Abstract
Although vitamin A was recognized as an “anti-infective vitamin” over 90 years ago, the mechanism of how vitamin A regulates immunity is only beginning to be understood. Early studies which focused on the immune responses in vitamin A-deficient (VAD) animals clearly demonstrated compromised immunity and consequently increased susceptibility to infectious disease. The active form of vitamin A, retinoic acid (RA), has been shown to have a profound impact on the homing and differentiation of leukocytes. Both pharmacological and genetic approaches have been applied to the understanding of how RA regulates the development and differentiation of various immune cell subsets, and how RA influences the development of immunity versus tolerance. These studies clearly show that RA profoundly impacts on cell- and humoral-mediated immunity. In this review, the early findings on the complex relationship between VAD and immunity are discussed as well as vitamin A metabolism and signaling within hematopoietic cells. Particular attention is focused on how RA impacts on T-cell lineage commitment and plasticity in various diseases.
I. INTRODUCTION
Nearly a century ago, vitamins were recognized as essential nutrients for human health. Among various vitamins, vitamin A was found to be essential for embryogenesis (215) and host defense against various microbial infections (209). Developmental biologists have extensively studied the active form of vitamin A, retinoic acid (RA), and have mapped how grandients of RA control the most exquisite processes during embryogenesis. Given the known correlation between vitamin A deficiency (VAD) and increased susceptibility to infectious diseases (ID) among preschool children in developing countries (20, 49), immunologists have focused on the understanding of how vitamin A, through RA, controls immunity. Initial clues into the molecular mechanisms of the “anti-infective” impact of vitamin A were provided in 2004, when Iwata et al. (84) demonstrated that RA imprints the homing of leukocytes to the gut through the upregulation of α4β7and chemokine (C-C motif) receptor 9 (CCR9). Our own studies (11, 140) and those of others (31, 139, 181) demonstrated that RA could enhance T-cell commitment and Treg differentiation. These seminal findings focused the immunological community on understanding the role of RA in the regulation of gut immunity and tolerance. The use of small molecule inhibitors or agonists of RA signaling as well as genetic-engineered mouse models has expanded our knowledge of how RA controls immune responses besides its known function in controlling gut homing. Numerous studies have elucidated the essential role of RA in modulating immune cell “lineage commitment” in various disease models in a context-dependent manner. RA is now appreciated as a pivotal mediator in promoting tolerance or immunity depending on what type of cell is acted upon. In this review, we focus on the known mechanism of how RA is synthesized by various antigen-presenting cells and how this is translated into signals that govern leukocyte lineage commitment.
II. VITAMIN A DEFICIENCY AND IMMUNITY
Vitamins by definition are organic compounds that cannot be synthesized by mammals and thus have to be obtained from food sources (52). The recognition that vitamin A plays an important role in immunity dates back to the 1920s when Drs. Green and Mellaby observed their colony of dogs on a VAD diet succumbed to an infection that ravaged through the animal facility (127). Since that time, the idea that vitamin A was an “anti-infective vitamin” instead of “growth-producing vitamin” (62) grew in recognition. Following this study, a small clinical trial was initiated in women with septicemia, in which only 2 out of 24 recovered without vitamin A treatment, and 5 out of 5 recovered with vitamin A treatment (128). An even larger clinical trial of puerperal sepsis reinforced the positive correlation between vitamin A treatment and clinical outcome by showing a statistical improvement in a wide range of ID of those on vitamin A-rich diets (63). At least 30 uncontrolled clinical trials led by Drs. Mellanby and Green demonstrated that vitamin A supplementation, in the form of cod-liver oil, reduced the morbidity and mortality caused by respiratory inflammation, measles, puerperal sepsis, and other ID (as reviewed in Ref. 165). A litany of studies have focused on the analysis of VAD and immunity, with the increasing knowledge of immunity and better-controlled experiments than in the early days. VAD animals are characteristic of a wide range of immunological changes, including pathological disintegrity in mucosal area (2), decreased antibody (Ab) response, changes in lymphocyte populations, and T- and B-cell dysfunction, which can be restored by treatment with retinol, a derivative of vitamin A (154).
The importance of vitamin A in human disease is highlighted further by the increased burden of ID in VAD children and adults. Presently, VAD is still a common health problem in some developing countries and a leading cause of childhood morbidity and mortality. An estimated 250 million children under the age of 5 yr have VAD (190), defined as serum retinol <0.7 μM (37), and a further 120 million are estimated to have significant VAD in the absence of clinical signs such as xerophthalmia. Malnourished children have increased susceptibility to diarrhea due to ID (49) and an increased risk of acute respiratory infections (20) and measles (54, 122). Some clinical data also indicate a correlation between VAD and the disease progression (166), mortality (166), and maternal-infant disease transfer (64, 167). Infection also leads to enhanced vitamin A depletion (21). Since the first randomized and controlled trial in the 1980s, a number of clinical trials in children from endemic areas have demonstrated a dramatic reduction in severity of diarrheal disease and measles morbidity with vitamin A supplementation (78, 81, 172). In addition, supplementation has led to a lower incidence of respiratory tract infections in some studies (9). Of note, high-dose vitamin A supplementation even reduced mortality and morbidity in children without clinical evidence of VAD (32, 78). Based on these clinical outcomes, the World Health Organization recommends that preschool children in developing countries receive high-dose vitamin A supplementation every 4–6 mo, often administered at the time of vaccination for clinical convenience.
In addition to the role of vitamin A sufficiency to efficiently fight against infectious diseases, evidence also suggests that vtiamin A supplementation can improve vaccine efficacy (92). The study by Sudfeld et al. (180) showed that at least two doses of 200,000 IU (60 mg retinol equivalent) for children ≥1 yr of age and 100,000 IU (30 mg retinol equivalent) for infants reduced measles mortality by 62%. In the study, vitamin A is administrated orally in an oil-based preparation of retinyl palmitate or retinyl acetate. However, the relationship of vitamin A supplementation and mechanistic impact on immunity is not always apparent. Chicks fed on a high-vitamin A diet had a significantly lower primary IgG and IgM Ab response to keyhole limpet hemocyanin (KLH), and also a lower secondary IgG response. In addition, administering retinyl palmitate did not change the Ab response in low-, regular-, or high-vitamin A diet-fed chicks (108). VAD diminished and high-level vitamin diet enhanced the development of experimental asthma in mice (162). Furthermore, vitamin A supplementation proved unpredictable in some clinical trials. In a clinical trial including 471 at-birth children, vitamin A supplementation (50,000 IU retinyl palmitate in vegetable oil) was associated with lower tumor necrosis factor (TNF)-α and interleukin (IL)-10 production in girls without and boys with diphtheria-tetanus-pertussis vaccination, indicating a complicated interaction between vitamin A sufficiency, gender, and vaccination (88).
As discussed above, the early epidemiological data and effectiveness of vitamin A supplementation to boost immunity shed light on a key role of vitamin A for development of host defense and opened the door to a wealth of research into the mechanisms through which vitamin A regulates immune responses. On the other hand, the insignificant improvement by vitamin A or RA supplementation in some diseases makes it clear that vitamin A effects are not always predictable.
The importance of vitamin A to the ontogenic development of immune system was recognized in 1930s, when VAD children were found to have atrophic thymi, spleens, and lymphoid tissues (15). Numerous studies in mice have demonstrated that vitamin A causes this impact on immune system through various immune cell components, such as NK cells (39), T cells (110), and B cells (16). Recently, the importance of vitamin A in development of immune system was further understood at the molecular level by van de Pavert et al. (197). In this study, the authors established that the secondary lymphoid organ formation during embryogenesis depends on hematopoietic cell-autonomous RA signaling in utero, by control of a subset of type 3 innate lymphoid cells (ILC3) differentiation. Therefore, maternal vitamin A intake controls the efficiency of immune response in the adult offspring. It further illustrated the essential role of vitamin A in the development of efficient immune system.
III. VITAMIN A METABOLISM AND RA SYNTHESIS
Both genetic and pharmacological approaches have been applied to elucidate the pathway of metabolizing vitamin A to RA. As retinoids (all vitamin A derivatives) cannot be synthesized de novo, the only source is diet derived. Earlier studies have illustrated the major proteins and enzymes involved in retinol storage are in the liver and the uptake of vitamin A is in the intestine (reviewed in Ref. 33). In mammals, retinol is the major circulating retinoid. Retinol is typically bound to retinol-binding protein 4 (RBP4) and transthyretin (TTR), and taken up by target tissues (10). Previous studies demonstrated that retinol-RBP-TTR complex (holo-RBP) uptake was facilitated by STRA6 (stimulated by retinoic acid gene 6) in various tissues (93). However, recent studies in stra6-null mice illustrated that STRA6 facilitates retinol uptake in the eye but couples holo-RBP to cell signaling in other tissues (12). Two sequential reactions are then carried out for the conversion of retinol to RA. First, retinol is converted by cytosolic alcohol dehydrogenase (ADHs, including ADH1, ADH5, and ADH7) (133) and microsomal retinol dehydrogenase (RDH) (158) to retinaldehyde. Adh1−/− mice are more sensitive to vitamin A embryotoxicity (134), but reveal no obvious phenotype when maintained on vitamin A-sufficient diet (38). In contrast, Rdh10 loss-of-function is lethal between embryonic day 10.5 (E10.5) and E14.5 and exhibits abnormalities characteristic of RA deficiency (158). These studies indicated that ADHs may control the removal of excessive retinol, whereas RDHs oxidize retinol to retinaldehyde (also called retinal). Second, retinaldehyde is irreversibly converted to all-trans-retinoic acid (ATRA) by three retinaldehyde dehydrogenases, RALDH1 (65), RALDH2 (43, 144) and RALDH3 (182) (encoded by Raldh1–3, respectively), in mice. In this article, RA refers to ATRA unless specificed otherwise. Of note, RALDH2 can also catalyze the dehydrogenation of other aldehydes, such as octanal and decanal, even if less efficiently compared with retinaldehyde (208).
Even though vitamin A has long been known to be essential for a competent immune system, the underlying mechanisms whereby RA regulates immunity are still incompletely understood. Substantial efforts have focused on the metabolism and synthesis of RA by various APCs for the homeostatic regulation of immune migration and differentiation of various hematopoietic compartments.
A. RA Synthesis in the Gut
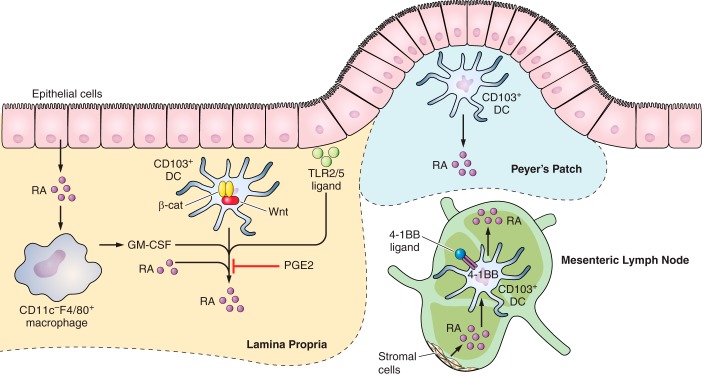
While ADHs are widely and constitutively expressed by multiple cell types, RALDH expression is dynamically regulated in a temporally and spatially restricted manner. Expression of RALDH is viewed as a key enzyme defining the ability of specific cell types or tissues to produce RA (43, 65, 144, 182). The discovery that RA induces the migration of leukocytes to the gut (84, 137, 223) has focused much of the attention of RA synthesis to mucosal sites. As such, researchers have begun to elucidate the factors and cells that govern the expression of RALDHs and RA synthesis in immune relevant cells in the gut and in the periphery. In situ hybridization and immunocytochemical analysis have confirmed RALDH1 expression in epithelial cells of mucosa, including small and large intestines (55). In the small intestine, epithelial cells express Raldh1 and produce RA constitutively by taking retinol from food sources (14, 55, 104, 192). Further studies found that monocyte-derived dendritic cells (MoDC) differentiated with RA or the supernatant of human intestinal epithelial cells acquire the ability to produce RA and induce regulatory T cell (Treg) differentiation in the gut (80). It has also been shown that direct contact between dendritic cells (DC) and intraepithelial lymphocytes (IELs) endowed the former to express Raldh2 and produce RA (47, 79). Many studies have suggested that CD103+ DCs in the small intestine are responsible for the high level of RA in mesenteric lymph node (MLN) (RALDH2), Peyer's Patch (PP) (RALDH1), and lamina propria (LP) (RALDH2) of the small intestine (84). Recent studies have shown that not only are there more CD103+ DCs in murine small intestine lamina propria (SI-LP) and draining MLN than in other lymphoid tissues, but CD103+ DCs in SI-LP and MLN showed higher Raldh2 expression and activity (132).
Our recent study demonstrated that both the expression and enzymatic activity of RALDH1 and RALDH2 in donor DCs and macrophages of MLN and LP lymphocytes were elevated in graft-versus-host-disease (GVHD) mice (5). The increase of RA synthesis in active GVHD mice is responsible for donor T cells differentiation into pathogenic Th1/Th17 cells and their migration to the instestine for disease progression.
Another study showed that RALDH2 is also expressed in MLN stromal cells, consistent with their ability to imprint proliferating T cells with α4β7 and CCR9. This study suggested that RA-producing stromal cells and DCs in MLN warrant the gut-tropism of activated T cells in the musoca area (131). In one elegant study of implanting MLN and peripheral LN fragments into the mesenteric region of host rat and mouse, it was established that stromal cells in MLN but not in peripheral LN provided a unique microenvironment allowing for the acquisition of a gut-tropism phenotype of T and B cells during antigen-induced activation (3, 72). In addition to gut mucosa, CD103+ DCs was also found in cutaneous and lung-draining LN, suggesting an important role in controlling T cell homing in those sites (67). A recent study demonstrated that lung CD103+ and CD24+CD11b+DCs express RALDH activity and can induce gut-homing receptors α4β7 and CCR9 (156). Even though no specific RALDH was identified, T cells primed by lung DCs via intranasal immunization efficiently induced migration to the gut and control enteric challenge of highly pathogenic Salmonella. While these correlations of expression and function within the CD103+ DC subset are compelling, it remains to be determined whether RA synthesis by these cells are causally imperative for the function of RA in the gut.
B. RA Synthesis in the Periphery
Various peripheral DC subsets acquired RA-producing potential in both allogenic skin transplantation (151) and melanoma model (69). As such, our studies have established that RA is a critical immune mediator outside the gut, in sustaining and controlling leukocyte differentiation in the periphery. RA production by DCs in peripheral tissues and lymphoid organs is consistent with the corresponding role of RA in regulating adaptive immunity in periphery. Furthermore, CD8α+CD8β− and CD8α+CD8β+ but not CD8α−CD8β− plasmacytoid DCs (pDC) were able to produce RA, and induce the conversion of naive CD4+ T cells to Tregs in combination with transforming growth factor (TGF)-β in vitro and in vivo during lung inflammation-mediated airway hypersensitivity (109).
As mentioned in section II, the intake of retinoids by the mother determines the development of secondary lymphoid organs during embryogenesis through the regulation of lymphoid tissue inducer (LTi) cells and CD3−IL7Rα+α4β7+ID2+c-Kit+CD11c−CD4− innate lymphoid cells (ILCs) as early as E10.5 (197). RALDH2 is responsible for RA synthesis until E8.5 (130, 144, 145), and thereafter RALDH1 and RALDH3 control RA synthesis (46, 125). The authors proposed that RA produced in utero operates in a cell-autonomous fashion to regulate retinoic acid receptor-related orphan receptor gamma (RORγT), a key transcription factor for the development of LTi cells. It remains unknown which RALDH is required for RA production and provision for LTi cell differentiation and secondary lymphoid organs generation.
No studies have yet reported RALDHs expression in T cells or RA synthesis by activated T cells in vitro or in vivo. The exclusive synthesis of RA by various APC subsets and impact on T cell differentiation raised the question of whether RA, as a lipophilic molecule, permissively diffuses across cell membranes of DCs and T cells during antigen presentation and thus determines the phenotype of corresponding T cells. Most likely, RA-producing DCs (or other APCs), in response to different stimuli, can generate an RA-rich microenvironment, which is also rich in stimuli-specific cytokines/chemokines, and determine the T-cell lineage commitment. Lineage-specific deletion of Raldh(s) will facilitate the understanding of the role of each RALDH in DCs or macrophages in the regard of determining T-cell differentiation and migration. Such studies may lead to the therapeutic inhibition of RALDH activity in specific cell subsets for control of disease progression. For instance, Crohn's disease and ulcerative colitis are driven by enhanced recruitment of effector macrophages, neutrophils, and effector T cells (159). Thus RALDH inhibition may block effector T-cell recruitment into the intestine and ameliorates the disease progression.
C. Regulation of RA Synthesis in the Immune System
Interest in understanding what inflammatory mediators control RA synthesis by the immune system has been paramount. The prominent targets for regulation involve the transcriptional regulation of each of the RALDHs as well as the expression of the RA catabolic pathways, involving mainly cytochrome P-450, CYP26 enzymes (CYP26A1-C1), and possibly retinal reductases (89). The following section focuses on our current understanding of the molecules involved in the expression and activity of RALDH1–3 and CYP26 in different immune cells.
1. Granulocyte-macrophage colony stimulating factor and IL-4
Granulocyte-macrophage colony stimulating factor (GM-CSF) and IL-4 have been shown to synergistically induce Raldh2 expression in both bone marrow-derived DCs (BMDC) and splenic DCs, thus enabling them to produce RA (6). Even though 1 μM ATRA minimally induced Raldh2 expression in BMDC or splenic DC, it moderately enhanced GM-CSF and/or IL-4-induced Raldh2 expression on BMDCs. The authors proposed a positive feedback loop of RA production in the intestine. That is, GM-CSF produced by CD11c−F4/80+ macrophages in the intestinal tissues induces Raldh2 expression and RA production by DCs. Raldh2+ DCs then provide RA to macrophages and induce further GM-CSF production. A recent study further elucidated that RA controls the generation of gut-tropic migratory DC precursors in the bone marrow (223). Therefore, high RA in the intestinal microenvironment is maintained through the generation of gut-homing DC precursors in bone marrow and further enhancement of RA production by GM-CSF in the intestine. Consistent with the finding that GM-CSF and IL-4 induced Raldh2 expression on BMDCs, IL-4 was shown to induce Raldh2 expression in MLN-DC (50).
