, 1998. [DOI] [PubMed] [Google Scholar]
- 54.Di Giusto G, Anzai N, Ruiz ML, Endou H, Torres AM. Expression and function of Oat1 and Oat3 in rat kidney exposed to mercuric chloride. Arch Toxicol 83: 887–897, 2009. [DOI] [PubMed] [Google Scholar]
- 55.Di Virgilio F, Steinberg TH, Silverstein SC. Inhibition of Fura-2 sequestration and secretion with organic anion transport blockers. Cell Calcium 11: 57–62, 1990. [DOI] [PubMed] [Google Scholar]
- 56.DiNatale BC, Murray IA, Schroeder JC, Flaveny CA, Lahoti TS, Laurenzana EM, Omiecinski CJ, Perdew GH. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci 115: 89–97, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duan P, Li S, Ai N, Hu L, Welsh WJ, You G. Potent inhibitors of human organic anion transporters 1 and 3 from clinical drug libraries: discovery and molecular characterization. Mol Pharm 9: 3340–3346, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duan P, Li S, You G. Regulation of human organic anion transporter 4 by parathyroid hormone-related protein and protein kinase A. Int J Biochem Mol Biol 3: 322–327, 2012. [PMC free article] [PubMed] [Google Scholar]
- 59.Duan P, Li S, You G. Transmembrane peptide as potent inhibitor of oligomerization and function of human organic anion transporter 1. Mol Pharmacol 79: 569–574, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duan P, You G. Short-term regulation of organic anion transporters. Pharmacol Ther 125: 55–61, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dunham I, Kundaje A, Aldred SF, Collins PJ, Davis CA, Doyle F, Epstein CB, Frietze S, Harrow J, Kaul R, Khatun J, Lajoie BR, Landt SG, Lee BK, Pauli F, Rosenbloom KR, Sabo P, Safi A, Sanyal A, Shoresh N, Simon JM, Song L, Trinklein ND, Altshuler RC, Birney E, Brown JB, Cheng C, Djebali S, Dong X, Ernst J, Furey TS, Gerstein M, Giardine B, Greven M, Hardison RC, Harris RS, Herrero J, Hoffman MM, Iyer S, Kelllis M, Kheradpour P, Lassmann T, Li Q, Lin X, Marinov GK, Merkel A, Mortazavi A, Parker SC, Reddy TE, Rozowsky J, Schlesinger F, Thurman RE, Wang J, Ward LD, Whitfield TW, Wilder SP, Wu W, Xi HS, Yip KY, Zhuang J, Bernstein BE, Green ED, Gunter C, Snyder M, Pazin MJ, Lowdon RF, Dillon LA, Adams LB, Kelly CJ, Zhang J, Wexler JR, Good PJ, Feingold EA, Crawford GE, Dekker J, Elinitski L, Farnham PJ, Giddings MC, Gingeras TR, Guigo R, Hubbard TJ, Kellis M, Kent WJ, Lieb JD, Margulies EH, Myers RM, Starnatoyannopoulos JA, Tennebaum SA, Weng Z, White KP, Wold B, Yu Y, Wrobel J, Risk BA, Gunawardena HP, Kuiper HC, Maier CW, Xie L, Chen X, Mikkelsen TS, Gillespie S, Goren A, Ram O, Zhang X, Wang L, Issner R, Coyne MJ, Durham T, Ku M, Truong T, Eaton ML, Dobin A, Tanzer A, Lagarde J, Lin W, Xue C, Williams BA, Zaleski C, Roder M, Kokocinski F, Abdelhamid RF, Alioto T, Antoshechkin I, Baer MT, Batut P, Bell I, Bell K, Chakrabortty S, Chrast J, Curado J, Derrien T, Drenkow J, Dumais E, Dumais J, Duttagupta R, Fastuca M, Fejes-Toth K, Ferreira P, Foissac S, Fullwood MJ, Gao H, Gonzalez D, Gordon A, Howald C, Jha S, Johnson R, Kapranov P, King B, Kingswood C, Li G, Luo OJ, Park E, Preall JB, Presaud K, Ribeca P, Robyr D, Ruan X, Sammeth M, Sandu KS, Schaeffer L, See LH, Shahab A, Skancke J, Suzuki AM, Takahashi H, Tilgner H, Trout D, Walters N, Wang H, Hayashizaki Y, Reymond A, Antonarakis SE, Hannon GJ, Ruan Y, Carninci P, Sloan CA, Learned K, Malladi VS, Wong MC, Barber GP, Cline MS, Dreszer TR, Heitner SG, Karolchik D, Kirkup VM, Meyer LR, Long JC, Maddren M, Raney BJ, Grasfeder LL, Giresi PG, Battenhouse A, Sheffield NC, Showers KA, London D, Bhinge AA, Shestak C, Schaner MR, Kim SK, Zhang ZZ, Mieczkowski PA, Mieczkowska JO, Liu Z, McDaniell RM, Ni Y, Rashid NU, Kim MJ, Adar S, Zhang Z, Wang T, Winter D, Keefe D, Iyer VR, Sandhu KS, Zheng M, Wang P, Gertz J, Vielmetter J, Partridge EC, Varley KE, Gasper C, Bansal A, Pepke S, Jain P, Amrhein H, Bowling KM, Anaya M, Cross MK, Muratet MA, Newberry KM, McCue K, Nesmith AS, Fisher-Aylor KI, Pusey B, DeSalvo G, Parker SL, Balasubramanian S, Davis NS, Meadows SK, Eggleston T, Newberry JS, Levy SE, Absher DM, Wong WH, Blow MJ, Visel A, Pennachio LA, Elnitski L, Petrykowska HM, Abyzov A, Aken B, Barrell D, Barson G, Berry A, Bignell A, Boychenko V, Bussotti G, Davidson C, Despacio-Reyes G, Diekhans M, Ezkurdia I, Frankish A, Gilbert J, Gonzalez JM, Griffiths E, Harte R, Hendrix DA, Hunt T, Jungreis I, Kay M, Khurana E, Leng J, Lin MF, Loveland J, Lu Z, Manthravadi D, Mariotti M, Mudge J, Mukherjee G, Notredame C, Pei B, Rodriguez JM, Saunders G, Sboner A, Searle S, Sisu C, Snow C, Steward C, Tapanari E, Tress ML, van Baren MJ, Washieti S, Wilming L, Zadissa A, Zhengdong Z, Brent M, Haussler D, Valencia A, Raymond A, Addleman N, Alexander RP, Auerbach RK, Bettinger K, Bhardwaj N, Boyle AP, Cao AR, Cayting P, Charos A, Cheng Y, Eastman C, Euskirchen G, Fleming JD, Grubert F, Habegger L, Hariharan M, Harmanci A, Iyenger S, Jin VX, Karczewski KJ, Kasowski M, Lacroute P, Lam H, Larnarre-Vincent N, Lian J, Lindahl-Allen M, Min R, Miotto B, Monahan H, Moqtaderi Z, Mu XJ, O'Geen H, Ouyang Z, Patacsil D, Raha D, Ramirez L, Reed B, Shi M, Slifer T, Witt H, Wu L, Xu X, Yan KK, Yang X, Struhl K, Weissman SM, Tenebaum SA, Penalva LO, Karmakar S, Bhanvadia RR, Choudhury A, Domanus M, Ma L, Moran J, Victorsen A, Auer T, Centarin L, Eichenlaub M, Gruhl F, Heerman S, Hoeckendorf B, Inoue D, Kellner T, Kirchmaier S, Mueller C, Reinhardt R, Schertel L, Schneider S, Sinn R, Wittbrodt B, Wittbrodt J, Jain G, Balasundaram G, Bates DL, Byron R, Canfield TK, Diegel MJ, Dunn D, Ebersol AK, Frum T, Garg K, Gist E, Hansen RS, Boatman L, Haugen E, Humbert R, Johnson AK, Johnson EM, Kutyavin TM, Lee K, Lotakis D, Maurano MT, Neph SJ, Neri FV, Nguyen ED, Qu H, Reynolds AP, Roach V, Rynes E, Sanchez ME, Sandstrom RS, Shafer AO, Stergachis AB, Thomas S, Vernot B, Vierstra J, Vong S, Weaver MA, Yan Y, Zhang M, Akey JA, Bender M, Dorschner MO, Groudine M, MacCoss MJ, Navas P, Stamatoyannopoulos G, Stamatoyannopoulos JA, Beal K, Brazma A, Flicek P, Johnson N, Lukk M, Luscombe NM, Sobral D, Vaquerizas JM, Batzoglou S, Sidow A, Hussami N, Kyriazopoulou-Panagiotopoulou S, Libbrecht MW, Schaub MA, Miller W, Bickel PJ, Banfai B, Boley NP, Huang H, Li JJ, Noble WS, Bilmes JA, Buske OJ, Sahu AO, Kharchenko PV, Park PJ, Baker D, Taylor J, Lochovsky L. An integrated encyclopedia of DNA elements in the human genome. Nature 489: 57–74, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eder K, Ringseis R. The role of peroxisome proliferator-activated receptor alpha in transcriptional regulation of novel organic cation transporters. Eur J Pharmacol 628: 1–5, 2010. [DOI] [PubMed] [Google Scholar]
- 63.Ekaratanawong S, Anzai N, Jutabha P, Miyazaki H, Noshiro R, Takeda M, Kanai Y, Sophasan S, Endou H. Human organic anion transporter 4 is a renal apical organic anion/dicarboxylate exchanger in the proximal tubules. J Pharmacol Sci 94: 297–304, 2004. [DOI] [PubMed] [Google Scholar]
- 64.Emami Riedmaier A, Nies AT, Schaeffeler E, Schwab M. Organic anion transporters and their implications in pharmacotherapy. Pharmacol Rev 64: 421–449, 2012. [DOI] [PubMed] [Google Scholar]
- 65.Engelbrecht AP. Computational Intelligence: An Introduction. Chichester, UK: Wiley, 2007, p. 628. [Google Scholar]
- 66.Engstrom K, Ameer S, Bernaudat L, Drasch G, Baeuml J, Skerfving S, Bose-O'Reilly S, Broberg K. Polymorphisms in genes encoding potential mercury transporters and urine mercury concentrations in populations exposed to mercury vapor from gold mining. Environ Health Perspect 121: 85–91, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, Hosoyamada M, Takeda M, Sekine T, Igarashi T, Matsuo H, Kikuchi Y, Oda T, Ichida K, Hosoya T, Shimokata K, Niwa T, Kanai Y, Endou H. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 417: 447–452, 2002. [DOI] [PubMed] [Google Scholar]
- 68.Eraly SA, Blantz RC, Bhatnagar V, Nigam SK. Novel aspects of renal organic anion transporters. Curr Opin Nephrol Hypertens 12: 551–558, 2003. [DOI] [PubMed] [Google Scholar]
- 69.Eraly SA, Bush KT, Sampogna RV, Bhatnagar V, Nigam SK. The molecular pharmacology of organic anion transporters: from DNA to FDA? Mol Pharmacol 65: 479–487, 2004. [DOI] [PubMed] [Google Scholar]
- 70.Eraly SA, Hamilton BA, Nigam SK. Organic anion and cation transporters occur in pairs of similar and similarly expressed genes. Biochem Biophys Res Commun 300: 333–342, 2003. [DOI] [PubMed] [Google Scholar]