2. TLR signaling
The RA-producing ability of CD103+ DCs in the gut mucosa is dependent on MyD88 signaling, in particular TLR2-mediated signaling (48, 206). TLR2 signaling also stimulated RALDH2 and IL-10 expression on splenic DCs, thus inducing Treg development but suppressing Th1/Th17 cell differentiation (119). Another study showed that TLR5 ligand, flagellin, specifically stimulated CD103+ LP-DCs to produce RA (194). The MyD88 and TLR signaling-dependent RALDH2 expression indicate the potential ability of both commensals and pathogens to induce RA synthesis by gut-associated DCs. Consistent with this, infection with Candida albicans was also shown to induce Raldh2 expression in splenic DCs (119). Surprisingly, both MyD88−/−TrifLps2/Lps2 mice (that lack central adaptors used by all TLRs and thus lack all signals via TLR) and wild-type C57BL/6 mice kept under specific pathogen-free or germ-free conditions showed moderately diminished RALDH activity in MLN DCs, indicating a redundant or distinctive pathway regulating the steady-state RALDH2 expression on MLN DCs (67). Thus regulation of RALDH2 in steady-state DCs may involve distinct pathways from RALDH2 expression induced by inflammation. More recent studies found that infection with the helminth Shistosoma mansoni, which provokes Th2 response, led to massive RALDH2 upregulation within alternatively activated macrophages (17). Interestingly, human mast cell-derived IL-3 can induce RALDH2 expression in basophils, thus inducing Th2 response in allergic diseases (174). However, no RALDHs induction was observed following infection with lymphocytic choriomeningitis virus (LCMV), highlighting the pathogen-specific induction of RA synthesis (17).
3. 4-1BB and prostaglandin E2
A recent study found that 4-1BB+CD103+ DCs in MLN displayed higher RALDH activity than 4-1BB−CD103+ DCs. 4-1BB stimulation allowed TLR2-pretreated splenic DCs to be more potent Treg inducers. The essential role of 4-1BB for steatdy-state RALDH2 expression on MLN-DCs was confirmed further by weak RALDH2 expression and Treg-promoting activity in MLN-DCs of 4-1BB-deficient mice (105). As it is essential for the gut-associated lymphoid organs to fight against foreign pathogens while maintaining tolerance towards food antigens, there needs to be negative regulation of RA synthesis by MLN DCs, thus eliciting anti-inflammatory response. Prostaglandin E2 was indeed shown to block GM-CSF-induced RALDH2 expression in both murine and human DCs, providing a potential mechanism for suppression of RA synthesis by DCs at steady state, since prostaglandin E2 is produced by stromal cells as wells as DCs (178). The coordinated RA synthesis by proinflammatory and anti-inflammatory signals highlights the essential role of RA playing in maintaining the balance between tolerance and immunity.
4. Wnt signaling
β-Catenin-stimulated Wnt signaling was upregulated in LPDCs compared with splenic DCs. Further studies illustrated that β-catenin is required for steady-state Raldh1 and Raldh2 expression at mRNA level in intestinal DCs and macrophages. Therefore, specific deletion of β-catenin in DCs led to lower Treg but higher Th1/Th17 differentiation, and the host is more prone to inflammatory bowel disease (120).
We summarize the different molecules for stimulating/maintaining RA synthesis in various cells in the gastrointestinal (GI) tract in Figure 1.
FIGURE 1.
Retinoic acid (RA) synthesis pathway in the gut. CD103+ DCs are major RA producers in LP, Peyer's patch, and MLN. Epithelial cells in the small intesine produce RA spontaneously. Intestinal epithelial cell-derived RA enhances CD11bc−F4/80+ macrophages to produce GM-CSF, which in combination with RA promotes CD103+ DCs in LP to produce more RA in SI-LP. Wnt/β-catenin signaling is required for RA production in LP CD103+ DCs. TLR2/5 ligands derived from dietary digestion products enhance RA synthesis in CD103+ DCs. In contrast, PGE2 inhibits RA production. 4-1BB l is required for MLN-DCs to produce RA. In addition, in MLN, stromal cells also produce RA, which in turn drives RA production by CD103+ DCs.
5. RA catabolism
To maintain RA tissue concentrations and gradients, regulation of catabolism is coupled with synthesis. A family of Cyp26A1-C1 can transform ATRA into more polar metabolite 4-hydroxy-RA or 4-oxo-RA, which is subject to further metabolism and elimination from cells (117, 185, 212). A recent study suggested that dehydrogenase/reductase-3 may also be involved in the catabolism of RA by reducing the level of all-trans-retinal (89). Thus the expression and activity of RALDHs, CYP26, and dehydrogenase/reductase-3 in combination determine the intracellular and extracellular RA levels. Cyp26A1 and Cyp26B1 show specific activity for ATRA (211, 213), whereas Cyp26C1 shows activity for both ATRA and 9-cis-RA (186). During early embryogenesis, RA levels decrease in the regions where CYP26 are expressed, thus Cyp26a1−/− or Cyp26b1−/− mice die in utero or immediately after birth (1, 219). The key role of CYP26 in regulating RA concentration was also demonstrated in immune cells. For instance, peroxisome proliferator-activated receptor gamma (PPARγ) agonists induced human DCs to produce RA, which then leads to the upregulation of CD1d, an antigen-presenting glycoprotein. This effect was enhanced by a CYP26 inhibitor (R115866), illustrating the essential role of CYP26 in RA catabolism in the immune system (184).
The classical RA response element (RARE) in the Cyp26a1 promoter region and a G-rich element synergistically allow for RA-induced Cyp26a1 expression (111, 112). In contrast, Takeuchi et al. (188) found that expression of Cyp26b1 instead of Cyp26a1 or Cyp26c1 can be induced by RA in naive T cells upon activation, indicating an indirect regulation of Cyp26b1 by RA in T cells. This study also illustrated that RA-induced Cyp26b1 expression was suppressed by TGF-β, but enhanced by proinflammatory cytokines IL-12, IL-4, and TNF-α. These findings are consistent with the effect of RA in 1) suppressing IL-12-mediated Th1, 2) enhancing IL-4-mediated Th2 response (83), and 3) enhancing TGF-β-induced Treg differentiation (11, 139, 181). To better understand the role of CYP26 in regulating regional RA level and thus differentiation or homing phenotype of immune cells, clear expression patterns of Cyp26 isoforms across hematopoietic cell lineages need to be defined. Also, conditional overexpression of specific Cyp26 isoforms in specific immune cell lineages will provide a better understanding of whether CYP26 activity determines cellular RA level in vivo. Due to the side effects of RA isomers in therapeutic applications, CYP26 inhibitors were synthesized and evaluated in preclinical models (60). The small molecules can potentially target certain specific cells through drug-Ab conjugates (193).
IV. RA SIGNALING PATHWAY
There are three different retinoic acid receptor (RAR) subtypes: RARα, RARβ, and RARγ (44, 222), each consisting of at least two isoforms generated by alternative splicing at the NH2 terminus (58, 91, 106). There are three major domains in RARs required for its function, including NH2-terminal domain (NTD), central DNA-binding domain (DBD), and COOH-terminal ligand binding domain (LBD) (27). RARs form heterodimer with retinoid X receptor (RXR) to regulate target genes expression. RAR-RXR heterodimer binds to RARE composed of two direct repeats of a core hexameric motif, PuG (G/T) TCA separated by 5 bp (DR5), 2 bp (DR2), or 1 bp (DR1) to regulate target gene transcription (8). The subsequent ligand-induced conformational changes in LBD direct the dissociation of corepressors and association of coactivator complexes, thus inducing gene transcription (27). Specifically, in the absence of retinoid ligand (such as RA), RAR-RXR heterodimer recruit and maintain corepressors to the promoter region of target genes, prevent other transcriptional machinery such as RNA polymerase binding to the promoter, and thus repress the transcription of these genes. On the other hand, however, RA binding to the RAR-RXR heterodimer releases the corepressors from the promoter, and recruits the coactivators and the transcription machinery so that target genes can be transcribed. ATRA can bind to RAR, but 9-cis-RA can bind to both RAR and RXR (57, 101, 224).
In addition to functioning through RAR-RXR heterodimer, RA was also shown to be a ligand for PPARdelta. The inconsistency between the prosurvival effect of RA and the proapoptotic function through RAR led to the discovery of PPARdelta as another receptor for RA signaling in specific cell types (161). Another study also illustrated that PPARdelta activation by RA leads to expression of the insulin-signaling gene PDK1, thus stimulating lipolysis and reducing triglyceride content (13).
In the immune system, some direct evidence has shown that RA can regulate target genes expression at transcription level (188). The cross-talk between RARα and nuclear factor of activated T cells, cytoplasmic, calcineurin-dependent 1 (NFATc1)/NFATc2 proved essential for CCR9 induction in response to transient TCR stimulation (148). In addition to the direct impact of RA on various genes transcription, RA was found to control microRNAs in different T-cell subsets, which enables RA to function through posttranscriptional regulation of multiple genes (86, 187). Therefore, given the large number of potential RAREs in the genome, RA may (in)directly regulate target genes through various mechanisms.
Various genetic models have been used to elucidate the role of RA in controlling the immune response from whole body to cellular and molecular level. DR5-luciferase mice have been used to visualize immune stimulation-induced RA signaling at both the whole body and cellular levels (5, 69, 151, 183). Various studies have used transcriptional profiling to determine the target of RA signaling in T cells (76), DCs, and other cell subsets. Conditional deletion of RARs in specific tissues or cell subsets has also been used to reveal the role of various RARs in controlling different aspects of immune response. In addition, overexpression of a dominant negative RARα (dnRARα) has been used to reveal the function of RA signaling in controlling T-cell differentiation (4, 5, 69, 151). This serves the purpose of recapitulating VAD in the immune system. The following section will focus on the known mechanism of RA signaling in control of T-cell differentiation and lineage commitment.
V. RA IN REGULATION OF T CELLS
A. RA Regulates T-Cell Homing Potential
The essential role of RA in gut immunity had led researchers to investigate the role of RA in the gut-tropism for T cells, an essential component in gut immunity. The interaction between intergrin α4β7 and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) is essential for T-cell homing to intestine endothelium (19), whereas CCR9 mediates T-cell migration to intestinal epithelial cells via interaction with chemokine (C-C motif) ligand 25 (CCL25) (100). T cells gain α4β7 and CCR9 expression during antigen stimulation by MLN-DC or PP-DC, or αCD3 and αCD28 stimulation in the presence of RA, while downregulating E- and P-selectin ligands simultaneously (84). The essential role of RA in regulating T-cell gut-tropism was further demonstrated by the fact that α4β7+ memory/activated T cells were significantly reduced in lymphoid organs and depleted of intestinal LP in VAD mice (84).
Multiple studies have confirmed the direct effect that RA imposes on α4β7 and CCR9 expression in both CD4+ and CD8+ T cells. Using in vitro culture system, Mora et al. (136) found that CD8+ T cells activated by DCs from peripheral LN gain skin-homing tropism by expressing E- and P-selectin ligands by default, while RA-producing DCs from the intestine allows for α4β7 and CCR9 expression (136). Other groups proved that RA induced α4β7 and CCR9 expression on CD4+ T cells while inducing simultaneous FoxP3 expression (11, 139, 181).
1. RA regulation of α4β7
Kang et al. (90) found that RA treatment enhanced transcription of both integrin α4 and CCR9 on T cells, whereas both integrin αE and the constitutive expression of β7 can be enhanced by TGF-β. Both integrin α4 and αE bind to β7. αEβ7 binds E-cadherin on epithelial cells (25). Even though there are seven potential RAREs in α4 promoter region, only one RARE was confirmed by the authors through binding to RARα in ChIP assay. Therefore, coordination between TGF-β and RA determines T cell migratory ability. Another study further demonstrated that increasing the relative amount of α4 to integrin β1 is essential for RA-induced α4β7 expression, as α4 predominantly binds to β1 in both naive and activated CD4+ T cells and only binds to β7 when highly expressed (41). α4β1 is involved in leukocyte migration to mucosal tissues, bone marrow, splenic follicles, and inflamed tissues (71, 199). The active participation of β1 in RA-regulating α4β7 expression on T cells proved to be physiologically important as β1−/− mice showed lower memory CD4+ T cells in bone marrow but elevated in PP.
2. RA regulation of CCR9
By measuring CCR9 mRNA level in activated CD4+ T cells in the presence of RA, and assessing the binding between NFAT and RAR-RXR complex in CCR9 promoter regions, Ohoka et al. (148) demonstrated that transient TCR-mediated signaling and CD28 costimulatory signals induced transcription factor NFATc1 and NFATc2 nuclear translocation. NFATc2 enhanced but NFATc1 inhibited RAR-RXR binding to RARE in CCR9 promoter region (148). Only transient T-cell activation allows for CCR9 upregulation as prolonged TCR signaling retains inhibitory NFATc1 in the nucleus. This finding confirms that the cross-talk between RAR-RXR and other TCR signaling-induced transcription factors is essential for the delicate regulation of RA-responsive genes expression. Another elegant study by Agace group (183) showed that high antigen dose decreased RA-induced CCR9 expression on activated CD8+ T cells, but induced E-selectin ligand expression. High antigen dose signal seems to function independently of or downstream of RA signaling. Thus T-cell receptor (TCR) signaling strength seems to determine CCR9 expression level. It remains unknown how RA signaling and TCR activation pathway cooperatively determine the tissue-tropism of CD8+ T cells.
Of note, CCR9 is constitutively expressed in naive CD4+CD8+ and CD8+ thymocytes, and on a subset of CD8+ T cells exiting the thymus into lymphoid organs (24). It remains unknown whether the steady-state CCR9 expression in these cells is regulated by RA signaling. There have not been studies addressing the mechanism of how CCR9 expression is lost on naive CD8+ T cells activated in vitro in the absence of RA.
The essential role of RA in inducing α4β7 and CCR9 expression was not only demonstrated pharmacologically by administration of specific RARs agonists/antagonist (including RA), but also by genetic approaches to manipulate RA signaling mediated in whole mouse or specific cell types. In line with previously published data using RARα antagonist, both our lab (68, 69, 151) and Hall et al. (70) showed that intrinsic RA signaling is required for gut-imprinting of T cells. Downregultion of RA-induced α4β7 and CCR9 expression was observed in both dnRARα-expressing and RARα-deficient CD8+ T cells and illustrated the requirement of RARα-RXR for CCR9 upregulation (68). In line with our studies, previous research showed that RARγ deficiency did not lead to any loss of RA-induced α4β7 or CCR9 expression on CD4+ or CD8+ T cells when activated in vitro (82).
B. RA-Dependent Induction of FoxP3 Treg Cells to Mediate Tolerance
It has been proposed that constitutitve RA presence in the gut is essential for tolerance to commensal microbes and dietary antigens by inducing Tregs to dampen the potential proinflammatory responses. To maintain immunity against foreign pathogens versus tolerance against food antigens, gut-associated DCs or other APCs have to distinguish, process, and present various antigens to induce inflammatory T-cell response or Treg differentiation, respectively.
1. Gut-associated Treg differentiation
Numerous studies have demonstrated that RA in combination with TGF-β can induce naive CD4+ T cells differentiation into stable FoxP3+ T cells both in vitro and in vivo (11, 31, 139, 181). Benson et al. (11) demonstrated that in vitro RA promoted induced TGF-β-dependent generation of inducible Tregs (iTreg) generation even in the presence of high-level costimulation. In vivo, these Tregs were very stable under CFA-driven inflammation (11). We will refer to Tregs generated in the presence of RA as RA-Tregs. Another group reported that oral administration of protein antigen or lymphopenic host induced conversion of naive CD4+ T cells into Tregs in the small intestine. This induction was dependent on RA produced by LP-DCs, because RAR-specific antagonists LE540 and LE135 inhibited RA-induced FoxP3 expression (181).
Further studies elucidated that different types of APCs are responsible for inducing RA-dependent Treg differentiation. It was observed that CD103+ and not CD103− MLN-DCs promoted the conversion of naive T cells into FoxP3+ Tregs. This conversion was dependent on both TGF-β and RA (31). Further study showed that indeed bile retinoids could also imprint intestinal CD103+ but not CD103− DCs with the ability to generate gut tolerance (85). Consistently, CD103+ DCs showed higher transcription of genes related to the activation and secretion of TGF-β (tgfb2, plat, itbp3, and Raldh2) than CD103− DCs (31). The addition of RA to CD103− DCs partially allowed these APCs to induce Foxp3 expression in T cells, indicating that RA is not the unique factor produced by these APCs for inducing Treg conversion.
A recent study showed that a deficient production of GM-CSF [encoded by colony-stimulating factor (Csf2)] from granulocyte-macrophages can affect the phagocytic functions of APCs, leading to reduced Treg numbers and impaired oral tolerance (138). Exogenous GM-CSF can restore the immunoregulatory potential of Csf2−/− APCs ex vivo. Notably, blockade of RA abrogated the ability of exogenous GM-CSF to rescue Treg induction in vitro by Csf2−/− APCs, whereas addition of RA rescued Treg induction in these cultures. Consistent with these findings, injection of a RAR antagonist compromised GM-CSF-mediated rescue of Tregs in Csf2−/− mice in vivo, whereas injection of RA restored Treg frequency in Csf2−/− mice in vivo.