- 71.Eraly SA, Monte JC, Nigam SK. Novel slc22 transporter homologs in fly, worm, and human clarify the phylogeny of organic anion and cation transporters. Physiol Genomics 18: 12–24, 2004. [DOI] [PubMed] [Google Scholar]
- 72.Eraly SA, Nigam SK. Novel human cDNAs homologous to Drosophila Orct and mammalian carnitine transporters. Biochem Biophys Res Commun 297: 1159–1166, 2002. [DOI] [PubMed] [Google Scholar]
- 73.Eraly SA, Vallon V, Rieg T, Gangoiti JA, Wikoff WR, Siuzdak G, Barshop BA, Nigam SK. Multiple organic anion transporters contribute to net renal excretion of uric acid. Physiol Genomics 33: 180–192, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eraly SA, Vallon V, Vaughn DA, Gangoiti JA, Richter K, Nagle M, Monte JC, Rieg T, Truong DM, Long JM, Barshop BA, Kaler G, Nigam SK. Decreased renal organic anion secretion and plasma accumulation of endogenous organic anions in OAT1 knock-out mice. J Biol Chem 281: 5072–5083, 2006. [DOI] [PubMed] [Google Scholar]
- 75.Filakovic P, Petek Eric A, Radanovic-Grguric L. Metabolic syndrome and psychotropic medications. Med Glas 9: 180–188, 2012. [PubMed] [Google Scholar]
- 76.Forrest LR, Kramer R, Ziegler C. The structural basis of secondary active transport mechanisms. Biochim Biophys Acta 1807: 167–188, 2011. [DOI] [PubMed] [Google Scholar]
- 77.Fujita T, Brown C, Carlson EJ, Taylor T, de la Cruz M, Johns SJ, Stryke D, Kawamoto M, Fujita K, Castro R, Chen CW, Lin ET, Brett CM, Burchard EG, Ferrin TE, Huang CC, Leabman MK, Giacomini KM. Functional analysis of polymorphisms in the organic anion transporter, SLC22A6 (OAT1). Pharmacogenet Genomics 15: 201–209, 2005. [DOI] [PubMed] [Google Scholar]
- 78.Gallegos TF, Martovetsky G, Kouznetsova V, Bush KT, Nigam SK. Organic anion and cation SLC22 ”drug“ transporter (Oat1, Oat3, and Oct1) regulation during development and maturation of the kidney proximal tubule. PloS One 7: e40796, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L. Membrane transporters in drug development. Nat Rev Drug Discov 9: 215–236, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gutgesell A, Ringseis R, Brandsch C, Stangl GI, Hirche F, Eder K. Peroxisome proliferator-activated receptor alpha and enzymes of carnitine biosynthesis in the liver are down-regulated during lactation in rats. Metabolism 58: 226–232, 2009. [DOI] [PubMed] [Google Scholar]
- 81.Hagos Y, Krick W, Braulke T, Muhlhausen C, Burckhardt G, Burckhardt BC. Organic anion transporters OAT1 and OAT4 mediate the high affinity transport of glutarate derivatives accumulating in patients with glutaric acidurias. Pflügers Arch 457: 223–231, 2008. [DOI] [PubMed] [Google Scholar]
- 82.Hagos Y, Stein D, Ugele B, Burckhardt G, Bahn A. Human renal organic anion transporter 4 operates as an asymmetric urate transporter. J Am Soc Nephrol 18: 430–439, 2007. [DOI] [PubMed] [Google Scholar]
- 83.Han YF, Fan XH, Wang XJ, Sun K, Xue H, Li WJ, Wang YB, Chen JZ, Zhen YS, Zhang WL, Zhou X, Hui R. Association of intergenic polymorphism of organic anion transporter 1 and 3 genes with hypertension and blood pressure response to hydrochlorothiazide. Am J Hypertens 24: 340–346, 2011. [DOI] [PubMed] [Google Scholar]
- 84.Hao CM, Breyer MD. Physiological regulation of prostaglandins in the kidney. Annu Rev Physiol 70: 357–377, 2008. [DOI] [PubMed] [Google Scholar]
- 85.Hardwick JP, Eckman K, Lee YK, Abdelmegeed MA, Esterle A, Chilian WM, Chiang JY, Song BJ. Eicosanoids in metabolic syndrome. Adv Pharmacol 66: 157–266, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hasegawa M, Kusuhara H, Endou H, Sugiyama Y. Contribution of organic anion transporters to the renal uptake of anionic compounds and nucleoside derivatives in rat. J Pharmacol Exp Ther 305: 1087–1097, 2003. [DOI] [PubMed] [Google Scholar]
- 87.Hatano R, Mukouchi H, Matsumoto Y, Kawaguchi K, Kazama I, Endo Y, Toyama H, Ejima Y, Kurosawa S, Kanai Y, Matsubara M, Asano S. Glucocorticoid mediates the transcription of OAT-PG, a kidney-specific prostaglandin transporter. Pflügers Arch 466: 925–935, 2014. [DOI] [PubMed] [Google Scholar]
- 88.Hatano R, Onoe K, Obara M, Matsubara M, Kanai Y, Muto S, Asano S. Sex hormones induce a gender-related difference in renal expression of a novel prostaglandin transporter, OAT-PG, influencing basal PGE2 concentration. Am J Physiol Renal Physiol 302: F342–F349, 2012. [DOI] [PubMed] [Google Scholar]
- 89.Heise M, Lautem A, Knapstein J, Schattenberg JM, Hoppe-Lotichius M, Foltys D, Weiler N, Zimmermann A, Schad A, Grundemann D, Otto G, Galle PR, Schuchmann M, Zimmermann T. Downregulation of organic cation transporters OCT1 (SLC22A1) and OCT3 (SLC22A3) in human hepatocellular carcinoma and their prognostic significance. BMC Cancer 12: 109, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hilgendorf C, Ahlin G, Seithel A, Artursson P, Ungell AL, Karlsson J. Expression of thirty-six drug transporter genes in human intestine, liver, kidney, and organotypic cell lines. Drug Metab Dispos 35: 1333–1340, 2007. [DOI] [PubMed] [Google Scholar]
- 91.Hirsch GH, Hook JB. Maturation of renal organic acid transport: substrate stimulation by penicillin and p-aminohippurate (PAH). J Pharmacol Exp Ther 171: 103–108, 1970. [PubMed] [Google Scholar]
- 92.Holland IB. ABC transporters, mechanisms and biology: an overview. Essays Biochem 50: 1–17, 2011. [DOI] [PubMed] [Google Scholar]
- 93.Hong M, Xu W, Yoshida T, Tanaka K, Wolff DJ, Zhou F, Inouye M, You G. Human organic anion transporter hOAT1 forms homooligomers. J Biol Chem 280: 32285–32290, 2005. [DOI] [PubMed] [Google Scholar]
- 94.Hong YH, Nishimura Y, Hishikawa D, Tsuzuki H, Miyahara H, Gotoh C, Choi KC, Feng DD, Chen C, Lee HG, Katoh K, Roh SG, Sasaki S. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology 146: 5092–5099, 2005. [DOI] [PubMed] [Google Scholar]
- 95.Hook JB, Hewitt WR. Development of mechanisms for drug excretion. Am J Med 62: 497–506, 1977. [DOI] [PubMed] [Google Scholar]
- 96.Hosoya K, Makihara A, Tsujikawa Y, Yoneyama D, Mori S, Terasaki T, Akanuma S, Tomi M, Tachikawa M. Roles of inner blood-retinal barrier organic anion transporter 3 in the vitreous/retina-to-blood efflux transport of p-aminohippuric acid, benzylpenicillin, and 6-mercaptopurine. J Pharmacol Exp Ther 329: 87–93, 2009. [DOI] [PubMed] [Google Scholar]
- 97.Hosoyamada M, Takiue Y, Morisaki H, Cheng J, Ikawa M, Okabe M, Morisaki T, Ichida K, Hosoya T, Shibasaki T. Establishment and analysis of SLC22A12 (URAT1) knockout mouse. Nucleosides Nucleotides Nucleic Acids 29: 314–320, 2010. [DOI] [PubMed] [Google Scholar]
- 98.Hu QH, Jiao RQ, Wang X, Lv YZ, Kong LD. Simiao pill ameliorates urate underexcretion and renal dysfunction in hyperuricemic mice. J Ethnopharmacol 128: 685–692, 2010. [DOI] [PubMed] [Google Scholar]
- 99.Huang ZL, Urade Y, Hayaishi O. Prostaglandins and adenosine in the regulation of sleep and wakefulness. Curr Opin Pharmacol 7: 33–38, 2007. [DOI] [PubMed] [Google Scholar]
- 100.Hwang JS, Park EY, Kim WY, Yang CW, Kim J. Expression of OAT1 and OAT3 in differentiating proximal tubules of the mouse kidney. Histol Histopathol 25: 33–44, 2010. [DOI] [PubMed] [Google Scholar]
- 101.Iacobucci I, Lonetti A, Candoni A, Sazzini M, Papayannidis C, Formica S, Ottaviani E, Ferrari A, Michelutti A, Simeone E, Astolfi A, Abbenante MC, Parisi S, Cattina F, Malagola M, Russo D, Damiani D, Gherlinzoni F, Gottardi M, Baccarani M, Fanin R, Martinelli G. Profiling of drug-metabolizing enzymes/transporters in CD33+ acute myeloid leukemia patients treated with Gemtuzumab-Ozogamicin and Fludarabine, Cytarabine and Idarubicin. Pharmacogenomics J 13: 335–341, 2012. [DOI] [PubMed] [Google Scholar]
- 102.Imai S, Kikuchi R, Kusuhara H, Sugiyama Y. DNA methylation and histone modification profiles of mouse organic anion transporting polypeptides. Drug Metab Dispos 41: 72–78, 2013. [DOI] [PubMed] [Google Scholar]
- 103.Imamura Y, Murayama N, Okudaira N, Kurihara A, Okazaki O, Izumi T, Inoue K, Yuasa H, Kusuhara H, Sugiyama Y. Prediction of fluoroquinolone-induced elevation in serum creatinine levels: a case of drug-endogenous substance interaction involving the inhibition of renal secretion. Clin Pharmacol Ther 89: 81–88, 2011. [DOI] [PubMed] [Google Scholar]