The studies mentioned above describe the importance of RA in promoting the conversion and stability of Tregs. However, RA can additionally inhibit the differentiation of inflammatory Th17 cells. Mucida et al. (139) showed that TGF-β induced Th17 differentiation in the presence of IL-6, but RA inhibited Th17 differentiation allowing Treg differentiation. Exogenous RA was shown to inhibit induction of Th17 cells in vivo using an infection model, whereas injection of RAR antagonists resulted in a decrease in Foxp3+Treg cells in the LP (139). In addition, another study found that intestinal macrophages, by secreting large amounts of IL-10 and RA, might limit their own production of IL-6 and IL-23. Thus, in the TGF-β-rich intestinal microenvironment, LP macrophages would continuously favor the generation of Treg cells (40).
In addition to the prominent role that RA plays in gut tolerance, it also plays an important role in the immune privilege of the eye. Retinal pigment epithelial cells were found to produce both TGF-β and RA, thus converting bystander CD4+ T cells into FoxP3+ Treg. Both pretreatment with RAR antagonists and silencing of RAR function in retinal pigment epithelial cells prevented Treg conversion. As anticipated, experimental autoimmune uveitis developed in VAD but not in vitamin A-sufficient mice (94).
2. Role of RA in the induction of FoxP3+CD8+ Treg
The first observation of RA-induced FoxP3 expression in CD8+ T cells was reported by Kishi et al. using an autoimmune diabetes model (98). The authors generated islet-specific CD8+FoxP3+CD103+ Tregs by stimulating naive CD8+ T cells with splenic DCs in the presence of the specific peptide, TGF-β, and RA in vitro. The CD8+FoxP3+ Tregs generated in vitro inhibited diabetogenic CD8+ T cells proliferation in vitro and completely prevented diabetes onset when transferred together with diabetogenic splenocytes from non-obese diabetic (NOD) mice. Using a transgenic mouse model, which express hemagglutinin (HA) antigen on intestinal epithelial cells, Fleissner et al. (53) found that CD8+FoxP3+ cells can be induced in the MLN. These CD8+FoxP3+ cells suppressed both CD4+ and CD8+ T cell proliferation (53). Further studies illustrated that RA in combination with TGF-β can induce FoxP3 expression on HA-specific CD8+ T cell when stimulated by MLN DCs in the presence of HA peptide. In the presence of IL-2, TGF-β, and RA, donor DCs induced and expanded alloantigen-specific CD8+FoxP3+ suppressive cells with enhanced expression of CCR4, CTLA4 and CD103. The induced CD8+FoxP3+ cells protected skin allografts in vivo via suppressing the upregulation of costimulatory molecules on DCs, and the production of proinflammatory cytokines by both CD4+ and CD8+ T cells (107). Even though FoxP3+CD8+ Tregs are not yet well recognized as a major suppressive population, the above studies illustrate that RA-dependent induction of FoxP3 expression was similar in CD4+ and CD8+ T cells, highlighting its role in maintaining immune tolerance.
3. RA-dependent induction of human Tregs
There have been contradictory findings in the regard of how RA regulates FoxP3 induction in human cells. Studies using human peripheral blood cells proved that ATRA and TGF-β converted naive but not memory CD4+ T cells into stable and suppressive FoxP3+ Tregs. Furthermore, ATRA treatment enhanced the suppressive capability of natural Tregs, reinforcing the key role that RA plays in tolerance mediated by both natural and induced Tregs (205). Lu et al. (115) found that RA enhanced and stabilized FoxP3 expression in CD4+CD45RA+ naive human T cells activated with suboptimal αCD3 and αCD28 in the presence of IL-2 and TGF-β in vitro. These induced Tregs showed suppressive activity in vitro and prevented immunodeficient mice from human-anti-mouse GVHD (115). Another group revealed the synergistic effect between ATRA and rapamycin in maintaining FoxP3 expression and suppression activity of human Tregs (59). ATRA alone only maintains transient FoxP3 expression during the expansion of Tregs purified from adult human peripheral blood. This study suggests the potential requirement for both rapamycin and ATRA in generating a large number of stable Tregs for therapeutic infusion into patients.
4. Molecular mechanism of RA-induced FoxP3 expression
The molecular mechanism of how RA controls FoxP3 expression has been investigated at transcriptional and epigenetic levels. It is well established that the enhancement of FoxP3 expression by RA depends on TGF-β, as RA alone is insufficient to induce Foxp3 expression (11, 31, 139). Studies using neonatal mouse cells and luciferase reporter assasys showed that TGF-β triggered the activation of Foxp3 gene, via the binding of Smad3 and NFAT to an enhancer in the intronic conserved non-coding sequence (CNS1) (195). Recent reports showed that CNS1 contains binding sites for transcription factors NFAT, Smad3, and RAR/RXR. Further studies revealed that RA enhances TGF-β signaling by increasing the expression and phosphorylation of Smad3 (146, 217). Indeed, RA actions involve the binding of RAR/RXR to CNS1. This leads to the increased histone acetylation in the region of the Smad3 binidng site and increased binding of phosphorylated Smad3, leading to increased FoxP3 expression (218).
However, other researchers reported that RA could induce FoxP3 expression even in CD4+ T cells isolated from Smad3 or Smad2 knockout mice. These observations indicate that RA upregulates an alternative signaling axis of TGF-β, leading to ERK1/2 rather than Smad2/3 activation (114). The contradictory findings may be due to redundant or overlapping signaling pathways in the control of FoxP3 transcription. These findings are also consistent with recent observations that Smad3 binding to CNS-1 is dispensable for FoxP3 expression except in the gut (160).
Recent studies by two different groups have shown that miR10a expression is a signature of RA-induced Tregs. One study showed that miR10a is highly expressed in natural Tregs compared with other T-cell subsets and can be induced by TGF-β and RA. MiR10a in these RA-induced Tregs can target both Bcl-6 and Ncor2, and attenuate the conversion of inducible Tregs into follicular helper T cells (187). Another study confirmed that miR10a is selectively upregulated in TGF-β-induced Tregs only in the presence of RA, and allows for stable FoxP3 expression; however, genetic ablation of miR10a had no impact on FoxP3 expression. These studies open the question as to how RA regulates various microRNAs, which have been discovered to regulate various aspects of the immune response. It will be interesting to elucidate the pathways that RA regulates through fine-tuning of miRNAs so that it may determine the stability and plasticity of various T-cell subsets.
C. RA in Regulation of Other Aspects of T-Cell Differentiation
1. Th17 differentiation
The impact of RA on Th17 survival and differenftaition is highly RA concentration dependent. Various studies have shown that RA suppresses Th17 differentiation while promoting TGF-β-depenent Treg differentiation (139, 217). Xiao et al. (217) showed that RA inhibited IL-6R, interferon (IFN) regulatory factor 4 (IRF4), and IL-23R, which are all required for Th17 differnetiation, induced by TGF-β during Th17 differentiation, thus suppressing Th17 development (217). Additional studies addressed the molecular mechanism of how RA regulates Th17 differentiation. In naive mice, the steady-state ATRA in mouse sera is 1–10 nM (1–10 μg in 25 g adult mice) (126, 143). Therefore, we propose that exogenous RA above 100 nM should be considered a pharmacological concentration. The following discussion will focus on how the physiological and pharmacological concentration of RA may deliver different conclusions.
A detailed study demonstrated that flagellin-activated LP-DCs produce RA to promote both Th17 and Th1 cells differentiation in vitro (196). IL-17 instead of IFN-γ production is inhibited by RA inhibitor LE540, illustrating the specific role of endogenous RA produced by LP-DCs in driving Th17 differentiation. Unsurprisingly, 1 nM RA is sufficient to promote Th17 differentiation in the presence of splenic DCs. The reduced IL-17 and IFN-γ production by 10 μM RA (∼1,000-fold higher than physiological concentration) may indeed be caused by high toxicity. Similarly, Elias et al. (51) also showed that 1 μM Pan-RAR agonist, 4-[E-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)-1-propenyl]benzoic acid (TTNBP), inhibited IL-17 differentiation in vitro (51). We suggest that the concentration may have been too high for evaluating the physiological role of RA in Th17 differentiation. Th17 cells are decreased in the SI of VAD mice, whereas DCs isolated from MLN, PP, or SI-LP of VAD mice, enhanced Th17 cells differentiation in vitro (26). The discrepany ascribed to the reduced commensal microbes in the gut of VAD mice, highlighting that Th17 differentiation is fine-tuned by RA in the gut via various mechanisms. Another study further argued against the general conclusion that RA suppressed Th17 cell differentiation, illustrating that although slightly reduced, Th17 differentiation still occurred in the presence of <30 nM RA, and these Th17 cells generated in vitro proved proinflammatory in vivo and migrated to the intestine (203). In the same study, pharmacological levels of RA (>30 nM) inhibited Th17 differentiation in vitro. In other words, the experimental outcome is largely dependent on the concentration of RA used in the culture. Therefore, the controversy between various studies may be due to the concentration of RA and other cytokines applied. To more accurately assess the role of RA in Th17 differentiation fate, new insights should be gained through the analysis of Th17 response via genetic blockade of RA signaling. In this regard, Hall et al. (70) and our group reported deficient Th17 cell differentiation in RARα-deficient CD4+ T cells. In the study of Hall et al. (70), defective TCR signaling was observed in the RARα-deficient CD4+ T cells, too. It remains unknown whether RARα deficiency has any impact on RORγT expression per se.
2. Th1/Th2 differentiation
Earlier studies revealed that VAD mice display excess Th1 and insufficient Th2 response via various mechanisms. One study followed to demonstrate that the RXR but not RAR signaling pathway activation enhanced Th2 development (177). These authors claimed that 9-cis-RA but not ATRA supplementation enhanced Th2 development from naive Th0 cells isolated from DO11.10 TCR transgenic mice. Further studies elucidated that RXR agonist AGN194204 but not RAR agonist TTNPB promoted Th2 development even under αCD3 and αCD28 stimulation in the absence of APC. Iwata et al. (83) demonstrated that RA at ≥1 nM directly suppressed Th1 but enhanced Th2 differentiation. This effect can be mimicked by RAR agonists Am80 and Tp80, but not RXR agonists HX600 or TZ335. These findings reinforced the role of RAR-dependent RA signaling in promoting Th2 but suppressing Th1 development (83). In human T cells, RA, RARα agonist AM580, and not RARβ/RARγ agonists decrease IFN-γ and TNF-α production while promoting IL-4, IL-5, and IL-13 synthesis (35).
3. CD8+ T-cell differentiation
In an oral immunization model of Listeria monocytogens, Huang et al. (77) found that RA and TGF-β produced by MLN DCs enhanced the expression of CD8αα on activated CD8αβ+ T cells. CD8αα can interact with a nonclassical MHCI molecule thymus leukemia antigen induced on DCs so that high-affinity memory precursor cells are geared towards the gut-imprinting effector cells (77). This study supports the concept of introducing RA into a vaccination to induce highly Ag-sensitive CD8+ memory cells in the intestine for defense against mucosal pathogens. Our own studies have illustrated that RA in the periphery is required for CD8+ T cell survival during expansion, thus allowing for the optimal antigen-specific CD8+ T-cell expansion and accumulation (69). Our further study suggested that RARα is the major isoform in determining CD8+ T-cell survival during systemic Listeria monocytogens infection (68).
Taken together with what has been discovered about Th17, Th1/Th2, and CD8+ T -differentiation, the contradictory observations seem to be due to the usage of various agonists/antagonists in experimental contexts. Caution should be taken when conclusions are drawn from studies with ∼10-fold above mouse sera RA. In vitro, the presence or absence of APC in culture may also influence the final outcome of RA signaling on T-cell differentiation phenotype. More genetic approaches should be harnessed to understand whether RA signaling-deficient CD4+ T cells are less efficient in differentiation into Th2 cells.
D. RA in Regulation of T-Cell Activation and Effector Functions
Emerging data suggest that RA is critical for the development of T-cell-mediated immunity. Hall et al. (70) have discovered that RA is essential for TCR signaling in early CD4+ T-cell activation and controls CD4+ T-cell proliferation and differentiation into Th1 and Th17 cells in vitro. In this study, the authors used T cells from RARα knockout (KO) mice and showed that RARα-deficient CD4+ T cells are defective in calcium influx and ERK phosphorylation and displayed insufficient activation. The RARα KO mice showed diminished Th1 and Th17 responses in Toxoplasma gondi infection and failure to control the infection. Our studies using another transgenic model by selectively overexpressing a dominant negative RARα (dnRARα) in T cells have demonstrated that the RA signaling-deficient CD4+ T cells can proliferate, but fail to differentiate into Th1 and Th17 cells in vivo (151). The differentiation failure accounts for the delay in allogenic skin graft rejection. The discrepancy between the two studies is presently unclear and is likely due to the means with which RAR signaling was ablated.
E. RA Regulates T-Cell Survival
In addition to the requirement of RA for the development of effector T-cell immunity, our own studies have shown that RA is essential for the survival of effector CD8+ T cells during expansion phase (69). In both tumor and neo-antigen immunization model, our studies showed for the first time that in vivo, RA signaling is essential for CD8+ T cells survival during expansion at effector phase. Since RA signaling in these studies was selectively disrupted in CD8+ T cells, it proves that it is an intrinsic need of T cells to trigger RA signaling pathway to survive. As anticipated, RA signaling-sufficient CD8+ T cells are essential to control tumor growth. This was confirmed Listeria monocytogenesis infection model, in which we demonstrated that RARα-deficient CD8+ T cells failed to accumulate and control bacterial expansion in the spleen (68).
In addition to RARα, RARγ has also been implicated in CD8+ T-cell biology. It has been shown that overexpression of human RARγ, under control of the Lck promoter, elevated the number of CD4−/CD8+ single positive cells in the thymus. These data suggest that RARγ may be involved in CD8+ T-cell development in the thymus (152). However, when RARγ is deleted from hematopoietic compartment, it is without effect on CD8 ontogeny (82). Therefore, in our point of view, RARγ does not regulate CD8+ T-cell survival.
VI. RA REGULATION OF T-CELL IMMUNITY IN DISEASES
A. RA in T-Cell-Mediated Anti-tumor Immunity
1. Extrinsic effect of RA on anti-tumor T-cell immunity
Early studies have shown that a vitamin A-rich diet or RA treatment can enhance the frequency of tumor-specific cytotoxic effector killer T cells elicited by the vaccination with irradiated tumor cells (118). Other studies found that in both tumor-bearing mice (102) and cancer patients (220), high-dose RA treatment can induce the differentiation of immature myeloid-derived suppressor cells (MDSC) into mature DCs, thus diminishing the tolerance of T cells and generating greater T-cell responses against the tumor. An earlier study showed that liposomal RA treatment induced regression of murine acute promyelocytic leukemia in cooperation with adaptive immunity (210). In line with these findings, combination of a DNA vaccine and RA administration induced robust CD4+ and CD8+ T-cell immunity and enabled long-term survival in an acute promyelocytic leukemia mouse model, although ATRA alone failed to elicit pronounced T-cell immunity (56). While the studies above were not specifically designed to understand the role of RA in mediating enhanced anti-tumor T-cell immunity, they support the notion that ATRA may be important for the development of anti-tumor T cell response, thus contributing to the observed efficacy in tumor control.
2. Intrinsic effect of RA on anti-tumor T-cell immunity
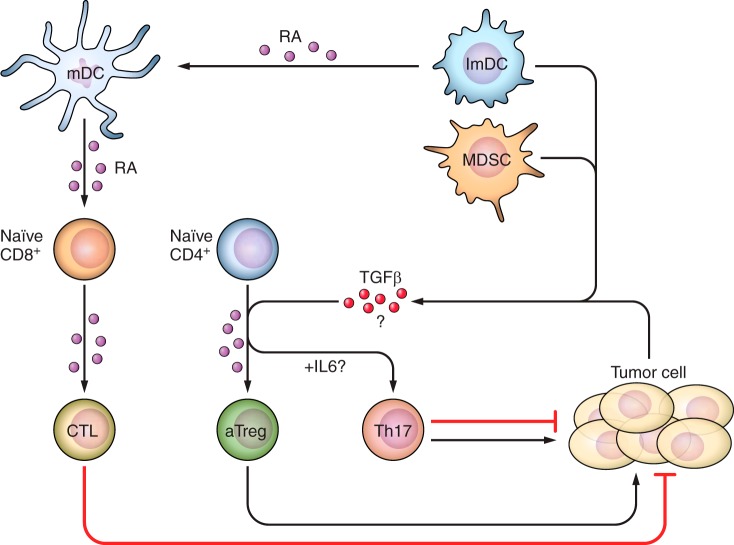
Our own studies demonstrated that T cell-intrinsic RA signaling is essential for CD8+ T-cell expansion and differentiation to IFN-γ secretion (69) in response to tumor antigen. Our studies have shown that as tumors progress, there is a progressive increase in the tissue concentration of RA at the tumor site (69). One may question as to whether RA in the tumor microenvironment (TME) contributes to the tolerant TME by facilitating the development of Treg or is it essential for the development of T effector functions (11, 31, 139, 181). One may speculate that RA produced by host DCs in TME can indeed induce Treg conversion, given heightened suppressive cytokines such as TGF-β production by malignant cells (45, 191) or host APCs (28) at late stage of tumor growth. However, we did not observe dramatic Treg changes in TME when RA signaling was blocked by dnRARα overexpression in T cells. Additional effort should be given to the study of RA signaling-deficient Tregs in TME, to evaluate whether Treg functionality in TME may be indeed dependent on RA signaling. We summarize the function of RA in regulating various aspects of anti-tumor T-cell immunity in Figure 2.
FIGURE 2.
The role of RA in regulation of anti-tumor T-cell immunity. Mature DCs produce high amounts of RA in TME, whereas RA also enhances the maturation of both immature DCs and suppressive MDSCs. Immature DCs and/or tumor cells may also produce suppressive molecule TGF-β. RA signaling is required for the expansion/accumulation of tumor-reactive CD8+ T cells, thus promoting tumor surveillance. RA and TGF-β, however, may promote aTreg accumulation in TME. Depending on tumor types, the presence of IL-6 in TME may also allow for Th17 differentiation. However, the pro- and anti-tumor activity of Th17 cells in TME is still a question of debate.
B. RA in Anti-infection T-Cell Immunity
VAD in children leads to increased susceptibility to ID and thus higher morbidity and mortality (173). These epidemiological and clinical findings clearly established that RA is essential for host resistance to infection. T cells are an essential component in fighting against both primary infections and maintenance of long-term memory response against infectious agents (214). Presently, vitamin A supplementation is currently used in many developing countries to reduce childhood mortality (78, 81, 172). The essential role of both RA and T cells in immune responses against ID suggests that RA signaling may play a key role in the development of T-cell immunity against infection. However, while VAD is associated with increased susceptibility to ID, the effect of vitamin A supplementation on ID control has been beneficial (200)/neutral (141) or even deleterious (175). RA may thus impose different impact on anti-infection immunity in response to different infectious agents, depending on what immune subsets are targeted. For instance, one group showed that RA administration enhanced LPS-induced nitric oxide synthesis 2 (NOS2) activation in innate immunity, thus leading to increased mortality in treated rats (42). In contrast, such an effect was not observed in Listeria monocytogenesis-infected rats (163). Given the importance of adaptive T-cell immunity in host protection against infection, our following discussion will focus on how RA regulates anti-infection T-cell immunity in different infectious diseases.
1. RA regulation of host resistance and CD8+ T-cell immunity
a) ra controls host resistance and mucosa-resident cd8+ t-cell immunity. Pharmacological and genetic approaches have been used to elucidate the function of RA in control of CD8+ T-cell responses to ID. The gut-homing tropism, FoxP3 induction, and survival effects that RA exerts on CD8+ T cells can all influence host susceptibility to ID. Kaufman et al. (92) found that VAD diet diminished the mucosal CD8+ T-cell response elicited by intramuscular immunization with recombinant adenovirus vaccine vector expressing OVA (rAd5-OVA). As a consequence, this abolished the protection against oral challenge of Listeria monocytogenesis overexpressing OVA (92). As expected, retinyl palmitate or RA administration fully restored the vaccination-driven gastrointestinal CD8+ T-cell response and protection against challenge. Similarly, in VAD mice there was a reduced frequency of CD8+ T cells in the lower respiratory tract to a candidate human parainfluenza virus type 1 vaccine compared with control mice. This study suggested vitamin A (or RA) controls CD8+ T-cell response in mucosa other than the gut (157). Therefore, depending on specific infectious pathogens, RA signaling may impose quite different effects on systemic CD8+ T-cell response. It has been proposed that the gut-tropism of T cells imposed by ATRA has proven beneficial in eliciting more robust T-cell immunity in the mucosa and better control of both Salmonella (73) and LCMV infection (189). In both studies, more effector and memory CD8+ T cells were observed in the gut of ATRA-treated mice.
With the use of genetic models that allow conditional blockade of RA signaling, precise questions can be asked as to the role of RA on specific leukocyte lineages. In one study using transgenic mice in which RARγ is deleted from the hematopoietic compartment, defective primary and memory CD8+ T-cell response against Listeria monocytogenesis was observed. It was proposed that this was due to a deficiency in macrophages secreting proinflammatory cytokines such as TNF-α and IL-6 (82). Interestingly, a recent study showed that RA signaling-deficient CD8+ T cells are more prone to the development of memory precurcors instead of short-lived effectors in the context of vaccinia virus infection (4). Genetic deletion of specific RAR isoforms exclusively in T cells allowed for the dissection of RARα, RARβ, and RARγ in control of anti-infection CD8+ T-cell immunity. Our recent study demonstrated that RAR is essential for survival of CD8+ T cells in vivo following Listeria monocytogenesis infection. In contrast, RAR deletion leads to modest deficiency in Ag-specific CD8+ T-cell expansion during infection. The defective survival of RARα-deficient CD8+ T cells leads to a deficiency in control of Listeria monocytogenesis expansion in the spleen (68). No defects were observed in RARγ-deficient CD8+ T cells, further corroborating that lower TNF-α and IL-6 production by the RARγ-deficient macrophages caused defective CD8+ T-cell immunity in a host with RARγ-deficient hematopoietic comparment.
2. RA regulation of host resistance and CD4+ T-cell immunity
a) th1/th2 balance. The balance between Th1 and Th2 responses during CD4+ T-cell differentiation is critical for infection control as it determines the humoral response against pathogens. Previous studies revealed that VAD environment induced constitutive expression of Th1 cytokines, and limited the humoral response to infection, thus diminishing the host ability to contain infection through antibody (Ab) production (23). Investigating Trichinella spiralis infection in VAD mice, Cantorna et al. (22) found higher IFN-γ production and lower frequencies of IL-5-producing Th2 cells, which can be restored by the addition of RA in vitro. These data suggest that RA inhibits Th1 and promotes Th2 differentiation and survival (22). Even in animals with normal serum levels of RA, RA was shown to enhance Th2 but inhibit Th1 responses during tetanus toxoid immunization resulting in higher IL-4:IFN-γ ratio and enhanced all anti-tetanus toxoid IgG isotypes. These data suggest that RA can be used as an adjuvant in generating more robust humoral response via Th2 skewing in vivo (116). However, despite the skewed Th2 response discussed above (22, 83), RA treatment of Ascaris suum-infected swine promoted profound and enhanced Th1, Th2, and Treg differentiation in the liver, albeit some may be an indirect impact from innate immune cells, such as more recruitment of eosinophils and more inflammatory cytokines produced thereafter (34).
b) th1/th17 response. Previous studies using CD4+ T cells from both VAD and RARα KO mice showed increased frequencies of FoxP3+ Tregs, deficient proliferation, and differentiation into Th1 and Th17 cells in Toxoplasma gondi model, while no dramatic differences were reported in Th2 response (70). However, the lower Th1 and Th17 response in vivo led to impaired control of systemic Toxoplasma gondi systemically following oral infection, clearly demonstrating the essential role of intact RA signaling for protective CD4+ T-cell immunity. As RARα is also deleted APCs in these mice, such as DCs and myeloid cells, the defective CD4+ T-cell responses in vivo may be partially due to the intrinsic RA signaling deficiency in T cells. Therefore, additional studies in mice with lineage-specific control of RA signaling will help to address these issues. It also remains to be elucidated whether intrinsic RA signaling controls the balance between suppressive Tregs and inflammatory Th1/Th17 response in various ID circumstances. The immune system has apparently developed sophisticated mechanisms to control infection and avoid overt pathology by balancing the magnitude of the inflammatory response. No doubt, RA falls into the group of fine-tuning molecules to keep immune response in balance.
c) treg regulation. RA also regulates host resistance and immunity via the enhancement of stable and gut-tropic Tregs. RA-Tregs are more stable than Tregs generated in the absence of RA (11). Due to concomitant α4β7 and CCR9 expression, RA-Tregs showed preferential migration to the gut (11, 181). RA-Tregs are thus also superior in protection against colitis due to the preferential migration to the gut (139). The role of RA in generating superior Tregs holds true during chronic helminth infection. Chronic helminth infection induced tolerogenic CD11clow DCs which induced RA signaling-dependent Treg generation, and modulated the immune response. Depletion of CD11chi DCs favored the dominance of CD11clow DCs, leading to lower effector CD4+ T-cell proliferation and effector cytokine production (170). RA treatment also enhanced FoxP3 but inhibited IL-17 expression in colonic biopsies from patients with ulcerative colitis and colon tissues ex vivo. In addition, RA also downregulated colon inflammatory responses and ameliorated trinitrobenzene sulfonic acid-induced murine colitis (7). RA-Tregs are also superior in resolving CD8+ T cell-mediated acute small intestine inflammation (129). This study highlighted that the selective migration of RA imposed on Tregs is key to their local suppressive function. All the above studies support that RA may ameliorate the infection-relevant pathology in the gut via generating highly stable and gut-residential Treg.
C. RA and T-Cell Differentiation in Transplantation
1. Suppression of effector response
During solid organ transplantation, donor alloantigens are processed and presented to CD4+ T cells, and induce the latter to activate and produce IL-2 and IFN-γ. The inflammatory cytokines can then induce cytotoxic CD8+ effector T cells, B cells, and macrophages proliferation and differentiation, which can mediate allogeneic tissue and organ distruction (207). Even though numerous studies have confirmed that CD4+ and CD8+ effector T cells mediate the rejection of allografts (169), there is always counterregulation by Tregs (as reviewed in Ref. 202). Therefore, the balance between effector T cells and Tregs determines the kinetics of rejection or acceptance of allogenic tissues or organs. Direct regulation of RA on T cell allogenic in GVHD development has been reported. In a mouse model of acute GVHD, RARα-specific agonist ER-38925 administration dampened the cytotoxic T lymphocyte (CTL) response, and inhibited Th1-derived serum IL-12 and IFN-γ and macrophage-derived TNF-α. The substantial inhibition of antiallogenic T-cell responses in ER-38925-treated mice therefore prevented the development of acute GVHD (75).
2. RA promotes allogenic effector response
Our recent study revealed a novel function of RA as being essential for the development of T-cell effector response against an allogenic skin transplant. In a model where T cell-intrinsic RA signaling was ablated, deficient Th1/Th17 response led to significantly delayed rejection of allogenic skin grafts. Our failure to observe an overriding suppression of immunity by RA indicated the dominant role of endogenous RA in driving effector response than Treg development in this model. These studies provide insightful understanding of the function of RA in controlling the development of effector T cells in allogenic organ rejection.
3. RA regulates transplantation through Treg development
Despite the requirement of effector T cell-intrinsic RA signaling for allogenic skin rejection, the administration of RA agonists induces immune suppression in some circumstances. Prior to the discovery that RA can induce Treg development, a study in cardiac transplantation demonstrated the immunosuppressive efficacy of RARα agonist in organ transplantation (164). During acute cardiac transplantation, prophylactic treatment with RARα-specific agonist ER-38925 in combination with tacrolimus inhibited cytotoxic effector cells proliferation and alloantigen-specific inflammatory cytokines IL-2, IL-12 and IFN-γ production, and thus delayed the rejection of the mouse cardiac allograft (BALB/c→C3H/HeN). The inhibition of cytotoxic response may be due to the induction of Treg development in this model, although not stated by the authors. Another study revealed that combination of RA and TGF-β indeed attenuated acute cardiac rejection by promoting Treg and inhibiting Th17 differentiation (204).
Various studies have now proven that Tregs can be generated ex vivo, efficiently dampen the host immune response against graft-specific antigens, and thus prevent both acute and chronic allograft rejection in vivo (87, 216). Numerous studies have demonstrated the success of RA-Treg in the prevention of allogenic transplant rejection. Moore et al. (135) cultured naive T cells with allogenic APCs, TGF-β, IL-2, and RA to generate Tregs that were successful in preventing allogenic skin rejection, proving the suppressive function and potential for therapeutic application of RA-Treg in allogenic graft rejection (135). In a rat orthotopic tracheal transplantation model, adoptive transfer of RA-Tregs enhanced TGF-β and IL-10 production, whereas inhibited inflammatory cytokines IL-17, IFN-γ, IL-6, and MCP-1. RA-Tregs also inhibited the infiltration of effector T cells into the graft. The balance between anti- and proinflammatory response led to attenuated airway obliteration and graft fibrication, thus protecting rat trachea allograft rejection (168). More surprisingly, RA-induced CD8+FoxP3+ Tregs were found to induce conventional CD4+FoxP3+ Treg conversion and concurrently suppressed CD8+ T effector expansion in situ, thus protecting allogenic skin graft (107). Therefore, RA can induce FoxP3 expression on both CD4+ and CD8+ T cells, and promote immunosuppression required for allogenic grafts protection.
D. RA and T-Cell Differentiation in Autoimmunity
As RA regulates various aspects of T-cell function, it is not surprising that RA also modulates the progression of various autoimmune diseases through the regulation of CD4+ or CD8+ T cells. Specifically, RA can control the differentiation of CD4+ T cells into Th1, Th2, and Th17 which impact on the intensity and pathological manifestations of many autoimmune diseases. Furthermore, RA may also regulate the progression of autoimmune diseases via control of CD8+ T-cell differentiation. In the following section, we focus on how CD4+ T-cell differentiation is controlled by RA signaling towards immunity or tolerance, and thus determine the disease incidence and severity in different autoimmune diseases.
1. RA regulates experimental autoimmune encephalomyelitis
a) ra suppresses pathogenic t cells in experimental autoimmune encephalomyelitis. Early in 1991, long before the discovery of Tregs and Th17 cells, 13-cis-RA was demonstrated to prevent both active and passive experimental autoimmune encephalomyelitis (EAE) development through the inhibition of T-cell proliferation, IL-2 production, and effector function (123). The same therapeutic efficacy was observed in ATRA treatment of myelin basic protein (MBP)-specific cell-induced EAE. RA was found to increase IL-4 but decrease IFN-γ, IL-2, and TNF-α expression in addition to inhibition of T-cell proliferation (153). The shift of Th1 to Th2-like phenotype by RA treatment was also observed in an MBP-specific human CD4+ T cell line, supporting the rationale for retinoids in the treatment of multiple sclerosis (113). Further studies have revealed the role that RA plays in Th17 cells in the treatment of autoimmune diseases. One study showed that RA inhibited pathological Th1 and Th17 cells through downregulation of IRF4, IL6Rα, and IL23R without altering the frequency of Tregs development in EAE, thus inhibiting EAE onset and progression (217).
b) ra promotes treg in amelioration of eae. The role of RA in promoting different suppressive Treg subpopulations makes the rationale that RA modulates the balance between inflammatory and tolerant response during the development of autoimmunity. A novel CD8αα+ Treg lineage derived from naive CD4+ T cells has been recognized lately as an important suppressive population generated in situ. Interestingly, RA in combination with TGF-β can induce CD8αα+ Tregs development from naive CD4+ T cells in RUNX3-dependent manner, to protect mice against myelin oligodendrocyte glycoprotein-induced EAE (142). The timing of RA treatment, however, needs to be taken into account as long-term treatment also leads to other cytokine profile change, which may result in effects opposite from what may be predicted from the disease onset. Studies showed that retinoids such as AM80, RARα/β-specific ligand, are effective during the early phase of EAE development. Such an effect was correlated with lower IL-10 production during disease progression. However, continuous administration failed to inhibit late EAE symptoms (99).
Despite the therapeutic efficacy of retinoids in treatment of EAE, more studies are warranted to illustrate whether RA signaling-deficient T cells still facilitate EAE progression. It is of great importance to understand the role of RA signaling in balance between effector and regulatory T cells during the disease progression for better control of time-dependent RA signaling.
2. RA regulates rheumatoid arthritis
Studies have revealed the essential role of RA in progression of rheumatoid arthritis progression via balancing between suppressive Treg and pathogenic Th1/Th17 cell differentiation. An early study in rheumatoid arthritis patients suggested that RA treatment decreased IFN-γ but not IL-2 or IL-4 production by CD4+ T cells from both healthy and rheumatoid arthritis patients. These results indicated the potential application of RA in treatment of rheumatoid arthritis (147). A recent study showed that RA treatment decreased Th17 and increased Tregs in a murine model of collagen-induced arthritis (CIA), leading to the inhibition of both incidence and progression of CIA (103). Further studies revealed that concurrent FoxP3 upregulation and IL-17 downregulation is independent of STAT3 or STAT5 signaling pathway, consistent with previous observations using conditional deletion of stat5 and stat3 (51). A very recent study demonstrated that all-trans-retinaldehyde (retinal) treatment reciprocally suppressed Th17 and promoted Treg development at both mRNA (decrease of IL-17 and IL-21 but increase of FoxP3, SOCS3, and IL-10) and protein level in DBA1/J mice with CIA, thus partially contributing to the attenuation of arthritis (150). It remains to be elucidated how retinal regulates the balance between Th17 and Tregs in CIA. All-trans-retinaldehyde demonstrated a function through phospholipase C/1,4,5-trisphosphate/Ca2+ signaling independent of its conversion to RA in some cell types (29). However, the efficacy observed in the above study was likely via the conversion to RA. In addition to its direct impact on Treg induction, RA was found to maintain the stability of natural Tregs and prevent Th17 differentiation. This occurs as a result of the downregulation of IL-6R expression and pSTAT3 activation (228). Further in vivo studies elucidated that adoptive transfer of RA-treated nTreg suppressed the progression of established CIA in DBA1 mice. However, nontreated nTreg succumbed to Th17 differentiation in this chronic inflammatory environment and lost therapeutic efficacy. This proved true even if nTreg maintained stable FoxP3 expression in vivo and were originally isolated from CIA mice, indicating the therapeutic potential of RA in rheumatoid arthritis (228).
3. RA regulates uveitis
Eyes are one of the immune privileged sites in human body. Some studies have revealed the role of RA in maintaining the immune tolerant microenvironment in the eye through inhibition of Th1/Th17 response and promotion of Treg development. Immunization of C57BL/6 mice with human interphotoreceptor retinoid binding protein peptide 1 to 20 [IRBP(1–20)] elicited experimental autoimmune uveoretinitis (EAU). Keino et al. (95) reported that intraperitoneal RA administration dampened the severity of EAU when administrated at 200 μg/mouse (∼20-fold above normal sera RA concentration, every other day) before the disease onset. No therapeutic efficacy was observed when RA is administrated at effector phase (95). Oral administration of Am80 at 3 mg/kg (∼75 μg/25 g mouse, ∼7-fold above naive serum RA level) every other day, inhibited the severity of EAU, through downregulation of IL-6R, IL-17A, and IFN-γ, and reciprocal upregulation of FoxP3 (96). The same effect of IL-6R upregulation on CD4+ T cells and antigen-specific Th1/Th17 response were observed in RA-treated mice as in EAE studies despite an unaltered Treg population.