- 104.Ingelman-Sundberg M, Zhong XB, Hankinson O, Beedanagari S, Yu AM, Peng L, Osawa Y. Potential role of epigenetic mechanisms in the regulation of drug metabolism and transport. Drug Metab Dispos 41: 1725–1731, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Irukayama-Tomobe Y, Tanaka H, Yokomizo T, Hashidate-Yoshida T, Yanagisawa M, Sakurai T. Aromatic D-amino acids act as chemoattractant factors for human leukocytes through a G protein-coupled receptor, GPR109B. Proc Natl Acad Sci USA 106: 3930–3934, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ito S, Alcorn J. Xenobiotic transporter expression and function in the human mammary gland. Adv Drug Deliv Rev 55: 653–665, 2003. [DOI] [PubMed] [Google Scholar]
- 107.Jacobs LF. From chemotaxis to the cognitive map: the function of olfaction. Proc Natl Acad Sci USA 109Suppl 1: 10693–10700, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jacobsson JA, Haitina T, Lindblom J, Fredriksson R. Identification of six putative human transporters with structural similarity to the drug transporter SLC22 family. Genomics 90: 595–609, 2007. [DOI] [PubMed] [Google Scholar]
- 109.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet 33Suppl: 245–254, 2003. [DOI] [PubMed] [Google Scholar]
- 110.Jang WC, Nam YH, Ahn YC, Park SM, Yoon IK, Choe JY, Park SH, Her M, Kim SK. G109T polymorphism of SLC22A12 gene is associated with serum uric acid level, but not with metabolic syndrome. Rheumatol Int 32: 2257–2263, 2012. [DOI] [PubMed] [Google Scholar]
- 111.Jung KY, Takeda M, Kim DK, Tojo A, Narikawa S, Yoo BS, Hosoyamada M, Cha SH, Sekine T, Endou H. Characterization of ochratoxin A transport by human organic anion transporters. Life Sci 69: 2123–2135, 2001. [DOI] [PubMed] [Google Scholar]
- 112.Kaler G, Truong DM, Khandelwal A, Nagle M, Eraly SA, Swaan PW, Nigam SK. Structural variation governs substrate specificity for organic anion transporter (OAT) homologs. Potential remote sensing by OAT family members. J Biol Chem 282: 23841–23853, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kaler G, Truong DM, Sweeney DE, Logan DW, Nagle M, Wu W, Eraly SA, Nigam SK. Olfactory mucosa-expressed organic anion transporter, Oat6, manifests high affinity interactions with odorant organic anions. Biochem Biophys Res Commun 351: 872–876, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Karns R, Zhang G, Sun G, Rao Indugula S, Cheng H, Havas-Augustin D, Novokmet N, Rudan D, Durakovic Z, Missoni S, Chakraborty R, Rudan P, Deka R. Genome-wide association of serum uric acid concentration: replication of sequence variants in an island population of the Adriatic coast of Croatia. Ann Hum Genet 76: 121–127, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kato Y, Sai Y, Yoshida K, Watanabe C, Hirata T, Tsuji A. PDZK1 directly regulates the function of organic cation/carnitine transporter OCTN2. Mol Pharmacol 67: 734–743, 2005. [DOI] [PubMed] [Google Scholar]
- 116.Kazama I, Matsubara M, Kanai Y, Hatano R, Asano S, Endo Y, Toyama H, Ejima Y, Kurosawa S, Maruyama Y. Decreased expression of a novel prostaglandin transporter, OAT-PG, facilitates renocortical PGE2 accumulation during rat pregnancy. Gynecol Obstet Invest 76: 163–170, 2013. [DOI] [PubMed] [Google Scholar]
- 117.Kikuchi R, Kusuhara H, Hattori N, Kim I, Shiota K, Gonzalez FJ, Sugiyama Y. Regulation of tissue-specific expression of the human and mouse urate transporter 1 gene by hepatocyte nuclear factor 1 alpha/beta and DNA methylation. Mol Pharmacol 72: 1619–1625, 2007. [DOI] [PubMed] [Google Scholar]
- 118.Kikuchi R, Kusuhara H, Hattori N, Shiota K, Kim I, Gonzalez FJ, Sugiyama Y. Regulation of the expression of human organic anion transporter 3 by hepatocyte nuclear factor 1alpha/beta and DNA methylation. Mol Pharmacol 70: 887–896, 2006. [DOI] [PubMed] [Google Scholar]
- 119.Kimura H, Takeda M, Narikawa S, Enomoto A, Ichida K, Endou H. Human organic anion transporters and human organic cation transporters mediate renal transport of prostaglandins. J Pharmacol Exp Ther 301: 293–298, 2002. [DOI] [PubMed] [Google Scholar]
- 120.Kiselyuk A, Lee SH, Farber-Katz S, Zhang M, Athavankar S, Cohen T, Pinkerton AB, Ye M, Bushway P, Richardson AD, Hostetler HA, Rodriguez-Lee M, Huang L, Spangler B, Smith L, Higginbotham J, Cashman J, Freeze H, Itkin-Ansari P, Dawson MI, Schroeder F, Cang Y, Mercola M, Levine F. HNF4alpha antagonists discovered by a high-throughput screen for modulators of the human insulin promoter. Chem Biol 19: 806–818, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kittayaruksakul S, Soodvilai S, Asavapanumas N, Muanprasat C, Chatsudthipong V. Liver X receptor activation downregulates organic anion transporter 1 (OAT1) in the renal proximal tubule. Am J Physiol Renal Physiol 302: F552–F560, 2012. [DOI] [PubMed] [Google Scholar]
- 122.Klahr S, Buerkert J, Purkerson ML. Role of dietary factors in the progression of chronic renal disease. Kidney Int 24: 579–587, 1983. [DOI] [PubMed] [Google Scholar]
- 123.Klein K, Jungst C, Mwinyi J, Stieger B, Krempler F, Patsch W, Eloranta JJ, Kullak-Ublick GA. The human organic anion transporter genes OAT5 and OAT7 are transactivated by hepatocyte nuclear factor-1alpha (HNF-1alpha). Mol Pharm 78: 1079–1087, 2010. [DOI] [PubMed] [Google Scholar]
- 124.Kobayashi Y, Ohshiro N, Sakai R, Ohbayashi M, Kohyama N, Yamamoto T. Transport mechanism and substrate specificity of human organic anion transporter 2 (hOat2 [SLC22A7]). J Pharm Pharmacol 57: 573–578, 2005. [DOI] [PubMed] [Google Scholar]
- 125.Kobayashi Y, Ohshiro N, Shibusawa A, Sasaki T, Tokuyama S, Sekine T, Endou H, Yamamoto T. Isolation, characterization and differential gene expression of multispecific organic anion transporter 2 in mice. Mol Pharmacol 62: 7–14, 2002. [DOI] [PubMed] [Google Scholar]
- 126.Kobayashi Y, Ohshiro N, Tsuchiya A, Kohyama N, Ohbayashi M, Yamamoto T. Renal transport of organic compounds mediated by mouse organic anion transporter 3 (mOat3): further substrate specificity of mOat3. Drug Metab Dispos 32: 479–483, 2004. [DOI] [PubMed] [Google Scholar]
- 127.Koepsell H. The SLC22 family with transporters of organic cations, anions and zwitterions. Mol Aspects Med 34: 413–435, 2013. [DOI] [PubMed] [Google Scholar]
- 128.Koh AS, Simmons-Willis TA, Pritchard JB, Grassl SM, Ballatori N. Identification of a mechanism by which the methylmercury antidotes N-acetylcysteine and dimercaptopropanesulfonate enhance urinary metal excretion: transport by the renal organic anion transporter-1. Mol Pharmacol 62: 921–926, 2002. [DOI] [PubMed] [Google Scholar]
- 129.Kojima R, Sekine T, Kawachi M, Cha SH, Suzuki Y, Endou H. Immunolocalization of multispecific organic anion transporters, OAT1, OAT2, and OAT3, in rat kidney. J Am Soc Nephrol 13: 848–857, 2002. [DOI] [PubMed] [Google Scholar]
- 130.Kolz M, Johnson T, Sanna S, Teumer A, Vitart V, Perola M, Mangino M, Albrecht E, Wallace C, Farrall M, Johansson A, Nyholt DR, Aulchenko Y, Beckmann JS, Bergmann S, Bochud M, Brown M, Campbell H, Connell J, Dominiczak A, Homuth G, Lamina C, McCarthy MI, Meitinger T, Mooser V, Munroe P, Nauck M, Peden J, Prokisch H, Salo P, Salomaa V, Samani NJ, Schlessinger D, Uda M, Volker U, Waeber G, Waterworth D, Wang-Sattler R, Wright AF, Adamski J, Whitfield JB, Gyllensten U, Wilson JF, Rudan I, Pramstaller P, Watkins H, Doering A, Wichmann HE, Spector TD, Peltonen L, Volzke H, Nagaraja R, Vollenweider P, Caulfield M, Illig T, Gieger C. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet 5: e1000504, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kouznetsova VL, Tsigelny IF, Nagle MA, Nigam SK. Elucidation of common pharmacophores from analysis of targeted metabolites transported by the multispecific drug transporter-Organic anion transporter1 (Oat1). Bioorg Med Chem 19: 3320–3340, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kusch-Poddar M, Drewe J, Fux I, Gutmann H. Evaluation of the immortalized human brain capillary endothelial cell line BB19 as a human cell culture model for the blood-brain barrier. Brain Res 1064: 21–31, 2005. [DOI] [PubMed] [Google Scholar]
- 133.Kusuhara H, Sekine T, Utsunomiya-Tate N, Tsuda M, Kojima R, Cha SH, Sugiyama Y, Kanai Y, Endou H. Molecular cloning and characterization of a new multispecific organic anion transporter from rat brain. J Biol Chem 274: 13675–13680, 1999. [DOI] [PubMed] [Google Scholar]
- 134.Kuznetsov G, Nigam SK. Folding of secretory and membrane proteins. N Engl J Med 339: 1688–1695, 1998. [DOI] [PubMed] [Google Scholar]
- 135.Kwak JO, Kim HW, Oh KJ, Ko CB, Park H, Cha SH. Characterization of mouse organic anion transporter 5 as a renal steroid sulfate transporter. J Steroid Biochem Mol Biol 97: 369–375, 2005. [DOI] [PubMed] [Google Scholar]
- 136.Lamhonwah AM, Mai L, Chung C, Lamhonwah D, Ackerley C, Tein I. Upregulation of mammary gland OCTNs maintains carnitine homeostasis in suckling infants. Biochem Biophys Res Commun 404: 1010–1015, 2011. [DOI] [PubMed] [Google Scholar]
- 137.Li S, Zhang Q, You G. Three ubiquitination sites of organic anion transporter-1 synergistically mediate protein kinase C-dependent endocytosis of the transporter. Mol Pharm 84: 139–146, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ljubojevic M, Balen D, Breljak D, Kusan M, Anzai N, Bahn A, Burckhardt G, Sabolic I. Renal expression of organic anion transporter OAT2 in rats and mice is regulated by sex hormones. Am J Physiol Renal Physiol 292: F361–F372, 2007. [DOI] [PubMed] [Google Scholar]
- 139.Ljubojevic M, Herak-Kramberger CM, Hagos Y, Bahn A, Endou H, Burckhardt G, Sabolic I. Rat renal cortical OAT1 and OAT3 exhibit gender differences determined by both androgen stimulation and estrogen inhibition. Am J Physiol Renal Physiol 287: F124–F138, 2004. [DOI] [PubMed] [Google Scholar]
- 140.Ljungberg MC, Ali YO, Zhu J, Wu CS, Oka K, Zhai RG, Lu HC. CREB-activity and nmnat2 transcription are downregulated prior to neurodegeneration, while NMNAT2 over-expression is neuroprotective, in a mouse model of human tauopathy. Hum Mol Genet 21: 251–267, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lopez-Lopez E, Ballesteros J, Pinan MA, Sanchez de Toledo J, Garcia de Andoin N, Garcia-Miguel P, Navajas A, Garcia-Orad A. Polymorphisms in the methotrexate transport pathway: a new tool for MTX plasma level prediction in pediatric acute lymphoblastic leukemia. Pharmacogenet Genomics 23: 53–61, 2013. [DOI] [PubMed] [Google Scholar]
- 142.Lopez-Nieto CE, Nigam SK. Selective amplification of protein-coding regions of large sets of genes using statistically designed primer sets. Nat Biotechnol 14: 857–861, 1996. [DOI] [PubMed] [Google Scholar]
- 143.Lopez-Nieto CE, You G, Barros EJG, Beier DR, Nigam SK. Molecular cloning and characterization of a novel transporter protein with very high expression in the kidney (Abstract). J Am Soc Nephrol 7: 1301, 1996. [Google Scholar]
- 144.Lopez-Nieto CE, You G, Bush KT, Barros EJ, Beier DR, Nigam SK. Molecular cloning and characterization of NKT, a gene product related to the organic cation transporter family that is almost exclusively expressed in the kidney. J Biol Chem 272: 6471–6478, 1997. [DOI] [PubMed] [Google Scholar]
- 145.Lopez-Nieto CE, You G, Bush KT, Barros EJ, Beier DR, Nigam SK. Mus musculus kidney-specific transport protein mRNA. GenBank 1996www.ncbi.nlm.nih.gov. [Google Scholar]
- 146.Macfarlane GT, Macfarlane S. Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int 95: 50–60, 2012. [DOI] [PubMed] [Google Scholar]
- 147.Maher JM, Slitt AL, Callaghan TN, Cheng X, Cheung C, Gonzalez FJ, Klaassen CD. Alterations in transporter expression in liver, kidney, and duodenum after targeted disruption of the transcription factor HNF1alpha. Biochem Pharmacol 72: 512–522, 2006. [DOI] [PubMed] [Google Scholar]
- 148.Mann DL. Hands-On Systematic Innovation. Ieper, Belgium: CREAX Press, 2002, p. 470. [Google Scholar]
- 149.Martovetsky G, Tee JB, Nigam SK. Hepatocyte nuclear factors 4a and 1a (Hnf4a and Hnf1a) regulate kidney developmental expression of drug-metabolizing enzymes and drug transporters. Mol Pharmacol 84: 808–823, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Masereeuw R, van den Bergh EJ, Bindels RJ, Russel FG. Characterization of fluorescein transport in isolated proximal tubular cells of the rat: evidence for mitochondrial accumulation. J Pharmacol Exp Ther 269: 1261–1267, 1994. [PubMed] [Google Scholar]
- 151.Mathers JC, McKay JA. Epigenetics: potential contribution to fetal programming. Adv Exp Med Biol 646: 119–123, 2009. [DOI] [PubMed] [Google Scholar]
- 152.Matsumura H, Honda K, Choi WS, Inoue S, Sakai T, Hayaishi O. Evidence that brain prostaglandin E2 is involved in physiological sleep-wake regulation in rats. Proc Natl Acad Sci USA 86: 5666–5669, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.McAdams-Demarco MA, Maynard JW, Baer AN, Kao LW, Kottgen A, Coresh J. A urate gene-by-diuretic interaction and gout risk in participants with hypertension: results from the ARIC study. Ann Rheum Dis 72: 701–706, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Meeks JP, Arnson HA, Holy TE. Representation and transformation of sensory information in the mouse accessory olfactory system. Nat Neurosci 13: 723–730, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Miller DS, Stewart DE, Pritchard JB. Intracellular compartmentation of organic anions within renal cells. Am J Physiol Regul Integr Comp Physiol 264: R882–R890, 1993. [DOI] [PubMed] [Google Scholar]
- 156.Miyajima M, Kusuhara H, Fujishima M, Adachi Y, Sugiyama Y. Organic anion transporter 3 mediates the efflux transport of an amphipathic organic anion, dehydroepiandrosterone sulfate, across the blood-brain barrier in mice. Drug Metab Dispos 39: 814–819, 2011. [DOI] [PubMed] [Google Scholar]
- 157.Miyazaki H, Anzai N, Ekaratanawong S, Sakata T, Shin HJ, Jutabha P, Hirata T, He X, Nonoguchi H, Tomita K, Kanai Y, Endou H. Modulation of renal apical organic anion transporter 4 function by two PDZ domain-containing proteins. J Am Soc Nephrol 16: 3498–3506, 2005. [DOI] [PubMed] [Google Scholar]
- 158.Mizuno N, Takahashi T, Iwase Y, Kusuhara H, Niwa T, Sugiyama Y. Human organic anion transporters 1 (hOAT1/SLC22A6) and 3 (hOAT3/SLC22A8) transport edaravone (MCI-186; 3-methyl-1-phenyl-2-pyrazolin-5-one) and its sulfate conjugate. Drug Metab Dispos 35: 1429–1434, 2007. [DOI] [PubMed] [Google Scholar]
- 159.Moller JV, Sheikh MI. Renal organic anion transport system: pharmacological, physiological, and biochemical aspects. Pharmacol Rev 34: 315–358, 1982. [PubMed] [Google Scholar]
- 160.Monte JC, Nagle MA, Eraly SA, Nigam SK. Identification of a novel murine organic anion transporter family member, OAT6, expressed in olfactory mucosa. Biochem Biophys Res Commun 323: 429–436, 2004. [DOI] [PubMed] [Google Scholar]
- 161.Mori K, Ogawa Y, Ebihara K, Aoki T, Tamura N, Sugawara A, Kuwahara T, Ozaki S, Mukoyama M, Tashiro K, Tanaka I, Nakao K. Kidney-specific expression of a novel mouse organic cation transporter-like protein. FEBS Lett 417: 371–374, 1997. [DOI] [PubMed] [Google Scholar]
- 162.Mori S, Takanaga H, Ohtsuki S, Deguchi T, Kang YS, Hosoya K, Terasaki T. Rat organic anion transporter 3 (rOAT3) is responsible for brain-to-blood efflux of homovanillic acid at the abluminal membrane of brain capillary endothelial cells. J Cereb Blood Flow Metab 23: 432–440, 2003. [DOI] [PubMed] [Google Scholar]
- 163.Morrissey KM, Wen CC, Johns SJ, Zhang L, Huang SM, Giacomini KM. The UCSF-FDA TransPortal: a public drug transporter database. Clin Pharmacol Ther 92: 545–546, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Motojima M, Hosokawa A, Yamato H, Muraki T, Yoshioka T. Uraemic toxins induce proximal tubular injury via organic anion transporter 1-mediated uptake. Br J Pharmacol 135: 555–563, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mount DB. The kidney in hyperuricemia and gout. Curr Opin Nephrol Hypertens 22: 216–223, 2013. [DOI] [PubMed] [Google Scholar]
- 166.Mount DB, Kwon CY, Zandi-Nejad K. Renal urate transport. Rheum Dis Clin North Am 32: 313–331, 2006. [DOI] [PubMed] [Google Scholar]
- 167.Mulla H. Understanding developmental pharmacodynamics: importance for drug development and clinical practice. Paediatr Drugs 12: 223–233, 2010. [DOI] [PubMed] [Google Scholar]
- 168.Nagle M, Truong DM, Bhatnagar V, Kaler G, Bush KT, Wu W, Eraly SA, Nigam SK. Organic Anion Transporters. In: Drug Transporters: Molecular Characterization and Role in Drug Disposition, edited by You G, Morris ME. Hoboken, NJ: Wiley, 2006, p. 51–73. [Google Scholar]
- 169.Nagle MA, Truong DM, Dnyanmote AV, Ahn SY, Eraly SA, Wu W, Nigam SK. Analysis of three-dimensional systems for developing and mature kidneys clarifies the role of OAT1 and OAT3 in antiviral handling. J Biol Chem 286: 243–251, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nagle MA, Wu W, Eraly SA, Nigam SK. Organic anion transport pathways in antiviral handling in choroid plexus in Oat1 (Slc22a6) and Oat3 (Slc22a8) deficient tissue. Neurosci Lett 534: 133–138, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nakakariya M, Shima Y, Shirasaka Y, Mitsuoka K, Nakanishi T, Tamai I. Organic anion transporter OAT1 is involved in renal handling of citrulline. Am J Physiol Renal Physiol 297: F71–F79, 2009. [DOI] [PubMed] [Google Scholar]