Despite the therapeutic efficacy of RA and synthetic RAR ligands in EAU treatment, the molecular mechanism of ocular immune privilege was not fully understood until recently. The Caspi group was able to purify naive Foxp3-gfp−CD4+ T cells from FoxP3-GFP mice, and convert them into highly suppressive Tregs using aqueous humor (AH) in synergy with TGF-β and RA. The Tregs also showed the upregulation of CD25, GITR, CD103, and CTLA4 (226). More in-depth analysis revealed that AH stabilized TGF-β and RA-induced FoxP3 expression. This study indicated a dual role of RA in the eye: in vision and immune privilege. The same group further demonstrated for the first time that in vivo naive CD4+ T cells can be converted to Tregs in a RA-dependent manner in the eye during specific auto-antigen-induced proliferation. Cornea and retina proved to be the source of RA for Treg development as both are positive for all three Raldh isoforms expression and activity (227). More strikingly, either preimmunization of the host with IRBP(161–180) or priming of CD4+ T cells (CD44hiCD62L−) diminished RA-dependent Treg induction significantly, thus inducing severe uveitis. Regarding the source of RA, Kawazoe et al. (94) also demonstrated that retinal pigment epithelial cells produced RA and, in combination with TGF-β, induced bystander T cells conversion into Tregs, thus maintaining the ocular immune tolerance (94). This study further reinforced the key role of RA in prevention of autoimmune uveitis development.
All the above studies extended the important function of RA in both vision development and immune privilege (201). Such findings reinforced the importance of sufficient vitamin A intake in both vision development and the tolerant ocular microenvironment maintenance.
4. RA regulates T cell-mediated type 1 diabetes
Numerous studies have revealed the functional deficiency or decrease of Tregs in type I diabetes (T1D) patients (as reviewed in Ref. 18). Hence, manipulating the immune system to facilitate Treg expansion or shift from pathogenic effector T cells is predicted to be efficient in T1D treatment. Van et al. (198) showed the therapeutic efficacy of (500 μg/mouse, every other day) RA in T1D treatment. In their study, RA treatment of nonobese diabetic (NOD) mice at 10 wk of age age, when the destructive insulitis already occurred, increased systemic Treg accumulation, decreased activated CD8+ effector T cell and Th1 cell infiltration into the islet (198). This combinatorial effect on T cells led to the regression of diabetes. Of note, the efficacy was completely dependent on Treg increase as depletion of CD4+CD25+ T cells impaired the efficacy. This study sets the foundation that RA-induced Tregs are essential for suppressing the development of T1D even though the direct inhibition of ATRA on effector T cells proliferation cannot be excluded.
A subsequent study tested the efficacy of retinoids, etretinate, and ATRA in three different preclinical models of human T1D (179). In streptozotocin-induced diabetes in CBA/H mice, both drugs suppressed the diseases progression in terms of decreasing hyperglicemia, concomitant with lower CD4+CD25+ effector T cells in the periphery. ATRA also decreased expression of IFN-γ and IL-17 at mRNA level and secretion of IL-6, IFN-γ, and IL-17 upon restimulation ex vivo. The authors observed the same effect as in previous studies (198) when RA was administrated in spontaneous diabetes developed in NOD mice. However, no efficacy was observed when cyclophosphamide was administered to accelerate diabetogenesis, highlighting the Treg-dependent ATRA efficacy. The discovery of ATRA-induced CD8+ Tregs led to the finding that CD8+FoxP3+ Tregs generated in vitro were suppressive in vivo and effective in T1D treatment (98).
More importantly, recent studies shed light on the importance of conditioning DCs to promote Treg development via RA production in T1D treatment. Immunization with Schistosoma mansoni soluble antigen preparations was known to protect NOD mice from T1D development (30). Interestingly, ω-1, a well-characterized glycoprotein in S. mansoni antigen, was demonstrated to induce both TGF-β and RA production by DCs and induce Treg development in vitro (221). The mechanism emphasized the intrinsic requirement of RA-induced FoxP3 expression in the therapeutic efficacy of S. mansoni antigens in NOD mice.
5. RA regulates other autoimmune diseases
In addition to the well-known autoimmune diseases mentioned above, other studies have elucidated the mechanism of RA signaling in control or treatment of other autoimmune diseases. Sobel et al. (171) reported that CD4+ T cells in systemic lupus erythematosus patients are defective in both TGF-β and RA signaling, indicating the proinflammatory environment caused by Treg decrease. In another study on experiment autoimmune myositis, Ohyanagi et al. (149) found that oral administration of Am80 increased IL-4, IFN-γ, and IL-10 but not IL-17 production by T cells, and decreased T-cell infiltration into the muscle, leading to ameliorated inflammation. The multifaceted function of RA in T-cell differentiation and migration makes it a good candidate for the treatment of a wide spectrum of autoimmune diseases.
Despite the rapid progress in understanding both the mechanism and therapeutic application of RA in control of autoimmune diseases, additional insights are critical. At this time, we do not have a clear mechanistic understanding of the molecular impact of RA on key cellular elements (T, B, myeloid) in vivo and how it governs the development of tolerance and autoimmunity.
E. RA in T-Cell Regulation for Allergy Response
The promiscuous impacts of RA on T-cell differentiation make it an attractive mediator in control of allergy. Early studies established that T cells in VAD hosts were skewed towards IL-10+ Tregs but diverted from IFN-γ+ Th1 memory cells without any impact on IL-4 production (176). In the following section, we will discuss the role of RA signaling in the control of allergic response via regulation of different T-cell subsets.
1. RA regulates allergy response via Th1/Th2
Various studies have revealed that RA may affect allergy response by altering both Th1 and Th2 response. Studies have shown that both ATRA and 9-cis-RA induced Th2 cytokines IL-4 and IL-5 production by human CD4+ T cells, while inhibiting Th1 cytokine IFN-γ production (36). As discussed above, the timing of ATRA administration determines the impact on allergy progression, as CD4+ T-cell differentiation status is different at the onset and progression stage. For instance, liposomally encapsulated ATRA increased IgE response, bronchoalveolar lavage (BAL) eosinophilia, and accumulation of IL-5, TGF-β1, and CCL1 when given at the time of systemic sensitizing injections for asthma manifestation. However, such variables were not affected when administered at the time of intranasal challenges (121). In both cases, T cells were prone to IL-4/IL-5+ Th2 but not IFN-γ+ Th1 cells in vitro, exacerbating allergic and inflammatory response. The different observations at different time points suggest that the impact on T-cell differentiation, for instance, is dependent on the priming status (plasticity) of these cells and their migration into the lung. This was consistent with previous reports showing that in ATRA-treated acute promyelocytic leukemia patients, there was a hyperleukocytic response associated with asthmalike respiratory syndrome (66, 74), eosinophilia, basophilia and hyperhistaminemia, peripheral edema, and so on (74).
2. RA regulates allergy through Tregs
More recent studies have focused on the role of RA-Treg in regulation of T cell-mediated allergy. The ability of ATRA to induce FoxP3 expression was not only demonstrated in naive CD4+ T cells (11, 139, 181), but also proven true in Th2 memory cells generated in vivo. Kim et al. (97) discovered that OVA-specific Th2 memory cells generated in vivo can be converted into functional FoxP3+ Tregs in the presence of TGF-β and ATRA ± rapamycin under both Ag-specific stimulation and polyclonal stimulation (97). The “converted” Tregs downregulated GATA3 and IRF4; produced little IL-4, IL-5, and IL-13; and migrated efficiently into the lung. Thus Tregs efficiently suppressed Th2-mediated airway hyperreactivity, including eosinophil and IgE response. This finding supported the therapeutic potential of RA in allergy treatment.
In addition to pharmacological approaches, genetic-engineered mouse models were also used to elucidate molecular mechanisms of how endogenous RA signaling regulates allergy response. In complete Aspergillus allergen-immunized mice model, epithelial metalloproteinase 7 (MMP7) activated IL-25 to promote Th2 cells differentiation, but inhibited RALDH1 and thus Treg development (61). Therefore, MMP7−/− mice showed higher RALDH1 expression, lower IL-4 and IL-5 in bronchoalveolar fluid, and dampened airway hyperreactivity. As such, the RALDH inhibitor Citral restored asthma in MMP7−/− mice, whereas ATRA administration attenuated the asthma phenotype with a concomitant increase in Treg.
The key role of RA in modulating allergy via Treg induction was also demonstrated in Th17-dominated chronic allergic airway inflammation. During mucosal sensitization with OVA and TNF-α, prolonged ATRA treatment enhanced Treg activity in the lung and consequently ameliorated Th17-mediated disease, reinforcing the therapeutic potential of ATRA in asthma treatment by modulating Th17-Treg balance (225). Of note, ATRA concentration needs to be taken into account for allergy treatment. In both OVA immunization-OVA challenge and house dust mite immunization-house dust mite challenge models, different doses of ATRA changed the allergy response differentially (124). Even though administration of ATRA at different doses (50, 500, and 2,500 μg) all enhanced eosinophil recruitment to the BAL, fewer eosinophils were recruited into BAL of 2,500 μg ATRA-treated mice than those treated at lower doses. In addition, total IL-5 and IgE in BAL were decreased in 2,500 μg ATRA-treated but increased in lower dose-treated hosts. Given that 50 μg is about fivefold higher than the total RA concentration in the whole mouse body, 500 and 2,500 μg (∼50- and ∼500-fold higher than physiological concentration, respectively) may indeed have some off-target effects and/or even toxicity. The lower eosinophil counts in 2,500 μg treatent group may be due to high toxicity. Therefore, lower pharmacological doses of ATRA enhance Th2 response in vivo. The global adverse effect of high-dose treatment needs to be taken into account when evaluating the application of RA in allergy treatment.
More studies are certainly needed to understand the regulatory role of RA in Th2 or Th17-dominant allergy disease. The recruitment, proliferation, differentiation, and activity of various T-cell subsets are the key components that RA signaling may impose impact on during both disease development and treatment.
F. RA and T Cell-Dependent Humoral Response
As discussed above, VAD leads to increased susceptibility to ID among young children, causing higher mortality and morbidity (173). B cells and humoral response are vital to host protection and vaccination against many infectious pathogens. There are two types of Ab response, namely, T-dependent and -independent response, depending on the specific Ag. Due to the wide-sepctrum features that RA regulates in T-cell differentiation, RA may also modulate humoral response via the pathway of T-cell differentiation. For researchers interested in the direct impact of RA on B cells, B-cell differentiation, memory B-cell generation, and IgA class switching, we recommend other review articles (155).
VII. CONCLUDING REMARKS
Numerous studies have elucidated the mechanism of how vitamin A controls the immune response through RA signaling. During embryogenesis, maternally derived RA is essential for the development of secondary lymphoid organs (197). In adult mammals, RA controls various immune cells differentiation and thus the ultimate outcome of immune response and host protection (84, 137). The dual role of RA promoting tolerance (11, 31, 139) and immunity (69, 70, 151) in different disease contexts points to the key notion that RA functions as a regulator of immune response. The overall impact of vitamin A deprivation or RA signaling ablation depends on the microenvironment in which other cytokines/chemokines may interact with RA-mediated signaling to determine the cell differentiation fate.
Although Iwata et al. (84) provided the initial clues that the “anti-infective” effect of vitamin A is conveyed through the upregulating impact of the gut-homing molecules α4β7 and CCR9, the impact of RA on immunity is far more profound. In the periphery, RA controls the specification and fate of Th1s, Th17s, and Th2s. In this regard, many questions remain to be answered. First, does RA signaling control the plasticity and stability of effector T cells during differentiation as it stablizes FoxP3 expression in Tregs? That is, is RA signaling required for stable expression of GATA-3, T-bet, and RORγT expression possibly through epigenetic modification? Second, what is the difference between signaling pathways induced by physiological and pharmacological RA? Caution should be taken in the interpretation of data acquired through the administration of high-dose ATRA or 9-cis-RA due to possible toxicities. Third, what other transcription factors cooperate with RARs in determining the lineage commitment of different immune cells? Fourth, what genes in T cells are directly or indirectly regulated by RA that impact on their function and fate? To answer these two questions, advanced proteomic and genomic analyses need to be harnessed. Chromatin-immunoprecipitation (ChIP)-Seq analysis will help to identify novel target genes during T-cell differentiation. These mechanistic studies will lead to a better understanding of how RA exerts such profound control over the polarity of the immune response.
GRANTS
This work was supported by National Institutes of Health Grant 1R01AT005382.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
acknowledgments
Address for reprint requests and other correspondence: R. J. Noelle, Norris Cotton Cancer Center, One Medical Center Dr., Rubin Bldg., Rm. 730, Lebanon, NH 03755 (e-mail: RJN@Dartmouth.edu) or MRC Centre for Transplantation, King's College London, 5th Floor Tower Wing, Guy's Hospital, London SE1 9RT, UK (e-mail: Randolph.Noelle@kcl.ac.uk).
REFERENCES
- 1.Abu-Abed S, Dolle P, Metzger D, Beckett B, Chambon P, Petkovich M. The retinoic acid-metabolizing enzyme, CYP26A1, is essential for normal hindbrain patterning, vertebral identity, and development of posterior structures. Genes Dev 15: 226–240, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed F, Jones DB, Jackson AA. The interaction of vitamin A deficiency and rotavirus infection in the mouse. Br J Nutr 63: 363–373, 1990. [DOI] [PubMed] [Google Scholar]
- 3.Ahrendt M, Hammerschmidt SI, Pabst O, Pabst R, Bode U. Stromal cells confer lymph node-specific properties by shaping a unique microenvironment influencing local immune responses. J Immunol 181: 1898–1907, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Allie SR, Zhang W, Tsai CY, Noelle RJ, Usherwood EJ. Critical role for all-trans retinoic acid for optimal effector and effector memory CD8 T cell differentiation. J Immunol 190: 2178–2187, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aoyama K, Saha A, Tolar J, Riddle MJ, Veenstra RG, Taylor PA, Blomhoff R, Panoskaltsis-Mortari A, Klebanoff CA, Socie G, Munn DH, Murphy WJ, Serody JS, Fulton LM, Teshima T, Chandraratna RA, Dmitrovsky E, Guo Y, Noelle RJ, Blazar BR. Inhibiting retinoic acid signaling ameliorates graft-versus-host disease by modifying T-cell differentiation and intestinal migration. Blood 122: 2125–2134, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aya Yokota HT, Maeda N, Ohoka Y, Kato C, Song S- Y, Iwata M. GM-CSF and IL-4 synergistically trigger dendritic cells to acquire retinoic acid-producing capacity. Int Immunol 21: 361–377, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai A, Lu N, Guo Y, Liu Z, Chen J, Peng Z. All-trans retinoic acid down-regulates inflammatory responses by shifting the Treg/Th17 profile in human ulcerative and murine colitis. J Leukoc Biol 86: 959–969, 2009. [DOI] [PubMed] [Google Scholar]
- 8.Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res 43: 1773–1808, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Barreto ML, Santos LM, Assis AM, Araujo MP, Farenzena GG, Santos PA, Fiaccone RL. Effect of vitamin A supplementation on diarrhoea and acute lower-respiratory-tract infections in young children in Brazil. Lancet 344: 228–231, 1994. [DOI] [PubMed] [Google Scholar]
- 10.Bellovino D, Morimoto T, Tosetti F, Gaetani S. Retinol binding protein and transthyretin are secreted as a complex formed in the endoplasmic reticulum in HepG2 human hepatocarcinoma cells. Exp Cell Res 222: 77–83, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med 204: 1765–1774, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berry DC, Jacobs H, Marwarha G, Gely-Pernot A, O'Byrne SM, DeSantis D, Klopfenstein M, Feret B, Dennefeld C, Blaner WS, Croniger CM, Mark M, Noy N, Ghyselinck NB. The STRA6 receptor is essential for retinol-binding protein-induced insulin resistance but not for maintaining vitamin A homeostasis in tissues other than the eye. J Biol Chem 288: 24528–24539, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berry DC, Noy N. All-trans-retinoic acid represses obesity and insulin resistance by activating both peroxisome proliferation-activated receptor beta/delta and retinoic acid receptor. Mol Cell Biol 29: 3286–3296, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhat PV. Retinal dehydrogenase gene expression in stomach and small intestine of rats during postnatal development and in vitamin A deficiency. FEBS Lett 426: 260–262, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Blackfan KD, Wolbach SB. Vitamin A deficiency in infants: a clinical and pathological study. J Pediatr 3: 679–706, 1933. [Google Scholar]
- 16.Blomhoff HK, Smeland EB, Erikstein B, Rasmussen AM, Skrede B, Skjonsberg C, Blomhoff R. Vitamin A is a key regulator for cell growth, cytokine production, and differentiation in normal B cells. J Biol Chem 267: 23988–23992, 1992. [PubMed] [Google Scholar]
- 17.Broadhurst MJ, Leung JM, Lim KC, Girgis NM, Gundra UM, Fallon PG, Premenko-Lanier M, McKerrow JH, McCune JM, Loke P. Upregulation of retinal dehydrogenase 2 in alternatively activated macrophages during retinoid-dependent type-2 immunity to helminth infection in mice. PLoS Pathog 8: e1002883, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol 10: 849–859, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol 72: 209–253, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Cameron C, Dallaire F, Vezina C, Muckle G, Bruneau S, Ayotte P, Dewailly E. Neonatal vitamin A deficiency and its impact on acute respiratory infections among preschool Inuit children. Can J Public Health 99: 102–106, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campos FA, Flores H, Underwood BA. Effect of an infection on vitamin A status of children as measured by the relative dose response (RDR). Am J Clin Nutr 46: 91–94, 1987. [DOI] [PubMed] [Google Scholar]
- 22.Cantorna MT, Nashold FE, Hayes CE. In vitamin A deficiency multiple mechanisms establish a regulatory T helper cell imbalance with excess Th1 and insufficient Th2 function. J Immunol 152: 1515–1522, 1994. [PubMed] [Google Scholar]
- 23.Cantorna MT, Nashold FE, Hayes CE. Vitamin A deficiency results in a priming environment conducive for Th1 cell development. Eur J Immunol 25: 1673–1679, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Carramolino L, Zaballos A, Kremer L, Villares R, Martin P, Ardavin C, Martinez AC, Marquez G. Expression of CCR9 beta-chemokine receptor is modulated in thymocyte differentiation and is selectively maintained in CD8(+) T cells from secondary lymphoid organs. Blood 97: 850–857, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, Brenner MB. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature 372: 190–193, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Cha HR, Chang SY, Chang JH, Kim JO, Yang JY, Kim CH, Kweon MN. Downregulation of Th17 cells in the small intestine by disruption of gut flora in the absence of retinoic acid. J Immunol 184: 6799–6806, 2010. [DOI] [PubMed] [Google Scholar]
- 27.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J 10: 940–954, 1996. [PubMed] [Google Scholar]
- 28.Chang HL, Gillett N, Figari I, Lopez AR, Palladino MA, Derynck R. Increased transforming growth factor beta expression inhibits cell proliferation in vitro, yet increases tumorigenicity and tumor growth of Meth A sarcoma cells. Cancer Res 53: 4391–4398, 1993. [PubMed] [Google Scholar]
- 29.Chen Y, Okano K, Maeda T, Chauhan V, Golczak M, Maeda A, Palczewski K. Mechanism of all-trans-retinal toxicity with implications for stargardt disease and age-related macular degeneration. J Biol Chem 287: 5059–5069, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooke A, Tonks P, Jones FM, O'Shea H, Hutchings P, Fulford AJ, Dunne DW. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol 21: 169–176, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 204: 1757–1764, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coutsoudis A, Broughton M, Coovadia HM. Vitamin A supplementation reduces measles morbidity in young African children: a randomized, placebo-controlled, double-blind trial. Am J Clin Nutr 54: 890–895, 1991. [DOI] [PubMed] [Google Scholar]
- 33.D'Ambrosio DN, Clugston RD, Blaner WS. Vitamin A metabolism: an update. Nutrients 3: 63–103, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dawson H, Solano-Aguilar G, Beal M, Beshah E, Vangimalla V, Jones E, Botero S, Urban JF Jr. Localized Th1-, Th2-, T regulatory cell-, and inflammation-associated hepatic and pulmonary immune responses in Ascaris suum-infected swine are increased by retinoic acid. Infect Immun 77: 2576–2587, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dawson HD, Collins G, Pyle R, Key M, Taub DD. The retinoic acid receptor-alpha mediates human T-cell activation and Th2 cytokine and chemokine production. BMC Immunol 9: 16, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dawson HD, Collins G, Pyle R, Key M, Weeraratna A, Deep-Dixit V, Nadal CN, Taub DD. Direct and indirect effects of retinoic acid on human Th2 cytokine and chemokine expression by human T lymphocytes. BMC Immunol 7: 27, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Pee S, Dary O. Biochemical indicators of vitamin A deficiency: serum retinol and serum retinol binding protein. J Nutr 132: 2895S–2901S, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Deltour L, Foglio MH, Duester G. Metabolic deficiencies in alcohol dehydrogenase Adh1, Adh3, and Adh4 null mutant mice. Overlapping roles of Adh1 and Adh4 in ethanol clearance and metabolism of retinol to retinoic acid. J Biol Chem 274: 16796–16801, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Dennert G, Crowley C, Kouba J, Lotan R. Retinoic acid stimulation of the induction of mouse killer T-cells in allogeneic and syngeneic systems. J Natl Cancer Inst 62: 89–94, 1979. [PubMed] [Google Scholar]
- 40.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol 8: 1086–1094, 2007. [DOI] [PubMed] [Google Scholar]
- 41.DeNucci CC, Pagan AJ, Mitchell JS, Shimizu Y. Control of alpha4beta7 integrin expression and CD4 T cell homing by the beta1 integrin subunit. J Immunol 184: 2458–2467, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Devaux Y, Grosjean S, Seguin C, David C, Dousset B, Zannad F, Meistelman C, De Talance N, Mertes PM, Ungureanu-Longrois D. Retinoic acid and host-pathogen interactions: effects on inducible nitric oxide synthase in vivo. Am J Physiol Endocrinol Metab 279: E1045–E1053, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Dockham PA, Lee MO, Sladek NE. Identification of human liver aldehyde dehydrogenases that catalyze the oxidation of aldophosphamide and retinaldehyde. Biochem Pharmacol 43: 2453–2469, 1992. [DOI] [PubMed] [Google Scholar]
- 44.Dolle P, Ruberte E, Kastner P, Petkovich M, Stoner CM, Gudas LJ, Chambon P. Differential expression of genes encoding alpha, beta and gamma retinoic acid receptors and CRABP in the developing limbs of the mouse. Nature 342: 702–705, 1989. [DOI] [PubMed] [Google Scholar]
- 45.Dong M, Blobe GC. Role of transforming growth factor-beta in hematologic malignancies. Blood 107: 4589–4596, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dupe V, Matt N, Garnier JM, Chambon P, Mark M, Ghyselinck NB. A newborn lethal defect due to inactivation of retinaldehyde dehydrogenase type 3 is prevented by maternal retinoic acid treatment. Proc Natl Acad Sci USA 100: 14036–14041, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edele F, Molenaar R, Gutle D, Dudda JC, Jakob T, Homey B, Mebius R, Hornef M, Martin SF. Cutting edge: instructive role of peripheral tissue cells in the imprinting of T cell homing receptor patterns. J Immunol 181: 3745–3749, 2008. [DOI] [PubMed] [Google Scholar]
- 48.Eduardo Villablanca SW, de Calisto J, Gomes DCO, Kane MA, Napoli JL, Blaner WS, Kagechika H, Blomhoff R, Rosemblatt M, Bono MR, von Andrian UH, Mora JR. MyD88 and retinoic acid signaling pathways interact to modulate gastrointestinal activities of dendritic cells. Gastroenterology 141: 176–185, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El Bushra HE, Ash LR, Coulson AH, Neumann CG. Interrelationship between diarrhea and vitamin A deficiency: is vitamin A deficiency a risk factor for diarrhea? Pediatr Infect Dis J 11: 380–384, 1992. [DOI] [PubMed] [Google Scholar]
- 50.Elgueta R, Sepulveda FE, Vilches F, Vargas L, Mora JR, Bono MR, Rosemblatt M. Imprinting of CCR9 on CD4 T cells requires IL-4 signaling on mesenteric lymph node dendritic cells. J Immunol 180: 6501–6507, 2008. [DOI] [PubMed] [Google Scholar]
- 51.Elias KM, Laurence A, Davidson TS, Stephens G, Kanno Y, Shevach EM, O'Shea JJ. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood 111: 1013–1020, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fairfield KM, Fletcher RH. Vitamins for chronic disease prevention in adults: scientific review. JAMA 287: 3116–3126, 2002. [DOI] [PubMed] [Google Scholar]
- 53.Fleissner D, Hansen W, Geffers R, Buer J, Westendorf AM. Local induction of immunosuppressive CD8+ T cells in the gut-associated lymphoid tissues. PLos One 5: e15373, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frieden TR, Sowell AL, Henning KJ, Huff DL, Gunn RA. Vitamin A levels and severity of measles. Am J Dis Child 146: 182–186, 1992. [DOI] [PubMed] [Google Scholar]
- 55.Frota-Ruchon A, Marcinkiewicz M, Bhat PV. Localization of retinal dehydrogenase type 1 in the stomach and intestine. Cell Tissue Res 302: 397–400, 2000. [DOI] [PubMed] [Google Scholar]
- 56.Furugaki K, Pokorna K, Le Pogam C, Aoki M, Reboul M, Bajzik V, Krief P, Janin A, Noguera ME, West R, Charron D, Chomienne C, Pla M, Moins-Teisserenc H, Padua RA. DNA vaccination with all-trans retinoic acid treatment induces long-term survival and elicits specific immune responses requiring CD4+ and CD8+ T-cell activation in an acute promyelocytic leukemia mouse model. Blood 115: 653–656, 2010. [DOI] [PubMed] [Google Scholar]
- 57.Germain P, Iyer J, Zechel C, Gronemeyer H. Co-regulator recruitment and the mechanism of retinoic acid receptor synergy. Nature 415: 187–192, 2002. [DOI] [PubMed] [Google Scholar]
- 58.Ghyselinck NB, Wendling O, Messaddeq N, Dierich A, Lampron C, Decimo D, Viville S, Chambon P, Mark M. Contribution of retinoic acid receptor beta isoforms to the formation of the conotruncal septum of the embryonic heart. Dev Biol 198: 303–318, 1998. [PubMed] [Google Scholar]
- 59.Golovina TN, Mikheeva T, Brusko TM, Blazar BR, Bluestone JA, Riley JL. Retinoic acid and rapamycin differentially affect and synergistically promote the ex vivo expansion of natural human T regulatory cells. PLos One 6: e15868, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gomaa MS, Bridgens CE, Aboraia AS, Veal GJ, Redfern CP, Brancale A, Armstrong JL, Simons C. Small molecule inhibitors of retinoic acid 4-hydroxylase (CYP26): synthesis and biological evaluation of imidazole methyl 3-(4-(aryl-2-ylamino)phenyl)propanoates. J Med Chem 54: 2778–2791, 2011. [DOI] [PubMed] [Google Scholar]
- 61.Goswami S, Angkasekwinai P, Shan M, Greenlee KJ, Barranco WT, Polikepahad S, Seryshev A, Song LZ, Redding D, Singh B, Sur S, Woodruff P, Dong C, Corry DB, Kheradmand F. Divergent functions for airway epithelial matrix metalloproteinase 7 and retinoic acid in experimental asthma. Nat Immunol 10: 496–503, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Green HN, Mellanby E. Vitamin A as an anti-infective agent. Br Med J 2: 691–696, 1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Green HN, Pindar D, Davis G, Mellanby E. Diet as a prophylactic agent against puerperal sepsis. Br Med J 2: 595–598, 1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Greenberg BL, Semba RD, Vink PE, Farley JJ, Sivapalasingam M, Steketee RW, Thea DM, Schoenbaum EE. Vitamin A deficiency and maternal-infant transmissions of HIV in two metropolitan areas in the United States. AIDS 11: 325–332, 1997. [DOI] [PubMed] [Google Scholar]
- 65.Greenfield NJ, Pietruszko R. Two aldehyde dehydrogenases from human liver. Isolation via affinity chromatography and characterization of the isozymes. Biochim Biophys Acta 483: 35–45, 1977. [DOI] [PubMed] [Google Scholar]
- 66.Gruson D, Hilbert G, Boiron JM, Portel L, Cony-Makoul P, Reiffers J, Gbikpi-Benissan G, Cardinaud JP. Acute respiratory distress syndrome due to all-trans retinoic acid. Intensive Care Med 24: 642, 1998. [DOI] [PubMed] [Google Scholar]
- 67.Guilliams M, Crozat K, Henri S, Tamoutounour S, Grenot P, Devilard E, de Bovis B, Alexopoulou L, Dalod M, Malissen B. Skin-draining lymph nodes contain dermis-derived CD103(-) dendritic cells that constitutively produce retinoic acid and induce Foxp3(+) regulatory T cells. Blood 115: 1958–1968, 2010. [DOI] [PubMed] [Google Scholar]
- 68.Guo Y, Lee YC, Brown C, Zhang W, Usherwood E, Noelle RJ. Dissecting the role of retinoic Acid receptor isoforms in the CD8 response to infection. J Immunol 192: 3336–3344, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guo Y, Pino-Lagos K, Ahonen CA, Bennett KA, Wang J, Napoli JL, Blomhoff R, Sockanathan S, Chandraratna RA, Dmitrovsky E, Turk MJ, Noelle RJ. A retinoic acid-rich tumor microenvironment provides clonal survival cues for tumor-specific CD8+ T cells. Cancer Res 72: 5230–5239, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hall JA, Cannons JL, Grainger JR, Dos Santos LM, Hand TW, Naik S, Wohlfert EA, Chou DB, Oldenhove G, Robinson M, Grigg ME, Kastenmayer R, Schwartzberg PL, Belkaid Y. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity 34: 435–447, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hamann A, Andrew DP, Jablonski-Westrich D, Holzmann B, Butcher EC. Role of alpha 4-integrins in lymphocyte homing to mucosal tissues in vivo. J Immunol 152: 3282–3293, 1994. [PubMed] [Google Scholar]
- 72.Hammerschmidt SI, Ahrendt M, Bode U, Wahl B, Kremmer E, Forster R, Pabst O. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J Exp Med 205: 2483–2490, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hammerschmidt SI, Friedrichsen M, Boelter J, Lyszkiewicz M, Kremmer E, Pabst O, Forster R. Retinoic acid induces homing of protective T and B cells to the gut after subcutaneous immunization in mice. J Clin Invest 121: 3051–3061, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hatake K, Uwai M, Ohtsuki T, Tomizuka H, Izumi T, Yoshida M, Miura Y. Rare but important adverse effects of all-trans retinoic acid in acute promyelocytic leukemia and their management. Int J Hematol 66: 13–19, 1997. [DOI] [PubMed] [Google Scholar]
- 75.Hida T, Shikata K, Tokuhara N, Ishibashi A, Nagai M, Yamauchi T, Kobayashi S. Immunosuppressive effect of ER-38925, a retinoic acid receptor subtype alpha-selective agonist, in mouse models of human graft-vs-host disease. Drug Discov Ther 2: 35–44, 2008. [PubMed] [Google Scholar]