- 172.Naud J, Michaud J, Boisvert C, Desbiens K, Leblond FA, Mitchell A, Jones C, Bonnardeaux A, Pichette V. Down-regulation of intestinal drug transporters in chronic renal failure in rats. J Pharmacol Exp Ther 320: 978–985, 2007. [DOI] [PubMed] [Google Scholar]
- 173.Naud J, Michaud J, Leblond FA, Lefrancois S, Bonnardeaux A, Pichette V. Effects of chronic renal failure on liver drug transporters. Drug Metab Dispos 36: 124–128, 2008. [DOI] [PubMed] [Google Scholar]
- 174.Naud J, Nolin TD, Leblond FA, Pichette V. Current understanding of drug disposition in kidney disease. J Clin Pharmacol 52: 10S–22S, 2012. [DOI] [PubMed] [Google Scholar]
- 175.Nevitt GA. Sensory ecology on the high seas: the odor world of the procellariiform seabirds. J Exp Biol 211: 1706–1713, 2008. [DOI] [PubMed] [Google Scholar]
- 176.Nigam SK, Bhatnagar V. How much do we know about drug handling by SLC and ABC drug transporters in children. Clin Pharmacol Ther 94: 27–29, 2013. [DOI] [PubMed] [Google Scholar]
- 177.Nigam SK, Bush KT, Bhatnagar V. Drug and toxicant handling by the OAT organic anion transporters in the kidney and other tissues. Nat Clin Pract Nephrol 3: 443–448, 2007. [DOI] [PubMed] [Google Scholar]
- 178.Nishiwaki T, Daigo Y, Tamari M, Fujii Y, Nakamura Y. Molecular cloning, mapping, and characterization of two novel human genes, ORCTL3 and ORCTL4, bearing homology to organic-cation transporters. Cytogenet Cell Genet 83: 251–255, 1998. [DOI] [PubMed] [Google Scholar]
- 179.Niwa T, Tsukushi S, Ise M, Miyazaki T, Tsubakihara Y, Owada A, Shiigai T. Indoxyl sulfate and progression of renal failure: effects of a low-protein diet and oral sorbent on indoxyl sulfate production in uremic rats and undialyzed uremic patients. Miner Electrolyte Metab 23: 179–184, 1997. [PubMed] [Google Scholar]
- 180.Nodari F, Hsu FF, Fu X, Holekamp TF, Kao LF, Turk J, Holy TE. Sulfated steroids as natural ligands of mouse pheromone-sensing neurons. J Neurosci 28: 6407–6418, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nolin TD. Altered nonrenal drug clearance in ESRD. Curr Opin Nephrol Hypertens 17: 555–559, 2008. [DOI] [PubMed] [Google Scholar]
- 182.Nozaki Y, Kusuhara H, Kondo T, Hasegawa M, Shiroyanagi Y, Nakazawa H, Okano T, Sugiyama Y. Characterization of the uptake of organic anion transporter (OAT) 1 and OAT3 substrates by human kidney slices. J Pharmacol Exp Ther 321: 362–369, 2007. [DOI] [PubMed] [Google Scholar]
- 183.Ogasawara K, Terada T, Asaka J, Katsura T, Inui K. Hepatocyte nuclear factor-4α regulates the human organic anion transporter 1 gene in the kidney. Am J Physiol Renal Physiol 292: F1819–F1826, 2007. [DOI] [PubMed] [Google Scholar]
- 184.Ose A, Ito M, Kusuhara H, Yamatsugu K, Kanai M, Shibasaki M, Hosokawa M, Schuetz JD, Sugiyama Y. Limited brain distribution of [3R,4R,5S]-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate phosphate (Ro 64–0802), a pharmacologically active form of oseltamivir, by active efflux across the blood-brain barrier mediated by organic anion transporter 3 (Oat3/Slc22a8) and multidrug resistance-associated protein 4 (Mrp4/Abcc4). Drug Metab Dispos 37: 315–321, 2009. [DOI] [PubMed] [Google Scholar]
- 185.Palmieri F. The mitochondrial transporter family SLC25: identification, properties and physiopathology. Mol Aspects Med 34: 465–484, 2013. [DOI] [PubMed] [Google Scholar]
- 186.Patnaik PR. Synthesizing cellular intelligence and artificial intelligence for bioprocesses. Biotechnol Adv 24: 129–133, 2006. [DOI] [PubMed] [Google Scholar]
- 187.Pavlova A, Sakurai H, Leclercq B, Beier DR, Yu AS, Nigam SK. Developmentally regulated expression of organic ion transporters NKT (OAT1), OCT1, NLT (OAT2), and Roct. Am J Physiol Renal Physiol 278: F635–F643, 2000. [DOI] [PubMed] [Google Scholar]
- 188.Perry JL, Dembla-Rajpal N, Hall LA, Pritchard JB. A three-dimensional model of human organic anion transporter 1: aromatic amino acids required for substrate transport. J Biol Chem 281: 38071–38079, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pham TX, Lee J. Dietary regulation of histone acetylases and deacetylases for the prevention of metabolic diseases. Nutrients 4: 1868–1886, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Pluznick JL, Caplan MJ. Novel sensory signaling systems in the kidney. Curr Opin Nephrol Hypertens 21: 404–409, 2012. [DOI] [PubMed] [Google Scholar]
- 191.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA 110: 4410–4415, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pombrio JM, Giangreco A, Li L, Wempe MF, Anders MW, Sweet DH, Pritchard JB, Ballatori N. Mercapturic acids (N-acetylcysteine S-conjugates) as endogenous substrates for the renal organic anion transporter-1. Mol Pharmacol 60: 1091–1099, 2001. [DOI] [PubMed] [Google Scholar]
- 193.Pontoglio M, Barra J, Hadchouel M, Doyen A, Kress C, Bach JP, Babinet C, Yaniv M. Hepatocyte nuclear factor 1 inactivation results in hepatic dysfunction, phenylketonuria, and renal Fanconi syndrome. Cell 84: 575–585, 1996. [DOI] [PubMed] [Google Scholar]
- 194.Pritchard JB. Coupled transport of p-aminohippurate by rat kidney basolateral membrane vesicles. Am J Physiol Renal Fluid Electrolyte Physiol 255: F597–F604, 1988. [DOI] [PubMed] [Google Scholar]
- 195.Pritchard JB, Sweet DH, Miller DS, Walden R. Mechanism of organic anion transport across the apical membrane of choroid plexus. J Biol Chem 274: 33382–33387, 1999. [DOI] [PubMed] [Google Scholar]
- 196.Purohit A, Woo LW, Potter BV. Steroid sulfatase: a pivotal player in estrogen synthesis and metabolism. Mol Cell Endocrinol 340: 154–160, 2011. [DOI] [PubMed] [Google Scholar]
- 197.Rabe T, Hosch R, Runnebaum B. Diagnosis of intrauterine fetal growth retardation (IUGR) and placental insufficiency by a dehydroepiandrosterone sulfate (DHAS) loading test. Biol Res Pregnancy Perinatol 4: 130–136, 1983. [PubMed] [Google Scholar]
- 198.Rammelkamp CH, Keefer CS. The absorption, excretion, and distribution of penicillin. J Clin Invest 22: 425–437, 1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Reddy VS, Saier MH Jr.. The major facilitator superfamily (MFS) revisited. FEBS J 279: 2036–2046, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Rehmann H, Wittinghofer A, Bos JL. Capturing cyclic nucleotides in action: snapshots from crystallographic studies. Nat Rev Mol Cell Biol 8: 63–73, 2007. [DOI] [PubMed] [Google Scholar]
- 201.Reimer RJ. SLC17: a functionally diverse family of organic anion transporters. Mol Aspects Med 34: 350–359, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Reimer RJ, Edwards RH. Organic anion transport is the primary function of the SLC17/type I phosphate transporter family. Pflügers Arch 447: 629–635, 2004. [DOI] [PubMed] [Google Scholar]
- 203.Rizwan AN, Burckhardt G. Organic anion transporters of the SLC22 family: biopharmaceutical, physiological, and pathological roles. Pharm Res 24: 450–470, 2007. [DOI] [PubMed] [Google Scholar]
- 204.Rosines E, Sampogna RV, Johkura K, Vaughn DA, Choi Y, Sakurai H, Shah MM, Nigam SK. Staged in vitro reconstitution and implantation of engineered rat kidney tissue. Proc Natl Acad Sci USA 104: 20938–20943, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Roth M, Obaidat A, Hagenbuch B. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol 165: 1260–1287, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sailaja BS, Cohen-Carmon D, Zimmerman G, Soreq H, Meshorer E. Stress-induced epigenetic transcriptional memory of acetylcholinesterase by HDAC4. Proc Natl Acad Sci USA 109: E3687–3695, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Saito H. Pathophysiological regulation of renal SLC22A organic ion transporters in acute kidney injury: pharmacological and toxicological implications. Pharmacol Ther 125: 79–91, 2010. [DOI] [PubMed] [Google Scholar]
- 208.Sakiyama M, Matsuo H, Shimizu S, Nakashima H, Nakayama A, Chiba T, Naito M, Takada T, Suzuki H, Hamajima N, Ichida K, Shimizu T, Shinomiya N. A common variant of organic anion transporter 4 (OAT4/SLC22A11) gene is associated with renal underexcretion type gout. Drug Metab Pharmacokinet 29: 208–210, 2014. [DOI] [PubMed] [Google Scholar]