- 76.Hill JA, Hall JA, Sun CM, Cai Q, Ghyselinck N, Chambon P, Belkaid Y, Mathis D, Benoist C. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi cells. Immunity 29: 758–770, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang Y, Park Y, Wang-Zhu Y, Larange A, Arens R, Bernardo I, Olivares-Villagomez D, Herndler-Brandstetter D, Abraham N, Grubeck-Loebenstein B, Schoenberger SP, Van Kaer L, Kronenberg M, Teitell MA, Cheroutre H. Mucosal memory CD8(+) T cells are selected in the periphery by an MHC class I molecule. Nat Immunol 12: 1086–1095, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hussey GD, Klein M. A randomized, controlled trial of vitamin A in children with severe measles. N Engl J Med 323: 160–164, 1990. [DOI] [PubMed] [Google Scholar]
- 79.Iliev ID, Mileti E, Matteoli G, Chieppa M, Rescigno M. Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning. Mucosal Immunol 2: 340–350, 2009. [DOI] [PubMed] [Google Scholar]
- 80.Iliev ID, Spadoni I, Mileti E, Matteoli G, Sonzogni A, Sampietro GM, Foschi D, Caprioli F, Viale G, Rescigno M. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut 58: 1481–1489, 2009. [DOI] [PubMed] [Google Scholar]
- 81.Imdad A, Herzer K, Mayo-Wilson E, Yakoob MY, Bhutta ZA. Vitamin A supplementation for preventing morbidity and mortality in children from 6 months to 5 years of age. Cochrane Database Syst Rev CD008524, 2010. [DOI] [PubMed] [Google Scholar]
- 82.Ivan Dzhagalov PC, He Y-W. Regulation of CD8 T lymphocyte effector function and macrophage inflammatory cytokine production by retinoic acid receptor. J Immunol 178: 2113–2121, 2007. [DOI] [PubMed] [Google Scholar]
- 83.Iwata M, Eshima Y, Kagechika H. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. Int Immunol 15: 1017–1025, 2003. [DOI] [PubMed] [Google Scholar]
- 84.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity 21: 527–538, 2004. [DOI] [PubMed] [Google Scholar]
- 85.Jaensson-Gyllenback E, Kotarsky K, Zapata F, Persson EK, Gundersen TE, Blomhoff R, Agace WW. Bile retinoids imprint intestinal CD103+ dendritic cells with the ability to generate gut-tropic T cells. Mucosal Immunol 4: 438–447, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jeker LT, Zhou X, Gershberg K, de Kouchkovsky D, Morar MM, Stadthagen G, Lund AH, Bluestone JA. MicroRNA 10a marks regulatory T cells. PLos One 7: e36684, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Joffre O, Santolaria T, Calise D, Al Saati T, Hudrisier D, Romagnoli P, van Meerwijk JP. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med 14: 88–92, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jorgensen MJ, Fisker AB, Sartono E, Andersen A, Erikstrup C, Lisse IM, Yazdanbakhsh M, Aaby P, Benn CS. The effect of at-birth vitamin A supplementation on differential leucocyte counts and in vitro cytokine production: an immunological study nested within a randomised trial in Guinea-Bissau. Br J Nutr 1–11, 2012. [DOI] [PubMed] [Google Scholar]
- 89.Kam RK, Shi W, Chan SO, Chen Y, Xu G, Lau CB, Fung KP, Chan WY, Zhao H. Dhrs3 protein attenuates retinoic acid signaling and is required for early embryonic patterning. J Biol Chem 288: 31477–31487, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kang SG, Park J, Cho JY, Ulrich B, Kim CH. Complementary roles of retinoic acid and TGF-beta1 in coordinated expression of mucosal integrins by T cells. Mucosal Immunol 4: 66–82, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kastner P, Krust A, Mendelsohn C, Garnier JM, Zelent A, Leroy P, Staub A, Chambon P. Murine isoforms of retinoic acid receptor gamma with specific patterns of expression. Proc Natl Acad Sci USA 87: 2700–2704, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kaufman DR, De Calisto J, Simmons NL, Cruz AN, Villablanca EJ, Mora JR, Barouch DH. Vitamin A deficiency impairs vaccine-elicited gastrointestinal immunity. J Immunol 187: 1877–1883, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science 315: 820–825, 2007. [DOI] [PubMed] [Google Scholar]
- 94.Kawazoe Y, Sugita S, Keino H, Yamada Y, Imai A, Horie S, Mochizuki M. Retinoic acid from retinal pigment epithelium induces T regulatory cells. Exp Eye Res 94: 32–40, 2012. [DOI] [PubMed] [Google Scholar]
- 95.Keino H, Watanabe T, Sato Y, Okada AA. Anti-inflammatory effect of retinoic acid on experimental autoimmune uveoretinitis. Br J Ophthalmol 94: 802–807, 2010. [DOI] [PubMed] [Google Scholar]
- 96.Keino H, Watanabe T, Sato Y, Okada AA. Oral administration of retinoic acid receptor-alpha/beta-specific ligand Am80 suppresses experimental autoimmune uveoretinitis. Invest Ophthalmol Visual Sci 52: 1548–1556, 2011. [DOI] [PubMed] [Google Scholar]
- 97.Kim BS, Kim IK, Park YJ, Kim YS, Kim YJ, Chang WS, Lee YS, Kweon MN, Chung Y, Kang CY. Conversion of Th2 memory cells into Foxp3+ regulatory T cells suppressing Th2-mediated allergic asthma. Proc Natl Acad Sci USA 107: 8742–8747, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kishi M, Yasuda H, Abe Y, Sasaki H, Shimizu M, Arai T, Okumachi Y, Moriyama H, Hara K, Yokono K, Nagata M. Regulatory CD8+ T cells induced by exposure to all-trans retinoic acid and TGF-beta suppress autoimmune diabetes. Biochem Biophys Res Commun 394: 228–232, 2010. [DOI] [PubMed] [Google Scholar]
- 99.Klemann C, Raveney BJ, Klemann AK, Ozawa T, von Horsten S, Shudo K, Oki S, Yamamura T. Synthetic retinoid AM80 inhibits Th17 cells and ameliorates experimental autoimmune encephalomyelitis. Am J Pathol 174: 2234–2245, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kunkel EJ, Campbell JJ, Haraldsen G, Pan J, Boisvert J, Roberts AI, Ebert EC, Vierra MA, Goodman SB, Genovese MC, Wardlaw AJ, Greenberg HB, Parker CM, Butcher EC, Andrew DP, Agace WW. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med 192: 761–768, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kurokawa R, DiRenzo J, Boehm M, Sugarman J, Gloss B, Rosenfeld MG, Heyman RA, Glass CK. Regulation of retinoid signalling by receptor polarity and allosteric control of ligand binding. Nature 371: 528–531, 1994. [DOI] [PubMed] [Google Scholar]
- 102.Kusmartsev S, Cheng F, Yu B, Nefedova Y, Sotomayor E, Lush RM, Gabrilovich DI. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res 63: 4441–4449, 2003. [PubMed] [Google Scholar]
- 103.Kwok SK, Park MK, Cho ML, Oh HJ, Park EM, Lee DG, Lee J, Kim HY, Park SH. Retinoic acid attenuates rheumatoid inflammation in mice. J Immunol 189: 1062–1071, 2012. [DOI] [PubMed] [Google Scholar]
- 104.Lampen A, Meyer S, Arnhold T, Nau H. Metabolism of vitamin A and its active metabolite all-trans-retinoic acid in small intestinal enterocytes. J Pharmacol Exp Ther 295: 979–985, 2000. [PubMed] [Google Scholar]
- 105.Lee SW, Park Y, Eun SY, Madireddi S, Cheroutre H, Croft M. Cutting edge: 4-1BB controls regulatory activity in dendritic cells through promoting optimal expression of retinal dehydrogenase. J Immunol 189: 2697–2701, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Leroy P, Krust A, Zelent A, Mendelsohn C, Garnier JM, Kastner P, Dierich A, Chambon P. Multiple isoforms of the mouse retinoic acid receptor alpha are generated by alternative splicing and differential induction by retinoic acid. EMBO J 10: 59–69, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lerret NM, Houlihan JL, Kheradmand T, Pothoven KL, Zhang ZJ, Luo X. Donor-specific CD8+ Foxp3+ T cells protect skin allografts and facilitate induction of conventional CD4+ Foxp3+ regulatory T cells. Am J Transplant 12: 2335–2347, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Livingston KA, Klasing KC. Retinyl palmitate does not have an adjuvant effect on the antibody response of chicks to keyhole limpet hemocyanin regardless of vitamin A status. Poultry Sci 90: 965–970, 2011. [DOI] [PubMed] [Google Scholar]
- 109.Lombardi V, Speak AO, Kerzerho J, Szely N, Akbari O. CD8alpha(+)beta(-) and CD8alpha(+)beta(+) plasmacytoid dendritic cells induce Foxp3(+) regulatory T cells and prevent the induction of airway hyper-reactivity. Mucosal Immunol 5: 432–443, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lotan R, Dennert G. Stimulatory effects of vitamin A analogs on induction of cell-mediated cytotoxicity in vivo. Cancer Res 39: 55–58, 1979. [PubMed] [Google Scholar]
- 111.Loudig O, Babichuk C, White J, Abu-Abed S, Mueller C, Petkovich M. Cytochrome P450RAI(CYP26) promoter: a distinct composite retinoic acid response element underlies the complex regulation of retinoic acid metabolism. Mol Endocrinol 14: 1483–1497, 2000. [DOI] [PubMed] [Google Scholar]
- 112.Loudig O, Maclean GA, Dore NL, Luu L, Petkovich M. Transcriptional co-operativity between distant retinoic acid response elements in regulation of Cyp26A1 inducibility. Biochem J 392: 241–248, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lovett-Racke AE, Racke MK. Retinoic acid promotes the development of Th2-like human myelin basic protein-reactive T cells. Cell Immunol 215: 54–60, 2002. [DOI] [PubMed] [Google Scholar]
- 114.Lu L, Ma J, Li Z, Lan Q, Chen M, Liu Y, Xia Z, Wang J, Han Y, Shi W, Quesniaux V, Ryffel B, Brand D, Li B, Liu Z, Zheng SG. All-trans retinoic acid promotes TGF-beta-induced Tregs via histone modification but not DNA demethylation on Foxp3 gene locus. PLos One 6: e24590, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lu L, Zhou X, Wang J, Zheng SG, Horwitz DA. Characterization of protective human CD4CD25 FOXP3 regulatory T cells generated with IL-2, TGF-beta and retinoic acid. PLos One 5: e15150, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ma Y, Chen Q, Ross AC. Retinoic acid and polyriboinosinic:polyribocytidylic acid stimulate robust anti-tetanus antibody production while differentially regulating type 1/type 2 cytokines and lymphocyte populations. J Immunol 174: 7961–7969, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.MacLean G, Abu-Abed S, Dolle P, Tahayato A, Chambon P, Petkovich M. Cloning of a novel retinoic-acid metabolizing cytochrome P450, Cyp26B1, and comparative expression analysis with Cyp26A1 during early murine development. Mech Dev 107: 195–201, 2001. [DOI] [PubMed] [Google Scholar]
- 118.Malkovsky M, Doré C, Hunt R, Pamler L, Chandler P, Medawar PB. Enhancement of specific antitumor immunity in mice fed a diet enriched in vitamin A acetate. Proc Natl Acad Sci USA 80: 6322–6326, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Manicassamy S, Ravindran R, Deng J, Oluoch H, Denning TL, Kasturi SP, Rosenthal KM, Evavold BD, Pulendran B. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat Med 15: 401–409, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Manicassamy S, Reizis B, Ravindran R, Nakaya H, Salazar-Gonzalez RM, Wang YC, Pulendran B. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science 329: 849–853, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Maret M, Ruffie C, Periquet B, Campo AM, Menevret M, Phelep A, Dziewiszek K, Druilhe A, Pretolani M. Liposomal retinoic acids modulate asthma manifestations in mice. J Nutr 137: 2730–2736, 2007. [DOI] [PubMed] [Google Scholar]
- 122.Markowitz LE, Nzilambi N, Driskell WJ, Sension MG, Rovira EZ, Nieburg P, Ryder RW. Vitamin A levels and mortality among hospitalized measles patients, Kinshasa, Zaire. J Tropical Pediatr 35: 109–112, 1989. [DOI] [PubMed] [Google Scholar]
- 123.Massacesi L, Castigli E, Vergelli M, Olivotto J, Abbamondi AL, Sarlo F, Amaducci L. Immunosuppressive activity of 13-cis-retinoic acid and prevention of experimental autoimmune encephalomyelitis in rats. J Clin Invest 88: 1331–1337, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Matheu V, Berggard K, Barrios Y, Barrios Y, Arnau MR, Zubeldia JM, Baeza ML, Back O, Issazadeh-Navikas S. Impact on allergic immune response after treatment with vitamin A. Nutr Metab 6: 44, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Matt N, Dupe V, Garnier JM, Dennefeld C, Chambon P, Mark M, Ghyselinck NB. Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development 132: 4789–4800, 2005. [DOI] [PubMed] [Google Scholar]
- 126.McPhillips DM, Kalin JR, Hill DL. The pharmacokinetics of all-trans-retinoic acid and N-(2-hydroxyethyl)retinamide in mice as determined with a sensitive and convenient procedure. Solid-phase extraction and reverse-phase high performance liquid chromatography. Drug Metab Dispos 15: 207–211, 1987. [PubMed] [Google Scholar]
- 127.Mellanby E. A British Medical Association lecture on diet and disease. Br Med J 1: 515–519, 1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mellanby E, Green HN. Vitamin A as an anti-infective agent: its use in the treatment of puerperal septigaemia. Br Med J 1: 984–986, 1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Menning A, Loddenkemper C, Westendorf AM, Szilagyi B, Buer J, Siewert C, Hamann A, Huehn J. Retinoic acid-induced gut tropism improves the protective capacity of Treg in acute but not in chronic gut inflammation. Eur J Immunol 40: 2539–2548, 2010. [DOI] [PubMed] [Google Scholar]
- 130.Mic FA, Haselbeck RJ, Cuenca AE, Duester G. Novel retinoic acid generating activities in the neural tube and heart identified by conditional rescue of Raldh2 null mutant mice. Development 129: 2271–2282, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Molenaar R, Greuter M, van der Marel AP, Roozendaal R, Martin SF, Edele F, Huehn J, Forster R, O'Toole T, Jansen W, Eestermans IL, Kraal G, Mebius RE. Lymph node stromal cells support dendritic cell-induced gut-homing of T cells. J Immunol 183: 6395–6402, 2009. [DOI] [PubMed] [Google Scholar]
- 132.Molenaar R, Knippenberg M, Goverse G, Olivier BJ, de Vos AF, O'Toole T, Mebius RE. Expression of retinaldehyde dehydrogenase enzymes in mucosal dendritic cells and gut-draining lymph node stromal cells is controlled by dietary vitamin A. J Immunol 186: 1934–1942, 2011. [DOI] [PubMed] [Google Scholar]
- 133.Molotkov A, Fan X, Deltour L, Foglio MH, Martras S, Farres J, Pares X, Duester G. Stimulation of retinoic acid production and growth by ubiquitously expressed alcohol dehydrogenase Adh3. Proc Natl Acad Sci USA 99: 5337–5342, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Molotkov A, Fan X, Duester G. Excessive vitamin A toxicity in mice genetically deficient in either alcohol dehydrogenase Adh1 or Adh3. Eur J Biochem 269: 2607–2612, 2002. [DOI] [PubMed] [Google Scholar]
- 135.Moore C, Fuentes C, Sauma D, Morales J, Bono MR, Rosemblatt M, Fierro JA. Retinoic acid generates regulatory T cells in experimental transplantation. Transplant Proc 43: 2334–2337, 2011. [DOI] [PubMed] [Google Scholar]
- 136.Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J Exp Med 201: 303–316, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mora JR, von Andrian UH. Role of retinoic acid in the imprinting of gut-homing IgA-secreting cells. Semin Immunol 21: 28–35, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y, Merad M. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 343: 1249288, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317: 256–260, 2007. [DOI] [PubMed] [Google Scholar]
- 140.Mucida D, Pino-Lagos K, Kim G, Nowak E, Benson MJ, Kronenberg M, Noelle RJ, Cheroutre H. Retinoic acid can directly promote TGF-beta-mediated Foxp3(+) Treg cell conversion of naive T cells. Immunity 30: 471–473, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nacul LC, Arthur P, Kirkwood BR, Morris SS, Cameiro AC, Benjamin AF. The impact of vitamin A supplementation given during a pneumonia episode on the subsequent morbidity of children. Tropical Med Int Health 3: 661–666, 1998. [DOI] [PubMed] [Google Scholar]
- 142.Nambu Y, Hayashi T, Jang KJ, Aoki K, Mano H, Nakano K, Osato M, Takahashi K, Itoh K, Teramukai S, Komori T, Fujita J, Ito Y, Shimizu A, Sugai M. In situ differentiation of CD8alphaalpha Tau cells from CD4 T cells in peripheral lymphoid tissues. Scientific Rep 2: 642, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Napoli JL. Effects of ethanol on physiological retinoic acid levels. IUBMB Life 63: 701–706, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Niederreither K, McCaffery P, Drager UC, Chambon P, Dolle P. Restricted expression and retinoic acid-induced downregulation of the retinaldehyde dehydrogenase type 2 (RALDH-2) gene during mouse development. Mech Dev 62: 67–78, 1997. [DOI] [PubMed] [Google Scholar]
- 145.Niederreither K, Vermot J, Le Roux I, Schuhbaur B, Chambon P, Dolle P. The regional pattern of retinoic acid synthesis by RALDH2 is essential for the development of posterior pharyngeal arches and the enteric nervous system. Development 130: 2525–2534, 2003. [DOI] [PubMed] [Google Scholar]
- 146.Nolting J, Daniel C, Reuter S, Stuelten C, Li P, Sucov H, Kim BG, Letterio JJ, Kretschmer K, Kim HJ, von Boehmer H. Retinoic acid can enhance conversion of naive into regulatory T cells independently of secreted cytokines. J Exp Med 206: 2131–2139, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nozaki Y, Tamaki C, Yamagata T, Sugiyama M, Ikoma S, Kinoshita K, Funauchi M. All-trans-retinoic acid suppresses interferon-gamma and tumor necrosis factor-alpha; a possible therapeutic agent for rheumatoid arthritis. Rheumatol Int 26: 810–817, 2006. [DOI] [PubMed] [Google Scholar]
- 148.Ohoka Y, Yokota A, Takeuchi H, Maeda N, Iwata M. Retinoic acid-induced CCR9 expression requires transient TCR stimulation and cooperativity between NFATc2 and the retinoic acid receptor/retinoid X receptor complex. J Immunol 186: 733–744, 2011. [DOI] [PubMed] [Google Scholar]
- 149.Ohyanagi N, Ishido M, Suzuki F, Kaneko K, Kubota T, Miyasaka N, Nanki T. Retinoid ameliorates experimental autoimmune myositis, with modulation of Th cell differentiation and antibody production in vivo. Arthritis Rheum 60: 3118–3127, 2009. [DOI] [PubMed] [Google Scholar]
- 150.Park MK, Jhun JY, Lee SY, Oh HJ, Park MJ, Byun JK, Yoon BY, Park EM, Lee DG, Kwok SK, Park SH, Kim HY, Cho ML. Retinal attenuates inflammatory arthritis by reciprocal regulation of IL-17-producing T cells and Foxp3(+) regulatory T cells and the inhibition of osteoclastogenesis. Immunol Lett 148: 59–68, 2012. [DOI] [PubMed] [Google Scholar]
- 151.Pino-Lagos K, Guo Y, Brown C, Alexander MP, Elgueta R, Bennett KA, De Vries V, Nowak E, Blomhoff R, Sockanathan S, Chandraratna RA, Dmitrovsky E, Noelle RJ. A retinoic acid-dependent checkpoint in the development of CD4+ T cell-mediated immunity. J Exp Med 208: 1767–1775, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pohl J, LaFace D, Sands JF. Transcription of retinoic acid receptor genes in transgenic mice increases CD8 T-cell subset. Mol Biol Rep 17: 135–142, 1993. [DOI] [PubMed] [Google Scholar]