- 209.Sauvant C, Holzinger H, Gekle M. Prostaglandin E2 inhibits its own renal transport by downregulation of organic anion transporters rOAT1 and rOAT3. J Am Soc Nephrol 17: 46–53, 2006. [DOI] [PubMed] [Google Scholar]
- 210.Schaeffeler E, Hellerbrand C, Nies AT, Winter S, Kruck S, Hofmann U, van der Kuip H, Zanger UM, Koepsell H, Schwab M. DNA methylation is associated with downregulation of the organic cation transporter OCT1 (SLC22A1) in human hepatocellular carcinoma. Genome Med 3: 82, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Schnabolk GW, Gupta B, Mulgaonkar A, Kulkarni M, Sweet DH. Organic anion transporter 6 (Slc22a20) specificity and Sertoli cell-specific expression provide new insight on potential endogenous roles. J Pharmacol Exp Ther 334: 927–935, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Schnabolk GW, Youngblood GL, Sweet DH. Transport of estrone sulfate by the novel organic anion transporter Oat6 (Slc22a20). Am J Physiol Renal Physiol 291: F314–F321, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Schoog M, Arning R. Effect of benemid on p-aminohippuric acid blood level. Arzneimittelforschung 3: 377–379, 1953. [PubMed] [Google Scholar]
- 214.Schroeder JC, Dinatale BC, Murray IA, Flaveny CA, Liu Q, Laurenzana EM, Lin JM, Strom SC, Omiecinski CJ, Amin S, Perdew GH. The uremic toxin 3-indoxyl sulfate is a potent endogenous agonist for the human aryl hydrocarbon receptor. Biochemistry 49: 393–400, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Secky L, Svoboda M, Klameth L, Bajna E, Hamilton G, Zeillinger R, Jager W, Thalhammer T. The sulfatase pathway for estrogen formation: targets for the treatment and diagnosis of hormone-associated tumors. J Drug Deliv 2013: 957605, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sharma K, Karl B, Mathew AV, Gangoiti JA, Wassel CL, Saito R, Pu M, Sharma S, You YH, Wang L, Diamond-Stanic M, Lindenmeyer MT, Forsblom C, Wu W, Ix JH, Ideker T, Kopp JB, Nigam SK, Cohen CD, Groop PH, Barshop BA, Natarajan L, Nyhan WL, Naviaux RK. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J Am Soc Nephrol 24: 1901–1912, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Shimada H, Moewes B, Burckhardt G. Indirect coupling to Na+ of p-aminohippuric acid uptake into rat renal basolateral membrane vesicles. Am J Physiol Renal Fluid Electrolyte Physiol 253: F795–F801, 1987. [DOI] [PubMed] [Google Scholar]
- 218.Shin HJ, Anzai N, Enomoto A, He X, Kim do K, Endou H, Kanai Y. Novel liver-specific organic anion transporter OAT7 that operates the exchange of sulfate conjugates for short chain fatty acid butyrate. Hepatology 45: 1046–1055, 2007. [DOI] [PubMed] [Google Scholar]
- 219.Shiraya K, Hirata T, Hatano R, Nagamori S, Wiriyasermkul P, Jutabha P, Matsubara M, Muto S, Tanaka H, Asano S, Anzai N, Endou H, Yamada A, Sakurai H, Kanai Y. A novel transporter of SLC22 family specifically transports prostaglandins and co-localizes with 15-hydroxyprostaglandin dehydrogenase in renal proximal tubules. J Biol Chem 285: 22141–22151, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Shuster DL, Bammler TK, Beyer RP, Macdonald JW, Tsai JM, Farin FM, Hebert MF, Thummel KE, Mao Q. Gestational age-dependent changes in gene expression of metabolic enzymes and transporters in pregnant mice. Drug Metab Dispos 41: 332–342, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Silva P, Torretti J, Hayslett JP, Epstein FH. Relation between Na-K-ATPase activity and respiratory rate in the rat kidney. Am J Physiol 230: 1432–1438, 1976. [DOI] [PubMed] [Google Scholar]
- 222.Simonson GD, Vincent AC, Roberg KJ, Huang Y, Iwanij V. Molecular cloning and characterization of a novel liver-specific transport protein. J Cell Sci 107: 1065–1072, 1994. [DOI] [PubMed] [Google Scholar]
- 223.Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol 82: 1807–1821, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Smith CA, Want EJ, O'Maille G, Abagyan R, Siuzdak G. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal Chem 78: 779–787, 2006. [DOI] [PubMed] [Google Scholar]
- 225.Smith HW, Finkelstein N, Aliminosa L, Crawford B, Graber M. The renal clearances of substituted hippuric acid derivatives and other aromatic acids in dog and man. J Clin Invest 24: 388–404, 1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Stuart RO, Bush KT, Nigam SK. Changes in global gene expression patterns during development and maturation of the rat kidney. Proc Natl Acad Sci USA 98: 5649–5654, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sugawara M, Mochizuki T, Takekuma Y, Miyazaki K. Structure-affinity relationship in the interactions of human organic anion transporter 1 with caffeine, theophylline, theobromine and their metabolites. Biochim Biophys Acta 1714: 85–92, 2005. [DOI] [PubMed] [Google Scholar]
- 228.Sugiyama D, Kusuhara H, Shitara Y, Abe T, Meier PJ, Sekine T, Endou H, Suzuki H, Sugiyama Y. Characterization of the efflux transport of 17beta-estradiol-d-17beta-glucuronide from the brain across the blood-brain barrier. J Pharmacol Exp Ther 298: 316–322, 2001. [PubMed] [Google Scholar]
- 229.Sun W, Wu RR, van Poelje PD, Erion MD. Isolation of a family of organic anion transporters from human liver and kidney. Biochem Biophys Res Commun 283: 417–422, 2001. [DOI] [PubMed] [Google Scholar]
- 230.Sweeney DE, Vallon V, Rieg T, Wu W, Gallegos TF, Nigam SK. Functional maturation of drug transporters in the developing, neonatal, and postnatal kidney. Mol Pharmacol 80: 147–154, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sweet DH, Bush KT, Nigam SK. The organic anion transporter family: from physiology to ontogeny and the clinic. Am J Physiol Renal Physiol 281: F197–F205, 2001. [DOI] [PubMed] [Google Scholar]
- 232.Sweet DH, Chan LM, Walden R, Yang XP, Miller DS, Pritchard JB. Organic anion transporter 3 (Slc22a8) is a dicarboxylate exchanger indirectly coupled to the Na+ gradient. Am J Physiol Renal Physiol 284: F763–F769, 2003. [DOI] [PubMed] [Google Scholar]
- 233.Sweet DH, Eraly SA, Vaughn DA, Bush KT, Nigam SK. Organic anion and cation transporter expression and function during embryonic kidney development and in organ culture models. Kidney Int 69: 837–845, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sweet DH, Miller DS, Pritchard JB, Fujiwara Y, Beier DR, Nigam SK. Impaired organic anion transport in kidney and choroid plexus of organic anion transporter 3 [Oat3 (Slc22a8)] knockout mice. J Biol Chem 277: 26934–26943, 2002. [DOI] [PubMed] [Google Scholar]
- 235.Sweet DH, Wolff NA, Pritchard JB. Expression cloning and characterization of ROAT1. The basolateral organic anion transporter in rat kidney. J Biol Chem 272: 30088–30095, 1997. [DOI] [PubMed] [Google Scholar]
- 236.Sykes D, Sweet DH, Lowes S, Nigam SK, Pritchard JB, Miller DS. Organic anion transport in choroid plexus from wild-type and organic anion transporter 3 (Slc22a8)-null mice. Am J Physiol Renal Physiol 286: F972–F978, 2004. [DOI] [PubMed] [Google Scholar]
- 237.Tabara Y, Kohara K, Kawamoto R, Hiura Y, Nishimura K, Morisaki T, Kokubo Y, Okamura T, Tomoike H, Iwai N, Miki T. Association of four genetic loci with uric acid levels and reduced renal function: the J-SHIPP Suita study. Am J Nephrol 32: 279–286, 2010. [DOI] [PubMed] [Google Scholar]
- 238.Tachikawa M, Ozeki G, Higuchi T, Akanuma S, Tsuji K, Hosoya K. Role of the blood-cerebrospinal fluid barrier transporter as a cerebral clearance system for prostaglandin E2 produced in the brain. J Neurochem 123: 750–760, 2012. [DOI] [PubMed] [Google Scholar]
- 239.Tachikawa M, Tsuji K, Yokoyama R, Higuchi T, Ozeki G, Yashiki A, Akanuma S, Hayashi K, Nishiura A, Hosoya K. A clearance system for prostaglandin D2, a sleep-promoting factor, in cerebrospinal fluid: role of the blood-cerebrospinal barrier transporters. J Pharmacol Exp Ther 343: 608–616, 2012. [DOI] [PubMed] [Google Scholar]
- 240.Tahara H, Shono M, Kusuhara H, Kinoshita H, Fuse E, Takadate A, Otagiri M, Sugiyama Y. Molecular cloning and functional analyses of OAT1 and OAT3 from cynomolgus monkey kidney. Pharm Res 22: 647–660, 2005. [DOI] [PubMed] [Google Scholar]
- 241.Takeda M, Khamdang S, Narikawa S, Kimura H, Hosoyamada M, Cha SH, Sekine T, Endou H. Characterization of methotrexate transport and its drug interactions with human organic anion transporters. J Pharmacol Exp Ther 302: 666–671, 2002. [DOI] [PubMed] [Google Scholar]
- 242.Takemiya T. Prostaglandin E2 produced by microsomal prostaglandin E synthase-1 regulates the onset and the maintenance of wakefulness. Neurochem Int 59: 922–924, 2011. [DOI] [PubMed] [Google Scholar]
- 243.Takeuchi F, Yamamoto K, Isono M, Katsuya T, Akiyama K, Ohnaka K, Rakugi H, Yamori Y, Ogihara T, Takayanagi R, Kato N. Genetic impact on uric acid concentration and hyperuricemia in the Japanese population. J Atheroscler Thromb 20: 351–367, 2013. [DOI] [PubMed] [Google Scholar]
- 244.Taleb O, Maammar M, Brumaru D, Bourguignon JJ, Schmitt M, Klein C, Kemmel V, Maitre M, Mensah-Nyagan AG. Xanthurenic acid binds to neuronal G-protein-coupled receptors that secondarily activate cationic channels in the cell line NCB-20. PloS One 7: e48553, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Thiagarajan RD, Georgas KM, Rumballe BA, Lesieur E, Chiu HS, Taylor D, Tang DT, Grimmond SM, Little MH. Identification of anchor genes during kidney development defines ontological relationships, molecular subcompartments and regulatory pathways. PloS One 6: e17286, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Thiebaud N, Menetrier F, Belloir C, Minn AL, Neiers F, Artur Y, Le Bon AM, Heydel JM. Expression and differential localization of xenobiotic transporters in the rat olfactory neuro-epithelium. Neurosci Lett 505: 180–185, 2011. [DOI] [PubMed] [Google Scholar]
- 247.Thiebaud N, Sigoillot M, Chevalier J, Artur Y, Heydel JM, Le Bon AM. Effects of typical inducers on olfactory xenobiotic-metabolizing enzyme, transporter, and transcription factor expression in rats. Drug Metab Dispos 38: 1865–1875, 2010. [DOI] [PubMed] [Google Scholar]
- 248.Tin A, Woodward OM, Kao WH, Liu CT, Lu X, Nalls MA, Shriner D, Semmo M, Akylbekova EL, Wyatt SB, Hwang SJ, Yang Q, Zonderman AB, Adeyemo AA, Palmer C, Meng Y, Reilly M, Shlipak MG, Siscovick D, Evans MK, Rotimi CN, Flessner MF, Kottgen M, Cupples LA, Fox CS, Kottgen A. Genome-wide association study for serum urate concentrations and gout among African Americans identifies genomic risk loci and a novel URAT1 loss-of-function allele. Hum Mol Genet 20: 4056–4068, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Torres AM, Dnyanmote AV, Bush KT, Wu W, Nigam SK. Deletion of multispecific organic anion transporter Oat1/Slc22a6 protects against mercury-induced kidney injury. J Biol Chem 286: 26391–26395, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Truong DM, Kaler G, Khandelwal A, Swaan PW, Nigam SK. Multi-level analysis of organic anion transporters 1, 3, and 6 reveals major differences in structural determinants of antiviral discrimination. J Biol Chem 283: 8654–8663, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Tsigelny IF, Greenberg J, Kouznetsova V, Nigam SK. Modeling of glycerol-3-phosphate transporter suggests a potential ‘tilt’ mechanism involved in its function. J Bioinform Comput Biol 6: 885–904, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Tsigelny IF, Kouznetsova VL, Sweeney DE, Wu W, Bush KT, Nigam SK. Analysis of metagene portraits reveals distinct transitions during kidney organogenesis. Sci Signal 1: ra16, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Tsigelny IF, Kovalskyy D, Kouznetsova VL, Balinskyi O, Sharikov Y, Bhatnagar V, Nigam SK. Conformational changes of the multispecific transporter organic anion transporter 1 (OAT1/SLC22A6) suggests a molecular mechanism for initial stages of drug and metabolite transport. Cell Biochem Biophys 61: 251–259, 2011. [DOI] [PubMed] [Google Scholar]
- 254.Tsuchida H, Anzai N, Shin HJ, Wempe MF, Jutabha P, Enomoto A, Cha SH, Satoh T, Ishida M, Sakurai H, Endou H. Identification of a novel organic anion transporter mediating carnitine transport in mouse liver and kidney. Cell Physiol Biochem 25: 511–522, 2010. [DOI] [PubMed] [Google Scholar]
- 255.Tsujimoto M, Hatozaki D, Shima D, Yokota H, Furukubo T, Izumi S, Yamakawa T, Minegaki T, Nishiguchi K. Influence of serum in hemodialysis patients on the expression of intestinal and hepatic transporters for the excretion of pravastatin. Ther Apher Dial 16: 580–587, 2012. [DOI] [PubMed] [Google Scholar]
- 256.Uehara I, Kimura T, Tanigaki S, Fukutomi T, Sakai K, Shinohara Y, Ichida K, Iwashita M, Sakurai H. Paracellular route is the major urate transport pathway across the blood-placental barrier. Physiol Rep 2: e12013, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ueo H, Motohashi H, Katsura T, Inui K. Human organic anion transporter hOAT3 is a potent transporter of cephalosporin antibiotics, in comparison with hOAT1. Biochem Pharmacol 70: 1104–1113, 2005. [DOI] [PubMed] [Google Scholar]
- 258.Ugele B, Bahn A, Rex-Haffner M. Functional differences in steroid sulfate uptake of organic anion transporter 4 (OAT4) and organic anion transporting polypeptide 2B1 (OATP2B1) in human placenta. J Steroid Biochem Mol Biol 111: 1–6, 2008. [DOI] [PubMed] [Google Scholar]
- 259.Ugele B, St-Pierre MV, Pihusch M, Bahn A, Hantschmann P. Characterization and identification of steroid sulfate transporters of human placenta. Am J Physiol Endocrinol Metab 284: E390–E398, 2003. [DOI] [PubMed] [Google Scholar]
- 260.Ullrich KJ, Rumrich G. Renal transport mechanisms for xenobiotics: chemicals and drugs. Clin Invest 71: 843–848, 1993. [DOI] [PubMed] [Google Scholar]
- 261.Urquhart BL, Kim RB. Blood-brain barrier transporters and response to CNS-active drugs. Eur J Clin Pharmacol 65: 1063–1070, 2009. [DOI] [PubMed] [Google Scholar]
- 262.Uwai Y, Motohashi H, Tsuji Y, Ueo H, Katsura T, Inui K. Interaction and transport characteristics of mycophenolic acid and its glucuronide via human organic anion transporters hOAT1 and hOAT3. Biochem Pharmacol 74: 161–168, 2007. [DOI] [PubMed] [Google Scholar]
- 263.Uwai Y, Taniguchi R, Motohashi H, Saito H, Okuda M, Inui K. Methotrexate-loxoprofen interaction: involvement of human organic anion transporters hOAT1 and hOAT3. Drug Metab Pharmacokinet 19: 369–374, 2004. [DOI] [PubMed] [Google Scholar]
- 264.Vallon V, Eraly SA, Rao SR, Gerasimova M, Rose M, Nagle M, Anzai N, Smith T, Sharma K, Nigam SK, Rieg T. A role for the organic anion transporter OAT3 in renal creatinine secretion in mice. Am J Physiol Renal Physiol 302: F1293–F1299, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Vallon V, Eraly SA, Wikoff WR, Rieg T, Kaler G, Truong DM, Ahn SY, Mahapatra NR, Mahata SK, Gangoiti JA, Wu W, Barshop BA, Siuzdak G, Nigam SK. Organic anion transporter 3 contributes to the regulation of blood pressure. J Am Soc Nephrol 19: 1732–1740, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Vallon V, Rieg T, Ahn SY, Wu W, Eraly SA, Nigam SK. Overlapping in vitro and in vivo specificities of the organic anion transporters OAT1 and OAT3 for loop and thiazide diuretics. Am J Physiol Renal Physiol 294: F867–F873, 2008. [DOI] [PubMed] [Google Scholar]
- 267.Van den Anker JN, Schwab M, Kearns GL. Developmental pharmacokinetics. In: Pediatric Clinical Pharmacology, edited by Seyberth HW, Rane A, Schwab M. Berlin: Springer, 2011, p. 51–75. [DOI] [PubMed] [Google Scholar]
- 268.Van der Harst P, Bakker SJ, de Boer RA, Wolffenbuttel BH, Johnson T, Caulfield MJ, Navis G. Replication of the five novel loci for uric acid concentrations and potential mediating mechanisms. Hum Mol Genet 19: 387–395, 2010. [DOI] [PubMed] [Google Scholar]
- 269.VanWert AL, Bailey RM, Sweet DH. Organic anion transporter 3 (Oat3/Slc22a8) knockout mice exhibit altered clearance and distribution of penicillin G. Am J Physiol Renal Physiol 293: F1332–F1341, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.VanWert AL, Gionfriddo MR, Sweet DH. Organic anion transporters: discovery, pharmacology, regulation and roles in pathophysiology. Biopharm Drug Dispos 31: 1–71, 2010. [DOI] [PubMed] [Google Scholar]
- 271.VanWert AL, Sweet DH. Impaired clearance of methotrexate in organic anion transporter 3 (Slc22a8) knockout mice: a gender specific impact of reduced folates. Pharm Res 25: 453–462, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Villeneuve LM, Reddy MA, Natarajan R. Epigenetics: deciphering its role in diabetes and its chronic complications. Clin Exp Pharmacol Physiol 38: 451–459, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Vitetta L, Linnane AW, Gobe GC. From the gastrointestinal tract (GIT) to the kidneys: live bacterial cultures (probiotics) mediating reductions of uremic toxin levels via free radical signaling. Toxins 5: 2042–2057, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Wang J, Simonavicius N, Wu X, Swaminath G, Reagan J, Tian H, Ling L. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J Biol Chem 281: 22021–22028, 2006. [DOI] [PubMed] [Google Scholar]