- 153.Racke MK, Burnett D, Pak SH, Albert PS, Cannella B, Raine CS, McFarlin DE, Scott DE. Retinoid treatment of experimental allergic encephalomyelitis. IL-4 production correlates with improved disease course. J Immunol 154: 450–458, 1995. [PubMed] [Google Scholar]
- 154.Ross AC. Vitamin A deficiency and retinoid repletion regulate the antibody response to bacterial antigens and the maintenance of natural killer cells. Clin Immunol Immunopathol 80: S63–72, 1996. [DOI] [PubMed] [Google Scholar]
- 155.Ross AC, Chen Q, Ma Y. Vitamin A and retinoic acid in the regulation of B-cell development and antibody production. Vitam Horm 86: 103–126, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ruane D, Brane L, Reis BS, Cheong C, Poles J, Do Y, Zhu H, Velinzon K, Choi JH, Studt N, Mayer L, Lavelle EC, Steinman RM, Mucida D, Mehandru S. Lung dendritic cells induce migration of protective T cells to the gastrointestinal tract. J Exp Med 210: 1871–1888, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rudraraju R, Surman SL, Jones BG, Sealy R, Woodland DL, Hurwitz JL. Reduced frequencies and heightened CD103 expression among virus-induced CD8(+) T cells in the respiratory tract airways of vitamin A-deficient mice. Clin Vaccine Immunol 19: 757–765, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sandell LL, Sanderson BW, Moiseyev G, Johnson T, Mushegian A, Young K, Rey JP, Ma JX, Staehling-Hampton K, Trainor PA. RDH10 is essential for synthesis of embryonic retinoic acid and is required for limb, craniofacial, and organ development. Genes Dev 21: 1113–1124, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sartor RB. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nature Clin Pract Gastroenterol Hepatol 3: 390–407, 2006. [DOI] [PubMed] [Google Scholar]
- 160.Schlenner SM, Weigmann B, Ruan Q, Chen Y, von Boehmer H. Smad3 binding to the foxp3 enhancer is dispensable for the development of regulatory T cells with the exception of the gut. J Exp Med 209: 1529–1535, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell 129: 723–733, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Schuster GU, Kenyon NJ, Stephensen CB. Vitamin A deficiency decreases and high dietary vitamin A increases disease severity in the mouse model of asthma. J Immunol 180: 1834–1842, 2008. [DOI] [PubMed] [Google Scholar]
- 163.Seguin-Devaux C, Hanriot D, Dailloux M, Latger-Cannard V, Zannad F, Mertes PM, Longrois D, Devaux Y. Retinoic acid amplifies the host immune response to LPS through increased T lymphocytes number and LPS binding protein expression. Mol Cell Endocrinol 245: 67–76, 2005. [DOI] [PubMed] [Google Scholar]
- 164.Seino K, Yamauchi T, Shikata K, Kobayashi S, Nagai M, Taniguchi M, Fukao K. Prevention of acute and chronic allograft rejection by a novel retinoic acid receptor-alpha-selective agonist. Int Immunol 16: 665–673, 2004. [DOI] [PubMed] [Google Scholar]
- 165.Semba RD. Vitamin A as “anti-infective” therapy, 1920–1940. J Nutr 129: 783–791, 1999. [DOI] [PubMed] [Google Scholar]
- 166.Semba RD, Graham NM, Caiaffa WT, Margolick JB, Clement L, Vlahov D. Increased mortality associated with vitamin A deficiency during human immunodeficiency virus type 1 infection. Arch Intern Med 153: 2149–2154, 1993. [PubMed] [Google Scholar]
- 167.Semba RD, Miotti PG, Chiphangwi JD, Liomba G, Yang LP, Saah AJ, Dallabetta GA, Hoover DR. Infant mortality and maternal vitamin A deficiency during human immunodeficiency virus infection. Clin Infectious Dis 21: 966–972, 1995. [DOI] [PubMed] [Google Scholar]
- 168.Shi Q, Cao H, Liu J, Zhou X, Lan Q, Zheng S, Liu Z, Li Q, Fan H. CD4+ Foxp3+ regulatory T cells induced by TGF-beta, IL-2 and all-trans retinoic acid attenuate obliterative bronchiolitis in rat trachea transplantation. Int Immunopharmacol 11: 1887–1894, 2011. [DOI] [PubMed] [Google Scholar]
- 169.Shizuru JA, Gregory AK, Chao CT, Fathman CG. Islet allograft survival after a single course of treatment of recipient with antibody to L3T4. Science 237: 278–280, 1987. [DOI] [PubMed] [Google Scholar]
- 170.Smith KA, Hochweller K, Hammerling GJ, Boon L, MacDonald AS, Maizels RM. Chronic helminth infection promotes immune regulation in vivo through dominance of CD11cloCD103- dendritic cells. J Immunol 186: 7098–7109, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sobel ES, Brusko TM, Butfiloski EJ, Hou W, Li S, Cuda CM, Abid AN, Reeves WH, Morel L. Defective response of CD4(+) T cells to retinoic acid and TGFbeta in systemic lupus erythematosus. Arthritis Res Ther 13: R106, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sommer A, Tarwotjo I, Djunaedi E, West KP Jr., Loeden AA, Tilden R, Mele L. Impact of vitamin A supplementation on childhood mortality. A randomised controlled community trial. Lancet 1: 1169–1173, 1986. [DOI] [PubMed] [Google Scholar]
- 173.Sommer A, Tarwotjo I, Hussaini G, Susanto D. Increased mortality in children with mild vitamin A deficiency. Lancet 2: 585–588, 1983. [DOI] [PubMed] [Google Scholar]
- 174.Spiegl N, Didichenko S, McCaffery P, Langen H, Dahinden CA. Human basophils activated by mast cell-derived IL-3 express retinaldehyde dehydrogenase-II and produce the immunoregulatory mediator retinoic acid. Blood 112: 3762–3771, 2008. [DOI] [PubMed] [Google Scholar]
- 175.Stansfield SK, Pierre-Louis M, Lerebours G, Augustin A. Vitamin A supplementation and increased prevalence of childhood diarrhoea and acute respiratory infections. Lancet 342: 578–582, 1993. [DOI] [PubMed] [Google Scholar]
- 176.Stephensen CB, Jiang X, Freytag T. Vitamin A deficiency increases the in vivo development of IL-10-positive Th2 cells and decreases development of Th1 cells in mice. J Nutr 134: 2660–2666, 2004. [DOI] [PubMed] [Google Scholar]
- 177.Stephensen CB, Rasooly R, Jiang X, Ceddia MA, Weaver CT, Chandraratna RA, Bucy RP. Vitamin A enhances in vitro Th2 development via retinoid X receptor pathway. J Immunol 168: 4495–4503, 2002. [DOI] [PubMed] [Google Scholar]
- 178.Stock A, Booth S, Cerundolo V. Prostaglandin E2 suppresses the differentiation of retinoic acid-producing dendritic cells in mice and humans. J Exp Med 208: 761–773, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Stosic-Grujicic S, Cvjeticanin T, Stojanovic I. Retinoids differentially regulate the progression of autoimmune diabetes in three preclinical models in mice. Mol Immunol 47: 79–86, 2009. [DOI] [PubMed] [Google Scholar]
- 180.Sudfeld CR, Navar AM, Halsey NA. Effectiveness of measles vaccination and vitamin A treatment. Int J Epidemiol 39Suppl 1: i48–55, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med 204: 1775–1785, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Suzuki R, Shintani T, Sakuta H, Kato A, Ohkawara T, Osumi N, Noda M. Identification of RALDH-3, a novel retinaldehyde dehydrogenase, expressed in the ventral region of the retina. Mech Dev 98: 37–50, 2000. [DOI] [PubMed] [Google Scholar]
- 183.Svensson M, Johansson-Lindbom B, Zapata F, Jaensson E, Austenaa LM, Blomhoff R, Agace WW. Retinoic acid receptor signaling levels and antigen dose regulate gut homing receptor expression on CD8+ T cells. Mucosal Immunol 1: 38–48, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Szatmari I, Pap A, Ruhl R, Ma JX, Illarionov PA, Besra GS, Rajnavolgyi E, Dezso B, Nagy L. PPARgamma controls CD1d expression by turning on retinoic acid synthesis in developing human dendritic cells. J Exp Med 203: 2351–2362, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tahayato A, Dolle P, Petkovich M. Cyp26C1 encodes a novel retinoic acid-metabolizing enzyme expressed in the hindbrain, inner ear, first branchial arch and tooth buds during murine development. Gene Expr Patterns 3: 449–454, 2003. [DOI] [PubMed] [Google Scholar]
- 186.Taimi M, Helvig C, Wisniewski J, Ramshaw H, White J, Amad M, Korczak B, Petkovich M. A novel human cytochrome P450, CYP26C1, involved in metabolism of 9-cis and all-trans isomers of retinoic acid. J Biol Chem 279: 77–85, 2004. [DOI] [PubMed] [Google Scholar]
- 187.Takahashi H, Kanno T, Nakayamada S, Hirahara K, Sciume G, Muljo SA, Kuchen S, Casellas R, Wei L, Kanno Y, O'Shea JJ. TGF-beta and retinoic acid induce the microRNA miR-10a, which targets Bcl-6 and constrains the plasticity of helper T cells. Nat Immunol 13: 587–595, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Takeuchi H, Yokota A, Ohoka Y, Iwata M. Cyp26b1 regulates retinoic acid-dependent signals in T cells and its expression is inhibited by transforming growth factor-beta. PLos One 6: e16089, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tan X, Sande JL, Pufnock JS, Blattman JN, Greenberg PD. Retinoic acid as a vaccine adjuvant enhances CD8+ T cell response and mucosal protection from viral challenge. J Virol 85: 8316–8327, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tang G, Hu Y, Yin SA, Wang Y, Dallal GE, Grusak MA, Russell RM. beta-Carotene in Golden Rice is as good as beta-carotene in oil at providing vitamin A to children. Am J Clin Nutr 96: 658–664, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 191.Teicher BA. Malignant cells, directors of the malignant process: role of transforming growth factor-beta. Cancer Metastasis Rev 20: 133–143, 2001. [DOI] [PubMed] [Google Scholar]
- 192.Thomas S, Prabhu R, Balasubramanian KA. Retinoid metabolism in the rat small intestine. Br J Nutr 93: 59–63, 2005. [DOI] [PubMed] [Google Scholar]
- 193.Tian F, Lu Y, Manibusan A, Sellers A, Tran H, Sun Y, Phuong T, Barnett R, Hehli B, Song F, DeGuzman MJ, Ensari S, Pinkstaff JK, Sullivan LM, Biroc SL, Cho H, Schultz PG, DiJoseph J, Dougher M, Ma D, Dushin R, Leal M, Tchistiakova L, Feyfant E, Gerber HP, Sapra P. A general approach to site-specific antibody drug conjugates. Proc Natl Acad Sci USA 111: 1766–1771, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ting Feng YC, Katie Alexander Charles Elson O. Regulation of Toll-like Receptor 5 Gene Expression and Function on Mucosal Dendritic Cells. PLoS One 7: 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol 9: 194–202, 2008. [DOI] [PubMed] [Google Scholar]
- 196.Uematsu S, Fujimoto K, Jang MH, Yang BG, Jung YJ, Nishiyama M, Sato S, Tsujimura T, Yamamoto M, Yokota Y, Kiyono H, Miyasaka M, Ishii KJ, Akira S. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol 9: 769–776, 2008. [DOI] [PubMed] [Google Scholar]
- 197.Van de Pavert SA, Ferreira M, Domingues RG, Ribeiro H, Molenaar R, Moreira-Santos L, Almeida FF, Ibiza S, Barbosa I, Goverse G, Labao-Almeida C, Godinho-Silva C, Konijn T, Schooneman D, O'Toole T, Mizee MR, Habani Y, Haak E, Santori FR, Littman DR, Schulte-Merker S, Dzierzak E, Simas JP, Mebius RE, Veiga-Fernandes H. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature 508: 123–127, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Van YH, Lee WH, Ortiz S, Lee MH, Qin HJ, Liu CP. All-trans retinoic acid inhibits type 1 diabetes by T regulatory (Treg)-dependent suppression of interferon-gamma-producing T-cells without affecting Th17 cells. Diabetes 58: 146–155, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Vermeulen M, Le Pesteur F, Gagnerault MC, Mary JY, Sainteny F, Lepault F. Role of adhesion molecules in the homing and mobilization of murine hematopoietic stem and progenitor cells. Blood 92: 894–900, 1998. [PubMed] [Google Scholar]
- 200.Villamor E, Fawzi WW. Vitamin A supplementation: implications for morbidity and mortality in children. J Infect Dis 182Suppl 1: S122–133, 2000. [DOI] [PubMed] [Google Scholar]
- 201.Wakabayashi Y, Kawahara J, Iwasaki T, Usui M. Retinoic acid transport to lens epithelium in human aqueous humor. Jpn J Ophthalmol 38: 400–406, 1994. [PubMed] [Google Scholar]
- 202.Walsh PT, Taylor DK, Turka LA. Tregs and transplantation tolerance. J Clin Invest 114: 1398–1403, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wang C, Kang SG, HogenEsch H, Love PE, Kim CH. Retinoic acid determines the precise tissue tropism of inflammatory Th17 cells in the intestine. J Immunol 184: 5519–5526, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wang G, Zhong A, Wang S, Dong N, Sun Z, Xia J. Retinoic acid attenuates acute heart rejection by increasing regulatory T cell and repressing differentiation of Th17 cell in the presence of TGF-beta. Transplant Int 23: 986–997, 2010. [DOI] [PubMed] [Google Scholar]
- 205.Wang J, Huizinga TW, Toes RE. De novo generation and enhanced suppression of human CD4+CD25+ regulatory T cells by retinoic acid. J Immunol 183: 4119–4126, 2009. [DOI] [PubMed] [Google Scholar]
- 206.Wang S, Villablanca EJ, De Calisto J, Gomes DC, Nguyen DD, Mizoguchi E, Kagan JC, Reinecker HC, Hacohen N, Nagler C, Xavier RJ, Rossi-Bergmann B, Chen YB, Blomhoff R, Snapper SB, Mora JR. MyD88-dependent TLR1/2 signals educate dendritic cells with gut-specific imprinting properties. J Immunol 187: 141–150, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wang X, Hao J, Metzger DL, Ao Z, Meloche M, Verchere CB, Chen L, Ou D, Mui A, Warnock GL. B7–H4 pathway in islet transplantation and beta-cell replacement therapies. J Transplant 2011: 418902, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wang X, Penzes P, Napoli JL. Cloning of a cDNA encoding an aldehyde dehydrogenase and its expression in Escherichia coli. Recognition of retinal as substrate. J Biol Chem 271: 16288–16293, 1996. [DOI] [PubMed] [Google Scholar]
- 209.West KP Jr., Howard GR, Sommer A. Vitamin A and infection: public health implications. Annu Rev Nutr 9: 63–86, 1989. [DOI] [PubMed] [Google Scholar]
- 210.Westervelt P, Pollock JL, Oldfather KM, Walter MJ, Ma MK, Williams A, DiPersio JF, Ley TJ. Adaptive immunity cooperates with liposomal all-trans-retinoic acid (ATRA) to facilitate long-term molecular remissions in mice with acute promyelocytic leukemia. Proc Natl Acad Sci USA 99: 9468–9473, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.White JA, Beckett-Jones B, Guo YD, Dilworth FJ, Bonasoro J, Jones G, Petkovich M. cDNA cloning of human retinoic acid-metabolizing enzyme (hP450RAI) identifies a novel family of cytochromes P450. J Biol Chem 272: 18538–18541, 1997. [DOI] [PubMed] [Google Scholar]
- 212.White JA, Guo YD, Baetz K, Beckett-Jones B, Bonasoro J, Hsu KE, Dilworth FJ, Jones G, Petkovich M. Identification of the retinoic acid-inducible all-trans-retinoic acid 4-hydroxylase. J Biol Chem 271: 29922–29927, 1996. [DOI] [PubMed] [Google Scholar]
- 213.White JA, Ramshaw H, Taimi M, Stangle W, Zhang A, Everingham S, Creighton S, Tam SP, Jones G, Petkovich M. Identification of the human cytochrome P450, P450RAI-2, which is predominantly expressed in the adult cerebellum and is responsible for all-trans-retinoic acid metabolism. Proc Natl Acad Sci USA 97: 6403–6408, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol 25: 171–192, 2007. [DOI] [PubMed] [Google Scholar]
- 215.Wolf G. Multiple functions of vitamin A. Physiol Rev 64: 873–937, 1984. [DOI] [PubMed] [Google Scholar]
- 216.Xia G, He J, Leventhal JR. Ex vivo-expanded natural CD4+CD25+ regulatory T cells synergize with host T-cell depletion to promote long-term survival of allografts. Am J Transplant 8: 298–306, 2008. [DOI] [PubMed] [Google Scholar]
- 217.Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B, Kuchroo VK. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol 181: 2277–2284, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Xu L, Kitani A, Stuelten C, McGrady G, Fuss I, Strober W. Positive and negative transcriptional regulation of the Foxp3 gene is mediated by access and binding of the Smad3 protein to enhancer I. Immunity 33: 313–325, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yashiro K, Zhao X, Uehara M, Yamashita K, Nishijima M, Nishino J, Saijoh Y, Sakai Y, Hamada H. Regulation of retinoic acid distribution is required for proximodistal patterning and outgrowth of the developing mouse limb. Dev Cell 6: 411–422, 2004. [DOI] [PubMed] [Google Scholar]
- 220.Yulia Nefedova MF, Simon SX, Wang AA, Beg A, Gabrilovich DI. Mechanism of all-trans retinoic acid effect on tumor-associated myeloid-derived suppressor cells. Cancer Res 67: 11021–11028, 2007. [DOI] [PubMed] [Google Scholar]
- 221.Zaccone P, Burton OT, Gibbs SE, Miller N, Jones FM, Schramm G, Haas H, Doenhoff MJ, Dunne DW, Cooke TA. S. mansoni glycoprotein omega-1 induces Foxp3 expression in NOD mouse CD4(+) T cells. Eur J Immunol 41: 2709–2718, 2011. [DOI] [PubMed] [Google Scholar]
- 222.Zelent A, Krust A, Petkovich M, Kastner P, Chambon P. Cloning of murine alpha and beta retinoic acid receptors and a novel receptor gamma predominantly expressed in skin. Nature 339: 714–717, 1989. [DOI] [PubMed] [Google Scholar]
- 223.Zeng R, Oderup C, Yuan R, Lee M, Habtezion A, Hadeiba H, Butcher EC. Retinoic acid regulates the development of a gut-homing precursor for intestinal dendritic cells. Mucosal Immunol 6: 847–856, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhang XK, Lehmann J, Hoffmann B, Dawson MI, Cameron J, Graupner G, Hermann T, Tran P, Pfahl M. Homodimer formation of retinoid X receptor induced by 9-cis retinoic acid. Nature 358: 587–591, 1992. [DOI] [PubMed] [Google Scholar]
- 225.Zhao J, Lloyd CM, Noble A. Th17 responses in chronic allergic airway inflammation abrogate regulatory T-cell-mediated tolerance and contribute to airway remodeling. Mucosal Immunol 6: 335–346, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zhou R, Horai R, Mattapallil MJ, Caspi RR. A new look at immune privilege of the eye: dual role for the vision-related molecule retinoic acid. J Immunol 187: 4170–4177, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zhou R, Horai R, Silver PB, Mattapallil MJ, Zarate-Blades CR, Chong WP, Chen J, Rigden RC, Villasmil R, Caspi RR. The living eye “disarms” uncommitted autoreactive T cells by converting them to Foxp3(+) regulatory cells following local antigen recognition. J Immunol 188: 1742–1750, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zhou X, Kong N, Wang J, Fan H, Zou H, Horwitz D, Brand D, Liu Z, Zheng SG. Cutting edge: all-trans retinoic acid sustains the stability and function of natural regulatory T cells in an inflammatory milieu. J Immunol 185: 2675–2679, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]