- 275.Wang JS, Newport DJ, Stowe ZN, Donovan JL, Pennell PB, DeVane CL. The emerging importance of transporter proteins in the psychopharmacological treatment of the pregnant patient. Drug Metab Rev 39: 723–746, 2007. [DOI] [PubMed] [Google Scholar]
- 276.Wang L, Sweet DH. Interaction of natural dietary and herbal anionic compounds and flavonoids with human organic anion transporters 1 (SLC22A6), 3 (SLC22A8), and 4 (SLC22A11). Evid Based Complement Alternat Med 2013: 612527, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Wang Y, Borchert ML, Deluca HF. Identification of the vitamin D receptor in various cells of the mouse kidney. Kidney Int 81: 993–1001, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Wegner W, Burckhardt BC, Burckhardt G, Henjakovic M. Male-dominant activation of rat renal organic anion transporter 1 (Oat1) and 3 (Oat3) expression by transcription factor BCL6. PloS One 7: e35556, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Wikoff WR, Nagle MA, Kouznetsova VL, Tsigelny IF, Nigam SK. Untargeted metabolomics identifies enterobiome metabolites and putative uremic toxins as substrates of organic anion transporter 1 (Oat1). J Proteome Res 10: 2842–2851, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Wong CC, Botting NP, Orfila C, Al-Maharik N, Williamson G. Flavonoid conjugates interact with organic anion transporters (OATs) and attenuate cytotoxicity of adefovir mediated by organic anion transporter 1 (OAT1/SLC22A6). Biochem Pharmacol 81: 942–949, 2011. [DOI] [PubMed] [Google Scholar]
- 281.Wright AF, Rudan I, Hastie ND, Campbell H. A “complexity” of urate transporters. Kidney Int 78: 446–452, 2010. [DOI] [PubMed] [Google Scholar]
- 282.Wright SH, Dantzler WH. Molecular and cellular physiology of renal organic cation and anion transport. Physiol Rev 84: 987–1049, 2004. [DOI] [PubMed] [Google Scholar]
- 283.Wu W, Baker ME, Eraly SA, Bush KT, Nigam SK. Analysis of a large cluster of SLC22 transporter genes, including novel USTs, reveals species-specific amplification of subsets of family members. Physiol Genomics 38: 116–124, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Wu W, Dnyanmote AV, Nigam SK. Remote communication through Slc and Abc drug transporter pathways: an update on the remote sensing and signaling hypothesis. Mol Pharmacol 79: 795–805, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Wu W, Jamshidi N, Eraly SA, Liu HC, Bush KT, Palsson BO, Nigam SK. Multispecific drug transporter slc22a8 (oat3) regulates multiple metabolic and signaling pathways. Drug Metab Dispos 41: 1825–1834, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Xu G, Bhatnagar V, Wen G, Hamilton BA, Eraly SA, Nigam SK. Analyses of coding region polymorphisms in apical and basolateral human organic anion transporter (OAT) genes [OAT1 (NKT), OAT2, OAT3, OAT4, URAT (RST)]. Kidney Int 68: 1491–1499, 2005. [DOI] [PubMed] [Google Scholar]
- 287.Xue X, Gong LK, Maeda K, Luan Y, Qi XM, Sugiyama Y, Ren J. Critical role of organic anion transporters 1 and 3 in kidney accumulation and toxicity of aristolochic acid I. Mol Pharm 8: 2183–2192, 2011. [DOI] [PubMed] [Google Scholar]
- 288.Yamashita F, Ohtani H, Koyabu N, Ushigome F, Satoh H, Murakami H, Uchiumi T, Nakamura T, Kuwano M, Tsujimoto M, Sawada Y. Inhibitory effects of angiotensin II receptor antagonists and leukotriene receptor antagonists on the transport of human organic anion transporter 4. J Pharm Pharmacol 58: 1499–1505, 2006. [DOI] [PubMed] [Google Scholar]
- 289.Yang CH, Glover KP, Han X. Characterization of cellular uptake of perfluorooctanoate via organic anion-transporting polypeptide 1A2, organic anion transporter 4, and urate transporter 1 for their potential roles in mediating human renal reabsorption of perfluorocarboxylates. Toxicol Sci 117: 294–302, 2010. [DOI] [PubMed] [Google Scholar]
- 290.Yang J, Kalogerou M, Gallacher J, Sampson JR, Shen MH. Renal tumours in a Tsc1+/− mouse model show epigenetic suppression of organic cation transporters Slc22a1, Slc22a2 and Slc22a3, and do not respond to metformin. Eur J Cancer 49: 1479–1490, 2013. [DOI] [PubMed] [Google Scholar]
- 291.Yang Q, Kottgen A, Dehghan A, Smith AV, Glazer NL, Chen MH, Chasman DI, Aspelund T, Eiriksdottir G, Harris TB, Launer L, Nalls M, Hernandez D, Arking DE, Boerwinkle E, Grove ML, Li M, Linda Kao WH, Chonchol M, Haritunians T, Li G, Lumley T, Psaty BM, Shlipak M, Hwang SJ, Larson MG, O'Donnell CJ, Upadhyay A, van Duijn CM, Hofman A, Rivadeneira F, Stricker B, Uitterlinden AG, Pare G, Parker AN, Ridker PM, Siscovick DS, Gudnason V, Witteman JC, Fox CS, Coresh J. Multiple genetic loci influence serum urate levels and their relationship with gout and cardiovascular disease risk factors. Circ Cardiovasc Genet 3: 523–530, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Yee SW, Nguyen AN, Brown C, Savic RM, Zhang YC, Castro RA, Cropp CD, Choi JH, Singh D, Tahara H, Stocker SL, Huang Y, Brett CM, Giacomini KM. Reduced renal clearance of cefotaxime in asians with a low-frequency polymorphism of OAT3 (SLC22A8). J Pharm Sci 102: 3451–3457, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Yokoyama H, Anzai N, Ljubojevic M, Ohtsu N, Sakata T, Miyazaki H, Nonoguchi H, Islam R, Onozato M, Tojo A, Tomita K, Kanai Y, Igarashi T, Sabolic I, Endou H. Functional and immunochemical characterization of a novel organic anion transporter Oat8 (Slc22a9) in rat renal collecting duct. Cell Physiol Biochem 21: 269–278, 2008. [DOI] [PubMed] [Google Scholar]
- 294.You G. Membrane transporters in drug disposition. Pharm Res 25: 441–443, 2008. [DOI] [PubMed] [Google Scholar]
- 295.Youngblood GL, Sweet DH. Identification and functional assessment of the novel murine organic anion transporter Oat5 (Slc22a19) expressed in kidney. Am J Physiol Renal Physiol 287: F236–F244, 2004. [DOI] [PubMed] [Google Scholar]
- 296.Yun CC, Chen Y, Lang F. Glucocorticoid activation of Na+/H+ exchanger isoform 3 revisited. The roles of SGK1 and NHERF2. J Biol Chem 277: 7676–7683, 2002. [DOI] [PubMed] [Google Scholar]
- 297.Zalups RK. Molecular interactions with mercury in the kidney. Pharmacol Rev 52: 113–143, 2000. [PubMed] [Google Scholar]
- 298.Zalups RK, Ahmad S. Handling of the homocysteine S-conjugate of methylmercury by renal epithelial cells: role of organic anion transporter 1 and amino acid transporters. J Pharmacol Exp Ther 315: 896–904, 2005. [DOI] [PubMed] [Google Scholar]
- 299.Zalups RK, Ahmad S. Homocysteine and the renal epithelial transport and toxicity of inorganic mercury: role of basolateral transporter organic anion transporter 1. J Am Soc Nephrol 15: 2023–2031, 2004. [DOI] [PubMed] [Google Scholar]
- 300.Zhang Q, Hong M, Duan P, Pan Z, Ma J, You G. Organic anion transporter OAT1 undergoes constitutive and protein kinase C-regulated trafficking through a dynamin- and clathrin-dependent pathway. J Biol Chem 283: 32570–32579, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Zhang W, Yen GG, He Z. Constrained optimization via artificial immune system. IEEE Trans Cybern 44: 185–198, 2013. [DOI] [PubMed] [Google Scholar]
- 302.Zhang X, De la Cruz O, Pinto JM, Nicolae D, Firestein S, Gilad Y. Characterizing the expression of the human olfactory receptor gene family using a novel DNA microarray. Genome Biol 8: R86, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Zhang XJ, Lai TB, Kong RY. Biology of fluoro-organic compounds. Top Curr Chem 308: 365–404, 2012. [DOI] [PubMed] [Google Scholar]
- 304.Zhao YY. Metabolomics in chronic kidney disease. Clin Chim Acta 422: 59–69, 2013. [DOI] [PubMed] [Google Scholar]
- 305.Zhou F, Illsley NP, You G. Functional characterization of a human organic anion transporter hOAT4 in placental BeWo cells. Eur J Pharm Sci 27: 518–523, 2006. [DOI] [PubMed] [Google Scholar]
- 306.Zhou F, Xu W, Tanaka K, You G. Comparison of the interaction of human organic anion transporter hOAT4 with PDZ proteins between kidney cells and placental cells. Pharm Res 25: 475–480, 2008. [DOI] [PubMed] [Google Scholar]
- 307.Zlender V, Breljak D, Ljubojevic M, Flajs D, Balen D, Brzica H, Domijan AM, Peraica M, Fuchs R, Anzai N, Sabolic I. Low doses of ochratoxin A upregulate the protein expression of organic anion transporters Oat1, Oat2, Oat3 and Oat5 in rat kidney cortex. Toxicol Appl Pharmacol 239: 284–296, 2009. [DOI] [PubMed] [Google Scholar]